1. Introduction
NPs are a distinct class of material distinguished by their small size and high surface-to-volume ratio. They have many applications in electronics, catalysis, medicine, and energy [
1,
2,
3,
4]. The most common approaches for producing nanoparticles are chemical methods (bottom-up techniques), physical methods (top-down techniques), and biological methods (extracts of active biological substances) [
5,
6]. Chemical methods offer precise control of nanoparticle size, shape and composition [
7] but may involve toxic solvents and reducing agents [
8,
9]. Physical methods create stable nanoparticles with great crystallinity at large scales, but they are energy intensive and require specialized equipment and facilities [
10]. Biological methods using organisms such as bacteria have environmental benefits but are harder to control and unsuitable for large-scale synthesis [
11,
12].
The green synthesis of metallic nanoparticles using natural resources such as plant extracts, microorganisms, and minerals as reducing and stabilizing agents is an environmentally friendly and cost-effective alternative to conventional chemical methods [
13,
14]. The green synthesis of gold and silver nanoparticles involves the reduction of metal ions present in solution to their metallic form by reducing agents. The size and shape of the nanoparticles can be controlled by adjusting the reaction conditions, such as the temperature, time, and concentration of the reactants [
15,
16,
17]. The major advantages of the green synthesis of gold and silver nanoparticles include particle stability, improved biological activity, cost effectiveness and eco-friendliness [
18,
19,
20,
21,
22,
23]. However, green synthesis methods need to overcome some related problems, including a lack of standardization, reproducibility, and scalability [
13,
24,
25], if they are to be applied at scale, for example, in point-of-care diagnosis.
Approximately 75% of women experience at least one episode of vaginal candidiasis [
26], a fungal infection caused by the overgrowth of Candida species in the vaginal area. This fungal infection leads to symptoms such as itching, burning and discharge, which can be mild to severe depending on the degree of the infection [
27]. Furthermore, studies have suggested that vaginal candidiasis may increase the risk of sexually transmitted infections due to changes in the vaginal microbiome [
28]. Recurrent vulvovaginal candidiasis (RVVC) is defined as four or more episodes of vaginal candidiasis within a year; this disease is difficult to manage and may require prolonged antifungal therapy, leading to increased healthcare costs and medication-related side effects [
27,
29]. According to a systematic review published in The Lancet Infectious Diseases journal, the global burden of RVVC is estimated to be approximately 138 million cases annually [
29].
The most common causal agent for vaginal candidiasis is
Candida albicans (CA), although it can be caused by other Candida species, such as
Candida glabrata and
Candida tropicalis . CA features a cell wall that consists of an inner skeletal layer containing chitin and β-glucan ((1→3)-β-D-glucan and (1→6)-β-D-glucan), as well as an outer layer containing highly glycosylated mannoprotein, which consists of approximately 84% water-insoluble (1→3)-β-D-glucan [
30,
31]. The detection of (1→3)-β-D-glucans has shown good overall performance and high sensitivity (80-90%) in patients with candidiasis [
32,
33]. The glucan-specific Limulus amebocyte lysate (LAL) (G-test) is the most widely used method for (1→3)-β-D-glucan measurement and has a sensitivity of 1–10 pg/ml. However, this platform requires high-quality samples and may be prone to false-positive results [
31]. Using the systematic evolution of ligands by exponential enrichment (SELEX) technique, the AD1 aptamer is a DNA oligonucleotide that specifically binds to the (1→3)-β-D-glucans on the cell wall of CA with high affinity [
31]. In addition, AD1-conjugated nanoparticles have been demonstrated to bind to a variety of morphological forms of CA, including yeast cells, germ tubes, hyphal biofilms and extracellular matrix material [
31,
34]. Therefore, this promising ligand was chosen for CA detection in this study.
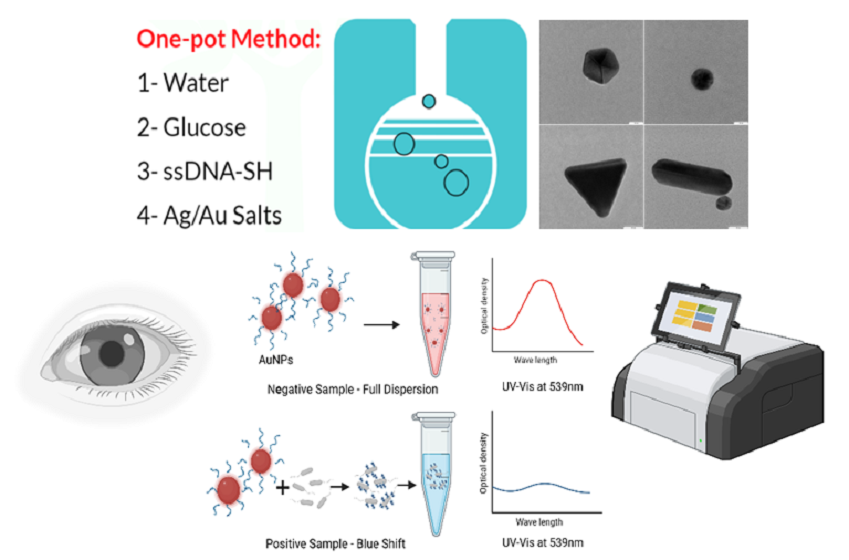
In this study, we used a novel combination of green chemical synthesis and modern nanotechnology processes to create gold nanoparticles for use as a straightforward solution for the early detection of vaginal candidiasis.
Figure 1 shows the working principle for detecting
Candida albicans (CA) in vaginal fluid via the green synthesis of gold nanoparticles (GNPs) functionalized with the anti-(1→3)-β-D-glucan (BDG) single-stranded DNA aptamer (ssDNA). This technology provides a more sustainable and environmentally friendly alternative to standard chemical synthesis methods, and it also produces metallic nanoparticles in a more efficient and cost-effective manner combined with high stability, reproducibility, and scalability.
Unlike some existing diagnostic methods for treating vaginal candidiasis, this approach is straightforward and accessible to a broader spectrum of healthcare providers. This approach overcomes these limitations by offering improved accuracy and sensitivity and rapid results, potentially outperforming traditional culture-based methods. Moreover, this noninvasive method, which requires minimal vaginal fluid sampling, holds promise for enhancing the speed and precision of CA infection diagnosis, ultimately facilitating prompt treatment initiation and improved patient care. This approach aims to overcome the limitations of the current detection methods while being simple to use and not requiring any additional training or knowledge.
2. Materials and Methods
2.1. Reagents, Microbiology Culture, and Apparatus
Auric chloride (Gold(III) chloride trihydrate–Tetra chloroauric acid, HAuCl4.3H2O, MW=393.83) and Tween-20 were obtained from Merck and prepared according to the manufacturer’s specifications. D-Glucose (MW=180.16) and NaOH (MW=40.00) were purchased from Merck Pty. Ltd., an affiliate of Merck KGaA, Darmstadt, Germany. Milli-Q water (≥18 MΩ·cm) was used for all reagent preparations. Candida albicans and Botrytis cinerea species were purchased from American Type Culture Collection (ATCC), Virginia, USA, and cultured following recommended protocols.
Thiol-modified single-stranded DNA (ssDNA-SH) anti- (1,3)-β-d-glucan (BDG) aptamer was purchased from Integrated DNA Technologies (IDT) and subjected to reduction as per the recommended procedure prior to usage. The detailed aptamer sequence was 5’-ThioMC6-D- GCG GAA TTC GAA CAG TCC GAG CCC ACA CGT GTG AGA AGG GTG TTA TCA TGT ATT TCG TGT TCC TTT CGT CAT TCC TTT GTC TGG GGT CAA TGC GTC ATA GGA TCC CGC -3’ [
31].
A vaginal fluid mimicking solution [
35] was prepared from the recipe used by Owen and Katz (1999). The solution consisted of NaCl (MW=58.44), KOH (MW=56.11), Ca(OH)
2 (MW=74.09), bovine serum albumin, lactic acid (L+) (MW=90.08), glacial acetic acid (MW=60.05), glycerol (MW=92.09), urea (MW=60.06) and D-glucose monohydrate (MW=198.17). The combined reagents were diluted to a total volume of 1 L with Milli-Q® water, and the pH of the solution was adjusted to 4.2 using HCl. The solution was then vacuum filtered, and UV sterilized before being aliquoted for later use.
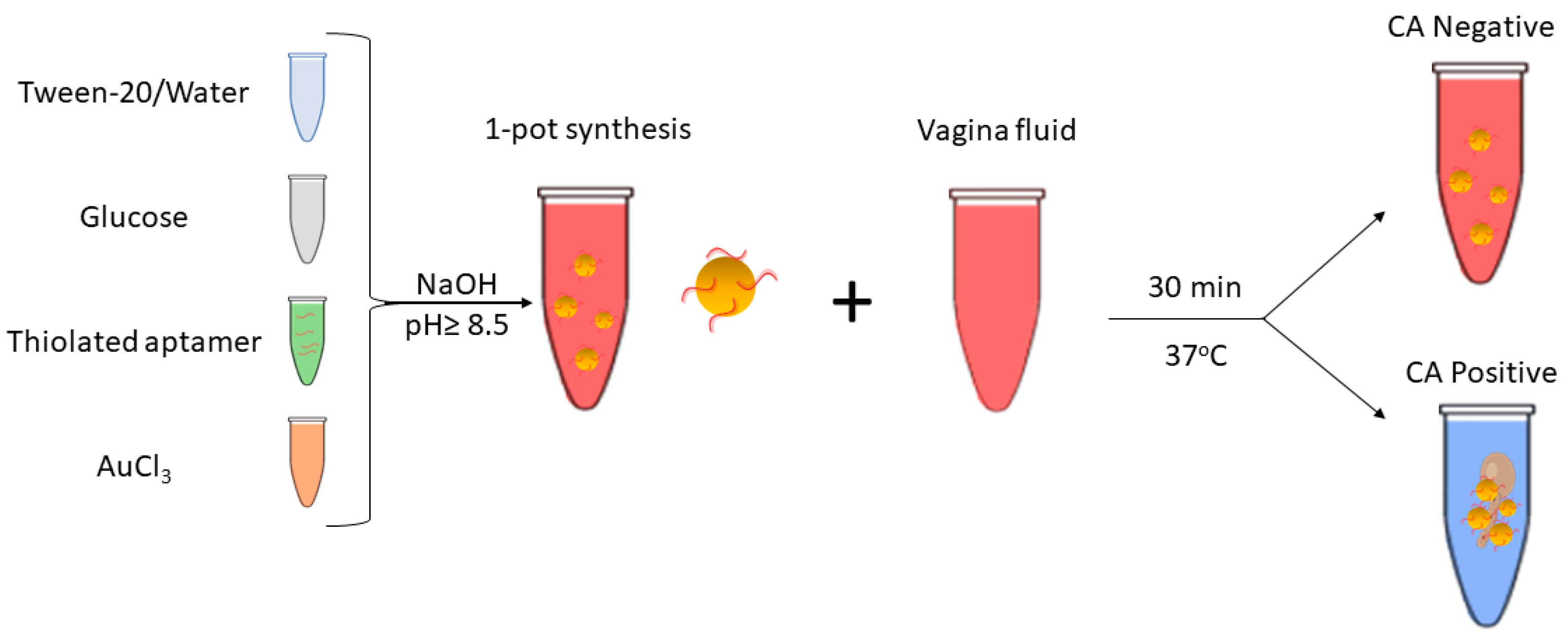
2.2. Instant Green Synthesis of Functional Ultrastable Gold Nanoparticles (USGNPs)
To functionalize the nanoparticles with the ssDNA-SH aptamer, 10 mL of particles were made within a glass vial according to the following protocol. First, 4,400 μL of 0.01% Tween-20 H2O was added to a glass vial, followed by the addition of 600 μL of 10 mM AuCl3 (gold(III) chloride). Then, 2,500 μL of 100 mM glucose was added, followed by the addition of 2,500 μL of 50 nM of the ssDNA-SH (anti-BDG) aptamer. The aptamer solution was prepared by mixing 1.25 μL of 100 μM aptamer stock solution with 2,498.75 μL of 0.01% Tween-20 H2O solution. The glass vial was placed onto a magnetic stirrer for 5 minutes at 500 rpm at room temperature, after which a strong reducing agent (sodium hydroxide solution 1.0 M) was added to form GNPs, for a final pH ≥8.5 for instant generation of a shiny pink solution of USGNP. The synthesized solution was further mixed at room temperature for an additional 5 minutes using a magnetic stirrer at 500 rpm to produce a high yield of uniformly sized, stable nanoparticles.
2.3. UV‒Vis Spectroscopy
The absorption of the ssDNA-SH aptamer-functionalized GNPs was characterized using UV‒visible spectroscopy (NanoDrop ND-1000 spectrophotometer, Thermo Scientific, MA, USA). Two microliters of particle sample was pipetted onto the measurement pedestal. Spectral measurements were then performed, monitoring the absorbance values at wavelengths ranging from 220 nm to 800 nm.
2.4. Particle Size and Zeta Potential Measurements Using Dynamic Light Scattering (DLS)
The hydrodynamic size and surface charge of the NPs were examined using dynamic light scattering (DLS) on an Anton Paar LiteSizer DLS Particle Size Analyzer. To determine the particle size of the functionalized GNPs, 100 µL of the nanoparticles were suspended in 900 µL of water and placed in a LiteSizer for analysis using a disposable cuvette (10×10×45 mm). A volume of 1 mL of the same mixture was measured in an omega cuvette utilizing the Zeta measurement mode for the Zeta potential analysis.
2.5. Particle Size Analysis and Concentration Determination via Nanoparticle Tracking Analysis (NTA)
Nanoparticle tracking analysis (NTA) was also used to compare the hydrodynamic diameter of the nanoparticles based on Brownian motion. For this purpose, 100 µL of nanoparticles was suspended in 900 µL of water, and the mixture was subsequently transferred to a 1-mL syringe on a NanoSight syringe pump. The measurements were obtained at 25°C with a violet laser (405 nm) on a Malvern Panalytical NanoSight NS300 instrument.
2.6. Particle Size and Shape Analysis by Scanning Electron Microscopy (SEM)
A TESCAN Mira3 scanning electron microscope at 10 kV was used to characterize the size and shape of the GNPs. The sample was dispersed onto a conductive substrate and sputter-coated with a thin layer of platinum.
2.7. Particle Size and Shape Analysis via Transmission Electron Microscopy (TEM)
To characterize the size and shape of the GNPs, a high-resolution imaging capability of a transmission electron microscope (TEM), specifically a JEOL 2100 operating at 200 kV, was employed.
2.8. Colorimetric Detection Assay of CA
As a nonspecific control, we used the fungus
Botrytis cinerea, which grows on and infects grapes. This fungus presents a problem within the wine industry because it produces the β-1,3-1,6-glucan polysaccharide that aggregates in the presence of ethanol in wine and blocks filtration during filtration [
36]. This fungal strain was selected as a control to rule out false-positive results due to its overexpression of the β-1,3-1,6-glucan polysaccharide.
For the detection assays, three setups were prepared: a blank sample (vaginal simulant mimic), a nonspecific control fungus (Botrytis cinerea), and a target fungus CA suspended in vaginal fluid mimicking solution. A total of 100 μL of the aptamer-functionalized nanoparticles was mixed with 100 μL of vaginal fluid mimicking solution and 50 μL of sample. All reaction tubes were incubated at 37°C for 30 minutes before UV‒Vis spectroscopy analysis.
3. Results and Discussion
3.1. Instant Green Synthesis and Characterization of Functional Ultrastable Gold Nanoparticles (USGNPs)
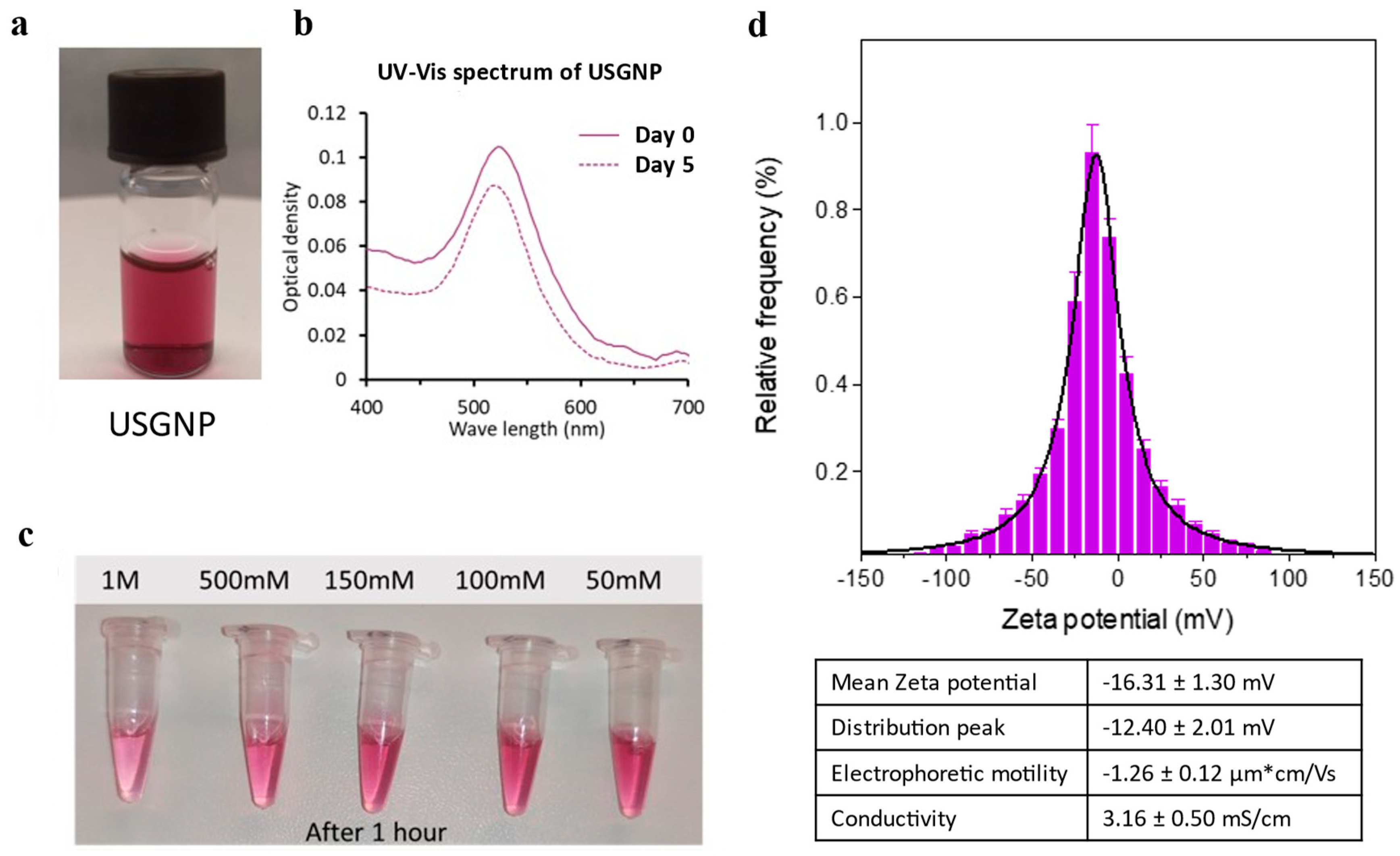
A unique green chemical technique was employed to synthesize anti-BDG aptamer (ssDNA-SH)-functionalized GNPs from an auric chloride solution, thereby obviating the necessity for citrate (which requires high-temperature reactions) or sodium borate (known for its toxicity). The novel particles, designated ultrastable gold nanoparticles (USGNPs), were formed instantly upon the addition of a reducing agent (
Figure 2A and Supplemental Video S1).
UV‒Vis spectroscopy measurements indicated that the novel nanoparticles had a peak at 541 nm, and the peak optical density decreased only slightly after 5 days at 4°C (
Figure 2B). More importantly, for various applications where a physiological saline concentration is required to mimic biological conditions, USGNPs were able to withstand up to 1 M NaCl for at least 1 hour (
Figure 2C). Furthermore, the zeta potential of the USGNPs in physiological PBS was measured via electrophoretic light scattering. The particles were observed to be slightly anionic, with a mean zeta potential of -16.31±1.30 mV and a distribution peak of -12.40 ± 2.01 mV (
Figure 2D). Notably, the zeta potential of human plasma falls within the range of -18 mV to -20 mV [
37,
38], indicating that USGNPs may be suitable for in vivo applications. Additionally, the electrophoretic mobility of the particles was -1.16±0.12 µm*cm/Vs, while the conductivity was 3.16±0.50 mS/cm. These data indicated a high degree of particle stability.
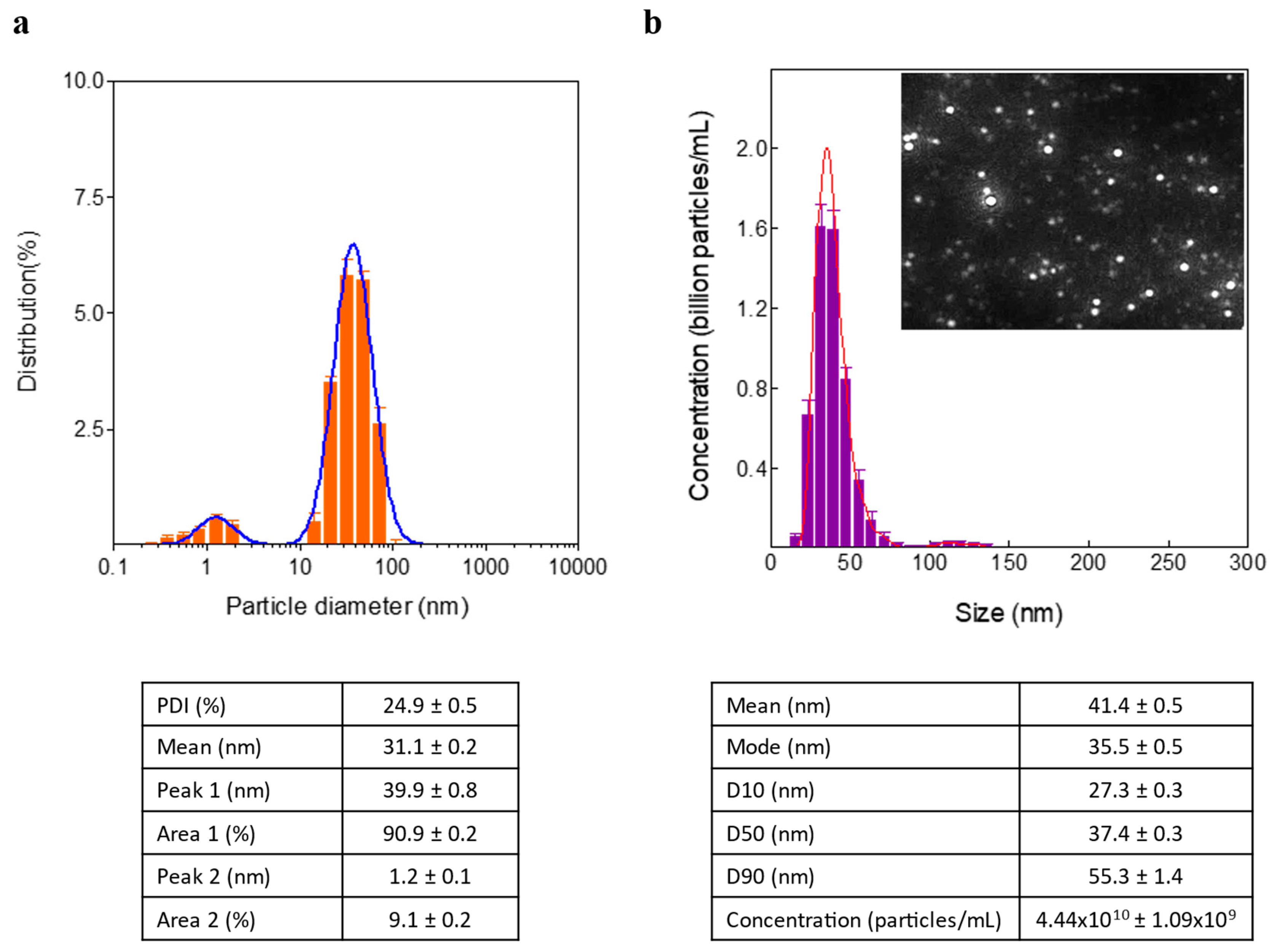
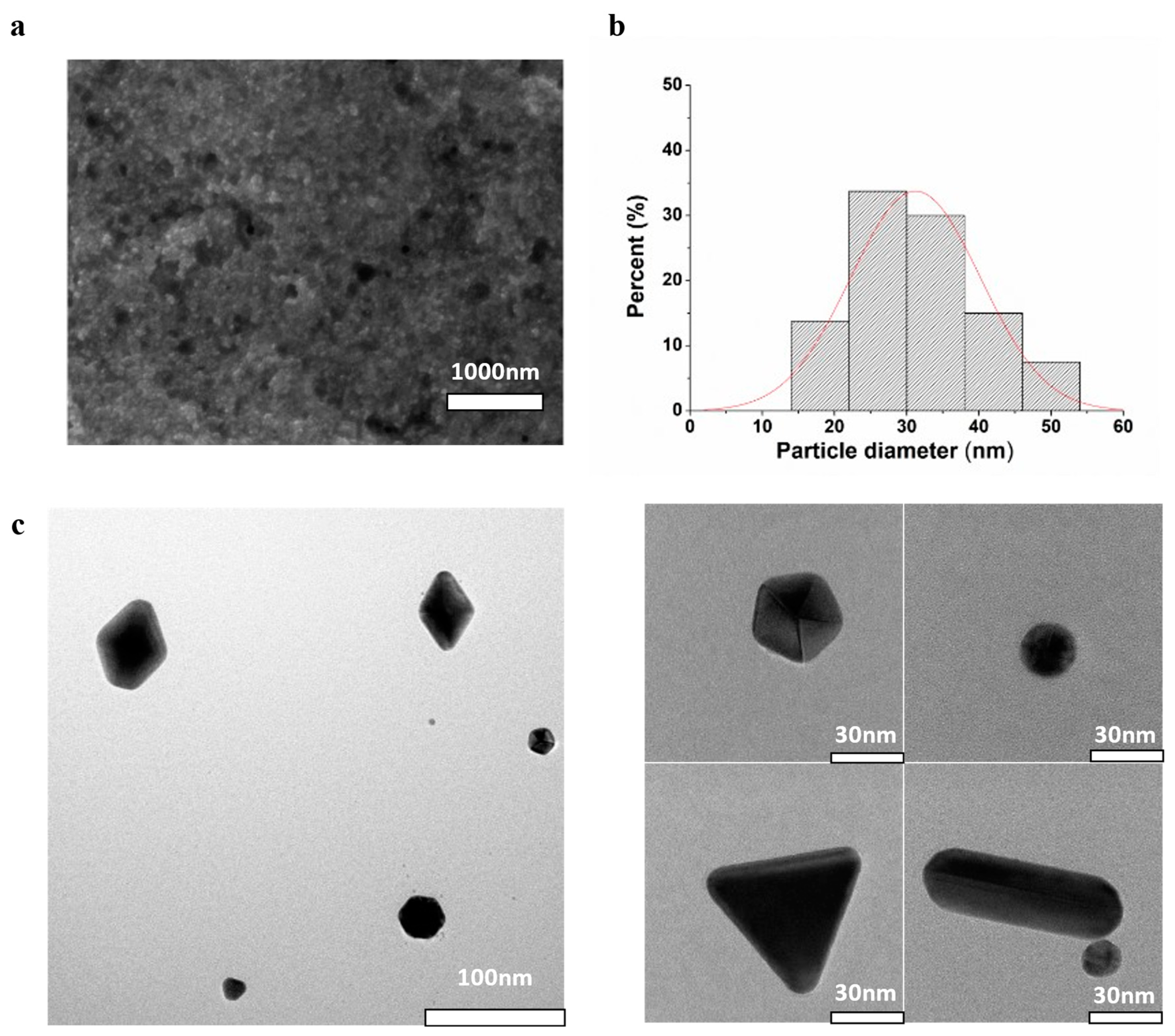
DLS analysis revealed that the mean hydrodynamic diameter of the USGNPs was 31.1±0.2 nm, with a polydispersity index of 24.9±0.5% (
Figure 3A). However, there were 2 populations of nanoparticles with peak hydrodynamic diameters of 39.9±0.8 nm and 1.2±0.1 nm. Therefore, nanoparticle tracking analysis (NTA) was employed to further investigate the size distribution of the USGNPs because of its greater resolution for determining the particle size distribution. The NTA results confirmed the dominance of the population of particles, with sizes ranging from 20 to 100 nm and an average size of approximately 41.4±0.5 nm (
Figure 3B).
Consistent with the findings from DLS and NTA, SEM analysis indicated that the USGNPs exhibited monodispersity with a diameter of 31.2±9.0 nm (
Figure 4A,B). However, observing the detailed morphology of USGNPs using HR-SEM was challenging due to the tiny size of the particles. Therefore, HR-TEM was utilized to achieve a more comprehensive understanding of the particle size, shape, and orientation. HR-TEM images showed that our USGNPs had a variety of morphologies, including nanostars, nanospheres, nanoprisms and nanorods (
Figure 4C). These images depicted distinct characteristics and intriguing crystallographic orientations of the particles, furnishing insights into their growth mechanisms and surface properties.
3.2. Candida Albicans Detection Assay
The effectiveness of the synthesized USGNPs as a detection tool for
Candida albicans (CA) was evaluated within a vaginal discharge-mimicking solution to test the stability of the particles while mimicking clinically relevant conditions. The vaginal discharge mimicking solution was first introduced by Owen and Katz in 1999 based on an intensive review of the composition of human vaginal fluid [
35]. Glycerol was incorporated to mimic the lubricating properties of the vagina [
39], and the medium was designed to mimic vaginal pH, ionic strength and other factors. of healthy, nonpregnant and premenopausal women.
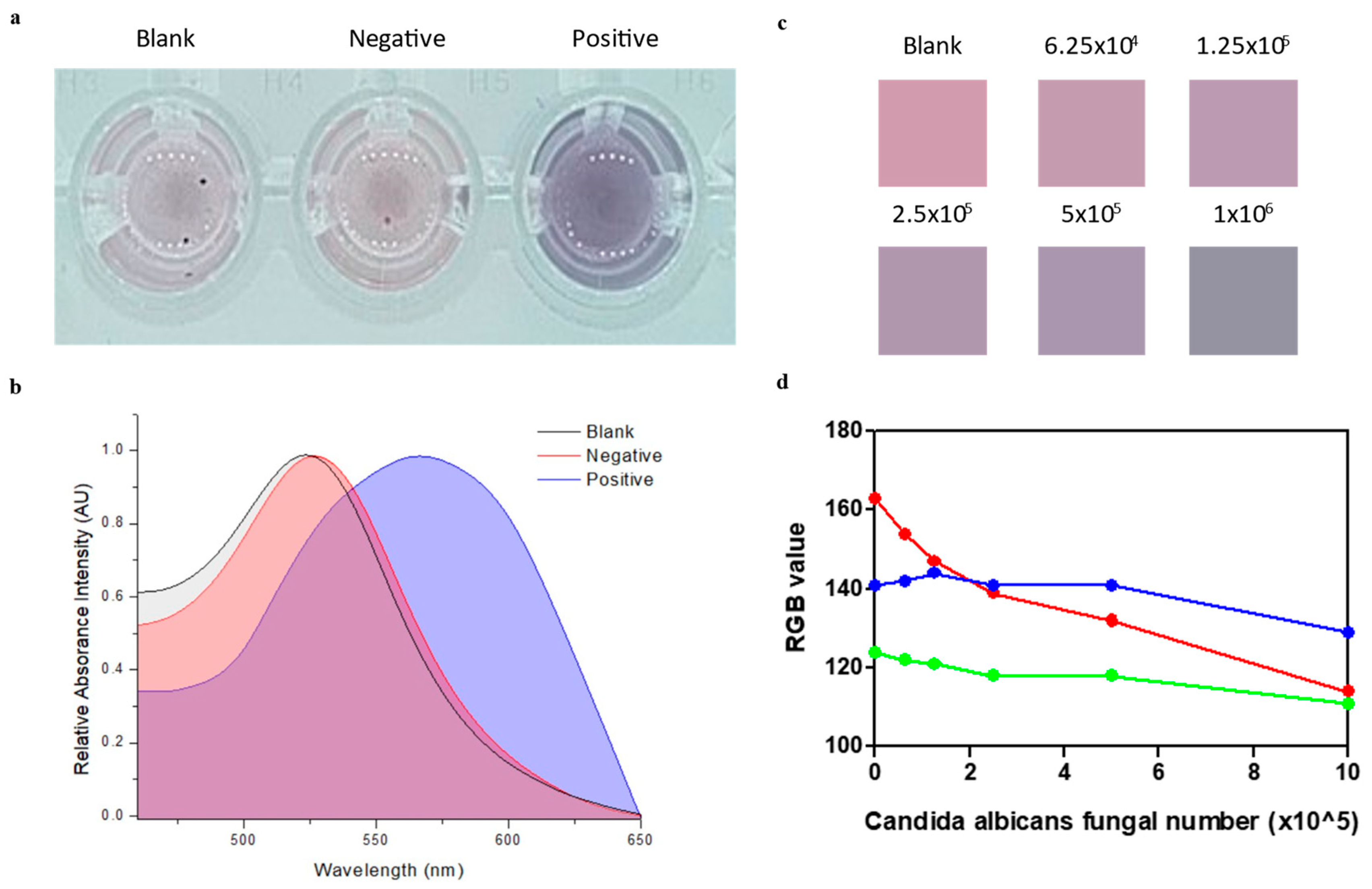
Figure 5 shows that the USGNPs were capable of specifically detecting CA in the vaginal discharge-mimicking solution. After 1 h of incubation at 37°C, while the negative control and blank samples maintained their original pink colors, only the positive samples developed a blue color due to the aggregation of the GNPs (
Figure 5A). UV‒Vis confirmed the visual observations by revealing a shift in the peak absorbance from 520 nm to 570 nm (
Figure 5B).
It is important to acknowledge the limitations of our colorimetric naked-eye platform when working with the quantification of serially diluted samples (
Figure 5C). Nevertheless, we could still observe changes in color from all the prepared
Candida albicans samples, even when as few as 6.25x10
4 fungal cells were present. An approach for quantification in colorimetric detection is to convert the color information into RGB values and construct a calibration curve of analyte concentrations [
40]. As shown in
Figure 5D, although the blue and green intensities fluctuated, there was a downward trend in the red intensity as the concentration of
C. albicans decreased.
4. Conclusions
In this study, we successfully developed a novel and environmentally friendly approach for the green synthesis of ultra-stable gold nanoparticles (USGNPs) functionalized with anti-(1→3)-β-D-glucan (BDG) aptamers. The synthetic pathway that was employed, utilizing ssDNA-SH aptamers as smart and biologically functional capping agents, allowed rapid (10 minutes) and high-yield (10 mL) synthesis of USGNPs. This innovative method created functional nanoparticles with exceptional stability, repeatability, and scalability while avoiding high-temperature or hazardous chemicals. The USGNPs exhibited remarkable stability, as demonstrated by UV‒Vis spectroscopy, which indicated consistent peak absorbance over several days of storage. The nanoparticles displayed resilience against high salt concentrations, further enhancing their suitability for various applications.
The successful evaluation of USGNPs as a detection tool for Candida albicans in a clinically relevant vaginal fluid-mimicking solution represents a significant advancement in the field of biomedical diagnostics. These findings provide valuable insights into the applicability of USGNPs for the detection of Candida albicans, a common fungal pathogen that causes vaginal infections. Furthermore, this detection method provides a practical and sustainable solution that could significantly improve the lives of many women by enabling early and precise detection, which is critical for effectively treating and controlling pregnancy conditions. To ensure the reliability and validity of the CA detection assay, the colorimetric results of a positive sample (Candida albicans), negative control (Botrytis cinerea), and blank (vaginal fluid mimic solution) were compared. The distinct colorimetric results observed in the positive samples for the (1,3)-β-d-glucan target of Candida albicans confirmed the effectiveness of USGNPs in detecting the presence of the pathogen. Conversely, negligible particle agglomeration and a visually persistent color were obtained for both the negative control and blank samples, further validating the specificity of our detection method.
The versatility of USGNPs, combined with their biocompatibility and ease of functionalization, make them ideal candidates for diagnostic assays in diverse clinical settings. The integration of USGNPs as a detection tool holds immense potential for improving the diagnosis and monitoring of various pathogens and is not limited to Candida albicans. Moreover, this green synthetic approach not only minimizes environmental impact but also enables the production of biologically functionalized nanoparticles, opening new possibilities for their integration into various biomolecular detection platforms and paving the way for the development of stable, rapid, sensitive, specific, and portable diagnostic devices.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
CRediT Authorship Contribution Statement
Mohamed Sallam: Conceptualization, Methodology, Writing – original draft , Writing – review & editing, Supervision, Project administration, Investigation, Formal analysis, Data curation. Kimberley Clack: Writing – original draft, Conceptualization. Ema Romão: Investigation, Formal analysis, Data curation. Cong Minh Nguyen: Formal analysis, Visualization, Data curation. Amandeep Singh Pannu: Formal analysis, Data curation. Tanzena Tanny: Investigation, Data curation. Frank Sainsbury: Writing – review & editing, Supervision, Data curation. Nam-Trung Nguyen: Supervision, Writing – review & editing. Pieter De Pauw: Conceptualization, Formal analysis. Nick Devoogdt: Supervision, Conceptualization. Nobuo Kimizuka: Supervision, Writing – review & editing. Serge Muyldermans: Supervision, Conceptualization, Writing – review & editing, Methodology, Project administration.
Data Availability
Data will be made available on request
Acknowledgements
This work was supported by the Griffith University Higher Degree Research Scholarship. The authors acknowledge the facilities and scientific and technical assistance provided by the Central Analytical Research Facility (CARF), the Institute of Health and Biomedical Innovation (IHBI), and The Queensland University of Technology (QUT). Graphical abstract figure was created with BioRender.com.
Declaration of Competing Interest
The authors declare no conflict of interest.
References
- Anselmo AC, Mitragotri S. Nanoparticles in the clinic: An update. Bioengineering & translational medicine. 2019; 4: e10143.
- Khan I, Saeed K, Khan I. Nanoparticles: Properties, applications and toxicities. Arabian journal of chemistry. 2019; 12: 908-31.
- Bundschuh M, Filser J, Lüderwald S, McKee MS, Metreveli G, Schaumann GE, et al. Nanoparticles in the environment: where do we come from, where do we go to? Environmental Sciences Europe. 2018; 30: 1-17.
- Sharma D, Kanchi S, Bisetty K. Biogenic synthesis of nanoparticles: a review. Arabian journal of chemistry. 2019; 12: 3576-600.
- Mourdikoudis S, Pallares RM, Thanh NT. Characterization techniques for nanoparticles: comparison and complementarity upon studying nanoparticle properties. Nanoscale. 2018; 10: 12871-934.
- Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nature Reviews Drug Discovery. 2021; 20: 101-24.
- Khodashenas B, Ghorbani HR. Synthesis of silver nanoparticles with different shapes. Arabian Journal of Chemistry. 2019; 12: 1823-38.
- Mittal D, Kaur G, Singh P, Yadav K, Ali SA. Nanoparticle-based sustainable agriculture and food science: Recent advances and future outlook. Frontiers in Nanotechnology. 2020; 2: 579954.
- Sani A, Cao C, Cui D. Toxicity of gold nanoparticles (AuNPs): A review. Biochemistry and biophysics reports. 2021; 26: 100991.
- Usman KAS, Maina JW, Seyedin S, Conato MT, Payawan Jr LM, Dumée LF, et al. Downsizing metal–organic frameworks by bottom-up and top-down methods. NPG Asia Materials. 2020; 12: 58.
- Pearce AK, Wilks TR, Arno MC, O’Reilly RK. Synthesis and applications of anisotropic nanoparticles with precisely defined dimensions. Nature Reviews Chemistry. 2021; 5: 21-45.
- Yaqoob AA, Umar K, Ibrahim MNM. Silver nanoparticles: various methods of synthesis, size affecting factors and their potential applications–a review. Applied Nanoscience. 2020; 10: 1369-78.
- Salem SS, Fouda A. Green synthesis of metallic nanoparticles and their prospective biotechnological applications: an overview. Biological trace element research. 2021; 199: 344-70.
- Zhang D, Ma X-l, Gu Y, Huang H, Zhang G-w. Green synthesis of metallic nanoparticles and their potential applications to treat cancer. Frontiers in Chemistry. 2020; 8: 799.
- Chandra H, Kumari P, Bontempi E, Yadav S. Medicinal plants: Treasure trove for green synthesis of metallic nanoparticles and their biomedical applications. Biocatalysis and Agricultural Biotechnology. 2020; 24: 101518.
- Singh A, Gautam PK, Verma A, Singh V, Shivapriya PM, Shivalkar S, et al. Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: A review. Biotechnology Reports. 2020; 25: e00427.
- Rana A, Yadav K, Jagadevan S. A comprehensive review on green synthesis of nature-inspired metal nanoparticles: Mechanism, application and toxicity. Journal of Cleaner Production. 2020; 272: 122880.
- Gour A, Jain NK. Advances in green synthesis of nanoparticles. Artificial cells, nanomedicine, and biotechnology. 2019; 47: 844-51.
- Ijaz I, Gilani E, Nazir A, Bukhari A. Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chemistry Letters and Reviews. 2020; 13: 223-45.
- Agarwal H, Shanmugam V. A review on anti-inflammatory activity of green synthesized zinc oxide nanoparticle: Mechanism-based approach. Bioorganic chemistry. 2020; 94: 103423.
- Agarwal H, Nakara A, Shanmugam VK. Anti-inflammatory mechanism of various metal and metal oxide nanoparticles synthesized using plant extracts: A review. Biomedicine & Pharmacotherapy. 2019; 109: 2561-72.
- Ishak NM, Kamarudin S, Timmiati S. Green synthesis of metal and metal oxide nanoparticles via plant extracts: an overview. Materials Research Express. 2019; 6: 112004.
- Dikshit PK, Kumar J, Das AK, Sadhu S, Sharma S, Singh S, et al. Green synthesis of metallic nanoparticles: Applications and limitations. Catalysts. 2021; 11: 902.
- Rónavári A, Igaz N, Adamecz DI, Szerencsés B, Molnar C, Kónya Z, et al. Green silver and gold nanoparticles: Biological synthesis approaches and potentials for biomedical applications. Molecules. 2021; 26: 844.
- Reguera J, Langer J, de Aberasturi DJ, Liz-Marzán LM. Anisotropic Metal Nanoparticles for Surface-Enhanced Raman Scattering. Colloidal Synthesis of Plasmonic Nanometals. 2020: 713-54.
- Phillips NA, Rocktashel M, Merjanian L. Ibrexafungerp for the Treatment of Vulvovaginal Candidiasis: Design, Development and Place in Therapy. Drug Design, Development and Therapy. 2023: 363-7.
- Denning DW, Kneale M, Sobel JD, Rautemaa-Richardson R. Global burden of recurrent vulvovaginal candidiasis: a systematic review. The Lancet infectious diseases. 2018; 18: e339-e47.
- De Seta F, Lonnee-Hoffmann R, Campisciano G, Comar M, Verstraelen H, Vieira-Baptista P, et al. The vaginal microbiome: III. The vaginal microbiome in various urogenital disorders. Journal of Lower Genital Tract Disease. 2022; 26: 85.
- Sobel JD. Recurrent vulvovaginal candidiasis. American journal of obstetrics and gynecology. 2016; 214: 15-21.
- Hua Y, Hu F, Ren X, Xiong Y, Hu J, Su F, et al. A novel aptamer-G-quadruplex/hemin self-assembling color system: rapid visual diagnosis of invasive fungal infections. Annals of Clinical Microbiology and Antimicrobials. 2023; 22: 1-15.
- Tang X-L, Hua Y, Guan Q, Yuan C-H. Improved detection of deeply invasive candidiasis with DNA aptamers specific binding to (1→ 3)-β-D-glucans from Candida albicans. European Journal of Clinical Microbiology & Infectious Diseases. 2016; 35: 587-95.
- Alexander BD, Smith PB, Davis RD, Perfect JR, Reller LB. The (1, 3) β-D-glucan test as an aid to early diagnosis of invasive fungal infections following lung transplantation. Journal of clinical microbiology. 2010; 48: 4083-8.
- Odabasi Z, Mattiuzzi G, Estey E, Kantarjian H, Saeki F, Ridge RJ, et al. β-D-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clinical Infectious Diseases. 2004; 39: 199-205.
- Hou Y, Yang M, Li J, Bi X, Li G, Xu J, et al. The enhancing antifungal effect of AD1 aptamer-functionalized amphotericin B-loaded PLGA-PEG nanoparticles with a low-frequency and low-intensity ultrasound exposure on C. albicans biofilm through targeted effect. NanoImpact. 2021; 21: 100275.
- Owen DH, Katz DF. A vaginal fluid simulant. Contraception. 1999; 59: 91-5.
- Jadhav SB, Gupta A. Studies on application of β-1, 3 glucanase in the degradation of glucans produced by Botrytis cinerea and inhibition of fungal growth. Biocatalysis and agricultural biotechnology. 2016; 7: 45-7.
- Nkanga CI, Chung YH, Shukla S, Zhou J, Jokerst JV, Steinmetz NF. The in vivo fate of tobacco mosaic virus nanoparticle theranostic agents modified by the addition of a polydopamine coat. Biomaterials Science. 2021; 9: 7134-50.
- Bondar OV, Saifullina D, Shakhmaeva I, Mavlyutova I, Abdullin T. Monitoring of the zeta potential of human cells upon reduction in their viability and interaction with polymers. Acta Naturae (англoязычная версия). 2012; 4: 78-81.
- Tietz K, Klein S. Simulated genital tract fluids and their applicability in drug release/dissolution testing of vaginal dosage forms. Dissolut. Technol. 2018; 25: 40-51.
- Shen L, Hagen JA, Papautsky I. Point-of-care colorimetric detection with a smartphone. Lab on a Chip. 2012; 12: 4240-3.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).