1. Introduction
Glutamate is a functional amino acid which plays an important physiological role in the growth of animal [
1,
2,
3]. Glutamate is the main source of energy for the animals due to it is an intermediate in the metabolism of amino acids [
4,
5]. Therefore, dietary supplementation with glutamate can improve the growth performance of animals. It has been reported that dietary 1.5 % glutamate effectively improved the weight gain of Atlantic salmon [
6]. Some similar studies were widely reported in Tilapia and Salmon or the other fish species [
7,
8]. In addition, glutamate can promote protein synthesis, thereby improving the efficiency of protein utilization in animals [
9,
10]. It has been reported that dietary glutamate improved the protein retention in godhead bream [
11]. Glutamate occupies a central position in the metabolism of amino acids, although it is a nonessential amino acid [
12,
13]. Diets supplemented with an appropriate amount of non-essential amino acids can save the catabolism of some essential amino acids and improve feed utilization [
14]. When there is insufficient protein in the diet or amino acid imbalance, alanine, glutamate, glutamine and aspartate in the gut of mice are preferentially used as energy substrates [
15,
16]. For example, juvenile herring preferentially use glutamate as an energy substrate, thereby saving the consumption of essential amino acids for protein synthesis [
4]. Therefore, diets supplementation with glutamate can improve the growth performance and save dietary protein in fishes and mammals.
Besides, glutamate is the metabolic precursor of glutathione which is the biologically active molecule [
3]. So, it plays an important role in the antioxidant capacity [
17]. Some studies have reported that dietary glutamate can provide materials for glutathione synthesis [
18,
19]. In addition, glutamine-derived glutamate can be converted to glutamate-γ-semialdehyde under the catalysis of dihydropyrrole-5-carboxylic acid synthase, which spontaneously generates dihydropyrrole-5-carboxylic acid and degrades to proline [
20]. Proline has been reported to eliminate free radicals, which can improve the antioxidant capacity of animals [
21]. Moreover, glutamate can increase the activities of several antioxidant enzymes in animals [
22]. A study reported that dietary glutamate reduced the activity of plasma alanine aminotransferase (ALT) and up-regulated the expression of antioxidation-related genes in Atlantic salmon (
Salmo salar L.), indicating that glutamate has a positive effect on the antioxidant capacity and liver health of Atlantic salmon [
6]. Similarly, glutamate increased the activities of SOD, GPX, and GST in muscle of
Cyprinus carpio var. Jian, thereby reducing muscle lipid peroxidation and improving muscle quality [
23]. However, most studies have focused on mammals or fishes, and it has not been reported in crustaceans.
In recent years, with the rapid development of aquaculture, feed protein resources such as fish meal and soybean meal have become increasingly scarce and their prices have been rising [
24,
25]. How to reduce the amount of feed protein and improve protein utilization is an important direction to maintain the sustainable development of aquaculture. Dietary protein deficiency can result in a large amount of essential amino acids being used for the synthesis of non-essential amino acids, and thereby reducing the utilization efficiency of amino acids [
26,
27]. Therefore, this study aimed to investigate the effects of glutamate on growth performance and antioxidant capacity of juvenile Chinese mitten crab
Eriocheir sinensis fed with low protein diets or normal protein diets.
2. Materials and Methods
2.1. Experimental Diets
Six experimental diets were formulated by gradient supplementation 0%, 1% and 2% glutamate were supplemented to low protein (30%) and normal protein (35%) diets respectively. The formulation and proximate composition of the experimental diets are shown in
Table 1.
The ingredients were finely ground and sieved through a 60-mesh strainer. The ingredients were weighed according to the formula and mixed using an electric mixer. The oil and distilled water were subsequently added to make a dough. Finally, the dough was pelleted using a screw-press pelletizer. The pellets were air-dried to the moisture content was < 10%. After drying, diets were stored at -20 °C.
2.2. Feeding Trial, Sampling and Growth Evaluation
Juvenile crabs were obtained from a farm in Huzhou. Crabs were acclimatized to the experimental conditions in 300 L tanks (100 × 80 × 60 cm) before the feeding trial. A total of 600 female crabs (0.4 ± 0.01 g, mean ± SE) were weighed and put into 30 tanks (100 × 80 × 60 cm) and each tank containing 20 crabs. Each experimental diet was randomly allotted to 5 tanks. Three plastic nets were placed in each tank as shelters to reduce attacking behavior. Diets with a daily ration of 4% body weight were hand-fed to crabs three times at 6:00, 18:00. Feces were removed in the morning (09:00), and the water of 30% tank volume was exchanged daily. Dead crabs were immediately removed from the tank, weighed and recorded. Feed intake of each tank was recorded throughout the trial period. During the experimental period, the experimental water temperature in the tanks varied from 25 °C to 27 °C, the dissolved oxygen concentration was > 7 mg/L, the ammonia nitrogen was < 0.05 mg/L.
After 56 d feeding trial, crabs were euthanized, five crabs were sampled randomly from each tank for proximate nutrient composition. Following, six crabs were sampled randomly and the hepatopancreases were frozen in liquid nitrogen and kept at ultra-low temperature freezer for enzyme activity, gene expression and nutrient composition analyses.
Weight gain, specific growth rate and hepatopancreas index were calculated using the formulas as below:
2.3. Chemical Composition Analysis
Chemical compositions of the experimental diets and crabs were measured according to the standard procedures for proximate composition analysis [
28]. Four duplicate samples were measured in each treatment. Moisture was determined after oven dry at 105 °C. Crude protein was quantified using the Kjeltec™ 8200 (Foss, Hoganas, Sweden). Crude lipid was extracted using a 1000 mL Soxhlet extraction tube (Fujian minbo toughened glass Co. Ltd., Fujian, China). Ash was analyzed using a muffle furnace (PCD-E3000 Serials, Peaks, Japan) at 550 °C for 6 h.
2.4. Analysis of Biochemical Parameters in the Hepatopancreas
The biochemical parameters in the hepatopancreas were measured using commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) in accordance with the instructions of the manufacturer. The source and information of each kit used in this study were as follows: total antioxidant capacity (T-AOC; Cat. No. A015-2), superoxide dismutase (SOD; Cat. No. A001-1), glutathione peroxidase (GPX; Cat. No. A005-1-2), glutathione S-transferase (GST; Cat. No. A004-1-1), pyruvate (Pyruvate; Cat. No. A081-1-1), glutaminase (GLS; Cat. No.A124-1-1), glutamine synthetase (GS; Cat. No. A047-1-1), glutamate dehydrogenase (GDH; Cat. No. A125-1-1).
2.5. Analysis of Gene Expression
Total RNA was extracted from the hepatopancreas using the RNAiso Plus (CAT # 9109, Takara, Japan). The total RNA concentration and quality were estimated using the Nano Drop 2000 spectrophotometer (Thermo, USA). If the ratio of A260/A280 was between 1.8 to 2.0, the sample was used for reverse transcription using a PrimeScript™ RT master mix reagent kit (Perfect Real Time, Takara, Japan). The specific primers for the genes of E. sinensis were designed based on the transcriptome sequencing results and NCBI data base using NCBI Primer BLAST (
Table 2). The RT-PCR amplification reactions were performed in a volume of 10 μL containing 5 μL 2×SYBR Premix Ex TaqTM, 0.25 μL of 10 mM forward primer, 0.25 μL of 10 mM reverse primer and 4.5 μL of diluted cDNA, using CFX96 Real-Time PCR system (Bio-rad, Richmond, CA). PCR conditions were as follows: 94 °C for 3 min, and following 40 cycles at 94 °C for 15 s and 60 °C for 50 s, and 72 °C for 20 s. Samples were run in quintuplicate and normalized with the control gene
β-actin and glyceraldehyde-phosphate dehydrogenase (
GAPDH). The gene expression levels were calculated by geometric averaging of multiple internal control genes [
29].
2.6. Statistical Analysis
Statistical analysis was performed using the SPSS 26.0 for Windows (SPSS, Michigan Avenue, Chicago, IL, USA). All data were subject to normality test and homogeneity of variance by using Shapiro-Wilk and Levene's equal variance tests, respectively. Data were analyzed by two-way analysis of variance (ANOVA) to determine if there was any interaction between dietary protein level and glutamate level. At the same protein condition, one-way analysis of variance (ANOVA) was used to analyze the significant differences among crabs fed the diets with different glutamate level after normality test and homogeneity of variance. When the means of each treatment were significantly different, Duncan’s multiple range test was used to compare means among these treatments. At the same glutamate level, independent-samples T test was used to determine significant differences between crabs cultivated at different protein levels. Significance was set at P < 0.05. The data were represented as the mean ± standard error of mean (S.E.).
3. Results
3.1. Growth Performance
At 30% protein level, diets supplemented with 1% and 2% Glutamate slightly increased the weight gain (WG) and Specific growth rate (SGR) of crabs (P > 0.05). However, at the 35% protein level, the WG and SGR of crabs significantly decreased with the increasing dietary glutamate, and the WG and SGR of crabs fed the 2% glutamate diet was significantly lower than the control crabs (P < 0.05). At 30% protein level, the hepatopancreas index (HSI) of crabs fed the 1% glutamate was significantly higher than the crabs fed the diets containing 0% and 2% glutamate (P < 0.05). At the 35% protein level, dietary glutamate did not significantly affect the HSI of crabs (P > 0.05). There were no significant interactions between dietary protein level and glutamate levels based on the WG, SGR and HSI (P > 0.05).
Table 3.
Effects of glutamate on the growth performance of juvenile Chinese mitten crab.
Table 3.
Effects of glutamate on the growth performance of juvenile Chinese mitten crab.
| |
Parameters |
| Diets |
Weight gain (%) |
Specific growth rate (% day-1) |
Hepatopancreas index (%) |
| 30% Protein-0% Glu |
283.59±76.72 |
2.29±0.37 |
8.62±2.72b
|
| 30% Protein-1% Glu |
327.91±73.45 |
2.48±0.32 |
12.4±0.95a
|
| 30% Protein-2% Glu |
328.53±67.74 |
2.49±0.29 |
8.46±1.63b
|
| 35% Protein-0% Glu |
375.2±59.48A
|
2.68±0.22A
|
8.98±1.6 |
| 35% Protein-1% Glu |
315.42±66.24AB
|
2.44±0.27AB
|
8.28±1.34 |
| 35% Protein-2% Glu |
265.83±106.38B
|
2.17±0.55B
|
9.83±0.75 |
| Two-way ANOVA (P value) |
|
|
| Protein |
NS |
NS |
NS |
| Glu |
NS |
NS |
NS |
| Protein × Glu |
NS |
NS |
NS |
The different lowercase letters in the table indicate that there are significant differences among crabs fed with different glutamate diets at 30% protein (P < 0.05). Different capital letters indicate that there are significant differences among crabs fed with different glutamate diets at 35% protein (P < 0.05), the same below.
3.2. Nutrient Composition of Crabs
As showed in the
Table 4, dietary glutamate did not significantly affect the moisture, ash, and crude lipid of crabs fed with the diets under both 30% and 35% protein levels (
P > 0.05). There was a significant main effect of dietary glutamate on crude protein content (
P < 0.05). At 30% protein level, the crude protein content of crabs fed the 2% glutamate diets was significantly higher than crabs fed the diets containing 0% and 1% glutamate (
P < 0.05). At 35% protein level, the crude protein content of crabs fed the 0% glutamate diets was significantly higher than crabs fed the diets containing 2% glutamate (
P < 0.05).
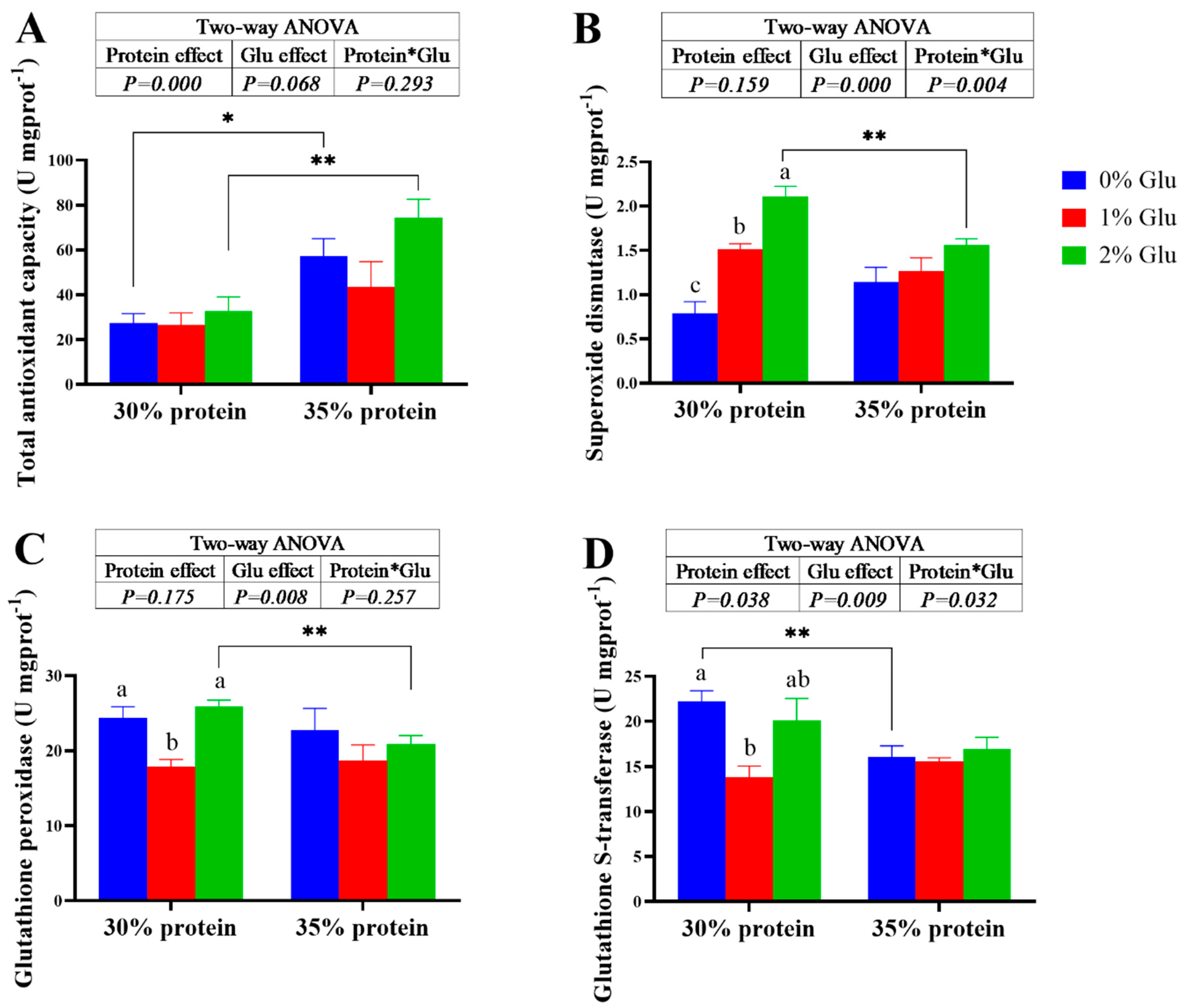
Figure 1.
Effects of glutamate on the antioxidant capacity in the hepatopancreas of juvenile Chinese mitten crab. The asterisk (*) indicates that there are significant differences among different protein level groups with the same amount of glutamate (* means P < 0.05, ** means P < 0.01, *** means P < 0.001), the same below.
Figure 1.
Effects of glutamate on the antioxidant capacity in the hepatopancreas of juvenile Chinese mitten crab. The asterisk (*) indicates that there are significant differences among different protein level groups with the same amount of glutamate (* means P < 0.05, ** means P < 0.01, *** means P < 0.001), the same below.
3.3. The Actiities of Enzymes Related to Antioxidant Capacity in the Hepatopancreas
Dietary glutamate did not significantly affect the total antioxidant capacity (T-AOC) of crabs fed with the diets under both 30% and 35% protein levels (P > 0.05). However, the crude protein content of diets has a significant main effect on the T-AOC (P < 0.05). The T-AOC of crabs fed the 30% protein diets was significantly lower than crabs fed the 35% protein diets (P < 0.05). At 30% protein level, the superoxide dismutase (SOD) activity significantly increased with the increase of glutamate content (P < 0.05). But At 30% protein level, dietary glutamate did not significantly affect the SOD of crabs (P > 0.05). Under the 2% glutamate condition, the SOD activity in the hepatopancreas of crabs fed the 30% protein was significantly higher than crabs fed the 35% protein (P < 0.05). At 30% protein level, the glutathione peroxidase (GPX) and glutathione S-transferase (GST) of crabs fed the 1% glutamate diets were significantly lower than crabs fed the 0% and 2% glutamate diets (P < 0.05). At 35% protein level, dietary glutamate did not significantly affect the activities of GPX and GST (P > 0.05).
3.4. The Amino Acid Metabolism in the Hepatopancreas
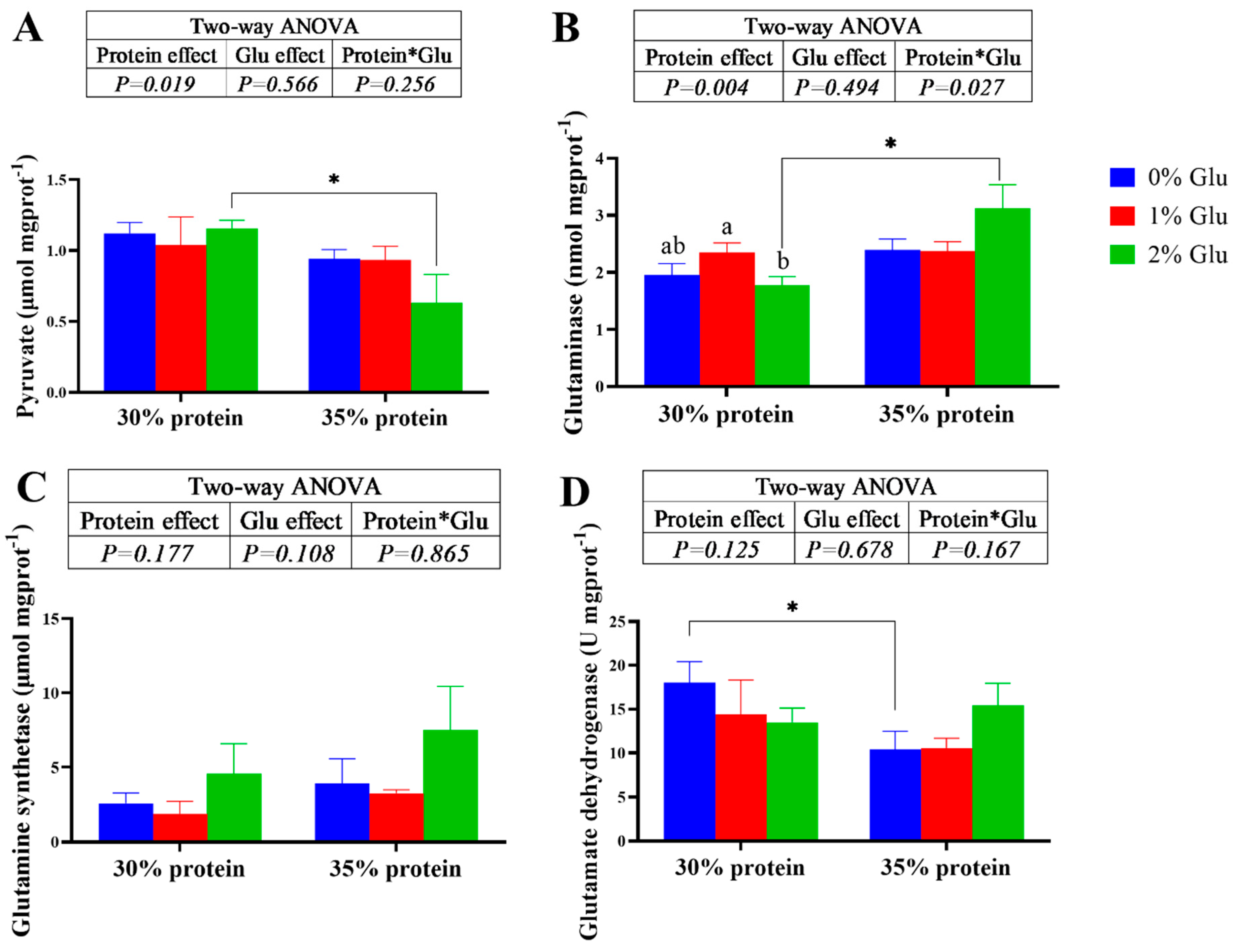
Dietary glutamate did not significantly affect the pyruvate, glutamine synthetase and glutamate dehydrogenase of crabs fed with the diets under both 30% and 35% protein levels (P > 0.05). However, the crude protein content of diets has a significant main effect on the pyruvate (P < 0.05). At 30% protein level, the activity of glutaminase was significantly higher in crabs fed the 1% glutamate diets (P < 0.05). At 35% protein level, dietary glutamate did not affect the activity of glutaminase (P > 0.05).
Figure 2.
Effects of glutamate on the amino acid metabolism in the hepatopancreas of juvenile Chinese mitten crab.
Figure 2.
Effects of glutamate on the amino acid metabolism in the hepatopancreas of juvenile Chinese mitten crab.
3.5. The Protein Metabolism in the Hepatopancreas
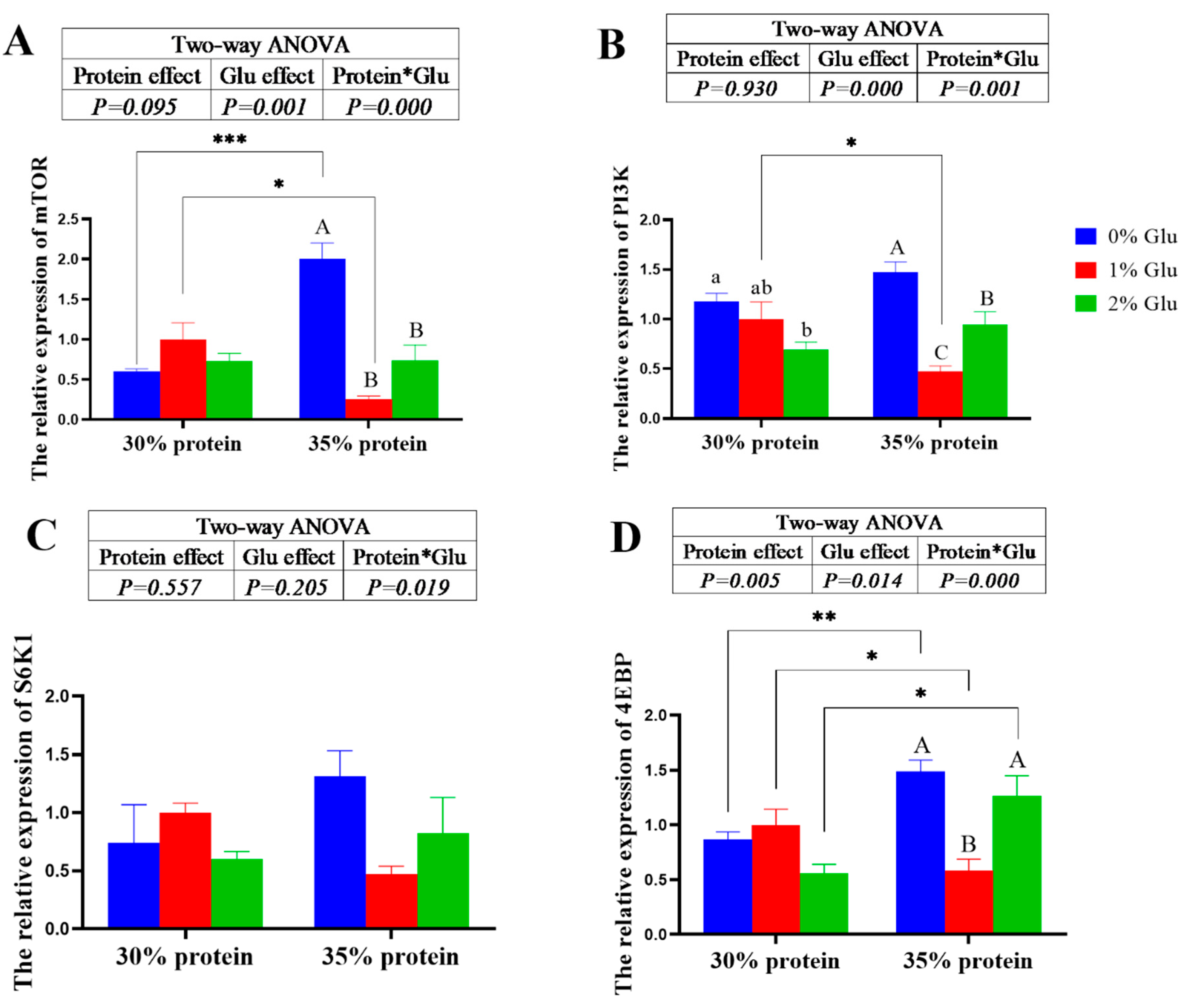
At 30% protein level, dietary glutamate did not affect the relative expressions of mTOR, S6K1 and 4EBP (P > 0.05). However, at 35% protein level, dietary glutamate significantly down-regulated the relative expressions of mTOR, PI3K, S6K1 and 4EBP (P < 0.05). At 30% protein level, dietary glutamate significantly down-regulated the relative expressions of PI3K (P < 0.05). The crude protein content of diets has a significant main effect on the relative expressions of 4EBP (P < 0.05). The relative expressions of 4EBP of crabs fed the 30% protein diets was significantly lower than crabs fed the 35% protein diets (P < 0.05).
Figure 3.
Effects of glutamate on the protein metabolism related genes in the hepatopancreas of juvenile Chinese mitten crab. mTOR: Mammalian target of rapamycin; PI3K: Phosphatidylinositide 3-kinases; S6K1: Ribosomal protein S6 kinase 1; 4EBP: e IF4E-binding protein.
Figure 3.
Effects of glutamate on the protein metabolism related genes in the hepatopancreas of juvenile Chinese mitten crab. mTOR: Mammalian target of rapamycin; PI3K: Phosphatidylinositide 3-kinases; S6K1: Ribosomal protein S6 kinase 1; 4EBP: e IF4E-binding protein.
4. Discussion
Glutamate plays an important physiological role in the growth of animal [
1,
2,
3]. Some previous studies reported that dietary glutamate can improve the weight gain of fishes [
6,
7,
8]. However, in the present study, dietary glutamate did not significantly affect the weight gain and specific growth rate. Moreover, dietary glutamate significantly decreased the growth of Chinese mitten crab. This result was different from grass carp [
30], golden head snapper [
11] and Atlantic salmon [
6]. The differences may be caused by species specificity. Some previous studies reported that glutamate can significantly increase the hepatosomic index of aquatic animals [
7]. Similar results were also found in the present study, where 1% glutamate supplemented to the diet with 30% protein level significantly increased the hepatopancreas index of juvenile Chinese mitten crab. Growth is closely related to the accumulation of nutrients in animals. A study reported that dietary glutamate increased the crude lipid content in the gold head snapper (
Sparus aurata) [
11]. Besides, it has been reported that dietary glutamate can improve protein content and lipid content of grass carp (
Ctenopharyngodon idella) [
30]. In the present study, dietary glutamate did not significantly affect the moisture, ash, and crude lipid of crabs fed with the diets under both 30% and 35% protein levels. Even worse, 2% glutamate significantly decreased the crude protein of Chinese mitten crab. The differences may be caused by species specificity. Unfortunately, glutamate has been poorly studied in crustaceans. Therefore, the expression of genes involved in protein synthesis in the hepatopancreas of the crab was further investigated in the present study. The results indicated that dietary glutamate significantly down-regulated the relative expressions of
mTOR,
PI3K,
S6K1 and
4EBP of crabs fed the diets containing 35% protein, which indicated that dietary glutamate inhibits the protein synthesis by inhibiting
mTOR pathway in Chinese mitten crab. These results were consistent with those of body composition. In summary, dietary glutamate decreased the growth and protein content of Chinese mitten crab by regulating the
mTOR pathway.
Previous studies have demonstrated that low protein diet can lead to oxidative stress in Chinese mitten crabs [
31]. In the present study, The T-AOC of crabs fed the 30% protein diets was significantly lower than crabs fed the 35% protein diets, which is consistent with those of other studies. GSH-PX and GSH-ST are required by the endogenous antioxidant defense system to scavengers oxygen free radicals and maintain cellular Redox balance [
32]. In the present study, dietary glutamate did not significantly affect the GSH-PX and GSH-ST, which indicated that glutamate did not improved the GSH enzyme system. However, SOD activity significantly increased with the increase of glutamate content. This result indicated that dietary glutamate can improve the activity of SOD, thereby increasing the antioxidant capacity in Chinese mitten crab under low protein condition.
5. Conclusions
Dietary glutamate cannot significantly increase the growth of Chinese mitten crab, but it can decrease the growth and protein content of Chinese mitten crab by regulating the mTOR pathway. However, dietary glutamate can improve the activity of SOD, thereby increasing the antioxidant capacity in Chinese mitten crab under low protein condition.
Author Contributions
Jiajun Zheng, writing – original draft and editing, Yisong He, writing—review and editing. Mengyu Shi, writing—review and editing. Li Jia, writing—review and editing. Yang Xu, writing—review and editing. Yue Tan, writing—review and editing. Changle Qi, supervision. Jinyun Ye, supervision.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
This research was supported by grants from the Zhejiang Province R&D Plan (2022C02058), Zhejiang Provincial Natural Science Foundation of China under Grant No. LTGN23C190003, and the Huzhou Natural Science Foundation (2021YZ14) and the Graduate Research Innovation Project of Huzhou University (2023KYCX68).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Brosnan, J.T.; Brosnan, M.E. Glutamate: A truly functional amino acid. Amino Acids 2013, 45, 413-418. [CrossRef]
- Lallès, J.P.; Bosi, P.; Smidt, H.; Stokes, C.R. Weaning—A challenge to gut physiologists. Livest. Sci. 2007, 108, 82-93. [CrossRef]
- Burrin, D.G.; Stoll, B. Metabolic fate and function of dietary glutamate in the gut. Am. J. Clin. Nutr. 2009, 90, 850-856. [CrossRef]
- Conceio, L.E.C.; Rnnestad, I.; Tonheim, S.K. Metabolic budgets for lysine and glutamate in unfed herring (Clupea harengus) larvae. Aquaculture 2002, 206, 305-312. [CrossRef]
- Waarde, V.; Aren. Aerobic and anaerobic ammonia production by fish. Comp. Biochem. Phys. B. 1983, 74, 675-684. [CrossRef]
- Larsson, T.; Koppang, E.O.; Espe, M.; Terjesen, B.F.; Krasnov, A.; Moreno, H.M.; Rørvik, K.A.; Thomassen, M.; Mørkøre, T.J.A. Fillet quality and health of Atlantic salmon (Salmo salar L.) fed a diet supplemented with glutamate. Aquaculture 2014, 426, 288-295. [CrossRef]
- Oehme, M.; Grammes, F.; Takle, H.; Zambonino-Infante, J.L.; Refstie, S.; Thomassen, M.S.; Rørvik, K.-A.; Terjesen, B.F. Dietary supplementation of glutamate and arginine to Atlantic salmon (Salmo salar L.) increases growth during the first autumn in sea. Aquaculture 2010, 310, 156-163. [CrossRef]
- Silva, L.C.R.d.; Furuya, W.M.; Natali, M.R.M.; Schamber, C.R.; Santos, L.D.d.; Vidal, L.V.O. Productive performance and intestinal morphology of Nile tilapia juvenile fed diets with L-glutamine and L-glutamate. Rev. Bras. Zootecn. 2010, 39, 1175-1179. [CrossRef]
- Maclennan, P.A.; Brown, R.; Rennie, M.J. A positive relationship between protein synthetic rate and intracellular glutamine concentration in perfused rat skeletal muscle. Febs. Lett. 1987, 215, 187-191. [CrossRef]
- Coffier, M.S.; Claeyssens, S.; Hecketsweiler, B.; Lavoinne, A.; Ducrotté, P.; Déchelotte, P. Enteral glutamine stimulates protein synthesis and decreases ubiquitin mRNA level in human gut mucosa. Am. J. Physiol. Gastr. L. 2003, 285, 266-279. [CrossRef]
- Caballero-Solares, A.; Viegas, I.; Salgado, M.C.; Siles, A.M.; Saez, A.; Metón, I.; Baanante, I.V.; Fernández, F.J.A. Diets supplemented with glutamate or glutamine improve protein retention and modulate gene expression of key enzymes of hepatic metabolism in gilthead seabream (Sparus aurata) juveniles. Aquaculture 2015, 444, 79-87. [CrossRef]
- Brosnan, M.E.; Brosnan, J.T. Hepatic glutamate metabolism: A tale of 2 hepatocytes. Am. J. Clin. Nutr. 2009, 90, 857-861. [CrossRef]
- Wu, G. Functional amino acids in growth, reproduction, and health. Adv. Nutr. 2010, 1, 31-37. [CrossRef]
- Abboudi, T.; Mambrini, M.; Larondelle, Y.; Rollin, X. The effect of dispensable amino acids on nitrogen and amino acid losses in Atlantic salmon (Salmo salar) fry fed a protein-free diet. Aquaculture 2009, 65, 345-353. [CrossRef]
- Tanaka; Shibata; Mori; Ogura. Metabolism of essential amino acids in growing rats at graded levels of soybean protein isolate. J. Nutr. Sci. Vitaminol. 1995, 41, 433-443. [CrossRef]
- Nakamura, H.; Kawamata, Y.; Kuwahara, T.; Torii, K.; Sakai, R. Nitrogen in dietary glutamate is utilized exclusively for the synthesis of amino acids in the rat intestine. Am. J. Physiol. Ph. 2013, 304, 100-108. [CrossRef]
- Espinosa, D.C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sánchez-Pérez, P.; Cadenas, S.; Lamas, S. Antioxidant responses and cellular adjustments to oxidative stress. Redox. Biol. 2015, 6, 183-197. [CrossRef]
- He, L.; Wu, J.; Tang, W.; Zhou, X.; Lin, Q.; Luo, F.; Yin, Y.; Li, T. Prevention of oxidative stress by α-ketoglutarate via activation of CAR signaling and modulation of the expression of key antioxidant-associated targets in vivo and in vitro. J. Agr. Food. Chem. 2018, 66, 11273-11283. [CrossRef]
- Cai, G.Y. Effect of glutamate on antioxoidant level and subseouent development of in vitro cultured mouse embryos in the blocking stage. Master's Thesis, Yanbian University, Jilin, China, 2017. (in Chinese).
- Fujita, T.; Yanaga, K. Association between glutamine extraction and release of citrulline and glycine by the human small intestine. Life Sci. 2007, 80, 1846-1850. [CrossRef]
- Kaul, S.; Sharma, S.S.; Mehta, I.K. Free radical scavenging potential of L-proline: Evidence from in vitro assays. Amino Acids 2008, 34, 315-320. [CrossRef]
- Meister, A. Glutathione-ascorbic acid antioxidant system in animals. J. Biol. Chem. 1994, 269, 9397-9402. [CrossRef]
- Zhao, Y.; Li, J.-Y.; Yin, L.; Feng, L.; Liu, Y.; Jiang, W.-D.; Wu, P.; Zhao, J.; Chen, D.-F.; Zhou, X.-Q.J.A. Effects of dietary glutamate supplementation on flesh quality, antioxidant defense and gene expression related to lipid metabolism and myogenic regulation in Jian carp (Cyprinus carpio var. Jian). Aquaculture 2019, 502, 212-222. [CrossRef]
- Canton, Helen. The Europa directory of international organizations 2021, 23rd ed.; Routledge: London, UK., 2021; pp. 297–305.
- Tao, S.; Zhang, Q.; Zhang, J. Feed market situation, prospect and countermeasures in 2021. CHN. J. Anim. Sci. 2022, 86, 45-49. (in Chinese).
- Huang, J.; Deng, H. Effects of low protein diet on nutrient digestion and nitrogen emission of growing pigs. Livest. Poult. Ind. 2017, 45, 21-28. (in Chinese).
- Gloaguen, M.; Floc'H, N.L.; Corrent, E.; Primot, Y.; Milgen, J.V. The use of free amino acids allows formulating very low crude protein diets for piglets. J. Anim. Sci. 2014, 92, 637-641. [CrossRef]
- AOAC. Official methods of analysis of AOAC International. Association of Official Analytical Chemists: Washington DC, USA 2005.
- Vandesompele, J.; Preter, K.D.; Pattyn, F.; Poppe, B.; Roy, N.V.; Paepe, A.D.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, 1-12. [CrossRef]
- Zhao, Y.; Hu, Y.; Zhou, X.Q.; Zeng, X.Y.; Feng, L.; Liu, Y.; Jiang, W.D.; Li, S.H.; Li, D.B.; Wu, X.Q. Effects of dietary glutamate supplementation on growth performance, digestive enzyme activities and antioxidant capacity in intestine of grass carp (C tenopharyngodon idella). Aquacult Nutr. 2015, 21, 935-941. [CrossRef]
- Zhu, S.; Long, X.; Turchini, G.M.; Deng, D.; Cheng, Y.; Wu, X.J.A. Towards defining optimal dietary protein levels for male and female sub-adult Chinese mitten crab, Eriocheir sinensis reared in earthen ponds: Performances, nutrient composition and metabolism, antioxidant capacity and immunity. Aquaculture 2021, 536, 736442. [CrossRef]
- Cabrini, L.; Bergami, R.; Fiorentini, D.; Marchetti, M.; Landi, L.; Tolomelli, B.J.I.L. Vitamin B6 deficiency affects antioxidant defences in rat liver and heart. Iubmb Life 1998, 46, 689-697. [CrossRef]
Table 1.
Formulation and proximate composition of the experimental diets (dry matter, %).
Table 1.
Formulation and proximate composition of the experimental diets (dry matter, %).
| Ingredients |
Experimental diets |
30% Protein
0% Glu |
30% Protein
1% Glu |
30% Protein
2% Glu |
35% Protein
0% Glu |
35% Protein
1% Glu |
35% Protein
2% Glu |
| Ingredients |
|
|
|
|
|
|
| Fish meal |
21 |
21 |
21 |
24.5 |
24.5 |
24.5 |
| Gelatin |
3 |
3 |
3 |
3.5 |
3.5 |
3.5 |
| Casein |
12 |
12 |
12 |
14 |
14 |
14 |
| Corn starch |
26 |
26 |
26 |
26 |
26 |
26 |
| Fish oil |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
| Soybean oil |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
| Arginine |
2 |
2 |
2 |
2 |
2 |
2 |
| Methionine |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
| Lysine |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
| Vitamin premix a
|
1.5 |
1.5 |
1.5 |
1.5 |
1.5 |
1.5 |
| Mineral premix b
|
1.5 |
1.5 |
1.5 |
1.5 |
1.5 |
1.5 |
| Soybean lecithin |
2 |
2 |
2 |
2 |
2 |
2 |
| Cholesterol |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
| Choline chloride |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
| Betaine |
2 |
2 |
2 |
2 |
2 |
2 |
| Butylated hydroxytoluene |
0.1 |
0.1 |
0.1 |
0.1 |
0.1 |
0.1 |
| Sodium carboxymethyl cellulose |
2 |
2 |
2 |
2 |
2 |
2 |
| Glutamate |
0 |
1 |
2 |
0 |
1 |
2 |
| Cellulose |
19.9 |
18.9 |
17.9 |
13.9 |
12.9 |
11.9 |
| Proximate analysis(%) |
|
|
|
|
|
|
| Moisture |
6.36 |
6.69 |
6.32 |
6.31 |
6.50 |
6.70 |
| Crude protein |
30.52 |
30.75 |
31.52 |
35.58 |
36.58 |
37.62 |
| Crude lipid |
8.68 |
8.59 |
8.63 |
9.56 |
9.47 |
9.62 |
| Ash |
4.81 |
4.92 |
5.00 |
5.53 |
5.61 |
5.29 |
Table 2.
Sequences of primers.
Table 2.
Sequences of primers.
| Primers name |
Sequences (5'-3') |
Product size |
References |
|
mTOR F |
AGGTCCTGTTATGCTGTGGC |
158 bp |
MT920347.1 |
|
mTOR R |
ATCTCGGGGATGTCCTGTGA |
|
PI3K F |
GCTGTCAGTCCAGTTCGACA |
111 bp |
c147204_g1 |
|
PI3K R |
ACAGTATGCTTGGTCAGGGC |
|
AMPD F |
CACAACGTCCACTCCGAGAA |
116 bp |
c143453_g1 |
|
AMPD R |
CGGAACAGGTTGTCGAGGAA |
|
AKT F |
ATAAGGACCCCAACAAGCGG |
134 bp |
KY709138.1 |
|
AKT R |
CACTTGGGGTTTGAAAGGCG |
|
S6K1 F |
TGACTACCCGGACCTGCTAA |
154 bp |
XM_050855088.1 |
|
S6K1 R |
TGCCACACCAATGAACCCTT |
|
4EBP F |
GCTGTCTGCTCCCTCACTTT |
163 bp |
XM_050856547.1 |
|
4EBP R |
ACCCGTCAGCTTCTTAAGCC |
|
GLDH F |
GGCAACGATGTAACGTGTGG |
116 bp |
XM_050832606.1 |
|
GLDH R |
CGAAGCATCTTGCCACCAAC |
Table 4.
Effect of glutamate on the whole-body composition of juvenile Chinese mitten crab.
Table 4.
Effect of glutamate on the whole-body composition of juvenile Chinese mitten crab.
| |
Parameters |
|
| Diets |
Moisture (%) |
Ash (%) |
Crude protein (%) |
Crude lipid (%) |
| 30% Protein-0% Glu |
66.18±2.16 |
11.88±0.21 |
14.19±0.13a
|
6.12±0.23 |
| 30% Protein-1% Glu |
66.54±3.15 |
12.01±0.42 |
14.14±0.33a
|
5.89±0.16 |
| 30% Protein-2% Glu |
66.28±1.11 |
11.96±0.25 |
13.34±0.24b
|
5.95±0.32 |
| 35% Protein-0% Glu |
66.82±3.74 |
12±0.33 |
13.94±0.26A
|
6.1±0.48 |
| 35% Protein-1% Glu |
66.13±1.65 |
12.08±0.49 |
13.76±0.11AB
|
6.03±0.23 |
| 35% Protein-2% Glu |
66.19±3.69 |
11.96±0.47 |
13.26±0.43B
|
5.81±0.67 |
| Two-way ANOVA (P value) |
|
|
|
| Protein |
NS |
NS |
NS |
< 0.05 |
| Glu |
NS |
NS |
< 0.01 |
NS |
| Protein × Glu |
NS |
NS |
NS |
NS |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).