Submitted:
03 June 2024
Posted:
04 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Description and Preparation of the Materials for the Experiment
2.2. Treatment Preparation
2.3. Physical, Chemical and Microbiological Characterization of the VC, S, and Treatments Materials
2.3.1. Vermicompost Analysis
2.3.2. Sand Analysis
2.3.3. Treatment Analysis
2.3.4. Extraction of Indoles from Vermicompost and S-VC Substrates
2.3.5. Indole Quantification by Spectrophotometry
2.3.6. Identification of Bacterial Strains in Vermciocompost
2.3.7. Bacterial Plate Count
2.4. Tomato Cultivation in Sand Substrates with Vermicompost
2.5. Statistical Analysis
3. Results
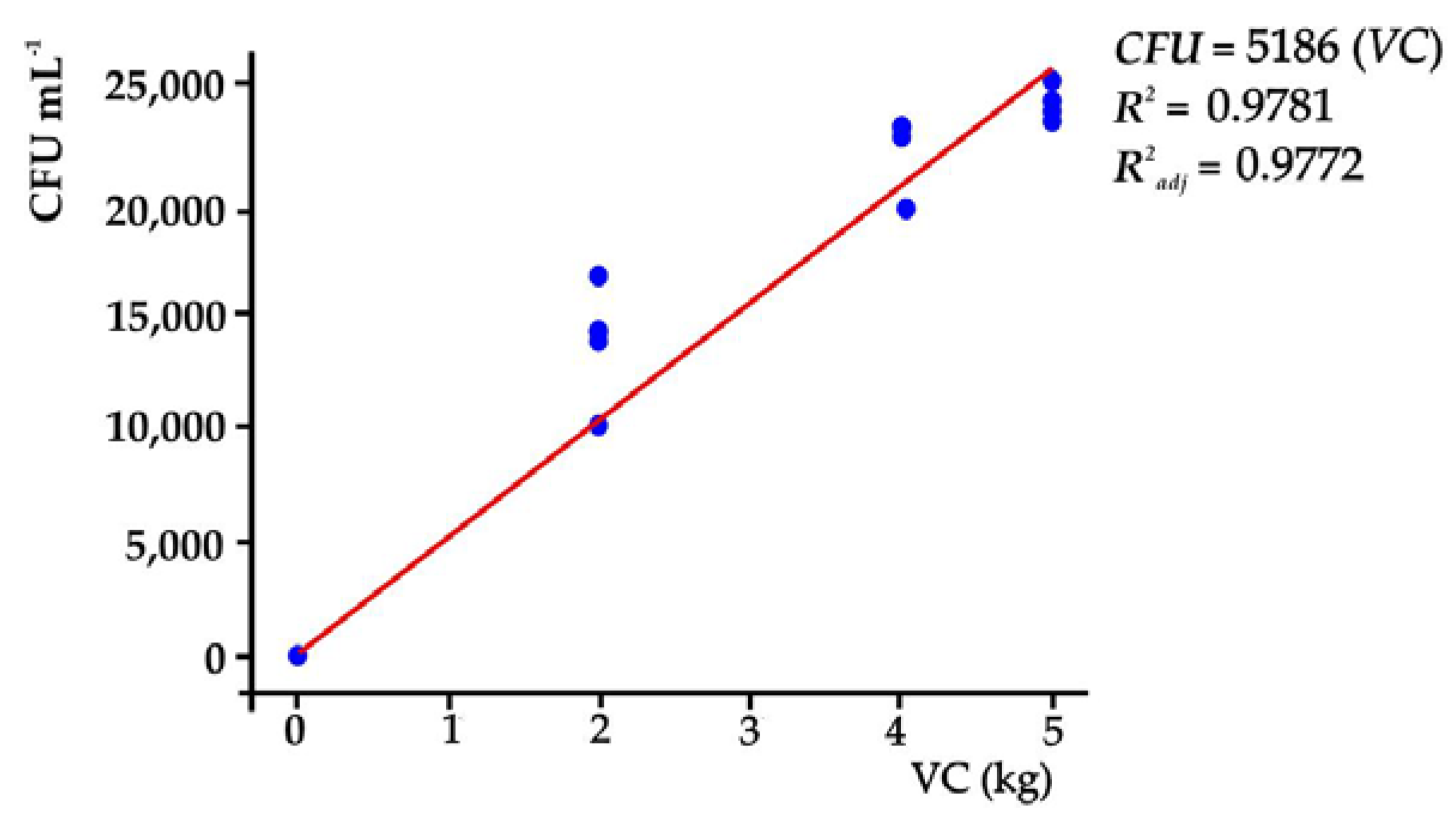
3.1. Characteristics of the Vermicompost
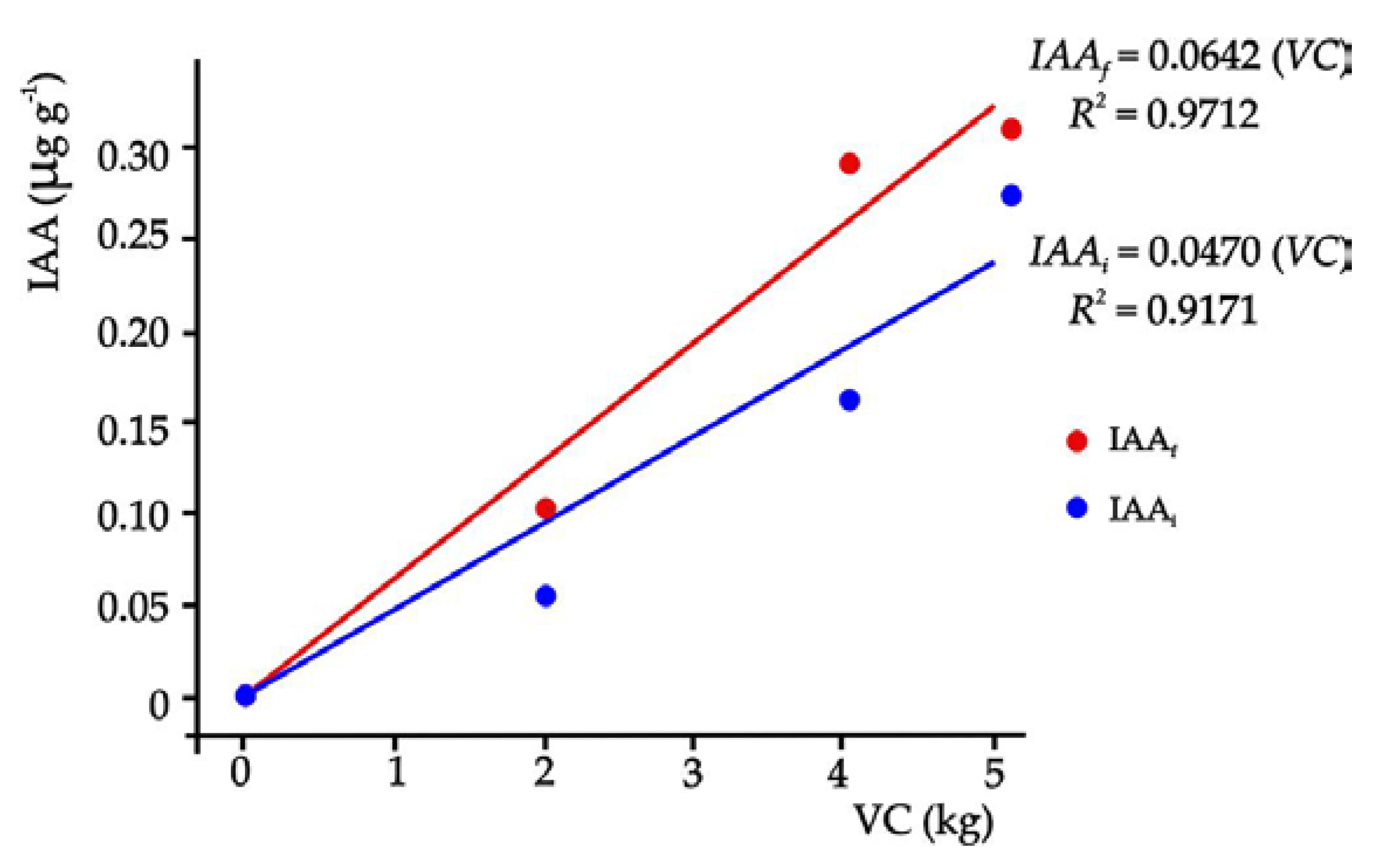
3.2. Physical, Chemical, and Biological Characteristics of the Treatments
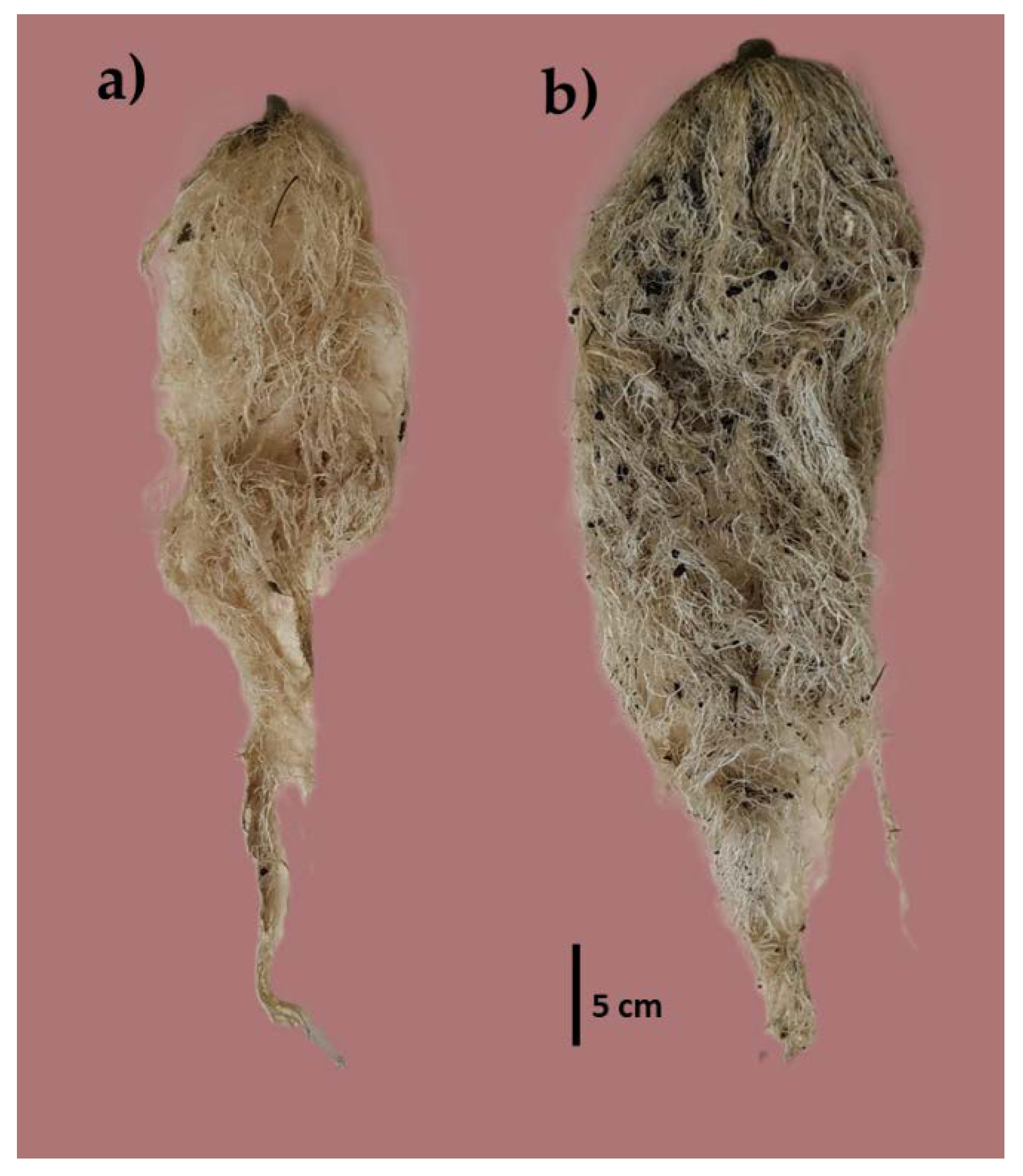
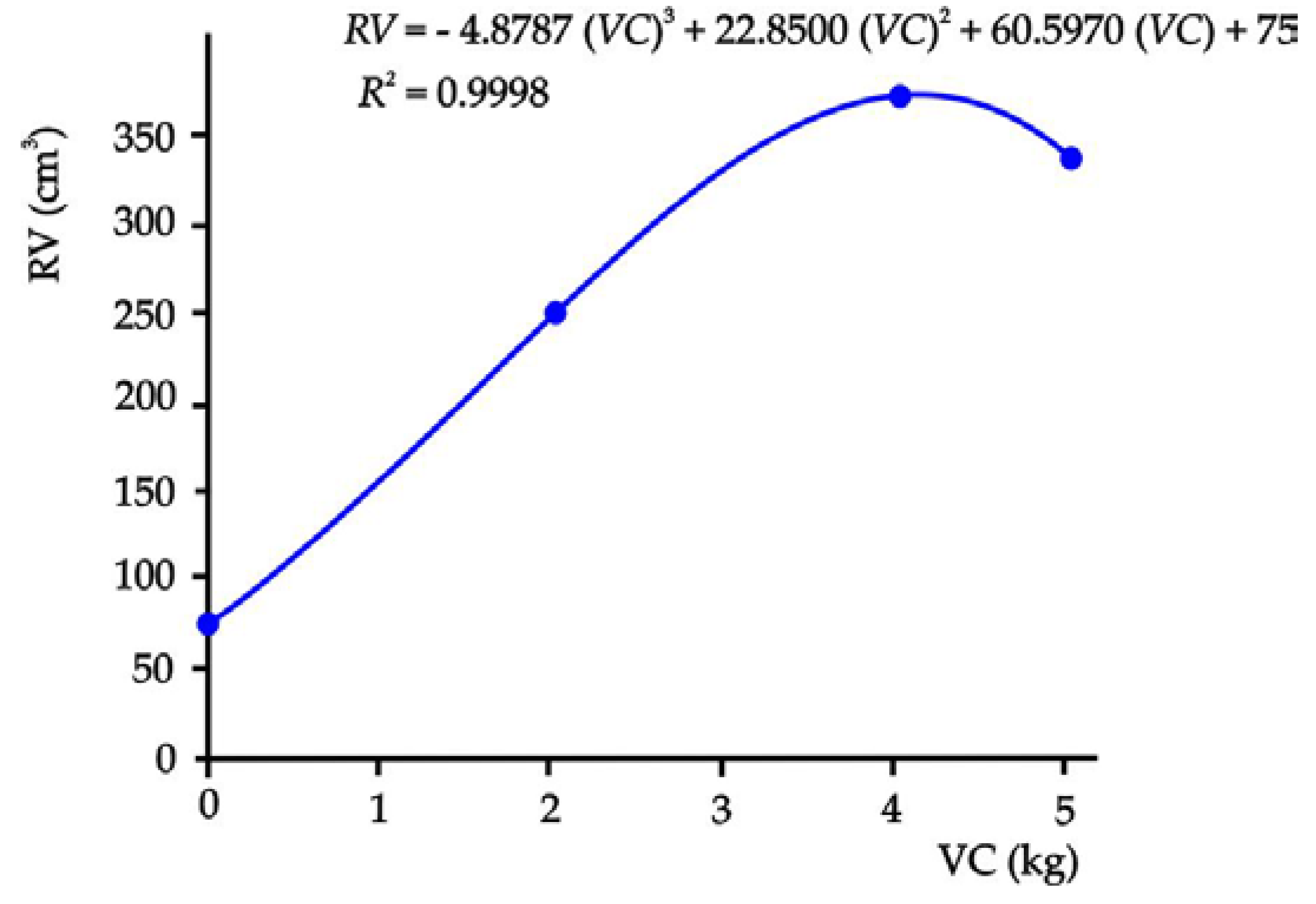
3.3. Tomato Crop Behavior in Substrates with Different Doses of Vermicompost
4. Discussion
4.1. Characteristics of the Vermicompost
4.2. Physical, Chemical, and Biological Characteristics of the Substrates
4.3. Crop Behavior in Sand Substrates with Vermicompost
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| Bd | Bulk density. |
| CEC | Cationic exchange capacity. |
| CFU | Colony-forming units. |
| Dat | days after transplanting. |
| EC | Electrical conductivity. |
| Eq | Equivalents. |
| EU | Experimental units. |
| FC | Floral clusters. |
| fS | Volume of sand. |
| fVC | Volume of vermicompost. |
| IAA | Indol-3-acetic acid. |
| NL | Leaf number. |
| OC | Organic carbon. |
| OM | Percentage of organic matter. |
| PH | Plant height. |
| Ps | Weight of substrate. |
| PsS | Weight of sand. |
| PsVC | Weight of vermicompost. |
| RL | Root length. |
| RV | Root volume. |
| RW | Root dry weight. |
| S | Sand. |
| SD | Stem diameter. |
| SHD | Significant honest difference. |
| SS | Secondary stems. |
| S-VC | Sand-based substrates with vermicompost. |
| Tmt | Treatment. |
| TN | Total nitrogen. |
| VC | Vermicompost. |
| VS | Volume of substrate. |
References
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J. 2016, 4, 162–176. [Google Scholar] [CrossRef]
- Napieraj, N.; Janicka, M.; Reda, M. Interactions of polyamines and phytohormones in plant response to abiotic stress. Plants 2023, 12, 1159. [Google Scholar] [CrossRef] [PubMed]
- Jini, D.; Joseph, B. Use of Phytohormones in improving abiotic stress tolerance to rice. In Advances in Rice Research for Abiotic Stress Tolerance. First ed.; Hasanuzzaman, M., Fujita, M., Nahar, K., Biswas, J.K., Eds.; Elsevier: Woodhead Publishing, Cambridge, United Kingdom, 2019; pp. 633–649. [Google Scholar]
- Zhang, X.; Niu, J.; Zhang, X.; Xiao, R.; Lu, M.; Cai, Z. Graphene oxide-SiO2 nanocomposite as the adsorbent for extraction and preconcentration of plant hormones for HPLC analysis. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1046, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, D.; Geelen, D.; Verstraeten, I. Control of endogenous auxin levels in plant root development. Int. J, Mol. Sci. 2017, 18, 12–2587. [Google Scholar] [CrossRef] [PubMed]
- Arancon, N.Q.; Lee, S.; Edwards, C.A.; Atiyeh, R. Effects of humic acids derived from cattle, food and paper-waste vermicomposts on growth of greenhouse plants: The 7th international symposium on earthworm ecology·Cardiff·Wales·2002. Pedobiologia 2003, 47, 741–744. [Google Scholar] [CrossRef]
- Ishii, T.; Soeno, K.; Asami, T.; Fujioka, S.; Shimada, Y. Arabidopsis seedlings over-accumulated indole-3-acetic acid in response to aminooxyacetic acid. Biosci. Biotechnol. Biochem. 2010, 74, 2345–2347. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bhanu, C.H.; Singh, N.; Kumar, B. Potential of vermicompost for sustainable crop production and soil health improvement in different cropping systems. Int. J. Curr. Microb. Appl. Sci. 2018, 7, 1042–1055. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, S.N.; Teo, C.H.; Yew, Y.R.; Ge, L.; Chen, X.; Yong, J.W.H. Analysis of phytohormones in vermicompost using a novel combinative sample preparation strategy of ultrasound-assisted extraction and solid-phase extraction coupled with liquid chromatography-tandem mass spectrometry. Talanta 2015, 139, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Morales, F.A.; García-Hernández, J.L.; Ruiz-Espinoza, F.H.; Preciado-Rangel, P.; Fortis-Hernández, M.; González-Zamora, A.; Valdez-Cepeda, D. Efectos de sustratos orgánicos en el crecimiento de seis variedades de chile jalapeño (Capsicum annuum L.). Ecosis. Recur. Agropec. 2016, 3, 143–149. [Google Scholar]
- Sivasankari, B. Indole -3-Acetic Acid production by the bacterial strains isolated from vermicomposts in the presence and absence of tryptophan. Int. J. Innov. Res. Sci. Eng. Technol. 2016, 5, 8698–8706. [Google Scholar]
- Lara-Mantilla, C.; Oviedo-Zumaqué, L.E.; Betancur-Hurtado, C.A. Bacterias nativas con potencial en la producción de ácido indolacético para mejorar los pastos. Zootecnia Trop. 2011, 29, 187–194. [Google Scholar]
- Mashiguchi, K.; Hisano, H.; Takeda-Kamiya, N.; Takebayashi, Y.; Ariizumi, T.; Gao, Y.; Ezura, H.; Sato, K.; Zhao, Y.; Hayashi, K.I.; Kasahara, H. Agrobacterium tumefaciens enhances biosynthesis of two distinct auxins in the formation of crown galls. Plant Cell Physiol. 2019, 60, 29–37. [Google Scholar] [CrossRef]
- Amhed, A.; Hasnain, S. Extraction and evaluation of indole acetic acid from indigenous auxin-producing rhizosphere bacteria. The J. Anim. Plant Sci. 2020, 30, 1024–1036. [Google Scholar] [CrossRef]
- Lázaro-De la Cruz, E.; Estrada-Botello, M.A.; Robledo-Torres, V.; Osorio-Osorio, R.; Márquez-Hernández, C.; Sánchez-Hernández, R. Producción de tomate en invernadero con composta y vermicomposta como sustrato. Universidad y Ciencia del trópico húmedo 2009, 25, 57–59. [Google Scholar]
- Glickmann, E.; Dessaux, Y. A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl. Environ. Microbiol. 1995, 61, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Wöhler, I. Auxin-indole derivatives in soils determined by a colorimetric method and by high performance liquid chromatography. Microbiol. Res. 1997, 152, 399–405. [Google Scholar] [CrossRef]
- Porfirio, S.; Gomes-da silva, D.R.M.; Peixe, A.; Cabrita, J.M. Current analytical methods for plant auxin quantification-A review. Anal. Chim. Acta. 2016, 902, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Balderas-Ruíz, K.A.; Gómez-Guerrero, C.I.; Trujillo-Roldán, M.A.; Valdez-Cruz, N.A.; Aranda-Ocampo, S.; Juárez, A.M.; Leyva, E.; Galindo, E.; Serrano-Carreón, L. Bacillus velezensis 83 increases productivity and quality of tomato (Solanum lycopersicum L.): Pre and postharvest assessment. Curr. Res. Microb. Sci. 2021, 2, 100076. [Google Scholar] [CrossRef] [PubMed]
- López-Zapata, S.P.; García-Jaramillo, D.J.; López, W.R.; Ceballos-Aguirre, N. Tomato (Solanum lycopersicum L.) and Fusarium oxysporum f. sp. lycopersici interaction. A review. Rev. U.D.C.A Act. & Div. Cient, 2021; 24, e1713. [Google Scholar]
- Ronga, D.; Caradonia, F.; Parisi, M.; Bezzi, G.; Parisi, B.; Allesina, G.; Pedrazzi, S.; Francia, E. Using Digestate and biochar as fertilizers to improve processing tomato production sustainability. Agronomy 2020, 10, 138. [Google Scholar] [CrossRef]
- Potencial-Jitomate. Available online: www.gob.mx/cms/uploads/attachment/file/257077/Potencial-Jitomate.pdf (accessed on 11 January 2024).
- Jaramillo-Noreña, J.E.; Sánchez-León, G.D.; Rodríguez, V.P.; Aguilar-Aguilar, P.A.; Gil-Vallejo, L.F.; Hío, J.C.; Pinzón-Perdomo, L.M.; García-Muñoz, M.C.; Quevedo-Garzón, D.; Zapata-Cuartas, M.Á.; Restrepo, J.F.; Guzmán-Arroyave, M. Tecnología para el cultivo de tomate bajo condiciones protegidas. 1ª ed. Corporación Colombiana de Investigación Agropecuaria CORPICA. Bogotá, Colombia, 2012; pp. 30–32.
- Ren, Z.; Liu, R.; Gu, W.; Dong, X. The Solanum lycopersicum auxin response factor SlARF2 participates inregulating lateral root formation and flower organ senescence. Plant Sci. 2017, 256, 103–111. [Google Scholar] [CrossRef]
- Pantoja-Guerra, M.; Valero-Valero, N.; Ramírez, C.A. Total auxin level in the soil–plant system as a modulating factor for the effectiveness of PGPR inocula: A review. Chem. Biol. Technol. Agric. 2023, 10, 6. [Google Scholar] [CrossRef]
- Agroactivocol. Ficha técnica de tomate variedad rio grande. Available online: https://agroactivocol.com/ (accessed on 13 January 2024).
- Rodríguez-Dimas, N.; Cano-Ríos, P.; Figueroa-Viramontes, U.; Favela-Chávez, E.; Moreno-Reséndez, A.; Márquez-Hernández, C.; Ochoa-Martínez, E.; Preciado-Rangel, P. Uso de abonos orgánicos en la producción de tomate en invernadero. Terra latinoam. 2009, 27, 319–327. [Google Scholar]
- SE (Secretaría de Economía). Norma Mexicana que establece los métodos y procedimientos para el tratamiento aerobio de la fracción orgánica de los residuos sólidos urbanos y de manejo especial, así como la información comercial y de sus parámetros de calidad de los productos finales. (NMX-AA-180-SCFI-2018). Diario Oficial, 21 de agosto de 2018. 52 p.
- SEMARNAT (Secretaria del Medio Ambiente y Recursos Naturales). Norma Oficial Mexicana que establece las especificaciones de fertilidad, salinidad y clasificación de suelos. Estudios, muestreos y análisis (NOM-021-RECNAT-2000). Diario Oficial de la Federación 31 de diciembre 2002. México, DF. 85 p.
- Celis-Bautista, L.X.; Gallardo, R.I. Estandarización de métodos de detección para promotores de crecimiento vegetal (Ácido indol acético y Giberelinas) en cultivos microbianos. Tesis de Licenciatura. Pontificia Universidad Javeriana, Facultad de Ciencias Microbiología Agrícola y Veterinaria. Bogotá, Colombia. Enero 2008. Recuperado de: http://hdl.handle.net/10554/8948. 1055. [Google Scholar]
- Mayer, A.M. Determination of indole acetic acid by the Salkowsky reaction. Nature 1958, 182, 1670–1671. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Ocampo, G.; Sánchez-González, M.E.; Portilla-López, N.; De Marmolejo-Santillán, Y.; Del Águila-Juárez, P.; Lugo, J. Densidad poblacional de actinomicetos en suelos florícolas, enmendados con vermicomposta. Terra Latinoam. 2020, 38, 745–753. [Google Scholar] [CrossRef]
- Allende, C.M.; Salinas, P.L. Olivares, P.N. Riquelme, S.J., Antúnez, B.A., Martínez, C.J.P., Felmer, E.S. Manual de cultivo de tomate en invernadero. Boletín No. 12. INIA-INDAP. Instituto de Investigaciones Agrícolas y Pecuarias: Santiago de Chile, Chile, 2017; 112p. [Google Scholar]
- Minitab Inc. Minitab® State College. Minitab Inc. Pennsylvania, EEUU, 2013. https://www.minitab.com/es-mx/ (Consulta: 15 December 2023).
- Gopal, M.; Alka, Gupta, A. ; Palaniswami, C.; Dhanapal, R.; Thomas, G. Coconut leaf vermiwash: a bio-liquid from coconut leaf vermicompost for improving the crop production capacities of soil. Curr. Sci. 2009, 98, 9–1202. [Google Scholar]
- Rekha, G.S.; Kaleena, P.K.; Elumalai, D.; Srikumaran, M.P.; Maheswari, V.N. Effects of vermicompost and plant growth enhancers on the exo-morphological features of Capsicum annum (Linn.) Hepper. Int. J. Recycl. Org. Waste Agricult. 2018, 7, 83–88. [Google Scholar] [CrossRef]
- Ravindran, B.; Lee, S.R.; Chang, S.W.; Nguyen, D.D.; Chung, W.J.; Balasubramanian, B.; Mupambwa, H.A.; Arasu, M.V.; Al-Dhabi, N.A.; Sekaran, G. Positive effects of compost and vermicompost produced from tannery waste-animal fleshing on the growth and yield of commercial crop-tomato (Lycopersicon esculentum L.) plant. J. Environ. Manag. 2019, 234, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Zulhipri; Erdawati; Purwanto, A. Development of technology vermicompost production for the coffee plant Industry. J. Phys. Conf. Ser. 2021, 1876, 012020. [Google Scholar] [CrossRef]
- Hemati, A.; Alikhani, H.A.; Ajdanian, L.; Babaei, M.; Asgari Lajayer, B.; van Hullebusch, E.D. Effect of different enriched vermicomposts, humic acid extract and indole-3-acetic acid amendments on the growth of Brassica napus. Plants 2022, 11, 227. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chen, C.; Liu, Y.; Zhang, G.; Yang, L. Vermicompost improves tomato yield and quality by promoting carbohydrate transport to fruit under salt stress. Horticulturae 2023, 9, 1015. [Google Scholar] [CrossRef]
- Alves-Pereira, M.M.; Moraes, L.C.; Mogollón, M.C.T.; Borja, C.J.F.; Duarte, M.; Buttrós, V.H.T.; Luz, J.M.Q.; Pasqual, M.; Dória, J. Cultivating biodiversity to harvest sustainability: Vermicomposting and inoculation of microorganisms for soil preservation and resilience. Agronomy 2023, 13, 103. [Google Scholar] [CrossRef]
- Villegas-Cornelio, V.M.; Laines-Canepa, J.R. Vermicompostaje: I avances y estrategias en el tratamiento de residuos sólidos orgánicos. Rev. Mex. Cienc. Agríc. 2017, 8, 393–406. [Google Scholar] [CrossRef]
- Weil, R.R.; Brady, N.C. The nature and properties of soils. 15th edition. Pearson Education Limited. London, England, 2017; 1104 p.
- Trinidad-Santos, A. El uso de abonos orgánicos en la producción agrícola. 1st ed. Colegio de Postgraduados, Montecillo, Estado de México, México, 1987; 45 p.
- Hernández-López, M.; Vidaña-Martínez, S.A.; Velázquez-Chávez, E.T. Características químicas y microbiológicas de vermicomposta producida en el ITSL. Ciencia Energía y Desarrollo Tec Lerdo 2020, 1, 35–39. [Google Scholar]
- Ahmad, A.; Aslam, Z.; Bellitürk, K.; Iqbal, N.; Idrees, M.; Nawaz, M.; Nawaz,M. Y.; Munir, M.K.; Kamal, A.; Ullah, E.; Jamil, M.A.; Akram, Y.; Abbas, T.; Aziz, M.M. Earth worms and vermicomposting: A review on the story of black gold. J. Innov. Sci. 2021, 7, 167–173. [Google Scholar] [CrossRef]
- Kaur, T. Vermicomposting: an effective option for recycling organic wastes. In Organic agriculture. First ed. Kumar Das, S. Ed. IntechOpen. London, United Kingdom. 2020. pp. 1–17. [CrossRef]
- Pathma, J.; Sakthivel, N. Molecular and functional characterization of bacteria isolated from straw and goat manure based vermicompost. Appl. Soil Ecol. 2013, 70, 33–47. [Google Scholar] [CrossRef]
- Vaz-Moreira, I.; Silva, M.E.; Manaia, C.M.; Nunes, O.C. Diversity of bacterial isolates from commercial and homemade composts. Microb. Ecol. 2008, 55, 714. [Google Scholar] [CrossRef] [PubMed]
- Diccionario Nahuatl. Available online: https://gdn.iib.unam.mx (accessed on 15 February 2024).
- Baldock, J.A.; Broos, K. Soil organic matter. In Hand book of soil science. 2nd ed. Huang, P.M., Li, Y., Sumner, M.E. eds. Vol. 1: Properties and processes. CRC Press (Taylor & Francis), Boca Raton, FL. EEUU, 2012; pp. 11.25–11.35.
- Segura-Castruita, M.A.; Preciado-Rangel, P.; González-Cervantes, G.; Frías-Ramírez, J.E.; García-Legaspi, G.; Orozco-Vidal, J.A.; Enríquez-Sánchez, M. Adición de material pomáceo a sustratos de arena para incrementar la capacidad de retención de humedad. Interciencia 2008, 33, 923. [Google Scholar]
- Gómez-Godínez, L.J.; Martínez-Romero, E.; Banuelos, J.; Arteaga-Garibay, R.I. Tools and challenges to exploit microbial communities in agriculture. Curr. Res. Microb. Sci. 2021, 2, 100062. [Google Scholar] [CrossRef] [PubMed]
- Aremu, A.O.; Stirk, W.A.; Kulkarni, M.G.; Tarkowská, D.; Turecková, V.; Gruz, J.; Subrtová, M.; Pnèík, A.; Novák, O.; Doleal, K.; Strnad, M.; Staden, L.V. Evidence of phytohormones and phenolic acids variability in garden-waste-derived vermicompost leachate, a well-known plant growth stimulant. Plant Growth Regul. 2015, 75, 483–492. [Google Scholar] [CrossRef]
- Steffen, G.P.K.; Maldaner, J.; de Morais, R.M.; Saldanha, C.W.; Missio, E.L.; Steffen, R.B.; Osorio-Filho, B.D. The vermicompost anticipates flowering and increases tomato productivity. Agrocienc. Urug. 2019, 23. [Google Scholar] [CrossRef]
- Rehman, S.U.; De Castro, F.; Aprile, A.; Benedetti, M.; Fanizzi, F.P. Vermicompost: enhancing plant growth and combating abiotic and biotic stress. Agronomy 2023, 13, 1134. [Google Scholar] [CrossRef]
- Puga-Freitas, R.; Abbad, S.; Gigon, A.; Garnier-Zarli, E.; Blouin, M. Control of cultivable IAA-producing bacteria by the plant Arabidopsis thaliana and the earthworm Aporrectodea caliginosa. Appl. Environ. Soil Sci. 2012, 307415. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Li, Y.; Li, G.; Liu, D.; Zhao, M.; Cai, N. Growth promotion of Yunnan pine early seedlings in response to foliar application of IAA and IBA. Inter. J. Mol. Sci. 2012, 13, 6507–6520. [Google Scholar] [CrossRef] [PubMed]
- Enders, T.A.; Strader, L.C. Auxin activity: Past, present, and future. Am. J. Bot. 2015, 102, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Ribnicky, D.M.; Ilic, N.; Cohen, J.D.; Cooke, T.J. The effects of exogenous auxins on endogenous indole-3-acetic acid metabolism (the implications for carrot somatic embryogenesis). Plant Physiology 1996, 112, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Sabri, A.N.; Ljung, K.; Hasnain, S. Quantification of indole-3-acetic acid from plant associated Bacillus spp. and their phytostimulatory effect on Vigna radiata (L.). World J. Microbiol. Biotechnol. 2009, 25, 519–526. [Google Scholar] [CrossRef]
- Cai, T.; Meng, X.; Liu, X.; Liu, T.; Wang, H.; Jia, Z.; Yang, D.; Ren, X. Exogenous hormonal application regulates the occurrence of wheat tillers by changing endogenous hormones. Front. Plant Sci. 2018, 9, 1886. [Google Scholar] [CrossRef]
| Tmt 1 | S (%) | VC (%) |
|---|---|---|
| T0 | 100 | 0 |
| T1 | 80 | 20 |
| T2 | 60 | 40 |
| T3 | 50 | 50 |
| Tmt 1 | Sand | Vermicompost | |||
|---|---|---|---|---|---|
| fS (L) |
PsS (kg) |
fVC (L) |
PsVC (kg) |
||
| T0 | 14.00 | 15.86 | 0.00 | 0.00 | |
| T1 | 11.20 | 12.76 | 2.80 | 2.01 | |
| T2 | 8.40 | 9.57 | 5.60 | 4.03 | |
| T3 | 7.00 | 7.98 | 7.00 | 5.04 | |
| Characteristics | Reference range 1 |
VC Data |
|---|---|---|
| TN 2 (%) | 1.00 – 4.00 | 2.24 |
| OM (%) | 20.00 – 50.00 | 48.15 |
| C/N | ≤ 20.00 | 12.36 |
| Moisture (%) | 20.00 – 40.00 | 30.00 |
| pH | 5.50 – 8.50 | 7.30 |
| EC (dS m-1) | ≤ 4.00 | 2.10 |
| CEC (cmol(+) kg-1) | > 40.00 | 130.00 |
| Ca (%) | 2.00 – 8.00 | 0.11 |
| Mg (%) | 1.00 – 2.50 | 0.45 |
| K (%) | 1.00 – 2.50 | 0.78 |
| P (%) | 2.00 – 8.00 | 1.20 |
| Bd (g cm-3) | 0.40 – 0.90 | 0.72 |
| Tmt 1 | OM | TN | pH | Bd | EC | CEC | K | Ca | Mg | P |
|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (g cm-3) | (dS m-3) | (cmol(+) kg-1) | (%) | (%) | (%) | (ppm) | ||
| T0 | 0.41 d2 | 0.01 c | 7.8 a | 1.16 a | 0.09 c | 24.89 d | 0.18 c | 0.01 c | 0.04 c | 0.75 d |
| T1 | 2.56 c | 0.21 c | 7.6 b | 1.06 b | 1.00 b | 30.89 c | 0.22 b | 0.02 b | 0.08 c | 28.1 c |
| T2 | 5.65 b | 0.44 a | 7.4 c | 1.04 b | 1.32 a | 45.01 b | 0.27 a | 0.03 a | 1.14 b | 68.7 b |
| T3 | 6.31 a | 0.47 a | 7.4 c | 1.04 b | 1.36 a | 51.77 a | 0.28 a | 0.03 a | 1.22 a | 88.1 a |
| SHD | 1.52 | 0.22 | 0.18 | 0.05 | 0.21 | 4.45 | 0.03 | 0.005 | 0.07 | 16.23 |
| Tmt 1 | Eq IAAi ± σ | Eq IAAf ± σ |
|---|---|---|
| (µg g-1) | (µg g-1) | |
| T0 | 0.0 ± 0.00 d 2 | 0.0 ± 0.00 c |
| T1 | 0.055 ± 0.05 c | 0.101 ± 0.50 c |
| T2 | 0.163 ± 0.50 b | 0.290 ± 0.50 a |
| T3 | 0.274 ± 0.02 a | 0.310 ± 1.02 a |
| SHD | 0.104 | 0.085 |
| Tmt 1 | PH | SD | NL | FC | SS | RV | RL | RW |
|---|---|---|---|---|---|---|---|---|
| (cm) | (cm) | (cm-3) | (cm) | (g) | ||||
| T0 | 56.50 c 2 | 1.03 b | 20.00 b | 5.33 c | 3.70 b | 75.00 d | 61.00 c | 7.70 c |
| T1 | 72.67 b | 1.43 a | 27.33 ab | 11.67 ab | 9.00 a | 249.50 c | 64.60 bc | 44.77 b |
| T2 | 97.53 a | 1.46 a | 33.33 a | 13.50 a | 10.00 a | 371.00 a | 77.25 a | 64.90 a |
| T3 | 95.25 a | 1.50 a | 33.80 a | 11.75 ab | 9.70 a | 336.25 b | 71.80 ab | 46.52 b |
| SHD | 10.48 | 0.26 | 7.21 | 6.05 | 3.58 | 15.65 | 9.69 | 9.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





