1. Introduction
Soybean isoflavone (SIF) is a natural phytoestrogen, which mainly exists in soybean and other leguminous plants. It has been widely reported that SIF can enhance non-specific immunity and alleviate human diseases [
1,
2]. Moreover, it can improve the lipid metabolism, and associate with gonadal development of animal. Therefore, SIF could be a potential feed additive for improve animal growth and health.
In mammals, a large number of studies have shown that soy isoflavone is an effective additive to regulate lipid metabolism. Dietary supplementation with SIF can alleviate the lipid accumulation by reducing triglyceride (TG) and low-density lipoprotein cholesterol (LDL-C) levels in the liver of obese rats [
3,
4]. The further studies suggested that SIF improved lipocatabolic metabolism by up-regulating the relative expression of peroxisome proliferate-activated receptor α (PPARα) [
5] and sterol regulatory element-binding proteins 1 (srebp-1) [
6]. There were other studies also reported SIF regulated lipid catabolism by stimulating liver X receptor α or liver X receptor β phosphorylation [
7]. In addition to promoting lipolysis, SIF can also alleviate lipid accumulation through reducing fat synthesis. Some previous studies reported that dietary SIF inhibited fat synthesis of obese Zucker rats by down-regulating the expression of peroxisom-proliferator activated receptor γ2 (PPARγ2) and adipose-specific protein 27 (FSP27) [
8]. In aquatic animals. Diets supplemented with 100 mg/kg and 500 mg/kg genistein significantly down-regulated the genes associated with lipid synthesis of
Cyprinus carpio [
9]. Some similar results were widely reported in
Oncorhynchus mykiss and
Paralichthys olivaceus [
10,
11]. However, some other studies shown the opposite results. Dietary SIF significantly increased TG and TC contents in the serum of
Allogynogenetic crucian carp and
Paralichthys olivaceus [
12,
13]. Unfortunately, it is difficult to understand the reason that soy isoflavone showed the different effects in different aquatic animals due to limited research. Therefore, it is necessary to do some insight studies to specified the functions of soy isoflavones.
The Chinese mitten crab (Eriocheir sinensis) is an economic crustacean that has been widely farmed. Different from mammals, shrimp and crabs do not have specific adipose tissue, and the lipid metabolism pattern is also different from that of mammals. Whether soy isoflavones can regulate fat metabolism and ovarian development of crabs is still poorly understood. Therefore, this study aimed to examine the effects of soy isoflavones on the growth performance and lipid metabolism of the Chinese mitten crab.
2. Material and Methods
2.1. Experimental Diets
Six experimental diets were formulated by gradient supplementation 0%, 0.004% and 0.008% soybean isoflavones at different dietary lipid levels (10% and 15%), which were named to NF-0 group (10% fat and 0% SIF), NF-0.004 group (10% fat and 0.004% SIF), NF-0.008 group (10% fat and 0.008% SIF), HF-0 group (15% fat and 0% SIF), HF-0.004 group (15% fat and 0.004% SIF) and HF-0.008 group (15% fat and 0.008% SIF). The formulation and proximate composition of the experimental diets are shown in
Table 1.
The ingredients were finely ground and sieved through a 60-mesh strainer. The ingredients were weighed according to the formula and mixed using an electric mixer. The oil and distilled water were subsequently added to make a dough. Finally, the dough was pelleted using a screw-press pelletizer. The pellets were air-dried to the moisture content was < 10%. After drying, diets were stored at -20 °C.
2.2. Feeding Trial, Sampling and Growth Evaluation
Adult crabs were obtained from a farm in Huzhou. Crabs were acclimatized to the experimental conditions in 300 L tanks (100 × 80 × 60 cm) before the feeding trial. A total of 1050 female crabs (0.4 ± 0.03 g, mean ± SE) were weighed and put into 30 tanks (100 × 80 × 60 cm) and each tank containing 35 crabs. Each experimental diet was randomly allotted to 4 tanks. Three plastic nets were placed in each tank as shelters to reduce attacking behavior. Diets with a daily ration of 4% body weight were hand-fed to crabs three times at 6:00, 18:00. Feces were removed in the morning (09:00), and the water of 30% tank volume was exchanged daily. Dead crabs were immediately removed from the tank, weighed and recorded. Feed intake of each tank was recorded throughout the trial period. During the experimental period, the experimental water temperature in the tanks varied from 18 °C to 24 °C, the dissolved oxygen concentration was >7 mg/L, the ammonia nitrogen was <0.05 mg/L.
At the end, crabs were euthanized, after which the hepatopancreaseses were frozen in liquid nitrogen and kept at ultra-low temperature freezer for enzyme activity, gene expression and nutrient composition analyses.
Weight gain, specific growth rate, survival and hepatopancreas index were calculated using the formulas as below:
Weight gain (WG, %) = (final crab weight - initial crab weight) × 100/initial crab weight;
Specific growth rate (SGR, %) = 100 × (LN final weight - LN initial weight) / days;
Survival (%) = 100 × final number / initial number.
Hepatopancreas index (%) = hepatopancreas weight of crab/whole crab weight × 100.
2.3. Chemical Composition Analysis
Chemical compositions of the experimental diets and crabs were measured according to the standard procedures for proximate composition analysis [
14]. Four duplicate samples were measured in each treatment (n = 4). Moisture was determined after oven dry at 105 °C. Crude protein was quantified using the Kjeltec™ 8200 (Foss, Hoganas, Sweden). Crude lipid was extracted using a 1000 mL Soxhlet extraction tube (Fujian minbo toughened glass Co. Ltd., Fujian, China). Ash was analyzed using a muffle furnace (PCD-E3000 Serials, Peaks, Japan) at 550 °C for 6 h.
2.4. Analysis of Biochemical Parameters in the Hepatopancreas
The biochemical parameters in the hepatopancreas were measured using commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) in accordance with the instructions of the manufacturer. The source and information of each kit used in this study were as follows: non-esterified fatty acid (NEFA; Cat. No. A042-1-1), lipase (LPS; Cat. No. A054-1-1), total cholesterol (TC; Cat. No. A111-2-1) and triglyceride (TG; Cat. No. A110-2-1).
2.5. Analysis of Gene Expression
Total RNA was extracted from the hepatopancreas using the RNAiso Plus (CAT # 9109, Takara, Japan). The total RNA concentration and quality were estimated using the Nano Drop 2000 spectrophotometer (Thermo, USA). If the ratio of A260 / A280 was between 1.8 to 2.0, the sample was used for reverse transcription using a PrimeScript™ RT master mix reagent kit (Perfect Real Time, Takara, Japan). The specific primers for the genes of
E. sinensis were designed based on the transcriptome sequencing results and NCBI data base using NCBI Primer BLAST (
Table 2). The RT-PCR amplification reactions were performed in a volume of 10 μL containing 5 μL 2×SYBR Premix Ex TaqTM, 0.25 μL of 10 mM forward primer, 0.25 μL of 10 mM reverse primer and 4.5 μL of diluted cDNA, using CFX96 Real-Time PCR system (Bio-rad, Richmond, CA). PCR conditions were as follows: 94 °C for 3 min, and following 40 cycles at 94 °C for 15 s and 60 °C for 50 s, and 72 °C for 20 s. Samples were run in quintuplicate and normalized with the control gene β-actin and glyceraldehyde-phosphate dehydrogenase (GAPDH). The gene expression levels were calculated by geometric averaging of multiple internal control genes [
15].
Table 2.
Sequences of primers.
Table 2.
Sequences of primers.
| Primers name |
Sequences(5’ - 3’) |
Product length |
|
β-actin F |
TGGGTATGGAATCCGTTGGC |
101 bp |
|
β-actin R |
AGACAGAACGTTGTTGGCGA |
|
GAPDH F |
CACCGTGCATGCTGTTACTG |
108 bp |
|
GAPDH R |
ACCAGTGGAGGATGGGATGA |
|
tgl F |
CCCTGTGGCGTACTTGGATT |
169 bp |
|
tgl R |
GGCGTAGGTGCTGTTGTCGT |
|
srebp-1 F |
TCTTCACACCCTCTGGACGC |
162 bp |
|
srebp-1 R |
CCAAGGTTGTAATGGCACGC |
|
fabp3 F |
CCACCGAGGTCAAGTTCAAGC |
195 bp |
|
fabp3 R |
TCACACCATCACACTCCGACAC |
|
fatp4 F |
GACGGCAGACACGGAAAGAGA |
101 bp |
|
fatp4 R |
CAGGTGGAGGCAAGCAAACTC |
|
fabp10 F |
TGCTGATTGGCTCAGTGCTGTG |
115 bp |
|
fabp10 R |
CGTGGTCTTGATGACGATGTCG |
|
elovl6 F |
TGAGAAGCGGCAATGGATGAAG |
164 bp |
|
elovl6 R |
TGGAGAAGAGGGCCAGGAAGAC |
|
Δ9 fad F |
TGGCACAACTACCACCACGTCT |
160 bp |
|
Δ9 fad R |
TCCTCTTCTCGATCATCTCCGG |
|
cpt-1a F |
CATCTGGACACCCACCTCCA |
183 bp |
|
cpt-1a R |
ATCTCCTCACCCGGCACTCT |
|
cpt-1b F |
GGCATTCTCCTTTGCCATCAC |
138 bp |
|
cpt-1b R |
ACACCACACCGCACATTGTTC |
|
cpt-2 F |
AGCAGGCAGTGGCTCAGTTTA |
169 bp |
|
cpt-2 R |
AAGGCAAGGAAGGGGTTGTAG |
|
caat F |
CATCAAGAGCCAGGAGCCCA |
172 bp |
|
caat R |
CTTCAACAGCAGCCCGCAAA |
|
mttp F |
TAGGACAAGCAGGACTTTCCTCA |
138 bp |
|
mttp R |
CCACATCCACAAACACATCAACA |
2.6. Statistical Analysis
Statistical analysis was performed using the SPSS 26.0 for Windows (SPSS, Michigan Avenue, Chicago, IL, USA). All data were subject to normality test and homogeneity of variance by using Shapiro-Wilk and Levene’s equal variance tests, respectively. Data were analyzed by two-way analysis of variance (ANOVA) to determine if there was any interaction between dietary lipid level and SIF level. At the same lipid condition, one-way analysis of variance (ANOVA) was used to analyze the significant differences among crabs fed the diets with different SIF level after normality test and homogeneity of variance. When the means of each treatment were significantly different, Duncan’s multiple range test was used to compare means among these treatments. At the same SIF level, independent-samples T test was used to determine significant differences between crabs cultivated at different lipid levels. Significance was set at P < 0.05. The data were represented as the mean ± standard error of mean (S.E.).
3. Results
3.1. Growth Performance
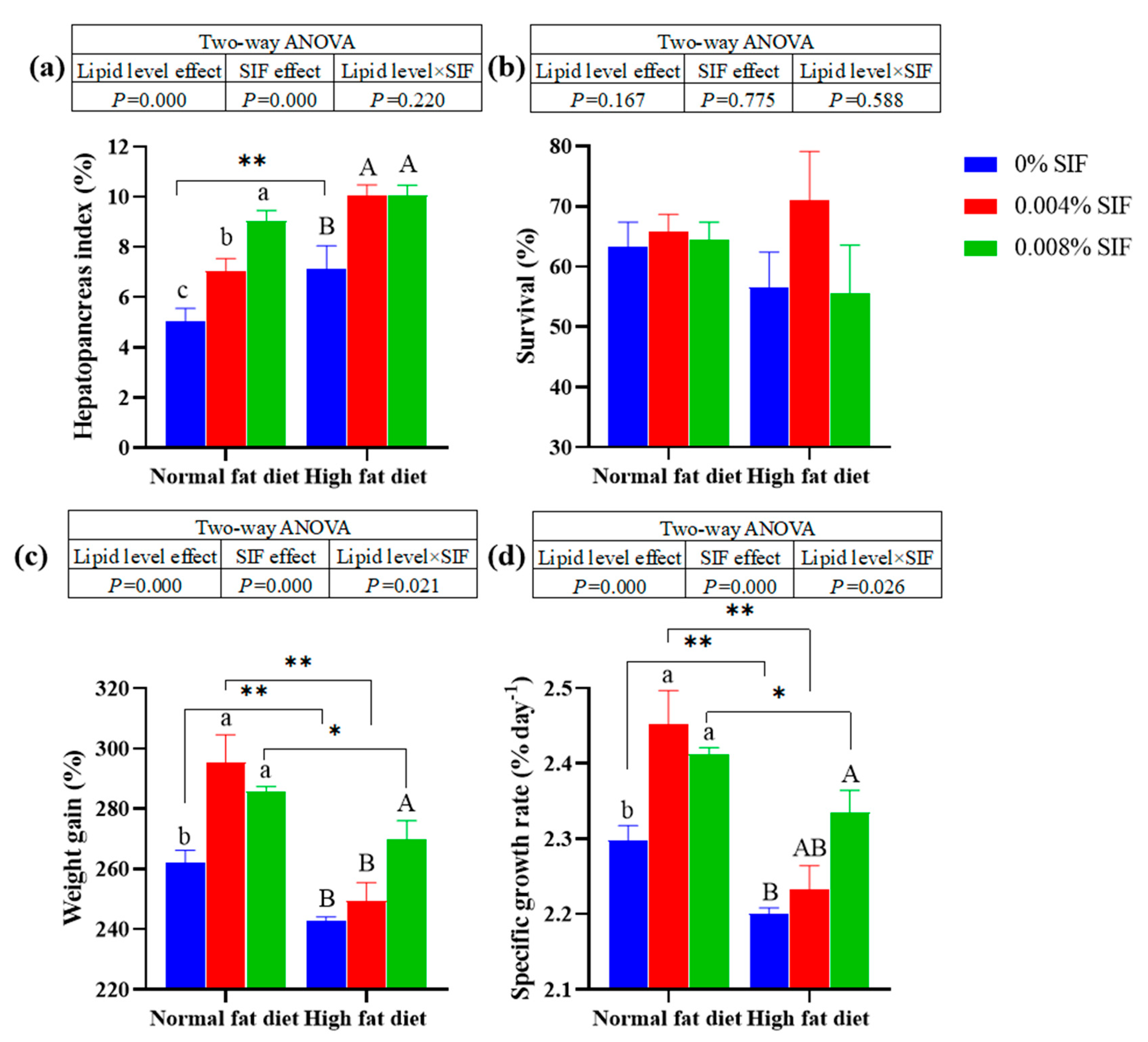
Both at normal fat diets and at high fat diets, dietary SIF significantly increased the weight gain (WG), specific growth rate (SGR) and hepatopancreas index (HSI) (P < 0.01). At normal fat level, the WG, SGR and HSI of crabs fed the diets supplemented with 0.004% and 0.008% SIF were significantly higher than crabs fed 0% SIF (P < 0.01). At high fat level, the WG, SGR and HSI of crabs fed the diets supplemented with 0.008% SIF were significantly higher than crabs fed 0% SIF (P < 0.01). In the absence of SIF, the WG and SGR of the crabs in the NF-0 group was significantly higher than that in the HF-0 group (P < 0.01). Dietary lipid level and SIF did not significantly affect the survival of crabs (P > 0.01).
Figure 1.
Effects of soybean isoflavones on the growth performance of juvenile Chinese mitten crab. Note: (a) Hepatopancreas index, (b) Survival, (c) Weight gain, (d) Specific growth rate. The different superscripts on the columns represent significant differences (P < 0.05, one-way ANOVA and Duncan multiple comparisons). The lines connecting the columns represent significant differences (P < 0.05 (marked *) or P < 0.01 (marked * *), independence T-test and Duncan multiple comparison). The table above the column represents the results of the two-factor analysis of variance, the same below.
Figure 1.
Effects of soybean isoflavones on the growth performance of juvenile Chinese mitten crab. Note: (a) Hepatopancreas index, (b) Survival, (c) Weight gain, (d) Specific growth rate. The different superscripts on the columns represent significant differences (P < 0.05, one-way ANOVA and Duncan multiple comparisons). The lines connecting the columns represent significant differences (P < 0.05 (marked *) or P < 0.01 (marked * *), independence T-test and Duncan multiple comparison). The table above the column represents the results of the two-factor analysis of variance, the same below.
3.2. Biochemical Parameters in the Hepatopancreas
At normal fat level, diets supplemented with 0.004% and 0.008% SIF significantly decreased the NEFA and TG contents in the hepatopancreas (P < 0.01). In the absence of SIF, the NEFA and TG contents in the hepatopancreas of crabs in the NF-0 group was significantly higher than that in the HF-0 group (P < 0.01). Dietary lipid level and SIF did not significantly affect the LPS activities of crabs (P > 0.01).
Table 3.
Effects of soybean isoflavones on the biochemical parameters in the hepatopancreas of Chinese mitten crab.
Table 3.
Effects of soybean isoflavones on the biochemical parameters in the hepatopancreas of Chinese mitten crab.
| |
Parameters |
| Diets |
NEFA
(µmol/gprot) |
LPS
(U/gprot) |
TC
(mmol/gprot) |
TG
(mmol/gprot) |
| NF-0 |
36.56±16.49a
|
1.49±0.74 |
3.98±0.42* |
34.13±2.26a
|
| NF-0.004 |
20.75±7.75b
|
1.83±0.38 |
4.01±0.29 |
30.29±2.13b* |
| NF-0.008 |
17.47±9.50b
|
2.13±0.35 |
4.01±0.74 |
26.80±3.53c* |
| HF-0 |
26.42±6.00A
|
2.44±0.65 |
3.15±0.36* |
33.61±4.66 |
| HF-0.004 |
22.65±3.48A
|
1.70±0.59 |
3.46±0.55 |
38.03±3.75* |
| HF-0.008 |
21.16±9.12B
|
2.73±0.88 |
3.70±0.54 |
33.31±2.61* |
| Two-way ANOVA (P value) |
|
|
| Lipid level |
<0.01 |
NS |
<0.01 |
<0.01 |
| SIF |
<0.01 |
NS |
NS |
<0.01 |
| Lipid level × SIF |
<0.01 |
NS |
NS |
<0.01 |
3.3. The mRNA Expressions of Genes Related to Lipid Synthesis in the Hepatopancreas
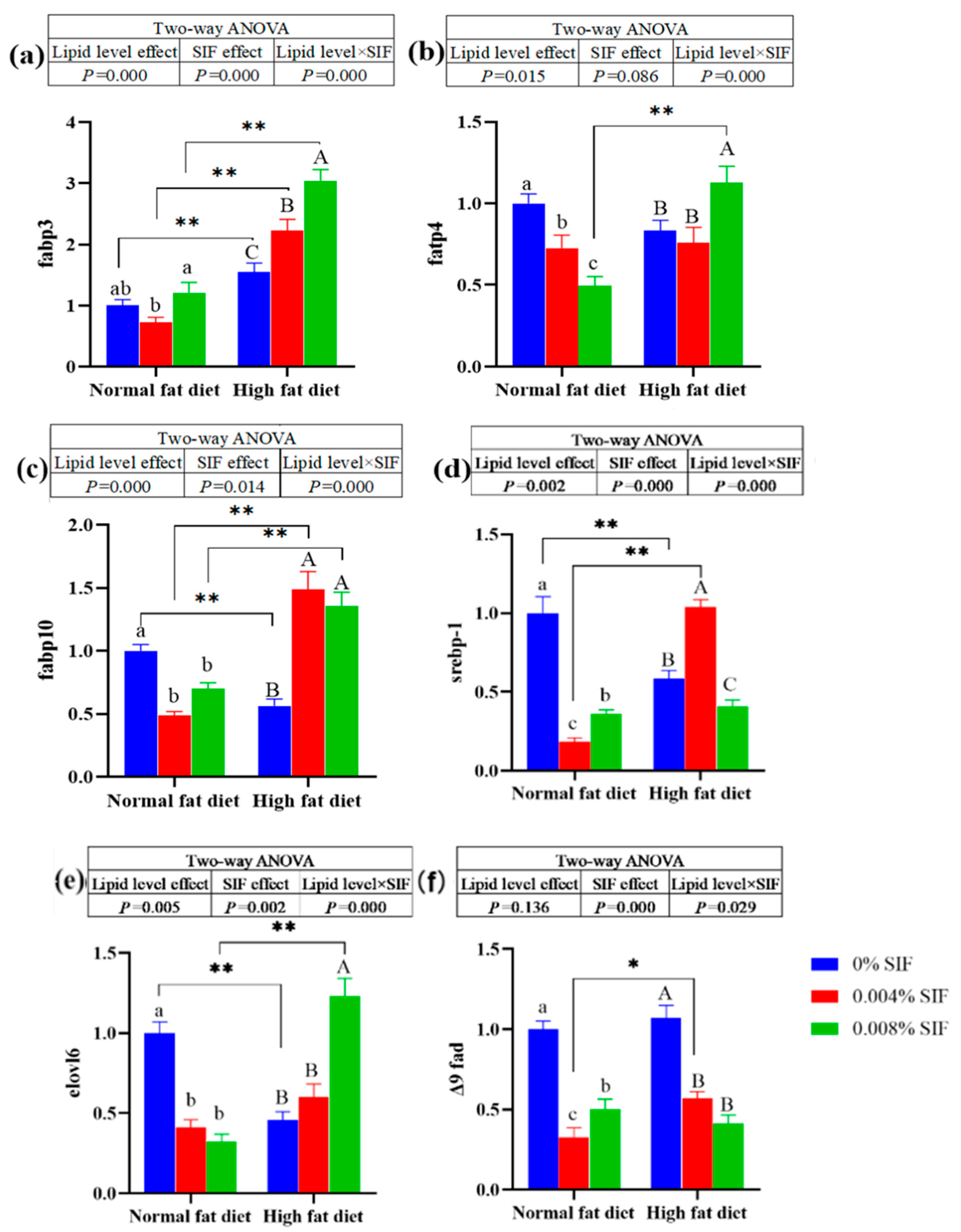
At normal fat level, diets supplemented with 0.004% SIF significantly down-regulated the mRNA expression of fabp 3 in the hepatopancreas (P < 0.01). However, at high fat level, diets supplemented with 0.004% and 0.008% SIF significantly up-regulated the mRNA expression of fabp 3 in the hepatopancreas (P < 0.01). The mRNA expression of fabp 3 of crabs fed the high fat diets were significantly higher than crabs fed the normal fat diets (P < 0.01). At normal fat level, dietay SIF significantly down-regulated the mRNA expression of fabp 4, fabp 10, srebp-1, elovl6 and Δ9 fad in the hepatopancreas (P < 0.01). At high fat level, diets supplemented with 0.008% SIF significantly up-regulated the mRNA expression of fabp 4 and elovl6 in the hepatopancreas (P < 0.01). Diets supplemented with 0.004% and 0.008% SIF significantly up-regulated the mRNA expression of fabp 10 in the hepatopancreas of crabs under high fat conditions (P < 0.01). Diets supplemented with 0.004% SIF significantly up-regulated the mRNA expression of srebp-1 in the hepatopancreas of crabs under high fat conditions (P < 0.01). At high fat level, diets supplemented with 0.004% SIF significantly up-regulated the mRNA expression of srebp-1 in the hepatopancreas (P < 0.01). On the contrary, diets supplemented with 0.004% and 0.008% SIF significantly down-regulated the mRNA expression of Δ9 fad in the hepatopancreas of crabs under high fat conditions (P < 0.01).
Figure 2.
Effects of soybean isoflavones on the mRNA expressions of genes related to lipid synthesis in the hepatopancreas of juvenile Chinese mitten crab. Note: (a) fatty acid binding protein 3; (b) fatty acid transport protein 4; (c) fatty acid binding protein 10; (d) sterol-regulatory element binding protein 1; (e) elongase of very long-chain fatty acids 6, (f) Δ9 fatty acyl desaturase.
Figure 2.
Effects of soybean isoflavones on the mRNA expressions of genes related to lipid synthesis in the hepatopancreas of juvenile Chinese mitten crab. Note: (a) fatty acid binding protein 3; (b) fatty acid transport protein 4; (c) fatty acid binding protein 10; (d) sterol-regulatory element binding protein 1; (e) elongase of very long-chain fatty acids 6, (f) Δ9 fatty acyl desaturase.
3.4. the mRNA Expressions of Lipolysis Related Genes in the Hepatopancreas
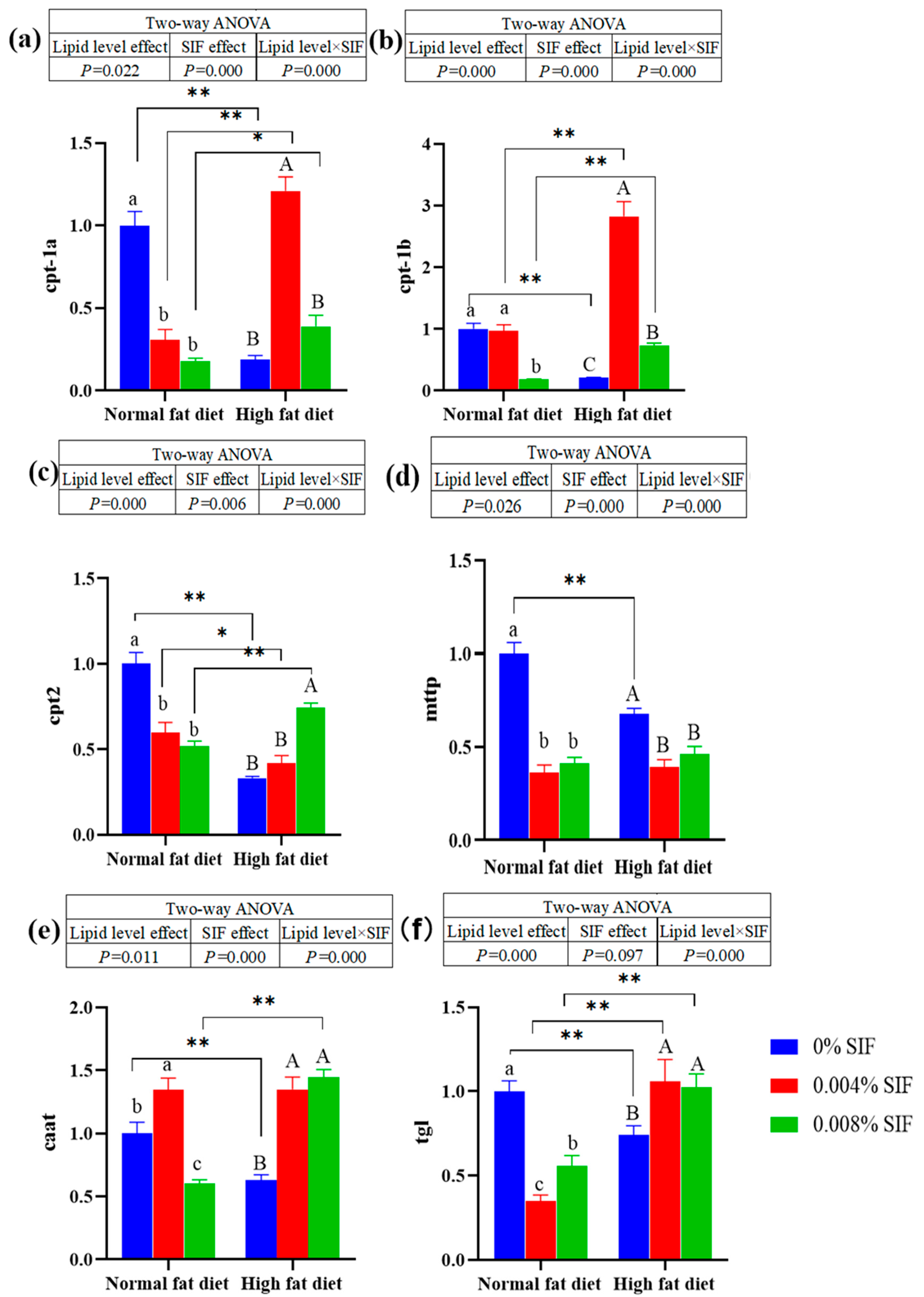
At normal fat level, diets supplemented with 0.004% SIF significantly down-regulated the mRNA expression of cpt-1a、cpt-2, mttp and tg1 in the hepatopancreas (P < 0.01). However, at high fat level, diets supplemented with 0.004% SIF significantly up-regulated the mRNA expression of cpt-1a and cpt-1b in the hepatopancreas (P < 0.01). Diets supplemented with 0.004% and 0.008% SIF significantly up-regulated the mRNA expression of caat and tg1 in the hepatopancreas of crabs under high fat conditions (P < 0.01). On the contrary, diets supplemented with 0.004% and 0.008% SIF significantly down-regulated the mRNA expression of mttp in the hepatopancreas of crabs under high fat conditions (P < 0.01). Diets supplemented with 0.008% SIF significantly down-regulated the mRNA expression of caat in the hepatopancreas of crabs under high fat conditions (P < 0.01). In the absence of SIF, the mRNA expression of cpt-1a and cpt-1b in the hepatopancreas of the crabs in the NF-0 group was significantly higher than that in the HF-0 group (P < 0.01). However, Dietary SIF significantly up-regulated the mRNA expression of cpt-1a and cpt-1b of crabs fed the high fat diets (P < 0.01). The similar results were observed in cpt-2, caat and tg1.
Figure 3.
Effects of soybean isoflavones on the mRNA expressions of lipolysis related genes in the hepatopancreas of juvenile Chinese mitten crab. Note: (a) carnitine palmitoyl transterase 1a, (b) carnitine palmitoyl transterase 1b, (c) carnitine palmitoyl transterase 2, (d) microsomal triglyceride transfer protein, (e) carnitine acetyltransferase, (f) triacylglycerol lipase.
Figure 3.
Effects of soybean isoflavones on the mRNA expressions of lipolysis related genes in the hepatopancreas of juvenile Chinese mitten crab. Note: (a) carnitine palmitoyl transterase 1a, (b) carnitine palmitoyl transterase 1b, (c) carnitine palmitoyl transterase 2, (d) microsomal triglyceride transfer protein, (e) carnitine acetyltransferase, (f) triacylglycerol lipase.
4. Discussion
Lipid is an important nutrient for aquatic animals, and an optimal dietary lipid level can ensure growth and development of aquatic animals. In the present study, 15% lipid diets significantly reduced the growth performance of juvenile Chinese mitten crab. The similar result was also reported in
Cyprinus carpio [
16]. These results showed that an excessive dietary lipid level has a negative impact on aquatic animals. Soy isoflavones play an important role in improving the immunity and lipid metabolism. It has been reported that diets supplemented with SIF improved the growth performance of pigs [
17] and chickens [
18] at normal fat diets. Moreover, high fat diets supplementation with SIF was able to promote the insulin resistant and growth of rats [
19]. There were some contrary results reported that SIF had no significant effect on the growth performance of animal [
20]. Even more, 4 g/kg SIF significantly inhibited the growth of fish [
21]. In the present study, the high fat diet decreased WG and SGR of juvenile crabs, but dietary SIF increased the growth performance of juvenile crabs. The possible reason for these results was that dietary SIF promoted the utilization of high-fat diet by optimizing the lipid metabolism process, thereby improving the growth performance of juvenile Chinese mitten crab.
A large number of studies have reported that SIF can perform lipid-decreasing effects in humans and animals [
22,
23]. Studies have reported that dietary SIF can reduce the blood glucose, serum TG and LDL-C levels in the mouse model of type 2 diabetes induced by high-fat and high-sugar diet [
24]. In the present study, at the 10% lipid level, dietary SIF significantly reduced the contents of NEFA and TG in the hepatopancreas of crab, which indicating that SIF may be involved in lipolysis and provide energy for the growth of juvenile Chinese mitten crab. However, 15% lipid diet decreased the TG and NEFA content in the hepatopancreas when the diet was not supplemented with SIF, but when diets supplementation with SIF, the TC content tended to increasing, which indicate that SIF may improve dietary fat utilization in juvenile crabs.
To date, the regulatory mechanisms of SIF on lipid metabolism in crustaceans is still unknown. Previous studies have shown that SIF can affect the activities of enzymes and β-oxidation involved in fat metabolism [
25,
26,
27,
28]. In the present study, the results showed SIF supplementation significantly up-regulated the relative expression of genes related to fat synthesis and lipolysis of crabs fed the high fat diets, which indicated that SIF could improve lipid synthesis and oxidation in the Chinese mitten crab. The similar result was reported in the rainbow trout, which reported that SIF enhanced the expression of lipid synthesis genes and lipid binding protein genes [
11]. The function of FABPs is targeted transporting the hepatic fat to catabolic and anabolic sites, which is an important indicator for fat anabolism [
29]. In the present study, SIF supplementation significantly increased the expression of FABPs and elovl6 of crabs fed with 15% fat diets. This result indicated that SIF can improve the lipid synthesis in Chinese mitten crab fed with high fat diets. In contrast, some other studies found that dietary SIF significantly inhibited the expression of genes related to fat synthesis in rats [
30]. The reason for these divergent results may be related to species differences.
Lipid oxidation is one of the key steps in lipid metabolism in mammals, fish and crabs, and β-oxidation is the main pathway of fatty acid oxidation [
31,
32]. CPT1 and CPT2 form the mitochondrial carnitine palmitoyltransferase system, which plays an important role in the transfer of long-chain fatty acids from cytosolic compartments to the cytoplasm [
33]. In the present study, the results showed that SIF increased the relative expression of genes related to lipolysis (
cpt-1a,
cpt-1b,
cpt-2 and
caat) in crabs fed the high fat diets. These results suggested that SIF supplemented to the high-fat diet may accelerate the lipid oxidation and improve the utilization of dietary lipid in the Chinese mitten crab.
5. Conclusions
Diets supplementation with 0.004% SIF or 0.008% SIF could inhibit the expression of fatty acid synthesis related genes srebp-1 and Δ9 fad, and promote the expression of lipolysis related genes caat, tgl, cpt-1a, cpt-1b and cpt2. Thereby, improving the growth performance and lipid utilization in juvenile Chinese mitten crab.
Acknowledgments
This research was supported by grants from the Zhejiang Provincial Natural Science Foundation of China under Grant No. LTGN23C190003, and the Huzhou Natural Science Foundation (2021YZ14), the National Natural Science Foundation of China (No. 32072986) and Zhejiang Province R&D Plan (2022C02058).
References
- Sacks, F.M.; Lichtenstein, A.; Horn, D.; Kris-Etherton, P.; Winston, M. Soy protein isoflavones and cardiovascular health. Circulation 2006, 113, 1689–1692. [Google Scholar] [CrossRef]
- Anthony, M.S.; Clarkson, T.B.; Koudy, W.J. Effectsof soy isoflavones on atherosclerosis: potential mechanisms. Am. J. of Clin. Nutr. 1998, 68, 1390–1393. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.O.; Renouf, M.; Ye, Z.; Murphy, P.A.; Hendrich, S. Isoflavone glycitein diminished plasma cholesterol in female golden Syrian hamsters. J. Agr. Food Chem. 2007, 55, 11063–11067. [Google Scholar] [CrossRef]
- Ali, A.A.; Velasquez, M.T.; Hansen, C.T.; Mohamed, A.I.; Bhathena, S.J. Effects of soybean isoflavones, probiotics, and their interactions on lipid metabolism and endocrine system in an animal model of obesity and diabetes. J. Nutr. Biochem. 2004, 15, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.T. Effects of soy isoflavones on serum FFA and hepatic PPARα mRNA expression in rats with feeding-induced metabolic syndrome. Heilongjiang University of Chinese Medicine, 2011.
- Kalaiselvan, V.; Kalaivani, M.; Vijayakumar, A.; Sureshkumar, K.; Venkateskumar, K. Current knowledge and future direction of research on soy isoflavones as a therapeutic agents. Phcog. Rev. 2010, 4, 111. [Google Scholar] [CrossRef]
- González-Granillo, M.; Steffensen, K.; Granados, O.; Torres, N.; Korach-André, M.; Ortíz, V.; Aguilar-Salinas, C.; Jakobsson, T.; Díaz-Villaseñor, A.; Loza-Valdes, A.J.D. Soy protein isoflavones differentially regulate liver X receptor isoforms to modulate lipid metabolism and cholesterol transport in the liver and intestine in mice. Diabetologia 2012, 55, 2469–2478. [Google Scholar] [CrossRef]
- Mezei, O.; Banz, W.J.; Steger, R.W.; Peluso, M.R.; Winters, T.A.; Shay, N. Soy isoflavones exert antidiabetic and hypolipidemic effects through the PPAR pathways in obese Zucker rats and murine RAW 264.7 cells. J. Nutr. 2003, 133, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, W.; Zhi, S.; Zhao, M.; Liu, M.; Qin, C.; Feng, J.; Yan, X.; Nie, G. Evaluation of dietary genistein on the antioxidant capacity, non-specific immune status, and fatty acid composition of common carp (Cyprinus carpio. L). Aquaculture 2022, 550, 737822. [Google Scholar] [CrossRef]
- Grgic, D.; Varga, E.; Novak, B.; Müller, A.; Marko, D. Isoflavones in animals: metabolism and effects in livestock and occurrence in feed. Toxins 2021, 13, 836. [Google Scholar] [CrossRef]
- Cleveland, B.M.; Manor, M.L. Effects of phytoestrogens on growth-related and lipogenic genes in rainbow trout (Oncorhynchus mykiss). Comp. Biochem. 2015, 170, 28–37. [Google Scholar] [CrossRef]
- Deng, J.; Mai, K.; Ai, Q.; Zhang, W.; Wang, X.; Xu, W.; Liufu, Z.; Cai, Y.; Chen, W. Effects of antinutritional factors on plasma lipoprotein levels in Japanese flounder Paralichthys olivaceus. J. Fish Biol. 2012, 80, 286–300. [Google Scholar] [CrossRef]
- Zhang, W. Effects of soybean saponins and soybean isoflavones on growth, physiology and intestinal health of Carassius auratus. Suzhou University, 2010.
- AOAC. Official methods of analysis of AOAC International. Association of Official Analytical Chemists, Washington DC, USA 2005.
- Vandesompele, J.; Preter, K.D.; Pattyn, F.; Poppe, B.; Roy, N.V.; Paepe, A.D.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Wan, T.T.; Liu, X.X.; Jia, J.L.; Qin, C.B.; Nie, G.X. Regulative effects of Rehmanniae rehmanniae or Yam on growth, serum biochemical indexes and lipid metabolism of common Carp in high fat diet. Chin. Acad. Fish. Sci. 2023, 30, 48–59. [Google Scholar]
- Li, Y.; Jiang, X.; Wei, Z.; Cai, L.; Yin, J.; Li, X. Effects of soybean isoflavones on the growth performance, intestinal morphology and antioxidative properties in pigs. Animal 2020, 14, 2262–2270. [Google Scholar] [CrossRef]
- Jiang, Z.; Jiang, S.; Lin, Y.; Xi, P.; Yu, D.; Wu, T. Effects of soybean isoflavone on growth performance, meat quality, and antioxidation in male broilers. Poult. Sci. 2007, 86, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Chen, S.W.; Zhang, L.S.; Feng, X.F. The effects of soy isoflavone on insulin sensitivity and adipocytokines in insulin resistant rats administered with high-fat diet. Nat. Prod. Res. 2008, 22, 1637–1649. [Google Scholar] [CrossRef]
- Payne, R.; Bidner, T.; Southern, L.; Geaghan, J. Effects of dietary soy isoflavones on growth, carcass traits, and meat quality in growing-finishing pigs. J. Anim. Sci. 2001, 79, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Mai, K.; Zhang, Y.; Chen, W.; Xu, W.; Ai, Q.; Zhang, W. Effects of dietary soy isoflavones on feed intake, growth performance and digestibility in juvenile Japanese flounder (Paralichthys olivaceus). J. Ocean Univer. Chin. 2012, 11, 511–516. [Google Scholar] [CrossRef]
- Nestel, P.J.; Yamashita, T.; Sasahara, T.; Pomeroy, S.; Dart, A.; Komesaroff, P.; Owen, A.; Abbey, M. Soy isoflavones improve systemic arterial compliance but not plasma lipids in menopausal and perimenopausal women. Arteriosclerosis, Thrombosis, and Vasc. Bio. 1997, 17, 3392–3398. [Google Scholar] [CrossRef]
- Ørgaard, A.; Jensen, L. The effects of soy isoflavones on obesity. Exp. Bio. Med. 2008, 233, 1066–1080. [Google Scholar] [CrossRef]
- Wan, H.M. Effects of soy isoflavones on lipid metabolism and inflammatory factors in type 2 diabetic mice. Shanxi Medical University, 2021.
- Park, S.A.; Choi, M.S.; Cho, S.Y.; Seo, J.S.; Jung, U.J.; Kim, M.J.; Sung, M.K.; Park, Y.B.; Lee, M.K. Genistein and daidzein modulate hepatic glucose and lipid regulating enzyme activities in C57BL/KsJ-db/db mice. Life Sci. 2006, 79, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Tsurugasaki, W.; Nakamura, S.; Osada, K. Comparison of regulative functions between dietary soy isoflavones aglycone and glucoside on lipid metabolism in rats fed cholesterol. J. Nutr. Biochem. 2005, 16, 205–212. [Google Scholar] [CrossRef]
- Mezei, O.; Li, Y.; Mullen, E.; Ross-Viola, J.S.; Shay, N.F. Dietary isoflavone supplementation modulates lipid metabolism via PPARα-dependent and-independent mechanisms. Physiol. Genomics 2006, 26, 8–14. [Google Scholar] [CrossRef]
- Lee, Y.M.; Choi, J.S.; Kim, M.H.; Jung, M.H.; Lee, Y.S.; Song, J. Effects of dietary genistein on hepatic lipid metabolism and mitochondrial function in mice fed high-fat diets. Nutrition 2006, 22, 956–964. [Google Scholar] [CrossRef]
- Storch, J.; Thumser, A.E. Tissue-specific functions in the fatty acid-binding protein family. J. Biol. Chem. 2010, 285, 32679–32683. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.W.; Wood, C.M.; Weber, D.; Aziz, S.A.; Mehta, R.; Griffin, P.; Cockell, K.A. Dietary supplementation with soy isoflavones or replacement with soy proteins prevents hepatic lipid droplet accumulation and alters expression of genes involved in lipid metabolism in rats. Genes Nutr. 2014, 9, 1–12. [Google Scholar] [CrossRef]
- Nguyen, P.; Leray, V.; Diez, M.; Serisier, S.; Bloc’h, J.L.; Siliart, B.; Dumon, H. Liver lipid metabolism. J. Anim. Physiol. Anim. Nutr. 2008, 92, 272–283. [Google Scholar] [CrossRef]
- Greene, D.H.; Selivonchick, D.P. Lipid metabolism in fish. Progress in Lipid Research 1987, 26, 53–85. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Long, X.; Deng, D.; Cheng, Y.; Wu, X.; Loor, J.J. Molecular characterization and tissue distribution of carnitine palmitoyltransferases in Chinese mitten crab (Eriocheir sinensis) and the effect of dietary fish oil replacement on their expression in the hepatopancreas. Plos One 2018, 13, e0201324. [Google Scholar] [CrossRef]
Table 1.
Formulation and proximate composition of the experimental diets (dry matter, %).
Table 1.
Formulation and proximate composition of the experimental diets (dry matter, %).
| |
Normal Fat |
High Fat |
| |
0% SIF |
0.004% SIF |
0.008% SIF |
0% SIF |
0.004% SIF |
0.008% SIF |
| Ingredients |
NF-0 |
NF-0.004 |
NF-0.008 |
HF-0 |
HF-0.004 |
HF-0.008 |
| Fish meal |
20 |
20 |
20 |
20 |
20 |
20 |
| Casein |
21 |
21 |
21 |
21 |
21 |
21 |
| Gelatin |
7 |
7 |
7 |
7 |
7 |
7 |
| Corn starch |
23 |
23 |
23 |
23 |
23 |
23 |
| Soybean lecithin |
2 |
2 |
2 |
2 |
2 |
2 |
| Cholesterol |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
| Choline chloride a
|
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
| Fish oil |
3 |
3 |
3 |
7 |
7 |
7 |
| Soybean oil |
3 |
3 |
3 |
7 |
7 |
7 |
| Arginine |
1.8 |
1.8 |
1.8 |
1.8 |
1.8 |
1.8 |
| Methionine |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
| Lysine |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
| Vitamin premix a
|
1.5 |
1.5 |
1.5 |
1.5 |
1.5 |
1.5 |
| Mineral premix b
|
1.5 |
1.5 |
1.5 |
1.5 |
1.5 |
1.5 |
| Sodium carboxymethyl cellulose |
2 |
2 |
2 |
2 |
2 |
2 |
| Attractant |
3 |
3 |
3 |
3 |
3 |
3 |
| Butylated hydroxytoluene |
0.1 |
0.1 |
0.1 |
0.1 |
0.1 |
0.1 |
| SIF |
0 |
0.004 |
0.008 |
0 |
0.004 |
0.008 |
| Microcrystalline Cellulose |
9.1 |
9.096 |
9.092 |
1.1 |
1.096 |
1.092 |
| Proximate analysis(%)d
|
|
|
|
|
|
| Crude protein |
45.18 |
45.52 |
44.63 |
43.68 |
43.97 |
45.71 |
| Crude lipid |
9.76 |
9.28 |
9.57 |
15.80 |
16.15 |
16.55 |
| Moisture |
9.13 |
10.32 |
10.80 |
9.63 |
9.97 |
10.25 |
| Ash |
7.56 |
7.59 |
7.59 |
7.55 |
7.48 |
7.49 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).