Submitted:
22 May 2024
Posted:
23 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Population
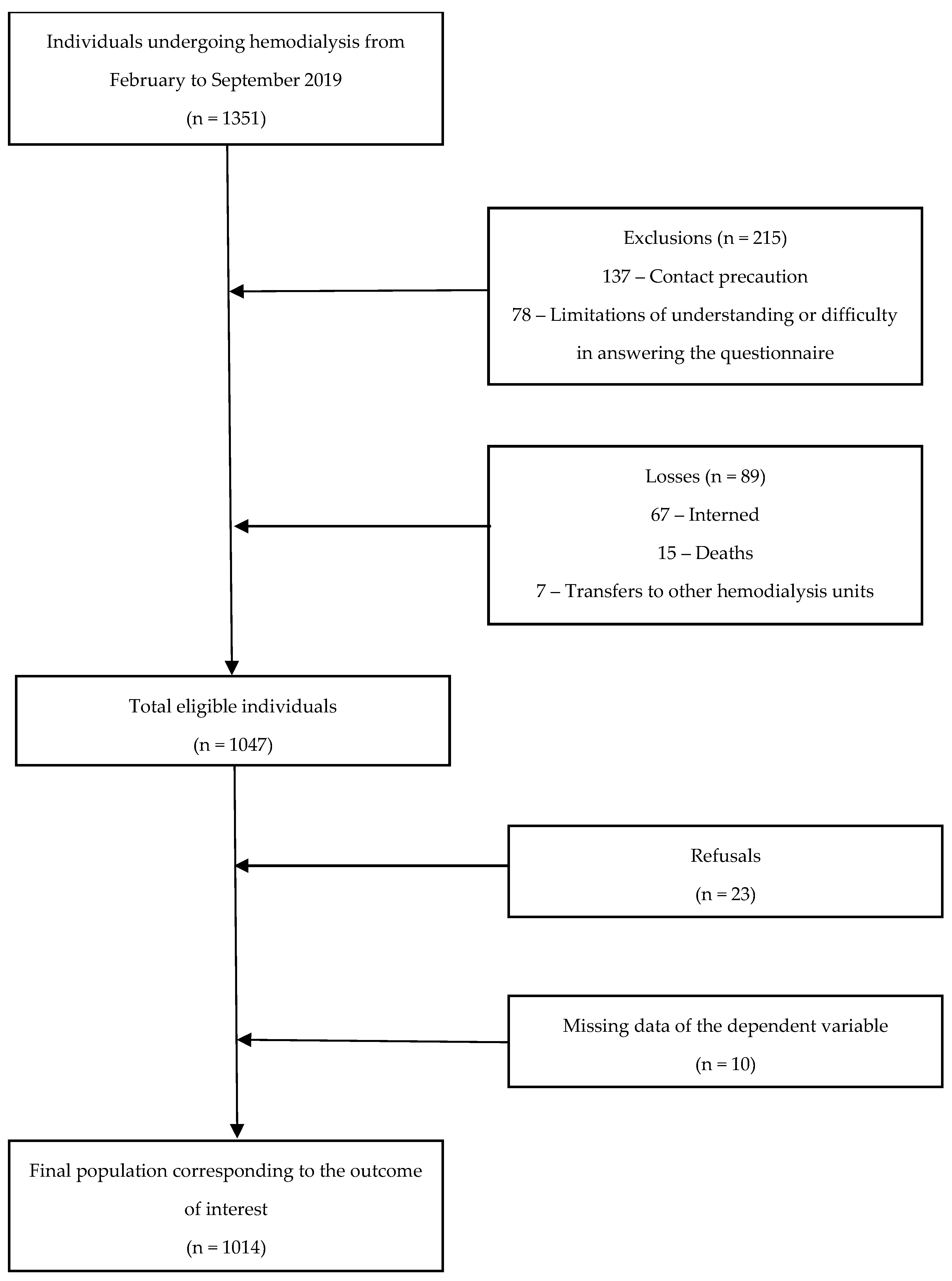
2.3. Data Collection
2.4. Sociodemographic and Clinical Characteristics
2.5. Anthropometry
2.6. Consumption of Ultra-Processed Foods
2.7. Ethical Aspects
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Monteiro, C.A.; Cannon, G.; Levy, R.; Moubarac, J.-C.; Jaime, P.; Martins, A.P.; Canela, D.; Louzada, M.; Parra, D. NOVA. The Star Shines Bright. World Nutr. 2016, 7, 28–38. [Google Scholar]
- Baker, P.; Machado, P.; Santos, T.; Sievert, K.; Backholer, K.; Hadjikakou, M.; Russell, C.; Huse, O.; Bell, C.; Scrinis, G.; et al. Ultra-processed Foods and the Nutrition Transition: Global, Regional and National Trends, Food Systems Transformations and Political Economy Drivers. Obes. Rev. 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Moubarac, J.C.; Cannon, G.; Ng, S.W.; Popkin, B. Ultra-Processed Products Are Becoming Dominant in the Global Food System. Obes. Rev. 2013, 14, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Instituto Brasileiro de Geografia e Estatística. Pesquisa de Orçamentos Familiares: 2017-2018: Avaliação Nutricional da Disponibilidade Domiciliar de Alimentos no Brasil / IBGE, Coordenação de Trabalho e Rendimento; Instituto Brasileiro de Geografia e Estatística: Rio de Janeiro, 2020. Available online: https://biblioteca.ibge.gov.br/index.php/biblioteca-catalogo?view=detalhes&id=2101704.
- Caballero, B. Humans Against Obesity: Who Will Win? Adv. Nutr. 2019, 10 (Suppl. 1), S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.P.C.; Vergara, C.M.A.C.; Sampaio, H.A.d.C.; Vasconcelos Filho, J.E.; Strozberg, F.; Ferreira Neto, J.F.R.; Mafra, M.L.P.; Garcia Filho, C.; Carioca Filho, A.A.F. Food and Nutrition Surveillance System: Temporal Trend of Coverage and Nutritional Status of Adults Registered on the System, Brazil, 2008-2019. Epidemiologia Serv. Saude 2022, 31. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-l.; Lv, J.; Du, Z.-p.; Feng, S.; Sheng, J.; Jin, Z.-x.; Liu, K.-y.; Gao, H.; Li, X.-d.; Cao, H.-j.; et al. The Levels of Phthalate Exposure and Associations With Obesity in an Elderly Population in China. Ecotoxicol. Environ. Saf. 2020, 201, 110749. [Google Scholar] [CrossRef] [PubMed]
- Perez-Campos, E.; Mayoral, L.-C.; Andrade, G.; Mayoral, E.-C.; Huerta, T.; Canseco, S.; Rodal Canales, F.; Cabrera-Fuentes, H.; Cruz, M.; Pérez Santiago, A.; et al. Obesity Subtypes, Related Biomarkers & Heterogeneity. Indian J. Med. Res. 2020, 151, 11. [Google Scholar] [CrossRef] [PubMed]
- Eickemberg, M.; Amorim, L.D.A.F.; Almeida, M.d.C.C.d.; Pitanga, F.J.G.; Aquino, E.M.L.d.; Fonseca, M.d.J.M.d.; Matos, S.M.A. Obesidade abdominal no ELSA-Brasil: Construção de padrão-ouro latente e avaliação da acurácia de indicadores diagnósticos. Cienc. & Saude Coletiva 2020, 25, 2985–2998. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, D.; Sharma, S. Abdominal Obesity, Adipokines and Non-Communicable Diseases. J. Steroid Biochem. Mol. Biol. 2020, 203, 105737. [Google Scholar] [CrossRef]
- Bosomworth, N.J. Normal-Weight Central Obesity: Unique Hazard of the Toxic Waist. Can. Fam. Physician 2019, 65, 399–408. [Google Scholar]
- Fang, H.; Berg, E.; Cheng, X.; Shen, W. How to Best Assess Abdominal Obesity. Curr. Opin. Clin. Nutr. & Metab. Care 2018, 21, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Raynor, H.A.; Champagne, C.M. Position of the Academy of Nutrition and Dietetics: Interventions for the Treatment of Overweight and Obesity in Adults. J. Acad. Nutr. Diet. 2016, 116, 129–147. [Google Scholar] [CrossRef] [PubMed]
- Balkau, B.; Deanfield, J.E.; Després, J.-P.; Bassand, J.-P.; Fox, K.A.A.; Smith, S.C.; Barter, P.; Tan, C.-E.; Van Gaal, L.; Wittchen, H.-U.; et al. International Day for the Evaluation of Abdominal Obesity (IDEA). Circulation 2007, 116, 1942–1951. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Katzmarzyk, P.T.; Ross, R. Waist Circumference and Not Body Mass Index Explains Obesity-Related Health Risk. Am. J. Clin. Nutr. 2004, 79, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Juul, F.; Martinez-Steele, E.; Parekh, N.; Monteiro, C.A.; Chang, V.W. Ultra-Processed Food Consumption and Excess Weight Among US Adults. Br. J. Nutr. 2018, 120, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Rauber, F.; Steele, E.M.; Louzada, M.L.d.C.; Millett, C.; Monteiro, C.A.; Levy, R.B. Ultra-Processed Food Consumption and Indicators of Obesity in the United Kingdom Population (2008-2016). PLOS ONE 2020, 15, e0232676. [Google Scholar] [CrossRef] [PubMed]
- Machado, P.P.; Steele, E.M.; Levy, R.B.; da Costa Louzada, M.L.; Rangan, A.; Woods, J.; Gill, T.; Scrinis, G.; Monteiro, C.A. Ultra-Processed Food Consumption and Obesity in the Australian Adult Population. Nutr. & Diabetes 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Park, J.M.; Oh, S.U.; Ha, K.; Joung, H. Consumption of Ultra-Processed Foods Increases the Likelihood of Having Obesity in Korean Women. Nutrients 2021, 13, 698. [Google Scholar] [CrossRef] [PubMed]
- Canhada, S.L.; Luft, V.C.; Giatti, L.; Duncan, B.B.; Chor, D.; Fonseca, M.d.J.M.d.; Matos, S.M.A.; Molina, M.d.C.B.; Barreto, S.M.; Levy, R.B.; et al. Ultra-Processed Foods, Incident Overweight and Obesity, and Longitudinal Changes in Weight and Waist Circumference: The Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Public Health Nutr. 2019, 23, 1076–1086. [Google Scholar] [CrossRef]
- KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am. J. Kidney Dis. 2012, 60, 850–886. [CrossRef]
- Neves, P.D.M. d. M.; Sesso, R.d.C.C.; Thomé, F.S.; Lugon, J.R.; Nascimento, M.M. Brazilian Dialysis Survey 2019. Braz. J. Nephrol. 2021, 43, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic Kidney Disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef] [PubMed]
- Nerbass, F.B.; Lima, H. d. N.; Thomé, F.S.; Vieira Neto, O.M.; Lugon, J.R.; Sesso, R. Brazilian Dialysis Survey 2020. Braz. J. Nephrol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.T.; Araujo, R.M.; Vogt, B.P.; Barretti, P.; Caramori, J.C.T. Most Consumed Processed Foods by Patients on Hemodialysis: Alert for Phosphate-Containing Additives and the Phosphate-to-Protein Ratio. Clin. Nutr. ESPEN 2016, 14, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Block, G.; Hulbert-Shearon, T.; Levin, N.; Port, F. Association of Serum Phosphorus and Calcium X Phosphate Product With Mortality Risk in Chronic Hemodialysis Patients: A National Study. Am. J. Kidney Dis. 1998, 31, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Foley, R.; Parfrey, P.; Sarnak, M. Clinical Epidemiology of Cardiovascular Disease in Chronic Renal Disease. Am. J. Kidney Dis. 1998, 32, S112–S119. [Google Scholar] [CrossRef] [PubMed]
- Kestenbaum, B.; Sampson, J.N.; Rudser, K.D.; Patterson, D.J.; Seliger, S.L.; Young, B.; Sherrard, D.J.; Andress, D.L. Serum Phosphate Levels and Mortality Risk Among People With Chronic Kidney Disease. J. Am. Soc. Nephrol. 2004, 16, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Block, G.A. Mineral Metabolism, Mortality, and Morbidity in Maintenance Hemodialysis. J. Am. Soc. Nephrol. 2004, 15, 2208–2218. [Google Scholar] [CrossRef] [PubMed]
- Parpia, A.S.; L’Abbé, M.; Goldstein, M.; Arcand, J.; Magnuson, B.; Darling, P.B. The Impact of Additives on the Phosphorus, Potassium, and Sodium Content of Commonly Consumed Meat, Poultry, and Fish Products Among Patients With Chronic Kidney Disease. J. Ren. Nutr. 2018, 28, 83–90. [Google Scholar] [CrossRef]
- Osté, M.C.J.; Duan, M.J.; Gomes-Neto, A.W.; Vinke, P.C.; Carrero., J.J.; Avesani., C.; Cai., Q.; Dekker, L.H.; Navis, G.J.; Bakker, S.J.L.; Corpeleijn, E. Ultra-Processed Foods and Risk of All-Cause Mortality in Renal Transplant Recipients. Am J Clin Nutr. 2022, 115. [Google Scholar] [CrossRef]
- Prasad, R.; Jha, R.K.; Keerti, A. Chronic Kidney Disease: Its Relationship With Obesity. Cureus 2022. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, Y.; Eriguchi, R.; Sato, Y.; Nakaya, Y. Chronic hemodialysis patients with visceral obesity have a higher risk for cardiovascular events. Asia Pac. J. Clin. Nutr. 2011, 20. [Google Scholar]
- El Said, H.W.; Mohamed, O.M.; El Said, T.W.; El Serwi, A.B. Central Obesity and Risks of Cardiovascular Events and Mortality in Prevalent Hemodialysis Patients. Int. Urol. Nephrol. 2017, 49, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Postorino, M.; Marino, C.; Tripepi, G.; Zoccali, C. Abdominal Obesity and All-Cause and Cardiovascular Mortality in End-Stage Renal Disease. J. Am. Coll. Cardiol. 2009, 53, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, J.; Sozio, S.M.; Jaar, B.G.; McAdams-DeMarco, M.A.; Estrella, M.M.; Tereshchenko, L.G.; Monroy-Trujillo, J.M.; Parekh, R.S. Association of Abdominal Adiposity With Cardiovascular Mortality in Incident Hemodialysis. Am. J. Nephrol. 2018, 48, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Han, K.-D.; Choi, H.S.; Bae, E.H.; Ma, S.K.; Kim, S.W. Association of Body Mass Index and Waist Circumference With All-Cause Mortality in Hemodialysis Patients. J. Clin. Med. 2020, 9, 1289. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, H.; Lan, X.; Qin, X.; Huang, Y.; Wang, J.; Luo, P.; Wen, Z.; Li, Y.; Kong, Y.; et al. Low BMI and High Waist-to-Hip Ratio Are Associated With Mortality Risk Among Hemodialysis Patients: A Multicenter Prospective Cohort Study. Clin. Kidney J. 2022. [Google Scholar] [CrossRef] [PubMed]
- Waxman, A. Who Global Strategy on Diet, Physical Activity and Health. Food Nutr. Bull. 2004, 25, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Lohman, T.J.; Roache, A.F.; Martorell, R. Anthropometric standardization reference manual; Human Kinetics Books; 1988. [Google Scholar]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation; World Health Organization: Geneva, 2000; Available online: https://apps.who.int/iris/handle/10665/42330.
- Hunter, G.R.; Kekes-Szabo, T.; Snyder, S.W.; Nicholson, C.; Nyikos, I.; Berland, L. Fat Distribution, Physical Activity, and Cardiovascular Risk Factors. Med. &Amp;amp Sci. Sports &Amp;amp Exerc. 1997, 29, 362–369. [Google Scholar] [CrossRef]
- Ministério da Saúde. Vigitel Brasil 2019 - Vigilância de Fatores de Risco e Proteção para Doenças Crônicas por Inquérito Telefônico: Estimativas sobre frequência e distribuição sociodemográfica de fatores de risco e proteção para doenças crônicas nas capitais dos 26 estados brasileiros e no Distrito Federal em 2019; Brasil, Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Análise em Saúde e Vigilância de Doenças não Transmissíveis; 2020. Available online: http://bvsms.saude.gov.br/bvs/publicacoes/vigitel_brasil_2019_vigilancia_fatores_risco.pdf.
- Marques, N.M.P.; Cattafesta, M.; Soares, F.L.P.; Petarli, G.B.; Paixão, M.P.C.P.; Martins, C.A.; Neto, E.T.S.N.; Salaroli, L.B. Consumption of Minimally Processed and Ultra-Processed Foods by Individuals on Hemodialysis in Southeastern Brazil. J. Hum. Growth Dev. 2022, 32, 237–251. [Google Scholar] [CrossRef]
- Evangelista, L.S.; Cho, W.-K.; Kim, Y. Obesity and Chronic Kidney Disease: A Population-Based Study Among South Koreans. PLOS ONE 2018, 13, e0193559. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, Z.; Moosa, M.R.; Blaauw, R. Obesity and Other Nutrition Related Abnormalities in Pre-Dialysis Chronic Kidney Disease (CKD) Participants. Nutrients 2020, 12, 3608. [Google Scholar] [CrossRef]
- Dierkes, J.; Dahl, H.; Lervaag Welland, N.; Sandnes, K.; Sæle, K.; Sekse, I.; Marti, H.-P. High Rates of Central Obesity and Sarcopenia in CKD Irrespective of Renal Replacement Therapy – An Observational Cross-Sectional Study. BMC Nephrol. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.T.V. d. S.; Vaz, I.M.F.; Ferraz, S.F.; Peixoto, M.d.R.G.; Campos, M.I.V.M.; Fornés, N.S. Prevalence and associated factors with abdominal obesity in hemodialysis patients in Goiânia - GO. J. Bras. Nefrol. 2013, 35, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.A.; Ferreira, J.R.S.; Cattafesta, M.; Neto, E.T.D.S.; Rocha, J.L.M.; Salaroli, L.B. Cut Points of the Conicity Index as an Indicator of Abdominal Obesity in Individuals Undergoing Hemodialysis: An Analysis of Latent Classes. Nutrition 2022, 111890. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Moubarac, J.-C.; Levy, R.B.; Louzada, M.L.C.; Jaime, P.C. The UN Decade of Nutrition, the NOVA Food Classification and the Trouble With Ultra-Processing. Public Health Nutr. 2017, 21, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.-C.; Louzada, M.L.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-Processed Foods: What They Are and How to Identify Them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Levy, R.B.; Claro, R.M.; de Castro, I.R.R.; Cannon, G. Increasing Consumption of Ultra-Processed Foods and Likely Impact on Human Health: Evidence From Brazil. Public Health Nutr. 2010, 14, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Moubarac, J.-C.; Martins, A.P.B.; Claro, R.M.; Levy, R.B.; Cannon, G.; Monteiro, C.A. Consumption of Ultra-Processed Foods and Likely Impact on Human Health. Evidence From Canada. Public Health Nutr. 2012, 16, 2240–2248. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V.; et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019, 30, 67–77.e3. [Google Scholar] [CrossRef]
- Shim, J.-S.; Shim, S.Y.; Cha, H.-J.; Kim, J.; Kim, H.C. Association Between Ultra-Processed Food Consumption and Dietary Intake and Diet Quality in Korean Adults. J. Acad. Nutr. Diet. 2021. [Google Scholar] [CrossRef] [PubMed]
- Poti, J.M.; Mendez, M.A.; Ng, S.W.; Popkin, B.M. Is the Degree of Food Processing and Convenience Linked With the Nutritional Quality of Foods Purchased by US Households? Am. J. Clin. Nutr. 2015, 101, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Rauber, F.; da Costa Louzada, M.L.; Steele, E.; Millett, C.; Monteiro, C.A.; Levy, R.B. Ultra-Processed Food Consumption and Chronic Non-Communicable Diseases-Related Dietary Nutrient Profile in the UK (2008–2014). Nutrients 2018, 10, 587. [Google Scholar] [CrossRef] [PubMed]
- Martínez Steele, E.; Popkin, B.M.; Swinburn, B.; Monteiro, C.A. The Share of Ultra-Processed Foods and the Overall Nutritional Quality of Diets in the US: Evidence From a Nationally Representative Cross-Sectional Study. Popul. Health Metr. 2017, 15. [Google Scholar] [CrossRef] [PubMed]
- Louzada, M.L. d. C.; Ricardo, C.Z.; Steele, E.M.; Levy, R.B.; Cannon, G.; Monteiro, C.A. The Share of Ultra-Processed Foods Determines the Overall Nutritional Quality of Diets in Brazil. Public Health Nutr. 2017, 21, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Marrón-Ponce, J.A.; Flores, M.; Cediel, G.; Monteiro, C.A.; Batis, C. Associations Between Consumption of Ultra-Processed Foods and Intake of Nutrients Related to Chronic Non-Communicable Diseases in Mexico. J. Acad. Nutr. Diet. 2019, 119, 1852–1865. [Google Scholar] [CrossRef] [PubMed]
- Wee, M.S.M.; Goh, A.T.; Stieger, M.; Forde, C.G. Correlation of Instrumental Texture Properties From Textural Profile Analysis (TPA) With Eating Behaviours and Macronutrient Composition for a Wide Range of Solid Foods. Food & Funct. 2018, 9, 5301–5312. [Google Scholar] [CrossRef]
- Fardet, A. Minimally Processed Foods Are More Satiating and Less Hyperglycemic Than Ultra-Processed Foods: A Preliminary Study With 98 Ready-to-Eat Foods. Food & Funct. 2016, 7, 2338–2346. [Google Scholar] [CrossRef]
- Fardet, A.; Méjean, C.; Labouré, H.; Andreeva, V.A.; Feron, G. The Degree of Processing of Foods Which Are Most Widely Consumed by the French Elderly Population Is Associated With Satiety and Glycemic Potentials and Nutrient Profiles. Food & Funct. 2017, 8, 651–658. [Google Scholar] [CrossRef]
- de Graaf, C.; Kok, F.J. Slow Food, Fast Food and the Control of Food Intake. Nat. Rev. Endocrinol. 2010, 6, 290–293. [Google Scholar] [CrossRef]
- Forde, C.G.; van Kuijk, N.; Thaler, T.; de Graaf, C.; Martin, N. Texture and Savoury Taste Influences on Food Intake in a Realistic Hot Lunch Time Meal. Appetite 2013, 60, 180–186. [Google Scholar] [CrossRef] [PubMed]
- McCrickerd, K.; Lim, C.M.; Leong, C.; Chia, E.M.; Forde, C.G. Texture-Based Differences in Eating Rate Reduce the Impact of Increased Energy Density and Large Portions on Meal Size in Adults. J. Nutr. 2017, 147, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Forde, C.G.; Mars, M.; de Graaf, K. Ultra-Processing or Oral Processing? A Role for Energy Density and Eating Rate in Moderating Energy Intake From Processed Foods. Curr. Dev. Nutr. 2020, 4. [Google Scholar] [CrossRef] [PubMed]
- Zinöcker, M.; Lindseth, I. The Western Diet–Microbiome-Host Interaction and Its Role in Metabolic Disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.N.; Chassard, C.; Lacroix, C. Gut Microbial Adaptation to Dietary Consumption of Fructose, Artificial Sweeteners and Sugar Alcohols: Implications for Host-Microbe Interactions Contributing to Obesity. Obes. Rev. 2012, 13, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Pearlman, M.; Obert, J.; Casey, L. The Association Between Artificial Sweeteners and Obesity. Curr. Gastroenterol. Rep. 2017, 19. [Google Scholar] [CrossRef]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial Sweeteners Induce Glucose Intolerance by Altering the Gut Microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Miclotte, L.; Van de Wiele, T. Food Processing, Gut Microbiota and the Globesity Problem. Crit. Rev. Food Sci. Nutr. 2019, 60, 1769–1782. [Google Scholar] [CrossRef] [PubMed]
- Shannon, M.; Green, B.; Willars, G.; Wilson, J.; Matthews, N.; Lamb, J.; Gillespie, A.; Connolly, L. The Endocrine Disrupting Potential of Monosodium Glutamate (MSG) on Secretion of the Glucagon-Like Peptide-1 (GLP-1) Gut Hormone and GLP-1 Receptor Interaction. Toxicol. Lett. 2017, 265, 97–105. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary Emulsifiers Impact the Mouse Gut Microbiota Promoting Colitis and Metabolic Syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, T.; Hu, W.; Wang, X.; Xu, B.; Lin, Z.; Hofer, T.; Stefanoff, P.; Chen, Y.; Wang, X.; et al. Association Between Exposure to a Mixture of Phenols, Pesticides, and Phthalates and Obesity: Comparison of Three Statistical Models. Environ. Int. 2019, 123, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Kolena, B.; Hlisníková, H.; Kečkéšová, Ľ.; Šidlovská, M.; Trnovec, T.; Petrovičová, I. Risk of Abdominal Obesity Associated With Phthalate Exposure of Nurses. Toxics 2022, 10, 143. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, M.; Chen, B.; Xu, M.; Xu, Y.; Huang, Y.; Lu, J.; Chen, Y.; Wang, W.; Li, X.; et al. Urinary Bisphenol a (BPA) Concentration Associates With Obesity and Insulin Resistance. J. Clin. Endocrinol. & Metab. 2012, 97, E223–E227. [Google Scholar] [CrossRef] [PubMed]
- Ko, A.; Hwang, M.-S.; Park, J.-H.; Kang, H.-S.; Lee, H.-S.; Hong, J.-H. Association Between Urinary Bisphenol a and Waist Circumference in Korean Adults. Toxicol. Res. 2014, 30, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Savastano, S.; Tarantino, G.; D’Esposito, V.; Passaretti, F.; Cabaro, S.; Liotti, A.; Liguoro, D.; Perruolo, G.; Ariemma, F.; Finelli, C.; et al. Bisphenol-a Plasma Levels Are Related to Inflammatory Markers, Visceral Obesity and Insulin-Resistance: A Cross-Sectional Study on Adult Male Population. J. Transl. Med. 2015, 13. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lehmler, H.-J.; Sun, Y.; Xu, G.; Liu, Y.; Zong, G.; Sun, Q.; Hu, F.B.; Wallace, R.B.; Bao, W. Bisphenol a Substitutes and Obesity in US Adults: Analysis of a Population-Based, Cross-Sectional Study. Lancet Planet. Health 2017, 1, e114–e122. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Ding, L.; Xuan, L.; Wang, T.; Li, M.; Zhao, Z.; Lu, J.; Xu, Y.; Chen, Y.; Wang, W.; et al. Urinary Bisphenol a Concentration and the Risk of Central Obesity in Chinese Adults: A Prospective Study. J. Diabetes 2017, 10, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-J.; Oh, H. Trends and Inequalities in Overall and Abdominal Obesity by Sociodemographic Factors in Korean Adults, 1998–2018. Int. J. Environ. Res. Public Health 2021, 18, 4162. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.F.; Clegg, D.J. The Sexual Dimorphism of Obesity. Mol. Cell. Endocrinol. 2015, 402, 113–119. [Google Scholar] [CrossRef]
- Valencak, T.G.; Osterrieder, A.; Schulz, T.J. Sex Matters: The Effects of Biological Sex on Adipose Tissue Biology and Energy Metabolism. Redox Biol. 2017, 12, 806–813. [Google Scholar] [CrossRef]
- Peters, S.A.E.; Bots, S.H.; Woodward, M. Sex Differences in the Association Between Measures of General and Central Adiposity and the Risk of Myocardial Infarction: Results From the UK Biobank. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Karastergiou, K.; Smith, S.R.; Greenberg, A.S.; Fried, S.K. Sex Differences in Human Adipose Tissues – The Biology of Pear Shape. Biol. Sex Differ. 2012, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Barzin, M.; Piri, Z.; Serahati, S.; Valizadeh, M.; Azizi, F.; Hosseinpanah, F. Incidence of Abdominal Obesity and Its Risk Factors Among Tehranian Adults. Public Health Nutr. 2018, 21, 3111–3117. [Google Scholar] [CrossRef] [PubMed]
- Løvsletten, O.; Jacobsen, B.K.; Grimsgaard, S.; Njølstad, I.; Wilsgaard, T.; Løchen, M.-L.; Eggen, A.E.; Hopstock, L.A. Prevalence of General and Abdominal Obesity in 2015–2016 and 8-Year Longitudinal Weight and Waist Circumference Changes in Adults and Elderly: The Tromsø Study. BMJ Open 2020, 10, e038465. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Peng, Q.; Yang, Y.; Zheng, S.; Wang, Y.; Lu, W. The Prevalence and Increasing Trends of Overweight, General Obesity, and Abdominal Obesity Among Chinese Adults: A Repeated Cross-Sectional Study. BMC Public Health 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Pahor, M.; Tylavsky, F.; Kritchevsky, S.B.; Cauley, J.A.; Newman, A.B.; Blunt, B.A.; Harris, T.B. One- And Two-Year Change in Body Composition as Measured by DXA in a Population-Based Cohort of Older Men and Women. J. Appl. Physiol. 2003, 94, 2368–2374. [Google Scholar] [CrossRef] [PubMed]
- Kuk, J.L.; Saunders, T.J.; Davidson, L.E.; Ross, R. Age-Related Changes in Total and Regional Fat Distribution. Ageing Res. Rev. 2009, 8, 339–348. [Google Scholar] [CrossRef]
- Tchkonia, T.; Morbeck, D.E.; Von Zglinicki, T.; Van Deursen, J.; Lustgarten, J.; Scrable, H.; Khosla, S.; Jensen, M.D.; Kirkland, J.L. Fat Tissue, Aging, and Cellular Senescence. Aging Cell 2010, 9, 667–684. [Google Scholar] [CrossRef] [PubMed]
- Sardinha, L.B.; Santos, D.A.; Silva, A.M.; Coelho-e-Silva, M.J.; Raimundo, A.M.; Moreira, H.; Santos, R.; Vale, S.; Baptista, F.; Mota, J. Prevalence of Overweight, Obesity, and Abdominal Obesity in a Representative Sample of Portuguese Adults. PLoS ONE 2012, 7, e47883. [Google Scholar] [CrossRef]
- López-Sobaler, A.M.; Rodríguez-Rodríguez, E.; Aranceta-Bartrina, J.; Gil, Á.; González-Gross, M.; Serra-Majem, L.; Varela-Moreiras, G.; Ortega, R.M. General and Abdominal Obesity Is Related to Physical Activity, Smoking and Sleeping Behaviours and Mediated by the Educational Level: Findings From the ANIBES Study in Spain. PLOS ONE 2016, 11, e0169027. [Google Scholar] [CrossRef]
- Hajian-Tilaki, K.O.; Heidari, B. Association of Educational Level With Risk of Obesity and Abdominal Obesity in Iranian Adults. J. Public Health 2009, 32, 202–209. [Google Scholar] [CrossRef]
- Malik, K.S.; Kouame, J.; Gbane, M.; Coulibaly, M.; Ake, D.M.; Ake, O. Prevalence of Abdominal Obesity and Its Correlates Among Adults in a Peri-Urban Population of West Africa. AIMS Public Health 2019, 6, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Dagne, S.; Menber, Y.; Petrucka, P.; Wassihun, Y. Prevalence and Associated Factors of Abdominal Obesity Among the Adult Population in Woldia Town, Northeast Ethiopia, 2020: Community-Based Cross-Sectional Study. PLOS ONE 2021, 16, e0247960. [Google Scholar] [CrossRef]
- Czernichow, S.; Bertrais, S.; Preziosi, P.; Galan, P.; Hercberg, S.; Oppert, J. Indicators of Abdominal Adiposity in Middle-Aged Participants of the SU.VI.MAX Study: Relationships With Educational Level, Smoking Status and Physical Inactivity. Diabetes & Metab. 2004, 30, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Vernay, M.; Malon, A.; Oleko, A.; Salanave, B.; Roudier, C.; Szego, E.; Deschamps, V.; Hercberg, S.; Castetbon, K. Association of Socioeconomic Status With Overall Overweight and Central Obesity in Men and Women: The French Nutrition and Health Survey 2006. BMC Public Health 2009, 9. [Google Scholar] [CrossRef]
- Cisse, K.; Samadoulougou, S.; Ouedraogo, M.; Kouanda, S.; Kirakoya-Samadoulougou, F. Prevalence of Abdominal Obesity and Its Association With Cardiovascular Risk Among the Adult Population in Burkina Faso: Findings From a Nationwide Cross-Sectional Study. BMJ Open 2021, 11, e049496. [Google Scholar] [CrossRef] [PubMed]
- Dagne, S.; Menber, Y.; Petrucka, P.; Wassihun, Y. Prevalence and Associated Factors of Abdominal Obesity Among the Adult Population in Woldia Town, Northeast Ethiopia, 2020: Community-Based Cross-Sectional Study. PLOS ONE 2021, 16, e0247960. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, T.S.; Zeleke, T.M.; Alemu, W.; Argaw, D.; Bedane, T.K. Dietary Diversity and Physical Activity as Risk Factors of Abdominal Obesity Among Adults in Dilla Town, Ethiopia. PLOS ONE 2020, 15, e0236671. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. The Diseasome of Physical Inactivity - And the Role of Myokines in Muscle-Fat Cross Talk. J. Physiol. 2009, 587, 5559–5568. [Google Scholar] [CrossRef]
- Slentz, C.A.; Houmard, J.A.; Kraus, W.E. Exercise, Abdominal Obesity, Skeletal Muscle, and Metabolic Risk: Evidence for a Dose Response. Obesity 2009, 17, S27–S33. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C.J. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Forceon Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World HeartFederation; International Atherosclerosis Society; And International Association for the Study of Obesity. Obes. Metab. 2010, 7, 63–65. [Google Scholar] [CrossRef]
- Pan American Health Organization. Ultra-Processed Food and Drink Products in Latin America: Trends, Impact on Obesity, Policy Implications; Washington, Pan American Health Organization; 2015; Available online: https://iris.paho.org/handle/10665.2/7699.
| Variables | Abdominal obesity | |||
|---|---|---|---|---|
| ABSENCE n = 231 (22.78%) |
PRESENCE n = 783 (77.22%) |
p-value a | Total n = 1014 (100%) |
|
| Sex n = 1014 | < 0.001 | |||
| Female | 65 (6.41) | 374 (36.88) | 439 (43.29) | |
| Male | 166 (16.37) | 409 (40.34) | 575 (56.71) | |
| Age range n = 1014 | < 0.001 | |||
| Adult | 168 (16.57) | 414 (40.83) | 582 (57.4) | |
| Elderly | 63 (6.21) | 369 (36.39) | 432 (69.22) | |
| Marital status n = 1014 | 0.919 | |||
| With partner | 128 (12.62) | 439 (43.29) | 567 (55.91) | |
| No partner | 103 (10.16) | 344 (33.93) | 447 (44.09) | |
| Race/Color n = 1013 | 0.494 | |||
| White | 57 (5.63) | 214 (21.12) | 271 (26.75) | |
| No white | 173 (17.08) | 569 (56.17) | 742 (73.25) | |
| Income (Minimum Wages) n = 971 | 0.179 | |||
| < 1 | 31 (3.19) | 74 (7.62) | 105 (10.81) | |
| 1–5 | 165 (17) | 570 (58.7) | 5.7) | |
| > 5–10 | 21 (2.16) | 66 (6.8) | 8.96) | |
| > 10 | 6 (0.62) | 38 (3.91) | 44 (4.53) | |
| Education (years) n = 1012 | 0.004 | |||
| < 8 | 95 (9.39%) | 425 (42) | 520 (51.39) | |
| 8 – 11 | 105 (10.37) | 231 (22.83) | 336 (33.2) | |
| > 11 | 31 (3.06) | 125 (12.35) | 156 (15.41) | |
| Work activity n = 1000 | 0.083 | |||
| With paid activity | 127 (12.7) | 365 (36.5) | 492 (49.2) | |
| No paid activity | 11 (1.1) | 37 (3.7) | 48 (4.8) | |
| Retired or on sick leave | 91 (9.1) | 369 (36.9) | 460 (46) | |
| Smoking n = 1008 | 0.329 | |||
| Smoker | 12 (1.19) | 41 (4.07) | 53 (5.26) | |
| Former smoker | 74 (7.34) | 293 (29.07) | 367 (36.41) | |
| Never smoked | 143 (14.18) | 445 (44.15) | 588 (58.33) | |
| CKD b Time (years) n = 1009 | 0.01 | |||
| ≤ 5 | 121 (11.99) | 483 (47.87) | 604 (59.86) | |
| > 5 | 110 (10.9) | 295 (29.24) | 405 (40.14) | |
| Hemodialysis time (years) n = 958 | 0.02 | |||
| < 1 | 10 (1.04) | 49 (5.11) | 59 (5.15) | |
| 1–5 | 107 (11.17) | 449 (46.87) | 8.04) | |
| > 5–10 | 54 (5.64) | 141 (14.72) | 195 (20.36) | |
| > 10 | 41 (4.28) | 107 (11.17) | 148 (15.45) | |
| Diabetes n = 1014 | < 0.001 | |||
| Absence | 175 (17.26) | 468 (46.15) | 643 (63.41) | |
| Presence | 56 (5.52) | 315 (31.07) | 371 (36.59) | |
| Hypertension n = 1014 | 0.323 | |||
| Absence | 42 (4.14) | 119 (11.74) | 161 (15.88) | |
| Presence | 189 (18.64) | 664 (65.48) | 853 (84.12) | |
| Physical activity n = 1013 | < 0.001 | |||
| Below recommended | 40 (3.95) | 71 (7.01) | 111 (10.96) | |
| Within the recommended | 34 (3.35) | 83 (8.19) | 117 (11.54) | |
| Does not practice | 156 (15.4) | 629 (62.1) | 785 (77.5) | |
| BMI c n = 951 | < 0.001 | |||
| No overweight | 195 (20.5) | 293 (30.8) | 488 (51.3) | |
| Overweight | 24 (2.5) | 439 (46.2) | 463 (48.7) | |
| UPF d Consumption n = 1014 | < 0.001 | |||
| Q1 + Q2 e | 102 (10.06) | 485 (47.83) | 587 (57.89) | |
| Q3 + Q4 e | 129 (12.72) | 298 (29.39) | 427 (42.11) | |
| Variables | Model 1 a | Model 2 b | Model 3 c | Final model d | ||||
|---|---|---|---|---|---|---|---|---|
| p-value e | OR f (CI95% g) | p-value e | OR f (CI95% g) | p-value e | OR f (CI95% g) | p-value e | OR f (CI95% g) | |
| UPF Consumption | ||||||||
| Q1 + Q2 i | 1 | 1 | 1 | 1 | ||||
| Q3 + Q4 i | < 0.001 | 1.77 (1.30–2.41) | 0.001 | 1.83 (1.34–2.50) | 0.001 | 1.72 (1.23–2.39) | 0.001 | 1.72 (1.23–2.39) |
| Sex | ||||||||
| Female | 1 | 1 | 1 | 1 | ||||
| Male | < 0.001 | 2.36 (1.70–3.28) | < 0.001 | 2.42 (1.72–3.38) | < 0.001 | 2.21 (1.55–3.16) | < 0.001 | 2.21 (1.55–3.16) |
| Age range | ||||||||
| Adult | < 0.001 | 2.31 (1.65–3.22) | < 0.001 | 2.23 (1.57–3.17) | < 0.001 | 2.00 (1.38–2.91) | < 0.001 | 2.00 (1.38–2.91) |
| Elderly | 1 | 1 | 1 | 1 | ||||
| Education (years) | ||||||||
| < 8 | 0.525 | 1.16 (0.73–1.84) | 0.498 | 1.17 (0.73–1.87) | 0.532 | 1.16 (0.71–1.90) | 0.532 | 1.16 (0.71–1.90) |
| 8–11 | 0.036 | 1.66 (1.03–2.67) | 0.034 | 1.67 (1.03–2.71) | 0.035 | 1.70 (1.03–2.79) | 0.035 | 1.70 (1.03–2.79) |
| > 11 | 1 | 1 | 1 | 1 | ||||
| Work activity | ||||||||
| With paid activity | 0.470 | 1.13 (0.80–1.58) | 0.507 | 1.12 (0.79–1.59) | ||||
| No paid activity | 0.812 | 1.10 (0.49–2.43) | 0.920 | 0.95 (0.40–2.28) | ||||
| Retired or on sick leave | 1 | 1 | ||||||
| Physical activity | ||||||||
| Below recommended | 1 | 1 | ||||||
| Within the recommended | 0.004 | 1.97 (1.23–3.14) | 0.004 | 1.97 (1.23–3.14) | ||||
| Does not practice | 0.389 | 1.23 (0.76–2.00) | 0.389 | 1.23 (0.76–2.00) | ||||
| CKD j time (years) | ||||||||
| ≤ 5 | 1 | |||||||
| > 5 | 0.698 | 1.29 (0.73–2.28) | ||||||
| Hemodialysis time (years) | ||||||||
| < 1 | 1 | |||||||
| 1–5 | 0.698 | 0.86 (0.40–1.81) | ||||||
| > 5–10 | 0.906 | 1.05 (0.44–2.51) | ||||||
| > 10 | 0.888 | 0.93 (0.38–2.30) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





