Submitted:
22 May 2024
Posted:
23 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
| 5.7 μM |
2. Marine Compounds with Antibacterial, Antifungal, Antiprotozoal, Antituberculosis and Antiviral Activities
2.1. Antibacterial Activity
2.2. Antifungal Activity
2.3. Antiprotozoal and Antituberculosis Activity
2.4. Antiviral Activity
3. Marine Compounds with Antidiabetic and Anti-Inflammatory Activity, and Affecting the Immune and Nervous System
3.1. Antidiabetic Activity
3.2. Anti-Inflammatory Activity
3.3. Marine Compounds with Activity on the Immune System
3.4. Marine Compounds Affecting the Nervous System
4. Marine Compounds with Miscellaneous Mechanisms of Action
5. Reviews on Marine Pharmacology and Pharmaceuticals
6. Conclusions
Author Contributions
Acknowledgments
Declarations of Interest
References
- Mayer, A.M.S.; Lehmann, V.K.B. Marine pharmacology in 1998: marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, anthelmintic, antiplatelet, antiprotozoal, and antiviral activities;with actions on the cardiovascular, endocrine, immune, and nervous systems; and other miscellaneous mechanisms of action. The Pharmacologist 2000, 42, 62-69.
- Mayer, A.M.; Hamann, M.T. Marine pharmacology in 1999: compounds with antibacterial, anticoagulant, antifungal, anthelmintic, anti-inflammatory, antiplatelet, antiprotozoal and antiviral activities affecting the cardiovascular, endocrine, immune and nervous systems, and other miscellaneous mechanisms of action. Comp Biochem Physiol C Toxicol Pharmacol 2002, 132, 315-339. [CrossRef]
- Mayer, A.M.S.; Hamann, M.T. Marine pharmacology in 2000: marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antituberculosis, and antiviral activities; affecting the cardiovascular, immune, and nervous systems and other miscellaneous mechanisms of action. Mar. Biotechnol. (NY) 2004, 6, 37-52. [CrossRef]
- Mayer, A.M.; Hamann, M.T. Marine pharmacology in 2001-2: marine compounds with anthelmintic, antibacterial, anticoagulant, antidiabetic, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems and other miscellaneous mechanisms of action. Comp Biochem. Physiol C. Toxicol. Pharmacol 2005, 140, 265-286. [CrossRef]
- Mayer, A.M.; Rodriguez, A.D.; Berlinck, R.G.; Hamann, M.T. Marine pharmacology in 2003-4: marine compounds with anthelmintic antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Comp Biochem. Physiol C Toxicol. Pharmacol 2007, 145, 553-581.
- Mayer, A.M.; Rodriguez, A.D.; Berlinck, R.G.; Hamann, M.T. Marine pharmacology in 2005-6: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Biochim. Biophys. Acta 2009, 1790, 283-308. [CrossRef]
- Mayer, A.M.; Rodriguez, A.D.; Berlinck, R.G.; Fusetani, N. Marine pharmacology in 2007-8: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous system, and other miscellaneous mechanisms of action. Comp Biochem. Physiol C. Toxicol. Pharmacol 2011, 153, 191-222. [CrossRef]
- Mayer, A.M.; Rodriguez, A.D.; Taglialatela-Scafati, O.; Fusetani, N. Marine Pharmacology in 2009-2011: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis, and Antiviral Activities; Affecting the Immune and Nervous Systems, and other Miscellaneous Mechanisms of Action. Mar. Drugs 2013, 11, 2510-2573. [CrossRef]
- Mayer, A.M.S.; Rodriguez, A.D.; Taglialatela-Scafati, O.; Fusetani, N. Marine Pharmacology in 2012-2013: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis, and Antiviral Activities; Affecting the Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action. Mar Drugs 2017, 15, 273. [CrossRef]
- Mayer, A.M.S.; Guerrero, A.J.; Rodriguez, A.D.; Taglialatela-Scafati, O.; Nakamura, F.; Fusetani, N. Marine Pharmacology in 2014-2015: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis, Antiviral, and Anthelmintic Activities; Affecting the Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action. Mar Drugs 2019, 18. [CrossRef]
- Mayer, A.M.S.; Guerrero, A.J.; Rodriguez, A.D.; Taglialatela-Scafati, O.; Nakamura, F.; Fusetani, N. Marine Pharmacology in 2016-2017: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis and Antiviral Activities; Affecting the Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action. Mar Drugs 2021, 19. [CrossRef]
- Mayer, A.M.S.; Pierce, M.L.; Howe, K.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Nakamura, F.; Fusetani, N. Marine pharmacology in 2018: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Pharmacol Res 2022, 183, 106391. [CrossRef]
- F.J. Schmitz, B.F. Bowden, Toth, S.I., Antitumor and Cytotoxic Compounds from Marine Organisms, in: D.H. Attaway, O.R. Zaborsky (Eds.) Plenum Press, New York and London, 1993, pp. 197-308.
- Gomez-Rodriguez, L.; Schultz, P.J.; Tamayo-Castillo, G.; Dotson, G.D.; Sherman, D.H.; Tripathi, A. Adipostatins E-J, New Potent Antimicrobials Identified as Inhibitors of Coenzyme-A Biosynthesis. Tetrahedron Lett 2020, 61. [CrossRef]
- Elliott, A.G.; Huang, J.X.; Neve, S.; Zuegg, J.; Edwards, I.A.; Cain, A.K.; Boinett, C.J.; Barquist, L.; Lundberg, C.V.; Steen, J.; et al. An amphipathic peptide with antibiotic activity against multidrug-resistant Gram-negative bacteria. Nat Commun 2020, 11, 3184. [CrossRef]
- Paderog, M.J.V.; Suarez, A.F.L.; Sabido, E.M.; Low, Z.J.; Saludes, J.P.; Dalisay, D.S. Anthracycline Shunt Metabolites From Philippine Marine Sediment-Derived Streptomyces Destroy Cell Membrane Integrity of Multidrug-Resistant Staphylococcus aureus. Front Microbiol 2020, 11, 743. [CrossRef]
- Wang, M.; Zhao, L.; Wu, H.; Zhao, C.; Gong, Q.; Yu, W. Cladodionen Is a Potential Quorum Sensing Inhibitor Against Pseudomonas aeruginosa. Mar Drugs 2020, 18. [CrossRef]
- Gowrishankar, S.; Pandian, S.K.; Balasubramaniam, B.; Balamurugan, K. Quorum quelling efficacy of marine cyclic dipeptide -cyclo(L-leucyl-L-prolyl) against the uropathogen Serratia marcescens. Food Chem Toxicol 2019, 123, 326-336. [CrossRef]
- de Figueiredo, C.S.; Menezes Silva, S.M.P.; Abreu, L.S.; da Silva, E.F.; da Silva, M.S.; Cavalcanti de Miranda, G.E.; Costa, V.C.O.; Le Hyaric, M.; Siqueira Junior, J.P.; Barbosa Filho, J.M.; et al. Dolastane diterpenes from Canistrocarpus cervicornis and their effects in modulation of drug resistance in Staphylococcus aureus. Nat Prod Res 2019, 33, 3231-3239. [CrossRef]
- Davison, J.R.; Bewley, C.A. Antimicrobial Chrysophaentin Analogs Identified from Laboratory Cultures of the Marine Microalga Chrysophaeum taylorii. J Nat Prod 2019, 82, 148-153. [CrossRef]
- Wang, Y.; Zhang, J.; Sun, Y.; Sun, L. A Crustin from Hydrothermal Vent Shrimp: Antimicrobial Activity and Mechanism. Mar Drugs 2021, 19. [CrossRef]
- Campana, R.; Favi, G.; Baffone, W.; Lucarini, S. Marine Alkaloid 2,2-Bis(6-bromo-3-indolyl) Ethylamine and Its Synthetic Derivatives Inhibit Microbial Biofilms Formation and Disaggregate Developed Biofilms. Microorganisms 2019, 7. [CrossRef]
- Liang, X.; Matthew, S.; Chen, Q.Y.; Kwan, J.C.; Paul, V.J.; Luesch, H. Discovery and Total Synthesis of Doscadenamide A: A Quorum Sensing Signaling Molecule from a Marine Cyanobacterium. Org Lett 2019, 21, 7274-7278. [CrossRef]
- Jabila Mary, T.R.; Kannan, R.R.; Muthamil Iniyan, A.; Carlton Ranjith, W.A.; Nandhagopal, S.; Vishwakarma, V.; Prakash Vincent, S.G. beta-lactamase inhibitory potential of kalafungin from marine Streptomyces in Staphylococcus aureus infected zebrafish. Microbiol Res 2021, 244, 126666. [CrossRef]
- Maynard, A.; Butler, N.L.; Ito, T.; da Silva, A.J.; Murai, M.; Chen, T.; Koffas, M.A.G.; Miyoshi, H.; Barquera, B. Antibiotic Korormicin A Kills Bacteria by Producing Reactive Oxygen Species. J Bacteriol 2019, 201. [CrossRef]
- Chung, B.; Kwon, O.S.; Shin, J.; Oh, K.B. Antibacterial Activity and Mode of Action of Lactoquinomycin A from. Mar Drugs 2020, 19. [CrossRef]
- Thulshan Jayathilaka, E.H.T.; Liyanage, T.D.; Rajapaksha, D.C.; Dananjaya, S.H.S.; Nikapitiya, C.; Whang, I.; De Zoysa, M. Octominin: An antibacterial and anti-biofilm peptide for controlling the multidrug resistance and pathogenic Streptococcus parauberis. Fish Shellfish Immunol 2021, 110, 23-34. [CrossRef]
- Yu, X.; Li, L.; Sun, S.; Chang, A.; Dai, X.; Li, H.; Wang, Y.; Zhu, H. A Cyclic Dipeptide from Marine Fungus. ACS Omega 2021, 6, 7693-7700. [CrossRef]
- Pan, Y.; Zheng, L.B.; Mao, Y.; Wang, J.; Lin, L.S.; Su, Y.Q.; Li, Y. The antibacterial activity and mechanism analysis of piscidin 5 like from Larimichthys crocea. Dev Comp Immunol 2019, 92, 43-49. [CrossRef]
- Kim, Y.G.; Lee, J.H.; Lee, S.; Lee, Y.K.; Hwang, B.S.; Lee, J. Antibiofilm Activity of Phorbaketals from the Marine Sponge. Mar Drugs 2021, 19. [CrossRef]
- Kizhakkekalam, V.C., K.; Joy, M. Antibacterial and antioxidant aryl-enclosed macrocyclic polyketide from intertidal macroalgae associated heterotrophic bacterium Shewanella algae. Med. Chem. Res. 2020, 29, 145-155. [CrossRef]
- Chakraborty, K.; Kizhakkekalam, V.K.; Joy, M. Macrocyclic polyketides with siderophore mode of action from marine heterotrophic Shewanella algae: Prospective anti-infective leads attenuate drug-resistant pathogens. J Appl Microbiol 2021, 130, 1552-1570. [CrossRef]
- Hansen, K. Antimicrobial activity of securamines from the bryozoan Securiflustra securifrons 2021.
- Hansen, I.K.O.; Isaksson, J.; Poth, A.G.; Hansen, K.O.; Andersen, A.J.C.; Richard, C.S.M.; Blencke, H.M.; Stensvag, K.; Craik, D.J.; Haug, T. Isolation and Characterization of Antimicrobial Peptides with Unusual Disulfide Connectivity from the Colonial Ascidian Synoicum turgens. Mar Drugs 2020, 18. [CrossRef]
- Reina, J.C.; Perez-Victoria, I.; Martin, J.; Llamas, I. A Quorum-Sensing Inhibitor Strain of Vibrio alginolyticus Blocks Qs-Controlled Phenotypes in Chromobacterium violaceum and Pseudomonas aeruginosa. Mar Drugs 2019, 17. [CrossRef]
- Elsadek, L.A.; Matthews, J.H.; Nishimura, S.; Nakatani, T.; Ito, A.; Gu, T.; Luo, D.; Salvador-Reyes, L.A.; Paul, V.J.; Kakeya, H.; et al. Genomic and Targeted Approaches Unveil the Cell Membrane as a Major Target of the Antifungal Cytotoxin Amantelide A. Chembiochem 2021, 22, 1790-1799. [CrossRef]
- Yang, B.; He, Y.; Lin, S.; Zhang, J.; Li, H.; Wang, J.; Hu, Z.; Zhang, Y. Antimicrobial Dolabellanes and Atranones from a Marine-Derived Strain of the Toxigenic Fungus Stachybotrys chartarum. J Nat Prod 2019, 82, 1923-1929. [CrossRef]
- Tang, X.X.; Yan, X.; Fu, W.H.; Yi, L.Q.; Tang, B.W.; Yu, L.B.; Fang, M.J.; Wu, Z.; Qiu, Y.K. New beta-Lactone with Tea Pathogenic Fungus Inhibitory Effect from Marine-Derived Fungus MCCC3A00957. J Agric Food Chem 2019, 67, 2877-2885. [CrossRef]
- Kim, H.; Hwang, J.Y.; Chung, B.; Cho, E.; Bae, S.; Shin, J.; Oh, K.B. 2-Alkyl-4-hydroxyquinolines from a Marine-Derived Streptomyces sp. Inhibit Hyphal Growth Induction in Candida albicans. Mar Drugs 2019, 17. [CrossRef]
- Dalisay, D.S.; Rogers, E.W.; Molinski, T.F. Oceanapiside, a Marine Natural Product, Targets the Sphingolipid Pathway of Fluconazole-Resistant. Mar Drugs 2021, 19. [CrossRef]
- Tripathi, S.K.; Feng, Q.; Liu, L.; Levin, D.E.; Roy, K.K.; Doerksen, R.J.; Baerson, S.R.; Shi, X.; Pan, X.; Xu, W.H.; et al. Puupehenone, a Marine-Sponge-Derived Sesquiterpene Quinone, Potentiates the Antifungal Drug Caspofungin by Disrupting Hsp90 Activity and the Cell Wall Integrity Pathway. mSphere 2020, 5. [CrossRef]
- Meng, L.; Sun, C.; Zhang, C.; Song, S.; Sun, X.; Ju, J.; Deng, Y. Efficacy of Compounds Isolated from Streptomyces olivaceus against the Morphogenesis and Virulence of Candida albicans. Mar Drugs 2019, 17. [CrossRef]
- Alhadrami, H.A.; Thissera, B.; Hassan, M.H.A.; Behery, F.A.; Ngwa, C.J.; Hassan, H.M.; Pradel, G.; Abdelmohsen, U.R.; Rateb, M.E. Bio-Guided Isolation of Antimalarial Metabolites from the Coculture of Two Red Sea Sponge-Derived Actinokineospora and Rhodococcus spp. Mar Drugs 2021, 19. [CrossRef]
- Sweeney-Jones, A.M.; Gagaring, K.; Antonova-Koch, J.; Zhou, H.; Mojib, N.; Soapi, K.; Skolnick, J.; McNamara, C.W.; Kubanek, J. Antimalarial Peptide and Polyketide Natural Products from the Fijian Marine Cyanobacterium Moorea producens. Mar Drugs 2020, 18. [CrossRef]
- Knestrick, M.A.; Wilson, N.G.; Roth, A.; Adams, J.H.; Baker, B.J. Friomaramide, a Highly Modified Linear Hexapeptide from an Antarctic Sponge, Inhibits Plasmodium falciparum Liver-Stage Development. J Nat Prod 2019, 82, 2354-2358. [CrossRef]
- Wright, A.E.; Collins, J.E.; Roberts, B.; Roberts, J.C.; Winder, P.L.; Reed, J.K.; Diaz, M.C.; Pomponi, S.A.; Chakrabarti, D. Antiplasmodial Compounds from Deep-Water Marine Invertebrates. Mar Drugs 2021, 19. [CrossRef]
- Rodríguez-Expósito, R.L.; Nocchi, N.; Reyes-Batlle, M.; Sifaoui, I.; Suárez-Gómez, B.; Díaz-Marrero, A.R.; Souto, M.L.; Piñero, J.E.; Fernández, J.J.; Lorenzo-Morales, J. Antiamoebic effects of sesquiterpene lactones isolated from the zoanthid Palythoa aff. clavata. Bioorg Chem 2021, 108, 104682. [CrossRef]
- Huang, H.N.; Chuang, C.M.; Chen, J.Y.; Chieh-Yu, P. Epinecidin-1: A marine fish antimicrobial peptide with therapeutic potential against Trichomonas vaginalis infection in mice. Peptides 2019, 112, 139-148. [CrossRef]
- Lima, M.L.; Romanelli, M.M.; Borborema, S.E.T.; Johns, D.M.; Migotto, A.E.; Lago, J.H.G.; Tempone, A.G. Antitrypanosomal activity of isololiolide isolated from the marine hydroid Macrorhynchia philippina (Cnidaria, Hydrozoa). Bioorg Chem 2019, 89, 103002. [CrossRef]
- Lorenzo-Morales, J.; Diaz-Marrero, A.R.; Cen-Pacheco, F.; Sifaoui, I.; Reyes-Batlle, M.; Souto, M.L.; Hernandez Daranas, A.; Pinero, J.E.; Fernandez, J.J. Evaluation of Oxasqualenoids from the Red Alga Laurencia viridis against Acanthamoeba. Mar Drugs 2019, 17. [CrossRef]
- Boudreau, P.D.; Miller, B.W.; McCall, L.I.; Almaliti, J.; Reher, R.; Hirata, K.; Le, T.; Siqueira-Neto, J.L.; Hook, V.; Gerwick, W.H. Design of Gallinamide A Analogs as Potent Inhibitors of the Cysteine Proteases Human Cathepsin L and Trypanosoma cruzi Cruzain. J Med Chem 2019, 62, 9026-9044. [CrossRef]
- Cartuche, L.; Reyes-Batlle, M.; Sifaoui, I.; Arberas-Jimenez, I.; Pinero, J.E.; Fernandez, J.J.; Lorenzo-Morales, J.; Diaz-Marrero, A.R. Antiamoebic Activities of Indolocarbazole Metabolites Isolated from Streptomyces sanyensis Cultures. Mar Drugs 2019, 17. [CrossRef]
- Casertano, M.; Imperatore, C.; Luciano, P.; Aiello, A.; Putra, M.Y.; Gimmelli, R.; Ruberti, G.; Menna, M. Chemical Investigation of the Indonesian Tunicate Polycarpa aurata and Evaluation of the Effects Against Schistosoma mansoni of the Novel Alkaloids Polyaurines A and B. Mar Drugs 2019, 17. [CrossRef]
- Liu, Z.; Wang, Q.; Li, S.; Cui, H.; Sun, Z.; Chen, D.; Lu, Y.; Liu, H.; Zhang, W. Polypropionate Derivatives with Mycobacterium tuberculosis Protein Tyrosine Phosphatase B Inhibitory Activities from the Deep-Sea-Derived Fungus Aspergillus fischeri FS452. J Nat Prod 2019, 82, 3440-3449. [CrossRef]
- Sudomova, M.; Shariati, M.A.; Echeverria, J.; Berindan-Neagoe, I.; Nabavi, S.M.; Hassan, S.T.S. A Microbiological, Toxicological, and Biochemical Study of the Effects of Fucoxanthin, a Marine Carotenoid, on Mycobacterium tuberculosis and the Enzymes Implicated in Its Cell Wall: A Link Between Mycobacterial Infection and Autoimmune Diseases. Mar Drugs 2019, 17. [CrossRef]
- Liu, D.; Li, Y.; Guo, X.; Ji, W.; Lin, W. Chartarlactams Q-T, Dimeric Phenylspirodrimanes with Antibacterial and Antiviral Activities. Chem Biodivers 2020, 17, e2000170. [CrossRef]
- Li, B.; Li, L.; Peng, Z.; Liu, D.; Si, L.; Wang, J.; Yuan, B.; Huang, J.; Proksch, P.; Lin, W. Harzianoic acids A and B, new natural scaffolds with inhibitory effects against hepatitis C virus. Bioorg Med Chem 2019, 27, 560-567. [CrossRef]
- Lin, S.C.; Lehman, C.W.; Stewart, A.K.; Panny, L.; Bracci, N.; Wright, J.L.C.; Paige, M.; Strangman, W.K.; Kehn-Hall, K. Homoseongomycin, a compound isolated from marine actinomycete bacteria K3-1, is a potent inhibitor of encephalitic alphaviruses. Antiviral Res 2021, 191, 105087. [CrossRef]
- Tan, S.; Yang, B.; Liu, J.; Xun, T.; Liu, Y.; Zhou, X. Penicillixanthone A, a marine-derived dual-coreceptor antagonist as anti-HIV-1 agent. Nat Prod Res 2019, 33, 1467-1471. [CrossRef]
- Izumida, M.; Suga, K.; Ishibashi, F.; Kubo, Y. The Spirocyclic Imine from a Marine Benthic Dinoflagellate, Portimine, Is a Potent Anti-Human Immunodeficiency Virus Type 1 Therapeutic Lead Compound. Mar Drugs 2019, 17. [CrossRef]
- Das, S.K.; Samantaray, D.; Sahoo, S.K.; Pradhan, S.K.; Samanta, L.; Thatoi, H. Bioactivity guided isolation of antidiabetic and antioxidant compound from Xylocarpus granatum J. Koenig bark. 3 Biotech 2019, 9, 198. [CrossRef]
- Luo, J.; Jiang, B.; Li, C.; Jia, X.; Shi, D. CYC27 Synthetic Derivative of Bromophenol from Red Alga Rhodomela confervoides: Anti-Diabetic Effects of Sensitizing Insulin Signaling Pathways and Modulating RNA Splicing-Associated RBPs. Mar Drugs 2019, 17. [CrossRef]
- Zaharudin, N.; Staerk, D.; Dragsted, L.O. Inhibition of alpha-glucosidase activity by selected edible seaweeds and fucoxanthin. Food Chem 2019, 270, 481-486. [CrossRef]
- Kawee-Ai, A.K., A.; Kim, S. . Inhibitory activities of microalgal fucoxanthin against α-amylase, α-glucosidase, and glucose oxidase in 3T3-L1 cells linked to type 2 diabetes. J. Oceanol. Limnol. 2019, 37, 928-937. [CrossRef]
- Hudlikar, R.R.; Sargsyan, D.; Li, W.; Wu, R.; Zheng, M.; Kong, A.N. Epigenomic, Transcriptomic, and Protective Effect of Carotenoid Fucoxanthin in High Glucose-Induced Oxidative Stress in Mes13 Kidney Mesangial Cells. Chem Res Toxicol 2021, 34, 713-722. [CrossRef]
- Chakraborty, K.A., T. First report of antioxidative abeo-oleanenes from red seaweed Gracilaria salicornia as dual inhibitors of starch digestive enzymes. Med. Chem. Res. 2019, 28, 696-710. [CrossRef]
- Yang, H.W.; Son, M.; Choi, J.; Oh, S.; Jeon, Y.J.; Byun, K.; Ryu, B. Effect of Ishophloroglucin A, A Component of Ishige okamurae, on Glucose Homeostasis in the Pancreas and Muscle of High Fat Diet-Fed Mice. Mar Drugs 2019, 17. [CrossRef]
- Paudel, P.; Seong, S.H.; Park, H.J.; Jung, H.A.; Choi, J.S. Anti-Diabetic Activity of 2,3,6-Tribromo-4,5-Dihydroxybenzyl Derivatives from Symphyocladia latiuscula through PTP1B Downregulation and alpha-Glucosidase Inhibition. Mar Drugs 2019, 17. [CrossRef]
- Seong, S.H.; Nguyen, D.H.; Wagle, A.; Woo, M.H.; Jung, H.A.; Choi, J.S. Experimental and Computational Study to Reveal the Potential of Non-Polar Constituents from Hizikia fusiformis as Dual Protein Tyrosine Phosphatase 1B and alpha-Glucosidase Inhibitors. Mar Drugs 2019, 17. [CrossRef]
- Lopez, D.; Cherigo, L.; Mejia, L.C.; Loza-Mejia, M.A.; Martinez-Luis, S. alpha-Glucosidase inhibitors from a mangrove associated fungus, Zasmidium sp. strain EM5-10. BMC Chem 2019, 13, 22. [CrossRef]
- Pereira, R.B.; Pereira, D.M.; Jimenez, C.; Rodriguez, J.; Nieto, R.M.; Videira, R.A.; Silva, O.; Andrade, P.B.; Valentao, P. Anti-Inflammatory Effects of 5alpha,8alpha-Epidioxycholest-6-en-3beta-ol, a Steroidal Endoperoxide Isolated from Aplysia depilans, Based on Bioguided Fractionation and NMR Analysis. Mar Drugs 2019, 17. [CrossRef]
- Wen, H.; Chen, C.; Sun, W.; Zang, Y.; Li, Q.; Wang, W.; Zeng, F.; Liu, J.; Zhou, Y.; Zhou, Q.; et al. Phenolic C-Glycosides and Aglycones from Marine-Derived Aspergillus sp. and Their Anti-Inflammatory Activities. J Nat Prod 2019, 82, 1098-1106. [CrossRef]
- Keeler, D.M.; Grandal, M.K.; McCall, J.R. Brevenal, a Marine Natural Product, is Anti-Inflammatory and an Immunomodulator of Macrophage and Lung Epithelial Cells. Mar Drugs 2019, 17. [CrossRef]
- Alvarino, R.; Alonso, E.; Lacret, R.; Oves-Costales, D.; Genilloud, O.; Reyes, F.; Alfonso, A.; Botana, L.M. Caniferolide A, a Macrolide from Streptomyces caniferus, Attenuates Neuroinflammation, Oxidative Stress, Amyloid-Beta, and Tau Pathology in Vitro. Mol Pharm 2019, 16, 1456-1466. [CrossRef]
- Van Thanh, N.; Jang, H.J.; Vinh, L.B.; Linh, K.T.P.; Huong, P.T.T.; Cuong, N.X.; Nam, N.H.; Van Minh, C.; Kim, Y.H.; Yang, S.Y. Chemical constituents from Vietnamese mangrove Calophyllum inophyllum and their anti-inflammatory effects. Bioorg Chem 2019, 88, 102921. [CrossRef]
- Ding, Y.; An, F.; Zhu, X.; Yu, H.; Hao, L.; Lu, Y. Curdepsidones B(-)G, Six Depsidones with Anti-Inflammatory Activities from the Marine-Derived Fungus Curvularia sp. IFB-Z10. Mar Drugs 2019, 17. [CrossRef]
- Ku, S.K.; Jeong, S.Y.; Yang, S.; Kim, K.M.; Choi, H.; Bae, J.S. Suppressive effects of collismycin C on polyphosphate-mediated vascular inflammatory responses. Fitoterapia 2019, 134, 447-453. [CrossRef]
- Oh, S.; Son, M.; Byun, K.A.; Jang, J.T.; Choi, C.H.; Son, K.H.; Byun, K. Attenuating Effects of Dieckol on High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease by Decreasing the NLRP3 Inflammasome and Pyroptosis. Mar Drugs 2021, 19. [CrossRef]
- Hu, T.Y.; Zhang, H.; Chen, Y.Y.; Jiao, W.H.; Fan, J.T.; Liu, Z.Q.; Lin, H.W.; Cheng, B.H. Dysiarenone from Marine Sponge Dysidea arenaria Attenuates ROS and Inflammation via Inhibition of 5-LOX/NF-kappaB/MAPKs and Upregulation of Nrf-2/OH-1 in RAW 264.7 Macrophages. J Inflamm Res 2021, 14, 587-597. [CrossRef]
- Kim, E.N.; Nabende, W.Y.; Jeong, H.; Hahn, D.; Jeong, G.S. The marine-derived natural product epiloliolide isolated from Sargassum horneri regulates NLRP3 via PKA/CREB, promoting proliferation and anti-inflammatory effects of human periodontal ligament cells. Mar Drugs 2021, 19. [CrossRef]
- Su, J.; Guo, K.; Huang, M.; Liu, Y.; Zhang, J.; Sun, L.; Li, D.; Pang, K.L.; Wang, G.; Chen, L.; et al. Fucoxanthin, a Marine Xanthophyll Isolated From Conticribra weissflogii ND-8: Preventive Anti-Inflammatory Effect in a Mouse Model of Sepsis. Front Pharmacol 2019, 10, 906. [CrossRef]
- Zheng, J.; Tian, X.; Zhang, W.; Zheng, P.; Huang, F.; Ding, G.; Yang, Z. Protective Effects of Fucoxanthin against Alcoholic Liver Injury by Activation of Nrf2-Mediated Antioxidant Defense and Inhibition of TLR4-Mediated Inflammation. Mar Drugs 2019, 17. [CrossRef]
- Ha, Y.J.; Choi, Y.S.; Oh, Y.R.; Kang, E.H.; Khang, G.; Park, Y.B.; Lee, Y.J. Fucoxanthin Suppresses Osteoclastogenesis via Modulation of MAP Kinase and Nrf2 Signaling. Mar Drugs 2021, 19. [CrossRef]
- Li, X.; Huang, R.; Liu, K.; Li, M.; Luo, H.; Cui, L.; Huang, L.; Luo, L. Fucoxanthin attenuates LPS-induced acute lung injury via inhibition of the TLR4/MyD88 signaling axis. Aging (Albany NY) 2020, 13, 2655-2667. [CrossRef]
- Li, Y.; Liu, L.; Sun, P.; Zhang, Y.; Wu, T.; Sun, H.; Cheng, K.W.; Chen, F. Fucoxanthinol from the Diatom Nitzschia Laevis Ameliorates Neuroinflammatory Responses in Lipopolysaccharide-Stimulated BV-2 Microglia. Mar Drugs 2020, 18. [CrossRef]
- Jan, J.S.; Yang, C.H.; Wang, M.H.; Lin, F.L.; Yen, J.L.; Hsieh, I.; Khotimchenko, M.; Lee, T.H.; Hsiao, G. Hirsutanol A Attenuates Lipopolysaccharide-Mediated Matrix Metalloproteinase 9 Expression and Cytokines Production and Improves Endotoxemia-Induced Acute Sickness Behavior and Acute Lung Injury. Mar Drugs 2019, 17. [CrossRef]
- Chen, H.; Yang, H.; Zhi, Y.; Yao, Q.; Liu, B. Evaluation of pyrrolidine-based analog of jaspine B as potential SphK1 inhibitors against rheumatoid arthritis. Bioorg Med Chem Lett 2021, 34, 127754. [CrossRef]
- Daskalaki, M.G.; Vyrla, D.; Harizani, M.; Doxaki, C.; Eliopoulos, A.G.; Roussis, V.; Ioannou, E.; Tsatsanis, C.; Kampranis, S.C. Neorogioltriol and Related Diterpenes from the Red Alga Laurencia Inhibit Inflammatory Bowel Disease in Mice by Suppressing M1 and Promoting M2-Like Macrophage Responses. Mar Drugs 2019, 17. [CrossRef]
- Herath, K.; Kim, H.J.; Jang, J.H.; Kim, H.S.; Kim, H.J.; Jeon, Y.J.; Jee, Y. Mojabanchromanol Isolated from Sargassum horneri Attenuates Particulate Matter Induced Inflammatory Responses via Suppressing TLR2/4/7-MAPK Signaling in MLE-12 Cells. Mar Drugs 2020, 18. [CrossRef]
- Ha, T.M.; Kim, D.C.; Sohn, J.H.; Yim, J.H.; Oh, H. Anti-Inflammatory and Protein Tyrosine Phosphatase 1B Inhibitory Metabolites from the Antarctic Marine-Derived Fungal Strain Penicillium glabrum SF-7123. Mar Drugs 2020, 18. [CrossRef]
- Kim, Y.N.; Ji, Y.K.; Kim, N.H.; Van Tu, N.; Rho, J.R.; Jeong, E.J. Isoquinolinequinone Derivatives from a Marine Sponge (Mar Drugs 2021, 19. [CrossRef]
- Chu, Y.C.; Chang, C.H.; Liao, H.R.; Cheng, M.J.; Wu, M.D.; Fu, S.L.; Chen, J.J. Rare Chromone Derivatives from the Marine-Derived Penicillium citrinum with Anti-Cancer and Anti-Inflammatory Activities. Mar Drugs 2021, 19. [CrossRef]
- Lee, S.M.; Kim, N.H.; Lee, S.; Kim, Y.N.; Heo, J.D.; Jeong, E.J.; Rho, J.R. Deacetylphylloketal, a New Phylloketal Derivative from a Marine Sponge, Genus Phyllospongia, with Potent Anti-Inflammatory Activity in In Vitro Co-Culture Model of Intestine. Mar Drugs 2019, 17. [CrossRef]
- Liu, Z.; Qiu, P.; Liu, H.; Li, J.; Shao, C.; Yan, T.; Cao, W.; She, Z. Identification of anti-inflammatory polyketides from the coral-derived fungus Penicillium sclerotiorin: In vitro approaches and molecular-modeling. Bioorg Chem 2019, 88, 102973. [CrossRef]
- Kim, M.J.; Jeong, S.M.; Kang, B.K.; Kim, K.B.; Ahn, D.H. Anti-Inflammatory Effects of Grasshopper Ketone from Sargassum fulvellum Ethanol Extract on Lipopolysaccharide-Induced Inflammatory Responses in RAW 264.7 Cells. J Microbiol Biotechnol 2019, 29, 820-826. [CrossRef]
- Abdelfattah, M.S.; Elmallah, M.I.Y.; Ebrahim, H.Y.; Almeer, R.S.; Eltanany, R.M.A.; Abdel Moneim, A.E. Prodigiosins from a marine sponge-associated actinomycete attenuate HCl/ethanol-induced gastric lesion via antioxidant and anti-inflammatory mechanisms. PLoS One 2019, 14, e0216737. [CrossRef]
- Hwang, J.; Kim, D.; Park, J.S.; Park, H.J.; Shin, J.; Lee, S.K. Photoprotective Activity of Topsentin, A Bis(Indole) Alkaloid from the Marine Sponge Spongosorites genitrix, by Regulation of COX-2 and Mir-4485 Expression in UVB-Irradiated Human Keratinocyte Cells. Mar Drugs 2020, 18. [CrossRef]
- Kim, E.A.K., S.Y.; Kim, J.; Oh, J.Y.; Kim, H.S., Yoon, W.J.; Kang, D.H.; Heo, S.J. Tuberatolide B isolated from Sargassum macrocarpum inhibited LPS-stimulated inflammatory response via MAPKs and NF-κB signaling pathway in RAW264.7 cells and zebrafish model. J. Funct. Foods 2019, 52, 109-115. [CrossRef]
- Yin, Y.; Xu, N.; Shi, Y.; Zhou, B.; Sun, D.; Ma, B.; Xu, Z.; Yang, J.; Li, C. Astaxanthin Protects Dendritic Cells from Lipopolysaccharide-Induced Immune Dysfunction. Mar Drugs 2021, 19. [CrossRef]
- Lin, C.C.; Chang, Y.K.; Lin, S.C.; Su, J.H.; Chao, Y.H.; Tang, K.T. Crassolide Suppresses Dendritic Cell Maturation and Attenuates Experimental Antiphospholipid Syndrome. Molecules 2021, 26. [CrossRef]
- Li, W.; Ye, S.; Zhang, Z.; Tang, J.; Jin, H.; Huang, F.; Yang, Z.; Tang, Y.; Chen, Y.; Ding, G.; et al. Purification and Characterization of a Novel Pentadecapeptide from Protein Hydrolysates of Cyclina sinensis and Its Immunomodulatory Effects on RAW264.7 Cells. Mar Drugs 2019, 17. [CrossRef]
- Oh, S.; Shim, M.; Son, M.; Jang, J.T.; Son, K.H.; Byun, K. Attenuating Effects of Dieckol on Endothelial Cell Dysfunction via Modulation of Th17/Treg Balance in the Intestine and Aorta of Spontaneously Hypertensive Rats. Antioxidants (Basel) 2021, 10. [CrossRef]
- Park, G.B.; Kim, M.J.; Vasileva, E.A.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; Han, J.; Lee, H.S.; Kim, D.; Jeong, J.Y. Echinochrome A Promotes Ex Vivo Expansion of Peripheral Blood-Derived CD34(+) Cells, Potentially through Downregulation of ROS Production and Activation of the Src-Lyn-p110delta Pathway. Mar Drugs 2019, 17. [CrossRef]
- Oh, S.J.; Seo, Y.; Ahn, J.S.; Shin, Y.Y.; Yang, J.W.; Kim, H.K.; Han, J.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; et al. Echinochrome A Reduces Colitis in Mice and Induces In Vitro Generation of Regulatory Immune Cells. Mar Drugs 2019, 17. [CrossRef]
- Park, G.T.; Yoon, J.W.; Yoo, S.B.; Song, Y.C.; Song, P.; Kim, H.K.; Han, J.; Bae, S.J.; Ha, K.T.; Mishchenko, N.P.; et al. Echinochrome A Treatment Alleviates Fibrosis and Inflammation in Bleomycin-Induced Scleroderma. Mar Drugs 2021, 19. [CrossRef]
- Han, E.J.; Kim, H.S.; Sanjeewa, K.K.A.; Herath, K.; Jeon, Y.J.; Jee, Y.; Lee, J.; Kim, T.; Shim, S.Y.; Ahn, G. Eckol from Ecklonia cava Suppresses Immunoglobulin E-mediated Mast Cell Activation and Passive Cutaneous Anaphylaxis in Mice. Nutrients 2020, 12. [CrossRef]
- Tai, H.C.; Lee, T.H.; Tang, C.H.; Chen, L.P.; Chen, W.C.; Lee, M.S.; Chen, P.C.; Lin, C.Y.; Chi, C.W.; Chen, Y.J.; et al. Phomaketide A Inhibits Lymphangiogenesis in Human Lymphatic Endothelial Cells. Mar Drugs 2019, 17. [CrossRef]
- Yang, M.; Li, H.; Zhang, Q.; Wu, Q.H.; Li, G.; Chen, K.X.; Guo, Y.W.; Tang, W.; Li, X.W. Highly diverse cembranoids from the South China Sea soft coral Sinularia scabra as a new class of potential immunosuppressive agents. Bioorg Med Chem 2019, 27, 3469-3476. [CrossRef]
- Laborde, R.J.; Ishimura, M.E.; Abreu-Butin, L.; Nogueira, C.V.; Grubaugh, D.; Cruz-Leal, Y.; Luzardo, M.C.; Fernández, A.; Mesa, C.; Pazos, F.; et al. Sticholysins, pore-forming proteins from a marine anemone can induce maturation of dendritic cells through a TLR4 dependent-pathway. Mol Immunol 2021, 131, 144-154. [CrossRef]
- Manzo, E.; Gallo, C.; Sartorius, R.; Nuzzo, G.; Sardo, A.; De Berardinis, P.; Fontana, A.; Cutignano, A. Immunostimulatory Phosphatidylmonogalactosyldiacylglycerols (PGDG) from the Marine Diatom Thalassiosira weissflogii: Inspiration for a Novel Synthetic Toll-Like Receptor 4 Agonist. Mar Drugs 2019, 17. [CrossRef]
- Wang, H.L.; Li, R.; Li, J.; He, J.; Cao, Z.Y.; Kurtan, T.; Mandi, A.; Zheng, G.L.; Zhang, W. Alternarin A, a Drimane Meroterpenoid, Suppresses Neuronal Excitability from the Coral-Associated Fungi Alternaria sp. ZH-15. Org Lett 2020, 22, 2995-2998. [CrossRef]
- Andrud, K.; Xing, H.; Gabrielsen, B.; Bloom, L.; Mahnir, V.; Lee, S.; Green, B.T.; Lindstrom, J.; Kem, W. Investigation of the Possible Pharmacologically Active Forms of the Nicotinic Acetylcholine Receptor Agonist Anabaseine. Mar Drugs 2019, 17. [CrossRef]
- Copmans, D.; Kildgaard, S.; Rasmussen, S.A.; Slezak, M.; Dirkx, N.; Partoens, M.; Esguerra, C.V.; Crawford, A.D.; Larsen, T.O.; de Witte, P.A.M. Zebrafish-Based Discovery of Antiseizure Compounds from the North Sea: Isoquinoline Alkaloids TMC-120A and TMC-120B. Mar Drugs 2019, 17. [CrossRef]
- Yang, W.C.; Zhang, Y.Y.; Li, Y.J.; Nie, Y.Y.; Liang, J.Y.; Liu, Y.Y.; Liu, J.S.; Zhang, Y.P.; Song, C.; Qian, Z.J.; et al. Chemical Composition and Anti-Alzheimer’s Disease-Related Activities of a Functional Oil from the Edible Seaweed Hizikia fusiforme. Chem Biodivers 2020, 17, e2000055. [CrossRef]
- Han, J.H.; Lee, Y.S.; Im, J.H.; Ham, Y.W.; Lee, H.P.; Han, S.B.; Hong, J.T. Astaxanthin Ameliorates Lipopolysaccharide-Induced Neuroinflammation, Oxidative Stress and Memory Dysfunction through Inactivation of the Signal Transducer and Activator of Transcription 3 Pathway. Mar Drugs 2019, 17. [CrossRef]
- Taksima, T.; Chonpathompikunlert, P.; Sroyraya, M.; Hutamekalin, P.; Limpawattana, M.; Klaypradit, W. Effects of Astaxanthin from Shrimp Shell on Oxidative Stress and Behavior in Animal Model of Alzheimer’s Disease. Mar Drugs 2019, 17. [CrossRef]
- Lee, J.; Jun, M. Dual BACE1 and Cholinesterase Inhibitory Effects of Phlorotannins from Ecklonia cava-An In Vitro and in Silico Study. Mar Drugs 2019, 17. [CrossRef]
- Konoki, K.; Baden, D.G.; Scheuer, T.; Catterall, W.A. Molecular Determinants of Brevetoxin Binding to Voltage-Gated Sodium Channels. Toxins (Basel) 2019, 11. [CrossRef]
- Jin, A.H.; Cristofori-Armstrong, B.; Rash, L.D.; Roman-Gonzalez, S.A.; Espinosa, R.A.; Lewis, R.J.; Alewood, P.F.; Vetter, I. Novel conorfamides from Conus austini venom modulate both nicotinic acetylcholine receptors and acid-sensing ion channels. Biochem Pharmacol 2019, 164, 342-348. [CrossRef]
- Niu, C.; Leavitt, L.S.; Lin, Z.; Paguigan, N.D.; Sun, L.; Zhang, J.; Torres, J.P.; Raghuraman, S.; Chase, K.; Cadeddu, R.; et al. Neuroactive Type-A γ-Aminobutyric Acid Receptor Allosteric Modulator Steroids from the Hypobranchial Gland of Marine Mollusk,. J Med Chem 2021, 64, 7033-7043. [CrossRef]
- Guo, M.; Yu, J.; Zhu, X.; Zhangsun, D.; Luo, S. Characterization of an α 4/7-Conotoxin LvIF from. Mar Drugs 2021, 19. [CrossRef]
- Qiang, Y.; Wu, Y.; Zhao, D.; Zhao, B.; Wang, F.; Ren, S.; Wen, Y.; Gu, J.; Zhang, L.; Liu, K.; et al. Discovery and characterization of the novel conotoxin Lv1d from Conus lividus that presents analgesic activity. Toxicon 2021, 194, 70-78. [CrossRef]
- Liu, Z.; Yu, Z.; Yu, S.; Zhu, C.; Dong, M.; Mao, W.; Hu, J.; Prorok, M.; Su, R.; Dai, Q. A Conantokin Peptide Con-T[M8Q] Inhibits Morphine Dependence with High Potency and Low Side Effects. Mar Drugs 2021, 19. [CrossRef]
- Wu, J.; Xi, Y.; Li, G.; Zheng, Y.; Wang, Z.; Wang, J.; Fang, C.; Sun, Z.; Hu, L.; Jiang, W.; et al. Hydroazulene Diterpenes from a. J Nat Prod 2021, 84, 1306-1315. [CrossRef]
- Kim, R.; Hur, D.; Kim, H.K.; Han, J.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; Chang, W. Echinochrome A Attenuates Cerebral Ischemic Injury through Regulation of Cell Survival after Middle Cerebral Artery Occlusion in Rat. Mar Drugs 2019, 17. [CrossRef]
- Paudel, P.; Seong, S.H.; Wu, S.; Park, S.; Jung, H.A.; Choi, J.S. Eckol as a Potential Therapeutic against Neurodegenerative Diseases Targeting Dopamine D(3)/D(4) Receptors. Mar Drugs 2019, 17. [CrossRef]
- Silva, J.; Alves, C.; Pinteus, S.; Susano, P.; Simoes, M.; Guedes, M.; Martins, A.; Rehfeldt, S.; Gaspar, H.; Goettert, M.; et al. Disclosing the potential of eleganolone for Parkinson’s disease therapeutics: Neuroprotective and anti-inflammatory activities. Pharmacol Res 2021, 168, 105589. [CrossRef]
- Chalorak, P.; Sanguanphun, T.; Limboonreung, T.; Meemon, K. Neurorescue Effects of Frondoside A and Ginsenoside Rg3 in. Molecules 2021, 26. [CrossRef]
- Gan, S.Y.; Wong, L.Z.; Wong, J.W.; Tan, E.L. Fucosterol exerts protection against amyloid beta-induced neurotoxicity, reduces intracellular levels of amyloid beta and enhances the mRNA expression of neuroglobin in amyloid beta-induced SH-SY5Y cells. Int J Biol Macromol 2019, 121, 207-213. [CrossRef]
- Hannan, M.A.; Dash, R.; Sohag, A.A.M.; Moon, I.S. Deciphering Molecular Mechanism of the Neuropharmacological Action of Fucosterol through Integrated System Pharmacology and In Silico Analysis. Mar Drugs 2019, 17. [CrossRef]
- Chen, S.J.; Lee, C.J.; Lin, T.B.; Peng, H.Y.; Liu, H.J.; Chen, Y.S.; Tseng, K.W. Protective Effects of Fucoxanthin on Ultraviolet B-Induced Corneal Denervation and Inflammatory Pain in a Rat Model. Mar Drugs 2019, 17. [CrossRef]
- Wu, W.; Han, H.; Liu, J.; Tang, M.; Wu, X.; Cao, X.; Zhao, T.; Lu, Y.; Niu, T.; Chen, J.; et al. Fucoxanthin Prevents 6-OHDA-Induced Neurotoxicity by Targeting Keap1. Oxid Med Cell Longev 2021, 2021, 6688708. [CrossRef]
- Kalina, R.S.; Koshelev, S.G.; Zelepuga, E.A.; Kim, N.Y.; Kozlov, S.A.; Kozlovskaya, E.P.; Monastyrnaya, M.M.; Gladkikh, I.N. APETx-Like Peptides from the Sea Anemone Heteractis crispa, Diverse in Their Effect on ASIC1a and ASIC3 Ion Channels. Toxins (Basel) 2020, 12. [CrossRef]
- Tangrodchanapong, T.; Sornkaew, N.; Yurasakpong, L.; Niamnont, N.; Nantasenamat, C.; Sobhon, P.; Meemon, K. Beneficial Effects of Cyclic Ether 2-Butoxytetrahydrofuran from Sea Cucumber Holothuria scabra against Abeta Aggregate Toxicity in Transgenic Caenorhabditis elegans and Potential Chemical Interaction. Molecules 2021, 26. [CrossRef]
- Fan, T.T.; Zhang, H.H.; Tang, Y.H.; Zhang, F.Z.; Han, B.N. Two New Neo-debromoaplysiatoxins-A Pair of Stereoisomers Exhibiting Potent Kv1.5 Ion Channel Inhibition Activities. Mar Drugs 2019, 17. [CrossRef]
- Jiao, Y.; Wang, G.; Li, D.; Li, H.; Liu, J.; Yang, X.; Yang, W. Okadaic Acid Exposure Induced Neural Tube Defects in Chicken (Gallus gallus) Embryos. Mar Drugs 2021, 19. [CrossRef]
- Benoit, E.; Couesnon, A.; Lindovsky, J.; Iorga, B.I.; Araoz, R.; Servent, D.; Zakarian, A.; Molgo, J. Synthetic Pinnatoxins A and G Reversibly Block Mouse Skeletal Neuromuscular Transmission In Vivo and In Vitro. Mar Drugs 2019, 17. [CrossRef]
- Seong, S.H.; Paudel, P.; Choi, J.W.; Ahn, D.H.; Nam, T.J.; Jung, H.A.; Choi, J.S. Probing Multi-Target Action of Phlorotannins as New Monoamine Oxidase Inhibitors and Dopaminergic Receptor Modulators with the Potential for Treatment of Neuronal Disorders. Mar Drugs 2019, 17. [CrossRef]
- Lee, J.P.; Kang, M.G.; Lee, J.Y.; Oh, J.M.; Baek, S.C.; Leem, H.H.; Park, D.; Cho, M.L.; Kim, H. Potent inhibition of acetylcholinesterase by sargachromanol I from Sargassum siliquastrum and by selected natural compounds. Bioorg Chem 2019, 89, 103043. [CrossRef]
- Chen, L.; Liu, Y.C.; Tan, H.; Zhang, Y.; Xu, J.; Liu, W.L.; Li, Z.Y.; Li, W.P. Santacruzamate A Ameliorates AD-Like Pathology by Enhancing ER Stress Tolerance Through Regulating the Functions of KDELR and Mia40-ALR in vivo and in vitro. Front Cell Neurosci 2019, 13, 61. [CrossRef]
- Jiang, C.S.; Ru, T.; Yao, L.G.; Miao, Z.H.; Guo, Y.W. Four new cembranoids from the Chinese soft coral Sinularia sp. and their anti-Abeta aggregation activities. Fitoterapia 2019, 136, 104176. [CrossRef]
- Paudel, P.; Park, S.E.; Seong, S.H.; Jung, H.A.; Choi, J.S. Bromophenols from Symphyocladia latiuscula Target Human Monoamine Oxidase and Dopaminergic Receptors for the Management of Neurodegenerative Diseases. J Agric Food Chem 2020, 68, 2426-2436. [CrossRef]
- Liu, Y.; Jin, W.; Deng, Z.; Zhang, Q.; Wang, J. Glucuronomannan GM2 from. Mar Drugs 2021, 19. [CrossRef]
- Paudel, P.; Seong, S.H.; Zhou, Y.; Park, H.J.; Jung, H.A.; Choi, J.S. Anti-Alzheimer’s Disease Activity of Bromophenols from a Red Alga, Symphyocladia latiuscula (Harvey) Yamada. ACS Omega 2019, 4, 12259-12270. [CrossRef]
- Feng, C.W.; Chen, N.F.; Wen, Z.H.; Yang, W.Y.; Kuo, H.M.; Sung, P.J.; Su, J.H.; Cheng, S.Y.; Chen, W.F. In Vitro and In Vivo Neuroprotective Effects of Stellettin B Through Anti-Apoptosis and the Nrf2/HO-1 Pathway. Mar Drugs 2019, 17. [CrossRef]
- Sheng, L.; Lu, B.; Chen, H.; Du, Y.; Chen, C.; Cai, W.; Yang, Y.; Tian, X.; Huang, Z.; Chi, W.; et al. Marine-Steroid Derivative 5alpha-Androst-3beta, 5alpha, 6beta-triol Protects Retinal Ganglion Cells from Ischemia(-)Reperfusion Injury by Activating Nrf2 Pathway. Mar Drugs 2019, 17. [CrossRef]
- Liang, X.; Luo, D.; Yan, J.L.; Rezaei, M.A.; Salvador-Reyes, L.A.; Gunasekera, S.P.; Li, C.; Ye, T.; Paul, V.J.; Luesch, H. Discovery of Amantamide, a Selective CXCR7 Agonist from Marine Cyanobacteria. Org Lett 2019, 21, 1622-1626. [CrossRef]
- Chakraborty, K.; Krishnan, S.; Joy, M. Macrocyclic lactones from seafood Amphioctopus neglectus: Newly described natural leads to attenuate angiotensin-II induced cardiac hypertrophy. Biomed Pharmacother 2019, 110, 155-167. [CrossRef]
- Shi, H. Two novel antioxidant peptides derived from Arca subcrenata against oxidative stress and extend lifespan in Caenorhabditis elegans. J. Funct. Foods 2021, 81, 104462. [CrossRef]
- Park, S.C.; Chung, B.; Lee, J.; Cho, E.; Hwang, J.Y.; Oh, D.C.; Shin, J.; Oh, K.B. Sortase A-Inhibitory Metabolites from a Marine-Derived Fungus Aspergillus sp. Mar Drugs 2020, 18. [CrossRef]
- Ohshiro, T.; Kobayashi, K.; Suzuki, A.; Yamazaki, H.; Uchida, R.; Namikoshi, M.; Tomoda, H. Inhibition of neutral lipid synthesis by avarols from a marine sponge. Bioorg Med Chem Lett 2019, 29, 2283-2285. [CrossRef]
- Son, M.; Oh, S.; Jang, J.T.; Son, K.H.; Byun, K. Pyrogallol-Phloroglucinol-6 6-Bieckol on Attenuates High-Fat Diet-Induced Hypertension by Modulating Endothelial-to-Mesenchymal Transition in the Aorta of Mice. Oxid Med Cell Longev 2021, 2021, 8869085. [CrossRef]
- Ryu, Y.S.; Fernando, P.; Kang, K.A.; Piao, M.J.; Zhen, A.X.; Kang, H.K.; Koh, Y.S.; Hyun, J.W. Marine Compound 3-bromo-4,5-dihydroxybenzaldehyde Protects Skin Cells against Oxidative Damage via the Nrf2/HO-1 Pathway. Mar Drugs 2019, 17. [CrossRef]
- Chen, H.; Xu, Z.; Fan, F.; Shi, P.; Tu, M.; Wang, Z.; Du, M. Identification and mechanism evaluation of a novel osteogenesis promoting peptide from Tubulin Alpha-1C chain in Crassostrea gigas. Food Chem 2019, 272, 751-757. [CrossRef]
- Chen, H.; Cheng, S.; Fan, F.; Tu, M.; Xu, Z.; Du, M. Identification and molecular mechanism of antithrombotic peptides from oyster proteins released in simulated gastro-intestinal digestion. Food Funct 2019, 10, 5426-5435. [CrossRef]
- Utkina, N.K.; Likhatskaya, G.N.; Balabanova, L.A.; Bakunina, I.Y. Sponge-derived polybrominated diphenyl ethers and dibenzo-p-dioxins, irreversible inhibitors of the bacterial alpha-d-galactosidase. Environ Sci Process Impacts 2019, 21, 1754-1763. [CrossRef]
- Pyeon, D.B.; Lee, S.E.; Yoon, J.W.; Park, H.J.; Park, C.O.; Kim, S.H.; Oh, S.H.; Lee, D.G.; Kim, E.Y.; Park, S.P. The antioxidant dieckol reduces damage of oxidative stress-exposed porcine oocytes and enhances subsequent parthenotes embryo development. Mol Reprod Dev 2021, 88, 349-361. [CrossRef]
- Wang, L.; Je, J.G.; Yang, H.W.; Jeon, Y.J.; Lee, S. Dieckol, an Algae-Derived Phenolic Compound, Suppresses UVB-Induced Skin Damage in Human Dermal Fibroblasts and Its Underlying Mechanisms. Antioxidants (Basel) 2021, 10. [CrossRef]
- Ding, Y.; Wang, L.; Im, S.; Hwang, O.; Kim, H.S.; Kang, M.C.; Lee, S.H. Anti-Obesity Effect of Diphlorethohydroxycarmalol Isolated from Brown Alga Ishige okamurae in High-Fat Diet-Induced Obese Mice. Mar Drugs 2019, 17. [CrossRef]
- Kang, M.C.; Ding, Y.; Kim, H.S.; Jeon, Y.J.; Lee, S.H. Inhibition of Adipogenesis by Diphlorethohydroxycarmalol (DPHC) through AMPK Activation in Adipocytes. Mar Drugs 2019, 17. [CrossRef]
- Lu, Y.A.; Jiang, Y.; Yang, H.W.; Hwang, J.; Jeon, Y.J.; Ryu, B. Diphlorethohydroxycarmalol Isolated from. Int J Mol Sci 2021, 22. [CrossRef]
- Manandhar, B.; Wagle, A.; Seong, S.H.; Paudel, P.; Kim, H.R.; Jung, H.A.; Choi, J.S. Phlorotannins with Potential Anti-tyrosinase and Antioxidant Activity Isolated from the Marine Seaweed Ecklonia stolonifera. Antioxidants (Basel) 2019, 8. [CrossRef]
- Zhen, A.X.; Hyun, Y.J.; Piao, M.J.; Fernando, P.; Kang, K.A.; Ahn, M.J.; Yi, J.M.; Kang, H.K.; Koh, Y.S.; Lee, N.H.; et al. Eckol Inhibits Particulate Matter 2.5-Induced Skin Keratinocyte Damage via MAPK Signaling Pathway. Mar Drugs 2019, 17. [CrossRef]
- Jia, X.; Xu, M.; Yang, A.; Zhao, Y.; Liu, D.; Huang, J.; Proksch, P.; Lin, W. Reducing Effect of Farnesylquinone on Lipid Mass in C. elegans by Modulating Lipid Metabolism. Mar Drugs 2019, 17. [CrossRef]
- Ohno, Y. Effect of phlorotannins isolated from Eisenia bicyclis on melanogenesis in mouse B16 melanoma cells Nat. Prod. Commun. 2021, 16.
- Raji, V.; Loganathan, C.; Sadhasivam, G.; Kandasamy, S.; Poomani, K.; Thayumanavan, P. Purification of fucoxanthin from Sargassum wightii Greville and understanding the inhibition of angiotensin 1-converting enzyme: An in vitro and in silico studies. Int J Biol Macromol 2020, 148, 696-703. [CrossRef]
- Yang, G.; Jin, L.; Zheng, D.; Tang, X.; Yang, J.; Fan, L.; Xie, X. Fucoxanthin Alleviates Oxidative Stress through Akt/Sirt1/FoxO3alpha Signaling to Inhibit HG-Induced Renal Fibrosis in GMCs. Mar Drugs 2019, 17. [CrossRef]
- Kim, D.C.; Minh Ha, T.; Sohn, J.H.; Yim, J.H.; Oh, H. Protein tyrosine phosphatase 1B inhibitors from a marine-derived fungal strain aspergillus sp. SF-5929. Nat Prod Res 2020, 34, 675-682. [CrossRef]
- Wang, Z.; Li, Z.X.; Zhao, W.C.; Huang, H.B.; Wang, J.Q.; Zhang, H.; Lu, J.Y.; Wang, R.N.; Li, W.; Cheng, Z.; et al. Identification and characterization of isocitrate dehydrogenase 1 (IDH1) as a functional target of marine natural product grincamycin B. Acta Pharmacol Sin 2021, 42, 801-813. [CrossRef]
- Wang, Y.; Sun, L.; Yu, G.; Qi, X.; Zhang, A.; Lu, Z.; Li, D.; Li, J. Identification of a novel non-ATP-competitive protein kinase inhibitor of PGK1 from marine nature products. Biochem Pharmacol 2021, 183, 114343. [CrossRef]
- Oh, Y.; Ahn, C.B.; Je, J.Y. Cytoprotective Role of Edible Seahorse (Mar Drugs 2021, 19. [CrossRef]
- Lee, H.G.; Kim, H.S.; Je, J.G.; Hwang, J.; Sanjeewa, K.K.A.; Lee, D.S.; Song, K.M.; Choi, Y.S.; Kang, M.C.; Jeon, Y.J. Lipid Inhibitory Effect of (-)-loliolide Isolated from. Mar Drugs 2021, 19. [CrossRef]
- Bakunina, I.; Likhatskaya, G.; Slepchenko, L.; Balabanova, L.; Tekutyeva, L.; Son, O.; Shubina, L.; Makarieva, T. Effect of Pentacyclic Guanidine Alkaloids from the Sponge Monanchora pulchra on Activity of alpha-Glycosidases from Marine Bacteria. Mar Drugs 2019, 17. [CrossRef]
- Nagabhishek, S.N.K., A.M. ; B, S. ; Balakrishnan, A. ; Katakia, Y.T., Chatterjee, S. ; Nagasundaram, N. . A marine sponge associated fungal metabolite monacolin X suppresses angiogenesis by down regulating VEGFR2 signaling. RSC Adv 2019, 9, 26646-26667, doi:DOI: 10.1039/C9RA05262C.
- D’Aniello, E.; Iannotti, F.A.; Falkenberg, L.G.; Martella, A.; Gentile, A.; De Maio, F.; Ciavatta, M.L.; Gavagnin, M.; Waxman, J.S.; Di Marzo, V.; et al. In Silico Identification and Experimental Validation of (-)-Muqubilin A, a Marine Norterpene Peroxide, as PPARalpha/gamma-RXRalpha Agonist and RARalpha Positive Allosteric Modulator. Mar Drugs 2019, 17. [CrossRef]
- Hayashi-Takanaka, Y.; Kina, Y.; Nakamura, F.; Yamazaki, S.; Harata, M.; Soest, R.; Kimura, H.; Nakao, Y. Effect of mycalolides isolated from a marine sponge Mycale aff. nullarosette on actin in living cells. Sci Rep 2019, 9, 7540. [CrossRef]
- Xu, Z.; Chen, H.; Fan, F.; Shi, P.; Tu, M.; Cheng, S.; Wang, Z.; Du, M. Bone formation activity of an osteogenic dodecapeptide from blue mussels (Mytilus edulis). Food Funct 2019, 10, 5616-5625. [CrossRef]
- Chen, J.; Gong, F.; Chen, M.F.; Li, C.; Hong, P.; Sun, S.; Zhou, C.; Qian, Z.J. In Vitro Vascular-Protective Effects of a Tilapia By-Product Oligopeptide on Angiotensin II-Induced Hypertensive Endothelial Injury in HUVEC by Nrf2/NF-kappaB Pathways. Mar Drugs 2019, 17. [CrossRef]
- Kong, F.D.; Fan, P.; Zhou, L.M.; Ma, Q.Y.; Xie, Q.Y.; Zheng, H.Z.; Zheng, Z.H.; Zhang, R.S.; Yuan, J.Z.; Dai, H.F.; et al. Penerpenes A-D, Four Indole Terpenoids with Potent Protein Tyrosine Phosphatase Inhibitory Activity from the Marine-Derived Fungus Penicillium sp. KFD28. Org Lett 2019, 21, 4864-4867. [CrossRef]
- Dai, J.; Chen, A.; Zhu, M.; Qi, X.; Tang, W.; Liu, M.; Li, D.; Gu, Q.; Li, J. Penicisulfuranol A, a novel C-terminal inhibitor disrupting molecular chaperone function of Hsp90 independent of ATP binding domain. Biochem Pharmacol 2019, 163, 404-415. [CrossRef]
- Wang, J.; Liang, Z.; Li, K.; Yang, B.; Liu, Y.; Fang, W.; Tang, L.; Zhou, X. Ene-yne Hydroquinones from a Marine-derived Strain of the Fungus Pestalotiopsis neglecta with Effects on Liver X Receptor Alpha. J Nat Prod 2020, 83, 1258-1264. [CrossRef]
- Tang, W.Z.; Liu, J.T.; Hu, Q.; He, R.J.; Guan, X.Q.; Ge, G.B.; Han, H.; Yang, F.; Lin, H.W. Pancreatic Lipase Inhibitory Cyclohexapeptides from the Marine Sponge-Derived Fungus Aspergillus sp. 151304. J Nat Prod 2020, 83, 2287-2293. [CrossRef]
- Wu, Y.; Liao, H.; Liu, L.Y.; Sun, F.; Chen, H.F.; Jiao, W.H.; Zhu, H.R.; Yang, F.; Huang, G.; Zeng, D.Q.; et al. Phakefustatins A-C: Kynurenine-Bearing Cycloheptapeptides as RXRalpha Modulators from the Marine Sponge Phakellia fusca. Org Lett 2020, 22, 6703-6708. [CrossRef]
- Kim, J.H.; Lee, S.; Park, S.; Park, J.S.; Kim, Y.H.; Yang, S.Y. Slow-Binding Inhibition of Tyrosinase by Ecklonia cava Phlorotannins. Mar Drugs 2019, 17. [CrossRef]
- Heo, S.Y.; Jeong, M.S.; Lee, H.S.; Kim, Y.J.; Park, S.H.; Jung, W.K. Phlorofucofuroeckol A from Ecklonia cava ameliorates TGF-beta1-induced fibrotic response of human tracheal fibroblasts via the downregulation of MAPKs and SMAD 2/3 pathways inactivated TGF-beta receptor. Biochem Biophys Res Commun 2020, 522, 626-632. [CrossRef]
- Oh, J.H.; Ahn, B.N.; Karadeniz, F.; Kim, J.A.; Lee, J.I.; Seo, Y.; Kong, C.S. Phlorofucofuroeckol A from Edible Brown Alga Ecklonia Cava Enhances Osteoblastogenesis in Bone Marrow-Derived Human Mesenchymal Stem Cells. Mar Drugs 2019, 17. [CrossRef]
- Guo, X.C.; Zhang, Y.H.; Gao, W.B.; Pan, L.; Zhu, H.J.; Cao, F. Absolute Configurations and Chitinase Inhibitions of Quinazoline-Containing Diketopiperazines from the Marine-Derived Fungus Penicillium polonicum. Mar Drugs 2020, 18. [CrossRef]
- Ko, S.C.; Ding, Y.; Kim, J.; Ye, B.R.; Kim, E.A.; Jung, W.K.; Heo, S.J.; Lee, S.H. Bromophenol (5-bromo-3,4-dihydroxybenzaldehyde) isolated from red alga Polysiphonia morrowii inhibits adipogenesis by regulating expression of adipogenic transcription factors and AMP-activated protein kinase activation in 3T3-L1 adipocytes. Phytother Res 2019, 33, 737-744. [CrossRef]
- Park, J.S.; Quang, T.H.; Thi Thanh Ngan, N.; Sohn, J.H.; Oh, H. New preaustinoids from a marine-derived fungal strain Penicillium sp. SF-5497 and their inhibitory effects against PTP1B activity. J Antibiot (Tokyo) 2019, 72, 629-633. [CrossRef]
- Lee, M.K.; Choi, J.W.; Choi, Y.H.; Nam, T.J. Protective Effect of Pyropia yezoensis Peptide on Dexamethasone-Induced Myotube Atrophy in C2C12 Myotubes. Mar Drugs 2019, 17. [CrossRef]
- Tseng, C.C.; Lai, Y.C.; Kuo, T.J.; Su, J.H.; Sung, P.J.; Feng, C.W.; Lin, Y.Y.; Chen, P.C.; Tai, M.H.; Cheng, S.Y.; et al. Rhodoptilometrin, a Crinoid-Derived Anthraquinone, Induces Cell Regeneration by Promoting Wound Healing and Oxidative Phosphorylation in Human Gingival Fibroblast Cells. Mar Drugs 2019, 17. [CrossRef]
- Kwon, M.L., B. ; Lim, S.J. ; Choi, J.S. ; Kim, H.R. Sargahydroquinoic acid, a major compound in Sargassum serratifolium (C. Agardh) C. Agardh, widely activates lipid catabolic pathways, contributing to the formation of beige-like adipocytes. J. Funct. Foods 2019, 58, 355-366. [CrossRef]
- Halkias, C.; Darby, W.G.; Feltis, B.N.; McIntyre, P.; Macrides, T.A.; Wright, P.F.A. Marine Bile Natural Products as Agonists of the TGR5 Receptor. J Nat Prod 2021, 84, 1507-1514. [CrossRef]
- Ji-Yeon, H.; Beomkoo, C.; Oh-Seok, K.; sung, P.; Eunji, C.; Dong, O.; Jongheon, S.; Ki-bong, O. Inhibitory effects of epipolythiodioxopiperazine fungal metabolites on isocitrate lyase in the glyoxylate cycle of Candida albicans. Mar drugs 2021, 19, 295. [CrossRef]
- Zhang, H.; Li, R.; Ba, S.; Lu, Z.; Pitsinos, E.N.; Li, T.; Nicolaou, K.C. DNA Binding and Cleavage Modes of Shishijimicin A. J Am Chem Soc 2019, 141, 7842-7852. [CrossRef]
- Zheng, J.; Manabe, Y.; Sugawara, T. Siphonaxanthin, a carotenoid from green algae Codium cylindricum, protects Ob/Ob mice fed on a high-fat diet against lipotoxicity by ameliorating somatic stresses and restoring anti-oxidative capacity. Nutr Res 2020, 77, 29-42. [CrossRef]
- Paudel, P.; Wagle, A.; Seong, S.H.; Park, H.J.; Jung, H.A.; Choi, J.S. A New Tyrosinase Inhibitor from the Red Alga Symphyocladia latiuscula (Harvey) Yamada (Rhodomelaceae). Mar Drugs 2019, 17. [CrossRef]
- Qiao, X.; Gan, M.; Wang, C.; Liu, B.; Shang, Y.; Li, Y.; Chen, S. Tetracenomycin X Exerts Antitumour Activity in Lung Cancer Cells through the Downregulation of Cyclin D1. Mar Drugs 2019, 17. [CrossRef]
- Keller, L.; Canuto, K.M.; Liu, C.; Suzuki, B.M.; Almaliti, J.; Sikandar, A.; Naman, C.B.; Glukhov, E.; Luo, D.; Duggan, B.M.; et al. Tutuilamides A-C: Vinyl-Chloride-Containing Cyclodepsipeptides from Marine Cyanobacteria with Potent Elastase Inhibitory Properties. ACS Chem Biol 2020, 15, 751-757. [CrossRef]
- Feng, X.; Liao, D.; Sun, L.; Wu, S.; Lan, P.; Wang, Z.; Li, C.; Zhou, Q.; Lu, Y.; Lan, X. Affinity Purification of Angiotensin Converting Enzyme Inhibitory Peptides from Wakame (Undaria Pinnatifida) Using Immobilized ACE on Magnetic Metal Organic Frameworks. Mar Drugs 2021, 19. [CrossRef]
- El-Baz, F.K.; Hussein, R.A.; Saleh, D.O.; Abdel Jaleel, G.A.R. Zeaxanthin Isolated from Dunaliella salina Microalgae Ameliorates Age Associated Cardiac Dysfunction in Rats through Stimulation of Retinoid Receptors. Mar Drugs 2019, 17. [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat Prod Rep 2021, 38, 362-413. [CrossRef]
- Liang, X.; Luo, D.; Luesch, H. Advances in exploring the therapeutic potential of marine natural products. Pharmacol Res 2019, 147, 104373. [CrossRef]
- Nunez-Pons, L.; Shilling, A.; Verde, C.; Baker, B.J.; Giordano, D. Marine Terpenoids from Polar Latitudes and Their Potential Applications in Biotechnology. Mar Drugs 2020, 18. [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat Prod Rep 2022, 39, 1122-1171. [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat Prod Rep 2023, 40, 275-325. [CrossRef]
- Mateos, R.; Perez-Correa, J.R.; Dominguez, H. Bioactive Properties of Marine Phenolics. Mar Drugs 2020, 18. [CrossRef]
- Martins, B.T.; Correia da Silva, M.; Pinto, M.; Cidade, H.; Kijjoa, A. Marine natural flavonoids: chemistry and biological activities. Nat Prod Res 2019, 33, 3260-3272. [CrossRef]
- Braddock, A.A.; Theodorakis, E.A. Marine Spirotetronates: Biosynthetic Edifices That Inspire Drug Discovery. Mar Drugs 2019, 17. [CrossRef]
- Vil, V.A.; Gloriozova, T.A.; Terent’ev, A.O.; Savidov, N.; Dembitsky, V.M. Hydroperoxides derived from marine sources: origin and biological activities. Appl Microbiol Biotechnol 2019, 103, 1627-1642. [CrossRef]
- Althagbi, H.I.; Alarif, W.M.; Al-Footy, K.O.; Abdel-Lateff, A. Marine-Derived Macrocyclic Alkaloids (MDMAs): Chemical and Biological Diversity. Mar Drugs 2020, 18. [CrossRef]
- Dahiya, R.; Dahiya, S.; Fuloria, N.K.; Kumar, S.; Mourya, R.; Chennupati, S.V.; Jankie, S.; Gautam, H.; Singh, S.; Karan, S.K.; et al. Natural Bioactive Thiazole-Based Peptides from Marine Resources: Structural and Pharmacological Aspects. Mar Drugs 2020, 18. [CrossRef]
- El-Demerdash, A.; Kumla, D.; Kijjoa, A. Chemical Diversity and Biological Activities of Meroterpenoids from Marine Derived-Fungi: A Comprehensive Update. Mar Drugs 2020, 18. [CrossRef]
- Li, G.; Dickschat, J.S.; Guo, Y.W. Diving into the world of marine 2,11-cyclized cembranoids: a summary of new compounds and their biological activities. Nat Prod Rep 2020, 37, 1367-1383. [CrossRef]
- Zhang, H.; Zou, J.; Yan, X.; Chen, J.; Cao, X.; Wu, J.; Liu, Y.; Wang, T. Marine-Derived Macrolides 1990-2020: An Overview of Chemical and Biological Diversity. Mar Drugs 2021, 19. [CrossRef]
- Demay, J.; Bernard, C.; Reinhardt, A.; Marie, B. Natural Products from Cyanobacteria: Focus on Beneficial Activities. Mar Drugs 2019, 17. [CrossRef]
- Fidor, A.; Konkel, R.; Mazur-Marzec, H. Bioactive Peptides Produced by Cyanobacteria of the Genus Nostoc: A Review. Mar Drugs 2019, 17. [CrossRef]
- Huang, I.S.; Zimba, P.V. Cyanobacterial bioactive metabolites-A review of their chemistry and biology. Harmful Algae 2019, 86, 139-209. [CrossRef]
- Li, Y.; Naman, C.B.; Alexander, K.L.; Guan, H.; Gerwick, W.H. The Chemistry, Biochemistry and Pharmacology of Marine Natural Products from Leptolyngbya, a Chemically Endowed Genus of Cyanobacteria. Mar Drugs 2020, 18. [CrossRef]
- Tan, L.T.; Phyo, M.Y. Marine Cyanobacteria: A Source of Lead Compounds and their Clinically-Relevant Molecular Targets. Molecules 2020, 25. [CrossRef]
- Qamar, H.; Hussain, K.; Soni, A.; Khan, A.; Hussain, T.; Chénais, B. Cyanobacteria as Natural Therapeutics and Pharmaceutical Potential: Role in Antitumor Activity and as Nanovectors. Molecules 2021, 26. [CrossRef]
- Lauritano, C.; Ferrante, M.I.; Rogato, A. Marine Natural Products from Microalgae: An -Omics Overview. Mar Drugs 2019, 17. [CrossRef]
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A.; Abd Allah, E.F. Microalgae metabolites: A rich source for food and medicine. Saudi J Biol Sci 2019, 26, 709-722. [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Seca, A.M.L.; Pinto, D.; Michalak, I.; Trincone, A.; Mishra, A.P.; Nigam, M.; Zam, W.; Martins, N. Current Trends on Seaweeds: Looking at Chemical Composition, Phytopharmacology, and Cosmetic Applications. Molecules 2019, 24. [CrossRef]
- Cikos, A.M.; Jurin, M.; Coz-Rakovac, R.; Jokic, S.; Jerkovic, I. Update on Monoterpenes from Red Macroalgae: Isolation, Analysis, and Bioactivity. Mar Drugs 2019, 17. [CrossRef]
- Freile-Pelegrin, Y.T., D. Seaweeds to the rescue of forgotten diseases: a review. Bot. Mar. 2019, 62, 211-226. [CrossRef]
- Hannan, M.A.; Sohag, A.A.M.; Dash, R.; Haque, M.N.; Mohibbullah, M.; Oktaviani, D.F.; Hossain, M.T.; Choi, H.J.; Moon, I.S. Phytosterols of marine algae: Insights into the potential health benefits and molecular pharmacology. Phytomedicine 2020, 69, 153201. [CrossRef]
- Rosa, G.P.; Tavares, W.R.; Sousa, P.M.C.; Pages, A.K.; Seca, A.M.L.; Pinto, D. Seaweed Secondary Metabolites with Beneficial Health Effects: An Overview of Successes in In Vivo Studies and Clinical Trials. Mar Drugs 2019, 18. [CrossRef]
- Rushdi, I.A.-R., I.; Saber, H.; Attia, E.; Abdelraheem, W.; Madkour, H.; Hassan, H.; Elmaidomv, A.; Abdelmohsen, U. Pharmacological and natural products diversity of the brown algae genus Sargassum. RSC Adv. 2020, 10, 24951-24972. [CrossRef]
- Purcell-Meyerink, D.; Packer, M.A.; Wheeler, T.T.; Hayes, M. Aquaculture Production of the Brown Seaweeds. Molecules 2021, 26. [CrossRef]
- Shannon, E.; Conlon, M.; Hayes, M. Seaweed Components as Potential Modulators of the Gut Microbiota. Mar Drugs 2021, 19. [CrossRef]
- Thangaraj, S.B., S. ; Gokula, V. Bioactive compounds of sea anemones: a review. Int. J. Pept. Res. Ther. 2019, 25, 1405-1416. [CrossRef]
- Tinta, T.; Kogovsek, T.; Klun, K.; Malej, A.; Herndl, G.J.; Turk, V. Jellyfish-Associated Microbiome in the Marine Environment: Exploring Its Biotechnological Potential. Mar Drugs 2019, 17. [CrossRef]
- Ciavatta, M.L.; Lefranc, F.; Vieira, L.M.; Kiss, R.; Carbone, M.; van Otterlo, W.A.L.; Lopanik, N.B.; Waeschenbach, A. The Phylum Bryozoa: From Biology to Biomedical Potential. Mar Drugs 2020, 18. [CrossRef]
- Guillen, P.O.; Jaramillo, K.B.; Genta-Jouve, G.; Thomas, O.P. Marine natural products from zoantharians: bioactivity, biosynthesis, systematics, and ecological roles. Nat Prod Rep 2020, 37, 515-540. [CrossRef]
- Youssef, D.T.A.; Almagthali, H.; Shaala, L.A.; Schmidt, E.W. Secondary Metabolites of the Genus Didemnum: A Comprehensive Review of Chemical Diversity and Pharmacological Properties. Mar Drugs 2020, 18. [CrossRef]
- Barone, G.V., S.; Tangherlini, M.; Rastelli, E.; Dell’Anno, A.; Danovaro, R.; Corinaldesi, C. . Marine fungi: biotechnological perspectives from deep-hypersaline anoxic basins. Diversity 2019, 11, 113. [CrossRef]
- Youssef, F.S.; Ashour, M.L.; Singab, A.N.B.; Wink, M. A Comprehensive Review of Bioactive Peptides from Marine Fungi and Their Biological Significance. Mar Drugs 2019, 17. [CrossRef]
- Youssef, F.S.; Simal-Gandara, J. Comprehensive Overview on the Chemistry and Biological Activities of Selected Alkaloid Producing Marine-Derived Fungi as a Valuable Reservoir of Drug Entities. Biomedicines 2021, 9. [CrossRef]
- Pandey, A. Pharmacological significance of marine microbial bioactive compounds. Environ Chem Lett 2019, 17, 1741-1751. [CrossRef]
- Sang, V.T.; Dat, T.T.H.; Vinh, L.B.; Cuong, L.C.V.; Oanh, P.T.T.; Ha, H.; Kim, Y.H.; Anh, H.L.T.; Yang, S.Y. Coral and Coral-Associated Microorganisms: A Prolific Source of Potential Bioactive Natural Products. Mar Drugs 2019, 17. [CrossRef]
- Santos, J.D.; Vitorino, I.; Reyes, F.; Vicente, F.; Lage, O.M. From Ocean to Medicine: Pharmaceutical Applications of Metabolites from Marine Bacteria. Antibiotics (Basel) 2020, 9. [CrossRef]
- Sayed, A.M.; Abdel-Wahab, N.M.; Hassan, H.M.; Abdelmohsen, U.R. Saccharopolyspora: an underexplored source for bioactive natural products. J Appl Microbiol 2020, 128, 314-329. [CrossRef]
- Xu, J.L.; Liu, Z.F.; Zhang, X.W.; Liu, H.L.; Wang, Y. Microbial Oligosaccharides with Biomedical Applications. Mar Drugs 2021, 19. [CrossRef]
- Shady, N.H.; Fouad, M.A.; Salah Kamel, M.; Schirmeister, T.; Abdelmohsen, U.R. Natural Product Repertoire of the Genus Amphimedon. Mar Drugs 2018, 17. [CrossRef]
- Varijakzhan, D.; Loh, J.Y.; Yap, W.S.; Yusoff, K.; Seboussi, R.; Lim, S.E.; Lai, K.S.; Chong, C.M. Bioactive Compounds from Marine Sponges: Fundamentals and Applications. Mar Drugs 2021, 19. [CrossRef]
- Nabeelah Bibi, S.; Fawzi, M.M.; Gokhan, Z.; Rajesh, J.; Nadeem, N.; Kannan, R.R.R.; R, D.D.G.A.; Pandian, S.K. Ethnopharmacology, Phytochemistry, and Global Distribution of Mangroves-A Comprehensive Review. Mar Drugs 2019, 17. [CrossRef]
- Islam, M.T.; Sharifi-Rad, J.; Martorell, M.; Ali, E.S.; Asghar, M.N.; Deeba, F.; Firoz, C.K.; Mubarak, M.S. Chemical profile and therapeutic potentials of Xylocarpus moluccensis (Lam.) M. Roem.: A literature-based review. J Ethnopharmacol 2020, 259, 112958. [CrossRef]
- Jia, S.L.; Chi, Z.; Liu, G.L.; Hu, Z.; Chi, Z.M. Fungi in mangrove ecosystems and their potential applications. Crit Rev Biotechnol 2020, 40, 852-864. [CrossRef]
- Hanif, N.; Murni, A.; Tanaka, C.; Tanaka, J. Marine Natural Products from Indonesian Waters. Mar Drugs 2019, 17. [CrossRef]
- El-Hossary, E.M.; Abdel-Halim, M.; Ibrahim, E.S.; Pimentel-Elardo, S.M.; Nodwell, J.R.; Handoussa, H.; Abdelwahab, M.F.; Holzgrabe, U.; Abdelmohsen, U.R. Natural Products Repertoire of the Red Sea. Mar Drugs 2020, 18. [CrossRef]
- Sun, W.; Wu, W.; Liu, X.; Zaleta-Pinet, D.A.; Clark, B.R. Bioactive Compounds Isolated from Marine-Derived Microbes in China: 2009-2018. Mar Drugs 2019, 17. [CrossRef]
- Pech-Puch, D.; Perez-Povedano, M.; Gomez, P.; Martinez-Guitian, M.; Lasarte-Monterrubio, C.; Vazquez-Ucha, J.C.; Novoa-Olmedo, M.L.; Guillen-Hernandez, S.; Villegas-Hernandez, H.; Bou, G.; et al. Marine Organisms from the Yucatan Peninsula (Mexico) as a Potential Natural Source of Antibacterial Compounds. Mar Drugs 2020, 18. [CrossRef]
- Shinde, P.; Banerjee, P.; Mandhare, A. Marine natural products as source of new drugs: a patent review (2015-2018). Expert Opin Ther Pat 2019, 29, 283-309. [CrossRef]
- Pereira, F. Have marine natural product drug discovery efforts been productive and how can we improve their efficiency? Expert Opin Drug Discov 2019, 14, 717-722. [CrossRef]
- Newman, D.J. Natural Product Based Antibody Drug Conjugates: Clinical Status as of November 9, 2020. J Nat Prod 2021, 84, 917-931. [CrossRef]
- Andryukov, B.M., V. ; Besednova, N. The biotechnological potential of secondary metabolites from marine bacteria. J. Mar. Sci. Eng 2019, 7, 176. [CrossRef]
- Stincone, P.; Brandelli, A. Marine bacteria as source of antimicrobial compounds. Crit Rev Biotechnol 2020, 40, 306-319. [CrossRef]
- Datta, S.R.; Roy, A. Antimicrobial peptides as potential therapeutic agents: a review. Int. J. Pept. Res. Ther 2021, 27, 555-577. [CrossRef]
- Zhang, S.; Liang, X.; Gadd, G.M.; Zhao, Q. Marine Microbial-Derived Antibiotics and Biosurfactants as Potential New Agents against Catheter-Associated Urinary Tract Infections. Mar Drugs 2021, 19. [CrossRef]
- Borges, A.; Simoes, M. Quorum Sensing Inhibition by Marine Bacteria. Mar Drugs 2019, 17. [CrossRef]
- Chen, J.; Wang, B.; Lu, Y.; Guo, Y.; Sun, J.; Wei, B.; Zhang, H.; Wang, H. Quorum Sensing Inhibitors from Marine Microorganisms and Their Synthetic Derivatives. Mar Drugs 2019, 17. [CrossRef]
- Torres, M.; Dessaux, Y.; Llamas, I. Saline Environments as a Source of Potential Quorum Sensing Disruptors to Control Bacterial Infections: A Review. Mar Drugs 2019, 17. [CrossRef]
- Zhao, J.; Li, X.; Hou, X.; Quan, C.; Chen, M. Widespread Existence of Quorum Sensing Inhibitors in Marine Bacteria: Potential Drugs to Combat Pathogens with Novel Strategies. Mar Drugs 2019, 17. [CrossRef]
- Liu, Y.; Ding, S.; Shen, J.; Zhu, K. Nonribosomal antibacterial peptides that target multidrug-resistant bacteria. Nat Prod Rep 2019, 36, 573-592. [CrossRef]
- Barbosa, F.; Pinto, E.; Kijjoa, A.; Pinto, M.; Sousa, E. Targeting antimicrobial drug resistance with marine natural products. Int J Antimicrob Agents 2020, 56, 106005. [CrossRef]
- Liu, M.; El-Hossary, E.M.; Oelschlaeger, T.A.; Donia, M.S.; Quinn, R.J.; Abdelmohsen, U.R. Potential of marine natural products against drug-resistant bacterial infections. Lancet Infect Dis 2019, 19, e237-e245. [CrossRef]
- Casertano, M.; Menna, M.; Imperatore, C. The Ascidian-Derived Metabolites with Antimicrobial Properties. Antibiotics (Basel) 2020, 9. [CrossRef]
- Neshani, A.; Zare, H.; Akbari Eidgahi, M.R.; Khaledi, A.; Ghazvini, K. Epinecidin-1, a highly potent marine antimicrobial peptide with anticancer and immunomodulatory activities. BMC Pharmacol Toxicol 2019, 20, 33. [CrossRef]
- Portelinha, J.; Duay, S.S.; Yu, S.I.; Heilemann, K.; Libardo, M.D.J.; Juliano, S.A.; Klassen, J.L.; Angeles-Boza, A.M. Antimicrobial Peptides and Copper(II) Ions: Novel Therapeutic Opportunities. Chem Rev 2021, 121, 2648-2712. [CrossRef]
- Willems, T.; De Mol, M.L.; De Bruycker, A.; De Maeseneire, S.L.; Soetaert, W.K. Alkaloids from Marine Fungi: Promising Antimicrobials. Antibiotics (Basel) 2020, 9. [CrossRef]
- Wang, C.; Tang, S.; Cao, S. Antimicrobial compounds from marine fungi. Phytochem. Rev 2021, 20. [CrossRef]
- Karpinski, T.M. Marine Macrolides with Antibacterial and/or Antifungal Activity. Mar Drugs 2019, 17. [CrossRef]
- Alves, E.; Dias, M.; Lopes, D.; Almeida, A.; Domingues, M.D.R.; Rey, F. Antimicrobial Lipids from Plants and Marine Organisms: An Overview of the Current State-of-the-Art and Future Prospects. Antibiotics (Basel) 2020, 9. [CrossRef]
- Almeida, M.C.; Resende, D.; da Costa, P.M.; Pinto, M.M.M.; Sousa, E. Tryptophan derived natural marine alkaloids and synthetic derivatives as promising antimicrobial agents. Eur J Med Chem 2021, 209, 112945. [CrossRef]
- Arockianathan, P.M.; Mishra, M.; Niranjan, R. Recent Status and Advancements in the Development of Antifungal Agents: Highlights on Plant and Marine Based Antifungals. Curr Top Med Chem 2019, 19, 812-830. [CrossRef]
- Christy, M.P.; Uekusa, Y.; Gerwick, L.; Gerwick, W.H. Natural Products with Potential to Treat RNA Virus Pathogens Including SARS-CoV-2. J Nat Prod 2021, 84, 161-182. [CrossRef]
- Hamoda, A.M.; Fayed, B.; Ashmawy, N.S.; El-Shorbagi, A.A.; Hamdy, R.; Soliman, S.S.M. Marine Sponge is a Promising Natural Source of Anti-SARS-CoV-2 Scaffold. Front Pharmacol 2021, 12, 666664. [CrossRef]
- Khan, M.T.; Ali, A.; Wang, Q.; Irfan, M.; Khan, A.; Zeb, M.T.; Zhang, Y.J.; Chinnasamy, S.; Wei, D.Q. Marine natural compounds as potents inhibitors against the main protease of SARS-CoV-2-a molecular dynamic study. J Biomol Struct Dyn 2021, 39, 3627-3637. [CrossRef]
- El-Tantawy, W.H.; Temraz, A. Natural products for the management of the hepatitis C virus: a biochemical review. Arch Physiol Biochem 2020, 126, 116-128. [CrossRef]
- Sun, T.T.; Zhu, H.J.; Cao, F. Marine Natural Products as a Source of Drug Leads against Respiratory Viruses: Structural and Bioactive Diversity. Curr Med Chem 2021, 28, 3568-3594. [CrossRef]
- Sansone, C.; Brunet, C.; Noonan, D.M.; Albini, A. Marine Algal Antioxidants as Potential Vectors for Controlling Viral Diseases. Antioxidants (Basel) 2020, 9. [CrossRef]
- Pagarete, A.; Ramos, A.S.; Puntervoll, P.; Allen, M.J.; Verdelho, V. Antiviral Potential of Algal Metabolites-A Comprehensive Review. Mar Drugs 2021, 19. [CrossRef]
- Besednova, N.N.; Andryukov, B.G.; Zaporozhets, T.S.; Kryzhanovsky, S.P.; Fedyanina, L.N.; Kuznetsova, T.A.; Zvyagintseva, T.N.; Shchelkanov, M.Y. Antiviral Effects of Polyphenols from Marine Algae. Biomedicines 2021, 9. [CrossRef]
- Diaz-Marrero, A.R.; Lopez-Arencibia, A.; Bethencout-Estrella, C.J.; Cen-Pacheco, F.; Sifaoui, I.; Hernandez Creus, A.; Duque-Ramirez, M.C.; Souto, M.L.; Hernandez Daranas, A.; Lorenzo-Morales, J.; et al. Antiprotozoal activities of marine polyether triterpenoids. Bioorg Chem 2019, 92, 103276. [CrossRef]
- Lee, S.M.; Kim, M.S.; Hayat, F.; Shin, D. Recent Advances in the Discovery of Novel Antiprotozoal Agents. Molecules 2019, 24. [CrossRef]
- Zhang, S.K., I.; Brimble, M. Naturally occurring antitubercular cyclic peptides. Tetrahedron Lett. 2019, 60, 151339. [CrossRef]
- Alvarez-Bardon, M.; Perez-Pertejo, Y.; Ordonez, C.; Sepulveda-Crespo, D.; Carballeira, N.M.; Tekwani, B.L.; Murugesan, S.; Martinez-Valladares, M.; Garcia-Estrada, C.; Reguera, R.M.; et al. Screening Marine Natural Products for New Drug Leads against Trypanosomatids and Malaria. Mar Drugs 2020, 18. [CrossRef]
- Nweze, J.A.; Mbaoji, F.N.; Li, Y.M.; Yang, L.Y.; Huang, S.S.; Chigor, V.N.; Eze, E.A.; Pan, L.X.; Zhang, T.; Yang, D.F. Potentials of marine natural products against malaria, leishmaniasis, and trypanosomiasis parasites: a review of recent articles. Infect Dis Poverty 2021, 10, 9. [CrossRef]
- Aguiar, A.C.C.; Parisi, J.R.; Granito, R.N.; de Sousa, L.R.F.; Renno, A.C.M.; Gazarini, M.L. Metabolites from Marine Sponges and Their Potential to Treat Malarial Protozoan Parasites Infection: A Systematic Review. Mar Drugs 2021, 19. [CrossRef]
- Hou, X.M.; Wang, C.Y.; Gerwick, W.H.; Shao, C.L. Marine natural products as potential anti-tubercular agents. Eur J Med Chem 2019, 165, 273-292. [CrossRef]
- Khan, M.T.; Kaushik, A.C.; Bhatti, A.I.; Zhang, Y.J.; Zhang, S.; Wei, A.J.; Malik, S.I.; Wei, D.Q. Marine Natural Products and Drug Resistance in Latent Tuberculosis. Mar Drugs 2019, 17. [CrossRef]
- Zhao, M.M.Z., K. W. . Marine natural products with anti-inflammation effects. Trad. Med. Res. 2020, 5, 252-260. [CrossRef]
- Bilal, M.; Qindeel, M.; Nunes, L.V.; Duarte, M.T.S.; Ferreira, L.F.R.; Soriano, R.N.; Iqbal, H.M.N. Marine-Derived Biologically Active Compounds for the Potential Treatment of Rheumatoid Arthritis. Mar Drugs 2020, 19. [CrossRef]
- Souza, C.R.M.; Bezerra, W.P.; Souto, J.T. Marine Alkaloids with Anti-Inflammatory Activity: Current Knowledge and Future Perspectives. Mar Drugs 2020, 18. [CrossRef]
- Xu, J.; Yi, M.; Ding, L.; He, S. A Review of Anti-Inflammatory Compounds from Marine Fungi, 2000-2018. Mar Drugs 2019, 17. [CrossRef]
- Di Costanzo, F.; Di Dato, V.; Ianora, A.; Romano, G. Prostaglandins in Marine Organisms: A Review. Mar Drugs 2019, 17. [CrossRef]
- Kemp, D.C.; Kwon, J.Y. Fish and Shellfish-Derived Anti-Inflammatory Protein Products: Properties and Mechanisms. Molecules 2021, 26. [CrossRef]
- Ahmad, S.; Saleem, M.; Riaz, N.; Lee, Y.S.; Diri, R.; Noor, A.; Almasri, D.; Bagalagel, A.; Elsebai, M.F. The Natural Polypeptides as Significant Elastase Inhibitors. Front Pharmacol 2020, 11, 688. [CrossRef]
- Zhang, Y.R.M.J.X.Z.L.J. Anti-inflammatory and immunomodulatory effects of marine n-3 polyunsaturated fatty acids on human health and diseases. J. Ocean Univ. China 2019, 18, 481-492. [CrossRef]
- Rausch, J.; Gillespie, S.; Orchard, T.; Tan, A.; McDaniel, J.C. Systematic review of marine-derived omega-3 fatty acid supplementation effects on leptin, adiponectin, and the leptin-to-adiponectin ratio. Nutr Res 2021, 85, 135-152. [CrossRef]
- Wei, Y.; Meng, Y.; Li, N.; Wang, Q.; Chen, L. The effects of low-ratio n-6/n-3 PUFA on biomarkers of inflammation: a systematic review and meta-analysis. Food Funct 2021, 12, 30-40. [CrossRef]
- Liu, M.; Li, W.; Chen, Y.; Wan, X.; Wang, J. Fucoxanthin: A promising compound for human inflammation-related diseases. Life Sci 2020, 255, 117850. [CrossRef]
- Alharbi, R. Antioxidant properties of marine algae: an overview. Biosci. Res. 2019, 16, 986-996.
- Saraswati; Giriwono, P.E.; Iskandriati, D.; Tan, C.P.; Andarwulan, N. Sargassum Seaweed as a Source of Anti-Inflammatory Substances and the Potential Insight of the Tropical Species: A Review. Mar Drugs 2019, 17. [CrossRef]
- Riccio, G.; Lauritano, C. Microalgae with Immunomodulatory Activities. Mar Drugs 2019, 18. [CrossRef]
- Dolmatova, L.S.; Dolmatov, I.Y. Different Macrophage Type Triggering as Target of the Action of Biologically Active Substances from Marine Invertebrates. Mar Drugs 2020, 18. [CrossRef]
- Rubilar, T.; Barbieri, E.S.; Gazquez, A.; Avaro, M. Sea Urchin Pigments: Echinochrome A and Its Potential Implication in the Cytokine Storm Syndrome. Mar Drugs 2021, 19. [CrossRef]
- Sanina, N. Vaccine Adjuvants Derived from Marine Organisms. Biomolecules 2019, 9. [CrossRef]
- Cao, Q.; Zhao, J.; Xing, M.; Xiao, H.; Zhang, Q.; Liang, H.; Ji, A.; Song, S. Current Research Landscape of Marine-Derived Anti-Atherosclerotic Substances. Mar Drugs 2020, 18. [CrossRef]
- Zhao, J.; Cao, Q.; Xing, M.; Xiao, H.; Cheng, Z.; Song, S.; Ji, A. Advances in the Study of Marine Products with Lipid-Lowering Properties. Mar Drugs 2020, 18. [CrossRef]
- Dwivedi, R.; Pomin, V.H. Marine Antithrombotics. Mar Drugs 2020, 18. [CrossRef]
- Doshi, G.; Nailwal, N. A Review on Molecular Mechanisms and Patents of Marine-derived Anti-thrombotic Agents. Curr Drug Targets 2021, 22, 318-335. [CrossRef]
- Carvalhal, F.; Cristelo, R.R.; Resende, D.; Pinto, M.M.M.; Sousa, E.; Correia-da-Silva, M. Antithrombotics from the Sea: Polysaccharides and Beyond. Mar Drugs 2019, 17. [CrossRef]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for High-Value Products Towards Human Health and Nutrition. Mar Drugs 2019, 17. [CrossRef]
- Yang, H.W.; Fernando, K.H.N.; Oh, J.Y.; Li, X.; Jeon, Y.J.; Ryu, B. Anti-Obesity and Anti-Diabetic Effects of Ishige okamurae. Mar Drugs 2019, 17. [CrossRef]
- Chen, L.; Liu, R.; He, X.; Pei, S.; Li, D. Effects of brown seaweed polyphenols, a class of phlorotannins, on metabolic disorders via regulation of fat function. Food Funct 2021, 12, 2378-2388. [CrossRef]
- Pradhan, B.; Nayak, R.; Patra, S.; Jit, B.P.; Ragusa, A.; Jena, M. Bioactive Metabolites from Marine Algae as Potent Pharmacophores against Oxidative Stress-Associated Human Diseases: A Comprehensive Review. Molecules 2020, 26. [CrossRef]
- Rayapu, L.; Chakraborty, K.; Valluru, L. Marine Algae as a Potential Source for Anti-diabetic Compounds - A Brief Review. Curr Pharm Des 2021, 27, 789-801. [CrossRef]
- Sachithanandam, V.; Lalitha, P.; Parthiban, A.; Mageswaran, T.; Manmadhan, K.; Sridhar, R. A Review on Antidiabetic Properties of Indian Mangrove Plants with Reference to Island Ecosystem. Evid Based Complement Alternat Med 2019, 2019, 4305148. [CrossRef]
- Bae, M.; Kim, M.B.; Park, Y.K.; Lee, J.Y. Health benefits of fucoxanthin in the prevention of chronic diseases. Biochim Biophys Acta Mol Cell Biol Lipids 2020, 1865, 158618. [CrossRef]
- Landon, R.; Gueguen, V.; Petite, H.; Letourneur, D.; Pavon-Djavid, G.; Anagnostou, F. Impact of Astaxanthin on Diabetes Pathogenesis and Chronic Complications. Mar Drugs 2020, 18. [CrossRef]
- Gabbia, D.; De Martin, S. Brown Seaweeds for the Management of Metabolic Syndrome and Associated Diseases. Molecules 2020, 25. [CrossRef]
- Gunathilaka, T.L.; Samarakoon, K.; Ranasinghe, P.; Peiris, L.D.C. Antidiabetic Potential of Marine Brown Algae-a Mini Review. J Diabetes Res 2020, 2020, 1230218. [CrossRef]
- Balasa, A.F.; Chircov, C.; Grumezescu, A.M. Marine Biocompounds for Neuroprotection-A Review. Mar Drugs 2020, 18. [CrossRef]
- Catanesi, M.; Caioni, G.; Castelli, V.; Benedetti, E.; d’Angelo, M.; Cimini, A. Benefits under the Sea: The Role of Marine Compounds in Neurodegenerative Disorders. Mar Drugs 2021, 19. [CrossRef]
- Ahmmed, M.A., F.; Tian, H.; Carne, A.; Bekhit, A. Marine omega-3 (n-3) phospholipids: a comprehensive review of their properties, sources, bioavailability, and relation to brain health. Compr. Rev. Food Sci. Food Saf. 2019, 19, 64-123. [CrossRef]
- Jin, A.H.; Muttenthaler, M.; Dutertre, S.; Himaya, S.W.A.; Kaas, Q.; Craik, D.J.; Lewis, R.J.; Alewood, P.F. Conotoxins: Chemistry and Biology. Chem Rev 2019, 119, 11510-11549. [CrossRef]
- Jimenez, E.C. Post-translationally modified conopeptides: Biological activities and pharmacological applications. Peptides 2021, 139, 170525. [CrossRef]
- Baj, A.; Bistoletti, M.; Bosi, A.; Moro, E.; Giaroni, C.; Crema, F. Marine Toxins and Nociception: Potential Therapeutic Use in the Treatment of Visceral Pain Associated with Gastrointestinal Disorders. Toxins (Basel) 2019, 11. [CrossRef]
- Barbalace, M.C.; Malaguti, M.; Giusti, L.; Lucacchini, A.; Hrelia, S.; Angeloni, C. Anti-Inflammatory Activities of Marine Algae in Neurodegenerative Diseases. Int J Mol Sci 2019, 20. [CrossRef]
- Olasehinde, T.A.; Olaniran, A.O.; Okoh, A.I. Macroalgae as a Valuable Source of Naturally Occurring Bioactive Compounds for the Treatment of Alzheimer’s Disease. Mar Drugs 2019, 17. [CrossRef]
- Hannan, M.A.; Dash, R.; Haque, M.N.; Mohibbullah, M.; Sohag, A.A.M.; Rahman, M.A.; Uddin, M.J.; Alam, M.; Moon, I.S. Neuroprotective Potentials of Marine Algae and Their Bioactive Metabolites: Pharmacological Insights and Therapeutic Advances. Mar Drugs 2020, 18. [CrossRef]
- Rathnayake, A.U.; Abuine, R.; Kim, Y.J.; Byun, H.G. Anti-Alzheimer’s Materials Isolated from Marine Bio-resources: A Review. Curr Alzheimer Res 2019, 16, 895-906. [CrossRef]
- Ciccone, L.; Vandooren, J.; Nencetti, S.; Orlandini, E. Natural Marine and Terrestrial Compounds as Modulators of Matrix Metalloproteinases-2 (MMP-2) and MMP-9 in Alzheimer’s Disease. Pharmaceuticals (Basel) 2021, 14. [CrossRef]
- Kabir, M.T.; Uddin, M.S.; Jeandet, P.; Emran, T.B.; Mitra, S.; Albadrani, G.M.; Sayed, A.A.; Abdel-Daim, M.M.; Simal-Gandara, J. Anti-Alzheimer’s Molecules Derived from Marine Life: Understanding Molecular Mechanisms and Therapeutic Potential. Mar Drugs 2021, 19. [CrossRef]
- Rahman, M.A.; Dash, R.; Sohag, A.A.M.; Alam, M.; Rhim, H.; Ha, H.; Moon, I.S.; Uddin, M.J.; Hannan, M.A. Prospects of Marine Sterols against Pathobiology of Alzheimer’s Disease: Pharmacological Insights and Technological Advances. Mar Drugs 2021, 19. [CrossRef]
- Silva, M.; Seijas, P.; Otero, P. Exploitation of Marine Molecules to Manage Alzheimer’s Disease. Mar Drugs 2021, 19. [CrossRef]
- Castaneda, A.; Ferraz, R.; Vieira, M.; Cardoso, I.; Vasconcelos, V.; Martins, R. Bridging Cyanobacteria to Neurodegenerative Diseases: A New Potential Source of Bioactive Compounds against Alzheimer’s Disease. Mar Drugs 2021, 19. [CrossRef]
- Huang, C.; Zhang, Z.; Cui, W. Marine-Derived Natural Compounds for the Treatment of Parkinson’s Disease. Mar Drugs 2019, 17. [CrossRef]
- Fakhri, S.; Aneva, I.Y.; Farzaei, M.H.; Sobarzo-Sanchez, E. The Neuroprotective Effects of Astaxanthin: Therapeutic Targets and Clinical Perspective. Molecules 2019, 24. [CrossRef]
- Sorrenti, V.; Davinelli, S.; Scapagnini, G.; Willcox, B.J.; Allsopp, R.C.; Willcox, D.C. Astaxanthin as a Putative Geroprotector: Molecular Basis and Focus on Brain Aging. Mar Drugs 2020, 18. [CrossRef]
- Bahbah, E.I.; Ghozy, S.; Attia, M.S.; Negida, A.; Emran, T.B.; Mitra, S.; Albadrani, G.M.; Abdel-Daim, M.M.; Uddin, M.S.; Simal-Gandara, J. Molecular Mechanisms of Astaxanthin as a Potential Neurotherapeutic Agent. Mar Drugs 2021, 19, 201. [CrossRef]
- Liao, Q.; Feng, Y.; Yang, B.; Lee, S.M. Cnidarian peptide neurotoxins: a new source of various ion channel modulators or blockers against central nervous systems disease. Drug Discov Today 2019, 24, 189-197. [CrossRef]
- Finol-Urdaneta, R.K.; Belovanovic, A.; Micic-Vicovac, M.; Kinsella, G.K.; McArthur, J.R.; Al-Sabi, A. Marine Toxins Targeting Kv1 Channels: Pharmacological Tools and Therapeutic Scaffolds. Mar Drugs 2020, 18. [CrossRef]
- Stonik, V.A.; Stonik, I.V. Marine Excitatory Amino Acids: Structure, Properties, Biosynthesis and Recent Approaches to Their Syntheses. Molecules 2020, 25. [CrossRef]
- Hong, A.; Tu, L.C.; Yang, I.; Lim, K.M.; Nam, S.J. Marine natural products with monoamine oxidase (MAO) inhibitory activity. Pharm Biol 2020, 58, 716-720. [CrossRef]
- Barbosa, A.J.M.; Roque, A.C.A. Free Marine Natural Products Databases for Biotechnology and Bioengineering. Biotechnol J 2019, 14, e1800607. [CrossRef]
- van Santen, J.A.; Kautsar, S.A.; Medema, M.H.; Linington, R.G. Microbial natural product databases: moving forward in the multi-omics era. Nat Prod Rep 2021, 38, 264-278. [CrossRef]
- Stuart, K.A.; Welsh, K.; Walker, M.C.; Edrada-Ebel, R. Metabolomic tools used in marine natural product drug discovery. Expert Opin Drug Discov 2020, 15, 499-522. [CrossRef]
- Williams, D.E.; Andersen, R.J. Biologically active marine natural products and their molecular targets discovered using a chemical genetics approach. Nat Prod Rep 2020, 37, 617-633. [CrossRef]
- Wang, L.; Umezawa, K. Cellular Signal Transductions and Their Inhibitors Derived from Deep-Sea Organisms. Mar Drugs 2021, 19. [CrossRef]
- Juarez-Portilla, C.; Olivares-Banuelos, T.; Molina-Jimenez, T.; Sanchez-Salcedo, J.A.; Moral, D.I.D.; Meza-Menchaca, T.; Flores-Munoz, M.; Lopez-Franco, O.; Roldan-Roldan, G.; Ortega, A.; et al. Seaweeds-derived compounds modulating effects on signal transduction pathways: A systematic review. Phytomedicine 2019, 63, 153016. [CrossRef]
- Kim, S.H.; Kim, H. Astaxanthin Modulation of Signaling Pathways That Regulate Autophagy. Mar Drugs 2019, 17. [CrossRef]
- Giannaccare, G.; Pellegrini, M.; Senni, C.; Bernabei, F.; Scorcia, V.; Cicero, A.F.G. Clinical Applications of Astaxanthin in the Treatment of Ocular Diseases: Emerging Insights. Mar Drugs 2020, 18. [CrossRef]
- Li, T.; Wang, N.; Zhang, T.; Zhang, B.; Sajeevan, T.P.; Joseph, V.; Armstrong, L.; He, S.; Yan, X.; Naman, C.B. A Systematic Review of Recently Reported Marine Derived Natural Product Kinase Inhibitors. Mar Drugs 2019, 17. [CrossRef]
- Parate, S.; Kumar, V.; Lee, G.; Rampogu, S.; Hong, J.C.; Lee, K.W. Marine-Derived Natural Products as ATP-Competitive mTOR Kinase Inhibitors for Cancer Therapeutics. Pharmaceuticals (Basel) 2021, 14. [CrossRef]
- Raghuvanshi, R.; Bharate, S.B. Preclinical and Clinical Studies on Bryostatins, A Class of Marine-Derived Protein Kinase C Modulators: A Mini-Review. Curr Top Med Chem 2020, 20, 1124-1135. [CrossRef]
- Wu, R.; Chen, H.; Chang, N.; Xu, Y.; Jiao, J.; Zhang, H. Unlocking the Drug Potential of the Bryostatin Family: Recent Advances in Product Synthesis and Biomedical Applications. Chemistry 2020, 26, 1166-1195. [CrossRef]
- Luesch, H.; Paavilainen, V.O. Natural products as modulators of eukaryotic protein secretion. Nat Prod Rep 2020, 37, 717-736. [CrossRef]
- Nishimura, S.; Matsumori, N. Chemical diversity and mode of action of natural products targeting lipids in the eukaryotic cell membrane. Nat Prod Rep 2020, 37, 677-702. [CrossRef]
- Risinger, A.L.; Du, L. Targeting and extending the eukaryotic druggable genome with natural products: cytoskeletal targets of natural products. Nat Prod Rep 2020, 37, 634-652. [CrossRef]
- Carazo, A.; Mladenka, P.; Pavek, P. Marine Ligands of the Pregnane X Receptor (PXR): An Overview. Mar Drugs 2019, 17. [CrossRef]
- Vasilopoulou, M.; Ioannou, E.; Roussis, V.; Chondrogianni, N. Modulation of the ubiquitin-proteasome system by marine natural products. Redox Biol 2021, 41, 101897. [CrossRef]
- González-Andrés, P.; Fernández-Peña, L.; Díez-Poza, C.; Villalobos, C.; Nuñez, L.; Barbero, A. Marine Heterocyclic Compounds That Modulate Intracellular Calcium Signals: Chemistry and Synthesis Approaches. Mar Drugs 2021, 19. [CrossRef]
- Kageyama, H.; Waditee-Sirisattha, R. Antioxidative, Anti-Inflammatory, and Anti-Aging Properties of Mycosporine-Like Amino Acids: Molecular and Cellular Mechanisms in the Protection of Skin-Aging. Mar Drugs 2019, 17. [CrossRef]
- Rosic, N.N. Mycosporine-Like Amino Acids: Making the Foundation for Organic Personalised Sunscreens. Mar Drugs 2019, 17. [CrossRef]
- Nowruzi, B.S., G.; Blanco, S. The cosmetic application of cyanobacterial secondary metabolites. Algal Res. 2020, 49, 101959. [CrossRef]
- Jesumani, V.; Du, H.; Aslam, M.; Pei, P.; Huang, N. Potential Use of Seaweed Bioactive Compounds in Skincare-A Review. Mar Drugs 2019, 17. [CrossRef]
- SHANNON, E.A.-G., N. Seaweeds as nutraceuticals for health and nutrition. Phycologia 2019, 58, 563-577. [CrossRef]
- Thiyagarasaiyar, K.; Goh, B.H.; Jeon, Y.J.; Yow, Y.Y. Algae Metabolites in Cosmeceutical: An Overview of Current Applications and Challenges. Mar Drugs 2020, 18. [CrossRef]
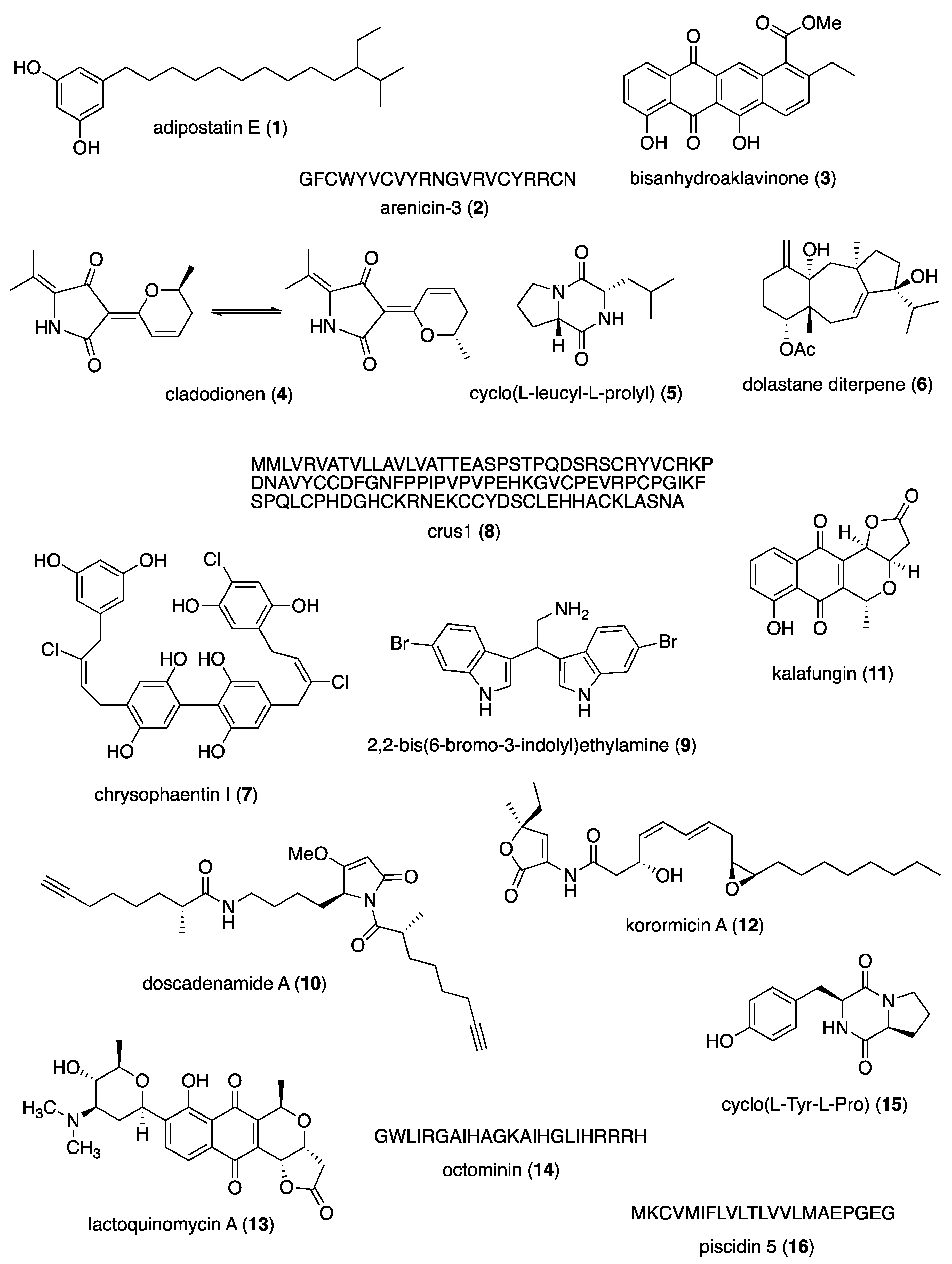
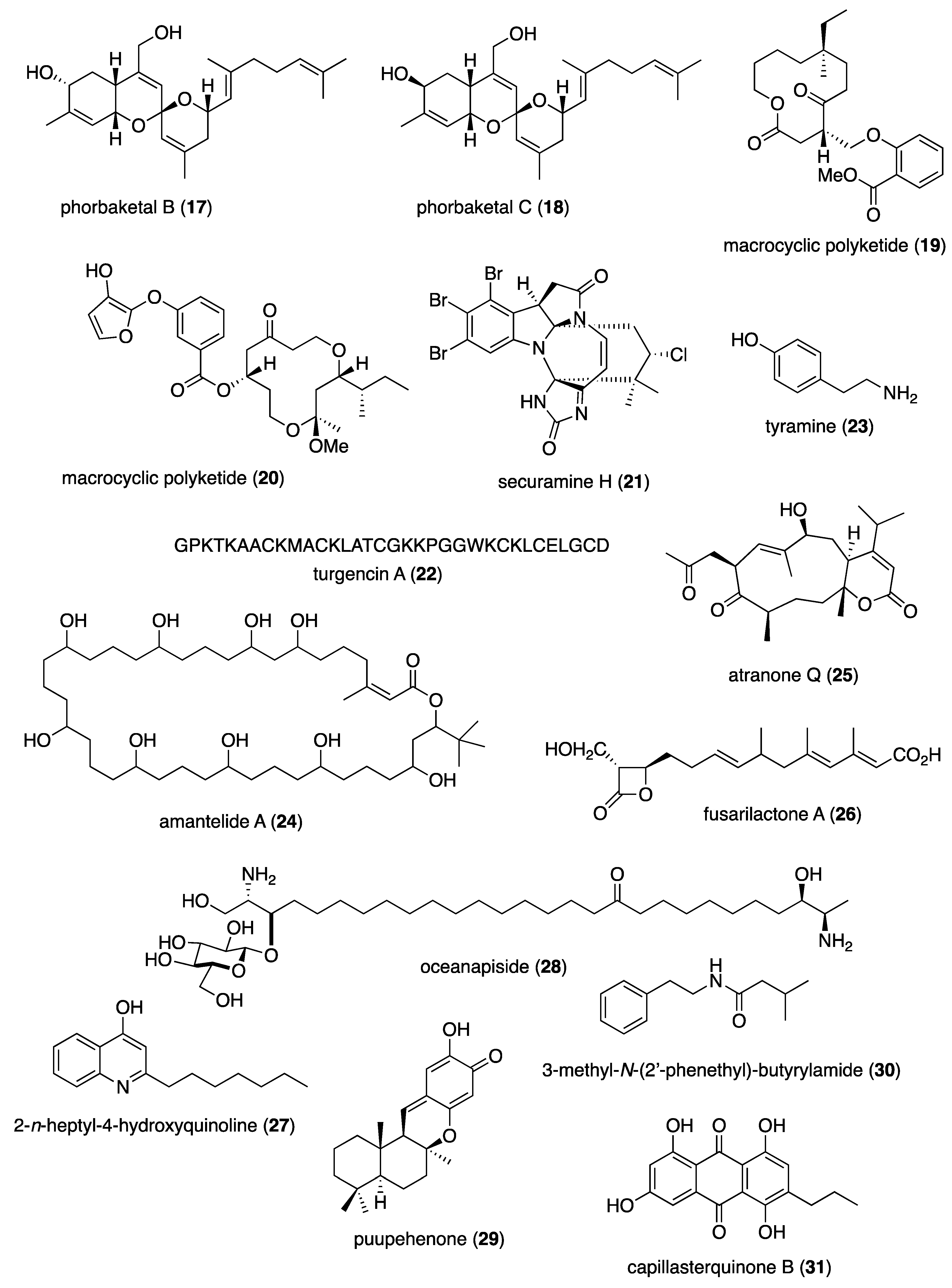
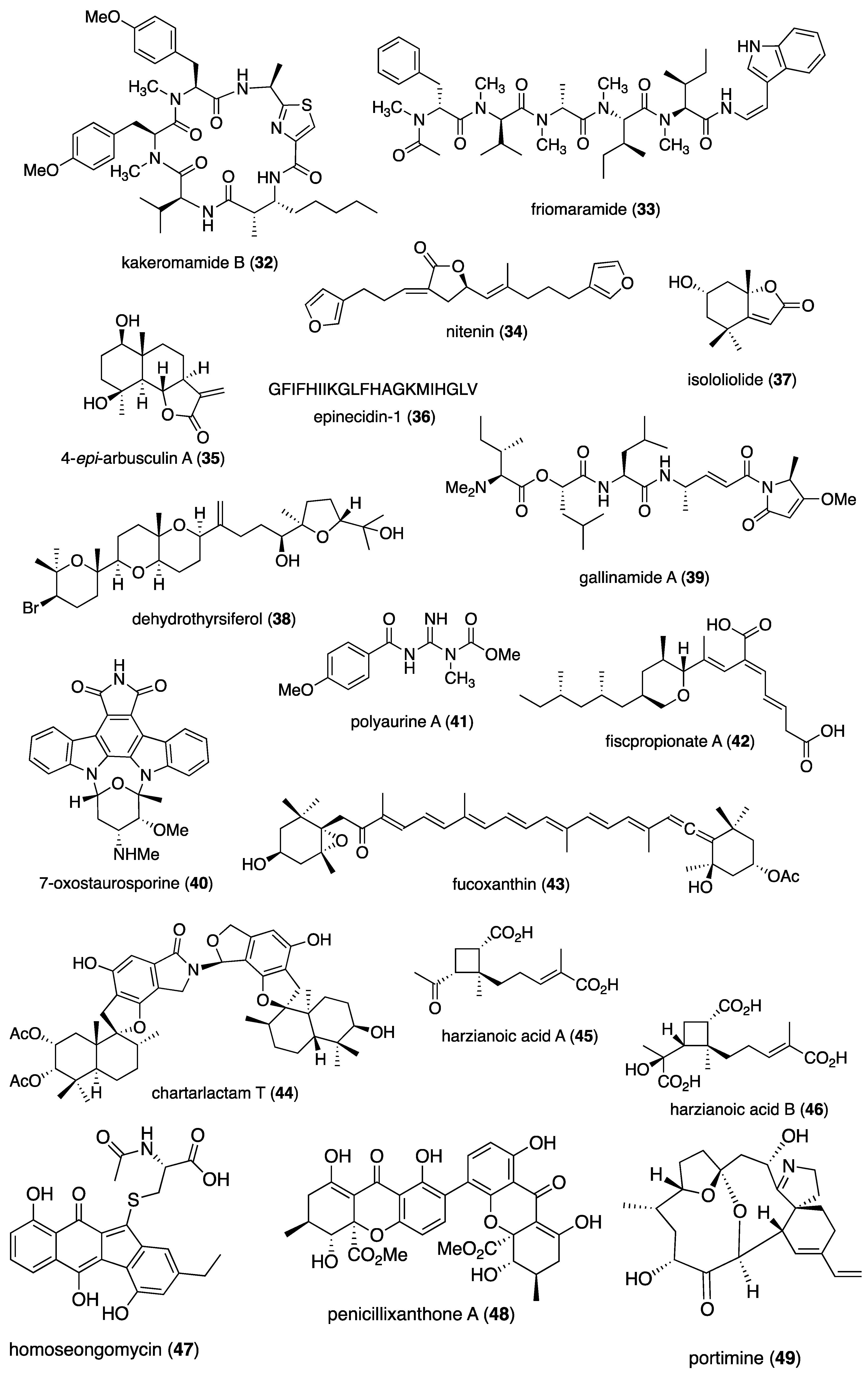

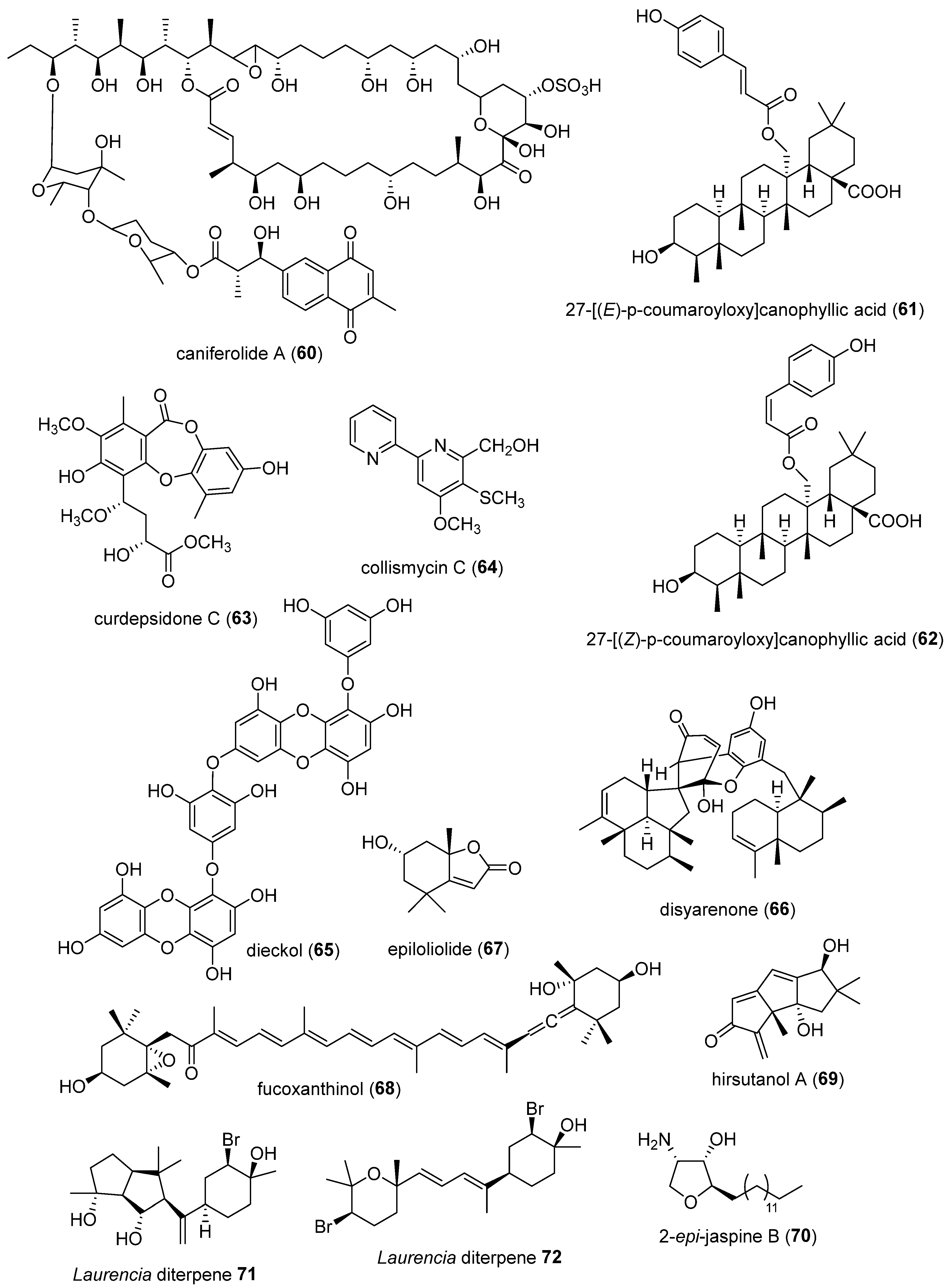
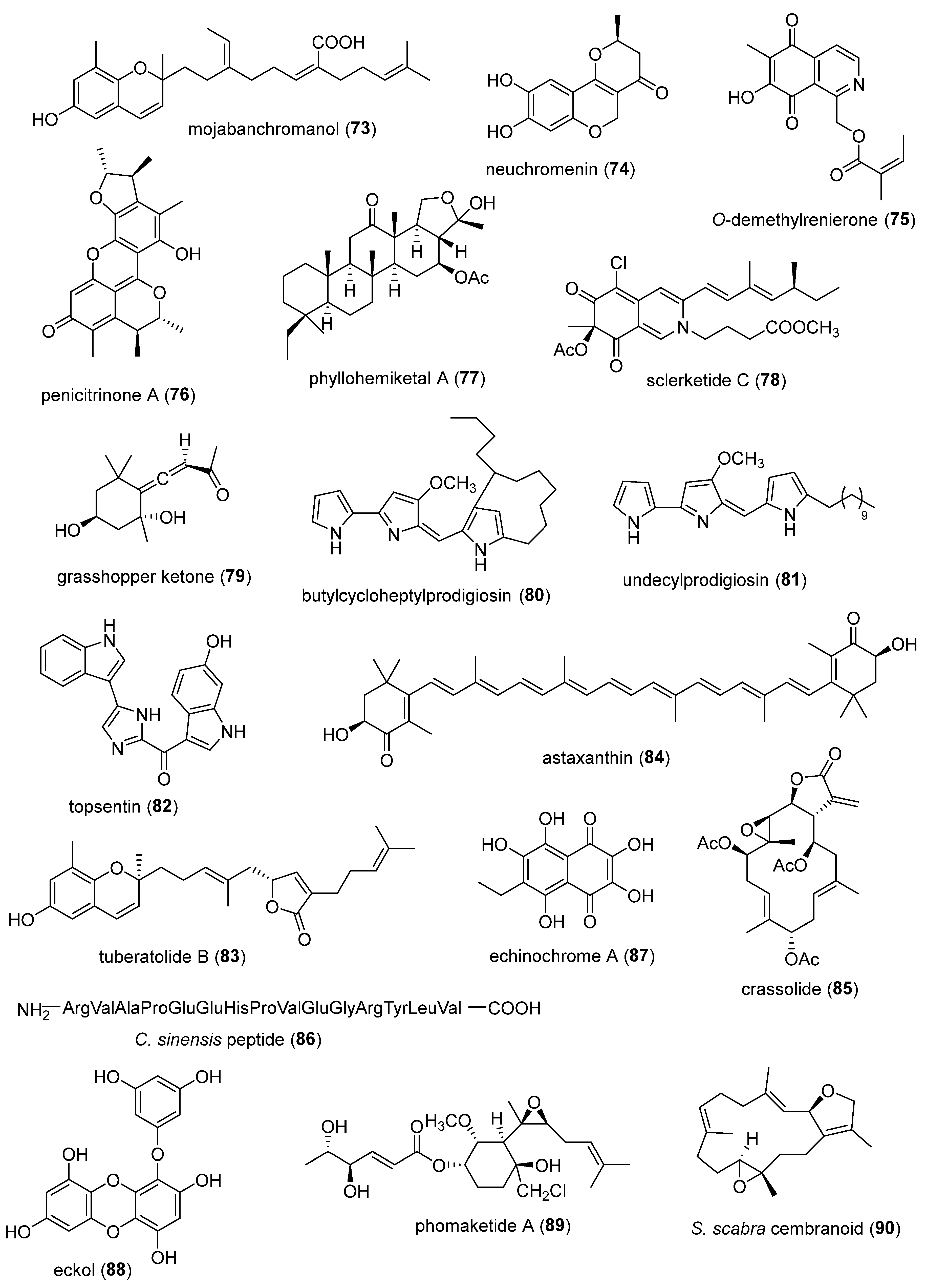
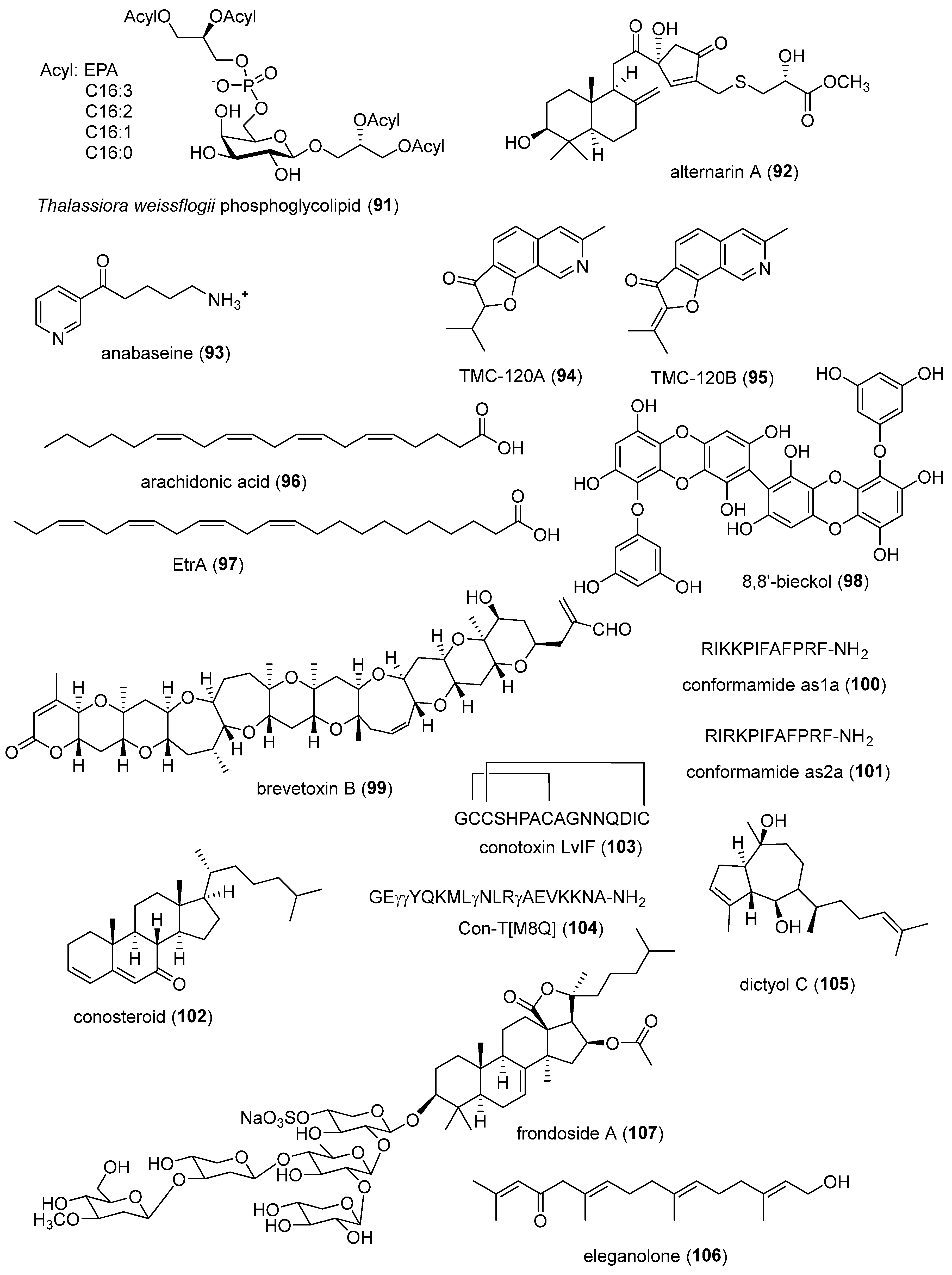

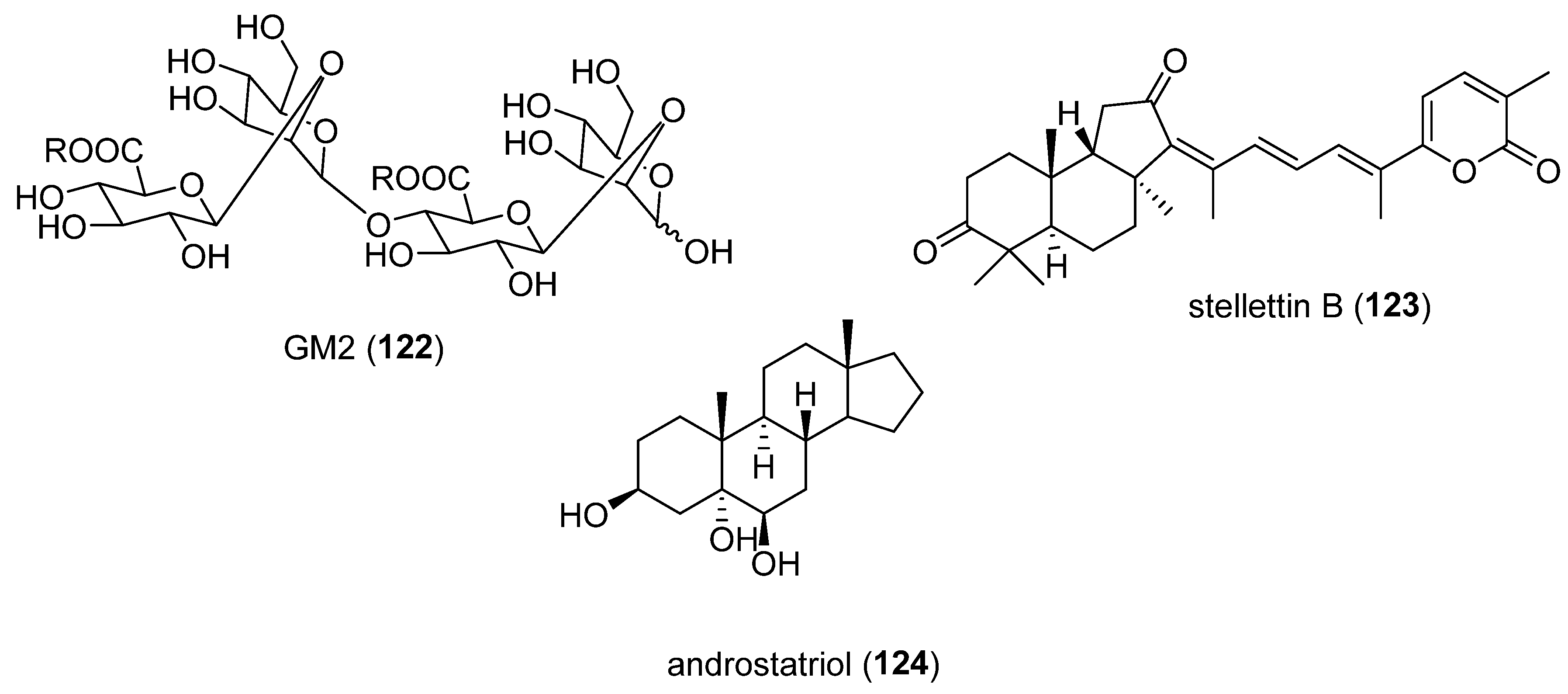
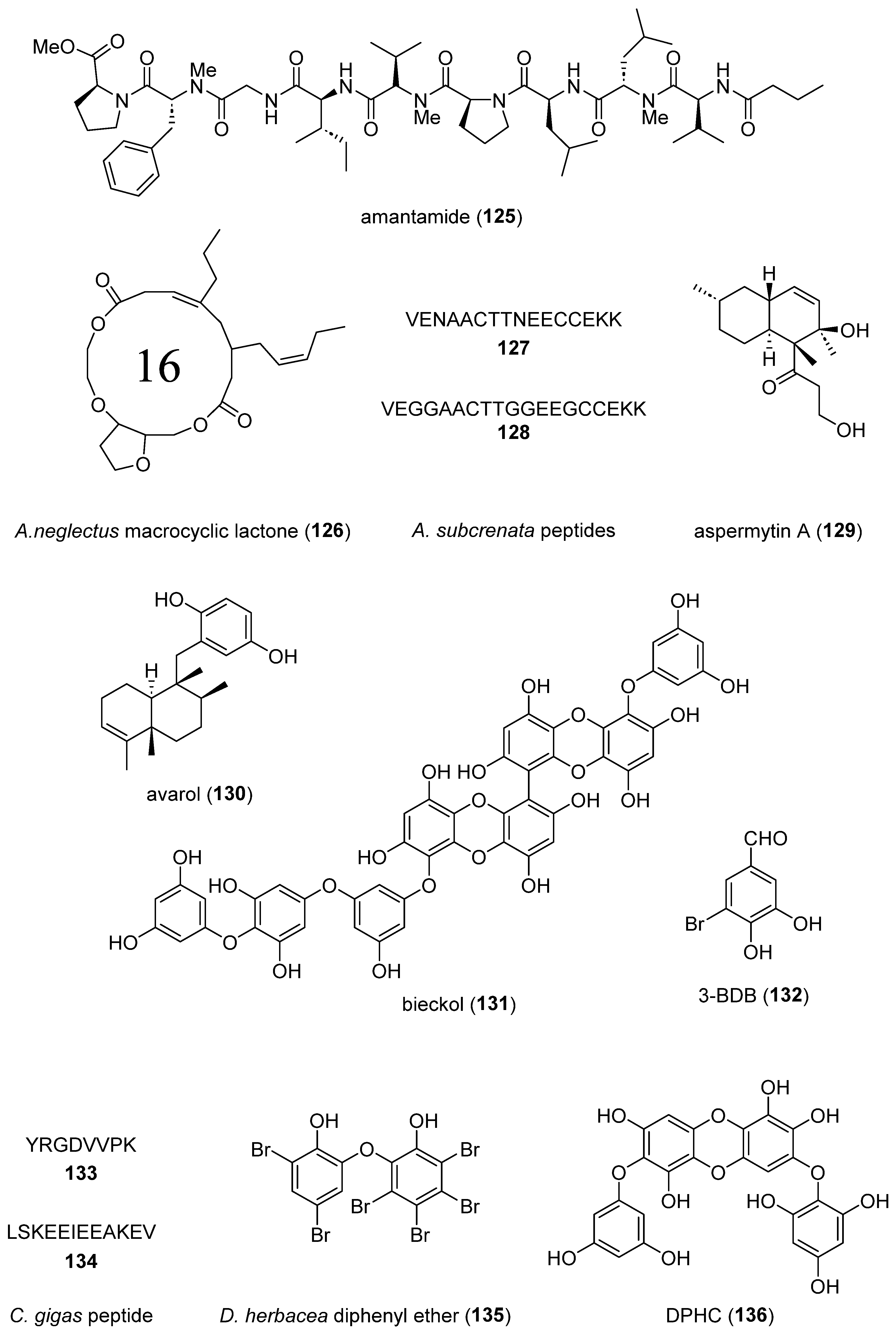
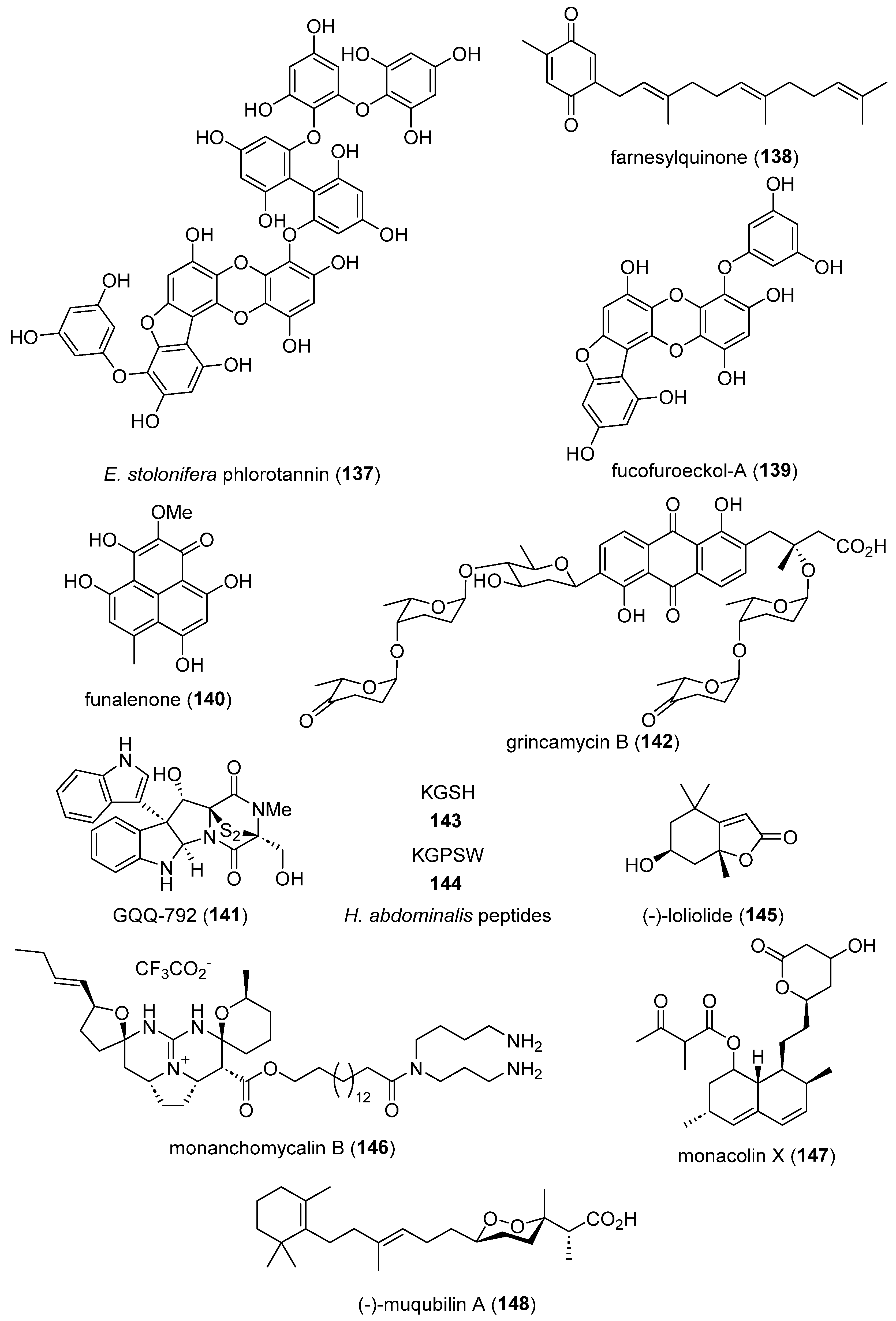
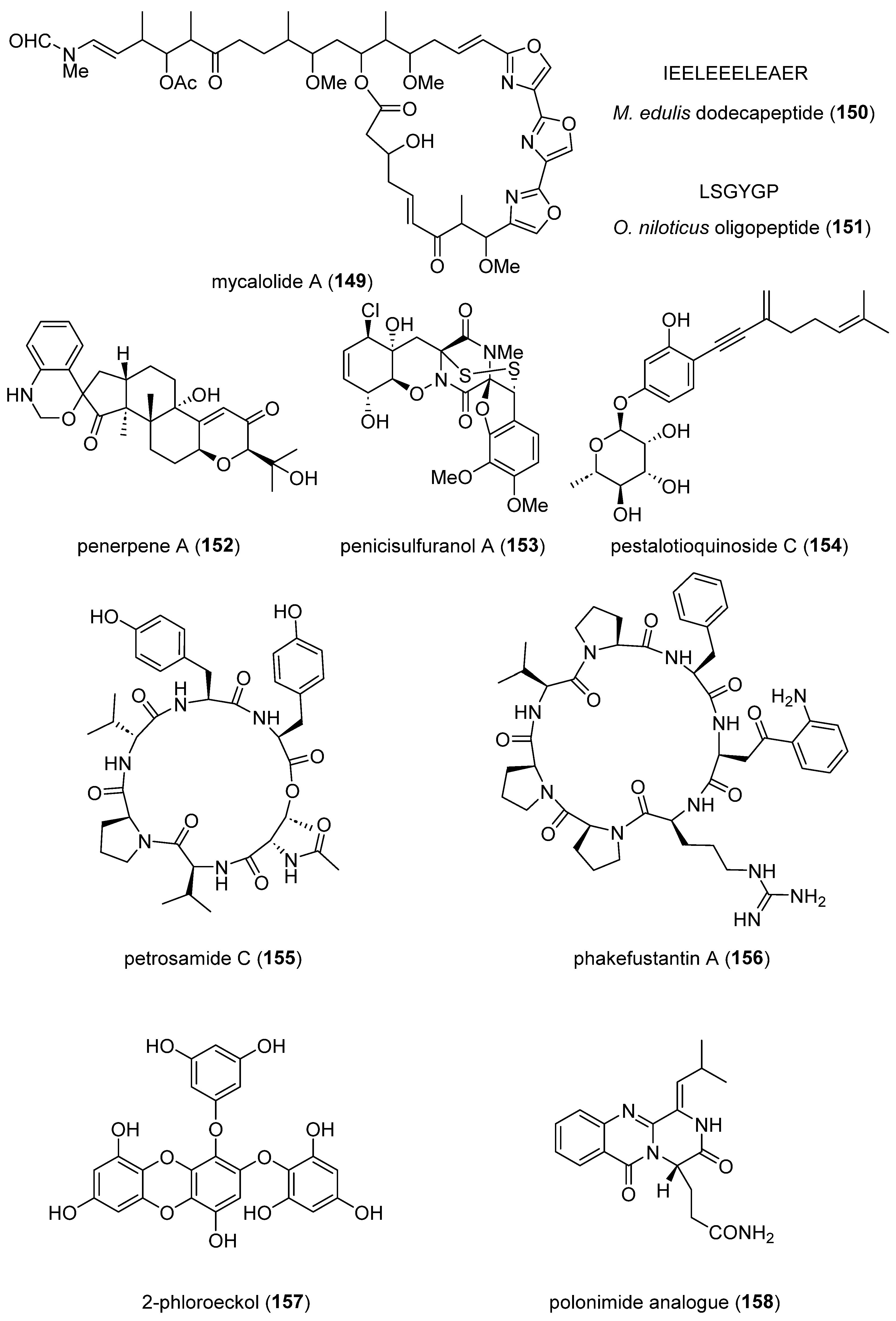
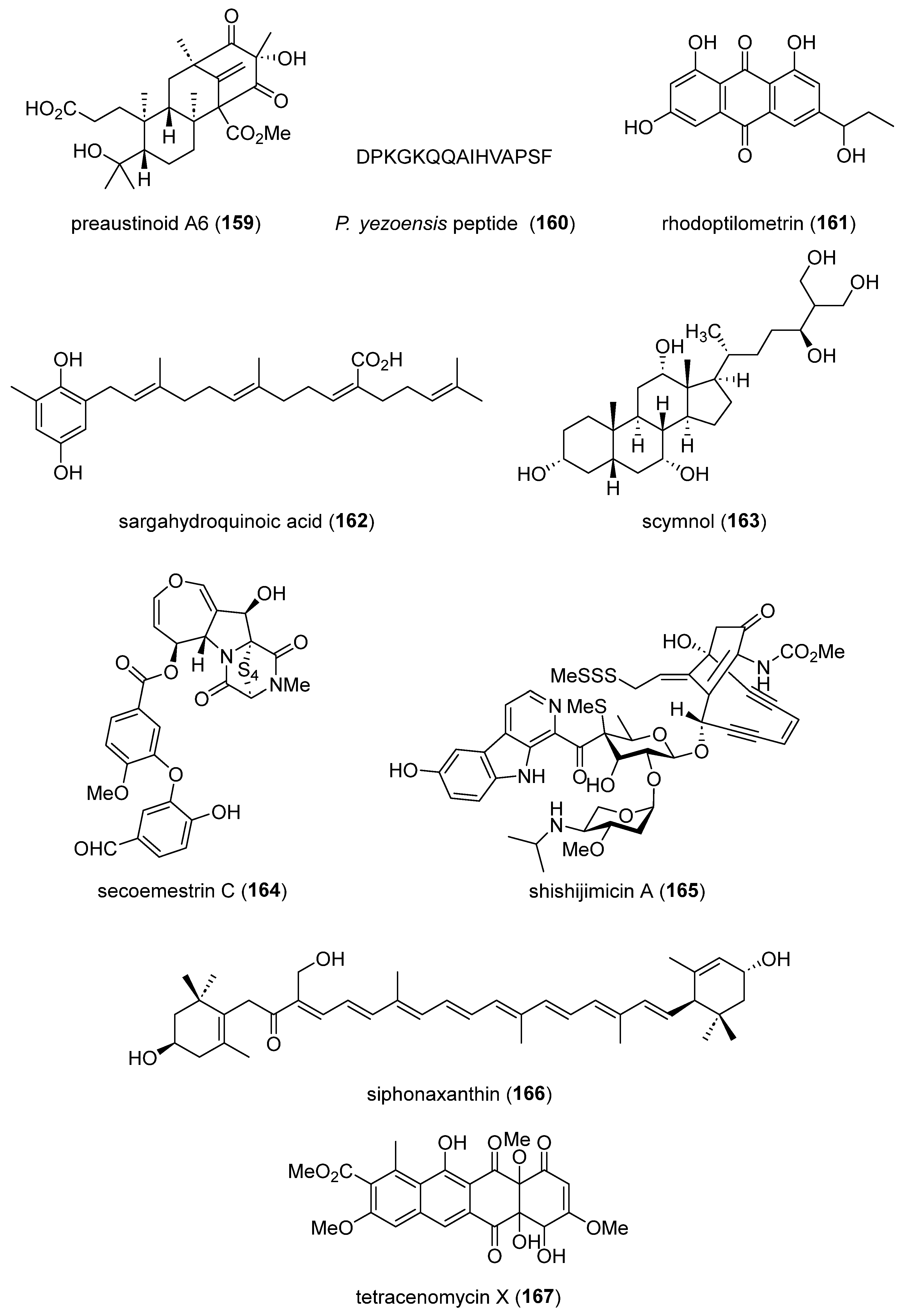
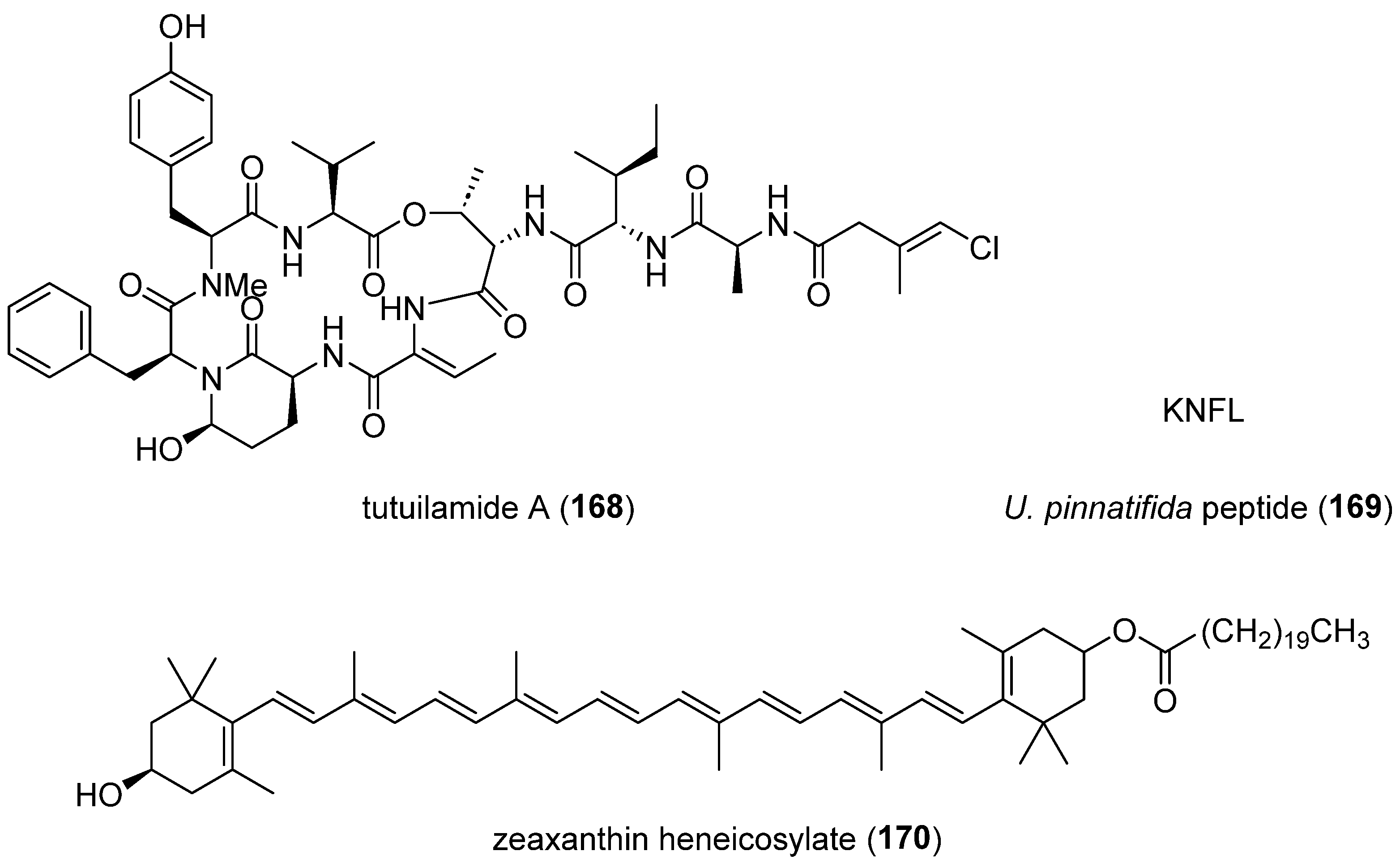
| Drug Class | Compound/Organism a | Chemistry | Pharmacologic Activity | IC50 b | MMOA c | Country d | References |
|---|---|---|---|---|---|---|---|
| Antibacterial | adipostatin E (1)/bacterium | Polyketide d | B. subtilis and L monocytogenes inhibition | 3.4, 5.9 µM | PPCS inhibition | CRI, USA | [14] |
| Antibacterial | arenicin-3 (2)/worm | Peptide f | E. coli & K. pneumoniae inhibition | 1-2 μg/mL+ | Cell membrane disruption and ATP release | AUS, CHE, CHN, DNK, DEU, GBR, IRL | [15] |
| Antibacterial | bisanhydroaklavinone (3)/bacterium | Polyketide d | S. aureus inhibition | 6.25 μg/mL+ | Cell membrane damage and DNA leakage | PHL, SGP | [16] |
| Antibacterial | cladodionen (4)/fungus | Polyketide d | P. aeruginosa quorum sensing inhibition | <400 µM | Downregulation of quorum sensing genes | CHN | [17] |
| Antibacterial | cyclo(L-leucyl-L-prolyl) (5)/bacterium | Peptide f | S. marcescens inhibition | 200 μg/mL+ | Biofilm formation inhibition | IND | [18] |
| Antibacterial | C. cervicornis diterpene (6)/alga | Terpenoid e | MR S. aureus inhibition | 8 μg/mL+ | Inhibition of efflux pump | BRA | [19] |
| Antibacterial | chrysophaentin I (7)/alga | Polyketide d | S. aureus inhibition | 10 μg/mL+ | Cytoskeletal protein FtsZ inhibition | USA | [20] |
| Antibacterial | crustin (8)/shrimp | Peptide f | M. luteus inhibition | 2.5 μM+ | Membrane disruption and depolarization | CHN | [21] |
| Antibacterial | D. candidum alkaloid (9)/ascidian | Alkaloid f | S. aureus, E. Coli, K. pneumoniae inhibition | 8 μg/mL+ | Biofilm formation inhibition | ITA | [22] |
| Antibacterial | doscadenamide A (10)/cyanobacterium | Peptide f -Polyketide d | P. aeruginosa quorum sensing activation | <10 µM | AHL-binding site | USA | [23] |
| Antibacterial | kalafungin (11)/bacterium | Polyketide d | S. aureus inhibition | 8, 16 μg/mL+ | Non-competitive β-lactamase inhibition | IND | [24] |
| Antibacterial | korormicin A (12)/bacterium | Polyketide d | V. cholerae and P. aeruginosa inhibition | 10-30 μM+ | Reactive oxygen species production | BRA, JPN, USA | [25] |
| Antibacterial | lactoquinomycin A (13)/bacterium | Polyketide d | MR S. aureus and S. enterica inhibition | 0.03-0.25 μg/mL+ | Induction of DNA damage | S. KOR | [26] |
| Antibacterial | octominin(14)/octopus | Peptide f | S. parauberis inhibition | 50 μg/mL+ | Membrane disruption and chromosomal DNA binding | S. KOR | [27] |
| Antibacterial | P. chrysogenum dipeptide (15)/fungus | Peptide f | C. violaceum and P. aeruginosa inhibition | 6.2 mg/mL+ | Anti-quorum sensing activity | CHN | [28] |
| Antibacterial | piscidin 5 (16)/fish | Peptide f | V. parahaemolyticus & P. damselae inhibition | 1.5-6.2 µM | Membrane disruption and DNA binding | CHN | [29] |
| Antibacterial | phorbaketal B and C (17, 18)/sponge | Terpenoid e | S. aureus biofilm inhibition | < 50 μg/mL | Downregulation of hemolysin-related genes | S. KOR | [30] |
| Antibacterial | S. algae polyketide (19)/bacterium | Polyketide d | E. coli and MR S. aureus inhibition | 3.75 μg/mL+ | MRSA penicillin-binding protein active site docking | IND | [31] |
| Antibacterial | S. algae polyketide (20)/bacterium | Polyketide d | VR E. faecalis and MR S. aureus inhibition | 1-3 μg/mL+ | Siderophore mechanism of action | IND | [32] |
| Antibacterial | securamine H (21)/bryozoan | Alkaloid f | S. aureus inhibition | 3.13 μM+ | Reduction of metabolic activity | NOR | [33] |
| Antibacterial | turgencin A(22)/ascidian | Peptide f | C. glutamicum and B. subtilis inhibition | 0.4 μM+ | Cell membrane disruption | AUS, NOR | [34] |
| Antibacterial | tyramine (23)/bacterium | Alkaloid f | P. aeruginosa quorum sensing inhibition | 1 mg/mL+ | Pyoverdine production inhibition | ESP | [35] |
| Antifungal | amantelide A (24)/cyanobacterium | Polyketide d | S. cervisiae inhibition | 12.5, 50 μM | Ergosterol binding and actin polymerization promotion | JPN, PHL, USA | [36] |
| Antifungal | atranone Q (25)/fungus | Terpenoid e | C. albicans growth inhibition | 8 μg/mL | Cytoplasm agglutination and cell membrane alterations | CHN | [37] |
| Antifungal | fusarilactone A (26)/fungus | Polyketide d | P. theae growth inhibition | 38.1 μg/mL | HMG-CoA inhibition | CHN | [38] |
| Antifungal | 2-n-heptyl-4-hydroxyquinoline (27)/bacterium | Alkaloid f | C. albicans hyphal growth inhibition | 11.4 μg/mL | cAMP-Efg1 pathway inhibition | S. KOR | [39] |
| Antifungal | oceanapiside (28)/sponge | Polyketide d | C. glabrata inhibition | 10 μg/mL | Sphingolipid synthesis inhibition | PHL, USA | [40] |
| Antifungal | puupehenone (29)/sponge | Terpenoid e | CAS- insensitive C. neoformans inhibition | 2.5-5 μg/mL+ | CWI integrity pathway disruption | USA | [41] |
| Antifungal | S. olivaceus butyrylamide (30)/bacterium | Shikimate h | C. albicans hyphal growth inhibition and adhesion | 100 μg/mL+ | Downregulation of hyphal formation genes | CHN | [42] |
| Antimalarial | capillasterquinone B (31)/bacterium | Polyketide d | P. falciparum 3D7 inhibition | 9.2 µg/mL | Lysyl-tRNA synthetase binding | DEU, EGY, GBR, SAU | [43] |
| Antimalarial | kakeromamide B (32)/cyanobacterium | Peptide f | Blood-stage P. falciparum inhibition | 8.9 μM | Binding to Plasmodium actin and sortilin | USA | [44] |
| Antimalarial | friomaramide (33)/sponge | Peptide f | P. falciparum sporozoites liver infection inhibition | <6.1 μM* | Hepatocyte nuclei viability confirmed | AUS, USA | [45] |
| Antimalarial | nitenin (34)/sponge | Terpenoid e | P. falciparum inhibition | 0.29 μM | Ring to trophozoite transition | USA | [46] |
| Antiprotozoal | 4-epi-arbusculin A (35)/zoanthid | Terpenoid e | A. castellanii inhibition | 26 μM | Programmed cell death induction | ESP | [47] |
| Antiprotozoal | epinecidin-1 (36)/fish | Peptide f | Trichomonas vaginalis inhibition | < 62.5 µg/mL | Membrane disruption | TWN | [48] |
| Antiprotozoal | isololiolide (37)/hydroid | Terpenoid e | T. cruzi trypomastigotes and amastigotes inhibition | 32, 40 μM | Disruption of membrane integrity | BRA, USA | [49] |
| Antiprotozoal | dehydrothyrsiferol (38)/alga | Terpenoid e | A. castellanii growth inhibition | 5.3 μM | Mitochondrial malfunction | MEX, ESP | [50] |
| Antiprotozoal | gallinamide A (39)/cyanobacterium | Peptide f | T. cruzi amastigote inhibition | 14.7 nM | Recombinant cruzain inhibition | USA | [51] |
| Antiprotozoal | 7-oxostaurosporine (40)/bacterium | Alkaloid f | A. castellanii growth inhibition | 0.8, 0.9, 5.5 μM | Mitochondrial malfunction | ECU, ESP | [52] |
| Antiprotozoal | polyaurine A (41)/ ascidian | Alkaloid f | S. mansoni inhibition | > 100 μM | Egg production impairment in vitro | IDN, ITA | [53] |
| Antituberculosis | fiscpropionate A (42) /fungus | Polyketide d | M. tuberculosis MptpB inhibition | 5.1 μM | Noncompetitive inhibition | CHN | [54] |
| Antituberculosis | fucoxanthin (43)/alga | Terpenoid e | M. tuberculosis strains inhibition | 2.8-4.1 μM+ | TBNAT inhibition | CHL, CZE, IRN, ROU | [55] |
| Antiviral | chartarlactam T (44)/fungus | Alkaloid f | Zika virus inhibition | 10 μM* | Protein E inhibition | CHN | [56] |
| Antiviral | harzianoic acids A & B (45, 46)/fungus | Terpenoid e | HCV inhibition | 35,43 μM | Virus replication and entry inhibition | CHN, DEU | [57] |
| Antiviral | homoseongomycin (47)/bacterium | Polyketide d | VEEV and EEEV inhibition | 8.6 μM | Viral replication inhibition | TWN, USA | [58] |
| Antiviral | penicillixanthone A (48)/fungus | Polyketide d | HIV-1 replication inhibition | 0.36 μM | CCR5/CXCR4 receptor antagonist | CHN | [59] |
| Antiviral | portimine (49)/dinoflagellate | Polyketide d | HIV-1 replication inhibition | 4.1 nM | Reverse transcriptase inhibition | JPN | [60] |
| Drug Class | Compound/organism a+ | Chemistry | Pharmacological activity | IC50 b | MMOA c | Country d | References |
|---|---|---|---|---|---|---|---|
| Antidiabetic | xyloccensin-1 (50)/mangrove | Terpenoid f | α-glucosidase inhibition | 0.16 mg/mL | Docking studies completed | IND | [61] |
| Antidiabetic | CYC27 (51)/alga | Shikimate h | Reduction in blood glucose | 50 mg/kg/day** | Insulin signaling pathways enhanced | CHN | [62] |
| Antidiabetic | fucoxanthin (43)/alga | Terpenoid f | α-amylase and α-glucosidase inhibition | 80 µg/mL | Mixed-type inhibition kinetics | DNK, MYS, S. KOR, THA | [63,64] |
| Antidiabetic | fucoxanthin (43)/alga | Terpenoid f | Decrease ROS production in kidney mensangial cell line | 0.5 µM* | Epigenomic and transcriptomic effects | USA | [65] |
| Antidiabetic | abeo-oleanene (52)/alga | Terpenoid f | α-amylase and α-glucosidase inhibition | 0.29 mM | Docking studies completed | IND | [66] |
| Antidiabetic | isophloroglucin A (53)/alga | Polyketide d | Glucose homeostasis improvement | 1.35 mg/kg/day** | GLUT4 levels increased | S. KOR | [67] |
| Antidiabetic | S. latiuscula bromophenol (54)/ alga | Shikimate h | α-glucosidase inhibition | 1.92 µM | PTP1B competitive inhibition | S. KOR | [68] |
| Antidiabetic | H. fusiformis fatty acid (55)/alga | Fatty Acids | α-glucosidase inhibition | 48 µM | PTP1B inhibition | S. KOR | [69] |
| Antidiabetic | tripalmitin (56)/fungus | Fatty Acids | α-glucosidase inhibition | 3.75 µM | Mixed-type inhibition kinetics | PAN | [70] |
| Anti-inflammatory | A. depilans EnP(5,8) (57)/sea hare | Terpenoid f | Macrophage NO, COX-2, IL-6 and TNF-α | 18.4 μM | Nos2 and COX-2 expression decrease | ESP, PRT | [71] |
| Anti-inflammatory | Aspergillus sp. aglycone (58)/fungus | Polyketide d | Macrophage NO release inhibition | 6 μM | NF-kB inhibition | CHN | [72] |
| Anti-inflammatory | brevenal (59)/dinoflagellate | Polyketide d | Macrophage TNF-α inhibition | 0.1 ng | Macrophage activation inhibition | USA | [73] |
| Anti-inflammatory | caniferolide A (60)/ bacterium | Polyketide d | Microglia NO, IL-1β, IL-6 release inhibition | 0.01 μM* | iNOS, ERK, JNK expression inhibition | ESP | [74] |
| Anti-inflammatory | C. inophyllum terpenoids (61,62)/mangrove | Terpenoid f | Macrophage NO and IL-1β release inhibition | 2.4, 7 μM | iNOS induction and NF-kB inhibition | VNM, S. KOR | [75] |
| Anti-inflammatory | curdepsidone C (63)/ fungus | Polyketided/Shikimateh | Human macrophage IL-1β release inhibition | 7.5 μM | JNK and ERK inhibition | CHN | [76] |
| Anti-inflammatory | collismycin C (64)/bacterium | Alkaloid g | Murine sepsis inhibition and survival | 4 mg/kg** | NF-kB and p38 inhibition | S. KOR | [77] |
| Anti-inflammatory | dieckol (65)/alga | Polyketide d | Decreased murine liver NLRP3 synthesis | 2.5 mg/kg/day** | NF-kB and NLRP3 inhibition | S. KOR | [78] |
| Anti-inflammatory | dysiarenone (66)/sponge | Terpenoid f | Macrophage IL-6, TNF-α and LTB4 release inhibition | 2-8 μM* | NF-kB, p38, ERK, Akt inhibition | CHN | [79] |
| Anti-inflammatory | epiloliolide (67)/alga | Terpenoid f | Human periodontal ligament cell iNOS, IL-1, IL-6, and TNF-α inhibition | >10 μM* | NLRP3 decrease and PKA/CREB increase | S. KOR | [80] |
| Anti-inflammatory | fucoxanthin (43)/diatom | Terpenoid f | Murine sepsis inhibition and survival | 1 mg/kg** | NF-kB inhibition | CHN, TWN, USA | [81] |
| Anti-inflammatory | fucoxanthin (43)/diatom | Terpenoid f | Murine liver inflammation inhibition | 10-40 mg/kg** | NF-kB inhibition and NRF2 increase | CHN | [82] |
| Anti-inflammatory | fucoxanthin (43)/alga | Terpenoid f | Macrophage osteoclastogenesis inhibition | < 5 μM* | ERK, p38 inhibition and NRF2 increase | S. KOR | [83] |
| Anti-inflammatory | fucoxanthin (43)/alga | Terpenoid f | Macrophage iNOS and COX-2 expression inhibition | 5,10 μM* | NF-kB inhibition | CHN, USA | [84] |
| Anti-inflammatory | fucoxanthinol (68)/ diatom | Terpenoid f | Microglia NO and PGE2 expression inhibition | 20 μM* | NF-kB, Akt, MAPK inhibition and NRF2 increase | CHN | [85] |
| Anti-inflammatory | hirsutanol A (69)/fungus | Terpenoid f | LPS-induced MMP-9 release and lung injury attenuation | 30 mg/kg** | NF-kB, STAT3, ERK inhibition | RUS, TWN | [86] |
| Anti-inflammatory | 2-epi-jaspine B (70)/sponge | Polyketide d | Rat arthritis inhibition | 30 mg/kg** | SphK1 inhibition | CHN | [87] |
| Anti-inflammatory | L. glandulifera diterpenes (71,72)/ alga | Terpenoid f | Macrophage NO release inhibition | 2.3, 2.9 μM | iNOS induction inhibition | GRC | [88] |
| Anti-inflammatory | mojabanchromanol (73)/alga | Terpenoid f | Murine alveolar epithelial cell line lipid peroxidation inhibition | 62.5 µg/mL* | ERK, JNK inhibition | S. KOR | [89] |
| Anti-inflammatory | neuchromenin (74)/fungus | Polyketide d | Microglia NO and PGE2 inhibition | 2.7, 3.2 μM | NF-kB and p38 inhibition | S. KOR | [90] |
| Anti-inflammatory | O-demethylrenierone (75)/sponge | Alkaloid g | Human macrophage NO and PGE2, inhibition | 10 µM* | NF-kB inhibition and increase | S. KOR, VNM | [91] |
| Anti-inflammatory | penicitrinone A(76)/fungus | Polyketide d | Human neutrophil superoxide anion inhibition | 2.7 µM | caspase-3 dependent apoptosis induction | TWN | [92] |
| Anti-inflammatory | phyllohemiketal A (77)/sponge | Terpenoid f | Human macrophage NO and PGE2, inhibition | 5 µM* | NF-kB, p38, ERK and JNK inhibition and NRF2 increase | S. KOR | [93] |
| Anti-inflammatory | sclerketide C (78)/fungus | Polyketide d | Macrophage NO release inhibition | 2.7 µM | iNOS and COX-2 mRNA expression decrease | CHN | [94] |
| Anti-inflammatory | grasshopper ketone (79)/alga | Terpenoid f | Macrophage NO, IL-1β, IL-6 release inhibition | 1-10 µg/mL* | NF-kB, p38, ERK, JNK inhibition | S. KOR | [95] |
| Anti-inflammatory | S. mastoidea prodigiosins (80,81)/bacterium | Alkaloid g | Rat gastric inflammation inhibition | > 100 mg/kg** | NF-kB inhibition and HO-1 increase | EGY | [96] |
| Anti-inflammatory | topsentin (82)/sponge | Alkaloid g | Human keratinocyte COX-2 expression inhibition | 1.2 µM | AP-1, p38, JNK, and Erk inhibition | S. KOR | [97] |
| Anti-inflammatory | tuberatolide B (83)/alga | Polyketide d/ Terpenoid f | Macrophage NO, IL-1β, IL-6 release inhibition | 12.5 µg/mL* | NF-kB, p38, ERK, JNK inhibition | S. KOR | [98] |
| Immune system | astaxanthin (84)/alga | Terpenoid f | Inhibition of LPS-induced dendritic cell dysfunction | 5-20 µM* | HO-1 and NRF-2 increase | CHN | [99] |
| Immune system | crassolide (85)/soft coral | Terpenoid f | Suppression of dendritic cell maturation and T cell responses | 2.5 µM* | DC maturation and pro-inflammatory cytokines inhibition | TWN | [100] |
| Immune system | C. sinensis peptide (86)/mollusk | Peptide g | Increased murine macrophage phagocytosis | 25 µM* | NF-kB and NLRP3 increase | CHN | [101] |
| Immune system | dieckol (65)/alga | Polyketide d | Decreased intestinal Th17 cells and increased Treg cells | 2.5 mg/kg/day | NF-kB and IL-6 decrease | S. KOR | [102] |
| Immune system | echinochrome A (87)/sea urchin | Polyketide d | Expansion of PBMC-derived CD34+ cells | 10 µM* | ROS and p38MAPK/JNK phosphorylation decrease | S. KOR, RUS | [103] |
| Immune system | echinochrome A (87)/sea urchin | Polyketide d | Protection against murine inflammatory bowel disease | 10 mg/kg | Regulatory T cell production increase | S. KOR, RUS | [104] |
| Immune system | echinochrome A (87)/sea urchin | Polyketide d | Inhibition of murine bleomycin-induced scleroderma | 1 µM* | STAT3 phosphorylation decrease | S. KOR, RUS | [105] |
| Immune system | eckol (88)/alga | Polyketide d | Inhibition murine IgE-mediated PCA reaction | 50 µg/mouse | FCεR and NF-kB activation decrease | S. KOR | [106] |
| Immune system | phomaketide A (89)/fungus | Polyketide d/ Terpenoid f | Lymphangiogenesis inhibition | 3.7 µM | VEGFR-3 phosphorylation and PKCδ activation decrease | TWN | [107] |
| Immune system | S. scabra cembranoid (90)/soft coral | Terpenoid f | LPS-induced B lymphocyte proliferation | 4.4 µM | B cell proliferation decrease and IL-10 increase | CHN | [108] |
| Immune system | sticholysins I & II(proteins of about 20KD)/sea anemone | Peptide g | Maturation of dendritic cells | 1 µg/mL* | TLR4 and MYD88 activation decrease | BRA, CUB, USA | [109] |
| Immune system | T. weissflogii phosphoglycolipid (91)/diatom | Polyketide d | Immunestimulation of human monocyte-derived dendritic cells | 10 µg/mL* | TLR4 and NF-kB activation decrease | ITA | [110] |
| Nervous system | alternarin A (92)/fungus | Terpenoid f | Neuronal spontaneous Ca2+ oscillations (SCO) inhibition | 3.2 µM | SCO frequency and amplitude decreased | CHN, HU | [111] |
| Nervous system | anabaseine (93)/ worm | Alkaloid g | α7 nAChR inhibition | 1.85-3.85 µM | Membrane depolarization | USA | [112] |
| Nervous system | A. insuetus TMC-120Ac& TMC-120B (94,95)/fungus | Alkaloid g | Mouse focal seizure duration reduction | 10 mg/kg** | undetermined | BEL, DNK, NOR | [113] |
| Nervous system | Ara and ETrA (96,97)/alga | Fatty Acids | AChE inhibition | 0.5-0.78 mg/mL | Non-competitive inhibition | CHN | [114] |
| Nervous system | astaxanthin (84)/shrimp | Terpenoid f | Reduction of LPS-induced memory impairment | 30 or 50 mg/kg** | Inhibits STAT3 phosphorylation | S. KOR, USA | [115] |
| Nervous system | astaxanthin (84)/shrimp | Terpenoid f | Cognitive dysfunction protection | 10 mg/kg** | ROS reduction and decreased Ab | THA | [116] |
| Nervous system | 8,8’-bieckol (98)/alga | Polyketide d | BACE1 and AChE inhibition | 1.6-4.6 µM | Non-competitive or competitive inhibition | S. KOR | [117] |
| Nervous system | brevetoxin (99)/dinoflagellate | Polyketide d | VGSC activator | 2.4 nM | Shifts voltage dependence, slows inactivation | JPN, USA | [118] |
| Nervous system | C. austini conorfamides (100,101)/ cone snail | Peptide g | α7 nAChR inhibition | 0.68-0.76 µM | Inhibition of Ca2+ ion flow | AUS, MEX | [119] |
| Nervous system | C. geographus conosteroid (102)/ cone snail | Terpenoid f | Hot plate murine pain model inhibition | 2-10 mg/kg** | GABAAR negative allosteric modulator | USA | [120] |
| Nervous system | C. lividus conotoxin Lv1F(103)/cone snail | Peptide g | α3β2 nAChR inhibition; hotplate and formalin murine pain inhibition | 0.0089 µM; 25-100 µg/kg** | Competitive binding; unknown | CHN | [121,122] |
| Nervous system | Con-T[M8Q] (104)/ | Peptide g | Inhibition of murine morphine dependence | 15 nmol/kg | NMDAR GluN2B antagonist | CHN, USA | [123] |
| Nervous system | dictyol C (105)/alga | Terpenoid f | Neuroprotection of rat CIRI | 80 µg/kg | Increased Nrf2/ARE signaling pathway | CHN | [124] |
| Nervous system | echinochrome A (87)/sea urchin | Polyketide d | Mitigation of cerebral ischemic injury | 10 µM** | Decreases pro-apoptotic factors; increased survival factors | S. KOR, RUS | [125] |
| Nervous system | eckol (88)/alga | Polyketide d | Dopamine D3/D4 agonist | 42,43 µM | GPCR-signaling | S. KOR | [126] |
| Nervous system | eleganolone (106)/alga | Terpenoid f | Human neuroblastoma cells neurotoxicity inhibition | 0.1-1 µM* | Decreases ROS levels and apoptotic factors | BRA, ESP, PRT | [127] |
| Nervous system | frondoside A (107)/sea cucumber | Terpenoid f | Dopaminergic degeneration inhibition | 0.1,0.5 µM* | Increase in protein degradation pathway, decrease apoptotic factors | THA | [128] |
| Nervous system | fucosterol (108)/alga | Terpenoid f | Aβ-induced neuronal apoptosis | 10 µM* | Decreased pro-apoptotic factors; decreased APP mRNA | MYS | [129] |
| Nervous system | fucosterol (108)/alga | Terpenoid f | Neurodegenerative disorders system pharmacology | NA | Neuronal survival pathways | S.KOR, | [130] |
| Nervous system | fucoxanthin (43)/alga | Terpenoid f | Reduced corneal denervation | 10 mg/kg** | Increased Nrf2 expression | TWN | [131] |
| Nervous system | fucoxanthin (43)/alga | Terpenoid f | Reduction of PC12 neurons intracellular ROS | 1 µM* | Binds to Keap1 | CHN | [132] |
| Nervous system | H. crispa peptides (109-111)/sea anemone | Peptide g | Inhibition of ASIC ion channels | 1.25-4.95 µM | rASIC1a ion channel inhibition | RUS | [133] |
| Nervous system | H. scabra 2-BTHF (112)/sea cucumber | Polyketide d | Aβ-induced C. elegans paralysis inhibition | 1 µg/mL* | Decreased the formation of Ab oligomers and fibrils | THA | [134] |
| Nervous system | neo-debromoaplysiatoxins E and F (113, 114)/cyanobacterium | Terpenoid f/ Shikimateh | Kv1.5 inhibition | 1.22-2.85 µM | Binding to Kv1.5 S6 domain | CHN | [135] |
| Nervous system | okadaic acid (115)/ dinoflagellate | Polyketide d | Chick embryos neural tube defects | 0.5 µM* | Increased ROS, decreased Nrf2-signaling pathway | CHN | [136] |
| Nervous system | pinnatoxins A and G (116, 117)/dinoflagellate | Polyketide d | Synaptic transmission block at neuromuscular junction | 2.8-3.1 nmol/kg** | AChE inhibition | FRA, USA | [137] |
| Nervous system | PFF-A (118)/alga | Polyketide d | hMAO-A inhibition | 9.2 µM | Noncompetitive inhibition | S. KOR | [138] |
| Nervous system | sargachromanol (119)/alga | Terpenoid f | AChE inhibition | 0.79 µM | Mixed reversible inhibition | S. KOR | [139] |
| Nervous system | santacruzamate A (120)/cyanobacterium | Alkaloid g | Amelioration of AD-like pathology | 10 mg/kg** | Increased KDELR, decreased ER stress | CHN | [140] |
| Nervous system | Sinularia sp. cembranoid (121)/soft coral | Terpenoid f | Aβ42 inhibition | >10 µM | Binds to c-terminal of Ab monomer | CHN | [141] |
| Nervous system | S. latiuscula bromophenol (54)/alga | Shikimateh | HD3R inhibition | 18.7 µM | Binding to HD3R orthosteric site | S. KOR | [142] |
| Nervous system | S. japonica GM2 (122)/alga | Sugar | PC12 neurons increased viability | 200,400 µg/mL | Increased autophagy factors; decreased pro-apoptotic factors | CHN | [143] |
| Nervous system | S. latiuscula bromophenol (54)/alga | Shikimateh | BACE1, AChE and BChe inhibition | 2.3-4.03 µM | Non-competitive or competitive inhibition | S. KOR | [144] |
| Nervous system | stelletin B (123)/sponge | Terpenoid f | Reversal of zebrafish locomotor deficiency | 1 nM * | Increased Nrf2/ARE signaling; decreased pro-apoptotic factors | TWN | [145] |
| Nervous system | androstatriol (124)/soft coral | Terpenoid f | Retinal ganglion cells protection | 80 µg/eye** | Negative regulation of Keap1 | CHN | [146] |
| Compound/Organism a | Chemistry | Pharmacological Activity | IC50 b | MMOA c | Country d | References |
|---|---|---|---|---|---|---|
| amantamide (125)/cyanobacterium | Peptide g | CXCR7 stimulation | 2.5 µM | Erk1/2 phosphorylation increase | CHN, PHL, USA | [147] |
| A.neglectus macrocyclic lactone (126)/octopus | Polyketide e | DPPH radical scavenging | 0.9 mM | ACE-1 non-competitive inhibition | IND | [148] |
| A. subcrenata peptides (127, 128)/shellfish | Peptide g | DPPH radical scavenging | 1 mM | Insulin/IGF-1 signaling modulation | CHN | [149] |
| aspermytin A (129)/fungus | Polyketide e | S. aureus-derived SrtA inhibition | 0.146 mM | Reversible mixed inhibition | S. KOR | [150] |
| avarol (130)/sponge | Terpenoid f | Cholesteryl ester synthesis inhibition | 5.7 μM | SOAT inhibition | JPN | [151] |
| bieckol (131)/alga | Polyketide e | Murine cholesterol, LDL and triglyceride decrease | 2.5 mg/kg/day** | Aortic LOX-1 and PKC-α expression decreased | S. KOR | [152] |
| 3-BDB (132)/alga | Shikimate h | HO-1 antioxidant enzyme upregulation | 10 µM* | Nrf2/HO-1 pathway activation | S. KOR | [153] |
| C. gigas peptide (133)/oyster | Peptide g | Osteogenesis induction | 0.1 µM* | Integrin α5β1 binding | CHN | [154] |
| C. gigas peptide (134)/oyster | Peptide g | Thrombin inhibition | 5 mg/mL* | Competitive inhibition | CHN | [155] |
| D. herbacea diphenyl ether (135)/sponge | Polyketide e | Bacterial α-D-galactosidase inhibition | 4.26 µM | Irreversible active-site inactivation | RUS | [156] |
| dieckol (65)/alga | Shikimate h | ROS inhibition | 0.5 µM* | Enhanced NFE2L and SOD1 gene expression | S. KOR | [157] |
| dieckol (65)/alga | Shikimate h | UVB-induced skin damage reduction | 25 µM* | Enhanced collagen synthesis and pro-inflammatory cytokines reduction | S. KOR | [158] |
| DPHC (136)/alga | Polyketide e | High-fat diet-induced adiposity inhibition | 25, 50 mg/kg/day** | Lipogenesis enzymes inhibition | S. KOR | [159,160] |
| DHPC (136)/alga | Polyketide e | NO stimulation | 20 µM* | AchR and VEGFR2 expression activation | S. KOR | [161] |
| eckol (88)/alga | Polyketide e | ROS inhibition | 30 µM* | MAPK signaling inhibition | S. KOR | [163] |
| E. stolonifera phlorotannin (137)/alga | Polyketide e | Tyrosinase inhibition | 1.6 µM | Competitive inhibition | S. KOR | [162] |
| farnesylquinone (138)/fungus | Polyketide e | Lipid-lowering activity | 0.5 mM | Mitochondrial β-oxidation enhancement | CHN, DEU | [164] |
| fucofuroeckol-A (139)/ alga | Polyketide e | Melanogenesis inhibition | 25 µM* | Tyrosinase-related protein-activity inhibition | JPN | [165] |
| fucoxanthin (43)/alga | Terpenoid f | ACE inhibition | 0.8 mM | Non-competitive inhibition | IND | [166] |
| fucoxanthin (43)/alga | Terpenoid f | Reduction of GMC’s collagen IV and fibronectin | 2 μM* | Akt/Sirt1/FoxO3α signaling regulation | CHN | [167] |
| funalenone (140)/fungus | Polyketide e | PTP1B inhibition | 6.1 μM | Non-competitive inhibition | S. KOR | [168] |
| GQQ-792 (141)/fungus | Alkaloid g | PGK1 inhibition | 1.2 μM | Non-competitive inhibition | CHN | [170] |
| grincamycin B (142)/fungus | Polyketide e | IDH1 inhibition | 1.25 μM* | Increased CHOP and GADD34 gene expression | CHN, USA | [169] |
| H. abdominalis peptides (143, 144)/seahorse | Peptide g | ROS inhibition in HUVEC | 100 µg/mL* | Nrf2 signaling activation | S. KOR | [171] |
| (-)-loliolide (145)/alga | Terpenoid g | Lipid accumulation suppresion | 62 μM* | Decreased adipogenic protein expression | S. KOR | [172] |
| monanchomycalin B (146) /sponge | Alkaloid g | α-PsGal inhibition | Not shown | Slow-biding irreversible inhibition | RUS | [173] |
| monacolin X (147)/fungus | Polyketide e | HUVEC tube formation inhibition | 30 μM* | VEGFR2 signaling modulation | IND, SGP | [174] |
| (-)-muqubilin A (148)/sponge | Terpenoid f | RXRα and PPARα agonist | 10 μM* | Positive RARα allosteric modulation | CAN, ITA, USA | [175] |
| mycalolide A (149)/sponge | Polyketide e | Cytokinesis inhibition | 0.01 µg/mL | F actin inhibition and binucleation induction | JPN | [176] |
| M. edulis dodecapeptide (150)/mussel | Peptide g | Osteoblast growth stimulation | 100 µg/mL | Binding to cellular 1L5G and 3V14 integrins | CHN | [177] |
| O. niloticus oligopeptide (151)/fish | Peptide g | NO and ROS inhibition | 10 μM* | NF-kB pathway suppression | CHN | [178] |
| penerpene A (152))/fungus | Terpenoid f | PTP inhibition | 1.7 μM | Docking studies completed | CHN | [179] |
| penicisulfuranol A (153)/fungus | Alkaloid g | Hsp90 inhibition | 0.5 μM | Binding to Hsp90α C-terminus | CHN | [180] |
| pestalotioquinoside C (154)/fungus | Polyketide e | ABCA1 mRNA upregulation | 50 μM | LXRα receptor binding | CHN | [181] |
| petrosamide C (155)/fungus | Peptide g | Pancreatic lipase inhibition | 0.5 μM | Competitive inhibition | CHN | [182] |
| phakefustantin A (156) sponge | Peptide g | Akt expression inhibition | 10 µM* | RXR-α binding | CHN | [183] |
| 2-phloroeckol (157)/alga | Polyketide e | Tyrosinase inhibition | 7 µM | Slow-binding competitive inhibition | S. KOR | [184] |
| phlorofucofuroeckol A (118)/alga | Polyketide e | Collagen type 1 expression inhibition | 25 µM* | MAPK and SMAD 2/3 pathway downregulation | S. KOR | [185] |
| phlorofucofuroeckol A (118)/alga | Polyketide e | Osteoblastogenesis stimulation | 5 µM* | BMP and Wnt/β catenin- signaling activation | S. KOR | [186] |
| polonimide analogue (158)/fungus | Alkaloid g | Insect GH18 chitinase ofChi-h inhibition | <1 µM* | Docking studies completed | CHN | [187] |
| P. morrowii bromophenol (132)/alga | Shikimate h | Adipogenesis inhibition | 25 µM* | PPAR-γ, C/EBPα, leptin inhibition and AMPK enhancement | S. KOR | [188] |
| preaustinoid A6 (159)/fungus | Terpenoid f | PTP inhibition | 17.6 µM | Non-competitive inhibition | S. KOR, VNM | [189] |
| P. yezoensis peptide (160)/alga | Peptide g | Dexamethasone-induced atrophy protection | 0.5 µg/mL | IFG-1 signaling activation | S. KOR | [190] |
| rhodoptilometrin (161)/crinoid | Polyketide e | Wound healing and cell migration stimulation | 1 µM* | FAK, fibronectin and type 1 collagen increased | TWN | [191] |
| sargahydroquinoic acid (162)/alga | Terpenoid f | Activation of lipid catabolism | 2.5 µM* | PPAR-γ and AMPKα activation | S. KOR | [192] |
| scymnol (163)/shark | Terpenoid f | Activation of TGR5 receptor | 0.5 mM* | Sustained intracellular Ca2+ release | AUS | [193] |
| secoemestrin C (164)/fungus | Alkaloid g | ICL inhibition | 4.77 µM | ICL mRNA expression inhibition | S. KOR | [194] |
| shishijimicin A (165)/ascidian | Alkaloid g | DNA cleavage | 0.014 µM | Binding to double-stranded DNA minor groove | GRC, SGP, USA, | [195] |
| siphonaxanthin (166)/alga | Terpenoid f | Cellular Nrf2 protein expression activation | 1 μM* | Nrf2 signaling activation | JPN | [196] |
| S. latiuscula bromophenol (54)/alga | Polyketide e | Tyrosinase inhibition | 2.9 µM | Competitive inhibition | S. KOR | [197] |
| tetracenomycin X (167)/bacterium | Polyketide e | Cyclin D1 downregulation | 2.5 µM* | Cyclin D1 proteosomal degradation | CHN | [198] |
| tutuilamide A (168)/cyanobacterium | Peptide g | Elastase inhibition | 0.001 μM | Docking studies completed | BRA, CHN, DEU, USA | [199] |
| U. pinnatifida peptide (169)/alga | Peptide g | ACE inhibition | 225 μM | Mixed-type inhibition | CHN | [200] |
| zeaxanthin heneicosylate (170)/alga | Terpenoid f | In vivo inhibition of age-associated cardiac dysfunction | 250 µg/kg** | RXR-α activation | EGY | [201] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





