Submitted:
14 May 2024
Posted:
15 May 2024
You are already at the latest version
Abstract
Keywords:
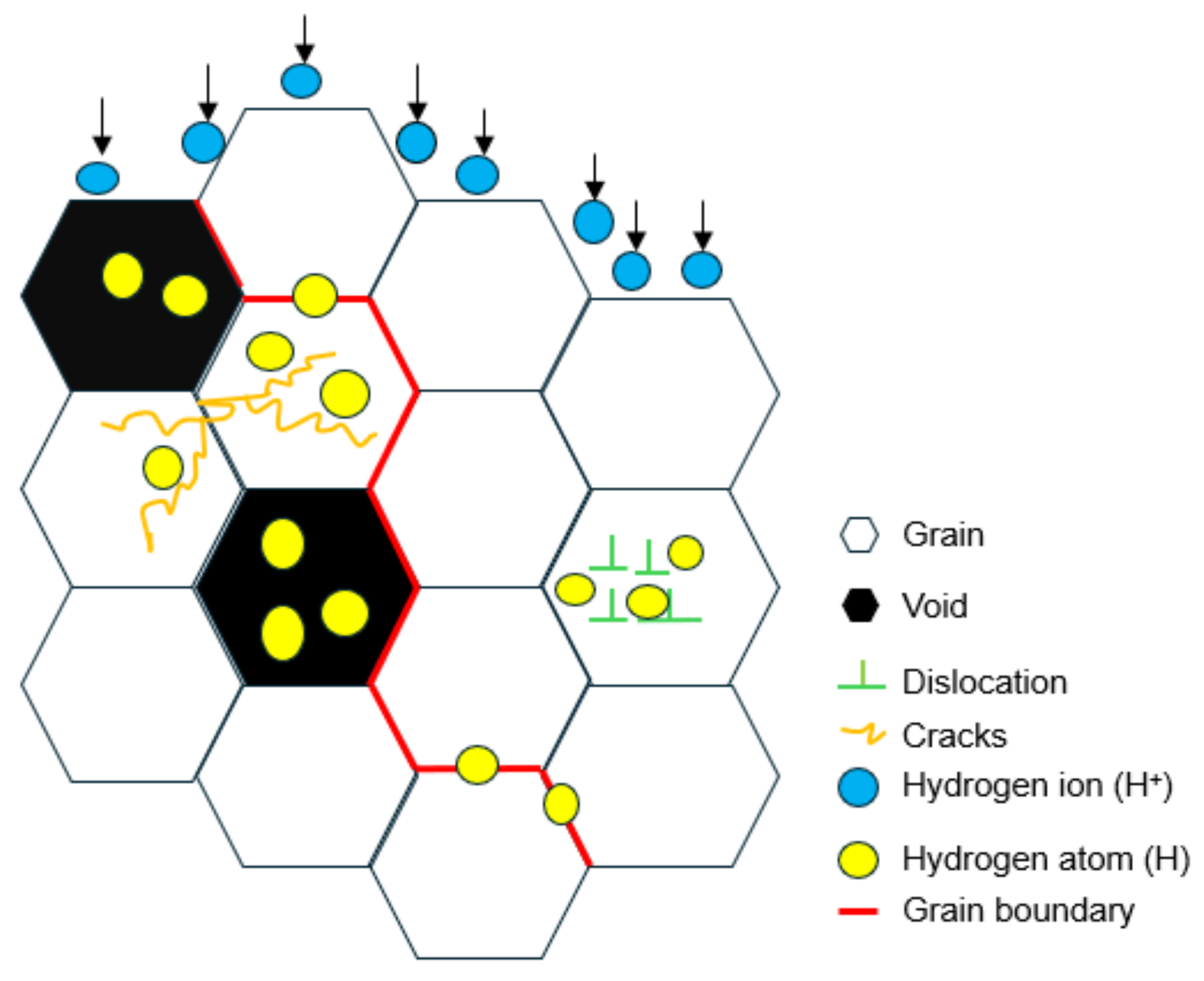
1. Introduction
2. Experimental Method
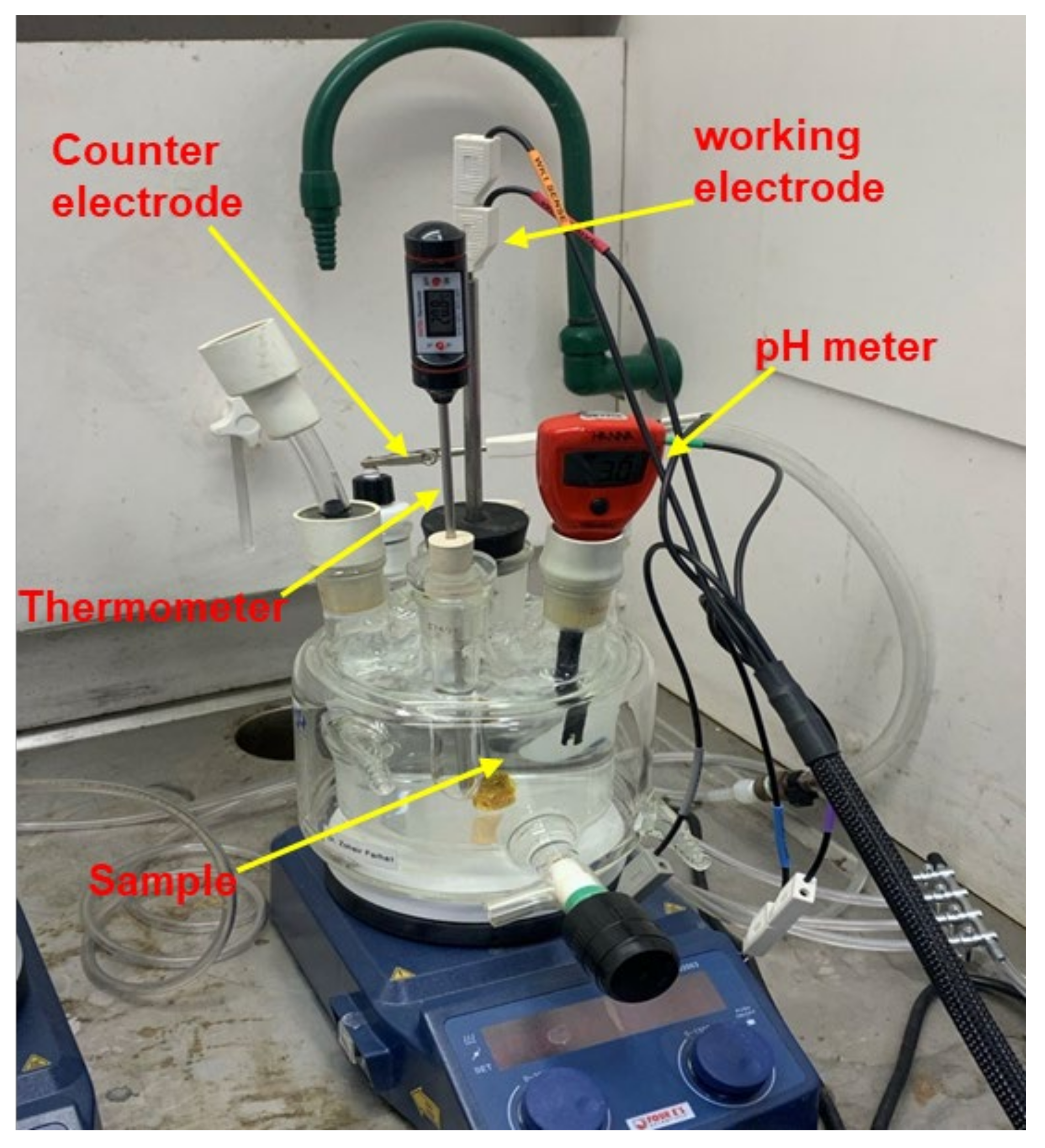
2.1. Electrochemical Charging
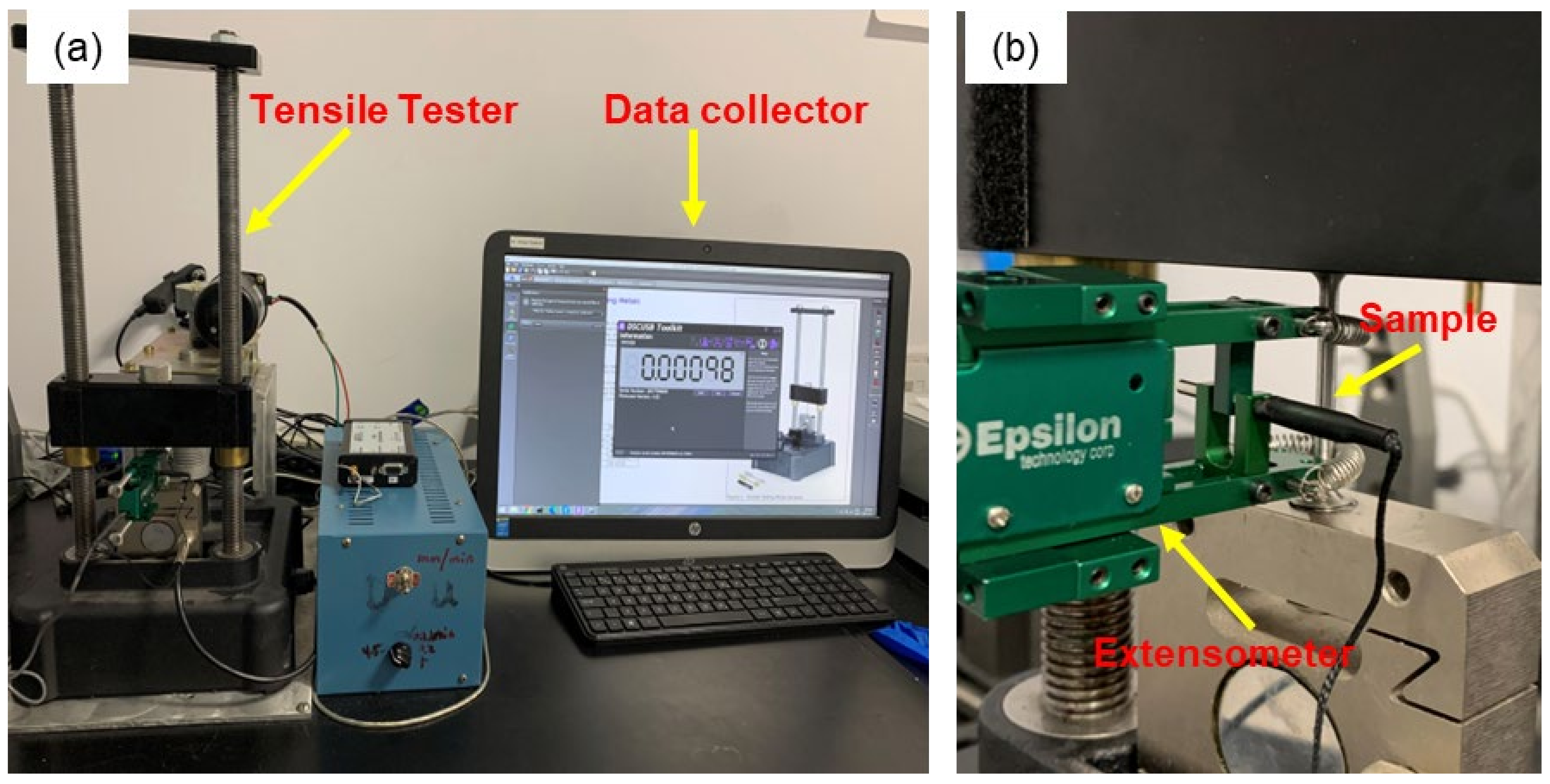
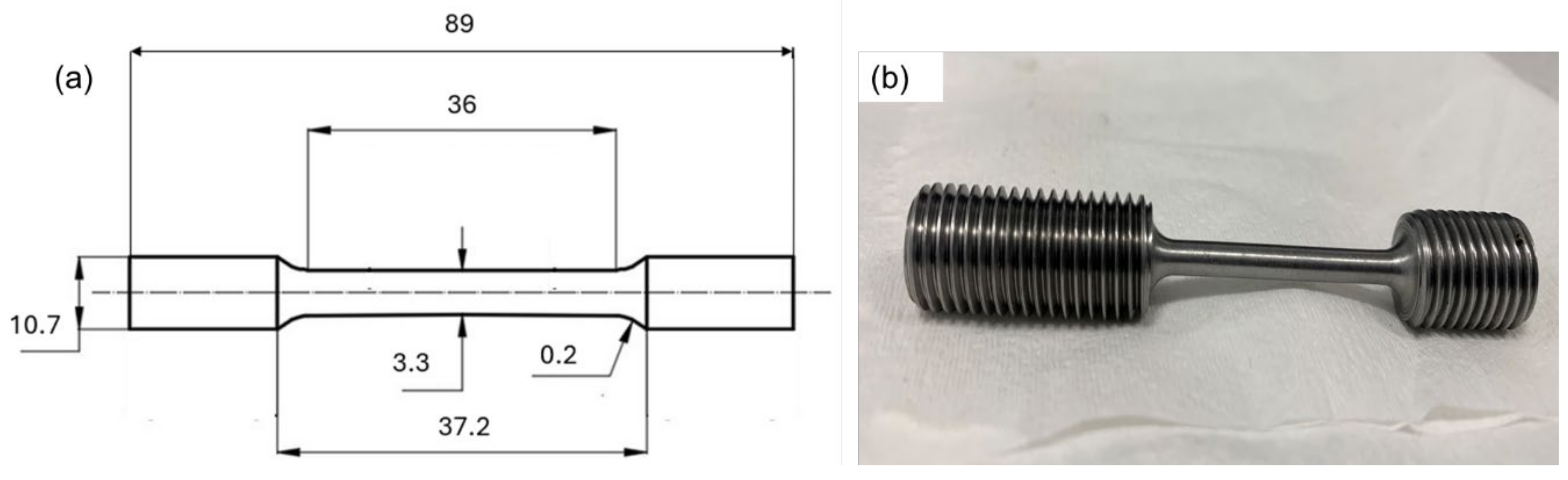
2.2. Tensile Testing
2.3. Characterization Techniques
2.3.1. Chemical Composition Analysis
| Element | Fe | C | Cr | Mg | Ni | S | Cu | Si | Mn |
| Composition (wt%) | 98.14 | 0.14 | 0.07 | 0.0008 | 0.04 | 0.004 | 0.08 | 0.25 | 0.50 |
| Element | C | Si | Mn |
| Compostion (wt%) | ≤ 0.25 | 0.1-0.5 | 0.4-0.7 |
2.3.2. Metallography
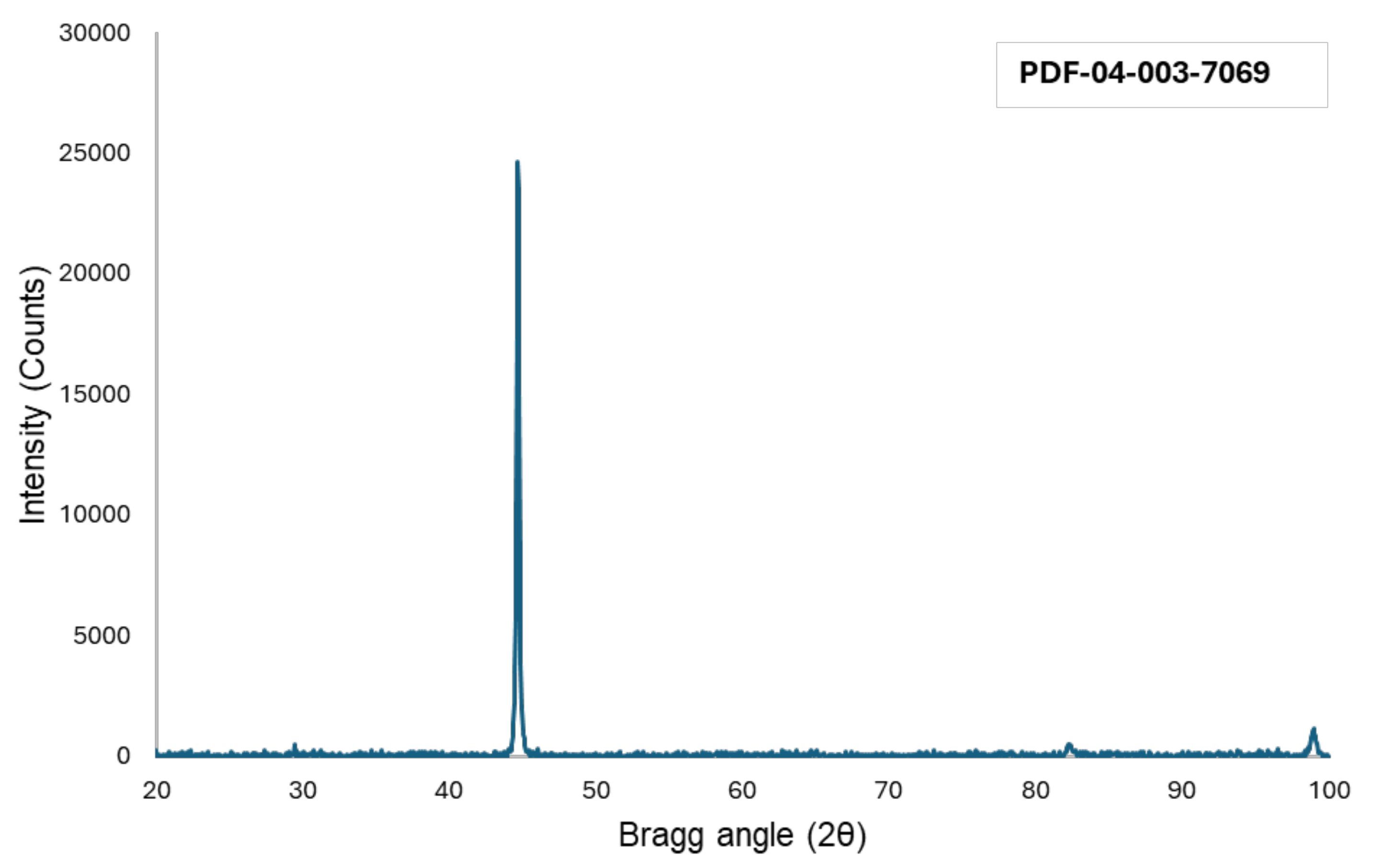
2.3.3. X-Ray Diffraction Analysis
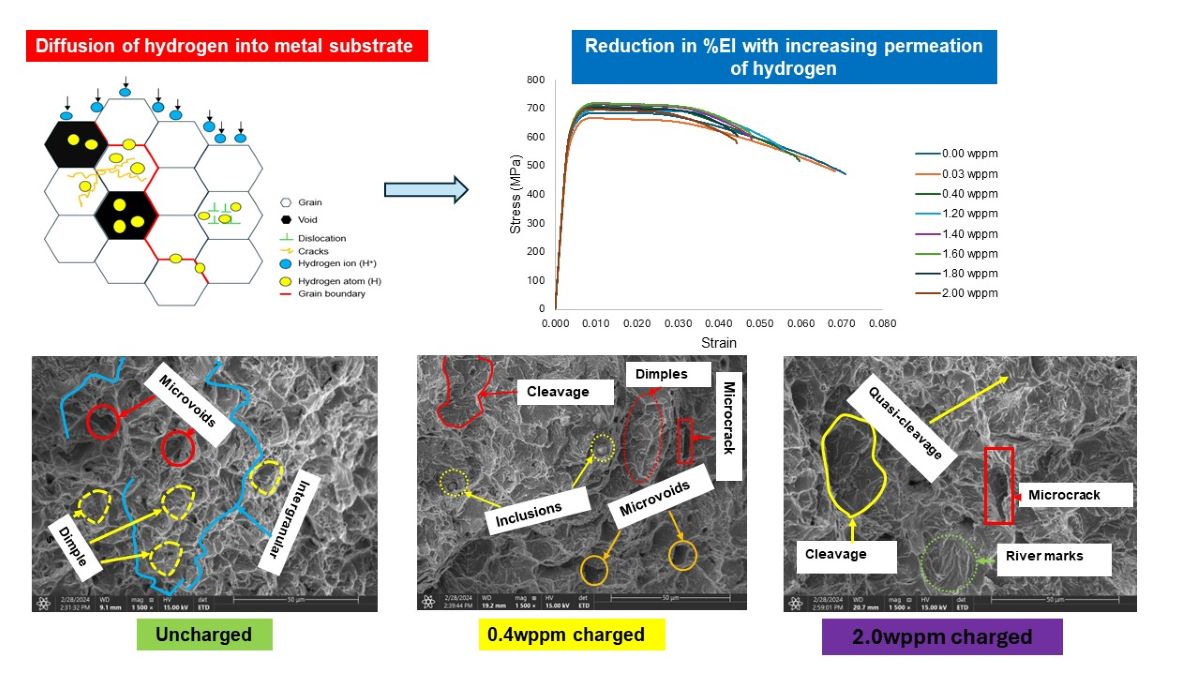
3. Results and Discussion
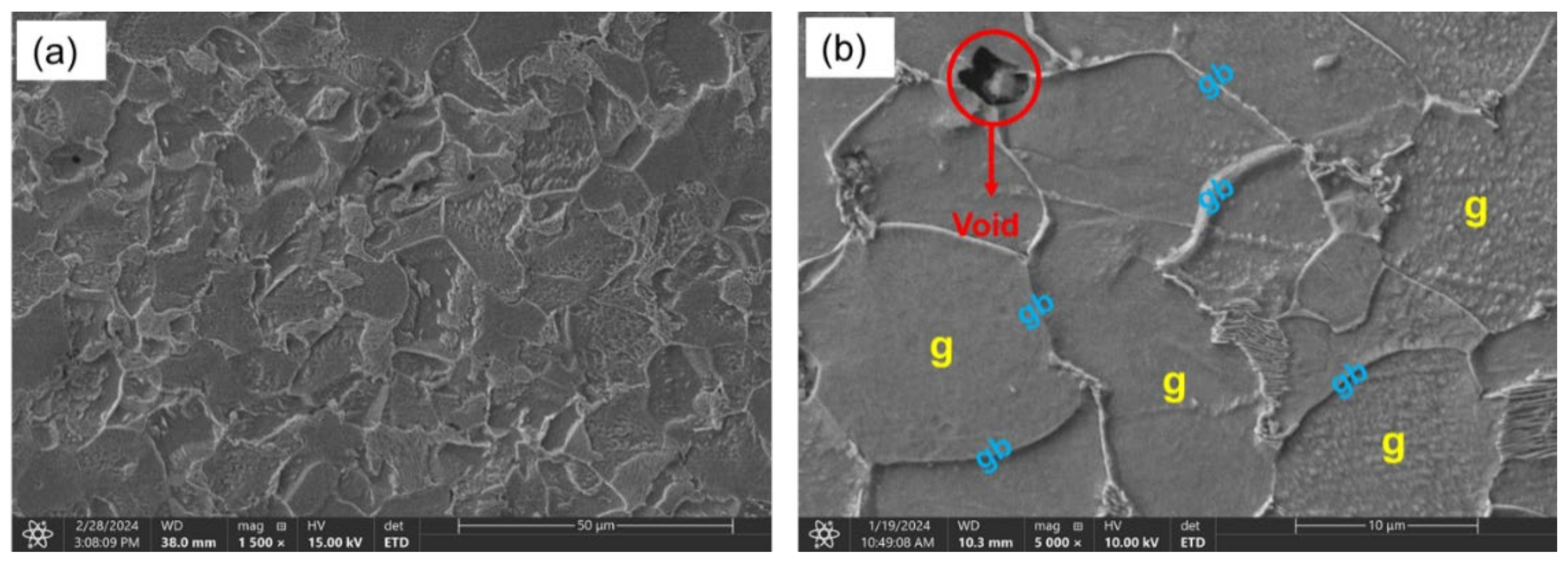
3.1. Microstructure (XRD, Rockwell Hardness, Confocal, SEM)
| Point | Hardness value (HRB) |
| 1 | 96.06 |
| 2 | 97.57 |
| 3 | 96.57 |
| 4 | 97.98 |
| 5 | 98.72 |
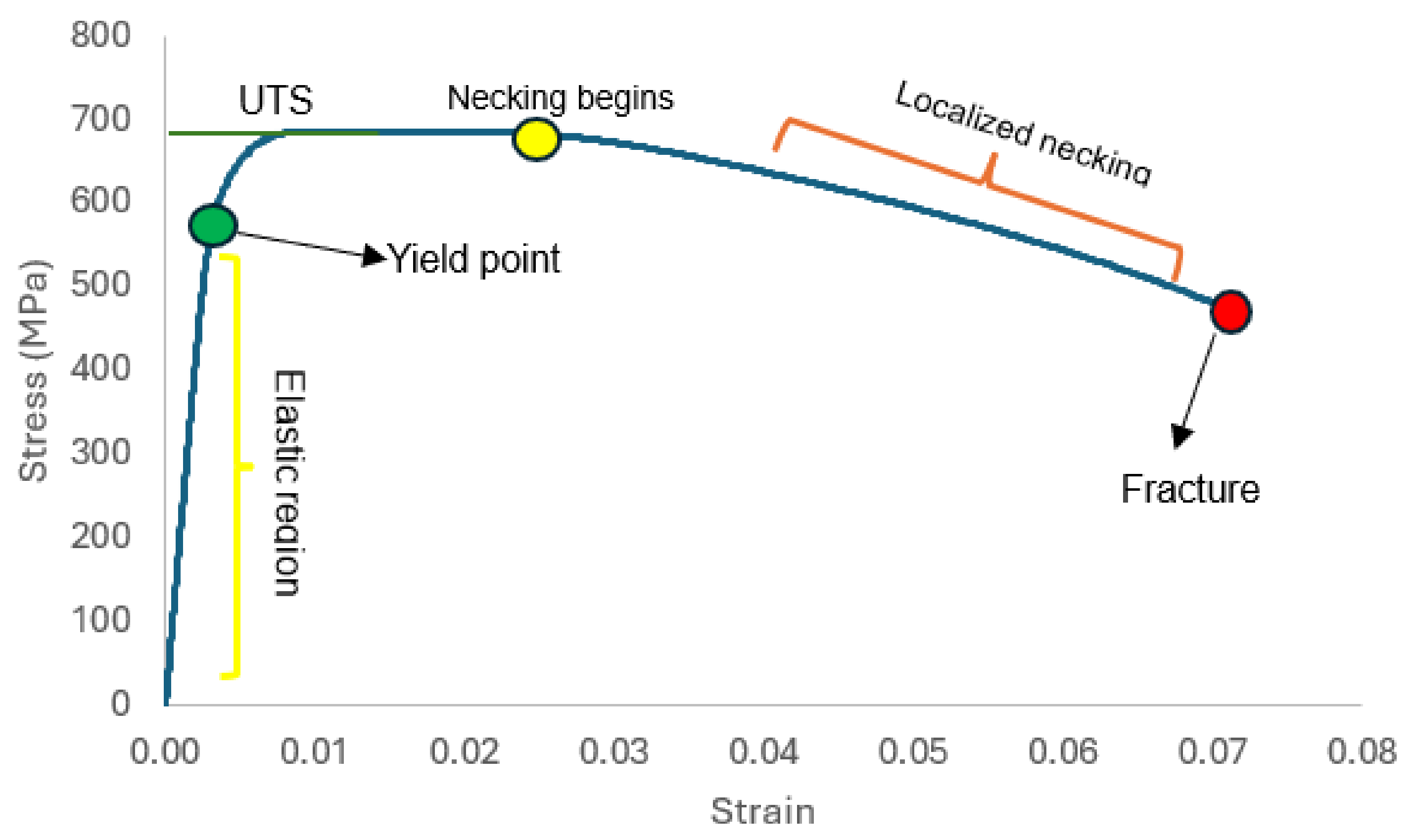
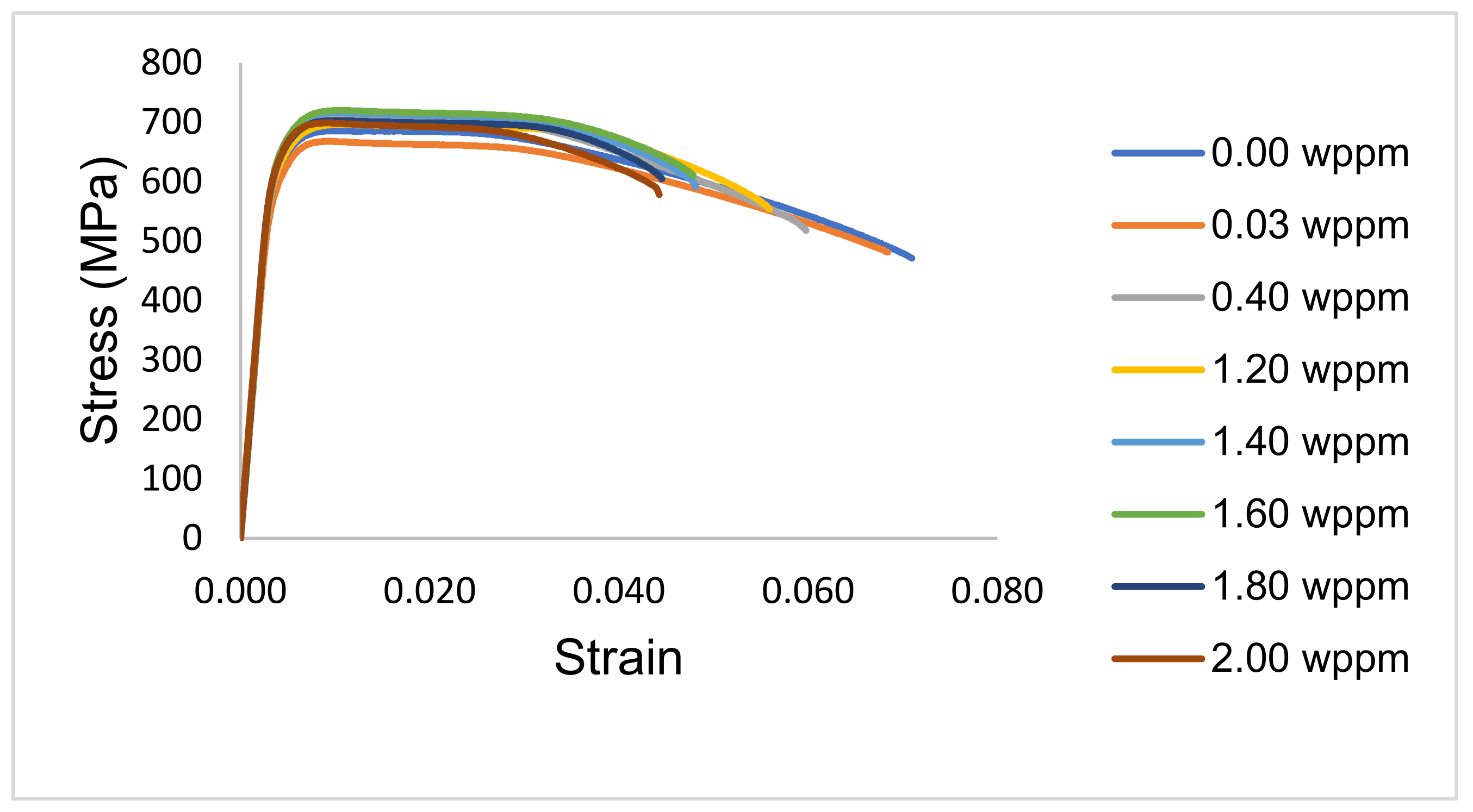
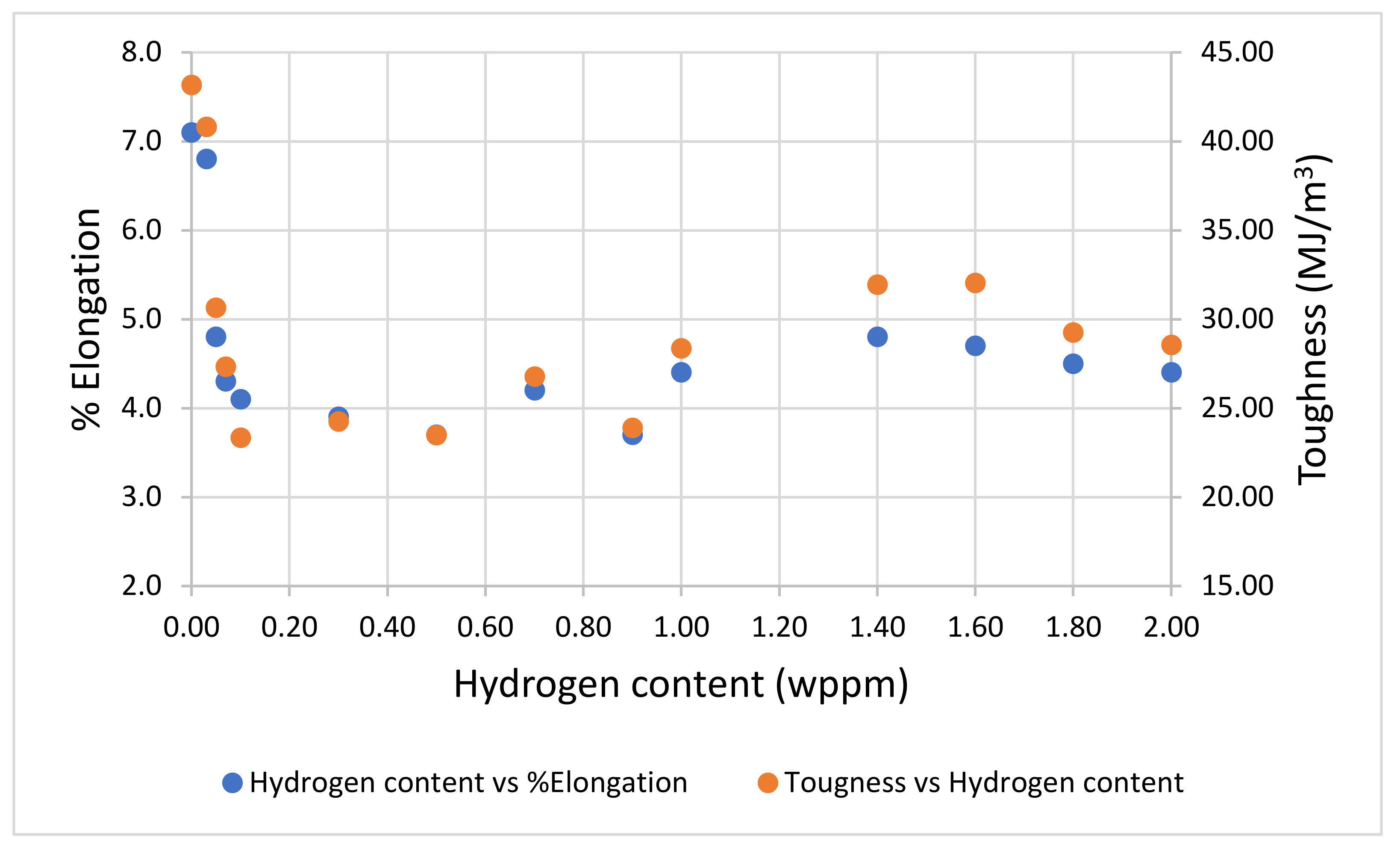
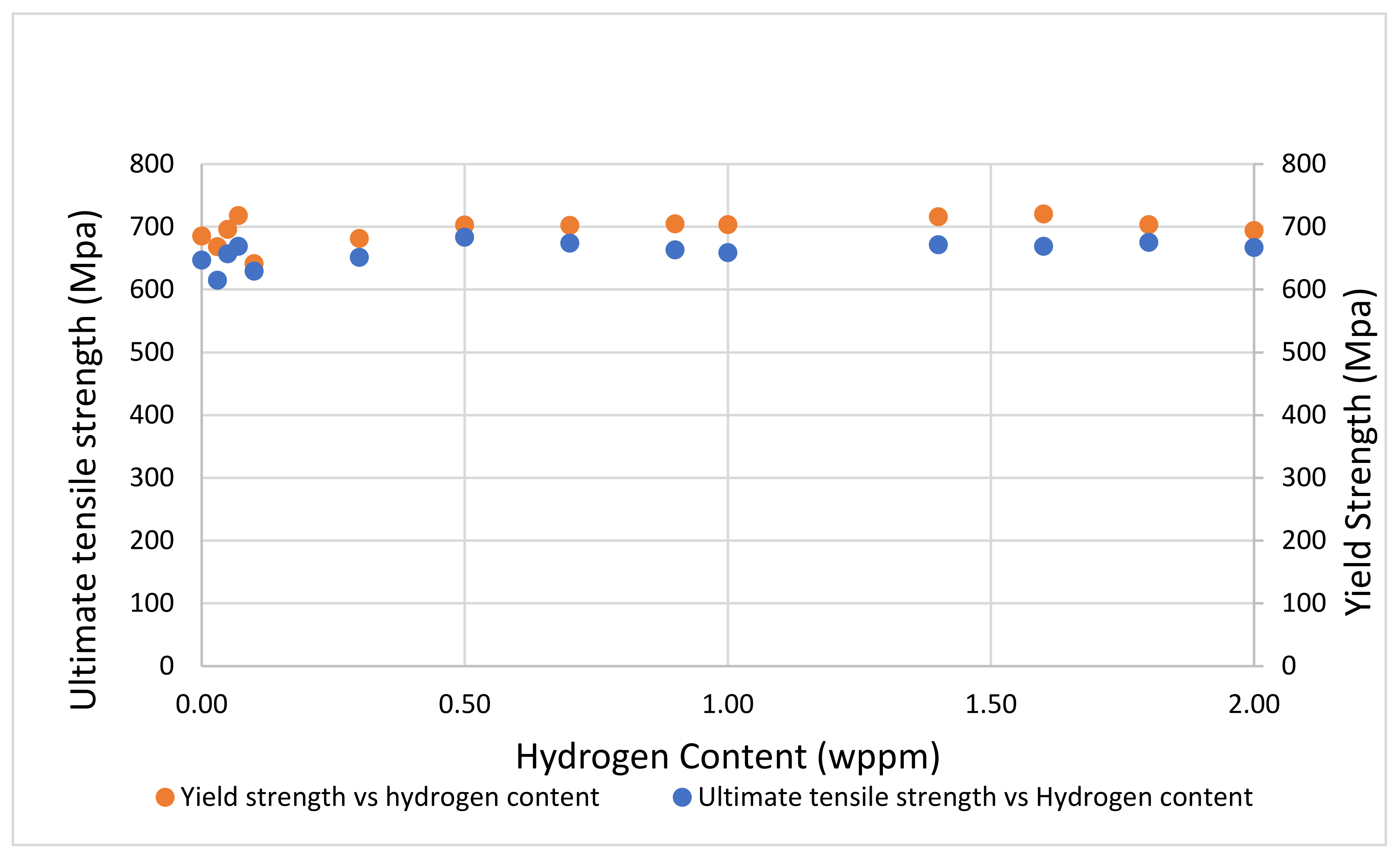
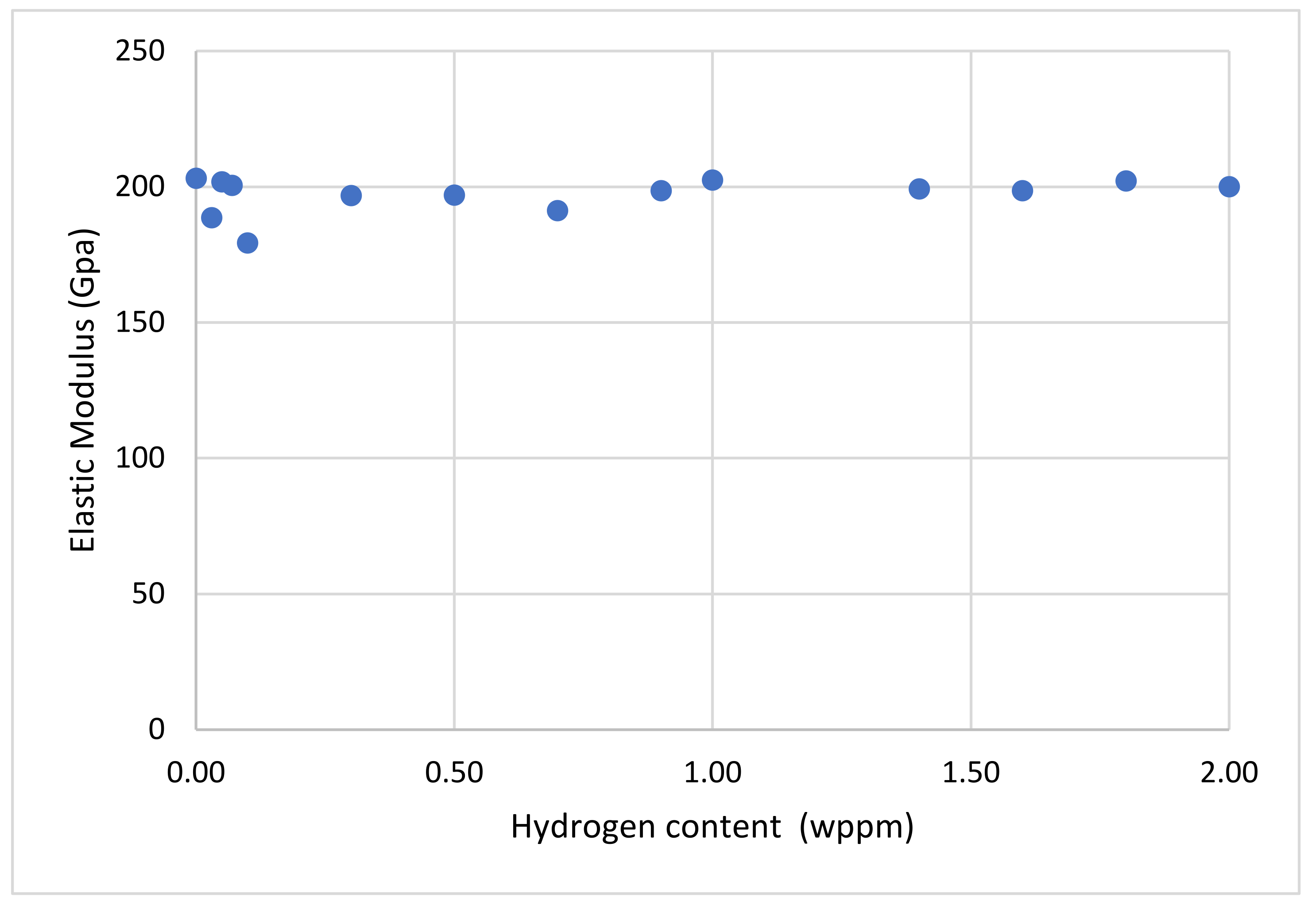
3.2. Tensile Properties
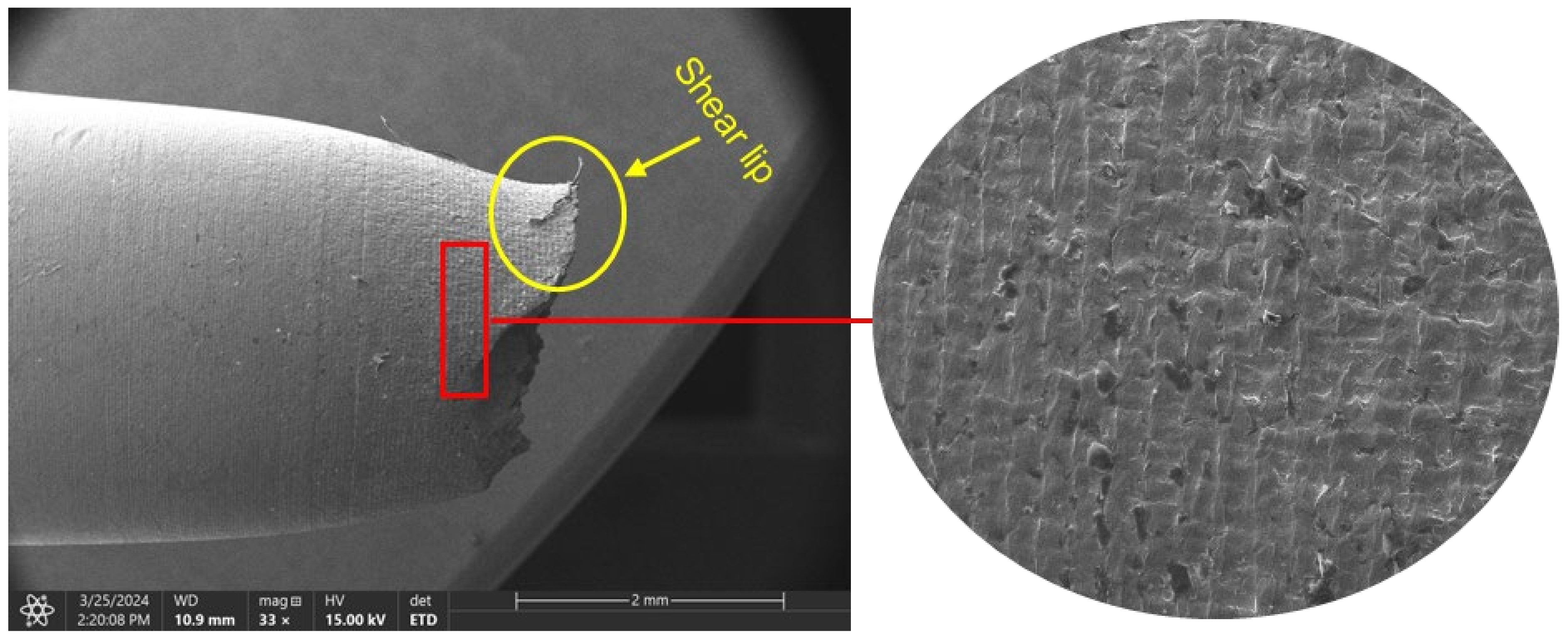
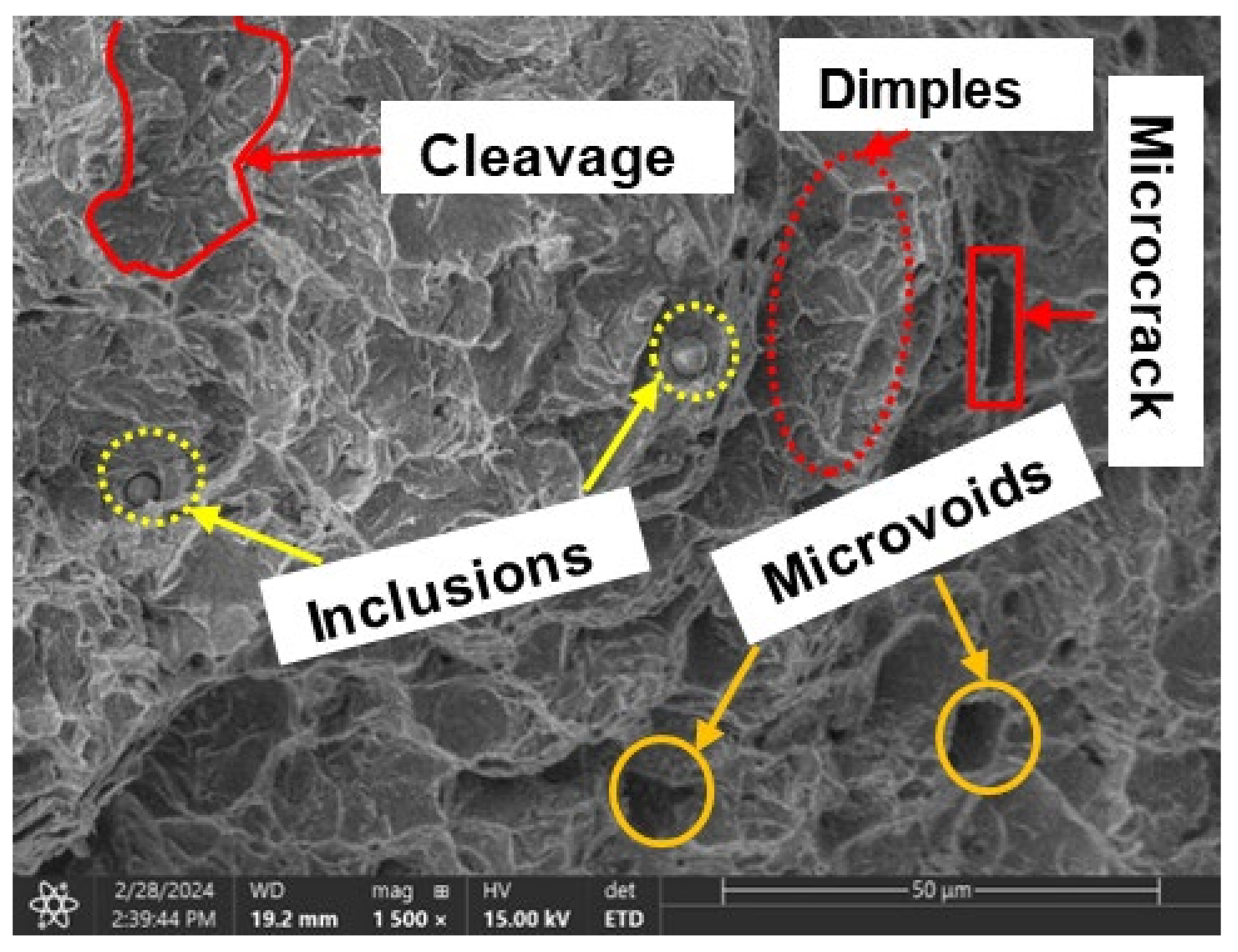
3.3. Fractography
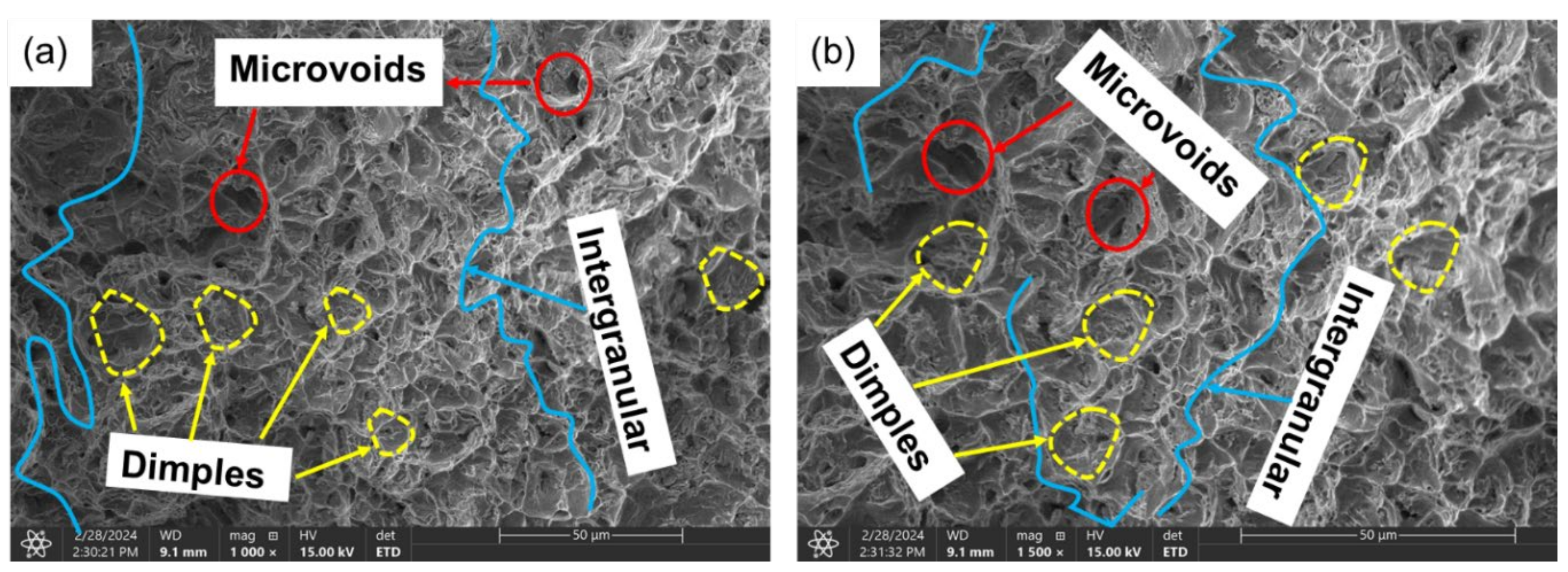
3.3.1. Ductile Failure (Uncharged Sample)
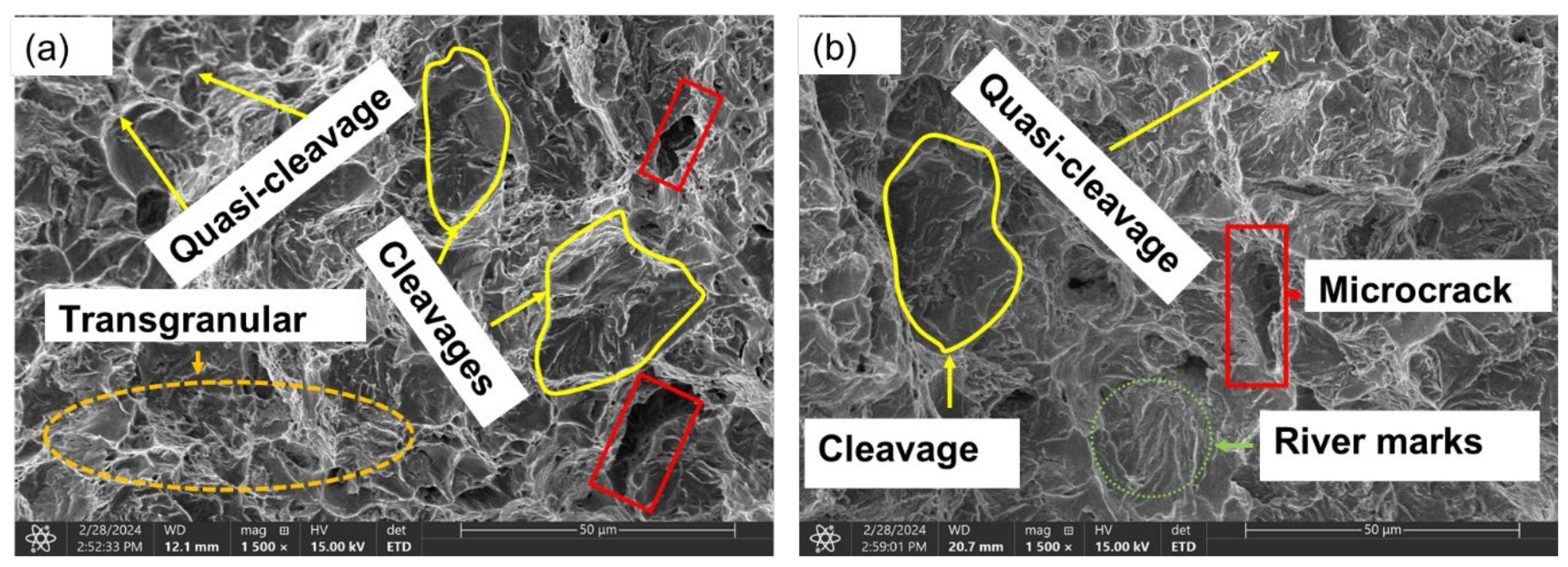
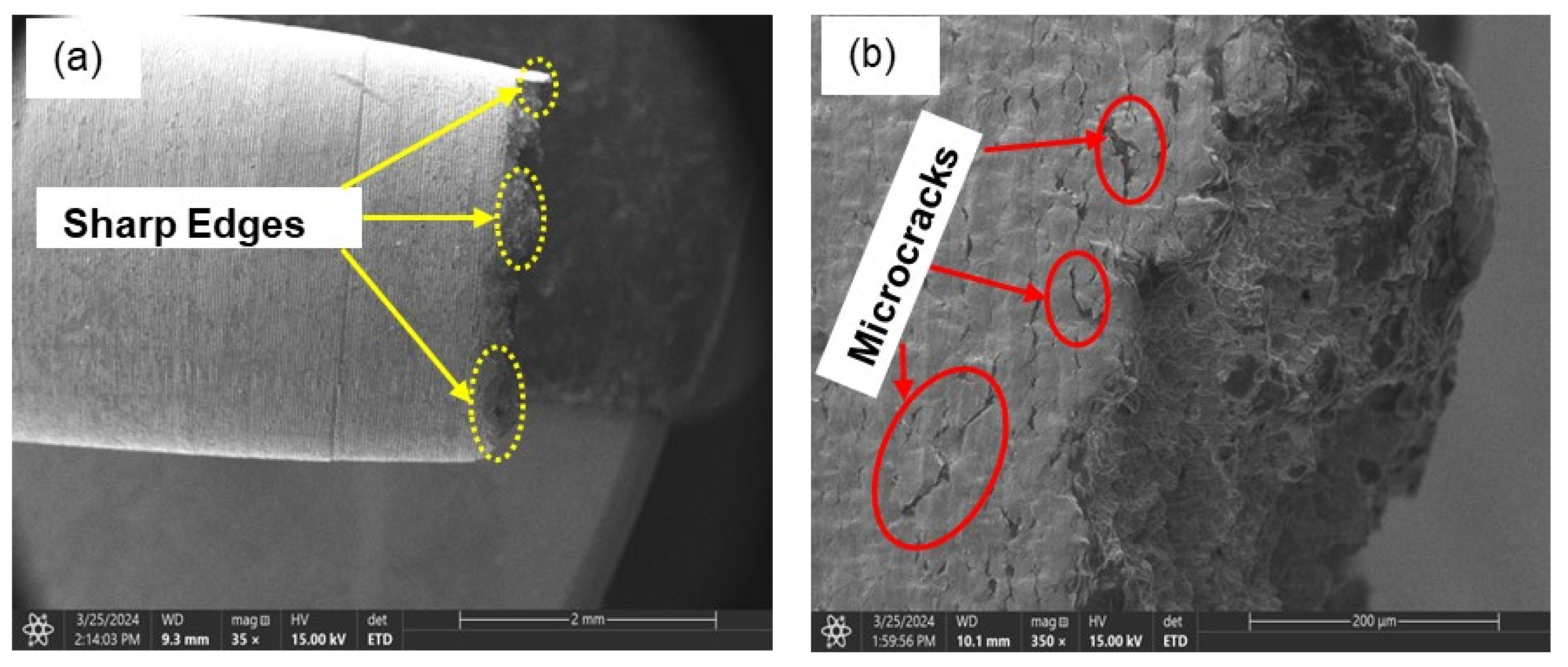
3.3.2. Brittle Fracture/Failure (Charged Samples)
4. Conclusions
- Sample under study had a simple microstructure primarily containing ferrite and pearlite phases with some microvoids. The stress-strain behavior of tensile testing showed a gradual reduction in the percentage elongation and toughness with increasing hydrogen content signifying embrittling from hydrogen diffusion into samples with extremities of 7.1% and 4.4% for uncharged and 2.0 wppm respectively.
- It is evidential from the fracture surface analysis where uncharged samples showed ductile features of dimpling, microvoid coalescence, and internagranular fracture as opposed to charged samples of 1.2 wppm and 2.0 wppm which indicated embrittling with feature like cleavages, microcracks, and trangranullar fracture.
- It was also observed that at lower hydrogen concentration levels, ie. 0.4wppm, samples showed both ductile and brittle fracture features.
Acknowledgments
References
- Li, X.; Ma, X.; Zhang, J.; Akiyama, E.; Wang, Y.; Song, X. Review of Hydrogen Embrittlement in Metals: Hydrogen Diffusion, Hydrogen Characterization, Hydrogen Embrittlement Mechanism and Prevention. Acta Metall. Sin.-Engl. Lett. 2020, 33, 759–773. [Google Scholar] [CrossRef]
- Rao, J.; Lee, S.; Dehm, G.; Duarte, M.J. Hardening Effect of Diffusible Hydrogen on BCC Fe-Based Model Alloys by in Situ Backside Hydrogen Charging. Mater. Des. 2023, 232. [Google Scholar] [CrossRef]
- Lu, Z.; Takeda, Y.; Shoji, T. Some Fundamental Aspects of Thermally Activated Processes Involved in Stress Corrosion Cracking in High Temperature Aqueous Environments. J. Nucl. Mater. 2008, 383, 92–96. [Google Scholar] [CrossRef]
- Pal, S.; Singh Raman, R.K. Determination of Threshold Stress Intensity Factor for Stress Corrosion Cracking (KISCC) of Steel Heat Affected Zone. Corros. Sci. 2009, 51, 2443–2449. [Google Scholar] [CrossRef]
- De Pannemaecker, A.; Fouvry, S.; Brochu, M.; Buffiere, J.Y. Identification of the Fatigue Stress Intensity Factor Threshold for Different Load Ratios R: From Fretting Fatigue to C(T) Fatigue Experiments. In Proceedings of the Int. J. Fatigue; Elsevier Ltd., January 1 2016; Vol. 82, pp. 211–225.
- Li, X.; Zhang, J.; Fu, Q.; Song, X.; Shen, S.; Li, Q. A Comparative Study of Hydrogen Embrittlement of 20SiMn2CrNiMo, PSB1080 and PH13-8Mo High Strength Steels; 2018;
- Zhang, P.; Laleh, M.; Hughes, A.E.; Marceau, R.K.W.; Hilditch, T.; Tan, M.Y. Effect of Microstructure on Hydrogen Embrittlement and Hydrogen-Induced Cracking Behaviour of a High-Strength Pipeline Steel Weldment. Corros. Sci. 2024, 227, 111764. [Google Scholar] [CrossRef]
- Li, X.; Liu, C.; Wang, X.; Dai, Y.; Zhan, M.; Liu, Y.; Yang, K.; He, C.; Wang, Q. Effect of Microstructure on Small Fatigue Crack Initiation and Early Propagation Behavior in Super Austenitic Stainless Steel 654SMO. Int. J. Fatigue 2024, 179. [Google Scholar] [CrossRef]
- Shin, J.W.; Bertocci, U.; Stafford, G.R. In Situ Stress Measurement During Hydrogen Sorption on Ultrathin (111)-Textured Pd Films in Alkaline Electrolyte. J Electrochem. Soc. 2011, 158, F127. [Google Scholar] [CrossRef]
- Peisl, H. 3. Lattice Strains Due to Hydrogen in Metals.
- Nagao, A.; Dadfarnia, M.; Somerday, B.P.; Sofronis, P.; Ritchie, R.O. Hydrogen-Enhanced-Plasticity Mediated Decohesion for Hydrogen-Induced Intergranular and “Quasi-Cleavage” Fracture of Lath Martensitic Steels. J. Mech. Phys. Solids 2018, 112, 403–430. [Google Scholar] [CrossRef]
- Katzarov, I.H.; Paxton, A.T. Hydrogen Embrittlement II. Analysis of Hydrogen-Enhanced Decohesion across (111) Planes in α -Fe. Phys. Rev. Mater. 2017, 1. [CrossRef]
- Lu, X.; Ma, Y.; Peng, D.; Johnsen, R.; Wang, D. In Situ Nanomechanical Characterization of Hydrogen Effects on Nickel-Based Alloy 725 under Different Metallurgical Conditions. J. Mater. Sci. Technol. 2023, 135, 156–169. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.; Toda, H.; Shimizu, K.; Su, H.; Uesugi, K.; Takeuchi, A.; Watanabe, Y. The Role of Hydrogen on the Local Fracture Toughness Properties of 7XXX Aluminum Alloys. Metall. Mater. Trans. A Phys. Metall. Mater. Sci 2018, 49, 5368–5381. [Google Scholar] [CrossRef]
- Su, H.; Yoshimura, T.; Toda, H.; Bhuiyan, M.S.; Uesugi, K.; Takeuchi, A.; Sakaguchi, N.; Watanabe, Y. Influences of Hydrogen Micropores and Intermetallic Particles on Fracture Behaviors of Al-Zn-Mg-Cu Aluminum Alloys. Metall. Mater. Trans. A Phys. Metall. Mater. Sci 2016, 47, 6077–6089. [Google Scholar] [CrossRef]
- Wilson, B.T.; Robson, J.D.; Shanthraj, P.; Race, C.P. Simulating Intergranular Hydrogen Enhanced Decohesion in Aluminium Using Density Functional Theory. Model. Simul. Mat. Sci. Eng. 2022, 30. [Google Scholar] [CrossRef]
- New Trends and Developments in Automotive System Engineering; InTech, 2012.
- Zheng, S.; Qin, Y.; Li, W.; Huang, F.; Qiang, Y.; Yang, S.; Wen, L.; Jin, Y. Effect of Hydrogen Traps on Hydrogen Permeation in X80 Pipeline Steel —— a Joint Experimental and Modelling Study. Int. J. Hydrogen Energy 2023, 48, 4773–4788. [Google Scholar] [CrossRef]
- He, Y.; Su, Y.; Yu, H.; Chen, C. First-Principles Study of Hydrogen Trapping and Diffusion at Grain Boundaries in γ-Fe. Int. J. Hydrogen Energy 2021, 46, 7589–7600. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, Y.; Wang, H.; Mi, Z.; Liu, Z.; Gao, L.; Yan, Y.; Su, Y.; Qiao, L. A First-Principles Study on the Hydrogen Trap Characteristics of Coherent Nano-Precipitates in α-Fe. Int. J. Hydrogen Energy 2020, 45, 27941–27949. [Google Scholar] [CrossRef]
- Turk, A.; Joshi, G.R.; Gintalas, M.; Callisti, M.; Rivera-Díaz-del-Castillo, P.E.J.; Galindo-Nava, E.I. Quantification of Hydrogen Trapping in Multiphase Steels: Part I – Point Traps in Martensite. Acta Mater. 2020, 194, 118–133. [Google Scholar] [CrossRef]
- Si, X.; Lu, R.; Zhao, Z.; Yang, X.; Wang, F.; Jiang, H.; Luo, X.; Wang, A.; Feng, Z.; Xu, J.; et al. Catalytic Production of Low-Carbon Footprint Sustainable Natural Gas. Nat. Commun. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Shrestha, E.; Hamburg, S.P.; Kupers, R.; Ocko, I.B. Climate Impacts of Hydrogen and Methane Emissions Can Considerably Reduce the Climate Benefits across Key Hydrogen Use Cases and Time Scales. Environ. Sci. Technol. 2023. [CrossRef] [PubMed]
- Li, Q.; Ghadiani, H.; Jalilvand, V.; Alam, T.; Farhat, Z.; Islam, M.A. Hydrogen Impact: A Review on Diffusibility, Embrittlement Mechanisms, and Characterization. Materials 2024, 17. [Google Scholar] [CrossRef] [PubMed]
- Michael Allen Adjelian Allen Rubeli Ltd., C.; Avery Consultant, H.; Babu Caterpillar, P.; Bayer Teledyne Vasco, A.M.; Boehm Nucor Steel, K.W.; Brentnall Solar Turbines, W.; Brinkman, C.; Bueche USS, E.J.; Steel Company, K.; Burrier, H.; et al. Volume 1 Publication Information and Contributors Wil Danesi Garrett Processing DivisionAllied-Signal Aerospace Company.
- Hanning, F.; Engelberg, D.L. Metallographic Screening of Grain Boundary Engineered Type 304 Austenitic Stainless Steel. Mater. Charact. 2014, 94, 111–115. [Google Scholar] [CrossRef]
- Örnek, C.; Şeşen, B.M.; Ürgen, M.K. Understanding Hydrogen-Induced Strain Localization in Super Duplex Stainless Steel Using Digital Image Correlation Technique. Met. Mater. Int. 2022, 28, 475–486. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, H.; Zhang, C.; Wang, G.; Lu, S.; Chen, Z.; Zhao, A. Effect of Hydrogen Charging Intensities and Times on Hydrogen Embrittlement of Q&P980 Steel. Mater. Res. Express 2024, 11. [Google Scholar] [CrossRef]
- Wang, H.; Wang, T.; Yang, S.; Gao, J.; Yu, Y.; Tao, H. bing Ductile Burst Behavior of High Pressure X100 Steel Pipe Considering Hydrogen Damage. Int. J. Hydrogen Energy 2024, 58, 362–379. [Google Scholar] [CrossRef]
- Troiano, A.R. The Role of Hydrogen and Other Interstitials in the Mechanical Behavior of Metals. Metallogr. Microstruct. Anal. 2016, 5, 557–569. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Shen, S.; Wang, Y.; Song, X. Effect of Tempering Temperature and Inclusions on Hydrogen-Assisted Fracture Behaviors of a Low Alloy Steel. Materials Science and Engineering: A 2017, 682, 359–369. [Google Scholar] [CrossRef]
- Wang, D.; Hagen, A.B.; Wan, D.; Lu, X.; Johnsen, R. Probing Hydrogen Effect on Nanomechanical Properties of X65 Pipeline Steel Using In-Situ Electrochemical Nanoindentation. Mater. Sci. Eng.: A 2021, 824, 141819. [Google Scholar] [CrossRef]
- Ranjan, R.; Meena, A. Effect of Martensite-Austenite Island Decomposition during Two-Step Tempering on the Fracture Surface Morphology of Impact and Tensile Tested Mn-Ni-Mo Steel. Eng. Fail. Anal. 2024, 161. [Google Scholar] [CrossRef]
- Ramazani, A.; Abbasi, M.; Prahl, U.; Bleck, W. Failure Analysis of DP600 Steel during the Cross-Die Test. In Proceedings of the Comput. Mater. Sci.; November 2012; Vol. 64, pp. 101–105.
- Li, W.; Zhou, Y.; Zhou, Q.; Li, J. Analysis of the Delayed Cracking Mechanism of an Industrial Hot-Dip Galvanized DP1180GI Steel Coil. Eng. Fail. Anal. 2024, 160. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





