Submitted:
22 May 2024
Posted:
22 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Model Adaptation
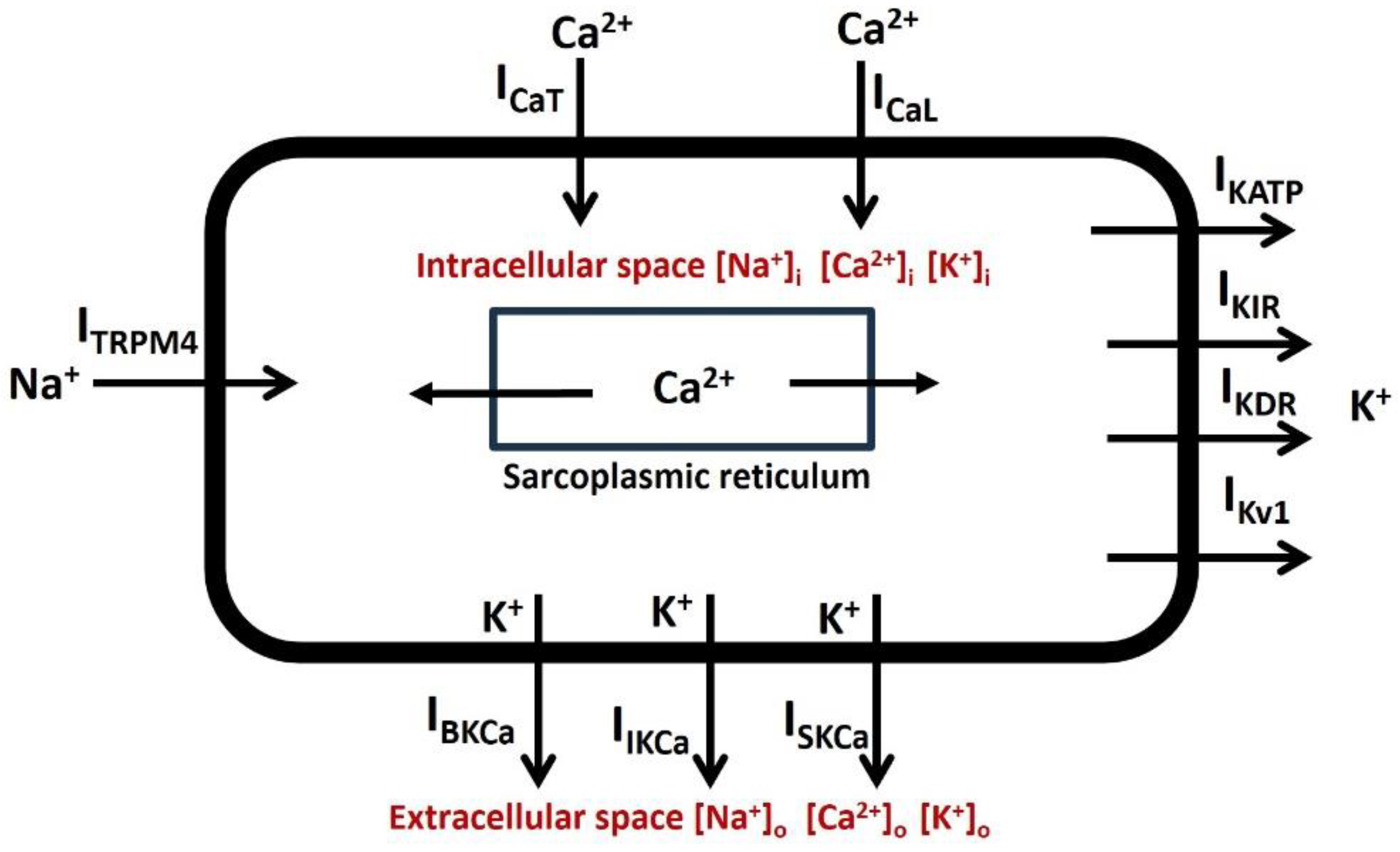
2.2. General Membrane Current Descriptions
KC3O3 = 0.000881 * a, KC4O4 = 0.054324 * a, KO0C0 = 328.1084 * b,
KO1C1 = 154. 1736 * b, KO2C2 = 33.6594 * b, KO3C3= 0.097312 *b,KO4C4=0.000406*b*cai
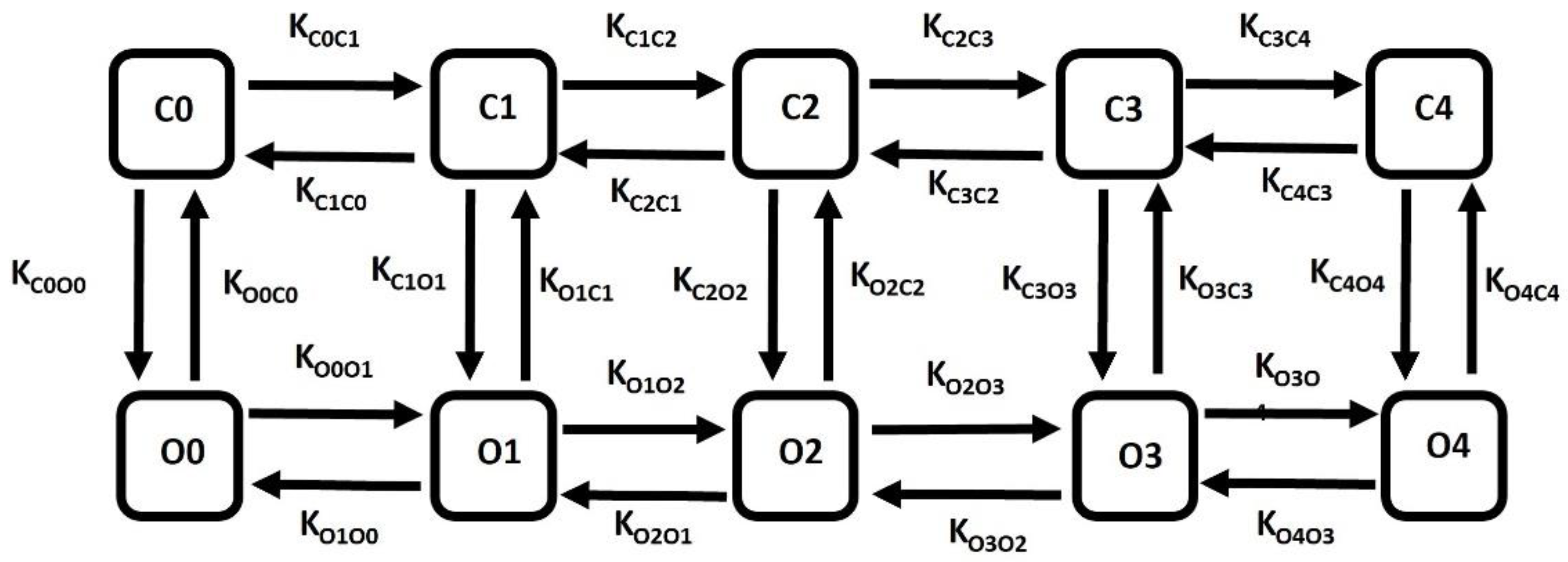
KC4C3= 3 * Kcoff * cai, KC3C2= 4 * Kcoff * cai, KC2C1= 3 * Kcoff * cai, KC1C0= Kcoff * cai
KO0O1= 3 * Kon * cai, KO1O2= 4 * Kon * cai, KO2O3= 3 * Kon * cai, KO3O4= Kon * cai
KO4O3= 3 * Kooff * cai, KO3O2= 4 * Kooff * cai, KO2O1= 3 * Kooff * cai, KO1O0 = Kooff * cai
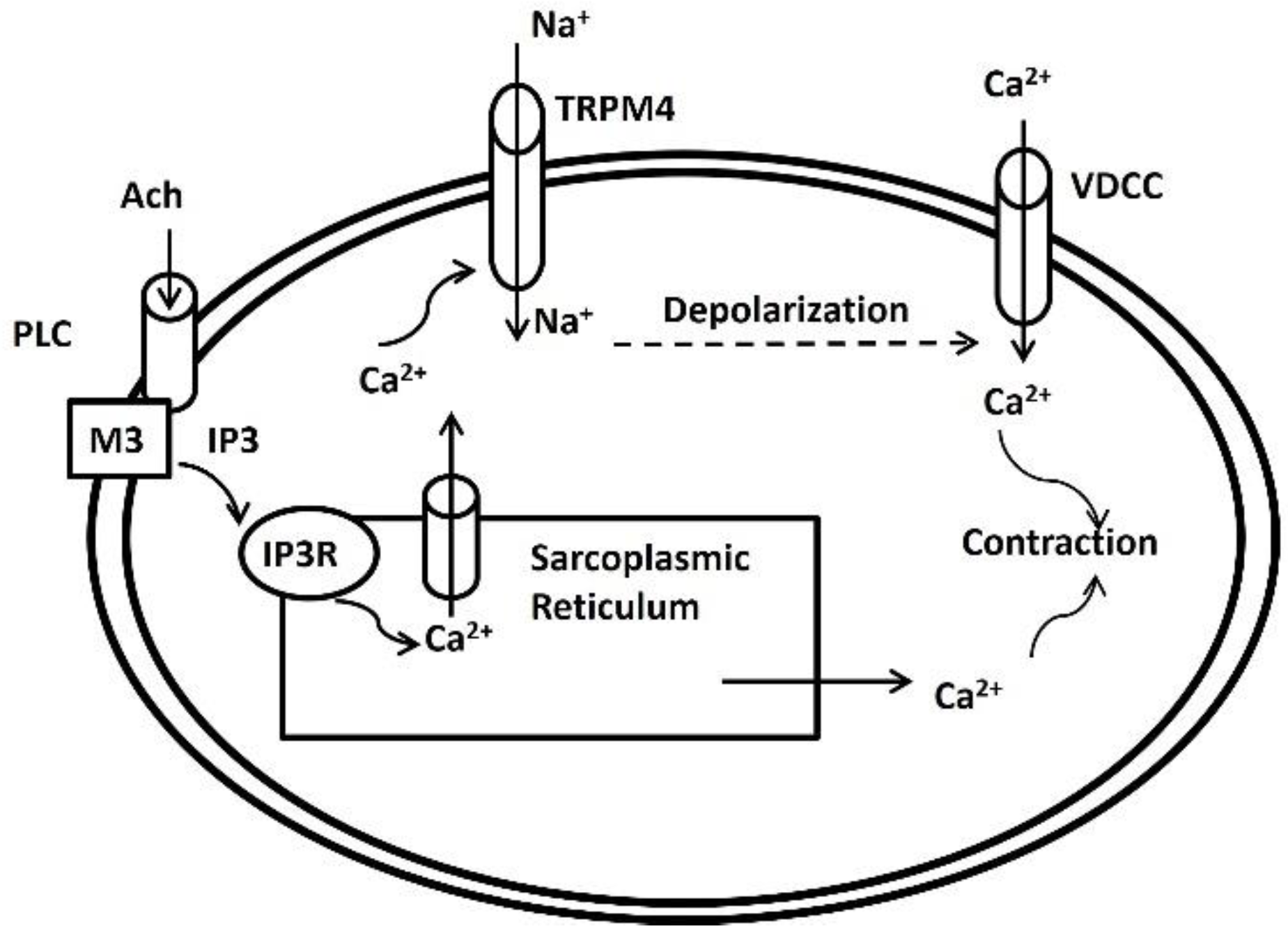
2.3. TRPM 4 Channel with Ca2+ Sensing Mechanism
2.4. Whole DSM Cell Model and Simulation
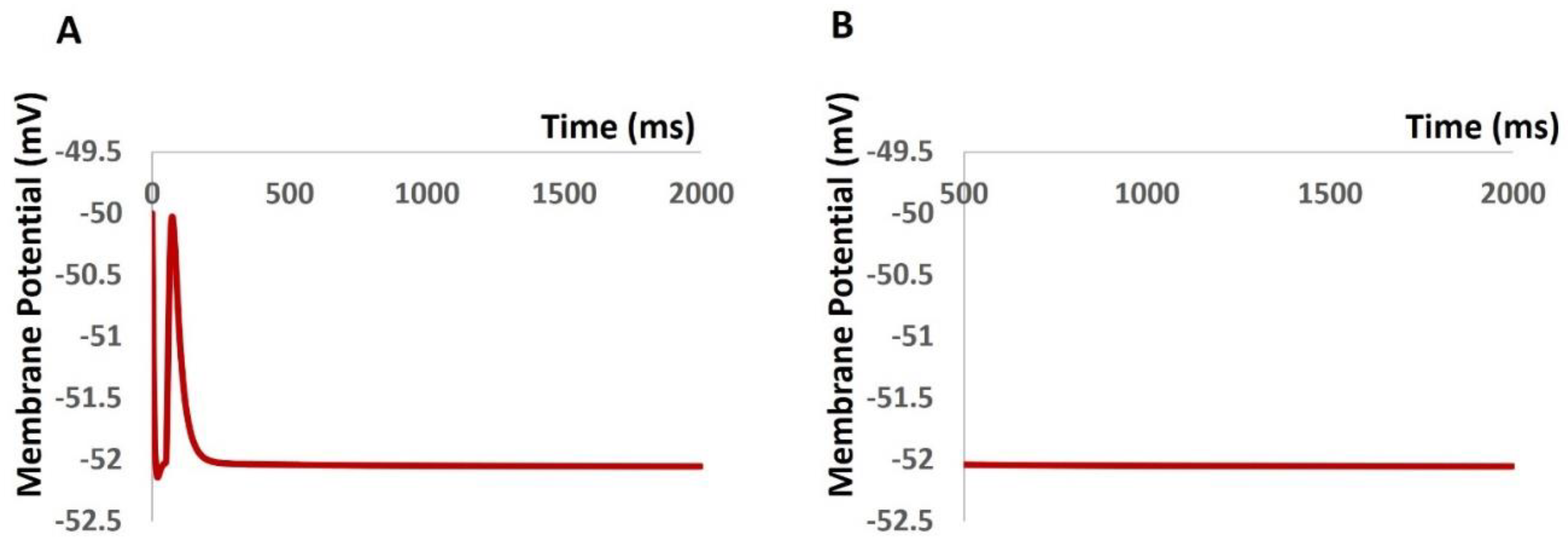
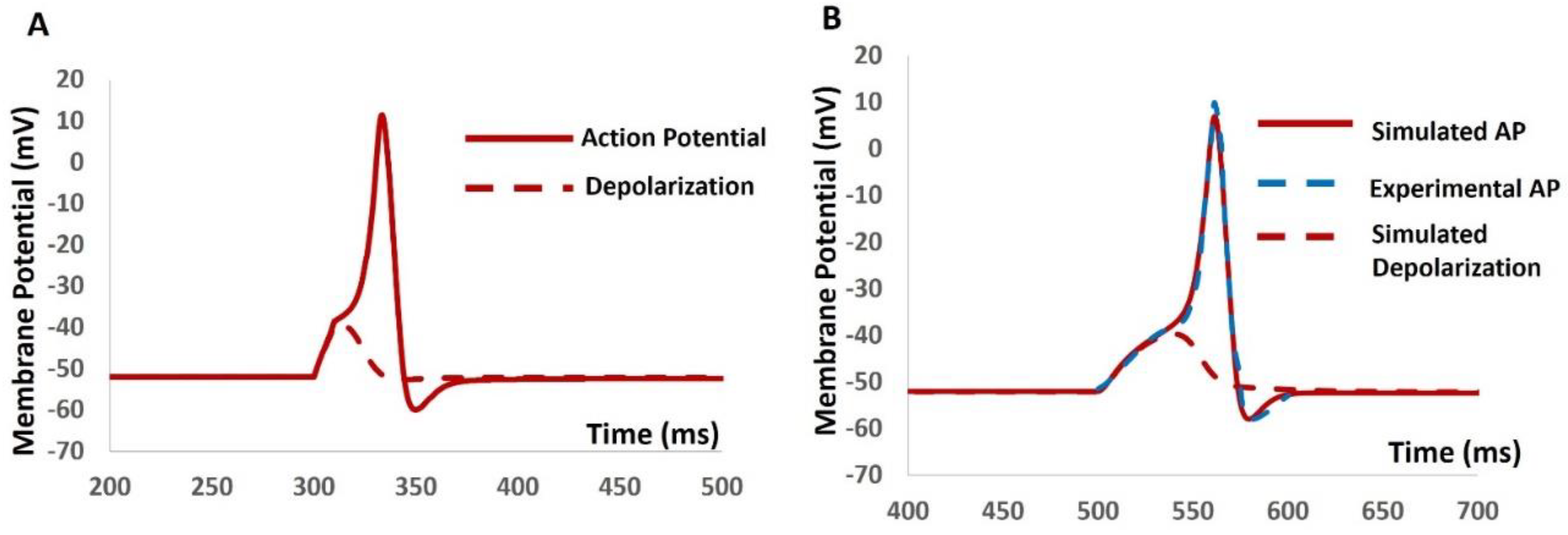
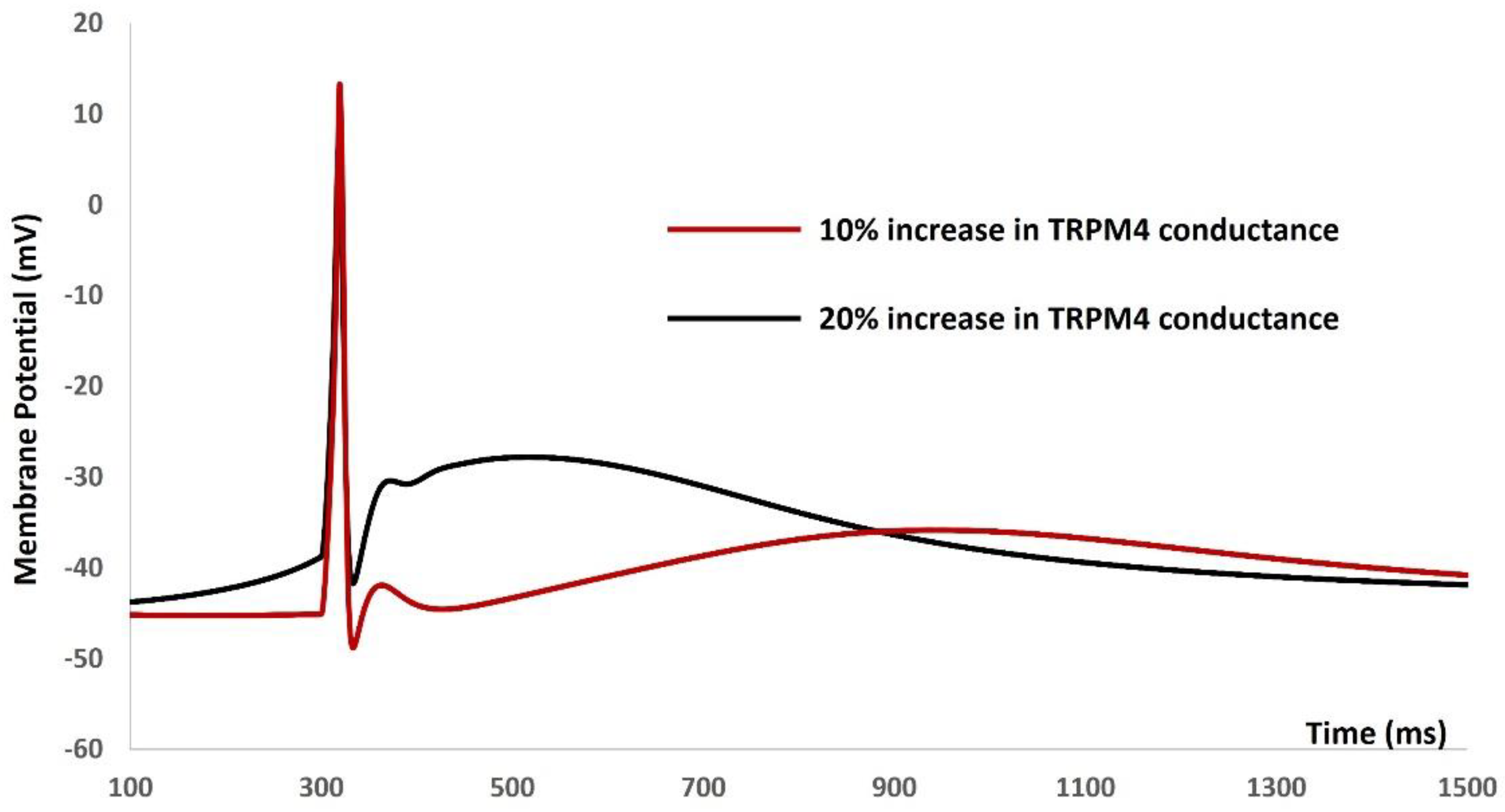
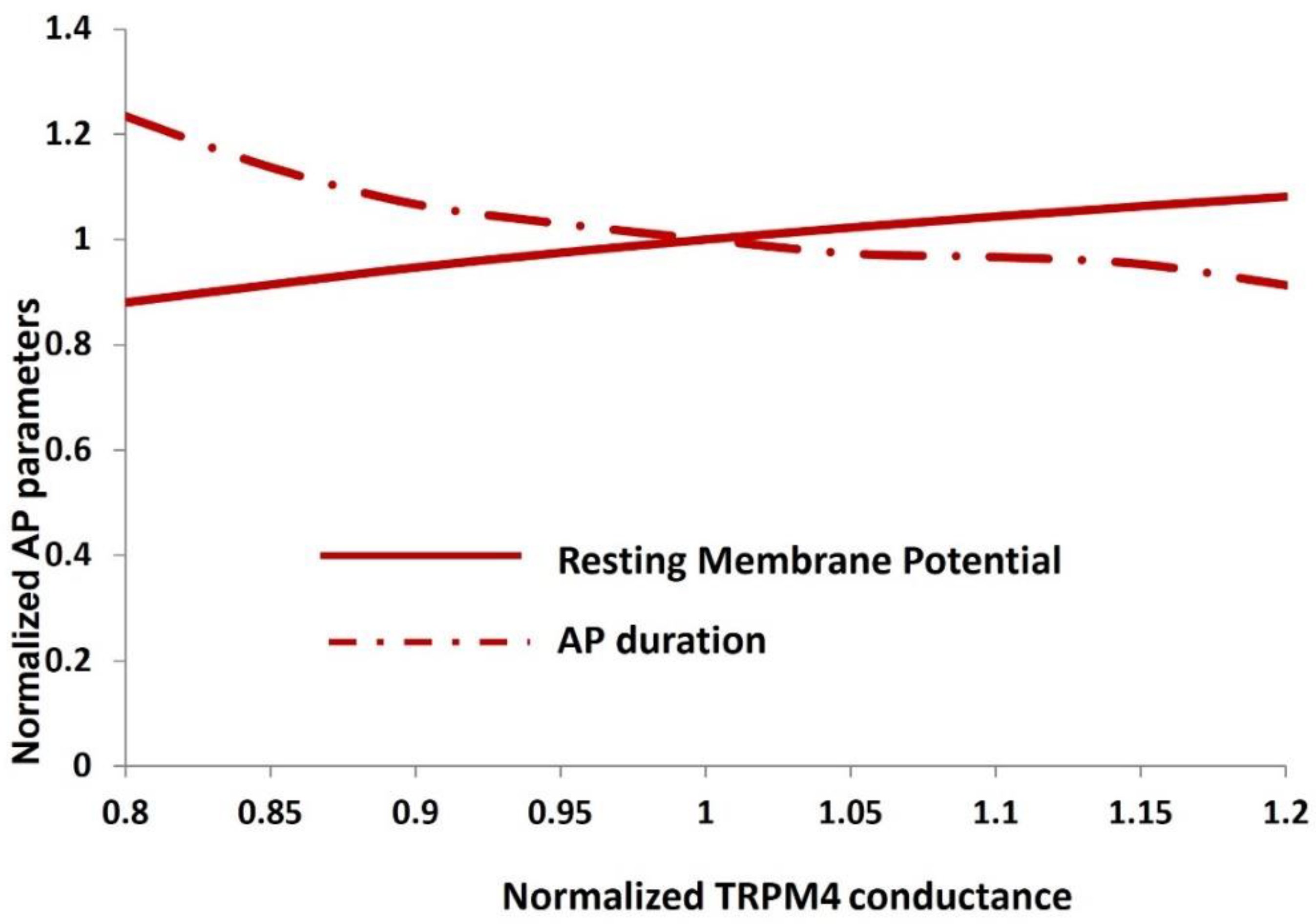
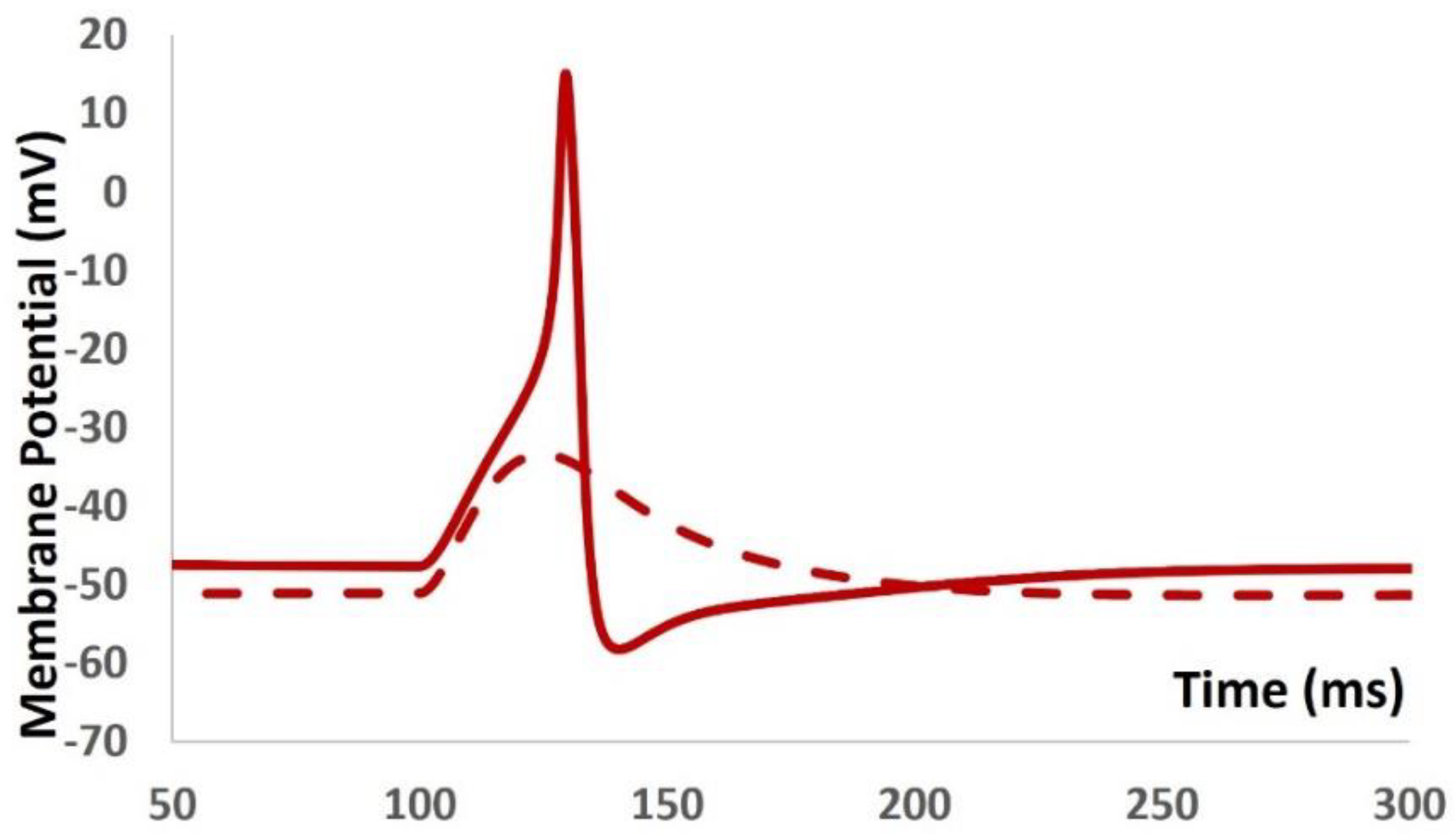
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Groat, William C., and N. A. O. K. I. Yoshimura. "Anatomy and physiology of the lower urinary tract." Handbook of clinical neurology 130 (2015): 61-108. [CrossRef]
- Tyagi, Shachi, Pradeep Tyagi, Naoki Yoshimura, and Michael B. Chancellor. "Physiology of micturition." In Textbook of Female Urology and Urogynecology-Two-Volume Set, pp. 232-246. CRC Press, 2017.
- Abrams, Paul. "Describing bladder storage function: overactive bladder syndrome and detrusor overactivity." Urology 62, no. 5 (2003): 28-37. [CrossRef]
- Wein, Alan J., and Raymond R. Rackley. "Overactive bladder: a better understanding of pathophysiology, diagnosis, and management." The Journal of urology 175, no. 3 (2006): S5-S10. [CrossRef]
- Drake, Marcus J. "The Overactive Bladder." In Textbook of Female Urology and Urogynecology-Two-Volume Set, pp. 584-593. CRC Press, 2017.
- Chapple, Christopher R., and Altaf Mangera. "Urgency incontinence and overactive bladder." Oxford Textbook of Urological Surgery (2017): 282.
- Dwyer, Peter L., and Anna Rosamilia. "Evaluation and diagnosis of the overactive bladder." Clinical obstetrics and gynecology 45, no. 1 (2002): 193-204. [CrossRef]
- Brown, Jeanette S., William F. McGhan, and Sudhansu Chokroverty. "Comorbidities associated with overactive bladder." Am J Manag Care 6, no. 11 Suppl (2000): S574-9.
- Palma-Zamora, Isaac D., and Humphrey O. Atiemo. "Understanding the economic impact of neurogenic lower urinary tract dysfunction." Urologic Clinics 44, no. 3 (2017): 333-343. [CrossRef]
- Lin, Kuan-Yin, Ka-Chun Siu, and Kuan-Han Lin. "Impact of lower urinary tract symptoms on work productivity in female workers: A systematic review and meta-analysis." Neurourology and Urodynamics 37, no. 8 (2018): 2323-2334. [CrossRef]
- Pierce, Heather, Lin Perry, Pauline Chiarelli, and Robyn Gallagher. "A systematic review of prevalence and impact of symptoms of pelvic floor dysfunction in identified workforce groups." Journal of Advanced nursing 72, no. 8 (2016): 1718-1734. [CrossRef]
- Chakrabarty, Basu, J. Crook, Marcus Drake, Niall Gilliland, Dev Gulur, Darryl Kitney, Aditya Manjunath, Pavlo Somov, and Bahareh Vahabi. "The Urinary Tract: Form and Function." Lower Urinary Tract Symptoms in Adults: A Clinical Approach (2020): 1-17.
- Kitney, Darryl Graham. Bladder spontaneous activity: influence of mild heating and inert injectables. University of Surrey (United Kingdom), 2016.
- Bortolini, Maria Augusta T., Andreisa PM Bilhar, and Rodrigo A. Castro. "Neural control of lower urinary tract and targets for pharmacological therapy." International urogynecology journal 25 (2014): 1453-1462. [CrossRef]
- Afrashteh, Behnaz. "Identification of lower urinary tract dysfunction after spinal cord injury at lumbosacral level by developing a clinical relevance rat model." PhD diss., University of Salzburg, 2023.
- Asl, Seyed Omid Komari Zadeh. Analysis and modeling of the roles of actin-myosin interactions in bladder smooth muscle biomechanics. Virginia Commonwealth University, 2014.
- Guntu, Vinay Sandeep Kumar. "Biophysical modeling to reverse engineer two mammalian neural circuits lower urinar Y tract and hippocampus." PhD diss., University of Missouri--Columbia, 2020.
- Wyman, J. F., K. L. Burgio, and D. K. Newman. "Practical aspects of lifestyle modifications and behavioural interventions in the treatment of overactive bladder and urgency urinary incontinence." International journal of clinical practice 63, no. 8 (2009): 1177-1191. [CrossRef]
- Willis-Gray, Marcella G., Alexis A. Dieter, and Elizabeth J. Geller. "Evaluation and management of overactive bladder: strategies for optimizing care." Research and reports in urology (2016): 113-122. [CrossRef]
- Smith, Louise E., Rebecca K. Webster, and G. James Rubin. "A systematic review of factors associated with side-effect expectations from medical interventions." Health Expectations 23, no. 4 (2020): 731-758. [CrossRef]
- Mostafaei, Hadi, Shahrokh F. Shariat, Hanieh Salehi-Pourmehr, Florian Janisch, Keiichiro Mori, Fahad Quhal, and Sakineh Hajebrahimi. "The clinical pharmacology of the medical treatment for overactive bladder in adults." Expert review of clinical pharmacology 13, no. 7 (2020): 707-720. [CrossRef]
- Hutchinson, Alexander, Alexander Nesbitt, Andre Joshi, Adrian Clubb, and Marlon Perera. "Overactive bladder syndrome: management and treatment options." Australian journal of general practice 49, no. 9 (2020): 593-598. [CrossRef]
- Fogaing, Cora, Abubakr H. Mossa, and Lysanne Campeau. "Are beta 3 adrenergic agonists now the preferred pharmacologic management of overactive bladder?." Current urology reports 21 (2020): 1-12. [CrossRef]
- Duong, Vi, Aya Iwamoto, Jon Pennycuff, Bela Kudish, and Cheryl Iglesia. "A systematic review of neurocognitive dysfunction with overactive bladder medications." International Urogynecology Journal 32, no. 10 (2021): 2693-2702. [CrossRef]
- Goodridge, Sophia Delpe, and Leslie M. Rickey. "Medical Therapy with Antimuscarinics and ß3-Agonists." In Female Urinary Incontinence, pp. 147-164. Cham: Springer International Publishing, 2022.
- Drake, Marcus J., Christopher H. Fry, Hikaru Hashitani, Ruth Kirschner-Hermanns, Mohammad S. Rahnama'i, John E. Speich, Hikaru Tomoe, Anthony J. Kanai, and Karen D. McCloskey. "What are the origins and relevance of spontaneous bladder contractions? ICI-RS 2017." Neurourology and urodynamics 37, no. S4 (2018): S13-S19. [CrossRef]
- Mitsui, Retsu, Ken Lee, Aoi Uchiyama, Shunta Hayakawa, Fumio Kinoshita, Shunichi Kajioka, Masatoshi Eto, and Hikaru Hashitani. "Contractile elements and their sympathetic regulations in the pig urinary bladder: a species and regional comparative study." Cell and tissue research 379 (2020): 373-387. [CrossRef]
- Burdyga, T. V., and Susan Wray. "The relationship between the action potential, intracellular calcium and force in intact phasic, guinea-pig uretic smooth muscle." The Journal of Physiology 520, no. 3 (1999): 867-883. [CrossRef]
- Ter Keurs, Henk EDJ, and Penelope A. Boyden. "Calcium and arrhythmogenesis." Physiological reviews 87, no. 2 (2007): 457-506. [CrossRef]
- Bolton, T. B., D. V. Gordienko, V. Pucovský, S. Parsons, and O. Povstyan. "Calcium release events in excitation–contraction coupling in smooth muscle." In Role Of The Sarcoplasmic Reticulum In Smooth Muscle: Novartis Foundation Symposium 246, vol. 246, pp. 154-173. Chichester, UK: John Wiley & Sons, Ltd, 2002.
- Sanders, Kenton M. "Spontaneous electrical activity and rhythmicity in gastrointestinal smooth muscles." Smooth muscle spontaneous activity: Physiological and pathological modulation (2019): 3-46. [CrossRef]
- Boopathi, Ettickan, Cristiano Gomes, Stephen A. Zderic, Bruce Malkowicz, Ranjita Chakrabarti, Darshan P. Patel, Alan J. Wein, and Samuel Chacko. "Mechanical stretch upregulates proteins involved in Ca2+ sensitization in urinary bladder smooth muscle hypertrophy." American Journal of Physiology-Cell Physiology 307, no. 6 (2014): C542-C553. [CrossRef]
- Poley, Rainer N., Christopher R. Dosier, John E. Speich, Amy S. Miner, and Paul H. Ratz. "Stimulated calcium entry and constitutive RhoA kinase activity cause stretch-induced detrusor contraction." European journal of pharmacology 599, no. 1-3 (2008): 137-145. [CrossRef]
- Kajioka, Shunichi, Shinsuke Nakayama, Gordon McMurray, Kihachiro Abe, and Alison F. Brading. "Ca2+ channel properties in smooth muscle cells of the urinary bladder from pig and human." European journal of pharmacology 443, no. 1-3 (2002): 19-29. [CrossRef]
- Wu, C., G. Sui, and C. H. Fry. "The role of the L-type Ca2+ channel in refilling functional intracellular Ca2+ stores in guinea-pig detrusor smooth muscle." The Journal of Physiology 538, no. 2 (2002): 357-369. [CrossRef]
- Petkov, Georgi V. "14 Role of Ion Channels in Urinary Bladder Smooth Muscle Function." Signal Transduction and Smooth Muscle (2018): 281. [CrossRef]
- Wray, Susan, and Theodor Burdyga. "Sarcoplasmic reticulum function in smooth muscle." Physiological reviews 90, no. 1 (2010): 113-178. [CrossRef]
- Wu, C., and C. H. Fry. "Na+/Ca2+ exchange and its role in intracellular Ca2+ regulation in guinea pig detrusor smooth muscle." American Journal of Physiology-Cell Physiology 280, no. 5 (2001): C1090-C1096. [CrossRef]
- Herrera, Gerald M., and Mark T. Nelson. "Sarcoplasmic reticulum and membrane currents." In Role Of The Sarcoplasmic Reticulum In Smooth Muscle: Novartis Foundation Symposium 246, vol. 246, pp. 189-207. Chichester, UK: John Wiley & Sons, Ltd, 2002.
- Provence, Aaron. "Kv7 Channels Of The Urinary Bladder Smooth Muscle: Functional Roles And Therapeutic Potential." (2018).
- Takagi, Hiroaki, and Hikaru Hashitani. "Effects of K+ channel openers on spontaneous action potentials in detrusor smooth muscle of the guinea-pig urinary bladder." European journal of pharmacology 789 (2016): 179-186. [CrossRef]
- Hayase, Masa, Hikaru Hashitani, Kenjiro Kohri, and Hikaru Suzuki. "Role of K+ channels in regulating spontaneous activity in detrusor smooth muscle in situ in the mouse bladder." The Journal of urology 181, no. 5 (2009): 2355-2365. [CrossRef]
- Xin, Wenkuan, Qiuping Cheng, Rupal P. Soder, and Georgi V. Petkov. "Inhibition of phosphodiesterases relaxes detrusor smooth muscle via activation of the large-conductance voltage-and Ca2+-activated K+ channel." American Journal of Physiology-Cell Physiology 302, no. 9 (2012): C1361-C1370. [CrossRef]
- Hristov, Kiril L., Shankar P. Parajuli, Rupal P. Soder, Qiuping Cheng, Eric S. Rovner, and Georgi V. Petkov. "Suppression of human detrusor smooth muscle excitability and contractility via pharmacological activation of large conductance Ca2+-activated K+ channels." American Journal of Physiology-Cell Physiology 302, no. 11 (2012): C1632-C1641. [CrossRef]
- Petkov, Georgi V. "Role of potassium ion channels in detrusor smooth muscle function and dysfunction." Nature Reviews Urology 9, no. 1 (2012): 30-40. [CrossRef]
- Hristov, Kiril L., Amy C. Smith, Shankar P. Parajuli, John Malysz, and Georgi V. Petkov. "Large-conductance voltage-and Ca2+-activated K+ channel regulation by protein kinase C in guinea pig urinary bladder smooth muscle." American Journal of Physiology-Cell Physiology 306, no. 5 (2014): C460-C470. [CrossRef]
- Parajuli, Shankar P., and Georgi V. Petkov. "Activation of muscarinic M3 receptors inhibits large-conductance voltage-and Ca2+-activated K+ channels in rat urinary bladder smooth muscle cells." American Journal of Physiology-Cell Physiology 305, no. 2 (2013): C207-C214. [CrossRef]
- Jenkins, David Paul. Modulators of Small and Intermediate Conductance Calcium-Activated Potassium Channel: Mechanism of Action and In Vivo Effects. University of California, Davis, 2012.
- Hristov, Kiril L., Amy C. Smith, Shankar P. Parajuli, John Malysz, Eric S. Rovner, and Georgi V. Petkov. "Novel regulatory mechanism in human urinary bladder: central role of transient receptor potential melastatin 4 channels in detrusor smooth muscle function." American Journal of Physiology-Cell Physiology 310, no. 7 (2016): C600-C611. [CrossRef]
- Hamilton, Kirk L. "New life in overactive bladder. Focus on “Novel regulatory mechanism in human urinary bladder: central role of transient receptor potential melastatin 4 channels in detrusor smooth muscle function”." American Journal of Physiology-Cell Physiology 310, no. 7 (2016): C597-C599. [CrossRef]
- Smith, Amy C., Kiril L. Hristov, Qiuping Cheng, Wenkuan Xin, Shankar P. Parajuli, Scott Earley, John Malysz, and Georgi V. Petkov. "Novel role for the transient potential receptor melastatin 4 channel in guinea pig detrusor smooth muscle physiology." American Journal of Physiology-Cell Physiology 304, no. 5 (2013): C467-C477. [CrossRef]
- Smith, Amy C., Shankar P. Parajuli, Kiril L. Hristov, Qiuping Cheng, Rupal P. Soder, Serge AY Afeli, Scott Earley, Wenkuan Xin, John Malysz, and Georgi V. Petkov. "TRPM4 channel: a new player in urinary bladder smooth muscle function in rats." American Journal of Physiology-Renal Physiology 304, no. 7 (2013): F918-F929. [CrossRef]
- Provence, Aaron, Eric S. Rovner, and Georgi V. Petkov. "Regulation of transient receptor potential melastatin 4 channel by sarcoplasmic reticulum inositol trisphosphate receptors: Role in human detrusor smooth muscle function." Channels 11, no. 5 (2017): 459-466. [CrossRef]
- Launay, Pierre, Andrea Fleig, Anne-Laure Perraud, Andrew M. Scharenberg, Reinhold Penner, and Jean-Pierre Kinet. "TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization." Cell 109, no. 3 (2002): 397-407. [CrossRef]
- Parajuli, Shankar P., Kiril L. Hristov, Michelle N. Sullivan, Wenkuan Xin, Amy C. Smith, Scott Earley, John Malysz, and Georgi V. Petkov. "Control of urinary bladder smooth muscle excitability by the TRPM4 channel modulator 9-phenanthrol." Channels 7, no. 6 (2013): 537-540. [CrossRef]
- Malysz, John, and Georgi V. Petkov. "Urinary bladder smooth muscle ion channels: expression, function, and regulation in health and disease." American Journal of Physiology-Renal Physiology 319, no. 2 (2020): F257-F283. [CrossRef]
- Hegde, Sharath S., and Richard M. Eglen. "Muscarinic receptor subtypes modulating smooth muscle contractility in the urinary bladder." Life sciences 64, no. 6-7 (1999): 419-428. [CrossRef]
- Ehlert, Frederick J. "Contractile role of M2 and M3 muscarinic receptors in gastrointestinal, airway and urinary bladder smooth muscle." Life sciences 74, no. 2-3 (2003): 355-366. [CrossRef]
- Andersson, Karl-Erik. "Potential benefits of muscarinic M3 receptor selectivity." European Urology Supplements 1, no. 4 (2002): 23-28. [CrossRef]
- Nausch, Bernhard, Thomas J. Heppner, and Mark T. Nelson. "Nerve-released acetylcholine contracts urinary bladder smooth muscle by inducing action potentials independently of IP3-mediated calcium release." American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 299, no. 3 (2010): R878-R888. [CrossRef]
- Goaillard, Jean-Marc, and Eve Marder. "Ion channel degeneracy, variability, and covariation in neuron and circuit resilience." Annual review of neuroscience 44 (2021): 335-357. [CrossRef]
- Walpole, Joseph, Jason A. Papin, and Shayn M. Peirce. "Multiscale computational models of complex biological systems." Annual review of biomedical engineering 15 (2013): 137-154. [CrossRef]
- Aliev RR, Richards W, Wikswo JP. A simple nonlinear model of electrical activity in the intestine. Journal of theoretical biology. 2000 May 7;204(1):21-8. [CrossRef]
- Bursztyn L, Eytan O, Jaffa AJ, Elad D. Mathematical model of excitation-contraction in a uterine smooth muscle cell. American Journal of Physiology-Cell Physiology. 2007 May 1;292(5):C1816-29. [CrossRef]
- Tong WC, Choi CY, Karche S, Holden AV, Zhang H, Taggart MJ. A computational model of the ionic currents, Ca2+ dynamics and action potentials underlying contraction of isolated uterine smooth muscle. PloS one. 2011 Apr 29;6(4):e18685. [CrossRef]
- Rihana S, Terrien J, Germain G, Marque C. Mathematical modeling of electrical activity of uterine muscle cells. Medical and Biological Engineering and Computing. 2009 Jun 1;47(6):665-75. [CrossRef]
- Mahapatra, C. (2023). # 2696 IN SILICO ELECTROPHYSIOLOGICAL STUDY REVEALS TAMSULOSIN MEDIATES URETER SMOOTH MUSCLE CONTRACTION BY INCREASING POTASSIUM CURRENT. Nephrology Dialysis Transplantation, 38(Supplement_1), gfad063c_2696. [CrossRef]
- Mahapatra, C., & Manchanda, R. (2019, July). Computational studies on ureter smooth muscle: modeling ion channels and their role in generating electrical activity. In Proceedings of the 2019 Summer Simulation Conference (pp. 1-6).
- Mahapatra, C., & Pradhan, A. (2022). FC025: Physiological Role of KV Channel in Ureter Smooth Muscle Cell Investigated Quantitatively by Electrophysiological Modeling. Nephrology Dialysis Transplantation, 37(Supplement_3), gfac100-001. [CrossRef]
- Poh YC, Corrias A, Cheng N, Buist ML. A quantitative model of human jejunal smooth muscle cell electrophysiology. PloS one. 2012 Aug 17;7(8): e42385. [CrossRef]
- Mahapatra, C., & Manchanda, R. (2022). In-Silico Investigation of Castration on Vas Deferens Smooth Muscle Electrophysiology. The FASEB Journal, 36. [CrossRef]
- Mahapatra, C., & Manchanda, R. (2019). Modeling Vas Deferens Smooth Muscle Electrophysiology: Role of Ion Channels in Generating Electrical Activity. In Soft Computing for Problem Solving: SocProS 2017, Volume 2 (pp. 655-663). Springer Singapore.
- Mahapatra, C. (2021). MODULATING EFFECTS OF CASTRATION ON VAS DEFERENS SMOOTH MUSCLE ELECTRICAL ACTIVITIES: INSIGHTS FROM A QUANTITATIVE STUDY. Fertility and Sterility, 116(3), e349. [CrossRef]
- Mahapatra, C., & Manchanda, R. (2020) Modeling VAS Deferens Smooth Muscle Electrophysiology: Role of Ion Channels in Generating Electrical Activity. Biophysical Journal, 118(3), 259a-260a. [CrossRef]
- Corrias A, Buist ML. A quantitative model of gastric smooth muscle cellular activation. Annals of biomedical engineering. 2007 Sep 1;35(9):1595-607. [CrossRef]
- Corrias A, Buist ML. Quantitative cellular description of gastric slow wave activity. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2008 Apr 1;294(4): G989-95. [CrossRef]
- Kapela A, Bezerianos A, Tsoukias NM. A mathematical model of Ca2+ dynamics in rat mesenteric smooth muscle cell: agonist and NO stimulation. Journal of theoretical biology. 2008 Jul 21;253(2):238-60. [CrossRef]
- Miftakhov RN, Abdusheva GR, Wingate DL. Model predictions of myoelectrical activity of the small bowel. Biological cybernetics. 1996 Jan 7;74(2):167-79. [CrossRef]
- Mahapatra, C. (2021). Computational Study of Action Potential Generation in Urethral Smooth Muscle Cell. In Computational Advances in Bio and Medical Sciences: 10th International Conference, ICCABS 2020, Virtual Event, December 10-12, 2020, Revised Selected Papers 10 (pp. 26-32). Springer International Publishing.
- Mahapatra, C., Adam, S., & Gupta, A. (2021, December). Computational modeling of electrophysiological properties in urethral smooth muscle cell. In JOURNAL OF COMPUTATIONAL NEUROSCIENCE (Vol. 49, No. SUPPL 1, pp. S81-S83). VAN GODEWIJCKSTRAAT 30, 3311 GZ DORDRECHT, NETHERLANDS: SPRINGER.
- Cha CY, Earm KH, Youm JB, Baek EB, Kim SJ, Earm YE. Electrophysiological modelling of pulmonary artery smooth muscle cells in the rabbits—special consideration to the generation of hypoxic pulmonary vasoconstriction. Progress in biophysics and molecular biology. 2008 Apr 30;96(1):399-420. [CrossRef]
- Mahapatra, C., & Samuilik, I. (2024). A Mathematical Model of Spontaneous Action Potential Based on Stochastics Synaptic Noise Dynamics in Non-Neural Cells. Mathematics, 12(8), 1149. [CrossRef]
- Mahapatra, C., Brain, K. L., & Manchanda, R. (2018). A biophysically constrained computational model of the action potential of mouse urinary bladder smooth muscle. PloS one, 13(7), e0200712. [CrossRef]
- Mahapatra, C., Dave, V., & Manchanda, R. (2017). A Mathematical Modeling of Voltage-gated Calcium ion channel-based Calcium Transient Response in UrinaryBladder Smooth Muscle Cell. International Journal of Pure and Applied Mathematics, 117(9), 71-75. [CrossRef]
- Mahapatra, C., & Manchanda, R. (2017). Simulation of In Vitro-Like Electrical Activities in Urinary Bladder Smooth Muscle Cells. Journal of Biomimetics, Biomaterials and Biomedical Engineering, 33, 45-51. [CrossRef]
- Mahapatra, C., & Manchanda, R. (2019). Modulating Properties of Hyperpolarization-Activated Cation Current in Urinary Bladder Smooth Muscle Excitability: A Simulation Study. In Recent Findings in Intelligent Computing Techniques: Proceedings of the 5th ICACNI 2017, Volume 1 (pp. 261-266). Springer Singapore.
- Mahapatra, C., Brain, K. L., & Manchanda, R. (2018, October). Computational study of Hodgkin-Huxley type calcium-dependent potassium current in urinary bladder over activity. In 2018 IEEE 8th international conference on computational advances in bio and medical sciences (ICCABS) (pp. 1-4). IEEE.
- Dave, V., Mahapatra, C., & Manchanda, R. (2015, August). A mathematical model of the calcium transient in urinary bladder smooth muscle cells. In 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) (pp. 5359-5362). IEEE.
- Hodgkin, A.L.; Huxley, A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952, 117, 500. [CrossRef]
- Noble, Denis. "A modification of the Hodgkin—Huxley equations applicable to Purkinje fibre action and pacemaker potentials." The Journal of physiology 160, no. 2 (1962): 317. [CrossRef]
- Fry, C. H., M. Cooklin, J. Birns, and A. R. Mundy. "Measurement of intercellular electrical coupling in guinea-pig detrusor smooth muscle." The Journal of urology 161, no. 2 (1999): 660-664.
- Geng, Yanyan, and Karl L. Magleby. "Single-channel kinetics of BK (Slo1) channels." Frontiers in physiology 5 (2015): 129016. [CrossRef]
- Heeger, David. "Synaptic Input." (2000).
- Hines, Michael L., and Nicholas T. Carnevale. "The NEURON simulation environment." Neural computation 9, no. 6 (1997): 1179-1209. [CrossRef]
- Carnevale, Ted. "Neuron simulation environment." Scholarpedia 2, no. 6 (2007): 1378. [CrossRef]
- Spiess, Andrej-Nikolai, and Natalie Neumeyer. "An evaluation of R 2 as an inadequate measure for nonlinear models in pharmacological and biochemical research: a Monte Carlo approach." BMC pharmacology 10 (2010): 1-11. [CrossRef]
- Demion, Marie, Patrick Bois, Pierre Launay, and Romain Guinamard. "TRPM4, a Ca2+-activated nonselective cation channel in mouse sino-atrial node cells." Cardiovascular research 73, no. 3 (2007): 531-538. [CrossRef]
- Marom, Shimon, and Eve Marder. "A biophysical perspective on the resilience of neuronal excitability across timescales." Nature Reviews Neuroscience 24, no. 10 (2023): 640-652. [CrossRef]
- Chow, K-Y., C. Wu, G. P. Sui, and C. H. Fry. "Role of the T-type Ca2+ current on the contractile performance of guinea pig detrusor smooth muscle." Neurourology and Urodynamics: Official Journal of the International Continence Society 22, no. 1 (2003): 77-82. [CrossRef]
- Jaggar, Jonathan H., Valerie A. Porter, W. Jonathan Lederer, and Mark T. Nelson. "Calcium sparks in smooth muscle." American Journal of Physiology-Cell Physiology 278, no. 2 (2000): C235-C256. [CrossRef]


| Ion Channel | Conductance (S/cm2) |
| T- type Ca2+ channel | 0.0002 |
| L- type Ca2+ channel | 0.0003 |
| Voltage-gated K+ channel –Kv1 | 0.0006 |
| Voltage-gated K+ channel-KDR | 0.0009 |
| Calcium-dependent K+ channel (BK) | 0.0008 |
| Calcium-dependent K+ channel (IK) | 0.0007 |
| Calcium-dependent K+ channel (SK) | 0.0001 |
| ATP-dependent K+ channel | 0.0001 |
| Inward-rectifying channel | 0.0001 |
| TRPM4 Channel | 0.0002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





