Submitted:
20 May 2024
Posted:
21 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
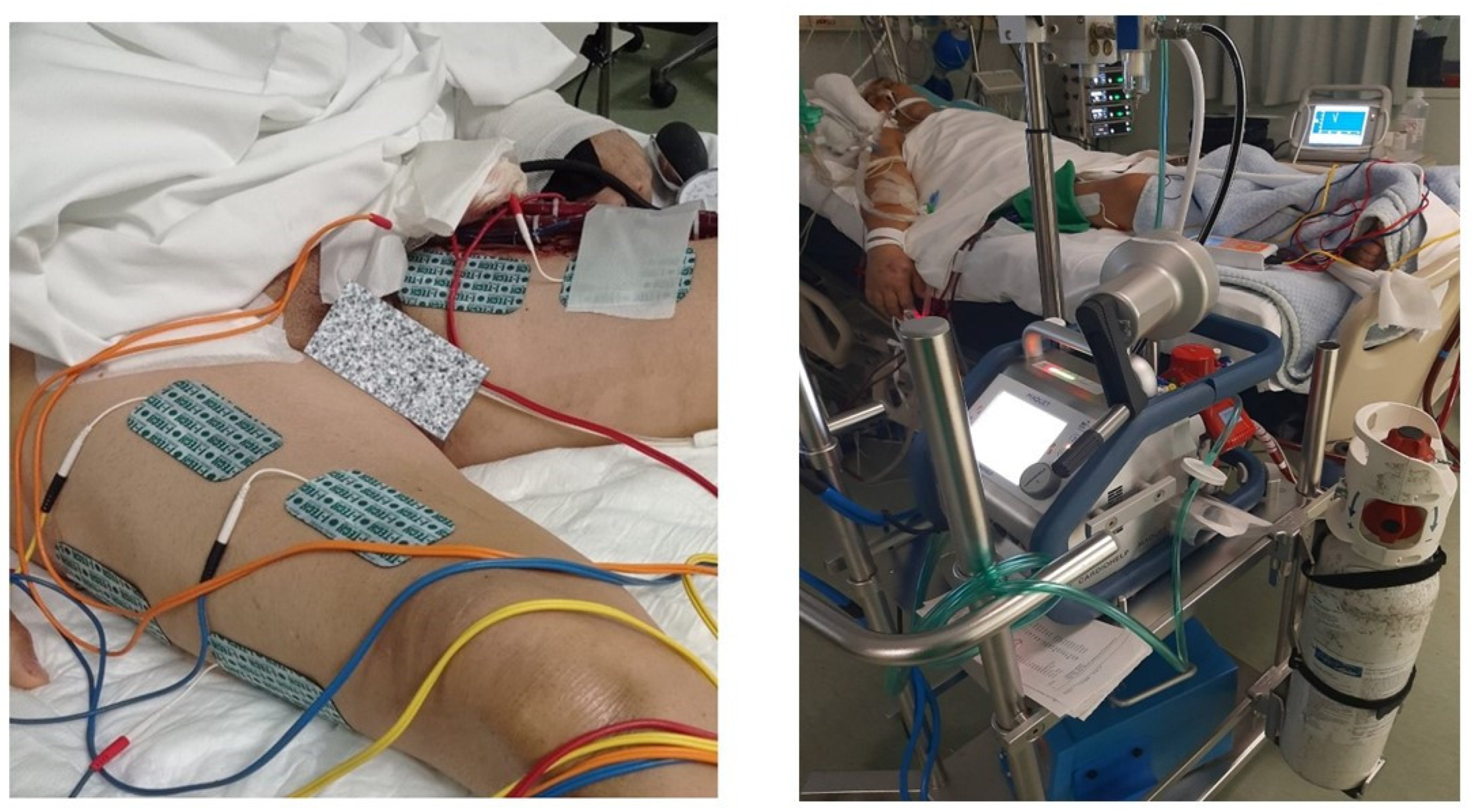
2.3. NMES Protocol
2.4. Outcomes
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nussbaum, E.L.; Houghton, P.; Anthony, J.; Rennie, S.; Shay, B.L.; Hoens, A.M. Neuromuscular Electrical Stimulation for Treatment of Muscle Impairment: Critical Review and Recommendations for Clinical Practice. Physiother. Can. 2017, 69, 1–76. [Google Scholar] [CrossRef] [PubMed]
- The Association of Paediatric Charted Physiotherapists. Publications. Available from: https://apcp.csp.org.uk/content/guide-use-electrical-stimulation-paediatric-neurodisabilityn (accessed 18/12/2022).
- Osman, H.; Siu, R.; Makowski, N.S.; Knutson, J.S.; Cunningham, D.A. Neurostimulation After Stroke. Phys. Med. Rehabil. Clin. N. Am. 2024, 35, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Ploesteanu, R.L.; Nechita, A.C.; Turcu, D.; Manolescu, B.N.; Stamate, S.C.; Berteanu, M. Effects of neuromuscular electrical stimulation in patients with heart failure - review. J. Med. Life 2018, 11, 107–118. [Google Scholar] [PubMed]
- LoMauro, A.; Gervasoni, F. 20 years of neuromuscular electrical stimulation in COPD. Eur. Respir. Rev. 2024, 33, 220247. [Google Scholar] [CrossRef] [PubMed]
- Gerovasili, V.; Stefanidis, K.; Vitzilaios, K.; Karatzanos, E.; Politis, P.; Koroneos, A.; Chatzimichail, A.; Routsi, C.; Roussos, C.; Nanas, S. Electrical muscle stimulation preserves the muscle mass of critically ill patients: a randomized study. Crit. Care 2009, 13, R161. [Google Scholar] [CrossRef] [PubMed]
- Karatzanos, E.; Gerovasili, V.; Zervakis, D.; Tripodaki, E.S.; Apostolou, K.; Vasileiadis, I.; Papadopoulos, E.; Mitsiou, G.; Tsimpouki, D.; Routsi, C.; et al. Electrical muscle stimulation: an effective form of exercise and early mobilization to preserve muscle strength in critically ill patients. Crit. Care Res. Pract. 2012, 2012, 432752. [Google Scholar] [CrossRef]
- Rodriguez, P.O.; Setten, M.; Maskin, L.P.; Bonelli, I.; Vidomlansky, S.R.; Attie, S.; Frosiani, S.L.; Kozima, S.; Valentini, R. Muscle weakness in septic patients requiring mechanical ventilation: protective effect of transcutaneous neuromuscular electrical stimulation. J. Crit. Care 2012, 27, 319.e1-8. [Google Scholar] [CrossRef] [PubMed]
- Kourek, C.; Kanellopoulos, M.; Raidou, V.; Antonopoulos, M.; Karatzanos, E.; Patsaki, I.; Dimopoulos, S. Safety and effectiveness of neuromuscular electrical stimulation in cardiac surgery: A systematic review. World J. Cardiol. 2024, 16, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Hayes, K.; Holland, A.E.; Pellegrino, V.A.; Mathur, S.; Hodgson, C.L. Acute skeletal muscle wasting and relation to physical function in patients requiring extracorporeal membrane oxygenation (ECMO). J. Crit. Care 2018, 48, 1–8. [Google Scholar] [CrossRef]
- Chen, X.; Lei, X.; Xu, X.; Zhou, Y.; Huang, M. Intensive Care Unit-Acquired Weakness in Patients With Extracorporeal Membrane Oxygenation Support: Frequency and Clinical Characteristics. Front. Med. (Lausanne) 2022, 9, 792201. [Google Scholar] [CrossRef]
- Segers, J.; Hermans, G.; Bruyninckx, F.; Meyfroidt, G.; Langer, D.; Gosselink, R. Feasibility of neuromuscular electrical stimulation in critically ill patients. J. Crit. Care 2014, 29, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Parry, S.M.; Berney, S.; Warrillow, S.; El-Ansary, D.; Bryant, A.L.; Hart, N.; Puthucheary, Z.; Koopman, R.; Denehy, L. Functional electrical stimulation with cycling in the critically ill: a pilot case-matched control study. J. Crit. Care 2014, 29, 695–e1. [Google Scholar] [CrossRef]
- McCormack, P.F.; Tronstad, O.; Walsh, J.R. Does exercising the quadriceps muscle of patients on extracorporeal membrane oxygenation (ECMO) with electrical stimulation affect the blood flow to their feet? A feasibility study. J. Intensive Care Soc. 2023, 24, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Herridge, M.S.; Tansey, C.M.; Matté, A.; Tomlinson, G.; Diaz-Granados, N.; Cooper, A.; Guest, C.B.; Mazer, C.D.; Mehta, S.; Stewart, T.E.; et al. Functional disability 5 years after acute respiratory distress syndrome. N. Engl. J. Med. 2011, 364, 1293–304. [Google Scholar] [CrossRef]
- Nanas, S.; Kritikos, K.; Angelopoulos, E.; Siafaka, A.; Tsikriki, S.; Poriazi, M.; Kanaloupiti, D.; Kontogeorgi, M.; Pratikaki, M.; Zervakis, D.; et al. Predisposing factors for critical illness polyneuromyopathy in a multidisciplinary intensive care unit. Acta Neurol. Scand. 2008, 118, 175–181. [Google Scholar] [CrossRef] [PubMed]
- de Jonghe, B.; Lacherade, J.C.; Sharshar, T.; Outin, H. Intensive care unit-acquired weakness: risk factors and prevention. Crit. Care Med. 2009, 37, S309–315. [Google Scholar] [CrossRef]
- Deem, S. Intensive-care-unit-acquired muscle weakness. Respir. Care 2006, 51, 1042–1052; discussion 1052-1053. [Google Scholar] [PubMed]
- Lee, C.M.; Fan, E. ICU-acquired weakness: what is preventing its rehabilitation in critically ill patients? BMC Med. 2012, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Kourek, C.; Nanas, S.; Kotanidou, A.; Raidou, V.; Dimopoulou, M.; Adamopoulos, S.; Karabinis, A.; Dimopoulos, S. Modalities of Exercise Training in Patients with Extracorporeal Membrane Oxygenation Support. J. Cardiovasc. Dev. Dis. 2022, 9, 34. [Google Scholar] [CrossRef]
- Routsi, C.; Gerovasili, V.; Vasileiadis, I.; Karatzanos, E.; Pitsolis, T.; Tripodaki, E.; Markaki, V.; Zervakis, D.; Nanas, S. Electrical muscle stimulation prevents critical illness polyneuromyopathy: a randomized parallel intervention trial. Crit. Care 2010, 14, R74. [Google Scholar] [CrossRef]
- Baldwin, E.R.; Klakowicz, P.M.; Collins, D.F. Wide-pulse-width, high-frequency neuromuscular stimulation: implications for functional electrical stimulation. J. Appl. Physiol. (1985) 2006, 101, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Bowman, B.R.; Baker, L.L. Effects of waveform parameters on comfort during transcutaneous neuromuscular electrical stimulation. Ann. Biomed. Eng. 1985, 13, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Panizza, M.; Nilsson, J.; Roth, B.J.; Basser, P.J.; Hallett, M. Relevance of stimulus duration for activation of motor and sensory fibers: implications for the study of H-reflexes and magnetic stimulation. Electroencephalogr. Clin. Neurophysiol. 1992, 85, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulos, E.; Karatzanos, E.; Dimopoulos, S.; Mitsiou, G.; Stefanou, C.; Patsaki, I.; Kotanidou, A.; Routsi, C.; Petrikkos, G.; Nanas, S. Acute microcirculatory effects of medium frequency versus high frequency neuromuscular electrical stimulation in critically ill patients - a pilot study. Ann. Intensive Care 2013, 3, 39. [Google Scholar] [CrossRef] [PubMed]
- Stefanou, C.; Karatzanos, E.; Mitsiou, G.; Psarra, K.; Angelopoulos, E.; Dimopoulos, S.; Gerovasili, V; Boviatsis, E. ; Routsi, C.; Nanas, S. Neuromuscular electrical stimulation acutely mobilizes endothelial progenitor cells in critically ill patients with sepsis. Ann. Intensive Care 2016, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Peckham, P.H.; Mortimer, J.T.; Marsolais, E.B. Alteration in the force and fatigability of skeletal muscle in quadriplegic humans following exercise induced by chronic electrical stimulation. Clin. Orthop. Relat. Res. 1976, 114, 326–33. [Google Scholar] [CrossRef]
- Gondin, J.; Brocca, L.; Bellinzona, E.; D’Antona, G.; Maffiuletti, N.A.; Miotti, D.; Pellegrino, M.A.; Bottinelli, R. Neuromuscular electrical stimulation training induces atypical adaptations of the human skeletal muscle phenotype: a functional and proteomic analysis. J. Appl. Physiol. (1985) 2011, 110, 433–450. [Google Scholar] [CrossRef]
- Arija-Blázquez, A.; Ceruelo-Abajo, S.; Díaz-Merino, M.S.; Godino-Durán, J.A.; Martínez-Dhier, L.; Martin, J.L.; Florensa-Vila, J. Effects of electromyostimulation on muscle and bone in men with acute traumatic spinal cord injury: A randomized clinical trial. J. Spinal Cord Med. 2014, 37, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Meijer, J.W.; Voerman, G.E.; Santegoets, K.M.; Geurts, A.C. Short-term effects and long-term use of a hybrid orthosis for neuromuscular electrical stimulation of the upper extremity in patients after chronic stroke. J. Rehabil. Med. 2009, 41, 157–161. [Google Scholar] [CrossRef]
- Wang, J.S.; Chen, S.Y.; Lan, C.; Wong, M.K.; Lai, J.S. Neuromuscular electric stimulation enhances endothelial vascular control and hemodynamic function in paretic upper extremities of patients with stroke. Arch. Phys. Med. Rehabil. 2004, 85, 1112–1116. [Google Scholar] [CrossRef]
- Rushton, D.N. Functional electrical stimulation and rehabilitation--an hypothesis. Med. Eng. Phys. 2003, 25, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Harper, N.J.; Greer, R.; Conway, D. Neuromuscular monitoring in intensive care patients: milliamperage requirements for supramaximal stimulation. Br. J. Anaesth. 2001, 87, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Burgess, L.C.; Immins, T.; Swain, I.; Wainwright, T.W. Effectiveness of neuromuscular electrical stimulation for reducing oedema: A systematic review. J. Rehabil. Med. 2019, 51, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Gerovasili, V.; Tripodaki, E.; Karatzanos, E.; Pitsolis, T.; Markaki, V.; Zervakis, D.; Routsi, C.; Roussos, C.; Nanas, S. Short-term systemic effect of electrical muscle stimulation in critically ill patients. Chest 2009, 136, 1249–1256. [Google Scholar] [CrossRef]
- Georgopoulos, C.; Katsogianni, A.; Patsaki, E.; Sidiras, G.; Vasileiadis, I.; Magira, E.; Nanas, S.; Karatzanos, E. High- vs medium-frequency neuromuscular electrical stimulation protocols on muscle mass in Intensive Care Unit patients, a pilot study. Health Res. J. 2023, 9, 219–236. [Google Scholar] [CrossRef]
| Demographic characteristics | |
|---|---|
| Number of patients (N) | 16 |
| Gender (Females) | 10 (63%) |
| Age (years)a | 46.6 ± 13.7 |
| BMI (kg/m2)a | 25.8 ± 3.1 |
| Cause of ECMO | |
| Cardiogenic shock | 11 (69%) |
| Respiratory failure | 5 (31%) |
| ECMO parameters | |
| FiO2 (%)a | 71.6 ± 19.1 |
| LPM (L/min)a | 2.62 ± 1.03 |
| RPM (rounds/min)a | 2478.0 ± 611.5 |
| Type of ECMO | |
| VA-ECMO (femo-femoral) | 13 (81%) |
| VV-ECMO (femo-jugular) | 3 (19%) |
| Duration of ECMO (days)b | 8 (5-28.75) |
| Ventilation during NMES | 13 (81%) |
| Sedation during NMES | 9 (56%) |
| Type of muscle contractionb | |
| Warm up | 4 (3-4) |
| Main phase | 5 (4-5) |
| Recovery phase | 4 (2-4) |
| Amplitude of NMESb | |
| Warm up (mA) | 30 (20-55) |
| Main phase (mA) | 53 (30-86) |
| Recovery phase (mA) | 43 (36-74) |
| Hemodynamic parametersa | During NMES | ||||
|---|---|---|---|---|---|
| Before NMES | 5 min | 35 min | 40 min | P value | |
| Systolic BP (mmHg) | 114.1 ± 26.7 | 114.8 ± 22.3 | 114.4 ± 25.2 | 112.2 ± 21.1 | 0.484 |
| Diastolic BP (mmHg) | 68.4 ± 15.4 | 67.6 ± 17.5 | 66.8 ± 15.9 | 65.9 ± 16.1 | 0.181 |
| Mean BP (mmHg) | 82.1 ± 18.2 | 82.6 ± 15.3 | 82.7 ± 16.4 | 82.0 ± 16.0 | 0.964 |
| HR (beats/min) | 102.4 ± 19.7 | 101.1 ± 20.1 | 101.4 ± 20.5 | 100.2 ± 19.5 | 0.475 |
| SpO2 (%) | 95.1 ± 10.4 | 96.7 ± 3.8 | 97.2 ± 3.4 | 97.2 ± 3.2 | 0.442 |
| RR (breaths/min) | 17.3 ± 6.2 | 18.3 ± 5.5 | 18.9 ± 6.7 | 17.9 ± 6.2 | 0.457 |
| ABG indicesa | Before NMES | After NMES | P value | ||
| Arterial blood pH | 7.43 ± 0.07 | 7.45 ± 0.06 | 0.468 | ||
| Lactate (mmol/L) | 1.68 ± 0.78 | 1.44 ± 0.60 | 0.242 | ||
| Biochemical parametersb | Before NMES | After NMES | P value | ||
| CPK (IU/L) | 327.5 (113.0-1234.5) | 257.5 (129.5-637.5) | 0.657 | ||
| AST-SGOT (IU/L) | 70.0 (32.0-118.8) | 57.5 (33.0-110.3) | 0.470 | ||
| ALT-SGPT (IU/L) | 50.0 (25.3-280.5) | 43.0 (23.8-136.3) | 0.006 | ||
| LDH (IU/L) | 488.0 (288.0-776.5) | 556.5 (321.5-756.8) | 0.972 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





