Submitted:
21 May 2024
Posted:
22 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
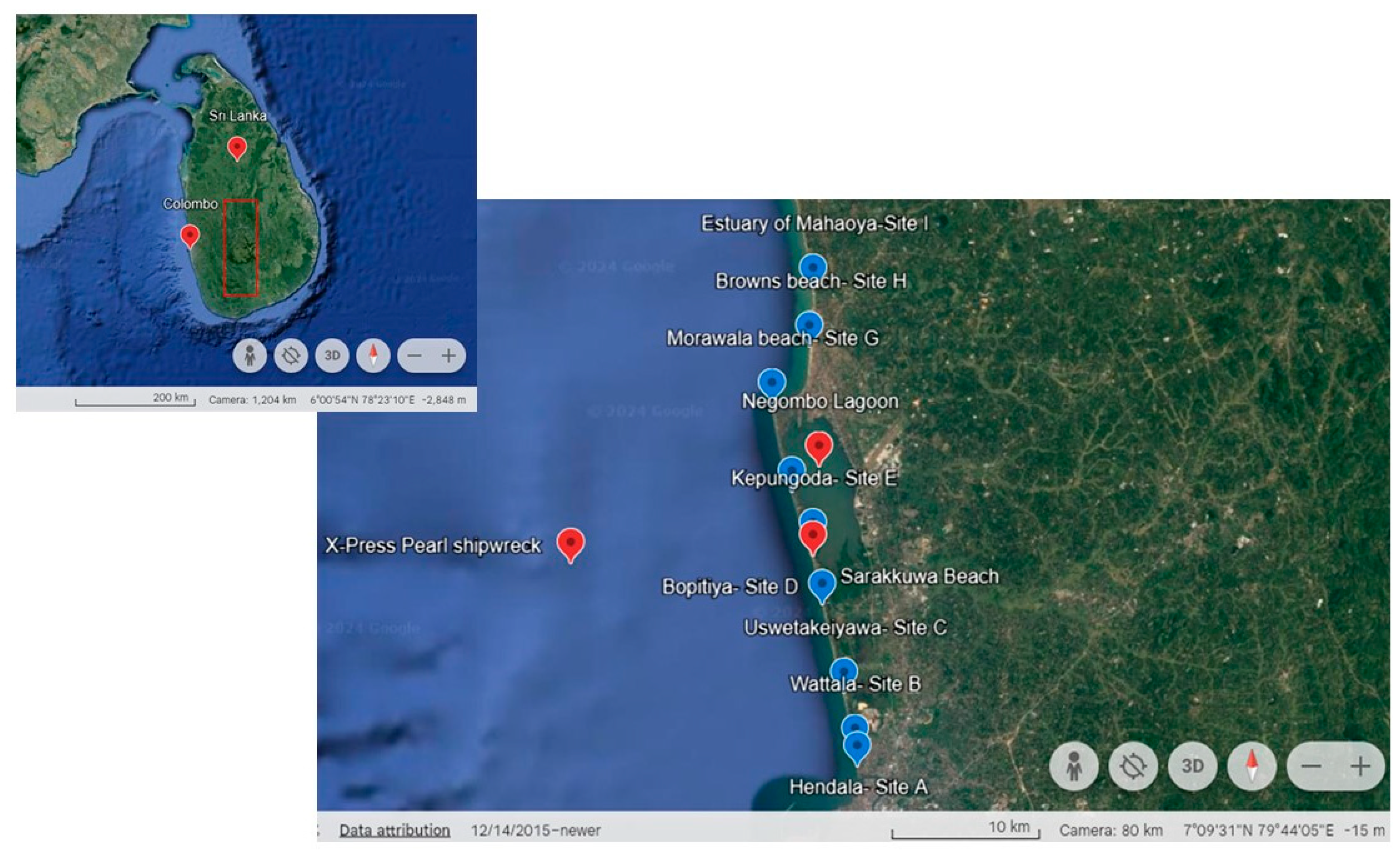
2.1. Study Area and Sample Collection
2.2. Microplastic Analysis: An improved NOAA Method
2.2.1. Extraction of MPs
2.2.2. Identification and Characterization of MPs
2.3. Method Validation and Contamination Sources
2.4. Determination of Pellet Pollution Index (PPI)
2.5. Data Analysis
3. Results
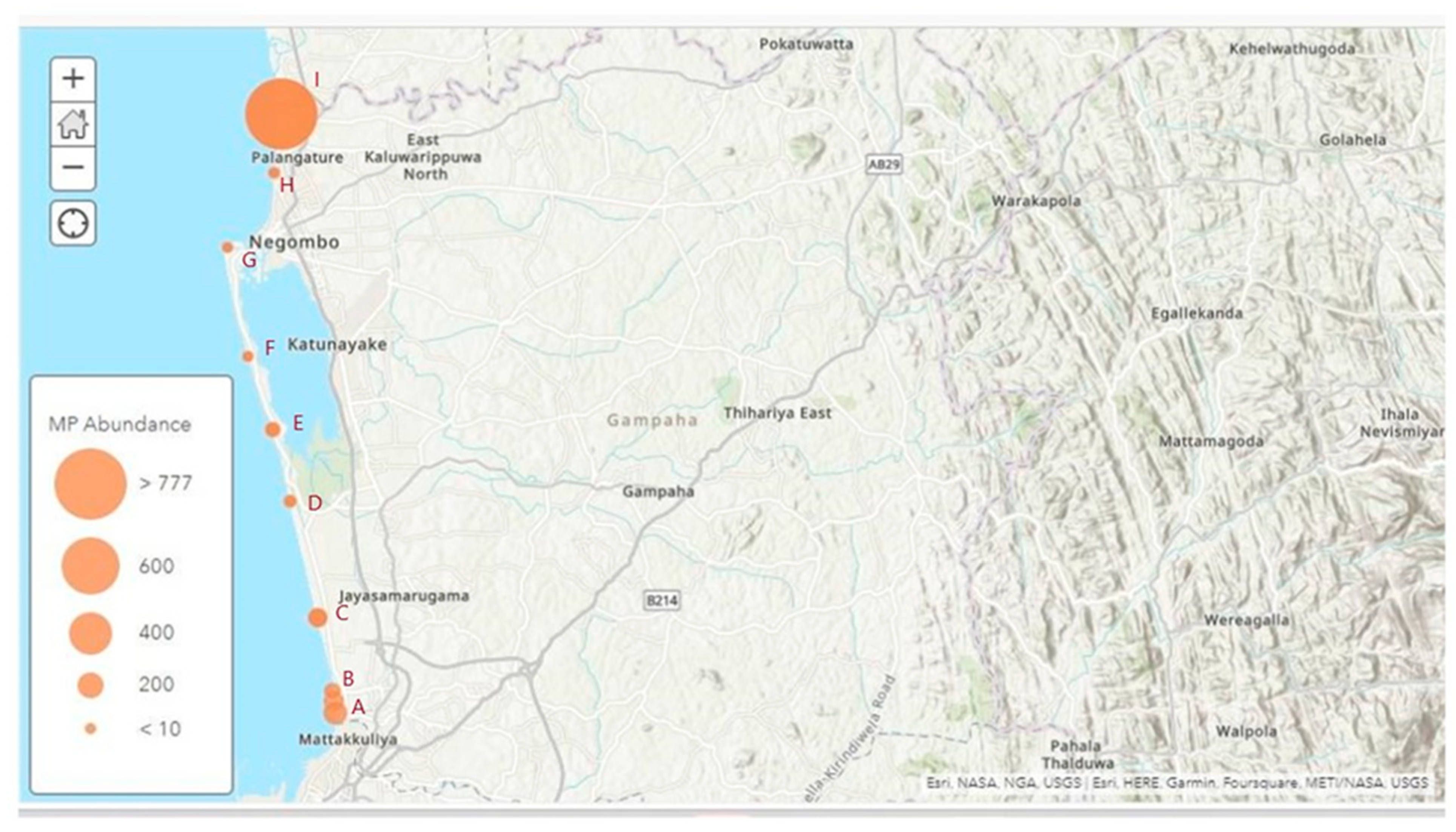
3.1. Abundance and Spatial Distribution of MPs in sampling sites
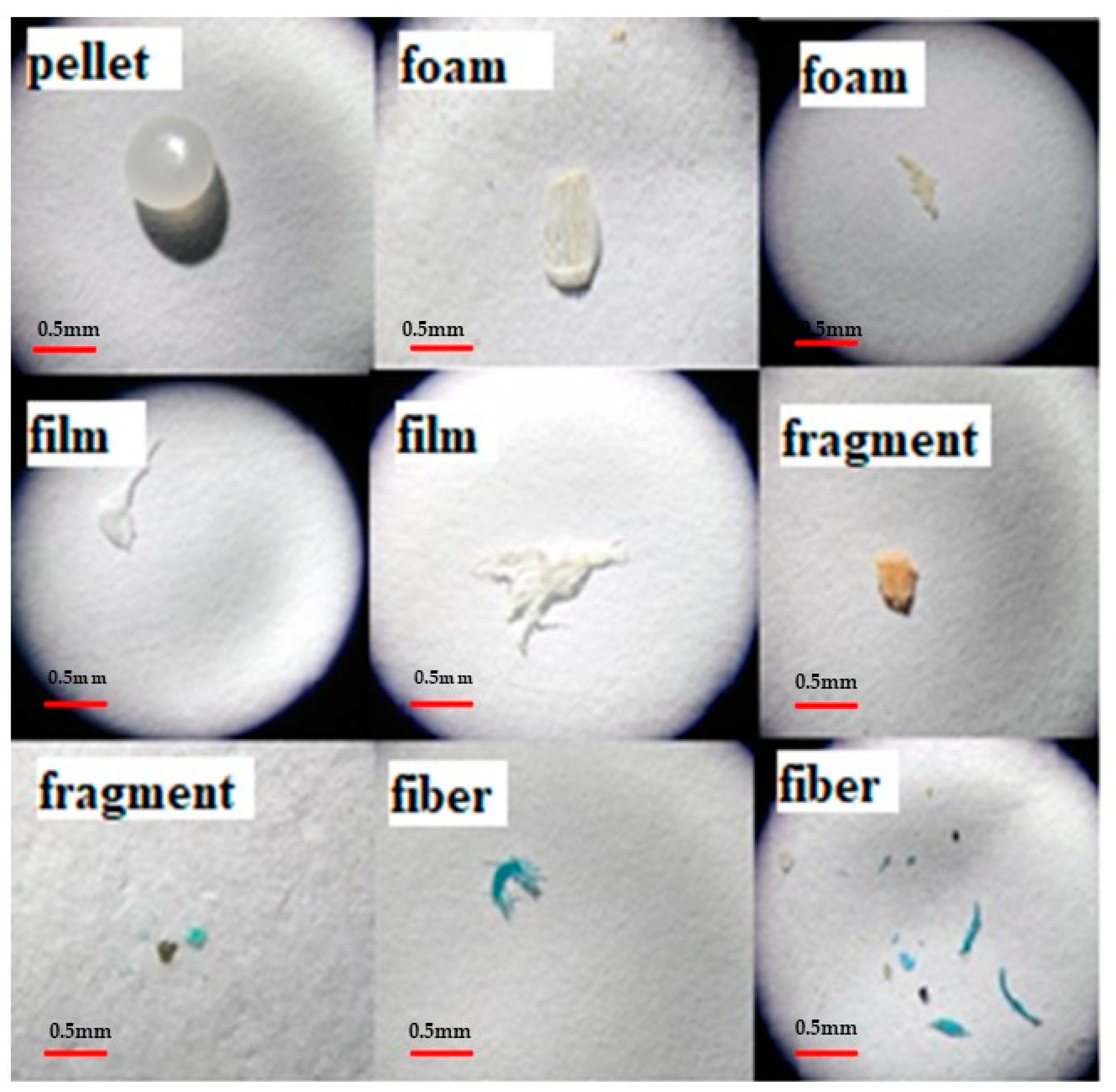
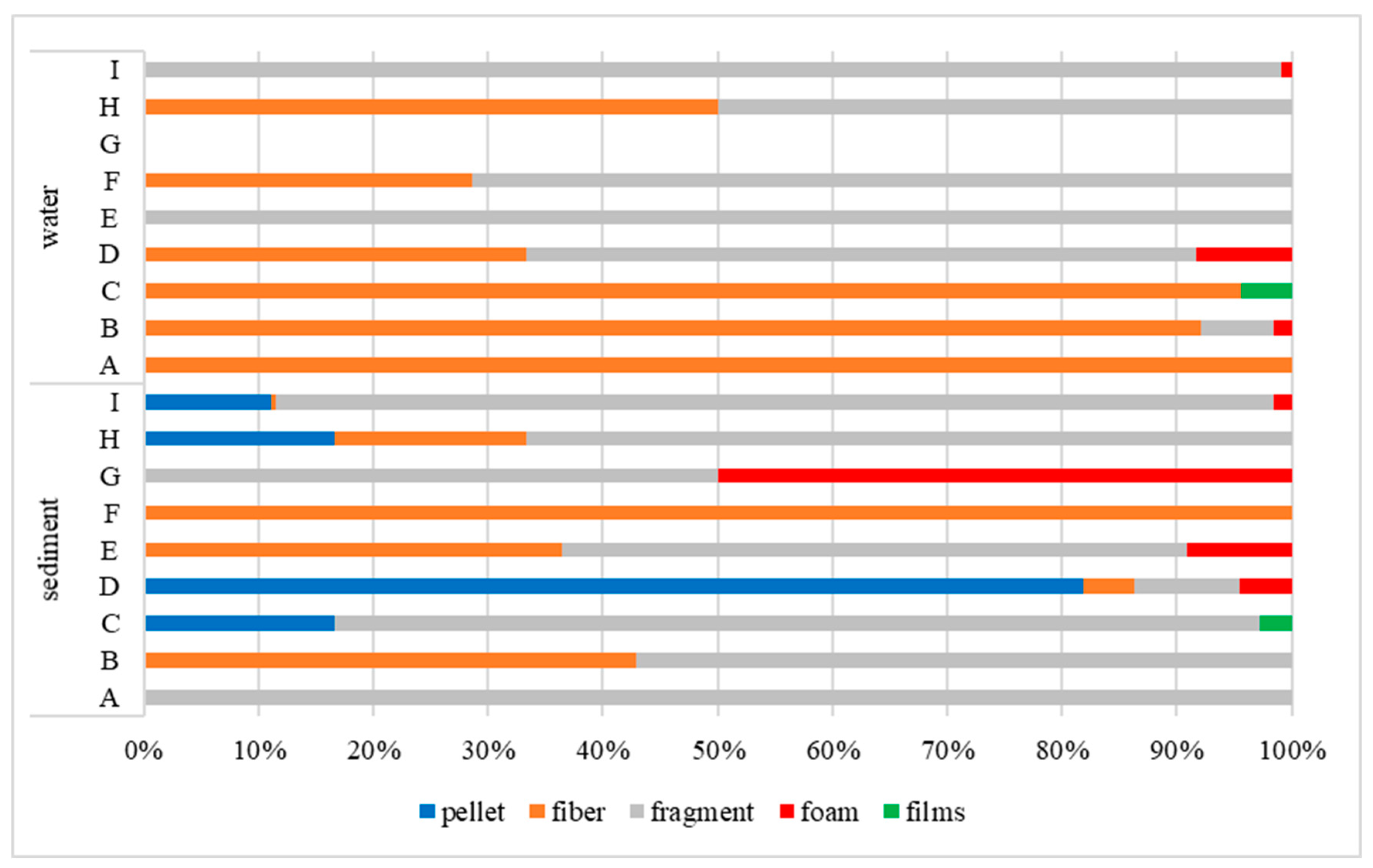
3.2. Morphology of MPs
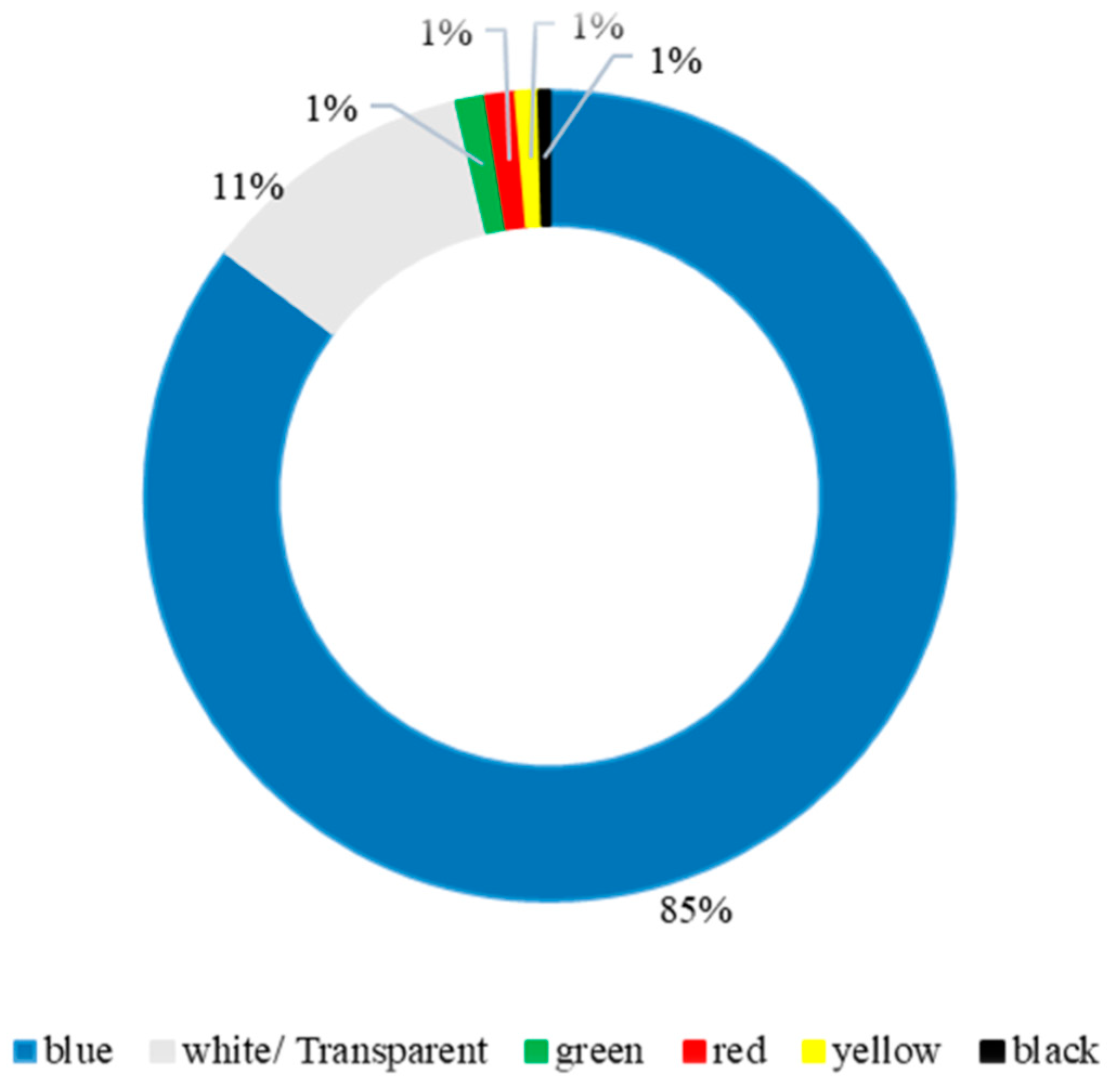
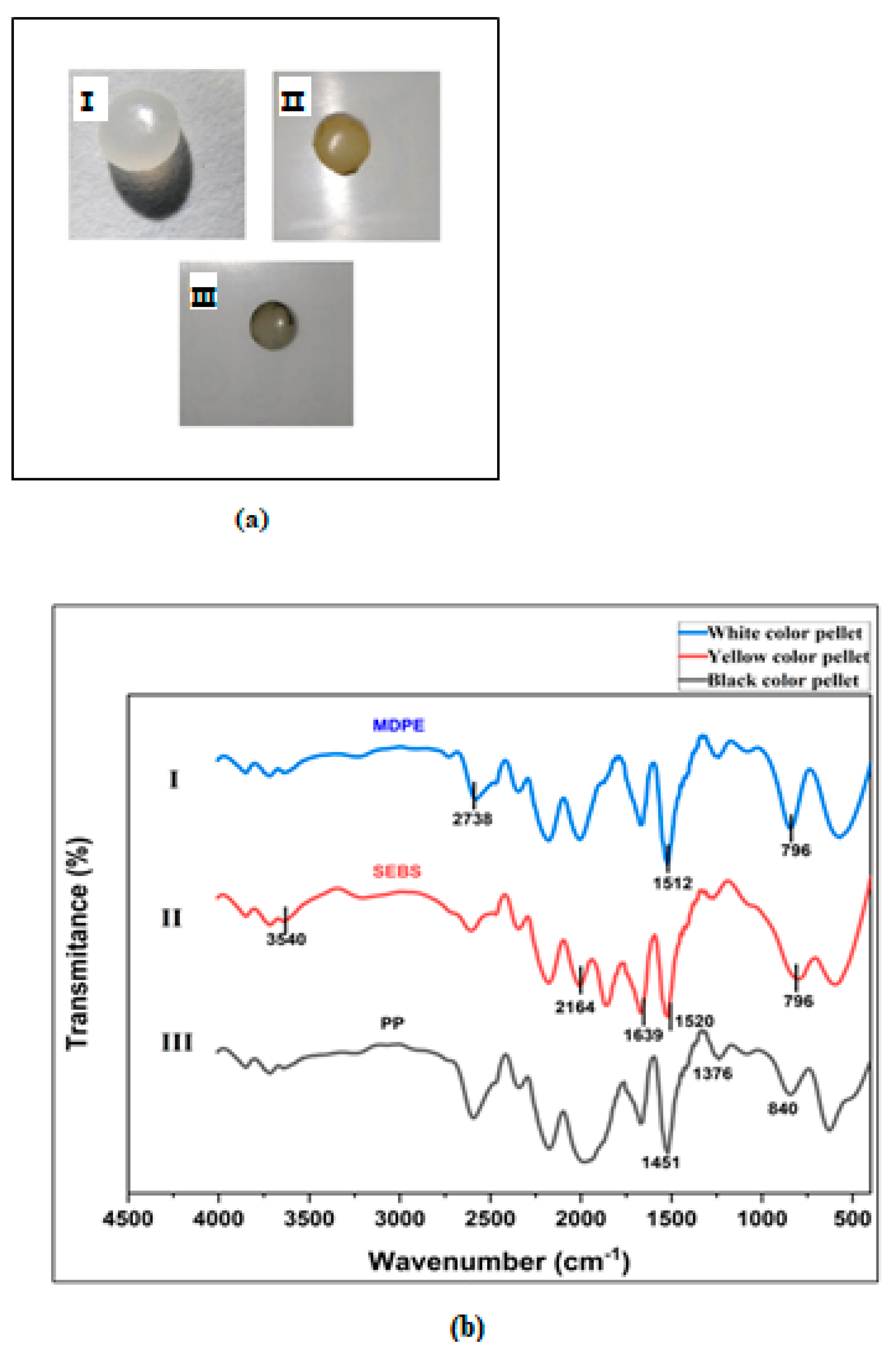
3.3. Color of MPs
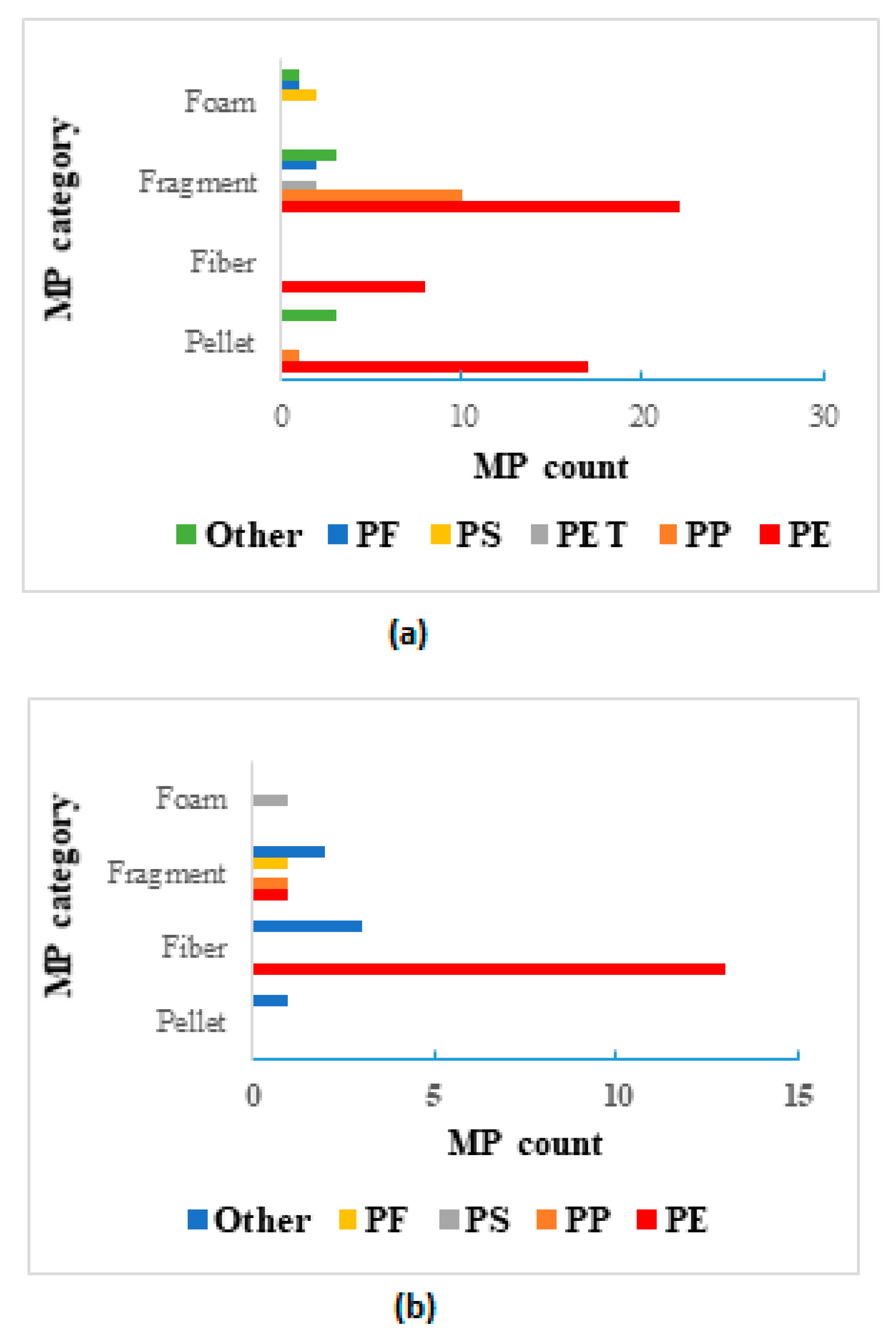
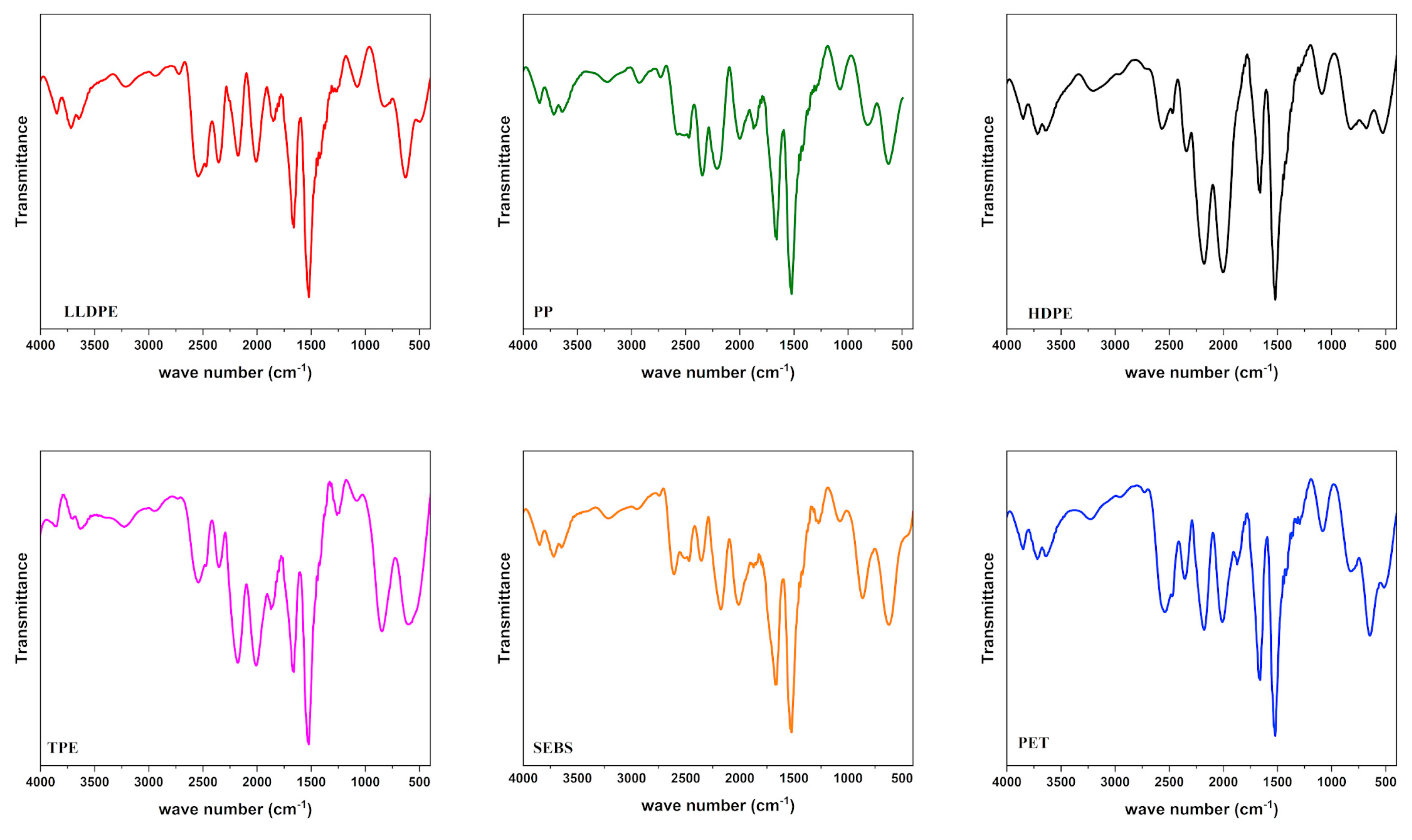
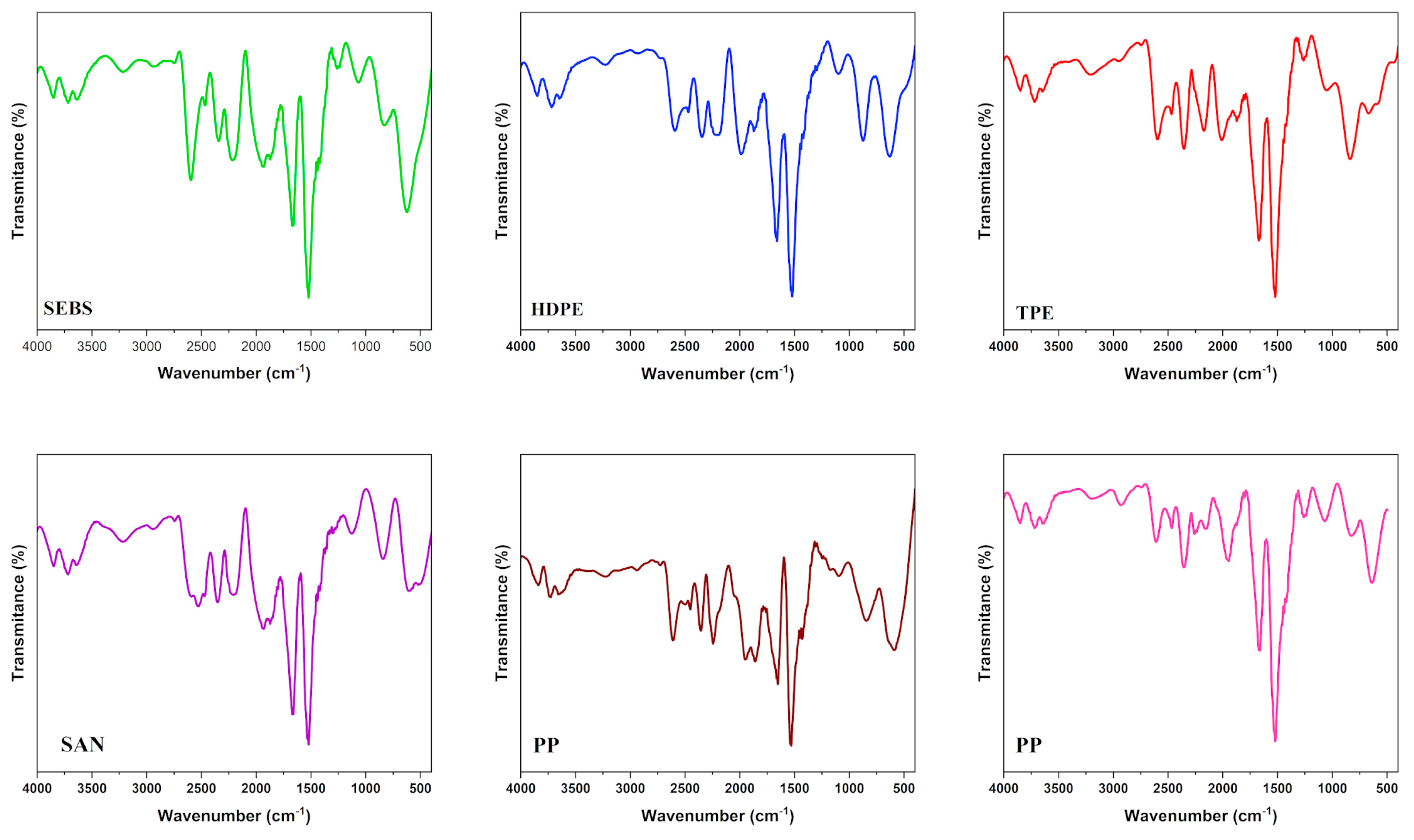
3.4. Polymer Characterization of MPs
3.5. Pellet Distribution
3.6. Plastic Pellet Pollution Index
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- J. H. Dekiff, D. Remy, J. Klasmeier, and E. Fries, “Occurrence and spatial distribution of microplastics in sediments from Norderney,” Environmental Pollution, vol. 186, pp. 248–256, Mar. 2014. [CrossRef]
- © Iucn, “IUCN website IUCN issues briefs: Twitter: @IUCN www.iucn.org www.iucn.org/issues-briefs.” [Online]. Available: www.iucn.org/issues-briefs.
- L. Yang, Y. Zhang, S. Kang, Z. Wang, and C. Wu, “Microplastics in soil: A review on methods, occurrence, sources, and potential risk,” Science of the Total Environment, vol. 780. Elsevier B.V., Aug. 01, 2021. [CrossRef]
- C. Campanale, C. Massarelli, G. Bagnuolo, I. Savino, and V. F. Uricchio, “The Problem of Microplastics and Regulatory Strategies in Italy,” in Handbook of Environmental Chemistry, vol. 112, 2022. [CrossRef]
- K. Ziani et al., “Microplastics: A Real Global Threat for Environment and Food Safety: A State of the Art Review,” Nutrients, vol. 15, no. 3. MDPI, Feb. 01, 2023. [CrossRef]
- H. Park and B. S. Park, “Review of microplastic distribution, toxicity, analysis methods, and removal technologies,” Water (Switzerland), vol. 13, no. 19. MDPI, Oct. 01, 2021. [CrossRef]
- A. L. Andrady, “Microplastics in the marine environment,” Marine Pollution Bulletin, vol. 62, no. 8. pp. 1596–1605, Aug. 2011. [CrossRef]
- S. Mariano, S. Tacconi, M. Fidaleo, M. Rossi, and L. Dini, “Micro and Nanoplastics Identification: Classic Methods and Innovative Detection Techniques,” Frontiers in Toxicology, vol. 3. Frontiers Media S.A., 2021. [CrossRef]
- D. K. A. Barnes, F. Galgani, R. C. Thompson, and M. Barlaz, “Accumulation and fragmentation of plastic debris in global environments,” Philosophical Transactions of the Royal Society B: Biological Sciences, vol. 364, no. 1526, pp. 1985–1998, Jul. 2009. [CrossRef]
- M. Claessens, L. Van Cauwenberghe, M. B. Vandegehuchte, and C. R. Janssen, “New techniques for the detection of microplastics in sediments and field collected organisms,” Mar Pollut Bull, vol. 70, no. 1–2, pp. 227–233, 2013. [CrossRef]
- C. B. Crawford and B. Quinn, “Microplastic identification techniques,” in Microplastic Pollutants, Elsevier, 2017, pp. 219–267. [CrossRef]
- M. Ateia, G. Ersan, M. G. Alalm, D. C. Boffito, and T. Karanfil, “Emerging investigator series: Microplastic sources, fate, toxicity, detection, and interactions with micropollutants in aquatic ecosystems-a review of reviews,” Environmental Science: Processes and Impacts, vol. 24, no. 2. Royal Society of Chemistry, pp. 172–195, Feb. 01, 2022. [CrossRef]
- N. Ali et al., “Environmental fate, aging, toxicity and potential remediation strategies of microplastics in soil environment: Current progress and future perspectives,” Science of the Total Environment, vol. 906. Elsevier B.V., Jan. 01, 2024. [CrossRef]
- L. Cutroneo et al., “Correction to: Microplastics in seawater: sampling strategies, laboratory methodologies, and identification techniques applied to port environment (Environmental Science and Pollution Research, (2020), 27, 9, (8938-8952), 10.1007/s11356-020-07783-8),” Environmental Science and Pollution Research, vol. 27, no. 16. 2020. [CrossRef]
- K. O. Babaremu et al., “Sustainable plastic waste management in a circular economy,” Heliyon, vol. 8, no. 7. Elsevier Ltd, Jul. 01, 2022. [CrossRef]
- E. Watt et al., “Ocean plastics: environmental implications and potential routes for mitigation - a perspective,” RSC Advances, vol. 11, no. 35. Royal Society of Chemistry, pp. 21447–21462, Jun. 17, 2021. [CrossRef]
- A. Hasan Anik, S. Hossain, M. Alam, M. Binte Sultan, M. T. Hasnine, and M. M. Rahman, “Microplastics pollution: A comprehensive review on the sources, fates, effects, and potential remediation,” Environmental Nanotechnology, Monitoring and Management, vol. 16. Elsevier B.V., Dec. 01, 2021. [CrossRef]
- T. Parsai, N. Figueiredo, V. Dalvi, M. Martins, A. Malik, and A. Kumar, “Implication of microplastic toxicity on functioning of microalgae in aquatic system,” Environmental Pollution, vol. 308. 2022. [CrossRef]
- S. Ghosh, J. K. Sinha, S. Ghosh, K. Vashisth, S. Han, and R. Bhaskar, “Microplastics as an Emerging Threat to the Global Environment and Human Health,” Sustainability (Switzerland), vol. 15, no. 14. Multidisciplinary Digital Publishing Institute (MDPI), Jul. 01, 2023. [CrossRef]
- G. Caruso, E. Bergami, N. Singh, and I. Corsi, “Plastic occurrence, sources, and impacts in Antarctic environment and biota,” Water Biology and Security, vol. 1, no. 2. Institute of Hydrobiology, Chinese Academy of Sciences, May 01, 2022. [CrossRef]
- A. Cózar et al., “Plastic debris in the open ocean,” Proc Natl Acad Sci U S A, vol. 111, no. 28, pp. 10239–10244, Jul. 2014. [CrossRef]
- A. M. Saley et al., “Microplastic accumulation and biomagnification in a coastal marine reserve situated in a sparsely populated area,” Mar Pollut Bull, vol. 146, pp. 54–59, Sep. 2019. [CrossRef]
- A. Isobe, K. Uchiyama-Matsumoto, K. Uchida, and T. Tokai, “Microplastics in the Southern Ocean,” Mar Pollut Bull, vol. 114, no. 1, pp. 623–626, Jan. 2017. [CrossRef]
- A. M. Saley et al., “Microplastic accumulation and biomagnification in a coastal marine reserve situated in a sparsely populated area,” Mar Pollut Bull, vol. 146, pp. 54–59, Sep. 2019. [CrossRef]
- H. Yu, Y. Zhang, W. Tan, and Z. Zhang, “Microplastics as an Emerging Environmental Pollutant in Agricultural Soils: Effects on Ecosystems and Human Health,” Frontiers in Environmental Science, vol. 10. Frontiers Media S.A., Mar. 09, 2022. [CrossRef]
- S. Wang et al., “Microplastic abundance, distribution and composition in the mid-west Pacific Ocean,” Environmental Pollution, vol. 264, p. 114125, Sep. 2020. [CrossRef]
- M. Eriksen et al., “Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea,” PLoS One, vol. 9, no. 12, Dec. 2014. [CrossRef]
- A. Adamopoulou, C. Zeri, F. Garaventa, C. Gambardella, C. Ioakeimidis, and E. Pitta, “Distribution Patterns of Floating Microplastics in Open and Coastal Waters of the Eastern Mediterranean Sea (Ionian, Aegean, and Levantine Seas),” Front Mar Sci, vol. 8, Sep. 2021. [CrossRef]
- M. P. de Almeida et al., “The Complex Dynamics of Microplastic Migration through Different Aquatic Environments: Subsidies for a Better Understanding of Its Environmental Dispersion,” Microplastics, vol. 2, no. 1, pp. 62–77, Jan. 2023. [CrossRef]
- Y. Wang et al., “Occurrence Characteristics and Ecotoxic Effects of Microplastics in Environmental Media: a Mini Review,” Appl Biochem Biotechnol, Dec. 2023. [CrossRef]
- J. C. Prata, J. P. da Costa, A. C. Duarte, and T. Rocha-Santos, “Methods for sampling and detection of microplastics in water and sediment: A critical review,” TrAC - Trends in Analytical Chemistry, vol. 110. Elsevier B.V., pp. 150–159, Jan. 01, 2019. [CrossRef]
- I. Lise, N. Bråte, I. J. Allan, and K. V. Thomas, Report made for the Norwegian Environment Agency: Microplastics in marine environments; Occurrence, distribution and effects. [Online]. AAvailable: https://www.researchgate.net/publication/273089847.
- A. L. Andrady, “Microplastics in the marine environmentAndrady, A. L. (2011). Microplastics in the marine environment. Marine Pollution Bulletin, 62(8), 1596–1605. [CrossRef]
- M. Long et al., “Interactions between microplastics and phytoplankton aggregates: Impact on their respective fates,” Mar Chem, vol. 175, pp. 39–46, Oct. 2015. [CrossRef]
- J. C. Prata, J. P. da Costa, I. Lopes, A. C. Duarte, and T. Rocha-Santos, “Environmental exposure to microplastics: An overview on possible human health effects,” Science of the Total Environment, vol. 702. Elsevier B.V., Feb. 01, 2020. [CrossRef]
- G. Renner, A. Nellessen, A. Schwiers, M. Wenzel, T. C. Schmidt, and J. Schram, “Data preprocessing & evaluation used in the microplastics identification process: A critical review & practical guide,” TrAC - Trends in Analytical Chemistry, vol. 111. Elsevier B.V., pp. 229–238, Feb. 01, 2019. [CrossRef]
- G. Erni-Cassola, V. Zadjelovic, M. I. Gibson, and J. A. Christie-Oleza, “Distribution of plastic polymer types in the marine environment; A meta-analysis,” J Hazard Mater, vol. 369, pp. 691–698, May 2019. [CrossRef]
- W. L. S. Sevwandi Dharmadasa, A. L. Andrady, P. B. T. P. Kumara, T. Maes, and C. S. Gangabadage, “Microplastic pollution in Marine Protected Areas of Southern Sri Lanka,” Mar Pollut Bull, vol. 168, p. 112462, Jul. 2021. [CrossRef]
- J. Bimali Koongolla, A. L. Andrady, P. B. Terney Pradeep Kumara, and C. S. Gangabadage, “Evidence of microplastics pollution in coastal beaches and waters in southern Sri Lanka,” Mar Pollut Bull, vol. 137, pp. 277–284, Dec. 2018. [CrossRef]
- Y. C. Jang et al., “Composition and abundance of marine debris stranded on the beaches of Sri Lanka: Results from the first island-wide survey,” Mar Pollut Bull, vol. 128, pp. 126–131, Mar. 2018. [CrossRef]
- “Weerakoonetal.2019I”.
- M. Sewwandi, O. Hettithanthri, S. M. Egodage, A. A. D. Amarathunga, and M. Vithanage, “Unprecedented marine microplastic contamination from the X-Press Pearl container vessel disaster,” Science of the Total Environment, vol. 828, Jul. 2022. [CrossRef]
- M. Sewwandi, A. A. D. Amarathunga, H. Wijesekara, K. Mahatantila, and M. Vithanage, “Contamination and distribution of buried microplastics in Sarakkuwa beach ensuing the MV X-Press Pearl maritime disaster in Sri Lankan sea,” Mar Pollut Bull, vol. 184, p. 114074, Nov. 2022. [CrossRef]
- A. De Vos et al., “The M/V X-Press Pearl Nurdle Spill: Contamination of Burnt Plastic and Unburnt Nurdles along Sri Lanka’s Beaches,” ACS Environmental Au, vol. 2, no. 2, pp. 128–135, Mar. 2022. [CrossRef]
- H. Partow, “X-PRESS PEARL MARITIME DISASTER SRI LANKA REPORT OF THE UN ENVIRONMENTAL ADVISORY MISSION,” 2021.
- N. Marine Debris Program, “Laboratory Methods for the Analysis of Microplastics in the Marine Environment: Recommendations for quantifying synthetic particles in waters and sediments,” 2015.
- A. Bakir et al., “A spatial and temporal assessment of microplastics in seafloor sediments: A case study for the UK,” Front Mar Sci, vol. 9, Jan. 2023. [CrossRef]
- G. Fernandino, C. I. Elliff, I. R. Silva, and A. C. S. P. Bittencourtc, “How many pellets are too many? the pellet pollution index as a tool to assess beach pollution by plastic resin pellets in Salvador, Bahia, Brazil,” Journal of Integrated Coastal Zone Management, vol. 15, no. 3, pp. 325–332, Sep. 2015. [CrossRef]
- S. Bolduc et al., “Banana fiber/low-density polyethylene recycled composites for third world eco-friendly construction applications – Waste for life project Sri Lanka,” Journal of Reinforced Plastics and Composites, vol. 37, no. 21, pp. 1322–1331, Nov. 2018. [CrossRef]
- “Final Report Jordi Labs LLC Case Study FTIR for Identification of Contamination.
- A. Naranjo, T. Osswald, A. Roldán-Alzate, J. Diego, S. María, and D. P. Noriega, “Plastics Testing and Characterization.
- D. Dilara Atas and S. Makkonen-Craig, “Sampling Microplastics in Beach Sediments and Analysis Using FTIR Spectroscopy Title: Sampling microplastics in beach sediments and analysis us-ing FTIR spectroscopy Supervisor (Arcada).
- T. G. Sunitha et al., “Micro-plastic pollution along the Bay of Bengal coastal stretch of Tamil Nadu, South India,” Science of the Total Environment, vol. 756, Feb. 2021. [CrossRef]
- A. M. G. A. D. Athawuda, H. B. Jayasiri, G. G. N. Thushari, and K. P. G. K. P. Guruge, “Quantification and morphological characterization of plastic litter (0.30–100 mm) in surface waters of off Colombo, west coast of Sri Lanka,” Environ Monit Assess, vol. 192, no. 8, Aug. 2020. [CrossRef]
- C. Li et al., “Pelagic microplastics in surface water of the Eastern Indian Ocean during monsoon transition period: Abundance, distribution, and characteristics,” Science of the Total Environment, vol. 755, Feb. 2021. [CrossRef]
- W. L. S. Sevwandi Dharmadasa, A. L. Andrady, P. B. T. P. Kumara, T. Maes, and C. S. Gangabadage, “Microplastics pollution in Marine Protected Areas of Southern Sri Lanka,” Mar Pollut Bull, vol. 168, Jul. 2021. [CrossRef]
- A. M. A. I. K. Athapaththu et al., “Plastics in surface water of southern coastal belt of Sri Lanka (Northern Indian Ocean): Distribution and characterization by FTIR,” Mar Pollut Bull, vol. 161, Dec. 2020. [CrossRef]
- K. U. D. N. Hansani et al., “Contamination of microplastics in tropical coral reef ecosystems of Sri Lanka,” Mar Pollut Bull, vol. 194, 2023. [CrossRef]
- P. Goswami, N. V. Vinithkumar, and G. Dharani, “Microplastics particles in seafloor sediments along the Arabian Sea and the Andaman Sea continental shelves: First insight on the occurrence, identification, and characterization,” Mar Pollut Bull, vol. 167, Jun. 2021. [CrossRef]
- J. Bimali Koongolla, A. L. Andrady, P. B. Terney Pradeep Kumara, and C. S. Gangabadage, “Evidence of microplastics pollution in coastal beaches and waters in southern Sri Lanka,” Mar Pollut Bull, vol. 137, pp. 277–284, Dec. 2018. [CrossRef]
- A. M. A. I. K. Athapaththu et al., “Plastics in surface water of southern coastal belt of Sri Lanka (Northern Indian Ocean): Distribution and characterization by FTIR,” Mar Pollut Bull, vol. 161, Dec. 2020. [CrossRef]
- B. S. Mayoma, C. Sørensen, Y. Shashoua, and F. R. Khan, “Microplastics in beach sediments and cockles (Anadara antiquata) along the Tanzanian coastline,” Bull Environ Contam Toxicol, vol. 105, no. 4, pp. 513–521, Oct. 2020. [CrossRef]
- J. Mu et al., “Microplastics abundance and characteristics in surface waters from the Northwest Pacific, the Bering Sea, and the Chukchi Sea,” Mar Pollut Bull, vol. 143, pp. 58–65, Jun. 2019. [CrossRef]
- S. Wang et al., “Microplastic abundance, distribution and composition in the mid-west Pacific Ocean,” Environmental Pollution, vol. 264, Sep. 2020. [CrossRef]
- Z. Pan et al., “Microplastics in the Northwestern Pacific: Abundance, distribution, and characteristics,” Science of the Total Environment, vol. 650, pp. 1913–1922, Feb. 2019. [CrossRef]
- X. Wang, C. Li, K. Liu, L. Zhu, Z. Song, and D. Li, “Atmospheric microplastic over the South China Sea and East Indian Ocean: abundance, distribution and source,” J Hazard Mater, vol. 389, May 2020. [CrossRef]
- A. M. G. A. D. Athawuda, H. B. Jayasiri, G. G. N. Thushari, and K. P. G. K. P. Guruge, “Quantification and morphological characterization of plastic litter (0.30–100 mm) in surface waters of off Colombo, west coast of Sri Lanka,” Environ Monit Assess, vol. 192, no. 8, Aug. 2020. [CrossRef]
- J. Li, C. Green, A. Reynolds, H. Shi, and J. M. Rotchell, “Microplastics in mussels sampled from coastal waters and supermarkets in the United Kingdom,” Environmental Pollution, vol. 241, pp. 35–44, Oct. 2018. [CrossRef]
- H. Taherdoost, “Sampling Methods in Research Methodology; How to Choose a Sampling Technique for Research,” 2016. [Online]. Available: https://ssrn.com/abstract=3205035.
- J. Park, S. Hong, W. J. Shim, J. S. Khim, and J. Park, “Distribution, compositional characteristics, and historical pollution records of microplastics in tidal flats of South Korea,” Mar Pollut Bull, vol. 189, 2023. [CrossRef]
- M. Cocca, E. Di Pace, M. E. Errico, G. Gentile, A. Montarsolo, and R. Mossotti, “Springer Water Proceedings of the International Conference on Microplastic Pollution in the Mediterranean Sea.” [Online]. Available: http://www.springer.com/series/13419.

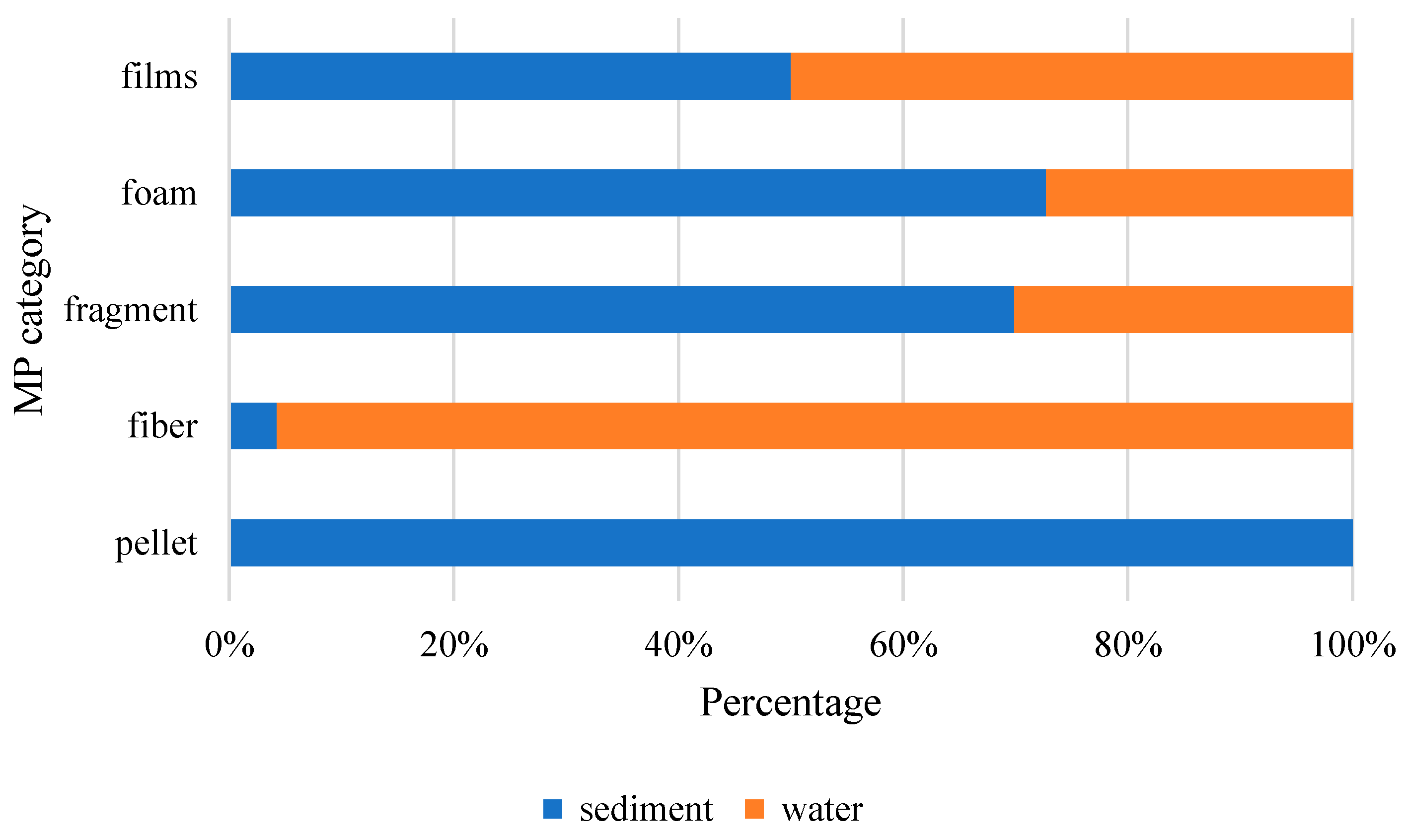
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




