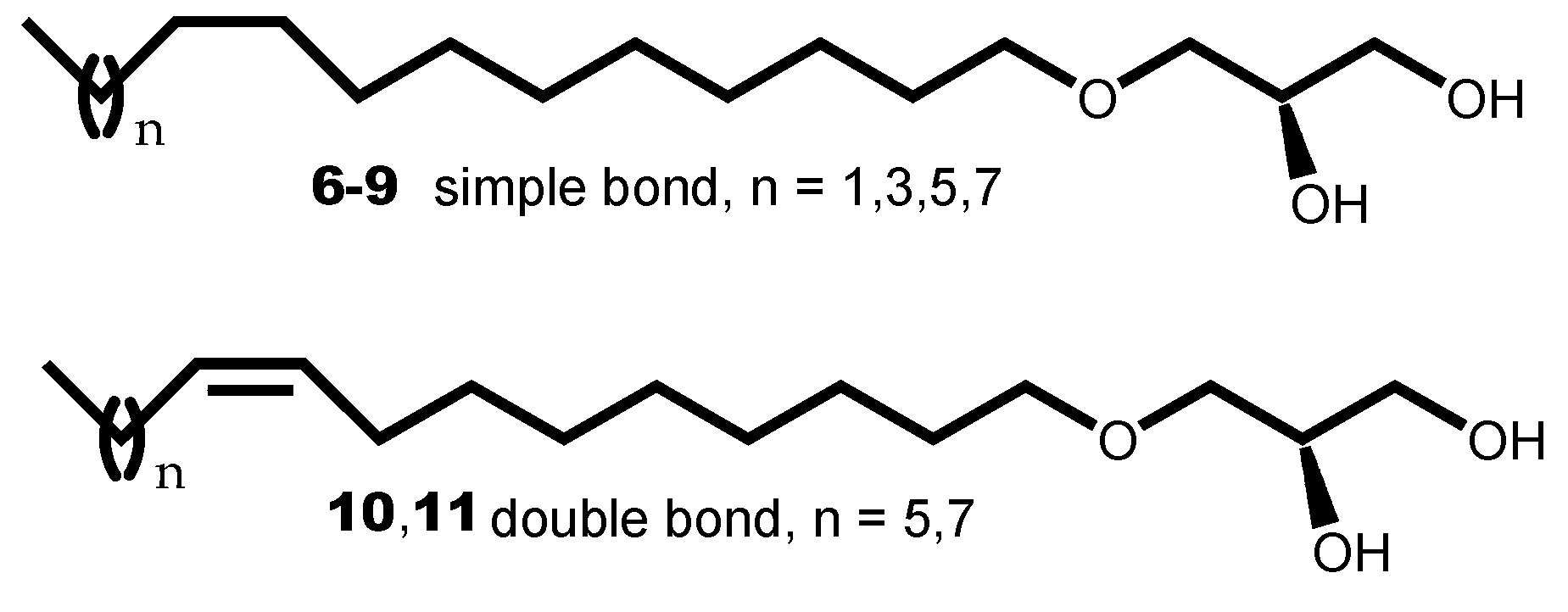
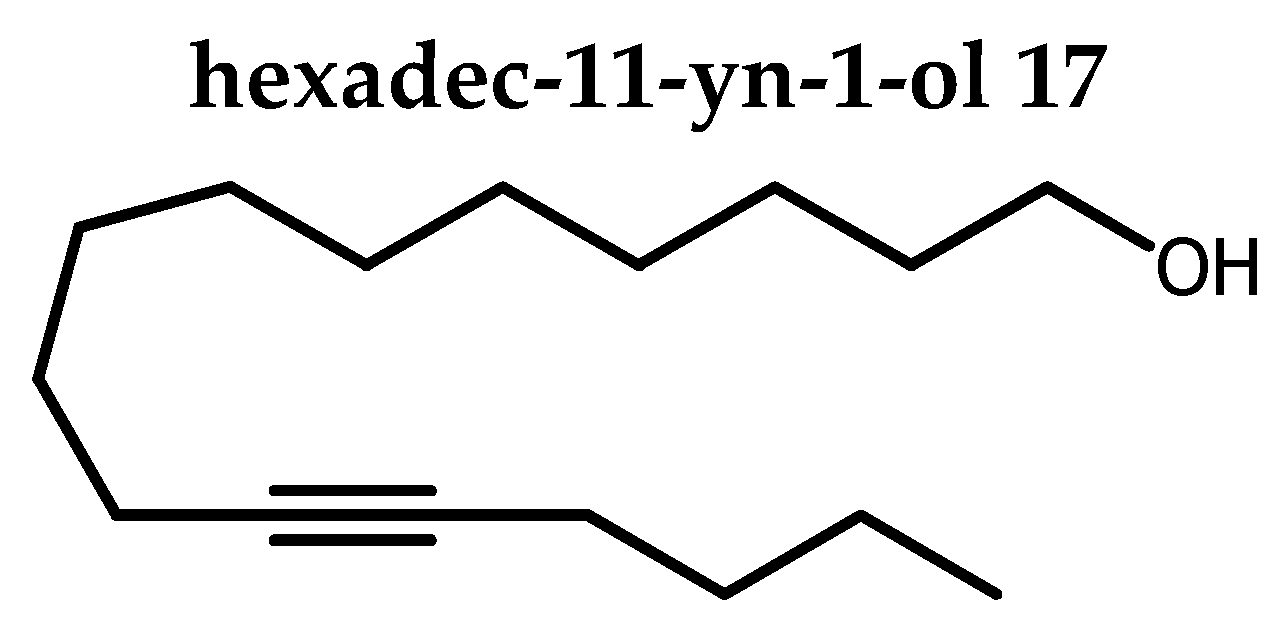
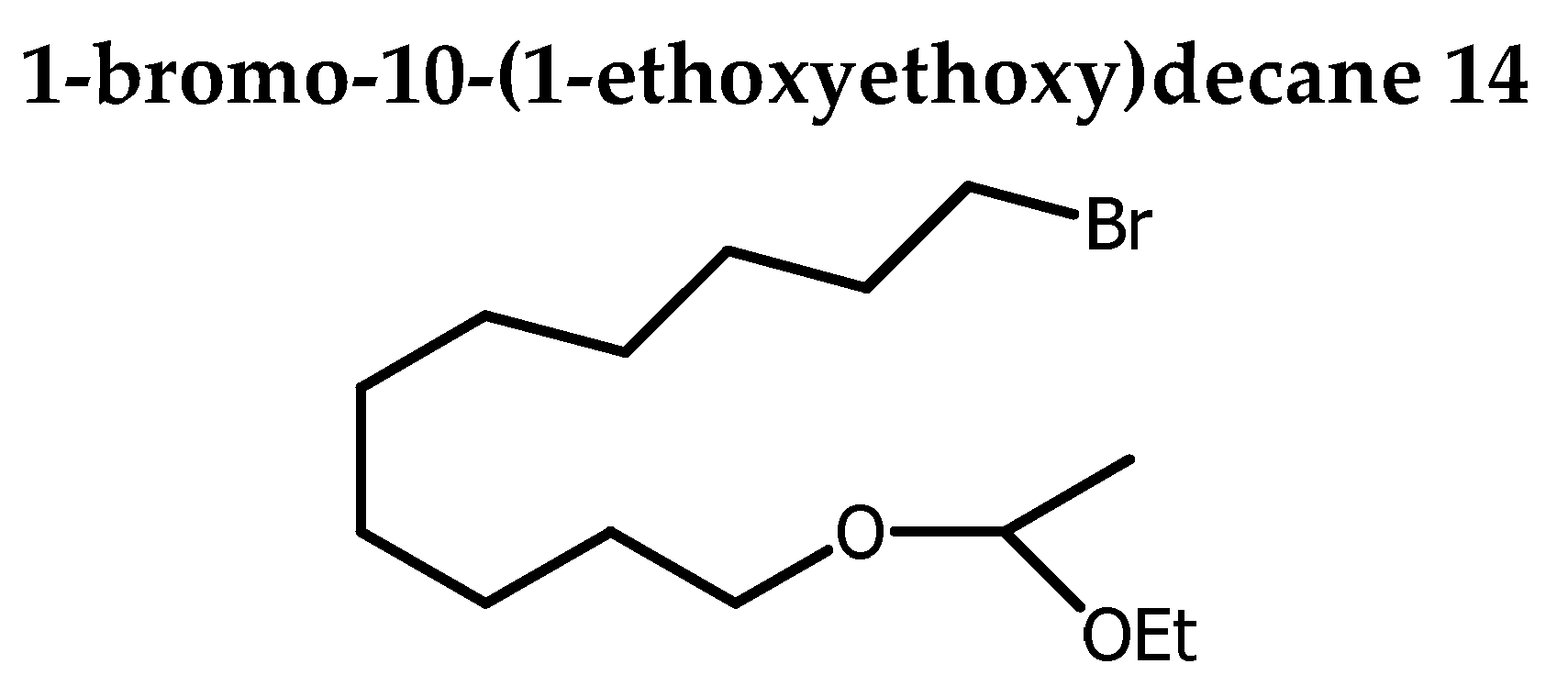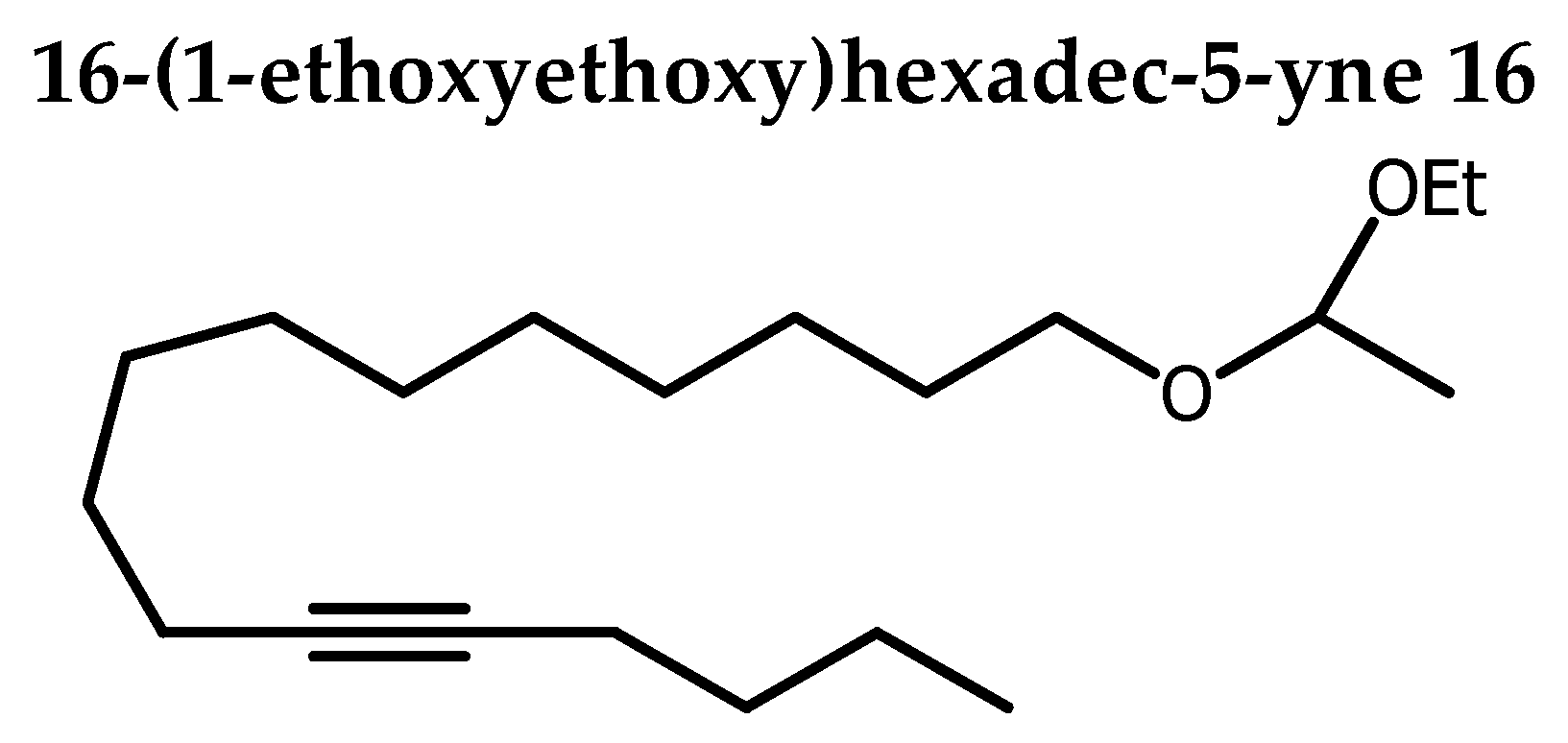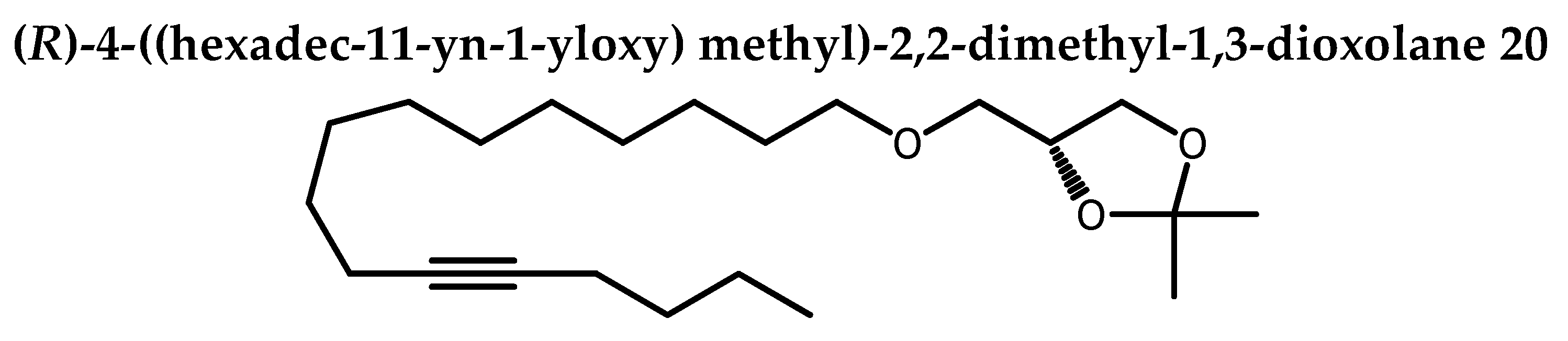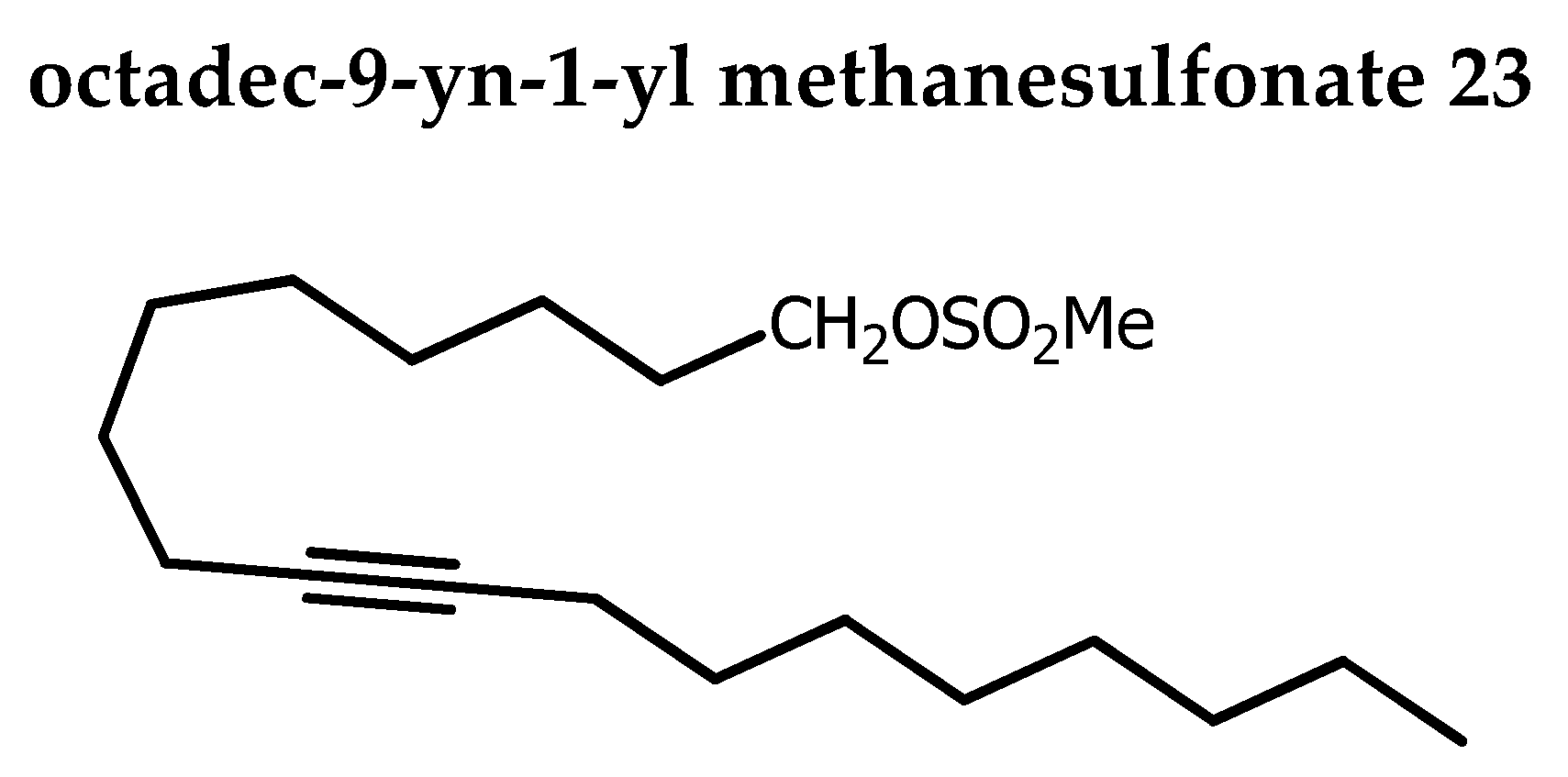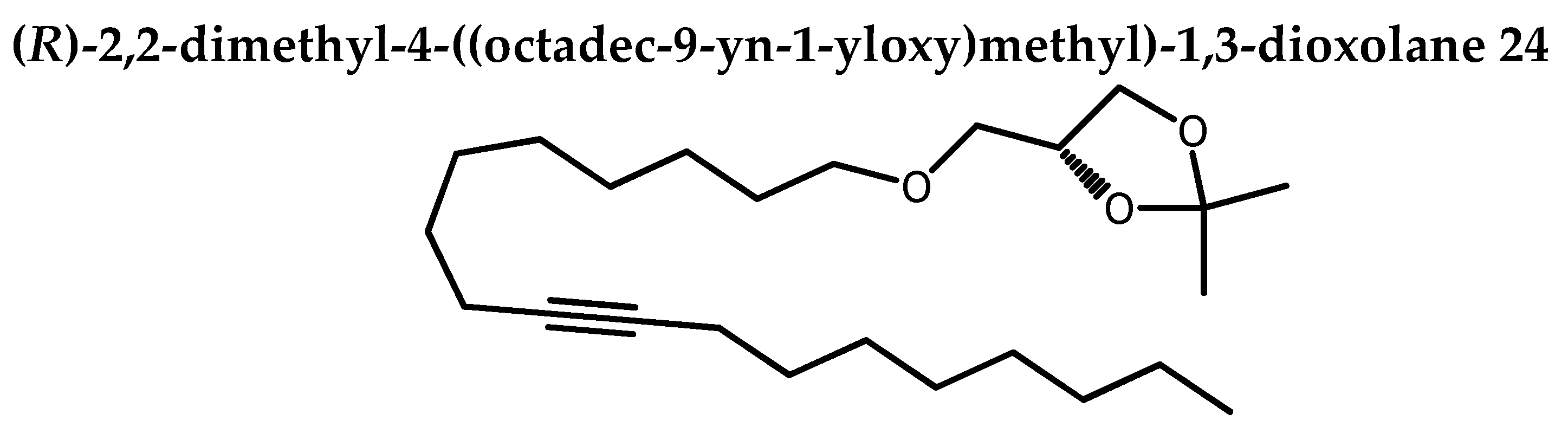4.1. General Information
Moisture sensitive reactions were performed under nitrogen. Anhydrous THF and diethyl ether were obtained by percolating through a column of a drying resin or by distilling over Na / benzophenone. Anhydrous DMF over molecular sieves was used as commercially supplied (Acros). Room temperature (rt) means a temperature generally in the interval 15-20°C. Basic alumina used for column chromatographies was purchased from Fluka. TLC plates were visualized by UV inspection followed by staining with an acidic ethanolic solution of p-anisaldehyde or with a solution of phosphomolybdic acid (5 g in 100 mL 95% ethanol). IR spectra were measured as films between KBr plates for liquids or as KBr disks for solids on a Thermo Nicolet Avatar 250 FTIR spectrometer. 1H NMR spectra (400.13 and 300.13 MHz) and 13C NMR spectra (100.61 and 75.47 MHz) were recorded on Avance 400 and 300 Bruker spectrometers using TMS as an internal standard. Optical rotations were measured using a Perkin Elmer 341 polarimeter (concentration in g/100 mL). High resolution mass spectra were recorded using a MicrO-Tof-Q II spectrometer under electrospray using methanol as solvent. Microanalyses were performed with a CHNS analyzer. 2,3-Isopropylidene-sn-glycerol 19 (≥ 95% pure) was purchased from Alfa Aesar.
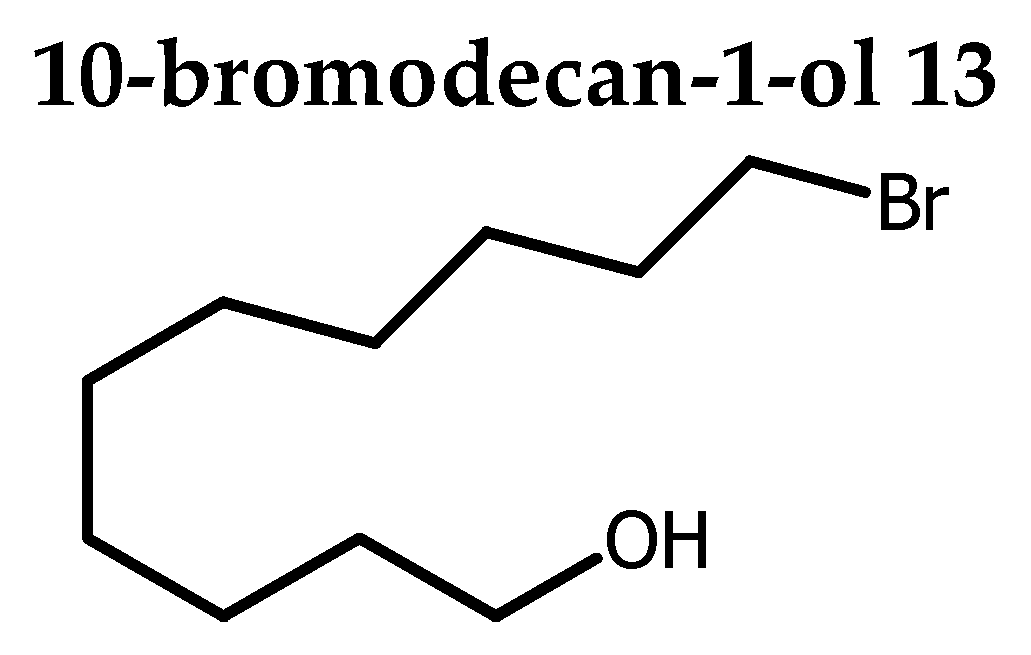
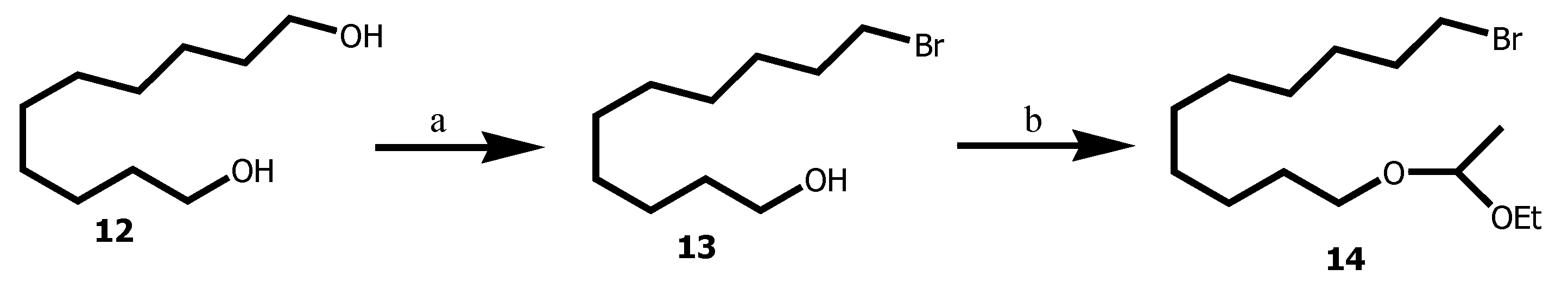
To a solution of 1,10-decandiol 12 (5 g, 28.69 mmol) in toluene (100 mL) under stirring, was added 48% aqueous solution of HBr (4.5 mL, 40.5 mmol, 1.41 eq). The corresponding mixture was refluxed overnight (18 h) and TLC monitoring showed completion of the reaction and cooled at rt. The crude was purified by Kugelrohr distillation (140 °C / 2 Torr) and afforded 13 as colorless oil (5.4 g, 80%). Rf 0.56 (petroleum ether / acetone 70:30).
1H NMR (400 MHz, CDCl3): δ= 3.64 (t, 2H, J = 6.6 Hz), 3.40 (t, 2H, J = 6.9 Hz), 1.85 (tt, 2H, J = 7.4, 6.9, Hz), 1.60 (tt, 2H, J = 7.3, 6.7 Hz), 1.37-1.19 (m, 12H)
13C NMR (100 MHz, CDCl3): δ= 63.15 (CH2), 34.06 (CH2Br), 32.82 (CH2), 32.76 (CH2), 29.47 (CH2), 29.37 (CH2), 29.35 (CH2), 28.74 (CH2), 28.15 (CH2), 25.71 (CH2)
In a flame-dried two necked flask containing a solution of 13 (592 mg, 2.5 mmol) in DCM (5 mL) under stirring and N2 cooled at 0 oC, was added a solution of ethyl vinyl ether (290 µL, 3.0 mmol, 1.2 eq) and p-toluenesulfonic acid (31.2 mg, 0.125 mmol, 0.5 eq). The stirring was maintained 30 min at the same temperature. A saturated solution of NaHCO3 was added to the mixture and the stirring was continued for 10 mn. Extraction was done with DCM (3 x). Organic layer was dried over Na2SO4, concentrated and purified by column chromatography on silica gel (0→1 % acetone in petroleum ether) afforded 14 as a colorless oil (672.1 mg, 87%). Rf 0.59 (petroleum ether / acetone 70:30).
1H NMR (400 MHz, CDCl3): δ= 4.68 (q, 1H, J = 5.3 Hz, CH-CH3), 3.65 (dq, 1H, J = 9.4, 7.1 Hz, OCH2CH3), 3.56 (dt, 1H, J = 9.4, 6.7 Hz, CH2O), 3.48 (dq, 1H, J = 9.4, 7.1 Hz, OCH2CH3), 3.41 (dt, 1H, J = 9.4, 6.6 Hz, CH2O), 3.40 (t, 2H, J = 6.9 Hz, CH2Br), 1.85 (tdd, 2H, J = 7.7, 6.7, 6.6 Hz, CH2CH2Br), 1.56 (tt, 2H, J = 7.0, 6.9 Hz, CH2CH2O), 1.47-1,38 (m, 2H, CH2), 1,38-1,24 (m, 10H, 5 CH2), 1.30 (d, 3H, J = 5.3 Hz, CH-CH3), 1.21 (t, 3H, J = 7.1 Hz, CH2CH3).
13C NMR (100 MHz, CDCl3): δ= 99.52 (CH-CH3), 65.27 (CH2CH2O), 60,65 (OCH2CH3), 34.02 (CH2Br), 32.83 (CH2), 29.89 (CH2), 29.47 (CH2), 29.42 (CH2), 29.38 (CH2), 28.75 (CH2), 28.16 (CH2), 26.24 (CH2), 19.89 (CH-CH3), 15.34 (OCH2CH3).
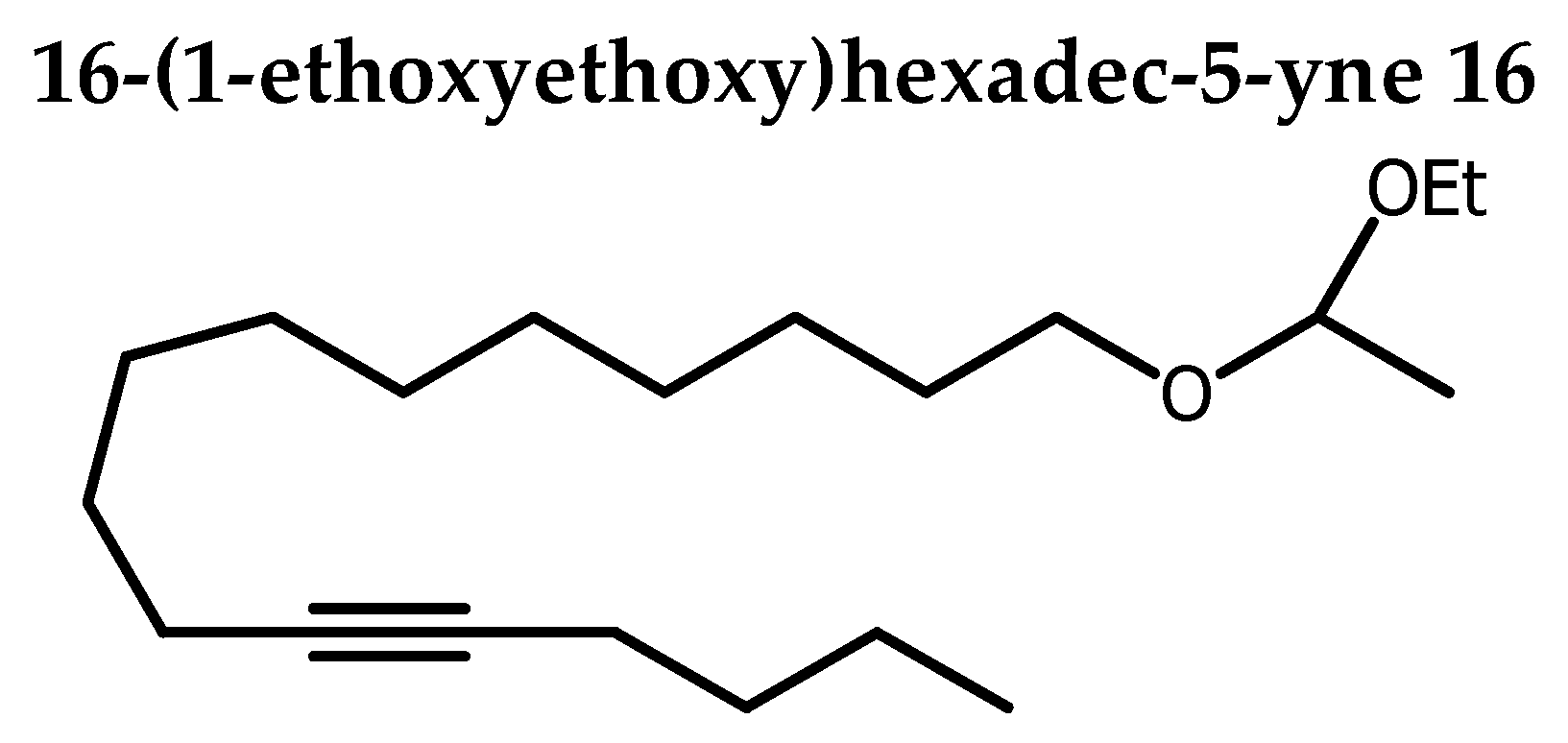
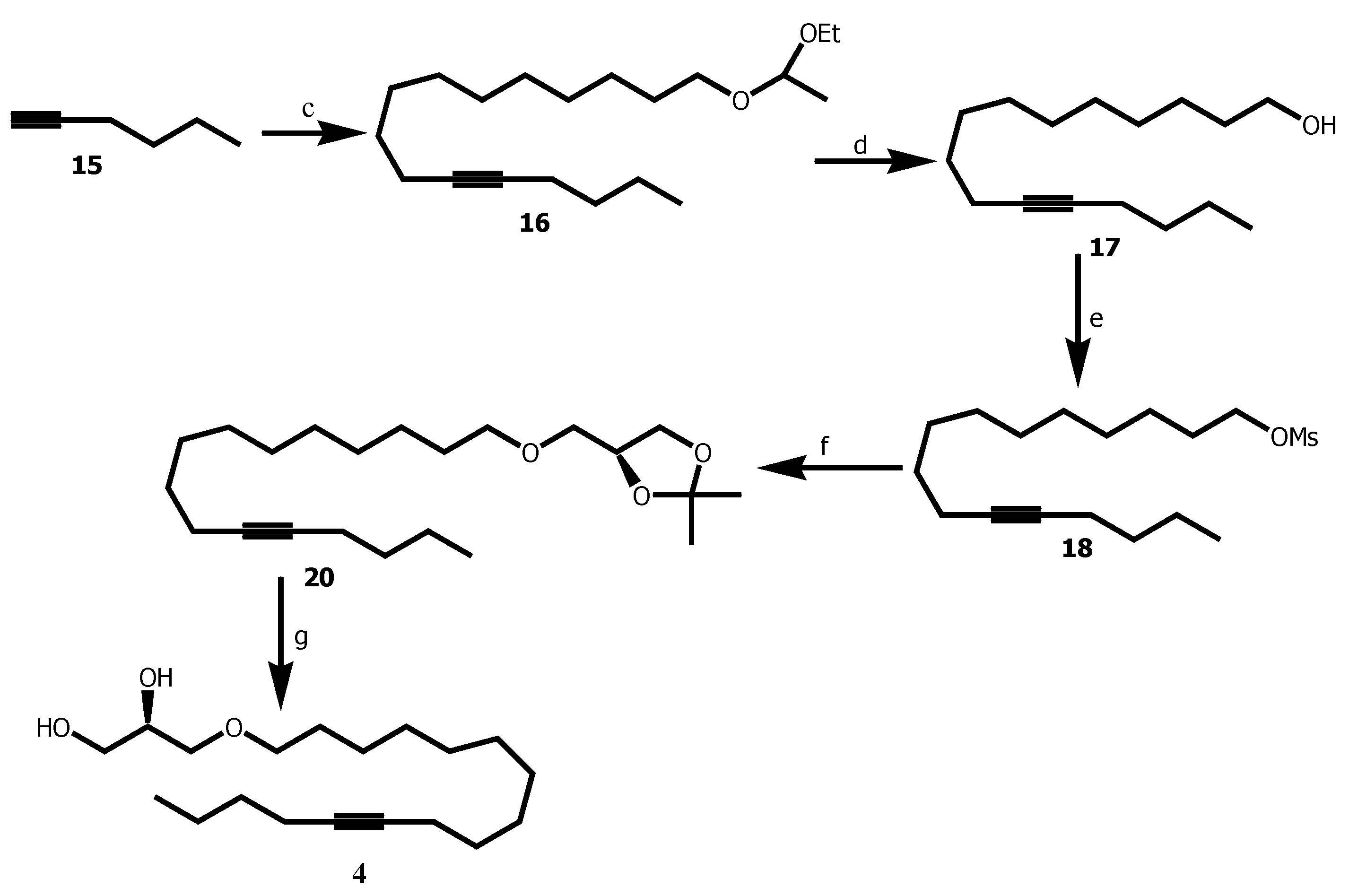
In a flamed-dried flash containing a solution of 1-hexyne 15 (350 µL, 3.0 mmol) in THF (2.5 mL) and HMPA (1 mL) cooled at -80°C , was added a solution of n-Buli (3.26 mL) under N2 and the stirring was continued for 1 h at -78°C. Thereafter, a solution of 14 (427 mg, 1.5 mmol ) in THF (1 mL) was added and the transfer was completed by THF (2 x 0.2 mL). The corresponding mixture was allowed to stir overnight (16 h) at rt. TLC monitoring showed completion of the reaction. Extraction was done with ethyl acetate and organic layer washed with distilled water and dried over Na2SO4. Solvent was removed under reduced pressure to obtain the crude product as yellow light oil. The crude product was purified by silica-gel column chromatography with petroleum ether / Et3N (99/1) and gave 16 as colorless oil (600 mg, 60%). Rf 0.57 (petroleum ether / acetone 90:10).
IR (film) νmax 2930, 2855, 2213, 1466, 1456, 1379, 1338, 1134, 1100, 1086, 1061, 951 cm-1
1H NMR (400 MHz, CDCl3): δ=4.68 (q, 1H, J = 5.3 Hz, CH-CH3), 3.65 (dq, 1H, J = 9.4, 7.1 Hz, OCH2CH3), 3.56 (dt, 1H, J = 9.3, 6.7 Hz, CH2O), 3.48 (dq, 1H, J = 9.4, 7.1 Hz, OCH2CH3), 3.41 (dt, 1H, J = 9.3, 6.7 Hz, CH2O), 2.18-2.10 (m, 4H, CH2CδCCH2), 1,56 (ddt, 2H, J = 8.3, 6.3, 6.7 Hz, CH2CH2O), 1.51-1.24 (m, 18H, 9 CH2), 1.31 (d, 3H, J = 5.3 Hz, CH-CH3), 1.21 (t, 3H, J = 7.1 Hz, OCH2CH3), 0.90 (t, 3H, J = 7.2 Hz, CH3).
13C NMR (100 MHz, CDCl3): δ=99.50 (CH-CH3), 80.21-80.17 (CδC), 65.27 (CH2CH2O), 60.63 (OCH2CH3), 31.27 (CH2), 29.90 (CH2), 29.56 (CH2), 29.50 (CH2), 29.48 (CH2), 29.17 (CH2), 29.16 (CH2), 28.86 (CH2), 26.26 (CH2), 21.94 (CH2, C15), 19.88 (CH-CH3), 18.76 (CH2CδC), 18.45 (CH2CδC), 15.33 (OCH2CH3), 13.65 (CH3).
To a solution of 16 (219.1 mg, 0.7 mmol,) in methanol (3.6 mL), was added camphosulfonic acid (9.4 mg, 0.04 mmol, 0.05 eq). The flask was then purged under nitrogen, stoppered and. the stirring was maintained 18 h at rt. TLC monitoring confirmed completion of the reaction and triethylamine (6 drops) was added to neutralise the acid. Methanol was evaporated under reduced pressure and the crude was purified by column chromatography on silica gel (0→2 % acetone in petroleum ether) and afforded 17 as colorless oil (150.5 mg, 89 % yield). Rf 0.16 (petroleum ether / acetone 90:10).
1H NMR (400 MHz, CDCl3): δ=3.64 (t, 2H, J = 6.7 Hz, CH2OH), 2.19-2.10 (m, 4H, CH2CδCCH2), 1.56 (tt, 2H, J = 7.3, 6.7 Hz, CH2CH2OH), 1.52-1.23 (m, 19H, 9 CH2 et OH), 0.90 (t, 3H, J = 7.2 Hz, CH3)
13C NMR (100 MHz, CDCl3): δ=80.21-80.20 (CδC), 63.07 (CH2OH), 32.80 (CH2), 31.27 (CH2), 29.56 (CH2), 29.48 (CH2), 29.43 (CH2), 29.17 (CH2), 29.15 (CH2), 28.85 (CH2), 25.74 (CH2), 21.94 (CH2), 18.76 (CH2CδC), 18.45 (CH2CδC) 13.65 (CH3).
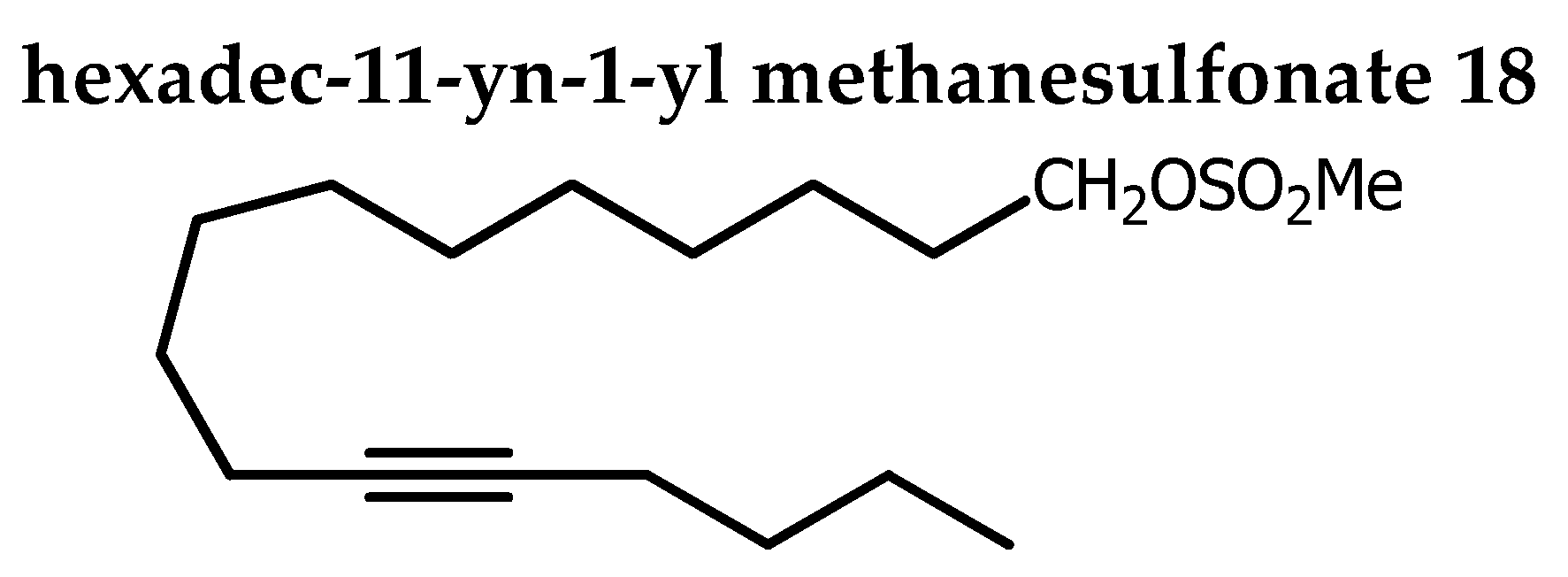
To the stirred solution at -50oC of 17 (121.1 mg, 0.51 mmol), Et3N (0.21 mL, 1.5 mmol, 2.95 eq) in DCM (2.5 mL) under N2, mesyl chloride (94 µL, 1.2 mmol, 1.25 eq) in DCM (9 mL) was added drop wise and the reaction mixture was stirred for an additional 5 h at the same temperature. Distilled water (25 mL) was added to quench the reaction and extraction was done with DCM (3 x). Organic phase was washed with brine and dried over Na2SO4. Solvent was removed under reduced pressure to obtain the crude product as yellow light oil. The crude product was purified by silica-gel column chromatography (0→1 % acetone in petroleum ether) and gave 18 as white solid (114.1 mg, 94%). Rf 0.48 (petroleum ether / acetone 85:15).
1H NMR (400 MHz, CDCl3): δ= 4.22 (t, 2H, J = 6.6 Hz, CH2OMs), 3.01 (s, 3H, OSO2CH3) 2.19-2.10 (m, 4H, CH2CδCCH2), 1.75 (ddt, 2H, J = 8.1, 6.8, 6.6 Hz, CH2CH2OMs), 1.52-1.23 (m, 18H, 9 CH2)
13C NMR (100 MHz, CDCl3): δ=80.20-80.17 (CδC), 70.20 (CH2OMs), 37.35 (OSO2CH3), 31.26 (CH2), 29.40 (CH2), 29.37 (CH2), 29.14 (CH2), 29.12 (CH2),
29.10 (CH2), 29.02 (CH2), 28.81 (CH2), 25.42 (CH2), 21.93 (CH2), 18.74 (CH2CδC), 18.44 (CH2CδC), 13.65 (CH3).
Melting point: 28-30°C.
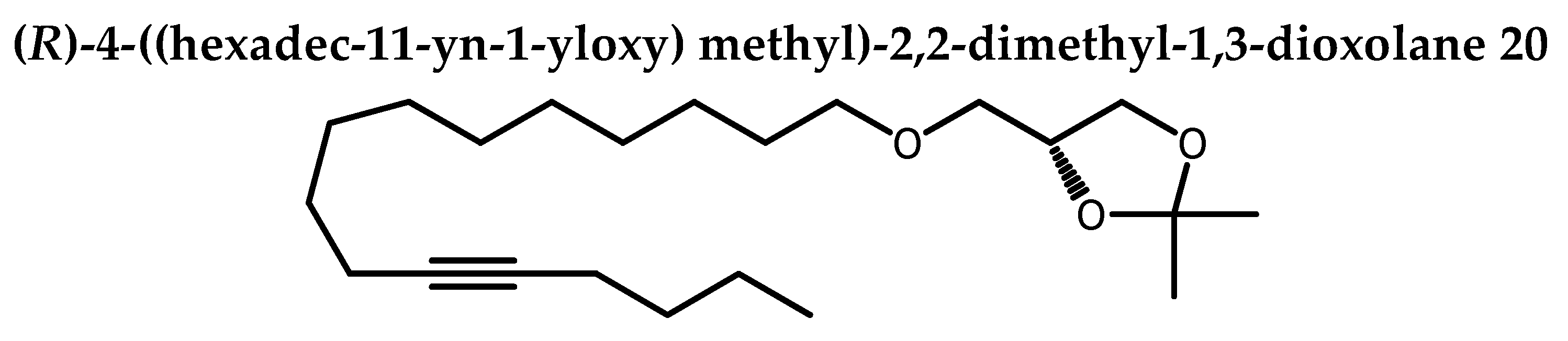
A 60% dispersion of sodium hydride in mineral oil (44 mg, 0.85 mmol, 2.5 eq) was washed three times with petroleum ether under argon. Anhydrous NMP (100 µL) was added and the mixture was cooled at 0°C. A solution of 2,3- isopropylidene-sn-glycerol 19 (56 µL, 0.46 mmol, 1.0 eq) in NMP (50 µL)was added dropwise followed by NMP (2 x 0.2 mL) to complete the transfer of 19. After stirring 10 min at 0°C, a solution of 18 (133.6 mg, 0.42 mmol) in NMP (0.4 mL) was added to the resulting white suspension. Transfer of 18 was completed by rinsing with NMP (2 x 0.2 mL). This mixture was left under good stirring overnight (18 h) at rt. 10 % solution of ammonium acetate (4 mL) and petroleum ether (5 mL) were added to the mixture and the stirring continued for 10 min. Extraction was done with petroleum ether (3 x). Organic phase was washed with brine and dried over Na2SO4. Solvent was removed under reduced and the crude product was purified by silica-gel column chromatography (0→1 % acetone in petroleum ether) afforded 20 as colorless oil (108.1 mg, 81%). Rf 0.56 (petroleum ether / acetone 80:20).
IR (film) νmax 2986, 2928, 2856, 1464, 1456, 1379, 1369, 1255, 1214, 1120, 1056, 847, 514 cm-1.
1H NMR (400 MHz, CDCl3): δ=4.26 (dddd, 1H, J = 6.4, 6.4, 5.7, 5.6 Hz, CHOCMe2), 4.06 (dd, 1H, J = 8.2, 6.4 Hz, CH2OCMe2), 3.72 (dd, 1H, J = 8.2, 6.4 Hz, CH2OCMe2) 3.52 (dd, 1H, J = 9.9, 5.7 Hz, CH2O((CH2)10), 3.50-3.42 (m, 2H, OCH2(CH2)9), 3.42 (dd, 1H, J = 9.9, 5.6 Hz, CH2O(CH2)10), 2.18-2.10 (m, 4H, CH2CδCCH2), 1.57 (tt, 2H, J = 7.3, 6.7 Hz, OCH2CH2), 1.52-1.23 (m, 18H, 9 CH2), 1.42 (s, 3H, C-CH3), 1.36 (s, 3H, C-CH3), 0.90 (t, 3H, J = 7.2 Hz, CH3).
13C NMR (100 MHz, CDCl3): δ=109.36 (C(CH3)2), 80.21-80.18 (CδC), 74.77 (CH α de O), 71.89 (CH2 α de O), 71.83 (CH2 α de O), 66.95 (CH2 α de O), 31.28 (CH2), 29.57 (CH2), 9.54 (CH2), 29.49 (CH2), 29.46 (CH2), 29.18 (CH2), 29.15 (CH2), 28.86 (CH2), 26.78 (C-CH3), 26.06 (CH2), 25.43 (C-CH3), 21.94 (CH2), 18.76 (CH2CδC), 18.45 (CH2CδC), 13.64 (CH3).
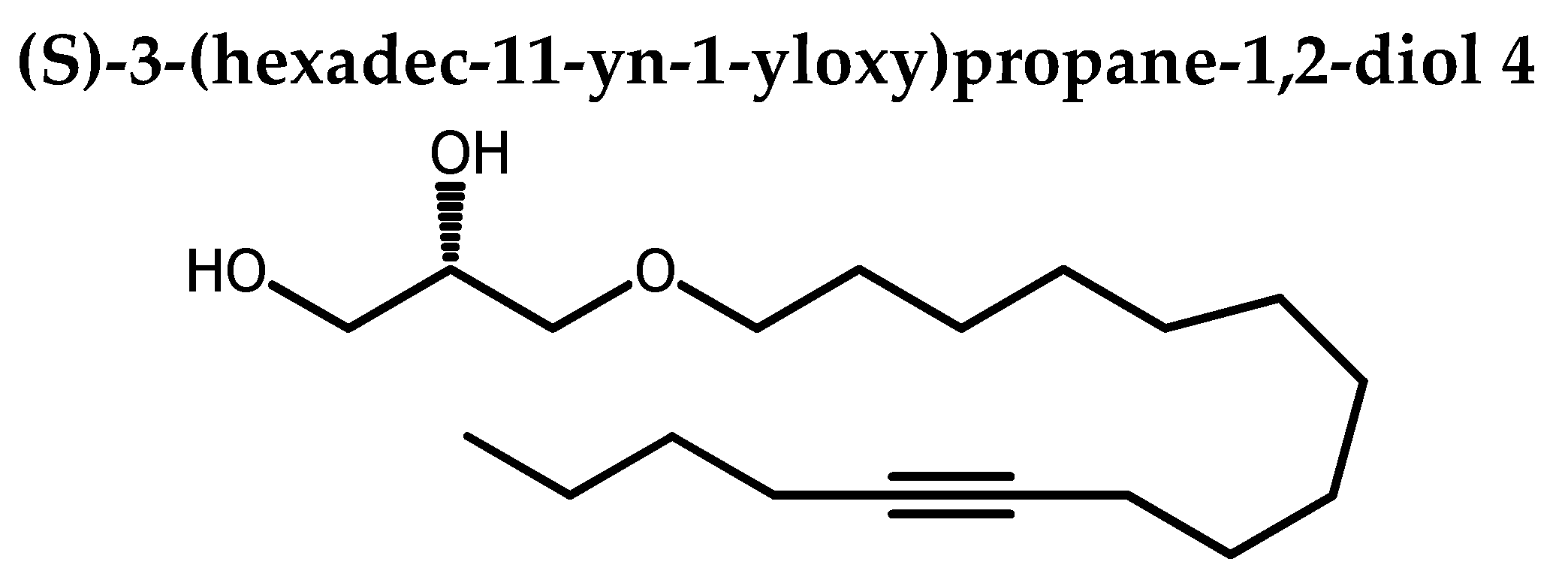
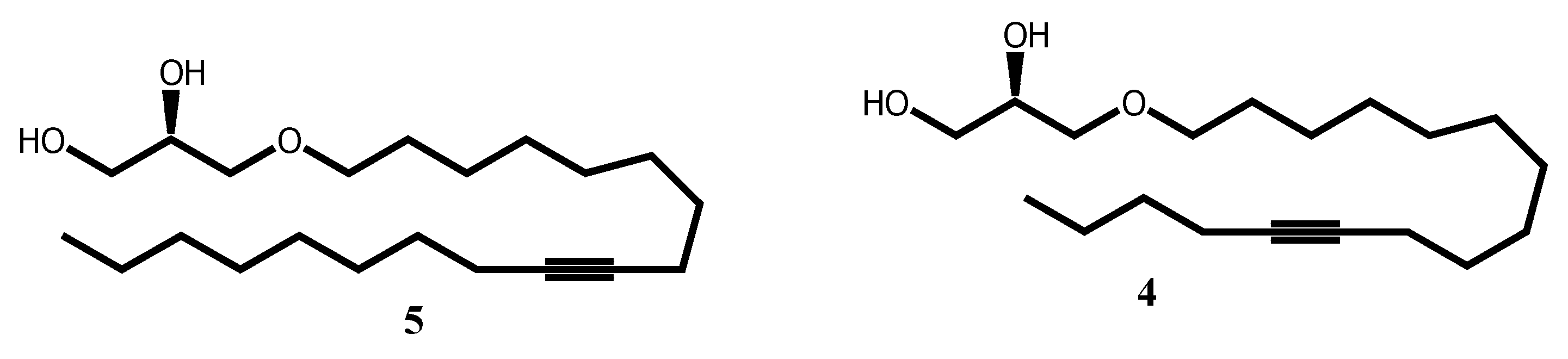
To a solution of 20 (60.4 mg, 0.17 mmol,) in THF (1.2 mL), was added p-toluenesulfonic acid monohydrate (4.8 mg, 0.03 mmol, 0.15 eq) and distilled water (0.51 mL). The flask was then purged under nitrogen, stoppered and dipped in a preheated bath at 80oC. The stirring was maintained 8 h at the same temperature. Sodium carbonate (5.8 mg, 0.07 mmol, 0.04 eq) was added and the stirring was continued for 1 h at 80oC. Extraction was done with EtOAC (3 x). Organic phase was washed with brine and dried over Na2SO4. Solvent was removed under reduced and the crude product was purified by silica-gel column chromatography (0→10 % EtOAC in petroleum ether) afforded 4 as white solid (46.3 mg, 87%). Rf 0.06 (petroleum ether / EtOAC 80:20).
1H NMR (400 MHz, CDCl3): δ=3.85 (ddt, 1H, J = 5.6, 5.5, 4.0 Hz, CHOCMe2), 3.71 (dd, 1H, J = 11.4, 3.8Hz, CH2OCMe2), 3.63 (dd, 1H, J = 11.4, 5.2 Hz, CH2OCMe2), 3.54 (dd, 1H, J = 9.9, 4.0 Hz ), 3.53-3.46 (m, 3H), 2.20-2.08 (m, 4H), 1.56 (ddt, 2H, J = 7.0, 6.8, 6.7 Hz), 1.52-1.18 (m, 21H), 0.89 (t, 3H, J = 6.9 Hz, CH3).
13C NMR (100 MHz, CDCl3): δ=80.21-80.19 (CδC), 72.52 (CH2), 71.85 (CH2), 70.41 (CH ), 64.30 (CH2), 31.27 (CH2), 29.58 (CH2), 29.52 (CH2), 29.48 (CH2), 29.44 (CH2), 29.17 (CH2), 29.14 (CH2), 28.85 (CH2), 26.08 (CH2), 21.93 (CH2), 18.75 (CH2), 18.44 (CH2), 13.65 (CH3)
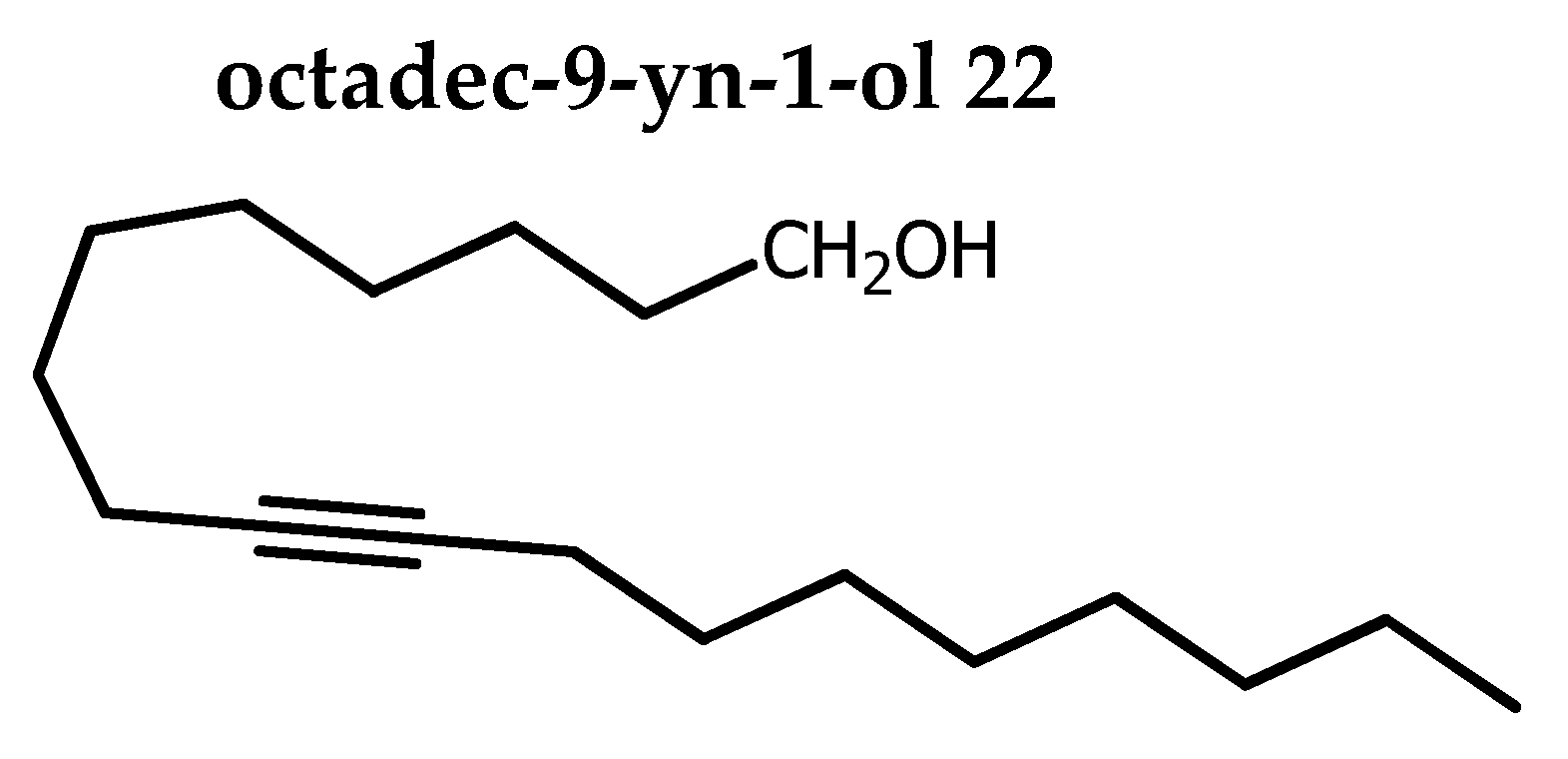
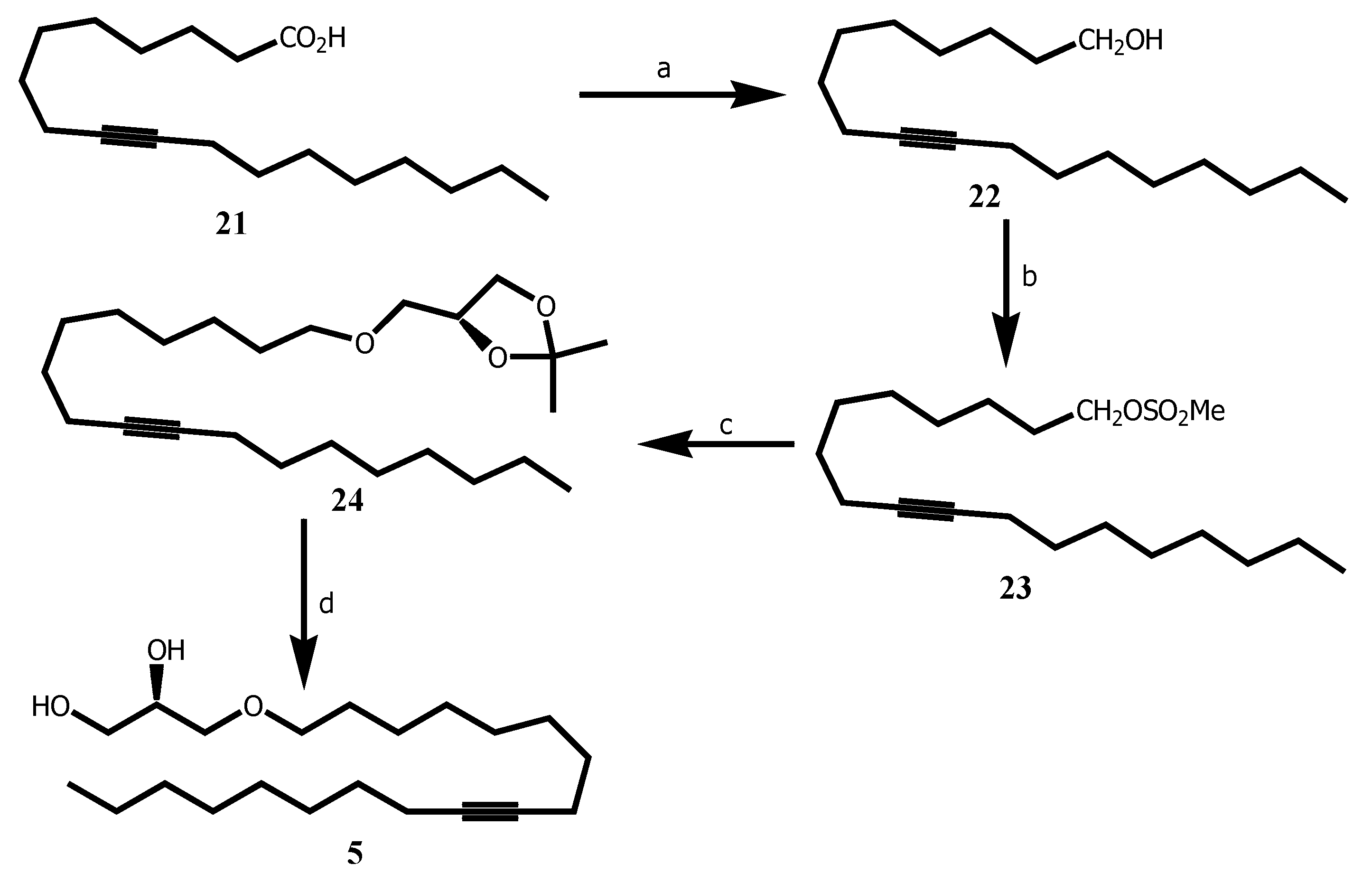
Red-Al (10.2 mL, 33.9 mmol, 1.75 eq) was added dropwise to a solution of 21 (5.43 g, 19.4 mmol) in THF (48 mL) cooled at 0oC under stirring and N2. The stirring was continued overnight at rt. TLC monitoring confirmed completion of the starting material. Citric acid (5.03 g) was added and distilled water (45 mL) and the stirring was continued for 30 mn. Extraction was done with petroleum ether / EtOAC (80:20). Organic layer was dried over Na2SO4, concentrated and purified by column chromatography on alumina gel (0→3 % acetone in petroleum ether) afforded 22 as a colorless oil (4. 69 g, 91 %). Rf 0.48 (petroleum ether / acetone 85:15).
1H NMR (400 MHz, CDCl3): δ=3.64 (t, 2H, J = 6.6 Hz), 2.17-2.10 (m, 4H, CH2CδCCH2), 1.56 (tt, 2H, J = 7.5, 6.8 Hz), 1.54-1.45 (m, 4H), 1.43-1.25 (m, 18H), 0.89 (pseudo t, 3H, J = 6.9 Hz)
13C NMR (100 MHz, CDCl3): δ=80.29 and 80.16 (CδC), 63.06 (CH2), 32.77 (CH2), 31.84 (CH2), 29.31 (CH2), 29.21 (CH2), 29.17 (CH2), 29.14(CH2), 29.13 (2CH2), 28.86 (CH2), 28.77(CH2), 25.69 (CH2), 22.66 (CH2), 18.74 (2CH2CδC), 14.09 (CH3).
To the stirred solution of 22 (5.14 g, 14.92 mmol), Et3N (4.03 mL, 28.97 mmol, 1.5 eq) in DCM (58 mL) under N2 at -40oC, mesyl chloride (1.94 mL, 25.11 mmol, 1.3 eq) in DCM (8.36 mL) was added drop wise. After addition, the reaction mixture was stirred for an additional 2 h at the same temperature. Distilled water (80 mL) was added to quench the reaction and extraction was done with DCM. Organic phase was washed with brine and dried over Na2SO4. Solvent was removed under reduced pressure and the crude product was purified by silica-gel column chromatography (0→1 % acetone in petroleum ether) gave 23 as colorless oil (6.2 g, 93 %). Rf 0.49 (petroleum ether / acetone 85:15).
1H NMR (400 MHz, CDCl3): δ=4.23 (t, 2H, J = 6.6 Hz, CH2OMs), 3.00 (s, 3H, OSO2CH3) 2.17-2.10 (m, 4H, CH2CδCCH2), 1.75 (tt, 2H, J = 13.2, 6.6 Hz, CH2CH2OMs), 1.52-1.43 (m, 4H), 1.41-1.22 (m, 18H), 0.89 (pseudo t, 3H, J = 6.9 Hz)
13C NMR (100 MHz, CDCl3): δ=80.37 and 80.07 (CδC), 70.14 (CH2OMs), 37.38 (OSO2CH3), 31.86 (CH2), 29.24 (CH2), 29.18 (CH2), 29.14 (CH2), 29.13 (CH2), 29.08 (CH2), 28.95 (2CH2), 28.89 (CH2), 28.70 (CH2), 25.40 (CH2), 22.68 (CH2), 18.76 (CH2CδC), 18.73 (CH2CδC), 14.12 (CH3).
To a stirred solution of 23 (6.19 g, 17.95 mmol), n-Bu4NBr (2.32 g, 7.18 mmol, 0.5 eq) in DMSO (45 mL), and potassium hydroxide (4.74 g, 71.8 mmol, 4 eq), was added a solution of 2,3- isopropylidene-sn-glycerol 19 (2.7 g, 20.64 mmol, 1.15 eq). The corresponding mixture went under stirring overnight (15h) at 45oC. Distilled water was added and extraction was done with petroleum ether / EtOAC (80:20). Organic phase was washed again with brine, dried over Na2SO4. Solvent was removed under reduced pressure. Chromatography of the crude on silica-gel (0→0.2 % acetone in petroleum ether) gave 24 as white solid (6.4 g, 93%). Rf 0.39 (petroleum ether / acetone 80:20).
1H NMR (400 MHz, CDCl3): δ=4.26 (ddt, 1H, J = 6.4, 6.3, 5.7Hz, CHOCMe2), 4.06 (dd, 1H, J = 8.2, 6.4 Hz, CH2OCMe2), 3.73 (dd, 1H, J = 8.2, 6.4 Hz, CH2OCMe2) 3.52 (dd, 1H, J = 10.0, 5.7 Hz), 3.48 (dd, 1H, J = 6.7, 2.7 Hz), 3.45 (dd, 1H, J = 7.0, 6.3 Hz), 3.41(dd, 1H, J = 10.0, 5.7 Hz), 2.16-2.10 (m, 4H, CH2CδCCH2), 1.62-1.53 (m, 2H), 1.52-1.41 (m, 4H), 1.47(q, 3H, J = 0.6 Hz, C-CH3 ), 1.36 (q, 3H, J = 0.6 Hz, C-CH3), 1.40-1.20 (m, 20H), 0.90 (t, 3H, J = 6.9 Hz, CH3).
13C NMR (100 MHz, CDCl3): δ=109.37 (C(CH3)2), 80.29 and 80.19 (CδC), 74.76 (CH α to O), 71.88 (CH2 α to O), 71.84 (CH2 α to O), 66.95 (CH2 α to O), 31.86 (CH2), 29.55 (CH2), 29.37 (CH2), 29.24 (CH2), 29.18 (CH2), 29.16 (CH2), 29.14 (CH2), 29.12 (CH2), 28.89 (CH2), 28.81 (CH2), 26.78 (C-CH3), 26.03 (CH2), 25.43 (C-CH3), 22.68 (CH2), 18.77 (CH2CδC), 18.76 (CH2CδC), 14.12 (CH3).
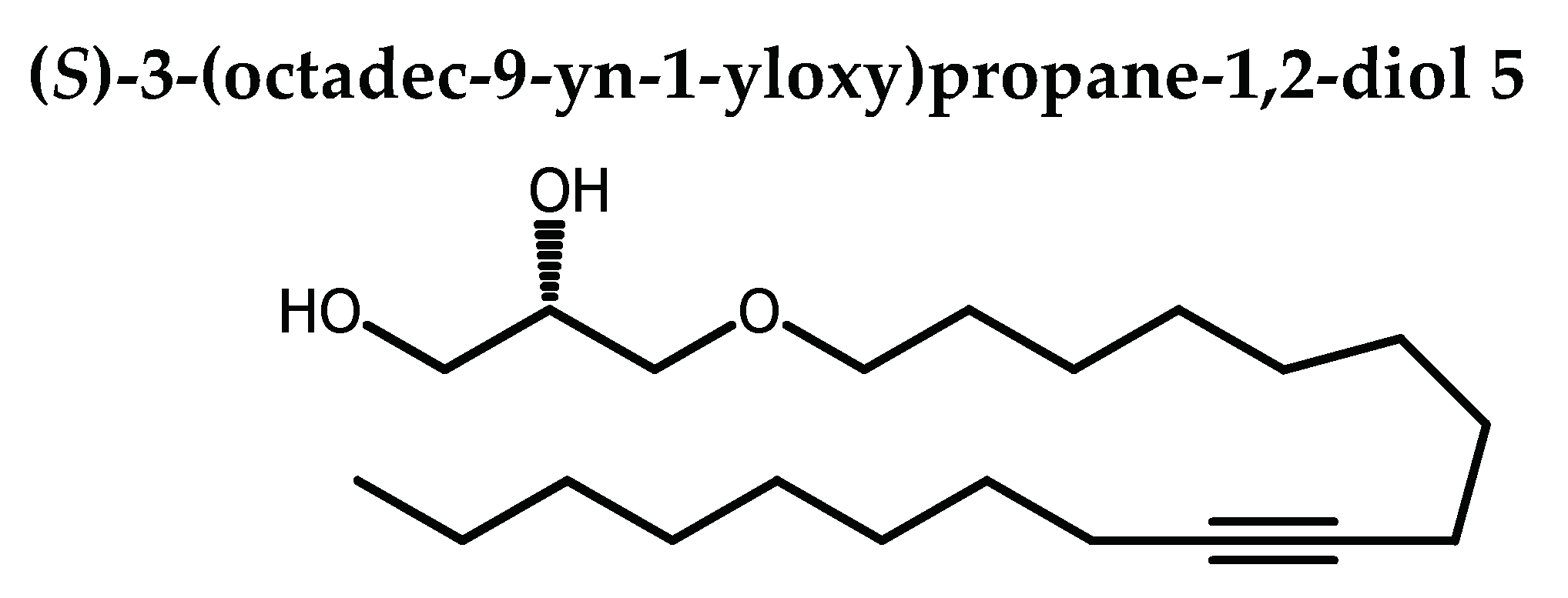
To a solution of 24 (60.4 mg, 0.17 mmol,) in THF (1.2 mL), was added p-toluenesulfonic acid monohydrate (4.8 mg, 0.03 mmol, 0.15 eq) and distilled water (0.51 mL). The flask was then purged under nitrogen, stoppered, and dipped in a preheated bath at 80oC. The stirring was maintained 8 h at the same temperature. Sodium carbonate (5.8 mg, 0.07 mmol, 0.04 eq) was added and the stirring was continued for 1 h at 80oC. Extraction was done with EtOAC (3 x). Organic phase was washed with brine and dried over Na2SO4. Solvent was removed under reduced and the crude product was purified by silica-gel column chromatography (0→10 % EtOAC in petroleum ether) afforded 5 as white solid (46.2 mg, 85%). Rf 0.05 (petroleum ether / EtOAC 80:20).
1H NMR (400 MHz, CDCl3): δ=3.85 (ddt, 1H, J = 5.9, 5.2, 3.9 Hz), 3.71 (dd, 1H, J = 11.4, 3.8 Hz), 3.63 (dd, 1H, J = 11.4, 5.2 Hz), 3.54 (dd, 1H, J = 10.0, 5.7 Hz), 3.48 (dd, 1H, J = 6.7, 2.7 Hz), 3.45 (dd, 1H, J = 7.0, 6.3 Hz), 3.41(dd, 1H, J = 9.7, 4.0 Hz), 3.49 (dd, 9.7, 6.0 Hz), 3.47 (dd, 1H, J = 6.7, 2.5 Hz), 3.44 (dd, 1H, J = 9.5, 6.8 Hz),
2.56-2.52 (enveloppe, 1H, OH), 2.40-2.20 (enveloppe, 1H, OH), 2.17-2.10 (m, 4H, CH2CδCCH2), 1.62-1.54 (m, 2H), 1.52-1.41 (m, 4H), 1.41-1.22 (m, 18H), 0.89 (t, 3H, J = 6.9 Hz, CH3)
13C NMR (100 MHz, CDCl3): δ=80.32 and 80.17 (CδC), 72.52 and 71.84 (CH2OCH2C17H31), 70.44 (CHOH), 64.30 (CH2OH), 31.86 (CH2), 29.57 (CH2), 29.35 (CH2), 29.24 (CH2), 29.18 (CH2), 29.14 (2CH2), 29.10 (CH2), 28.89 (CH2), 28.80 (CH2), 26.05 (CH2), 22.68 (CH2), 18.77 (CH2CδC), 18.76 (CH2CδC), 14.12 (CH3)