1. Introduction
Glaucoma is an ocular pathology characterized by producing a series of both structural and functional alterations at the level of the optic nerve and the structures of the posterior pole in a progressive, chronic, and irreversible manner. Among the alterations it produces are death of retinal ganglion cells, increased optic disc cupping, thinning of the neuroretinal ring (ANR), and the retinal nerve fiber layer (RNFL), which translates into functional damage. such as visual field loss and blindness (1-3). Although it has always been believed that the increase in intraocular pressure (IOP) was the cause of glaucoma, we now know that it is one of the risk factors that the patient may or may not be present, since there are types of glaucoma with normal IOPs. However, it is the only treatable risk factor (4,5).
Other risk factors are age and the presence of myopia. Considering that life expectancy is increasing as well as the prevalence of myopia, it is not surprising that the prevalence of glaucoma is increasing. Glaucoma is considered the first cause of irreversible blindness worldwide. It is estimated that in 2020 there were around 79.6 million people with glaucoma, with a total of 111.8 million by 2040. Of all of them, 50% are considered undiagnosed. One of the problems with this pathology is that it does not present symptoms in its initial stages until the first signs of vision loss begin, by which time it is too late (6,7). For this reason, the lines of research focus on developing instruments that allow early detection of the anatomical changes that glaucoma produces in its beginnings, thus being able to apply a treatment, carry out a close follow-up of each patient, and be able to stop or prevent the appearance of glaucoma. and blindness (8–10).
Due to this diagnostic need, OCT has become the most widely used instrument, along with computerized perimetry. The development of the OCT has meant a great advance, especially in recent times. It is a non-invasive and fast technique. It is based on the principle of low coherence using light beams (4,11–13). We can find mainly two types: temporal domain (TD) or spectral domain (SD), the latter being the most innovative and developed to improve both the resolution of the images and decrease the time for obtaining them. It allows evaluation of fundus structures such as retinal nerve fiber layers, changes in the optic nerve head (ON), or the thickness of retinal ganglion cells (11,14,15).
There are numerous models on the market, with the OCT Spectralis from Heidelberg being the one we used for our study due to the great advantages it offers over the rest. This model has a tracking system called TruTrack that corrects involuntary eye movements. It also has an anatomical positioning system (APS) that takes two reference points: the center of the fovea and the opening of the Bruch to always perform each shot image with the same points and thus reduce the error between measurements. We have also implemented the "Glaucoma Premium" program that allows us to perform circumpapillary scans of the RNFL thickness with a diameter of 3.5mm, 4.1mm, and 4.7mm, although at first the 3.5mm measurement was taken as a reference. There are studies that show that this diameter is not completely reliable because it does not take into account the inter-individual variability of the size of the optic disc (4,16-18).
Other characteristics of this program are that it performs deviation maps both at the RNFL and at the macular level; it can combine all the results and classifying them according to whether they are within the green limits, in yellow if they are borderline, or in red if they are out of all these features. The OCT Spectralis is the most accurate instrument on the market.
With our study, we intend to carry out an assessment of the RGC and the RNFL of the NO, with the OCT Spectralis and the "Glaucoma Premium" program in groups of healthy patients, with myopia, with glaucoma, and with myopia and glaucoma together to identify the structural changes .
2. Materials and Methods
Patients
The subjects of the study are a total of 160 patients, who were chosen from among the patients who attended the consultation of the reference center and divided into 4 study groups.
It is an observational-analytical cohort study conducted with four groups of patients: A) healthy, B) patients with myopia between -9.25 to-0.25 dp, C) patients with POAG, and D) patients with both myopia and refractions from -16.75 to 0.25 dp as POAG.
All of them underwent IOP measurement using a tonometer, refraction measurement, corneal thickness measurement, and Heidelberg OCT Spectralis, a fundus scanner was performed to analyze the thickness of the RNFL, RGC, and the BMO area.
The inclusion criteria to form the 4 study groups were:
Healthy patients without ocular pathology.
Myopic patients with no other ophthalmological abnormalities.
Patients suffering from POAG.
People who have POAG and myopia
The exclusion criteria were: patients undergoing any type of surgery to control glaucoma, such as iridotomy, trabeculectomy, or application of valves to drain the aqueous humor; and those patients undergoing refractive surgery to correct myopia.
The study variables were: Bruch's membrane opening (BMO) size area, BMO-minimum ring width (BMO-MRW) size, RNFL and RGC layer thickness using the Glaucoma program Premium of the OCT Spectralis of Heidelberg. The secondary variables of the study were: ocular refraction, pachymetry, and IOP.
The patients were selected by simple random sampling, applying the inclusion and exclusion criteria, from which they were divided into four groups:
Healthy patients without ocular pathology were made up of a total of 40 patients without myopia and without POAG. Confirming that they do not have ocular pathology and therefore meet the criteria for inclusion in the group and in the study.
Patients with myopia who have no other ophthalmological changes, a total of 40 patients with myopia. The patients in this group present a myopic refraction with a range between -0.25dp to -9.25dp. These are the diopters that most of the patients found in our database present.
Patients diagnosed with POAG, made up of 40 patients who are diagnosed with POAG and do not have myopia
Patients diagnosed with POAG and myopia, made up of a total of 40 patients who have POAG and myopia, presenting diopters between 0.25dp to -16.75dp.
All the patients in the four study groups underwent different diagnostic tests. IOP and pachymetry were taken with the Topcon CT1 tonometer. The refraction was taken with the KR-1 Auto-Keratorefractometer from Topcon. The Topcon company and the fundus scan were performed with the OCT Spectralis from Heidelberg.
Data Collect
For taking the refraction, we have used the KR-1 Auto kerato-refractometer from Topcon, which performs the measurement automatically. It is one of the most accurate on the market when it comes to taking data.
The Topcon CT1 tonometer-pachymeter was used for both intraocular pressure measurement and corneal thickness measurement. It is performed with the patient sitting, who centers the pupil through a touch screen, eliminating the joystick, making it much more precise. This model, thanks to its software, is capable of adjusting the amount of air according to the characteristics of each cornea.
Finally, for taking the area of the BMO, of the BMO-MRW, the analysis of the RNFL and CGR, the OCT Spectralis from Heidelberg was used. Similarly, it is performed with the patient seated, and a scanning scan of the fundus of the eye is performed, for which pupil mydriasis is not necessary. Carrying out this scanner allows us to visualize the retina layer by layer and accurately study the RNFL, NO, and the macular area. With the Glaucoma Premium module, which includes the eye-tracking system and the Anatomical Positioning System (APS), that corrects the measurements according to the patient's anatomy, it is much more precise than other OCTs on the market.
Statistic analysis
The data has been tabulated and analyzed by the SPSS 23 computer package (licensed for the Central Computer Service of the University of Malaga).
Continuous variables, such as mean SD, and categorical variables, such as frequencies and percentages, are expressed as mean SD. Bivariate analysis: a) continuous outcome variable: linear regression and Pearson's correlation. Multivariate analysis (controlling for confounding factors) ANOVA.
Data is expressed as the mean (SD and 95% CI) or percentage. A value of p<0.05 will be considered statistically significant.
3. Results
The mean age of the sample is 55 years (±15.72 years), of which 39.7% are men and the other 60.63% are women. They were divided into 4 groups, with each group having the same number of patients to ensure good reliability of the results.
Table 1 shows the data on the BMO area values, with the myopia and glaucoma groups having the smallest area. With respect to the BMO-MRW, the glaucoma and glaucoma with myopia groups present the lowest values. It should be noted that in the temporal sector, all the groups present a large decrease with respect to the rest of the sectors.
With respect to the mean values of RNFL thickness, we can see in the
Table 1 how patients with glaucoma and myopia present lower thicknesses. This occurs for all the RNFL sectors studied. In addition, the ISNT rule is met in all study groups.
About the RGC thickness, the patients in the glaucoma and glaucoma with myopia groups are the lowest RGC thickness compared to the other groups, even with respect to the group that includes patients with glaucoma and myopia, all these results we can see in the
Table 1.
Regarding the correlation analysis between age and RNFL thickness, there are significant results in all study groups and, furthermore, the correlation is negative, that is, the older the age, the smaller the RNFL thickness, as can be seen in
Figure 1A corresponding to the healthy group. As we can see in the glaucoma and myopia group, there is a relationship between age and the thickness of the RGC layer, as we can see in
Figure 1B, while for the rest of the groups, no significant results have been obtained.
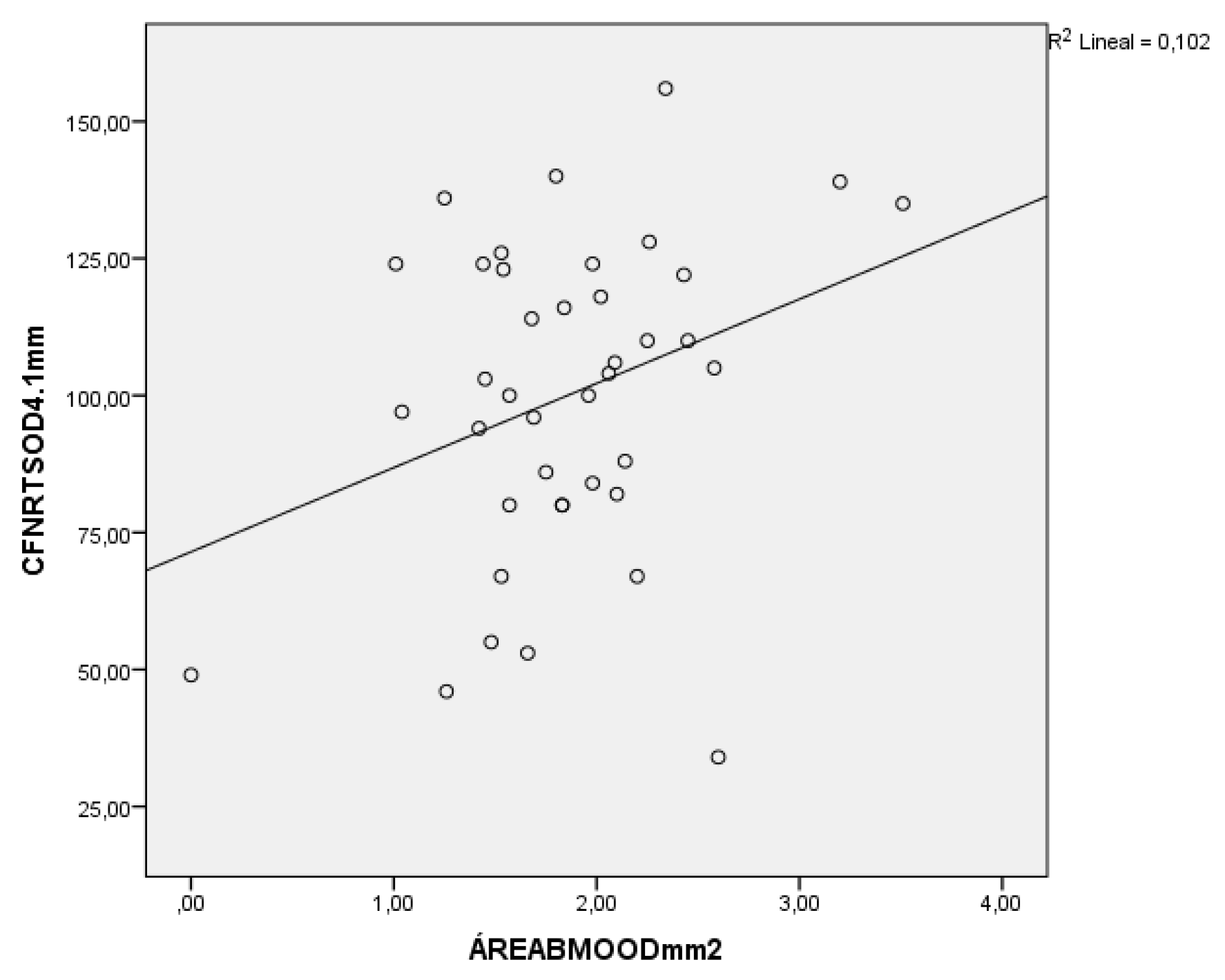
In the analysis between the area of the BMO and the thickness of the RNFL, it should be noted that both in the healthy, myopic, and glaucoma with myopia group, significant results have also been obtained with positive correlations; that is, the lower the thickness of the RNFL, the lower the area of the BMO, as we can see in
Figure 2 (which corresponds to the glaucoma and myopic group).
In the study of the RGC thickness with secondary variables such as age, IOP, ring area, and pachymetry, a positive correlation was found with respect to age; the older the person, the less the RGC thickness. Although no significant results have been obtained for the area of the BMO, the correlations obtained are positive for all groups.
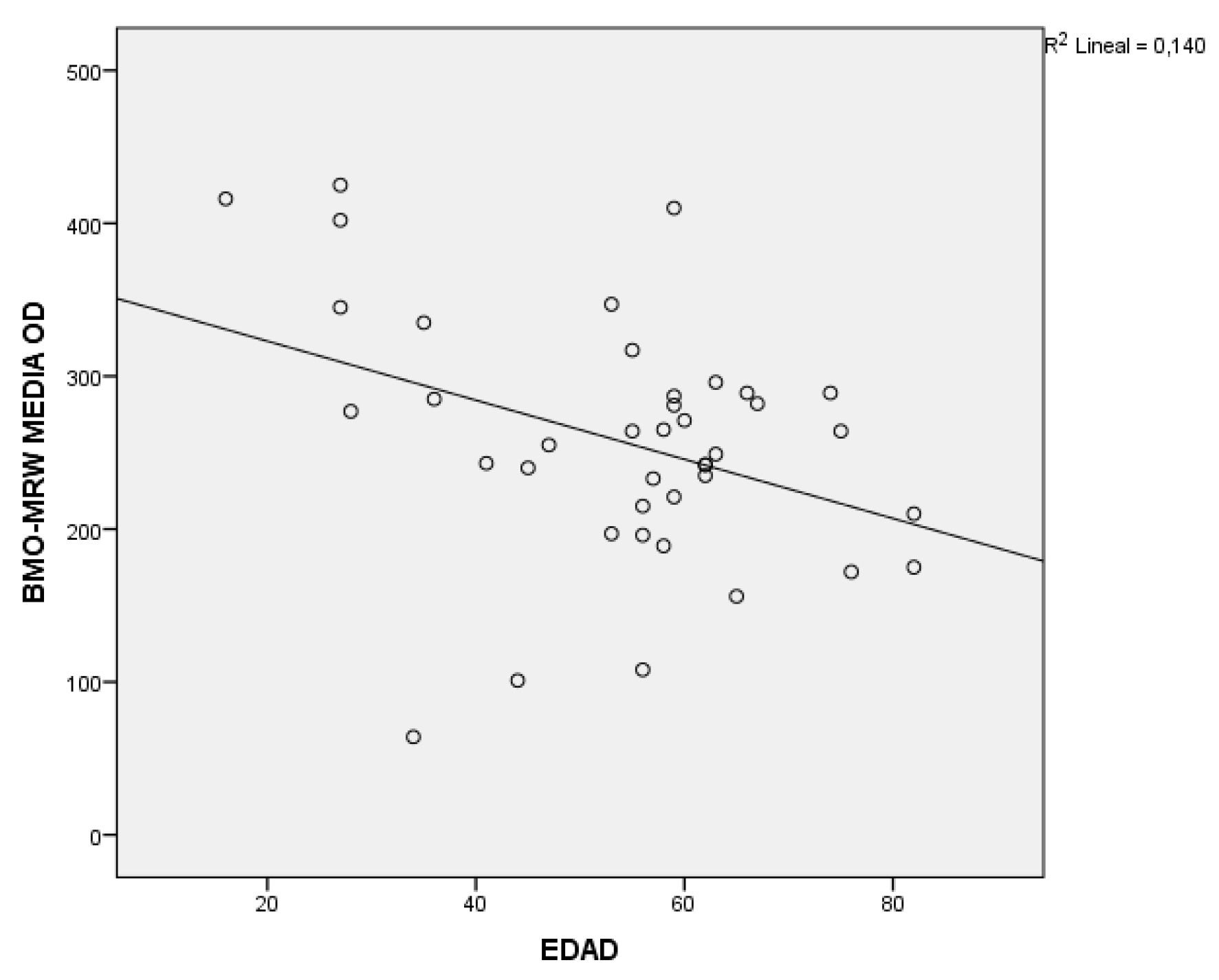
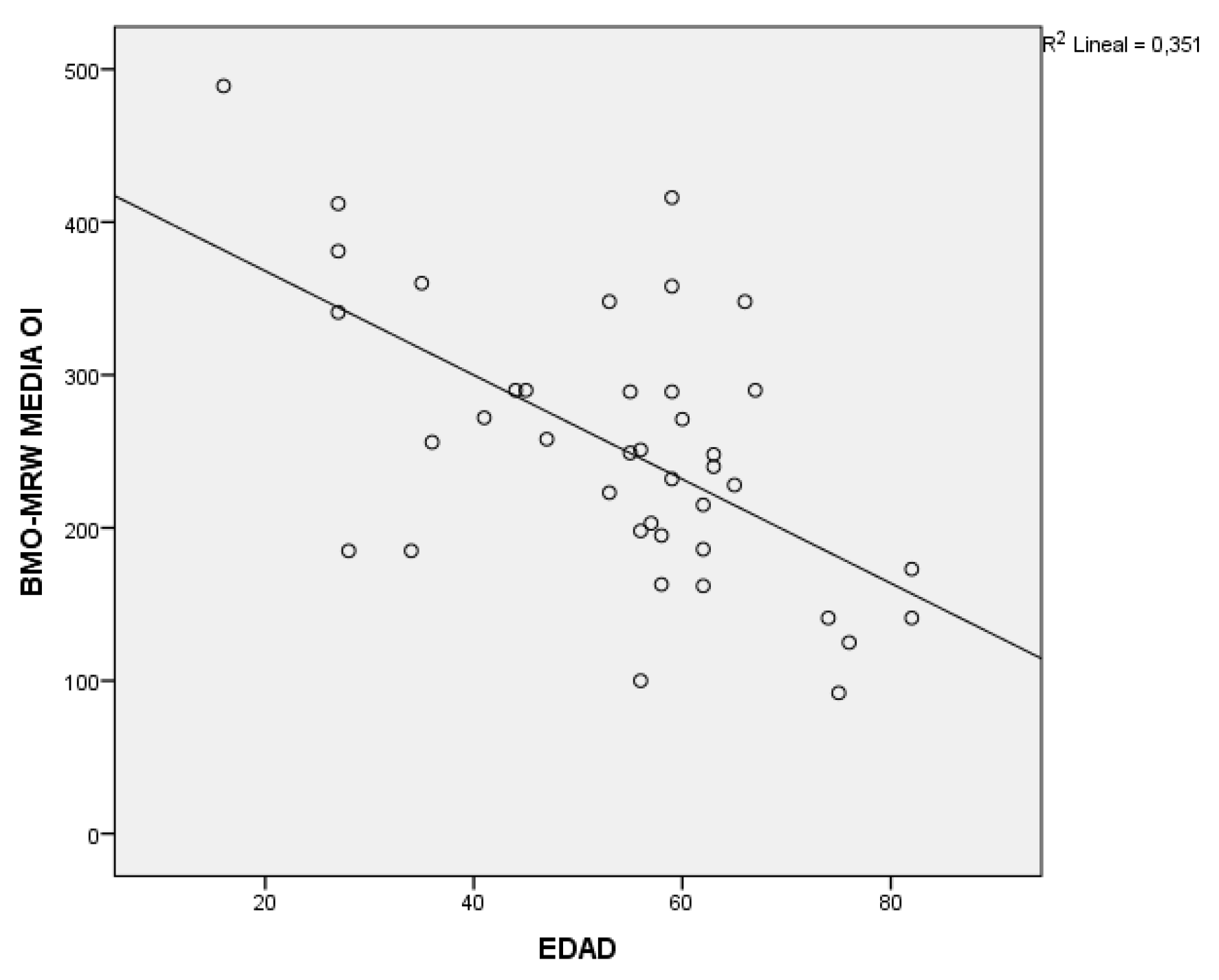
In the analysis of the BMO-MRW with the secondary variables, significant results were obtained with respect to age in all groups, in addition to a negative correlation as seen in
Figure 3. With respect to the rest of the study variables, it should be noted that in the glaucoma group there is a relationship with pachymetry and with the BMO area, with both variables being positively correlated. And for the glaucoma and myopia group, to say that significant results have been obtained with pachymetry and with IOP, with the latter being a negative correlation; the higher the IOP, the smaller the thickness of the BMO-MRW; and although with refraction there is no relationship, yes, the trend is positive; that is, the greater myopia, the smaller the thickness.
With respect to the area of the BMO and the BMO-MRW, no results have been found that relate them except in the glaucoma group, with a positive correlation.
When performing the Pearson correlation analysis of the thickness of the RNFL together with the BMO-MRW, it should be noted that there is a relationship in all the study groups. In addition, with a positive correlation, the lower the thickness of the RNFL, the lower the BMO-MRW, as can be seen in
Table 1.
The following table (
Table 2) is a summary of the Pearson Correlation analysis between the thickness variables of the RNFL, the RGC, and the BMO-MRW in the four study groups, where the significant results can be seen in yellow, that is, those in which the variables are related to each other. Thus, it is highlighted that the group of glaucoma and myopia is the one that presents the greatest degree of damage in the different structures of the eye.
4. Discussion
As the need arises to diagnose early those pathologies related to the retina and as there is increasing evidence that anatomical damage occurs before functional damage (19-23), scientific-technological development in the area is essential in ophthalmology, specifically, in relation to diagnostic instruments responsible for the study of the anatomical structures of the eye and the retina.
For this purpose, various instruments were developed, including the spectral domain (SD) OCT, which performs a scan using light emitted by superluminescence with a wavelength of 850 nm and is 73 times faster than time domain (TD) OCT. In the market we can find numerous OCTs, among which the OCT Spectralis was the one used for this study. Both measure RNFL thickness, NO head structures, and RGC structures with very high accuracy, but the turning point between the two is that fundus tracking and mapping software is included in the Spectralis , called TruTrack, that compensates for the involuntary movement of the eye and the anatomical positioning system (APS), that makes a map so that each time the patient comes for the exam, the same areas are scanned, reducing the technical measurement error (17, 24–28).
Other advantages of OCT Spectralis are the implementation of the "Glaucoma Premium" program, which we have used in this study, whose main novelty is that it performs circumpapillary RNFL measurements, taking 3 shots in diameters of 3.5 mm, 4.1 mm, and 4.7mm. Thanks to these shots, there are studies where the glaucomatous damage is reflected first in the larger diameters by affecting the more peripheral cells. There are also studies that show that the measurements in the 3.5 mm diameter can be affected by the size of the papilla, giving not very reliable thicknesses (17,29,30).
In this study, we wanted to assess the different structures of the posterior pole of the eye using the Heidelberg OCT Spectralis equipment with the "Glaucoma Premium" program (Heidelberg Engineering GmbH, Heidelberg, Germany), in order to make an early detection of glaucoma at a structural level. to determine the differences in the different study groups and thus be able to prevent functional or campimetric damage in the different groups.
Regarding the results obtained, as we have pointed out, the ISNT standard is fulfilled in all the groups.
In general terms, in a first analysis of the results, the relationship between the thickness of the RNFL and age was verified, despite not obtaining significant results. After carrying out the correlation study, the trend of the results coincides with the results obtained by other authors (5,31-34), that with the suspicion of glaucoma it intensifies, and with age the thickness of the RNFL decreases, making it one of the non-treatable risk factors. In terms of age, we found no significant differences in RGC thickness, but the trend is that the older a person is, the thinner the RGC (35,36).
Several studies that have analyzed the relationship between the thickness of the cornea with refraction and IOP have obtained the same results as ours; that is, there are no statistically significant differences between these variables, corneal thickness does not influence refraction or IOP(37–42).
When analyzing the relationship between refraction and BMO area, there are several studies such as Mei-Chin et al, Biswas et al, and Kim et al. (8,43,44) that state that in eyes with high myopia, the optic disc is larger and the ANR is smaller. Although the results obtained in this regard are not significant data, they do show a trend that in myopic eyes, the area of the ANR decreases by reducing the number of nerve fibers that are lost because the RNFL is thinner in these patients (36,45) In addition, studies such as the one by Savini et al. show that large discs, as occurs in the case of myopic patients, have a smaller ANR area as the nerve fibers are less grouped (30). In graph 5, in the myopic group, the BMO area is smaller than in the healthy or glaucoma groups.
The data obtained after Pearson's correlation analysis between refraction and the average thickness of the RNFL do not show a clear relationship between the two in any group, except in the glaucoma and myopia group in which significant results have been obtained. In other words, the thickness of the RNFL is less the more myopic the patient has, as occurs in the studies and bibliography consulted in this regard, such as in the study by Xu Xiao, in which they state that the damage at the level of the RNFL is due to the axial elongation that these patients present (8,43). Despite this, there are studies such as the one by Huang et al., which corroborate the results obtained for the rest of the groups in which there is no relationship between the RNFL thickness and refraction (47).
In various studies, such as those of the Güerr Moncls, an increase in IOP can be a direct cause of cell death at the retinal fiber level, either due to ischemia, an increase in cytotoxic factors, or a blockage of the transport of neurotrophic factors; in the case of the IOP relationship with the ANR area, the data obtained are significant in the glaucoma group, supporting the theory that prolonged exposure to high IOP levels leads to deafness The same does not happen at the level of the thickness of the RGC layer, which is not affected by the increase in IOP. In these cases, the damage may be caused by other factors such as smoking, having had a stroke or being hypertensive, as shown in the study by Belzunce et al. (49).
As a result, the study prefers the analysis of the RNFL thickness because, as the Pollet-Villard et al. study shows, the thickness of the RNFL with the BMO-MRW presents a good relationship when diagnosing glaucoma (45) and would also support Hammel's theory that damage at the level of the thickness of the RGC in myopic eyes is slower than damage in the RNFL (29). However, although in the glaucoma and myopia group the relationship is not significant, the correlation is positive. That is, the more myopia, the thinner the thickness of the RGC layer, which may be largely due to the fact that with glaucoma the damage occurs first at the level of the RGCs.
When talking about the relationship between the thickness of the RNFL and the RGC, in the myopia and glaucoma groups separately and in the glaucoma with myopia group, there is a significant relationship between both variables; in addition to positive correlations, the lower the RGC thickness, the lower the CFNR; in the case of the myopia group, this difference is found in the temporal, superior temporal, and inferior temporal sectors; although as Yu-Xu says in the study, RGC thickness is not very reliable for diagnosing glaucoma in myopic patients, if damage occurs at this level, it would begin in the IT sector, coinciding with our results (45). In the glaucoma group, all sectors present a positive correlation, with the majority being statistically significant results, thus confirming that both the decrease in RNFL and the RGC affect each other (50–52), although we do not know what happens first if the damage is in the CFNR or in the CGR. There are studies in which RGC damage is slower (29).
5. Conclusions
In summary, the eye of the patient with glaucoma is the one with the least thickness of the RGC and is closely related to a loss of thickness of the RNFL. However, in the eyes of patients with glaucoma and myopia, damage at the functional, both the thickness of the RNFL, the RCG and the BMO-MRW are greater than other groups. All these alterations can be evaluated and monitored with the OCT Spectralis thanks to the programs it includes, making this instrument one of the best for an early diagnosis of glaucoma and the damage produced in myopic patients.
References
- Bowling B. Kanski. Oftalmología clínica: en un enfoque sistemático. 8a Edición. Elsevier España D., editor. Barcelona; 2016.
- Corcostegui B. El fondo del ojo en la medicina práctica. 1a Edición. Espaxs, editor. Barcelona; 1983.
- Divakar Gupta M, Philip P. Chen M. Glaucoma. Clin Evid (Online). 2011;2011(May 2010):1-22.
- Gros Otero J. ESTUDIO DE LA PROGRESIÓN PERIMÉTRICA EN DISTINTOS TIPOS DE GLAUCOMA<monospace> </monospace>: SISTEMAS DE DIAGNÓSTICO Y FACTORES DE RIESGO. Universidad de Alcalá; 2012.
- Güerrí Monclús N. Relación estructura-función en el glaucoma: estudio topográfico de correlación entre los espesores de la capa de fibras nerviosas de la retina y la perimetría automatizada convencional. Universidad de Zaragoza; 2009.
- Agrawal P, Bradshaw SE. Systematic Literature Review of Clinical and Economic Outcomes of Micro-Invasive Glaucoma Surgery (MIGS) in Primary Open-Angle Glaucoma. Ernest & Young [Internet]. 2016;2020(2):7. [CrossRef]
- Traverso CE, Walt JG, Kelly SP, Hommer AH, Bron AM, Denis P, et al. Direct costs of glaucoma and severity of the disease: A multinational long term study of resource utilisation in Europe. Br J Ophthalmol. 2005;89(10):1245-9. [CrossRef]
- Mei Chin, T. Diagnostic capability of peripapillary retinal nerve fiber layer parameters in time-domain versus spectral-domain optical coherence tomography for assessing glaucoma in high myopia. Int J Ophthalmol. 2017. [CrossRef] [PubMed]
- Chansangpetch S, Panpruk R, Manassakorn A, Tantisevi V, Rojanapongpun P, Hurst CP, et al. Impact of myopia on corneal biomechanics in glaucoma and nonglaucoma patients. Investig Ophthalmol Vis Sci. 2017. [CrossRef] [PubMed]
- Saínz RIB, Triana II, Ii C. Miopía: factor de riesgo del glaucoma de ángulo abierto. Rev ciencias medicas La Habana. 2013;19(1).
- Enfoque para la Optometría Clínica U, Peña Martínez V, Paola Espinosa Castañeda RESUMEN A. DESCRIPCIÓN Y ANÁLISIS DEL OCT, HRT Y GDX EN GLAUCOMA.
- CÓMO PUEDEN AYUDAR LOS ANALIZADORES DE IMAGEN (HRT, OCT Y GDx-VCC) EN LA PRÁCTICA CLÍNICA DIARIA DEL OFTALMÓLOGO FRENTE AL PACIENTE GLAUCOMATOSO_.
- Griñó C, Lugo F, León M, Ligero S, Ruiz JM, Montero J. Tomografía de Coherencia Óptica (OCT) Funcionamiento y utilidad en patología macular (I). Gac Óptica. 2008;427(I):12-4.
- Alías Alegre EG, Borque Rodríguez E, Larrosa Poves JM, Polo Llorens V, Honrubia López FM. ¿Cómo pueden ayudar los analizadores de imagen (HRT, OCT y GDx-VCC) en la práctica clínica diaria del oftalmólogo frente al paciente glaucomatoso? Thea Innovación. 2008;58:26.
- Peña Martínez V, Espinosa Castañeda AP. DescripcPeña Martínez, V., & Espinosa Castañeda, A. P. (2015). Descripción y análisis del OCT, HRT y GDX en glaucoma. Un enfoque para la optometría clínica., 1–28.ión y análisis del OCT, HRT y GDX en glaucoma. Un enfoque para la optometría clínica. 2015;1-28.
- Engineering H. Spectralis Glaucoma Module Premium Edition [Internet]. Disponible en: http://www.heidelbergengineering.com/media/e-learning/Totara/Dateien/pdf-tutorials/210030-002_SPECTRALIS_PDF Tutorial_BMO Positioning and OCT Interpretation in Glaucoma_IT.pdf.
- Ghassibi MP, Chien JL, Patthanathamrongkasem T, Abumasmah RK, Rosman MS, Skaat A, et al. Glaucoma Diagnostic Capability of Circumpapillary Retinal Nerve Fiber Layer Thickness in Circle Scans with Different Diameters. J Glaucoma. 2017;26(4):335-42.
- Lee EJ, Lee KM, Kim H, Kim TW. Glaucoma diagnostic ability of the new circumpapillary retinal nerve fiber layer thickness analysis based on bruch’s membrane opening. Investig Ophthalmol Vis Sci. 2016;57(10):4194-204. [CrossRef]
- Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86(2):238-42. [CrossRef]
- Morgan JE. Optic nerve head structure in glaucoma: Astrocytes as mediators of axonal damage. Eye. 2000;14(3):437-44. [CrossRef]
- Castejón Cervero M. Análisis morfológico con OCT del daño glaucomatoso en distintos tipos de glaucoma de ángulo abierto. Universidad de Alcalá; 2011.
- Gutiérrez-Ortiz C, Ángel M, Guezala T. Patología del nervio óptico.
- Quispe Fuentes JE, García López A, Ortega Santana JF. Correlación entre parámetros estructurales del nervio óptico: distancia mínima al borde BMO–MRW y promedio de capa de fibras nerviosas RNFL medido por tomografía de coherencia óptica en pacientes con daño campimétrico por glaucoma. Rev Mex Oftalmol. 2017;91(4):203-8. [CrossRef]
- Hernández EA, Díaz IP, González FF. Uso clínico del tomógrafo de coherencia óptica Spectralis en la evaluación del glaucoma Use of the Spectralis optical coherence tomography scanner to evaluate glaucoma. 2017;30(4):1-11.
- Álvarez Bulnes O. Descripción y análisis del grosor de capa de fibras nerviosas retinianas obtenidos mediante tomografía de coherencia óptica en pacientes sometidos a cirugía combinada de glaucoma. 2010.
- Heidelberg Engineering [Internet]. Disponible en: https://business-lounge.heidelbergengineering.com/es/en/products/spectralis/glaucoma-module/#product-details.
- Chauhan P. Segmented retinal thickness values BMO-MRW and RNFLT in optic atrophies. 2016;(November):34-5. Disponible en: https://media.heidelbergengineering.com/uploads/News/2016/12/Glaucoma-OCT-phenotyping-the-fundus-in-glaucoma_Ophthalmology-Times-Europe.pdf.
- SPECTRALIS Glaucoma Toolkit. [Internet]. Disponible en: https://business-lounge.heidelbergengineering.com/us/en/products/spectralis/glaucoma-module/downloads/#downloads.
- Hammel N, Belghith A, Weinreb RN, Medeiros FA, Mendoza N, Zangwill LM. Comparing the Rates of Retinal Nerve Fiber Layer and Ganglion Cell–Inner Plexiform Layer Loss in Healthy Eyes and in Glaucoma Eyes. Am J Ophthalmol. 2017. [CrossRef]
- Savini G, Zanini M, Carelli V, Sadun AA, Ross-Cisneros FN, Barboni P. Correlation between retinal nerve fibre layer thickness and optic nerve head size: An optical coherence tomography study. Br J Ophthalmol. 2005;89(4):489-92. [CrossRef]
- Hernández Martínez F. Nuevos factores de riesgo para la progresión del glaucoma. Universidad de Valencia; 2015.
- Chan TCW, Bala C, Siu A, Wan F, White A. Risk Factors for Rapid Glaucoma Disease Progression. Am J Ophthalmol. 2017;180(17):151-7. [CrossRef]
- Azcona-Cruz M, Ríos Lobo M, Amador-Jiménez S. Glaucoma: Aspectos relevantes para la detección oportuna. Salud y Adm. 2015;2(4):23-35.
- McMonnies CW. Historial de glaucoma y factores de riesgo. Journal of Optometry. 2017.
- Alasil T, Wang K, Keane PA, Lee H, Baniasadi N, De Boer JF, et al. Analysis of normal retinal nerve fiber layer thickness by age, sex, and race using spectral domain optical coherence tomography. J Glaucoma [Internet]. septiembre de 2013 [citado 21 de junio de 2021];22(7):532-41. Disponible en: https://pubmed.ncbi.nlm.nih.gov/22549477/. [CrossRef]
- Bendschneider D, Tornow RP, Horn FK, Laemmer R, Roessler CW, Juenemann AG, et al. Spectral-Domain Optical Coherence Tomography for Glaucoma Diagnosis. Investig Ophthalmol Vis Sci [Internet]. septiembre de 2017 [citado 21 de junio de 2021];23(1):1-14. Disponible en: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3624763/pdf/nihms412728.pdf.
- Gros-Otero J, Arruabarrena-Sánchez C, Teus M. Espesor corneal central en una población sana española. Arch Soc Esp Oftalmol. 2011;86(3):73-6. [CrossRef]
- Chen M. Central Corneal Thickness and its Association with Ocular Parameters. Med Res Arch. 2017;5(1). [CrossRef]
- Luna-Martínez I, Brechtel-Bindel M, de la Fuente-Torres MA. Relación del espesor corneal central y la variación en la presión intraocular con daño al nervio óptico en pacientes mexicanos con glaucoma. Rev Mex Oftalmol. 2009;83(4):193-6.
- Del Buey MA, Lavilla L, Ascaso FJ, Lanchares E, Huerva V, Cristóbal JA. Assessment of corneal biomechanical properties and intraocular pressure in myopic Spanish healthy population. J Ophthalmol. 2014. [CrossRef]
- Chen M, Yin-Tzu L, Chia-Chen T, Yen-Cheng C, Ching-Kuang C, Shu-Mei L. Relationship Between Central Corneal Thickness, Refractive Error, Corneal Curvature, Anterior Chamber Depth and Axial Length | Elsevier Enhanced Reader [Internet]. [citado 22 de junio de 2021]. Disponible en: https://reader.elsevier.com/reader/sd/pii/S1726490109700383?token=F52A824938635C89C527577C124D569D86A602522BD32F5973A524F32C38C3D60AD868F1FD2EEE1E38A4F74F26D63E4B&originRegion=eu-west-1&originCreation=20210621222751. [CrossRef]
- Zhang H, Xu L, Chen C, Jonas JB. Central corneal thickness in adult Chinese. Association with ocular and general parameters. The Beijing eye study. Graefe’s Arch Clin Exp Ophthalmol [Internet]. 12 de abril de 2008 [citado 22 de junio de 2021];246(4):587-92. [CrossRef]
- Biswas S, Lin C, Leung CKS. Evaluation of a myopic normative database for analysis of retinal nerve fiber layer thickness. JAMA Ophthalmol. 2016;134(9):1032-9. [CrossRef]
- Kim EK, Park H-YL, Park CK. Posterior scleral deformations around optic disc are associated with visual field damage in open-angle glaucoma patients with myopia. PLoS One. 2019;14(3):e0213714. [CrossRef]
- Xu X, Xiao H, Luo J, Liu X. Evaluation of spectral domain optical coherence tomography parameters in discriminating preperimetric glaucoma from high myopia. Int J Ophthalmol. 2019. 2019. [CrossRef]
- Pollet-Villard F, Chiquet C, Romanet JP, Noel C, Aptel F. Structure-function relationships with spectral-domain optical coherence tomography retinal nerve fiber layer and optic nerve head measurements. Investig Ophthalmol Vis Sci. 1 de abril de 2014;55(5):2953-62. [CrossRef]
- Huang D, Chopra V, Lu ATH, Tan O, Francis B, Varma R. Does optic nerve head size variation affect circumpapillary retinal nerve fiber layer thickness measurement by optical coherence tomography? Investig Ophthalmol Vis Sci [Internet]. 1 de julio de 2012 [citado 22 de junio de 2021];53(8):4990-7. Disponible en: www.AIGStudy. [CrossRef]
- Sommer A, Tielsch JM, Katz J, Quigley HA, Gottsch JD, Javitt J, et al. Relationship Between Intraocular Pressure and Primary Open Angle Glaucoma Among White and Black Americans: The Baltimore Eye Survey. Arch Ophthalmol [Internet]. 1991 [citado 22 de junio de 2021];109(8):1090-5. Disponible en: https://pubmed.ncbi.nlm.nih.gov/1867550/. [CrossRef]
- A.B, M. C. Vascular risk factors in primary open angle glaucoma. An Sist Sanit Navar [Internet]. 2004;27(3):335-44. Disponible en: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L40095249%5Cnhttp://sfx.library.uu.nl/utrecht?sid=EMBASE&issn=11376627&id=doi:&atitle=Vascular+risk+factors+in+primary+open+angle+glaucoma&stitle=An.+Sist.+Sanit.+Navarra&title=Anale.
- Lara SM, Lázaro F, Isabel M, Monclús G. Estudio y evaluación de la Capa de Fibras Nerviosas de la Retina y de las Células Ganglionares mediante dispositivo OCT en pacientes diagnosticados de glaucoma . Autor: 2016.
- Seong M, Sung KR, Choi EH, Kang SY, Cho JW, Um TW, et al. Macular and peripapillary retinal nerve fiber layer measurements by spectral domain optical coherence tomography in normal-tension glaucoma. Investig Ophthalmol Vis Sci. 1 de marzo de 2010;51(3):1446-52. [CrossRef]
- Mwanza JC, Oakley JD, Budenz DL, Chang RT, Knight OJ, Feuer WJ. Macular ganglion cell-inner plexiform layer: Automated detection and thickness reproducibility with spectral domain-optical coherence tomography in glaucoma. Investig Ophthalmol Vis Sci. octubre de 2011;52(11):8323-9. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).