Submitted:
23 May 2024
Posted:
28 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Copper Nanoparticles (NPs) Synthesis
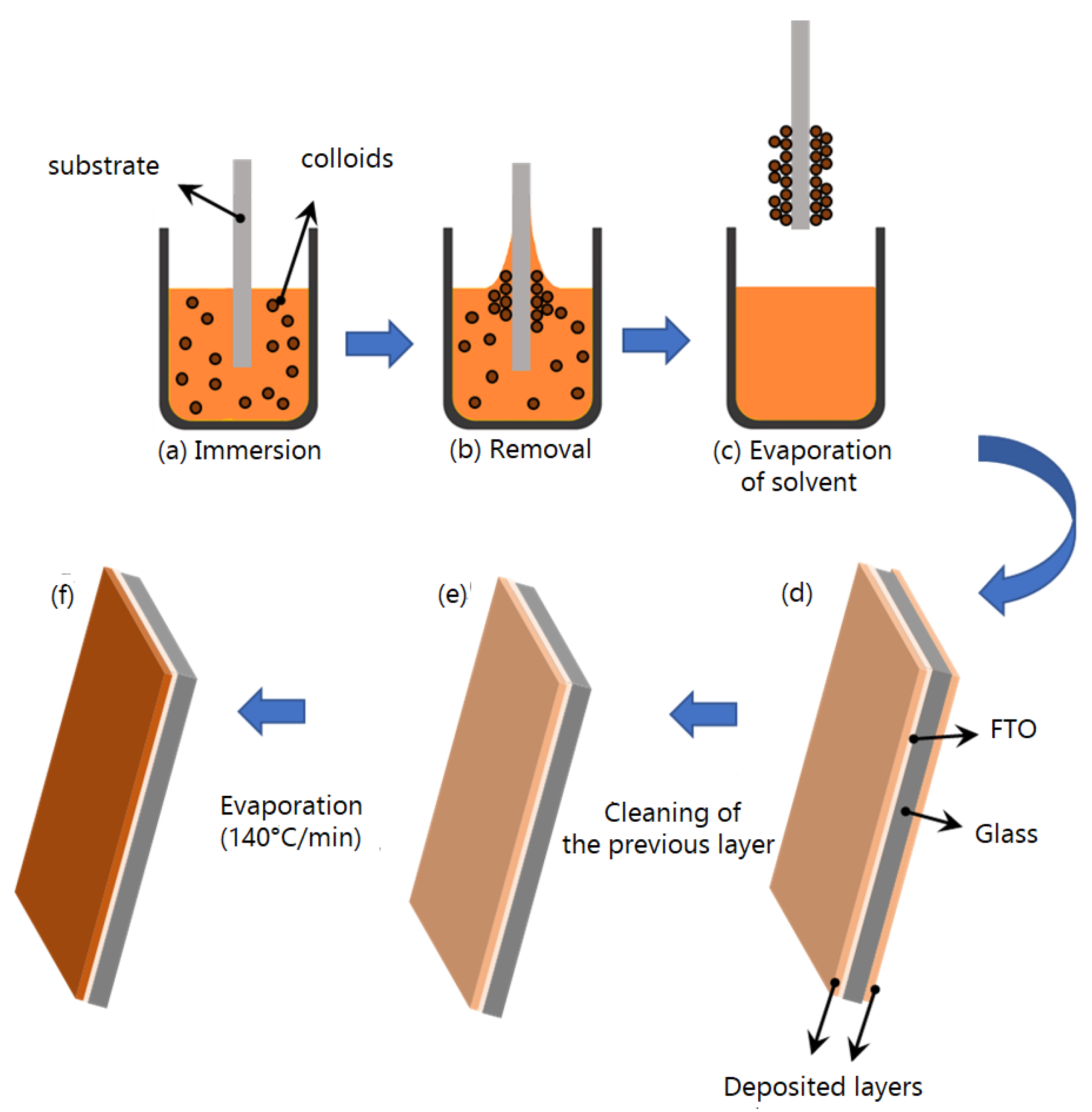
2.3. Development of Electrocatalysts
2.4. Characterization
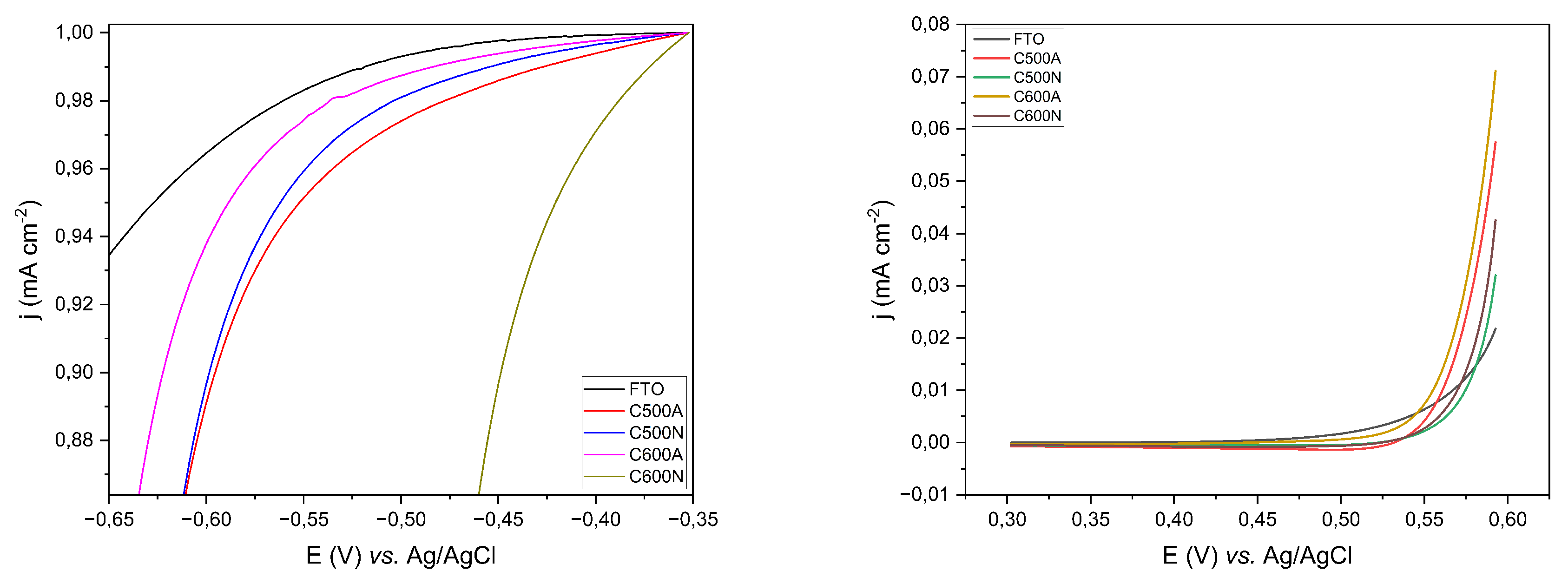
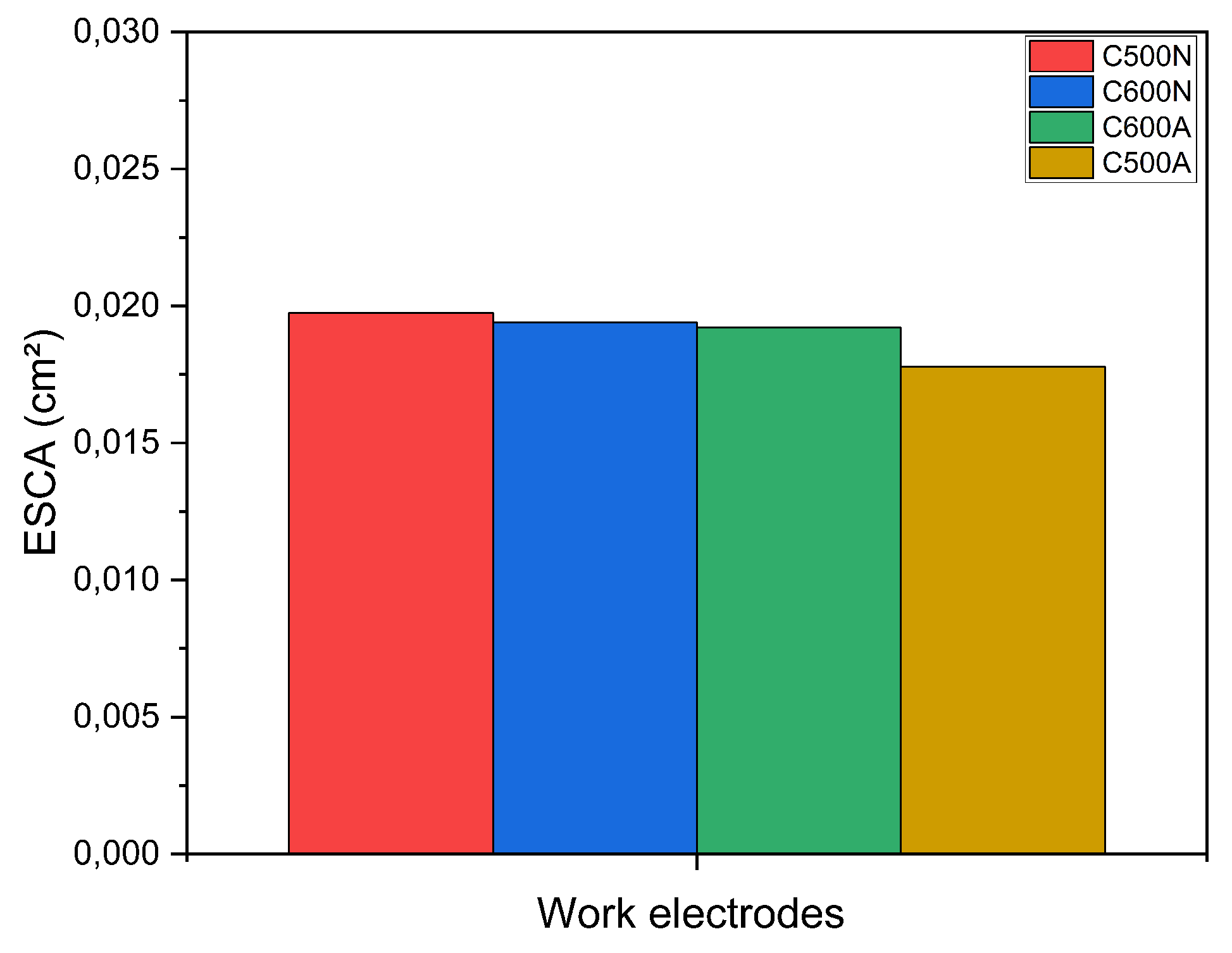
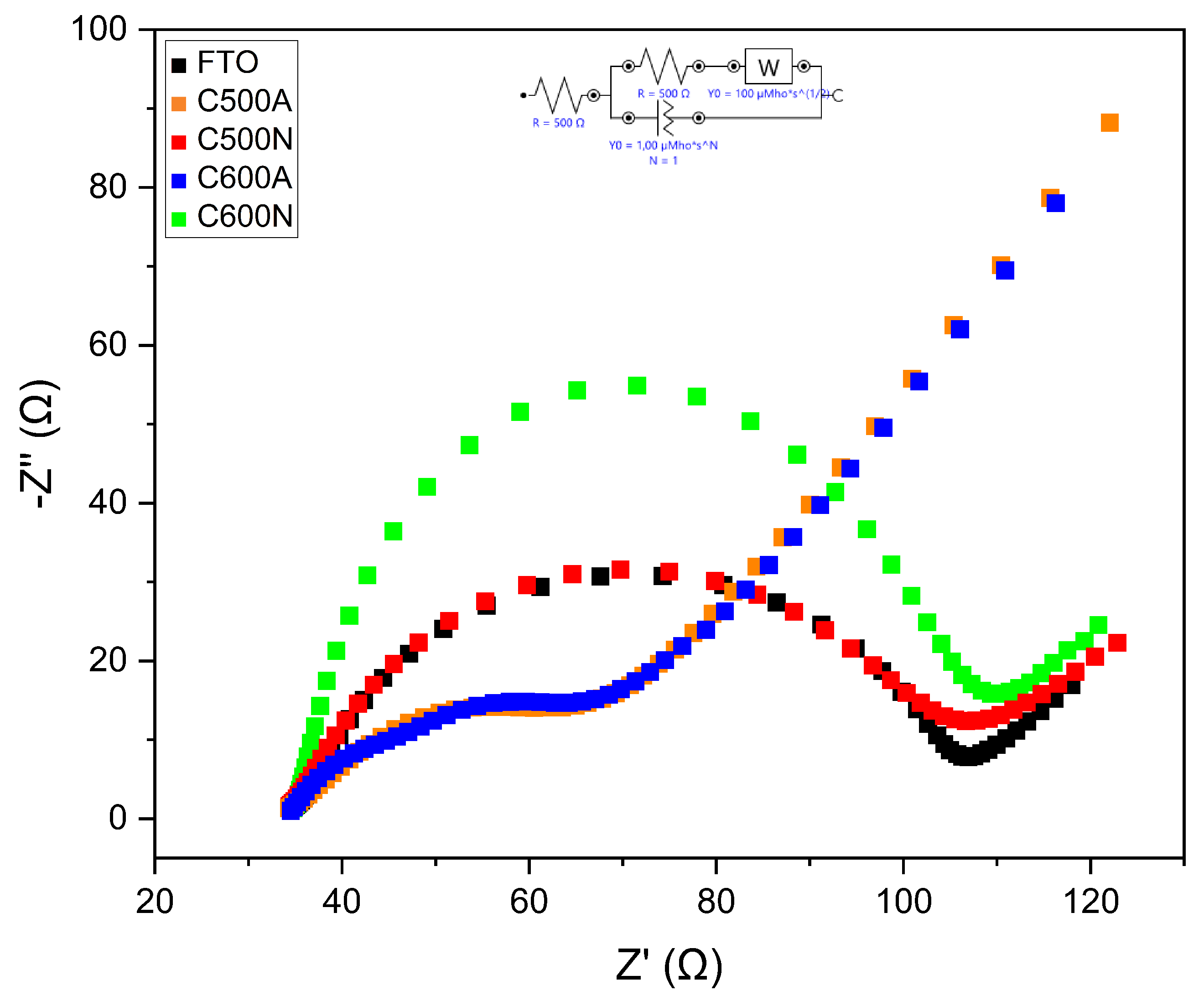
3. Results and Discussion
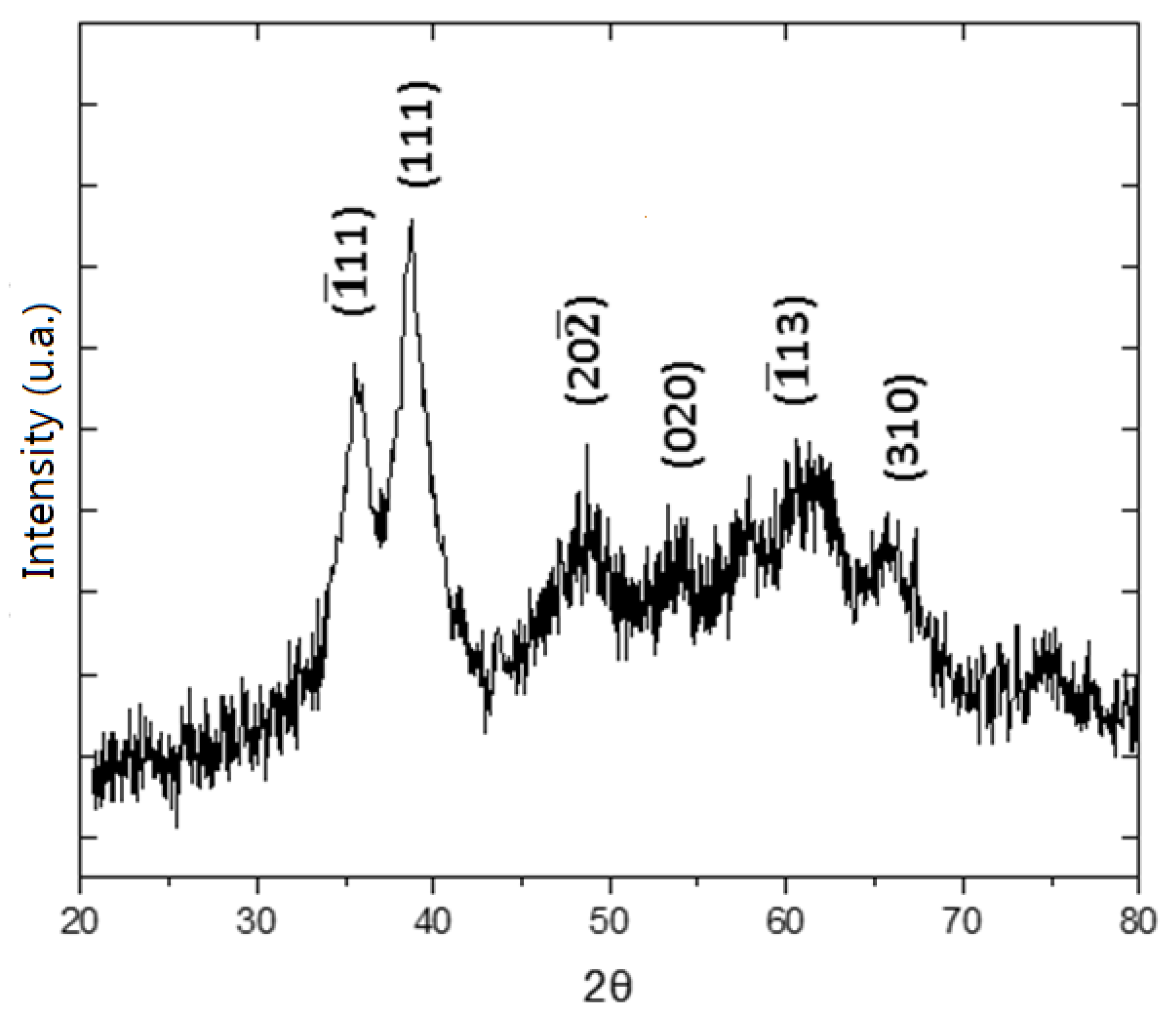
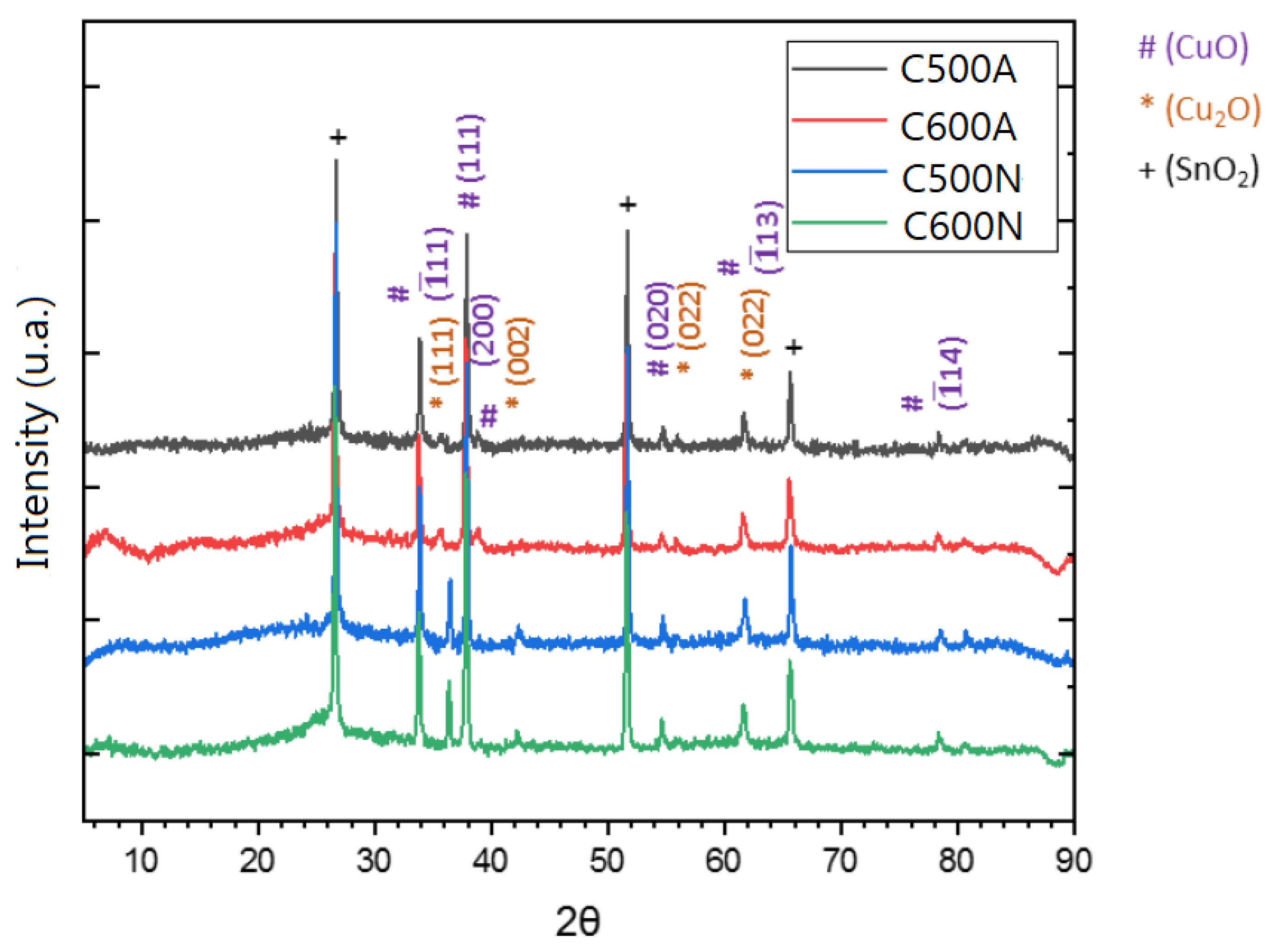
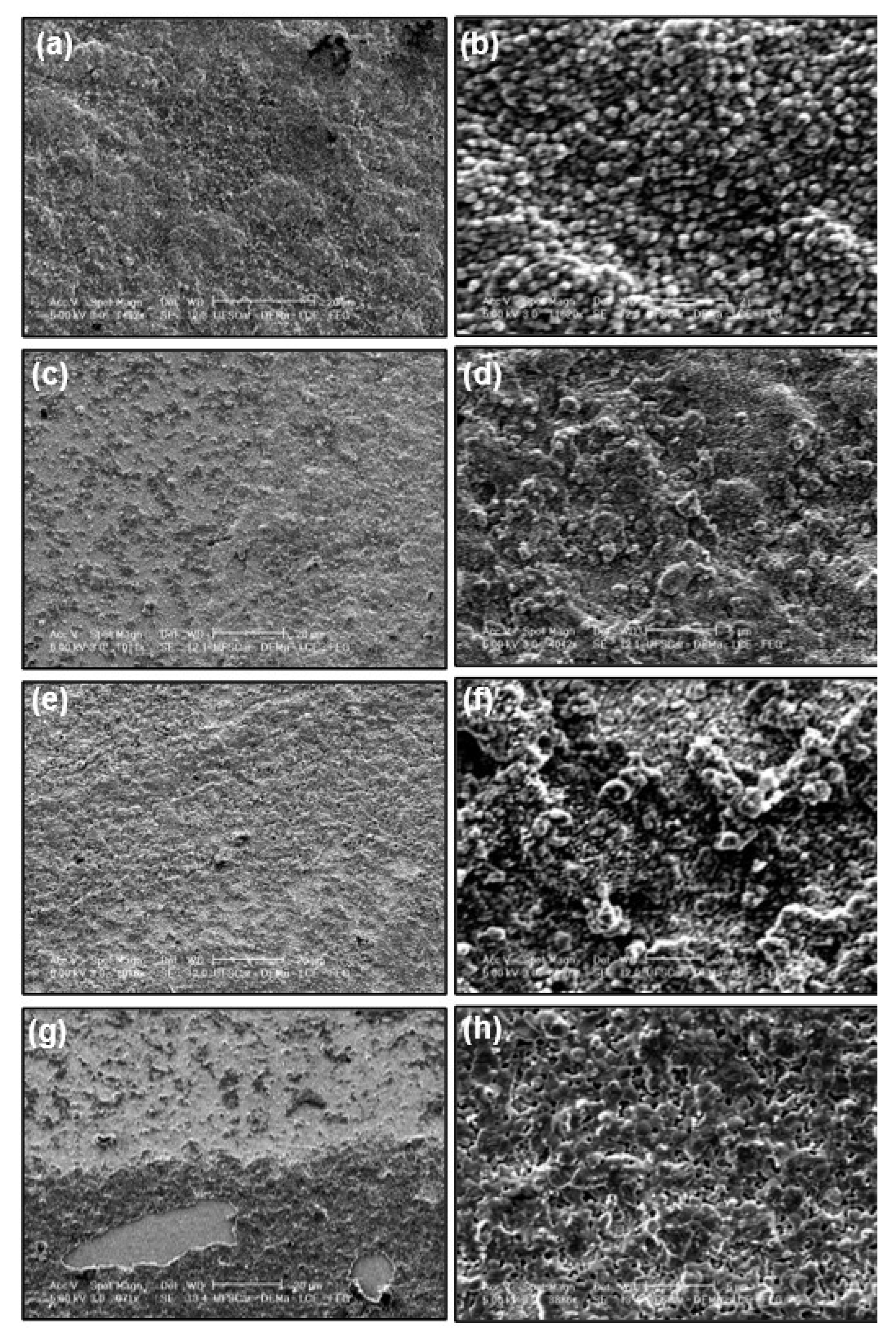
3.1. General Features of the Electrodic Materials
4. Conclusions
Conflicts of Interest
Acknowledgments
References
- Greguš, J. Sustainability, Population and Reproductive Ethics. Česká gynekologie 2023, 88, 190–199. [Google Scholar] [CrossRef] [PubMed]
- IEA, P. World Energy Outlook 2021. https://www.iea.org/reports/world-energy-outlook-2021 2021.
- Detz, R.J.; Reek, J.N.H.; van der Zwaan, B.C.C. The future of solar fuels: when could they become competitive? Energy Environ. Sci. 2018, 11, 1653–1669. [Google Scholar] [CrossRef]
- Poizot, P.; Dolhem, F. Clean energy new deal for a sustainable world: from non-CO2 generating energy sources to greener electrochemical storage devices. Energy Environ. Sci. 2011, 4, 2003–2019. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Kanan, M.W.; Surendranath, Y.; Nocera, D.G. Cobalt–phosphate oxygen-evolving compound. Chem. Soc. Rev. 2009, 38, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.P.; Mejía, E.; Friedrich, A.; Pazidis, A.; Junge, H.; Surkus, A.E.; Jackstell, R.; Denurra, S.; Gladiali, S.; Lochbrunner, S.; Beller, M. Photocatalytic water reduction with copper-based photosensitizers: a noble-metal-free system. Angewandte Chemie (International ed. in English) 2013, 52, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Buttler, A.; Spliethoff, H. Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: A review. Renewable and Sustainable Energy Reviews 2018, 82, 2440–2454. [Google Scholar] [CrossRef]
- Nandy, S.; Banerjee, A.; Fortunato, E.; Martins, R. A Review on Cu2O and CuI-Based p-Type Semiconducting Transparent Oxide Materials: Promising Candidates for New Generation Oxide Based Electronics. Reviews in Advanced Sciences and Engineering (ISSN: 2157-9121) 2013, 2, 273–304. [Google Scholar] [CrossRef]
- Bi, X.; Wang, H.; Yang, Z.; Zhao, Y.; Ma, Z.; Liu, T.; Wu, M. Localized Surface Plasmon Resonance Enhanced Continuous Flow Photoelectrocatalytic CO 2 Conversion to CO. Energy & Fuels 2022, 36. [Google Scholar] [CrossRef]
- Kunturu, P.P.; Huskens, J. Efficient Solar Water Splitting Photocathodes Comprising a Copper Oxide Heterostructure Protected by a Thin Carbon Layer. ACS Applied Energy Materials 2019, 2, 7850–7860. [Google Scholar] [CrossRef]
- Effenberger, F.; Sulca, M.; Machini, M.T.; Couto, R.; Kiyohara, P.; Machado, G.; Rossi, L. Copper nanoparticles synthesized by thermal decomposition in liquid phase: the influence of capping ligands on the synthesis and bactericidal activity. Journal of Nanoparticle Research 2014, 16, 2588. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



