1. Introduction
Lung cancer is the leading cause of cancer deaths globally [
1], with Hungary being in the mid-league among European countries at an overall incidence rate of 52.4 [
2]. Due to late-appearing symptoms, only 16% of lung cancers are diagnosed early, while most cases are diagnosed at a metastatic stage in which treatment options are ineffective and the five-year survival rate is 8% [
3,
4].
In the last two decades, low-dose computed tomography (LDCT) has gained attention internationally as a tool for screening and diagnosing lung cancer at curable stages. The US National Lung Screening Trial (NLST) and the European NELSON trial provided evidence that screening by LDCT reduces lung cancer mortality by 20% and 25%, respectively [
5,
6]. In Eastern-Europe, the HUNCHEST pilot trial has demonstrated that LDCT screening facilitates early lung cancer diagnosis [
7]. However, there has been debate on which LDCT screening protocols are the most efficient in diagnosing lung cancer, and whether these protocols are sufficiently cost-effective to warrant routine screening for the millions at risk.
In Hungary, annual LDCT screening for smokers was demonstrated to be cost-effective [
8]. Various independent evaluations of the NLST have also demonstrated cost-effectiveness for the US [
9,
10]. However, there are inherent differences in the screening protocols currently practiced by Hungary, which are similar to those used in the NELSON trial, and the screening protocols used in the NLST. Most notable is the option for an indeterminate result in Hungary for a scan potentially suspicious for lung cancer, which is confirmed as positive or negative in three months by a repeat LDCT scan [
7]. In the NLST, all screens are either deemed positive or negative, with no option for a repeat LDCT scan [
5]. Regarding the clinical diagnosis of lung cancer, Hungary and the rest of Europe diagnose cases based on nodule volume and volume doubling-time, while the US assesses nodule diameter [
11]. These differences in screening procedures can have implications in diagnosis rates and timelines, resource consumption, and patient outcomes.
With many countries considering the introduction of LDCT screening for lung cancer, it is important to assess the different screening protocols practiced internationally. Despite several independent evaluations of cost-effectiveness in the US and Europe, few studies have directly compared the cost-effectiveness of different screening policies. In this paper, we aim to directly compare the cost-effectiveness of the NELSON and NLST screening strategies applied in the same healthcare environment.
2. Materials and Methods
2.1. Model Development
A decision-analytic model analyzing the cost-effectiveness of Hungarian lung cancer screening protocols, based on the NELSON protocol, was originally developed by Nagy et. al. 2023 [
8]. We manipulated this model to reflect the protocols of the NLST, while maintaining features specific to the Hungarian healthcare setting. This was done to estimate the costs and patient outcomes of annual LDCT screening had Hungary followed the screening protocols used by the NLST. Throughout the paper, the “Hungarian screening pathway” or the “Hungarian model” refers to the original model reflecting Hungarian protocols used in the Hungarian setting. The “US screening pathway” or the “US model” refers to the manipulated model reflecting NLST protocols used in the Hungarian setting.
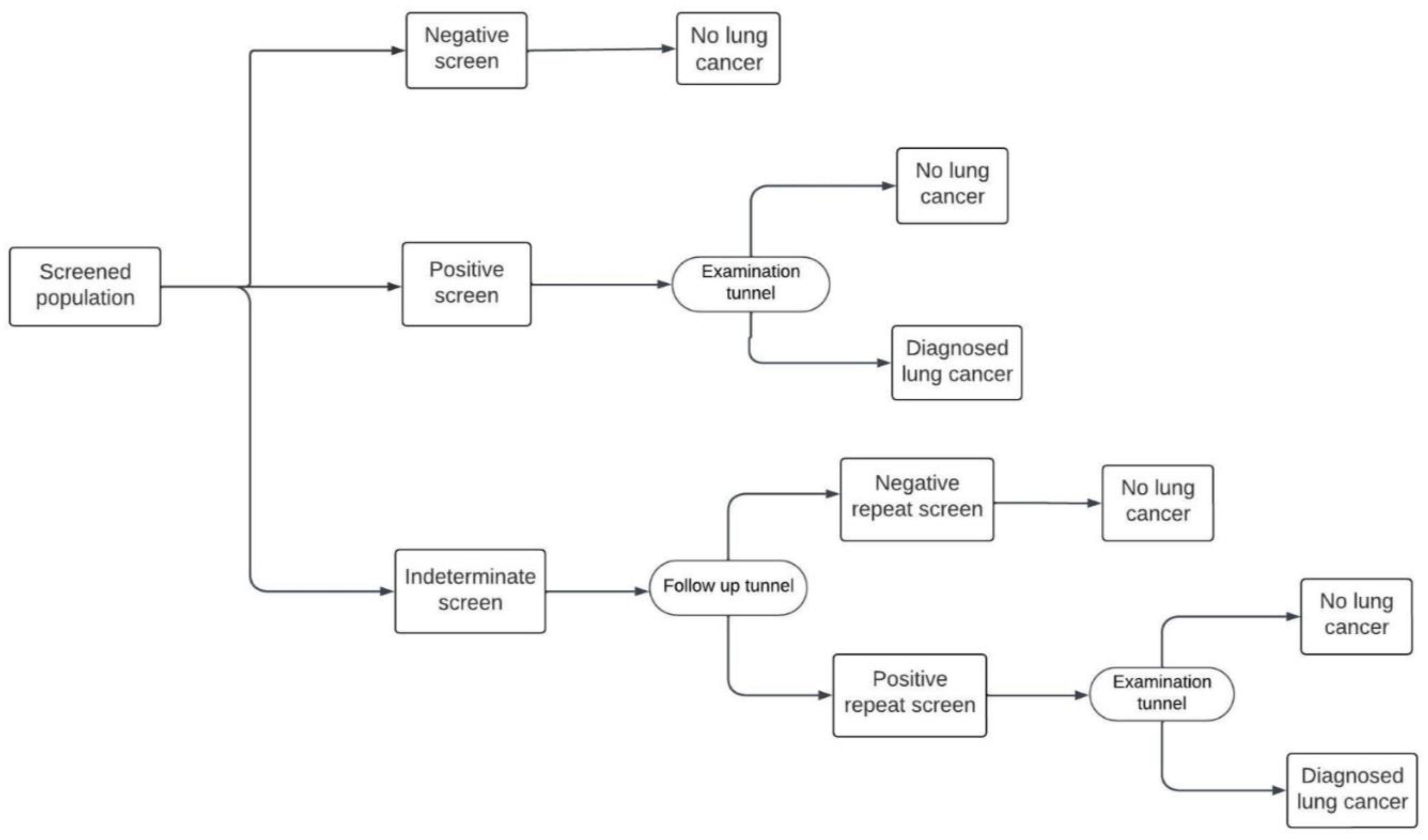
Both the Hungarian and US models include an initial baseline screening followed by annual screening from age 55 up to the age of 74. In the Hungarian model, there are three possible outcomes to the initial round of screening: positive, negative, or indeterminate, indicating an uncertain degree of suspicion for lung cancer (
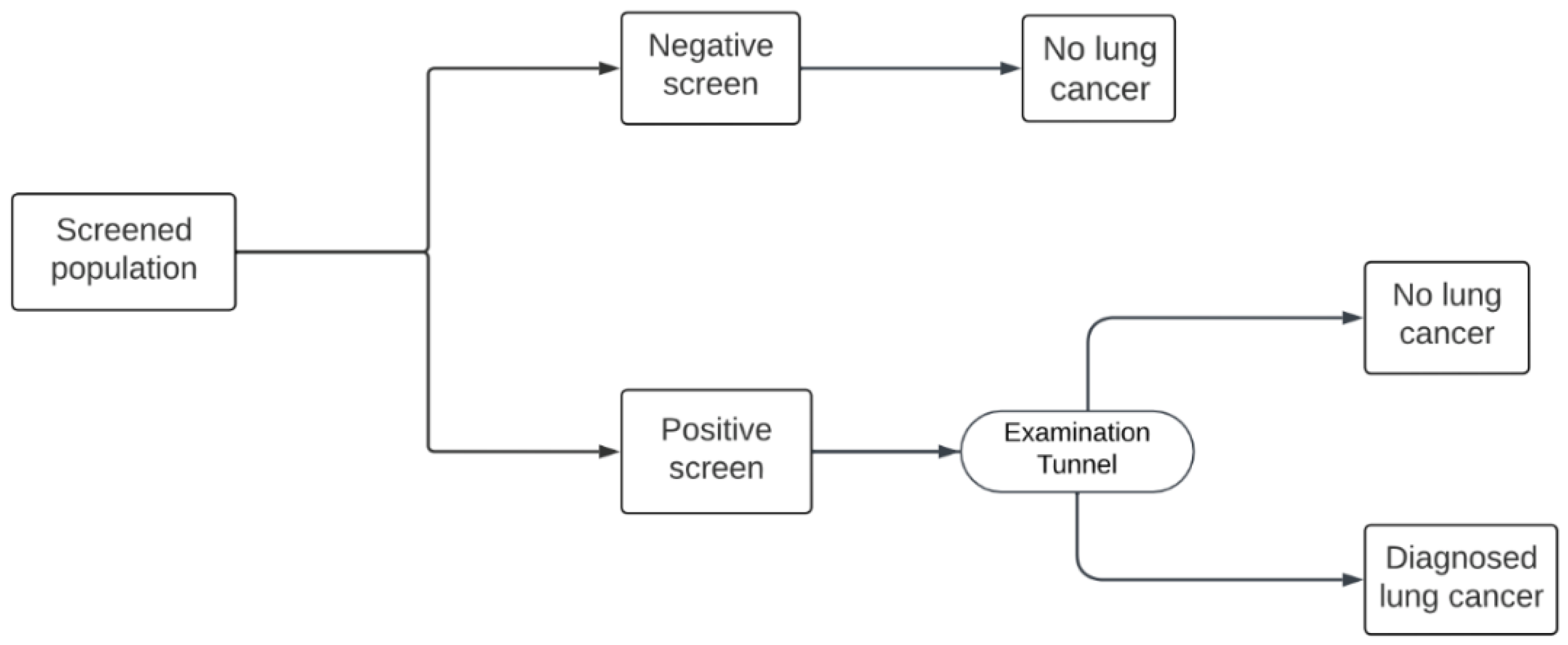
Figure 1). This protocol differs from the US model, in which the only possible screening outcomes are positive or negative, with no indeterminate option (
Figure 2). In both models, patients with a positive result enter an “Examination tunnel”, entailing more intensive examinations for lung cancer diagnosis, such as a chest or abdominal CT scan, bronchoscopy, or biopsy. Meanwhile, patients with an indeterminate result in the Hungarian model are subject to a follow-up LDCT screen in three months; then they are assigned a final negative result, or a positive result and subsequently enter the “Examination tunnel”. The cycle length of both models is 1 month. Patient pathways after diagnosis and subsequent (beyond year 1) screening years are simulated in the decision analytic model as explained in detail by Nagy et. al [
8].
2.2. Effectiveness Data and Model Inputs
Effectiveness data for screening in the Hungarian model was based on data from the HUNCHEST study [
7], the NELSON trial [
12,
13,
14], and Hungarian incidence and prevalence data [
15,
16]. To develop the US model, data from the NLST was derived from the National Institute of Health’s Cancer Data Access System [
17]. Effectiveness data for screening in the US model was calculated from the NLST’s baseline and year 1 LDCT screening results and used as model inputs for patient pathways, as can be seen in
Table 1 and
Table 2. The NLST cohort included either current or former smokers (who had quit within the past 15 years) aged 55-74 years with a 30+ pack year history. This is comparable to the Hungarian model’s cohort of current smokers aged 55-74 years with a 25+ pack year history.
Given the absence of an indeterminate status in the NLST, the rate of indeterminate screens was 0% in the US model; hence all screens were either positive or negative (
Table 1 and
Table 2). This contrasts with the Hungarian model, in which an indeterminate result was assigned for 14.35% and 6.58% of baseline and year 1 screens, respectively. A greater proportion of patients were therefore screened positive in the US model (27.33%) compared to the Hungarian model (3.33%).
The rates of diagnostic examinations for the “Examination tunnel” in the US model are derived from the procedure rates conducted in the NLST’s baseline year (
Table 3). The diagnostic examination rates in the Hungarian model are based on published literature and expert opinion [
18]. Health care costs associated with LDCT screening and diagnosis are based on Hungarian national health insurance data and were unchanged in the Hungarian and US models [
19,
20,
21]. Treatment data for diagnosed cases was unchanged between models to maintain Hungarian medical practices.
2.3. Utility and ICER Calculation
The models estimate patients’ utility combined with their life trajectory to yield quality adjusted life years (QALY). In both models, these were calculated using Hungarian EuroQol 5D index values and international data sources that were adjusted to age and gender specific utility tariffs [
22]. A 0.03 utility decrement was associated with the uncertainty of waiting for a repeat LDCT in indeterminate cases in the Hungarian model [
23,
24].
The models calculate costs and incremental cost-effectiveness ratio (ICER). All costs and benefits were discounted at a yearly rate of 3.7% [
25]. Results are presented in Euros based on the average conversion rate of 2023 for Hungarian Forints to Euros (1 € = 381.74 HUF) [
26]. The willingness to pay threshold for this particular indication according to the latest Hungarian guideline is 26,761 € (1.5 x GDP/capita) [
25].
2.4. Deterministic Sensitivity Analysis
One-way deterministic sensitivity analysis was used to test parameter uncertainty. Parameters were changed one-by-one by +/-10% of the default value: a tornado diagram presented the most sensitive parameters (
Figure 1 and
Figure 2). Additionally, a stratified analysis of age was carried out, accounting for differences in lung cancer screening effectiveness among different age bands in the NLST (
Table 4).
2.5. Overdiagnosis
Both models track screen-detected and clinically-detected cases separately. As such, we could estimate overdiagnosis (defined as the probability that a screen–detected cancer would not have been diagnosed clinically in the patient’s lifetime without screening) for both the US and the Hungarian pathway using the method proposed by Patz et al. [
27]: number of excess lung cancer cases in the LDCT arm compared to the no screening arm / total number of screen-detected lung cancer cases in the LDCT arm.
3. Results
3.1. Diagnosis Rate
Our results showed that if smokers aged 55-74 years were screened annually under the Hungarian pathway, the proportion of diagnosed lung cancer cases would be 3.53% after five years (
Table 5). If screened annually under the US pathway, the proportion of diagnosed cases would be greater at 3.61%, which yielded a 2.21% relative increase in the proportion of detected cases.
3.2. ICER
The average lifetime treatment cost (the cost of diagnostic examinations and subsequent lung cancer treatment) of the population of smokers aged 55-74 years screened annually under the Hungarian pathway was previously calculated to be 2317 €, and the average lifetime screening cost (the cost of initial and repeat LDCT screening) was calculated to be 226 € [
8]. Annual screening for the same population under the US screening pathway resulted in an increased average treatment cost of 2375 € and a decreased average screening cost of 211 €. Consequently, the US pathway resulted in a 43 € increase in total average lifetime costs (from 2543 € to 2586 €). The US screening pathway for smokers aged 55-74, compared to the Hungarian screening pathway, resulted in a lifetime gain of 0.006 QALYs. The incremental costs and QALY yield an ICER of 7875 €/QALY (
Table 6).
3.3. Overdiagnosis
The probability of overdiagnosis under the US pathway was found to be 8.39%, in comparison to the Hungarian pathway’s overdiagnosis rate of 7.93%, which reflects a 5.80% increase in overdiagnosis rates.
3.4. Deterministic Sensitivity Analysis
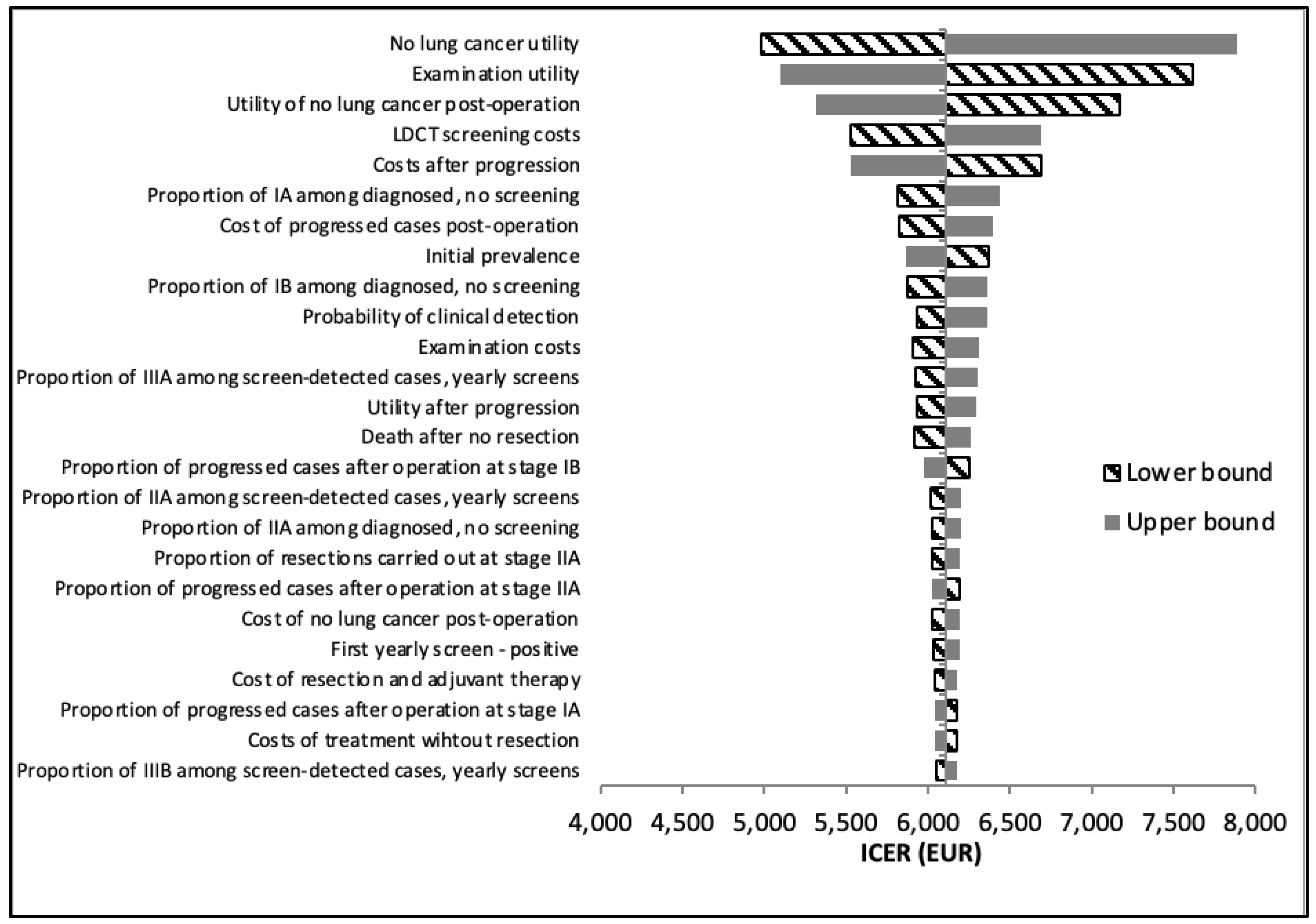
One-way deterministic sensitivity analysis demonstrated that when compared to no lung cancer screening at all, the most influential parameters in the US model are the utility value of not having lung cancer, utility of undergoing diagnostic examinations, utility of no lung cancer post-operation, LDCT screening costs, and treatment costs after progression of lung cancer (
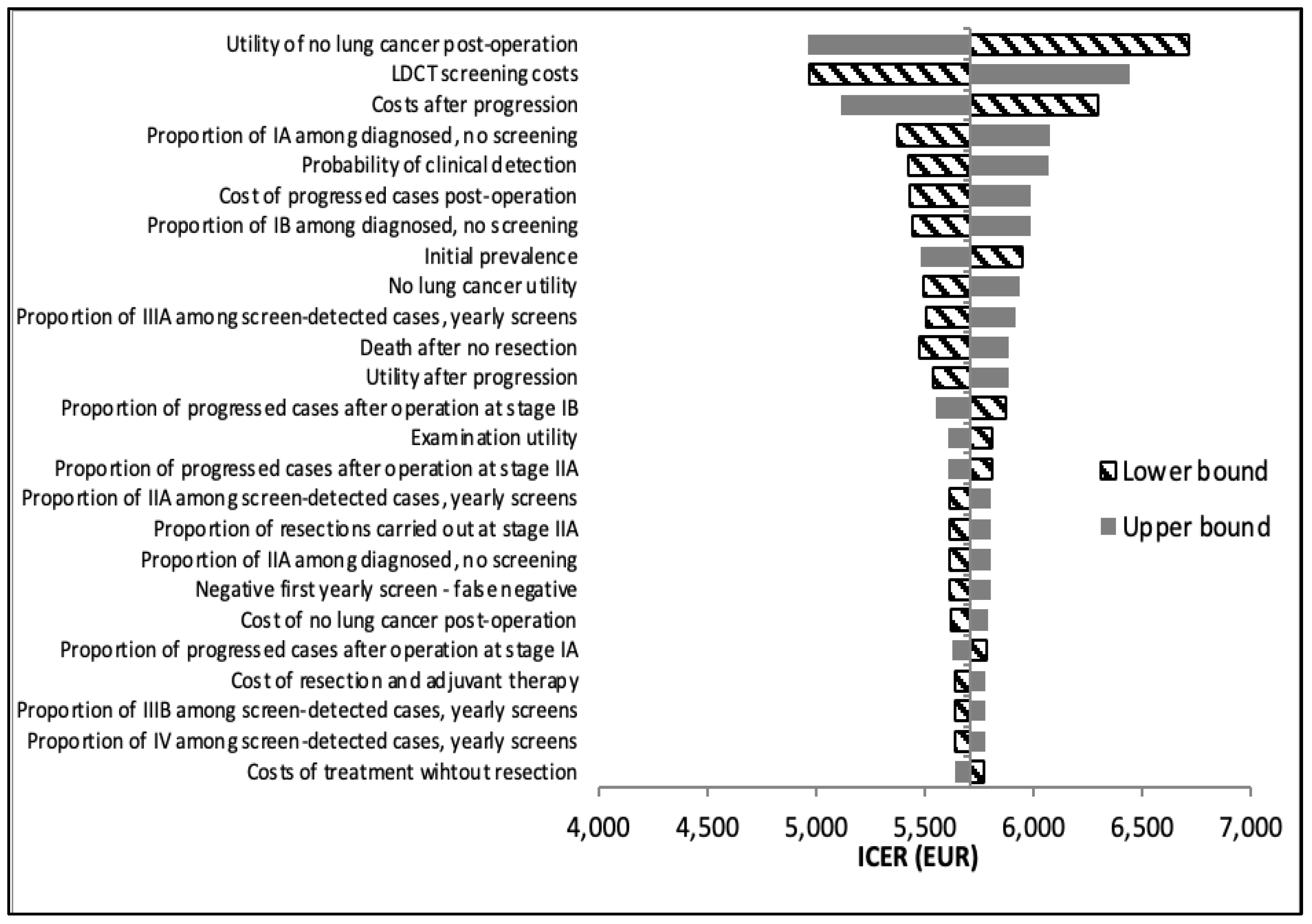
Figure 3). The Hungarian model demonstrated the same most influential parameters when compared to no screening, apart from the utility of not having lung cancer and utility of diagnostic examinations, which were the 9th and 14th most influential, respectively (
Figure 4). Rather, the probability of clinical detection was in the top influential parameters in the Hungarian model only.
3.5. Stratified Analysis by Age
Stratified analysis of age bands (
Table 7) demonstrated that when selectively screening ages 55-64, there is a QALY gain of 0.803 and cost decrease of 173 €, yielding cost-savings when compared to the base case cohort of 55-74 years. Selectively screening ages 55-69 also demonstrated a QALY gain and cost savings, but to a lesser extent than the 55-64 age group. We see a switch in the age bands 60-74 and 65-74, where there are less QALYs and more costs compared to the base case. Screening a younger subset of the population was deemed more efficient than screening the 55-74 year-old population.
4. Discussion
In this study, we explore the economic impact of applying the US (NLST) protocol for lung cancer screening in the Hungarian healthcare setting compared to the currently applied European (NELSON) protocol. A fundamental difference between the two programs is the way uncertain cases are handled. The NELSON protocol assigns uncertain cases to an indeterminate health state and repeats LDCT screening before undergoing a final diagnosis. In contrast, the NLST protocol directs uncertain cases straight through the standard process of lung cancer diagnosis (termed “Examination tunnel” in our analysis).
Our results demonstrate that assigning any suspicious LDCT screen as a positive result (US model) rather than indeterminate (Hungarian model) can yield a slight QALY gain that is worth the additional use of resources according to Hungary’s willingness to pay threshold. In other words, the Hungarian health system may benefit by forgoing a repeat LDCT screen at three months and instead jumping to a more advanced diagnostic test such as a CT scan or a bronchoscopy. However, it is important to note that compared to no screening, both the NLST and the NELSON screening protocols were deemed cost-effective in the Hungarian setting.
Applicable to the Hungarian model only, patients with an indeterminate screen have a utility decrement of 0.03 while waiting for their repeat LDCT. This follows the logic that the state of uncertainty associated with waiting an additional three months for final screening results is less desirable than receiving an initial, definitive result [
23,
28]. Patients in the US model do not experience this utility loss, as they receive a confirmed positive or negative result in the initial screening round. Therefore, as there is no follow-up LDCT screen in the US model, the elimination of uncertainty largely explains the QALY gain demonstrated in favor of the US model.
National screening programs have been implemented due to the importance of early detection in treating and surviving lung cancer [
29]. It is assumed that in our study’s US model, lung cancer can be diagnosed earlier, as suspicious or uncertain cases immediately undergo diagnostic examinations (abdominal or chest CT, bronchoscopy, or biopsy). In terms of diagnostic timelines, the US model has an approximately three-month advantage over the Hungarian model due to not requiring a repeat LDCT screen for indeterminate cases. Treatment can therefore begin earlier in the US case, potentially increasing chances of survival [
30]. More patients in the US model are thus captured in the “Examination tunnel” and undergo advanced diagnostic examinations. Our deterministic sensitivity analysis validates this (
Figure 3), as the utility of advanced diagnostic examinations was among the top influential parameters in the US model only. This translates to increased spending necessitated by the NLST protocol, as evidenced by the increased average treatment cost in the US model. However, our results demonstrate that the slight overall increase in total average lifetime costs is worth it, i.e. the cost of gaining one QALY is below Hungary’s societal willingness to pay threshold.
It is also important to consider minimizing resource overutilization and unnecessary patient radiation, as overdiagnosis and high false-positive rates are often a point of criticism in routine LDCT screening [
31,
32]. Overdiagnosis rates in the US model increased in comparison to the Hungarian model, which is expected given the higher positive rate among first screens. Notable in the input tables (
Table 1 and
Table 2) is also the greater rate of false-positive cases in the US model compared to the Hungarian model (96.25% vs 51.02% in the baseline year and 97.57% vs 52.67% in year 1), due to the lack of an indeterminate screening result in the US model. In line with these findings, our deterministic sensitivity analysis shows that the probability of clinical detection is a top parameter for only the Hungarian model. This confirms that clinical detection, i.e., cases caught without screening, plays a larger role in lung cancer diagnosis in the Hungarian protocol than in the US, influenced by the lower positive rate in the Hungarian model. Identification of appropriate target groups for screening is an important step in limiting overdiagnosis and false-positives.
Hungary and the rest of Europe examine nodule volume and volume doubling-time for diagnosing suspicious nodules, and studies have shown that these volume-based methods have higher predictive values and reduced false-positive rates compared to diameter-based methods [
11,
33]. Throughout the NLST, however, a diameter-based analysis for nodule diagnosis was employed, and the US continues to use this method today. Therefore, the method of diagnosis may also be partially responsible for the high false-positive rates observed in the NLST trial. Ongoing studies are evaluating the impact of the US transitioning to volume-based diagnosis [
34,
35]. If false-positive rates can be reduced by changing to the volume-based diagnostic method, the cost-effectiveness of LDCT screening under US screening protocols may be even greater than what we demonstrate in this study.
Our model accounted for initial incidence and mortality data specific to various age groups, allowing us to compare their LDCT screening outcomes and costs. Stratified analysis shows that a more targeted approach to screening based on age decreases costs incurred and increases QALY gain. Even if false-positive rates are lower for the older age bands (mainly due to higher incidence of lung cancer), this population’s detection has less gains as their lifespans are shorter and they receive less benefit from treatment [
36]. These results are in line with published literature suggesting that the value of routine LDCT screening may begin decreasing over 65 years of age [
37], and here we demonstrate the decreased economic value, too.
While we were able to stratify based on age, incidence and mortality data, for other factors known to influence lung cancer outcomes, including COPD, status as a current versus former smoker, and number of pack years smoked [
38,
39], were not included in the model and thus we were unable to stratify based on these factors. A future version of the model can account for these risk factors to comprehensively evaluate cost-effectiveness for various cohorts. Furthermore, risk prediction models for LDCT screening can allow for personalization to individual needs, as is being assessed in the 4-IN-THE-LONG-RUN trial [
40].
Lastly, the NLST trial was conducted from 2002 to 2004 and set the framework for a more robust national screening program in the US. In 2014, the Lung CT Screening and Reporting Data System (Lung-RADS) was implemented to guide physicians and standardize the lung cancer screening process in the US [
41]. This includes recommendations for follow-up screening tailored to nodule size and growth over time. Therefore, we expect to see an improvement in false-positive and overdiagnosis rates today in the US. However, it is important to reflect on the NLST itself, as the trial continues to be a guiding force internationally for the many countries just now implementing their own trials and screening programs.
It is important to emphasize that our results reflect the healthcare setting of Hungary, with Hungarian healthcare costs and utilities applicable to the Hungarian population. While we demonstrate that a less conservative and more rapid diagnostic strategy to screening can be cost-effective for Hungary, this may not be the case for all countries. Individual countries should assess what works best within the capacities of their own healthcare infrastructures and budget as well as their populations’ needs. Nevertheless, our study provides insight on the cost-effectiveness, advantages, and disadvantages of two influential screening trials and can be used as a resource for other countries beginning their national screening programs.
5. Conclusions
Employing an appropriate lung cancer screening strategy is imperative to maximizing clinical and cost-effectiveness. Our results demonstrate the cost-effectiveness of accelerating screening timelines while minimizing patient uncertainty in Hungary. However, balancing these benefits with the drawbacks of overdiagnosis and resource overutilization remains a challenge. As screening with low-dose computed tomography continues to demonstrate reductions in lung cancer mortality, focus should shift toward comparing the clinical efficacy and economic impact of various methods in individual healthcare settings.
Author Contributions
Conceptualization, T.R., B.N., L.S., D.G., and Z.V.; methodology, T.R., B.N., L.S., D.G., and Z.V. ; model creation, B.N., L.S., D.G., and M.T.; data curation, T.R.; validation, T.R., B.N., L.S., and M.T.; formal analysis and interpretation, T.R., B.N., M.T., L.S., and Z.V.; investigation, T.R., B.N., and L.S.; writing—original draft preparation, T.R. and B.N.; writing—review and editing, T.R., B.N., and Z.V.; supervision, B.N. and Z.V.; funding acquisition, T.R. All authors have read and agreed to the published version of the manuscript.
Funding
Tanya Rajabi received funding from the Fulbright U.S. Student Program and the Hungarian Fulbright Commission.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data from the National Lung Screening Trial can be requested from the National Institute of Health Cancer Access System at
https://cdas.cancer.gov/nlst/.
Acknowledgments
Tanya Rajabi gratefully acknowledges the support for this research by the Fulbright U.S. Student Program and Hungarian Fulbright Commission. The authors also thank the National Cancer Institute for access to the data collected by the NLST. The contents contained herein are solely those of the authors and do not represent the official views of the above organizations.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cancer Stat Facts: Common Cancer Sites. Available online: https://seer.cancer.gov/statfacts/html/common.html. (accessed on 21 May 2024).
- Bogos, K.; Kiss, Z.; Galffy, G.; Tamasi, L.; Ostoros, G.; Muller, V.; Urban, L.; Bittner, N.; Sarosi, V.; Vastag, A.; et al. Revising Incidence and Mortality of Lung Cancer in Central Europe: An Epidemiology Review From Hungary. Front Oncol 2019, 9, 1051. [Google Scholar] [CrossRef] [PubMed]
- Babar, L.; Modi, P.; Anjum, F. Lung Cancer Screening. In StatPearls; Treasure Island (FL), 2024.
- Lung Cancer Key Findings. Available online: https://www.lung.org/research/state-of-lung-cancer/key-findings. (accessed on 21 May 2024).
- National Lung Screening Trial Research, T.; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011, 365, 395–409. [Google Scholar] [CrossRef]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med 2020, 382, 503–513. [Google Scholar] [CrossRef]
- Kerpel-Fronius, A.; Monostori, Z.; Kovacs, G.; Ostoros, G.; Horvath, I.; Solymosi, D.; Pipek, O.; Szatmari, F.; Kovacs, A.; Markoczy, Z.; et al. Nationwide lung cancer screening with low-dose computed tomography: implementation and first results of the HUNCHEST screening program. Eur Radiol 2022, 32, 4457–4467. [Google Scholar] [CrossRef] [PubMed]
- Nagy, B.; Szilberhorn, L.; Gyorbiro, D.M.; Moizs, M.; Bajzik, G.; Kerpel-Fronius, A.; Voko, Z. Shall We Screen Lung Cancer With Low-Dose Computed Tomography? Cost-Effectiveness in Hungary. Value Health Reg Issues 2023, 34, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Criss, S.D.; Cao, P.; Bastani, M.; Ten Haaf, K.; Chen, Y.; Sheehan, D.F.; Blom, E.F.; Toumazis, I.; Jeon, J.; de Koning, H.J.; et al. Cost-Effectiveness Analysis of Lung Cancer Screening in the United States: A Comparative Modeling Study. Ann Intern Med 2019, 171, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Toumazis, I.; de Nijs, K.; Cao, P.; Bastani, M.; Munshi, V.; Ten Haaf, K.; Jeon, J.; Gazelle, G.S.; Feuer, E.J.; de Koning, H.J.; et al. Cost-effectiveness Evaluation of the 2021 US Preventive Services Task Force Recommendation for Lung Cancer Screening. JAMA Oncol 2021, 7, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Heuvelmans, M.A.; Oudkerk, M. Volume versus diameter assessment of small pulmonary nodules in CT lung cancer screening. Transl Lung Cancer Res 2017, 6, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Horeweg, N.; Scholten, E.T.; de Jong, P.A.; van der Aalst, C.M.; Weenink, C.; Lammers, J.W.; Nackaerts, K.; Vliegenthart, R.; ten Haaf, K.; Yousaf-Khan, U.A.; et al. Detection of lung cancer through low-dose CT screening (NELSON): a prespecified analysis of screening test performance and interval cancers. Lancet Oncol 2014, 15, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Horeweg, N.; van der Aalst, C.M.; Thunnissen, E.; Nackaerts, K.; Weenink, C.; Groen, H.J.; Lammers, J.W.; Aerts, J.G.; Scholten, E.T.; van Rosmalen, J.; et al. Characteristics of lung cancers detected by computer tomography screening in the randomized NELSON trial. Am J Respir Crit Care Med 2013, 187, 848–854. [Google Scholar] [CrossRef]
- Horeweg, N.; van Rosmalen, J.; Heuvelmans, M.A.; van der Aalst, C.M.; Vliegenthart, R.; Scholten, E.T.; ten Haaf, K.; Nackaerts, K.; Lammers, J.W.; Weenink, C.; et al. Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncol 2014, 15, 1332–1341. [Google Scholar] [CrossRef]
- Central Statistics Office [Központi Statisztikai Hivatal]. Health Survey 2009 [Egészségfelmérés (ELEF), 2009]. Statisztikai tükör. 2010;4:4.
- Central Statistics Office [Központi Statisztikai Hivatal]. Population of Hungary by sex and age [Magyarország népességének száma nemek és életkor szerint]. Available online: https://www.ksh.hu/interaktiv/korfak/orszag.html (accessed on 21 May 2024).
- National Cancer Institute Cancer Data Access System. Available online: https://cdas.cancer.gov/nlst/ (accessed on 21 May 2024).
- Kelsey, C.R.; Marks, L.B.; Hollis, D.; Hubbs, J.L.; Ready, N.E.; D'Amico, T.A.; Boyd, J.A. Local recurrence after surgery for early stage lung cancer: an 11-year experience with 975 patients. Cancer 2009, 115, 5218–5227. [Google Scholar] [CrossRef]
- Affidea. Cost of Low-dose Chest CT [CT Mellkas natív vizsgálat - csökkentett sugárterheléssel]. Available online: https://www.affidea.hu/privat-arlista/. (accessed on 3 August 2021).
- Medicover. Cost of Low-dose Chest CT [Melkas natív vizsgálat]. Available online: https://medicover.hu/arlista/szakrendelesek-arlista/#ct-vizsgalat. (accessed on 3 August 2021).
- NEFMI. 11/2012. (II. 28.) NEFMI Decree on anti-cancer therapies financed from the Health Insurance Fund according to homogeneous disease groups 959A-L and 9511-9515 [11/2012. (II. 28.) NEFMI rendelet az Egészségbiztosítási Alapból a 959A-L, valamint 9511- 9515 homogén betegségcsoportok szerint finanszírozott daganatellenes terápiákról]. 2012.
- In Self-Reported Population Health: An International Perspective based on EQ-5D, Szende, A., Janssen, B., Cabases, J., Eds.; Dordrecht (NL), 2014.
- Papatheofanis, F.J. Utility evaluations for Markov states of lung cancer for PET-based disease management. Q J Nucl Med 2000, 44, 186–190. [Google Scholar] [PubMed]
- Mahadevia, P.J.; Fleisher, L.A.; Frick, K.D.; Eng, J.; Goodman, S.N.; Powe, N.R. Lung cancer screening with helical computed tomography in older adult smokers: a decision and cost-effectiveness analysis. JAMA 2003, 289, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Human Capacities. Professional guideline of Ministry of Human Capacities on conducting health-economic analyses [Az Emberi Erıforrások Minisztériuma szakmai irányelve az egészség-gazdaságtani elemzések készítéséhez és értékeléséhez]. Egészségügyi Közlöny. 2021;5(21).
- European Central Bank. Available online: https://www.ecb.europa.eu/stats/policy_and_exchange_rates/euro_reference_exchange_rates/html/eurofxref-graph-huf.en.html (accessed on 21 May 2024).
- Patz, E.F., Jr.; Pinsky, P.; Gatsonis, C.; Sicks, J.D.; Kramer, B.S.; Tammemagi, M.C.; Chiles, C.; Black, W.C.; Aberle, D.R.; Team, N.O.M.W. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med 2014, 174, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Damhus, C.S.; Quentin, J.G.; Malmqvist, J.; Siersma, V.; Brodersen, J. Psychosocial consequences of a three-month follow-up after receiving an abnormal lung cancer CT-screening result: A longitudinal survey. Lung Cancer 2021, 155, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, H.L.; Heuvelmans, M.A.; Oudkerk, M. Low-dose computed tomography lung cancer screening: Clinical evidence and implementation research. J Intern Med 2022, 292, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Schabath, M.B.; Cote, M.L. Cancer Progress and Priorities: Lung Cancer. Cancer Epidemiol Biomarkers Prev 2019, 28, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Pinsky, P.F. Assessing the benefits and harms of low-dose computed tomography screening for lung cancer. Lung Cancer Manag 2014, 3, 491–498. [Google Scholar] [CrossRef]
- Yankelevitz, D.F.; Henschke, C.I. Overdiagnosis in lung cancer screening. Transl Lung Cancer Res 2021, 10, 1136–1140. [Google Scholar] [CrossRef]
- Heuvelmans, M.A.; Walter, J.E.; Vliegenthart, R.; van Ooijen, P.M.A.; De Bock, G.H.; de Koning, H.J.; Oudkerk, M. Disagreement of diameter and volume measurements for pulmonary nodule size estimation in CT lung cancer screening. Thorax 2018, 73, 779–781. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, A.; van Ginneken, B.; Nair, A.; Baldwin, D. Use of Volumetry for Lung Nodule Management: Theory and Practice. Radiology 2017, 284, 630–644. [Google Scholar] [CrossRef] [PubMed]
- Revel, M.P.; Bissery, A.; Bienvenu, M.; Aycard, L.; Lefort, C.; Frija, G. Are two-dimensional CT measurements of small noncalcified pulmonary nodules reliable? Radiology 2004, 231, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Presley, C.J.; Reynolds, C.H.; Langer, C.J. Caring for the Older Population With Advanced Lung Cancer. Am Soc Clin Oncol Educ Book 2017, 37, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Pinsky, P.F.; Gierada, D.S.; Hocking, W.; Patz, E.F., Jr.; Kramer, B.S. National Lung Screening Trial findings by age: Medicare-eligible versus under-65 population. Ann Intern Med 2014, 161, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Durham, A.L.; Adcock, I.M. The relationship between COPD and lung cancer. Lung Cancer 2015, 90, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Tindle, H.A.; Stevenson Duncan, M.; Greevy, R.A.; Vasan, R.S.; Kundu, S.; Massion, P.P.; Freiberg, M.S. Lifetime Smoking History and Risk of Lung Cancer: Results From the Framingham Heart Study. J Natl Cancer Inst 2018, 110, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Ten Haaf, K.; van der Aalst, C.M.; de Koning, H.J.; Kaaks, R.; Tammemagi, M.C. Personalising lung cancer screening: An overview of risk-stratification opportunities and challenges. Int J Cancer 2021, 149, 250–263. [Google Scholar] [CrossRef]
- Pinsky, P.F.; Gierada, D.S.; Black, W.; Munden, R.; Nath, H.; Aberle, D.; Kazerooni, E. Performance of Lung-RADS in the National Lung Screening Trial: a retrospective assessment. Ann Intern Med 2015, 162, 485–491. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).