Submitted:
18 May 2024
Posted:
21 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction: Peculiarities of Phosphorus as a Macronutrient
1.1. Global Perspective on P: A Nutrient That is “Abundant but Scarce” and Mostly Wasted
1.2. P Acquisition and Storage in the Cell
1.2.1. Cell P quota and P uptake Capacity
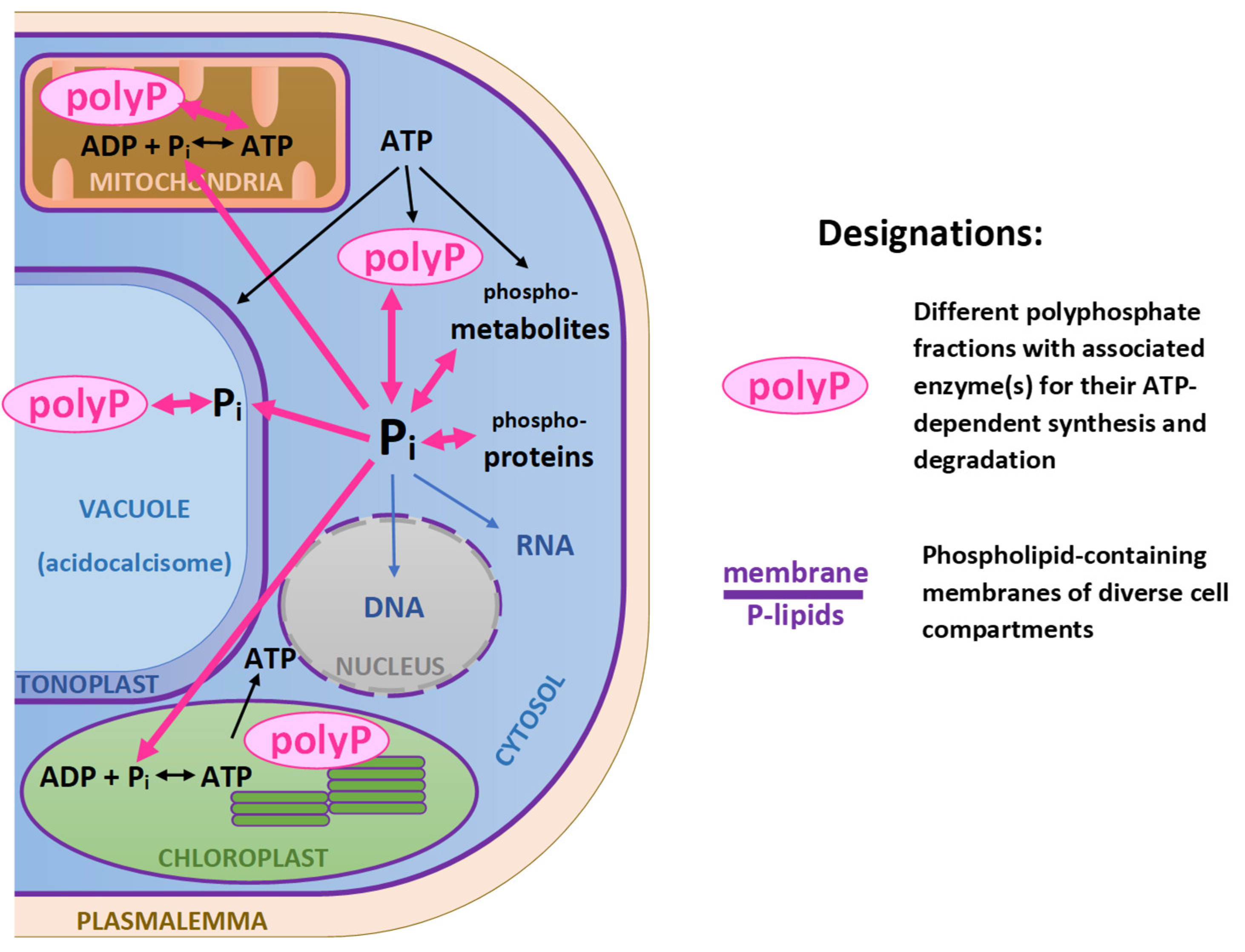
1.1.2. Phosphorus Pools in the Cell
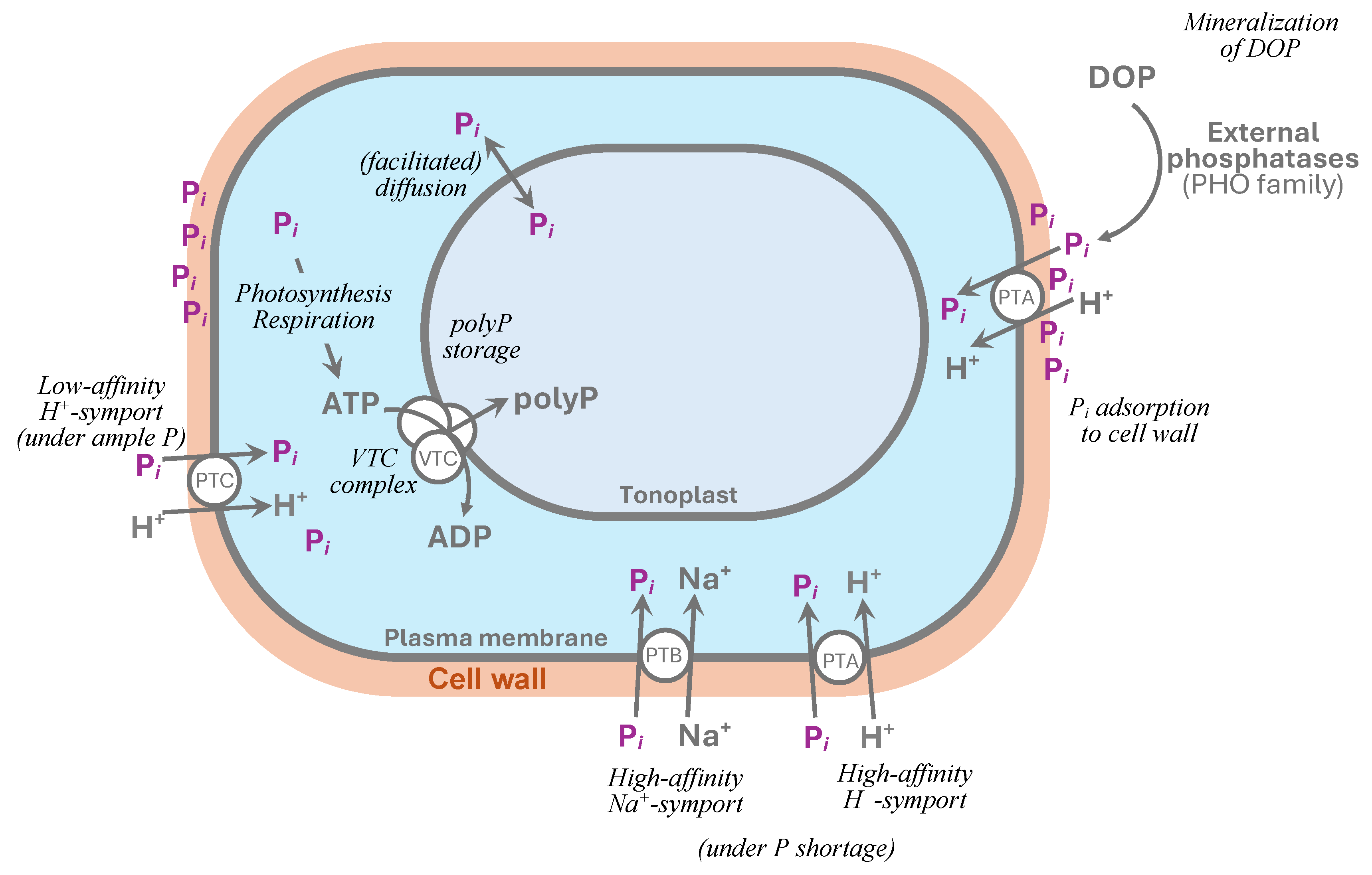
1.2.3. Phosphorus Uptake
1.1.4. PolyPhosphate Turnover and Its Regulation
2. Between Scylla and Charybdis: P-Starvation and P-Toxicity
2.1. Phosphorus Starvation
2.2. Mobilization of External DOP
2.3. Phosphate Toxicity and Resilience to Elevated Pi Concentrarions
3. Biotechnological Implications
3.1. Microalgae: The Curse of Eutrophication and the Boon of Biosequestration
3.2. Microalgae-Mediated Biocapture of P
3.2.1. Cultivation Conditions and P Nutrition History of the Culture
3.2.2. Algal-Bacterial Communities and P acquizition
3.2.3. Phosphorus Load and Nutrient Balancing
3.2.4. PolyP and Stress Resilience in the Context of Waste Stream Phycoremediation
3.3. Microalgal Biomass Is an Efficient and Environtally Friendly Biofertilizer
3.4. PolyP as a Valuable Commodity
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lauer, M.J.; Blevins, D.G.; Sierzputowska-Gracz, H. 31P-nuclear magnetic resonance determination of phosphate compartmentation in leaves of reproductive soybeans (Glycine max L.) as affected by phosphate nutrition. Plant Physiology 1989, 89, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Plaxton, W.C. Phosphorus: Back to the Roots. In Annual Plant Reviews Volume 48, John Wiley & Sons, Inc.: 2015; pp. 1-22. [CrossRef]
- Tiessen, H. Phosphorus in the global environment. In The Ecophysiology of Plant-Phosphorus Interactions, White, P.J., Hammond, J.P., Eds. Springer Netherlands: Dordrecht, 2008; pp. 1-7. [CrossRef]
- Bennett, E.; Elser, J. A broken biogeochemical cycle. Nature 2011, 478, 29–31. [Google Scholar]
- Cembella, A.D.; Antia, N.J.; Harrison, P.J. The utilization of inorganic and organic phosphorous compounds as nutrients by eukaryotic microalgae: A multidisciplinary perspective: Part I. Critical reviews in microbiology 1982, 10, 317–391. [Google Scholar] [CrossRef] [PubMed]
- Blank, L.M. (Poly)phosphate biotechnology: Envisaged contributions to a sustainable P future. Microb Biotechnol 2023, 16, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Solovchenko, A.; Verschoor, A.M.; Jablonowski, N.D.; Nedbal, L. Phosphorus from wastewater to crops: An alternative path involving microalgae. Biotechnology advances 2016, 34, 550–564. [Google Scholar] [CrossRef] [PubMed]
- Cordell, D.; White, S. Life’s Bottleneck: Implications of Global Phosphorus Scarcity and Pathways for a Sustainable Food System. Annual Review of Environment and Resources 2014, 39. [Google Scholar]
- Fixen, P.E.; Johnston, A.M. World fertilizer nutrient reserves: a view to the future. Journal of the Science of Food and Agriculture 2012, 92, 1001–1005. [Google Scholar] [CrossRef]
- Simpson, R.J.; Oberson, A.; Culvenor, R.A.; Ryan, M.H.; Veneklaas, E.J.; Lambers, H.; Lynch, J.P.; Ryan, P.R.; Delhaize, E.; Smith, F.A. Strategies and agronomic interventions to improve the phosphorus-use efficiency of farming systems. Plant and Soil 2011, 349, 89–120. [Google Scholar] [CrossRef]
- Hallegraeff, G.; Anderson, D.; Cembella, A. Manual on harmful marine microalgae; UNESCO Publising:, 2003; p. 770. [Google Scholar]
- Smith, V.H.; Schindler, D.W. Eutrophication science: where do we go from here? Trends in ecology & evolution 2009, 24, 201–207. [Google Scholar]
- Grossman, A.R.; Aksoy, M. Algae in a phosphorus-limited landscape. In Annual Plant Reviews, Phosphorus Metabolism in Plants, Plaxton, W., Lambers, H., Eds.; Wiley-Blackwell: 2015; Vol. 48, pp. 337–374.
- Solovchenko, A.E.; Ismagulova, T.T.; Lukyanov, A.A.; Vasilieva, S.G.; Konyukhov, I.V.; Pogosyan, S.I.; Lobakova, E.S.; Gorelova, O.A. Luxury phosphorus uptake in microalgae. Journal of Applied Phycology 2019, 31, 2755–2770. [Google Scholar] [CrossRef]
- Solovchenko, A.; Khozin-Goldberg, I.; Selyakh, I.; Semenova, L.; Ismagulova, T.; Lukyanov, A.; Mamedov, I.; Vinogradova, E.; Karpova, O.; Konyukhov, I.; et al. Phosphorus starvation and luxury uptake in green microalgae revisited. Algal Research 2019, 43, 101651. [Google Scholar] [CrossRef]
- Redfield, A.C. The biological control of chemical factors in the environment. American scientist 1958, 46, 230A–221. [Google Scholar]
- Yu, D.; Yan, L.; Shi, J.; Liu, Y.; Zhang, A.; Wang, Y.; Zhang, Y.; Xie, T. Phosphorus Removal and Recovery During Microalgae-Based Wastewater Treatment: A Mini-review. International Journal of Environmental Research 2024, 18, 34. [Google Scholar] [CrossRef]
- Cao, T.N.-D.; Mukhtar, H.; Le, L.-T.; Tran, D.P.-H.; Ngo, M.T.T.; Nguyen, T.-B.; Bui, X.-T. Roles of microalgae-based biofertilizer in sustainability of green agriculture and food-water-energy security nexus. Science of The Total Environment 2023, 870, 161927. [Google Scholar] [CrossRef]
- Crimp, A.; Brown, N.; Shilton, A. Microalgal luxury uptake of phosphorus in waste stabilization ponds – frequency of occurrence and high performing genera. Water Science and Technology 2017, 78, 165–173. [Google Scholar] [CrossRef] [PubMed]
- You, K.; Ge, F.; Wu, X.; Song, K.; Yang, Z.; Zhang, Q.; Liu, Y.; Ruan, R.; Zheng, H. Nutrients recovery from piggery wastewater and starch wastewater via microalgae-bacteria consortia. Algal Research 2021, 60, 102551. [Google Scholar] [CrossRef]
- Wang, S.; Li, N.; Yuan, Q.; Liang, D.; Chang, J.; Wang, X.; Ren, N. Vivianite recovery from high concentration phosphorus wastewater with mine drainage as iron sources. Science of The Total Environment 2023, 858, 160098. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Dyhrman, S.T.; Lomas, M.W.; Poulton, N.J.; Van Mooy, B.A.S. Accumulation and enhanced cycling of polyphosphate by Sargasso Sea plankton in response to low phosphorus. Proceedings of the National Academy of Sciences 2014, 111, 8089–8094. [Google Scholar] [CrossRef]
- Diaz, J.M.; Björkman, K.M.; Haley, S.T.; Ingall, E.D.; Karl, D.M.; Longo, A.F.; Dyhrman, S.T. Polyphosphate dynamics at Station ALOHA, North Pacific subtropical gyre. Limnology and Oceanography 2016, 61, 227–239. [Google Scholar] [CrossRef]
- Bolier, G.; de Koningh, M.C.J.; Schmale, J.C.; Donze, M. Differential luxury phosphate response of planktonic algae to phosphorus removal. Hydrobiologia 1992, 243, 113–118. [Google Scholar] [CrossRef]
- Li, J.; Plouchart, D.; Zastepa, A.; Dittrich, M. Picoplankton accumulate and recycle polyphosphate to support high primary productivity in coastal Lake Ontario. Scientific Reports 2019, 9, 19563. [Google Scholar] [CrossRef] [PubMed]
- Weihrauch, C.; Opp, C. Ecologically relevant phosphorus pools in soils and their dynamics: The story so far. Geoderma 2018, 325, 183–194. [Google Scholar] [CrossRef]
- Fang, Z.; Shao, C.; Meng, Y.; Wu, P.; Chen, M. Phosphate signaling in Arabidopsis and Oryza sativa. Plant Science 2009, 176, 170–180. [Google Scholar] [CrossRef]
- Hentrich, S.; Hebeler, M.; Grimme, L.H.; Leibfritz, D.; Mayer, A. P-31 NMR saturation transfer experiments in Chlamydomonas reinhardtii : evidence for the NMR visibility of chloroplastidic Pi. European Biophysics Journal 1993, 22, 31–39. [Google Scholar] [CrossRef]
- Filippelli, G.M. The global phosphorus cycle: past, present, and future. Elements 2008, 4, 89–95. [Google Scholar] [CrossRef]
- Gross, M. Where is all the phosphorus? Current Biology 2017, 27, R1141–R1144. [Google Scholar] [CrossRef]
- Smil, V. Phosphorus in the environment: natural flows and human interferences. Annual review of energy and the environment 2000, 25, 53–88. [Google Scholar] [CrossRef]
- Shen, A.; Gao, S.; Jiang, J.; Hu, Q.; Wang, H.; Yuan, S. Oscillations of algal cell quota: Considering two-stage phosphate uptake kinetics. J Theor Biol 2024, 581, 111739. [Google Scholar] [CrossRef] [PubMed]
- Solovchenko, A.; Gorelova, O.; Karpova, O.; Selyakh, I.; Semenova, L.; Chivkunova, O.; Baulina, O.; Vinogradova, E.; Pugacheva, T.; Scherbakov, P. Phosphorus feast and famine in cyanobacteria: is luxury uptake of the nutrient just a consequence of acclimation to its shortage? Cells 2020, 9, 1933. [Google Scholar] [CrossRef]
- Lobakova, E.S.; Selyakh, I.O.; Semenova, L.R.; Scherbakov, P.N.; Fedorenko, T.A.; Chekanov, K.A.; Chivkunova, O.B.; Baulina, O.I.; Vasilieva, S.G.; Solovchenko, A.E. Hints for understanding microalgal phosphate-resilience from Micractinium simplicissimum IPPAS C-2056 (Trebouxiophyceae) isolated from a phosphorus-polluted site. Journal of Applied Phycology 2022, 34, 2409–2422. [Google Scholar] [CrossRef]
- Brown, N.; Sells, M.; Jayamaha, N.; Shilton, A. Predicting phosphorus accumulation and proposing conditions needed for an algal-based phosphorus uptake process. Environmental Technology 2018, 132, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Sells, M.D.; Brown, N.; Shilton, A.N. Determining variables that influence the phosphorus content of waste stabilization pond algae. Water Research 2018, 132, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Powell, N.; Shilton, A.N.; Pratt, S.; Chisti, Y. Factors influencing luxury uptake of phosphorus by microalgae in waste stabilization ponds. Environmental Science & Technology 2008, 42, 5958–5962. [Google Scholar]
- Lavrinovičs, A.; Mežule, L.; Juhna, T. Microalgae starvation for enhanced phosphorus uptake from municipal wastewater. Algal Research 2020, 52, 102090. [Google Scholar] [CrossRef]
- Sforza, E.; Calvaruso, C.; La Rocca, N.; Bertucco, A. Luxury uptake of phosphorus in Nannochloropsis salina: Effect of P concentration and light on P uptake in batch and continuous cultures. Biochemical Engineering Journal 2018, 134, 69–79. [Google Scholar] [CrossRef]
- Grobbelaar, J.U. Inorganic Algal Nutrition. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2 ed.; Richmond, A., Hu, Q., Eds.; John WIley and Sons: Chichester, UK, 2013; pp. 123–133. [Google Scholar]
- Brown, N.; Shilton, A. Luxury uptake of phosphorus by microalgae in waste stabilisation ponds: current understanding and future direction. Reviews in Environmental Science and Bio/Technology 2014, 13, 321–328. [Google Scholar] [CrossRef]
- Plouviez, M.; Bolot, P.; Shilton, A.; Guieysse, B. Phosphorus uptake and accumulation in Chlamydomonas reinhardtii: Influence of biomass concentration, phosphate concentration, phosphorus depletion time, and light supply. Algal Research 2023, 71. [Google Scholar] [CrossRef]
- Jensen, T.E.; Sicko, L.M. Phosphate metabolism in blue-green algae. I. Fine structure of the” polyphosphate overplus” phenomenon in Plectonema boryanum. Canadian journal of microbiology 1974, 20, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Grillo, J.F.; Gibson, J. Regulation of phosphate accumulation in the unicellular cyanobacterium Synechococcus. Journal of bacteriology 1979, 140, 508–517. [Google Scholar] [CrossRef]
- Aitchison, P.; Butt, V. The relation between the synthesis of inorganic polyphosphate and phosphate uptake by Chlorella vulgaris. Journal of Experimental Botany 1973, 24, 497–510. [Google Scholar] [CrossRef]
- Schaedig, E.; Cantrell, M.; Urban, C.; Zhao, X.; Greene, D.; Dancer, J.; Gross, M.; Sebesta, J.; Chou, K.J.; Grabowy, J.; et al. Isolation of phosphorus-hyperaccumulating microalgae from revolving algal biofilm (RAB) wastewater treatment systems. Front Microbiol 2023, 14, 1219318. [Google Scholar] [CrossRef] [PubMed]
- Christ, J.J.; Willbold, S.; Blank, L.M. Methods for the Analysis of Polyphosphate in the Life Sciences. Analytical Chemistry 2020, 92, 4167–4176. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.R.; Kornberg, A. Inorganic polyphosphate in the origin and survival of species. Proceedings of the National Academy of Sciences of the United States of America 2004, 101, 16085–16087. [Google Scholar] [CrossRef] [PubMed]
- Rasala, B.; Mayfield, S. Photosynthetic biomanufacturing in green algae; production of recombinant proteins for industrial, nutritional, and medical uses. Photosynthesis Research 2015, 123, 227–239. [Google Scholar] [CrossRef]
- Blank, L.M. The cell and P: from cellular function to biotechnological application. Current Opinion in Biotechnology 2012, 23, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Wang, X.; Tong, Y.; Chen, X.; Liao, H. Bioengineering and management for efficient phosphorus utilization in crops and pastures. Current Opinion in Biotechnology 2012, 23, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Cook, R.; Lupette, J.; Benning, C. The Role of Chloroplast Membrane Lipid Metabolism in Plant Environmental Responses. Cells 2021, 10, 706. [Google Scholar] [CrossRef] [PubMed]
- Mühlroth, A.; Winge, P.; El Assimi, A.; Jouhet, J.; Maréchal, E.; Hohmann-Marriott, M.F.; Vadstein, O.; Bones, A.M. Mechanisms of Phosphorus Acquisition and Lipid Class Remodeling under P Limitation in a Marine Microalga. Plant Physiology 2017, 175, 1543–1559. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A. Interactions between nitrogen and phosphorus metabolism. Annual Plant Reviews Volume 48: Phosphorus Metabolism in Plants 2015, 48, 187–214. [Google Scholar]
- Raghothama, K. Phosphate acquisition. Annual review of plant biology 1999, 50, 665–693. [Google Scholar] [CrossRef]
- Rao, N.N.; Gómez-García, M.R.; Kornberg, A. Inorganic polyphosphate: essential for growth and survival. Annual review of biochemistry 2009, 78, 605–647. [Google Scholar] [CrossRef] [PubMed]
- Kamennaya, N.A.; Geraki, K.; Scanlan, D.J.; Zubkov, M.V. Accumulation of ambient phosphate into the periplasm of marine bacteria is proton motive force dependent. Nat Commun 2020, 11, 2642. [Google Scholar] [CrossRef] [PubMed]
- Grossman, A.; Takahashi, H. Macronutrient utilization by photosynthetic eukaryotes and the fabric of interactions. Annual Review of Plant Biology 2001, 52, 163–210. [Google Scholar] [CrossRef] [PubMed]
- Shimogawara, K.; Wykoff, D.D.; Usuda, H.; Grossman, A.R. Chlamydomonas reinhardtii Mutants Abnormal in Their Responses to Phosphorus Deprivation. Plant Physiology 1999, 120, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiao, L.; Yang, H.; Chen, G.; Zeng, H.; Zhao, H.; Zhu, Y. Genome-Wide Identification, Expression Profiling, and Evolution of Phosphate Transporter Gene Family in Green Algae. Front Genet 2020, 11, 590947. [Google Scholar] [CrossRef] [PubMed]
- Falkner, G.; Falkner, R. The complex regulation of the phosphate uptake system of cyanobacteria. In Bioenergetic Processes of Cyanobacteria; Springer: 2011; pp. 109–130.
- Achbergerová, L.; Nahálka, J. Polyphosphate-an ancient energy source and active metabolic regulator. Microb Cell Fact 2011, 10, 14170–14175. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Luque, E.; Bhaya, D.; Grossman, A.R. Polyphosphate: A Multifunctional Metabolite in Cyanobacteria and Algae. Frontiers in Plant Science 2020, 11. [Google Scholar] [CrossRef]
- Kulaev, I.; Vagabov, I.; Kulakovskaya, T. The Biochemistry of Inorganic Polyphosphates, 2 ed.; John Wiley & Sons, Ltd.: Chichester, England, 2004. [Google Scholar]
- Ruiz, F.A.; Marchesini, N.; Seufferheld, M.; Govindjee, *!!! REPLACE !!!*; Docampo, R. The Polyphosphate Bodies of Chlamydomonas reinhardtii Possess a Proton-pumping Pyrophosphatase and Are Similar to Acidocalcisomes. Journal of Biological Chemistry 2001, 276, 46196–46203. [Google Scholar] [CrossRef]
- Goodenough, U.; Heiss, A.A.; Roth, R.; Rusch, J.; Lee, J.-H. Acidocalcisomes: Ultrastructure, Biogenesis, and Distribution in Microbial Eukaryotes. Protist 2019, 170, 287–313. [Google Scholar] [CrossRef]
- Guan, Z.; Chen, J.; Liu, R.; Chen, Y.; Xing, Q.; Du, Z.; Cheng, M.; Hu, J.; Zhang, W.; Mei, W.; et al. The cytoplasmic synthesis and coupled membrane translocation of eukaryotic polyphosphate by signal-activated VTC complex. Nat Commun 2023, 14, 718. [Google Scholar] [CrossRef]
- Desfougeres, Y.; Gerasimaite, R.U.; Jessen, H.J.; Mayer, A. Vtc5, a Novel Subunit of the Vacuolar Transporter Chaperone Complex, Regulates Polyphosphate Synthesis and Phosphate Homeostasis in Yeast. J Biol Chem 2016, 291, 22262–22275. [Google Scholar] [CrossRef] [PubMed]
- Müller, O.; Neumann, H.; Bayer, M.J.; Mayer, A. Role of the Vtc proteins in V-ATPase stability and membrane trafficking. Journal of Cell Science 2003, 116, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Cliff, A.; Guieysse, B.; Brown, N.; Lockhart, P.; Dubreucq, E.; Plouviez, M. Polyphosphate synthesis is an evolutionarily ancient phosphorus storage strategy in microalgae. Algal Research 2023. [Google Scholar] [CrossRef]
- Plouviez, M.; Abyadeh, M.; Hasan, M.; Mirzaei, M.; Paulo, J.A.; Guieysse, B. The proteome of Chlamydomonas reinhardtii during phosphorus depletion and repletion. Algal Research 2023, 71. [Google Scholar] [CrossRef]
- Plouviez, M.; Fernandez, E.; Grossman, A.R.; Sanz-Luque, E.; Sells, M.; Wheeler, D.; Guieysse, B. Responses of Chlamydomonas reinhardtii during the transition from P-deficient to P-sufficient growth (the P-overplus response): The roles of the vacuolar transport chaperones and polyphosphate synthesis. J Phycol 2021. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Garcia, M.R.; Fazeli, F.; Grote, A.; Grossman, A.R.; Bhaya, D.J.J.o.b. Role of polyphosphate in thermophilic Synechococcus sp. from microbial mats. 2013, 195, 3309–3319. [Google Scholar]
- Ota, S.; Yoshihara, M.; Yamazaki, T.; Takeshita, T.; Hirata, A.; Konomi, M.; Oshima, K.; Hattori, M.; Bisova, K.; Zachleder, V.; et al. Deciphering the relationship among phosphate dynamics, electron-dense body and lipid accumulation in the green alga Parachlorella kessleri. Scienific Reports 2016, 6, 25731. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.B.; Kang, M.J.; Choi, K.M.; Yang, I.K.; Hong, S.J.; Lee, C.G. Inhibition of Polyphosphate Degradation in Synechocystis sp. PCC6803 through Inactivation of the phoU Gene. J Microbiol Biotechnol 2024, 34, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Sebesta, J.; Cantrell, M.; Schaedig, E.; Hou, H.J.M.; Pastore, C.; Chou, K.J.; Xiong, W.; Guarnieri, M.T.; Yu, J. Polyphosphate kinase deletion increases laboratory productivity in cyanobacteria. Front Plant Sci 2024, 15, 1342496. [Google Scholar] [CrossRef]
- Voronkov, A.; Sinetova, M. Polyphosphate accumulation dynamics in a population of Synechocystis sp. PCC 6803 cells under phosphate overplus. Protoplasma, 2019. [Google Scholar] [CrossRef]
- Paytan, A.; McLaughlin, K. The oceanic phosphorus cycle. Chemical Reviews 2007, 107, 563–576. [Google Scholar] [CrossRef]
- Moseley, J.L.; Chang, C.-W.; Grossman, A.R.J.E.c. Genome-based approaches to understanding phosphorus deprivation responses and PSR1 control in Chlamydomonas reinhardtii. 2006, 5, 26–44.
- Falkner, R.; Falkner, G. Distinct adaptivity during phosphate uptake by the cyanobacterium Anabaena variabilis reflects information processing about preceding phosphate supply. Journal of trace and microprobe techniques 2003, 21, 363–375. [Google Scholar] [CrossRef]
- Vítová, M.; Bišová, K.; Kawano, S.; Zachleder, V. Accumulation of energy reserves in algae: From cell cycles to biotechnological applications. Biotechnology Advances 2015, 33, 1204–1218. [Google Scholar] [CrossRef] [PubMed]
- Bertilsson, S.; Berglund, O.; Karl, D.M.; Chisholm, S.W. Elemental composition of marine Prochlorococcus and Synechococcus: Implications for the ecological stoichiometry of the sea. Limnology and oceanography 2003, 48, 1721–1731. [Google Scholar] [CrossRef]
- Abida, H.; Dolch, L.-J.; Meï, C.; Villanova, V.; Conte, M.; Block, M.A.; Finazzi, G.; Bastien, O.; Tirichine, L.; Bowler, C.; et al. Membrane Glycerolipid Remodeling Triggered by Nitrogen and Phosphorus Starvation in Phaeodactylum tricornutum. Plant Physiology 2014, 167, 118–136. [Google Scholar] [CrossRef]
- Canavate, J.P.; Armada, I.; Hachero-Cruzado, I. Aspects of phosphorus physiology associated with phosphate-induced polar lipid remodelling in marine microalgae. Journal of Plant Physiology 2017, 214, 28–38. [Google Scholar] [CrossRef]
- Bolik, S.; Schlaich, A.; Mukhina, T.; Amato, A.; Bastien, O.; Schneck, E.; Demé, B.; Jouhet, J. Lipid bilayer properties potentially contributed to the evolutionary disappearance of betaine lipids in seed plants. BMC biology 2023, 21, 275. [Google Scholar] [CrossRef]
- Gomez, R.E.; Castets, J.; Lupette, J.; Chambaud, C.; Joubès, J.; Bernard, A. Phosphatidylinositol-4-phosphate joins the dance of plant autophagosome formation. Autophagy 2023, 19, 1609–1610. [Google Scholar] [CrossRef]
- Kokabi, K.; Gorelova, O.; Ismagulova, T.; Itkin, M.; Malitsky, S.; Boussiba, S.; Solovchenko, A.; Khozin-Goldberg, I. Metabolomic foundation for differential responses of lipid metabolism to nitrogen and phosphorus deprivation in an arachidonic acid-producing green microalga. Plant Science 2019, 283, 95–115. [Google Scholar] [CrossRef] [PubMed]
- Shemi, A.; Schatz, D.; Fredricks, H.F.; Van Mooy, B.A.; Porat, Z.; Vardi, A. Phosphorus starvation induces membrane remodeling and recycling in Emiliania huxleyi. New Phytologist 2016, 211, 886–898. [Google Scholar] [CrossRef]
- Martin, P.; Dyhrman, S.T.; Lomas, M.W.; Poulton, N.J.; Van Mooy, B.A. Accumulation and enhanced cycling of polyphosphate by Sargasso Sea plankton in response to low phosphorus. Proc Natl Acad Sci U S A 2014, 111, 8089–8094. [Google Scholar] [CrossRef]
- Gorelova, O.; Baulina, O.; Ismagulova, T.; Kokabi, K.; Lobakova, E.; Selyakh, I.; Semenova, L.; Chivkunova, O.; Karpova, O.; Scherbakov, P. Stress-induced changes in the ultrastructure of the photosynthetic apparatus of green microalgae. Protoplasma 2019, 256, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Brányiková, I.; Maršálková, B.; Doucha, J.; Brányik, T.; Bišová, K.; Zachleder, V.; Vítová, M. Microalgae—novel highly efficient starch producers. Biotechnology and bioengineering 2011, 108, 766–776. [Google Scholar] [CrossRef] [PubMed]
- de Mazancourt, C.; Schwartz, M.W. Starve a competitor: evolution of luxury consumption as a competitive strategy. Theoretical Ecology 2010, 5, 37–49. [Google Scholar] [CrossRef]
- Tetu, S.G.; Brahamsha, B.; Johnson, D.A.; Tai, V.; Phillippy, K.; Palenik, B.; Paulsen, I.T. Microarray analysis of phosphate regulation in the marine cyanobacterium Synechococcus sp. WH8102. The ISME Journal 2009, 3, 835–849. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Martin, M.A.; Mateo, P.; Leganes, F.; Fernandez-Pinas, F. Novel cyanobacterial bioreporters of phosphorus bioavailability based on alkaline phosphatase and phosphate transporter genes of Anabaena sp. PCC 7120. Anal Bioanal Chem 2011, 400, 3573–3584. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhang, H.; Huang, B.; Lin, S. Alkaline phosphatase gene sequence and transcriptional regulation by phosphate limitation in Amphidinium carterae (Dinophyceae) 1. Journal of phycology 2011, 47, 1110–1120. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Litaker, R.W.; Sunda, W.G. Phosphorus physiological ecology and molecular mechanisms in marine phytoplankton. Journal of Phycology 2016, 52, 10–36. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.R.; Fees, J.; Christ, J.J.; Hofmann, I.; Block, C.; Herzberg, D.; Bröring, S.; Reckels, B.; Visscher, C.; Blank, L.M.; et al. Biotechnological production of food-grade polyphosphate from deoiled seeds and bran. EFB Bioeconomy Journal 2023, 3, 100048. [Google Scholar] [CrossRef]
- Lobakova, E.; Gorelova, O.; Selyakh, I.; Semenova, L.; Scherbakov, P.; Vasilieva, S.; Zaytsev, P.; Shibzukhova, K.; Chivkunova, O.; Baulina, O. Failure of Micractinium simplicissimum Phosphate Resilience upon Abrupt Re-Feeding of Its Phosphorus-Starved Cultures. International Journal of Molecular Sciences 2023, 24, 8484. [Google Scholar] [CrossRef]
- Li, Q.; Fu, L.; Wang, Y.; Zhou, D.; Rittmann, B.E. Excessive phosphorus caused inhibition and cell damage during heterotrophic growth of Chlorella regularis. Bioresource Technology 2018, 268, 266–270. [Google Scholar] [CrossRef]
- Fu, L.; Li, Q.; Yan, G.; Zhou, D.; Crittenden, J.C. Hormesis effects of phosphorus on the viability of Chlorella regularis cells under nitrogen limitation. Biotechnol Biofuels 2019, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Cogliatti, D.H.; Clarkson, D.T. Physiological changes in, and phosphate uptake by potato plants during development of, and recovery from phosphate deficiency. Physiologia Plantarum 1983, 58, 287–294. [Google Scholar] [CrossRef]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S.J.N. Agricultural sustainability and intensive production practices. 2002, 418, 671.
- Martín, H.G.; Ivanova, N.; Kunin, V.; Warnecke, F.; Barry, K.W.; McHardy, A.C.; Yeates, C.; He, S.; Salamov, A.A.; Szeto, E. Metagenomic analysis of two enhanced biological phosphorus removal (EBPR) sludge communities. Nature biotechnology 2006, 24, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, R.; Guieysse, B. Algal-bacterial processes for the treatment of hazardous contaminants: A review. Water research 2006, 40, 2799–2815. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.L.; Rodrigues, C.M.; Pires, J.C.M.; Simões, M. The effect of increasing CO2 concentrations on its capture, biomass production and wastewater bioremediation by microalgae and cyanobacteria. Algal Research 2016, 14, 127–136. [Google Scholar] [CrossRef]
- de Siqueira Castro, J.; Calijuri, M.L.; Mattiello, E.M.; Ribeiro, V.J.; Assemany, P.P. Algal biomass from wastewater: soil phosphorus bioavailability and plants productivity. Science of the total environment 2020, 711, 135088. [Google Scholar] [CrossRef]
- Kandasamy, S.; Narayanan, M.; Raja, R.; Devarayan, K.; Kavitha, R. The current state of algae in wastewater treatment and energy conversion: a critical review. Current Opinion in Environmental Science & Health 2023, 100469. [Google Scholar]
- Li, T.; Xu, L.; Li, W.; Wang, C.; Gin, K.Y.; Chai, X.; Wu, B. Dissolved organic carbon spurs bacterial-algal competition and phosphorus-paucity adaptation: Boosting Microcystis’ phosphorus uptake capacity. Water Res 2024, 255, 121465. [Google Scholar] [CrossRef] [PubMed]
- Odibo, A.; Janpum, C.; Pombubpa, N.; Monshupanee, T.; Incharoensakdi, A.; Ur Rehman, Z.; In-Na, P. Microalgal-bacterial immobilized co-culture as living biofilters for nutrient recovery from synthetic wastewater and their potential as biofertilizers. Bioresour Technol 2024, 398, 130509. [Google Scholar] [CrossRef]
- Jakhwal, P.; Daneshvar, E.; Skalska, K.; Matsakas, L.; Patel, A.; Park, Y.; Bhatnagar, A. Nutrient removal and biomass production of marine microalgae cultured in recirculating aquaculture systems (RAS) water with low phosphate concentration. J Environ Manage 2024, 358, 120859. [Google Scholar] [CrossRef]
- Tang, C.C.; Hu, Y.R.; Zhang, M.; Chen, S.L.; He, Z.W.; Li, Z.H.; Tian, Y.; Wang, X.C. Role of phosphate in microalgal-bacterial symbiosis system treating wastewater containing heavy metals. Environ Pollut 2024, 123951. [Google Scholar] [CrossRef]
- Pick, U.; Bental, M.; Chitlaru, E.; Weiss, M. Polyphosphate-hydrolysis-a protective mechanism against alkaline stress? FEBS Letters 1990, 274, 15–18. [Google Scholar]
- Bental, M.; Pick, U.; AVRON, M.; Degani, H. The role of intracellular orthophosphate in triggering osmoregulation in the alga Dunaliella salina. European journal of biochemistry 1990, 188, 117–122. [Google Scholar] [CrossRef]
- Leitão, J.M.; Lorenz, B.; Bachinski, N.; Wilhelm, C.; Müller, W.E.; Schröder, H.C. Osmotic-stress-induced synthesis and degradation of inorganic polyphosphates in the alga Phaeodactylumtricornutum. Marine Ecology Progress Series 1995, 121, 279–288. [Google Scholar] [CrossRef]
- Miranda, A.M.; Hernandez-Tenorio, F.; Villalta, F.; Vargas, G.J.; Sáez, A.A. Advances in the Development of Biofertilizers and Biostimulants from Microalgae. Biology 2024, 13, 199. [Google Scholar] [CrossRef]
- Ammar, E.E.; Aioub, A.A.A.; Elesawy, A.E.; Karkour, A.M.; Mouhamed, M.S.; Amer, A.A.; El-Shershaby, N.A. Algae as Bio-fertilizers: Between current situation and future prospective. Saudi Journal of Biological Sciences 2022, 29, 3083–3096. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.L.; Weyers, S.L.; Goemann, H.M.; Peyton, B.M.; Gardner, R.D. Microalgae, soil and plants: A critical review of microalgae as renewable resources for agriculture. Algal Research 2021, 54, 102200. [Google Scholar] [CrossRef]
- Schreiber, C.; Schiedung, H.; Harrison, L.; Briese, C.; Ackermann, B.; Kant, J.; Schrey, S.D.; Hofmann, D.; Singh, D.; Ebenhöh, O.; et al. Evaluating potential of green alga Chlorella vulgaris to accumulate phosphorus and to fertilize nutrient-poor soil substrates for crop plants. Journal of Applied Phycology 2018, 30, 2827–2836. [Google Scholar] [CrossRef]
- Alvarez-Gonzalez, A.; Uggetti, E.; Serrano, L.; Gorchs, G.; Ferrer, I.; Diez-Montero, R. Can microalgae grown in wastewater reduce the use of inorganic fertilizers? J Environ Manage 2022, 323, 116224. [Google Scholar] [CrossRef]
- Kublanovskaya, A.; Khapchaeva, S.; Zotov, V.; Zaytsev, P.; Lobakova, E.; Solovchenko, A. The effect of the microalga Chlorella vulgaris Ippas C-1 biomass application on yield, biological activity, and the microbiome of the soil during bean growing. Moscow University Biological Sciences Bulletin 2019, 74, 227–234. [Google Scholar] [CrossRef]
- Solovchenko, A.; Zaitsev, P.; Zotov, V. Phosphorus biofertilizer from microalgae. Biofertilizers 2021, 57–68. [Google Scholar]
- de Souza, M.H.B.; Calijuri, M.L.; Assemany, P.P.; Castro, J.d.S.; de Oliveira, A.C.M. Soil application of microalgae for nitrogen recovery: A life-cycle approach. Journal of Cleaner Production 2019, 211, 342–349. [Google Scholar] [CrossRef]
- Castro, J.d.S.; Calijuri, M.L.; Ferreira, J.; Assemany, P.P.; Ribeiro, V.J. Microalgae based biofertilizer: A life cycle approach. Science of The Total Environment 2020, 724, 138138. [Google Scholar] [CrossRef]
- Sharma, G.K.; Khan, S.A.; Shrivastava, M.; Bhattacharyya, R.; Sharma, A.; Gupta, D.K.; Kishore, P.; Gupta, N. Circular economy fertilization: Phycoremediated algal biomass as biofertilizers for sustainable crop production. Journal of Environmental Management 2021, 287, 112295. [Google Scholar] [CrossRef]
- Cao, M.; Wang, F.; Zhou, B.; Chen, H.; Yuan, R.; Ma, S.; Geng, H.; Li, J.; Lv, W.; Wang, Y.; et al. Nanoparticles and antibiotics stress proliferated antibiotic resistance genes in microalgae-bacteria symbiotic systems. J Hazard Mater 2023, 443, 130201. [Google Scholar] [CrossRef]
- Eheneden, I.; Wang, R.; Zhao, J. Antibiotic removal by microalgae-bacteria consortium: Metabolic pathways and microbial responses. Sci Total Environ 2023, 891, 164489. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Jin, P.; Xie, S.; Beardall, J.; Feng, Y.; Guo, C.; Ma, Z.; Gao, G. Micro- and nanoplastics interact with conventional pollutants on microalgae: Synthesis through meta-analysis. Environ Pollut 2023. [Google Scholar] [CrossRef]
- Behera, B.; Venkata Supraja, K.; Paramasivan, B. Integrated microalgal biorefinery for the production and application of biostimulants in circular bioeconomy. Bioresource Technology 2021, 339, 125588. [Google Scholar] [CrossRef]
- Demling, P.; Baier, M.; Deitert, A.; Fees, J.; Blank, L.M. Biotechnological polyphosphate as an opportunity to contribute to the circularization of the phosphate economy. Current Opinion in Biotechnology 2024, 87, 103107. [Google Scholar] [CrossRef] [PubMed]
- Bowlin, M.Q.; Gray, M.J. Inorganic polyphosphate in host and microbe biology. Trends Microbiol 2021, 29, 1013–1023. [Google Scholar] [CrossRef]
- Kulakovskaya, T.V.; Vagabov, V.M.; Kulaev, I.S. Inorganic polyphosphate in industry, agriculture and medicine: Modern state and outlook. Process Biochemistry 2012, 47, 1–10. [Google Scholar] [CrossRef]
- Feng, G.; Dong, S.; Huang, M.; Zeng, M.; Liu, Z.; Zhao, Y.; Wu, H. Biogenic Polyphosphate Nanoparticles from a Marine Cyanobacterium Synechococcus sp. PCC 7002: Production, Characterization, and Anti-Inflammatory Properties In Vitro. Mar Drugs 2018, 16. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A. Phosphorus and the future. In The Ecophysiology of Plant-Phosphorus Interactions. Springer, Dordrecht, The Netherlands, White, P., Hammond, J., Eds. Springer: Dordrecht, 2008; pp. 271-283. [CrossRef]
| Habitat or source | P or Pi range | Ref. |
|---|---|---|
| Wastewater | 3–330 mg L–1 (3 µM–3 mM) Pi | [19] |
| Domestic: 0.5 – 8.6 mg-P L-1 (0.02-0.3 mM) | [20] | |
| Industrial e.g., mine drainage: 186 – 558 mg-P L-1 (6 – 18 mM) | [21] | |
| Deep aphotic ocean | Soluble reactive P: 0.8 – 5.4 µg-P L-1 (0.025 – 0.175 µM) | [22,23] |
| River | Total P 0.04-0.4 mg-P L–1 (0.001-0.01 mM ) | [24] |
| Lakes | Soluble reactive P: 0.01-0.85 mg-P–1 (0.3 µM – 0.3 mM)) | [25] |
| Unfertilized soil | 12.4 – 341 µg-P L-1 (0.4––11 μM)* | [2] |
| Fertilized soil | 0.1–5.0 mg-P L-1 (0.003-0.16 mM) | [26] |
| Intracellular concentration | Crop plants: 155 – 620 mg-P L–1 (5–20 µM) | [27] |
| Microalgae: C. reinhardtii: 152 ± 37.0 µg free Pi (4.9±1.2 µmol mostly in the chloroplast) | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





