1. Introduction
Peripheral nerve defects often occur after trauma or tumor resection, which require reconstruction to prevent loss of motor function and sensation. Autologous nerve transplantation is considered the most effective method for recovery; however, its use is limited by the availability of donor tissue and potential donor site morbidity [
1,
2,
3,
4]. Allografts have been identified as an effective alternative [
5,
6,
7], with studies demonstrating that treated allografts can restore nerve function comparably to autografts in animal models [
8,
9]. However, the scarcity of donors prompts the exploration of xenogeneic nerve transplantation as a substitute, which encounters challenges, particularly immune rejection [
10,
11].
To address the aforementioned, strategies such as administering immunosuppressants or reducing antigenicity have been considered [
12]. However, the systemic side effects of immunosuppressants often negate the benefits of nerve transplantation [
13]. Alternative approaches involve various methods including cold preservation, freeze-thawing, and chemically treated nerves. The detergent-based decellularization, which removes cells and myelin that act as donor antigens, has been shown to effectively diminish immunogenicity and is widely used [
14].
Therefore, this study aimed to assess whether applying a detergent-based method to xenograft nerves would decrease antigenicity, thereby rendering xenograft nerve transplantation as effective as allograft transplantation. The experimental approach involved harvesting the sciatic nerve of a rabbit to create an acellular xenograft, as well as chemical processing. This graft was then transplanted into a 1-cm gap in a sciatic nerve of a rat to reconstruct the defect. The outcomes, including axon regeneration and functional recovery, were evaluated via immunohistochemical and electrophysiological analyses, visual observations, and motor function tests, and compared with those of negative and positive control groups. The previous findings related to acellular nerve allografts demonstrate the effective repair of nerve defects, which can exhibit characteristics similar to those of nerve xenografts owing to their structure.
Additionally, the rationale behind decellularization is that the extracellular matrix (ECM) components are highly conserved across different species and do not provoke significant immune responses [
15].
The present methodology was formulated based on the hypothesis that such nerve xenografts would yield favorable results because their structure exhibits characteristics similar to those of nerve allografts, and is well established by various methods.
2. Materials and Methods
2.1. Subjects & Methods
2.1.1. Experimental Subjects
This study received approval from the Institutional Animal Care and Use Committee (IACUC). Group 1 (n = 4) consisted of subjects whose xenogeneic nerves were transplanted after decellularizing using detergent. Serving as the negative control, Group 2 (n = 7) included subjects whose nerves were severed without repair of the defect. Group 3 (n = 5) functioned as the positive control group. Additionally, an allograft control group was established, wherein grafts were harvested from one rat and immediately transplanted into another. For the xenograft group, 10-mm nerve segments were collected from New Zealand White Rabbits (body weight: 4-5 kg) and used as grafts for male Sprague-Dawley rats weighing 250-300 g.
2.1.2. Experimental methods
a) Xenogeneic nerve pre-processing process
A New Zealand White Rabbit was intramuscularly injected with Ketamine-HCL (40 mg/kg). Subsequently, the surgical area was locally anesthetized using lidocaine, which contained 1:100,000 epinephrine. An incision of approximately 1 cm in length was made in the skin from the buttocks to the posterior thigh. Dissection of the biceps femoris muscle exposed the sciatic nerve. A 2-cm segment of the nerve was removed, and the remaining ends were trimmed to a length of 1 cm under sterile conditions. Subsequently, the nerve was treated using Sondell’s protocol, which involves a decellularization process using 3% Triton X- 100 and 4% sodium deoxycholate. This method removes the cellular components, leaving only the extracellular matrix (ECM), which supports axonal growth.
The harvested sciatic nerve was initially immersed in distilled water for 7 h, which was followed by a 12-h immersion in 3% Triton X-100, and then a 24 h immersion in 4% sodium deoxycholate. This process was repeated with another 12-h immersion in 3% Triton X-100, and a subsequent 24-h immersion in 4% sodium deoxycholate. Finally, the nerve was thoroughly rinsed in PBS with agitation and stored in Roswell Park Memorial Institute (RPMI) 1640 medium at 3-4℃ for one week.
b) Immediate transplantation of nerve allografts for the positive control group
After administering anesthesia via intraperitoneal injection of Ketamine-HCL (5 mg/kg) and local anesthesia of the nerve area with Lidocaine (containing epinephrine 1:100,000), an incision was made in the thigh to expose the sciatic nerve. A 1-cm defect was created in the sciatic nerve, after which the animal was sacrificed.
c) Reconstruction using the sciatic nerve xenograft
After administering anesthesia in the same manner as indicated in method b) above, the following three groups were established: Group 1 (n = 4) consisted of the decellularized xenograft nerve transplant group; Group 2 (n = 7) served as a negative control group, involving amputation with no repair of the defect; and Group 3 (n = 4) functioned as a positive control group, using an allograft obtained directly from one rat and immediately transplanted into another. Following resection of a 1-cm segment of the sciatic nerve in the recipient area, transplantation was performed. This procedure utilized a 10-0 Ethylon suture for both the experimental and positive control groups; all sutures were performed under microscopic observation. Ibuprofen (30 mg/kg) was intraperitoneally administered to manage postoperative pain.
2.2. Evaluation of nerve regeneration
1) Assessment of Motor Function for Evaluating Nerve Regeneration
To perform a “Walking Track Analysis”, the extent of nerve regeneration was evaluated by analyzing the footprints made as the subjects traversed an acrylic tunnel measuring 20 x 12 x 200 cm. The results were quantified using the Sciatic Function Index (SFI), which is a specific formula designed for functional assessment. The SFI is derived from three parameters measured during the experiment, which compares the experimental and non-experimental sides using an established equation. An SFI score approaching –100 indicates complete impairment, whereas a score near 0 suggests normal function.
a) Incorporation of the SFI into the equation:
i) Parameters used during the measurement
① Distance from the heel to the top of the third toe
(print length; PL)
② Distance between the first and fifth toes (toe spread; TS)
③ Distance between the second and fourth toes
(mid-toe spread; IT)
ii) For the analysis, the parameters from the non-tested
(normal) feet were designated as NPL, NTS, and NIT, whereas
those from the tested feet were labeled EPL, ETS, and EIT,
respectively.
iii) The parameters were computed using the following
equation developed:
SFI = (-38.3 x ① PLF) + (109.5 x ② TSF) + (13.3 X ③ ITF) -8.8
① PLF = (EPL-NPL)/NPL
② TSF = (ETS-NTS)/NTS
③ ITF = (EIT-NIT)/NIT
Figure 1.
Values based on the footprints of the rat were substituted into the formula.
Figure 1.
Values based on the footprints of the rat were substituted into the formula.
b) Statistical methods
To objectively assess the extent of nerve regeneration, the SFI was calculated for all the samples. Group 1 (n = 4) consisted of the decellularized xenograft nerve grafts, whereas Group 2 (n = 7) served as the negative control, where the defect remained unrepaired. Group 3 (n = 5) was categorized as the positive control and the allograft group, which involves the immediate transplantation of a nerve from one rat to another. The homogeneity of variance was ensured before conducting measurements to compare the mean differences among the groups at each time point. The data analysis involved one-way analysis of variance (ANOVA) to identify differences across the groups. The Scheffe method was employed for post hoc testing. Following data organization, statistical analyses were conducted using SPSS software (version 22.0, IBM, USA). The results are presented as the mean ± standard deviation, with p < 0.05 indicating a statistically significant level of difference.
2) Visual observation
Adhesion between the nerve and surrounding tissue including the alterations in the gloss and color of the nerve were observed.
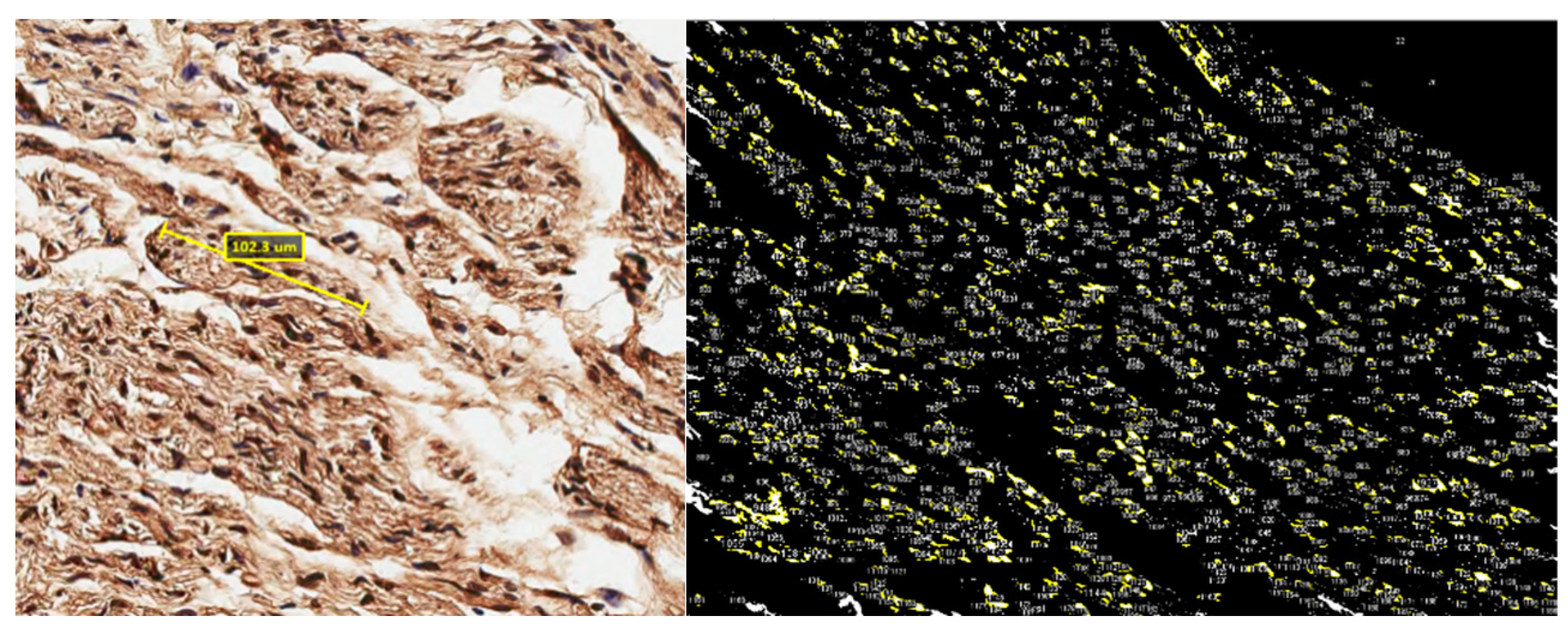
3) Immunohistochemical analysis using Images stained with S-100
-Staining with immunohistochemical factor S-100
The samples were segmented into proximal, graft, and distal sections. Each section was collected and stained with s-100. The distribution of the Schwann cell nuclei per area was quantified using ImageJ software. A one-way ANOVA was conducted to determine if there were significant differences in the means across the groups.
Figure 2.
Harvested nerves were dissected into proximal, graft, and distal parts before immunohistochemical staining.
Figure 2.
Harvested nerves were dissected into proximal, graft, and distal parts before immunohistochemical staining.
Figure 3.
Images stained with s-100 were calculated by the actual ratio of the image and computed the area occupied by the nuclei counted.
Figure 3.
Images stained with s-100 were calculated by the actual ratio of the image and computed the area occupied by the nuclei counted.
4) Motor-Nerve Conduction Study
Peripheral nerve stimulation was administered at the proximal end of the peripheral nerve, and the resulting compound muscle action potentials (CMAPs) from the innervated muscles were recorded, which were used to assess the maximum peak amplitude, latency, and conduction velocity. The iWorx IX-TA-220 system facilitated these measurements.
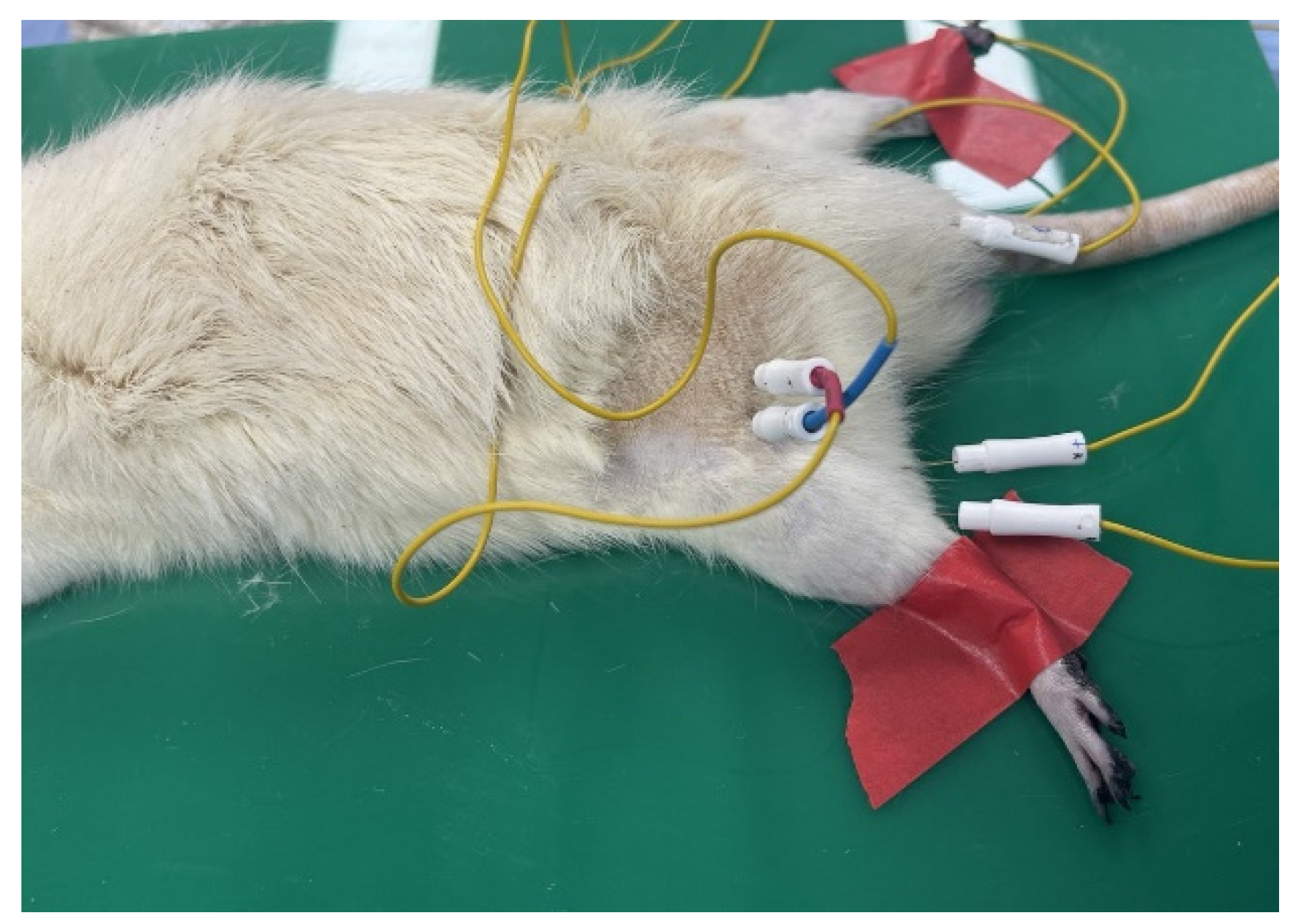
Anesthesia was administered via an intraperitoneal injection of Ketamine–HCl (5 mg/kg). Subsequently, after removing its hair, the rat was secured in a supine position for the procedure. After administering Lidocaine (1:10 local anesthetic with epinephrine) to anesthetize the nerve area, an incision in the thigh was made to expose the sciatic nerve.
The electrode configuration was as follows: Electrode 1 was used for the bipolar stimulation (both positive and negative), Electrode 2 was used for bipolar recording (both positive and negative), Electrodes 3 and 4 were used for recording at the muscle belly, and Electrode 5 served as the ground electrode positioned at the tail.
Electrodes 1 and 2 were placed 1 cm apart at the nerve reconstruction site, whereas Electrodes 3 and 4 were positioned subcutaneously in the non-muscle area.
Figure 4.
Rat fixation in the supine position and insertion of each stimulating and recording electrode needle.
Figure 4.
Rat fixation in the supine position and insertion of each stimulating and recording electrode needle.
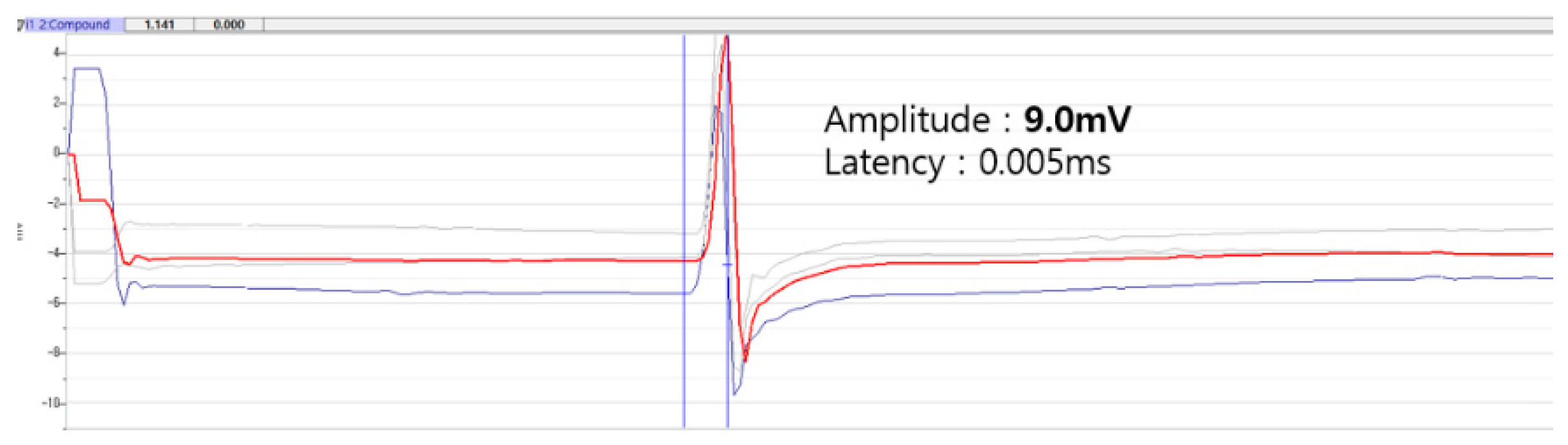
Figure 5.
Recording and analysis of motor nerve conduction study.
Figure 5.
Recording and analysis of motor nerve conduction study.
The nerve was then stimulated on both the normal and surgical sides for comparison. Stimulation occurred at identical locations relative to the recording site, specifically proximal and distal to the suture site.
Following a 16-week period, the CMAPs were recorded, including the measurements of both the latency and amplitude. The nerve was stimulated at intensities ranging from 2 to 5 V to elicit maximum CMAPs in the muscle, which was repeated three times. Latency indicates the rate of axon depolarization, whereas amplitude indicates the number of motor units activated. The motor-nerve conduction velocity was determined by dividing the distance (mm) between the proximal and distal stimulation sites by the difference in latency (ms), thus representing the maximal conduction velocity of the fastest nerve fibers.
An electromyography of the non-operated sciatic nerve was performed to establish normal control values for comparison. Paired-sample t-tests were utilized to assess the interaction between the normal and operated sides of each group in terms of the compound muscle action potentials, including the amplitude, latency, and conduction velocity. All the data are presented as the mean ± standard deviation, with p < 0.05 indicating statistically significant differences between groups.
3. Results
3.1. Motor Function Examination for Evaluating Nerve Regeneration
Table 1.
Intergroup mean and standard deviation of sciatic functional index (*p < .05, **p < .01, ***p < .001).
Table 1.
Intergroup mean and standard deviation of sciatic functional index (*p < .05, **p < .01, ***p < .001).
| The postoperative state date |
Group |
n |
Mean |
Standard
deviation |
F |
p |
Scheffe |
| 3 months |
1(a) |
4 |
-72.9 |
7.292 |
4.715* |
0.015 |
b<a<c |
| 2(b) |
7 |
-82.35 |
4.984 |
| 3(c) |
5 |
-63.28 |
8.073 |
| 2 months |
1(a) |
4 |
-73.29 |
6.688 |
7.037** |
0.003 |
b<a<c |
| 2(b) |
7 |
-83.75 |
7.548 |
| 3(c) |
5 |
-63.35 |
9.985 |
| 1 month |
1(a) |
4 |
-79.45 |
10.553 |
0.641 |
0.6 |
- |
| 2(b) |
7 |
-83.07 |
7.026 |
| 3(c) |
5 |
-78.62 |
8.7 |
| 4 weeks |
1(a) |
4 |
-61.75 |
10.2 |
4.446* |
0.019 |
b<c<a |
| 2(b) |
7 |
-78.05 |
4.611 |
| 3(c) |
5 |
-70.01 |
10.157 |
| 3 weeks |
1(a) |
4 |
-61.03 |
6.746 |
2.024 |
0.151 |
- |
| 2(b) |
7 |
-70.63 |
11.738 |
| 3(c) |
5 |
-76.12 |
8.703 |
| 2 weeks |
1(a) |
4 |
-61.52 |
7.211 |
2.248 |
0.128 |
- |
| 2(b) |
7 |
-75.44 |
11.167 |
| 3(c) |
5 |
-79.58 |
11.861 |
| at 1 week |
1(a) |
4 |
-53.43 |
11.534 |
2.858 |
0.07 |
- |
| 2(b) |
7 |
-66.61 |
12.243 |
| 3(c) |
5 |
-76.85 |
5.704 |
A one-way ANOVA was performed to evaluate whether significant differences existed in the mean sciatic nerve index between the different groups over time. The analysis revealed significant differences at four weeks (F = 4.446, p < 0.05), two months (F = 7.037, p < 0.01), and three months (F = 4.715, p < 0.05) post operation. No significant differences were found at one month, three weeks, two weeks, and one week post operation. A subsequent post-hoc analysis using Scheffe’s test indicated that at four weeks post operation, Group 1 had a significantly higher mean sciatic nerve index than that of Group 2. At two and three months post operation, Group 3 demonstrated a significantly higher mean compared to Group 2.
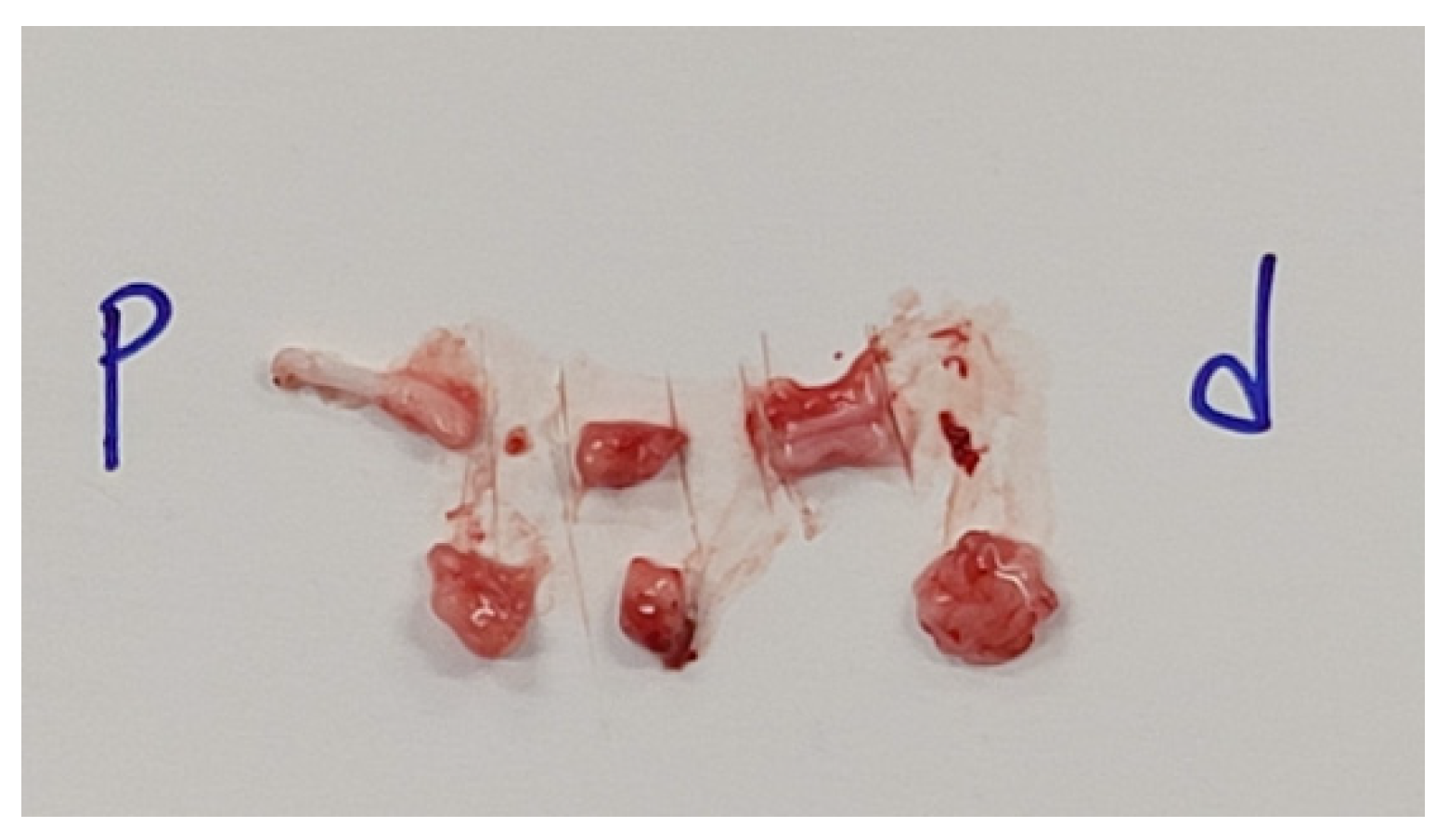
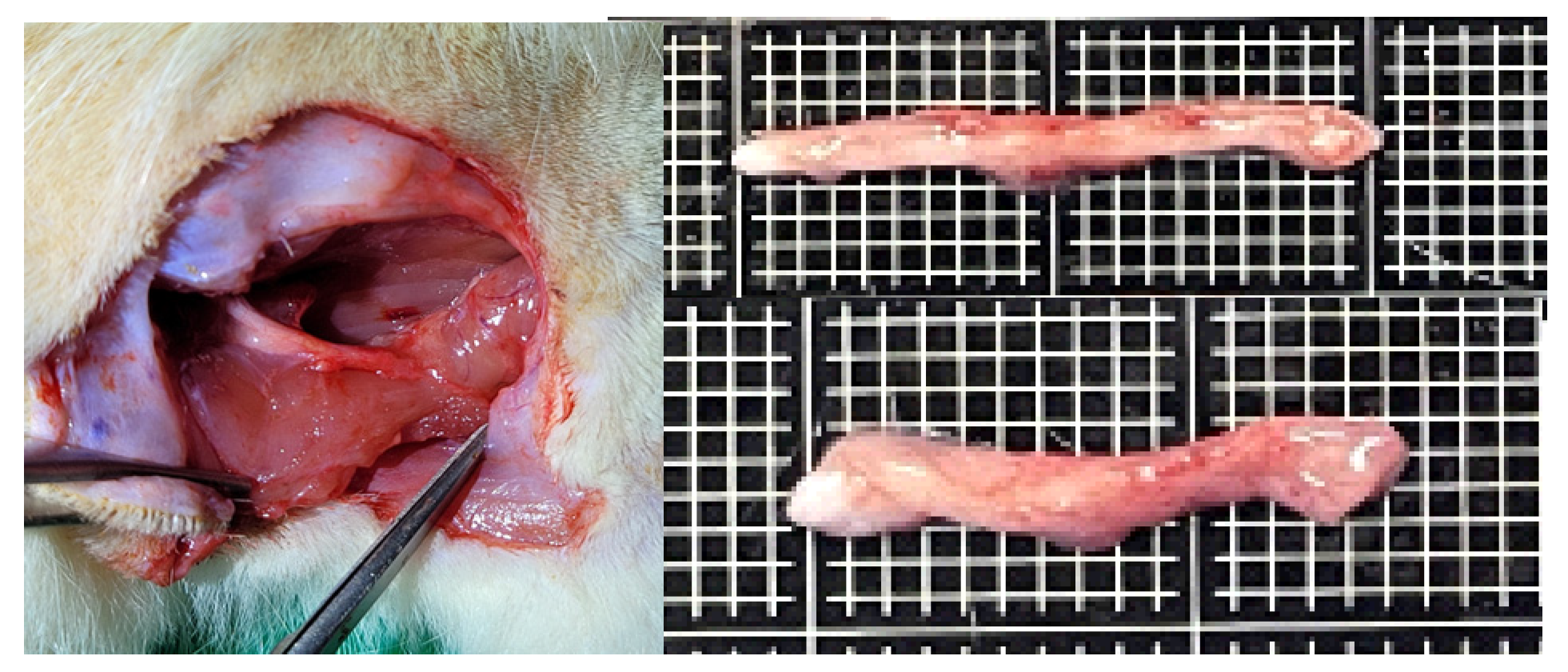
3.2. Visual observation
Except for the negative control group, there was no adhesion with the surrounding tissue. Both the positive control and experimental groups retained their original color and gloss.
Figure 6.
Appearance of the nerve harvested and dissected after sacrificing the rats for visual observation.
Figure 6.
Appearance of the nerve harvested and dissected after sacrificing the rats for visual observation.
3.3. Immunohistochemical Analysis Using S-100
Table 2.
Distribution mean and standard deviation of Schwann cells in each group via S-100 immunohistochemical staining.
Table 2.
Distribution mean and standard deviation of Schwann cells in each group via S-100 immunohistochemical staining.
Dependent
variable |
Group |
n |
Mean(%) |
Standard deviation |
F |
p |
Scheffe |
Distribution of Schwann
cell |
1
(Proximal) |
4 |
3.34 |
1.80 |
0.908 |
0.514 |
- |
1
(Graft) |
4 |
4.16 |
2.62 |
1
(Distal) |
4 |
4.99 |
2.52 |
2
(Proximal) |
6 |
4.02 |
2.51 |
2
(Distal) |
4 |
3.89 |
1.27 |
3
(Proximal) |
5 |
4.16 |
4.01 |
3
(Graft) |
5 |
3.89 |
1.07 |
3
(Distal) |
5 |
6.93 |
2.90 |
Statistical analysis revealed no significant differences within the groups. The results from the Schwann cell transplant test were as follows:
Table 3.
Distribution mean and standard deviation of Schwann cells in grafts of Groups 1 and 3 via immunohistochemical staining using S-100.
Table 3.
Distribution mean and standard deviation of Schwann cells in grafts of Groups 1 and 3 via immunohistochemical staining using S-100.
| Dependent variable |
Group |
n |
Mean |
Standard deviation |
F |
p |
Scheffe |
| Distribution of Schwann cell |
1
(Graft) |
4 |
4.16 |
2.62 |
0.044 |
0.839 |
- |
3
(Graft) |
5 |
3.89 |
1.07 |
The immuno-stained results in the proximal, graft, and distal segments were analyzed. The Schwann cells in the graft segment were similar for the experimental (E) (Schwann cell % area : 4.16±2.62) and positive control (PC) groups (Schwann cell % area : 3.86±1.07).
3.4. Motor Nerve Conduction Study
1) Comparison of the amplitude during the nerve conduction study
E-group
Table 4.
Comparison of the amplitude during the nerve conduction study in the experimental group.
Table 4.
Comparison of the amplitude during the nerve conduction study in the experimental group.
| Amplitude |
n |
Mean |
Standard
deviation |
t |
p |
| Normal side |
4 |
5.53 |
7.49 |
1.05 |
0.353 |
| Operated side |
4 |
1.7 |
1.38 |
b) PC-group
Table 5.
Comparison of the amplitude during the nerve conduction study in the positive control group.
Table 5.
Comparison of the amplitude during the nerve conduction study in the positive control group.
| Amplitude |
n |
Mean |
Standard
deviation |
t |
p |
| Normal side |
5 |
3.73 |
3.35 |
0.42 |
0.715 |
| Operated side |
5 |
2.58 |
1.49 |
The experimental group displayed an average amplitude of 5.53 ± 7.49 on the normal side and 1.7 ± 1.38 on the surgical side. Conversely, the positive control group had an average amplitude of 3.73 ± 3.35 on the normal side and 2.58 ± 1.49 on the surgical side. No significant differences were observed between the normal and surgical sides across the groups (p > 0.05). On average, the amplitude in the positive control group (M = 2.58) was higher than that in the experimental group (M = 1.38).
2) Comparison of latency during the nerve conduction study
a) E-group
Table 6.
Comparison of Latency during the nerve conduction study in the experimental group.
Table 6.
Comparison of Latency during the nerve conduction study in the experimental group.
| Latency |
n |
Mean |
Standard Deviation |
t |
P |
| Normal side |
4 |
0.24 |
0.05 |
-1 |
0.37 |
| Operated side |
4 |
0.25 |
0.05 |
Table 7.
Comparison of Latency during the nerve conduction study in the positive control group.
Table 7.
Comparison of Latency during the nerve conduction study in the positive control group.
| Latency |
n |
Mean |
Standard
Deviation |
t |
p |
| Normal side |
5 |
0.18 |
0.05 |
1 |
0.423 |
| Operated side |
5 |
0.16 |
0.02 |
b) PC-group
In the E-group, the mean latency values were 0.24 ± 0.05 ms on the normal side and 0.25 ± 0.05 ms on the surgical side. For the PC-group, the mean latency values were 0.18 ± 0.05 ms on the normal side and 0.16 ± 0.02 ms on the surgical side. No significant differences were observed between the normal and surgical sides within each group (p > 0.05). However, a comparison of the mean values revealed that the experimental group (M = 0.25 ms) exhibited a slower latency compared to the positive control group (M = 0.16 ms), with the positive control group demonstrating the fastest latency among the groups.
3) Comparison of the conduction velocity during the nerve conduction study
a) E-group
Table 8.
Comparison of the conduction velocity during the nerve conduction study in the experimental group.
Table 8.
Comparison of the conduction velocity during the nerve conduction study in the experimental group.
| Conduction velocity (m/s) |
n |
Mean |
Standard Deviation |
t |
p |
| Normal side |
4 |
8.64 |
1.86 |
1 |
0.37 |
| Operated side |
4 |
8.24 |
1.7 |
Table 9.
Comparison of the conduction velocity during the nerve conduction study in the positive control group.
Table 9.
Comparison of the conduction velocity during the nerve conduction study in the positive control group.
| Conduction velocity (m/s) |
n |
Mean |
Standard Deviation |
t |
p |
| Normal side |
5 |
11.53 |
3.05 |
-1 |
0.423 |
| Operated side |
5 |
11.2 |
1.9 |
b) PC-group
In the experimental group, the mean nerve conduction velocity was 8.64 ± 1.86 ms on the normal side and 8.24 ± 1.7 ms on the surgical side. For the positive control group, these values were 11.53 ± 3.05 ms and 12.2 ± 1.90 ms, respectively. A statistical analysis revealed no significant differences between the normal and surgical sides within each group (p > 0.05). However, a comparison of the group means indicated that the experimental group (M = 8.64) demonstrated a faster conduction velocity than that of the positive control group (M = 11.53)
4. Discussion
The optimal treatment for peripheral nerve regeneration is autologous nerve transplantation [
16]. However, this method is constrained by the limited availability of suitable donor nerves and the potential sensory loss resulting from the sacrifice of the donor nerve. Therefore, research is being conducted to address these challenges. Consequently, investigations regarding allogeneic transplantations are also essential. However, the primary concern with this approach is the immune rejection response against the donor tissue [
17].
Efforts to mitigate immune responses against allogeneic nerve grafts have been explored; however, the scarcity of these grafts demands the consideration of xenogeneic nerve transplantation. Consequently, this study employs a well-established decellularization technique using surfactants on xenogeneic nerves. Following Sondell's protocol [
18], nerve tissue from rabbits was repeatedly treated with water, Triton X-100, and sodium deoxycholate (SDC). This method effectively removes most cellular components, including myelin and Schwann cells. However, owing to the potential toxicity of residual detergents, the treated nerves were extensively rinsed with agitation in PBS to eliminate cytotoxicity. Subsequently, the processed xenogeneic nerves were preserved in RPMI media at 4 °C for one week prior to the transplantation. This preservation method was selected based on studies comparing various nerve preservation strategies, which demonstrated that storage at 4 °C for up to seven days in RPMI media not only maintains biomechanical properties such as the Young’s modulus and tensile strength, but also preserves the structural and ECM composition, including laminin, collagen, and sGAG, as reported by the RPMI [
19].
The xenogeneic nerve harvested from rabbits, as previously described, was used to reconstruct a 10-mm defect in the sciatic nerve of recipient rats. Both positive and negative control groups were established to assess the extent of nerve regeneration. Various methods were employed to confirm peripheral nerve regeneration. Morphological methods involve the observation and analysis of changes in the nerve cells, peripheral nerve fibers, and nerve terminals, either by counting or measuring these structures. Electromyography (EMG) measures the nerve conduction velocity or assesses changes in the compound action potentials to evaluate the nerve regeneration and functional recovery.
A biochemical analysis includes the examination of components such as the myelin sheath and neurofilaments to assess nerve degeneration and regeneration. Functional testing methods, which measure the responses elicited by stimulating experimental animals, are also utilized. These diverse methods [
20] are selected based on their respective strengths and purposes; when combined, they complement one another, leading to more accurate interpretations [
21]. Consequently, in this study, motor function testing, immunohistochemical examinations, visual observations, and nerve conduction testing were conducted.
Motor function testing included a footprint analysis conducted at intervals of 1, 2, 3, and 4 weeks, and subsequently at 1, 2, and 3 months. Up to the 4th week, the experimental group statistically demonstrated significantly higher regeneration values. However, by the 2nd and 3rd months, the group receiving allogenic transplantation exhibited the highest regeneration values. Although concerns regarding the applicability and limitations of the SFI have been presented, it remains the most commonly employed functional test. Shenaq et al. reported discrepancies between the SFI, histological findings, and clinical observations, indicating potential inconsistencies [
22].
Despite gross and histological observations indicating that nerve regeneration was comparable to or less than that observed in the positive control group, the SFI values remained consistently low across all groups. This discrepancy is consistent with findings from another study [
23], which reported a limited functional recovery of motor nerves following nerve transplantation. This may have resulted from the significant deformity and paralysis of the operated paw, typically characterized by a dropped foot and clawing of the toes. Based on the SFI values; the minimal recovery can be primarily attributed to the deterioration of gait patterns resulting from axotomy and denervation, which is consistent with the findings from other studies [
14]. The unsatisfactory results following peripheral nerve repair have led to the efforts of gaining a better understanding of the molecular and cellular processes involved in nerve regeneration [
24].
Second, Schwann cells create a conducive environment for nerve regeneration following peripheral nerve injury, which is essential for the success of non-cellular nerve allografts that rely on Schwann cell proliferation [
25]. These cells provide a bioactive matrix that supports axonal migration and secrete molecules that influence axonal growth, thereby serving as significant prognostic indicators for assessing nerve regeneration [
26]. However, the process of decellularization, which is used to prevent rejection, eliminates all cellular components, including Schwann cells, from the peripheral nerves [
27].
Several studies [
5,
18,
28] have detected remnants of Schwann cells in tissues using S-100 staining following detergent-based decellularization, revealing negligible staining in regions where Schwann cells had been removed. Considering that nerve regeneration primarily occurs via the extension of Schwann cell processes rather than axonal growth [
29], monitoring the migration of host Schwann cells toward transplanted grafts post-transplantation can offer valuable insights into the dynamics of regeneration and recovery. Consequently, in this study, nerve tissues were segmented into proximal, graft, and distal sections and analyzed using S-100 immunohistochemistry. The proximal and distal segments were excised approximately 2 mm from the nerve stump towards the graft site.
In a study conducted by Sondell [
18], Schwann cells were not detected within the grafts at 5 days post-operation; however, intense S-100 positive staining was noted at the proximal and distal ends of the nerve stumps. Similarly, the timing of Schwann cell detection varies with the experimental animal used. Previous research [
30] indicates that in rats, Schwann cell proliferation is observable from the third week, with a pronounced proliferation of regenerative fibers evident between 10 to 14 weeks. Consequently, animals were sacrificed at 16 weeks for conducting the immunohistochemical analysis. In this study, the results confirmed that Schwann cells extended throughout the graft, reflecting the pattern of regenerated axons, as demonstrated by the positive S-100 staining. Note, the experimental group demonstrated that staining for the S-100 positive cells was comparable to the positive control group, suggesting that Schwann cell proliferation is similar. Previous research regarding xenogeneic cell transplantation [
31] has demonstrated that Schwann cells are capable of migrating to regenerate axons and form myelin sheaths in both xenogeneic transplantation and non-cellular nerve graft groups. However, these studies reported a lower number of Schwann cells in these groups compared to those in autologous transplantation, although the specific levels of the S-100 positive cell staining were not presented. Additionally, conventional approaches for evaluating nerve recovery after peripheral nerve injury and repair, such as electrophysiology and histomorphometry, which are commonly used in studies regarding neural regeneration, may not consistently align with the restoration of the motor and sensory functions [
32]. Therefore, quantifying the distribution of Schwann cells using S-100 staining, and expressing this quantification as a percentage, is considered valuable. It may suggest that other factors affect both the function and morphology.
Third, nerve conduction studies were conducted to assess the function of the nervous system within the scope of electro-diagnostic testing, which aimed to elucidate the patho-physiological status of nerves. Although the methods used to assess nerve regeneration may not always correlate precisely with the recovery of nerve function, they provide the benefits of objectivity and quantifiability with minimal error. This process involves the application of electrical stimulation to designated regions of peripheral nerves and the subsequent recording of the resulting action potentials in nerves or muscles to identify abnormalities in the nerve conduction pathways.
Measurements typically include the latency, amplitude, velocity, duration, morphology, and area of the action potentials, which are compared against established normal ranges for assessment. Nerve conduction studies are performed on motor, sensory, and mixed nerves [
33]. Sensitive recording techniques are essential for assessing disease progression and particularly the effectiveness of treatments in nerve injury models. The needle electrodes for the measurements were employed based on prior research [
34], which facilitates a rapid, reproducible evaluation of nerves, while avoiding axonal loss or desiccation commonly associated with conventional methods. Note, animal nerve conduction studies do not follow the stringent protocols observed in human studies, and various techniques for conducting these studies have been reported in literature.
In this experiment, motor nerve conduction studies (MNCS) were performed in a manner similar to those conducted on humans. A supramaximal stimulation was applied to the proximal segment of the peripheral nerve, and the resulting CMAPs were recorded from the muscles that the nerve innervates, such as the gastrocnemius muscle. Supramaximal stimulation is defined as the level of stimulation at which further increases in the stimulus intensity do not produce an increase in the amplitude of the CMAP.
After inserting the needle electrodes into the animal muscles and conducting a weak contraction test, the intensity of the muscle contractions increased. As this intensity progressively increased, the firing frequency of the motor units also accelerated. With a sustained strong contraction, interference effects emerged as additional motor units joined the contraction, which hindered the distinct identification of waveform shapes.
Therefore, we analyzed the waveform shapes, focusing on parameters such as the amplitude, latency, and conduction velocity. The amplitude, which reflects the number of conducting fibers, decreases in cases of axonal loss. Latency, defined as the time elapsed from the stimulation to the onset of the evoked response, represents the duration required for the fastest nerve fibers to respond.
The conduction velocity was derived by segmenting the distance between the stimulation and recording sites by latency, which serves as a critical indicator of the myelin sheath integrity in nerve conduction studies. In cases of segmental demyelination, the conduction velocity decreases owing to the disruption of saltatory conduction in demyelinated fibers. Although the control group consistently exhibited faster conduction velocities compared with those of the experimental group, the differences were not statistically significant (p > 0.05).