1. Introduction
Sea turtles are highly important in marine ecosystems, as they contribute to the health and maintenance of coral reefs, estuaries, rocky shores and sandy beaches and are widely distributed throughout tropical and subtropical regions [
1]. Of the seven species known worldwide, five occur on the Brazilian coast: the loggerhead turtle (
Caretta caretta), the hawksbill turtle (
Eretmochelys imbricata), the green turtle (
Chelonia mydas), the olive turtle (
Lepidochelys olivacea) and the leatherback turtle (
Dermochelys coriacea).
They are found in different regions of Brazil, including the feeding, growth, resting, reproduction and spawning areas. The population stocks, conservation status and biological and behavioral conditions vary among the species and are related to factors such as predatory fishing, contamination and pollution of the seas, and maintenance of the availability and quality of breeding and feeding areas [
2,
3].
Despite the recent update of the extinction risk classification of sea turtles, which included the green turtle (
C. mydas) in the “near threatened” (NT) category, according to MMA Ordinance No. 2022, some species, such as the hawksbill turtle (
E. imbricata) (EN), loggerhead turtle (
C. caretta) (VU), leatherback turtle (
D. coriacea) (CR) and the olive turtle (
L. olivacea) (VU), remain critically endangered (CR), endangered (EN) or vulnerable (VU). This finding justifies the maintenance of conservation and monitoring programs for all species of sea turtles that occur off the Brazilian coast [
3,
4,
5].
There are several factors that pose risks to sea turtles, such as incidental fishing, the ingestion of solid waste, interactions with fishing gear or boats, parasitic diseases, and FP, which is a debilitating disease of multifactorial nature that can be fatal [
6,
7]. Even with the efforts of the Tamar-ICMBio Project and several other entities that work and provide information on these animals on the Brazilian coast, there are still gaps in knowledge about the occurrence and health status of these species in some coastal regions, particularly in the northern region of the country [
8].
Hamede et al. [
9] emphasize the crucial need to enhance disease surveillance and data collection to address the alarming rise of wildlife illnesses linked to human activities and rapid environmental changes. Specifically in the case of FP, challenges in conducting aquatic research and acquiring high-quality relevant data have hindered the effectiveness of surveillance and information gathering efforts. When discussing research on the prevalence of FP in sea turtles, it is crucial to emphasize the importance of implementing efficient study methods to obtain data that accurately reflect the true situation. Previous research has shown that the approach taken in data collection can significantly impact data interpretation.
These gaps in knowledge make it difficult to determine the proportion of turtles with FP, given that sea turtles are migratory animals that circulate along the entire Brazilian coast and even in other regions of the world [
8]. Thus, this review aims to contribute to the understanding of various aspects of FP in sea turtles in Brazil context, from its characterization to its significance for the conservation of the species, since these animals play a fundamental role in the environment, and FP may present a risk to affected individuals. Without a solid information base, it becomes increasingly difficult to gather the necessary resources to establish programs and initiatives to minimize impacts on turtles and mitigate environmental factors that could contribute to the development of FP in individuals.
2. Research Findings on Knowledge of Sea Turtles and FP on the Northern Coast of Brazil
Since 1980, the breeding areas of marine species have been systematically identified in Brazil, with the active participation of the TAMAR-ICMBio Project in the main locations identified. In recent years, various institutions have conducted research and monitoring to increase our knowledge about other breeding beaches. However, there are still areas in northern Brazil that lack sufficient information for a complete understanding [
8,
10].
In the state of Pará, there is a lack of research on marine turtles, particularly on the incidence of FP in these animals. This highlights the urgent need for more information to assist in the preservation of these invaluable species for the ecosystem. Especially in an area with such great potential, where these animals are known to be incidentally captured in fishing gear such as gillnets, longlines, fish traps, and are also seen on beaches, potentially for feeding, resting, or even breeding for the five species of marine turtles found in Brazil [
8,
11,
12,
13].
The study conducted by Martini et al. [
10] revealed a stark lack of interest in obtaining permits from the Biodiversity Authorization and Information System (SISBIO) to study marine turtles in the northern region of Brazil, in comparison to other regions of the country. Furthermore, a significant number of research projects initiated in the region failed to produce publishable results due to a myriad of challenges, such as limited financial resources to support the research and the difficulty of effectively monitoring the vast areas in the region.
The breeding season of marine turtles varies depending on the region and the species being studied. In Brazil, it is widely known that the nesting season begins in september and continues until April along the coastal beaches, while on the oceanic islands, it begins in December and the turtles remain in reproductive activity until june [
14]. The nesting period in the Pará region is described as occurring between march and july [
15].
In a study conducted in an extractive reserve in the municipality of Curuçá, on the coast of Pará, Brazil, the presence of individuals of green turtles, loggerhead turtles, hawksbill turtles, and olive ridley turtles was documented through incidental fishing captures. Additionally, nests with hatchlings of the species
C. mydas, E. imbricata, and
L. olivacea were monitored [
15]. The latter species was also recorded in the Para region in a study by Silva [
16], where a specimen was brought in for veterinary care due to a lacerative injury on its flipper, indicative of entanglement in fishing gear. These findings confirm the species’ presence in the region and its interaction with fishing activities. The leatherback sea turtle (
Dermochelys coriacea) has not been recorded with adult individuals or nests being monitored. However, reports from survey participants indicate the capture of an adult female with eggs present, who unfortunately died after being caught in a beach seine fishing net. Another adult individual was also reported to have become entangled in a fishing net in the same year, 2009 [
15].
The peak period for the capture of turtles in the fishing enclosures along the coast of Pará extends from february to june [
15], aligning with the pattern observed in the northeast region, specifically in the state of Ceará, where a higher incidence of captures in fishing enclosures was also noted during the first half of the year [
17,
18,
19].
A retrospective study conducted at a Wildlife Screening and Rehabilitation Center (WSRC) in the state of Pará, Brazil, recorded the occurrence of 20 individuals, with
E. imbricata, C. mydas and
C. caretta species being the most prevalent, predominantly consisting of juvenile specimens, followed by juvenile animals and only one adult. The majority of rescues were identified in the regions of Pará, Brazil, (
Table 1), with the presence of
C. mydas species indicating intensive use of the area by this species in the state [
20].
In a study conducted by Silva [
20], the presence of turtles with lesions compatible with FP was observed in 3 juvenile specimens of
C. mydas, found in the mesoregion of northeastern Pará, Brazil in the cities of Curuçá, Salinópolis, and Maracanã (specifically on the island of Algodoal) respectively. The characteristics of the tumors followed the pattern described by other authors, mainly located in soft tissues, with distribution in the fin regions, around the eyes, neck, inguinal area, cervical region, axillary region, base of the tail, and cloaca.
The Northern region of Brazil lacks comprehensive studies on marine turtles, particularly in relation to the incidence of FP in the population. This highlights the need for investments in monitoring programs to identify priority areas for reproduction, feeding, and resting for these species. Understanding the health status of individuals in the region is crucial in developing effective conservation strategies. This information will guide efforts to protect and preserve these endangered species [
10].
2. FP in Sea Turtles
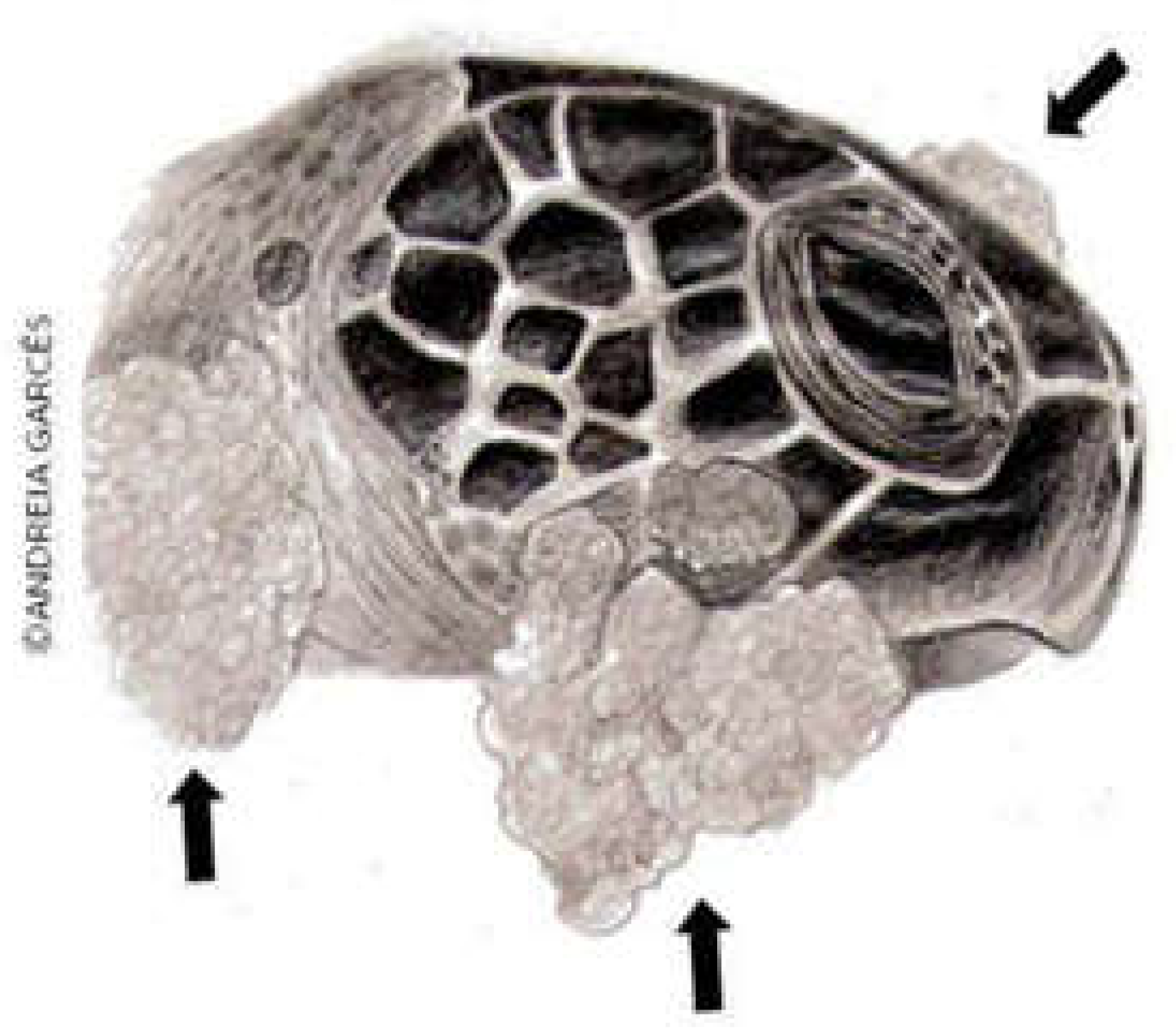
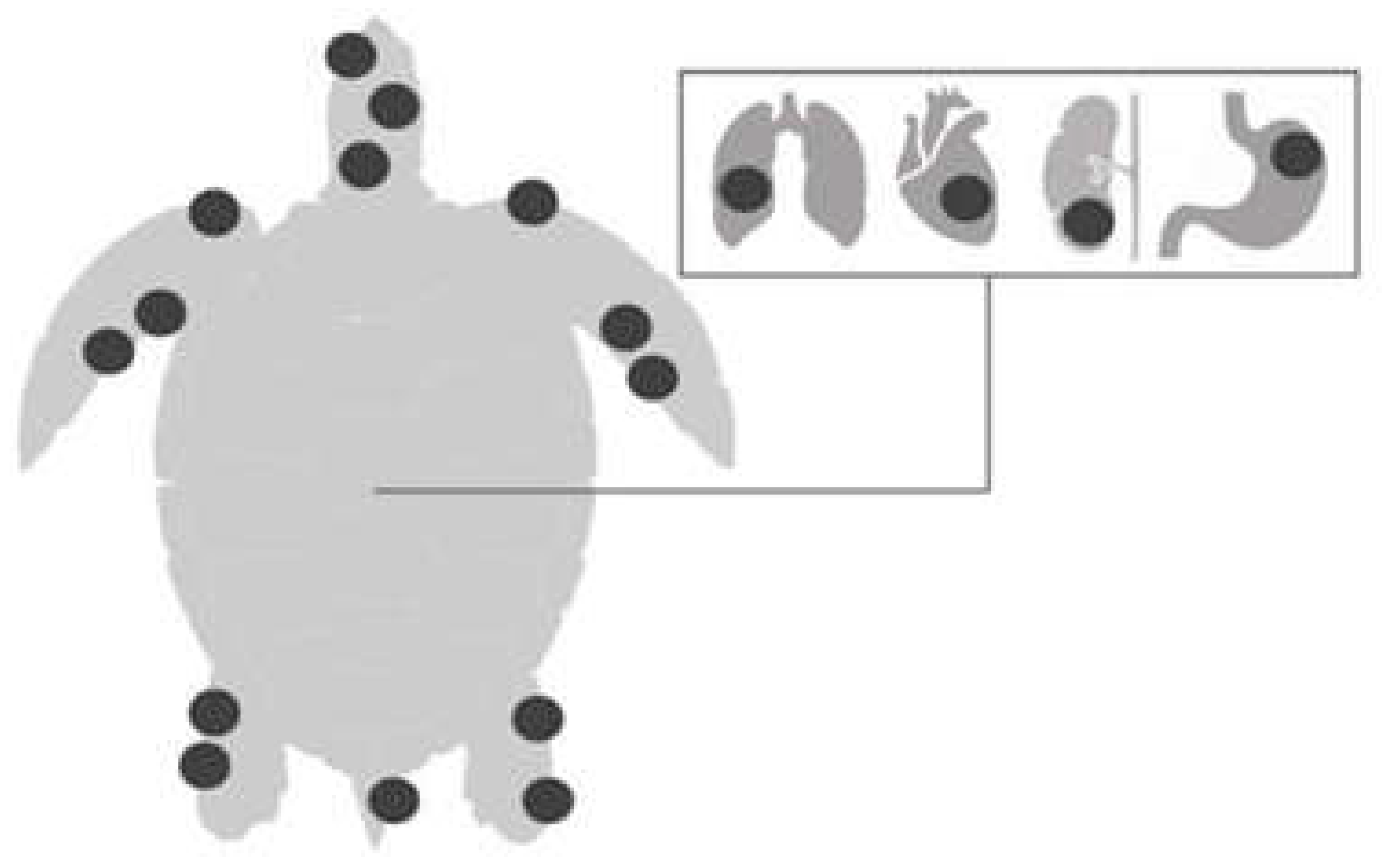
FP is characterized by single or multiple tumors (
Figure 1) that develop in various regions of the body, especially at the base of the fins and tail and on the neck, head and eyes; they may also occur on internal organs such as the lungs, liver, kidneys, ovaries, and heart (
Figure 2). Tumors can also develop in tissues of the carapace and plastron, as well as in the cornea of affected animals [
21]. The neoformations may have a verrucous, smooth or rough appearance and have different colors, depending on the pigment of the area where the tumor originated [
22]. Clinical evaluations have shown that these injuries have detrimental effects on the vision of turtles, impacting their ability to search for food, evade predators, and interact with other individuals [
23].
These fibromas, cutaneous papillomas and fibropapillomas, despite their benign course, can impair locomotion, vision, breathing or even the apprehension of food, and depending on the stage of the disease, the animal can be weak and anemic; therefore, it is a debilitating and potentially fatal disease for sea turtles [
6,
7,
24,
25]. When associated with the presence of Chelonid Alphaherpesvirus 5 (ChHV5) as the causative agent of the disease, it is also possible to detect viral particles in oral, ocular and cloacal secretions, blood, urine and plasma [
26,
27,
28]. In a loggerhead turtle (
C. caretta), bilateral mucoid secretion, chemosis, conjunctival hyperemia and bilateral eyeball retraction were observed [
29].
There are records of the occurrence of fibropapilloma in all species of sea turtles, and there is evidence that the green turtle (
C. mydas) has the highest prevalence of this disease among sea turtles [
30]. The curved carapace length (CCL) is a significant factor in the occurrence of the disease, being the most frequent disease in turtles with CCLs greater than 30 cm, with a significant decrease in prevalence in animals with CCLs equal to or greater than 80 cm [
7]. As noted in a study conducted in Australia, it was observed that turtles affected by FP were often young animals [
31]. Therefore, it is frequently observed in young animals, but it has also been diagnosed in turtles near the adult stage and less commonly in adults [
32].
This difference in size between affected animals may be because younger individuals die and disappear from the locality or acquire immunity and mature to adults without the disease; alternatively, healthy a dult individuals may have never been exposed to the potential infectious agent [
30]. Sex is not a determining factor for the disease, and no significant difference in prevalence was observed between males and females [
33].
The first case of FP in Brazil occurred in 1986 in Espírito Santo, and the monitoring of FP was included in the national database of the TAMAR project beginning in 2000; the presence or absence of tumors characteristic of the disease are recorded in the database. Based on this monitoring, it was possible to characterize the prevalence of the disease in different regions along the Brazilian coast, and until 2015, it was possible to observe disease-free areas, such as the oceanic islands of Atol das Rocas and Trindade [
7,
34,
35].
Studies have sought to elucidate the relationship between the prevalence of FP and environmental quality, including heavy pollution in coastal areas, high human density, agricultural runoff and/or biotoxin-producing algae [
36]. The island of Fernando de Noronha was among the areas free of FP included in TAMAR until December of 2015, when a green turtle (
C. mydas) with a bilateral ocular nodule, characterized as fibropapilloma, was reported. This case was associated with ChHV5, revealing the importance of constant monitoring of the disease [
24].
Despite the damage caused to individuals affected by this disease, there are still conflicting discussions about whether FP is of significant importance to the survival of sea turtles. Authors such as Hamann et al. [
37] emphasize that deepening knowledge and effectively addressing this disease is a top priority in research focused on the conservation of sea turtles. Ongoing surveillance of FP is essential for identifying changes in the spread, incidence, and severity of the disease, as stated by specialists [
38]. Without a complete understanding of the disease, one cannot rule out FP as an obstacle to the survival of the species [
30].
3. Diagnosis, Tratament and Control of FP in Sea Turtles
The diagnosis of the disease relies on physical examination of an affected animal. Tumors are found via macroscopic visualization and can be confirmed via histopathology after incision of a fragment of the lesion [
39]. Developing sensitive and specific diagnostic tools for detecting ChHV5 in sea turtles, including rapid PCR assays and serological tests.
Investigating non-invasive diagnostic methods, such as thermal imaging and biomarker detection, for early detection of FP in sea turtles [
9,
30,
37].
Histopathological analyses performed by Rodenbusch et al. [
40] demonstrated that fibropapilloma had a papillary pattern, with the presence of melanocytes, epithelial hyperplasia, hyperkeratosis and a nuclear halo, in addition to moderate stromal hyperplasia and dyskaryosis. In the analyses performed by Gattamorta et al. [
24], fibropapilloma was characterized by hyperplastic epidermal and stromal proliferation, epithelial cells with cytoplasmic vacuolization, degeneration of epidermal cells and fibroblast proliferation.
One of the main challenges in managing FP is the lack of a specific cure or vaccine. Current treatment options are limited to surgical removal of tumors and supportive care, which may help improve the quality of life for affected turtles but do not address the underlying cause of the disease. Research into antiviral therapies and immunomodulatory drugs holds promise for improving treatment outcomes for FP. However, further studies are needed evaluating the effectiveness of antiviral drugs, immunomodulatory therapies, and supportive care in treating FP and improving sea turtle health. Developing novel treatment strategies, such as gene therapy or immunotherapy, for controlling tumor growth and viral replication in affected turtles [
9,
30,
37].
To prevent and control of FP it is necessary to prevent pollution, habitat degradation, and other human activities that can weaken sea turtle immune systems and increase their susceptibility to FP. Implementing quarantine and biosecurity measures in sea turtle rehabilitation facilities and captive breeding programs to prevent the spread of FP. And educating the public about the importance of sea turtle conservation and the risks of FP to help reduce human activities that can contribute to the spread of the disease [
9,
30,
37].
4. Association of FP with the ChHV5 Virus in Sea Turtles
The first studies conducted by Smith and Coates [
41] did not identify viral elements in histological exams, but recent studies have suggested that FP has an infectious origin [
6,
7,
25,
42,
43]. The most prevalent theory indicates that the virus involved in FP is ChHV5, with a taxonomic position in the
Herpesviridae family, the Alphaherpesvirinae subfamily, and the
Scutavirus genus [
30,
44]. Sequence analysis of fragments of the DNA polymerase region revealed the presence of ChHV5 in fibropapillomas, and several other regions of the ChHV5 genome have been studied [
6,
32,
40,
41,
42,
43,
44,
45,
46,
47,
48,
49].
According to Ene et al. [
25], it is not clear at which stage of sea turtle development the transmission of infectious agents occurs, with the prenatal period, hatching or time spent in the pelagic zone being possibilities. Sea turtles have a complex life history, beginning when the young are born and continuing during their migration to pelagic environments and return to coastal areas for foraging and later for reproduction and nesting, making it difficult to identify the life stage and location where virus transmission occurs [
50]. There is speculation about the potential spread of the virus through the dispersion and distribution of juvenile turtles to aggregation areas, driven by ocean currents. These turtles come into contact with a high density of infected marine turtles [
29], possibly spreading the disease when they return to their original beach [
51,
52,
53]. This transmission can occur through direct contact with tumors/secretions or through vectors such as marine leeches, cleaner fish, or through infected individuals and waters [
50,
54,
55,
56].
There are currently two hypotheses: horizontal transmission through direct contact with tumors/secretions or through vectors and water [
50] and vertical transmission, as recent analyses have reported the presence of ChHV5 in pups [
57]. However, in another study, the pups tested negative despite coming from mothers that tested positive using the same method [
58].
The first studies to detect ChHV5 in tissues of turtles with and without FP showed that amplification of target gene sequences was rare in animals without the disease [
46]. Subsequently, Quackenbush et al. [
47] amplified ChHV5 in samples collected from turtles without FP, showing that the virus may be present in animals without clinical signs; i.e., the absence of clinical signs of the disease does not confirm the absence of viral infection. It is possible that active or latent virus may be more common in turtles than previously imagined and that perhaps the percentage of sick animals is lower than that of turtles infected with the virus [
48]. Results from a study conducted in the coastal waters of Granada in 2020 revealed a significant finding, an adult green turtle underwent necropsy due to severe emaciation and cutaneous fibropapillomas, upon further testing, it was confirmed that several tumors tested positive for ChHV5, marking the first case of FP associated with this virus in Granada. In this region, active ChHV5 infection is considered rare [
59].
Several other studies have reported the presence of ChHV5 in tissues and animals not affected by FP, which may suggest that the presence of the virus alone is not a determinant for the development of the disease and that other factors, such as the condition of the host, the presence of the virus and environmental conditions, may be involved [
7,
60].
Although studies have shown the presence of the ChHV5 virus in the tumor tissues of animals with FP, this result may not be surprising, as these tumors are known to have a higher concentration of viral DNA; however, in the presence of the virus in latent infections, i.e., in animals that do not present clinical manifestations of disease, the viral DNA load in tissues tends to be lower, which represents a challenge for the detection of the presence and prevalence of the virus under these circumstances [
30].
Considering this, Alfaro-Núñez and Gilbert [
61] sought to demonstrate an effective molecular testing method by studying animals that did not present clinical manifestations of the disease and apparently healthy tissues. The authors detected the presence of ChHV5 in 100% of the samples analyzed, suggesting latent infections and corroborating the assumption that the presence of this virus in tissues, fluids and secretions of sea turtles without tumors is indicative of the multifactorial nature of FP [
48].
5. Multifactorial Nature of FP in Sea Turtles
Even with the great likelihood of herpesvirus involvement, there are other potentially correlated factors involved in infection and symptom manifestation, such high nitrogen levels in watersheds that contribute to coastal eutrophication. This results in eutrophication events and the proliferation of microalgae, causing a nutritional imbalance in foraging areas, altering the immune system of turtles and thus favoring the formation of tumors, parasitism, genetic susceptibility, and biotoxins and adverse effects from prolonged exposure to ultraviolet light, chemical pollutants, and environmental and immunological factors [
7,
62,
63].
Water temperature can also affect the development and growth of lesions, with higher temperatures probably being more favorable for FP, as observed by Herbst [
29] in Florida, where the change in water temperature is significant because the seasons are well defined. Studies have shown that the increased incidence of ultraviolet radiation is responsible for the onset of the disease. This is due to the ability of radiation to damage DNA and thus the increase in mutations that cause tumors in animals. In addition, in captive turtles, tumor growth was more evident in warmer times of the year, indicating that climate change can directly impact FP [
64,
65,
66].
In a study conducted by Garefino [
67], plasma levels of vitamin D in turtles with and without visible FP tumors were analyzed. Vitamin D plays a crucial role in immune function and overall health, and the results indicated that sunlight exposure significantly affects the levels of this vitamin in marine turtles brought to a rehabilitation center. It was found that prolonged exposure to sunlight impacts vitamin D levels in marine turtles that are brought to a rehabilitation center. Additionally, turtles with tumors were observed to have lower levels of vitamin D and calcium, and higher levels of parathyroid hormone compared to those without tumors. Turtles housed in tanks with higher exposure to ultraviolet light showed greater increases in vitamin D levels and more effective recovery. These findings suggest that sun exposure can benefit the health and recovery of green turtles with FP in rehabilitation centers.
Jones et al. [
31] conducted a study focusing on water quality variables that could directly or indirectly impact turtles, such as metals and pesticides with potential toxic effects on turtles, as well as dissolved inorganic nitrogen and total suspended solids that could harm seagrass growth, a vital food source for green turtles [
68]. This could indirectly affect the health and presence of green turtles in their feeding areas. However, the study yielded data that, while relevant, did not show significant importance in indicating a relationship between FP and the water quality parameters analyzed. Overall, Jones et al. [
31] research findings shed light on the potential impacts of certain water quality variables on turtle health, but further investigation is needed to determine the exact relationship between FP and water quality factors.
Based on James’ data analysis [
59], it is clear that the genetics of ChHV5 does not appear to be a determining factor in the evolution or variation of the disease. Over two decades of sampling, it was observed that the virus strains remained constant and highly similar. Therefore, it is likely that environmental or host processes play a key role in the development and progression of the disease.
Sea turtles are considered sentinel species, representing the health status of marine ecosystems [
36]. Molecular studies [
42,
47,
54,
69] have demonstrated the presence of ChHV5 in animals with and without FP, and due to the possible relationship between the development of FP and the quality of the environment where the turtles live and because the disease may be stimulated by environmental factors, it is extremely important to understand both the prevalence of the disease in these species and the quality of the water where these animals live so that an efficient management plan can be developed to support the maintenance and conservation of the environment and sea turtles [
22,
70,
71].
Thus, monitoring FP is essential for understanding its epidemiology, distribution pattern, occurrence and severity [
24,
71]. As proposed by Jones et al. [
30], future studies should also take into account environmental factors, such as water quality, and evaluate not only the presence of FP but also possible latent infections without apparent clinical signs. Another important aspect of FP research is understanding its epidemiology and transmission dynamics. Factors such as water temperature, pollution, and habitat degradation may influence the prevalence and severity of FP outbreaks. By identifying these factors, conservation efforts can be targeted towards reducing the impact of these environmental stressors on sea turtle populations. Future research on FP should also focus on improving diagnostic tools for early detection of the disease. Rapid and accurate diagnosis is crucial for implementing timely treatment and control measures. Advances in molecular biology and imaging techniques may help improve diagnostic accuracy and efficiency. In conclusion, FP remains a significant threat to sea turtle populations worldwide, highlighting the need for continued research and conservation efforts. By improving our understanding of the disease and developing effective management strategies, we can help protect these iconic marine reptiles for future generations.
3. Conclusion
Studies that demonstrate the current status and the constant changes in the state of research on FP in sea turtles are of vital importance for the conservation of these species and the environment in which they live since there are strong relationships between these elements, in addition to the presence of ChHV5. Studies such as this one are of great importance for developing a comprehensive understanding of the disease and reveal that the search for more information about the interaction of animals with viruses, transmission modes, and the mechanisms that lead to the development of the disease should be prioritized to support the establishment of management methods and species conservation. Encouraging research in areas with limited information on the presence of sea turtles and the incidence of FP, such as the northern region of Brazil, is crucial. As these cosmopolitan creatures inhabit various locations, their conservation, especially in breeding grounds, is essential. Let’s continue to explore and protect these endangered species for a sustainable future.
Author Contributions
Conceptualization, K.P.P.d.C.; and F.M.S.; methodology, K.P.P.d.C and F.M.S.; formal analysis, K.P.P.d.C and F.M.S.; investigation, K.P.P.d.C.; and F.M.S; data curation, K.P.P.d.C.; and F.M.S.; writing—original draft preparation, K.P.P.d.C..; writing—review and editing, A.d.M.C.L.; M.A.G., E.R.M. and F.M.S.; supervision, M.A.G., E.R.M and F.M.S.; project administration, F.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to Programa de Pós-graduação em Reprodução Animla na Amazônia (ReproAmazon); CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), FAPESPA (Fundação Amazônia de Amparo a Estudos e Pesquisas do Estado do Pará), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Finance Code 001) and PROPESP-UFPA (Pró-Reitoria de Pesquisa e Pós-Graduação da Universidade Federal do Pará) for funding the publication of this article via the Programa Institucional de Apoio à Pesquisa—PAPQ/2024.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rossi S., Zwarg T., Sanches T.C., César M.O.,Werneck M.R. & Matushima E.R. 2009. Hematological profile of Chelonia mydas (Testudines, Cheloniidae) according to the severity of fibropapillomatosis or its absence. Pesq. Vet. Bras. 2009, 29, 974–978. [CrossRef]
- Brasil, Ministério do Meio Ambiente. Plano de ação nacional para a conservação das tartarugas marinhas. Available online: https://www.gov.br/icmbio/pt-br/assuntos/biodiversidade/pan/pan-tartarugas-marinhas (accessed on 16 April 2024).
- International Union for Conservation of Nature (IUCN). Red list of threatened species. Available online: https://www.iucnredlist.org/ (accessed on 16 April 2024).
- Brasil, Ministério do Meio Ambiente. Portaria MMA nº 148, de 7 de junho de 2022. DOU Nº 108 Seção 1, 08 de junho 2022. Available online: https://www.in.gov.br/en/web/dou/-/portaria-mma-n-148-de-7-de-junho-de-2022-406272733 (accessed on 16 April 2024).
- Brasil, Ministério do Meio Ambiente. Nota de esclarecimento do centro TAMAR ICMBio sobre a nova classificação do status de conservação da tartaruga verde Chelonia mydas no Brasil. Available online: https://www.icmbio.gov.br/centrotamar/ultimas-noticias/53-nota-de-esclarecimento-do-centro-tamar-icmbio-sobre-a-nova-classificacao-do-status-de-conservacao-da-tartaruga-verde-chelonia-mydas-no-brasil (accessed on 16 April 2024).
- Matushima, E.R., Filho, A.L., Di Loretto, C., Kanamura, C.T., Sinhorini, I.L., Gallo, B., Baptistolle, C. Cutaneous papillomas of green turtles: a morphological, ultra-structural and immunohistochemical study in Brazilian specimens. Braz. J. Vet. Res. Anim. Sci. 2001, 38, 51–54. [CrossRef]
- Cubas, Z.S., Silva, J.C.R., Dias, J.L.C. Tratado de animais selvagens: 2nd. ed.; Rocca: São Paulo, SP, Brazil. 2014.
- Carvalho, G.D.; Fósse, K.M.; Souza, M.; Reis, N.G.R. A importância ecológica da conservação das tartarugas marinhas. In: Anais do Congresso Brasileiro Interdisciplinar em Ciências e Tecnologias, UFVJM, 2021.
- Hamede, R.; Owen, R.; Siddle, H.; Peck, S.; Jones, M.; Dujon, A.; Giraudeau, M.; Roche, B.; Ujvari, B.; Thomas, F. The ecology and evolution of wildlife cancers: Applications for management and conservation. Evo. Applicat. 2020; 13, 1719–173. [CrossRef]
- Martini, E.A.; Teixeira, F.L.; Soares, J.S.; Bonach, K. Perfil das Pesquisas Científicas Envolvendo Tartarugas Marinhas No Mar Do Norte Brasileiro. Rev. Multid. Educ. Meio Amb. 2020, 1, 145–146. [Google Scholar]
- Brasil, Ministério do Meio Ambiente. Portaria MMA nº 09, de 23 de janeiro de 2007. Available online: https://antigo.mma.gov.br/estruturas/chm/_arquivos/biodiversidade31.pdf (accessed on 16 April 2024).
- Instituto Chico Mendes (ICMBio). 2012. Available online: https://www.gov.br/icmbio/pt-br/assuntos/biodiversidade/unidade-de-conservacao/unidades-de-biomas/marinho/lista-de-ucs/resex-marinha-de-caete-taperacu/arquivos/resex_caete_taperacu_pm_plan.pdf (accessed on 16 April 2024).
- Castilho, D B.A. Interlocução de Saberes na Antropologia:1st ed; Atena, São Paulo, SP, Brazil. 2019. [CrossRef]
- Marcovaldi, M.A.; Lopez, G.G..; Sorares, L.S.; López-Mendilaharsu, M. Satellite tracking of hawksbill turtles Eretmochelys imbricata nesting in northern Bahia, Brazil: turtle moviments and foraging destinations. Endang. Species Res. 2012, 17, 123–132. [CrossRef]
- Abrantes, M.M.R. Interação de tartarugas marinhas com a pesca artesanal na Reserva Extrativista Marinha Mãe Grande de Curuçá, Pará, Brasil. Master Thesis, Universidade Federal do Pará, Pará, 2011. [Google Scholar]
- Silva, A. L. Atendimento clínico de tartaruga marinha no ambulatório de animais selvagens (HOVET/UFRA): o caso da tartaruga oliva (Lepidochelys olivacea). RCW (Residency Completion Work) - Multiprofessional Health Residency Program, Universidade Federal Rural da Amazônia, Belém, 2018.
- Paiva, P.P.; Fonteles Filho, A.A. Sobre a produção pesqueira de alguns currais de pesca do Ceará – dados de 1965 a 1967. Arq. Cien. Mar, 1965, 5, 175–214. [Google Scholar]
- Collyer, E.C.; Aguiar, D.U. Sobre a produção pesqueira de alguns currais de pesca do Ceará dados de 1968 a 1970. Arq. Cien. Mar, 1972, 24, 1–9. [Google Scholar]
- Lima, E. H. S. M. Captura acidental de tartarugas marinhas em currais de pesca na Praia de Almofala – Itarema/CE: subsídios para a preservação dos quelônios marinhos em áreas de alimentação. Master Thesis, Universidade Federal do Ceará, 1999.
- Silva, S. F. Tartarugas marinhas do litoral paraense: ocorrência e aspectos clínicos de animais recebidos no Centro de Triagem e Reabilitação de Animais Selvagens da UFRA. CCW (Course Completion Work) – Bachelor’s Degree in Veterinary Medicine, Universidade Federal Rural da Amazônia, Pará, 2023.
- Rossi, S.; Zamana, R. R.; Santos, P. P. de A.; Bomfim, A.C.; Farias, D. S. D.; Freire, A.C.B.; Oliveira, R. M.; Gattamorta, M A.; Matushima. E. R.; Pires, J. M. de L.; Sacristán, C.; Silva-Júnior, E. S. da; Silva, F. J. de L.; Gavilan, S. A. Visceral neoplasms and Chelonid alphaherpesvirus 5 in green turtles with FP. Arch. Vet. Sc. 2021; 26. [CrossRef]
- Brooks, D.E., Ginn, P.E., Miller, T.R., Bramson, L., Jacobson, E.R. Ocular fibropapillomas of Green turtles (Chelonia mydas). Veterinary Pathology, 1994; v. 31, p. 335-339. [CrossRef]
- Garcês, A.; Pires, I. FP on sea turtles, a sentinel of ecosystem health? Envi. Sc.Proceed. 2022, 24. [Google Scholar] [CrossRef]
- Gattamorta, M.A., Gavilan, S.A., Silva, F.J.L, Zamana, R.R., Setim, F.E., Rossi, S., Farias, D.S.D., Oliveira, R.E.M., Freire, A.C.B., Santos, A.J B., Lara, P H., Matushima, E.R. First report of FP (FP) and Chelonid alphaherpesvirus 5 (ChHV5) in a green sea turtle (Chelonia mydas) from the historically FP-free Fernando de Noronha Archipelago, Northeastern Brazil. Braz. J. Vet. Res. Anim. Sci. 2022; 59, p. e181776. [CrossRef]
- Ene, A.; Su, M.; Lemaire,S.; Rose, C.; Sharff, S.; Moretti.; Lenz,J.; Herbst, L.H. Distribution of chelonid FP associated herpesvirus variants in Florida: molecular genetic evidence for infection of turtles following recruitment to neritic developmental habitats. J. Wildl.Dis. 2005, 41489–41497. [CrossRef]
- Monezi, T.A., Mehnert, D.U., de Moura, E.M., Müller, N.M., Garrafa, P., Matushima, E.R., Werneck, M.R., Borella, M.I. Chelonid herpesvirus 5 in secretions and tumor tissues from green turtles (Chelonia mydas) from Southeastern Brazil: A ten-year study. Vet. Microbiol. 2016, 186, 150–156. [CrossRef]
- Page-Karjian, A.; Gottdenker, N.L.; Whitfield, J.; Herbst, L.; Norton, T.M.; Ritchie, B. Potential Non-Cutaneous sites of Chelonid Herpesvirus 5 Persistence and shedding in green sea turtles (Chelonia mydas). J Aquat. Ani. Heal. 2017, 29, 136–142. [Google Scholar] [CrossRef]
- Silva-Júnior, E.S. ; Rossi, S., Zamana, R.R.; Gattamorta, M.A.; Freire, A.C.B.; Revorêdo, R.Â.; Farias, D.S.D.; Sacristán, C.; Bonfim, A.C.; Matushima, E.R.; Silva, F.J.L.; Gavilan, S.A. Chelonid alphaherpesvirus 5 in cutaneous tumors of green turtles (Chelonia mydas) stranded in Northeastern Brazil. Arch. Vet. Sci., 2021,. 26, 1-16. [CrossRef]
- Herbst, L.H. FP of marine turtles. Ann. Review Fish Dis. 1994, 4, 389–425. [Google Scholar] [CrossRef]
- Jones, K.; Ariel, E.; Burgess, G.; Read, M. A review of FP in green turtles (Chelonia mydas). The Vet. J. 2016, 212, 48–57. [Google Scholar] [CrossRef]
- Jones, K.; Limpus, C J.; Brodie, J.; Jones, R.; Read, M.; Shum, E.; Bell, I.P.; Ariel, E. Spatial distribution of FP in green turtles along the Queensland coast and an investigation into the influence of water quality on prevalence. Conser. Sci. Pract, 2022, e12755. [CrossRef]
- Oriá, A.P., Silva, D.N., Raposo, A.C., Estrela-Lima, A., Pires, T.T., Gattamorta, M.A., Zamana, R.R., Matushima, E.R., Ofri, R. Atypical ocular Chelonoid herpesvirus manifestations in a captive Loggerhead turtle (Caretta caretta). Vet. Ophthalmol. 2021. 24, 97-102. [CrossRef]
- Work, T.M., Balazs, G.H., Rameyer, R.A., Morris, R.A. Retrospective pathology survey of Green turtles Chelonia mydas with FP in the Hawaiian Islands. Dis.Aquatic Org. 2004, 62, 163–176. [CrossRef]
- Lutz, P.L.; Musick, J.A. The Biology of Sea Turtles. Marine Science Series: 1st ed.CRC Press: Boca Raton, FL, USA, 1997.
- Baptistotte, C.; Rieth, D. B.; Becker, J. H.; Lopez, G.; Castilhos, J. C.; Lima, E. H. S. M.; Belini, C.; Matushima, E. R.; Barata, P. C. R. Prevalência de fibropapilomatose em tartarugas marinhas nas áreas de alimentação no Brasil. In: Anais do V Congresso e X Encontro da Associação Brasileira de Veterinários de Animais Selvagens - São Paulo, 2001; p. 29.
- Aguirre, AA.; Lutz, P. L. Marine turtles as sentinels of ecosystem health: is FP an indicator? EcoHealt. 2004, 1, 275–283. [Google Scholar] [CrossRef]
- Hamann, M., Godfrey, M., Seminoff, J., Arthur, K., Barata, P., Bjorndal, K., Bolten, A., Broderick, A., Campbell, L., Carreras, C., Et Al. Global research priorities for sea turtles: Informing management and conservation in the 21st century. End. Species Resear. 2010, 11, 245–269. [CrossRef]
- Hargrove, S. A.; Work, T.M.; Brunson, S.; Foley, A.M.; Balazs, G. Proceedings of the 2015 International Summit on FP: global status, trends, and population impacts. NOAA Technical Memorandum, 2016, 1-85. [CrossRef]
- Rhodes, K.H. Dermatologia de Pequenos Animais Consulta em 5 minutos: 1st ed. Revinter: Rio de Janeiro, RJ, BR., 2002.
- Rodenbusch C.R., Almeida L.L., Marks F.S., Ataíde M.W., Alievi M.M., Tavares M., Pereira R.A. and Canal C.W. Detection and characterization of fibropapilloma associated herpesvirus of marine turtles in Rio Grande do Sul, Brazil. Pesq. Vet. Bras. 2012, 32, 1179–1183. [CrossRef]
- Smith, G.M., Coates, C.W. Fibro-epithelial growths of the skin in large marine turtles, Chelonia mydas (Linnaeus). Zool. 1938, 23,. 93-98. [CrossRef]
- Alfaro-Núñez A; Bojesen, A.M.; Bertelsen, M.F.; Wales, N.; Balazs, G.H.; Gilbert, M.T.P. Further evidence of Chelonid herpesvirus 5 (ChHV5) latency: high levels of ChHV5 DNA detected in clinically healthy marine turtles. Peer., 2016, 4, e2274. [CrossRef]
- Zamana, R.R; Gattamorta, M.A.; Cruz Ochoa, P.F.; Navas-Suáres, P.E.; Sacristán, C.; Rossi, S.; Grisi-Filho, J.H.H.; Silva, I.S.; Matushima, E.R. High Occurrence of Chelonid Alphaherpesvirus 5 (ChHV5) in Green Sea Turtles Chelonia mydas with and without FP in Feeding Areas of the São Paulo Coast, Brazil. J. Aquatic Animal Heal. 33252–263. [CrossRef]
- Mashkour, N.; Jones, K.; Kophamel, S.; Hipolito, T.; Ahasan, S.; Walker, G.; Jakob-Hoff, R.; Whittaker, M.; Hamann, M.; Bell, I.; Elliman, J.; Owens, L.; Saladin, C.; Crespo-Picazo, L.; Gardner, B.; Loganathan, A.L.; Bowater, R.; Young, E.; Robinson, D.; Baverstock, W.; Blyde, D.; March, D.; Eghbali, M.; Mohammadi, M.; Freggi, D.; Giliam, J.; Hale, M.; Nicolle, N.; Spiby, K.; Wrobel, D.; Parga, M.; Mobaraki, A.; Rajakaruna, R.; Hyland, K.P.; Read, M.; Ariel, E. Disease risk analysis in sea turtles: A baseline study to inform conservation efforts. PLoS ONE, 2020;. 15, p. e0230760. [CrossRef]
- Gattamorta,M.A. Ecologia, prevalência e caracterização molecular de Chelonid fibropapilloma-associated herpesvirus (CFPHV) em Tartarugas verdes (Chelonia mydas) em áreas da costa brasileira. Phd Thesis, Escola Superior de Agricultura “Luiz de Queiroz” - Centro de Energia Nuclear na Agricultura, Universidade de São Paulo, Piracicaba/SP, 2015; p. 121.
- Quackenbush, S.L., Bowser, P.R., Work, T.M., Balazs, G.H., Casey, R.N., Casey, J.W., Rovnak, J., Chaves, A., Dutoit, L., Baines, J.D., Parrish, C.R. Three closely related herpesviruses are associated with FP in marine turtles. Virolog. 1998, 246, 392–399. [CrossRef]
- Quackenbush, S.L., Aguirre, A.A., Spraker, T.R., Horrocks, J.A., Vermeer, L.A., Balazs, G.H., Casey, J.W., Casey, R.N., Murcek, R.J., Paul, T.A., Et Al. Quantitative analysis of herpesvirus sequences from normal tissue and fibropapillomas of marine turtles with real time PCR. Virolog. 2001, 287105–111. [CrossRef]
- Alfaro-Núñez, A.; Bertelsen, F.M.; Bojesen, A.M.; Rasmussen, I.; Zepeda-Mendoza, L.; Olsen, M.T.; Gilbert, M.T.P. Global distribution of Chelonid fibropapilloma-associated herpesvirus among clinically healthy sea turtles. BMC Evol. Biol. 2014, 14. [Google Scholar] [CrossRef]
- Ackemann, M.; Koriabine, M.; Hartmann-Fritsch, F.; Jong, P.J.; Lewis, T.D.; Schetle, N.; Work, T.M.; Dagenais, J.; Balazs, G. H.; Leong, J. C. The genome of Chelonid herpesvírus 5 harbors atypical genes. PLoS ONE, 2012; 7, e46623. [CrossRef]
- Jones, K.; Burgess, G.; Budd, A.M.; Huerlimann, R.; Mashkour, N.; Ariel, E. Molecular evidence for horizontal transmission of Chelonid alphaherpesvirus 5 at green turtle (Chelonia mydas) foraging grounds in Queensland, Australia. PLoS ONE, 2020, 15,. e0227268. [CrossRef]
- Peare, T.; Parker, P.G. Local genetic structure within two rookeries of Chelonia mydas (the green turtle). Hered., 1996, 77, 619–628. [Google Scholar] [CrossRef]
- Lee, P.LM.; Luschi, P.; Hays, G.C. Detecting female precise natal philopatry in green turtles using assignment methods. Mol. Ecol. 2006, 16, 61–74. [Google Scholar] [CrossRef]
- Patrício, A.R.; Herbst, L. H.; Duarte, A.; Velez-Zuazo, X.; Santos Loureiro, N. S.; Pereira, N.; Tavares, L.; Toranzos, G. A. Global phylogeography and evolution of chelonid fibropapilloma-associated herpesvirus. J. Gen. Virol. 2012, 93, 1035–1045. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Yu, Q.; Aguirre, A.A.; Balazs, G.H.; Nerurkar, V.R.; Yanagihara, R. Detection of herpesviral sequences in tissues of green turtles with fibropapilloma by polymerase chain reaction. Arch. Virolog. 2000, 145, 1985–1893. [Google Scholar] [CrossRef]
- Herbst, L.H.; Jacobson, E.R.; Moretti, R.; Brown, T.; Sundberg, J.P.; Klein, P.A. Experimental transmission of green turtle fibropapillomatosis using cell-free tumor extracts. Dis. Aquat. Organ. 1995, 22, 1–12. [Google Scholar] [CrossRef]
- Greenblatt, R.J.; Work, T M.; Balazs, G.H.; Sutton, C.A.; Casey, R.N.; Casey, J.W. The Ozobranchus leech is a candidate mechanical vector for the fibropapilloma associated turtle herpesvirus found latently infecting skin tumors on Hawaiian green turtles (Chelonia mydas). Virolog. 2004, 321, 101–110. [CrossRef]
- Farrell, J.A.; Whitmore, L.; Mashkour, N.; Ramia, D RR.; Thomas, RS.; Eastman, C. B.; Burkhalter, B.; Yetsko, K.; Mott, C.; Wood, L.; Zirkelbach, B.; Meers, L.; Kleinsasser, P.; Stock, S.; Libert, E.; Herren, R.; Eastman, S.; Crowder, W.; Bovery, C.; Anderson, D.; Godfrey, D.; Condron, N.; Duffy, D.J. Detection and population genomics of sea turtle species via noninvasive environmental DNA analysis of nesting beach sand tracks and oceanic water. Mol. Ecol. Resour. 2022, 22, 2471–2493. [CrossRef]
- Page-Karjian, A.; Stacy, N I.; Morgan, A N.; Coppenrath, C.M.; Manire, C.A.; Herbst, L.H.; Perrault, J.R. Morphologic and physiologic characteristics of green sea turtle (Chelonia mydas) hatchlings in southeastern Florida, USA. J. Comp. Phys. 2022, 192, 751–764. [CrossRef]
- James, A.; Page-Karjian, A.; Charles, K.E.; Edwards, J.; Gregory, C.R.; Cheetham, S.; Buter, B.P.; Marancik, D.P. Chelonid Alphaherpesvirus 5 Prevalence and First Confirmed Case of Sea Turtle FP in Grenada, West Indies. Animals, 2021;.11, 1490. [CrossRef]
- García-Sastre, A., Sansonetti, P.J. Host-pathogen interactions. Current Opinion Immun. 2010, 22, 425–427. [CrossRef]
- Alfaro-Núñez A., Gilbert M.T.P. Validation of a sensitive PCR assay for the detection of Chelonid Fibropapilloma-associated Herpesvirus in Latent Turtle Infections. J. Virol. Met. 2014, 206, 38–41. [CrossRef]
- Dos Santos, R.G., Martins, A.S., Torezani, E., Baptistotte, C., Da Nóbrega, F.J., Horta, P.A., Work, T.M., Balazs, G.H. Relationship between FP and environmental quality: a case study with Chelonia mydas off Brazil. Dis. Aquat. Organ. 2010. 89, 87-95. [CrossRef]
- Van Houtan, K.S., Hargrove, S.K., Balazs, G.H. Land use, macroalgae, and a tumor-forming disease in marine turtles. PLoS One. 2010, 5, e12900. [CrossRef]
- Arthur, K.; Limpus, C.; Balaz, G.; Capper, A.; Udy, J.; Shaw, G.; Keuper-Bennet, U.; Bennet, P. The exposure of Green Turtle s (Chelonia mydas) to tumor promoting compounds produced by the Cyanobacterium lyngbya majuscula and their potencial role in the aetiology of fipropapillomatosis. Harmful Algae, 2008, 7, 114–125. [Google Scholar] [CrossRef]
- Page-Karjian, A.; Norton, T.M.; Krimer, P.; Groner, M.; Nelson Jr, S.E.; Gottdenker, N.L. Factors influencing survivorship of rehabilitating green sea turtles (Chelonia mydas) with FP. J. Zoo Wildl. Med. 2014, 45, 507–519. [Google Scholar] [CrossRef]
- Duffy, D. J.; Schnitzler, C.; Karpinski, L., Thomas, R.; Whilde, J.; Eastman, C.; Yang, C.; Krstic, A.; Rollinson, D.; Zirkelbach, B.; Sea turtle fibropapilloma tumors share genomic drivers and therapeutic vulnerabilities with human cancers. Commun. Biol. 2018, 1,. 63. [CrossRef]
- Garefino, V.E.; Milton, S L. Influence of Sunlight on Vitamin D and Health Status in Green (Chelonia mydas) Sea Turtles with FP. Animals, 2022, 12, 488. [Google Scholar] [CrossRef]
- Read, M. A.; Limpus, C. J. The green turtle, Chelonia mydas, in Queensland: Feeding ecology of immature turtles in Moreton Bay, southeastern Queensland. Memoirs Queensland Museum, 2002, 48, 207–214.
- Page-Karjian, A.; Torres, F., Zhang, J.; Rivera, S., Diez, C.; Moore, P.A.; Moore, D.; Brown, C. Presence of chelonid fibropapilloma-associated herpesvirus in tumored and non tumored Green turtles, as detected by polymerase chain reaction, in endemic and non-endemic aggregations, Puerto Rico. SpringerPlus, 2012, 1, 1–8. [CrossRef]
- Van Houtan, K.S.; Smith, C.M.; Dailer, M.L.; Kawachi, M. Eutrophication and the dietary promotion of sea turtle tumors. PeerJ. 2014, 2, e602. [Google Scholar] [CrossRef]
- Manes, C.; Carthy, R.R.; Hull, V. A Coupled Human and Natural Systems Framework to Characterize Emerging Infectious Diseases-The Case of Fibropapillomatosis in Marine Turtles. Animals, 2023, 13, 1441. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).