1. Introduction
Fraxinus mandshurica Rupr., a deciduous tree native to Northeast China and belonging to
Fraxinus Linn, is renowned for its robust growth and exceptional cold resistance [
1]. Among the distinctive Fraxinus species in the region, it stands out for its remarkable wood quality, which exhibits excellent dryness and suitability for diverse applications. Due to its versatility,
F. mandshurica finds extensive uses across various scenarios, owing to its desirable traits and adaptability [
2,
3,
4,
5].
When exposed to prolonged periods of low temperatures, plants undergo a spectrum of physiological, biochemical, and phenotypic changes, which collectively contribute to their cold resistance. Cold environments can impede tree growth to a notable degree, particularly impacting roots and young shoots, thus directly affecting tree productivity [
6,
7,
8]. In response to cold stress, plants exhibit a heightened accumulation of reactive oxygen species within the cell membrane system. While these species pose a threat to plants, enzymes like peroxidase (POD) and superoxide dismutase (SOD) play pivotal roles in their detoxification. Nevertheless, excessive reactive oxygen accumulation can lead to lipid membrane oxidation, resulting in the generation of malondialdehyde (MDA). MDA can interact with membrane proteins, causing protein denaturation, elevated membrane permeability, and increased relative conductivity.
Under the conditions of cold stress, plants augment their resilience by elevating their levels of soluble sugars and soluble proteins [
9]. Additionally, numerous genes encoding proteins with cold resistance properties are upregulated, further enhancing their expression [
9]. These adaptive mechanisms enable plants to effectively overcome the challenges presented by cold environments, thereby enhancing their chances of survival and growth [
11,
12,
13].
The NAC (NAM, no apical meristem; ATAF1/2, Arabidopsis transcription activation factor; CUC2, cup-shaped cotyledon) family of transcription factors is pivotal in plant development and hormonal signal responses [
14,
15,
16]. Representing one of the largest families of plant-specific transcription factors, NAC-domain proteins have been identified across various plant species including
Arabidopsis, rice [
17],
Populus [
18], chestnut [
19],
Cyclocarya paliurus [
20], and
Catharanthus roseus [
21]. These proteins feature a conserved N-terminal NAC domain, which is responsible for DNA and protein binding, serving as a distinguishing hallmark for their identification. Furthermore, they exhibit a diverse array of C-terminal domains. Unlike a classical helix–turn–helix motif, the NAC domain presents a unique transcription factor fold comprising distorted β-sheets encircled by helical elements [
22]. NAC proteins can be categorized into six significant branches: MAM/CUC3, secondary wall NAC (SWN), TIP, SNAC (stress-responsive NAC),
ANAC034, and
OsNAC4 [
23].
The involvement of NAC proteins spans across the transcriptional regulation of diverse plant processes, encompassing the development of shoot apical meristems, floral organs, and lateral roots. Additionally, they play a pivotal role in the plant's stress response, modulating pathways associated with stress tolerance, thus enhancing the plant's resilience to environmental challenges [
24,
25,
26,
27,
28]. Consequently, the NAC family of transcription factors is revealed to be indispensable in coordinating plant growth, development, and adaptation to fluctuating environmental conditions [
29].
The significance of NAC transcription factors in imparting resistance to abiotic stresses has gained increasing recognition, with numerous studies underscoring their importance across various plant species [
30,
31]. In tomato fruit, the overexpression of
MaNAC1 enhances cold resistance, leading to thicker cell walls and increased cellulose content. Similarly, in banana fruit,
MaNAC1 acts as a positive regulator in governing cellulose metabolism within the cell wall [
32]. Additionally, many NAC transcription factors are reportedly induced by cold stress and play pivotal roles in plant responses to such conditions [
32]. The identification of the
NAC056 transcription factor, which enhances plant cold tolerance by upregulating genes in the
CBF pathway and its association with the
CBF1-NIA1 regulatory module, underscores the critical role of
NAC056 in balancing plant growth and plant responses to cold stress [
34].
The interaction between the growth-hormone-inducible NAC1 protein and the RING structural domain protein SINAT5 mediates the involvement of the
NAC1 gene in regulating plant abscisic acid control. This interaction attenuates growth hormone signaling by facilitating the ubiquitination and degradation of
NAC1. In corn, a significant correlation was observed between the expression levels of
ZmNAC1 and the lateral root density. The overexpression of
ZmNAC1 in transgenic
Arabidopsis plants resulted in a higher number of lateral roots compared to the wild type, indicating the pivotal role of
ZmNAC1 in lateral root development [
35,
36,
37,
38].
Specific examples include the banana
NAC1 transcription factor, which has been demonstrated to enhance cold tolerance in banana plants [
39], and
PbeNAC1 in pears, which confers cold tolerance to their respective species [
40]. In tomato plants,
NAC3 is induced by low temperatures and can enhance the cold tolerance of transgenic plants [
41]. Moreover,
HuNAC20 and
HuNAC25 from Hylocereus undatus exhibited a positive effect, increasing cold tolerance in transgenic
Arabidopsis by promoting the expression of stress-responsive genes [
41]. It is noteworthy that, while extensive research has been conducted on the
NAC1 gene in herbaceous plants, relatively little attention has been paid to this gene in woody plants. It is therefore necessary to explore
NAC transcription factors in woody plant species to gain a better understanding of their roles in stress tolerance and their potential applications in enhancing the resilience of these important plant varieties.
Notably, the NAC1 gene in F. mandshurica has been underexplored, necessitating further investigations into its role and function within this specific species. This study addresses the primary challenges encountered in cultivating F. mandshurica, particularly the vulnerability of seedlings to cold damage. F. mandshurica is renowned for its unique flowering and leafing patterns, yet it must endure severe environmental conditions, particularly late-spring cold spells in northern regions, to ensure successful growth and development.
In this study, the FmNAC1 gene, a key regulator of plant growth and stress resistance from F. mandshurica, was identified. The full-length sequence of the FmNAC1 gene was obtained, and significant increases in its expression under cold stress and exogenous hormone treatment were also detected. Moreover, a transgenic validation experiment involving the overexpression of the FmNAC1 gene in tobacco plants was conducted. The results showed that the overexpression of FmNAC1 enhanced the cold tolerance of the transgenic tobacco plants and accelerated their growth. These findings provide valuable insights into the molecular mechanisms underlying the growth and cold stress responses of F. mandshurica and offer potential avenues for the future development of improved F. mandshurica varieties with enhanced cold tolerance and overall performance.
2. Results
2.1. Cloning and Sequence Analysis of FmNAC1 Genes and Prediction of the Protein Tertiary Structure
The gene sequence was screened from the
F. mandshurica transcriptome database and designated
FmNAC1 [
67]. Subsequently, the
FmNAC1 gene was successfully amplified via PCR using cDNA extracted from
F. mandshurica leaves as a template, following software analysis and primer design (
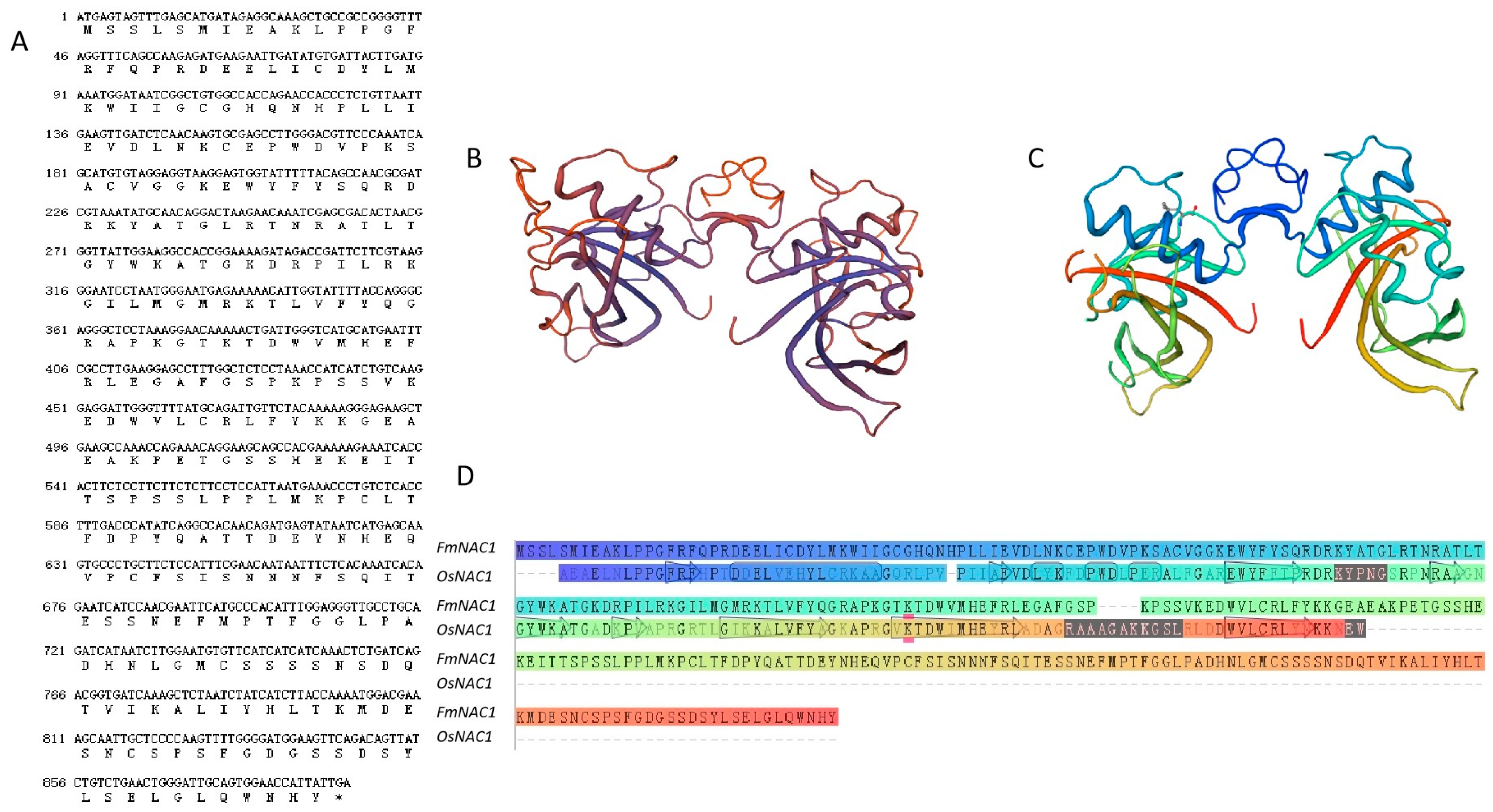
Figure 1A). Through NCBI comparison and analysis, it was determined that the total length of the coding region of the
FmNAC1 gene (MG269829.1) is 891 bp, encoding 296 amino acids. The analysis of the NAC1 protein revealed that the NAC1 domain coverage of the FmNAC1 protein was 51.88% (
Figure 1B,C).
2.2. Phylogenetic Analysis of the FmNAC1 Gene Reveals Relationships with Homologous NAC1 Sequences in Plants
For the homologous alignment, FmNAC1 and 30 other amino acid sequences were aligned using DANMAN. The results revealed that
FmNAC1 exhibited a high level of similarity with homologous genes from other species (
Figure 2A).
FmNAC1 and the 30 other genes shared a NAC conserved domain (
Figure 2A). To analyze the phylogenetic relationships between
FmNAC1 and homologous
NAC1 sequences in other plants, a phylogenetic tree was constructed using MEGA 5.0 software. The gene sequences of
FmNAC1 and the other genes were included in the analysis, which revealed that
FmNAC1 was most closely related to
OeNAC1 (XP_022848339.1) in
Olea europaea var., with a homology of 85.14% (
Figure 2B).
2.3. Subcellular Localization of the FmNAC1 Protein
Onion epidermal tissue was cocultured in MS media and subjected to GFP fluorescence observations (
Figure 3). Due to the absence of a localization signal in the empty vector, the fluorescence signal of the empty vector was clearly observed in the cell membrane and nuclei of the onion epidermal cells under the microscope. In addition, onion nuclei were stained with DAPI, and blue was used as a positive control. The fluorescence signal of the
FmNAC1-GFP fusion protein was exclusively detected in the nucleus of the onion epidermis, indicating the nuclear localization of
FmNAC1. These results confirm that
FmNAC1 is localized in the nucleus and functions as a transcription factor with transcriptional activation activity.
2.4. Expression Pattern of FmNAC1 in F. mandshurica
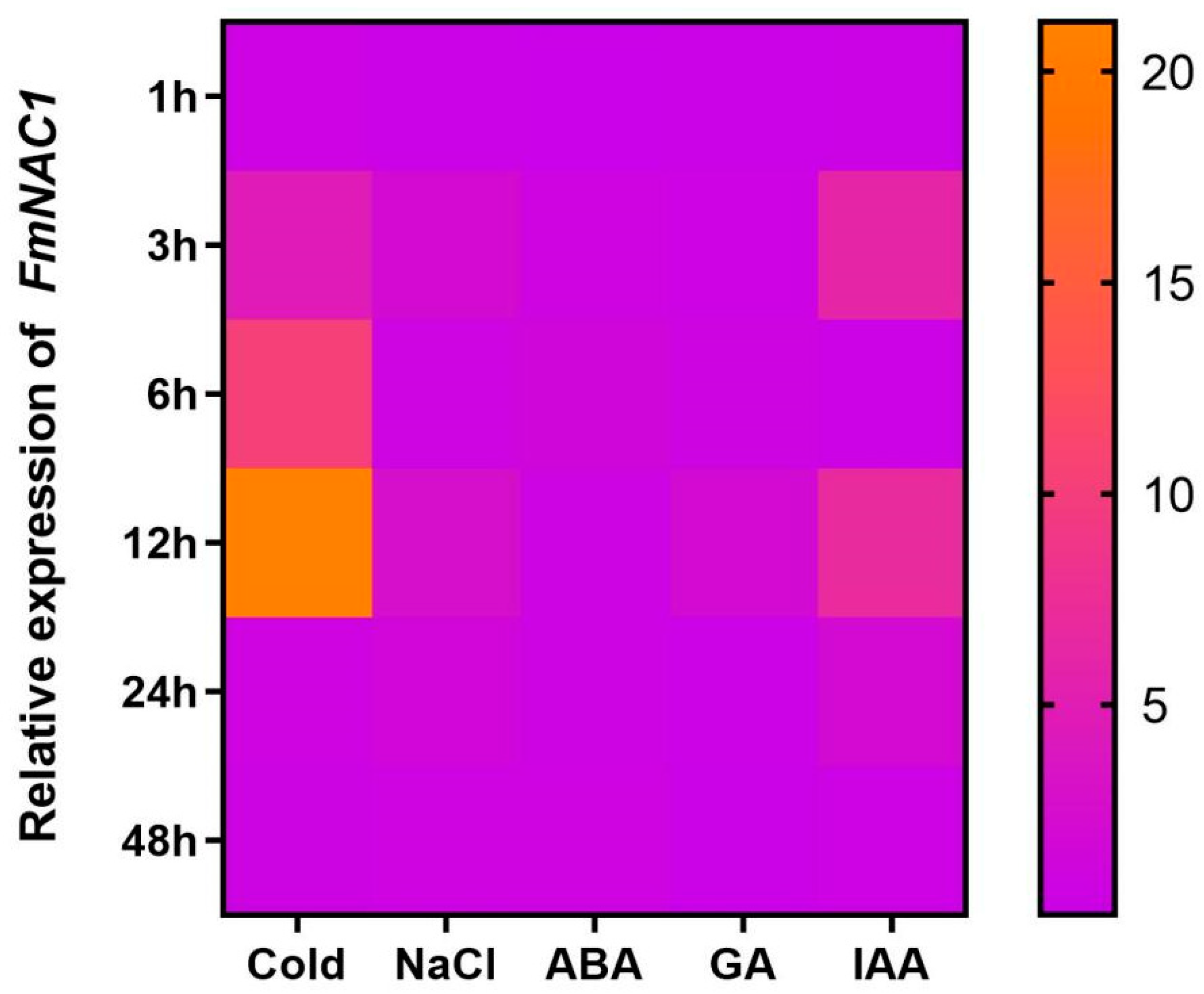
The expression pattern of the
FmNAC1 gene exhibited varying responses to different treatments (
Figure 4). After exposure to salt, the expression of this gene increased at 3 h, 12 h, and 24 h but decreased at 1 h, 6 h, and 48 h. The peak expression level was observed at 12 h, reaching 2.58 times that of the control group. Similarly, in response to the cold treatment, the gene expression increased at 3 h and 6 h and peaked at 12 h, reaching a value 21.16 times that of the control group. Conversely, gene expression decreased at the time points of 1 h, 24 h, and 48 h. Additionally, the
FmNAC1 gene exhibited responses to hormone signals such as IAA, GA, and ABA, with distinct upregulation observed at specific time points following treatment with these hormones. These findings highlight the dynamic nature of the
FmNAC1 gene and its responsiveness to various environmental stimuli and hormone signals.
2.5. Acquisition of Transgenic Tobacco Overexpressing FmNAC1
The transgenic tobacco plants overexpressing
FmNAC1 demonstrated successful tissue cultures and regeneration (
Figure 5). After three days of preculture, the material started to expand and exhibited signs of infection, which were effectively addressed through significant expansion and bacterial removal following three days of coculture in the dark. By day 15, the plates were nearly fully covered, with small buds emerging on the callus at the incision site; they were subsequently transferred to a rooting screening medium for further growth. These findings indicate favorable responses to genetic modification and provide valuable insights into the potential effects of
FmNAC1 overexpression on plant growth and development.
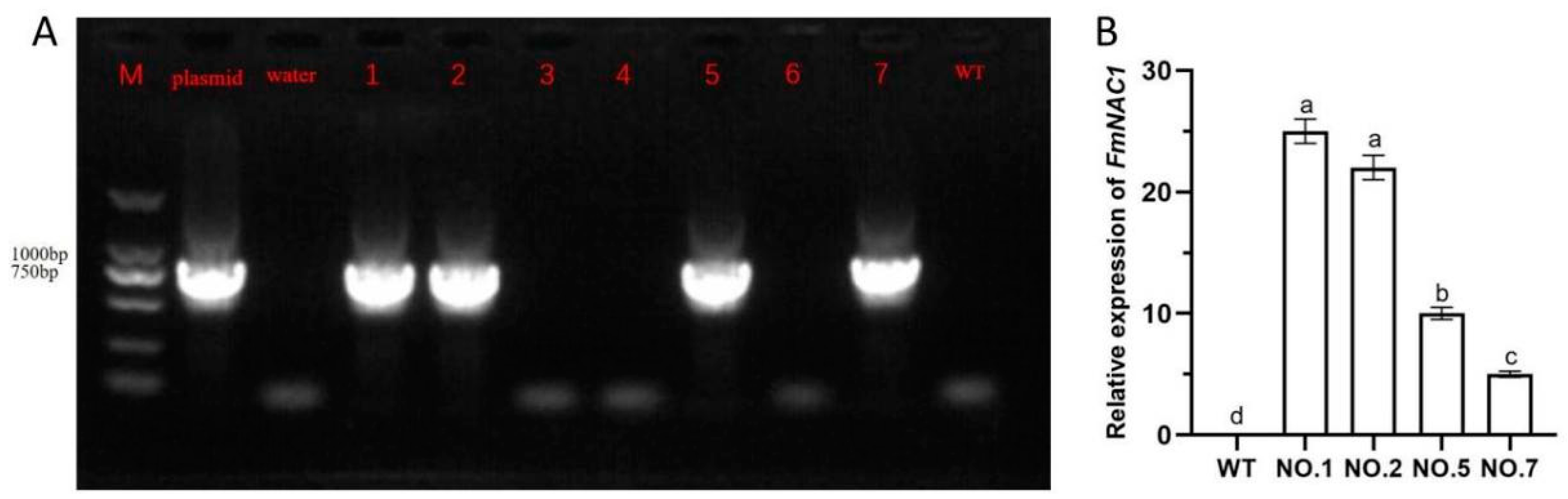
2.6. Identification of FmNAC1-OE Transgenic Tobacco Lines
The successful construction of the overexpression vector facilitated its transformation into wild-type (WT) tobacco tissue-culture-generated plants using Agrobacterium-mediated techniques (
Figure 6). The resulting transgenic tobacco plants overexpressing the gene of interest were screened for kanamycin resistance, which resulted in the generation of a significant number of robust and resistant plants. Utilizing tissue culture methodologies such as differentiation and rooting, a substantial quantity of transgenic tobacco material was produced. Subsequently, genomic DNA was extracted individually from each WT and transgenic tobacco strain; this was followed by verification using specific primers through PCR amplification. This analysis revealed four transgenic strains exhibiting amplified specific bands consistent with those of the positive plasmid, while the wild-type samples displayed no amplified bands. These results confirm the successful development of transgenic tobacco lines(T0
generation of transgenic tobacco plants)featuring the enhanced expression of the target gene; this constitutes a strong foundation for further exploration of the implications of
FmNAC1 overexpression in tobacco. Four transgenic tobacco lines were subjected to real-time-fluorescence quantitative PCR analysis. In this study, when detecting transgenic heterologous overexpression in tobacco, a tobacco gene was used as the internal reference gene instead of
F. mandshurica. The quantitative PCR results revealed varying degrees of expression of the
FmNAC1 gene across the four strains. Notably, strain 1 exhibited the highest level of heterologous overexpression, at 74.99%. Consequently, we selected the three strains with the highest expression: lines No. 1, 2, and 5(T2
generation of transgenic tobacco plants)were selected for further investigation.
2.7. Growth Phenotype Analysis of FmNAC1-OE Transgenic Tobacco
The
FmNAC1-overexpressing transgenic tobacco plants exhibited a faster growth phenotype (
Figure 7), with the height of the transgenic plants measuring 11.3 cm, which was 1.61 times greater than that of the WT plants. These results indicated that the overexpression of the
FmNAC1 gene accelerated the growth of the tobacco plants.
2.8. Cold Resistance Analysis of FmNAC1-OE Tobacco Plants
We conducted phenotypic observations of transgenic tobacco under low-temperature stress (
Figure 8). Tobacco plants that grew robustly, including both transgenic and wild-type plants, were carefully selected for this experiment. The plants were subjected to a 24-hour incubation at -2°C to induce low-temperature stress, after which the response of the plants was assessed. The white arrow indicates the overexpression of the transgenic tobacco, while the other three pots represent the wild-type controls at the same time. After the low-temperature treatment, the conditions of the overexpression lines were similar to those before treatment. In contrast, the leaves of the wild-type tobacco (WT) plants displayed obvious wilting, with main stem yellowing and softening and the branches withering. These results strongly indicate that the
FmNAC1 gene has a robust function in conferring cold resistance.
2.9. Analysis of the Physiological Indexes of FmNAC1-OE Transgenic Tobacco Plants under Low-Temperature Stress
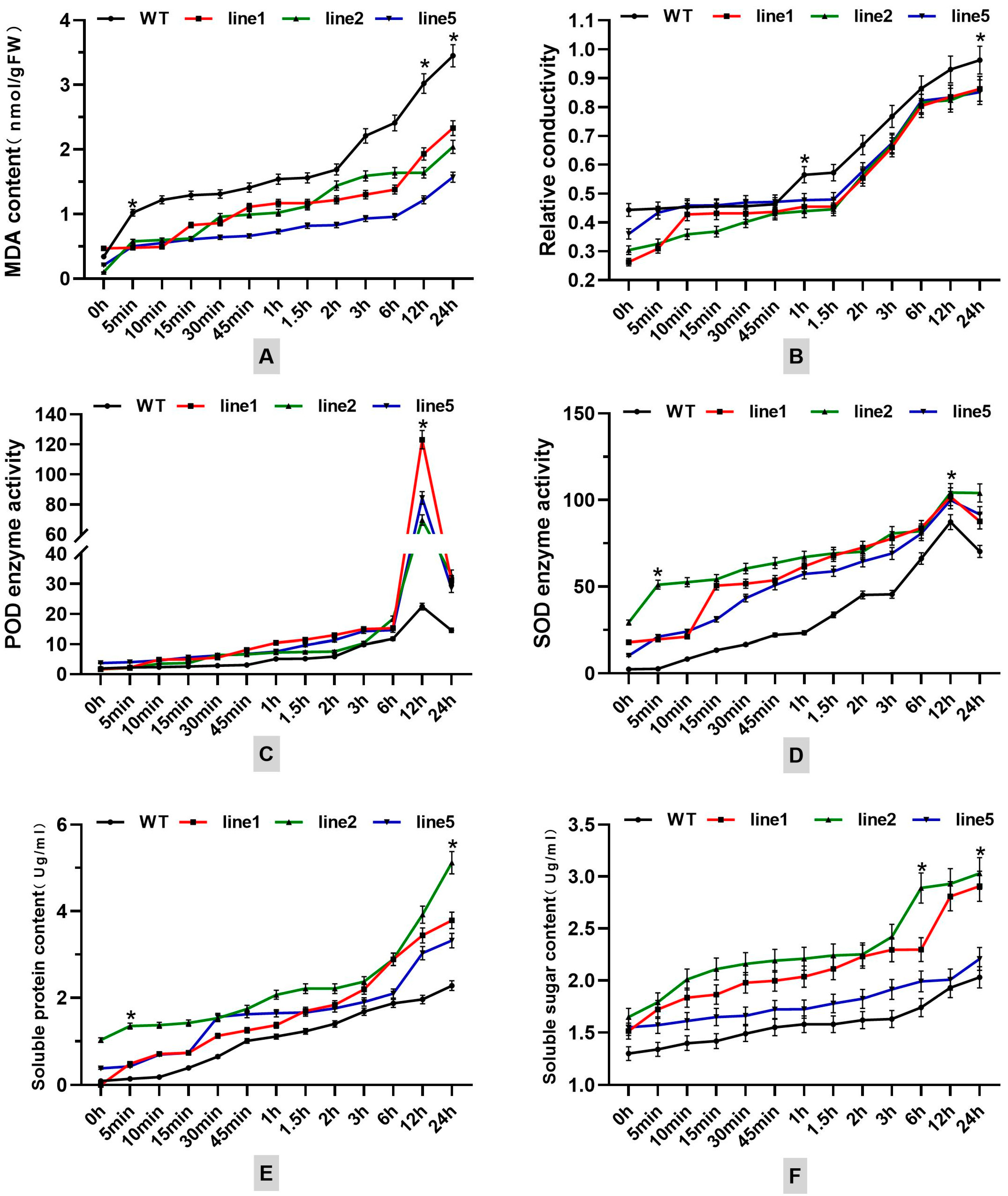
Under cold stress conditions, the FmNAC1 transgenic tobacco plants exhibited consistently lower levels of MDA and relative conductivity in their membrane system compared to wild-type (WT) plants. These levels gradually increased and peaked after 24 hours, showcasing reductions of 39.9% and 38.4%, respectively, in comparison to WT tobacco. This observation suggests that the enhanced resistance of FmNAC1 transgenic tobacco to cold stress, coupled with the maintenance of membrane integrity over a prolonged period, contributes to its heightened tolerance to cold stress.
Additionally, the soluble protein and soluble sugar contents in the osmotic system of the FmNAC1 transgenic tobacco plants consistently exceeded those in the WT tobacco plants under cold stress. These levels displayed an increasing trend and reached peak values after 24 hours, with increases of 34.1% and 65.5%, respectively, compared to those of WT tobacco. This suggests that the solute concentration in the osmotic system can increase in FmNAC1 transgenic tobacco plants, decreasing the freezing point and increasing the tolerance of these plants to cold stress.
Moreover, the activities of POD and SOD in the ROS scavenging system of transgenic tobacco consistently surpassed those of WT tobacco under cold stress. These activities exhibited an increasing trend and peaked after 24 hours, showing increases of 275.7% and 3.6%, respectively, compared to those of WT tobacco. This indicates that
FmNAC1 transgenic tobacco can endure cold stress and effectively eliminate accumulated ROS, thereby enhancing its tolerance to cold stress.
Figure 9.
Analysis of the physiological indexes of transgenic tobacco plants under low-temperature stress: (A) MDA content, (B) relative conductivity, (C) POD activities, (D) SOD activities, (E) soluble protein content, (F) soluble sugar content. The black line represents the wild type, the red line represents transgenic line 1, the green line represents transgenic line 2, and the blue line represents transgenic line 5. * Indicates a significant difference between the control and the transgenic tobacco (p < 0.05).
Figure 9.
Analysis of the physiological indexes of transgenic tobacco plants under low-temperature stress: (A) MDA content, (B) relative conductivity, (C) POD activities, (D) SOD activities, (E) soluble protein content, (F) soluble sugar content. The black line represents the wild type, the red line represents transgenic line 1, the green line represents transgenic line 2, and the blue line represents transgenic line 5. * Indicates a significant difference between the control and the transgenic tobacco (p < 0.05).
2.10. Gene Expression in FmNAC1-OE Transgenic Tobacco Plants
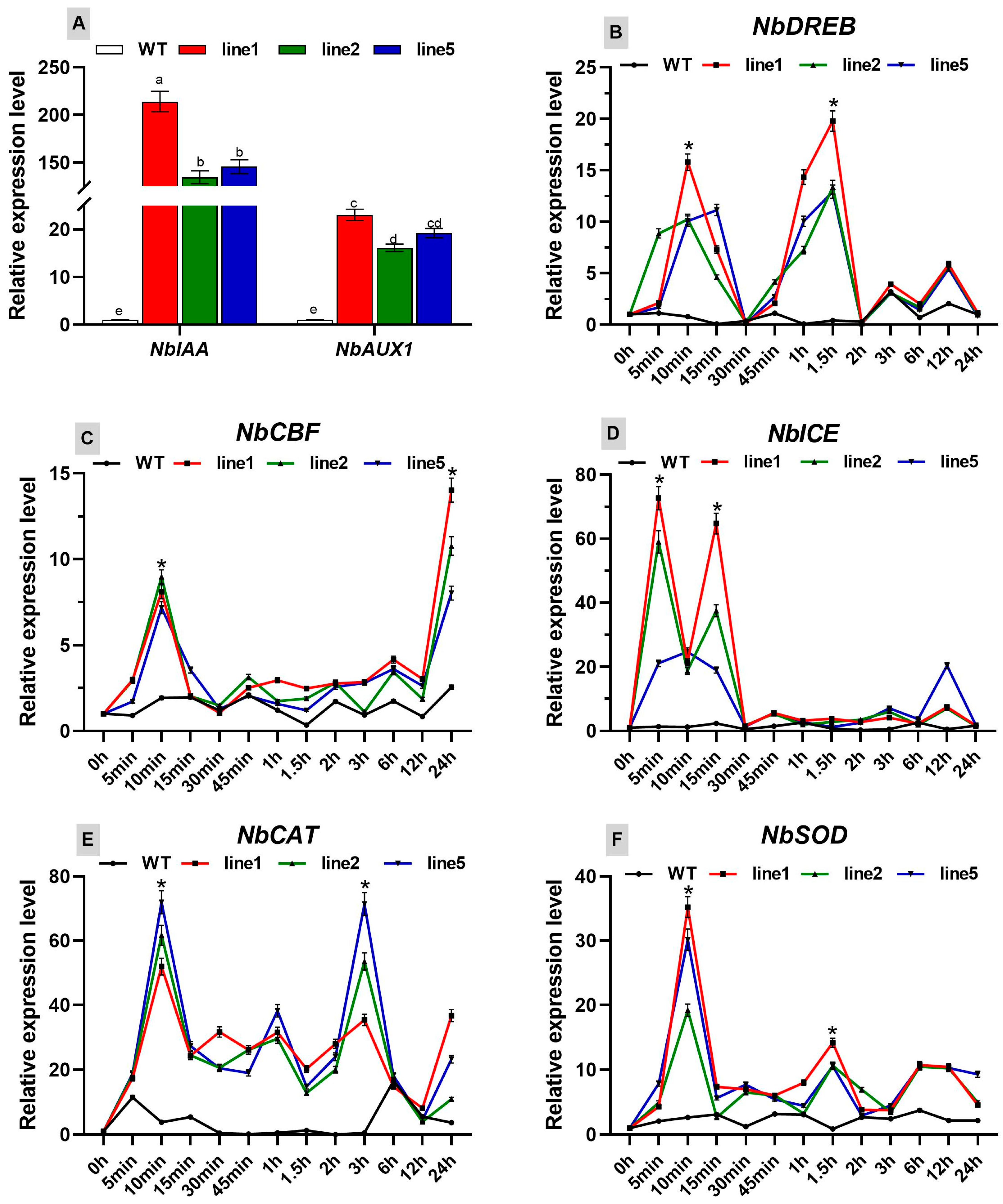
The expression levels of the IAA and AUX1 genes, which are associated with the growth of FmNAC1 transgenic tobacco, were significantly elevated compared to those of the WT, showing increases of 164.8-fold and 19.8-fold, respectively. This indicates a regulatory association between the FmNAC1 transcription factor and the growth-related IAA and AUX1 genes, resulting in the heightened expression of these genes and the promotion of rapid growth in transgenic tobacco.
Under cold stress, the expression of the ICE, DREB and CBF genes, which are linked to cold resistance in FmNAC1 transgenic tobacco, surpassed that in the WT, exhibiting a pattern of an initial increase, a subsequent decrease, and a subsequent increase. Specifically, the expression of ICE peaked after 5 minutes of cold treatment, reaching 54.61 times that of the WT, which decreased to 1.21 times after 30 minutes of cold treatment. Similarly, DREB reached a minor peak after 10 minutes of cold treatment, showing a 19.65-fold increase compared to that of the WT; it reached its highest expression level after 1.5 hours of cold treatment, which was 31.82 times greater than that of the WT. Additionally, the expression of CBF peaked after 10 minutes of cold treatment, exhibiting a 7.79-fold increase compared to that of the WT; it reached its highest level after 24 hours of cold treatment, with a value 10.94 times greater than that of the WT. These findings indicate that the expression of related genes in FmNAC1 transgenic tobacco can respond to cold signals, maintain prolonged high expression levels, and regulate the expression of related genes. This finding suggests that the FmNAC1 transcription factor has a regulatory relationship with the ICE, DREB, and CBF genes associated with cold resistance, resulting in the increased expression of these genes and enhanced cold stress tolerance in transgenic tobacco.
Furthermore, under cold stress, the expression of the
CAT and
SOD genes involved in the clearance of reactive oxygen species (ROS) in the transgenic tobacco plants was greater than that in the WT plants, displaying a pattern of an initial increase, a subsequent decrease, and a subsequent increase. The expression of both genes peaked after 10 minutes of treatment, showing increases of 23.65-fold and 6.04-fold compared to those in the WT, respectively. These results indicate that
FmNAC1 transgenic tobacco can promptly respond to cold signals, maintain prolonged high expression levels, and regulate the expression of ROS-clearance-related genes. This finding suggests that the
FmNAC1 transcription factor has a regulatory relationship with the
CAT and
SOD genes associated with ROS clearance, resulting in the increased expression of these genes, the enhanced ability of transgenic tobacco to scavenge reactive oxygen species, and its increased tolerance to cold stress.
Figure 10.
Gene expression levels in FmNAC1-OE transgenic tobacco plants under cold stress: (A) NbIAA gene; NbAUX1 gene; (B) NbDREB gene; (C) NbCBF gene; (D) NbICE gene; (E) NbCAT gene; and (F) NbSOD gene. The black line represents the wild type, the red line represents transgenic line 1, the green line represents transgenic line 2, and the blue line represents transgenic line 5. * Indicates a significant difference between the control and the transgenic tobacco (p < 0.05).
Figure 10.
Gene expression levels in FmNAC1-OE transgenic tobacco plants under cold stress: (A) NbIAA gene; NbAUX1 gene; (B) NbDREB gene; (C) NbCBF gene; (D) NbICE gene; (E) NbCAT gene; and (F) NbSOD gene. The black line represents the wild type, the red line represents transgenic line 1, the green line represents transgenic line 2, and the blue line represents transgenic line 5. * Indicates a significant difference between the control and the transgenic tobacco (p < 0.05).
3. Discussion
Transcription factors in the NAC family are well known to play a pivotal role in plant development and stress response [
44]. Introducing or augmenting key NAC transcription factors such as
NAC1 in plants has the potential to enhance wood quality, boost yields, and fortify plant resistance to diverse stressors. Our study aimed to elucidate the molecular mechanism underlying
FmNAC1's contribution to stress resilience in
F. mandshurica, a species of significant economic and ecological value.
Upon exposure to NaCl, cold stress, and hormonal signals such as IAA, GA, and ABA, the expression of FmNAC1 in F. mandshurica initially increased before subsequently decreasing. This pattern suggests that FmNAC1 can respond to a variety of stressors including cold, salinity, and hormonal fluctuations, indicating its crucial role in both stress adaptation and growth regulation in F. mandshurica. When exposed to stress, this species adjusts its hormonal balance to adapt to external environmental conditions. Notably, the expression of FmNAC1 was significantly more responsive to IAA than to GA or ABA. This heightened responsiveness to IAA may be due to the impact of cold stress on NAC1 gene expression, potentially enhancing cold resistance. The involvement of FmNAC1 in plant growth, particularly in response to IAA hormonal changes, appears to be pivotal, highlighting its integral role in the developmental processes and stress responses of F. mandshurica.
After the transgenic tobacco was transplanted, measurements were taken every 2 days for a total of 11 days to determine the plant height; this resulted in a final height of 11.3 cm, marking a 1.61-fold increase compared to their wild-type counterparts. These findings highlight the role of the overexpression of the
FmNAC1 gene in accelerating tobacco plant growth. Upon exposure to low temperatures (-5℃ for 24 hours), wild-type tobacco (WT) exhibited visible wilting, leaf yellowing, stem softening, and branch withering. Conversely, transgenic lines overexpressing
FmNAC1 showcased significantly enhanced growth compared to the WT, maintaining robustness with thick stems and green leaves. These observations underscore the robust cold resistance conferred by the
FmNAC1 gene. Furthermore, the heightened growth and structural integrity observed in transgenic tobacco plants bolster their resilience to environmental fluctuations. By stimulating root growth, the
FmNAC1 gene facilitates nutrient absorption, thereby promoting rapid growth and augmenting cold tolerance [
34].
In assessing the membrane damage caused by low temperatures, relative conductivity and MDA content serve as reliable indicators. In our investigation, we consistently observed a lower MDA content and lower relative conductivity in the membrane system of
FmNAC1 transgenic tobacco compared to regular tobacco when subjected to cold stress. Even after 24 hours of cold treatment, the transgenic plants overexpressing
FmNAC1 maintained a lower MDA content and lower relative conductivity compared to the control plants. With prolonged cold treatment, the MDA content gradually increased, indicating exacerbated cell damage. Notably, all samples exhibited a gradual rise in MDA content before 12 hours of cold treatment, which can potentially be attributed to elevated catalase and superoxide dismutase activity. However, despite the heightened activities of these enzymes after 24 hours of cold treatment, MDA accumulation persisted due to the extensive cellular damage induced by prolonged cold stress [
46,
47,
48,
49,
50].
Under cold stress,
FmNAC1 transgenic tobacco consistently exhibited elevated levels of soluble protein, soluble sugar, peroxidase (POD), and superoxide dismutase (SOD) in the osmotic system compared to conventional tobacco. The modification of osmoregulatory substances, such as soluble sugars and soluble proteins, enhances plants' ability to withstand extreme temperatures [
51,
52,
53]. Soluble sugars play a pivotal role in plant responses to cold stress, with an increased content being crucial in mitigating damage from low temperatures. Throughout our study, prolonged exposure to cold treatment led to increased levels of soluble sugars and soluble proteins. This rise in content could effectively enhance the cold resistance of transgenic plants overexpressing
FmNAC1. The enhanced cold stress tolerance observed in transgenic
FmNAC1 tobacco suggests the gene's potential to augment nutrient accumulation, promoting robust growth and influencing the plants' ability to scavenge active oxygen, thus bolstering cold resistance.
GmNAC20 functions by controlling the expression of stress-tolerance-enhancing
COR genes,
DREBIA/CBF3, and
DREB1C/CBF2. It directly interacts with the promoters of
DREB1A/CBF3 and
DREB1C/CBF2, leading to increased
CBF3 transcription and decreased
CBF2 transcription [
54]. In bananas,
MaNAC1 is regulated by
MaICE1, and this protein interacts with
MaCBF1 to govern cold tolerance in banana fruit [
55]. Furthermore, the
PbeNAC1 protein can enhance the transcription level of stress-related genes by interacting with
PbeDREB1 and
PbeDREB2A, thereby boosting the tolerance of transgenic tobacco [
56]. These findings underscore the involvement of NAC TEs in downstream
ICE1 and upstream
CBF regulation.
Similarly, in
F. mandshurica,
FmNAC1 has been shown to have a regulatory relationship with genes associated with growth (IAA and AUX1), cold resistance (
ICE, DREB, and
CBF1), and ROS clearance (CAT and SOD) in transgenic tobacco under cold treatment [
57,
58,
59,
60]. It is suggested that
FmNAC1 genes directly regulate
CBF and
DREB genes downstream, thereby promoting rapid growth, cold resistance, and ROS clearance in transgenic tobacco.
NAC1 is characterized as a regulator of fast plant growth and cold resistance in
F. mandshurica, potentially serving as a pivotal factor in balancing these two biological traits [
61]. However, the specific mechanism by which
NAC1 maintains this balance remains a primary focus of future research [
62,
63].
5. Materials and Methods
5.1. Plant Material and Growth Conditions
Nicotiana tabacum and F. mandshurica seeds from the Northeast Forestry University experimental forest farm were used. The seeds were surface sterilized and grown at 25 °C under long-day conditions (16h light/8h dark) on a standard field.
5.2. Cloning and Identification of the FmNAC1 Gene
A MiniBEST Plant RNA Extraction Kit (Takara Bio, Inc., Japan) was used for the total RNA extraction. The synthesized cDNA was created using the PrimeScript First Strand cDNA Synthesis Kit (Takara Bio Inc., Shiga, Japan). Then, a PCR was performed to amplify the
F. mandshurica cDNA (template) for 40 cycles using 2×Phanta Flash Master Mix (Dye Plus; Vazyme, Nanjing, China) and FmNAC1 primers (
Table 1). The PCR program was as follows: predenaturation at 94°C for 5 min; denaturation at 94°C for 30s; annealing at 53°C for 45s; extension at 72°C for 30s [
64]. After electrophoresis on 2% agarose gel, the PCR products were purified using the Zymoclea Gel DNA Recovery Kit (ZYMO), cloned into the pCE2 TA/Blunt-Zero Vector (Vazyme), and transformed into
Escherichia coli DH5a cells [
66]. The sequence of the FmNAC1 gene was submitted to GenBank with the accession number MG269829.1.
5.3. Experimental Approach for FmNAC1 Protein Subcellular Localization
The Thermo Scientific
TM Spel single enzyme-cut PBI121-GFP plasmid was incubated at 37°C for 15min and then heat-inactivated at 80°C for 5min. The linearized vector obtained from single-enzyme digestion was designed for the homologous recombination of the target gene according to the ClonExpress principle. Primers for the target gene were designed as follows: PBNAC1-GFP-F (5’-tacccgggtcgactgactagtATGAGTAGTTTGAGCATGATAGAGGC-3’) and PBNAC1-GFP-R (5’- gcccttgctcaccatactagtTCAATAATGGTTCCACTGCAATCC-3’). The insert fragment was obtained via high-fidelity enzyme PCR using the subcloned vector of the target gene as a template. Homologous recombination was performed using ClonExpress/Mut Express enzymes (Vazyme, Nanjing, China), and the resulting construct was transformed into
Escherichia coli DH5a cells (
Figure 11A).
A transient expression vector for PBI121-FmNAC1-GFP was constructed and transformed into Agrobacterium GV3103 cells. Five to six layers of onion scale were soaked in 75% ethanol for 10 min and sterile water was added 3–5 times; then, l cm2 of onion epidermis was cut with a sterile scalpel, spread in hypertonic medium, and cultured for 24 h. At OD = l, the cells were infected for 20 min, washed several times, and placed in coculture medium for 24–36 h.
5.4. Abiotic Stresses and Hormone Signal Treatments
Thirty-day-old uniformly growing manchurian seedlings were subjected to hormone and abiotic stress treatments. For the low-temperature stress treatment, manchurian seedlings were transferred to liquid MS medium and placed in a 4℃ low-temperature culture chamber [
68]. For the salt stress, seedlings were transferred to MS liquid medium containing 200 mmol/L NaCl. For the hormone treatments, seedlings were transferred to MS liquid medium containing 100 mmol/L ABA, 100 pmol/L GA
3, and 100 pmol/L IAA. For the control group, untreated seedlings were transferred to MS liquid medium and cultured at 25℃ under long-day photoperiod conditions (16h light/8h dark). All materials were treated for 48 hours, and samples of the treated manchurian seedlings were taken at 1 h, 3 h, 6 h, 12 h, 24 h, and 48 h before being held in liquid nitrogen and then stored in a -80℃ freezer for later use. Each treatment group was set up with 5 biological replicates.
5.5. Agrobacterium-Mediated Genetic Transformation, Identification, and Propagation of Nicotiana
The Thermo Scientific
TM XbaI and
KpnI double-enzyme-cut vector pROKII-GUS plasmid was incubated at 37°C for 15 min and then heat-inactivated at 80°C for 5 min. The linearized vector obtained from the double-enzyme digestion was designed for the homologous recombination of the target gene according to the ClonExpress principle. Primers for the target gene were designed as follows: pRNAC1-GUS-F (5’- gagaacacgggggactctagaATGAGTAGTTTGAGCATGATAGAGGC-3’) and pRNAC1-GUS-R (5’- catggtcaagagtccggtaccTCAATAATGGTTCCACTGCAATCC-3’). The insert fragment was obtained via a high-fidelity enzyme PCR, using the subcloned vector of the target gene as a template. Homologous recombination was performed using ClonExpress/Mut Express enzymes (Vazyme, Nanjing, China), and the resulting construct was transformed into
Escherichia coli DH5a cells (
Figure 11B). A transient expression vector for pROKII-FmNAC1-GUS was constructed and transformed into
Agrobacterium GV3103 cells.
Native tobacco seeds were disinfected on an ultraclean workbench, soaked in 30% 84 disinfection dilution solution for 30 min, and washed 3–5 times in sterile water. Afterwards, the seeds were disinfected with 75% alcohol for 30 s, and the triangular bottle was constantly shaken. The plants were quickly transferred to 0.1% mercury solution and shaken for 6–8 min, sterile water was removed many times, and the water on the seed surface was left to dry on sterilized filter paper. The bacteria were vaccinated in MS media and then cultured once after 28 days of sterile culture. The native tobacco plants were transformed via the leaf disc method as follows: high-quality tender green tobacco leaves were picked, the vein trunk was removed, the plants were cut into 1 cm
2 pieces on MS media, and the plants were precultured for 1–2 days. The engineered bacteria were inoculated into sterile Petri dishes, the leaves were soaked for 5 min, and then the leaf surface liquid was removed. The plants were transferred to a coculture media and grown in the dark for 2 days. After sterilization once a day for 5 d, the leaf explants were washed 3–5 times with 500 mg/L cephalosporin, dried for surface water, and transferred to sterile media (MS+0.05 mg/L NAA+0.5 mg/L 6-BA + 500 mg/L cephalosporin + 25 mg/L Kana, pH=6). Leaf explants were placed in differentiation screening media (MS+0.05 mg/L NAA+0.5 mg/L 6-BA + 500 mg/L cephalosporin + 50 mg/L Kana, pH=6) to select the resistant buds. Days 7 to 14 were selected for bud production so that the buds could fully contact the selection medium. The buds that were able to grow normally were resistant buds. When the resistant buds grew to approximately 3 cm, they were removed and placed in differentiation screening media. The resistant shoots were cultured for 7 d in rooting screening media (MS+0.25 mg/L NAA + 500 mg/L cephalosporin + 50 mg/L Kana, pH=6) for further transgenic testing of the positive transformed shoots [
64].&#;
Identification and propagation of transgenic tobacco: DNA was extracted from tobacco leaves using the cetyltrimethylammonium bromide (CTAB) method [
65]. DNA was extracted from positive tobacco plants selected on the resistance culture medium and was then amplified and identified using primers for cloning the
FmNAC1 gene and PCR conditions, with the recombinant plasmid as positive control. Part of the material was subsequently transplanted to a 1:1 mixture of chat soil/vermiculite matrix and cultured at a constant temperature and humidity.
5.6. Determination of Physiological Traits&#;&#;
POD activity was assayed using the photochemical guaiacol method. SOD activity was assayed using the photochemical NBT method. The concentration of MDA was assayed using the thiobarbituric acid method [
2]. The soluble sugar content was determined using the anthrone colorimetric method [
44]. The soluble protein content was determined using the Coomassie Brilliant Blue G-250 method [
43].
5.7. Analysis of Low-Temperature Tolerance in Transgenic Tobacco Plants
The plants in the transgenic tobacco group were subjected to a -2°C low-temperature treatment for 0 min, 5 min, 10 min, 15 min, 30 min, 45 min, 1 h, 1.5 h, 2 h, 3 h, 6 h, 12 h, and 24 h. The tobacco status was observed, and the plants were precooled in liquid nitrogen in a -80°C refrigerator. The physiological and biochemical indicators were subsequently measured, and qRT-PCR was used to detect changes in the expression of genes related to low-temperature-resistance-related phenotypes at the molecular level of the FmNAC1 protein.
5.8. Analysis of Gene Expression
The quantified RNA was reverse transcribed into cDNA using the PrimeScript™ RT reagent kit with a gDNA Eraser (Perfect Real-Time) (Takara Bio Inc., Shiga, Japan). Quantitative RT-PCR (qRTPCR) was conducted in a 7500 Real-Time PCR system (Applied Biosystems, Forster City, CA, USA) using the Takara SYBR
® Premix Ex Taq™ II (Perfect Real Time) (Takara Bio, Inc., Japan). All reactions were performed in triplicate to ensure technical and biological reproducibility, and the relative abundance of the transcripts was calculated using 7500 Software v 2.0.6 (Applied Biosystems, Forster City, CA, USA) using the comparative 2
−△△CT method. The qRT-PCR primer pairs are shown in
Table 2. Tubulin was used as an internal control to determine the expression levels of the target genes.
5.9. Statistical Analysis of Data
Data processing and statistics: all physiological and biochemical index (MDA, antioxidant enzyme activity, qPCR, phytohormone content) data are presented as the mean ± the standard deviation (n = 3). The effects of the cold treatment, the biotype, and the interactions between the two factors were determined via an analysis of variance (ANOVA) followed by Duncan’s multiple range test (with a probability level of 0.05 indicating statistical significance) using Excel and SPSS 20.0, Inc. (Chicago, IL, USA). The figures were drawn using Prism 8.0.
Figure 1.
Sequence analysis of FmNAC1 genes and prediction of the protein tertiary structure. (A) The FmNAC1 gene and its encoded amino acid sequence. (B) The tertiary structure of the FmNAC1 protein. (C) The tertiary structure of the conserved domain of the Oryza sativa L. stress-responsive NAC1 protein. (D) The different regions, identified with different colors, on the amino acid sequences of FmNAC1and OsNAC1.
Figure 1.
Sequence analysis of FmNAC1 genes and prediction of the protein tertiary structure. (A) The FmNAC1 gene and its encoded amino acid sequence. (B) The tertiary structure of the FmNAC1 protein. (C) The tertiary structure of the conserved domain of the Oryza sativa L. stress-responsive NAC1 protein. (D) The different regions, identified with different colors, on the amino acid sequences of FmNAC1and OsNAC1.
Figure 2.
Multiple sequence alignment and phylogenetic relationships of the
FmNAC1 protein. (A) Homolog alignment of the FmNAC1 protein. The red box represents the NAC conserved domain. (B) Construction of the FmNAC1 phylogenetic tree. The phylogenetic relationships of the FmNAC1 proteins were investigated by obtaining the NAC1 protein sequences from 30 plant species from the NCBI database (
http://www.ncbi.nlm.nih.gov/) using the BLASTP method. Sequence alignment was performed using the ClustalW method, and a phylogenetic tree was constructed using the neighbor-joining method with MEGA 5.0 software. In the constructed phylogenetic tree, FmNAC1 is represented by a red triangle. (Different colors indicate different clades, with distinct phyla marked on the outside).
Abrus precatorius (XP_027343628.1),
Beta vulgaris subsp. (XP_010666181.1),
Cajanus cajan (XP_020223961.1),
Camellia sinensis (XP_028081260.1),
Carya illinoinensis (XP_042951768.1),
Cicer arietinum (XP_004488843.1),
Coffea arabica (XP_027061236.1),
Fraxinus mandshurica (AXO66323.1),
Glycine soja (XP_028244150.1),
Herrania umbratica (XP_021288005.1),
Hevea brasiliensis (XP_021644348.1),
Jatropha curcas (XP_012072721.1),
Juglans microcarpa x Juglans regia (XP_040989265.1),
Juglans regia (XP_018838950.1),
Lupinus angustifolius (XP_019443758.1),
Manihot esculenta (XP_021606973.1),
Medicago truncatula (XP_003595973.1),
Nelumbo nucifera (XP_010258675.1),
Nicotiana tomentosiformis (XP_009605814.1),
Olea europaea var. (XP_022848339.1),
Phaseolus vulgaris (XP_007149262.1),
Populus alba (XP_034910133.1),
Populus euphratica (XP_011012784.1),
Populus trichocarpa (XP_002310688.1),
Prunus avium (XP_021832498.1),
Pyrus x bretschneideri (XP_009336675.1),
Quercus lobata (XP_030961789.1),
Ricinus communis (XP_002529954.1),
Sesamum indicum (XP_011095676.1),
Theobroma cacao (XP_007022298.1),
Vigna angularis (XP_017423259.1).
Figure 2.
Multiple sequence alignment and phylogenetic relationships of the
FmNAC1 protein. (A) Homolog alignment of the FmNAC1 protein. The red box represents the NAC conserved domain. (B) Construction of the FmNAC1 phylogenetic tree. The phylogenetic relationships of the FmNAC1 proteins were investigated by obtaining the NAC1 protein sequences from 30 plant species from the NCBI database (
http://www.ncbi.nlm.nih.gov/) using the BLASTP method. Sequence alignment was performed using the ClustalW method, and a phylogenetic tree was constructed using the neighbor-joining method with MEGA 5.0 software. In the constructed phylogenetic tree, FmNAC1 is represented by a red triangle. (Different colors indicate different clades, with distinct phyla marked on the outside).
Abrus precatorius (XP_027343628.1),
Beta vulgaris subsp. (XP_010666181.1),
Cajanus cajan (XP_020223961.1),
Camellia sinensis (XP_028081260.1),
Carya illinoinensis (XP_042951768.1),
Cicer arietinum (XP_004488843.1),
Coffea arabica (XP_027061236.1),
Fraxinus mandshurica (AXO66323.1),
Glycine soja (XP_028244150.1),
Herrania umbratica (XP_021288005.1),
Hevea brasiliensis (XP_021644348.1),
Jatropha curcas (XP_012072721.1),
Juglans microcarpa x Juglans regia (XP_040989265.1),
Juglans regia (XP_018838950.1),
Lupinus angustifolius (XP_019443758.1),
Manihot esculenta (XP_021606973.1),
Medicago truncatula (XP_003595973.1),
Nelumbo nucifera (XP_010258675.1),
Nicotiana tomentosiformis (XP_009605814.1),
Olea europaea var. (XP_022848339.1),
Phaseolus vulgaris (XP_007149262.1),
Populus alba (XP_034910133.1),
Populus euphratica (XP_011012784.1),
Populus trichocarpa (XP_002310688.1),
Prunus avium (XP_021832498.1),
Pyrus x bretschneideri (XP_009336675.1),
Quercus lobata (XP_030961789.1),
Ricinus communis (XP_002529954.1),
Sesamum indicum (XP_011095676.1),
Theobroma cacao (XP_007022298.1),
Vigna angularis (XP_017423259.1).
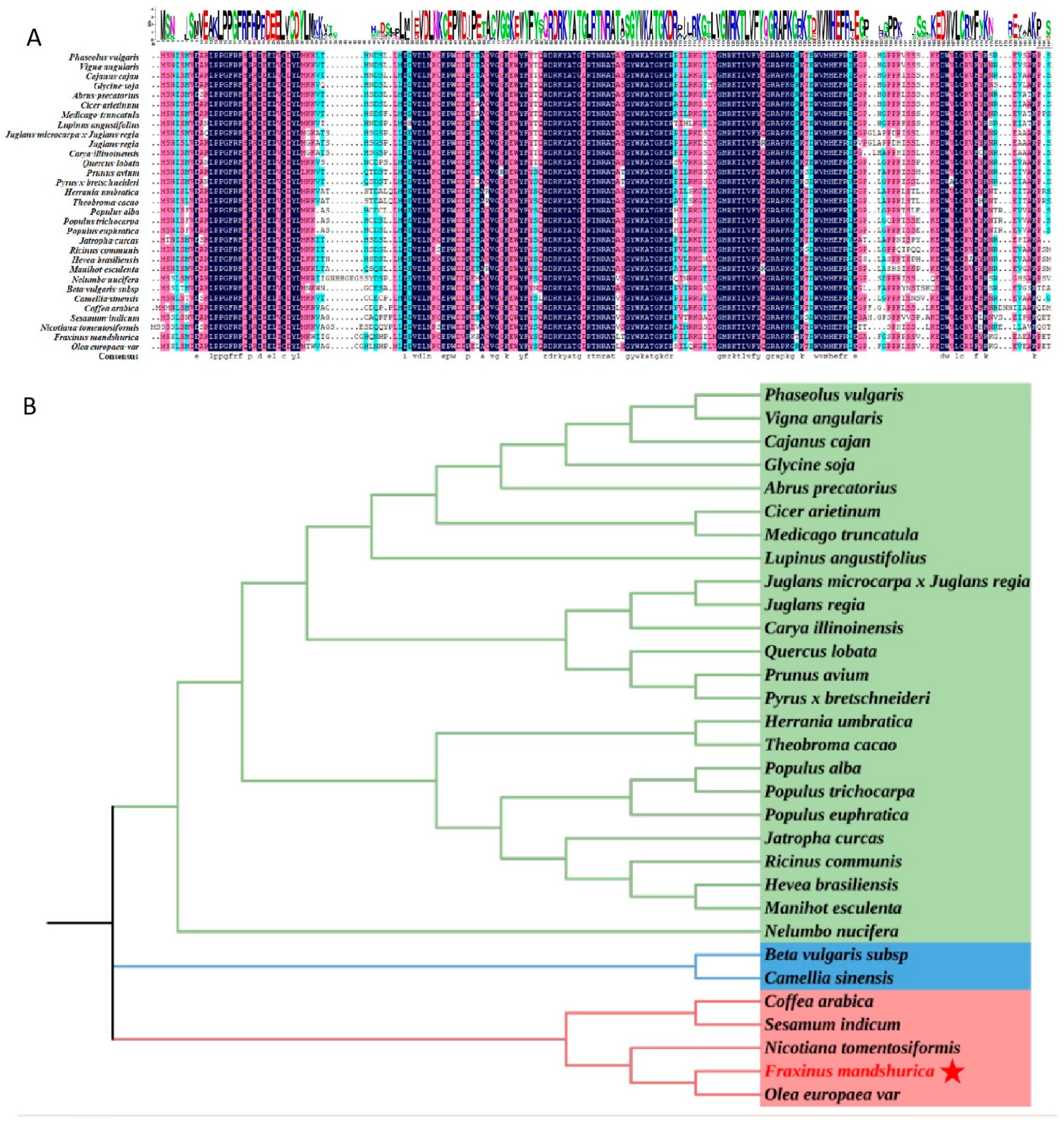
Figure 3.
Subcellular localization of FmNAC1. The photographs were taken under dark-field illumination for green fluorescence localization (GFP), bright-field illumination to examine the cell morphology (bright field), and merged-field illumination (merged). The bar represents 10 μm. The red arrows indicate that green fluorescence occurred in the nucleus.
Figure 3.
Subcellular localization of FmNAC1. The photographs were taken under dark-field illumination for green fluorescence localization (GFP), bright-field illumination to examine the cell morphology (bright field), and merged-field illumination (merged). The bar represents 10 μm. The red arrows indicate that green fluorescence occurred in the nucleus.
Figure 4.
The expression of the FmNAC1 gene fluctuated with the duration of the biological stress. Note: The horizontal axes represent the cold, salt, ABA, GA, and IAA treatments, and the vertical axes represent 1 hour, 3 hours, 6 hours, 12 hours, 24 hours, and 48 hours of treatment.
Figure 4.
The expression of the FmNAC1 gene fluctuated with the duration of the biological stress. Note: The horizontal axes represent the cold, salt, ABA, GA, and IAA treatments, and the vertical axes represent 1 hour, 3 hours, 6 hours, 12 hours, 24 hours, and 48 hours of treatment.
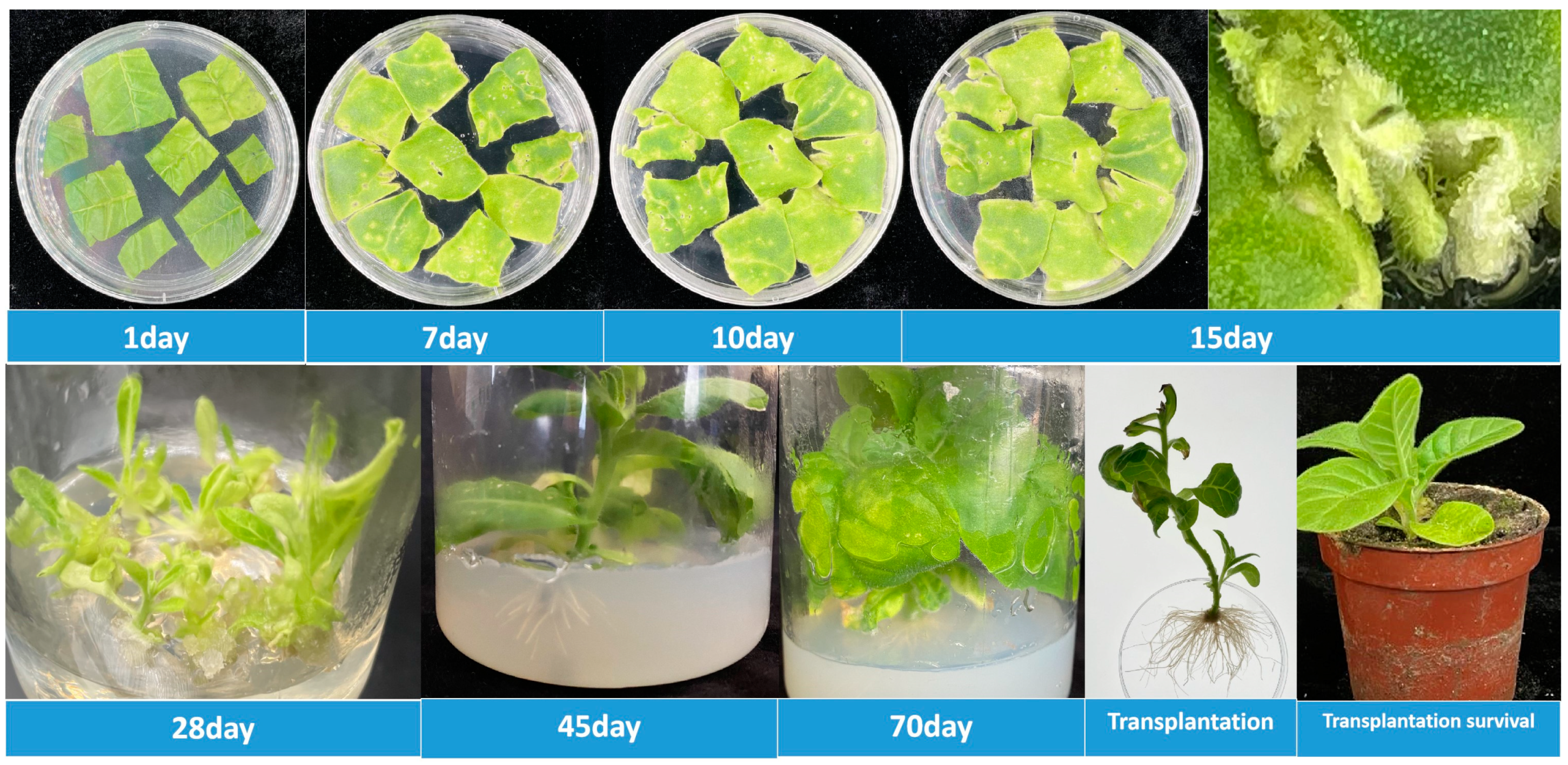
Figure 5.
Transgenic tobacco from FmNAC1-OE plants was obtained. Note: the above figure represents the growth status of transgenic tobacco plants cultured for 1 day, 7 days, 10 days, 15 days, 28 days, 45 days, 70 days, and 45 days after successive generations; the plants were finally transplanted into the greenhouse.
Figure 5.
Transgenic tobacco from FmNAC1-OE plants was obtained. Note: the above figure represents the growth status of transgenic tobacco plants cultured for 1 day, 7 days, 10 days, 15 days, 28 days, 45 days, 70 days, and 45 days after successive generations; the plants were finally transplanted into the greenhouse.
Figure 6.
Verification of the transgenic tobacco strain overexpressing FmNAC1. Note: (A) The columns are arranged as follows: the first column represents the marker, the second column represents the plasmid, the third column represents the water control, columns four to ten represent tobacco lines overexpressing FmNAC1, and the eleventh column represents the wild-type group. (B) The gene expression of FmNAC1 in transgenic tobacco. The first column represents the wild-type group, followed by columns for samples 1, 2, 5, and 7.
Figure 6.
Verification of the transgenic tobacco strain overexpressing FmNAC1. Note: (A) The columns are arranged as follows: the first column represents the marker, the second column represents the plasmid, the third column represents the water control, columns four to ten represent tobacco lines overexpressing FmNAC1, and the eleventh column represents the wild-type group. (B) The gene expression of FmNAC1 in transgenic tobacco. The first column represents the wild-type group, followed by columns for samples 1, 2, 5, and 7.
Figure 7.
Changes in the height of the FmNAC1 transgenic tobacco plants after transplanting. (A) Experimental plants, with a white arrow indicating the overexpression of transgenic tobacco, while the other three pots represent wild-type controls from the same time period. The height of the transgenic tobacco plants was measured one day after transplantation. (B) The x-axis represents time, the y-axis represents height, the black line represents the wild type, the red line represents transgenic line 1, the green line represents transgenic line 2, and the blue line represents transgenic line 5. * Indicates a significant difference between the control and transgenic tobacco (p < 0.05).
Figure 7.
Changes in the height of the FmNAC1 transgenic tobacco plants after transplanting. (A) Experimental plants, with a white arrow indicating the overexpression of transgenic tobacco, while the other three pots represent wild-type controls from the same time period. The height of the transgenic tobacco plants was measured one day after transplantation. (B) The x-axis represents time, the y-axis represents height, the black line represents the wild type, the red line represents transgenic line 1, the green line represents transgenic line 2, and the blue line represents transgenic line 5. * Indicates a significant difference between the control and transgenic tobacco (p < 0.05).
Figure 8.
Phenotypic changes in the FmNAC1 transgenic tobacco gene under cold stress. Note: from left to right, transgenic line 1, transgenic line 2, transgenic line 5, and the wild type.
Figure 8.
Phenotypic changes in the FmNAC1 transgenic tobacco gene under cold stress. Note: from left to right, transgenic line 1, transgenic line 2, transgenic line 5, and the wild type.
Figure 11.
The vector map of the FmNAC1 gene overexpression construct. (A) Vector map of the PBI121-FmNAC1-GFP overexpression construct. (B) Vector map of the pROKII-FmNAC1-GUS overexpression construct.
Figure 11.
The vector map of the FmNAC1 gene overexpression construct. (A) Vector map of the PBI121-FmNAC1-GFP overexpression construct. (B) Vector map of the pROKII-FmNAC1-GUS overexpression construct.
Table 1.
The primers corresponding to the coding region of the cloned FmNAC1 gene.
Table 1.
The primers corresponding to the coding region of the cloned FmNAC1 gene.
| Gene |
GenBank accession numbers |
Primer name |
Primer sequences |
Melting temperatures |
| FmNAC1 |
MG269829.1 |
FmNAC1-F |
5'-TCTATCACCTCCGCCATC -3' |
53.1℃ |
| |
|
FmNAC1-R |
5'-TCAATAATGGTTCCACTGC-3' |
52.3℃ |
Table 2.
Sequences of the primers used for the qRT-PCR.
Table 2.
Sequences of the primers used for the qRT-PCR.
| Gene |
GenBank accession numbers |
Primer Name |
Primer Sequence |
Melting temperatures |
| NbIAA |
XM_016592822.1 |
q NbIAA -F |
5' GCCTCCTTCCACATTCCC 3' |
57℃ |
| |
q NbIAA -R |
5' AGTCCACGTTTCGCACCA 3' |
57.4℃ |
| NbAUX1 |
NM_001326023.1 |
q NbAUX1-F |
5' AGGCTACTGCCCTACCAC 3' |
52℃ |
| |
qNbAUX1-R |
5' CAGCAACTGTATTCCCACA 3' |
51.8℃ |
| NbICE |
XM_016610394.1 |
q NbICE-F |
5' CCCATCATTCCATTTGCT 3' |
53.3℃ |
| |
q NbICE-R |
5' CCATTATCCCACTTCCTCT 3' |
51.1℃ |
| NbDREB |
NM_001325812.1 |
q NbDREB-F |
5' GGCTTGGCACTTTCCCTT 3' |
57.3℃ |
| |
qNbDREB-R |
5' AATAGCGCCTCCTCATCC 3' |
54.3℃ |
| NbCBF |
NM_001325227.1 |
q NbCBF-F |
5' GGGGAATAAGGAGGAGAA 3' |
51℃ |
| |
q NbCBF-R |
5' CTGAAGTCGGGATGGGTA 3' |
53.2℃ |
| NbCAT |
NM_001325412.1 |
q NbCAT-F |
5' AATCATAGCCACGCCACC 3' |
56.2℃ |
| |
q NbCAT-R |
5' CGAATAGTAAAGACCAGGGA 3' |
52.2℃ |
| NbSOD |
XM_016581519.1 |
q NbSOD -F |
5' GATCTGGGAAGAGGTGGA 3' |
51.7℃ |
| |
q NbSOD -R |
5' CAGCCCTAATGATAAACTGA 3' |
50.5℃ |
| NbTU |
XM_016640346.1 |
qNbTU-F |
5'ATGTTGTTAGGAAGGAGGCT3' |
53.2℃ |
| |
qNbTU-R |
5' ATCATGCGGTCAGGGTAT 3' |
52.6℃ |
| FmNAC1 |
MG269829.1 |
qFmNAC1-F |
5' AATGGATAATCGGCTGTG 3' |
50.9℃ |
| |
qFmNAC1-R |
5' CCTTACGAAGAATCGGTCTA 3' |
52.4℃ |
| FmTU |
qPT-PCR |
qFmTU-F |
5' AGGACGCTGCCAACAACTTT 3' |
59.8℃ |
| |
qFmTU-R |
5' TTGAGGGGAAGGGTAAATAGTG 3' |
58.0℃ |