Submitted:
16 May 2024
Posted:
17 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Acquisition
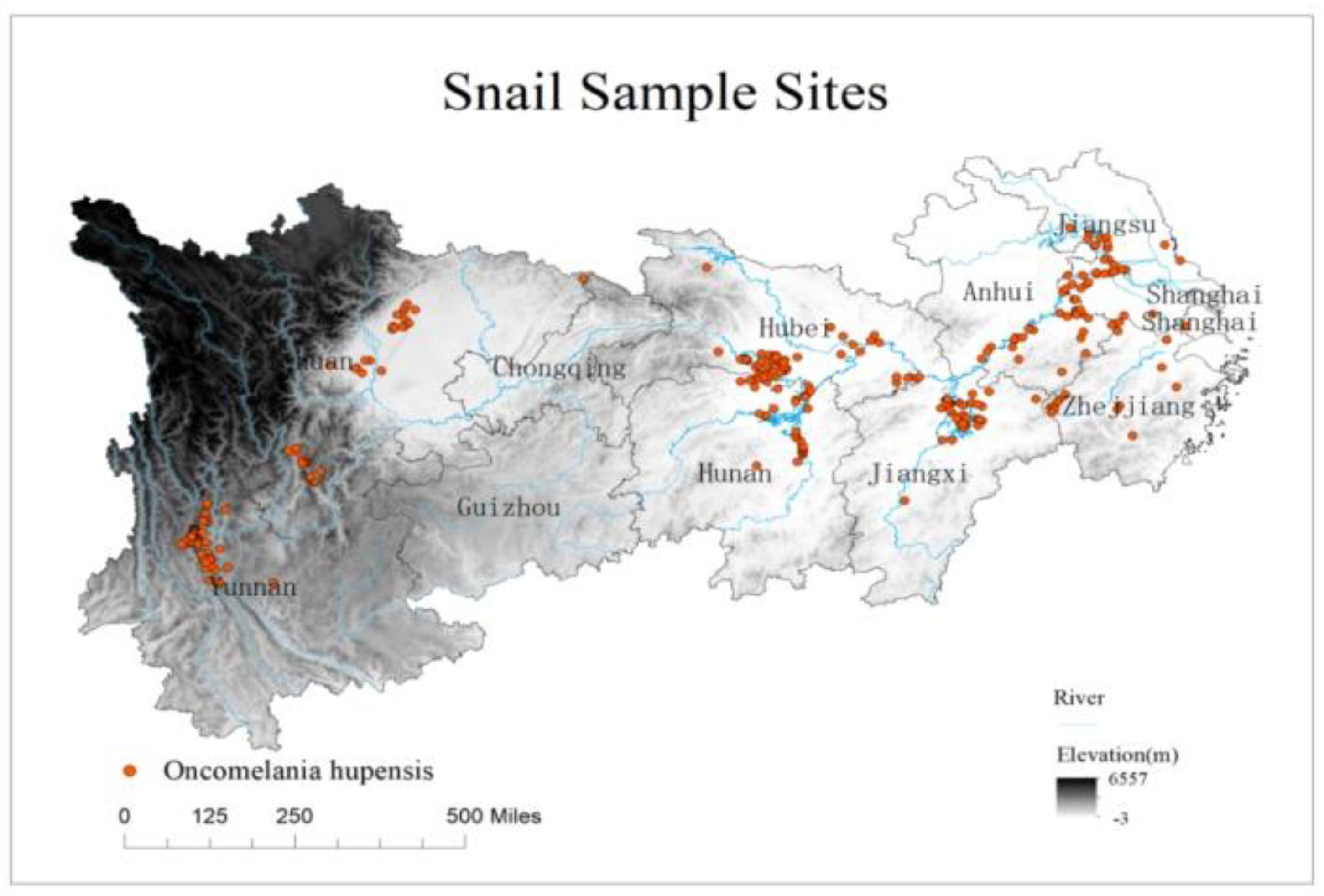
2.2.1. Occurrence Data for Oncomelania Hupensis
2.2.2. Environmental Data
2.3. Methodology
2.3.1. Establishment and Evaluation of the Species Distribution Model for O. hupensis
2.3.2. Change in Potential Distribution and Centroids
2.3.3. Regionalization and Analytical Methods
3. Results
3.1. The Establishment and Effect of the Model
3.1.1. Filtering of Environment Variables
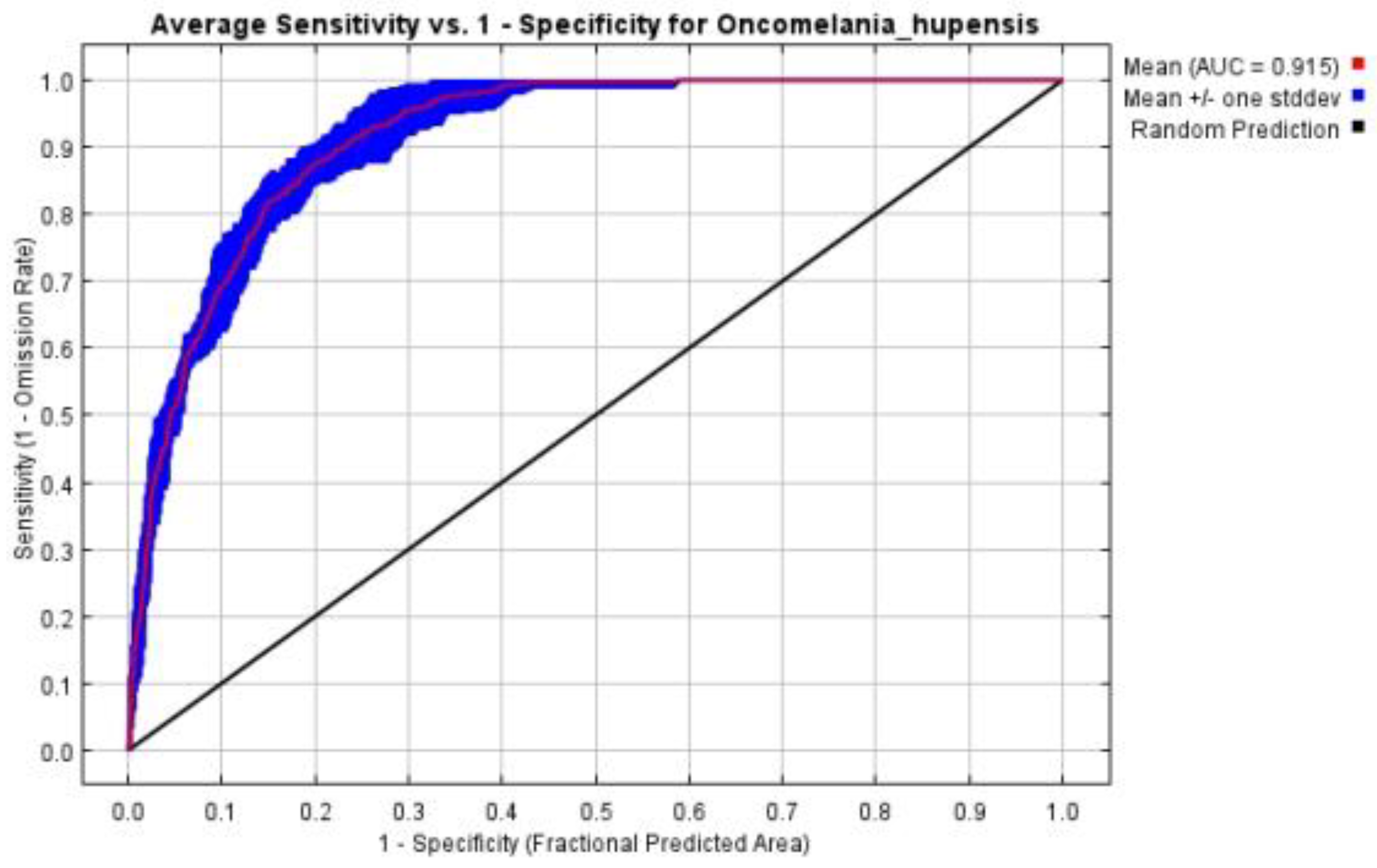
3.1.2. Evaluation of Modellings
3.2. The Influence of Environmental Factor
3.3. Current Prediction of O. hupensis
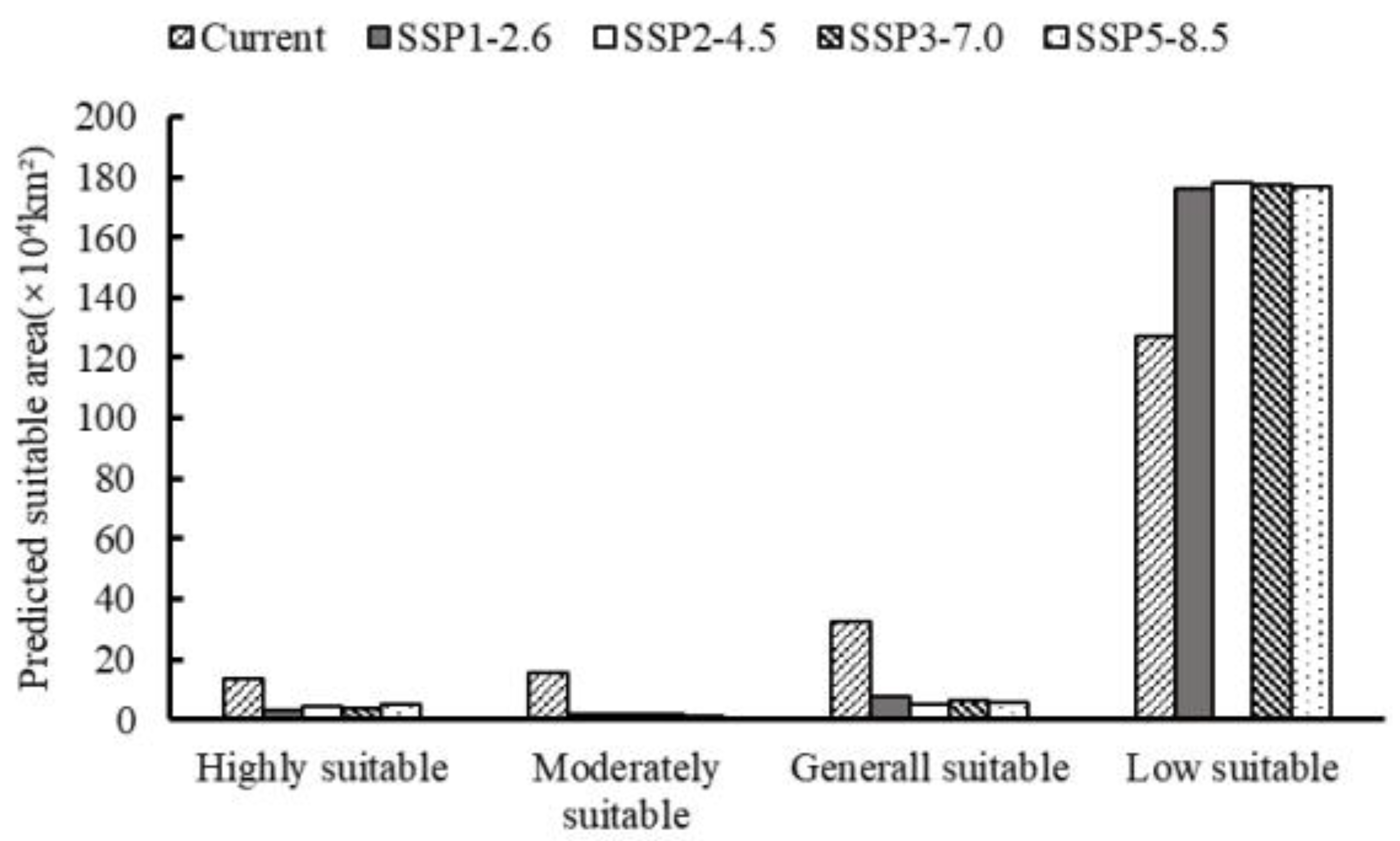
3.4. Future Prediction of O. hupensis
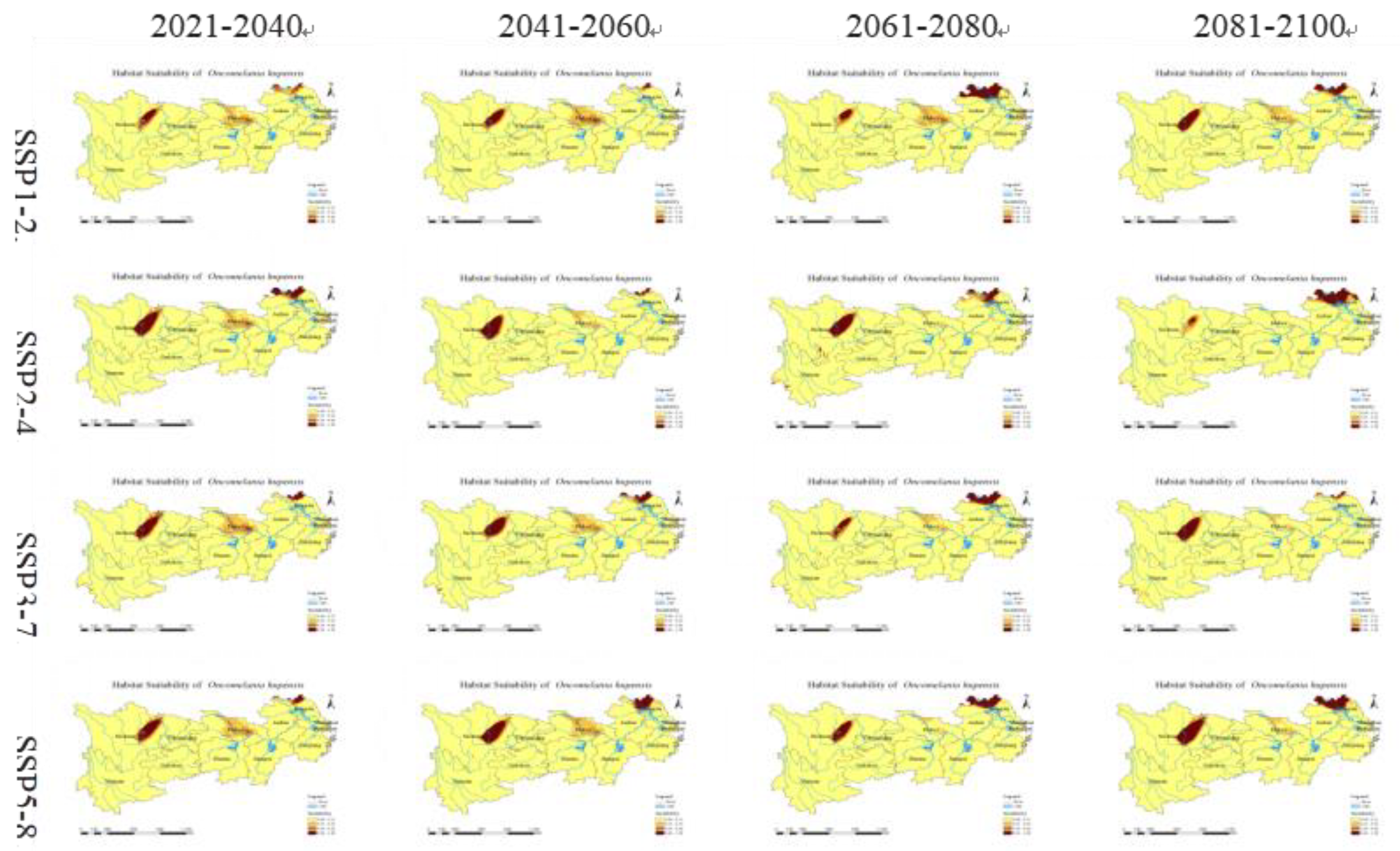
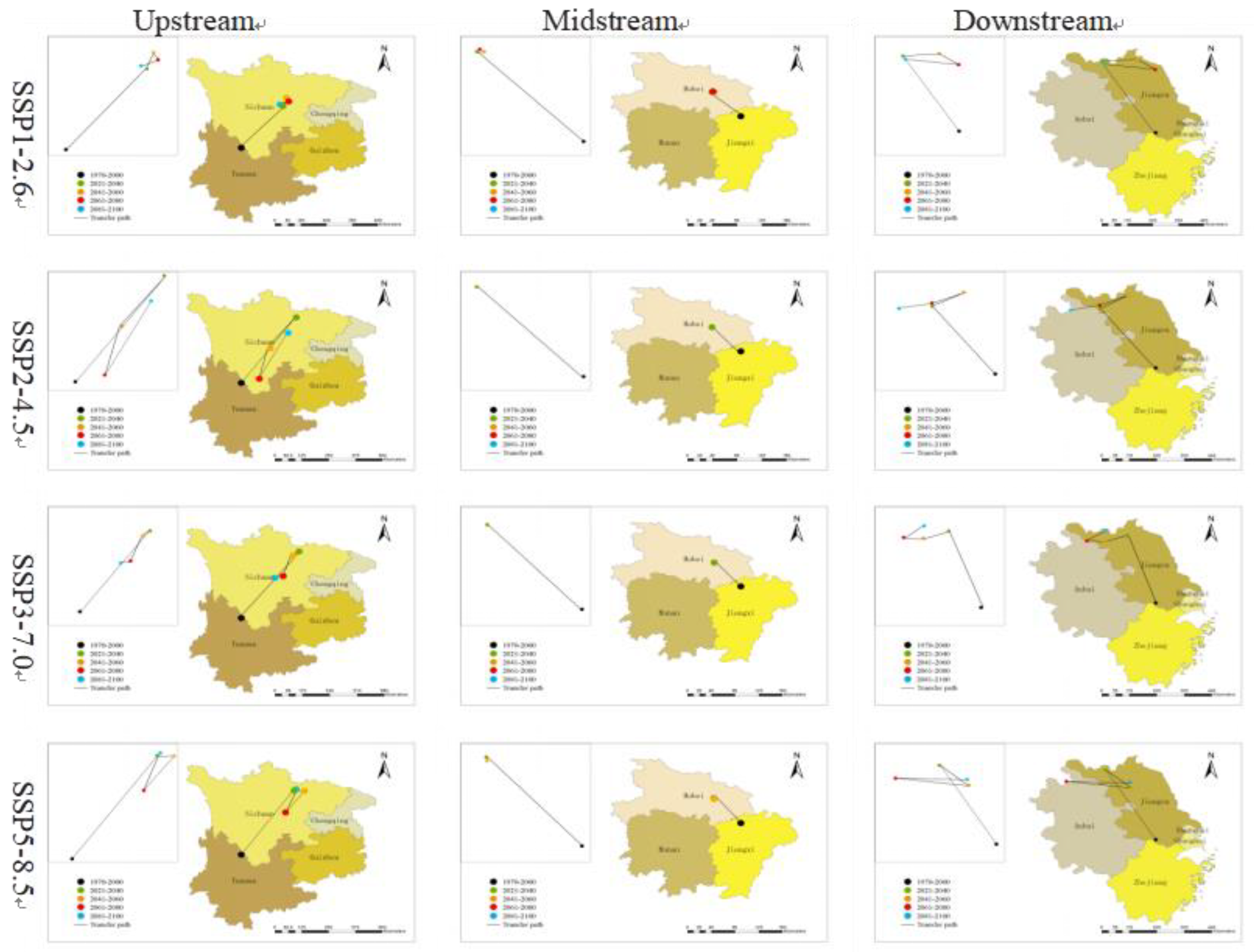
3.5. Directional Analysis of O. hupensis's Distribution Centroid
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, X. Distribution and control of schistosomiasis in China. Journal of Anhui Agricultural Sciences 2007, 3766-3768. [CrossRef]
- Moloo, A. Schistosomiasis elimination: refocusing on snail control to sustain progress. Available online: https://www.who.int/news/item/25-03-2020-schistosomiasis-elimination-refocusing-on-snail-control-to-sustain-progress (accessed on 2020-03-25).
- Qin, J.; Tan, Z.; Zhang, C. Environment factors and spatial characters of distribution of Oncomcelania Snails in islet and beach of Dongting Lake area. Journal of Natural Disasters 2008, 19-27.
- Yang, G.-J.; Zhou, X.-N.; Sun, L.-P.; Wu, F.; Zhong, B.; Qiu, D.-C.; Utzinger, J.; Bradshaw, C.J.A. Compensatory density feedback of Oncomelania hupensis populations in two different environmental settings in China. Parasites & Vectors 2011, 4, 133. [CrossRef]
- Zhou, X.; Xu, J.; Lü, S.; Li, S. Progress of the national programme and achievements of scientific researches on schistosomiasis elimination in China. Chinese Journal of Disease Control and Prevention 2019, 23, 749-753. [CrossRef]
- Wang, Z.; Liu, L.; Shi, L.; Wang, X.; Zhang, J.; Li, W.; Yang, K. Identifying the determinants of distribution of Oncomelania hupensis based on geographically and temporally weighted regression model along the Yangtze River in China. Pathogens 2022, 11, 970. [CrossRef]
- Peng, H.; Li, C. The measures of the environment construction to kill snails and their effect on controlling Schistosomiasis. Chinese Journal of Endemiology 2002, 92-94.
- Xu, J.; Lin, D.-D.; Wu, X.-H.; Zhu, R.; Wang, Q.-Z.; Lv, S.-B.; Yang, G.-J.; Han, Y.-Q.; Xiao, Y.; Zhang, Y. Retrospective investigation on national endemic situation of schistosomiasis. III. Changes of endemic situation in endemic rebounded counties after transmission of schistosomiasis under control or interruption. Zhongguo xue xi Chong Bing Fang zhi za zhi= Chinese Journal of Schistosomiasis Control 2011, 23, 350-357.
- Jiang, T.; Yang, K. Progresses of research on patterns and monitoring approaches of Oncomelania hupensis spread. Chinese Journal of Schistosomiasis Control 2020, 32, 208-212. [CrossRef]
- Yangtze River Protection Law of the People's Republic of China. China Water Resources 2021, 1-8.
- Li, L.; Zhang, L.; Zhang, S.; Xu, J. Challenges and prospects of Oncomelania snail control via mollusciciding in China in the new era. Journal of Tropical Diseases and Parasitology 2022, 20, 31-35.
- Chen, D., M.; Rojas, B.H.; Samset, K.; Cobb, A.; Diongue Niang, P.; Edwards, S.; Emori, S.H.; Faria, E.; Hawkins, P.; Hope, P.; et al. 2021: Framing, Context, and Methods. InClimate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change[Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)]. ; Cambridge, United Kingdom and New York, NY, USA, : Cambridge University Press, 2021; pp. 147-286.
- Li, L.; Xiong, L.; Jiang, C.; Zhang, H. Impact of air temperature on annual runoff of batang station in the headstream of Yangtze river. Resources and Environment in the Yangtze Basin 2015, 24, 1142-1149.
- Yang, H.; Pang, H.; Hu, H.; Xie, C.; Qiu, L.; Huang, J.; Sun, Y.; Hong, Q.; Zhou, X. Prediction of snail habitats in the marshland along the yangtze ri ver affected by flood in 1998 by remote sensing. Chinese Journal of Schistosomiasis Control 2000, 337-339+386. [CrossRef]
- Zhou, X.; Yang, K.; Hong, Q.; Sun, L.; Yang, G.; Liang, Y.; Huang, Y. Prediction of the Impact of Climate Warming on Transmission of Schistosomiasis in China. Chinese Journal of Parasitology and Parasitic Diseases 2004, 8-11+67-68.
- Li, Y. Impact of water level and climatic factors on the distribution of Schistosoma japonicum intermediate host Oncomelania hupensis and the identification of snail habitats in Eastern Dongting Lake Areas. master, Fudan University, 2011.
- Huang, J.; Liu, K.; Chang, A.; Peng, J. Research on the response of the intermediate host—snail to the meteorological factors in Dongting Lake area. Journal of Public Health and Preventive 2015, 26, 27-30.
- Liao, J.; Yi, Z.; Li, S.; Xiao, L. Maxent modeling for predicting the potentially geographical distribution of Miscanthus nudipes under different climate conditions. Acta Ecologica Sinica 2020, 40, 8297-8305.
- Du, Q.; Wei, C.; Liang, C.; Yu, J.; Wang, H.; Wang, W. Future climatic adaption of 12 dominant tree species in Northeast China under 3 climatic scenarios by using MaxEnt modeling. Acta Ecologica Sinica 2022, 42, 9712-9725.
- Zhang, X.; Jiang, Y.; Bi, Y.; Liu, X.; Li, X.; Sun, T.; Chen, H.; Li, J. Identification of potential distribution area for Hippophae rhamnoides subsp. sinensis by the MaxEnt model. Acta Ecologica Sinica 2022, 42, 1420-1428.
- Oso, O.G.; Sunday, J.O.; Odaibo, A.B. Models for predicting bulinids species habitats in southwestern Nigeria. Parasite Epidemiology and Control 2022, 18, e00256. [CrossRef]
- Hu, Y.; Zhang, Z.; Chen, Y.; Wang, Z.; Gao, J.; Tao, B.; Jiang, Q.; Jiang, Q. Spatial pattern of schistosomiasis in Xingzi, Jiangxi Province, China: the effects of environmental factors. Parasites & Vectors 2013, 6, 214. [CrossRef]
- Zhou, Y.-B.; Liang, S.; Chen, G.-X.; Rea, C.; Han, S.-M.; He, Z.-G.; Li, Y.-P.; Wei, J.-G.; Zhao, G.-M.; Jiang, Q.-W. Spatial-temporal variations of Schistosoma japonicum distribution after an integrated national control strategy: a cohort in a marshland area of China. BMC Public Health 2013, 13, 297. [CrossRef]
- Chen, Y.-Y.; Huang, X.-B.; Xiao, Y.; Jiang, Y.; Shan, X.-w.; Zhang, J.; Cai, S.-X.; Liu, J.-B. Spatial Analysis of Schistosomiasis in Hubei Province, China: A GIS-Based Analysis of Schistosomiasis from 2009 to 2013. PLOS ONE 2015, 10, e0118362. [CrossRef]
- Zhu, H.; Cai, S.-X.; Liu, J.-B.; Tu, Z.-W.; Xia, J.; Shan, X.-W.; Qiu, J.; Jiang, Y.; Xiao, Y.; Tang, L.; et al. A spatial analysis of human Schistosoma japonicum infections in Hubei, China, during 2009–2014. Parasites & Vectors 2016, 9, 529. [CrossRef]
- Zou, L.; Ruan, S. Schistosomiasis transmission and control in China. Acta Tropica 2015, 143, 51-57. [CrossRef]
- Ye, X.; Yang, X.; Liu, F.; Wu, J.; Liu, J. Spatio-temporal variations of land vegetation gross primary production in the Yangtze River Basin and correlation with meteorological factors. Acta Ecologica Sinica 2021, 41, 6949-6959.
- Yi, L.; Sun, Y.; Yin, S.; Wei, X.; Ou, Y. Spatial-temporal variations of vegetation coverage and its driving factors in the Yangtze River Basin from 2000 to 2019. Acta Ecologica Sinica 2023, 43, 798-811.
- Xiao, W.; Zuo, L.; Yang, W.; Li, C. Generating pseudo-absence samples of invasive species based on the similarity of geographical environment in the Yangtze River Economic Belt. Biodiversity Science 2023, 31, 218-228. [CrossRef]
- Li, Z.; Wang, Z.; Cao, W.; Yang, X. Spatial-temporal Dynamics and Influencing Factors of Ecological Security in the Yangtze River Economic Belt: Based on Three-dimensional Ecological Footprint Expansion Model. Journal of Natural Science of Hunan Normal University 2023, 46, 42-50.
- Gong, Y.; Tong, Y.; Jiang, H.; Xu, N.; Yin, J.; Wang, J.; Huang, J.; Chen, Y.; Jiang, Q.; Li, S.; et al. Three Gorges Dam: the changing trend of snail density in the Yangtze River basin between 1990 and 2019. Infectious Diseases of Poverty 2023, 12, 45. [CrossRef]
- Zhai, Q.; Sun, F.; Ao, X.; Geng, S.; Li, C.; Li, Y.; Li, M. Evaluation and projection of temperature and precipitation change in the Liaohe River Basin based on BCC-CSM2-MR model CMIP6 test. Journal of Meteorology and Environment 2022, 38, 27-36.
- Yang, H.; Jiang, Z.; Li, L. Biases and improvements in three dynamical downscaling climate simulations over China. Climate Dynamics 2016, 47, 3235-3251. [CrossRef]
- Shi, X.; Chen, X.; Dai, Y.; Hu, G. Climate Sensitivity and Feedbacks of BCC-CSM to Idealized CO2 Forcing from CMIP5 to CMIP6. Journal of Meteorological Research 2020, 34, 865-878. [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Diversity and Distributions 2011, 17, 43-57. [CrossRef]
- Cao, X.; Lin, T.; Zhang, Y.; Dong, Z. Study on the Movement of Population and Economic Gravity Center in Chongqing from 1999 to 2010. Journal of Chongqing Normal University(Natural Science) 2012, 29, 43-48.
- Zhang, L.; Wang, L.; Liu, S.; Sun, P.; Yu, Z.; Huang, S.; Zhang, X. An evaluation of four threshold selection methods in species occurrence modelling with random forest: Case studies with Davidia involucrata and Cunninghamia lanceolata. Chinese Journal of Plant Ecology 2017, 41, 387-395.
- Luo, Z.; Wei, W.; Li, Z.; Ding, L.; Yuan, L.; Xia, M.; Tang, L.; Ren, G.; Wang, J.; Wei, G. Impact of environmental changes on Oncomelania snail distribution in Dongting Lake beach. Chinese Journal of Schistosomiasis Control 2012, 24, 387-392. [CrossRef]
- Yang, Y.; Li, W.; Cheng, W.; Yang, Y.; Dong, S.; Li, L.; Liang, J.; Ang, Y.; Yang, D.; Cai, B.; et al. Relationship between natural extinction of Oncomelania hupensis snails and water chemical properties in Eastern Dongting Lake areas. Chinese Journal of Schistosomiasis Control 2019, 31, 126-133. [CrossRef]
- Chen, Z.; Zhou, X.-N.; Yang, K.; Wang, X.-H.; Yao, Z.-Q.; Wang, T.-P.; Yang, G.-J.; Yang, Y.-J.; Zhang, S.-Q.; Wang, J. Strategy formulation for schistosomiasis japonica control in different environmental settings supported by spatial analysis: a case study from China. Geospatial health 2007, 1, 223-231. [CrossRef]
- Li, Y.; Wang, H.; Zhang, J.; Zuo, Y.; Xiong, Y.; Luo, H.; Zhou, Y.; Xu, M. Distribution characteristics of snails in Wuhan and its relationship with elevation. Journal of Public Health and Preventive 2018, 29, 42-44.
- Zhu, H.R.; Liu, L.; Zhou, X.N.; Yang, G.J. Ecological Model to Predict Potential Habitats of Oncomelania hupensis, the Intermediate Host of Schistosoma japonicum in the Mountainous Regions, China. PLoS neglected tropical diseases 2015, 9, e0004028. [CrossRef]
- Cheng, G.; Li, D.; Zhuang, D.; Wang, Y. The influence of natural factors on the spatio-temporal distribution of Oncomelania hupensis. Acta Tropica 2016, 164, 194-207. [CrossRef]
- Tang, Y.; Liu, K.; Wei, F.; Ren, Y.; Zhang, L.; Xiao, W. Impact of Future Climate Change on Potential Distribution of Oncomelania in Hubei Province. Climate Change Research 2017, 13, 606-613.
- Mao, C. Biology of schistosome and control of schistosomiasis. People’s Health Press,Beijing 1990.
- Liu, Y.; Zhang, W.; Wang, Y. Medical Malacology. . Beijing: China Ocean Press. 1993, 157 p.
- Qin, H.; Gao, X.; Wang, H.; Xiao, J. Relative importance of meteorological and geographical factors in the distribution of Fasciola hepatica infestation in farmed sheep in Qinghai province, China. Parasite 2016, 23.
- Torgerson, P.; Claxton, J. Epidemiology and control. Fasciolosis 1999, 113, 149.
- Liu, W. Studies of Water, Heat and CO2, Fluxes over a Beach Snail Control and Schistosomiasis Prevention Forests in Yueyang city, Hunan province. Doctorate, Chinese Academy of Forestry, 2010.
- Cordellier, M.; Pfenninger, M. Inferring the past to predict the future: climate modelling predictions and phylogeography for the freshwater gastropod Radix balthica (Pulmonata, Basommatophora). Molecular Ecology 2009, 18, 534-544. [CrossRef]
- Yin, Y.; He, Q.; Pan, X.; Liu, Q.; Wu, Y.; Li, X. Predicting current potential distribution and the range dynamics of Pomacea canaliculata in China under global climate change. Biology 2022, 11, 110. [CrossRef]
- Liang, Y.; Xiao, R.; Dai, J.; Ye, J.; Song, H.; Chen, Z.; Jiang, B.; Liao, F.; Zhu, Y.; Zhu, J. Field observations on the survival and multiplication of Oncomelania snails in the different latitude regions in China. Chinese Journal of Schistosomiasis Control 1996, 259-262. [CrossRef]
- Zhou, X.; Yang, G.; Sun, L.; Hong, Q.; Yang, K.; Wang, R.; Hua, Z. Potential impact of global warming on schistosomiasis transmission. Chinese Journal of Epidemiology 2002, 8-11.
- Zhou, X. Effects of global warming on schistosomiasis transmission in China; 2004.
- Ge, Z. Response of Holocene flood to southwest monsoon change in upper reaches of Yangtze River. Geographical Research 2009, 28, 592-600.
- Shen, R.; Luo, S.; Chen, L. The relationship between monsoon circulation in midsummer and precipitation in China. Journal of Yunnan University(Natural Sciences Edition) 1982, 105-114.
- Zhang, S.; Cen, S.; Lai, X.; Zhang, G.; Zhang, Z.; Yao, S. Prediction of Rainstorm in the Upper Reaches of the Yangtze River Based on CMIP6 Multi-Model Ensemble. Plateau Meteorology 2024, 1-16.
- Li, Y.; Zhu, R.; Liu, T.; Chang, Y.; Yin, Z. Trend Analysis of Future Temperature and Precipitation in Shule River Basin based on BCC-CSM2-MR Model. Plateau Meteorology 2021, 40, 535-546.
- Li, S.; Quan, W.; Wang, Z.; Chen, Y.; Su, T.; Yan, P. Evaluation of the ability of BCC-CSM2-MR global climate model in simulating precipitation and temperature in East Asia. Journal of Arid Meteorology 2023, 41, 984-996.
- Li, Y.; Yan, D.; Peng, H.; Xiao, S. Evaluation of precipitation in CMIP6 over the Yangtze River Basin. Atmospheric Research 2021, 253, 105406.
- King, C.H.; Sturrock, R.F.; Kariuki, H.C.; Hamburger, J. Transmission control for schistosomiasis–why it matters now. Trends in parasitology 2006, 22, 575-582.
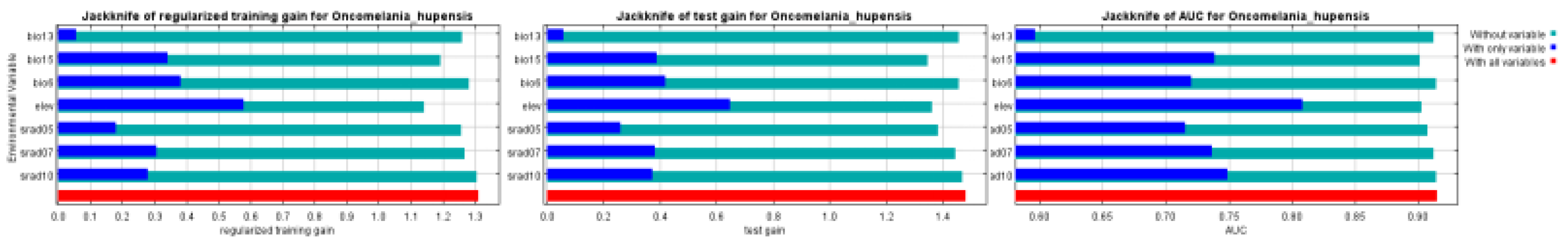
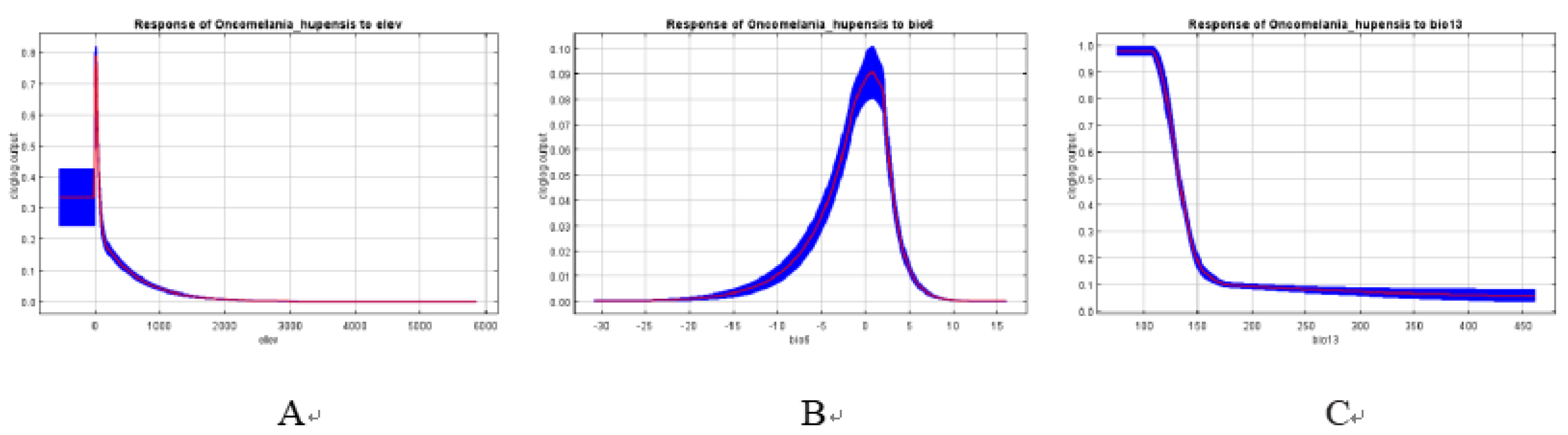

| Code | Environmental Variable | Percent contribution | Permutation importance |
|---|---|---|---|
| elev | Elevation | 40.1 | 46.8 |
| bio6 | Min Temperature of Coldest Month | 18.1 | 13.3 |
| bio13 | Precipitation of Wettest Month | 11.9 | 6.5 |
| srad07 | Solar radiation in July | 11.3 | 17.9 |
| srad05 | Solar radiation in May | 8.7 | 7.9 |
| bio15 | Precipitation Seasonality | 8.0 | 6.3 |
| srad10 | Solar radiation in October | 1.9 | 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





