1. Introduction
Despite undeniable improvements in the conservative and interventional management of coronary artery disease, clinicians are still faced with the daily management of patients with refractory angina (RA).
Refractory angina is defined as persistent symptoms (>3 months) due to established reversible ischemia that is not adequately controlled with optimal medical therapy and for which appropriate revascularization options are not available. The proportion of patients with RA is increasing, ranging from 2% to even up to 20 % [
1,
2], and unfortunately, there are no adequate therapeutic options.
The vast majority of cases of RA is associated with patients with advanced, diffuse CAD, yet the pathophysiology of RA is far more heterogeneous and involves coronary disease other than obstructive CAD [
3]. Patients with RA have significant morbidity and quality of life impairments. In addition, their treatment results in significantly increased healthcare costs [
4].
Several therapeutic agents aimed at improving myocardial perfusion (beyond traditional revascularization or anti-ischemic drugs) have been developed to overcome the current limitations of contemporary clinical practice in the management of the RA subpopulation [
5]. Since 2007 [
6], when the first-in-man study of the Coronary Sinus Reducer (CSR) device was conducted, we can observe a slowly growing body of scientific evidence supporting the use of the CSR in RA patients. The Neovasc Reducer (Neovasc Inc., Richmond, Canada) is a percutaneous, balloon-expandable, hourglass-shaped stainless steel stent that creates a focal narrowing of the CS and increases coronary venous pressure. It is postulated that the increased venous backward pressure in the cardiac microcirculation promotes blood redistribution, improves the endocardial/epicardial blood flow ratio, and alleviates the symptoms of angina [
7].
In this single-center prospective observational study, we evaluated the mid-term safety and efficiency of CSR in “real-life” cohort with RA.
2. Materials and Methods
Study Population
The study population consisted of all consecutive patients who underwent the procedure of implantation of the Coronary Sinus Reducer in the Department of Cardiology of the Copper Health Center in Lubin, Poland, between May 2022 and January 2024. All subjects enrolled in the study had a primary diagnosis of chronic refractory angina (Canadian Cardiovascular Society (CCS) classes 2-4) for at least 3 months before the procedure, in spite of maximum tolerable medical therapy for angina (at least 3 different drug groups). Only individuals without a recent history of acute coronary syndrome or recent coronary revascularization were included in the study (the blanking period for the recruitment process was 3 months). All patients prior to the procedure underwent evaluation by the local heart team for potential revascularization options. In addition, patients with chronic heart failure (New York Heart Association (NYHA) class 3-4), severe left ventricular systolic impairment (less than <25%), or high mean right atrial pressure (greater than 15 mm Hg) were excluded from the study.
All subjects signed a written consent for the procedure and participation in the study. The study was also approved by the local ethics committee (Bioethics Committee of the Lower Silesian Medical Board in Wroclaw- approval number 02/BOBD/2022). In addition, the study was registered and approved by clinicaltrials.gov (NCT06288165).
Coronary Sinus Reducer Device and the Implantation Procedure
The Coronary Sinus Reducer (Neovasc Inc., Richmond, Canada) is an hourglass-shaped stainless steel device mounted on an expendable balloon-catheter delivery system that operates in a standard “over-the-wire” fashion way. The delivery system has three radiopaque markers, two of which are attached to the distal edges of a scaffold, while the third additional marker is attached proximal to the balloon and is used to determine the exact position of the device during the implantation procedure.
The procedure is performed by vascular access located in the right Jugular vein (in selected cases, it is possible to perform the procedure through the femoral approach). Under local anesthesia introducer sheath (9 Fr) is inserted into the vein system. After the advancement of the multipurpose diagnostic catheter into the right atrium by the standard 0.035” wire the arterial pressure measurements are performed (mean pressure needs to be lower than 15 mm Hg). In the next step, the CS is engaged with a multipurpose catheter; a standard 0.035" wire is advanced distally into the CS and venography is performed to evaluate the potential implantation area (adequate dimensions for device deployment - proximal diameter <10 mm and >14 mm; absence of side branches or vascular anomalies). After switching to the 9 Fr guiding catheter, the CSR is implanted at 4-6 atmospheres with a device oversize of up to 20% relative to the CS size. A standard dose of unfractionated heparin (100 UI/kg) is administered during the procedure. Patients will receive additional dual antiplatelet therapy from the day of implantation for three months.
Follow-Up
All patients underwent an initial clinical evaluation by trained medical staff regarding past medical history. In addition, an in-depth analysis of angina symptoms was performed, including Canadian Cardiovascular Society (CCS) class assessment, Seattle Angina Questionnaire - 7 items (SAQ-7) scores; New York Heart Association (NYHA) functional class, and 6-minute walk test (6-MWT), along with transthoracic echocardiography (TTE). In addition, all patients were assessed for quality of life using the 36-item Short Form Health Survey (SF-36), the EQ-5D-5L questionnaire, and the EQ-VAS. At the end of the initial hospitalization, all clinical and procedural characteristics, including safety adverse events, were also collected. The primary time point for clinical re-evaluation (including all data collected during the initial evaluation) was set at 3 months.
Statistical Analysis
Data are presented as mean with standard deviation (SD) or median with interquartile range (IQR), depending on the normality of the distribution (assessed by Shapiro-Wilk test). Categorical data were analyzed using McNemar's test. Continuous data were analyzed using Student's paired t-test or Wilcoxon paired signed rank test, depending on the results of the Shapiro-Wilks test for normality. McNemar's test was used to compare changes in CCS levels. Post-hoc comparisons of CCS subgroups were performed using the McNemar's test, comparing a given category with other categories, with the Holm-Bonferroni correction for multiple testing. Sample mean and 95% confidence interval for mean were used for t-test, and sample pseudomedian and 95% confidence interval for pseudomedian were used for Wilcoxon test. All tests were performed at a significance level of alpha=0.05. The R statistical package was used for all analyses.
3. Results
The study includes a retrospective analysis of the mid-term results (3-month follow-up) of 55 consecutive patients who underwent implantation of the Sinus Coronary Reducer device between April 2022 and January 2024 at the Department of Cardiology, Cooper Health Centre Lubin, Poland. Baseline clinical characteristics are summarized in
Table 1. The vast majority of subjects enrolled in the study were men (87.3%) with a mean age of 73.1 ± 6.9 years.
We observed a high burden of cardiovascular risk factors in the study cohort - hypertension (100%), hyperlipidemia (89.1%), diabetes (68.2%), or prediabetes (9.1%). Nearly nine out of ten patients underwent revascularization by PCI (85.9%), and more than half of the study population (58.2%) had previously undergone CABG surgery. Only four patients out of the study cohort had non-obstructive CAD (microvascular dysfunction), the rest were presented with obstructive CAD. Of these, the majority (62.7%) were considered to have previously undergone optimal revascularization, while the remaining were considered to be ineligible for further revascularization.
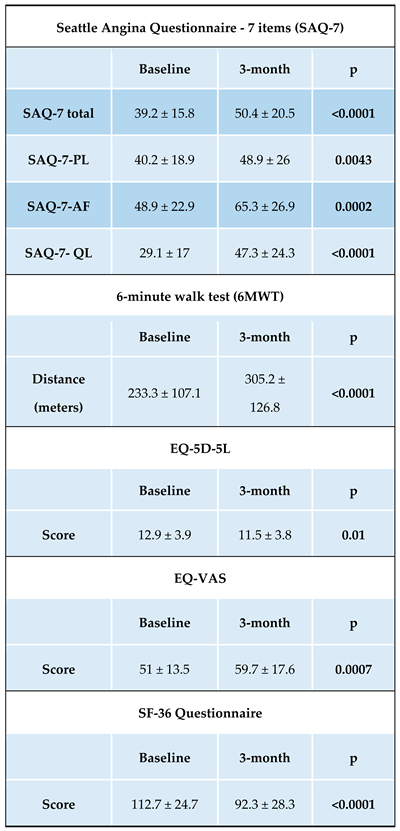
The mean CCS angina class at baseline was 3.15 ± 0.6. A high angina burden was reported in all patients. It was highlighted by relatively low scores on the SAQ-7 Total (39.2 ± 15.8) and poor exercise tolerance with a mean 6-minute walk test of 233.3 ± 107.1 m. Poor angina control also affected baseline quality of life as measured by EQ-5D-5L (12.9 ± 3.9), SF-36 (112.7 ± 24.7), and EQ-VAS (51 ± 13.5).
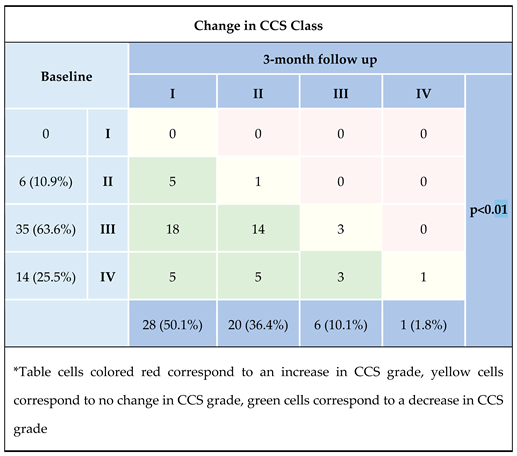
Three months after implantation 22 (40%) subjects noticed an improvement in symptom control at one CCS class. Furthermore 23 (41.8%) patients after Reducer implantation demonstrated a 2 CCS class reduction, and 5 (9.1%) patients reported a 3 CCS class reduction.
Table 2. These favorable outcomes were confirmed by significant improvement in angina control measured SAQ-7 Total (39.2 ± 15.8 vs 50.4 ± 20.5; p<0.0001) along with increased abolition of physical limitation – 6-MWT (233.3 ± 107.1 vs 305.2 ± 126.8; p<0.0001). In addition, patients reported a statistically significant improvement in quality of life 3 months after CSR implantation; all data on these clinical characteristics are summarized in
Table 3. Antianginal pharmacotherapy remained unchanged during the observation period. In terms of safety outcomes, all patients underwent successful device deployment with no access site complications. In one case, we observed migration of the Coronary Sinus Reducer into the pulmonary arteries. In this particular case, we were able to retrieve the lost device using percutaneous loops. After successful extraction of a device during the same procedure, the patient underwent a second successful attempt at CSR implantation.
4. Discussion
In the presented single-center observational study we observed good safety and efficiency of Coronary Sinus Reducer in a “real-life” population of patients with chronic disabling refractory angina pectoris without a revascularization option. The main findings of the study are 1) CS Reducer implantation was a relatively safe procedure without serious adverse events requiring bailout cardiac surgery; 2) Clinical efficacy of this therapy was documented in real-life by reducing disabling angina symptoms, improving quality of life and exercise tolerance; 3) Despite variability in preimplant clinical presentation with concomitant limited support of advanced imaging (MRI/PET) during the qualification process, favourable clinical and safety results were maintained at midterm.
Despite undeniable improvements in revascularization techniques and pharmacological management of coronary artery disease, clinicians are often faced with patients presenting with disabling chronic refractory angina although all therapeutic options have been applied in the treatment protocol. Paradoxically, as revascularization techniques and armamentarium evolve, the number of patients with severe refractory angina and a predictable long life expectancy is steadily increasing [
8,
9], and there is an urgent clinical need for novel therapies to alleviate symptoms and improve the quality of life in this group of patients.
Evidence for the safety and efficacy of SCR in non-optional patients with refractory angina pectoris is still accumulating, but the vast majority of it comes from small-number observational registries or few randomized trials [
10,
11,
12,
13,
14,
15,
16,
17,
18,
19], which is reflected in the relatively low level of recommendation in revascularization guidelines [
20].
In our experience, all attempts at device implantation have ultimately resulted in successful CSR implantation. This high percentage of success is still slightly higher than the average results observed to date, but seems to be in line with previously reported results [
18]. This finding is probably related to an advanced proctoring program applied in our cardiac center along with the fact that the implantation procedure is always performed by two operators, one of whom has extensive experience in CRT-D implantation. Another added value is the intensive development of the CSR implant program at our site - we performed a relatively high number of procedures during a short training period. Furthermore, in our study cohort, we noticed only one periprocedural compilation (temporary dislocation of the device from coronary sinus) such a low rate of complications (1,8%) is similar to other studies [
18] suggesting a good safety profile. Moreover, from a practical standpoint, it is noteworthy that we routinely perform vascular access under ultrasound guidance, which allows us to eliminate access site complications despite the high burden of patients on anticoagulant therapy in the study cohort (30.9%).
In our study cohort despite the fact that qualification for the procedure was based mainly on clinical evaluation without the use of sophisticated imaging methods (MRI or PET) implantation of the Reducer resulted in favorable results the majority of patients 50 (90.9%) obtained results seems to be in line with a previously reported response rate, which was 71-85%. 3 months after implantation, the average angina reduction reached a mean 1.6 ± 0.8 CCS class. These favorable results were confirmed by a significant improvement in angina control as measured by the SAQ-7 Total Questionnaire. Furthermore, these data were further objectified by an increase in physical activity capacity- 6-MWT distance increased significantly (233.3 ± 107.1 vs. 305.2 ± 126.8; p<0.0001).
The clinical benefit achieved in the significant reduction of refractory angina was reflected in an improvement in the overall quality of life by all of the assessment methods used - EQ-5D-5L, SF-36 and EQ-VAS. These favorable results have potentially important practical implications; given that patients with uncontrolled refractory angina are more likely to suffer from depression and anxiety disorders [
21,
22], CSR may be a key to addressing these unmet clinical needs in this subpopulation. Furthermore, over time, it may help reduce economic costs and the burden on the healthcare system by reducing the number of unnecessary hospitalizations (our study cohort had an average of up to 4 hospitalizations due to refractory angina during the year prior to implantation).
Limitations
Our study has several limitations. The main limitations of our study appear to be the lack of a control group, and the relatively small number of patients enrolled. Another important shortcoming of our study is the lack of objective measurement of myocardial ischemia reduction after Reducer implantation. Despite these limitations, this study appears to be valuable from a practical standpoint since it includes data from one of the largest numbers of patients treated in a single-center, real-world setting.
5. Conclusions
Data from our real-world, single-center registry appear to confirm that CS Reducer implantation is a safe and effective therapeutic option for patients with refractory angina who are not candidates for revascularization. CRS appears to be particularly effective in alleviating angina symptoms and improving quality of life. Further large, randomized trials with a control group are needed to fully investigate the impact of CRS on long-term clinical outcomes in this patient subpopulation.
Author Contributions
Conceptualization, Szymon Włodarczak, Piotr Rola and Adrian Włodarczak; Data curation, Szymon Włodarczak, Artur Jastrzębski, Piotr Włodarczak and Jan Jakub Kulczycki; Formal analysis, Szymon Włodarczak, Andrzej Korda, Jan Jakub Kulczycki and Łukasz Furtan; Funding acquisition, Karol Turkiewicz, Andrzej Korda, Mateusz Barycki and Maciej Lesiak; Investigation, Szymon Włodarczak, Piotr Rola, Artur Jastrzębski, Karol Turkiewicz, Andrzej Korda, Jan Jakub Kulczycki, Łukasz Furtan and Adrian Włodarczak; Methodology, Szymon Włodarczak, Piotr Rola, Adrian Włodarczak and Maciej Lesiak; Project administration, Szymon Włodarczak and Artur Jastrzębski; Resources, Szymon Włodarczak, Artur Jastrzębski and Adrian Włodarczak; Software, Karol Turkiewicz, Piotr Włodarczak and Mateusz Barycki; Supervision, Piotr Rola, Artur Jastrzębski, Adrian Włodarczak and Maciej Lesiak; Validation, Szymon Włodarczak, Piotr Włodarczak and Mateusz Barycki; Visualization, Szymon Włodarczak; Writing – original draft, Szymon Włodarczak; Writing – review & editing, Szymon Włodarczak, Piotr Rola, Adrian Włodarczak and Maciej Lesiak.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (Lower Silesian Medical Chamber – number of approval 02/BOBD/2022).
Informed Consent Statement
Informed consent for Coronary Sinus Reducer implantation procedure was obtained from all subjects involved in the study additionally patients consent to participate in study was waived due to local ethical board statement due to a nature of this study (prospective, observational, anonymized).
Data Availability Statement
All data not included in the manuscript are available after contacting the corresponding author in accordance with local legal regulations.
Acknowledgments
The authors are grateful to all the staff and the patients at the study centers who contributed to this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Povsic, T.J.; Henry, T.D.; Ohman, E.M. , et al. Therapeutic Approaches for the No-Option Refractory Angina Patient. Circ Cardiovasc Interv. 2021, 14, e009002. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Tradewell, M.; Kohl, L.P.; Garberich, R.F.; Traverse, J.H.; Poulose, A.; Brilakis, E.S.; Arndt, T.; Henry, T.D. Revascularization in "no option" patients with refractory angina: Frequency, etiology and outcomes. Catheter Cardiovasc Interv. 2018, 92, 1215–1219. [Google Scholar] [CrossRef]
- Sara, J.D.; Widmer, R.J.; Matsuzawa, Y. , et al. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv 2015, 8, 1445–53. [Google Scholar] [PubMed]
- Gallone, G.; Baldetti, L.; Tzanis, G.; Gramegna, M.; Latib, A.; Colombo, A.; Henry, T.D.; Giannini, F. Refractory Angina: From Pathophysiology to New Therapeutic Nonpharmacological Technologies. JACC Cardiovasc Interv. 2020, 13, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Gallone, G.; Baldetti, L.; Tzanis, G.; Gramegna, M.; Latib, A.; Colombo, A.; Henry, T.D.; Giannini, F. Refractory Angina: From Pathophysiology to New Therapeutic Nonpharmacological Technologies. JACC Cardiovasc Interv. 2020, 13, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Banai, S.; Ben Muvhar, S.; Parikh, K.H.; Medina, A.; Sievert, H.; Seth, A.; Tsehori, J.; Paz, Y.; Sheinfeld, A.; Keren, G. Coronary sinus reducer stent for the treatment of chronic refractory angina pectoris: a prospective, open-label, multicenter, safety feasibility first-in-man study. J Am Coll Cardiol. 2007, 49, 1783–9. [Google Scholar] [CrossRef] [PubMed]
- Konigstein, M.; Giannini, F.; Banai, S. The Reducer device in patients with angina pectoris: mechanisms, indications, and perspectives. Eur Heart J 2018, 39, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, M.; Chatterjee, A.; Acharya, D. Persistent or Recurrent Angina Following Percutaneous Coronary Revascularization. Curr Cardiol Rep. 2022, 24, 1837–1848. [Google Scholar] [CrossRef] [PubMed]
- Fezzi, S.; Ding, D.; Mahfoud, F.; Huang, J.; Lansky, A.J.; Tu, S.; Wijns, W. Illusion of revascularization: does anyone achieve optimal revascularization during percutaneous coronary intervention? Nat Rev Cardiol. 2024. [CrossRef]
- Giannini, F.; Baldetti, L.; Ponticelli, F.; Ruparelia, N.; Mitomo, S.; Latib, A.; Montorfano, M.; Jabbour, R.J.; Aurelio, A.; Ferri, L.; Mangieri, A.; Regazzoli, D.; Ancona, M.; Pagnesi, M.; Faccini, A.; Chieffo, A.; Azzalini, L.; Carlino, M.; Colombo, A. Coronary Sinus Reducer Implantation for the Treatment of Chronic Refractory Angina: A Single-Center Experience. JACC Cardiovasc Interv. 2018, 11, 784–792. [Google Scholar] [CrossRef]
- Verheye, S.; Agostoni, P.; Giannini, F.; Hill, J.M.; Jensen, C.; Lindsay, S.; Stella, P.R.; Redwood, S.; Banai, S.; Konigstein, M. Coronary sinus narrowing for the treatment of refractory angina: a multicentre prospective open-label clinical study (the REDUCER-I study). EuroIntervention. 2021, 17, 561–568. [Google Scholar] [CrossRef]
- Włodarczak, S.; Rola, P.; Jastrzębski, A.; Woitek, F.; Barycki, M.; Furtan, Ł.; Doroszko, A.; Włodarczak, A.; Grygier, M.; Lesiak, M. Coronary Sinus Reducer implantation in refractory angina: Short-term outcomes based on the Lower Silesia Sinus Reducer Registry (LSSRR). Kardiol Pol. 2023, 81, 508–511. [Google Scholar] [CrossRef]
- Silvis, M.J.M.; Dekker, M.; Zivelonghi, C.; Agostoni, P.; Stella, P.R.; Doevendans, P.A.; de Kleijn, D.P.V.; van Kuijk, J.P.; Leenders, G.E.; Timmers, L. The Coronary Sinus Reducer; 5-year Dutch experience. Neth Heart J. 2021, 29, 215–223. [Google Scholar] [CrossRef]
- Konigstein, M.; Ponticelli, F.; Zivelonghi, C.; Merdler, I.; Revivo, M.; Verheye, S.; Giannini, F.; Banai, S. Long-term outcomes of patients undergoing coronary sinus reducer implantation - A multicenter study. Clin Cardiol. 2021, 44, 424–428. [Google Scholar] [CrossRef]
- Ponticelli, F.; Tzanis, G.; Gallone, G.; Baldetti, L.; Mangieri, A.; Colombo, A.; Giannini, F. Safety and efficacy of Coronary Sinus Reducer implantation at 2-year follow-up. Int J Cardiol. 2019, 292, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Ferreira Reis, J.; Brízido, C.; Madeira, S.; Ramos, R.; Almeida, M.; Cacela, D. Coronary sinus Reducer device for the treatment of refractory angina: A multicenter initial experience. Rev Port Cardiol. 2023, 42, 413–420. [Google Scholar] [CrossRef]
- Ponticelli, F.; Giannini, F. Coronary sinus reducer for the treatment of chronic refractory angina pectoris. Future Cardiol. 2022, 18, 523–537. [Google Scholar] [CrossRef]
- Hochstadt, A.; Itach, T.; Merdler, I.; Ghantous, E.; Ziv-Baran, T.; Leshno, M.; Banai, S.; Konigstein, M. Effectiveness of Coronary Sinus Reducer for Treatment of Refractory Angina: A Meta-analysis. Can J Cardiol. 2022, 38, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Mrak, M.; Pavšič, N.; Žižek, D.; Ležaić, L.; Bunc, M. Effect of Coronary Sinus Reducer Implantation on Aerobic Exercise Capacity in Refractory Angina Patients—A CROSSROAD Study. J. Cardiovasc. Dev. Dis. 2023, 10, 235. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; Agewall, S.; Dickstein, K.; Edvardsen, T.; Escaned, J.; Gersh, B.J.; Svitil, P.; Gilard, M.; Hasdai, D.; Hatala, R.; Mahfoud, F.; Masip, J.; Muneretto, C.; Valgimigli, M.; Achenbach, S.; Bax, J.J. ; ESC Scientific Document Group. 41.
- Geovanini, G.R.; Gowdak, L.H.W.; Pereira, A.C.; Danzi-Soares, N.J.; Dourado, L.O.C.; Poppi, N.T.; Cesar, L.A.M.; Drager, L.F.; Lorenzi-Filho, G. OSA and depression are common and independently associated with refractory angina in patients with coronary artery disease. Chest. 2014, 146, 73–80. [Google Scholar] [CrossRef]
- Krantz, D.S.; Whittaker, K.S.; Francis, J.L.; Rutledge, T.; Johnson, B.D.; Barrow, G.; McClure, C.; Sheps, D.S.; York, K.; Cornell, C.; Bittner, V.; Vaccarino, V.; Eteiba, W.; Parashar, S.; Vido, D.A.; Merz, C.N. Psychotropic medication use and risk of adverse cardiovascular events in women with suspected coronary artery disease: outcomes from the Women's Ischemia Syndrome Evaluation (WISE) study. Heart. 2009, 95, 1901–6. [Google Scholar] [CrossRef] [PubMed]
| Baseline characteristics (n=55) |
|---|
| Age |
73.1± 6.9 |
| Male |
48 (87.3%) |
| BMI (kg/m2) |
28.6 ± 4.5 |
| Hypertension |
55 (100%) |
| Dyslipidaemia |
49 (89.1%) |
Diabetes mellitus
|
32 (58.2%)
5 (9.1%) |
| Impaired Glucose Tolerance |
5 (9.1%) |
| Nicotine addiction |
21 (38.2%) |
| Coronary Artery Disease |
55 (100%) |
| Years with CAD diagnosis (Q1-Q3) |
14 (10-20) |
| Ejection Fraction (%) (Q1-Q3) |
55 (45-60) |
Heart Failure
NYHA Class I NYHA Class II
|
13 (23.6%)
1 (1.8%)
12 (21.8%) |
| COPD |
8 (14.5%) |
Atrial fibrillation
|
17 (30.9%)
14 (25.5%)
3 (5.5%) |
| Cerebrovascular events: Stroke/TIA |
6 (10.9%) |
Chronic Renal Disorder
|
11 (20%)
1 (1.8%)
10 (18.2%) |
| Peripheral Arterial Disease |
20 (36.4%) |
| Coronary status |
| Obstructive CAD |
51 (92.7%) |
| Non-obstructive CAD |
4 (7.3%) |
| Microvascular dysfunction |
4 (14.5%) |
| Optimal revascularization status |
32 (58.2%) |
| Disqualified from revascularization |
19 (34.5%) |
Post MI
|
32 (58.2%)
15 (27.3%)
19 (34.5%) |
Post PCI
PCI LM PCI LAD PCI Cx PCI RCA PCI of bypass graft
|
47 (85.5%)
10 (18.2%)
23 (41.8%)
28 (50.9%)
36 (65.5%)
6 (10.9%) |
| Number of DES (Q1-Q3) |
7 (5-9) |
| Post CABG |
32 (58.2%) |
| LIMA-LAD |
27 (49.1%) |
| Ao-LAD |
4 (7.3%) |
| Ao-Dg |
46 (83.6%) |
| Ao-OM |
20 (36.4%) |
| Ao-RCA |
22 (40%) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).