1. Introduction
The significant rise in mining, pharmaceutical, industrial, and agricultural activities has raised concerns about the widespread introduction of emerging contaminants into aquatic ecosystems [
1,
2,
3,
4]. This problem not only threatens the health of ecosystems but also the health and well-being of communities that depend on water as a vital resource. In this context, accurate and timely detection of contaminants becomes crucial to mitigate associated risks and safeguard water quality for present and future generations.
Conventionally, sophisticated methods such as high-performance liquid chromatography (HPLC) and mass spectrometry have been used to detect emerging contaminants [
3,
4,
5,
6,
7]. Despite their effectiveness, these have significant drawbacks. They are expensive due to the high cost of equipment and reagents and require highly trained personnel. Additionally, these methods are labor-intensive and slow, limiting their use in situations requiring real-time or high-frequency monitoring.
Given these challenges, the search for more accessible and efficient alternatives is a priority in the field of contaminant detection. Surface-enhanced Raman spectroscopy (SERS) has emerged as a highly promising technique due to its selectivity, sensitivity, and non-destructive capacity, crucial in various applications, from environmental monitoring to medical diagnosis [
8,
9,
10,
11,
12,
13]. In this technique, the enhanced Raman signal is produced by the interaction of light with metallic nanostructures (e.g., Au, Ag and Cu), generating the excitation of surface plasmons [
10,
11,
14]. These excitations, known as surface plasmon resonances, increase the electromagnetic field on the surface of isolated nanoparticles or in the interparticle spaces (“hot spots”) [
10,
11,
15]. This phenomenon allows to increase the Raman signal of various analytes located in the vicinity of these hot spots by several orders of magnitude [
10,
11,
15,
16]. Consequently, obtaining nanostructured metallic, also known as SERS substrates, with controlled morphological and optical properties, greatly influence SERS performance [
10,
11,
15].
Typically, these substrates are produced via advanced nanofabrication techniques such as electron beam lithography, focused ion beam and UV photolithography [
11,
15,
17]. These methods offer high reproducibility and sensitivity, enabling precise control over the size, shape, and gap distance between metallic nanostructures [
11,
15,
17,
18,
19]. However, the procedures associated with these techniques are usually long, expensive, and difficult to scale. In this context, obtaining SERS substrates through solid-state dewetting stands out as a low-cost, simple, and fast alternative technique with low experimental complexity. This method involves the controlled heating of a thin layer of metallic material on a solid substrate to reduce the free energy of the environment-film-substrate system and transform the film into a set of nanoparticles or droplets [
20,
21,
22].
In this work, we demonstrate the fabrication of gold nanostructures films as SERS substrates by thermally treating gold thin films deposited on glass substrates. These SERS substrates, obtained under various synthesis conditions, were characterized for their morphological and optical properties. Subsequently, we evaluated the direct detection of rhodamine B using the SERS substrates and found that they enhance the Raman signal, achieving an intensity enhancement in a range 20 to 90 times to 6 and 3 hours, respectively.
2. Materials and Methods
2.1. Materials and Cleaning the Glass Substrate
All reagents were obtained from Chemical companies and used without further purification. The glass substrates underwent a two-stage cleaning process. Initially, a solution comprising acetone and isopropanol was prepared and introduced, along with the substrates, into a Branson M1800H ultrasonic cleaner for 15 minutes. Subsequently, the substrates were transferred to a solution of isopropanol and underwent a further 15 min ultrasonic cleaning cycle. This procedure aimed to eliminate any remaining impurities that might impact the sample quality.
2.2. Fabrication of SERS Substrate
5 mg of gold was evaporated onto glass substrates using the thermal evaporation method to produce gold thin films. The process utilized a current of 70 A under a vacuum of 10
-4 mbar. Subsequently, the prepared substrates underwent thermal treatment at 300 °C for 1 h, 3 h, 6 h, and 12 h to investigate the influence of annealing time on the SERS signal. Afterward, a 5 µL drop of Rhodamine B (RhB, C
28H
31C
lN
2O
3) solution with a concentration of 5 ppm was deposited onto the gold nanostructured films. To facilitate the adsorption of RhB onto the film surface, the solution droplet was left in contact with the film for 24 hours to air dry before spectrum acquisition. The choice of RhB was made due to its widespread use in evaluating SERS activity [
23,
24,
25,
26].
2.3. Characterization
The morphology of the films was examined using a Tescan Clara Field Emission Scanning Electron Microscope (FE-SEM). Optical characterization was performed with a Jasco V-770 UV-visible (UV-Vis) spectrometer to observe the surface plasmon resonance (SPR) of the gold nanostructures on glass substrates. SERS measurements were conducted using a Jasco RMS 4500 Raman spectrometer. The samples were irradiated with a line laser at 457.10 nm (2.72 eV) with a power of 3.5 mW. All Raman spectra were recorded with an exposure time of 5 s and 30 accumulations to facilitate visualization of the characteristic vibrational modes of RhB. Measurements were taken at three different points where the RhB droplet had dried, and an additional three spectra were obtained at zones on the film where no RhB was present.
3. Results and Discussion
3.1. Characterization of Gold SERS Substrates
3.1.1. Effect of Thermal Treatment Time
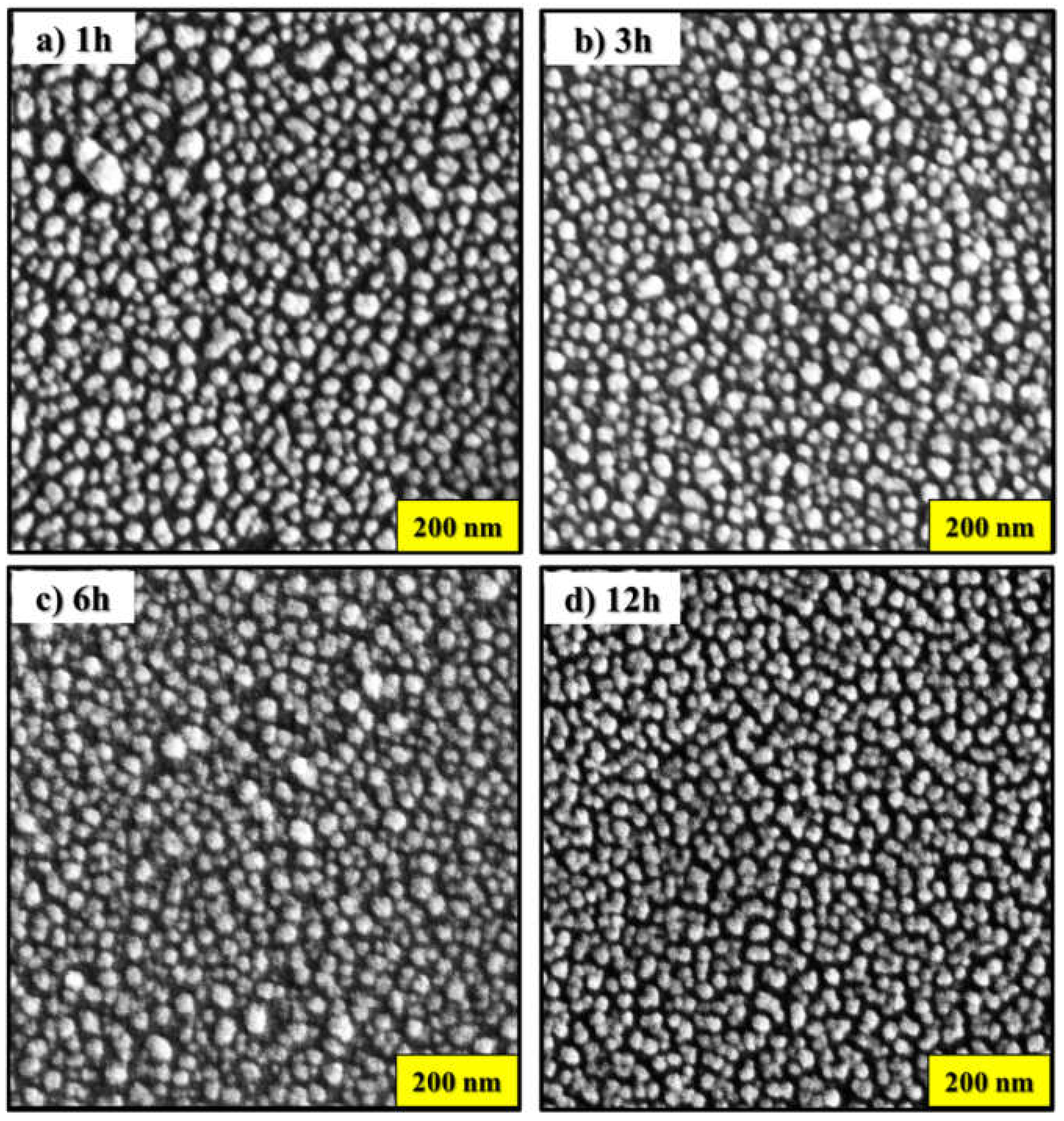
Figure 1 shows the scanning electron microscopy (SEM) images of gold nanostructures (AuNSs) deposited on glass substrates by thermal evaporation method. However, the glass substrates with AuNSs were annealed at a temperature of 300 °C. Here, nanostructure with spheroidal morphology is observed, as shown in
Figure 1a–c. Furthermore, as the time at the annealing temperature increases, some AuNSs tend to coalesce, as seen in
Figure 1d. Despite the coalescence, it can be seen from
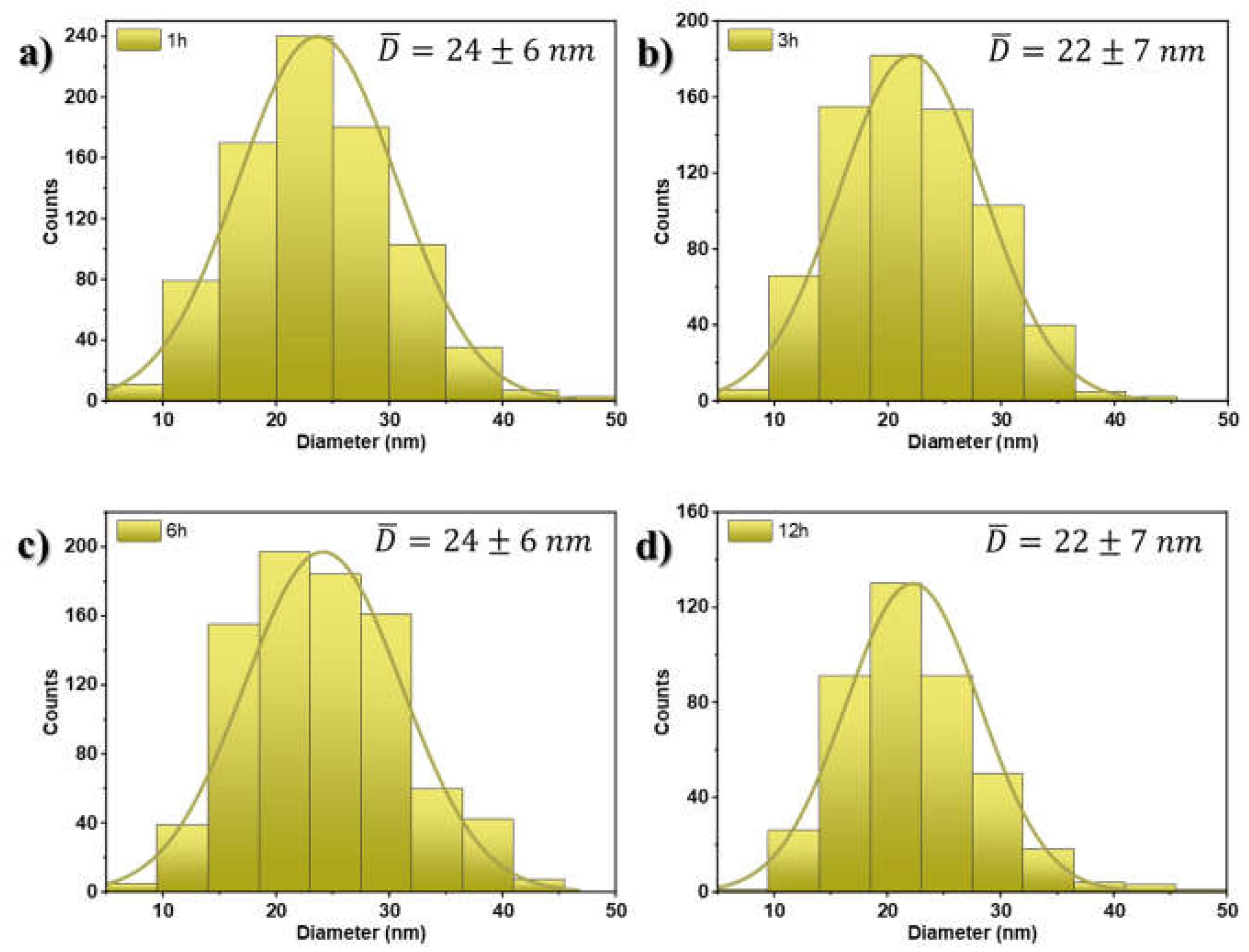
Figure 1 that there is no major and significant difference in the size and shape of AuNSs found for samples annealed at this temperature. This suggests consistency in the annealing process in terms of nanostructure morphology. Furthermore, the average particle size was estimated between 22 ± 7 nm and 24 ± 6 nm, respectively, without considering some nanoparticles with irregular shapes as the annealing time increases. The estimation of the size of the nanostructures was carried out using ImageJ Software. The histogram of the particle size distribution is shown in
Figure 2a–d. Here, the average diameters show a variation attributed to errors associated with the assumption of a spherical shape in the nanoparticles.
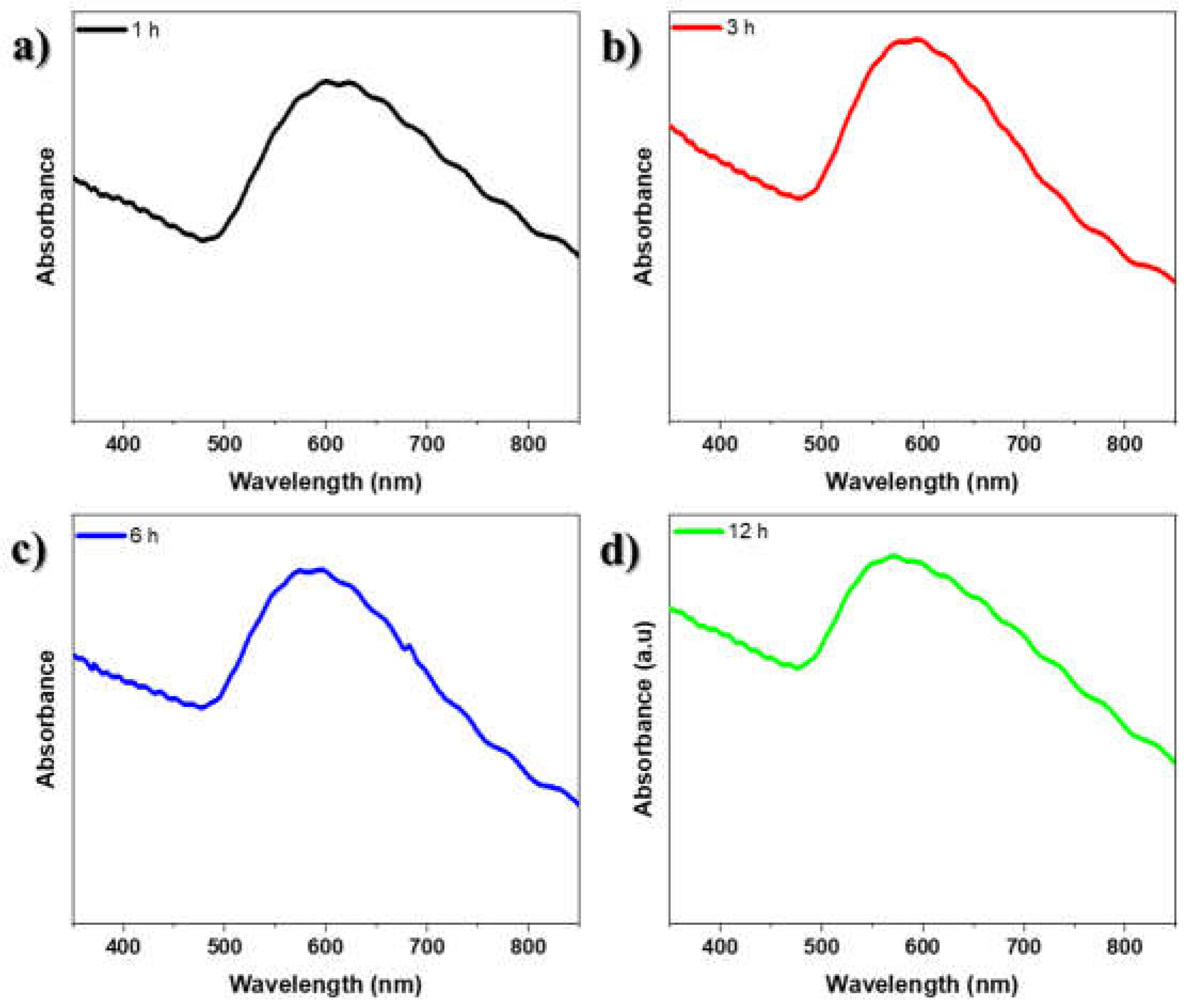
On the other hand, UV-visible absorption spectroscopy was carried out to determine the surface plasmon resonance (SPR) of the gold nanostructures on glass substrates. The SPR phenomenon exhibited by AuNSs is known to be influenced by their size and shape [
27]. Consequently, the absorption spectra of the film annealed for 1 h exhibit a strong SPR band around 600 nm (2.07 eV), attributed to AuNSs (
Figure 3a). However, for AuNSs annealed at 3 h, 6 h, and 12 h, the observed SPR bands were located around 588 nm (2.11 eV), 590 nm (2.10 eV), and 575 nm (2.16 eV), as depicted in
Figure 3c–d. Additionally, the SPR band indicated that the supported AuNSs on the glass substrate were approximately spheroidal in shape, as observed in the SEM images. Notably,
Figure 3 shows no significant difference in the absorption spectrum for the sample annealed for 12 h, consistent with the morphological findings. Furthermore, a similar trend is observed for the other samples. However, according to the shape and position of the SPR band, a shift is observed with increased annealed time. Moreover, the undulations observed in the absorbance spectra could be attributed to both the size of the gold nanostructures and the roughness of their surface.
3.2. SERS Spectra of RhB Adsorbed on AuNSs
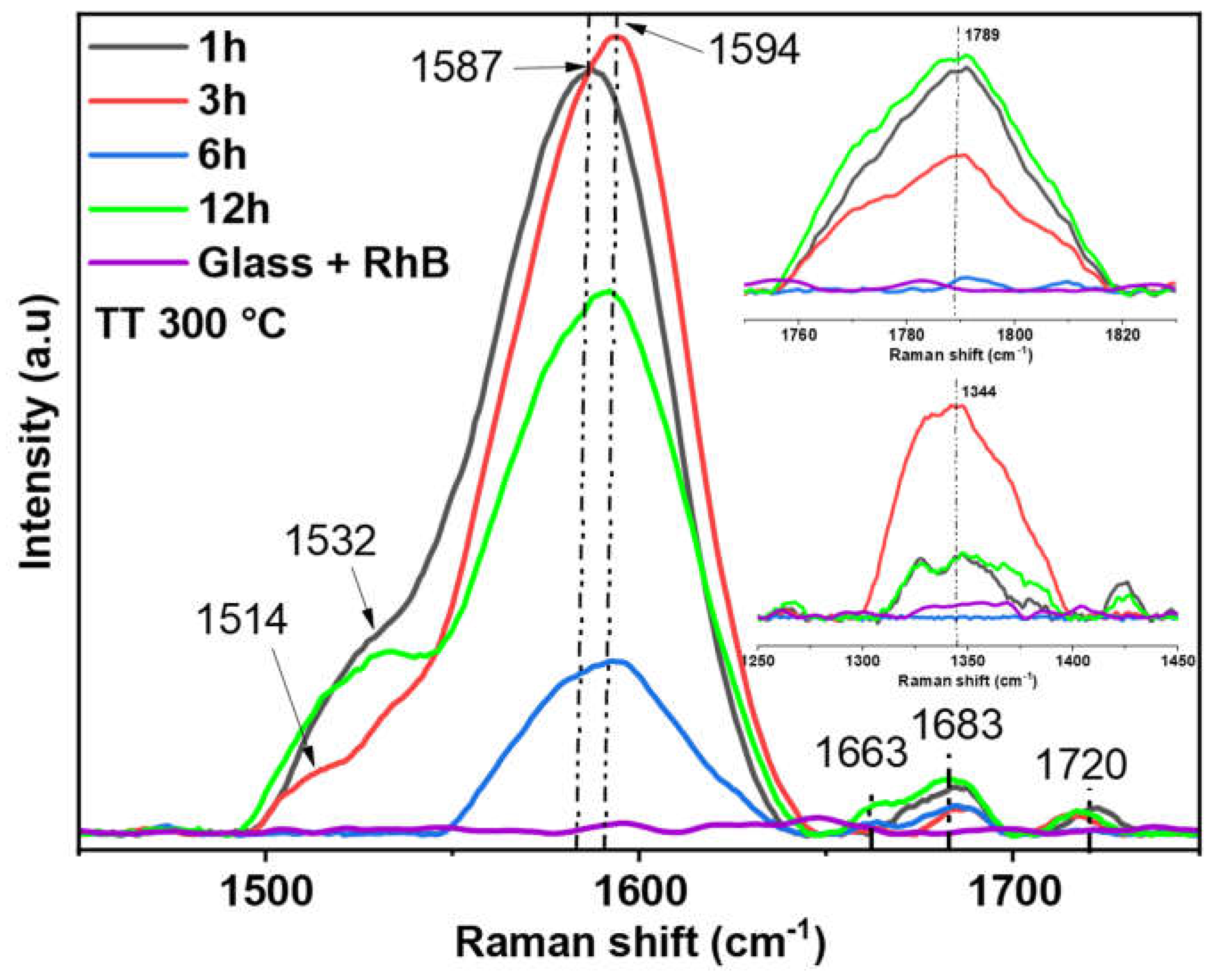
Micro-Raman spectroscopy was utilized to characterize the surface-enhanced Raman scattering (SERS) activities of gold nanostructures fabricated on glass substrates, with Rhodamine B employed as the labeling molecule.
Figure 4 displays the Raman scattering spectra of RhB adsorbed on AuNSs annealed at 300 °C for 1 h, 3 h, 6 h, and 12 h, respectively. Additionally, no SERS signals were detected for the AuNSs without RhB. It is observed that the SERS spectra exhibit similarities, although certain vibrational modes appear more intense than others. This discrepancy in intensity can be attributed to the spatial orientation of the RhB molecule relative to the substrate, which influences the Raman signal. Furthermore, by examining the annealing time of each SERS substrate with the Raman signal obtained from RhB, it is inferred that 1 h or 3 h is sufficient to achieve a good SERS substrate and, therefore, detect the molecule’s Raman signal. It is also important to consider that SERS sensing could be greatly affected by the size and morphology of the substrates used. Hence, the SERS response of this molecule could be attributed solely to the properties of the substrate, without additional contributions from the molecule itself [
28]. The bands observed in the SERS spectra closely match those reported in previous Raman studies [
23,
25,
28,
29,
30]; however, some bands exhibit a slight shift and broadening, attributed to the interaction between the analyte molecules and the AuNSs. Therefore, characteristic vibrational modes and their assignment are shown in
Figure 4 and
Table 1. However, a few new vibrational modes appear at around 1683, 1720 and 1789 cm–1 respectively. The new vibrational modes could be attributed to the interaction of the carboxyl group with Cl– [
23]. Furthermore, this suggests a possible change of the electronic state or local symmetry of the RhB molecule, given that the SERS spectrum can be influenced by AuNSs in the probed area owing to variations in plasmon resonance conditions and population of the hot spot. Therefore, considering the nature of the fabricated substrates, it is reasonable to observe fluctuations in the spectral shapes from the distinct experiments. Nevertheless, these spectral variations can yield unique features depending on the analysis conditions employed.
Taking the above into account, our results demonstrate that the intensity of the Raman signal varies from 20 to 90 times for the 6-hour and 3-hour treatments, respectively.
4. Conclusions
In this study, we illustrated the potential of thin films composed of gold nanostructures as highly efficient and appropriate substrates for fabricating Surface-Enhanced Raman Spectroscopy (SERS) platforms aimed at detecting emerging contaminants in water. A significant advantage of these AuNSs thin films lies in their ability to strongly enhance SERS signals, particularly for Rhodamine B detection. Our results show a range of variation of the intensity of the Raman signal from 20 to 90 times for 6 and 3 hours, respectively. Experimental findings underscored that the enhancement of SERS is closely linked to the coupling of particles at the nanoscale level. Furthermore, alterations observed in the SERS spectrum upon sample drying are attributed to the dynamic behavior of RhB molecules, potentially linked to changes in molecular adsorption and surface distribution, which manifest through distinct plasmonic resonances. Additionally, new vibrational modes were identified and documented in this study.
Author Contributions
Conceptualization, Wilkendry Cervantes, Lorena Marín, Jesús Diosa, Luis Rodríguez and Edgar Vargas; Formal analysis, Cristhian Visbal, Wilkendry Cervantes, Lorena Marín and Edgar Vargas; Funding acquisition, Jesús Diosa, Luis Rodríguez and Edgar Vargas; Investigation, Cristhian Visbal, Wilkendry Cervantes, Lorena Marín, John Betancourt, Angélica Pérez, Jesús Diosa, Luis Rodríguez and Edgar Vargas; Methodology, Cristhian Visbal, Wilkendry Cervantes, Lorena Marín, John Betancourt, Luis Rodríguez and Edgar Vargas; Project administration, Jesús Diosa; Resources, Lorena Marín, Jesús Diosa and Edgar Vargas; Supervision, Lorena Marín, Jesús Diosa, Luis Rodríguez and Edgar Vargas; Validation, Wilkendry Cervantes, Lorena Marín, Luis Rodríguez and Edgar Vargas; Writing – original draft, Cristhian Visbal, Wilkendry Cervantes, Lorena Marín and Edgar Vargas; Writing – review & editing, Cristhian Visbal, Wilkendry Cervantes, Lorena Marín and Edgar Vargas.. All authors have read and agreed to the published version of the manuscript.
Funding
This APC was funded by Sistema General de Regalías, through the projects with BPIN 2021000100424.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful for the financial support of the CTel Fund (Sistema General de Regalías) through the projects SGR BPIN 2021000100424, Fortalecimiento de la Alianza CENM - INCIMAR para incrementar la capacidad de investigación y desarrollo tecnológico en remediación de contaminantes emergentes en agua en el Valle del Cauca. We are also grateful to Universidad del Valle and the Centro de Excelencia en Nuevos Materiales (CENM) for providing laboratory support. E. Mosquera would like to thank Dr. Mario Millan for his valuable comments on the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- H. A. Khan and R. Barros, “Pharmaceuticals in Water: Risks to Aquatic Life and Remediation Strategies,” Hydrobiology, vol. 2, no. 2, pp. 395–409, Jun. 2023. [CrossRef]
- S. Sharma and A. Bhattacharya, “Drinking water contamination and treatment techniques,” Applied Water Science, vol. 7, no. 3. Springer Verlag, pp. 1043–1067, Jun. 01, 2017. [CrossRef]
- M. Patel, R. Kumar, K. Kishor, T. Mlsna, C. U. Pittman, and D. Mohan, “Pharmaceuticals of emerging concern in aquatic systems: Chemistry, occurrence, effects, and removal methods,” Chemical Reviews, vol. 119, no. 6. American Chemical Society, pp. 3510–3673, Mar. 27, 2019. [CrossRef]
- “Pharmaceuticals in drinking-water,” 2012.
- S. N. Zulkifli, H. A. Rahim, and W. J. Lau, “Detection of contaminants in water supply: A review on state-of-the-art monitoring technologies and their applications,” Sensors and Actuators, B: Chemical, vol. 255. Elsevier B.V., pp. 2657–2689, Feb. 01, 2018. [CrossRef]
- D. Kadadou, L. Tizani, H. Alsafar, and S. W. Hasan, “Analytical methods for determining environmental contaminants of concern in water and wastewater,” MethodsX, vol. 12, Jun. 2024. [CrossRef]
- D. Fatta, A. Achilleos, A. Nikolaou, and S. Meriç, “Analytical methods for tracing pharmaceutical residues in water and wastewater,” TrAC - Trends in Analytical Chemistry, vol. 26, no. 6, pp. 515–533, Jun. 2007. [CrossRef]
- L. Dykman and N. Khlebtsov, “Gold nanoparticles in biomedical applications: Recent advances and perspectives,” Chem Soc Rev, vol. 41, no. 6, pp. 2256–2282, Feb. 2012. [CrossRef]
- Z. Huang, A. Zhang, Q. Zhang, and D. Cui, “Nanomaterial-based SERS sensing technology for biomedical application,” Journal of Materials Chemistry B, vol. 7, no. 24. Royal Society of Chemistry, pp. 3755–3774, 2019. [CrossRef]
- K. Kneipp, “Surface-enhanced raman scattering,” Phys Today, vol. 60, no. 11, pp. 40–46, 2007. [CrossRef]
- L. Haynes, A. D. Mcfarland, and R. P. Van Duyne, “RAMAN SPECTROSCOPY,” 2005.
- Liu, D. Xu, X. Dong, and Q. Huang, “A review: Research progress of SERS-based sensors for agricultural applications,” Trends in Food Science and Technology, vol. 128. Elsevier Ltd., pp. 90–101, Oct. 01, 2022. [CrossRef]
- G. Barbillon and H. Cheap-Charpentier, “Advances in Surface-Enhanced Raman Scattering Sensors of Pollutants in Water Treatment,” Nanomaterials, vol. 13, no. 17. Multidisciplinary Digital Publishing Institute (MDPI), Sep. 01, 2023. [CrossRef]
- P. A. Mosier-Boss, “Review of SERS substrates for chemical sensing,” Nanomaterials, vol. 7, no. 6. MDPI AG, Jun. 08, 2017. [CrossRef]
- R. C. Maher, “SERS hot spots,” in Raman Spectroscopy for Nanomaterials Characterization, vol. 9783642206207, Springer-Verlag Berlin Heidelberg, 2011, pp. 215–260. [CrossRef]
- A. M. Michaels, M. Nirmal, and L. E. Brus, “Surface enhanced Raman spectroscopy of individual rhodamine 6G molecules on large Ag nanocrystals,” J Am Chem Soc, vol. 121, no. 43, pp. 9932–9939, Nov. 1999. [CrossRef]
- J. S. Hwang and M. Yang, “Sensitive and reproducible gold SERS sensor based on interference lithography and electrophoretic deposition,” Sensors (Switzerland), vol. 18, no. 11, 2018. [CrossRef]
- K. Wang, Q. Ma, C. X. Qu, H. T. Zhou, M. Cao, and S. D. Wang, “Review on 3D Fabrication at Nanoscale,” Autex Research Journal, vol. 23, no. 3. Sciendo, pp. 350–369, Sep. 01, 2023. [CrossRef]
- G. Schmid, Nanotechnology. Wiley-VCH, 2008.
- W. Jiang, Y. Wang, D. J. Srolovitz, and W. Bao, “Solid-state dewetting on curved substrates,” Phys Rev Mater, vol. 2, no. 11, Nov. 2018. [CrossRef]
- H. A. El-Sayed, C. A. Horwood, E. Owusu-Ansah, Y. J. Shi, and V. I. Birss, “Gold nanoparticle array formation on dimpled Ta templates using pulsed laser-induced thin film dewetting,” Physical Chemistry Chemical Physics, vol. 17, no. 16, pp. 11062–11069, Apr. 2015. [CrossRef]
- V. Thompson, “Solid-state dewetting of thin films,” Annual Review of Materials Research, vol. 42. pp. 399–434, Aug. 2012. [CrossRef]
- Y. Ma, W. Hu, X. N. Song, and C. K. Wang, “Density functional theory study on Raman spectra of rhodamine molecules in different forms,” Chinese Journal of Chemical Physics, vol. 27, no. 3, pp. 291–296, Jun. 2014. [CrossRef]
- H. Wang, X. Guo, S. Fu, T. Yang, Y. Wen, and H. Yang, “Optimized core-shell Au@Ag nanoparticles for label-free Raman determination of trace Rhodamine B with cancer risk in food product,” Food Chem, vol. 188, pp. 137–142, Apr. 2015. [CrossRef]
- C. H. Sun, M. L. Wang, Q. Feng, W. Liu, and C. X. Xu, “Surface-enhanced Raman scattering (SERS) study on Rhodamine B adsorbed on different substrates,” Russian Journal of Physical Chemistry A, vol. 89, no. 2, pp. 291–296, Feb. 2015. [CrossRef]
- M. Liu et al., “Fabrication, characterization, and high temperature surface enhanced Raman spectroscopic performance of SiO2 coated silver particles,” Nanoscale, vol. 10, no. 12, pp. 5449–5456, Mar. 2018. [CrossRef]
- V. Amendola and M. Meneghetti, “Size evaluation of gold nanoparticles by UV-vis spectroscopy,” Journal of Physical Chemistry C, vol. 113, no. 11, pp. 4277–4285, Mar. 2009. [CrossRef]
- Li, D.W. Li, Y. Li, J. S. Fossey, and Y. T. Long, “Cyclic electroplating and stripping of silver on Au@SiO2 core/shell nanoparticles for sensitive and recyclable substrate of surface-enhanced Raman scattering,” J Mater Chem, vol. 20, no. 18, pp. 3688–3693, 2010. [CrossRef]
- S. Lin et al., “Rapid and sensitive SERS method for determination of Rhodamine B in chili powder with paper-based substrates,” Analytical Methods, vol. 7, no. 12, pp. 5289–5294, Jun. 2015. [CrossRef]
- C. Fang et al., “DNA detection using nanostructured SERS substrates with Rhodamine B as Raman label,” Biosens Bioelectron, vol. 24, no. 2, pp. 216–221, Oct. 2008. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).