1. Introduction
Soil salinity is one of the key factors limiting global food production, severely impacting such common agricultural crops such as wheat (
Triticum aestivum) and rice (
Oryza sativa). Recent estimates suggest that globally ~33% of irrigated cropland is salinized as a result of fertilizer use and other management impacts [
1]. Substrate salt deposition and salinity / sodicity is also a major factor limiting plant establishment and growth in urban ecosystems [
2,
3], and in an ecological restoration context [
4]. Negative impacts of soil salinity on plants tend to be most pronounced in early seedling development (e.g., [
5,
6]). This suggests the strategy of targeting mitigation measures at the seedling stage.
Soil amendments, including fertilizers, organic matter, and cover crop residues, are long-standing methods for ameliorating soil salinity effects in agriculture [
7,
8]. A novel approach is the use of pyrolyzed organic materials, commonly referred to as biochar [
9,
10]. There has been great research interest in biochar generally in the last 10-15 years, with a large body of research indicating that biochar use as a soil amendment can improve soil properties and increase crop yields [
11], particularly in coarse-textured, acidic soils of low nutrient status [
12]. Biochar exhibits high sorption capacity for a wide variety of substances due to its negatively charged and porous surface, which can act to reduce bioavailability of toxic compounds when applied to soils [
13]; biochar has specifically been applied to mitigate effects of toxic metals and polycyclic aromatic compounds [
14,
15]. The potential for biochar to mitigate soil salinity has also been recently recognized [
9,
16], with promising results in agricultural systems [
17,
18]. Biochars generally sorb Na
+ ions [
19]; however, effects of biochar on salinity in situ also depend on soil porosity and hydraulic conductivity effects that determine leaching [
17]. Soil electrical conductivity (EC) is the most commonly used general indicator of soil salinity; several field studies have observed reduced soil EC with biochar additions to saline soils [
16,
20], although studies have also found increased soil EC at high biochar application rates (e.g., [
9,
21]). In addition to sorption and leaching effects, mitigation of salt stress effects may be enhanced by K supplied by biochar, which acts to reduce plant Na uptake [
20,
22].
Applications of biochar to agriculture at scale entail relatively high costs. Formal economic analyses suggest that costs of large-scale biochar amendments generally do not outweigh benefits, at least in high-input agriculture in the temperate zone [
23,
24]. An alternative to broadcast applications of biochar is its use in biochar-based seed coatings. This approach effectively concentrates biochar at the germination site of individual plants. To date, few published studies have examined the potential effects of biochar-based seed coatings on seedling development and plant growth [
25,
26,
27], though interest on this topic is growing [
28,
29]. The effects of biochar-based coating on the germination and growth of agricultural crops remain unexplored. Seed encapsulation necessitates use of a binding agent, with polyvinyl acetate (PVAc) being perhaps the most common [
30]; however, PVAc’s potential effect on plant development in this context remains unclear.
Here we present results of lab germination and greenhouse growth trials of radish (Raphanus sativa) seeds coated with biochar using PVAc as a binding agent, with and without salinity treatments at the seed germination and seedling establishment stages. We test the following hypotheses: (1) biochar-based seed coatings will enhance seed germination and seedling growth; however, PVAc may have adverse effects that offset these benefits; (2) salinity will suppress seed germination and seedling growth; (3) growth inhibition by salt will be mitigated by biochar-based seed coatings. We also present results from a meta-analysis of prior studies of biochar-based seed coatings to examine the broader utility of PVAc as a binding agent in this context.
2. Materials and Methods
2.1. Experimental Species
Radish (
Raphinus sativa L.) was used as the experimental species. Radish, part of the family Brassicaceae, is a root vegetable crop commonly cultivated around the world. Due to its rapid growth, radish is one of the longest-standing model systems for plant biology study [
31,
32]. Radish shows rapid germination and radicle growth as well as quick true leaf development after germination [
32] and has been widely used as a target species for studies of biochar effects on germination in relation to toxics (e.g., [
33,
34]). Radish seed was obtained from OSC Seeds (Kitchener, Ontario, Canada).
2.2. Biochar Encapsulation of Seeds
Seeds were coated with a mixed conifer feedstock biochar produced by BC Biocarbon (McBride, British Columbia, Canada) produced from coniferous feedstock using slow pyrolysis at ~600°C. Biochar ash content was determined following standard methodology (ASTM D1762-84): samples were first dried at 105°C, and ash determined based on remaining after combustion at 950°C. Total C and N determinations were made using a CN analyzer (628 Series, LECO Corporation, St. Joseph, MI, USA). Bulk density was determined using a graduated cylinder to determine volume of dried biochar samples of known mass. Measurements of pH and EC using a glass electrode pH meter (IQ Scientific Instruments, Carlsbad, CA, USA) and a conductivity meter (Orion Star A112, Thermo Scientific, Waltham, MA, USA), respectively on a 1:20 (v:v) ratio of sample to deionized water after being shaken on an oscillating table at 60 rpm for 24 h. Biochar P, K, Ca, and Mg were determined using inductively coupled plasma mass spectrometry (ICP-MS) following digestion with a mixture of hydrochloric, nitric, perchloric, and hydrofluoric acids, with analyses performed by Actlabs (Ancaster, ON, Canada). All measurements were made in triplicate. Basic physiochemical properties of the biochar used are listed in
Table 1 (with additional property data in Table S1).
Biochar-encapsulated radish seeds were provided by Seed the North, Ltd. (Hazelton, British Columbia, Canada). Biochar was sieved to a particle size <500 µm and PVAc was used as a binding agent in a coating pan process. Encapsulated seeds were coated to a final diameter of 5-7 mm, with ~2-3 mm of material surrounding each seed. Control seeds were obtained from the same seed lots used for coating purposes.
2.3. Experimental Design
2.3.1. Petri-Dish Experiments
Experiments were carried out in a laboratory at the University of Toronto. Germination trials used 90-mm diameter polymer Petri dishes containing Whatman grade #1 filter paper, with 5 ml of each treatment solution added to each replicate. The Petri dishes were kept under incandescent light bulbs simulating a 12-h day and night cycle at room temperature (∼24℃ day and ∼22℃ night temperatures).
In the first experiment, treatments consisted of a two-way factorial combination of biochar coating (biochar coated vs. uncoated seeds), and three salinity treatments (20 mM NaCl, 40 mM NaCl and a deionized water control); 10 seeds were added per Petri dish, with 2 replicates per treatment (i.e., 2 petri dishes, 20 seeds in total per treatment).
In the second experiment, there were three biochar coating treatments (biochar coated seeds, uncoated seeds, and leached biochar coated seeds); there were 10 seeds per dish and 5 replicates (50 seeds per treatment). The leaching treatment involved placement of seeds in deionized water on a shaker table at 60 rpm for 24 h immediately prior to starting the experiment. The other treatments (biochar-coated and uncoated seeds) were placed in treatments during the leaching process so that imbibition was synchronized across treatments.
The third experiment involved exposure of seeds to a 2% PVAc solution compared to deionized water controls; in this experiment there were 5 seeds per dish and 6 replicates (30 seeds per treatment).
2.3.2. Greenhouse Experiments
Greenhouse experiments were also conducted to measure treatment effects on later plant development stages. In greenhouse experiment 1, 9 seeds with and without biochar encapsulation were evenly distributed in three 4-inch pots (3 seeds/pot; N = 3 pots/treatment), containing a potting soil (Miracle-Gro All Purpose Potting Soil Mix). Plants were grown with an average temperature of 28℃ during the day, 24℃ at night and a 12-h day and night cycle. NaCl was applied to the soil to induce salinity stress, with a 40 mM solution of NaCl in distilled water used for plant irrigation (following [
35]). This experiment was run for 44 days from seed addition.
Greenhouse experiment 2 replicated the methods of experiment 1, but increased sample size (N = 12 pots/treatment); in experiment 2 plants were grown for 39 days. This experiment used 80 mM NaCl as a salinity treatment; however, mortality was close to 100% in the 80 mM NaCl treatments, so only the no-salt data are presented.
2.4. Seedling Performance and Growth Measurements
Germination was recorded on Day 4, 8, and 12 after the onset of Petri dish experiments, with seeds showing any exposed radicle scored as germinated. Radicle length measurements were taken at the same time to the nearest 1 mm. In both Petri dish and greenhouse experiments, plants were harvested at the end of the growing period and dried at 60°C for 24 h, and dry mass determined using an analytical balance.
2.5. Physiological Measurements
Leaf chlorophyll content was measured with a CCM-200plus Chlorophyll Meter (Opti-Sciences, Inc., Hudson, NH, USA). Photosynthetic fluorescence (Fv/Fm) was measured with a MINI-PAM-II Photosynthesis Yield Analyzer (Heinz Walz GmbH, Effeltrich, Germany), using a saturated pulse technique [
36]. Physiological measurements were made in the greenhouse experiments on day 15.
2.6. Statistical Analysis of Experimental Results
Both the Petri dish and greenhouse experiments included multiple seedlings per experimental unit; linear mixed models that included pot or Petri dish as a random effect variable were therefore initially used for analysis. The random effect was not significant in any of these analyses; therefore, data were analyzed using simple two-way analysis of variance, with seed coating and salinity treatments considered as main effects. Germination proportion data were analyzed using Petri dishes as replicates, using the final (day 12) germination data only. Germination proportions were transformed using a logit transformation prior to analysis, with an adjustment made to accommodate zero and one values [
37]. Contrasts to elucidate pairwise treatment differences were based on t-tests adjusted using a false discovery rate procedure (with p < 0.05 considered significant). All statistical analyses were conducted in the R programming environment [
38].
2.7. Meta-Analysis
Literature was searched using Google Scholar, with a cutoff date of Dec. 2023, with search terms including those pertaining to biochar (“biochar”, “black carbon”, “char”, “charcoal”, “hydrochar”), in conjunction with terms for seed coating (“seed coating(s)”, “seed treatment(s)”); relevant reviews [
28,
29] were also consulted. Performance measures used included percent seed germination in lab studies, and of seedling establishment in field studies. Data were extracted from where possible; graphical data were digitized using webplotdigitizer [
39]. The response ratio (R = ln(X
t/X
c) was used as the effect size statistic used, where X
t is the treatment mean and X
c is the control mean; pooled R values were weighted by the inverse of sampling variance. Where means were presented without error values, standard deviations were imputed from the observed average coefficient of variation across studies [
40]. Percent change values corresponding to R were calculated as 100 × (exp(R) − 1). Analyses were conducted in R version 4.3.2 [
38] using the metafor package [
41].
3. Results
3.1. Petri Dish Experiments
3.1.1. Germination and Early Seedling Growth
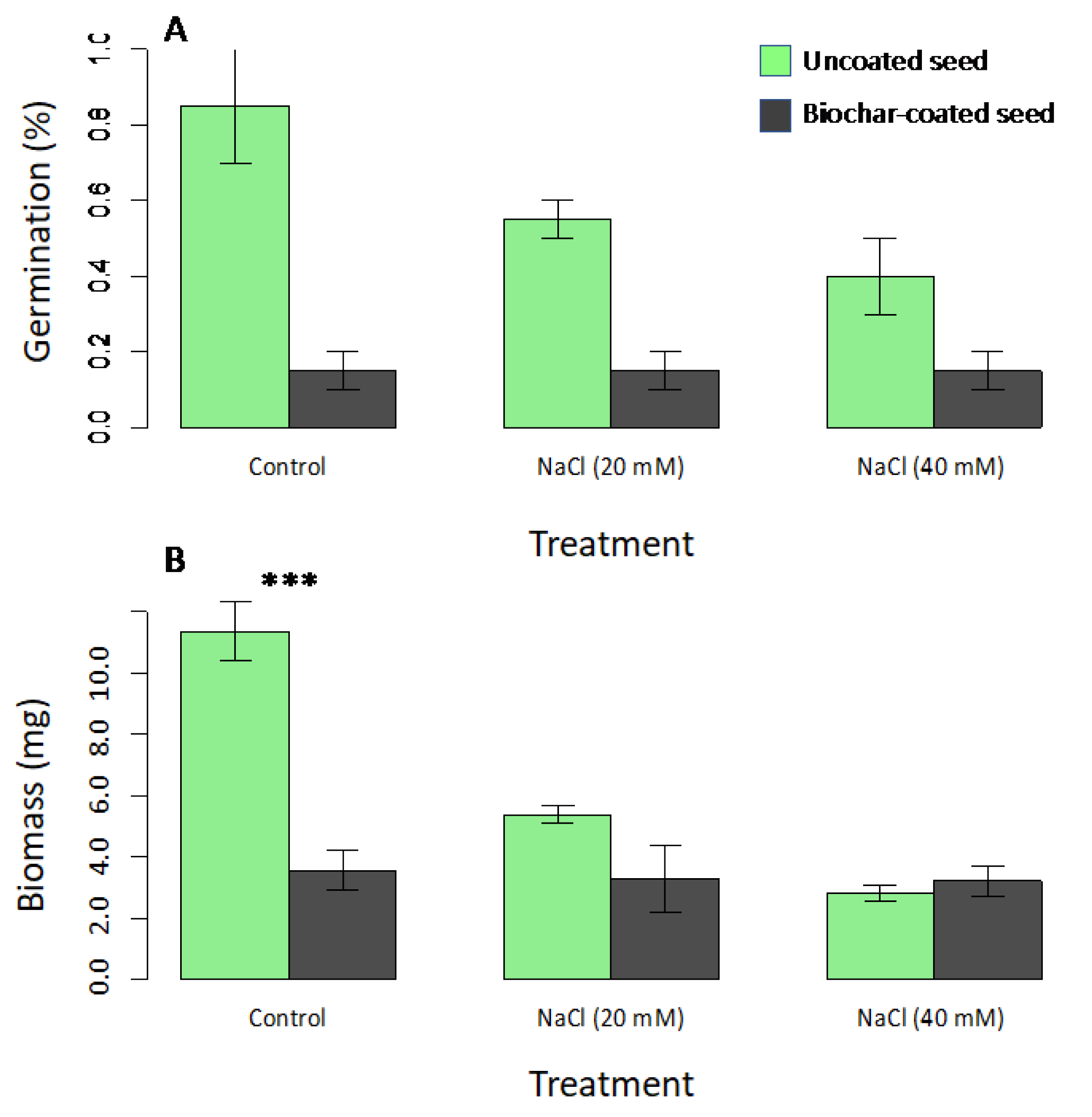
In the first Petri dish experiment, the seed coating treatment resulted in significantly reduced germination (F
1,6 = 13.3; p = 0.003:
Figure 1A), but there was not a significant effect of salinity or a coating x salinity interaction (p > 0.05 in both cases). The analysis for seedling biomass showed significant main effects of seed coating (F
1,26 = 35.1; p < 0.001) and salinity (F
2,26 = 22.1; p < 0.001), as well as a significant coating x salinity interaction term (F
2,26 = 13.5; p < 0.001), corresponding to a large negative effect of seed coating in the controls, but no effect on seedling biomass for the high salinity treatments (
Figure 1B).
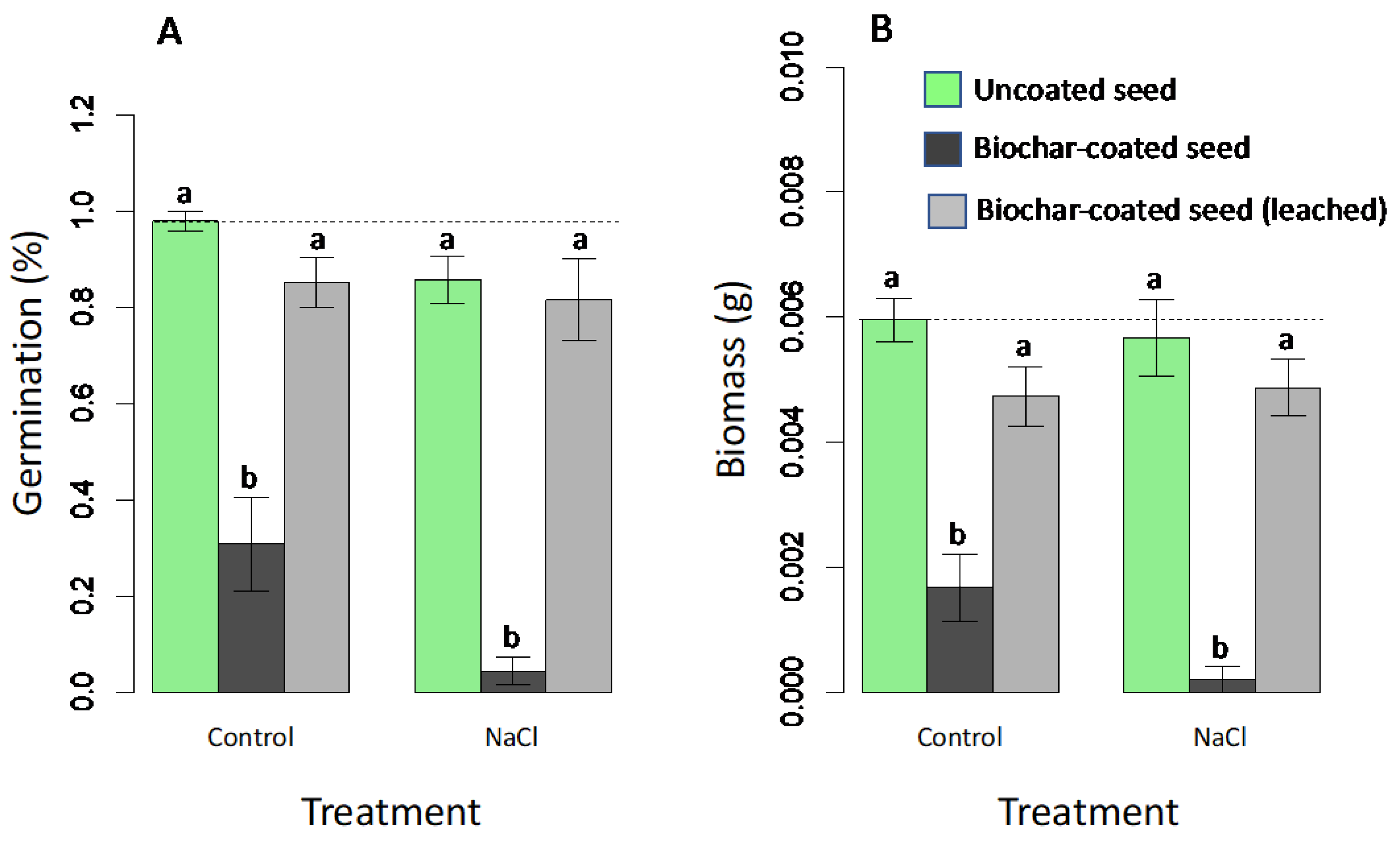
The second Petri dish experiment examined the hypothesis that negative effects of seed coating were due to soluble chemicals and included a leaching treatment for coated seeds, with only one salinity level used (40 mM NaCl). Results indicate that seed coating and salinity treatments had significant effects on germination (F
2,24 = 5.6; p < 0.001 and F
1,24 = 7.5; p = 0.011, respectively), but there was not a significant interaction (F
2,24 = 1.5; p = 0.242). Seed coating alone strongly decreased germination, with an average germination rate of 98% in the deionized water control and 31% in unleached coated seeds (
Figure 1A). This suppression of germination was almost completely counteracted by the leaching treatment, with an 85% germination rate in coated, leached seeds (
Figure 1A) (not significantly different from the control by the HSD test). Salt addition only had a modest effect, reducing germination rates from 98% to 86% in uncoated seeds, and with similar patterns observed in both coated seed treatments (
Figure 2A). Similar patterns were observed on seedling growth (
Figure 2B), with significant seed coating treatment effects on biomass of seedlings evaluated at day 5 (F
2,24 = 55.1; p < 0.001), though with no significant salt effect (F
1,24 = 2.8; p = 0.105) or salt x coating interaction (F
2,24 = 1.2; p = 0.315).
3.1.2. PVAc Effects on Germination
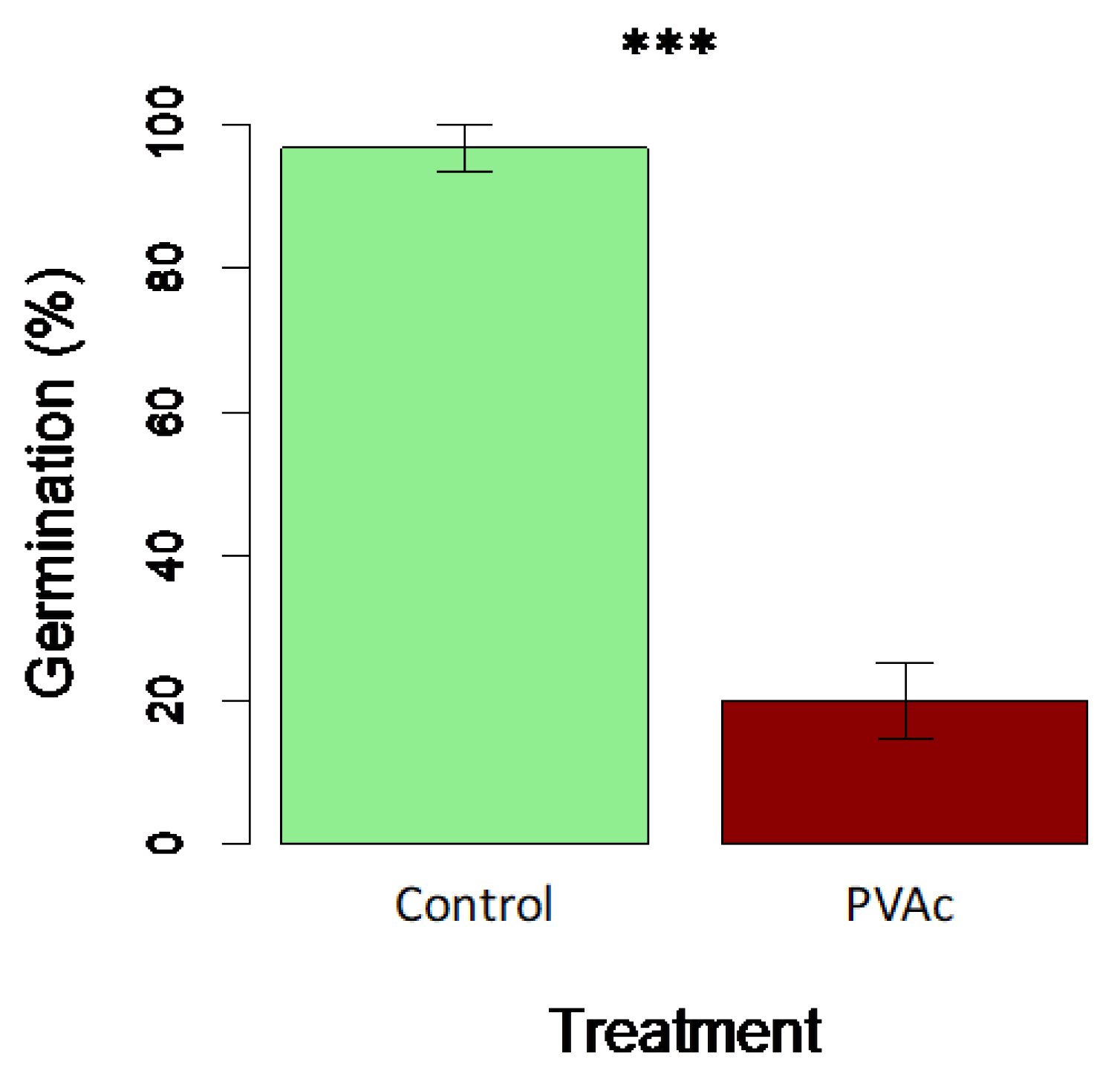
PVAc application alone (i.e., without a seed coating process) dramatically reduced the germination rate of radish seeds compared to the deionized water control (
Figure 3). The germination rate decreased from 96.7% to 20.0% in response to the PVAc treatment (F
1,10 = 76.7; p < 0.001).
3.2. Greenhouse Experiments
3.2.1. Biomass Responses
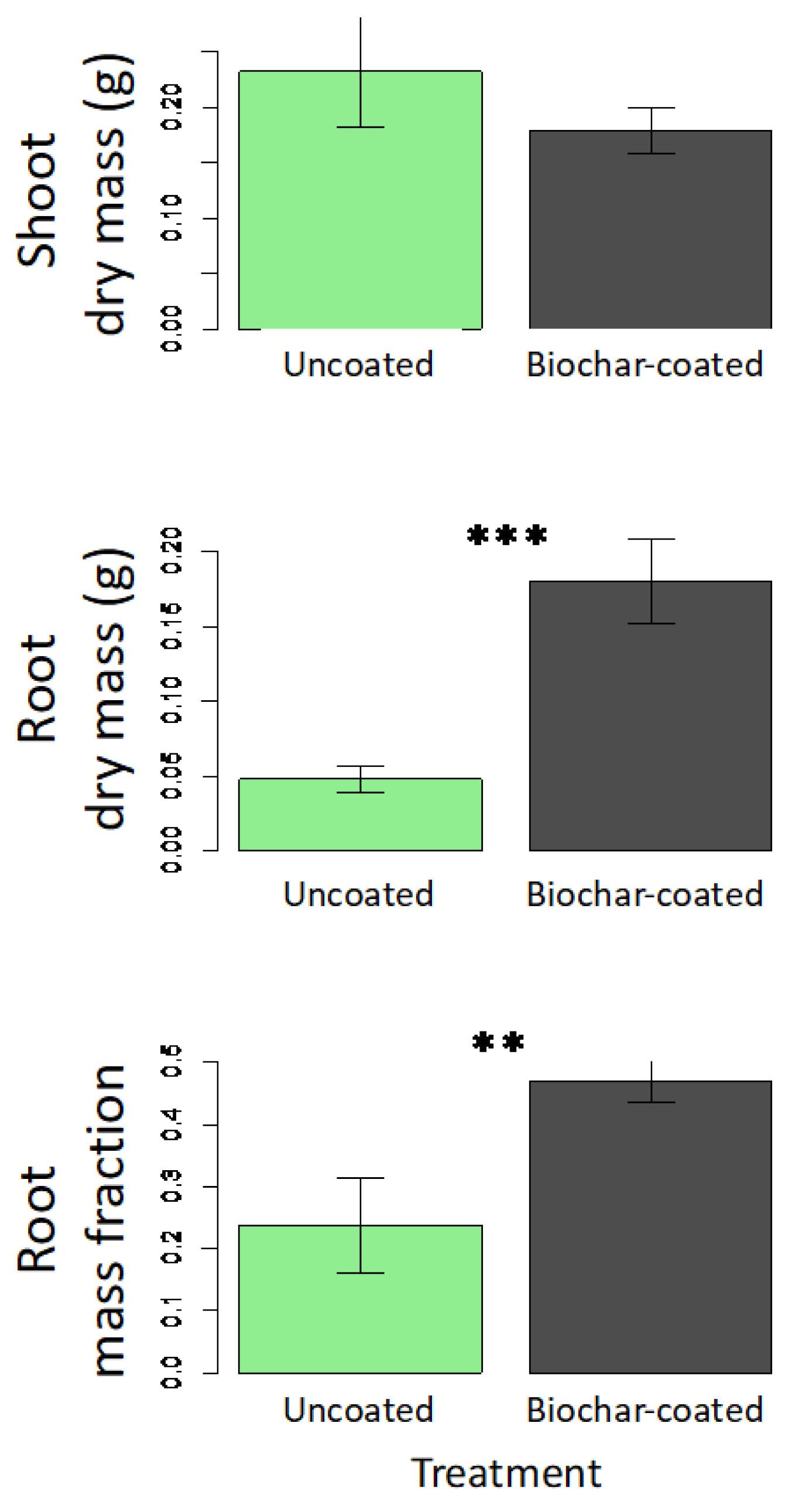
Since biomass was not measured in the first experiment, a second greenhouse experiment was conducted (
Figure 4). ANOVA results indicate a significant biochar treatment effect on root mass (F
1,22 = 18.5; p < 0.001), and mass fraction (F
1,22 = 8.1; p = 0.010), but no significant effect on shoot mass (F
1,22 = 1.1; p = 0.304). Plants developing from biochar-coated seed showed a 3.8-fold increase in root mass compared to the control (
Figure 4). The lack of an aboveground response indicates that this pattern is due to root-shoot partitioning rather than an overall growth stimulation.
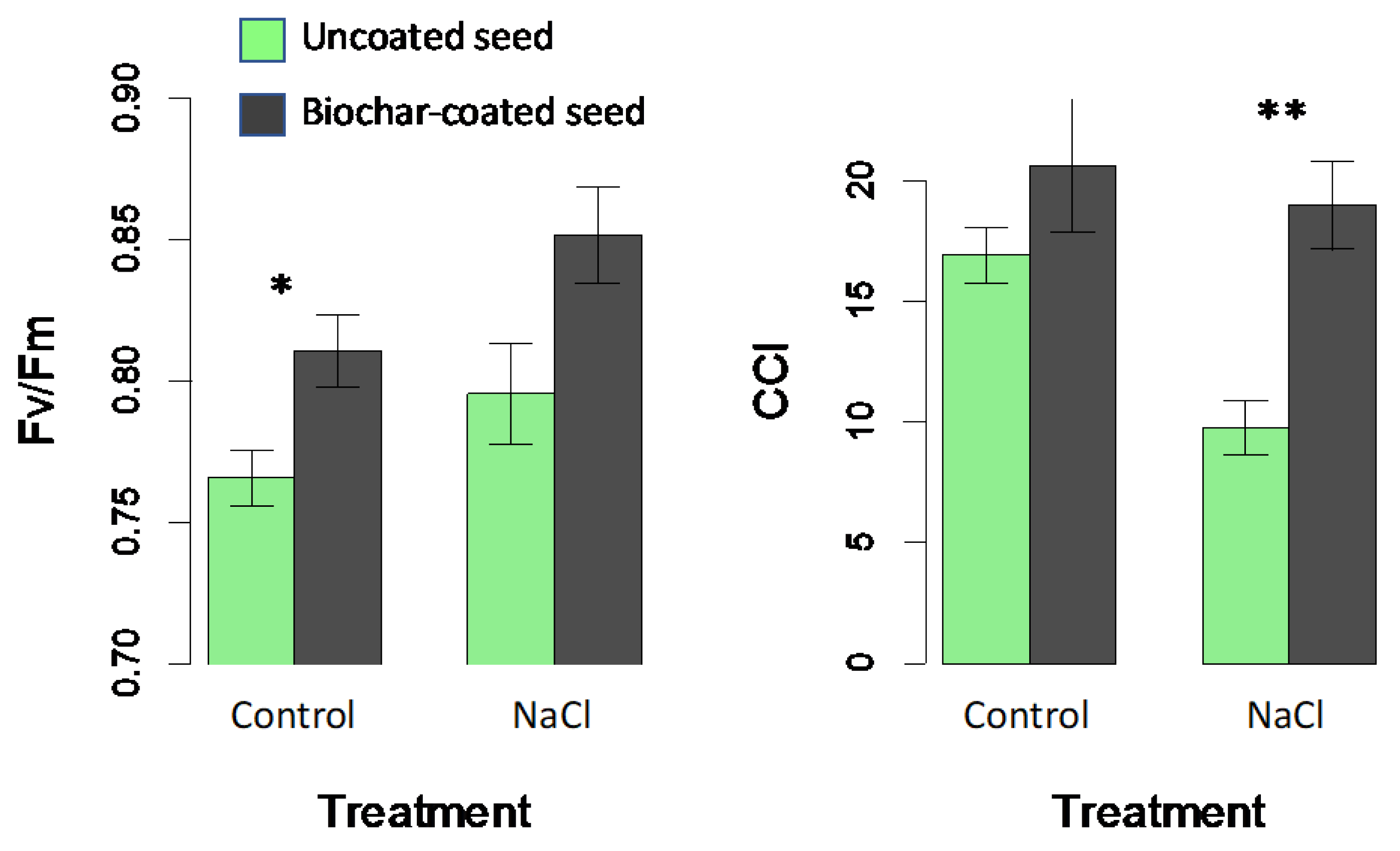
3.2.2. Chlorophyll Content and Fluorescence
Significant treatment effects were observed for chlorophyll fluorescence (Fv/Fm) and leaf chlorophyll content (CCI) in greenhouse experiment 1 (
Figure 5). Plants exhibited significantly reduced chlorophyll content in response to salinity treatments (F
1,16 = 5.8; p = 0.029), while biochar encapsulation increased chlorophyll content (F
1,16 = 12.3; p = 0.003) (
Figure 5). Biochar-encapsulated seed showed had higher Fv/Fm compared with leaves from non-encapsulated seeds (
Figure 5) (F
1,16 = 11.9; p = 0.003); however, the salinity treatment increased Fv/Fm values (F
1,16 = 2.2; p = 0.028). The biochar x salinity interaction was again not significant (F
1,16 = 0.2; p = 0.702)
4. Discussion
Our results indicate that biochar seed coatings can enhance performance of radish plants, both in general and specifically in soils affected by moderate salinity. Unexpectedly, Petri dish trials produced the opposite effect: biochar seed coatings strongly inhibited seed germination and early seedling development, unless biochar-coated seeds were pre-treated by leaching. These results are consistent with phytotoxicity of PVAc used as a binding agent, as was shown directly by a toxicity bioassay (
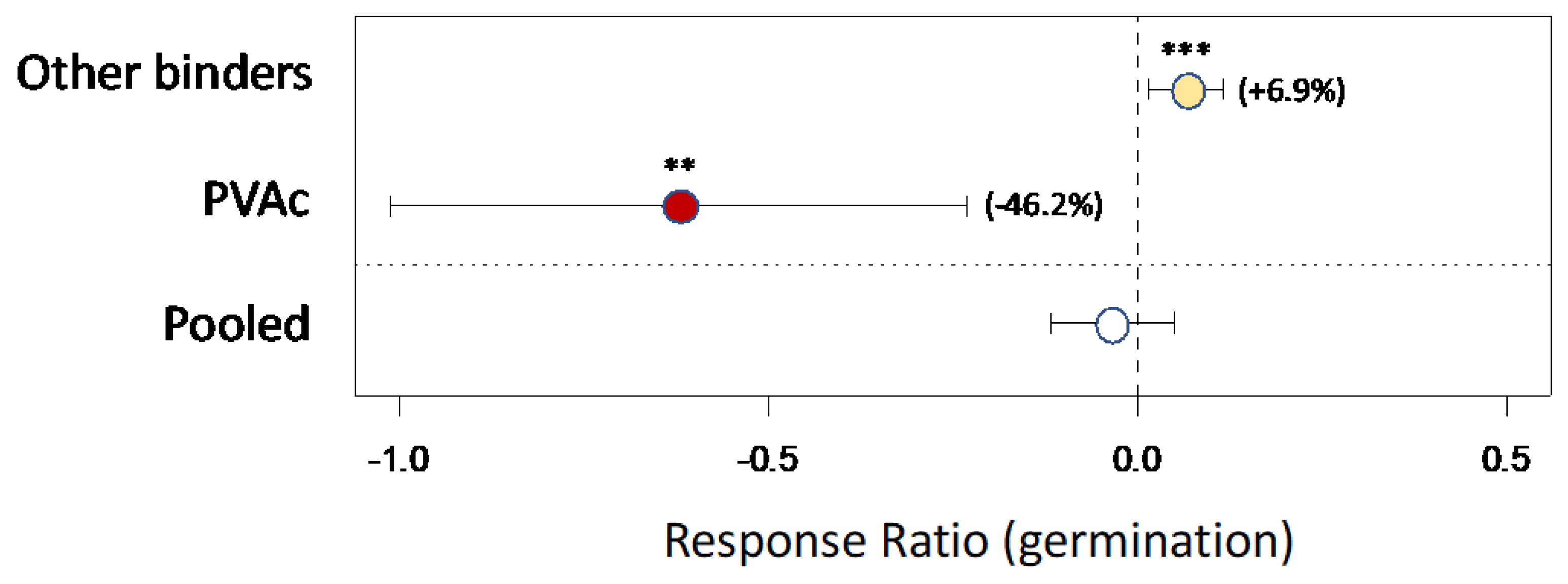
Figure 3). The widespread inimical effects of PVAc on seed germination were also supported by a meta-analysis of published studies of biochar-based seed coatings (
Figure 6). In the greenhouse trials, where there was an opportunity for transport of leachates away from seeds, biochar seed coatings showed positive effects on plant root growth and physiological performance. Positive effects on seedling performance were generally more pronounced in moderate salinity treatments, supporting use of biochar-based seed coatings in the case of saline/sodic soils.
4.1. Effects of Biochar-Based Seed Coatings
While seed coatings of various sorts are widely used in agriculture [
49,
50], biochar-based seed coatings have only recently received attention. A recent review [
29] noted 25 studies examining biochar-based seed coatings or seed co-amendments (e.g., seed dressings or pelletized products containing seed); however, most of these studies focused on combinations of biochar with microbial inocula or other amendments or actually examined activated carbon (generally made from coal) rather than biochar
sensu stricto. Among prior studies that specifically examine effects of biochar seed coatings alone (i.e., biochar plus a binding agent), there have been mixed results. Głodowska et al. [
26] found increased growth in response to biochar-based seed coatings in maize seedlings for one of two biochars tested, but inhibition by a second biochar. A study on rice seedlings found positive effects of a rice-straw feedstock biochar on seedling emergence and early growth [
47], and a study on black gram also found positive effects, with the largest highest biochar addition per seed showing the best results [
45]. Brown et al. [
48] also recently reported positive effects biochar-based pellets on the native Australian legume
Jacksonia furcellata; however, results varied with the size and form of pellets. Neutral effects of biochar-based seed coatings on seed germination and early seedling performance have also been reported in several studies [
27,
42,
49].
Our results indicate that a critical factor contributing to this variability in results is the binding agent used. Due to its availability and convenience, PVAc has been widely used as binding agent for seed coatings in agronomic and horticultural applications. PVAc (often in commercial formulations and sometimes mis-labeled as polyvinyl alcohol (PVA)) is the binding agent used in more than half of the studies listed by Zhang et al. [
29] and has been commonly used with both biochar- and activated-carbon-based seed coatings and pellets (e.g., [
43,
50,
51]). Our results indicate that PVAc strongly suppresses seed germination in radish (
Figure 3); in addition, the leaching experiment (
Figure 2) indicates that a water-soluble chemical inhibitor present in the biochar coatings is largely responsible for the inhibition observed in Petri dish trials. Meta-analysis results (
Figure 6) also suggest that PVAc use is responsible for many cases of negative effects of biochar-based seed coatings on seedling germination and establishment.
PVAc has generally been regarded as non-toxic; however, there is surprisingly little direct information on its phytotoxicity (e.g., no recorded records of plant toxicity trials with PVAc in the ECOTOX database [
52]). PVAc commonly includes trace quantities of the precursor monomer vinyl acetate, which is moderately phytotoxic (e.g., [
53]). It is also well known that PVAc reacts with bases to form PVA and acetic acid; in the context of biochar-based seed coatings this reaction would be expected due to the alkalinity of biochar (in particular the presence of CaCO
3). The acetic acid produced is relatively strongly phytotoxic [
54,
55], and acetic acid is also commonly already present in biochars as it is produced during pyrolysis and then sorbed [
55], especially in the case of fast pyrolysis biochars [
56]. In addition, PVAc is hydrophobic, and may thus reduce seed imbibition. A prior study that examined use of PCAc as a soil conditioner also documented large negative effects consistent with phytotoxicity [
57].
While the seed coatings used inhibited germination, positive effects on performance of established seedlings were observed. Our greenhouse experiment results indicate enhanced root development as a main response to biochar-based seed coatings, resulting in substantial increases in root mass fraction (
Figure 4). The broader literature on biochar responses suggests little average effect on root-shoot partitioning [
58], although increased root allocation has been observed in specific cases (e.g., [
44,
59]). Relatively high root allocation can be a useful predictor of success for transplanted seedlings in horticulture and forestry, particularly under conditions of water stress [
60], although this is not always the case [
61]. Increased root production is also a direct agronomic benefit in root crops such as radish.
4.2. Biochar-Based Seed Coatings and Salinity Mitigation
The salinity treatments used in experimental trials (up to 40 mM NaCl) resulted in pronounced reductions in plant biomass (
Figure 1,
Figure 2 and
Figure 4), consistent with prior studies that have found salinity levels as low as 30 mM can induce significant inhibitory effects [
62,
63]. Cytotoxicity by Na (and to a lesser extent Cl) is generally the most important mechanism for observed growth reduction in plants [
64]. High salinity also causes an imbalance in osmotic equilibrium which decreases plant water uptake. In the present study, salt-stressed plants exhibited physiological stress indicators, including reduced chlorophyll content and chlorophyll fluorescence (Fv/Fm). This is consistent with inhibition of chlorophyll synthesis and stress-induced chlorophyll degradation [
65].
Biochar-coating of radish seeds substantially alleviated these salinity stress responses, with higher root weight and elevated chlorophyll content than non-coated seeds (
Figure 4 and
Figure 5). These findings are consistent with prior studies showing alleviation of salt stress by soil applications of biochar [
9,
17]. The small amount of biochar used in seed coating suggests that the mechanism for this effect is not increased total nutrient availability, as in soil applications (e.g., [
22]). Rather, these results suggest carry-over effects that derive from alleviation of salt stress during seed germination and early seedling development.
5. Conclusions
The results of the present study support the assertion that biochar-based seed coatings have potential to enhance early seedling performance generally [
28,
29] and may be specifically advantageous under conditions of moderate soil salinity stress. However, it is clear that PVAc, the most commonly used binder used for this application, can have pronounced negative effects. Future research should be dedicated to testing and implementing alternative seed-coating binders that will not interfere with plant function; however, the fact that some positive effects were observed in spite of the use of PVAc itself emphasizes the promise of biochar-based seed coatings in general, since the positive net effects observed had to overcome negative impacts of PVAc. Future work should also include assessment of a range of common crop species to verify biochar’s general efficacy as a seed-coating agent, as well as field trials to document effects
in situ. In particular, potential broader applications of biochar-based seed coatings include use in drone-based precision seeding [
66] for reforestation and ecological restoration.
Author Contributions
Conceptualization, S.C.T. and Y.L.; methodology, S.C.T. and Y.L.; formal analysis, S.C.T. and Y.L.; investigation, Y.L. and E.T.; resources, S.C.T.; data curation, Y.L. and E.T.; writing—original draft preparation, S.C.T. and Y.L.; writing—review and editing, E.T.; visualization, S.C.T.; supervision, S.C.T.; project administration, S.C.T.; funding acquisition, S.C.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants from the Canadian Natural Sciences and Engineering Research Council, with partner contributions from Seed the North, Inc., and BC Biocarbon, Inc.
Data Availability Statement
Data for this article can be found in the University of Toronto Dataverse research data repository: link to be provided.
Acknowledgments
We thank Natasha Kuperman and Matthan Moktar of Seed the North, Inc., and and Phil Marsh of BC Biocarbon, Inc. for contributions of materials used.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Nachshon, U. Cropland soil salinization and associated hydrology: Trends, processes and examples. Water 2018, 10, 1030. [Google Scholar] [CrossRef]
- Cunningham, M.A.; Snyder, E.; Yonkin, D.; Ross, M.; Elsen, T. Accumulation of deicing salts in soils in an urban environment. Urban Ecosys. 2008, 11, 17–31. [Google Scholar] [CrossRef]
- Equiza, M.A.; Calvo-Polanco, M.; Cirelli, D.; Señorans, J.; Wartenbe, M.; Saunders, C.; Zwiazek, J.J. Long-term impact of road salt (NaCl) on soil and urban trees in Edmonton, Canada. Urban For. Urban Green. 2017, 21, 16–28. [Google Scholar] [CrossRef]
- Mendez, M.O.; Maier, R.M. Phytostabilization of mine tailings in arid and semiarid environments—an emerging remediation technology. Environ. Health Perspect. 2008, 116, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Dumbroff, E.B.; Cooper, A.W. Effects of salt stress applied in balanced nutrient solutions at several stages during growth of tomato. Botan. Gaz. 1974, 135, 219–224. [Google Scholar] [CrossRef]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. Changes in plant response to NaCl during development of rice (Oryza sativa L.) varieties differing in salinity resistance. J. Exper. Bot. 1995, 46, 1843–1852. [Google Scholar] [CrossRef]
- Qadir, M.; Oster, J.D. Crop and irrigation management strategies for saline-sodic soils and waters aimed at environmentally sustainable agriculture. Sci. Tot. Environ. 2004, 323, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Leogrande, R.; Vitti, C. Use of organic amendments to reclaim saline and sodic soils: a review. Arid Land Res. Manage. 2019, 33, 1–21. [Google Scholar] [CrossRef]
- Thomas, S.C.; Frye, S.; Gale, N.; Garmon, M.; Launchbury, R.; Machado, N.; Melamed, S.; Murray, J.; Petroff, A.; Winsborough, C. Biochar mitigates negative effects of salt additions on two herbaceous plant species. J. Environ. Manage. 2013, 129, 62–68. [Google Scholar] [CrossRef]
- Kanwal, S.; Ilyas, N.; Shabir, S.; Saeed, M.; Gul, R.; Zahoor, M.; Batool, N.; Mazhar, R. Application of biochar in mitigation of negative effects of salinity stress in wheat (Triticum aestivum L.). J. Plant Nutr. 2018, 41, 526–538. [Google Scholar] [CrossRef]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Yakov Kuzyakov, Y.; Luo, Y.; Ok, Y.S.; Palansooriya, K.N.; Shepherd, J.; Stephens, S.; Weng, Z.H.; Lehmann, J. How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. GCB Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Dai, Y.; Zheng, H.; Jiang, Z.; Xing, B. Combined effects of biochar properties and soil conditions on plant growth: a meta-analysis. Sci. Tot. Environ. 2020, 713, 136635. [Google Scholar] [CrossRef] [PubMed]
- Hale, S.E.; Cornelissen, G.; Werner, D. Sorption and remediation of organic compounds in soils and sediments by (activated) biochar. In Biochar for environmental management: science and technology, 2nd ed.; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2015; pp. 154–196. [Google Scholar]
- Anyika, C.; Abdul Majid, Z.; Ibrahim, Z.; Zakaria, M.P.; Yahya, A. The impact of biochars on sorption and biodegradation of polycyclic aromatic hydrocarbons in soils—a review. Environ. Sci. Pollut. Res. 2015, 22, 3314–3341. [Google Scholar] [CrossRef] [PubMed]
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Yong Sik Ok, Y.S.; Cao, X. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit. Rev. Environ. Sci. Technol. 2016, 46, 406–433. [Google Scholar] [CrossRef]
- Lashari, M.S.; Liu, Y.; Li, L.; Pan, W.; Fu, J.; Pan, G.; Zheng, J.; Zheng, J.; Zhang, X.; Yu, X. Effects of amendment of biochar-manure compost in conjunction with pyroligneous solution on soil quality and wheat yield of a salt-stressed cropland from Central China Great Plain. Field Crops Res. 2013, 144, 113–118. [Google Scholar] [CrossRef]
- Dahlawi, S.; Naeem, A.; Rengel, Z.; Naidu, R. Biochar application for the remediation of salt-affected soils: Challenges and opportunities. Sci. Tot. Environ. 2018, 625, 320–335. [Google Scholar]
- Yu, H.; Zou, W.; Chen, J.; Chen, H.; Yu, Z.; Huang, J.; Tang, H.; Wei, X.; Gao, B. Biochar amendment improves crop production in problem soils: A review. J. Environ. Manage. 2019, 232, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Rostamian, R.; Hedarpour, M.; Mousavi, S.F.; Afyuni, M. Characterization and sodium sorption capacity of biochar and activated carbon prepared from rice husk. J. Agr. Sci. Tech. 2015, 17, 1057–1069. [Google Scholar]
- Lashari, M.S.; Ye, Y.; Ji, H.; Li, L.; Kibue, G.W.; Lu, H.; Zheng, J.; Pan, G. Biochar–manure compost in conjunction with pyroligneous solution alleviated salt stress and improved leaf bioactivity of maize in a saline soil from central China: a 2-year field experiment. J. Sci. Food Agric. 2015, 95, 1321–1327. [Google Scholar] [CrossRef]
- She, D.; Sun, X.; Gamareldawla, A.H.D.; Nazar, E.A.; Hu, W.; Edith, K.; Yu, S. Benefits of soil biochar amendments to tomato growth under saline water irrigation. Sci Rep. 2018, 8, 14743. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Liu, F. Biochar mitigates salinity stress in potato. J. Agron. Crop Sci. 2015, 201, 368–378. [Google Scholar] [CrossRef]
- Dickinson, D.; Balduccio, L.; Buysse, J.; Ronsse, F.; Van Huylenbroeck, G.; Prins, W. Cost-benefit analysis of using biochar to improve cereals agriculture. GCB Bioenergy, 2015, 7, 850–864. [Google Scholar] [CrossRef]
- Bach, M.; Wilske, B.; Breuer, L. Current economic obstacles to biochar use in agriculture and climate change mitigation. Carbon Manage. 2016, 7, 183–190. [Google Scholar] [CrossRef]
- Williams, M.I.; Dumroese, R.K.; Page-Dumroese, D.S.; Hardegree, S.P. Can biochar be used as a seed coating to improve native plant germination and growth in arid conditions? J. Arid Environ. 2015, 125, 8–15. [Google Scholar] [CrossRef]
- Głodowska, M.; Husk, B.; Schwinghamer, T.; Smith, D. Biochar is a growth-promoting alternative to peat moss for the inoculation of corn with a pseudomonad. Agron. Sustain. Dev. 2016, 36, 21. [Google Scholar] [CrossRef]
- Głodowska, M.; Schwinghamer, T.; Husk, B.; Smith, D. Biochar Based Inoculants Improve Soybean Growth and Nodulation. Agric. Sci. 2017, 08, 1048–1064. [Google Scholar] [CrossRef]
- Brown, V.S.; Erickson, T.E.; Merritt, D.J.; Madsen, M.D.; Hobbs, R.J.; Ritchie, A.L. A global review of seed enhancement technology use to inform improved applications in restoration. Sci. Tot. Environ. 2021, 798, 149096. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Khan, Z.; Yu, Q.; Qu, Z.; Liu, J.; Luo, T.; Zhu, K.; Bi, j.; Hu, L.; Luo, L. Biochar coating is a sustainable and economical approach to promote seed coating technology, seed germination, plant performance, and soil health. Plants 2022, 11, 2864. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.E.H.; Ali, A.Y.A.; Elsiddig, A.M.I.; Zhou, G.; Nimir, N.E.A.; Ahmad, I.; Suliman, M.S.E.; Elradi, S.B.M.; Salih, E.G.I. Biochar improved sorghum germination and seedling growth under salinity stress. Agron. J. 2020, 112, 911–920. [Google Scholar] [CrossRef]
- Klinger, T.; Elam, D.R.; Ellstrand, N.C. Radish as a model system for the study of engineered gene escape rates via crop-weed mating. Cons. Biol. 1991, 5, 531–535. [Google Scholar] [CrossRef]
- Mitsui, Y.; Shimomura, M.; Komatsu, K.; Namiki, N.; Shibata-Hatta, M.; Imai, M.; Katayose, Y.; Mukai, Y.; Kanamori, H.; Kurita, K.; Kagami, T.; Wakatsuki, A.; Ohyanagi, H.; Ikawa, H.; Minaka, N.; Nakagawa, K.; Shiwa, Y.; Sasaki, T. The radish genome and comprehensive gene expression profile of tuberous root formation and development. Sci. Rep. 2015, 5, 10835. [Google Scholar] [CrossRef] [PubMed]
- Sujeeun, L.; Thomas, S.C. Potential of Biochar to Mitigate Allelopathic Effects in Tropical Island Invasive Plants: Evidence from Seed Germination Trials. Tropical Cons. Sci. 2017, 10, 1940082917697264. [Google Scholar] [CrossRef]
- Bieser, J.M.; Al-Zayat, M.; Murtada, J.; Thomas, S.C. Biochar mitigation of allelopathic effects in three invasive plants: evidence from seed germination trials. Can. J. Soil Sci. 2022, 102, 213–224. [Google Scholar] [CrossRef]
- Zamljen, T.; Medic, A.; Hudina, M.; Veberic, R.; Slatnar, A. Salt stress differentially affects the primary and secondary metabolism of peppers (Capsicum annuum L.) according to the genotype, fruit part, and salinity Level. Plants 2022, 11, 853. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.; Johnson, G.N. (2000). Chlorophyll fluorescence—a practical guide. J. Exp. Botan. 2022, 51, 659–668.
- Martín-Fernández, J.A.; Thió-Henestrosa, S. Rounded zeros: some practical aspects for compositional data. Geol. Soc. London, Special Pubs 2006, 264, 191–201. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2023. https://www.R-project.org/.
- Rohatgi, A. WebPlotDigitizer. 2019. https://apps.automeris.io/wpd/.
- Lajeunesse, M.J.; Koricheva, J.; Gurevitch, J.; Mengersen, K. Recovering missing or partial data from studies: a survey of conversions and imputations for meta-analysis. In Handbook of Meta-analysis in Ecology and Evolution; Gurevitch, J., Koricheva, J., Mengersen, K., Eds.; Princeton Univ. Press: Princeton, USA, 2013; pp. 195–206. [Google Scholar]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Elamparithi, R.; Sujatha, K.; Menaka, C.; Senthil, K. Impact of organic seaweed pelleting on seed quality and biochemical parameters in brinjal seeds. Pharma Innov. 2021, 10, 1466–1469. [Google Scholar]
- Terry, T.J.; Madsen, M.D.; Gill, R.A.; Anderson, V.J.; St. Clair, S.B. Selective herbicide control: Using furrows and carbon seed coatings to establish a native bunchgrass while reducing cheatgrass cover. Restor. Ecol 2021, 29, e13351. [Google Scholar] [CrossRef]
- Adelabu, D.B.; Franke, A.C. The beneficial effects of insect pollination and biochar seed coating on okra (Abelmoschus esculentus) seed quality at varying temperature conditions. Agriculture 2022, 12, 1690. [Google Scholar] [CrossRef]
- Parvin Banu, A.; Sujatha, K.; Geetha, R.; Kannan, P. Biochar coating and its effect on seed quality and biochemical parameters in black gram (Vigna mungo L.) var. VBN 11. Pharma Innov, 2022; 11, 2836–2840. [Google Scholar]
- Law, Y.K.; Lee, C.K.; Pang, C.C.; Hau, B.C.H.; Wu, J. Vegetation regeneration on natural terrain landslides in Hong Kong: direct seeding of native species as a restoration tool. Land Degrad. Dev. 2023, 34, 751–762. [Google Scholar] [CrossRef]
- Zhang, K.; Khan, Z.; Liu, J.; Luo, T.; Zhu, K.; Hu, L.; Bi, J.; Luo, L. Germination and Growth Performance of Water-Saving and Drought-Resistant Rice Enhanced by Seed Treatment with Wood Vinegar and Biochar under Dry Direct-Seeded System. Agronomy 2022, 12, 1223. [Google Scholar] [CrossRef]
- Brown, V.S.; Erickson, T.E.; Hobbs, R.J.; Mastrantonis, S.; Ritchie, A.L. Carbon-based pelleting, soil ripping and herbicide application can be used to overcome plant recruitment barriers in Grey Stinkwood (Jacksonia furcellata). Ecol. Manage. Restor. 2023, 24, 119–127. [Google Scholar] [CrossRef]
- Afzal, I.; Javed, T.; Amirkhani, M.; Taylor, A.G. Modern seed technology: Seed coating delivery systems for enhancing seed and crop performance. Agriculture 2020, 10, 526. [Google Scholar] [CrossRef]
- James, M.S. Effects of Biochar-Based Seed Coatings on Seed Germination and Seedling Vigor of California Brome (Bromus carinatus L.) and Blue Wildrye (Elymus glaucus L.). Master’s Thesis, Oregon State University, Corvallis, OR, USA, 2015. [Google Scholar]
- Pedrini, S.; Merritt, D.J.; Stevens, J.; Dixon, K. Seed coating: science or marketing spin? Trends Plant Sci. 2017, 22, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Xavier, P.B.; Vieira, H.D.; Guimarães, C.P. Physiological potential of Stylosanthes cv. Campo Grande seeds coated with different materials. J. Seed Sci. 2015, 37, 117–124. [Google Scholar] [CrossRef]
- Baughman, O.W.; Griffen, J.; Kerby, J.; Davies, K.W.; Clenet, D.; Boyd, C. Herbicide protection pod technology for native plant restoration: One size may not fit all. Restor. Ecol. 2021, 29, e13351. [Google Scholar] [CrossRef]
- Olker, J.H.; Elonen, C.M.; Pilli, A.; Anderson, A.; Kinziger, B.; Erickson, S.; Skopinski, M.; Pomplun, A.; LaLone, C.A.; Russom, C.L.; Hoff, D. The ECOTOXicology knowledgebase: A curated database of ecologically relevant toxicity tests to support environmental research and risk assessment. Environ. Toxicol. Chem. 2022, 41, 1520–1539. [Google Scholar] [CrossRef]
- Berdahl, J.D.; Barker, R.E. Germination and emergence of Russian wildrye seeds coated with hydrophilic materials. Agron. J. 1980, 72, 1006–1008. [Google Scholar] [CrossRef]
- Himanen, M.; Prochazka, P.; Hänninen, K.; Oikari, A. Phytotoxicity of low-weight carboxylic acids. Chemosphere 2012, 88, 426–431. [Google Scholar] [CrossRef]
- Thomas, S.C.; Ruan, R.; Gale, N.V.; Gezahegn, S. Phytotoxicity and hormesis in common mobile organic compounds in leachates of wood-derived biochars. Biochar 2024, in press. [Google Scholar] [CrossRef]
- Gezahegn, S.; Sain, M.; Thomas, S.C. Phytotoxic condensed organic compounds are common in fast but not slow pyrolysis biochars. Bioresour. Technol. Rep. 2021, 13, 100613. [Google Scholar] [CrossRef]
- Carr, C.E.; Greenland, D.J. Potential application of polyvinyl acetate and polyvinyl alcohol in the structural improvement of sodic soils. Soil Conditioners 1975, 7, 47–63. [Google Scholar]
- Xiang, Y.; Deng, Q.; Duan, H.; Guo, Y. Effects of biochar application on root traits: a meta-analysis. GCB Bioenergy 2017, 9, 1563–1572. [Google Scholar] [CrossRef]
- Li, X.; Yao, T.; Huang, X.; Li, P.; Du, S.; Wang, W.; Miao, S.; Wang, D.; Jin, F.; Shao, X. Biochar increases rice yield by improving root morphological and root physiological functions in heavily saline-sodic paddy soil of Northeast China. BioResources, 2022; 17, 1241. [Google Scholar]
- Davis, A.S.; Jacobs, D.F. Quantifying root system quality of nursery seedlings and relationship to outplanting performance. New For. 2005, 30, 295–311. [Google Scholar] [CrossRef]
- Sheridan, R.A.; Davis, A.S. Characterizing the utility of the root-to-shoot ratio in Douglas-fir seedling production. Forests 2021, 12, 1745. [Google Scholar] [CrossRef]
- Duan, D. Y., Li, W. Q., Liu, X. J., Ouyang, H., An, P., Duan, D. Y., & Liu, W. Q. (2007). Seed germination and seedling growth of Suaeda salsa under salt stress. Ann. Bot. Fenn. 2007, 44, 161–169.
- Sidari, M.; Santonoceto, C.; Anastasi, U.; Preiti, G.; Muscolo, A. Variations in four genotypes of lentil under NaCl-salinity stress. Am. J. Agric. Biol. Sci. 2008, 3, 410–416. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Santos, C.V. Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci. Hortic. 2004, 103, 93–99. [Google Scholar] [CrossRef]
- Mohan, M.; Richardson, G.; Gopan, G.; Aghai, M. M.; Bajaj, S.; Galgamuwa, G. P., et al. (2021). UAV-supported forest regeneration: Current trends, challenges and implications. Remote Sens. 2021, 13, 2596.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).