3. Results
In the analysis of the samples with the p-XRF technique, the elements copper and tin as well as the elements lead, antimony, iron and nickel, were recorded. Since the surfaces under examination consist solely of corrosion products crusts, no quantitative analyses of the measurements were conducted. Moreover, the lack of metal core does not allow for the determination of the chemical composition of the alloy. However, we can conclude that for the manufacture of the vase a binary copper-tin (Cu-Sn) alloy has been used.
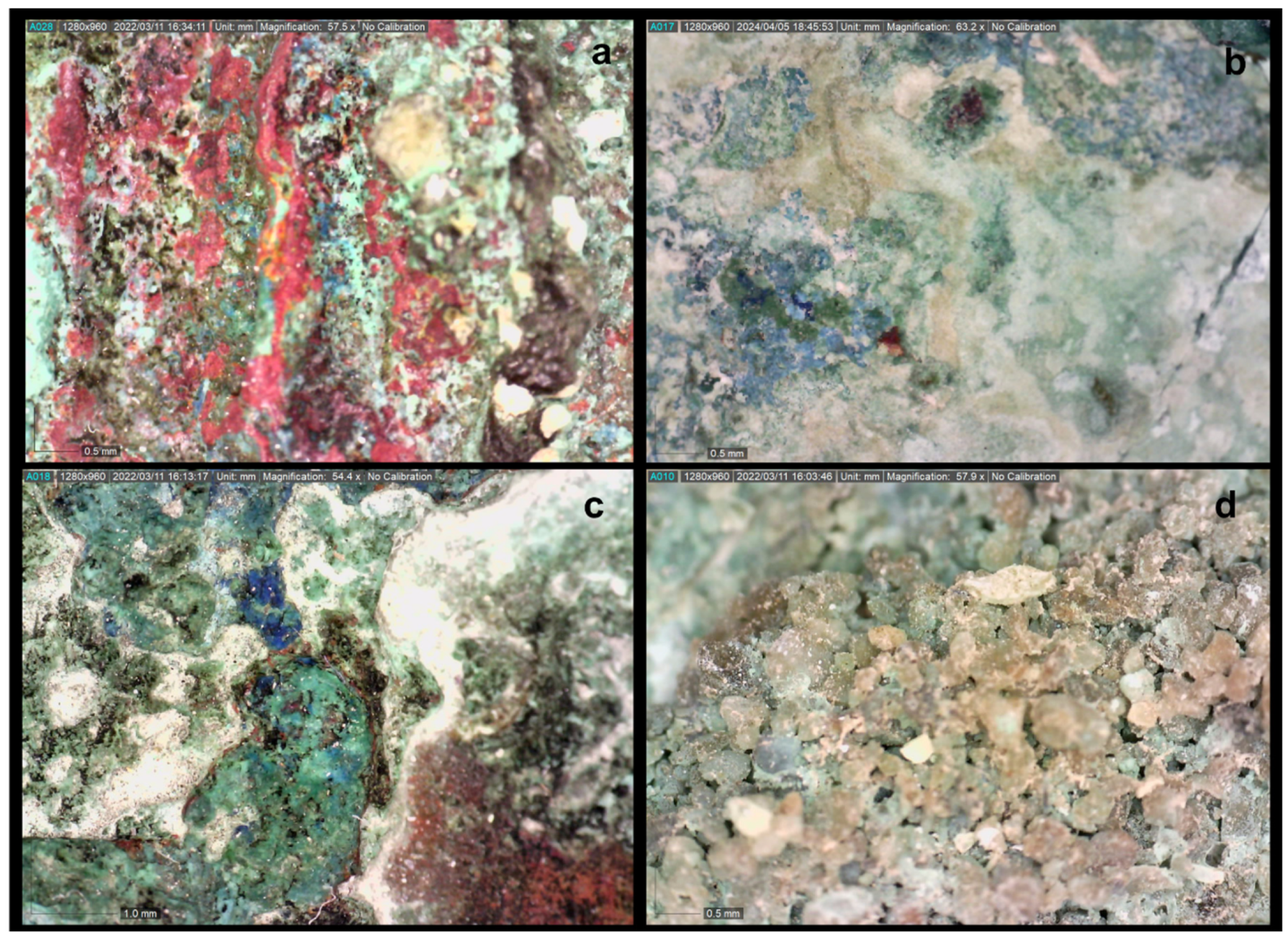
The observation of the fragments via optical microscopy showed that the entire artefact has been turned into corrosion products and there was no sign of metal core preservation in their section. The surface corrosion layers principally contained products of red, green and off-white/whitish colour, while, in places, there were additionally observed products of blue, dark-black and orange colour, as well as accretions of soil material remains (
Figure 10a-c).
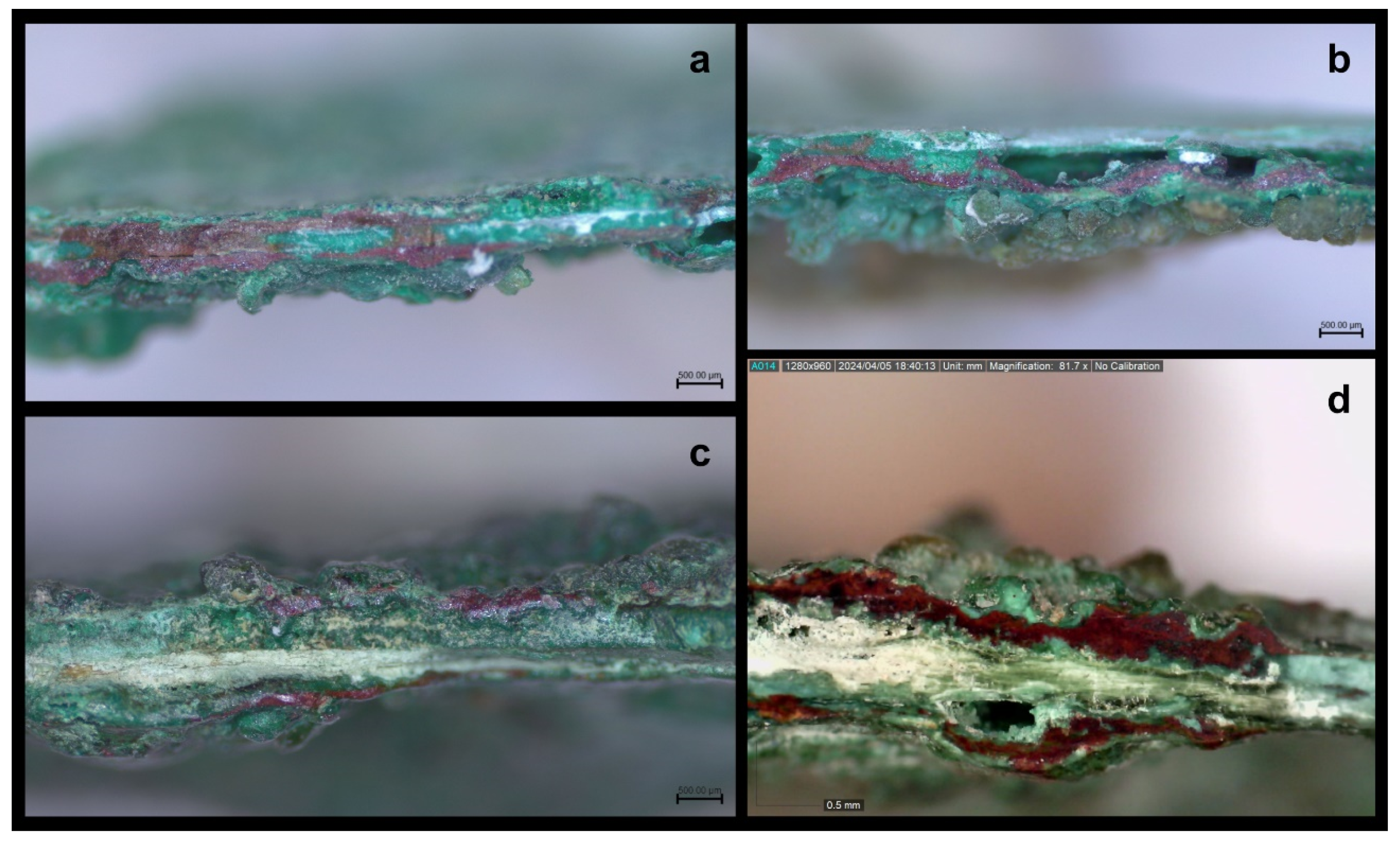
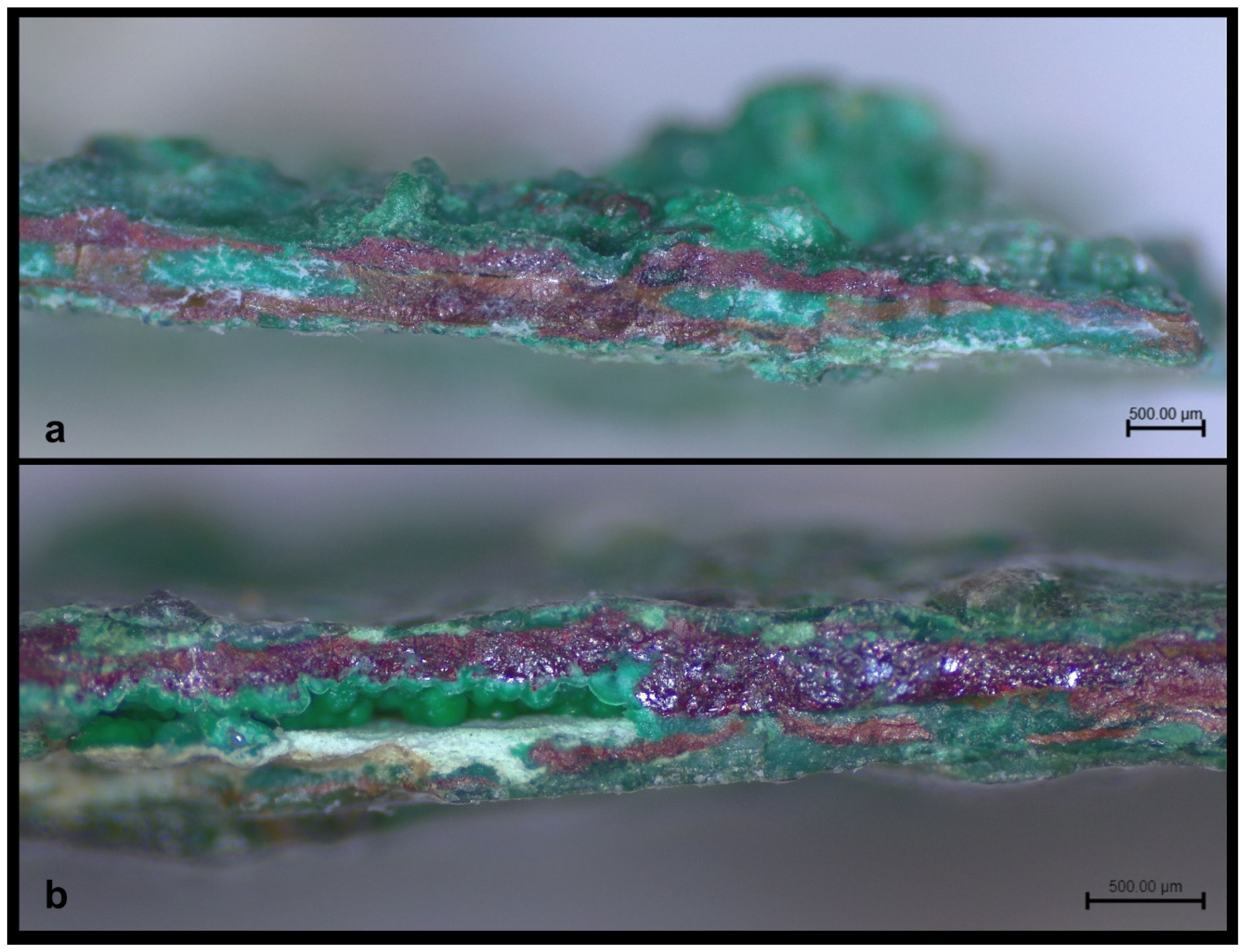
In the fracture sections the following were observed: a) In most cases, a layer of red colour and of small uneven thickness, was detected in the centre, which was surrounded by chiefly green corrosion products. This inner part, which also contained red and sometimes green corrosion products, was in reality the metal part that had been completely mineralized [
26]. Furthermore, in the compact red corrosion layers, colour gradations were observed, a parameter that indicates probable differentiation as to tin content in them, a fact that will be referred to in the discussion further below. b) Patches of green colour interrupted the red layer, in places. c) Soil remains had intruded, in some cases, at a greater depth in the corrosion layers. d) An off-white/whitish phase, which was surrounded by layers of red and green colour, was sometimes discerned in the centre of the fragments and sometimes between the corrosion layers. This phase was unstable and in powdery state, while, in some cases, seemed to have retreated interrupting the layer sequence of corrosion products and creating void spaces in the layering. These off-white layers also appear to have caused surface flaking, as their remains were observed, in places, in the areas where surface corrosion flaking had retreated. e) Corrosion products of light green vitreous colour, with evident bulges, were observed in the interior of the structure of corrosion layers (
Figure 11a-d).
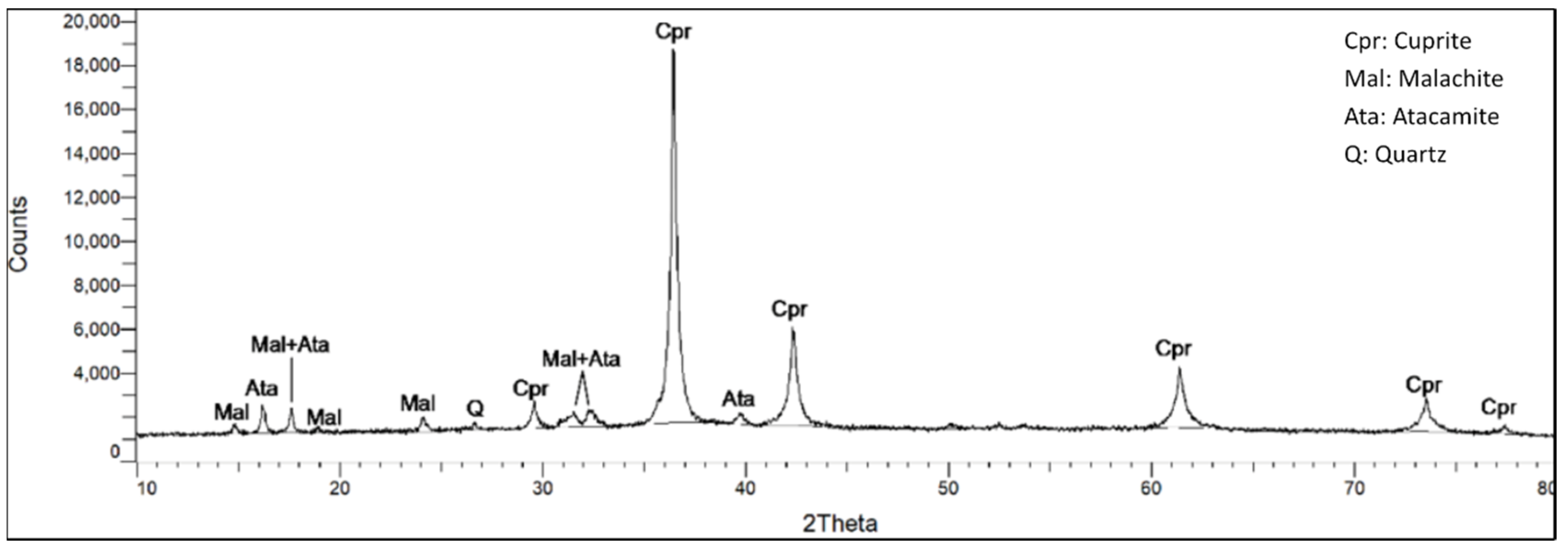
The qualitative analysis of two samples by the XRD technique showed that in the corrosion products of the bronze lebes from the Phaleron Delta there were detected cuprite (CuO
2), malachite (Cu
2CO
3(OH)
2) and atacamite (Cu
2(OH)
3Cl). The quartz (SiO
2), which was also recorded, derived from remains of soil material that had penetrated in the porous structure of the corrosion in the course of the long period of time the artefact remained buried. The absence of tin compounds, such as oxide of tin - cassiterite (SnO
2), from the diffractograms, is related to the difficulty in the detection of these compounds by the method of XRD, because of their very low crystallinity or amorphous structure in the bronze patinas [
23,
26,
28]. As a result, it was attested that the main corrosion products that were documented in the examined samples were copper (I) oxide (cuprite), basic copper carbonate hydroxide salt (malachite) and copper trihydroxychloride (atacamite). The diffractograms of the two samples are seen in the
Figure 12 and
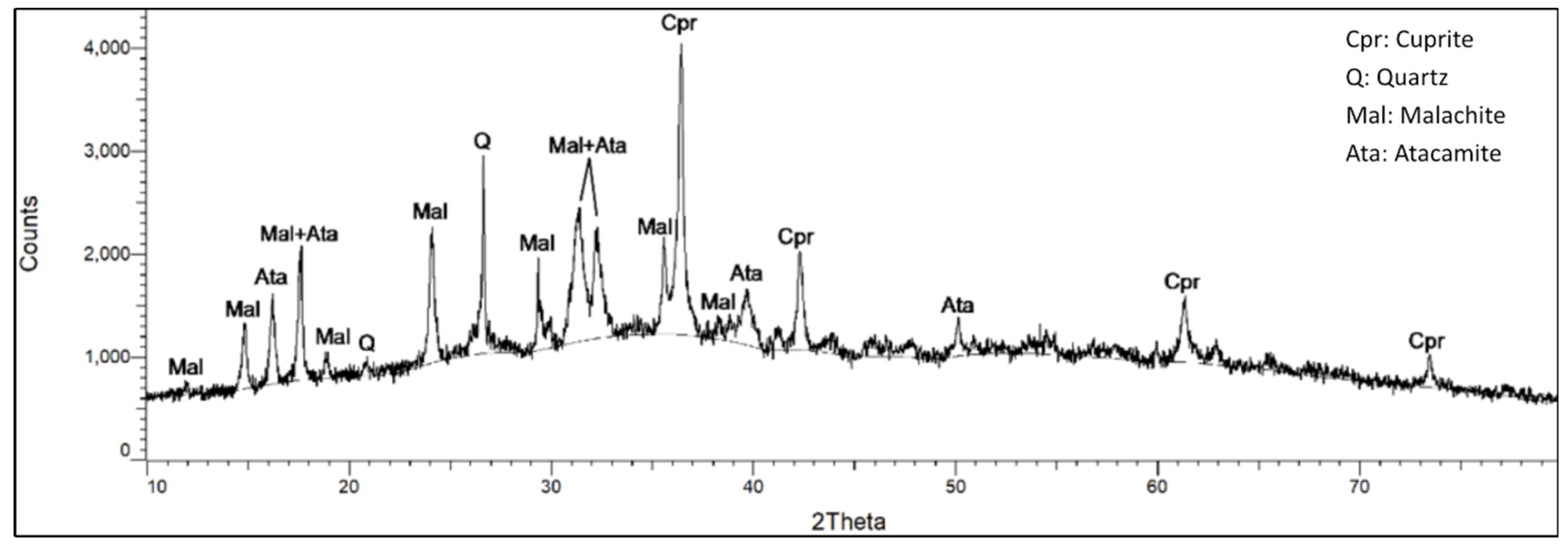
Figure 13.
4. Discussion
The corrosion of artifacts made of copper alloys in archaeological environments depends as much on endogenous factors, such as the nature of the alloy, the heterogeneity of its components and the method of processing, as on exogenous ones that determine the interaction of the finds with the burial environment, such as soil pH, conductivity, humidity and presence of ions [
29,
30]. Moreover, corrosion may affect to a greater extent certain areas of the artifact and manifests itself more intensively and at higher rates in areas where the metal is thinner, when there are discontinuities of the metallic surface, around cracks and micro-cracking as well as along the borders of the alloy grains and inclusions [
15,
29,
31] (p. 217, p.93, pp. 387-397). Hammering and cold forming process for the manufacture of objects made of copper alloys, causes the elongation of metal grains, via the intense compression forces they have been subjected to [
32,
33] (pp. 83-105, pp. 334-335), resulting in their becoming more prone to corrosion processes. Thus, oxidation processes are accelerated, while corrosion penetrates in depth and develops along the borders of the grains, causing pericrystalline corrosion and intergranular cracks [
24,
29,
34].
With regard to the condition the Phaleron bronze lebes was found in, the aforementioned factors have played an important role, along with the immediate burial environment, which is the sandy coast of the Phaleron bay. The Phaleron Delta, according to historical sources that are evidently confirmed by current geological studies, lies in the wider region of the Halipedon, a Greek name the etymology of which is analysed as follows: ἃλς (salt) + πεδίον (field). The Halipedon seems to have consisted of marshy areas, which, depending on the prevailing conditions, were drying up and re-emerging from time to time, with brackish waters and formations of coastal dunes, which extended across a large part of the narrow sandy strip of the bay, being a soil factor characterized by accumulation of quartz or limestone sedimentation sand [
35] (pp. 136-158). All this evidence leads directly to the conclusion that in the area where the examined bronze lebes was found, prevail two important corrosive factors, as to artefacts of copper alloys: presence of humidity and circulation of chloride ions in aqueous solutions. Furthermore, access to oxygen, an element necessary for the occurrence of the corrosion phenomenon, is favoured in sandy soil, while, differentiations in the quantity of oxygen, due to the different granulometry which can occur, in places, in corresponding soil types, enhance considerably the progress of the phenomenon [
36]. Furthermore, water as solvent and carrier of salts operates as an essential factor in the corrosion of archaeological finds. The impact of humidity and circulation of water increase the rate of corrosion, especially in cases of burial at small distance from the sea. In the present case, the depth level of the find and its proximity to the sea front intensify the phenomenon of corrosion, mainly because of the presence of chloride salts. Chloride ions facilitate the corrosion of bronze, as they pass through its protective oxides, forming microscopic, non-coherent atacamite–paratacamite crystals [
15,
37]. In conclusion, the sandy environment of the lebes burial place could be considered as “aggressive”, since it is characterized by the ability to retain humidity but also water from rainfall and from the water table, promoting the circulation of ions and hence salt solutions.
Regarding the manufacture alloy of the bronze lebes, the lack of metal core did not allow the determination of its chemical composition through quantitative analysis by the p-XRF method. Nevertheless, the qualitative analysis by this technique led us to the conclusion that this is a binary copper – tin alloy. These kinds of alloys are frequent in the Greek area and were in use for a long period of time [
38]. From the viewpoint of processing, they can be subjected to cold hammering, while the hardening of the alloy by this technique, requires a high tin content for the produced object to have durability and for deformations not to be caused during its use [
39] (pp. 283-284). As to the existence of lead, an element that was also detected by the p-XRF analysis, it is well known that for an alloy to be suitable to be processed by cold hammering, there is need for a uniform distribution of this element in the metal mass and its content should not exceed 2% [
38]. In relation to iron, the existence of which would have had a particularly adverse effect on the process of cold hammering [
33,
40] (pp. 51-54, pp. 673-676), its presence is usually reinforced owing to corrosion and the intrusion of soil material. Worth mentioning, at this point, is that in the Phaleron Delta cemetery a bronze double axe of the same chronological period has been uncovered, made of good quality copper and tin alloy [
41] (pp. 101-114). These two finds (the bronze double axe and the bronze lebes) are valuable artefacts and unique occurrences in this particular excavation, and play an important role in the reading of their archaeological context as a whole.
In the assessment of the lebes fragments’ condition, via optical microscopy, it was observed that the developing corrosion layers had formed rough and uneven crusts. In the areas, where corrosion had advanced at a faster pace, bulges of corrosion products had been produced, while often soil material remains were observed in hard to remove accretions of corrosion products. Through the XRD analysis, it was attested that the corrosion layers are composed of cuprite, malachite and atacamite, while there was also recognised the presence of quartz as a result of sand intrusion from the burial environment. As far as cuprite goes, apart from the characteristic red colour, which is its principal hue, can also appear in hues of different intensity, which vary from orange to yellow [
24,
28], a fact that was observed, as well, in the microscopic observation of the examined lebes fragments (
Figure 14a). Colour gradations in the cuprite layers occur depending on the alteration of the Cu/Sn ratio and, more precisely, the lighter red colour indicates corrosion areas or bands rich in tin, while, as the colour gets darker, the content in copper increases [
23,
24,
26]. Also microscopically, there were observed a) small areas of black to grey colour, between the cuprite layers, which may derive from bivalent copper oxides, tenorite and b) corrosion products of blue colour, which appear rarely, and are associated with copper carbonate salts, azurite [
15] (pp. 216-217). These two compounds (tenorite and azurite) did not produce a detectable sign in the XRD technique, probably due to their limited and fragmentary appearance in the examined bronze vase fragments. Moreover, no tin compounds were documented, despite the fact that the existence of this element in the examined fragments was recorded by the p-XRF technique. This is due to the scarcely crystalline to amorphous structure of these compounds, a fact that renders infeasible their characterization through X-ray diffraction analysis [
23,
26]. In general, in the completely corroded bronze artefacts, without metal core, as the bronze lebes from Phaleron, the composition of the inner corrosion layers depends on the alloy’s original composition, namely its content in tin, which, as mentioned above, is responsible for colour gradations in the red compact layers, and on the presence of chloride, the quantity of which influences the hue of the green layers [
25,
26]. In the course of the corrosion process, the layers of cuprite lose their coherence and break down because of the penetration of chloride ions and the formation of copper chloride compounds in the inner corrosion layer [
23], a phenomenon that was observed in several cases in the fragments under examination (
Figure 14b).
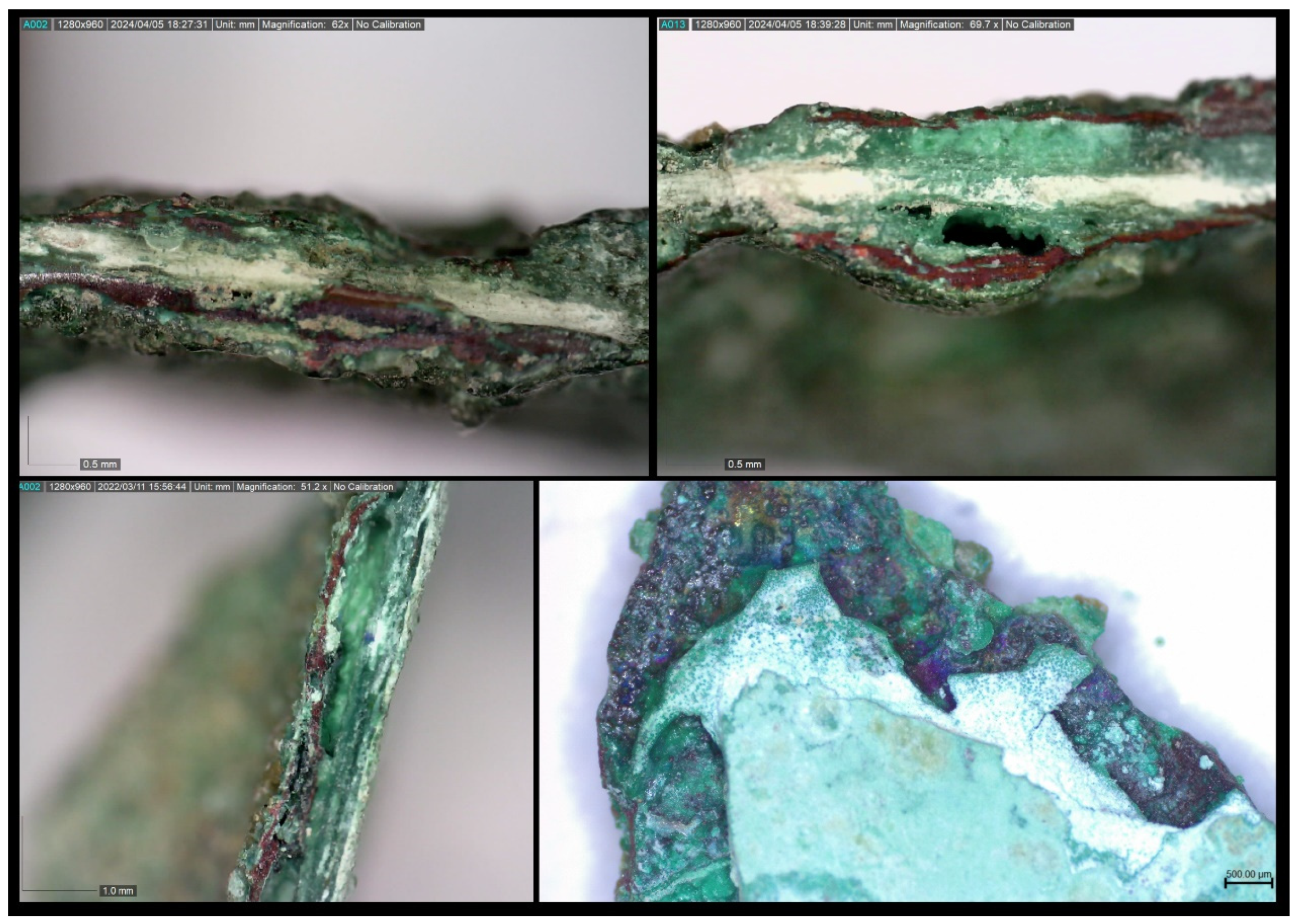
The microscopic observation, especially in fracture areas, ascertained, as well, the existence of unstable layers between the corrosion products, which show an off-white/whitish hue and which cause disruption of the material, surface detachments and segregation of the artefact (
Figure 15).
These off-white phases are tin-rich and their formation is due to the process of decuprification or selective leaching of Cu, in which the copper ions are dissolved and removed from the metallic structure, while, at the same time, the tin in the inner part of the alloy is oxidized [
23,
24,
25,
26,
28,
42]. The quantity of copper that will leach from the metal’s structure, will subsequently react with the anions contained in the soil (in our case chloride and carbonate ones) and will be deposited on the external surface of the artefact [
3]. The reaction of the dissolved copper with chloride ions leads to the formation of copper trihydroxichlorides, which seriously undermine the integrity of artefacts made of copper alloys, by causing rashes of powdery texture [
15,
37].
On the basis of the microscopic observations and analyses that were conducted, the corrosion mechanism of the bronze lebes from the Phaleron Delta is apparently an outcome of the interaction of copper with chloride anions, which come from the burial environment. This process is known as “bronze disease” and is correlated to the action of active corrosion on copper alloys. The more the concentration of chloride and humidity in the burial soil is, the more intensive the corrosion becomes, and, in this particular case, the high water table of the area the lebes was found in, and its proximity to the sea, attest to the presence of these two corrosive factors. The development of the above mentioned phenomenon has led to the complete corrosion of the artefact under examination, as it has caused the dissolution of a considerable quantity of copper, which is the main component of its alloy, through the process of decuprification. The ongoing process a) of dissolution – re-deposition of copper and b) of the reaction of copper with chloride ions from the burial environment, has increased the thickness of the corroded artefact with the formation of porous and friable layers of corrosion products [
24,
26].
For the upcoming research of the bronze lebes, scheduled also is the examination of the corrosion layers by other analytical techniques, such as SEM/EDS. Moreover, its comparative study with other bronze artefacts from the same cemetery, on one hand, will contribute to the understanding of the state of preservation and the causes of corrosion, and, on the other hand, to the choice of interventions suitable for the treatment of active corrosion and their conservation/preservation.
5. Conclusions
The method to be chosen for the lifting of the finds from the excavation field is considered to be of vital importance for the preservation of sensitive materials, evidence and information. For this reason, in the case of the bronze lebes we adopted the practice of lifting it en block from the sandy soil of the Phaleron Delta. Furthermore, the mechanical cleaning that was performed, is the only method that can be applied for the removal of soil remains and the revival of the surface of a completely corroded bronze artefact. On the other hand, in order to suppress the advance of corrosion and preserve the bronze vase, it was deemed absolutely necessary to conduct interventions, both of active conservation treatments with the use of corrosion inhibitors, and of passive conversation treatments by regulating relative humidity and by keeping or storing it in suitable environmental conditions. Furthermore, the uniqueness of an artefact within the archaeological context it has been found in, often necessitates its reconstruction, with the aim, on one hand, to study it, and, on the other hand, to potentially include it in an exhibition. In the present case, the resetting of the bronze lebes was conducted on the principle of minimum possible intervention, safeguarding its authenticity and, at the same time, its structural adequacy, enabling, as well, the general public to read and absorb information on its use, shape and size.
The preliminary research on the condition of the lebes from the Phaleron Delta showed that this is a completely mineralized bronze artefact, without metal core. The main elements that compose the corrosion products are cuprite, malachite and atacamite, while no compounds of tin oxides were identified, since their detection is difficult by the XRD method. Microscopically, there were observed exceptionally unstable and friable corrosion areas that indicate decuprification of the alloy, an activity that is connected to the phenomenon of active corrosion, which is called “bronze disease”. This form of corrosion is associated with the environment of the place where the bronze artefact was found, which is the Phaleron bay. The high level of humidity in the coastal burial environment, which, it should be noted, from time to time, in antiquity, was saturated with brackish waters and turned into a wetland, promoted the mobility of the copper from the alloy. Furthermore, the high concentration of chloride and the continual reactions of copper with chloride in the presence of humidity and oxygen caused the formation of copper trihydroxichlorides and have led to complete segregation of parts of the lebes. In conclusion, the water as a carrier of ions constitutes the principal corrosion factor for the bronze artefact, while, the depth level of the find and its proximity to the sea front, intensify the phenomenon of corrosion by accelerating the process of decuprification and active corrosion. The scheduled archaeometric analyses of the bronze lebes corrosion layers, including the SEM/EDS technique, and comparison of the corrosion with other bronze artefacts from the same excavation will contribute to further investigation of the state of preservation of the bronze finds that have been unearthed in the exceedingly aggressive environment of the Phaleron Delta cemetery, in Attica.
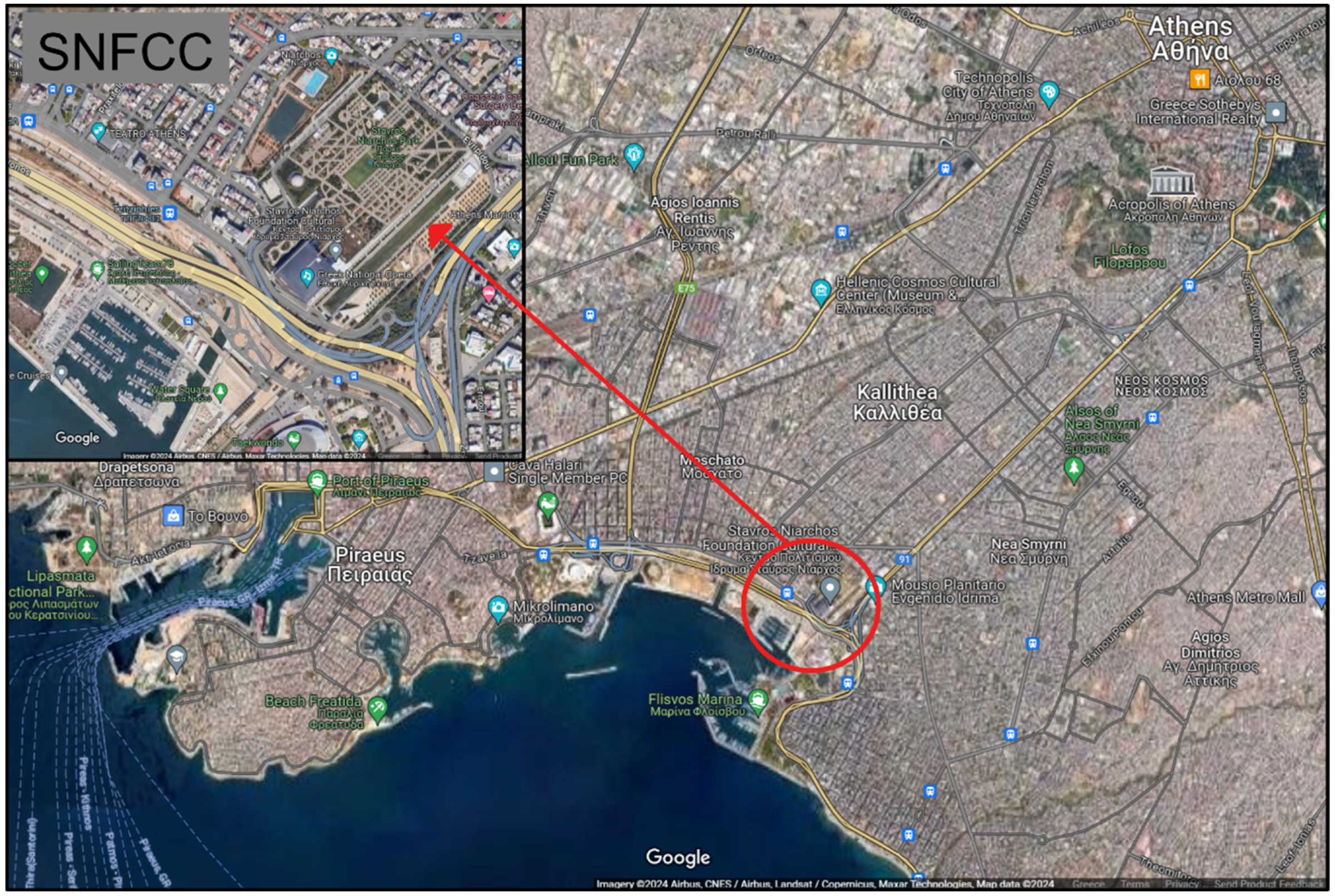
Figure 1.
Map showing the location of the Phaleron Delta in relation to Athens. The smaller map shows the place of the SNFCC.
Figure 1.
Map showing the location of the Phaleron Delta in relation to Athens. The smaller map shows the place of the SNFCC.
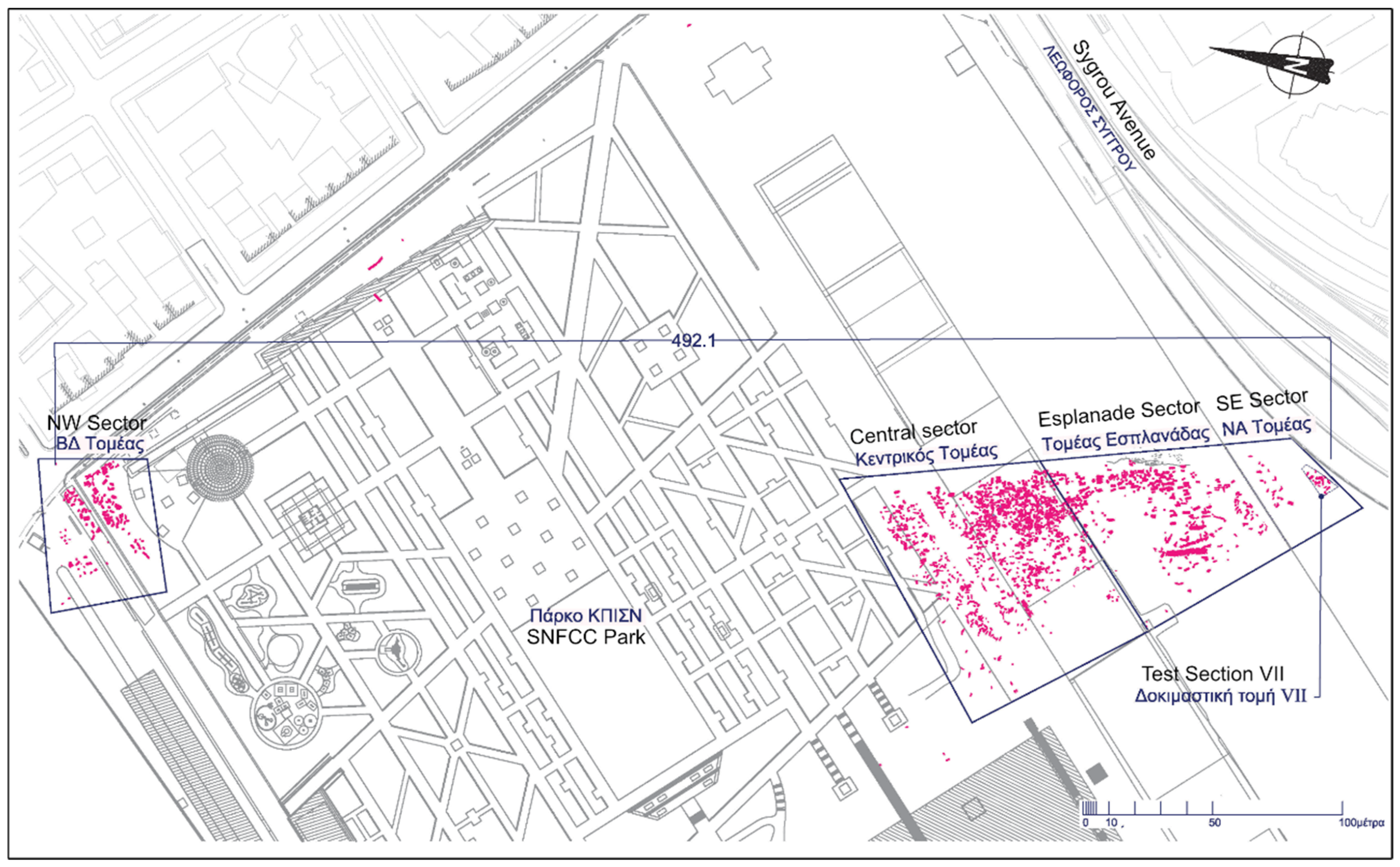
Figure 2.
Map of the cemetery part which was investigated from 2012 onwards and the excavation sectors (©Ephorate of Antiquities of Piraeus and Islands/Konstantina Deli).
Figure 2.
Map of the cemetery part which was investigated from 2012 onwards and the excavation sectors (©Ephorate of Antiquities of Piraeus and Islands/Konstantina Deli).
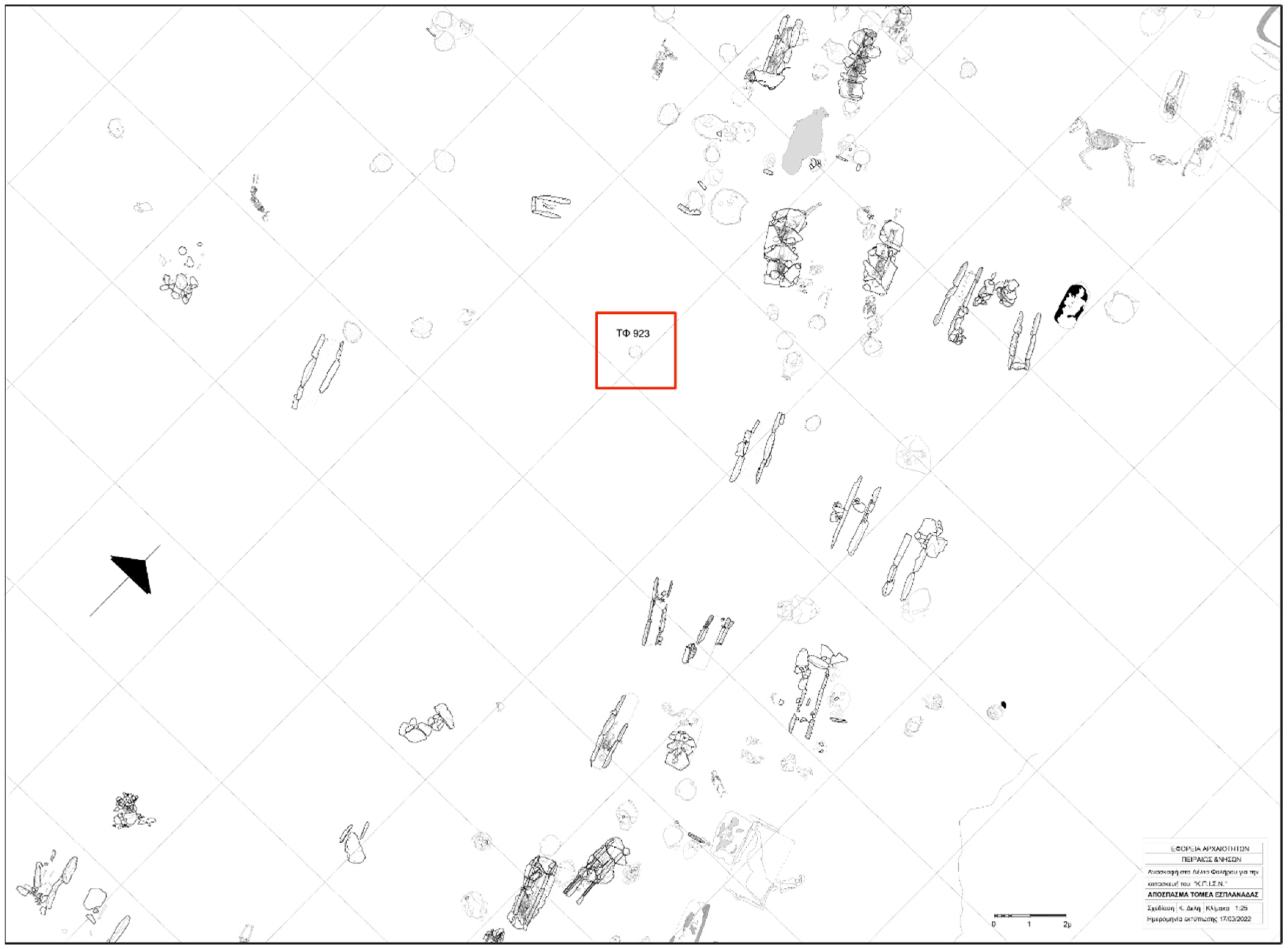
Figure 3.
Part of the Esplanade Sector and Burial 923 marked in red (©Ephorate of Antiquities of Piraeus and Islands).
Figure 3.
Part of the Esplanade Sector and Burial 923 marked in red (©Ephorate of Antiquities of Piraeus and Islands).
Figure 4.
Burial 923 in situ (left) and within the limits of the square it was located in (right) (©Ephorate of Antiquities of Piraeus and Islands).
Figure 4.
Burial 923 in situ (left) and within the limits of the square it was located in (right) (©Ephorate of Antiquities of Piraeus and Islands).
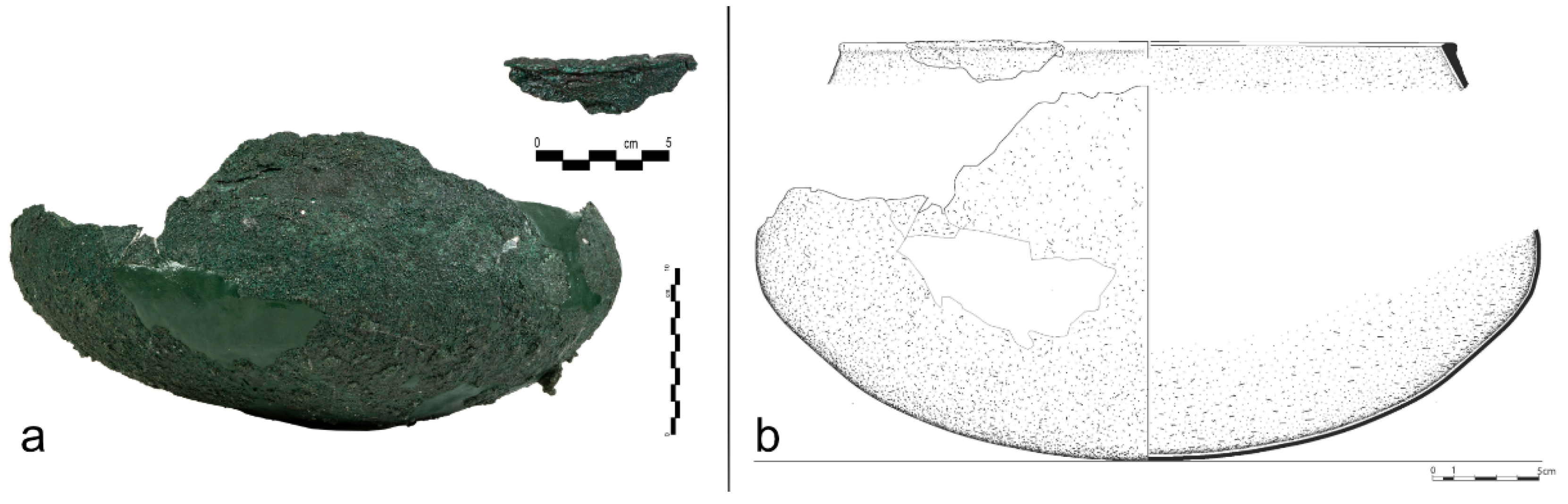
Figure 5.
a) The fragmentary lebes of Burial 923 and part of its rim (©Ephorate of Antiquities of Piraeus and Islands) and b) drawing illustration of the lebes in Burial 923 (©Ephorate of Antiquities of Piraeus and Islands /Konstantina Deli).
Figure 5.
a) The fragmentary lebes of Burial 923 and part of its rim (©Ephorate of Antiquities of Piraeus and Islands) and b) drawing illustration of the lebes in Burial 923 (©Ephorate of Antiquities of Piraeus and Islands /Konstantina Deli).
Figure 6.
Lifting stages: a) gradual removal of sandy soil material, b) application of a cast on the exterior, c) bandage with polyethylene sheet and d) preparation for the transportation to the lab (©Ephorate of Antiquities of Piraeus and Islands).
Figure 6.
Lifting stages: a) gradual removal of sandy soil material, b) application of a cast on the exterior, c) bandage with polyethylene sheet and d) preparation for the transportation to the lab (©Ephorate of Antiquities of Piraeus and Islands).
Figure 7.
In the course of the fill removal, remains of cremation and fragments of the vase come to light (left). Osteological material that was retrieved from the interior of the lebes. There are observed: diaphysis and epiphysis bone fragments, a few cranium and vertebra fragments, alterations in colour, diffusion of bronze corrosion products (right).
Figure 7.
In the course of the fill removal, remains of cremation and fragments of the vase come to light (left). Osteological material that was retrieved from the interior of the lebes. There are observed: diaphysis and epiphysis bone fragments, a few cranium and vertebra fragments, alterations in colour, diffusion of bronze corrosion products (right).
Figure 8.
During the fill removal and cleaning operations. On the outside, the cast kept to retain the vase fragments.
Figure 8.
During the fill removal and cleaning operations. On the outside, the cast kept to retain the vase fragments.
Figure 9.
Presentation of the bronze lebes and part of its rim in the temporary exhibition of the Archaeological Museum of Piraeus (©Ephorate of Antiquities of Piraeus and Islands).
Figure 9.
Presentation of the bronze lebes and part of its rim in the temporary exhibition of the Archaeological Museum of Piraeus (©Ephorate of Antiquities of Piraeus and Islands).
Figure 10.
Images from the surface of the fragments under examination. Colour gradations of corrosion products (a-c) and accretions of soil material and corrosion products (d) (Dino digital microscopy, magnification 57.5, 63.2, 54.4 and 57.9X).
Figure 10.
Images from the surface of the fragments under examination. Colour gradations of corrosion products (a-c) and accretions of soil material and corrosion products (d) (Dino digital microscopy, magnification 57.5, 63.2, 54.4 and 57.9X).
Figure 11.
Images of the fracture sections. a and b) Red and green corrosion layers in the centre of the fragments (Leica Microsystems – M80, Magnification camera 0.8X/Visual magnification 20X). c) Off-white phase in powdery state and soil remains among the corrosion products (Leica Microsystems – M80, Magnification camera 0.8X/Visual magnification 20X). d) Corrosion products of light green colour and unstable formations of off-white colour (Dino digital microscopy, magnification 81.7X).
Figure 11.
Images of the fracture sections. a and b) Red and green corrosion layers in the centre of the fragments (Leica Microsystems – M80, Magnification camera 0.8X/Visual magnification 20X). c) Off-white phase in powdery state and soil remains among the corrosion products (Leica Microsystems – M80, Magnification camera 0.8X/Visual magnification 20X). d) Corrosion products of light green colour and unstable formations of off-white colour (Dino digital microscopy, magnification 81.7X).
Figure 12.
Diffractogram of the first examined sample. It contains cuprite (Cpr), malachite (Mal), atacamite (Ata) and quartz (Q).
Figure 12.
Diffractogram of the first examined sample. It contains cuprite (Cpr), malachite (Mal), atacamite (Ata) and quartz (Q).
Figure 13.
Diffractogram of the second examined sample. It shows peaks that correspond to those of cuprite (Cpr), quartz (Q), malachite (Mal) and atacamite (Ata).
Figure 13.
Diffractogram of the second examined sample. It shows peaks that correspond to those of cuprite (Cpr), quartz (Q), malachite (Mal) and atacamite (Ata).
Figure 14.
a) Colour gradations in the cuprite layers (Leica Microsystems – M80, Magnification camera 0.8X/Visual magnification 20X). b) Atacamite formations of light green vitreous colour between corrosion layers (Leica Microsystems – M80, Magnification camera 1.3X/Visual magnification 30X).
Figure 14.
a) Colour gradations in the cuprite layers (Leica Microsystems – M80, Magnification camera 0.8X/Visual magnification 20X). b) Atacamite formations of light green vitreous colour between corrosion layers (Leica Microsystems – M80, Magnification camera 1.3X/Visual magnification 30X).
Figure 15.
Unstable off-white corrosion layers that cause disruption, flaking and segregation of the artefact.
Figure 15.
Unstable off-white corrosion layers that cause disruption, flaking and segregation of the artefact.