Introduction
The outcome of SARS-CoV-2 infection in patients is highly variable. As such, a wide spectrum of prognosis is observed in patients, and tissue damage and organ failure of the lungs, heart, and kidneys may be seen, leading to an unopposed multi-systemic inflammatory reaction in some patients [
1]. SARS-CoV-2 infection may cause acute respiratory failure but also remains responsible for many other pathologies, including electrolyte disorders [
2,
3,
4].
Changes in plasma sodium concentration are determined by changes in water balance, independent of the total body sodium amount. The two basic mechanisms responsible for regulating water metabolism are the antidiuretic hormone and the feeling of thirst [
5]. While SARS-CoV-2 infection can cause multiple organ failure, it can also cause dysfunction of the renin-angiotensin-aldosterone system and disrupt water hemostasis with thirst and appetite abnormalities [
6]. Various electrolyte disorders were observed in SARS-CoV2 patients, but hypo-hypernatremia was particularly striking. Severe hyponatremia alone may be the leading cause of death or cause of permanent neurological changes. The most important risk factors for death in patients presenting with hyponatremia were found to be hypoxia and sepsis [
7]. Hypernatremia is usually caused by either a deficit of total body water or by an inappropriately high sodium input. In general, however, even during infusion of large amounts of sodium-containing solutions (as during treatment of acute hypovolemia), hypernatremia is infrequently observed and less pronounced.
HOPE is an international study registry of 4664 hospitalized SARS-CoV-2 infection patients. In this study, 20.5% of patients were reported to have hyponatremia, and hypernatremia was observed in 3.7% of patients [
8]. Proinflammatory cytokines such as IL-1b and IL-6 are known to stimulate hypothalamic arginine vasopressin secretion [
9,
10,
11]. Supporting this, the study by Berni et al. reported that IL-6 levels were inversely proportional to serum sodium levels. The coexistence of hyponatremia and elevation of IL-6 levels has been shown in SARS-CoV2 patients [
12]. Therefore, it can be predicted that dysnatremia affects prognosis and may be associated with mortality in patients admitted to SARS-CoV2 intensive care with SARS-CoV-2.
The aim of this study is to determine the relationship of dysnatremia with prognosis and mortality in SARS-CoV-2 intensive care patients. Patients were classified as hyponatremic (Blood sodium level <135 mmol/L), eunatremic (Blood sodium level 135–145 mmol/L), and hypernatremic (Blood sodium level >145 mmol/L) on admission to intensive care unit (ICU).
Patients and Method
After the study protocol was approved by Ankara Ataturk Sanatorium Training and Research Hospital Ethics Committee (Ethical Decision No: E-53610172-799-206667331 dated 11.01.2023), patients whose admissions were made between April 12, 2021, and March 1, 2022, were retrospectively scanned from the hospital database and patient files. The time period was chosen as the intensive care unit was solely assigned to COVID-19 patients during the mentioned period.
The study included patients admitted to the ICU who were over 18 years old and diagnosed with SARS-CoV-2 infection by clinical and thoracic tomography findings or with a positive reverse transcription polymerase chain reaction (RT-PCR) test result.
Demographic data of the patients, body mass index, additional comorbidities if present, and laboratory sampling results at the time of admission were the main evaluated data. The laboratory markers included routine blood count, liver and renal function tests, inflammatory markers, and routine testing performed for COVID-19, which included ferritin, procalcitonin, LDH, and d-dimer levels. Treatment modalities in the ICU were also recorded, consisting of nutritional support requirements and types, COVID-19 treatment regimens, inotropic support, glucocorticoid treatment regimens, and respiratory support requirements. For outcome evaluation, length of stay in the ICU, total hospitalization duration, and mortality in ICU were recorded.
The laboratory marker comparison was made with the final assessment performed before the time of exitus or ICU discharge. Patients under the age of 18, who have PCR (-), and whose clinical and thoracic tomography findings are not suggestive of SARS-CoV-2 infection were excluded from the study. Those who had been re-evaluated at the ICU for other possible diagnoses and were later transferred to other ICUs, such as those admitted with clinical suspicion, were also removed from the study.
Statistical evaluation
The patients’ results were put into a Microsoft Excel file for overall evaluation. After investigating any mis-input and values, the data were moved to a statistics module (IBM Version 25th for Windows). The initial assessment was performed by descriptive analysis, for which values were given with mean and standard deviation or with median and percentiles as required. Parametric distribution was evaluated using a Q-Q plot analysis. Paired sample T-test comparisons were made between groups for parametric values. Correlation analyses were performed by Spearman correlation. Binomial regression analysis was performed to evaluate the role of any parameters as an independent factor. P values at or below 0.05 were accepted as statistically significant.
Results
A total of 209 patients were included in the study. The majority of the patients were male (n=114, 54.5%). The average age of the patients was 68.1 (±13.8) years, and the body mass index (BMI) was found to be 26.6 (±2.6). A median of 5 (2-10) days was observed between RT-PCR positivity and hospital admission. The median duration of total hospitalization, days in ICU, days on invasive mechanical ventilation (IMV), and non-invasive mechanical ventilation (NIMV) were reported to be 11 (5-18), 6 (2-12), 1 (0-6) and 2 (1-5) days, respectively. 78 patients (37.3%) showed culture positivity regarding additional bacterial involvement. Diabetes mellitus (n=66, 31.6%) and hypertension (n=86, 41.1%) were the most observed comorbidities. Regarding nutritional support requirements, 20 (9.6%) patients required total parenteral support, while nearly half of the patients (n=110, 52.6%) had enteral support requirements.
Favipiravir (n=102, 48.8%) and intravenous glucocorticoid regimens were the mainstay of the treatment given in ICU. Inotropic requirements among patients were high (n=124, 59.3%), which was reflected in the first-month mortality (n=124, 59.3%) (
Table 1).
Laboratory parameters comparison made at admission and last evaluation had shown that, in routine blood count, hemoglobin, white blood cell, lymphocyte and platelets had changed significantly (13.03 g/dl to 12.05 g/dl, p=0.001; 13.33 109/L to 15.06 109/L, p=0.002; 0.96 109/L to 1.25 109/L, p=0.001 and 258.23 109/L to 218.51 109/L, p=0.001 respectively). A treatment response favoring reduced inflammatory markers was also observed in C-reactive protein and sedimentation evaluation (127.22 mg/L to 92.3 mg/L, p=0.001 and 60.44 to 50.47, p=0.001, respectively). Ferritin, d-dimer, and procalcitonin levels did not show significant change.
Lactate dehydrogenase (LDH), creatinine, aspartate aminotransferase (AST) and alanine transaminase (ALT) were other parameters found to be statistically different (612.72 U/L to 877.96 U/L, p=0.01; 1.17 mg/dl to 1.43 mg/dl, p=0.001; 141 U/L to 308 U/L, p=0.041 and 109 U/L to 198 U/L, p=0.038 respectively). Sodium value also varied from the initial admission result to the last evaluation, with a mean of 139 mEq/L to 142 mEq/L and a p-score of 0.001 (
Table 2).
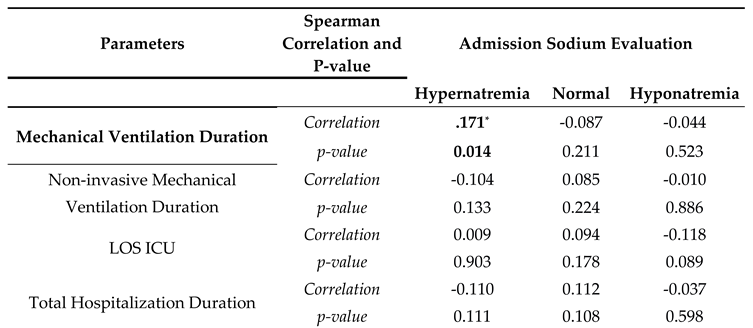
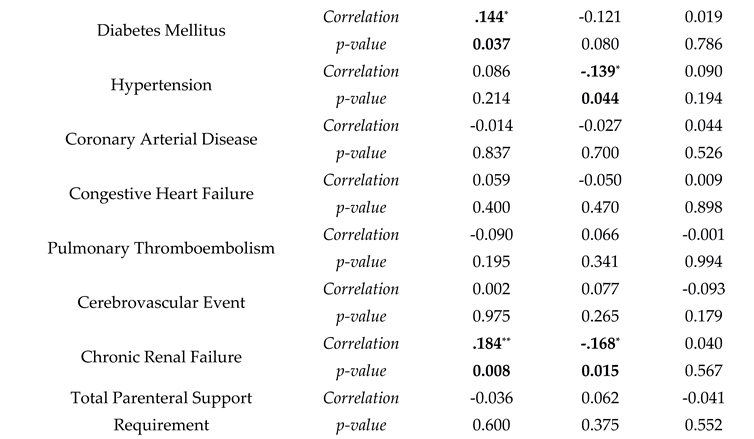
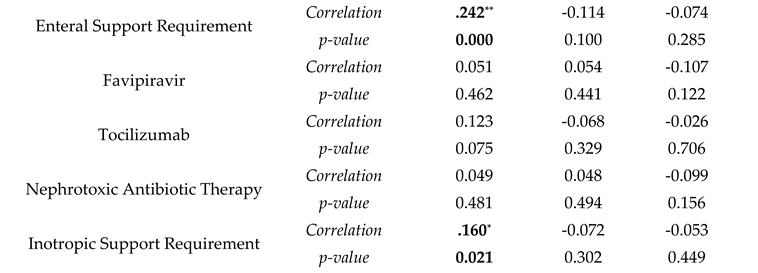
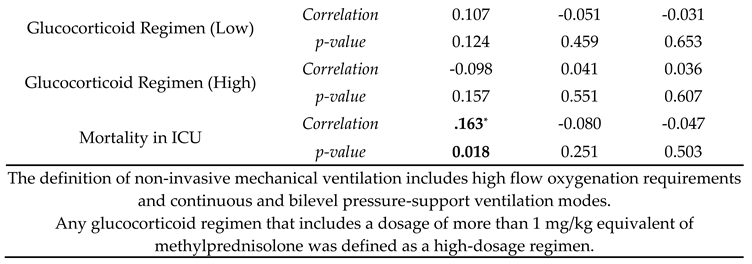
Correlation analyses were made with sodium levels being divided into three categories: hyponatremia, normal, and hypernatremia. Hypernatremia was correlated with diabetes mellitus, chronic renal failure, and a longer duration under mechanical ventilation ( r(209)=0.144, p=0.037; r(209)=0.184, p=0.008; r(209)=0.171, p=0.014 respectively). Hypertension and chronic renal failure were also negatively correlated with normal blood sodium levels (r(209)=-0.139, p=0.044 and r(209)=-0.168, p=0.015). For treatment correlation, hypernatremia presence was associated with an increase in enteral support and inotropic support requirement and mortality (r(209)=0.242, p=0.001 and r(209)=0.161, p=0.001). Hyponatremia did not have any correlation with comorbidities and treatment modalities (
Table 3).
Regression analyses were performed for the role of hypernatremia, with three models for mortality, enteral support, and inotropic support. All models had a value higher than 0.5 for the Hosmer and Lemeshow test (0.106, 0.085, and 0.053, respectively). The models’ Nagelkerke R square results were 0.706, 0.870, and 0.666, with each model correctly classifying at least 60% of the given data. Hypernatremia’s role in first-month mortality and inotropic support was not statistically significant in the regression analyses (p values of 0.339 and 0.417, respectively). However, the regression analysis was significant when hypernatremia and enteral support were evaluated (p:0.031) (
Table 4,
Table 5 and
Table 6).
Discussion
The study had included an adequate count of patients, with a varying range of NIMV and IMV requirements. Comorbidities were within expected ranges of what would be observed in an elderly patient group. Increased inotropic support requirement and elevated mortality support the assumption of patients being in a severe status and thus requiring ICU stay. The change in routine blood count, in favor of lessened inflammatory markers, was in favor of an overall treatment response, which was also present in CRP and sedimentation levels. An elevation of renal and liver function tests, on the other hand, leads to the presumption that patients were in an organ failure status. A change was evident in sodium levels, which could be attributed to the given enteral and parenteral support.
Regarding sodium level evaluation, hypernatremia’s correlation with a longer mechanical ventilation duration and renal failure could be attributed to the increased length of nutritional support. This was further strengthened by the correlation present between hypernatremia and enteral support. The same correlation was also present between inotropic support requirement and mortality. Thus, patients in a more severe condition who had required additional respiratory and cardiac support had a predisposition to hypernatremia. However, when evaluated by regression analysis, hypernatremia was not significant in terms of mortality and inotropic support, yet it maintained its significance in terms of enteral support. Unlike hypernatremia in our study, Hyponatremia was not observed as a significant risk factor.
Aggarwal et al., in a study performed in the USA, reported 50% of patients had hyponatremia [
13]. Similarly, in the HOPE study, 20.5% of the patients had reported hyponatremia, with hypernatremia being reported at 3.7%, and both statuses being defined as independent risk factors for mortality and sepsis in patients hospitalized with SARS-CoV2 pneumonia. This was a different finding compared to our study, in which hypernatremia was the predominant risk factor. Zimmel et al. stated similar findings to those in our study performed with 12 patients, with hypernatremia being related to a longer duration under mechanical ventilation and overall ICU duration [
14].
In the HOPE study done by Ruiz-Sanchez et al., patients admitted to the ICU with pneumonia had an overall disposition to hyponatremia compared to hypernatremia upon admission. This was attributed to the syndrome of inappropriate antidiuretic hormone secretion (SIADH) [
8]. Cuesta et al. had confirmed this observation only in the half of the patients [
15]. Although the mechanisms are not precise, tachypnea was independently associated with hyponatremia as well as hypernatremia, in which tachypnea contributed to insensitive body fluid loss. The fluid loss was further exacerbated by reduced overall oral intake, which worsened the situation [
16]. As mentioned in Cuesta’s study, Khann et al. had reported that hyponatremia could not be totally explained by SIADH, and other factors could play a role in patients with COVID-19 infection [
8,
17]. Yousaf et al. defined many factors contributing to SIADH by affecting the activation of secondary pathways of ADH, such as interleukin-6 [
18].
The limitation of the study mainly could be stated that the patients’ type of hyponatremia and hypernatremia was unknown, as the exact volume given to the patients was not stated, and the volume status of the patients was not present in the study design. Dysnatrmeia, similarly, could not be entirely excluded, as the glucose status of the patients was present in the same sampling type. However, repeated sampling of glucose was not performed for confirmation.
Our study had validated that hypernatremia was an important risk factor in ICU patients hospitalized for SARS-CoV-2 infection, which was also affected by the treatment regimens given itself. This complex relationship underlies the importance of proper electrolyte management, especially in patients who were under severe stress and organ failure.
Author Contributions
All of the authors declare that they have all participated in the concept, design, literature search, data collection, analyses, writing and that they have approved the final version. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics Committee Approval
Ethical Decision No: E-53610172-799-206667331 dated 11.01.2023, Ankara Ataturk Sanatorium Traning and Research Hospital, Clinical Research Ethics Committee.
Compliance with Ethical Standards
All the authors mentioned in the manuscript have agreed for authorship, read and approved the manuscript, and given consent for submission and subsequent publication of the manuscript. The manuscript in part or in full has not been submitted or published anywhere.
Data Access Statement
All relevant data are within the paper and its Supporting Information files.
Acknowledgments
We would like to thank all healthcare professionals who contributed to the SARS-CoV-2 pandemic, which was a difficult process.
Conflicts of Interest
The authors have declared that no competing interests exist.
References
- Martinez, A.F.; Galindo, D.B.; Sanchez, J.R. Management of hyponatraemia and hypernatraemia during the Covid-19 pandemic: a consensus statement of the Spanish Society for Endocrinology (Acqua Neuroendocrinology Group). Rev. Endocr. Metab. Disord. 2021, 22, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Richardson S, Hirsch JS, Narasimhan M, Crawford, J. M., McGinn, T., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–59.
- Lippi, G.; South, A.M.; Henry, B.M. Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19). Ann. Clin. Biochem. 2020, 57, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Bhave, G. , & Neilson, E. G. Body fluid dynamics: back to the future. Journal of the American Society of Nephrology. 2011; 22(12): 2166-81.
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Nzerue, C.M.; Baffoe-Bonnie, H.; You, W.; Falana, B.; Dai, S. Predictors of outcome in hospitalized patients with severe hyponatremia. J Natl Med Assoc. 2003, 95, 335–43. [Google Scholar] [PubMed]
- Ruiz-Sanchez, J.G.; Nunez-Gil, I.J.; Cuesta, M.; Rubio, M.A.; Maroun-Eid, C.; Arroyo-Espliguero, R.; Romero, R.; Becerra-Munoz, V.M.; Uribarri, A.; Feltes, G.; et al. Prognostic Impact of Hyponatremia and Hypernatremia in COVID-19 Pneumonia. A HOPE-COVID-19 (Health Outcome Predictive Evaluation for COVID-19) Registry Analysis. Front. Endocrinol. 2020, 11, 599255. [Google Scholar] [CrossRef] [PubMed]
- Swart, R.M.; Hoorn, E.J.; Betjes, M.G.; Zietse, R. Hyponatremia and Inflammation: The Emerging Role of Interleukin-6 in Osmoregulation. Nephron Physiol. 2010, 118, p45–p51. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, R.; Neumann, I.; Holsboer, F.; Pittman, Q.J. Interleukin-1β Stimulates both Central and Peripheral Release of Vasopressin and Oxytocin in the Rat. Eur. J. Neurosci. 1995, 7, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Mastorakos, G.; Weber, J.S.; A Magiakou, M.; Gunn, H.; Chrousos, G.P. Hypothalamic-pituitary-adrenal axis activation and stimulation of systemic vasopressin secretion by recombinant interleukin-6 in humans: potential implications for the syndrome of inappropriate vasopressin secretion. J. Clin. Endocrinol. Metab. 1994, 79, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Berni A, Malandrino D, Parenti G, Maggi M, Poggesi L, Peri A. Hyponatremia, IL-6, and SARS-CoV-2 (COVID-19) infection: may all fit together? J Endocrinol Invest, 2020;43:1137–9. [CrossRef]
- Aggarwal S, Garcia-Telles N, Aggarwal G, Lavie C, Lippi G, Henry BM. Clinical features, laboratory characteristics, and out comes of patients hospitalized with coronavirus disease 2019 (COVID-19): early report from the United States. Diagn Berl Ger. 2020;7(2):91–6.
- Zimmer, M.A.; Zink, A.K.; Weißer, C.W.; Vogt, U.; Michelsen, A.; Priebe, H.-J.; Mols, G. Hypernatremia—A Manifestation of COVID-19: A Case Series. A&A Pr. 2020, 14, e01295. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, M.; Slattery, D.; Goulden, E.L.; Gupta, S.; Tatro, E.; Sherlock, M.; Tormey, W.; O'Neill, S.; Thompson, C.J. Hyponatraemia in patients with community-acquired pneumonia; prevalence and aetiology, and natural history of SIAD. Clin. Endocrinol. 2019, 90, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Rong, L.; Nian, W.; He, Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment. Pharmacol. Ther. 2020, 51, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Ata, F.; Munir, W.; Yousaf, Z. Fluid Replacement Versus Fluid Restriction in COVID-19 Associated Hyponatremia. Cureus 2020, 12, e9059. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, Z.; Al-Shokri, S.D.; Al-Soub, H.; Mohamed, M.F.H. COVID-19-associated SIADH: a clue in the times of pandemic! Am. J. Physiol. Endocrinol. Metab. 2020, 318, E882–E885. [Google Scholar] [CrossRef] [PubMed]
Table 1.
Demographic Parameters, Comorbidities, and Treatment Modalities.
Table 1.
Demographic Parameters, Comorbidities, and Treatment Modalities.
| Demographic Parameters and Treatment Duration |
No of Patients (n=209) |
| Gender |
Male (%)
|
114 (54.5) |
| Female (%)
|
95 (45.5) |
| Age (years, SD)
|
68.15 (±13.81) |
| Body Mass Index (SD) |
26.66 (±2.66) |
| RT-PCR Positivity to Admission (Days, 25th-75th) |
5 (2-10) |
| Mechanical Ventilation Duration (Days, 25th-75th) |
1 (0-6) |
| Non-invasive Mechanical Ventilation Duration (Days, 25th-75th) |
2 (1-5) |
| LOS ICU (Days, 25th-75th) |
6 (2-12) |
| Total Hospitalization Duration (Days, 25th-75th) |
11 (5-18) |
| Culture Positivity (%) |
78 (37.3) |
| Comorbidities |
N, % |
| Diabetes Mellitus |
66 (31.6) |
| Hypertension |
86 (41.1) |
| Coronary Arterial Disease |
24 (11.5) |
| Congestive Heart Failure |
14 (6.7) |
| Pulmonary Thromboembolism |
10 (4.8) |
| Cerebrovascular Event |
7 (3.3) |
| Chronic Renal Failure |
3 (1.4) |
| Nutritional and Respiratory Support |
N, % |
| Total Parenteral Support Requirement |
20 (9.6) |
| Enteral Support Requirement |
110 (52.6) |
| Treatment Modalities and Overall Mortality |
N, % |
| Favipiravir |
102 (48.8) |
| Tocilizumab |
18 (8.6) |
| Nephrotoxic Antibiotic Therapy |
47 (22.5) |
| Inotropic Support Requirement |
124 (59.3) |
| Intravenous Glucocorticoid Requirement (Low) |
81 (38.8) |
| Intravenous Glucocorticoid Requirement (High) |
112 (53.6) |
| Mortality in ICU |
124 (59.3) |
SD: Standard deviation, RT-PCR: Reverse transcription polymerase chain reaction
The definition of non-invasive mechanical ventilation includes high flow oxygenation requirements and continuous and bilevel pressure-support ventilation modes.
The definition of culture positivity includes any positive bacterial and viral result taken from a patient upon intensive care unit admission, regardless of sampling origin.
Any glucocorticoid regimen that includes a dosage of more than 1 mg/kg equivalent of methylprednisolone was defined as a high-dosage regimen. |
Table 2.
Comparison Between Laboratory Parameters Upon Admission and Last Evaluation.
Table 2.
Comparison Between Laboratory Parameters Upon Admission and Last Evaluation.
| Paired Samples T-Test |
Sampling Time |
Mean |
SD |
t |
dF |
p |
| Sodium (mEq/L)
|
Admission |
139.44
|
6.80
|
-5.057
|
208
|
0.001 |
| Last Evaluation |
142.41
|
8.69
|
| Hemoglobin (g/dL)
|
Admission |
13.03
|
2.81
|
7.669
|
176
|
0.001 |
| Last Evaluation |
12.05
|
2.92
|
| White Blood Cell (109/L)
|
Admission |
13.33
|
7.09
|
-3.125
|
176
|
0.002 |
| Last Evaluation |
15.06
|
7.86
|
| Lymphocyte (109/L)
|
Admission |
0.96
|
1.71
|
-3.906
|
176
|
0.001 |
| Last Evaluation |
1.25
|
1.91
|
| Neutrophil (109/L)
|
Admission |
12.51
|
9.19
|
-1.229
|
176
|
0.221
|
| Last Evaluation |
13.38
|
7.39
|
| Platelets (109/L)
|
Admission |
258.23
|
119.17
|
4.618
|
176
|
0.001 |
| Last Evaluation |
218.51
|
128.36
|
| Ferritin (ng/ml)
|
Admission |
844.93
|
569.92
|
-1.516
|
176
|
0.131
|
| Last Evaluation |
898.79
|
588.70
|
| D-Dimer (mg/L)
|
Admission |
8.793
|
24.663
|
0.635
|
172
|
0.526
|
| Last Evaluation |
7.680
|
11.169
|
| Procalcitonin (ng/ml)
|
Admission |
3.8
|
13.5
|
-0.767
|
170
|
0.444
|
| Last Evaluation |
4.8
|
15.6
|
| Creatinine Kinase (U/L)
|
Admission |
196.07
|
287.86
|
-1.427
|
166
|
0.155
|
| Last Evaluation |
349.61
|
1410.65
|
| LDH (U/L)
|
Admission |
612.72
|
677.78
|
-2.597
|
174
|
0.010 |
| Last Evaluation |
877.96
|
1496.24
|
| Glomerular Filtration Rate |
Admission |
70.48
|
29.25
|
1.128
|
175
|
0.261
|
| Last Evaluation |
68.33
|
35.19
|
| Creatinine (mg/dL)
|
Admission |
1.17
|
0.83
|
-3.257
|
174
|
0.001 |
| Last Evaluation |
1.43
|
1.27
|
| Potassium (mEq/L)
|
Admission |
4.34
|
0.71
|
0.703
|
175
|
0.483
|
| Last Evaluation |
4.29
|
0.99
|
| AST (U/L)
|
Admission |
141.68
|
671.52
|
-2.061
|
175
|
0.041 |
| Last Evaluation |
308.71
|
943.93
|
| ALT (U/L)
|
Admission |
109.11
|
567.28
|
-2.086
|
173
|
0.038 |
| Last Evaluation |
189.24
|
641.40
|
| C-Reactive Protein (mg/L)
|
Admission |
127.22
|
86.89
|
5.112
|
174
|
0.001 |
| Last Evaluation |
92.30
|
82.57
|
| Albumin (g/L)
|
Admission |
28.30
|
5.23
|
1.600
|
169
|
0.112
|
| Last Evaluation |
26.74
|
13.09
|
| Sedimentation |
Admission |
60.44
|
29.04
|
3.773
|
155
|
0.001 |
| Last Evaluation |
50.47
|
33.00
|
SD: Standard deviation, LDH: Lactate dehydrogenase, AST: Aspartate aminotransferase, ALT: Alanine transaminase
The last evaluation time period includes the final testing performed before intensive care discharge or the last testing performed before exit. |
Table 3.
Correlation Between Admission Sodium Status, Treatment Modalities and Comorbidities.
Table 3.
Correlation Between Admission Sodium Status, Treatment Modalities and Comorbidities.
Table 4.
Binominal Regression Analysis between Hypernatremia and Mortality.
Table 4.
Binominal Regression Analysis between Hypernatremia and Mortality.
| Mortality in ICU |
B |
SE |
Wald |
Odds Ratio |
p-value |
| Constant |
-7.213
|
5.885
|
1.502
|
|
|
| Age |
0.052
|
0.026
|
3.959
|
1.053
|
0.047 |
| Body Mass Index |
0.211
|
0.093
|
5.214
|
1.235
|
0.022 |
| Gender |
-1.375
|
0.643
|
4.581
|
0.253
|
0.032 |
| Total Parenteral Support Requirement |
2.222
|
1.292
|
2.960
|
9.226
|
0.085
|
| Enteral Support Requirement |
2.293
|
0.694
|
10.923
|
9.906
|
0.001 |
| MV Duration |
0.135
|
0.233
|
0.334
|
1.144
|
0.563
|
| NIMV Duration |
0.040
|
0.236
|
0.028
|
1.040
|
0.867
|
| Intensive Care Admission Duration |
-0.128
|
0.229
|
0.311
|
0.880
|
0.577
|
| Diabetes Mellitus |
0.168
|
0.717
|
0.055
|
1.183
|
0.815
|
| Hypertension |
0.036
|
0.608
|
0.003
|
1.036
|
0.953
|
| Coronary Arterial Disease |
1.312
|
0.897
|
2.142
|
3.714
|
0.143
|
| Congestive Heart Failure |
0.354
|
1.061
|
0.111
|
1.424
|
0.739
|
| Pulmonary Thromboembolism |
0.280
|
1.431
|
0.038
|
1.322
|
0.845
|
| Cerebrovascular Event |
-2.934
|
1.976
|
2.204
|
0.053
|
0.138
|
| Glucocorticoid Requirement (Low) |
-2.080
|
1.230
|
2.860
|
0.125
|
0.091
|
| Glucocorticoid Requirement (High) |
-1.454
|
1.231
|
1.395
|
0.234
|
0.238
|
| Favipiravir |
0.382
|
0.581
|
0.431
|
1.465
|
0.512
|
| Hypernatremia |
0.882
|
0.923
|
0.914
|
2.416
|
0.339
|
| Hemoglobin |
-0.129
|
0.092
|
1.986
|
0.879
|
0.159
|
| White Blood Cell |
-0.020
|
0.051
|
0.160
|
0.980
|
0.689
|
| Platelets |
-0.001
|
0.003
|
0.275
|
0.999
|
0.600
|
| Ferritin |
0.001
|
0.001
|
4.746
|
1.001
|
0.029 |
| D-Dimer |
0.031
|
0.021
|
2.175
|
1.032
|
0.140
|
| Procalcitonin |
-0.002
|
0.025
|
0.006
|
0.998
|
0.938
|
| C-Reactive Protein |
0.009
|
0.004
|
6.422
|
1.009
|
0.011 |
| Sedimentation |
-0.011
|
0.010
|
1.202
|
0.989
|
0.273
|
| AST |
-0.007
|
0.004
|
2.248
|
0.993
|
0.134
|
| ALT |
0.010
|
0.008
|
1.588
|
1.011
|
0.208
|
SE: Standard Error, MV: Mechanical Ventilation, NIMV: Non-invasive mechanical ventilation
AST: Aspartate aminotransferase, ALT: Alanine transaminase. |
Table 5.
Binominal Regression Analysis between Hypernatremia and Enteral Support Requirement.
Table 5.
Binominal Regression Analysis between Hypernatremia and Enteral Support Requirement.
| Enteral Support Requirement |
B |
SE |
Wald |
Odds Ratio |
p-value |
| Constant |
-25.917
|
11.194
|
5.360
|
|
|
| Age |
0.061
|
0.050
|
1.497
|
1.062
|
0.221
|
| Body Mass Index |
0.283
|
0.156
|
3.293
|
1.328
|
0.070
|
| Gender |
-1.005
|
1.132
|
0.789
|
0.366
|
0.375
|
| NIMV Duration |
-1.704
|
0.369
|
21.291
|
0.182
|
0.001 |
| Intensive Care Admission Duration |
1.850
|
0.412
|
20.204
|
6.359
|
0.001 |
| Diabetes Mellitus |
0.431
|
1.009
|
0.183
|
1.539
|
0.669
|
| Hypertension |
0.995
|
1.247
|
0.637
|
2.704
|
0.425
|
| Coronary Arterial Disease |
2.442
|
1.497
|
2.660
|
11.499
|
0.103
|
| Congestive Heart Failure |
3.891
|
2.235
|
3.031
|
48.946
|
0.082
|
| Pulmonary Thromboembolism |
0.084
|
2.367
|
0.001
|
1.087
|
0.972
|
| Cerebrovascular Event |
-2.799
|
2.456
|
1.299
|
0.061
|
0.254
|
| Glucocorticoid Requirement (Low) |
-1.554
|
2.366
|
0.431
|
0.211
|
0.511
|
| Glucocorticoid Requirement (High) |
1.137
|
2.249
|
0.255
|
3.116
|
0.613
|
| Favipiravir |
0.619
|
1.058
|
0.342
|
1.857
|
0.559
|
| Hypernatremia |
4.153
|
1.923
|
4.662
|
63.602
|
0.031 |
| Hemoglobin |
-0.017
|
0.165
|
0.010
|
0.984
|
0.920
|
| White Blood Cell |
0.026
|
0.118
|
0.049
|
1.026
|
0.825
|
| Platelets |
-0.008
|
0.006
|
1.727
|
0.992
|
0.189
|
| Ferritin |
0.001
|
0.001
|
2.581
|
1.001
|
0.108
|
| D-Dimer |
0.009
|
0.010
|
0.881
|
1.010
|
0.348
|
| Procalcitonin |
-0.009
|
0.038
|
0.059
|
0.991
|
0.808
|
| C-Reactive Protein |
0.004
|
0.005
|
0.478
|
1.004
|
0.489
|
| Sedimentation |
0.002
|
0.016
|
0.018
|
1.002
|
0.894
|
| AST |
-0.001
|
0.010
|
0.006
|
0.999
|
0.938
|
| ALT |
0.011
|
0.020
|
0.337
|
1.011
|
0.562
|
|
SE: Standard Error, MV: Mechanical Ventilation, NIMV: Non-invasive mechanical ventilation AST: Aspartate aminotransferase, ALT: Alanine transaminase. |
Table 6.
Binominal Regression Analysis between Hypernatremia and Inotropic Support Requirement.
Table 6.
Binominal Regression Analysis between Hypernatremia and Inotropic Support Requirement.
| Inotropic Support Requirement |
B |
SE |
Wald |
Odds Ratio |
p-value |
| Constant |
-6.640
|
5.954
|
1.244
|
|
| Age |
0.054
|
0.028
|
3.815
|
1.055
|
0.051
|
| Body Mass Index |
0.226
|
0.098
|
5.267
|
1.253
|
0.022 |
| Gender |
-1.667
|
0.705
|
5.585
|
0.189
|
0.018 |
| Total Parenteral Support Requirement |
2.710
|
1.404
|
3.727
|
15.033
|
0.054
|
| Enteral Support Requirement |
3.128
|
0.815
|
14.738
|
22.832
|
0.001 |
| MV Duration |
1.232
|
0.575
|
4.593
|
3.429
|
0.032 |
| NIMV Duration |
1.124
|
0.564
|
3.967
|
3.076
|
0.046 |
| Intensive Care Admission Duration |
-1.230
|
0.569
|
4.664
|
0.292
|
0.031 |
| Diabetes Mellitus |
-0.072
|
0.758
|
0.009
|
0.930
|
0.924
|
| Hypertension |
-0.173
|
0.659
|
0.069
|
0.841
|
0.793
|
| Coronary Arterial Disease |
1.154
|
0.935
|
1.525
|
3.172
|
0.217
|
| Congestive Heart Failure |
-0.019
|
1.125
|
0.000
|
0.981
|
0.987
|
| Pulmonary Thromboembolism |
-0.030
|
1.533
|
0.000
|
0.971
|
0.985
|
| Cerebrovascular Event |
-3.651
|
2.086
|
3.062
|
0.026
|
0.080
|
| Glucocorticoid Requirement (Low) |
-2.244
|
1.232
|
3.317
|
0.106
|
0.069
|
| Glucocorticoid Requirement (High) |
-1.835
|
1.239
|
2.193
|
0.160
|
0.139
|
| Favipiravir |
0.460
|
0.625
|
0.541
|
1.584
|
0.462
|
| Hypernatremia |
0.785
|
0.968
|
0.659
|
2.193
|
0.417
|
| Hemoglobin |
-0.114
|
0.095
|
1.427
|
0.892
|
0.232
|
| White Blood Cell |
-0.060
|
0.057
|
1.128
|
0.941
|
0.288
|
| Platelets |
0.002
|
0.003
|
0.246
|
1.002
|
0.620
|
| Ferritin |
0.002
|
0.001
|
7.481
|
1.002
|
0.006 |
| D-Dimer |
0.029
|
0.021
|
1.870
|
1.029
|
0.172
|
| Procalcitonin |
-0.017
|
0.024
|
0.531
|
0.983
|
0.466
|
| C-Reactive Protein |
0.009
|
0.004
|
5.879
|
1.009
|
0.015 |
| Sedimentation |
-0.006
|
0.011
|
0.314
|
0.994
|
0.575
|
| AST |
-0.005
|
0.005
|
1.230
|
0.995
|
0.267
|
| ALT |
0.007
|
0.009
|
0.669
|
1.007
|
0.413
|
|
SE: Standard Error, MV: Mechanical Ventilation, NIMV: Non-invasive mechanical ventilation AST: Aspartate aminotransferase, ALT: Alanine transaminase. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).