1. Introduction
In patient with specific type of cancer, a life-threatening complication called Tumor lysis syndrome (TLS), can happen as a result of breakdown of tumor cells which may result in abrupt release of intracellular contents to the blood stream, due to patients’ exposure to cytotoxic treatment or spontaneous causes. Releasing intracellular contents may lead to series of electrolytes and other laboratory changes like hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia. Besides this, a serious consequence could be occurred due to these laboratory abnormalities such as cardiac arrhythmias, acute kidney injury, seizures and death if not treated immediately. Clinical significance of TLS occurs 12-72 hours after starting treatment with chemotherapy [
1,
2,
3,
4,
5].
Cairo and Bishop define laboratory TLS as the presence of two or more of the classic metabolic abnormalities in adult: uric acid 476 mmol/L (8 mg/dL) or 25% increase from baseline, Potassium 6.0 mmol/L or 25% increase from baseline, phosphorus 1.45 mmol/L (4.5 mg/dL) or 25% increase from baseline and calcium 1.75 mmol/L (7 mg/dL) or 25% decrease from baseline). While the clinical definition of TLS includes (1) Creatinine 1.5 Upper limit of normal (ULN) (age >12 years or age adjusted), (2) Cardiac arrhythmia/sudden death, (3) Seizure [
3]. Current treatment of TLS includes intensive hydration, forced diuresis, and use of allopurinol which is xanthine oxidase inhibitor that prevents formation of uric acid and/ or rasburicase which is a recombinant form of urate oxidase, catalyzes the conversion of uric acid to allantoin, allowing for greater urinary excretion. However, rasburicase doesn’t inhibit the formation of uric acid [
5].
Rasburicase was approved by US FDA for adults in 2009 which helped in the prevention and management of hyperuricemia of TLS in rapidly proliferating cancers. The approved dosage of Rasburicase is 0.2 mg/kg given intravenously once daily for 5 days to be administered within 4-24 hours of chemotherapy [
6]. Several published guidelines suggest using 0.2 mg/kg for 1-7 days [
7,
8,
9,
10,
11].
A retrospective single-center chart review study conducted by Khan MA et al. on 94 adult patients evaluated the effectiveness of a single 6-mg fixed dose of rasburicase in decreasing serum uric acid and creatinine levels for the prevention or management of hyperuricemia associated with TLS in adults with cancer. The study found that 79% (75) of the patients achieved normalization of serum uric acid levels (<7 mg/dL; median: 1.00 mg/dL) within 24 hours, and approximately 81% (77) achieved normalized levels (median: 1.96 mg/dL) within 48 hours. Furthermore, normal uric acid levels (median: 2.97 mg/dL) were maintained for up to 96 hours. The analysis also revealed significant cost savings of
$320,000 in the treatment of these patients using the single fixed dose compared to the multiple-day dosing recommended by the FDA, indicating both cost-effectiveness and clinical benefits [
12].
Marjoncu, D et al. carried out a multi-center, retrospective analysis of 79 adult cancer patients who were admitted to hospital and received rasburicase to test whether Rasburicase 3mg had similar rates of uric acid normalization (defined as uric acid 7.5mg/dL) within 24h in comparison to dose of 6 mg. The study results showed that while the baseline uric acid was lower in the 3 mg arm compared to the 6 mg arms, there was no difference in the uric acid normalization at 24h between the 3mg arm (95%) and 6mg arm (82%) (p=0.134). A cost-savings of over
$300,000 annually can be achieved with the proposed protocol. A single, fixed rasburicase dose of 3 mg was effective in treating hyperuricemia within 24hrs and is associated with remarkable cost-savings [
13].
Princess Nourah Oncology Center (PNOC) is one of largest tertiary care referral oncology facilities in the western province of Saudi Arabia and plays a crucial role in treating all kinds of cancer types which are generally associated with tumor lysis syndrome (TLS). Due to the Covid-19 pandemic, medical care had been negatively affected, including issues with pharmaceutical care and procurement, which had impacted the availability of rasburicase. To address this, PNOC implemented a policy to use a single fixed dose of 3 mg rasburicase for preventing or managing hyperuricemia in high-risk TLS patients during the COVID 19 pandemic.
To date a few studies investigated the effectiveness of a rasburicase single fixed dose 3 mg. Therefore, we planned this retrospective study to evaluate the effectiveness of single fixed dose of 3 mg rasburicase for prevention and management of hyperuricemia of tumor lysis syndrome in adult cancer patients, and to determine the cost-saving impact of use of a single fixed dose of 3 mg rasburicase.
Our primary objective was to assess the effectiveness of a single 3 mg-fixed dose of rasburicase to lower uric levels to 420 mmol/L (<7 mg/dL) from baseline to 24 hours and 48 hours after rasburicase administration. While our secondary objectives were to determine the effectiveness of a single 3-mg fixed dose of rasburicase to lower uric acid levels to <7 mg/dL at 72 hours and 96 hours after rasburicase administration; to lower serum creatinine level from baseline to 132 µmol/L (<1.5 mg/dL) at 96 hours after rasburicase administration; to determine the number of rasburicase doses that are required for the prevention or treatment of hyperuricemia associated with TLS and to determine the cost saving impact of use of single fixed dose of 3 mg rasburicase.
2. Materials and Methods
This is a retrospective, observational cohort single-center study which was conducted according to the guidelines of the Declaration of Helsinki and was approved by Institutional Review Board of King Abdullah International Medical Research Center, Saudi Arabia with IRB approval number IRB/1259/22 on July 18, 2022.
We examined the electronic medical records (EMR) at PNOC for eligible adult patients aged more than 14 years old (the Saudi healthcare system defines adults as aged more than 14 years) with confirmed diagnoses of any type of cancer and had received fixed dose 3 mg Rasburicase one or more doses for prevention or treatment of hyperuricemia between March 2020 to November 2022. Patients who didn’t have serum uric acid and serum creatinine levels measured before and after rasburicase administration or who received any different dosing of rasburicase, were excluded from the study. Patients were classified into two categories based on indication (treatment vs. prevention) considering UA level before rasburicase administration. Patients were included in treatment group if UA before rasburicase administration was more than 7 mg/dL. Similarly, patients with UA below 7 mg/dL before rasburicase administration having no other laboratory abnormalities were included in prevention group. Then each category was analyzed independently.
Demographic information, concurrent medications use, and laboratory parameters were collected for each patient. In addition, uric acid levels after rasburicase administration with repeated serum uric acid and serum creatinine levels recorded for up to 96 hours after rasburicase administration were collected. Our institution is using specific handling procedures for the measurement of serum uric acid after the administration of rasburicase as per our center’s existing TLS guidelines, because rasburicase causes enzymatic degradation of the uric acid in blood samples if left at room temperature, resulting in falsely low uric acid levels. Our TLS guidelines recommend accurate measurements of uric acid by collecting blood into prechilled tubes containing heparin anticoagulant, and then immediately immersing and maintaining in an ice-water bath; plasma samples are assayed within 4 hours of sample collection, as per our TLS guidelines and hence serum uric acid data collected retrospectively after rasburicase administration were reliable. Patients with serum uric acid level >7 mg/dL were defined as having hyperuricemia. Acute renal dysfunction was defined as Serum Creatinine > 1.5 mg/dL. As single fixed dose of 3 mg rasburicase is an off-label dosing, we compared the cost of single fixed dose of 3 mg rasburicase with FDA approved dosing of the rasburicase for the same indication i.e., 0.2 mg/Kg IV daily for 5 days using the actual weight of the patient included in the study to calculate the impact on the cost saving.
3. Data Collection and Analysis
There were several steps included in our study. We used a data collection sheet to record the variables and laboratory values for each patient as shown in
Table 1 and
Table 2. Recorded variables and laboratory values for each patient were patient demographics (age, sex, weight, height); type of cancer; medication (rasburicase, allopurinol); serum uric acid levels; serum creatinine levels; serum lactate dehydrogenase levels; serum potassium levels; serum phosphorus levels; and corrected serum calcium levels all at baseline, pre-rasburicase, and post-rasburicase at 24 hours, 48 hours, 72 hours, and 96 hours.
The patient charts in electronic medical record were accessed to obtain patient demographics, pertinent medication and laboratory values by using BestCare 2.0A which is our electronic hospital information system. Data was saved and analyzed by using Microsoft Office Excel 2010 protected by password key to ensure the confidentiality of data. Serum uric acid levels were assessed at baseline as pre-rasburicase administration, and at 24 to 96 hours post- rasburicase doses. Serum creatinine, serum potassium, serum phosphorus, corrected serum calcium, and serum lactate dehydrogenase were collected retrospectively, and the cost impact was calculated. The mean and standard deviations were reported for continuous variables; numbers and percentages were reported for nominal or categorical data.
4. Results
A total of 37 adult patients were included in this study which were divided into two groups prevention and treatment based on baseline serum uric acid at the time of rasburicase dose administration. We enrolled 17 (46%) patients in the treatment group and 20 (54%) patients in the prevention group. Baseline characteristics of the study population are described in
Table 1 and
Table 2 for treatment and prevention group respectively. While the laboratory parameters which are commonly influenced by TLS were described for study subjects in the
Table 3 and
Table 4 for treatment and prevention group respectively including serum uric acid, serum creatinine, serum potassium, serum lactate dehydrogenase, serum phosphorus, and corrected serum calcium.
4.1. Baseline Characteristics of the Study Population
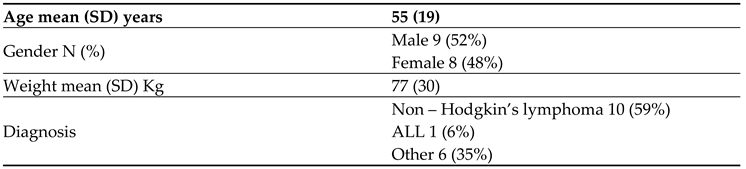
Table 1 represents the baseline characteristics of the treatment group (N=17). The mean age was 55 years (SD±19), with 52% males and 48% females. Non-Hodgkin’s lymphoma accounted for 59% of diagnoses, acute leukemia for 6%, and other solid tumors for 35%.
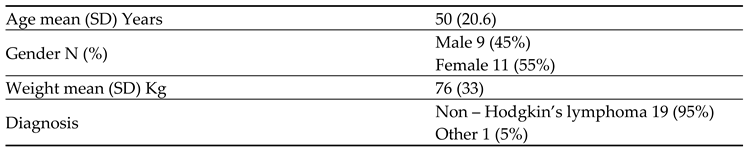
Table 2 displays the baseline characteristics of the prevention group (N=20). The mean age was 50 years (SD=20.6), with a gender distribution of 45% males and 55% females. Non-Hodgkin’s lymphoma constituted 95% of diagnoses, while other solid tumors made up the remaining 5%.
4.2. Baseline Laboratory Parameter
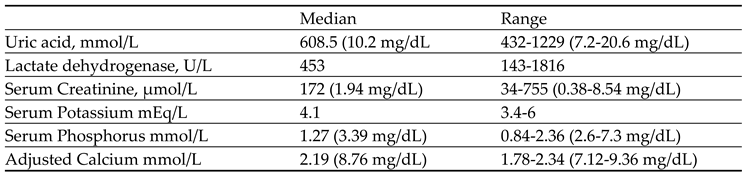
Table 3 showcases the baseline laboratory parameters of patients in the treatment group (N=17). Uric acid levels ranged from 7.2 mg/dL to 20.6 mg/dL (median:10.2 mg/dL), lactate dehydrogenase from 143 to 1816 U/L (median:453 U/L), serum creatinine from 0.38 to 8.54 mg/dL (median: 1.94 mg/dL), serum potassium from 3.4 to 6 mEq/L (median: 4.1 mEq/L), serum phosphorus from 2.6-7.3 mg/dL (median: 3.39 mg/dL), and adjusted calcium levels varied within a range of 7.12-9.36 mg/dL (median:8.76 mg/dL).
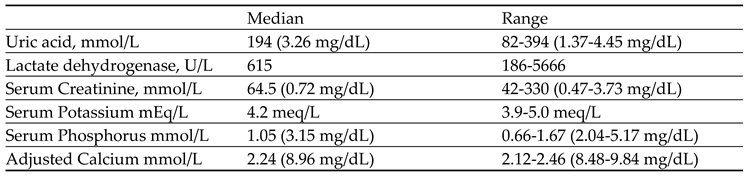
Table 4 presents the baseline laboratory parameters of patients in the prevention group (N=20). Uric acid levels ranged from 1.37 to 4.45 mg/dL (median; 3.26 mg/dL), lactate dehydrogenase from 186 to 5666 U/L (median: 615 U/L), serum creatinine from 0.47 to 3.73 mg/dL (median:0.72 mg/dL), serum potassium from 3.9 to 5 mEq/L (median: 4.2 mEq/L), serum phosphorus from 2.04 to 5.17 mg/dL (median:3.15 mg/dL), and adjusted calcium levels varied within a range of 8.48-9.84 mg/dL (median: 8.96 mg/dL).
All patients had received a 3 mg-fixed dose of rasburicase. We recorded all serum UA levels that were obtained pre and post rasburicase for upto 96 hours. At 24 hours from receiving the rasburicase dose 15 (88%) patients achieved normal UA level (< 7 mg/dL) in treatment group (
Table 5). The rest of study subjects in the treatment group achieved normal UA level within 72 hours. Whereas all 20 (100 %) patients maintained normal UA (< 7 mg/dL) level in prevention group during 96 hours (
Table 7). Normalization of serum creatinine below 1.5 mg/dL was achieved in 90% patients in the prevention group with a median of 0.62 mg/dL (range 0.39-3.29 mg/dL) and in 65% of the patients in the treatment group with a median of 0.97 mg/dL (range 0.39-3.72 mg/dL) at 96 hours after rasburicase administration. Sixteen (94 %) patients required single 3 mg-fixed dose of rasburicase in the treatment group to achieve normal UA (< 7 mg/dL) whereas 1 (6%) patient required 3 doses of 3 mg fixed dose of rasburicase in the treatment group (
Table 6). While in prevention group, 17 (85%) patients required single 3-mg fixed dose of rasburicase, 2 (10%) patients required 2 doses of 3-mg fixed dose of rasburicase and only 1 (5%) patient required 3 doses of 3-mg fixed dose of rasburicase to maintain normal UA of < 7 mg/dL (
Table 8).
4.3. Subgroup Analysis
Patients with serum uric acid higher than 7 mg/dL during the 96-hour period after receiving the first dose of rasburicase were considered appropriate candidates for a repeated dose of rasburicase. We found that a repeated dose of rasburicase used only in one patient in the treatment group was appropriate. However, 3 patients (15%) in the prevention group, received a repeated dose of rasburicase unnecessarily, because their serum uric acid was lower than the normal limit of the institution for 96 hours. Overall, approximately 4 out of 37 patients (10.8%) received a repeated 3-mg dose of rasburicase, but based on our findings, this was appropriate in only 1 of 4 patients (25%), meaning that it was unnecessary in approximately 75% (3) of these patients.
4.4. Cost Saving Benefit
As single fixed dose of 3 mg rasburicase is an off-label dosing, we compared the cost of single fixed dose of 3 mg rasburicase with FDA approved dosing of the rasburicase for the same indication i.e., 0.2 mg/Kg IV daily for 5 days using the actual weight of the patient included in the study to calculate the impact on the cost saving. Saudi food and drug authority (SFDA) cost of the rasburicase is 979.75 SAR (261.26 USD) per 1.5 mg vial and cost of institutional recommended dose during COVID-19 pandemic, 3 mg was 979.75 X 2 vials = SAR 1959.5 SAR (522.53 USD). Whereas the cost of the rasburicase therapy as per US FDA recommended dose/schedule is much higher. The median weight in our study was 77 Kg. Using US FDA recommended dose of 0.2 mg/Kg for 5 days would require 50 vials per patient (77 kg X 0.2 mg = 15.4 mg per day which was rounded to 10 vials X 5 days = 50 vials). The cost of the 50 vials of the rasburicase is 50X 979.75 = 48,987.5 SAR (13,063.33 USD) that means we potentially saved 48,987.5 – 1959.5 = 47,028 SAR (12,540.8 USD) per patient. Cost saving by using single 3 mg-fixed dose of rasburicase in 37 patients was 1,740,036 SAR (464,009 USD).
5. Discussion
Tumor lysis syndrome (TLS) is a fatal complication of hematologic malignancies and some solid tumors. TLS can occur after tumor cells break down spontaneously or after exposure to radiation or chemotherapy [
1]. Rasburicase is recommended for the management or prevention of hyperuricemia in patients at high risk for TLS as it significantly decreases uric acid levels in patients with hyperuricemia related to TLS [
4,
9]. Allopurinol, a xanthine oxidase inhibitor is an alternative treatment option for the management or prevention of hyperuricemia associated with TLS; but, this medication is mainly used for cancer patients at low- or intermediate-risk for TLS [
14]. Screening patients before rasburicase administration for glucose-6-phosphate dehydrogenase deficiency (G6PD) is very important. Deficiency of G6PD is a contraindication for use of rasburicase use, because patients may have severe complication of severe hemolytic anemia, within 2 to 4 days after rasburicase initiation in a patient with G6PD deficiency [
15]. US FDA had initially approved rasburicase in 2002 to prevent or treat the hyperuricemia of TLS in children receiving chemotherapy who are at high risk for TLS.3,6 Later on, FDA granted approval for use of rasburicase in the initial management of TLS in adults6, based on a randomized phase 3 study of patients with hematologic malignancies at risk for hyperuricemia and TLS [
16]. FDA recommended dose of rasburicase in adult cancer patients receiving chemotherapy, for prevention or management of hyperuricemia associated with TLS is 0.2 mg/kg administered intravenously once daily for ≤5 days [
6]. Many guidelines recommend the use of 0.2 mg/kg for 1 to 7 days [
9,
14,
17], but many published studies have reported efficacy and cost-savings with the use of rasburicase at lower doses of 3-7.5 mg single dose [
18,
19,
20].
In our study, we found that median daily serum uric acid and serum creatinine levels remained stable at low levels over a period of 24 to 96 hours. The study findings indicate that nearly 88% of patients in the treatment group achieved normalization of uric acid within 24 hours, with all reaching normal levels within 72 hours with sustained reduction until the 96-hour mark in both treatment and prevention groups (
Table 5 and
Table 7). Patients who received a single fixed dose of 3 mg should continue to be monitored for tumor lysis syndrome (TLS) and have their uric acid levels checked daily up to 96 hours to determine the need for additional doses. If the serum uric acid level remains above 7 mg/dL during this period, a repeated dose should be considered.
Overall, approximately 4 out of 37 patients (10.8%) received a repeated 3-mg dose of rasburicase, but based on our findings, this was appropriate in only 1 of 4 patients (25%), meaning that it was unnecessary in approximately 75% (3) of these patients. Additionally, our study revealed significant cost-savings associated with using a 3 mg single fixed dose of rasburicase compared to the FDA-recommended multiple-day dosing regimen.
The results of this study were presented as posters at two international meetings: the Hematology Oncology Pharmacy Assembly Meeting in Phoenix, USA (March 28 - April 1, 2023) and the 3rd Saudi Society of Clinical Pharmacy Meeting in Riyadh, Saudi Arabia (September 7-9, 2023). An oral presentation was also delivered at a local meeting “Saudi Pharmacy Resident Day” organized by the Saudi Commission for Health Specialists in August 2023. The results were shared with our oncology department as well as oncologists and hematologists nationwide, leading to a change in practice where rasburicase single fixed dose (3 mg) is now being used specifically for preventing hyperuricemia associated with TLS in lymphoma patients. This study has a significant impact on clinical practice across the country.
Limitations
One limitation of this study was its small sample size and being conducted at a single center.
6. Conclusions
In conclusion, our study demonstrated that a single fixed dose of 3 mg rasburicase was effective in preventing or managing hyperuricemia in high-risk patients and maintaining reduced uric acid levels for up to 96 hours. The use of a fixed 3-mg dose of rasburicase is also deemed to be cost-effective. Based on the data provided, we recommend implementing the single 3-mg fixed-dose strategy for the prevention or management of hyperuricemia associated with TLS in our institution and others. It is advised that patients who receive a single 3-mg fixed dose of rasburicase should undergo continued TLS monitoring and have their uric acid levels checked daily for up to 96 hours to assess whether repeated doses of rasburicase are necessary.
Author Contributions
SB: concept, design, literature search, data acquisition, data analysis, manuscript preparation, manuscript editing and review; MAK: concept, design, literature search, clinical studies, data acquisition, data analysis, statistical analysis, manuscript preparation, manuscript editing and review, MAS: concept, design, manuscript editing and review, RM: concept, manuscript editing and review, ANA: concept, design, manuscript editing and review, MM: concept, design, manuscript editing and review, AA: concept, design, manuscript editing and review, MA: manuscript editing and review, MA: manuscript editing and review.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by Institutional Review Board of King Abdullah International Medical Research Center, Saudi Arabia with IRB approval number IRB/1259/22 on July 18, 2022.
Conflicts of Interest
Sabirin Bakhsh, Mansoor Ahmed Khan, Majed Alshamrani, Roula Mufti, Anjum Naeem Ansari, Mubarak Al-Mansour, Ahmad Alsaeed, Mousa Alahmari and Mohammed Aseeri, have no conflicts of interest to report.
References
- Howard, S.C.; Jones, D.P.; Pui, C.H. The tumor lysis syndrome. N Engl J Med 2011, 364, 1844. [Google Scholar] [CrossRef] [PubMed]
- Bishop, M.R.; Cairo, M.S.; Coccia, P.F. Tumor lysis syndrome. In: Abeloff MD, ed. Clinical Oncology. 3rd ed. Orlando, Fl: Churchill Livingstone; 2004, 50.
- Cairo, M.S.; Bishop, M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol 2004, 127, 3. [Google Scholar] [CrossRef] [PubMed]
- Abu-Alfa, A.K.; Younes, A. Tumor lysis syndrome and acute kidney injury: evaluation, prevention, and management. Am J Kidney Dis 2010, 55, S1–S13. [Google Scholar] [CrossRef] [PubMed]
- Alakel, N.; Middeke, J.M.; Schetelig, J.; Bornhauser, M. Prevention and treatment of tumor lysis syndrome, and the efficacy and role of rasburicase. OncoTargets and therapy 2017, 10, 597. [Google Scholar] [CrossRef] [PubMed]
- Elitek (rasburicase) for injection for intravenous use [prescribing information]. Bridgewater, NJ: Sanofi-Aventis; September 2017.
- MCCN Tumor Lysis Syndrome Guidelines 2010 by NHS Cancer Network.
- Cairo MS, ASCO 2008 guidelines for the management of Pediatric and Adult tumor lysis syndrome.
- Cairo, M.S.; Coiffier, B.; Reiter, A.; Younes, A. and on behalf of the TLS Expert Panel. Recommendations for the evaluation of risk and prophylaxis of Tumor Lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. British Journal of Haematology 2010, 149, 578–586. [Google Scholar] [PubMed]
- Rasburicase . Lexi-Comp [version 1.5.0 (112)] Lexi-Comp, Inc, Hudson, OH Accessed March 10, 2013.
- Rasburicase. Drugdex Evaluation. Thomason Micromedex. Greenwood Village, CO. Accessed March 10, 2013.
- Khan, M.A.; Alshamrani, M.A.; Aseeri, M.A.; Al Saeed, A.S.; Alhamdan, H.S.; Masari, A.O. Effectiveness of a Single 6-mg Fixed Dose of Rasburicase for Prevention or Management of Hyperuricemia Associated with Tumor Lysis Syndrome in Adults with Cancer. Journal of Hematology Oncology Pharmacy 2019, 9. [Google Scholar]
- Marjoncu, D.; Holman, K. The efficacy and cost-impact of rasburicase 3 mg versus 6 mg for the management of tumor lysis syndrome: A multicenter analysis. Journal of Oncology Pharmacy 2022. [CrossRef] [PubMed]
- Cortes, J.; Seiter, K.; Maziarz, R.T., et al. Superiority of rasburicase versus allopurinol on serum uric acid control in adult patients with hematological malignancies at risk of developing tumor lysis syndrome: results of a randomized comparative phase III study. Blood (ASH Annual Meeting Abstracts). 2008, 112. Abstract 919. [CrossRef]
- Sonbol, M.B.; Yadav, H.; Vaidya, R., et al. Methemoglobinemia and hemolysis in a patient with G6PD deficiency treated with rasburicase. Am J Hematol. 2013, 88, 152–154. [CrossRef] [PubMed]
- PRNewswire. FDA approved Elitek (rasburicase) for management of plasma uric acid levels in adults with leukemia, lymphoma, solid tumors receiving anti-cancer therapy. October 16, 2009. www.news.sanofi.us/press-releases? item=118501. Accessed November 29, 2018.
- Coiffier, B.; Altman, A.; Pui, C.H., et al. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol. 2008, 26, 2767-2778. Erratum in: J Clin Oncol. 2010, 28, 708. [CrossRef] [PubMed]
- Coutsouvelis, J.; Wiseman, M.; Hui, L.; Poole, S.; Dooley, M.; Patil, S.; Avery, S.; Wei, A.; Spencer, A. Effectiveness of a single fixed dose of rasburicase 3 mg in the management of tumour lysis syndrome. Br J Clin Pharmacol. 2013, 75, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Marjoncu, D.; Holman, K. The efficacy and cost-impact of rasburicase 3 mg versus 6 mg for the management of tumor lysis syndrome: A multicenter analysis. J Oncol Pharm Pract. 2023, 29, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Liu, L.; Nie, X.; Li, J.; Zhang, J.; Zhao, L.; Wang, X. The optimal single-dose regimen of rasburicase for management of tumour lysis syndrome in children and adults: a systematic review and meta-analysis. J Clin Pharm Ther 2017, 42, 18–26. [Google Scholar] [CrossRef] [PubMed]
Table 1.
Baseline characteristic of treatment group (N=17).
Table 1.
Baseline characteristic of treatment group (N=17).
Table 2.
Baseline characteristic of prevention group (N=20).
Table 2.
Baseline characteristic of prevention group (N=20).
Table 3.
Baseline Laboratory parameter of patients in treatment group (N=17).
Table 3.
Baseline Laboratory parameter of patients in treatment group (N=17).
Table 4.
Baseline Laboratory parameter of patients in prevention group N=20.
Table 4.
Baseline Laboratory parameter of patients in prevention group N=20.
Table 5.
Patient achieved normal uric acid level in treatment group.
Table 5.
Patient achieved normal uric acid level in treatment group.
Table 6.
Total number of Rasburicase 3 mg fixed dose/s to lower uric acid in the treatment group.
Table 6.
Total number of Rasburicase 3 mg fixed dose/s to lower uric acid in the treatment group.
Table 7.
Patient achieved normal uric acid level in prevention group.
Table 7.
Patient achieved normal uric acid level in prevention group.
Table 8.
Total number of Rasburicase 3 mg fixed dose/s to lower uric acid in the prevention group.
Table 8.
Total number of Rasburicase 3 mg fixed dose/s to lower uric acid in the prevention group.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).