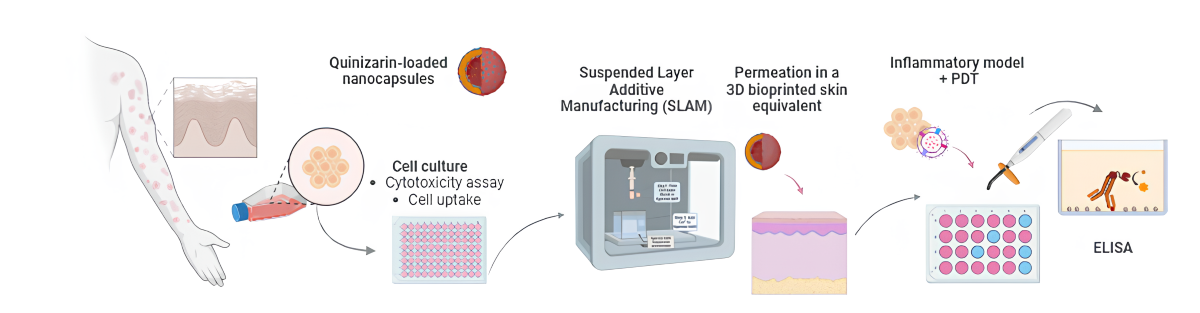
1. Introduction
The skin is the largest organ in the human body. It performs vital protective functions and has three layers: the epidermis, dermis and the subcutaneous tissue. The epidermis is the outermost layer and is mainly made up of keratinocytes (95%), as well as cells such as melanocytes, Langerhans cells and Merkel cells. Keratinocytes are fundamental cells in the skin's inflammatory process and related immune responses, interacting with immune system cells by releasing cytokines and chemokines [
1,
2,
3].
Epithelial cells are influenced by intrinsic genetic factors, as well as by the environment and the microbiome, which can have a direct influence on immune responses to potential allergens and pathogens. During an inflammatory response, keratinocytes release pro-inflammatory cytokines (TNF-α, IL-1α, IL-1β and IL-18. IL-1), inducing the expression of new inflammatory cytokines (IL-6, IL-8 and TNF-α) and adhesion molecules on endothelial cells, leading to the targeting of effector T cells to the site of inflammation, and each type of injury/inflammation leads to different activation and recruitment of the most convenient cell subset. Uncontrolled immune responses can lead to chronic inflammatory diseases such as psoriasis and atopic dermatitis [
4,
5].
Psoriasis, atopic dermatitis and rosacea are the most common chronic skin diseases, characterized by hyperproliferation of keratinocytes, involving multifactorial inflammatory responses linked to highly complex autoimmune responses [
6,
7]. Psoriasis it´s characterized by excessive growth and abnormal differentiation of keratinocytes leading to skin lesions, resulting from inflammatory and angiogenic stimuli, linked to increased expression of immune cells and inflammatory cytokines. Currently 2-4% of the world's population is affected, giving anti-psoriatic drugs a market worth close to 18.8 billion USD in 2021 [
8,
9,
10]. The inflammatory mechanism of psoriasis involves the presence of keratinocytes, leukocytes, T cells, macrophages, neutrophils, mast cells, dendritic, followed by the release of inflammatory cytokines [
11]. Keratinocytes are activated by pro-inflammatory cytokines such as TNF-α and IL-1, which are elevated in psoriatic lesions. Once activated, keratinocytes change their proliferation and differentiation, thickening the epidermis and leading to increased production of chemokines, leading to a positive feedback loop between keratinocytes and immune system cells [
12].
Considering psoriasis treatment, the biggest obstacle is the physiology of the skin, which becomes stiffer and more impermeable to medicines, limiting their bioavailability at the lower epidermis [
10]. The first line of treatment for psoriasis and others chronic skin diseases, consists of topical therapy. If this treatment is no longer effective, it is proceeded with phototherapy and systemic drug administration [
9]. However, these methods are associated with numerous side effects, such as nausea, irritation of the skin, hepatotoxicity, in addition to drug resistance. Combined, these factors define the need for more effective treatments, with greater drug targeting and bioavailability [
13,
14].
Photodynamic Therapy (PDT) is a widely used therapeutic modality, with promising results in patients with various types of superficial skin diseases such as psoriasis [
15]. Contrary to biological agents and other medications, PDT is a powerful and secure technique with no systemic side effects [
16]. The mechanism of action of PDT is based on photochemistry-controlled reactions, which can induce changes in the cellular metabolism, including induction of cell death via apoptosis and/or necrosis based on the cell type, light intensity, and PS type [
9,
17,
18]. One of the biggest challenges of PDT is the choice of the most suitable photosensitizer, which essential characteristics such as biological affinity, biodistribution and minimum photodynamic potential for application [
15]. Quinizarin, (QZ) (1,4 dihydroxyanthraquinone), an anthraquinone derivative compound, shows secondary biological activities characteristics as anti-inflammatory, antioxidant, antibacterial action [
19,
20,
21], and high potential as photosensitizing compounds [
22] showing high-density planar and quinoid conjugated aromatic groups electronics, which may contribute to the generation of reactive oxygen species (ROS), [
23,
24,
25,
26], with a high potential as photosensitizing compounds from a PDT perspective [
22].
However, there are some limitations to the use of Anthraquinones in PDT, as their low solubility in aqueous media, which can cause lack of selectivity in the treatment. Pharmaceutical nanotechnology is a valuable resource, which can significantly influence the use of anthraquinone derivatives in PDT, increasing their permeation at the required site, as well as their controlled and sustained release [
27]. Among the main colloidal nanocarriers with potential application, the polymeric and emulsified systems come in focus, especially polymeric nanocapsules (NC). They are mostly composed of biodegradable polymers, biocompatible, avoiding toxicity, and presenting high efficiency of encapsulation [
28]. In addition, the polymeric wrapping present in nanocapsules preserves the drug from the degradative effects of factors such as light, oxygen, and the acidic stomach environment, and controls the release of the internalized active into the biological targets of interest [
29].
The development of new therapies has been underpinned for years by two-dimensional culture (monolayer) or animal models, but limitations linked to reliability and reproducibility still exist. The concept of Tissue Engineering has evolved rapidly in this context, opening space for three-dimensional printing, an automated and highly efficient technology for creating porous and complex structures, capable of reliable systems for drug testing and disease modeling [
30]. Technologies, such as the Suspended layer additive manufacture (SLAM) method, aim to overcome the limitations of 3D bioprinting cell-laden constructs. The technology presents a solution for creating hydrogels with defined properties, making it a valuable tool for recapitulating in vivo interactions, mimicking the complexity of real tissues [
31,
32,
33].
The present study proposes the development and characterization of a 2D model of inflammatory process in human keratinocyte cells for the application of Photodynamic Therapy using polymeric quinizarin-loaded nanocapsules, an anthraquinone derivative with secondary biological activities, evaluating the joint action of nanotechnology and PDT in a new therapeutic modality, combined with Tissue Engineering.
2. Materials and Methods
2.1. Development of Polymeric Nanoparticles Loaded with Quinizarin
Polymeric NCs (QZ/NC) were prepared using the nanoprecipitation method as described by Mora-Huertas et al. [
24] with modifications by Siqueira-Moura et al. [
34], at the concentration of 0.1 mg.mL
-1 Quinizarin. The coating co-polymer poly (L-lactic acid-co-glycolic acid) - PLGA 50:50 (0.75% w/v) and lecithin (high purity soy phosphatidylcholine) at 1.75% m/v were dissolved in 15 mL of acetone at 40 °C (characterizing the organic phase) and slowly added by dripping at the rate of 1.0 g.s
−1, under the aqueous solution, containing Pluronic F-68 (1.25% m/v) in 30 mL of water under moderate and constant stirring (250 rpm) at 40°C. The emulsification and interfacial coating process was started after phase mixing directly in a 250 mL jacketed reactor coupled to a thermostatic bath under constant circulation. Subsequently, the organic solvent was removed to finalize the nanoemulsification process, through the route-evaporation step at 40 °C and 225 rpm rotation under reduced pressure, obtaining a final formulation volume of 10 mL.
2.2. Characterization of Particle Size, Polydispersity Index (PdI) and Zeta Potential
The physicochemical analyses were performed following the standard operating procedures of the equipment ZetasizerTM, model Nano ZS90 Malvern, obtaining parameters of Particle Size, Polydispersity Index (PdI) and Zeta Potential.
2.3. Atomic Force Microscopy
Atomic force microscopy allows the topographical surface of nanomaterials to be analyzed. Images of the QZ/NC were taken at room temperature using a Shimadzu Scanning Probe Microscope model SPM-9600. The sample was prepared on a mica surface, where a drop (5.0 µL) of the formulation was deposited. The images were obtained using the intermittent contact method, with a 124 µm long cantilever, operating at a resonance frequency in the range of 324-369 KHz, rigidity of 34-51 N/m and constant force.
2.4. 3D Fluorescence Emission Spectroscopy UV/Vis
The 3D fluorescence emission spectra of QZ in acetonitrile and after nanoencapsulation (QZ/NC) were obtained as part of the spectroscopic characterization. Analyses were carried out using a fluorimeter (Shimadzu model RF-6000) with the following parameters: emission wavelength (525 - 685nm); excitation wavelength (400 - 520nm); data interval: 2nm; excitation band: 1.5nm and emission band 3nm.
2.5. Cell Culture
The dermal fibroblast (HDFs – ATCCTM PCS-201-010™), adipose-derived stem cells (ADSCs – Thermo Fisher Scientific R7788115), human epidermal keratinocytes (NHEKs - Thermo Fisher Scientific C0015C), Immortalized human keratinocyte (HaCaT- Cell line service) and commercial murine fibroblast (NIH/3T3 - ATCCTM CRL-1658™) cell lines were cultivated using the standard cultivation protocol. The cell lines were cultured in an incubator with 5% CO2 at 37 °C, and the culture was conducted until the confluence stage was reached, where the cells were used for the subsequent assays.
2.6. Cellular Uptake
Immortalized human keratinocyte lineage cells (HaCaT - cell line service) were plated in 24-well plates (3x104 cells.well−1) and maintained at 37 °C in an atmosphere containing 5% CO2 for 24h. Subsequently, QZ/NC were placed at a concentration of 15 µg.mL-1 at three different times (3h, 6h and 24) in order to analyze the best time for cell internalization. After the mentioned times, the respective plates were quantified using the EnSpireTM microplate reader (PerkinElmer, USA) where the fluorescence intensity was analyzed.
2.7. Cytotoxicity Assay Using Resazurin Test
NIH/3T3 and HaCaT cells were seeded into a 96-wells plate (5 × 10
3 cells.well
−1), after 24 h was added free QZ and QZ/NC in range concentration of 2.5−70 μg.mL
−1 using mean DMEM for dilutions and incubated for 3 h for NIH/3T3 and 24h for HaCaT as suggested by ISO 10993-5. After 24 h of treatment, 20 μL of Resazurin solution (25 μg.mL
−1 in PBS) and 180 mL of DMEM were added and incubated for 4 h. Afterwards the wells analyses were carried out in the microplate reader EnSpire
TM (PerkinElmer, USA) in 570 nm and the basal absorbance was corrected in 590 nm. The percentage of viable cells was calculated following the Eq. (1).
2.8. Permeation Study in a New 3D Bioprinted Skin Equivalent: Suspended layer Additive Manufacturing (SLAM) Method
The 3D skin equivalents, and the printing media were prepared as described by Moakes et al [
33] following the method of suspended layer additive manufacturing. The layers were prepared from a blend of collagen to pectin: papillary layer (2:1 blend containing 3x10
6 cells.mL
-1 (HDFs—passage 8); reticular layer (2:1 blend containing 1.5x10
6 cells.mL
-1 (HDFs—passage 7) and 1:1 blend containing 5x10
5 cells.mL
-1 (ADCSs—passage 3). The construct's G-code was sent to the printing program to print a tri-layer with the following dimensions with a cylindrical shape: A=225mm²; H=1.2mm in a 0.5% w/v Agarose fluid gel support bath. Subsequently, the constructs were gelled through the addition of a 200 mmol.L
−1 CaCl
2(aq) solution to the support bath, followed by a 30-min incubation at 37
OC. The constructs were then transferred into clean wells for culture at 37 °C and 5% CO
2 with supplemented DMEM medium (10% v/v Fetal Bovine Serum, 1% v/v PenStrep; Sigma-Aldrich, UK) for 14 days. Keratinocyte cells (NHEKs 2.5x10
5 cells.mL
-1 — passage 4
) were placed on top of the constructs and cultured for another 7 days, forming the epidermal layer. The QZ/NC, at a concentration of 15µg.mL
−1, defined as the safety limit, and free QZ (15µg.mL
−1), both suspended in DMEM, were added to the top of the equivalents (80µL), inside a cylindrical support, which ensured that the sample would be concentrated at the top of the construct; a control was also prepared with DMEM only. After 24 hours, cross-sections were made and the samples were analyzed using a SPARK microplate reader (TECAN, SWI) and a Cytation imaging reader (BioTek, USA) where the fluorescence intensity was observed.
2.9. Induction of Inflammatory Process in HaCaT Cells and Application of Photodynamic Therapy
HaCaT cells were used as a biological model for the development of the inflammatory model. The cells were cultured as described in 2.7 and plated in 24-well plates. The cells were induced with bacterial lipopolysaccharide (LPS) at a concentration of 10 µg.mL-1 and incubated for 24h - 37°C, 5% CO2. After the mentioned period, QZ/NC was added at the concentration of 15 µg.mL-1. Subsequently, the cells still with LPS and QZ/NC were submitted to PDT, where a 24-well LED tabletop photoactivation system (IrradLED/Biopdi) emitting at 470 nm with 37.2 mW optical power was used. The energy densities (fluences) used were: 1.0 J.cm-2 (D1), 5.0 J.cm-2 (D2) and 10 J.cm-2 (D3) for the respective exposure times: 27s, 134s and 269s. Two plates were made for post-irradiation analysis.
2.10. Enzyme-Linked Immunosorbent Assay (ELISA)
For in vitro assays, IL-1β, IL-8 and MCP-1 levels of cell supernatants were detected using the commercially available human quantikine ELISA kit (BD Biosciences, California, USA), and according to the manufacturer's instructions, following the protocol previously described by Cardoso et al. [
35]. Briefly, 100 μL of each of the standards, controls, and samples were loaded into 96-well polystyrene microplates wells, containing 100 μL of assay diluent in triplicate. After incubation for 2h, the microplates were washed 4 times. Then, 200 μL of specific conjugate solutions were added to each well for 2h, washed, and 200 μL of a substrate solution was added. After 20 min of incubation, 50 μL of the stop solution stop solution was added and the optical density was read at 450 nm using a microplate reader microplate reader (Molecular Devices, Sunnyvale, CA) with a correction wavelength of 540 nm.
2.11. Statistical Analysis
Statistical analysis was performed using one-way analysis of variance (one-way ANOVA), with Tukey's test (Cytotoxicity) and Dunnett 's test (ELISA) as post-test for multiple comparisons analysis between the groups (*p<0,05; **p<0,001).
3. Results and Discussion
3.1. Physical-Chemical and Two-Dimensional Topography Analysis: Particle Size, PdI, Zeta Potential and AFM
Stability studies are essential for pharmaceutical and industrial application of nanomaterials [
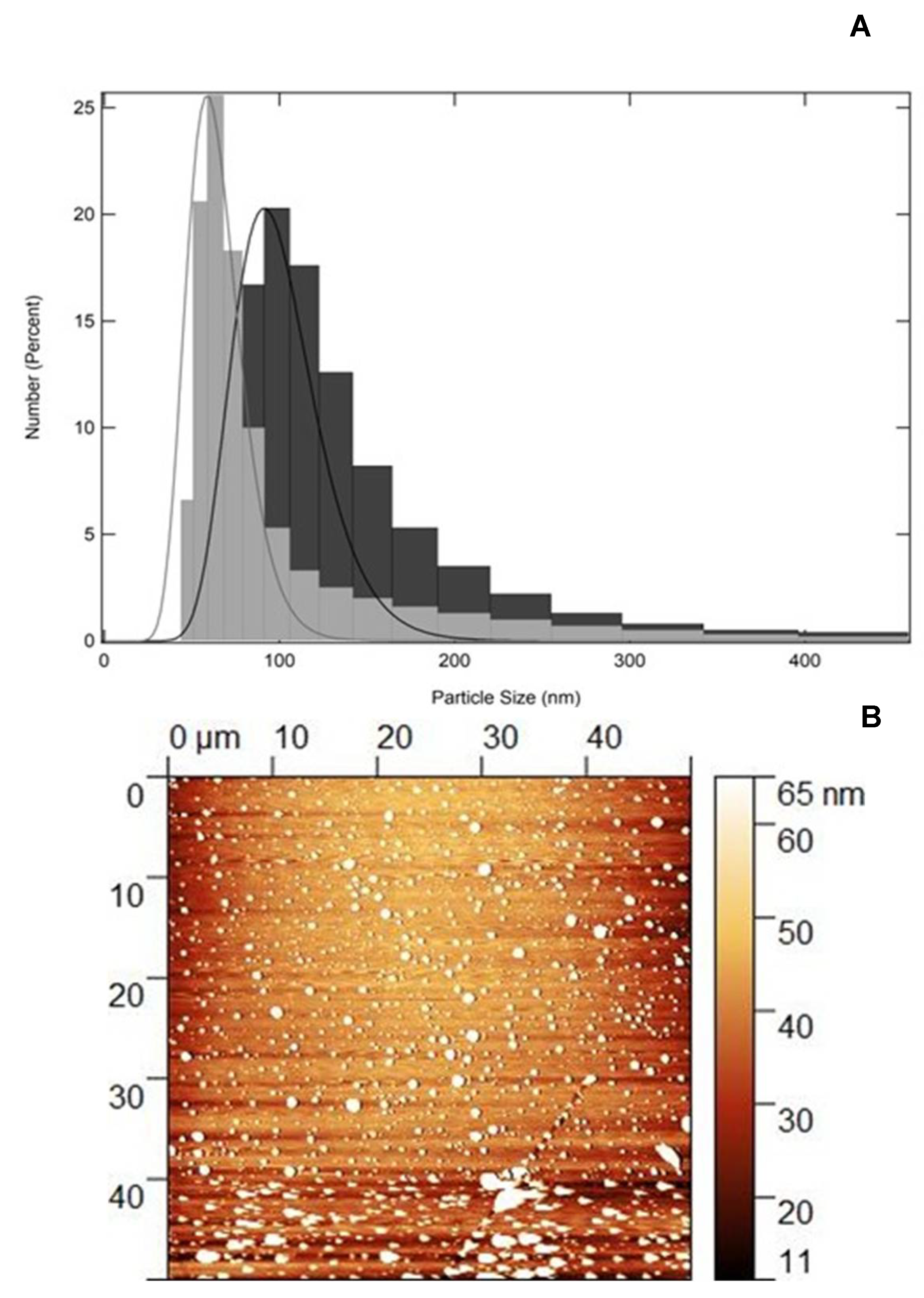
36]. The particle size, polydispersity index (PdI) and Zeta potential (ζ), which provides surface charge, were determined using the Dynamic Light Scattering (DLS) technique. The average of the particle number values obtained for QZ/NC and for Unloaded/NC were expressed as mean ± standard deviation and are shown in
Table 1.
Both synthesized nanomaterials (QZ/NC and Unloaded/NC) showed a satisfactory profile, which is expected for colloidal systems submicron polymers. The PdI values for show a polydispersion of the systems, indicating possible agglomeration and/or variation in particle size. However, the percentages of number of particles (97.9 %) in the region of the predominant average size, indicate a monodisperse system with variation relative to the larger particle distribution. The zeta potential values remained within the proposed stability range within the analysis period (+30mV, -30mV). Within the 61-day post-synthesis period, QZ/NC and Empty/QZ remained stable within the analyzed parameters, as can be seen in
Figure 1-A. The determination of physicochemical parameters is extremely important, describing the nanomaterial and evaluating its behavior when inserted in a biological environment. Particle size, polydispersity index, and Zeta potential are fundamental analyses that describe nanoparticles. The surface characteristics, indicated by the Zeta potential, influence the biological performance and safety of the nanomaterial, determining its biodistribution and special stability and, consequently, indicating the drug release in biological targets [
37]. These parameters, associated with the polydispersity index (PdI) indicate the stability of nanomaterials that are designed from better performance compared to macromaterials applied in biological use. Characteristics such as particle size are essential for the development of effective nanomaterials for topical application, considering the cutaneous permeation, where particles with submicron size tend to penetrate in a facilitated manner through the skin, reaching the systemic circulation [
38,
39].
The AFM analysis of the QZ/NC, shown in
Figure 1 - B, shows clear images of particles with a well-defined spherical shape, with a size within the expected, with an average around 65nm, since previous DLS analyses, indicating that the particles analyzed previously had a larger particle size than the MFA analysis, which is because DLS measures the particle's hydrodynamic radius or solvation radius. Thus, the analysis confirms the average particle size expected for nanostructured polymeric systems and shows the characteristic spherical morphology of the system developed, with excellent correlation with the hydrodynamic sizes obtained by the DLS technique, where the QZ/NC show no deformations on the surface, indicating a homogeneous particle formed only by the PLGA, which covers the nanocapsules [
27].
3.2. 3D Fluorescence Emission Spectroscopy UV/Vis
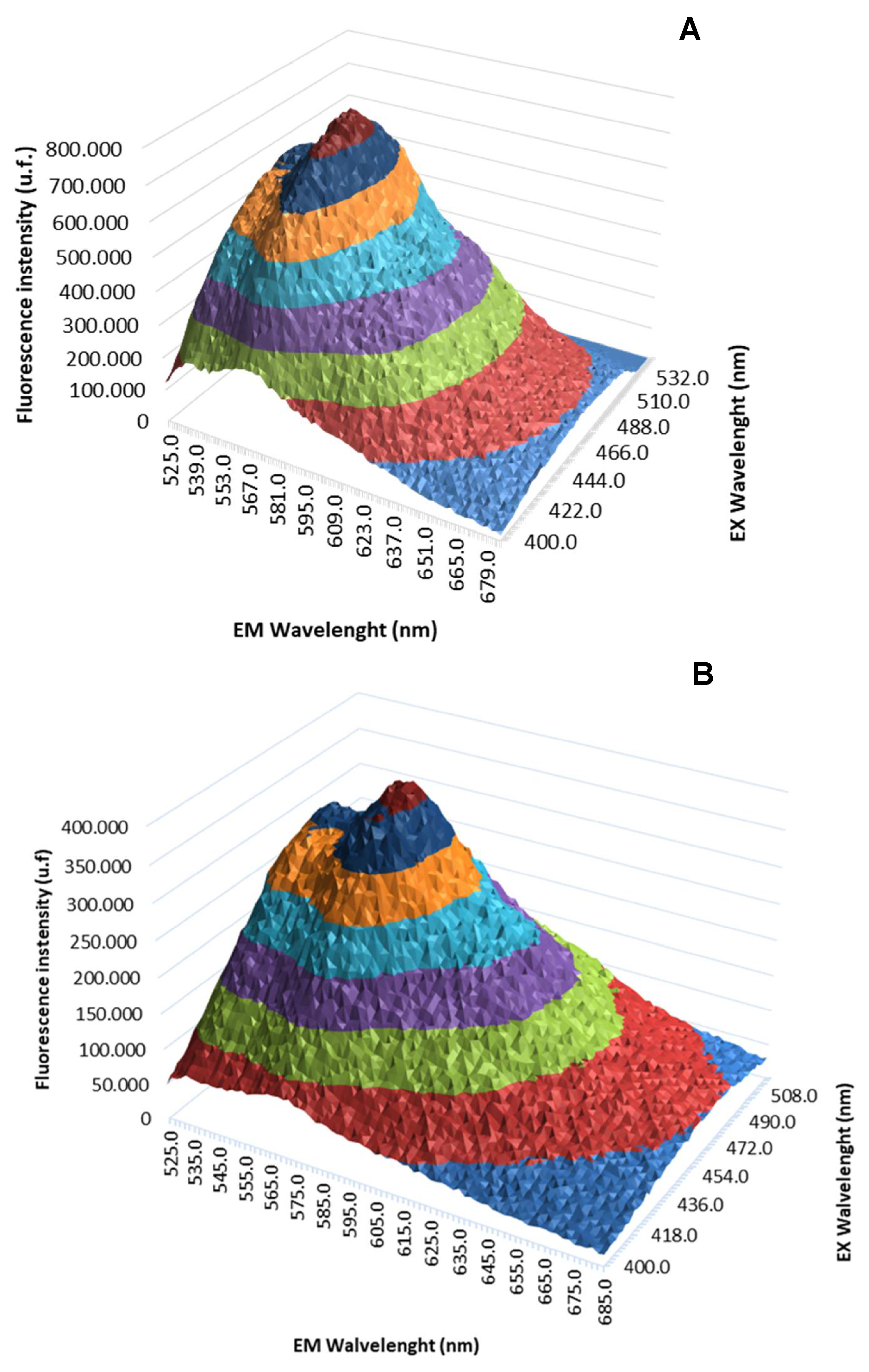
The 3D fluorescence emission spectra profiles of free QZ and QZ/NC were obtained by UV/Vis fluorescence emission spectroscopy studies, as shown in
Figure 2. The fluorescence intensity maximum profile was obtained at 569 nm from the excitation at 480 nm.
The analysis of compounds is of fundamental, facilitating their characterization and the identification of possible modifications. The fluorescence method, when compared to other spectral methods, has shown significant advantages, due to its simplicity, speed, high sensitivity, with emphasis on 3D fluorescence spectroscopy, which measures emission spectra over a range of excitation wavelengths, allowing a more extensive identification of samples, becoming a powerful tool in the classification and identification of substances [
40,
41].
There were no significant changes in fluorescence profile of free QZ when nanoencapsulated, with no reaction of the active with the components used to prepare the nanostructured system, promoting support for the use of QZ/NC in future biological tests. Previous studies carried out with quinizarin in a spray dryer nanosystem have shown favorable results and in agreement with the one presented. In particular, no changes were observed in the molecule's profile after encapsulation, with a high association rate (72.3%), which is satisfactory for subsequent in vitro studies [
42]. Considering the application in Photodynamic Therapy, maintaining the photophysical properties of the PS in the ground state is extremely important, ensuring absorption in the visible region of the electromagnetic spectrum at the optimal excitation wavelength.
3.3. Cytotoxicity Assay Using Resazurin Test – NIH/3T3 Cells
NIH/3T3 cells were used as preliminary assays to analyze the cytotoxicity of the material. QZ/NC was analyzed at concentrations of 2.5 - 70μg.mL-1, Unloaded/QZ 50% (v/v) and free QZ (50 and 70 μg.mL-1). These data are interesting and are in accordance with the incubation times suggested in the international standard ISO 10993-5 for cytotoxicity testing of nanomaterials.
Cytotoxicity studies are extremely important and necessary in drug development and synthesis of new medicines for assessing the in vitro biological compatibility profile of active ingredients and compounds with potential for biological application. There are also tests that help determine important nanotechnology development parameters, such as IC10 and IC50, which are exactly the inhibitory concentrations that induce a 10% and 50% reduction in cell viability respectively.
As evidenced in
Table 2, the absence of cytotoxicity was observed at the lower concentrations of QZ/NC, Unloaded/NC, and free QZ at both concentrations tested. At the higher concentrations of QZ/NC there was a decrease in cell viability, of approximately 30% (25 μg.mL
-1) and 90% (50 and 70 μg.mL
-1). The concentration of 15 μg.mL
-1 was chosen for the development of the sequential investigative biological assays, considered the in vitro safety threshold
3.4. Cell Internalization and Permeation Study
The processes of cell permeation and internalization are influenced by several variables, such as the size of the nanomaterial chosen, as the concentration, pharmaceutical form, surface characteristics and the internal structure [
43]. The greatest limitations involved in the development of drugs for topical and transdermal application is the permeation barrier provided by the stratum corneum of the skin, composed of rigid layers rich in keratin and lipid intercellular matrix, which directly influences the therapeutic result [
44,
45].
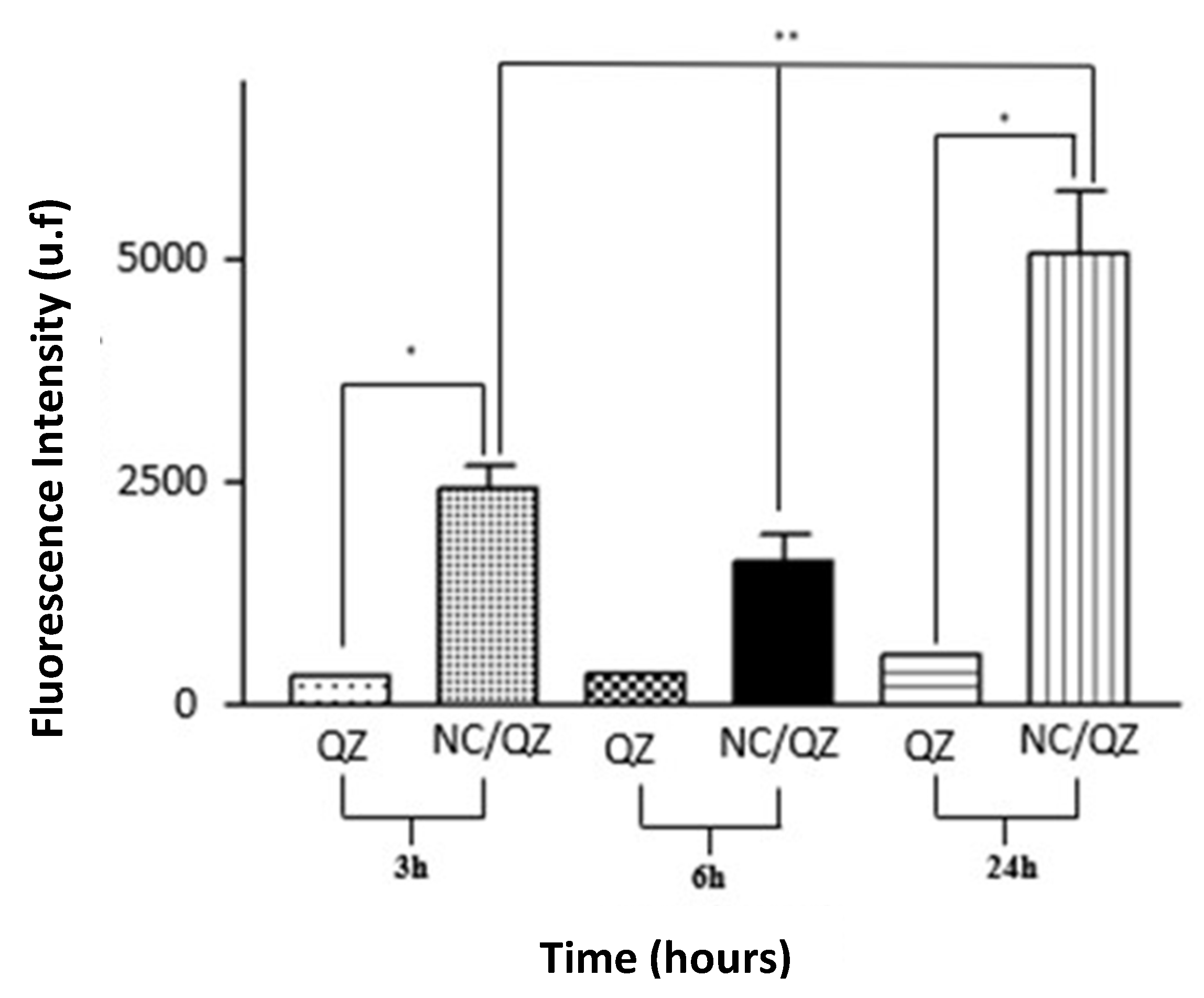
In order to determine the internalization of QZ/NC in HaCaT cells, three different incubation times (3, 6 and 24 hours) were analyzed QZ/NC at the concentration of 15 μg.mL
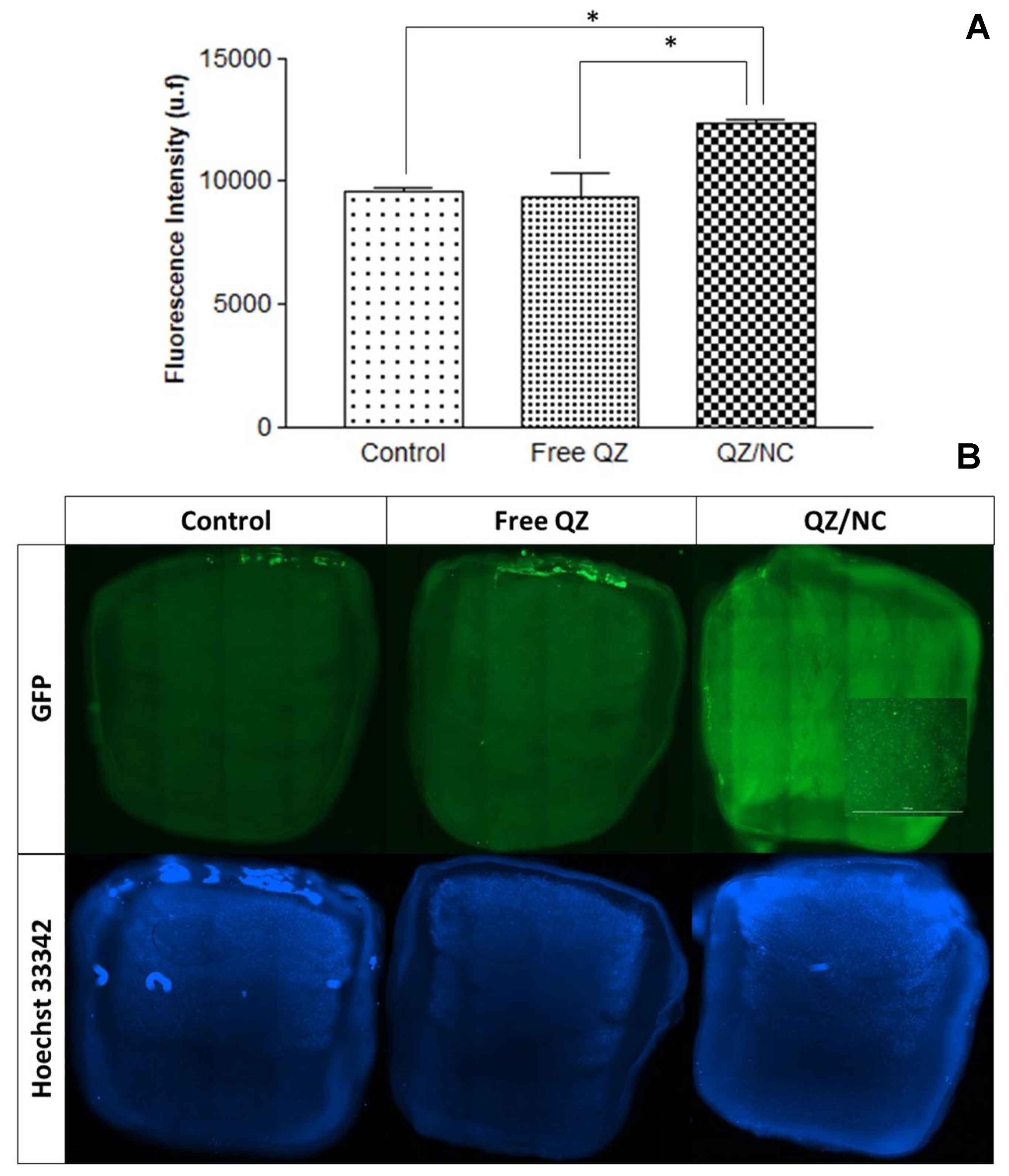
-1. Higher internalization was observed for QZ/NC when compared to free QZ (
Figure 3), demonstrating the desired effect of increased cytoplasmic internalization of the nanocarrier active.
Another data obtained in the assay was the best incubation time relative to the best internalization rates of the active. According to the following results, the time of 24h presented a fluorescence intensity of QZ much higher than the previous times, indicating an internalization 9x higher than the others. These data are in accordance with the incubation times suggested in the international standard ISO 10993-5 for cytotoxicity testing of nanomaterials.
The permeation of QZ/NC and free quinizarin was evaluated using a 3D bioprinted skin equivalent as a model, developed by the suspended layer additive manufacturing (SLAM) method, where computer-aided design (CAD) was used to create the design of the three layers, mimicking the skin. The composition of the bioink consisted of a mixture of pectin, which is similar to the polysaccharides found in native ECM, and collagen, mimicking the composition of skin [
33]. Analyses were conducted to assess permeation after 24 hours in cross-sections, evaluating permeation throughout the model (from top to bottom), using the previously determined excitation and fluorescence emission parameters of quinizarin. The concentration used was 15µg/mL, defined in the cytotoxicity experiment as the drug's safety threshold.
The fluorescence analyses, shown in
Figure 4, showed permeation of QZ/NC in all the layers of the three-dimensional model. Observed were fluorescence signals characteristic of the QZ/NC, which is one of the main desired effects and justifies the use of nanotechnology for biological applications Quantitative assessment of the fluorescent signals showed no statistically significant difference between the control (DMEM) and the free QZ, suggesting impaired permeability of free QZ through the skin model barrier. Conversely, the QZ/NC samples exhibited a statistically significant (*p<0.05) 1.25x increase in the fluorescent signal. Previous studies analyzing the permeation of nanoparticles in human cadaver skin have observed an increase and improvement in the permeation of nanostructured compounds
[44]. Also, considering the structure of the skin in inflammatory diseases such as psoriasis, where the hyperproliferation of keratinocytes and the inflammatory process make the skin rigid and impermeable, the use of polymeric nanoparticles is highly applicable, since they promote greater bioavailability and permeability in the stratum corneum of the skin [
10].
3.5. Cytotoxicity Assay Using Resazurin Test – HaCaT Cells
The modulation of the human epidermis through the use of monolayer keratinocytes has been studied extensively, making it possible to understand the functional characteristics of interactions within the cell [
46]. An additional cell viability test was carried out on human keratinocyte cells (HaCaT), assessing the toxicity of QZ/NC at different concentrations (2.5 - 50 µg.mL
-1), since the keratinocyte lineage was used to modulate the inflammatory process in vitro. The incubation time used was 24 hours, as determined in section 3.4.
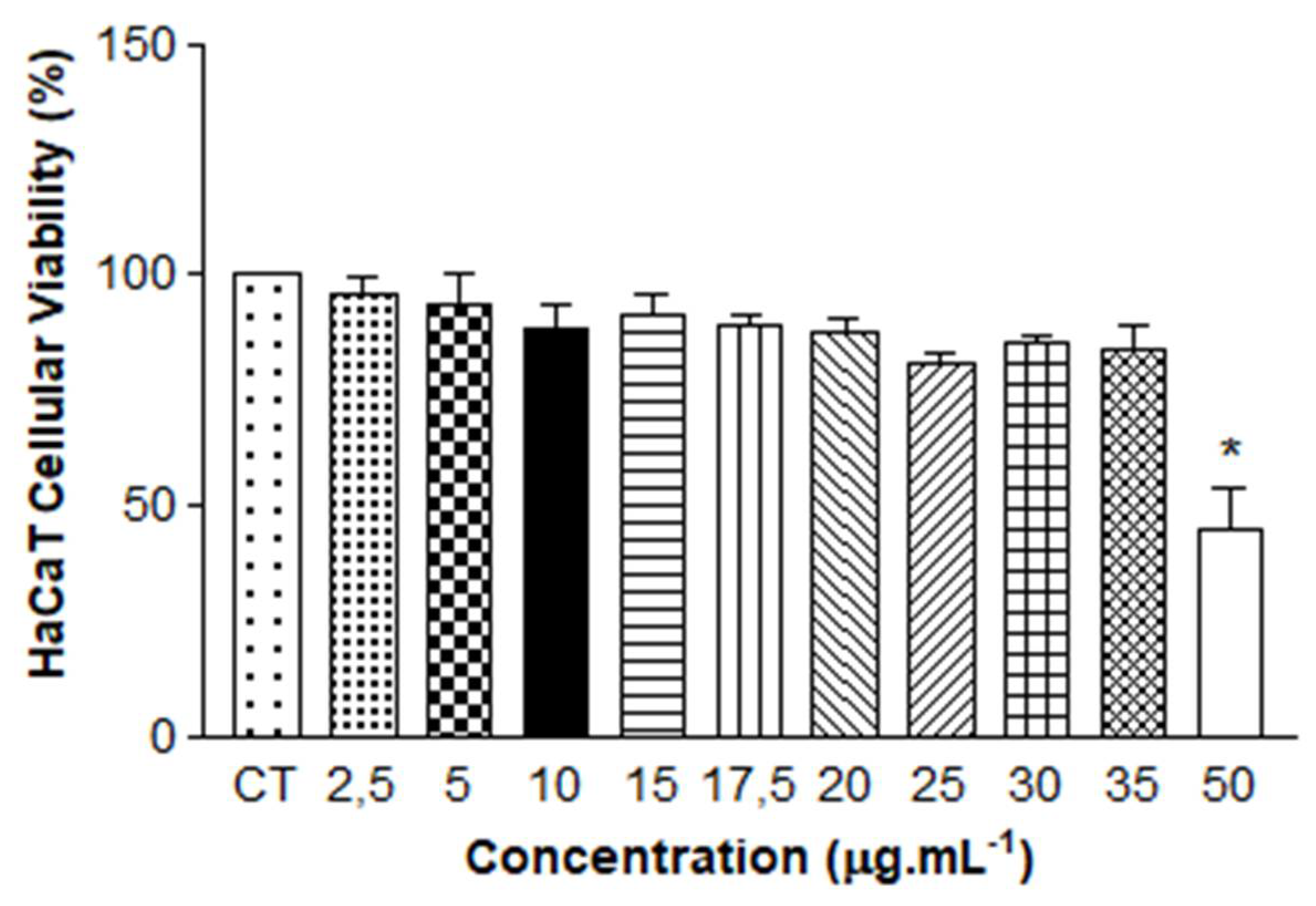
As shown in
Figure 5, the results of the One-way ANOVA statistical analysis show absence of cytotoxicity for concentrations between 2.5 µg.mL
-1 and 35 µg.mL
-1, with cell viability above 70%. The reduction in viability was only observed at the highest concentration of QZ/NC tested. The obtained results demonstrate that the QZ/NC presents compatibility in the specified concentration range. This makes it a potent candidate for use in protocols with biological application and future studies for evaluation of photodynamic activity in models of inflammatory process in vitro.
3.6. Induction of Inflammatory Process in Human Keratinocytes with Application of Photodynamic Therapy and Subsequent Immunoenzymatic Assay
After induction of the inflammatory process, further analyses were performed using the enzyme-linked immunosorbent assay - ELISA, described in 2.9. Cytokines are small proteins secreted in cascade by cells in response to an inflammatory process, influencing interactions and communication between cells. The following pro-inflammatory cytokines were analyzed in this study: Interleukin IL-1β (pro-inflammatory cytokine essential in the inflammatory response and defense of cells, and exacerbates damage in chronic diseases and acute tissue injury [
47]; Interleukin IL- 8 (chemokine responsible for the migratory stimulation of neutrophils and other immune cells to the site of inflammation [
48], in addition to stimulating the cell proliferation of keratinocytes [
48] and monocyte chemotactic protein-1 MCP-1 (responsible for regulating the release of monocytes/macrophages at the site of inflammation [
50].
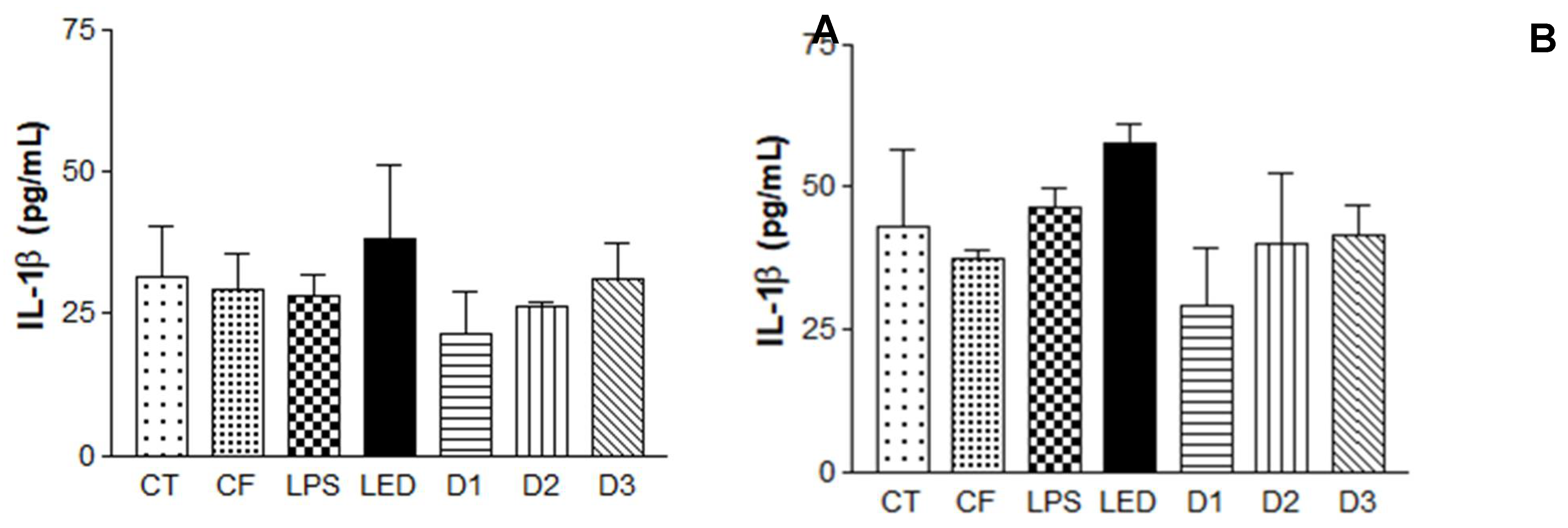
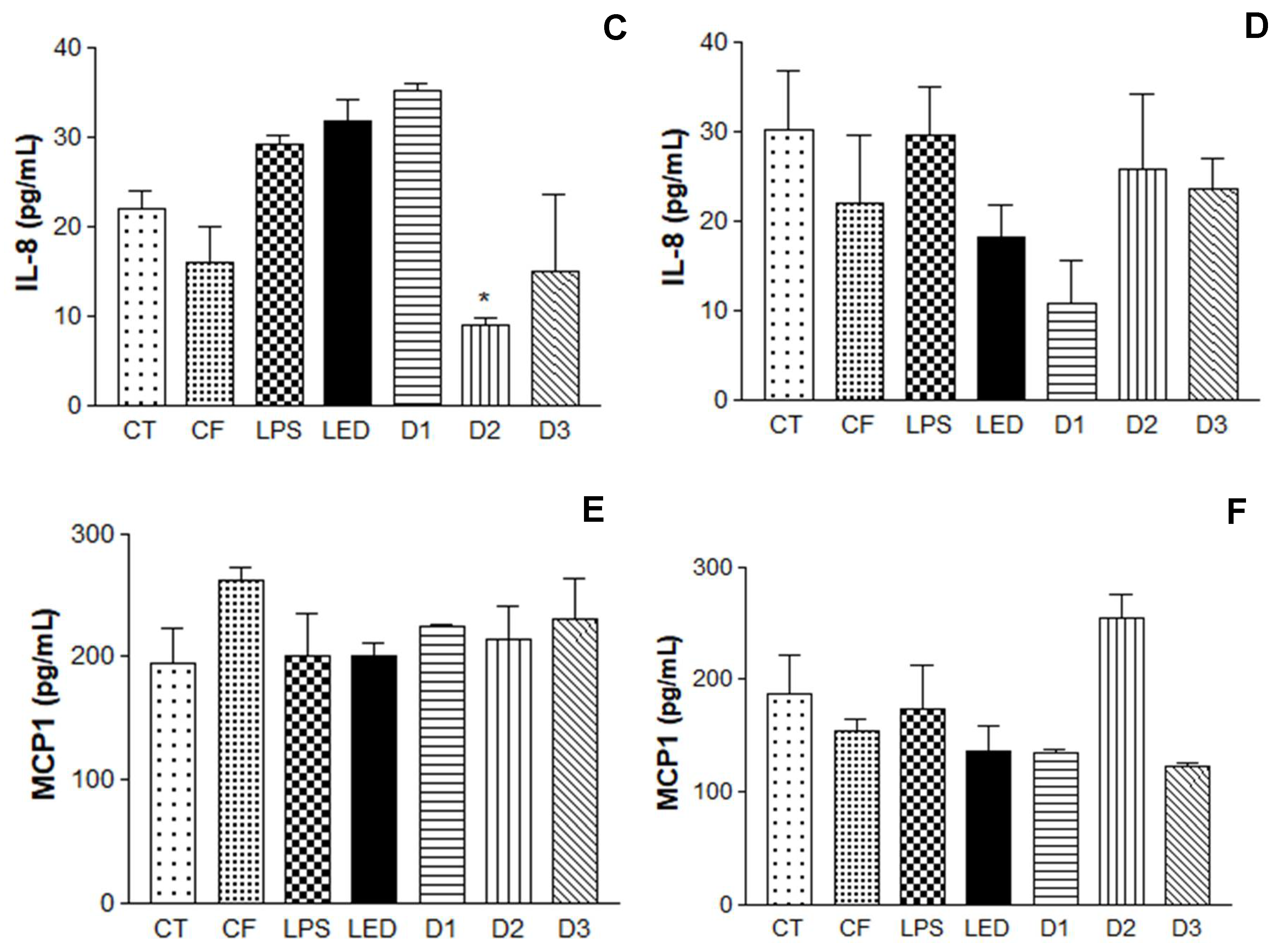
The results obtained are summarized in
Figure 6. It is possible to observe the induction of the inflammatory process, where LPS levels were significant compared to the control containing only HaCaT cells, leading to the expression of cytokines IL-1β, IL-8 and MCP1.
Once the inflammatory process is triggered in vitro, inflammatory mediators initiate the production of cytokines, which play key roles in the inflammatory response [
51]. Previous studies have analyzed the action of PDT as a therapeutic method through the production of cytokines, observing an increase in pro-inflammatory cytokines (IL-1β, IL-8, IL-6 and MCP1), highlighting that the action of PDT has three phases: generation of ROS; expression of local endothelial damage and the mechanisms of the disease, so patients who responded to PDT had their levels of pro-inflammatory cytokines increased, as a response to the inflammatory process [
52,
53,
54]. Also, in comparison with PDT and LED therapy, PDT proved to be more effective in reducing cytokine expression 24 hours after irradiation, when compared to the action of LED alone (therapeutic LED effect) [
55,
56].
In the analysis of the cytokines IL-1β and IL-8, after 24 hours of irradiation, the formulation had no influence on the level of cytokines, further corroborating the hypothesis that the association of the photosensitizer with the action of the LED is more effective (photodynamic effect). Regarding the energy doses used, the results varied depending on the cytokine analyzed in relation to the time observed. IL-1β, 6 hours after irradiation, showed no statistically significant between the doses analyzed. However, D1 had a considerable reduction in cytokine expression after 24 hours. IL-8, 6h after, D2 and D3 showed significant differences when compared to the control. After 24h, D1 had the most significant difference of the group. MCP1 at 6h after irradiation showed no difference between doses. However, after 24 hours, D1 and D3 showed a reduction in the expression of this cytokine.
The PDT showed greater potential, compared to LED therapy (LED use only) and the QZ/NC formulation applied without any irradiation. Considering the cytokines analyzed above are directly linked to the inflammatory process of autoimmune and inflammatory diseases, these results show PDT as an effective treatment with potential to be explored in the future.
Author Contributions
Stéphanie R. do Amaral: Writing - original draft, Methodology, Formal analysis, Validation, Data curation, Statistical analysis. Camila F. Amantino: Methodology, Writing review & editing. Aleksandar Atanasov: Methodology, Validation, Writing review & editing. Stefanie Souza: Methodology, Statistical analysis, Writing review & editing. Richard Moakes: Methodology. Sônia M. Oliani: Writing review & editing. Liam M. Grover: Writing review & editing. Fernando L. Primo: Writing - review & editing, Funding acquisition, Writing - original draft, Project administration, Resources, Formal analysis, Supervisor.
Figure 1.
A: Size distribution profile of the developed NC using dynamic light scattering technique. Unloaded/NC (gray) and QZ/NC (black) at 61 days after synthesis. B: Two-dimensional photomicrograph of QZ/NC obtained using the AFM technique.
Figure 1.
A: Size distribution profile of the developed NC using dynamic light scattering technique. Unloaded/NC (gray) and QZ/NC (black) at 61 days after synthesis. B: Two-dimensional photomicrograph of QZ/NC obtained using the AFM technique.
Figure 2.
3D fluorescence spectra of free Quinizarin (A) and Quinizarin nanoencapsulated (B).
Figure 2.
3D fluorescence spectra of free Quinizarin (A) and Quinizarin nanoencapsulated (B).
Figure 3.
Cellular uptake study in HaCaT cells at a concentration of 15 μg.mL-1 evaluating the cellular internalization of free Quinizarin (QZ) and nanoencapsulated form (QZ/NC) at 3, 6 and 24 hours. Statistical significance was determined using the One-way ANOVA test of variance, with 95% significance level, followed by Tukey post-test for multiple comparisons, where *statistical difference obtained between free QZ and QZ/NC, **statistical difference obtained between QZ/NC in the three tested times (p<0.05).
Figure 3.
Cellular uptake study in HaCaT cells at a concentration of 15 μg.mL-1 evaluating the cellular internalization of free Quinizarin (QZ) and nanoencapsulated form (QZ/NC) at 3, 6 and 24 hours. Statistical significance was determined using the One-way ANOVA test of variance, with 95% significance level, followed by Tukey post-test for multiple comparisons, where *statistical difference obtained between free QZ and QZ/NC, **statistical difference obtained between QZ/NC in the three tested times (p<0.05).
Figure 4.
Analysis of permeation in the three-dimensional skin model by evaluating fluorescence intensity (A) and cross-sectional images (B) using a SPARK microplate reader (TECAN, SWI) and an Cytation imagin reader (BioTek, USA) respectively. The control, containing only DMEM, free quinizarin and QZ/NC were analyzed. Statistical significance was determined using the analysis of variance test One-way ANOVA, at 95% significance level, followed by Tukey post-test for multiple comparisons (*p<0,05).
Figure 4.
Analysis of permeation in the three-dimensional skin model by evaluating fluorescence intensity (A) and cross-sectional images (B) using a SPARK microplate reader (TECAN, SWI) and an Cytation imagin reader (BioTek, USA) respectively. The control, containing only DMEM, free quinizarin and QZ/NC were analyzed. Statistical significance was determined using the analysis of variance test One-way ANOVA, at 95% significance level, followed by Tukey post-test for multiple comparisons (*p<0,05).
Figure 5.
Safety tests from in vitro cytotoxicity assay of QZ/NC on human keratinocyte HaCaT (HaCaT - cell line service) at concentrations of 2.5; 5; 10; 15; 17.5; 20; 25; 30; 35 and 50 μg.mL-1 (QZ/NC). Statistical significance was determined using the analysis of variance test One-way ANOVA, at 95% significance level, followed by Tukey post-test for multiple comparisons (*p<0,05).
Figure 5.
Safety tests from in vitro cytotoxicity assay of QZ/NC on human keratinocyte HaCaT (HaCaT - cell line service) at concentrations of 2.5; 5; 10; 15; 17.5; 20; 25; 30; 35 and 50 μg.mL-1 (QZ/NC). Statistical significance was determined using the analysis of variance test One-way ANOVA, at 95% significance level, followed by Tukey post-test for multiple comparisons (*p<0,05).
Figure 6.
Enzyme-Linked Immunosorbent Assay (ELISA) for the analysis of IL-1β, IL-8 cytokines and macrophages MCP1 in Human Keratinocytes (HaCaT - cell line service) where A, C and E: analysis 6h after irradiation and B, D and F: analysis 24h after irradiation, where: CT= Control; LPS = Lipopolysaccharide; LED = irradiation dose of 10 J. cm-2; D1 = QZ/NC irradiation dose of 1.0 J.cm-2; D2 = QZ/NC irradiation dose of 5 J.cm-2; D3 = QZ/NC irradiation dose of 10 J.cm-2; CF= Formulation control (QZ/NC 15μg.mL-1). Statistical significance was determined using the One-way ANOVA analysis of variance test, with a 95% significance level, followed by the Dunnett's post test.
Figure 6.
Enzyme-Linked Immunosorbent Assay (ELISA) for the analysis of IL-1β, IL-8 cytokines and macrophages MCP1 in Human Keratinocytes (HaCaT - cell line service) where A, C and E: analysis 6h after irradiation and B, D and F: analysis 24h after irradiation, where: CT= Control; LPS = Lipopolysaccharide; LED = irradiation dose of 10 J. cm-2; D1 = QZ/NC irradiation dose of 1.0 J.cm-2; D2 = QZ/NC irradiation dose of 5 J.cm-2; D3 = QZ/NC irradiation dose of 10 J.cm-2; CF= Formulation control (QZ/NC 15μg.mL-1). Statistical significance was determined using the One-way ANOVA analysis of variance test, with a 95% significance level, followed by the Dunnett's post test.
Table 1.
Data of average* Particle Size, PdI, and Zeta Potential of QZ/NC and Unloaded/NC.
Table 1.
Data of average* Particle Size, PdI, and Zeta Potential of QZ/NC and Unloaded/NC.
| Nanomaterial |
Average Particle Size (nm) |
PdI |
Zeta Potential (mV) |
| QZ/NC |
103.9 ± 34.5 |
0.4 ± 0.03 |
-31.8 ± 0.723 |
| Unloaded/NC |
137.2 ± 46.1 |
0.5 ± 0.06 |
-35.1 ± 1.107 |
Table 2.
Cellular viability§ obtained from in vitro cytotoxicity assay of QZ/NC, Unloaded/NC and free QZ on murine fibroblast cell lines NIH/3T3 (ATCC® CRL-1658) at concentrations of 2.5; 5; 15; 25; 50; 70 μg.mL-1 (QZ/NC), 50% (v/v) NC/empty and free QZ at concentrations of 50 and 70 μg.mL-1. Statistical significance was determined using the One-way ANOVA test of analysis of variance at 95% significance level, followed by Tukey post-test for multiple comparisons (*p<0.05, **p<0.001).
Table 2.
Cellular viability§ obtained from in vitro cytotoxicity assay of QZ/NC, Unloaded/NC and free QZ on murine fibroblast cell lines NIH/3T3 (ATCC® CRL-1658) at concentrations of 2.5; 5; 15; 25; 50; 70 μg.mL-1 (QZ/NC), 50% (v/v) NC/empty and free QZ at concentrations of 50 and 70 μg.mL-1. Statistical significance was determined using the One-way ANOVA test of analysis of variance at 95% significance level, followed by Tukey post-test for multiple comparisons (*p<0.05, **p<0.001).
Sample/Concentration
(µg.mL-1) |
3T3 - Cellular Viability (%) |
Significance level (*p<0.05, **p<0.001) |
| Control |
100 |
- |
| Unloaded Nanocapsule |
81.43 |
- |
| Free Quinizarin 50
|
100.06 |
- |
| Free Quinizarin 70 |
91.30 |
- |
| QZ/NC 2.5 |
98.32 |
- |
| QZ/NC 5.0 |
100.92 |
- |
| QZ/NC 15.0 |
96.69 |
- |
| QZ/NC 25.0 |
69.82 |
* |
| QZ/NC 50 |
9.66 |
** |
| QZ/NC 70 |
9.02 |
** |