Submitted:
20 May 2024
Posted:
21 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Endocannabinoid System and Its Association with Alzheimer’s Disease
3. Therapeutic Potential of Cannabinoids in Alzheimer’s Disease
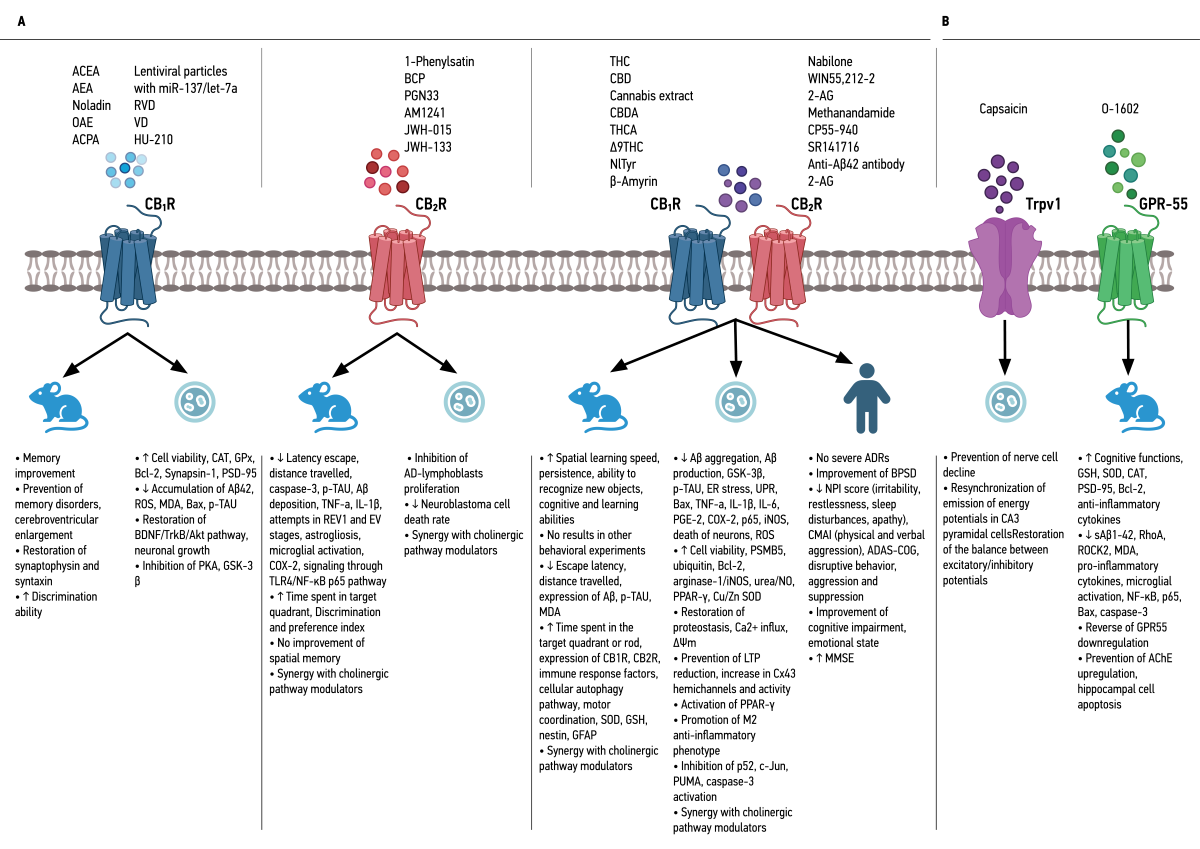
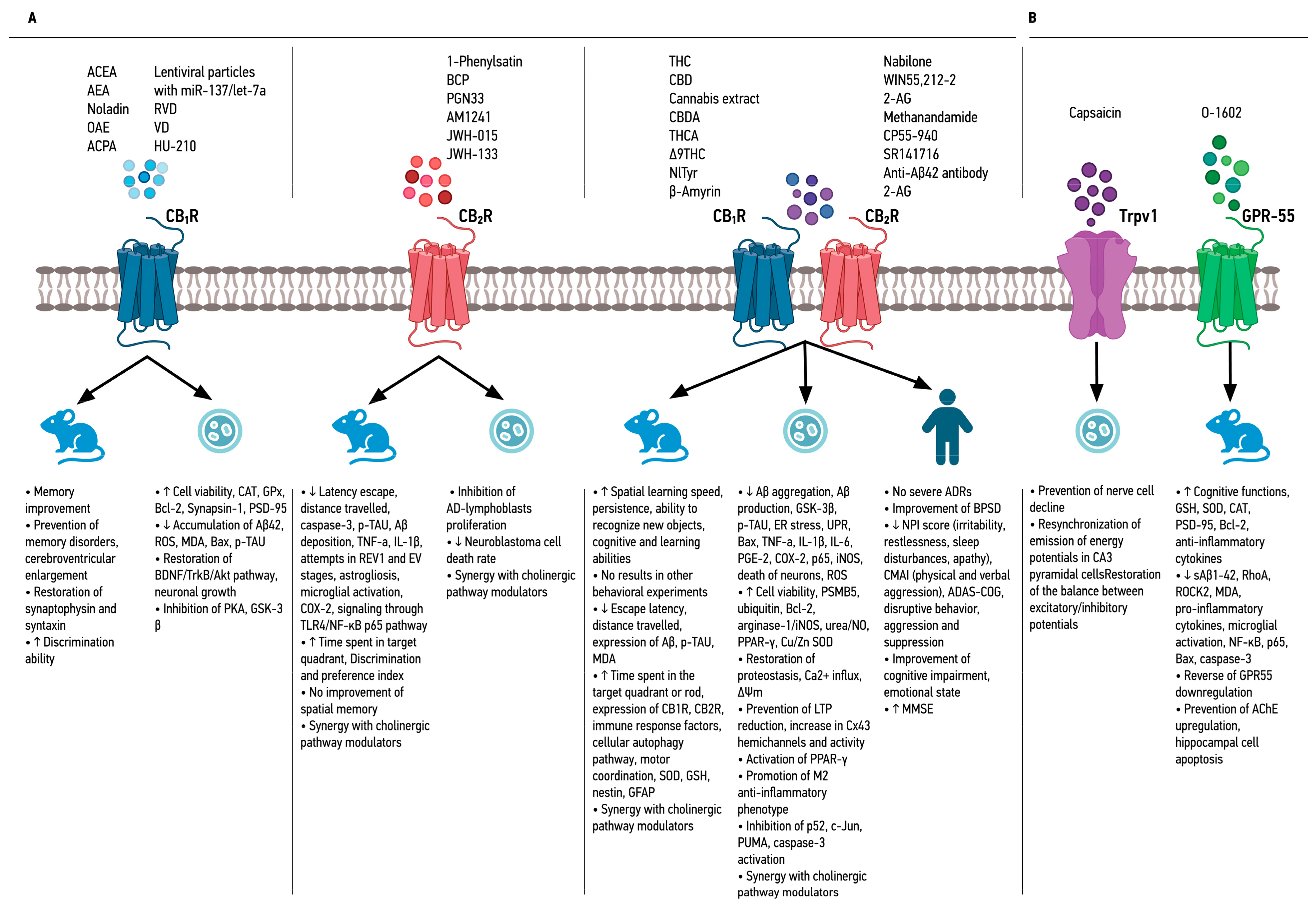
3.1. Selective Agonists of Cannabinoid Receptor 1 (CB1R)
3.2. Selective Agonists of Cannabinoid Receptor 2 (CB2R)
3.3. Agonists of Cannabinoid Receptor 2 (CB2R) Associated with Cholinergic Pathways
3.4. Non-Selective Agonists of Cannabinoid Receptors 1 and 2 (CB1R and CB2R)
3.5. Non-Selective Agonists of Cannabinoid Receptors 1 and 2 (CB1R and CB2R) Related to Cholinergic Pathways
3.6. Molecules That Act through Pathways Related to the Endocannabinoid System
3.7. Combination Studies of Agonists of Different Classes
4. Discussion
5. Conclusions and Future directions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders : DSM-5. 5th ed. American Psychiatric Association; 2013.
- Dunne, T.E. Alzheimer’s Disease: An Overview. Encyclopedia of Mental Health 2016. [CrossRef]
- Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s and Dementia. 2011;7(3):280-292. [CrossRef]
- Takahashi RH, Nagao T, Gouras GK. Plaque formation and the intraneuronal accumulation of β-amyloid in Alzheimer’s disease. Pathol Int. 2017;67(4):185-193. [CrossRef]
- Kim T, Vidal GS, Djurisic M, et al. Human LilrB2 is a β-amyloid receptor and its murine homolog PirB regulates synaptic plasticity in an Alzheimer’s model. Science (1979). 2013;341(6152):1399-1404. [CrossRef]
- Selkoe DJ. THE GENETICS AND MOLECULAR PATHOLOGY OF ALZHEIMER’S DISEASE: Roles of Amyloid and the Presenilins. Neurol Clin. 2000;18(4):903-921. [CrossRef]
- de Paula V de JR, Guimarães FM, Diniz BS, Forlenza OV. Neurobiological pathways to Alzheimer’s disease: Amyloid-beta, TAU protein or both? Dement Neuropsychol. 2009;3(3):188-194. [CrossRef]
- Meyer-Luehmann M, Spires-Jones TL, Prada C, et al. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature. 2008;451(7179):720-724. [CrossRef]
- Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL. Alzheimer’s disease. Nat Rev Dis Primers. 2015;1. [CrossRef]
- Rhein V, Eckert A. Effects of Alzheimer’s amyloid-beta and tau protein on mitochondrial function - Role of glucose metabolism and insulin signalling. Arch Physiol Biochem. 2007;113(3):131-141. [CrossRef]
- Wagner U, Utton M, Gallo JM, Miller CCJ. Cellular phosphorylation of tau by GSK-3β influences tau binding to microtubules and microtubule organisation. J Cell Sci. 1996;109(6):1537-1543.
- Gao Y, Tan L, Yu JT, Tan L. Tau in Alzheimer’s Disease: Mechanisms and Therapeutic Strategies. Curr Alzheimer Res. 2018;15(3):283-300. [CrossRef]
- Sheppard O, Coleman M. Alzheimer’s Disease: Etiology, Neuropathology and Pathogenesis. In: Alzheimer’s Disease: Drug Discovery. Exon Publications; 2020:1-22. [CrossRef]
- Gajardo-Gómez R, Labra VC, Maturana CJ, et al. Cannabinoids prevent the amyloid β-induced activation of astroglial hemichannels: A neuroprotective mechanism. Glia. 2017;65(1):122-137. [CrossRef]
- Deture MA, Dickson DW. The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener. 2019;14(1). [CrossRef]
- Wang JF, Chen S, Gao XD. Research Progress of Alzheimer’s Disease Targets and Related Drugs | 阿尔兹海默症靶点及相关药物研究进展. Chinese Journal of Pharmaceutical Biotechnology. 2021;28(3):323-330. [CrossRef]
- Clark MA, Harvey RA, Finkel R, Rey JA, Whalen K. Pharmacology. 5th ed. Lippincott Williams & Wilkins; 2011.
- Caraci F, Santagati M, Caruso G, et al. New antipsychotic drugs for the treatment of agitation and psychosis in Alzheimer’s disease: Focus on brexpiprazole and pimavanserin. F1000Res. 2020;9. [CrossRef]
- Shi M, Chu F, Zhu F, Zhu J. Impact of Anti-amyloid-β Monoclonal Antibodies on the Pathology and Clinical Profile of Alzheimer’s Disease: A Focus on Aducanumab and Lecanemab. Front Aging Neurosci. 2022;14. [CrossRef]
- Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4(8):1770-1804. [CrossRef]
- Boadu O, Gombolay GY, Caviness VS, El Saleeby CM. Intoxication From Accidental Marijuana Ingestion in Pediatric Patients: What May Lie Ahead. Pediatr Emerg Care. 2020;36(6):e349-e354. [CrossRef]
- Oberbarnscheidt T, Miller NS. Pharmacology of Marijuana. Published online 2016. [CrossRef]
- Ronan PJ, Wongngamnit N, Beresford TP. Molecular Mechanisms of Cannabis Signaling in the Brain. Prog Mol Biol Transl Sci. 2016;137:123-147. [CrossRef]
- Ruver-Martins AC, Bicca MA, de Araujo FS, et al. Cannabinoid extract in microdoses ameliorates mnemonic and nonmnemonic Alzheimer’s disease symptoms: A case report. J Med Case Rep. 2022;16(1):277. [CrossRef]
- Li S, Huang Y, Yu L, Ji X, Wu J. Impact of the Cannabinoid System in Alzheimer’s Disease. Curr Neuropharmacol. 2023;21(3):715-726. [CrossRef]
- Lafaye G, Karila L, Blecha L, Benyamina A. Cannabis, cannabinoids, and health. Dialogues Clin Neurosci. 2017;19(3):309-316. [CrossRef]
- Iuvone T, Esposito G, Esposito R, Santamaria R, Di Rosa M, Izzo AA. Neuroprotective effect of cannabidiol, a non-psychoactive component from Cannabis sativa, on beta-amyloid-induced toxicity in PC12 cells. J Neurochem. 2004;89(1):134-141. [CrossRef]
- Ruver-Martins AC, Bicca MA, de Araujo FS, et al. Cannabinoid extract in microdoses ameliorates mnemonic and nonmnemonic Alzheimer’s disease symptoms: a case report. J Med Case Rep. 2022;16(1):277. [CrossRef]
- Monteiro KLC, Dos Santos Alcântara MG, de Aquino TM, da Silva-Júnior EF. Cannabinoid pharmacology and its therapeutic uses in Alzheimer’s disease. Neural Regen Res. 2021;16(5):990-991. [CrossRef]
- Páez JA, Campillo NE. Innovative Therapeutic Potential of Cannabinoid Receptors as Targets in Alzheimer’s disease and Less Well-Known Diseases. Curr Med Chem. 2019;26(18):3300-3340. Available online: https://mc04.manuscriptcentral.com/crmchttps://mc04.manuscriptcentral.com/crmc.
- Tang Y, Bao JS, Su JH, Huang W. MicroRNA-139 modulates Alzheimer’s-associated pathogenesis in SAMP8 mice by targeting cannabinoid receptor type 2. Genetics and Molecular Research. 2017;16(1). [CrossRef]
- Aso E, Andrés-Benito P, Ferrer I. Genetic deletion of CB1 cannabinoid receptors exacerbates the Alzheimer-like symptoms in a transgenic animal model. Biochem Pharmacol. 2018;157:210-216. [CrossRef]
- Campillo NE, Páez JA. Cannabinoid system in neurodegeneration: New perspectives in Alzheimer’s disease. Mini Rev Med Chem. 2009;9(5):539-559. [CrossRef]
- Silvestri C, Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. 2013;17(4):475-490. [CrossRef]
- Komorowska-Müller JA, Schmöle AC. CB2 receptor in microglia: The guardian of self-control. Int J Mol Sci. 2021;22(1):1-27. [CrossRef]
- Crunfli F, Vrechi TA, Costa AP, Torrão AS. Cannabinoid Receptor Type 1 Agonist ACEA Improves Cognitive Deficit on STZ-Induced Neurotoxicity Through Apoptosis Pathway and NO Modulation. Neurotox Res. 2019;35(3):516-529. [CrossRef]
- Moreira-Silva D, Carrettiero DC, Oliveira ASA, et al. Anandamide effects in a streptozotocin-induced Alzheimer’s disease-like sporadic dementia in rats. Front Neurosci. 2018;12(SEP). [CrossRef]
- Khavandi M, Rao PPN, Beazely MA. Differential Effects of Endocannabinoids on Amyloid-Beta Aggregation and Toxicity. Int J Mol Sci. 2023;24(2). [CrossRef]
- Hosseininia M, Rostami F, Delphi L, Ghasemzadeh Z, Kouhkan F, Rezayof A. Memory impairment was ameliorated by corticolimbic microinjections of arachidonylcyclopropylamide (ACPA) and miRNA-regulated lentiviral particles in a streptozotocin-induced Alzheimer’s rat model. Exp Neurol. 2023;370:114560. [CrossRef]
- Zhang R san, He Z, Jin W dong, Wang R. Effects of the cannabinoid 1 receptor peptide ligands hemopressin, (m)RVD-hemopressin(α) and (m)VD-hemopressin(α) on memory in novel object and object location recognition tasks in normal young and Aβ 1–42 -treated mice. Neurobiol Learn Mem. 2016;134:264-274. [CrossRef]
- Zhang R, Lao K, Lu B, et al. (m)RVD-hemopressin (α) and (m)VD-hemopressin (α) improve the memory-impairing effect of scopolamine in novel object and object location recognition tasks in mice. Peptides (NY). 2021;136:170442. [CrossRef]
- Zhang R, Zheng Y, Hu F, et al. Effect of (m)VD-hemopressin against Aβ1-42-induced oxidative stress and apoptosis in mouse hippocampal neurons. Peptides (NY). 2020;124:170185. [CrossRef]
- Zhang R, He X, Cheng J, et al. (m) RVD-hemopressin (α) Ameliorated Oxidative Stress, Apoptosis and Damage to the BDNF/TrkB/Akt Pathway Induced by Scopolamine in HT22 Cells. Neurotox Res. 2023;41(6):627-637. [CrossRef]
- Zhang R, Luan J, Hu F, et al. Effect of (m)RVD-hemopressin against Aβ1-42-induced apoptosis and inhibition of neurite outgrowth in SH-SY5Y cells. Neuropeptides. 2020;81:102044. [CrossRef]
- Velikova M, Doncheva D, Tashev R. Subchronic effects of ligands of cannabinoid receptors on learning and memory processes of olfactory bulbectomized rats. Acta Neurobiol Exp (Wars). 2020;80(3):286-296. [CrossRef]
- Jayant S, Sharma BM, Bansal R, Sharma B. Pharmacological benefits of selective modulation of cannabinoid receptor type 2 (CB2) in experimental Alzheimer’s disease. Pharmacol Biochem Behav. 2016;140:39-50. [CrossRef]
- Cheng Y, Dong Z, Liu S. β-Caryophyllene Ameliorates the Alzheimer-Like Phenotype in APP/PS1 Mice through CB2 Receptor Activation and the PPARγ Pathway. Pharmacology. 2014;94(1-2):1-12. [CrossRef]
- del Cerro P, Alquézar C, Bartolomé F, et al. Activation of the Cannabinoid Type 2 Receptor by a Novel Indazole Derivative Normalizes the Survival Pattern of Lymphoblasts from Patients with Late-Onset Alzheimer’s Disease. CNS Drugs. 2018;32(6):579-591. [CrossRef]
- Abd El-Rahman SS, Fayed HM. Improved cognition impairment by activating cannabinoid receptor type 2: Modulating CREB/BDNF expression and impeding TLR-4/ NFκBp65/M1 microglia signaling pathway in D-galactose-injected ovariectomized rats. PLoS ONE. 2022;17(3 3). [CrossRef]
- Li C, Shi J, Wang B, Li J, Jia H. CB2 cannabinoid receptor agonist ameliorates novel object recognition but not spatial memory in transgenic APP/PS1 mice. Neurosci Lett. 2019;707:134286. [CrossRef]
- Çakır M, Tekin S, Doğanyiğit Z, et al. Cannabinoid type 2 receptor agonist JWH-133, attenuates Okadaic acid induced spatial memory impairment and neurodegeneration in rats. Life Sci. 2019;217:25-33. [CrossRef]
- Marta KS, Agnieszka D, Grazyna B. The Influence of CB2-Receptor Ligands on the Memory-Related Responses in Connection with Cholinergic Pathways in Mice in the Passive Avoidance Test. Molecules. 2022;27(13). [CrossRef]
- Montanari S, Mahmoud AM, Pruccoli L, et al. Discovery of novel benzofuran-based compounds with neuroprotective and immunomodulatory properties for Alzheimer’s disease treatment. Eur J Med Chem. 2019;178:243-258. [CrossRef]
- Spatz P, Steinmüller SAM, Tutov A, et al. Dual-Acting Small Molecules: Subtype-Selective Cannabinoid Receptor 2 Agonist/Butyrylcholinesterase Inhibitor Hybrids Show Neuroprotection in an Alzheimer’s Disease Mouse Model. J Med Chem. 2023;66(9):6414-6435. [CrossRef]
- Scheiner M, Dolles D, Gunesch S, et al. Dual-Acting Cholinesterase–Human Cannabinoid Receptor 2 Ligands Show Pronounced Neuroprotection in Vitro and Overadditive and Disease-Modifying Neuroprotective Effects in Vivo. J Med Chem. 2019;62(20):9078-9102. [CrossRef]
- Cao C, Li Y, Liu H, et al. The potential therapeutic effects of THC on Alzheimer’s disease. Journal of Alzheimer’s Disease. 2014;42(3):973-984. [CrossRef]
- Gugliandolo A, Blando S, Salamone S, et al. Δ8-THC Protects against Amyloid Beta Toxicity Modulating ER Stress In Vitro: A Transcriptomic Analysis. Int J Mol Sci. 2023;24(7):6598. [CrossRef]
- van den Elsen GAH, Ahmed AIA, Verkes RJ, et al. Tetrahydrocannabinol for neuropsychiatric symptoms in dementia. Neurology. 2015;84(23):2338-2346. [CrossRef]
- Coles M, Watt G, Kreilaus F, Karl T. Medium-Dose Chronic Cannabidiol Treatment Reverses Object Recognition Memory Deficits of APPSwe/PS1ΔE9 Transgenic Female Mice. Front Pharmacol. 2020;11. [CrossRef]
- Hughes B, Herron CE. Cannabidiol Reverses Deficits in Hippocampal LTP in a Model of Alzheimer’s Disease. Neurochem Res. 2019;44(3):703-713. [CrossRef]
- Amini M, Abdolmaleki Z. The Effect of Cannabidiol Coated by Nano-Chitosan on Learning and Memory, Hippocampal CB1 and CB2 Levels, and Amyloid Plaques in an Alzheimer’s Disease Rat Model. Neuropsychobiology. 2022;81(3):171-183. [CrossRef]
- Hao F, Feng Y. Cannabidiol (CBD) enhanced the hippocampal immune response and autophagy of APP/PS1 Alzheimer’s mice uncovered by RNA-seq. Life Sci. 2021;264:118624. [CrossRef]
- Alexandri F, Papadopoulou L, Tsolaki A, Papantoniou G, Athanasiadis L, Tsolaki M. The Effect of Cannabidiol 3% on Neuropsychiatric Symptoms in Dementia – Six-Month Follow-Up. Clin Gerontol. Published online May 8, 2023:1-8. [CrossRef]
- Palmieri B, Vadalà M. Oral THC: CBD cannabis extract in main symptoms of Alzheimer disease: Agitation and weight loss. Clin Ter. 2023;174(1):53-60. [CrossRef]
- Kim J, Choi P, Park YT, Kim T, Ham J, Kim JC. The Cannabinoids, CBDA and THCA, Rescue Memory Deficits and Reduce Amyloid-Beta and Tau Pathology in an Alzheimer’s Disease-like Mouse Model. Int J Mol Sci. 2023;24(7). [CrossRef]
- Defrancesco M, Hofer A. Cannabinoid as Beneficial Replacement Therapy for Psychotropics to Treat Neuropsychiatric Symptoms in Severe Alzheimer’s Dementia: A Clinical Case Report. Front Psychiatry. 2020;11. [CrossRef]
- Long C mei, Zheng Q xue, Zhou Y, et al. N-linoleyltyrosine exerts neuroprotective effects in APP/PS1 transgenic mice via cannabinoid receptor-mediated autophagy. J Pharmacol Sci. 2021;147(4):315-324. [CrossRef]
- Askari VR, Fereydouni N, Baradaran Rahimi V, et al. β-Amyrin, the cannabinoid receptors agonist, abrogates mice brain microglial cells inflammation induced by lipopolysaccharide/interferon-γ and regulates Mφ1/Mφ2 balances. Biomedicine & Pharmacotherapy. 2018;101:438-446. [CrossRef]
- Herrmann N, Ruthirakuhan M, Gallagher D, et al. Randomized Placebo-Controlled Trial of Nabilone for Agitation in Alzheimer’s Disease. The American Journal of Geriatric Psychiatry. 2019;27(11):1161-1173. [CrossRef]
- Aguirre-Rueda D, Guerra-Ojeda S, Aldasoro M, et al. WIN 55,212-2, agonist of cannabinoid receptors, prevents amyloid β<inf>1-42</inf> effects on astrocytes in primary culture. PLoS ONE. 2015;10(4). [CrossRef]
- Mahdi O, Chiroma SM, Baharuldin MTH, et al. Win55,212-2 attenuates cognitive impairments in alcl<inf>3</inf> + d-galactose-induced alzheimer’s disease rats by enhancing neurogenesis and reversing oxidative stress. Biomedicines. 2021;9(9). [CrossRef]
- Gajardo-Gómez R, Labra VC, Maturana CJ, et al. Cannabinoids prevent the amyloid β-induced activation of astroglial hemichannels: A neuroprotective mechanism. Glia. 2017;65(1):122-137. [CrossRef]
- Soto-Mercado V, Mendivil-Perez M, Jimenez-Del-Rio M, Velez-Pardo C. Multi-Target Effects of the Cannabinoid CP55940 on Familial Alzheimer’s Disease PSEN1 E280A Cholinergic-Like Neurons: Role of CB1 Receptor. Journal of Alzheimer’s Disease. 2021;82(s1):S359-S378. [CrossRef]
- Nuñez-Borque E, González-Naranjo P, Bartolomé F, et al. Targeting Cannabinoid Receptor Activation and BACE-1 Activity Counteracts TgAPP Mice Memory Impairment and Alzheimer’s Disease Lymphoblast Alterations. Mol Neurobiol. 2020;57(4):1938-1951. [CrossRef]
- Balleza-Tapia H, Crux S, Andrade-Talavera Y, et al. TrpV1 receptor activation rescues neuronal function and network gamma oscillations from Aβ-induced impairment in mouse hippocampus in vitro. Elife. 2018;7. [CrossRef]
- Muller C, Morales P, Reggio PH. Cannabinoid Ligands Targeting TRP Channels. Front Mol Neurosci. 2019;11. [CrossRef]
- Xiang X, Wang X, Jin S, et al. Activation of GPR55 attenuates cognitive impairment and neurotoxicity in a mouse model of Alzheimer’s disease induced by Aβ1–42 through inhibiting RhoA/ROCK2 pathway. Prog Neuropsychopharmacol Biol Psychiatry. 2022;112:110423. [CrossRef]
- Ryberg E, Larsson N, Sjögren S, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152(7):1092-1101. [CrossRef]
- Xiang X, Wang X, Wu Y, et al. Activation of GPR55 attenuates cognitive impairment, oxidative stress, neuroinflammation, and synaptic dysfunction in a streptozotocin-induced Alzheimer’s mouse model. Pharmacol Biochem Behav. 2022;214:173340. [CrossRef]
- Wang X, Xiang X, Hu J, et al. Pharmacological Activation of GPR55 Improved Cognitive Impairment Induced by Lipopolysaccharide in Mice. Journal of Molecular Neuroscience. 2022;72(8):1656-1669. [CrossRef]
- Elmazoglu Z, Rangel-López E, Medina-Campos ON, et al. Cannabinoid-profiled agents improve cell survival via reduction of oxidative stress and inflammation, and Nrf2 activation in a toxic model combining hyperglycemia+Aβ1-42 peptide in rat hippocampal neurons. Neurochem Int. 2020;140:104817. [CrossRef]
- Navarro-Dorado J, Villalba N, Prieto D, et al. Vascular dysfunction in a transgenic model of Alzheimer’s disease: Effects of CB1R and CB2R cannabinoid agonists. Front Neurosci. 2016;10(SEP). [CrossRef]
- Schubert D, Kepchia D, Liang Z, Dargusch R, Goldberg J, Maher P. Efficacy of Cannabinoids in a Pre-Clinical Drug-Screening Platform for Alzheimer’s Disease. Mol Neurobiol. 2019;56(11):7719-7730. [CrossRef]
- Brannigan A, Zwerman W. The real “Hawthorne effect.” Society. 2001;38(2):55-60. [CrossRef]
- Oyagawa CRM, Grimsey NL. Cannabinoid receptor CB1 and CB2 interacting proteins: Techniques, progress and perspectives. In: ; 2021:83-132. [CrossRef]
| Reference | Cannabinoid | Dosage | Major results | Model used | |
|---|---|---|---|---|---|
| Crunfli et al., 2019 [36] | ACEA | 3 mg/kg i.p. |
|
In vitro and in vivo experiments in AD models with STZ | |
| Moreira-Silva et al., 2018 [37] | AEA | 100 ng i.c.v. |
|
In vivo experiments in Wistar rats with STZ injection | |
| Khavandi, Rao and Beazely, 2023 [38] | AΕA | 10 μΜ |
|
In vitro experiments in mouse hippocampal HT22 cells and hamster ovary CHO cells expressing human CB1R | |
| Noladin | 10 μΜ |
|
|||
| OAΕ | 1 μM 10 μΜ |
|
|||
| Hosseininia et al., 2023 [39] | ACPA | 10 ng/0.5 μL (corticolimbic microinjection) |
|
In vivo experiments in Wistar rats after i.c.v. STZ | |
| Lentiviral particles with miR-137 or miR-let-7a | 0.5 μL/rat e.o.d. | ||||
| Zhang et al., 2016 [40] | RVD & VD | 1 nmol 2.5 nmol 5 nmol, i.c.v./i.p. |
|
In vivo experiments in mice infected with Aβ1→42 | |
| Zhang et al., 2021 [41] |
|
In vivo experiments in mice after i.p. scopolamine | |||
| Zhang et al., 2020a [42] | VD |
|
In vitro experiments on hippocampal neurons from mice infected with Aβ1→42 | ||
| Zhang et al., 2023 [43] | RVD |
|
In vitro experiments in HT22 cells treated with scopolamine | ||
| Zhang et al., 2020b [44] |
|
In vitro experiments in SH-SY5Y cells infected with Aβ1→42 | |||
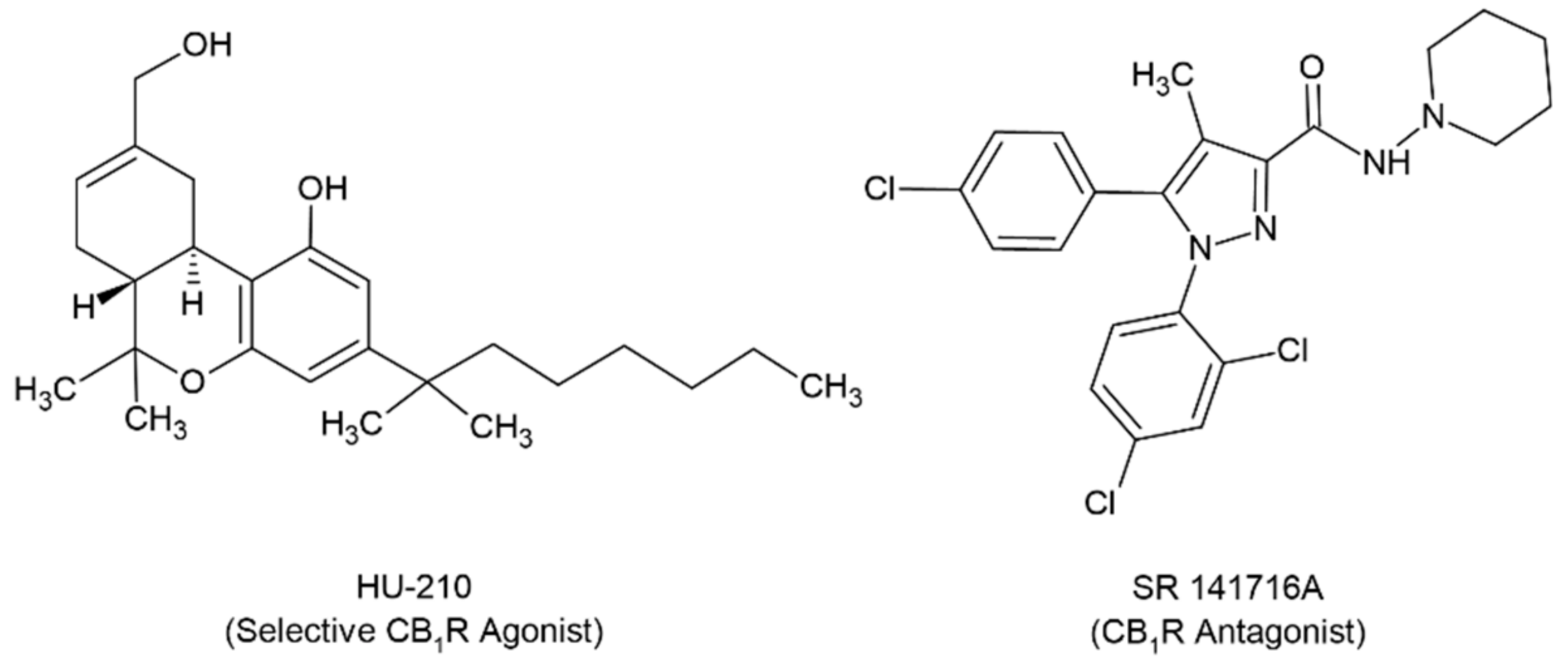
| Velikova, Doncheva and Tashev, 2020 [45] | HU-210 | 5 μg/day i.c.v. |
|
Direct correlation of CB1R with memory function (active and passive avoidance tests) | In vivo experiments in OBX rats |
| SR 141716A (CB1R Antagonist) | 3 μg/day i.c.v. |
|
|||
| Reference | Cannabinoid | Dosage | Major results | Model used | |
|---|---|---|---|---|---|
| Jayant et al., 2016 [46] | 1-Phenylsatin | 20 mg/kg p.o. | MWM test |
|
In vivo experiments in rats exposed to STZ or AlCl3 + D-Gal |
| Attentional set shifting test |
|
||||
| Cheng, Dong and Liu, 2014 [47] | BCP | 48 mg/kg p.o. |
|
In vivo experiments in APP/PS1 mice | |
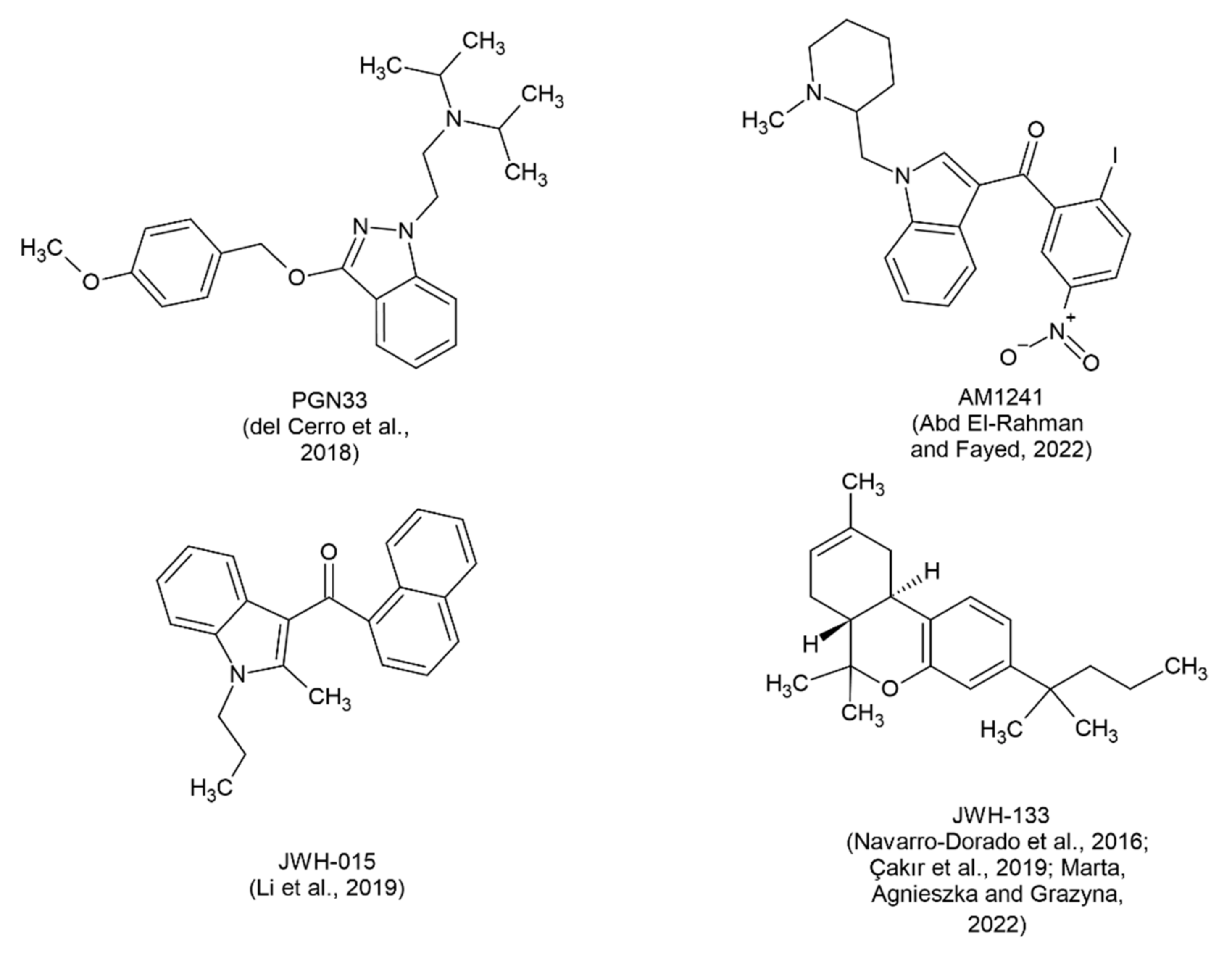
| del Cerro et al., 2018 [48] | PGN33 | 2.5 nM 5 nM 7.5 nM 10 nM |
|
In vitro experiments in lymphoblasts isolated from AD patients and in Aβ-treated SH-SY5Y cells | |
| Abd El-Rahman and Fayed, 2022 [49] | AM1241 | 3 mg 6 mg i.p. |
|
In vivo experiments in model AD female rats, treated with D-Gal after ovariectomy | |
| Li et al., 2019 [50] | JWH-015 | 0.5 mg/kg i.p. |
|
In vivo experiments in transgenic APP/PS1 mice | |
| Çakır et al., 2019 [51] | JWH-133 | 0.2 mg/kg i.p. |
|
In vivo experiments in a rat model with hyperphosphorylated TAU from OKA administration | |
| Reference | Cannabinoid | Dosage | Major results | Model used |
|---|---|---|---|---|
| Marta, Agnieszka and Grazyna, 2022 [52] | JWH-133 | 0.25 mg/kg i.p. |
|
In vivo experiments in Swiss mice after scopolamine administration |
| Nicotine (Cholinergic agonist) |
0.05 mg/kg s.c. | |||
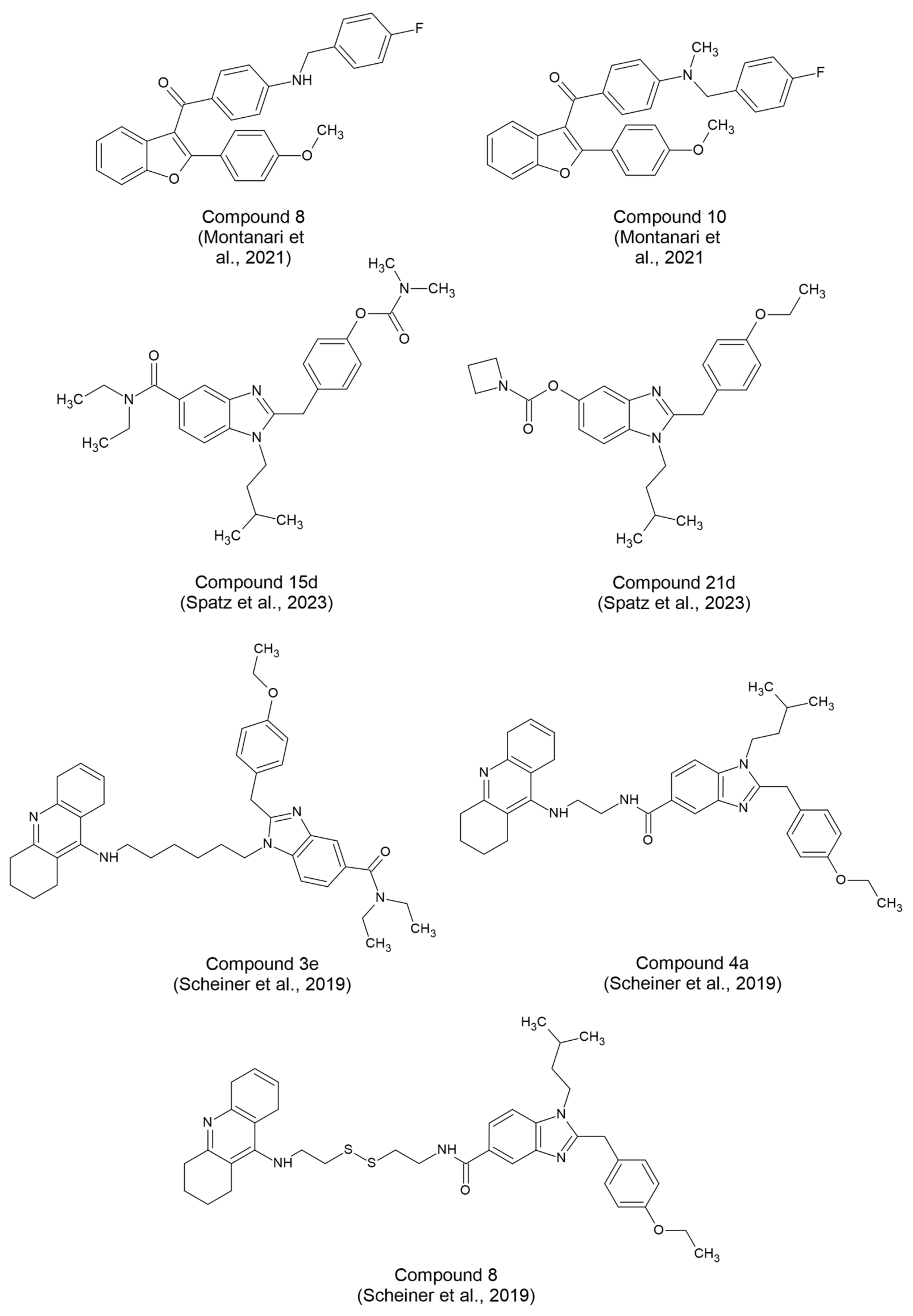
| Montanari et al., 2021 [53] | Compound 8 (CB2R agonist, BChE inhibitor) | 5 μΜ |
|
In vitro experiments in SH-SY5Y cells treated with Aβ1-42 |
| Compound 10 (CB2R inverse agonist) | 5 μΜ |
|
||
| Spatz et al., 2023 [54] | Compound 15d (CB2R agonist, BChE inhibitor) |
0.3-3 mg/kg/day i.p. |
|
In vitro experiments in LPS-treated N9 microglial cells and in vivo experiments in mice challenged with Aβ25-35 oligomers |
| Scheiner et al., 2019 [55] | Compound 3e (CB2R agonist, AChE inhibitor) | 0.3 mg/kg i.p., o.d. |
|
In vitro experiments in cellular model of neuronal oxidative stress in N9 microglial cells and in vivo experiments in Aβ25-35 injected AD mice |
| Compound 4a (CB2R agonist, AChE inhibitor) | 1 mg/kg i.p., o.d. | |||
| Compound 8 (CB2R agonist, AChE inhibitor) | 0.3 mg/kg i.p., o.d. |
| Reference | Cannabinoid | Dosage | Major results | Model used |
|---|---|---|---|---|
| Cao et al., 2014 [56] | THC | 0.25 nM – 2.5 μΜ every 6h or 24h or 48h |
|
In vitro experiments in N2a/AβPPswe cells |
| Gugliandolo et al., 2023 [57] | THC | 20 μΜ |
|
In vitro experiments in SH-SY5Y cells treated with Aβ1-42 |
| van den Elsen et al., 2015 [58] | THC | 1.5 mg/3 times/day |
|
Randomized controlled trial in 50 patients with dementia |
| Coles et al., 2020 [59] | CBD | 5 mg/kg i.p. |
|
In vivo experiments in female APP/PS1 mice |
| Hughes and Herron, 2019 [60] | CBD | 10 μΜ |
|
In vitro experiments in sections from the CA1 hippocampus region of C57Bl/6 mice after Aβ treatment |
| Amini and Abdolmaleki, 2022 [61] | CBD with nano-chitosan coating | 120 mg/kg p.o. |
|
In vivo experiments in rat AD model after injection of Aβ1-42 |
| Hao and Feng, 2021 [62] | CBD | 5 mg/kg/day i.p. |
|
In vivo study in APP/PS1 mice through analysis of DEGs |
| Alexandri et al., 2023 [63] | CBD oil drops | 3% p.o. for 6 months |
|
Comparative study in 20 patients with dementia between 3% CBD and usual treatment |
| Palmieri and Vadalà, 2023 [64] | Cannabins extract in oil | 1 mL/day (22% THC, 0.5% CBD) p.o. |
|
Limited-size cohort study in 30 patients with moderate-to-severe AD |
| Ruver-Martins et al., 2022 [28] | Cannabis extract | Microdoses (most often 500 µg p.o.) of 8:1 THC:CBD extract for 22 months |
|
Case report on a 75-year-old patient with mild AD (memory impairment, spatiotemporal disorientation) |
| Kim et al., 2023 [65] | CBDA | 6 μΜ, 3 μL (intrahippocampal injection) |
|
In vivo experiments in ICR mice after injection of Aβ1-42 |
| THCA | 12 μΜ, 3 μL (intrahippocampal injection) | |||
| Defrancesco and Hofer, 2020 [66] | Dronabinol drops (Δ9THC) | 4.9 mg – 6.7 mg/day p.o. |
|
Case report on a 69-year-old AD patient with severe NPS (depression, paranoid perception) |
| Long et al., 2021 [67] | NlTyr | 60 mg/kg p.o. |
|
In vivo experiments in AD APP/PS1 mouse model |
| Askari et al., 2018 [68] | β-Amyrin | 4-16 μΜ |
|
In vitro experiments in rat microglial cells treated with LPS/IFN-γ |
| Herrmann et al., 2019 [69] | Nabilone | 1-2 mg/day p.o. |
|
Randomized, double-blind, crossover clinical trial in 39 patients with moderate to severe AD |
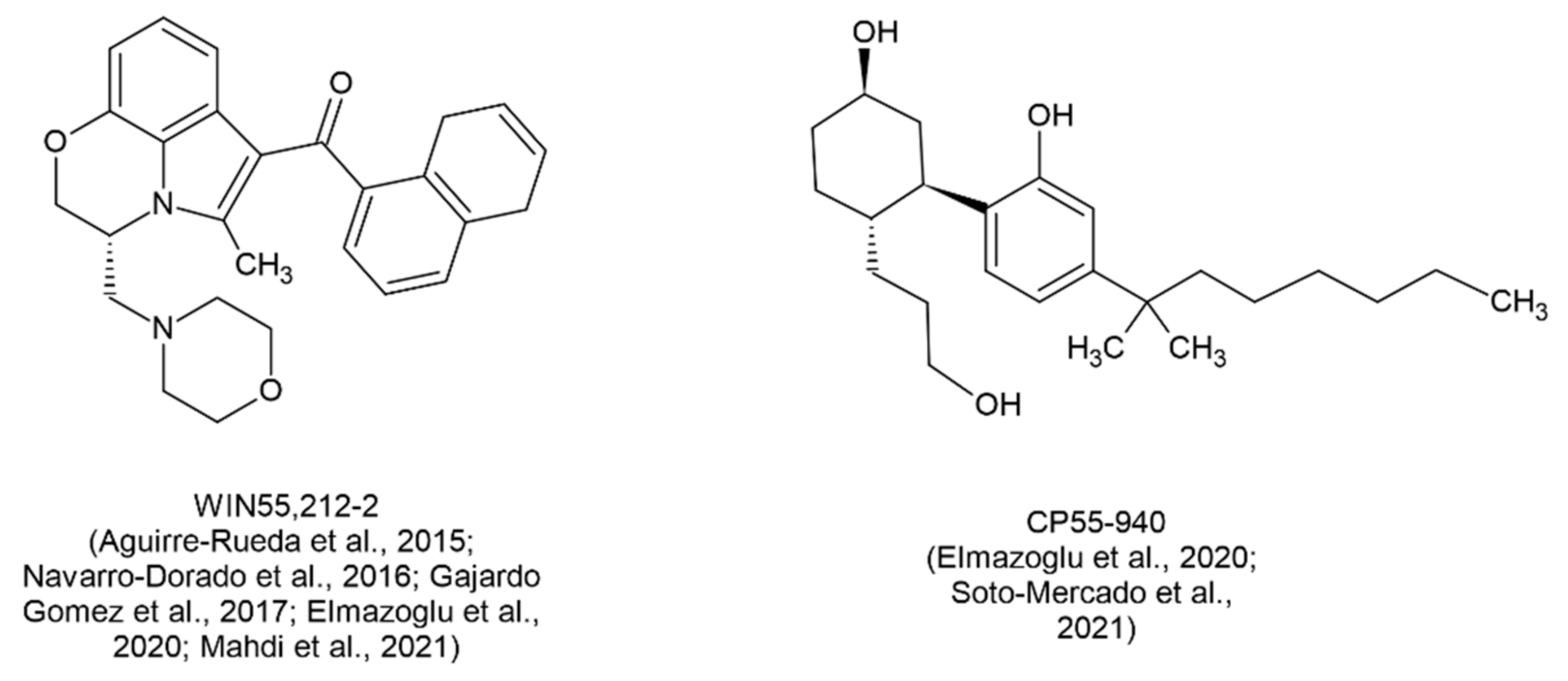
| Aguirre-Rueda et al., 2015 [70] | WIN55,212-2 | 10 μΜ |
|
In vitro experiments on rat cortical astrocytes treated with Aβ |
| Mahdi et al., 2021 [71] | WIN55,212-2 | 0.5 mg/kg 1 mg/kg 2 mg/kg |
|
In vivo experiments in a Wistar rat model after administration of AlCl3 + D-Gal |
| Gajardo-Gómez et al., 2017 [72] | WIN55,212-2 | 5 μΜ |
|
In vitro experiments on cells from rat hippocampal slices treated with Aβ25-35 |
| 2-AG | ||||
| Methanandamide | ||||
| Soto-Mercado et al., 2021 [73] | CP55-940 | 1 μΜ |
|
In vitro experiments in PSEN1 E280A cells (familial AD model) |
| SR141716 (CB1R inverse agonist) | ||||
| Anti-Aβ42 antibody | ||||
| 2-AG | ||||
| CP55-940 | ||||
| WIN55,212-2 | ||||
| URB597 (FAAH inhibitor) |
| Reference | Cannabinoid | Dosage | Major results | Model used |
|---|---|---|---|---|
| Non-selective agonists of CB1R and CB2R associated with cholinergic pathways | ||||
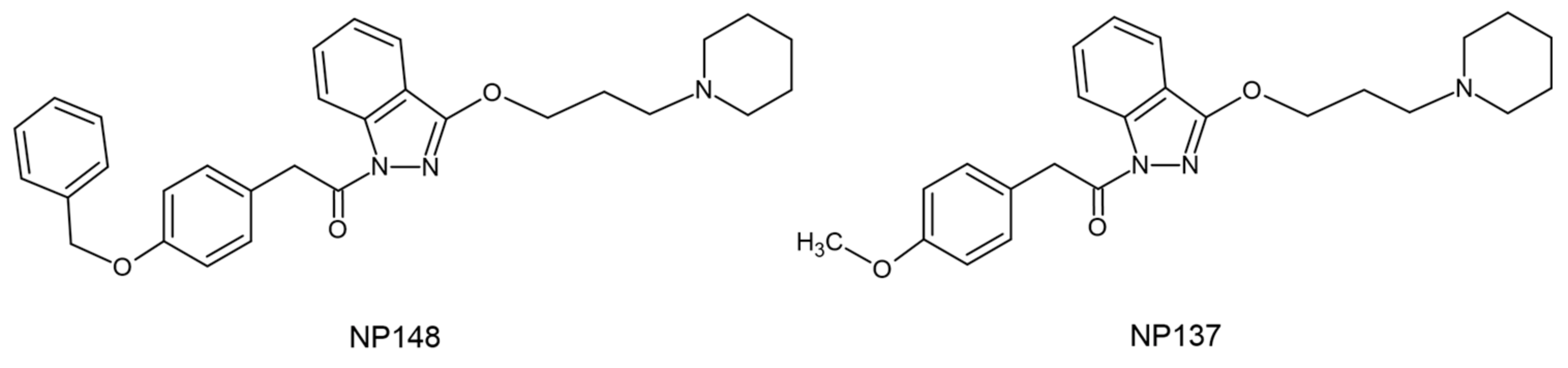
| Nuñez-Borque et al., 2020 [74] | NP137 | 2.5 μΜ/ 5 μΜ/ 1 mg/kg/day p.o. |
In vitro
|
In vitro experiments in immortalized lymphocytes of patients with delayed AD and in vivo experiments in TgAPP mice |
| NP148 | 5 μΜ | |||
| Modulator molecules that act through pathways related to the endocannabinoid system | ||||
| Balleza-Tapia et al., 2018 [75] | Capsaicin (Trpv-1 agonst) | 10 μΜ |
|
In vitro experiments in Aβ-treated hippocampal cells |
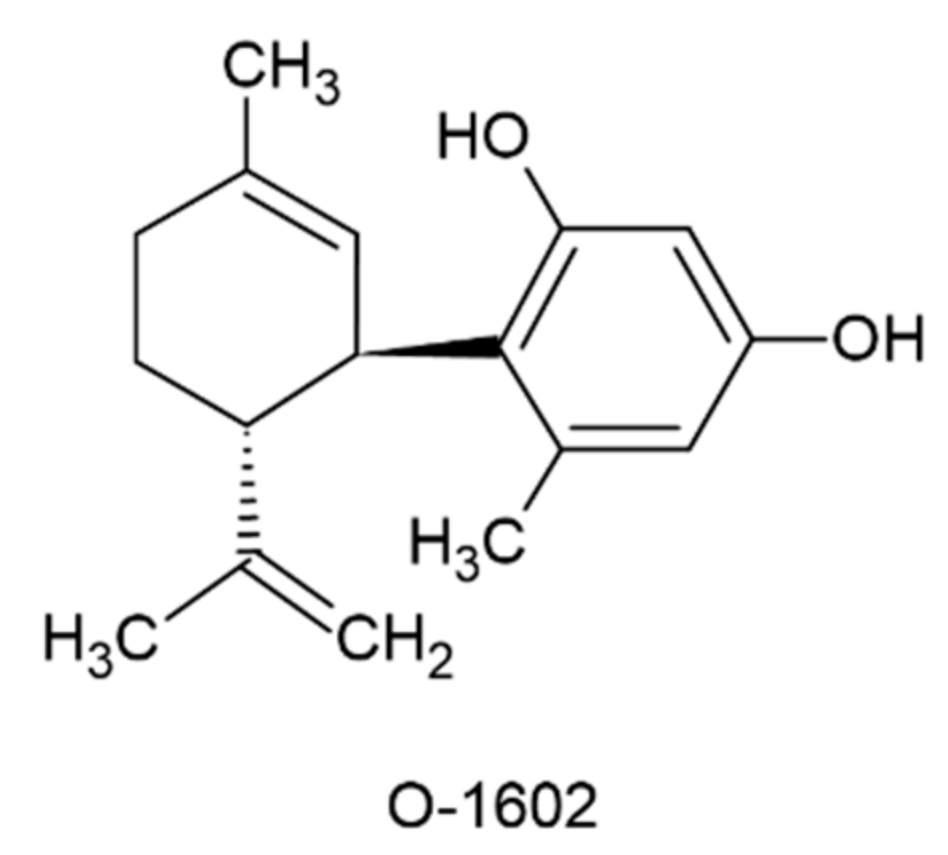
| Xiang et al., 2022a [77] | O-1602 (GPR-55 agonist) | 2 μg/mouse or 4 μg/mouse i.c.v. |
|
In vivo experiments in mice affected by Aβ1-42 |
| Xiang et al., 2022b [79] |
|
In vivo experiments in mice affected by STZ | ||
| Wang et al., 2022 [80] |
|
In vivo experiments in mice affected by LPS | ||
| Reference | Cannabinoid | Dosage | Major results | Model used |
|---|---|---|---|---|
| Elmazoglu et al., 2020 [81] | AEA | 1-1000 μΜ |
|
In vitro experiments in rat hippocampal neurons - model of combined toxic hyperglycemia and Aβ1-42 |
| 2-AG | ||||
| CP55-940 | ||||
| WIN55,212-2 | ||||
| URB597 (FAAH inhibitor) | ||||
| Navarro-Dorado et al., 2016 [82] | WIN55,212-2 | 0.2 mg/kg/day p.o. |
Combined action
|
In vivo experiments in TgAPP AD mice |
| JWH-133 | ||||
| Schubert et al., 2019 [83] | Δ8THC | 250 nΜ – 10 μM |
|
In vitro experiments in HT22 and MC65 cells after induction of C99 production |
| Δ9THC | ||||
| Δ9THCA | ||||
| CBD | ||||
| CBDA | ||||
| DMCBD | ||||
| CBDV | ||||
| CBG | ||||
| CBGA | ||||
| CBC | ||||
| CBN | ||||
| MCBN |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





