1. Introduction
1.1. The Structure and Function of the Otolithic Receptors
A normally functioning vestibular system is critical for spatial orientation, balance, and steadying gaze while the head is in motion. Unlike the semicircular canals that detect angular acceleration, the otolith organs detect linear displacement with translational movements of the head or tilting against the pull of gravity. This is a key function for sensing gravity as a frame of reference to maintain spatial orientation while interacting with the environment [
1]. Natural or electrical stimulation of otolith organs lead to eye movements and postural changes. The orientation of the sensory epithelium or the macula within each otolith organ allows detection of motion within the same plane. The utricular macula is aligned horizontally and the saccular macula is aligned vertically. The utricular macula has stronger projections to the ocular motor system and the saccular macula has stronger projections to the vestibulospinal system.
The macula of each otolith organ consists of a mix of type I and II hair cell receptors. Type 1 receptors are mainly located in the striola or central zone of the macula and are connected to afferents with irregular resting discharge sensitive to sound and vibration [
2]. Type II receptors are located on either side of the central zone with opposite polarities (i.e., hair cell deflections in opposite directions), and are connected to afferents with regular discharges sensitive to low frequency linear acceleration. This different pattern of stimulation is related to the morphology of the hair cell receptors. Type I receptors have short hair bundles that do not reach the gelatinous layer covering the hair cells, but type II receptors have long projections protruding into the gelatinous layer covered by the crystals of dense otoconia [
3]. Because of their short projections, type I receptors are sensitive to higher frequency stimulation like sound and vibration while type II receptors are more responsive to low frequency linear acceleration or static tilt against the constant pull of gravity because of the high density of otoconia on the hair cell projections (for review see [
2,
3]).
The unique anatomical characteristics of hair cells highlight the significance of otoconia in facilitating the function of type II afferents in particular. These afferents maintain a consistent baseline activity and are sensitive to gravity or low-frequency linear accelerations, forming what is known as the static or sustained otoconial system [
3]. A good example of a test using the sustained system is the response to maintained head tilt. Pathologies that affect otoconial mass such as head trauma or age-related degeneration may also affect the sustained pathway and reduce deflection of hair cell bundles by the pull of gravity [
4,
5,
6]. Studies suggest loss of otoconial mass of about 40% in the utricle and 70% in the saccule at the age of 80 [
7,
8,
9]. No difference, however, is found between type I or type II hair cells on human maculae utriculi or sacculi with aging and both are reduced by about 25% [
10]. According to Rauch et al., the ratio of type I to type II hair cells is about 1.3 in the macula based on 67 temporal bones from 49 individuals (age range from birth to 100 y/o) [
10]. Similarly, Gopen et al., found that the ratio of type I to type II hair cells was about 1.7 in few subjects with an age range of 42 to 96 y/o [
11]. Unlike hair cells in the macula receptors, hair cells in the cristae of the semicircular canals and the cochlea show a reduction of about 40% with aging [
10]. In humans, no specific pathology is associated with the exclusive loss of type II hair cells, although pronounced loss of type II hair cells has been reported in Meniere’s disease and following irradiation of the inner ear in animal models [
12,
13]. The key role of otoconia in vestibular physiology is also shown with mouse models of otoconial loss[
14,
15]. These animals have livelong balance problems despite having normal hair cell morphology.
The overall contribution of otolith receptors to eye movements, postural control and perceptual functions is the basis for clinical testing of otolith function. With such wide range of contributions, it is important to recognize that the functional outcomes of these tests may vary depending on the specific method employed to stimulate the hair cells. In the next section, we review common methods used for clinical evaluation of otolith function and how different aspects of physiology may affect the functional measurements in these tests.
2. Clinical Evaluation of the Otolith Organs
Common clinical tests for evaluation of otolith function include (i) the vestibular evoked myogenic potentials (VEMP) [
16], (ii) the video ocular counter-roll (vOCR) [
17], (iii) measurement of the torsional eye position or ocular torsion using fundus photography [
18], and (iv) the tests of subjective visual vertical (SVV) or horizontal (SVH) for assessment of perceived direction of gravity [
19]. Other less common tests are off-axis rotation (OVAR)[
20,
21] and translational VOR (tVOR) [
22].
As supported by recordings from type I hair cells, VEMP testing is based on activation of hair cells by high frequency air-conducted sound or bone-conducted vibration [
23]. VEMP responses from ocular muscles or oVEMP are used as a measure of utricular function, and responses from cervical muscles or cVEMP are used as a measure of saccular function. The common stimulus frequency for VEMP testing is 500 Hz, which results in selective activation of irregular neurons from the striola. At this frequency, the irregular afferents within the semicircular canals are not usually stimulated by sound or vibration [
23]. Because of the high density of otoconia, type II receptors are more responsive to low frequency linear acceleration or static tilt against the constant pull of gravity. Clinical tests of otolith function that involve static head tilt exploit this property. Tilting the head results in a torsional vestibular ocular reflex (VOR) or ocular counter roll (OCR), during which the eyes roll in the opposite direction of the head tilt. During the movement of the head, a combination of otolith and semicircular canal stimulation results in a torsional nystagmus with the slow phase in the opposite direction of the head tilt. During the static head tilt, however, the OCR is driven primarily by inputs from the utricle [
24].
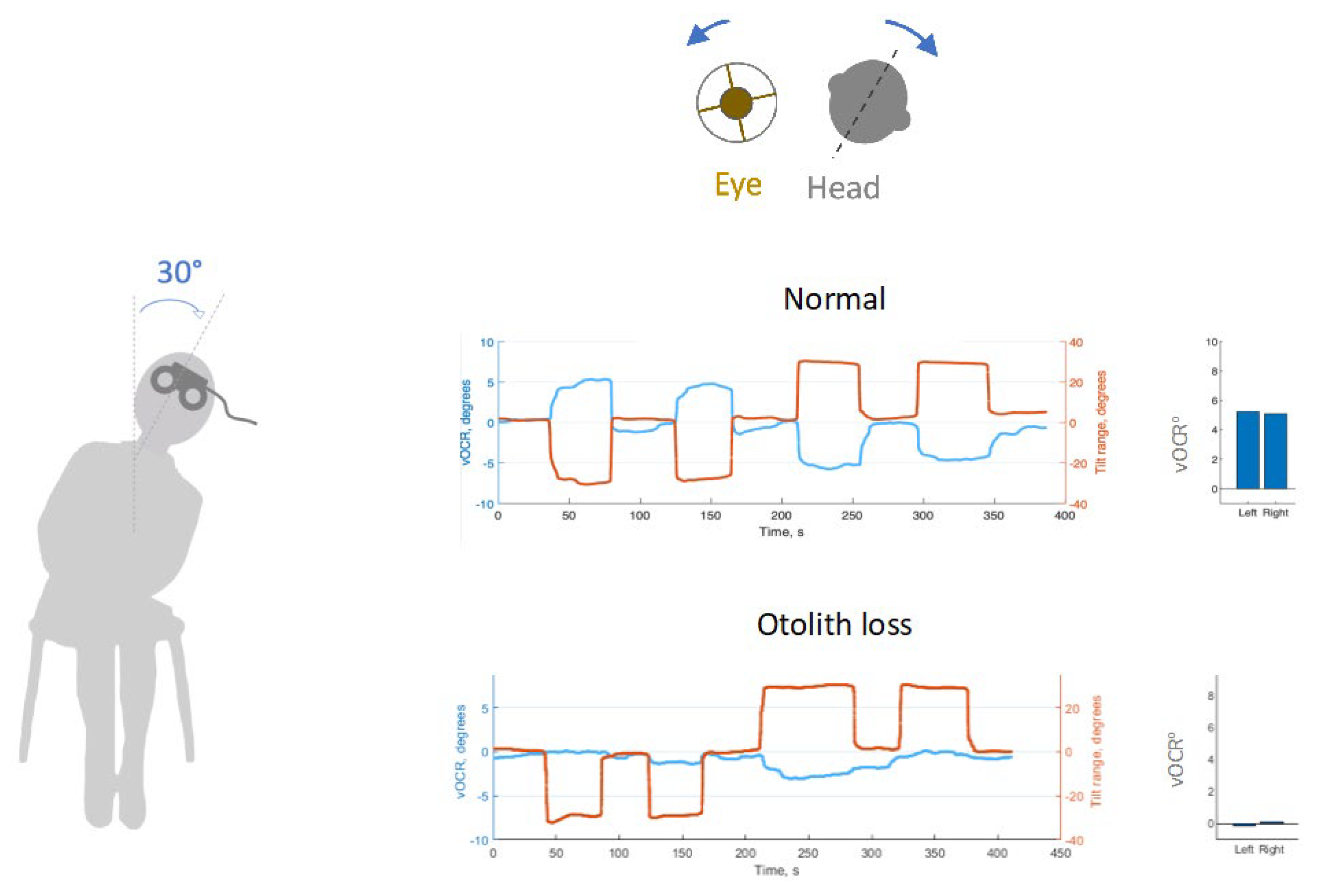
The static OCR has a normal gain (eye position divided by head position) of about 0.15 that can be measured using a video oculographic method known as the vOCR [
17] (
Figure 1).
While the vOCR is a test of torsional VOR, measurement of ocular torsion with the head upright can be used to evaluate otolith-ocular tone balance [
25]. In humans, ocular torsion is the major component of vestibular response during lateral head tilt known as the physiological ocular tilt reaction. This involves rolling of the eyes in the opposite direction of the head tilt to realign with the direction of gravity. Consider a motorcycle rider going around a tight bend to the right. The lateral tilt of the body excites the right utricle resulting in:1) A head tilt to the left, and 2) an ocular counter-roll with the top pole of each eye rotated toward left. and 3) a compensatory skew deviation with upward movement of the lowermost right eye and downward movement of the uppermost left eye. In cases where a pathology leads to utricular tone imbalance, an ocular tilt response may also emerge even with the head upright. This pathological or ‘false’ ocular tilt response consists of the same three components including a lateral head tilt, skew deviation, and pathological ocular-roll or torsional misalignment of the eyes with the top poles rotating toward the side of the lower eye.
The otolith-ocular pathway decussates within the brainstem, and as a result, lesions of the same pathway at different levels can lead to different direction of pathological ocular-roll. In lesions of the utricle and lower brainstem (i.e., prior to decussation), the top pole of each eye is rotated towards the ipsilateral side. Conversely, if the pathway is lesioned in the upper brainstem (i.e., after decussation), the top pole of each eye is rotated toward the contralateral side [
26,
27,
28]. This feature of the pathway is outlined in greater detail in the section on SVV.
The effect of isolated loss of utricular function is also shown in animal models. Unilateral loss of utricular function in guinea pig resulted in strong postural changes at the acute stage with head roll-tilt toward the affected side [
29]. These responses are similar to the ocular tilt reaction with complete unilateral vestibular loss in humans, gradually diminishing with time as recovery and vestibular compensation occur [
30].
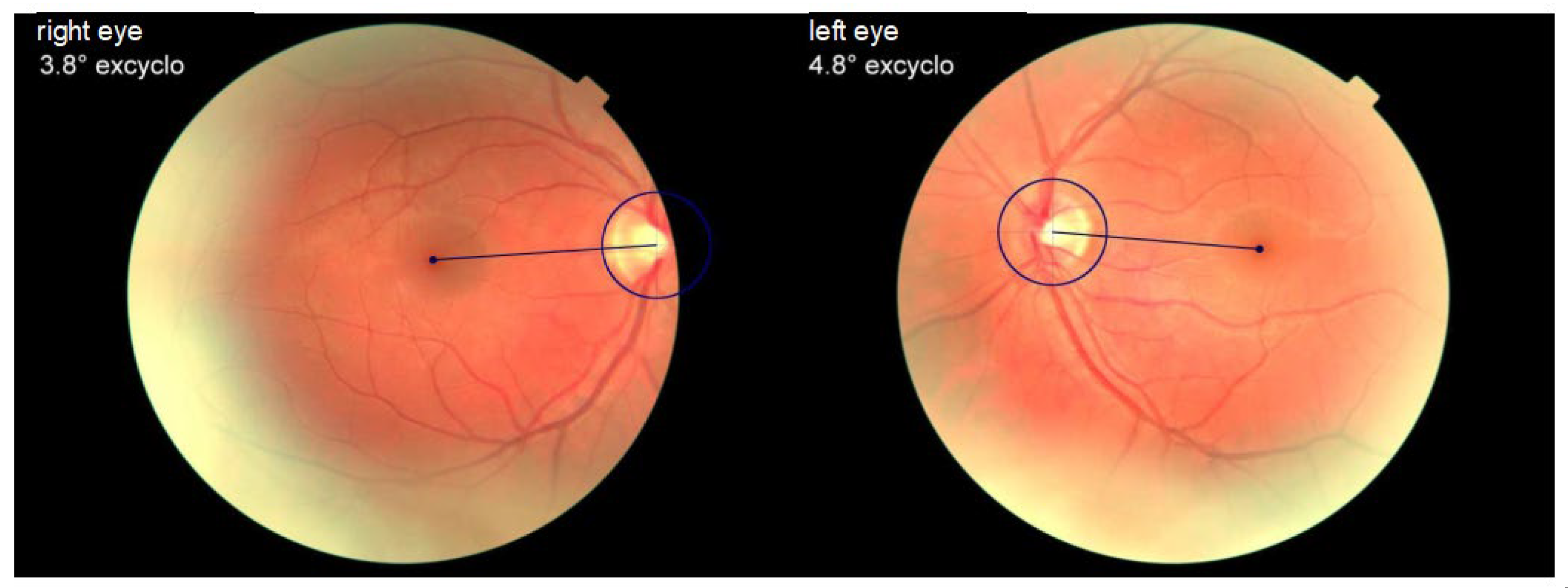
The pathological ocular-roll can be assessed on fundus photos by measuring the angle between the optic disc and fovea (
Figure 2) [
31]. The normative value for the disc foveal angle is within a range of 5-7
o [
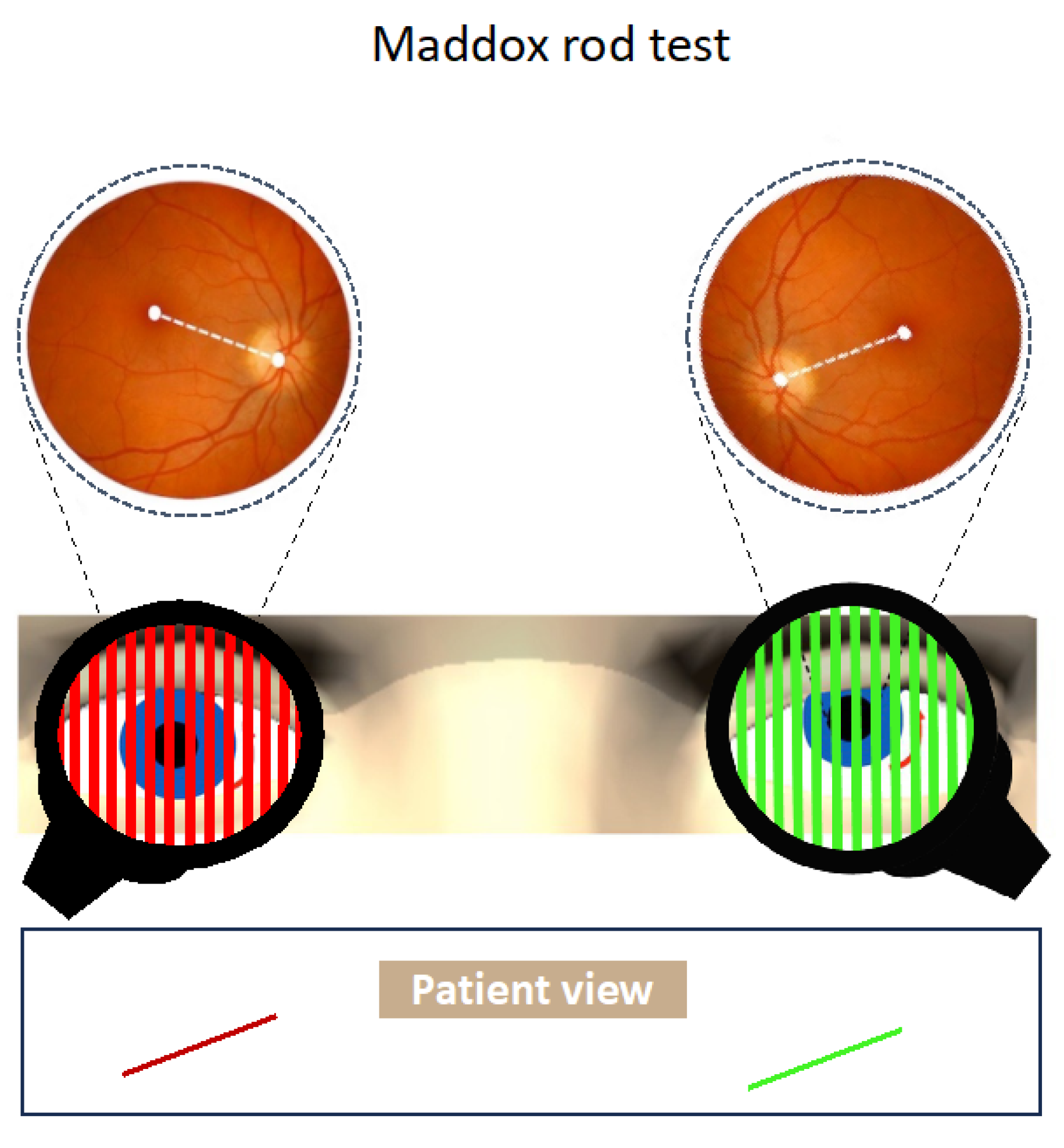
32]. Another method for evaluation of torsional alignment of the eyes is with the use of the double Maddox rod (
Figure 3) [
25]. This test can be easily performed at the bedside for assessment of otolith-ocular tone balance. The Maddox rod is a filter that converts a source of light into a line when held in front of the eye. The visual line orientation seen with each eye can be used to assess torsional alignment of the eyes. The degree of torsional misalignment with the pathological ocular roll is fairly uniform in both eyes (i.e., there is no difference between the two eyes and the deviation is comitant).
The perceptual consequence of the ocular counter roll is shift in the visual vertical (SVV), measured as the angle between their perceptual and gravitational (true) vertical. Consequently, SVV is another sensitive sign of a disturbance in the otolith--ocular pathways. Measurement of SVV can be done reliably and quickly at the bedside using the bucket test [
33]. In this test, the subject places their head inside a bucket to align a straight line visible on the bottom of a bucket to vertical orientation Visual cues are removed as the subject’s head is inside the bucket, and proprioceptive cues are removed as the bucket is randomly rotated to the right or left by the examiner. SVV can also be measured by presenting a visual line on portable devices in a completely dark room. In the upright position, normal individuals can position a visual line in an otherwise completely dark room within two degrees of true vertical [
34,
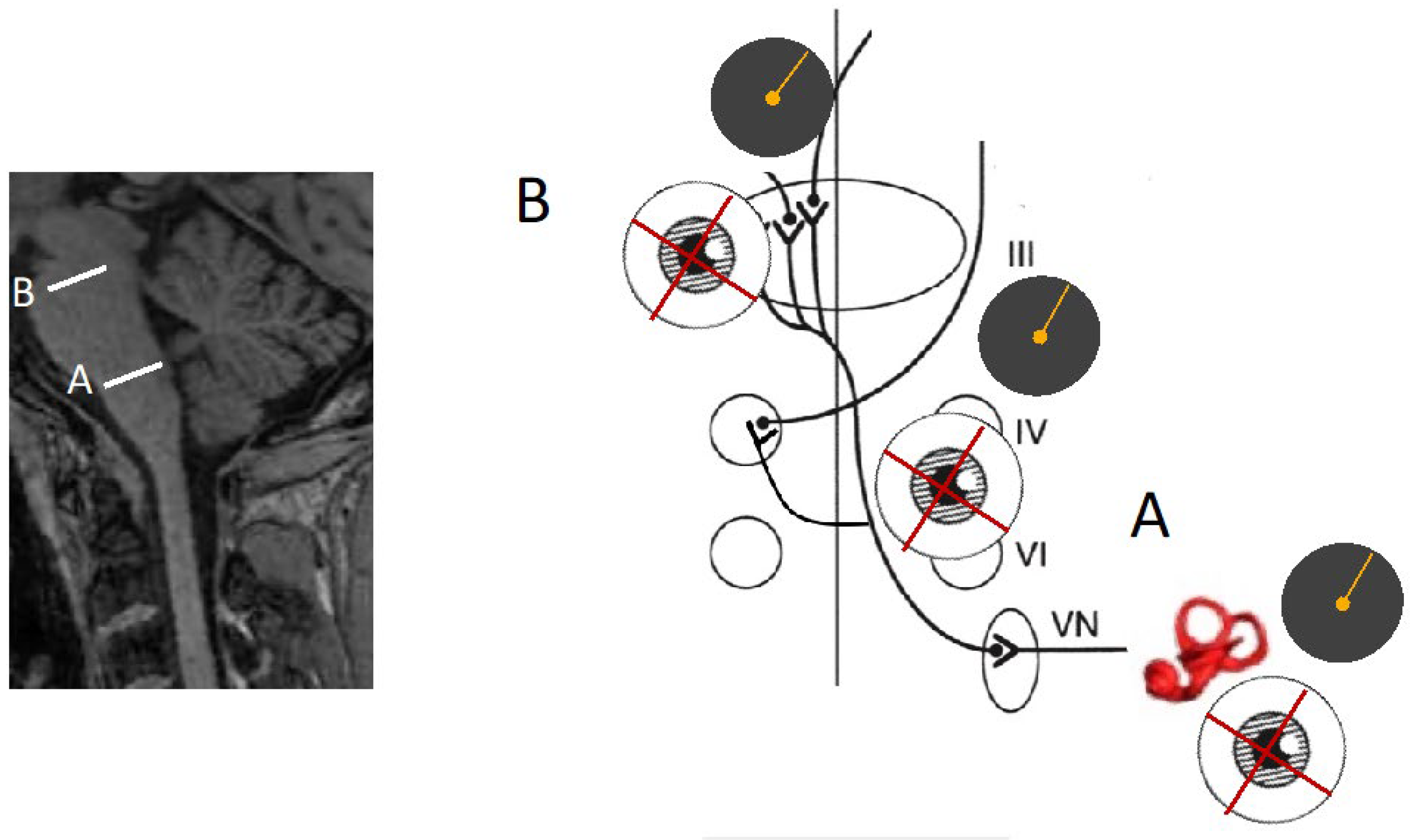
35]. Patients with acute peripheral or central vestibular lesions, however, have subjective vertical that deviates from the true vertical by several degrees. As a topographic rule, lesions affecting the labyrinth or the lower brainstem (caudal pons and rostral medulla) cause ipsilateral SVV deviations and lesions in the higher brainstem (rostral pons and midbrain) cause contralateral SVV deviations (
Figure 4) [
36] . As a measure of spatial orientation in reference to gravity, SVV measurement is also affected by other sensory modalities including vision and body proprioception. Because of such multisensory contribution, SVV deviation and ocular torsion can be dissociated [
37], and thus cannot be a specific test of utricular function. Therefore, while SVV deviation can be caused by vestibular imbalance, pathologies affecting other sensory modalities and their sensory integration may also lead to SVV abnormalities [
19,
38].
3. Comparisons of Clinical Tests of Otolith Function
When comparing different tests of otolith function, one must consider the difference between subjective and objective measurement methos, how they could be specifically affected by multisensory or otolith-specific contributions, and how these tests may vary in stimulation of hair cell populations.
Studies that have compared SVV and VEMP measurements have shown mixed results without clear association between VEMP and perception of gravity in pathologies such as Meniere’s disease or vestibular neuritis, with more discrepancies between acute and chronic patients [
39,
40,
41].These findings are in line with the multisensory nature of SVV compared to VEMP as a more specific and objective measure of otolith function [
42]. Similar dissociation is found between ocular torsion and SVV measurements in patients with vestibular pathologies [
41,
43]. The SVV deviation normalized in most patients within few months of recovery whereas ocular torsion remained abnormal even a year later [
40], suggesting some role for central compensation in patient’s perception of upright.
When analyzed separately, oVEMP and ocular torsion abnormalities were present in about 70% of cases with vestibular neuritis [
39,
41,
44,
45,
46]. However, when measured together in a same group of patients, the ocular torsion and oVEMP abnormalities overlapped in about 50% of cases [
43]. The oVEMP alone was abnormal in 40% of cases, and ocular torsion alone was abnormal alone in about 10% of cases [
43]. This discrepancy could be related to different aspects of utricular function captured differently by oVEMP and ocular torsion measurements. While oVEMP responses mainly reflect the activity of type I hair cells on the utricular macula that are sensitive to sound or vibration, ocular torsion reflects the overall balance of utricular inputs with the torsional deviation pointing towards the weaker side.
The oVEMP response to sound or vibration is primarily transmitted by irregular utricular afferents [
3,
47,
48]. These striolar type 1 hair cells and their irregular afferents are called the transient system, which may remain unaffected in cases with loss of otoconia. While the transient system is preferentially reacting to dynamic stimulation, i. e., high frequency linear acceleration, the sustained system reacts more to stimulation by lower frequencies. As a constant, low frequency acceleration, the pull of gravity is transmitted by the sustained system. Since the vOCR response is generated by the pull of gravity on the utricular macula, it may primarily reflect the function of type II hair cells, whereas the VEMP response would reflect the function of the type I hair cells sensitive to sound and vibration. Such distinction could be valuable to examine differential involvement of the transient and sustained pathways of utricular afferents. As a test of utricular-ocular function, vOCR demonstrates a sensitivity level comparable to VEMP in detecting vestibular loss [
49]. However, unlike VEMP responses, vOCR deficits often recover after vestibular injury [
17,
30,
50]. With acute vestibular loss, vOCR is mainly reduced on the affected side, but there is a symmetrical reduction on both sides with chronic vestibular loss [
49,
50]. In line with this finding, the largest vOCR deficit is observed in bilateral vestibular loss [
49].
4. Conclusions
The overall contribution of otolith receptors to eye movement, postural control and perceptual functions is the basis for clinical testing of otolith function. With such wide range of contributions, it is important to recognize that the functional outcomes of these tests may vary depending on the specific method employed to stimulate the hair cells. Within the otolithic maculae, different neural afferents have different selectivity; type 1 afferents are sensitive to sound and vibration while type II afferents are tuned to low frequency linear acceleration and static head tilt. The type 1 hair cells and their irregular afferents are called the transient system and type II hair cells with their constant baseline activity are called the sustained system. These unique anatomical characteristics of hair cells highlight the significance of otoconia in facilitating the function of type II afferents in sensing the pull of gravity.
Common clinical tests for evaluation of otolith function include VEMP, vOCR, assessment of ocular torsion using fundus photography, and SVV for assessment of perceived vertical. VEMP is an objective test that exploit a low-level reflex, mostly reflecting the function of type I hair cells. The new vOCR test assesses the physiologic ocular counter roll in response to a static head tilt, mostly consistent with type II hair cells and otoconial function. While the vOCR is a test of torsional VOR, measurement of ocular torsion with the head upright can be used to evaluate for otolith-ocular tone imbalance. The perceptual consequence of the ocular counter roll is shift of perceived vertical that can be measured as SVV error. When comparing different tests of otolith function, one must consider the difference between subjective and objective measurement methods, how they may vary in stimulation of hair cell populations, and the results be affected by multisensory or otolith-specific contributions. Further validation of, and comparison of, tests of bedside otolith function is an active area of research.2. Materials and Methods
The Materials and Methods should be described with sufficient details to allow others to replicate and build on the published results. Please note that the publication of your manuscript implicates that you must make all materials, data, computer code, and protocols associated with the publication available to readers. Please disclose at the submission stage any restrictions on the availability of materials or information. New methods and protocols should be described in detail while well-established methods can be briefly described and appropriately cited.
Research manuscripts reporting large datasets that are deposited in a publicly available database should specify where the data have been deposited and provide the relevant accession numbers. If the accession numbers have not yet been obtained at the time of submission, please state that they will be provided during review. They must be provided prior to publication.
Interventionary studies involving animals or humans, and other studies that require ethical approval must list the authority that provided approval and the corresponding ethical approval code.
References
- Smith, P.F. The Growing Evidence for the Importance of the Otoliths in Spatial Memory, Front. Neural Circuits 2019, 13. [Google Scholar] [CrossRef] [PubMed]
- Curthoys, I.S. The new vestibular stimuli: sound and vibration-anatomical, physiological and clinical evidence, Exp Brain Res 2017, 235, 957–972. [CrossRef]
- Curthoys, I.S.; MacDougall, H.G.; Vidal, P.-P.; de Waele, C. Sustained and Transient Vestibular Systems: A Physiological Basis for Interpreting Vestibular Function, Front Neurol 2017, 8, 117. 8. [CrossRef]
- Hegemann, S.C.A.; Weisstanner, C.; Ernst, A.; Basta, D.; Bockisch, C.J. Constant severe imbalance following traumatic otoconial loss: a new explanation of residual dizziness, Eur Arch Otorhinolaryngol 2020, 277, 2427–2435. [CrossRef]
- Hegemann, S.C.A.; Bockisch, C.J. Otoconial loss or lack of otoconia - An overlooked or ignored diagnosis of balance deficits, Med Hypotheses 2019, 128, 17–20. [CrossRef]
- Serrador, J.M.; Lipsitz, L.A.; Gopalakrishnan, G.S.; Black, F.O.; Wood, S.J. LOSS OF OTOLITH FUNCTION WITH AGE IS ASSOCIATED WITH INCREASED POSTURAL SWAY MEASURES, Neurosci Lett 2009, 465, 10–15. [CrossRef]
- Johnsson, L.G. Degenerative changes and anomalies of the vestibular system in man, Laryngoscope 1971, 81, 1682–1694. [CrossRef]
- Ross, M.D.; Peacor, D.; Johnsson, L.G.; Allard, L.F. Observations on normal and degenerating human otoconia, Ann Otol Rhinol Laryngol 1976, 85, 310–326. [CrossRef]
- Ishiyama, G. Imbalance and vertigo: the aging human vestibular periphery, Semin Neurol 2009, 29, 491–499. [CrossRef]
- Rauch, S.D.; Velazquez-Villaseñor, L.; Dimitri, P.S.; Merchant, S.N. Decreasing hair cell counts in aging humans, Ann N Y Acad Sci 2001, 942, 220–227. [CrossRef]
- Gopen, Q.; Lopez, I.; Ishiyama, G.; Baloh, R.W.; Ishiyama, A. Unbiased stereologic type I and type II hair cell counts in human utricular macula, Laryngoscope 2003, 113, 1132–1138. [CrossRef]
- Tsuji, K.; Velázquez-Villaseñor, L.; Rauch, S.D.; Glynn, R.J.; Wall, C.; Merchant, S.N. Temporal bone studies of the human peripheral vestibular system. Meniere’s disease, Ann Otol Rhinol Laryngol Suppl 2000, 181, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Winther, F.O. X-ray irradiation of the inner ear of the guinea pig. Early degenerative changes in the vestibular sensory epithelia, Acta Otolaryngol 1969, 68, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Erway, L.; Hurley, L.S.; Fraser, A.S. Congenital ataxia and otolith defects due to manganese deficiency in mice, J Nutr 1970, 100, 643–654. [CrossRef]
- Paffenholz, R.; Bergstrom, R.A.; Pasutto, F.; Wabnitz, P.; Munroe, R.J.; Jagla, W.; Heinzmann, U.; Marquardt, A.; Bareiss, A.; Laufs, J.; Russ, A.; Stumm, G.; Schimenti, J.C.; Bergstrom, D.E. Vestibular defects in head-tilt mice result from mutations in Nox3, encoding an NADPH oxidase, Genes Dev 2004, 18, 486–491. [CrossRef]
- Curthoys, I.S.; Manzari, L. Otolithic disease: clinical features and the role of vestibular evoked myogenic potentials, Semin Neurol 2013, 33, 231–237. [CrossRef]
- Yang, Y.; Tian, J.; Otero-Millan, J.; Kheradmand, A. Video ocular counter roll: A bedside test of otolith-ocular function, Annals of Clinical and Translational Neurology n/a (n.d.). [CrossRef]
- Otero-Millan, J.; Roberts, D.C.; Lasker, A.; Zee, D.S.; Kheradmand, A. Knowing what the brain is seeing in three dimensions: A novel, noninvasive, sensitive, accurate, and low-noise technique for measuring ocular torsion, J Vis 2015, 15,. [CrossRef]
- Kheradmand, A.; Winnick, A. Perception of Upright: Multisensory Convergence and the Role of Temporo-Parietal Cortex, Front Neurol 2017, 8,. [CrossRef]
- Furman, J.M.; Schor, R.H.; Schumann, T.L. Off-vertical axis rotation: a test of the otolith-ocular reflex, Ann Otol Rhinol Laryngol 1992, 101, 643–650. [CrossRef]
- Kingma, H. Clinical testing of the statolith-ocular reflex, ORL J Otorhinolaryngol Relat Spec 1997, 59, 198–208. [CrossRef]
- Kessler, P.; Tomlinson, D.; Blakeman, A.; Rutka, J.; Ranalli, P.; Wong, A. The high-frequency/acceleration head heave test in detecting otolith diseases, Otol. Neurotol. 2007, 28, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Curthoys, I.S.; Vulovic, V.; Burgess, A.M.; Cornell, E.D.; Mezey, L.E.; Macdougall, H.G.; Manzari, L.; McGarvie, L.A. The basis for using bone-conducted vibration or air-conducted sound to test otolithic function, Ann N Y Acad Sci 2011, 1233, 231–241. [CrossRef]
- De Graaf, B.; Bos, J.E.; Groen, E. Saccular impact on ocular torsion, Brain Res Bull 1996, 40, 321–326; discussion 326-330. [CrossRef]
- Kheradmand, A.; Zee, D.S. The bedside examination of the vestibulo-ocular reflex (VOR): an update, Rev. Neurol. (Paris) 2012, 168, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, M.C.; Donahue, S.P.; Vaphiades, M.; Brandt, T. ; Skew deviation revisited; Ophthalmol, S. 2006, 51, 105–128. [CrossRef]
- Halmagyi, G.M.; Curthoys, I.S.; Brandt, T.; Dieterich, M. Ocular tilt reaction: clinical sign of vestibular lesion, Acta Otolaryngol Suppl 1991, 481, 47–50. [CrossRef]
- Shah, M.; Primiani, C.T.; Kheradmand, A.; Green, K.E. Pearls & Oy-sters: Vertical Diplopia and Ocular Torsion: Peripheral vs Central Localization, Neurology 2022, 99, 212–215. [CrossRef]
- De Waele, C.; Graf, W.; Josset, P.; Vidal, P.P. A radiological analysis of the postural syndromes following hemilabyrinthectomy and selective canal and otolith lesions in the guinea pig, Exp Brain Res 1989, 77, 166–182. [CrossRef]
- Yang, Y.; Tian, J.; Otero-Millan, J.; Schubert, M.C.; Kheradmand, A. Video Ocular Counter-Roll (vOCR): Otolith-Ocular Function and Compensatory Effect of the Neck Following Vestibular Loss, Otolaryngol Head Neck Surg (2023). [CrossRef]
- Kang, H.; Lee, S.J.; Shin, H.J.; Lee, A.G. Measuring ocular torsion and its variations using different nonmydriatic fundus photographic methods, PLoS One 2020, 15, e0244230. [CrossRef]
- Lee, H.J.; Lim, K.H. The Range of Ocular Torsion in Mass Screening. Journal of the Korean Ophthalmological Society 2005, 46, 1684–1689. [Google Scholar]
- Zwergal, A.; Rettinger, N.; Frenzel, C.; Dieterich, M.; Brandt, T.; Strupp, M. A bucket of static vestibular function, Neurology 2009, 72, 1689–1692. [CrossRef]
- Howard, I.P.; Orientation, H.V. ; Wiley; York, N. 1982.
- Van Beuzekom, A.D.; Van Gisbergen, J.A.M. Properties of the internal representation of gravity inferred from spatial-direction and body-tilt estimates, Journal of Neurophysiology 2000, 84, 11–27.
- Brandt, T.; Dieterich, M. Vestibular syndromes in the roll plane: topographic diagnosis from brainstem to cortex, Ann Neurol 1994, 36, 337–347. [CrossRef]
- Otero-Millan, J.; Kheradmand, A. Upright Perception and Ocular Torsion Change Independently during Head Tilt, Front Hum Neurosci 2016, 10,. [CrossRef]
- Barra, J.; Marquer, A.; Joassin, R.; Reymond, C.; Metge, L.; Chauvineau, V.; Pérennou, D. Humans use internal models to construct and update a sense of verticality, Brain 2010, 133, 3552–3563. [CrossRef]
- Choi, J.W.; Kang, S.I.; Rhee, J.H.; Choi, B.Y.; Kim, J.-S.; Koo, J.-W. Clinical implication of ocular torsion in peripheral vestibulopathy, Eur Arch Otorhinolaryngol 2015, 272, 1613–1617. [CrossRef]
- Faralli, M.; Ricci, G.; Manzari, L.; Zambonini, G.; Lapenna, R.; Pettorossi, V.E. Different time course of compensation of subjective visual vertical and ocular torsion after acute unilateral vestibular lesion, Eur Arch Otorhinolaryngol 2021, 278, 2269–2276. [CrossRef]
- Choi, K.-D.; Oh, S.-Y.; Kim, H.-J.; Koo, J.-W.; Cho, B.M.; Kim, J.S. Recovery of vestibular imbalances after vestibular neuritis, Laryngoscope 2007, 117, 1307–1312. [CrossRef]
- Böhmer, A. The subjective visual vertical as a clinical parameter for acute and chronic vestibular (otolith) disorders, Acta Otolaryngol 1999, 119, 126–127. [CrossRef]
- Hösli, S.; Straumann, D. Independent Measures of Utricular Function: Ocular Vestibular Evoked Myogenic Potentials Do Not Correlate With Subjective Visual Vertical or Fundus Photographic Binocular Cyclorotation, Front. Neurol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-A.; Hong, J.-H.; Lee, H.; Yi, H.-A.; Lee, S.-R.; Lee, S.-Y.; Jang, B.-C.; Ahn, B.-H.; Baloh, R.W. Otolith dysfunction in vestibular neuritis: recovery pattern and a predictor of symptom recovery, Neurology 2008, 70, 449–453. [CrossRef]
- Magliulo, G.; Gagliardi, S.; Appiani, M.C.; Iannella, G.; Re, M. Vestibular Neurolabyrinthitis: A Follow-Up Study With Cervical and Ocular Vestibular Evoked Myogenic Potentials and the Video Head Impulse Test, Ann Otol Rhinol Laryngol 2014, 123, 162–173. [CrossRef]
- Taylor, R.L.; McGarvie, L.A.; Reid, N.; Young, A.S.; Halmagyi, G.M.; Welgampola, M.S. Vestibular neuritis affects both superior and inferior vestibular nerves, Neurology 2016, 87, 1704–1712. [CrossRef]
- Curthoys, I.S.; Kim, J.; McPhedran, S.K.; Camp, A.J. Bone conducted vibration selectively activates irregular primary otolithic vestibular neurons in the guinea pig, Exp Brain Res 2006, 175, 256–267. [CrossRef]
- Taylor, R.L.; Wise, K.J.; Taylor, D.; Chaudhary, S.; Thorne, P.R. Patterns of vestibular dysfunction in chronic traumatic brain injury, Front Neurol 2022, 13, 942349. [CrossRef]
- Otero-Millan, J.; Treviño, C.; Winnick, A.; Zee, D.S.; Carey, J.P.; Kheradmand, A. The video ocular counter-roll (vOCR): a clinical test to detect loss of otolith-ocular function, Acta Otolaryngol 2017, 137, 593–597. [CrossRef]
- Sadeghpour, S.; Fornasari, F.; Otero-Millan, J.; Carey, J.P.; Zee, D.S.; Kheradmand, A. Evaluation of the Video Ocular Counter-Roll (vOCR) as a New Clinical Test of Otolith Function in Peripheral Vestibulopathy, JAMA Otolaryngology–Head & Neck Surgery (2021). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).