1. Introduction
Peripheral aneurismal diseases are rare and encountered in less than 1% of the general population. Even though, popliteal artery aneurysms (PAA) are the most common ones accounting for 70% of all peripheral arterial aneurysms. Its epidemiology is relevant to the gender prevalence with 95% in the male population and its anatomical ones to its association with contralateral popliteal aneurysms in 20% of cases, and abdominal aortic aneurysms in 6.1% [
1,
2]. The etiology processes have not been identified yet. Some molecular studies suggest a combination of genetic and inflammatory factors. A decrease in the mechanical strength of the arterial wall associated with an infiltration of inflammatory cells appears to be implicated in aneurysm formation. Most PAA patients are asymptomatic at the time of detection [
3] with the increasing need for intervention within 2-3 years around 68% [
1]. The treatment of PAA has evolved considerably over the years, but there is equipoise and a lack of consensus about the comparative effectiveness of either approach in managing PAAs. The traditional management is open surgery with aneurysm ligation or exclusion bypass with an autologous vein or prosthetic conduit. However, the use of endovascular devices (endoprosthesis and stents) has increased in the last few years, quickly becoming one of the primary treatments for these peripheral diseases. In 2022 the Society for Vascular Surgery published clinical practice guidelines on popliteal artery aneurysms [
1] but it doesn’t point out evidence of the superiority of open PAA repair (OPAR) versus Endovascular PAA repair (EPAR). Although, all recent literature underlines stackable outcomes of these invasive approaches. The importance of choosing an appropriate treatment lies in the clinical significance of PAA with potentially limb-threatening sequelae that may arise from acute limb ischemia to major amputation [
4]. Thromboembolism for aneurysmal sac thrombosed and PAA ruptured are the first native complications of this limb disease, defining the major cause of occurrence in hospital and emergency treatment settings of PAA. Instead, postoperative complications of both OPAR and EPAR could define a more severe setting with peripheral artery compromise, insufficient collateral vessels, or diffuse arterial disease that underlines severe peripheral limb impairment and a clear possibility of limb loss.
In this contest, the infective complications are identified as the rarest postoperative complications, but when it occurs, it is burdened with high morbidity and mortality rates[
5]. Late-onset infections may present even years after the initial procedure and symptoms are often vague. This delayed and non-specific clinical presentation considerably affects the outcomes because misdiagnosis or delay in treatment is frequent. Management strategies depending on presentation severity, anastomotic graft involvement, stent graft involvement, and infectious microorganisms could include long-term antibiotic therapy along with maximal invasive surgery with open conversion.
The primary objective of this study is the evaluation of infectious complications related to endovascular implants in these aneurysmal diseases through a comprehensive review of the literature and reporting two cases from our vascular unit experience. Furthermore, considering the severity of the endograft infection, wished aim is to find the best treatment strategy to save the limb and the patient's life.
2. Case Reports
These cases report two male patients who underwent exclusion of popliteal aneurysm with different stent graft devices. Several years after the endovascular procedure, both patients developed severe abscesses in the treated popliteal region with pain, edema, and swelling symptoms. Computed Tomography Angiography (CTA) images clearly showed popliteal fluid collection surrounding the stent graft devices. After an appropriate discussion in a multidisciplinary team with the infective consultants of our department, both patients were treated by an open surgery treatment with explantation of the infected stent graft and femoropopliteal bypass. Follow-up control after 1 year using ultrasound revealed the complete patency of the bypass and downstream vessels.
2.1. Case A
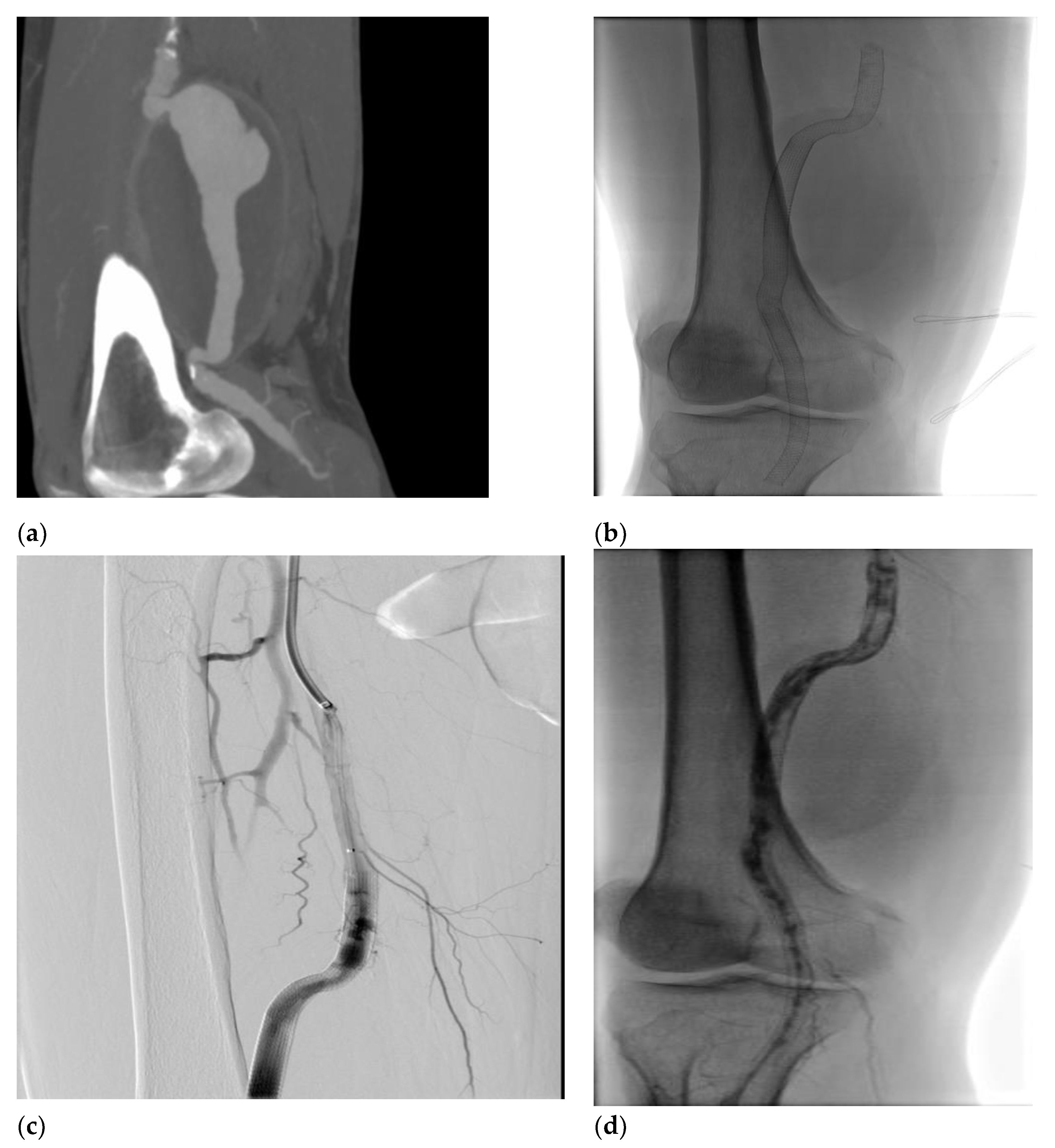
A 58-year-old man was admitted to the emergency room (ER) for right lower extremity pain and pallor, involving the right leg with motility and sensitivity reduced. An urgent CTA scan revealed a ruptured right PAA with poor below-the-knee (BTK) run-off vessels (
Figure 1a). The patient underwent emergent hybrid treatment, with popliteal artery and distal vessel embolectomy followed by an endovascular procedure with PAA exclusion using VIABHAN™ endoprosthesis and Supera Stent for distal landing zone (
Figure 1b). After two years, the patient complained of severe claudication; an ultrasound exam revealed a severe intrastent restenosis (
Figure 1c). The patient underwent an endovascular procedure with Percutaneous Transluminal Angioplasty (PTA) using a drug-eluting balloon, which occurred in intraoperative complication with distal vessel thrombosis (
Figure 1d), solved with thromboaspiration and intra-arterial thrombolysis treatment with urokinase for 48 hours. The postoperative image exams recorded a good recovery of artery patency, and the patient was discharged on fourth postoperative day. Five days later, the patient was re-admitted to the hospital for right inferior limb acute ischemia. Again, a conservative approach was done using 24h intra-arterial thrombolysis and two adjunctive VIABHAN™ endoprosthesis were implanted because of disconnection between the VIABHAN™ previously implanted. After two months, the patient came back again for the third time, presenting pain, hyperemia, and swelling of the right leg. CT scan showed a fluid-corpuscular collection surrounding the endoprosthesis related to aneurysmal abscess collection of the right superficial femoral (SFA) and popliteal arteries (
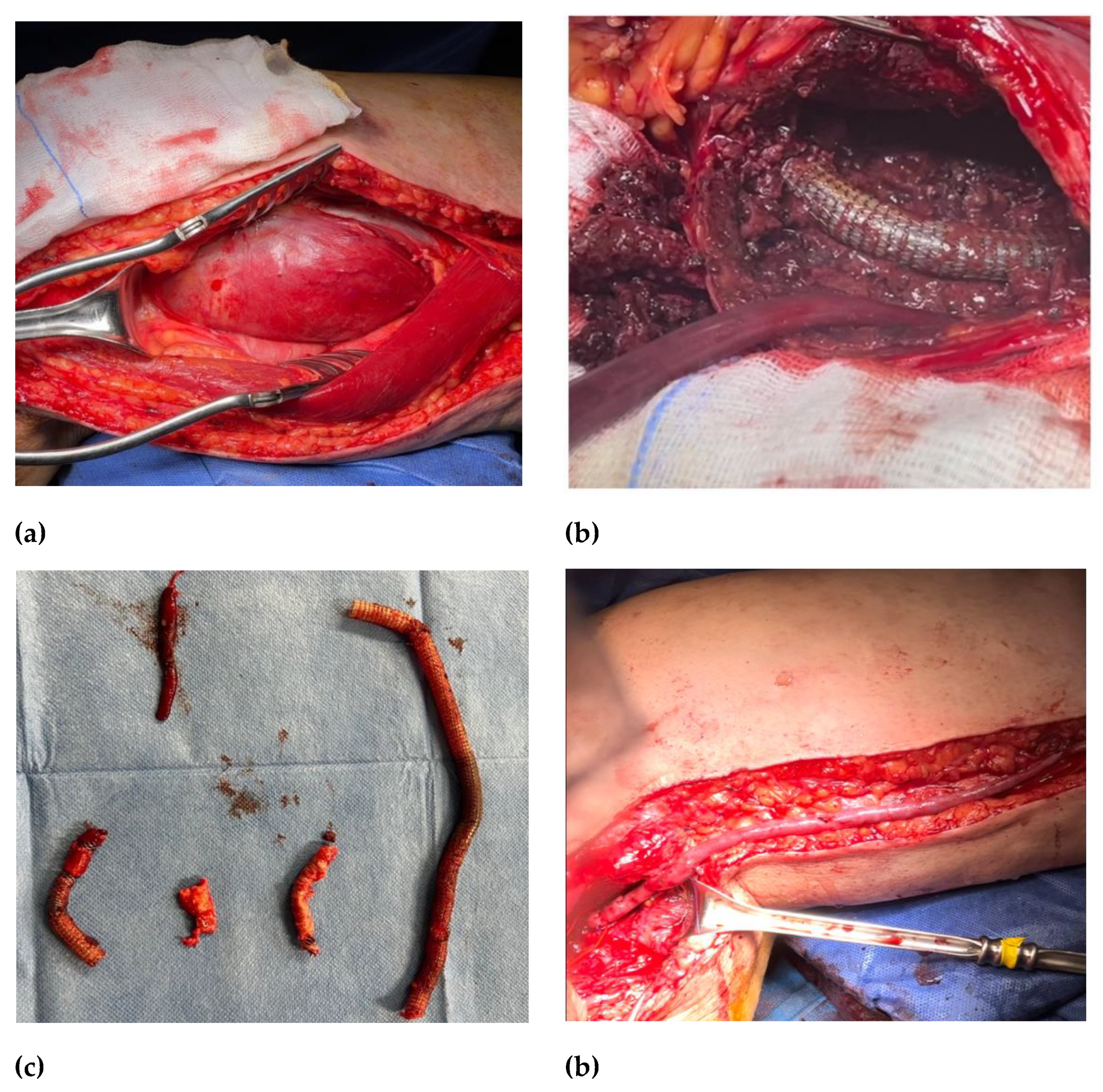
Figure 1e). Blood and abscess samples were positive for Staphylococcus Capitis. A multidisciplinary team approach deemed appropriate started antibiotic therapy with daptomycin and finalized for open surgery treatment. The patient underwent a surgical conversion with a complete explantation of endovascular material (
Figure 2b,c) and performed a femoropopliteal bypass using an autologous vein (
Figure 2d) with good patency of the downstream vessels one year after surgery.
2.2. Case B
A 76-year-old man was admitted to the ER for fever and swelling on the left leg. The left lower limb physical examination showed a hyperemic, and painful area from the third distal thigh to the ankle. In the medical history, the patient underwent an endovascular intervention in an emergent setting to exclude a left ruptured popliteal aneurysm by a VIABHAN™ endoprosthesis. After a short interview, the patients declared recent access to another hospital with the same fever symptoms a few months before, with a sepsis status and positive hemoculture for Staphylococcus Aureus. The patient was treated with antibiotic therapy and after three weeks was discharged.
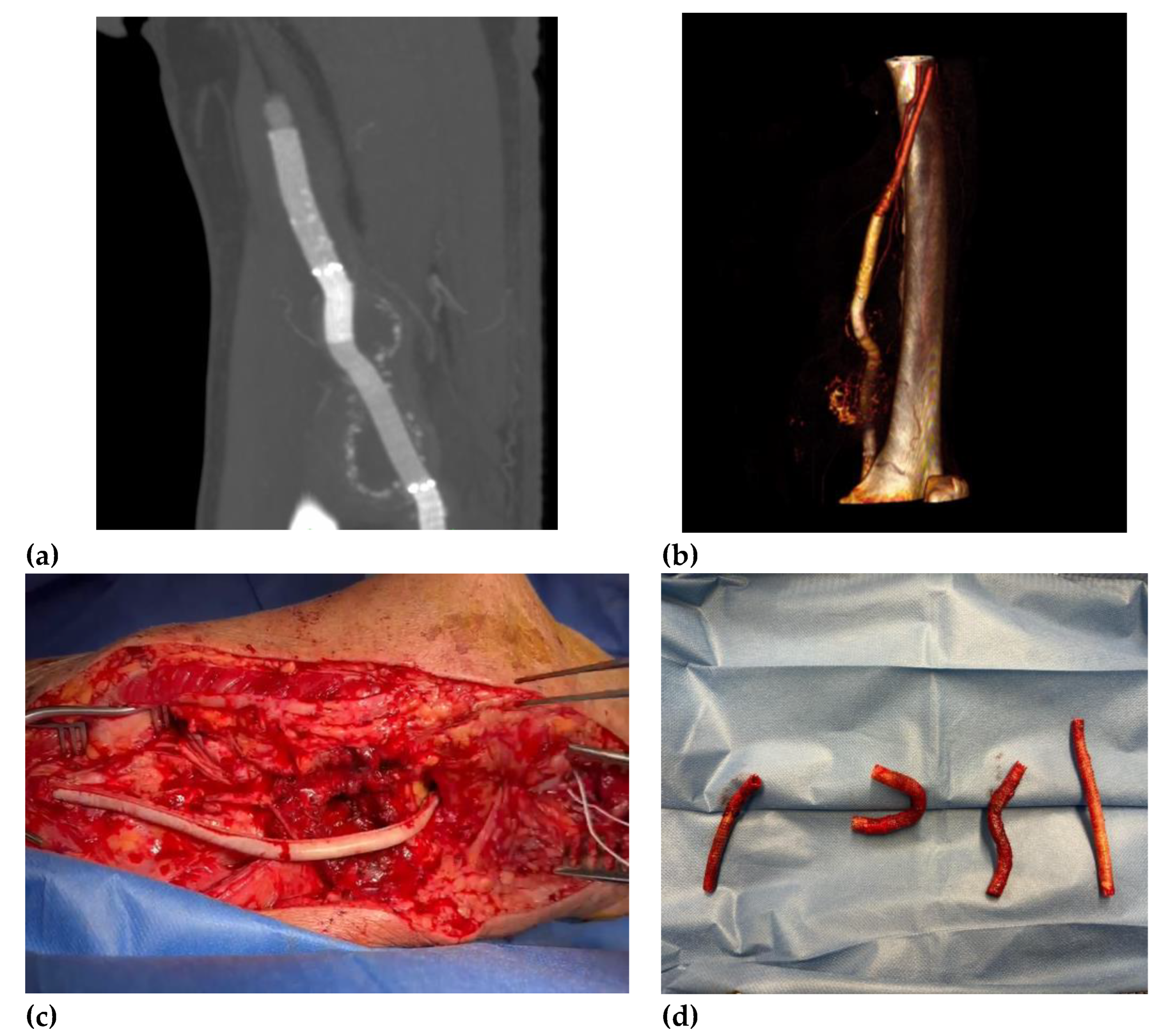
Deeply studying the ongoing popliteal status, we decided to perform a CTA that showed the presence of a peri-aneurysmatic fluid-corpuscular collection around the left popliteal artery (
Figure 3). Moreover, we deemed the infective status necessary and worthy of further investigation. Microbiological exams revealed the presence of Staphylococcus aureus from blood and abscess material samples. Multidisciplinary teams with infective consultant collaboration believed in joint antibiotical therapy and open surgical treatment by abscess drainage and popliteal region reclaiming. After one week of antibiotic therapy, the patient underwent to femoropopliteal bypass using a biological pericardium bovine prosthesis with the final complete perviousness of the downstream vessels. The choice of biological prosthesis was because of the poor quality of patient’s saphenous veins. One-year follow-up revealed an ultrasound patency of bypass and tibiofemoral vessels and complete remission from infective status.
3. Material and Methods
This study reported a comprehensive review of the literature over the last 50 years about cases of late-onset popliteal endograft infection. Data about risk factors, clinical presentation, medical and surgical treatment, outcomes, and mortality were collected and compared.
3.1. Literature Review
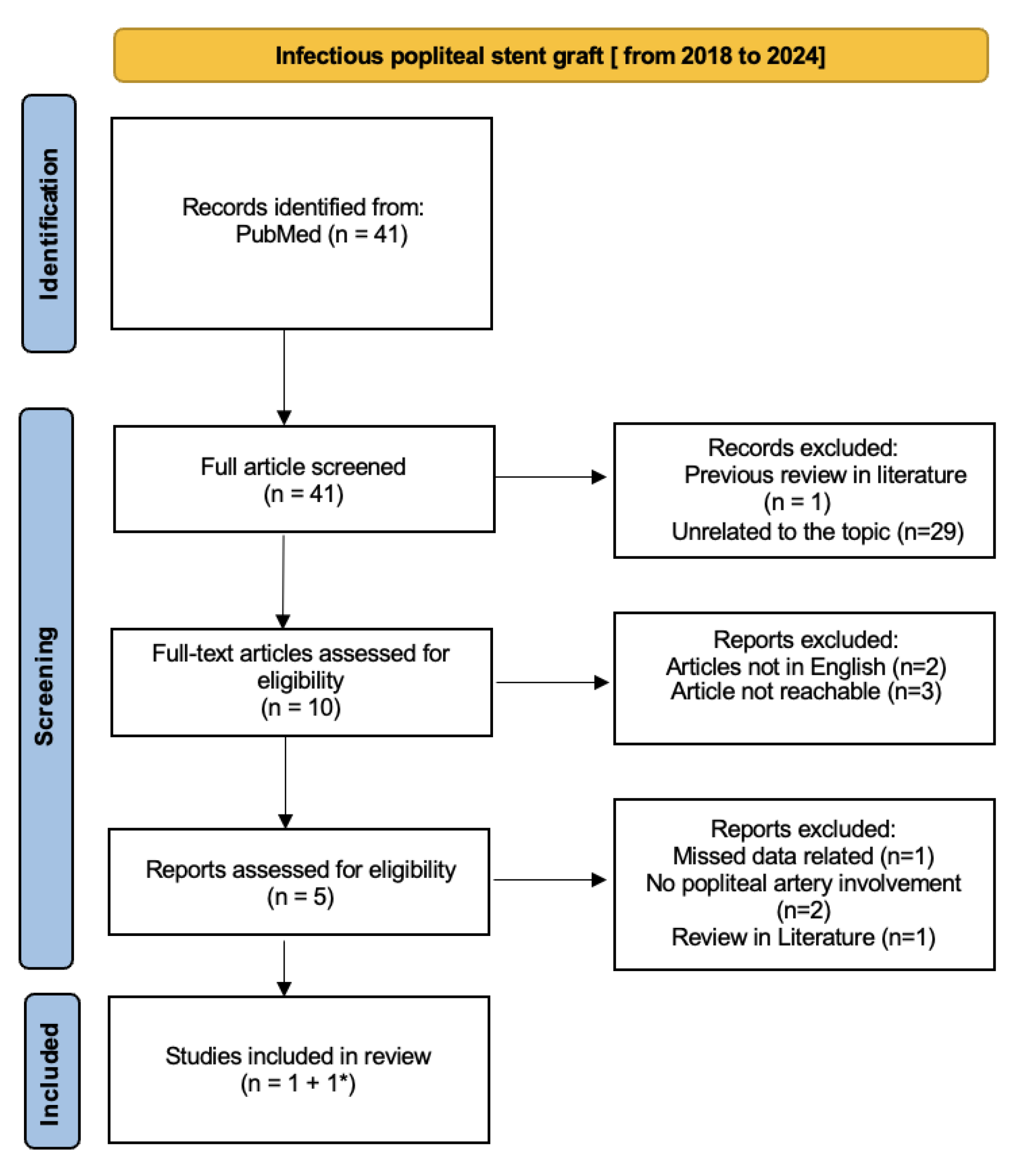
One author conducted a comprehensive review of literature from 1980 to 2024 through the Preferred Reporting Items for Systematic Review (PRISMA) to collect data about reported cases of late-onset infections over bare metal or covered popliteal artery stents. Search terms were [stent infection] AND [peripheral stent infection] AND [infectious popliteal stent graft] OR [infective popliteal stent graft]. The titles and abstracts were reviewed for appropriateness and articles without available full text or missing in the English international language or unrelated to the popliteal disease were excluded.
The Vascular and Endovascular Division of the University of Vermont [
6] published a comprehensive literature review about peripheral vascular stent graft infection in volume 51 of the Annals of Vascular Surgery Journal (AVSJ) in 2018. This complete review analyzed infective complication vascular-stent grafts in all peripheral arteries related from 1980 to 2018. Also, the European Journal of Vascular Surgery (EJVS) published a case report and review of literature about infections of intravascular bare metal stents in 2013 [
5] but we deemed it appropriate to consider the AVSJ review as more recent, complete, and exhaustive. Moreover, all popliteal infection EJVS-cited articles are present in the AVSJ review. To finalize our research, we decided to divide our review into two timeline sections: the first was based on all popliteal stent graft infection articles published from 1980 to 2018 including the previous Annals of Vascular Surgery 2018 review[
6] (
Figure 4), and the second one included articles from 2018 to 2024 (
Figure 5).
In the second section, we directly identified one case report article [
7] from free PubMed research; then the last case report article was indirectly found from a 2022 review of the literature’s general stent-graft infection [
8].
The author extracted data from each study using a predefined database form that included the following information: general data (author name, year, and type of study) and clinical data (risk factors, clinical presentation, imaging findings, responsible bacteria, medical and surgical treatment, complications and outcomes). The extracted data are reported in percentages and absolute values (
Table 1).
3.2. Results
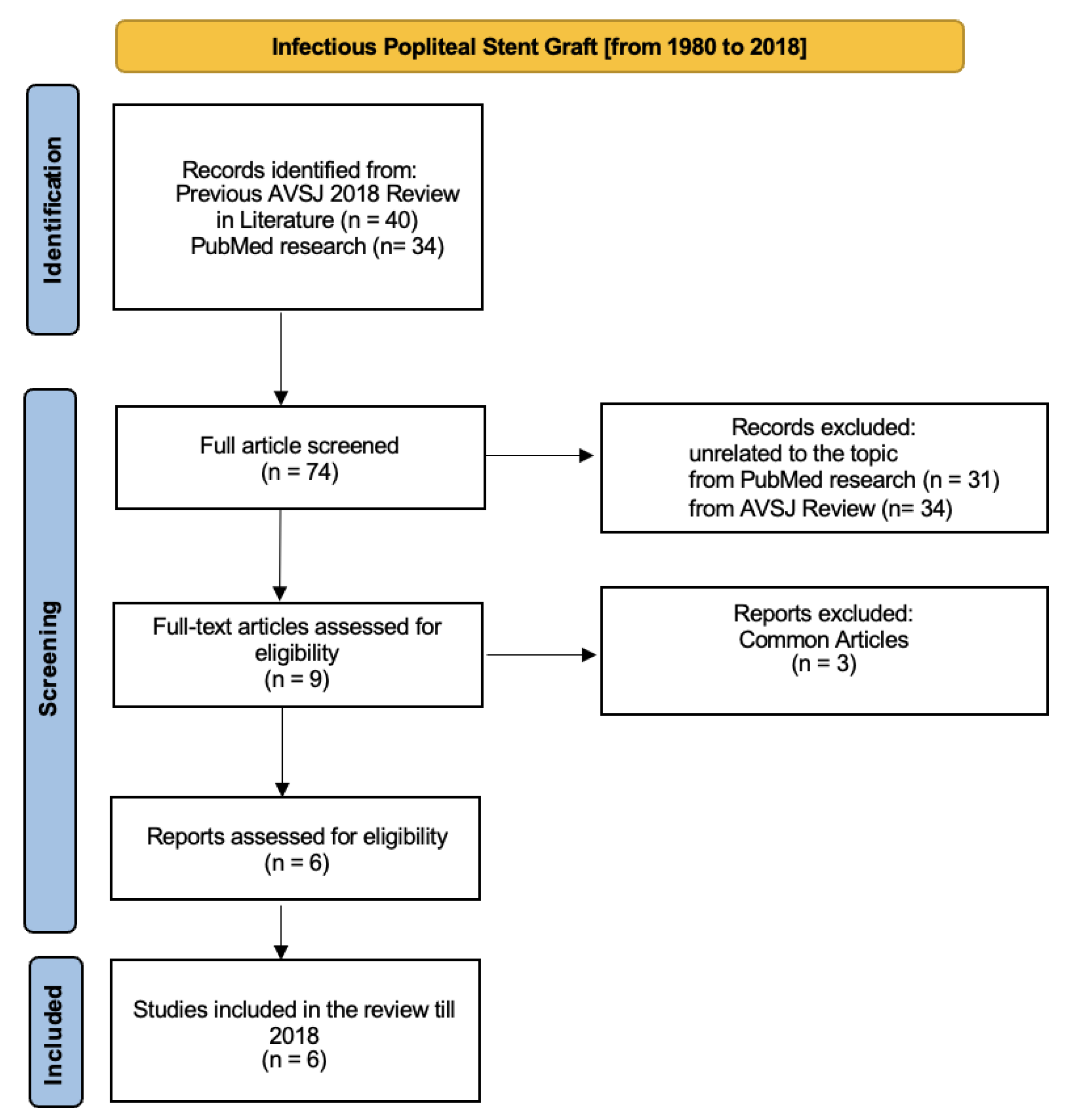
The first review section is based both on the 2018 AVSJ review
[6] and PubMed research using the criteria described before. We identified around 74 articles, divided into 40 articles from AVSJ review and 34 from PubMed research. From the AVSJ review, we chose 4 from 40 articles because specifying related to popliteal artery stent-graft infection
[9,10,11,12]; other articles describe cases of stent-graft artery infection involving other arteries. Of these four articles, three are case reports
[9,10,11] and one is a coup d’oeil
[12]. We also considered the case report described in the AVSJ review
[6]. Moreover, we identified four more articles from PubMed research: one was excluded because it was the AVSJ review, other two articles were excluded because there are the same articles already cited in the AVSJ review. The last one
[13] was considered pertinent to our field. We considered 6 final articles related to infectious popliteal stent grafts from 1980 to 2018.
Then, the second section analyzed articles from PubMed research from 2018 to 2024. We identified two pertinent articles. Firstly, we added a 2022 case report
[7]; then the second case report was indirectly found in a 2022 review of the literature about late-onset infection of covered and bare metal arterial stents
[8]. Even if it was a 2003 article
[14] laying outside the second timeline, it described a case report more pertinent to our research so we decided to consider it as a relative inclusion error of the first section, adding it to the second section.
Finally, our review of the literature from 1980 to 2024 included 8 articles mostly case reports referred to specific popliteal stent graft infections.
Table 1 describes and compares the principal data.
Table 1.
General information about review in literature. Article collected from 1980 to 2024.
Table 1.
General information about review in literature. Article collected from 1980 to 2024.
| Author |
Year |
Study Type |
Gender |
Stent Type |
Onset Infective Symptom Timeline |
| Giannoukas et al.[11] |
1999 |
Case report |
Male |
BMS BREAK(Strecker stent) |
16 days |
| Green et al.[10] |
2013 |
Case report |
Female |
BMS (Bard) |
3 weeks |
| Houthoofd et al.[9] |
2012 |
Case report |
Male |
Stent graft BREAK(Hemobahn) |
24 months |
| Walker et al.[12] |
2017 |
Coup d’oeil |
Male |
EndoprosthesisBREAK(VIABHAN™) |
12 months |
| Witcher et al.[6] |
2018 |
Case report and review in literature |
Female |
BMS (Bard) |
72 months |
| Gharacholou et al.[13] |
2017 |
Case report |
Male |
EndoprosthesisBREAK(VIABHAN™) |
13 days |
| Macheda et al.[14] |
2003 |
Case report |
Male |
BMS (Palmaz, Cordis) |
18 months |
| Watanabe et al.[7] |
2022 |
Case report |
Male |
EndoprosthesisBREAK(VIABHAN™) |
Intraoperative |
Table 2.
Clinical presentation and responsible microorganism.
Table 2.
Clinical presentation and responsible microorganism.
| Clinical Presentation |
Number |
% |
| Acute limb ischemia |
5 |
62 |
| skin ulceration/skin erythema |
5 |
62 |
| Distal necrosis/gangrene/petechiae |
3 |
37 |
| Claudication |
1 |
12 |
| Pseudoaneurysm (CT images) |
2 |
25 |
| Abscess/peri-arterial infiltration/fluid collection (CT images) |
3 |
37 |
| Microorganism |
Number |
% |
| Gram positive (Staphilococcus Aureus) |
5 |
62 |
| Gram positive (Staphilococcus epidermidis) |
1 |
12 |
| Gram negative (Proteus mirabilis) |
1 |
12 |
| Gram negative (Pseudomonas Aereuginosa) |
1 |
12 |
Table 3.
Treatment and outcomes.
Table 3.
Treatment and outcomes.
| Treatment |
Number |
% |
| Autologous vein bypass |
5 |
62 |
| Prosthesis bypass (ring stripper) |
1 |
12 |
| Tied artery |
1 |
12 |
| Conservative management |
1 |
12 |
| Outcomes |
Number |
% |
| Major amputation |
1 |
12 |
| Minor amputation |
3 |
37 |
| Death |
n/a |
|
| Good downstream vessel flow after 1 month |
5 |
62 |
| Claudication >100 mt |
1 |
12 |
Eight patients have been included in our literature database referring to stent graft infection involving only the popliteal artery. The studied population was mostly composed of males (6 males, 2 females). The main onset symptoms were acute limb ischemia in 5 cases and different skin manifestations from erythema (5 cases) to distal gangrene in 3 cases. The main instrumental report was CTA abscess documented as a periarterial collection in 3 cases; in two patients there were described as pseudoaneurysmal collection. The main risk factors recorded were as the same in the vascular population: hypertension (7 patients), diabetes mellitus (2); no reference to genetic disease (Marfan syndrome or Ehlers–Danlos syndrome), or immunological disease was found. Laboratory analysis and instrumental analysis were not available in almost all cases. Two cases clearly demonstrated a high elevation of white blood cells and inflammatory index (active C-protein).
The screening of the updated literature revealed the effectiveness of open surgery treatment in 7 cases, mostly using autologous vein bypass in 5 of 8 cases; only one conservative management was reported due to patient intervention denial. Outcomes clarify the effectiveness of autologous vein bypass and document the patency of the bypass with a good downstream vessel flow till one year from the surgery. Minor amputation (mostly toe amputation) was judged necessary to preserve the distal lower limb and prevent other possible causes of infection. Only one case underwent major amputation (below-the-knee amputation) because the lower limb was judged un-recoverable preventing a massive toxic system damage due to acute prolonged limb ischemia.
4. Discussion
Endovascular stent infection is a rare but most potentially lethal complication of interventional vascular procedures. It firstly involved the surrounding stent area causing local symptoms (pain, edema, swelling) but it quickly could become a systemic infection, compromising and failing multiple organs and leading to death. Therefore, it is very important to diagnose and eradicate infection as quickly as possible
[15]. The timeline of onset symptoms could suggest the principal causes of this terrible complication and as already described in our review of the literature, it is wide-ranging. Stent infection mainly occurs during the first 2 weeks after placement, suggesting probable contamination due to inadequate sterile technique. Late-onset symptoms could suggest an independent correlation with the primary vascular procedure probably related to other urological procedures, dental work, or general infections. As we already discussed in the result section of material and methods, most diagnoses of an infected stent may be clinically suggested by septic distal emboli causing acute limb ischemia or other manifestations such as skin erythema, petechiae, or distal gangrene. Instrumental diagnosis methods are also fundamental to identifying, quantifying, and defining other organ involvement stratifying surgical risk. In the CTA images, the infection is found in peri-stent soft tissue inflammation as a fluid collection. It could also be found a pseudoaneurysm formation at a stent site, even though it is a rare condition (in our review of literature only 2 patients out of eight).
Moreover, the microbiological samples are fundamental to understand the supporting infective mechanism. The different sample microbiology identification could monitor the infective state suggesting a restricted infectious area if only the peri-stent culture was positive, or systemic compromising if drainage and hemoculture were both positive. Also, blood tests could be a simple and clear but not specific systemic infective monitoring index. The most common organisms implicated in vascular graft infections are gram-positive and gram-negative bacteria such as Staphylococcus and Pseudomonas
[5,8] and it is supported also by our review of the literature indicating the same incidence of popliteal infection and the general vascular one. Microorganism identification is a fundamental step for the management of the disease because through the right antibiotic choice, the main attack route can be identified, and it could influence all patients’ outcomes.
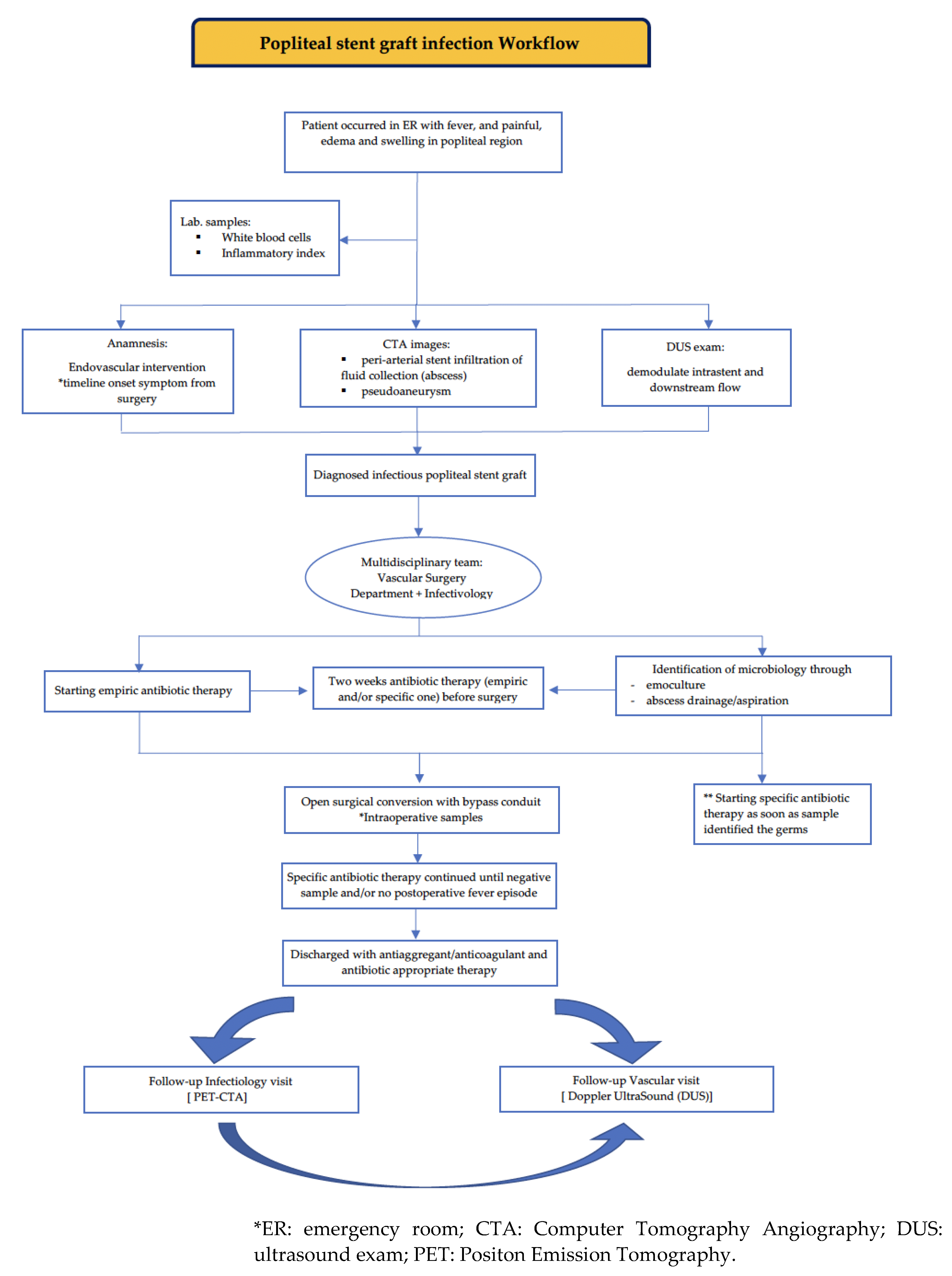
Above all, considering infective stent complications as fatal, it seems to be a priority for the identification of international management of these popliteal stent graft infections. Definitive treatment of an infected stent generally requires removal of the stent and vascular reconstruction as needed. One of the main aims of this study is to emphasize the necessity of a multidisciplinary team in which vascular surgeons could proactively discuss with the infectiology team to identify the best management for each patient. Choosing the right surgical interventional time is often the survival strategy with the best outcome rates even in emergency settings. A combination of prophylactic and post-operative antibiotic therapy and open surgical conversion represents the best management strategy to eradicate the infection, and drainage of the local infectious area and prevent possible systemic re-infection. In our cases, our infectiology team participated in every step of the disease management. They set up the same empiric antibiotic therapy (piperacillin/tazobactam + daptomycin) for both patients from their occurrence in the ER to the microorganism identification. Then, they started personalized antibiotic therapy with daptomycin in both cases one week before surgery. The multidisciplinary team identified as the best surgical approach to open surgery, considering it more efficacy and powerful for the drainage of local infectious areas. The surgical conversion was done two weeks after empiric therapy started and one week after the microbiological identification based on the abscess sampling followed by specific antibiotic therapy. The choice of bypass conduit is based on the patency and adequacy of autologous vein; when the vein tribute is considered poor, a pericardium bovine tube graft can be used, as in our second reported case.
After the surgical conversion, patients continued antibiotic therapy till to negative hemoculture and no fever episode occurred. Moreover, the infectiology team had deemed to appropriate the choice of different discharge antibiotic therapy. In the first case, the choice of antibiotic discharge therapy (trimethoprim/sulfamethoxazole) is suggested for from the discharge till to control visit after one month; in the second case, they chose a single dalbavancin dose before the discharge because of multiple patient comorbidities (previous coronary and carotid stent). Both patients were connected to the infective ambulatory and after one month there were normal range blood test results, no fever, and no other clinical suspected symptoms were referred.
During the infectivological follow-up, both patients underwent chronic antibiotic therapy using Dalbavancin until the PET-CTA scans result were negative.
However, the vascular follow-up visit after one year documented good patency bypass and downstream vessel, confirming no fever episode or other clinical symptoms during a year from the surgery. We propose our workflow to deeply understand our multidisciplinary decision process (
Figure 6).
5. Conclusions
Popliteal stent graft infection identifies a tragic post-operative complication with several major sequelae to probable fatal exitus. It seems to be necessary to identify an international management of this disease to prevent systemic involvement and save patients’ lives. Supporting an extensive literature review, this study suggests the fundamentals of the multidisciplinary team approach. The collaboration of vascular surgeons and infectiology team is necessary for the treatment of lower limb infection disease through open surgery and also to prevent systemic infective involvement with appropriate antibiotic therapy. Moreover, open surgical conversion of popliteal endograft infection with bypass conduit seems to be the best surgical strategy to manage peripheral infection of the popliteal stent graft.
In the future, it could be helpful to create an international register, aiming to identify the best worldwide approaches for these infectious peripherical aneurismal pathologies. The knowledge gained and the data collected will help to plan treatment strategies addressing and preventing several infectious diseases associated with the use of endovascular devices for the treatment of popliteal aneurysms on an international scale. We propose our multidisciplinary approach workflow as start for future collaboration.
Author Contributions
Conceptualization, M.A. and W.M.; methodology, A.M. and A.D.; software R.C. and A.M. (Andrea Molinari); validation, W.M.; formal analysis, M.A., A.D. and W.M.; investigation, M.A. and W.M.; resources, M.A.; data curation, M.A.; writing—original draft preparation, M.A.; writing—review and editing, M.A., A.D. and W.M.; visualization, A.D.G. and F.M.; supervision, L.D.M.; project administration, W.M.. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to case report and systematic review article since we obtained informed consent.
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Data sharing is not applicable; no new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Farber, A.; Angle, N.; Avgerinos, E.; Dubois, L.; Eslami, M.; Geraghty, P.; Haurani, M.; Jim, J.; Ketteler, E.; Pulli, R.; et al. The Society for Vascular Surgery Clinical Practice Guidelines on Popliteal Artery Aneurysms. J Vasc Surg 2022, 75, 109S-120S. [CrossRef]
- Diwan, A.; Sarkar, R.; Stanley, J.C.; Zelenock, G.B.; Wakefield, T.W. Incidence of Femoral and Popliteal Artery Aneurysms in Patients with Abdominal Aortic Aneurysms. J Vasc Surg 2000, 31, 863–869. [CrossRef]
- Tsilimparis, N.; Dayama, A.; Ricotta, J.J. Open and Endovascular Repair of Popliteal Artery Aneurysms: Tabular Review of the Literature. Ann Vasc Surg 2013, 27, 259–265. [CrossRef]
- Kropman, R.H.J.; Schrijver, A.M.; Kelder, J.C.; Moll, F.L.; de Vries, J.P.P.M. Clinical Outcome of Acute Leg Ischaemia Due to Thrombosed Popliteal Artery Aneurysm: Systematic Review of 895 Cases. European Journal of Vascular and Endovascular Surgery 2010, 39, 452–457. [CrossRef]
- Bosman, W.M.P.F.; Borger Van Der Burg, B.L.S.; Schuttevaer, H.M.; Thoma, S.; Hedeman Joosten, P.P. Infections of Intravascular Bare Metal Stents: A Case Report and Review of Literature. European Journal of Vascular and Endovascular Surgery 2014, 47, 87–99. [CrossRef]
- Whitcher, G.H.; Bertges, D.J.; Shukla, M. Peripheral Vascular Stent Infection: Case Report and Review of Literature. Ann Vasc Surg 2018, 51, 326.e9-326.e15. [CrossRef]
- Watanabe, S.; Morimoto, H.; Futagami, D.; Kitaura, J.; Mukai, S.; Kobayashi, T. Superficial Femoral Artery–Anterior Tibial Artery Bypass with Great Saphenous Vein Grafting via the Lateral Femoropopliteal Route for Infection after Viabahn Placement. Clin Case Rep 2023, 11. [CrossRef]
- Borghese, O.; Pisani, A.; Funaru, D.A.; Di Marzo, L.; Di Centa, I. Late Onset Infection of Covered and Bare Metal Arterial Stents. Vascular 2022, 30, 960–968.
- Houthoofd, S.; Yazar, O.; Topal, H.; Daenens, K.; Fourneau, I. Stent Graft Infection in the Lower Limb. Acta Chir Belg 2012, 112, 441–443. [CrossRef]
- Green, B.R.; McCaslin, J.; Wyatt, M.G. Infection of a Nitinol Popliteal Arterial Stent. European Journal of Vascular and Endovascular Surgery 2013, 46, 501. [CrossRef]
- Giannoukas, A.D.; Tsetis, D.K.; Touloupakis, E.; Alamanos, E.; Karniadakis, S.; Korakas, P.; Katsamouris, A.N. Suppurative Bacterial Endarteritis After Percutaneous Transluminal Angioplasty, Stenting and Thrombolysis for Femoropopliteal Arterial Occlusive Disease; 1999; Vol. 18;.
- Walker, S.R. PET CT to Confirm an Infected Popliteal Stent Graft Used to Treat Popliteal Artery Aneurysm. European Journal of Vascular and Endovascular Surgery 2017, 54, 612. [CrossRef]
- Gharacholou, S.M.; Dworak, M.; Dababneh, A.S.; Varatharaj Palraj, R.; Roskos, M.C.; Chapman, S.C. Acute Infection of Viabahn Stent Graft in the Popliteal Artery. J Vasc Surg Cases Innov Tech 2017, 3, 69–73. [CrossRef]
- Macheda, B.; Garcier, J.M.; Ravel, A.; Miguel, B.; Therre, T.; Boyer, L. Mycotic Pseudo-Aneurysm Occurring 22 Months after Popliteal Artery Stenting. EJVES Extra 2003, 5, 5–8. [CrossRef]
- Capoccia, L.; Mansour, W.; di Marzo, L.; Grimaldi, S.; Di Girolamo, A. Symptomatic Popliteal Artery Aneurysms in Recently SARS-CoV-2-Infected Patients: The Microangiopathic Thrombosis That Undermines Treatment. Diagnostics 2023, 13. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).