Modern agricultural intensification has significantly diminished non-rice field habitats, causing a notable decline in farmland biodiversity and facilitating the proliferation of crop pest populations [
1]. To promote sustainable agricultural practices, refined habitat regulation techniques have emerged as crucial elements in the ecological management of crop pests [
2,
3], particularly in the context of rice pest control [
4,
5,
6]. The objective of ecological pest control is to strategically leverage plant diversity to bolster the efficiency of natural pest enemies, thereby mitigating economic losses [
1,
4]. This entails safeguarding and amplifying the role of natural enemies by providing essential resources like shelter, host plants, alternative prey, and non-prey foods [
1,
6].
The conservation and utilization of natural enemies in rice fields have proven effective in rice planthopper control [
4]. Weeds in non-rice field habitats serve as shelters for
Anagrus nilaparvatae, a significant egg parasitoid of rice planthoppers, with delphacidae insects on weeds serving as crucial alternative hosts for
A. nilaparvatae [
7]. However, the protective effects on natural enemies vary across different host plants and alternative hosts [
8,
9], necessitating exploration to identify the most effective plant and alternative host systems to enhance conservation biology efficiency.
The bank plant system, a recent innovation in biological control technology, comprises bank plants, alternative food, and beneficial organisms, or combinations thereof [
10]. Bank plants provide food resources to alternative hosts or prey, aiming to establish self-sustaining propagation systems for beneficial organisms within crop systems [
10]. Various bank plants like cucumber, papaya, ornamental peppers, and oats have demonstrated success in pest control [
11], though research on bank plant systems for rice pests remains limited. The integration of nectar plants into agricultural systems to attract natural predators and enhance their egg production and pest control capacity warrants further investigation [
12]. Studies indicated that incorporating
Sesamum indicum into paddy field systems effectively boosts the population and pest control capabilities of key natural enemies of rice pests [
4].
Rice serves as the staple food for over 50% of the global population, with China alone relying on it for 60% of its dietary needs [
13]. The rice pest
Nilaparvata lugens Stål, has witnessed alarming outbreaks in recent years, posing a significant threat to global food security [
14,
15]. Current rice pest management heavily leans on broad-spectrum insecticides, despite their acknowledged side effects on natural enemies, the environment, and food safety [
16]. However, research on ecological pest control methods for sustainable rice pest management remains limited, despite the severity of the damage caused [
4].
Nilaparvata muiri, a homologous species of
N. lugens, is prevalent in China’s southern rice-growing regions [
17]
. Leersia sayanuka, a primary host of
N. muiri, exhibits a high population growth rate on this grass, making it a promising candidate as a bank plant for rice planthopper control [
18]. Previous studies have identified the potential of the
L. sayanuka,
N. muiri,
A. nilaparvatae system as a bank plant system for rice planthopper control, validating its efficacy in field applications [
18]. Further research by Zheng
et al. [
19] explored the feasibility of establishing a “
L. sayanuka, N. muiri, Tytthus chinensis” bank plant system in the field.
This study evaluates the potential of L. sayanuka as a bank plant system for rice planthopper control and assesses the feasibility of S. indicum as a functional plant to enhance the effectiveness of the bank plant system. The findings aim to provide a foundation for subsequent research and field implementation in this area.
1. Materials and Methods
1.1. Experimental Materials
1.1.1. Rice
Seeds of the insect-susceptible rice cultivar TN1 were obtained from the International Rice Research Institute (IRRI) and germinated in concrete tanks. After 15 days, the seedlings were transplanted into plastic pots, and those aged between 40 and 50 days were selected for testing. TN1 rice seedlings were sown at 15-day intervals.
Leersia sayanuka was collected from fields in the suburbs of Hangzhou, with tillers cut and directly transplanted into plastic pots. One stem was placed per pot for propagation, with transplantations performed every 15 days. Plants aged between 40 and 50 days were used for testing.
Both TN1 rice and L. sayanuka were cultivated in small plastic pots (10 cm in diameter and 12 cm in height) within insect-free mesh chambers to ensure the absence of insects before testing. Before introducing insects, any old or yellow leaves, along with their sheaths, were removed. For rice, three tillers were maintained per pot, while for L. sayanuka, five tillers were kept per pot to ensure uniform biomass distribution in each pot.
1.1.2. N. Lugens
The test N. lugens were collected from the rice fields of the Jinhua experimental base and subsequently reared in laboratory cages (90 cm×80 cm×80 cm). These cages contained 40 to 50-day-old TN1 rice seedlings. The insect-rearing greenhouse maintained an average temperature of 27 ± 0.5 ℃, with relative humidity ranging between 70-90%, and a light cycle of 12 hours of light followed by 12 hours of darkness. N. lugens were reared on TN1 rice for four generations prior to testing.
1.2. Experimental Methods
1.2.1. Site Description
The experimental site was chosen in the Rice Pest Ecological Engineering Pest Control Demonstration Area in Siping Village, Tangxi Town, Jinhua City, Zhejiang Province, China. The demonstration area was 10 hm2, and each field in the core area was regular in size and dimension (20 m × 50 m), containing three longitudinal and two transverse mechanized roads.
1.2.2. Experimental Design
Four treatments were established: 1) A 50 cm wide and 5 m long strip of L. sayanuka was planted in the rice field next to the ridge. S. indicum seeds were planted on the ridge; 2) A 50 cm wide and 5 m long strip of L. sayanuka was planted in the rice field next to the ridge; 3) A 50cm wide and 1 m long L. sayanuka strip was planted in the rice field next to the ridge; 4) Conventional ridges lacking L. sayanuka and S. indicum were utilized as a control. The experiment was a split-plot design with three replicates. The ridge length in each plot was 10 m, and the isolation distance was 10 m. The rice cultivar was an indica-japonica hybrid single-season rice Yongyou 1540. In early June, the ridge was planted with S. indicum seeds. The L. sayanuka was transplanted at the same time with rice on June 15, and L. sayanuka was transplanted as single stem tiller cuttings into the rice field near the ridge, with the spacing between plants and rows being 5 cm. On June 30, strips of L. sayanuka were planted with N. muiri at a density of 100 adult females per m2.
1.2.3. Survey of Rice Planthoppers and Their Parasitic Natural Enemies during the Growing Season of Rice
The egg trapping method was utilized to assess the parasitization of rice planthopper eggs by wasps. For each pot, three strong rice seedlings were placed with five egg-bearing N. lugens. After 24 hours of oviposition, the N. lugens were removed. Pots containing N. lugens eggs were positioned at distances of 1 m, 5 m, and 10 m from the ridge. After 48 hours, these pots were retrieved and brought to the laboratory. After five days of incubation in an insect-free artificial climate chamber (26.0 ± 1 ℃, 70% to 90% RH, 12-hour light cycle), they were dissected to determine the total number of N. lugens eggs and the number parasitized, enabling the calculation of the parasitism rate. This investigation was conducted once during the booting stage of rice. To assess the population of Anagrus spp., yellow sticky traps measuring 28.5 cm × 21 cm were deployed at distances of 1 m, 5 m, and 10 m from the ridge. These traps were wrapped in plastic wrap and retrieved after 48 hours. In the laboratory, they were examined using a stereoscope to record the population count of Anagrus spp. The population count of rice planthoppers (Laodelphax striatellus, Sogatella furcifera, N. lugens) and spiders in rice fields under different treatments was conducted using the patting method (with a 30 × 40 cm enamel tray) during the tillering and booting stages of rice. Each treatment was sampled using the five-point sampling method, with each point consisting of 5 trays of 2 rice bushes.
1.2.4. Statistical Analysis
Data were analyzed using SPSS 26.0 software to contrast the significant differences between treatments using analysis of variance (ANOVA) followed by a Tukey’s test for the number of rice planthoppers and rice planthoppers egg parasitoid populations. Parasitism rate data were subjected to inverse sinusoidal square root transformation prior to analysis.
2. Results and Discussion
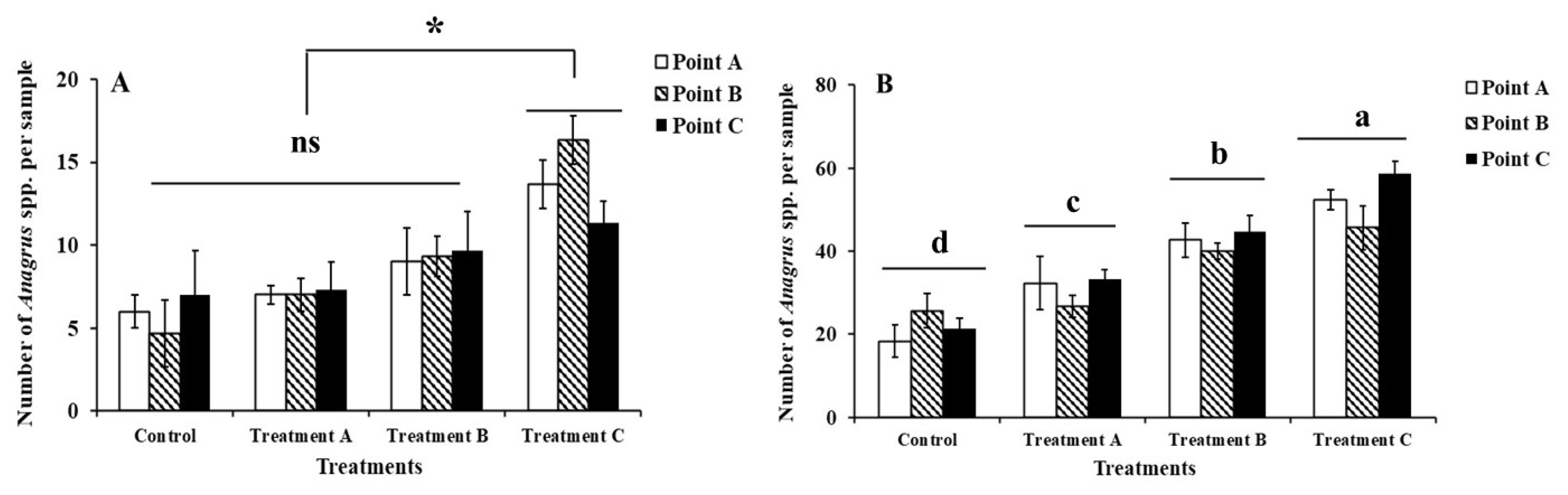
2.1. Effects of Different Treatments of L. Sayanuka Bank Plant System on the Population of Anagrus spp. in Rice Fields
There was no significant difference in the
Anagrus spp. Populations in the rice field within 10 m of the ridge (
df = 2,
F = 1.237,
P = 0.299) (
Figure 1A&1B). However, there was a significant difference between the different treatments of rice fields and the population number of
Anagrus spp. in rice fields. The population count of
Anagrus spp. in rice fields of the 5 m
L. sayanuka combined with
S. indicum treatment was significantly higher than the other three treatments at the tillering stage of rice (
df = 3,
F = 14.158,
P < 0.001) (
Figure 1A). Similarly, the population count of
Anagrus spp. was significantly higher in the rice field with 5 m spacing of
L. sayanuka combined with
S. indicum treatment than in the rice field with 5 m spaced
Leersia sayanuka treatment compared to 1 m spaced
L. sayanuka. The population count of
Anagrus spp. was significantly lower in the control rice field than in the other three treatments at the booting stage of rice (
df = 3,
F = 34.746,
P < 0.001) (
Figure 1B).
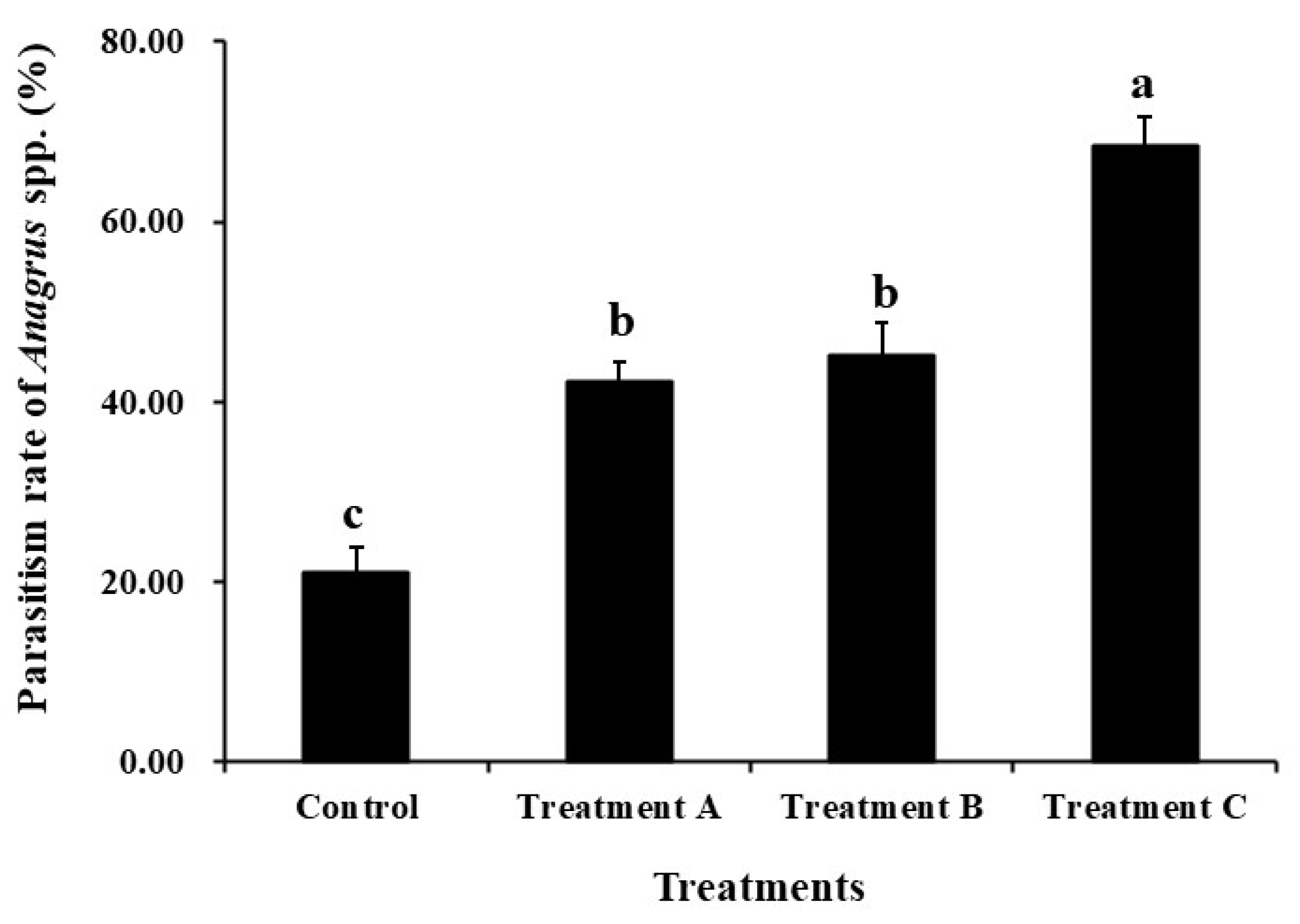
2.2. Effect of Different Treatments of the L. Sayanuka Bank Plant System on Parasitism Rate of Anagrus spp. in Rice Fields
The findings of the egg trapping test of rice planthoppers demonstrated no significant difference in the parasitism rate of the
Anagrus spp. in the rice field within 10 m from the ridge (
df = 2,
F = 0.061,
P = 0.941). There were significant differences in the parasitism rates of
Anagrus spp. in rice fields across different treatments, with parasitism rates of
Anagrus spp. in rice fields in the 5 m spaced
L. sayanuka combined with
S. indicum treatment being significantly higher than those in the other three treatments. Moreover, there was no significant difference between the 5 m spaced
L. sayanuka treatment and the 1 m spaced
L. sayanuka treatment in rice fields, but they were significantly higher than the control treatment (
df = 3,
F = 49.399,
P < 0.001) (
Figure 2).
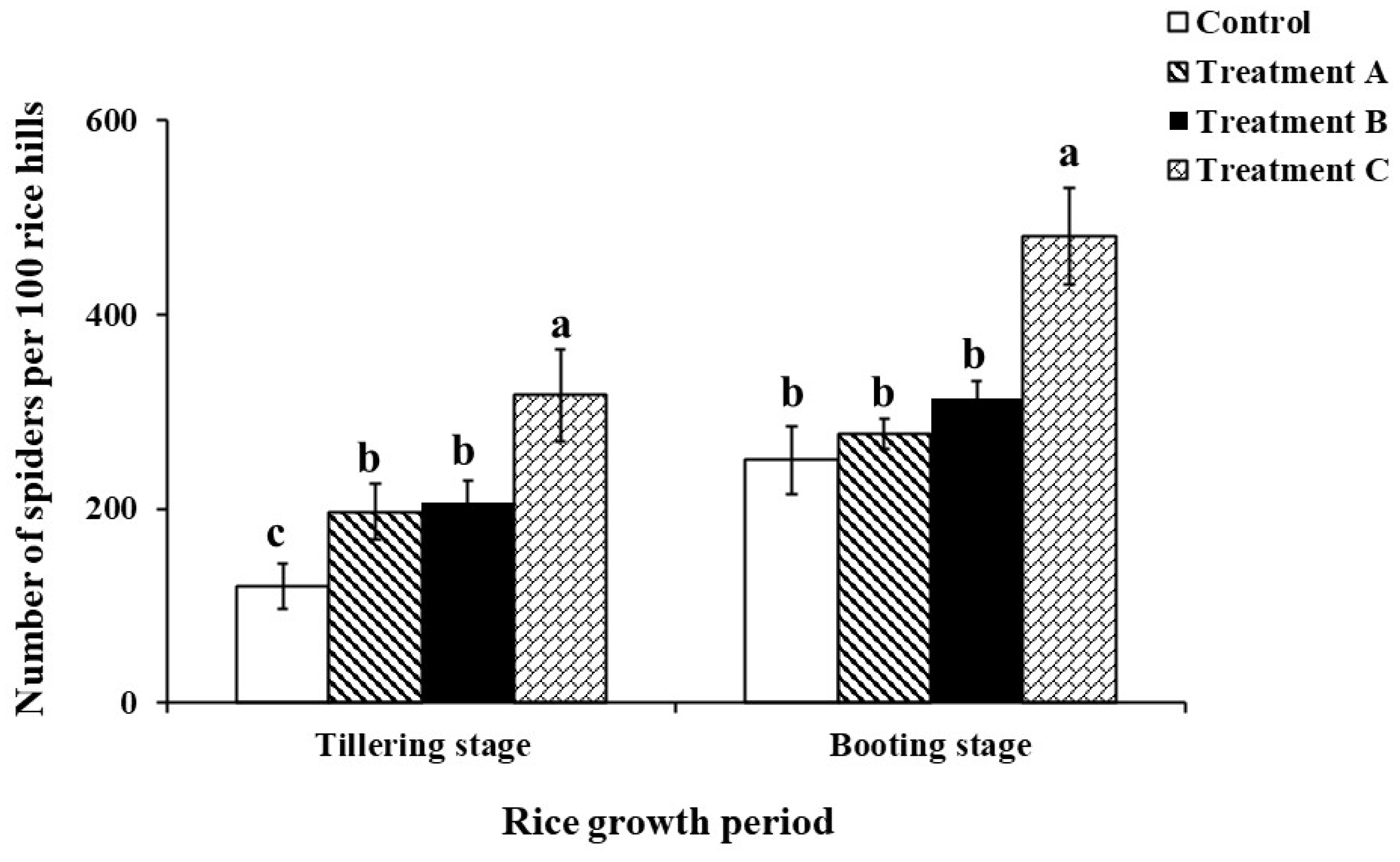
2.3. Effect of Various Treatments of the L. Sayanuka Bank Plant System on Spiders in Rice Fields
There were significant differences in the impacts of different treatments on the population count of spiders in the rice fields. The population count of spiders in the 5 m spaced
L. sayanuka plus
S. indicum treatment was significantly higher than the other three treatments, while there was no significant difference between the 5 m spaced
L. sayanuka treatment and the 1 m spaced
L. sayanuka treatment in the rice fields. However, these treatments had significantly higher counts than the control treatment at the tillering stage (tillering stage:
df = 3,
F = 6.265,
P = 0.001; grain filling stage:
df = 3,
F = 9.226,
P < 0.001) (
Figure 3).
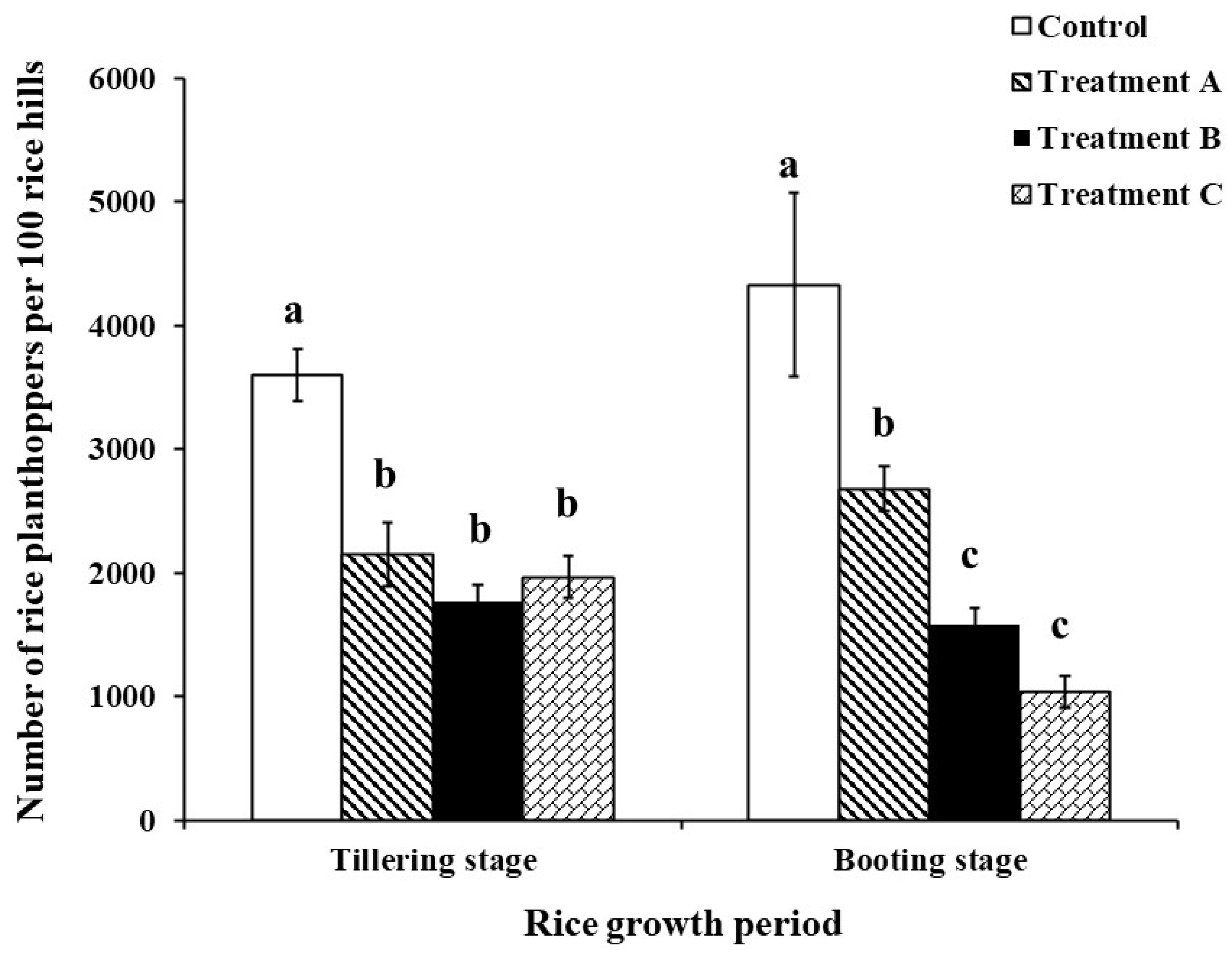
2.4. Effectiveness of Different Treatments of the L. Sayanuka Bank Plant System for Field Control of Rice Planthoppers
Significant differences existed in the field control efficacy of different treatments on rice planthoppers. At the rice tillering stage, the number of rice planthoppers was significantly lower in the 50 cm wide 5 m long
L. sayanuka combined with
S. indicum, 50 cm wide 5 m long
L. sayanuka, and 50 cm wide 1 m long
L. sayanuka treatments compared to the control treatment (
df = 3,
F = 17.547,
P < 0.001). At the rice tillering stage, there was no significant difference between the 50 cm wide 5 m long
L. sayanuka combined with
S. indicum treatment and the 50 cm wide 5 m long
L. sayanuka strip treatment paddy fields regarding the number of rice planthoppers counts in the paddy fields. The number of rice planthoppers in 50 cm wide 5 m long
L. sayanuka combined with
S. indicum treated paddy fields was lower than that of 1 m
L. sayanuka treated paddy fields with no significant difference but significantly lower levels than control paddy fields (
df = 3,
F = 30.624,
P < 0.001) (
Figure 4).
3. Discussion
As an innovative method of traditional biological control, bank plant systems include the artificial propagation of non-crop pests on non-cash crops as alternative hosts for natural enemies. This is accompanied by the mass expansion of natural enemies, aiming to achieve sustained control of outbreaks of major pests during the growing season and avoiding chemical pesticides [
20]. Even in environments with low or absent pest populations, natural enemies can maintain high population via alternative hosts. This characteristic is what makes bank plants more preventive and long-lasting [
10]. In addition, this strategy is more labor and cost-efficient than other biological control strategies [
18,
20]. Numerous studies have been conducted on bank plant systems, but most are focused on indoor or greenhouse conditions [
11,
21,
22]. Large areas of field crops must account for additional influencing factors and feasibility, including variable and unstable environmental factors, compatibility of the bank plant system with the crop system, and benefits to farmers.
Some studies have confirmed that
N. muiri cannot establish a sustained population on rice, although it can complete generational development on rice [
18]. The population of
N. muiri around the paddy ecosystem is very high, according to Luo
et al. [
17], who examined
N. lugens Stål under the forecasting light in five locations across four provinces of China, including Guangxi, Hunan, Jiangxi, and Zhejiang. They found that the proportion of
N. muiri in the
Nilaparvata spp. on the lamps throughout 2008 and 2009 was over 40%, and the number of
N. muiri exceeded the number of
N. lugens in Jiangxi, Hunan, and Zhejiang before September. Among weeds in non-rice habitats,
L. sayanuka is the most suitable host for
N. muiri[
23]. Integrating
L. sayanuka into the paddy ecosystem as an egg parasitoid bank can continuously release
A. nilaparvatae into the paddy field to control rice planthoppers. During overwintering,
L. sayanuka acts as an efficient overwintering host that can accommodate a higher density of overwintering natural enemies than other weeds [
18]. Moreover, the
N. muiri overwinters locally and early, with nymphs hatching in March and oviposition occurring in April, offering hosts for the overwintering
Anagrus spp., which builds up sizable populations prior to the mass migrations of rice planthoppers. This study demonstrated that planting 50 cm wide strips of
L. sayanuka for over 1 m along the edge of the paddy field had a significant controlling effect on rice planthoppers in the paddy field, indicating the efficiency of this as a bank plant system.
The spatial pattern of non-crop habitats impacts the composition, structure, diversity, and dynamics of natural enemies in crop habitats [
24,
25,
26]. Different species of natural enemies show varied responses to different complex habitats [
27,
28,
29]. The results of this study showed that the “
L. sayanuka,
N. muiri,
A. nilaparvatae” bank plant system combined with the nectar plant
S. indicum significantly increased the population of
Anagrus spp. in the paddy field, and enhanced its egg parasitism rate of the rice planthoppers, which in turn significantly enhancing the control effect of the rice planthoppers in the paddy field.
Takada et al. [
30] suggested that
Echinochloa crus-galli and
Schoenoplectus juncoides on the paddy field margins contribute to the spread of the rice pest
Stenotus rubrovittatus, which can exacerbate the damage to rice near these two grasses. This system may pose a risk as it may climb into the rice bushes and compete for water and fertilizer, affecting rice yield. However, in this experiment,
L. sayanuka did not grow as rapidly as
Leersia hexandra Swartz (a local noxious weed), and grew
in situ before rice plants elongation stage. In contrast, after rice elongation stage,
L. sayanuka growth was inhibited by rising temperatures, and it did not invade the rice.
In the practical application of the bank plant system of “
L. sayanuka,
N. muiri,
A. nilaparvatae”,
N. muiri is subject to predation by natural enemies (e.g., spiders). The use of this system will greatly impact the ecology of rice fields and the trophic relationships of the arthropod community.
L. sayanuka is also a host for
Cnaphalocrocis medinalis, and eggs laid by
C. medinalis and larvae on
L. sayanuka can act as a natural enemy host for rice Lepidopteran pests [
31]. This combines them with egg parasitoids and larvae parasitoids (e.g.,
Trichogramma japonicum,
Trichogramma chilonis, and
Apanteles cypris), offering another possible benefit of planting
L. sayanuka strips in paddy field margins as a bank for rice stem borer natural enemies. This means than
L. sayanuka is a bank plant for rice stem borers as well as a bank plant for rice planthopper control. Artificial release of
Trichogramma parasitoids for rice stem borer control is on the rise in China, but the high cost of unsustainable artificial release mode of
Trichogramma parasitoids impacts the widespread dissemination and control efficacy of artificial releases of
Trichogramma parasitoids. In contrast, planting strips of
L. sayanuka likely has an important field conservation role for
Trichogramma parasitoids released into rice fields, which is another possible positive influence of
L. sayanuka.
Author Contributions
X.G., Q.Z. and M.R. were mainly responsible for writing the full text; P.Z. was mainly responsible for the structure of the paper; X.G., Q.Z., M.R., B.C., X.H. and X.Z. were mainly responsible for the field experiment. X.G., X.Z. and P.Z. were mainly responsible for data processing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program, China (No. 2021YFD1401100 and No. 2023YFD1400804), the Zhejiang Key Research and Development Program, China (No. 2022C02047), the Zhejiang Collaborative Promotion Plan for Major Agricultural Technologies (No. 2023XTTGLY0101), and the Zhejiang Normal University ‘Shuanglong Scholar’ Research Start-up Fund (No. YS304021920).
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Gurr, G.M.; Wratten, S.D.; Landis, D.A.; You, M. Habitat management to suppress pest populations: progress and prospects. Annu. Rev. Entomol. 2017, 62, 91–109. [Google Scholar] [CrossRef] [PubMed]
- Allen, W.J.; Bufford, J.L.; Barnes, A.D.; Barratt, B.I.P.; Deslippe, J.R.; Dickie, L.A.; Goldson, S.L.; Howlett, B.G.; Hulme, P.E.; Lavorel, S.; et al. A network perspective for sustainable agroecosystems. Trends Plant Sci. 2022, 27, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Settele, J.; Biesmeijer, J.; Bommarco, R. Switch to ecological engineering would aid independence. Nature. 2008, 456, 570. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.Y.; Zheng, X.S.; Johnson, A.C.; Chen, G.H.; Xu, H.X.; Zhang, F.C.; Yao, X.M.; Heong, K.L.; Lü, Z.X.; Gurr, G.M. Ecological engineering for rice pest suppression in China. A review. Agron. Sustain. Dev. 2022, 42, 69. [Google Scholar] [CrossRef]
- Xu, H.X.; Zheng, X.S.; Tian, J.C.; Lai, F.X.; He, J.C.; Lv, Z.X. Advances in the development and application of control technologies for insect pest management in paddy fields in China. J. Plant Prot. 2017, 44, 925–939. [Google Scholar]
- Zhao, J.; Cai, W.L.; Shen, L.Y.; Zhu, H.Y.; Pu, L.; Xie, M.Q.; Zou, Y.L.; Hua, H.X. Current situation and prospect of green rice pest control technology. J. Huazhong Agric. Univ.
- Yu, X.P.; Hu, C.; Heong, K.L. Parasitization and preference characterisitics of egg parasitoids from various habitats to homopterans. Acta Entomol. Sin. 1998, 41, 41–47. [Google Scholar]
- Skellern, M.P.; Clark, S.J.; Ferguson, A.W.; Watts, N.P.; Cook, S.M. Banker plant bonuses? The benefits and risks of including brassicas in field margins to promote conservation biocontrol of specialist pests in oilseed rape. Insects 2023, 14, 349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ji, D.; Zhang, Q.; Jin, L. Evaluation of eleven plant species as potential banker plants to support predatory Orius sauteri in tea plant systems. Insects 2021, 12, 162. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jaworski, C.C.; Dai, H.J.; Liang, Y.Y.; Guo, X.J.; Wang, S.; Zang, L.S.; Desneux, N. Combining banker plants to achieve long-term pest control in multi-pest and multi-natural enemy cropping systems. J. Pest Sci. 2022, 95, 685–697. [Google Scholar] [CrossRef]
- Pan, M.Z.; Liu, T.X. Banker-plant system for biological control of pests in greenhouse-grown crops. Chin. J. Appl. Entomol. 2019, 56, 917–926. [Google Scholar]
- Zhu, P.Y.; Zheng, X.S.; Xie, G.; Chen, G.H.; Lu, Z.X.; Gurr, G. Relevance of the ecological traits of parasitoid wasps and nectariferous plants for conservation biological control: a hybrid meta-analysis. Pest Manag. Sci. 2020, 76, 1881–1892. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.P. Development of hybrid rice to ensure food security. Rice Sci. 2014, 21, 1–2. [Google Scholar] [CrossRef]
- Gurr, G.M.; Liu, J.; Read, D.M.Y.; Catindig, J.L.A.; Cheng, J.A.; Lan, L.P.; Heong, K.L. Parasitoids of Asian rice planthopper (Hemiptera: Delphacidae) pests and prospects for enhancing biological control by ecological engineering. Ann. Appl. Biol. 2011, 158, 149–176. [Google Scholar] [CrossRef]
- Shi, L.Q.; Liu, D.W.; Qiu, L.M.; Jiang, Z.W.; Zhan, Z.X. Evaluation of the parasitism capacity of a thelytoky egg parasitoid on a serious rice pest, Nilaparvata lugens (Stål). Animals 2023, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Stehle, S.; Schulz, R. Agricultural insecticides threaten surface waters at the global scale. Proc. Natl. Acad. Sci. 2015, 112, 5750–5755. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Fu, Q.; Lu, Z.J.; Wu, C.Q.; Li, Y.B.; Duan, D.K.; Liu, Y.K.; Zhang, Z.T. Population dynamics of Nilaparvata lugens and its two sibling species under black light trap. Chin. J. Rice Sci. 2010, 24, 315–319. [Google Scholar]
- Zheng, X.S.; Lu, Y.H.; Zhu, P.Y.; Zhang, F.C.; Tian, J.C.; Xu, H.X.; Chen, G.H.; Nansen, C.; Lv, Z.X. Use of banker plant system for sustainable management of the most important insect pest in rice fields in China. Sci. Rep. 2017, 7, 45581. [Google Scholar] [CrossRef]
- Zheng, X.S.; Tian, J.C.; Zhong, L.Q.; Xu, H.X.; Lü, Z.X. A banker plant system of ‘Leesia sayanuka-Nlilaparvata muiri-Tytthus chinensis’ to control rice planthoppers. Chin. J. Appl. Ecol. 2017, 28, 941–946. [Google Scholar]
- Frank, S.D. Biological control of arthropod pests using banker plant systems: past progress and future directions. Biol. Control. 2010, 52, 8–16. [Google Scholar] [CrossRef]
- Sullivan, C.F.; Davari, A.; Kim, J.S.; Parker, B.L.; Skinner, M. Evaluation of a guardian plant system to suppress Frankliniella occidentalis (Thysanoptera: Thripidae) in greenhouse ornamentals. Pest Manag. Sci. 2023, 79, 3559–3569. [Google Scholar] [CrossRef]
- Fauteux, A.; Fournier, M.; Soares, A.O.; Lucas, E. The right banker plant for the right application: Comparison of three candidates for aphid biocontrol, barley (Hordeum vulgare L.), corn (Zea mays L.), and finger millet (Eleusine coracana (L.) Gaertn). Pest Manag. Sci. [CrossRef]
- Cui, Y.L.; He, J.C.; Luo, J.; Lai, F.X.; Fu, Q. Host plants of Nilaparvata muiri China and N. bakeri (Muir), two sibling species of N. lugens (Stål). Chin. J. Rice Sci. 2013, 27, 105–110. [Google Scholar]
- Costamagna, A.C.; Landis, D.A.; Difonzo, C.D. Suppression of soybean aphid by generalist predators results in a trophic cascade in soybeans. Ecol. Appl. 2007, 17, 441–451. [Google Scholar] [CrossRef]
- Desneux, N.; Han, P.; Wang, S.; Li, S. Direct and indirect effects of banker plants on population establishment of Harmonia axyridis and aphid control on pepper crop. Front. Plant Sci. 2022, 13, 1083848. [Google Scholar]
- Sow, A.; Soti, V.; Thiaw, I.; Brévault, T. Non-crop habitats concurrently drive crop colonization by the millet head miner and regulation by natural enemies. Basic Appl. Ecol. 2022, 64, 45–56. [Google Scholar] [CrossRef]
- Gardiner, M.M.; Landis, D.A.; Gratton, C.; Schmidt, N.; O’Neal, M.; Mueller, E.; Chacon, J.; Heimpel, G.E. Landscape composition influences the activity density of Carabidae and Arachnida in soybean fields. Biol. Control. 2010, 55, 11–19. [Google Scholar] [CrossRef]
- Woodcock, B.A.; Redhead, J.; Vanbergen, A.J.; Hulmes, L.; Hulmes, S.; Peyton, J; Nowakowski, M. ; Pywell, R.F.; Heard, M.S. Impact of habitat type and landscape structure on biomass, species richness and functional diversity of ground beetles. Agricult. Ecosyst. Environ. 2010, 139, 181–186. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Bian, Z.X.; Wang, S.; Guo, X.Y.; Zhou, W. Effect of agricultural landscape pattern on the qualitative food web of epigaeic arthropods in low hilly areas of northern China. Ecol. Model. 2024, 488, 110574. [Google Scholar] [CrossRef]
- Takada, M.B.; Yoshioka, A.; Takagi, S.; Iwabuchi, S.; Washitani, I. Multiple spatial scale factors affecting mirid bug abundance and damage level in organic rice paddies. Biol. Control. 2012, 60, 169–174. [Google Scholar] [CrossRef]
- Zheng, X.S.; Tian, J.C.; Yang, Y.J.; Zhu, P.Y.; Li, K.; Xu, H.X.; Lü, Z.X. The feasibility of using graminaceous weeds as a functional plant for controlling rice leaffolder (Cnaphalocrocis medinalis). Sci. Agr. Sin. 2017, 50, 4129–4137. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).