1. Introduction
Intraoperative fluid management is crucial for maintaining adequate organ perfusion, with hypovolemia and excessive fluid administration posing potential complications [
1]. The predominant approach to fluid management involves monitoring parameters such as heart rate (HR), mean arterial pressure (MAP), and central venous pressure (CVP). Nevertheless, clinical research suggests that changes in MAP are inadequate for assessing stroke volume (SV) and cardiac output (CO), and that CVP measurement alone is insufficient for predicting fluid responsiveness [
2,
3]. An alternative method is goal-directed fluid management (GDFM), which entails tailored fluid administration utilizing both static parameters (HR, CVP, etc.) and dynamic indicators: stroke volume variation (PPV), arterial pulse pressure variation (PPV) [
4], systolic pressure variation (SPV), and plethysmographic waveform variation (PWI). In humane medicine, PPV during mechanical ventilation stands out as a robust tool for guiding volume therapy [
5,
6]. Respiratory variations in pulse oximeter waveform and pulse reliably predicted fluid responsiveness [
7,
8]. The pleth variability index (PVi), a dynamic variable that automatically and continuously measures the respiratory variations in the pulse oximeter waveform amplitude, is as effective as stroke volume variation in predicting fluid responsiveness [
9]. However, it remains unclear whether optimizing intraoperative PVi improves fluid management and circulation. Technologies like Masimo’s Signal Extraction Technology (SET) offer tools like the PVi to monitor respiratory variations in ventricular preload. PVi, calculated based on the perfusion index (Pi), provides continuous insights into circulatory perfusion and helps clinicians optimize fluid management.
In veterinary medicine, a fixed fluid rate may not suit all patients due to varied fluid needs based on preoperative conditions, clinical context (dehydration, hemorrhage, congestive heart failure, chronic renal failure), and surgical blood loss [
10,
11]. Exploring whether PVi can assess fluid status in anesthetized veterinary patients becomes essential. PVi demonstrates accuracy in discriminating between fluid responders (patients that respond positively to fluid loading by increasing their CO/SV by 10-15%) [
12] and non-responders, aiding in fluid optimization and hypotension prevention. Hypotension in anesthetized patients can be predicted early through PVi, allowing for precautionary measures [
13,
14] Yoshioka et al., 2011. However, even with normal values of MAP and HR, dogs under general anesthesia can exhibit preload dependency when subjected to a small-volume fluid challenge (FC) [
15]. This condition can be anticipated through the assessment of dynamic preload parameters, with PPV and PVi proving more accurate than SPV and SVV. It is noteworthy that MAP is an inadequate predictor of fluid responsiveness (FR), and preload dependency may not necessarily be linked to hypotension. The PVi higher than 14% demonstrated to be a cut-off value for discriminating fluid responders from non-responders in dogs [
15].
The use of PVi to guide fluid therapy has not been explored extensively in dogs so far. The aim of the study was to compare the use of PVi to guide the rate of intra-operative fluid therapy with conventional fluid management (CFM) in ASA 1-2 dogs undergoing surgery. The cut-off value of PVi distinguished dogs requiring increased fluid intake from those in normofluidic conditions. Dynamic adjustments to the fluid rate throughout the surgical procedure, guided by PVi, are hypothesized to prevent or reduce the need for bolus administration and potentially decrease hypotensive events. End points of the study were: total amount fluid administered, incidence of cardiovascular instability and use of cardiovascular supportive drugs.
2. Materials and Methods
This prospective, randomized, multicenter clinical study was approved by the Ethical Committee of the Clinical and Zootechnical Studies in Animals of the Department of Precision and Regenerative Medicine and Jonic Area of the University of Bari, Italy (Prot. n. 1082 III/13), and it was conducted in two centers: University of Bari (Bari, Italy), University of Bologna (Bologna, Italy). This article is reported in accordance with the Consolidated Standard of Reporting Trials (CONSORT) Statement for the reporting of randomized controlled trials [
16].
2.1. Animals
After the written owner consent, the study included dogs undergoing general anesthesia for surgical procedures. The inclusion criteria were as follows: bodyweight > 6 kg, ASA status 1 or 2 (based on history, physical examination and a complete blood count) [
17], surgical procedures were involved the limbs, abdomen, skin and eyes. Exclusion criteria were: patients affected by any other major diseases, MAP lower than 65 mmHg at the beginning of the study, thoracic surgical procedures, major cardiovascular and respiratory diseases, pregnant females, duration of anesthesia shorter than 30 minutes, impossibility to collect the data required.
Dogs were admitted to the clinic two hours before general anesthesia and housed individually in separate cages. Solid food was withheld for 6 hours, and water for 2 hours before the surgery.
2.2. Anesthetic Management
Dogs were premedicated with a different protocol (alfa-2 and/or opioids) chosen by the anesthetist before the procedure. After aseptic preparation, a catheter was inserted into a cephalic vein and general anesthesia was induced with intravenous (IV) propofol (Proposure 10 mg mL–1; Merial) administered to effect after at least two minutes of preoxygenation via facemask. The trachea was intubated with an appropriately sized cuffed endotracheal tube and connected to a circular breathing system. Anesthesia was maintained with isoflurane [end-tidal isoflurane percentage (EtISO) between 1.1 – 1.4%; [
18]] in oxygen (FiO
2 > 0.8). All animals received lactated Ringer’s solution (B.Braun, Ringer Lattato, B.Braun, Italy) infusion during the procedure at a rate based on the study protocol. Antibiotic therapy and anti-inflammatory therapy according to the surgical procedure were administered.
During anesthesia, dogs were ventilated in volume-controlled mode, using a tidal volume (TV) of 12 mL kg–1 with an inspiratory to expiratory ratio of 1:2, 25% end-inspiratory pause. Plateau airway pressure was maintained between 8 and 10 cmH2O adjusting the TV. Respiratory frequency was titrated based on the end-tidal CO2 (EtCO2) targeting a value between 35 and 45 mmHg, obtaining an adequate level of anesthesia (absence of palpebral reflex, ventral–medial rotation of the eye, and loss of the jaw tone) and confirming via pressure/volume loop and capnography observation that there was no patient/ventilator asynchrony.
Monitoring during general anesthesia included peripheral hemoglobin oxygen saturation (SpO2; %) assessed with a pulse oximetry probe on the tongue, heart rate (HR; beats/minute) obtained from lead II electrocardiography, non-invasive arterial blood pressure (systolic, diastolic, and mean arterial blood pressure; SAP, DAP, and MAP, respectively; mmHg) using an oscillometric method (Suntech® Vet20, USA), core body temperature (T; ºC) monitored through an esophageal probe, respiratory rate (RR), EtCO2 and FiO2, using a multiparametric monitor, PVi and PI was assessed using Massimo rainbow SET® pulse oximeter. At the procedure’s end, isoflurane or sevoflurane administration was stopped, and dogs were disconnected from the circuit once thoracic expansion and oxygen saturation were satisfactory. The orotracheal tube was removed upon the return of the swallowing reflex.
2.3. Study Protocol
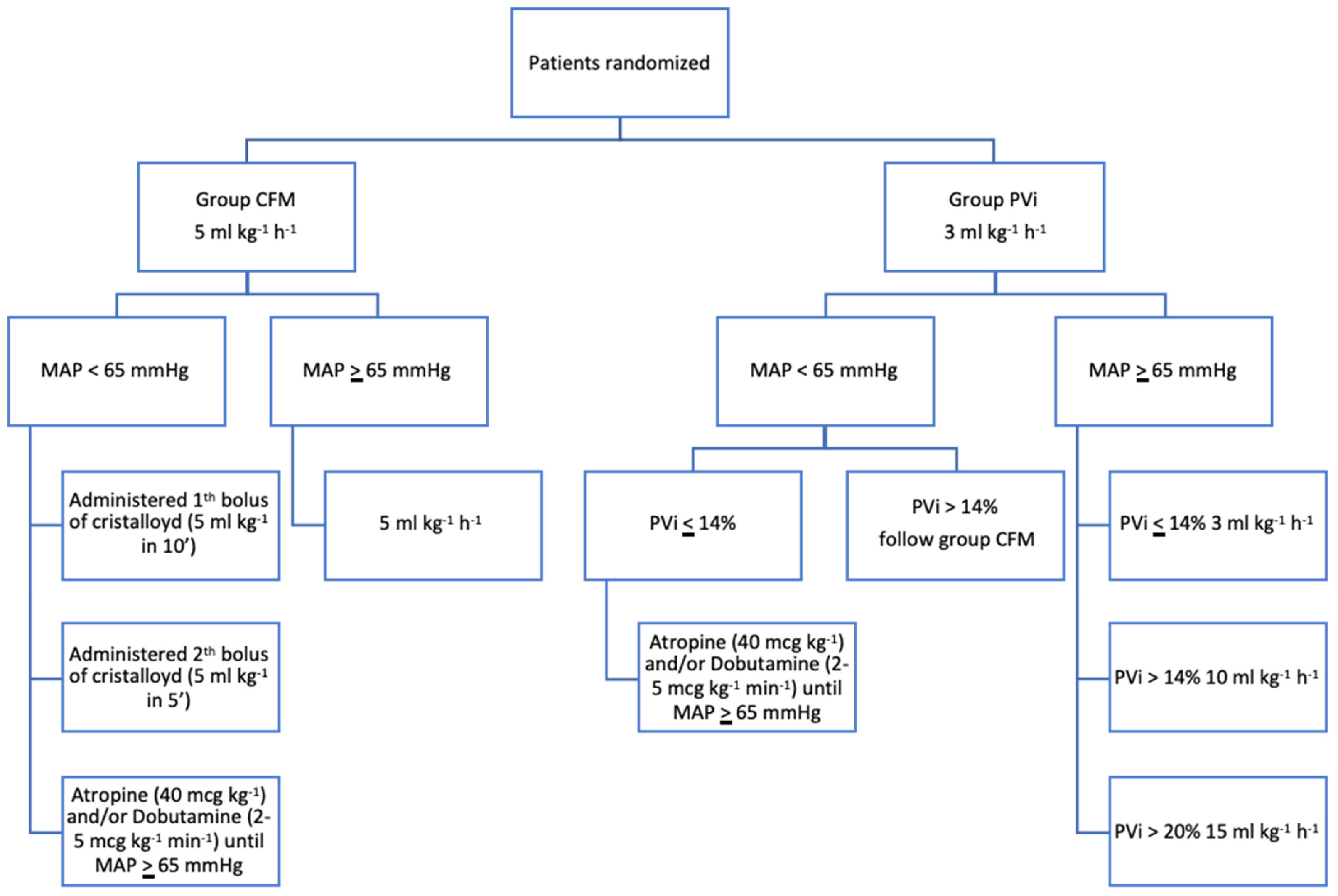
The dogs were randomized in two groups: classical fluid management (CFM) and PVi-guided fluid therapy (PVi), utilizing a sequence generator available at
http://www.random.org/.
For the study hypotension was defined as a condition in which the MAP was below 65 mmHg.
Fluids rate was started at 5 ml kg-1 h-1 since the induction of anesthesia, thereafter at the time of the beginning of the study the rate was adjusted in each dog based on the study group
In the CFM group, the fluid rate was maintained at 5 ml kg-1 h-1 throughout the procedure. In case of hypotension, one bolus of crystalloid at 5 ml kg-1 in 10 min was administered IV, in case of persistence of hypotension a second bolus of fluid was administered in 5 minutes. Thereafter, in cases of persistence of hypotension a constant rate infusion of dobutamine (2-5 mcg kg-1 min-1) was administered until MAP become higher than 65 mmHg. In case of bradycardia atropine (40 mcg kg-1 IV) was administered.
In the PVi group, a pulse oximetry probe (LNCS TC-I®; Masimo Corp., USA) was placed to the tongue for all patients. It was securely wrapped to prevent interference from external light sources. The pulse oximeter was connected to the Masimo Radical 7 monitor (Masimo SET; Masimo Corp., USA), which included the PVi software (version 7.0.3.3). PVi automatically and continuously calculates respiratory variations in the photoplethysmogram using data obtained non-invasively from a pulse oximetry sensor. PVi reflects the amplitude of the pulse oximeter waveform and is computed by indexing the pulsatile infrared signal (AC or variable component) against the non-pulsatile infrared signal (DC or constant component).
The calculation of PVi involves Perfusion Index (PI)
The Perfusion Index depicts the status of peripheral perfusion and gives information about strength of pulse signal at the site of measurement, it has a wide range (0.02% to 20%).
After the stabilization of mechanical ventilation, Ringer Lactate infusion was adjusted to 3 ml kg
-1 h
-1 in the patients of this group. In case of patient was not hypotensive and the PVi was higher than 14% for more than 5 min, fluid rate was increased to 10 ml kg h. If the PVi was higher than 20% fluid rate further increased to 15 ml kg
-1 h
-1. If PVi was less than or equal to 14 % fluid rate was reestablished at 3 ml kg h. In case of hypotension if PVi was less than or equal to 14 % constant rate infusion of dobutamine (2-5 mcg kg
-1 min
-1) was administered until MAP become higher than 65 mmHg, with concurrent bradycardia intravenous bolus of atropine (40 mcg kg
-1) was administered directly; with PVi over 14% was followed the same protocol used in CFM-group (
Figure 1).
In each group the infusion of dobutamine was stopped when MAP was higher than 80 mmHg. In case of MAP > 100 mmHg, the study was interrupted, and cases were managed according to clinical judgment.
The time of the end of the study was recorded and identified after the end surgery before starting the weaning of the dog from the MV.
The physiological parameters were collected every five minutes for the entire duration of the study. For data anaylsis were considered only the parameters recorded at the beginning (T0) the midle (T1/2) and the end (Tend) of the study. The number of hypotensive episodes and the total amount of christalloids, dobutamine and atropine administered during the study time (T0 - Tend), were recorded in each case. The total amount of fluids was normalised for BW and time of infusion to determine the average fluids rate (ml kg-1 min-1) for each group. The same was computed for dobutamine and atropine. Moreover the duration of surgery and anesthesia was also recorded.
2.4. Statistical Analysis
The statistical analysis was carried out with MedCalc 12.7.0.0 software. Normal distribution of the data was confirmed using the D’Agostino test. For all data, the mean/median, standard deviation (SD) and 95% confidence intervals (CI) were calculated. Data regarding age, bodyweight, total fluid amount, average fluid rate, and anesthesia duration were compared between groups with the one-way ANOVA test. The values of HR, SAP, MAP, DAP, RR, T, PVi, PI and SpO2 were analyzed by two-ways ANOVA test (time and treatment), using the value obtained at T0, T1/2 and Tend. The Tukay test was used for post-hoc analysis. The incidence of hypotension was compared between the groups with Chi-squared test. A P value < 0.05 was considered statistically significant.
3. Results
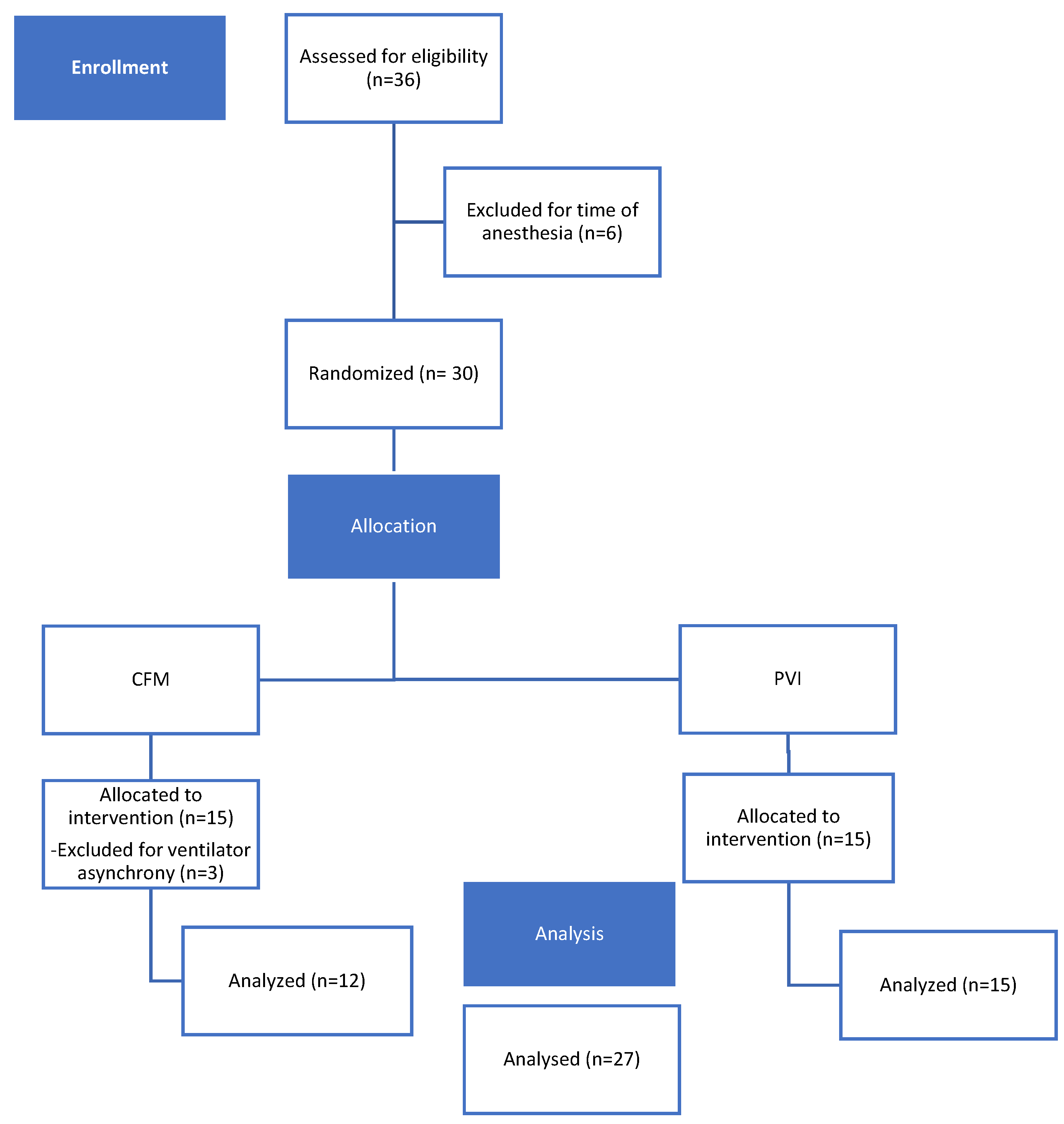
Thirty-six dogs were assessed for eligibility, in the center number 1 and in the center number 2. Six cases were excluded as they did not match the inclusion criteria (duration of anesthesia). Of the 30 dogs enrolled in the study, three were excluded because asynchrony with the ventilator. Twenty-seven dogs completed the study without any complications. The details regarding the allocation of dogs into the two groups is shown in
Figure 2.
The demographic and preoperative characteristics were similar in both groups (
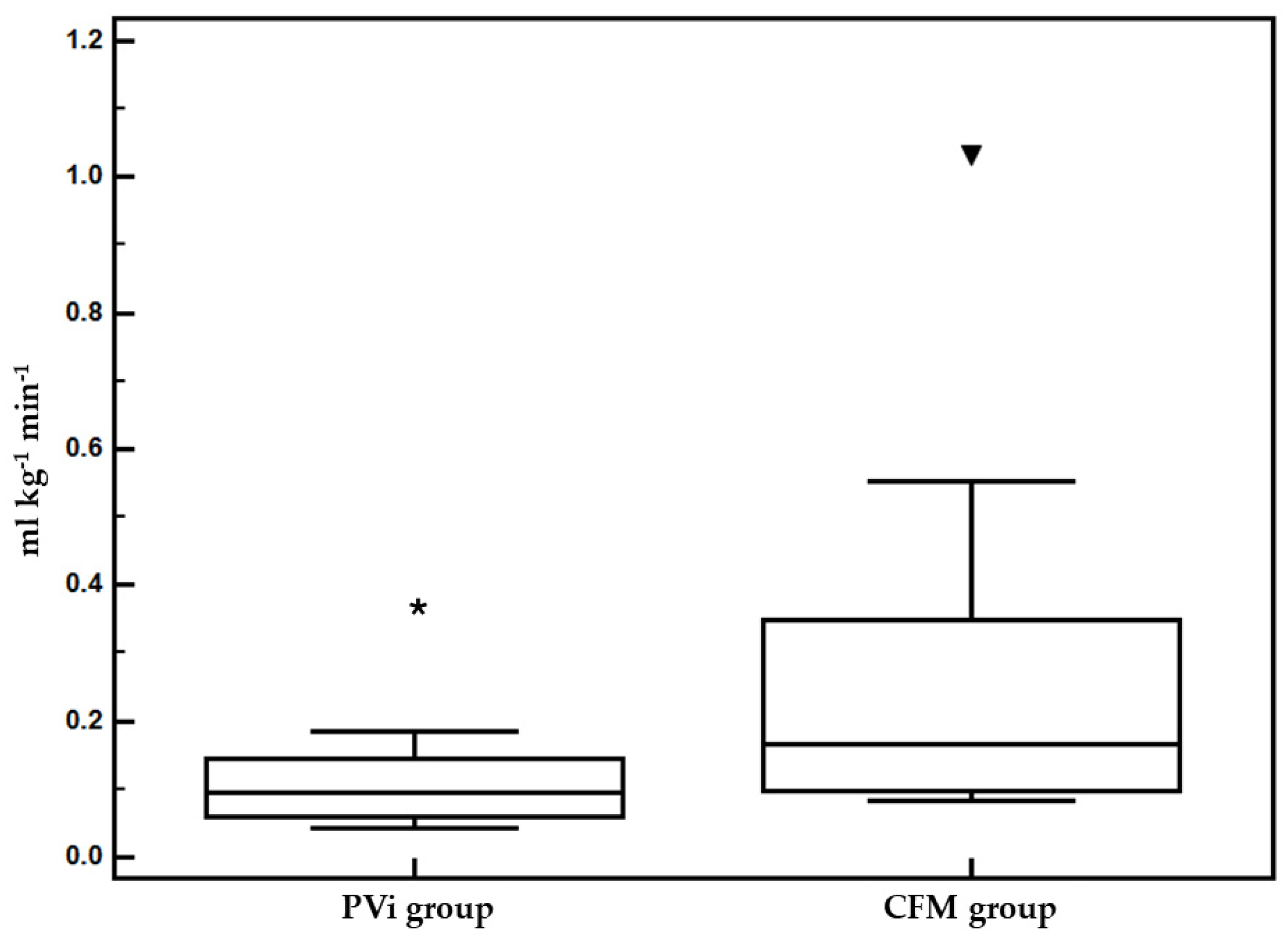
Table 1). The durations of anesthesia and surgery were similar as well. The average fluid rate was statistically significantly higher in CFM group (0.132 ± 0.115 ml kg
-1 min
-1) compared to the PVi group (0.056 ± 0.27 ml kg
-1 min
-1) during the study time (T0 – Tend) (
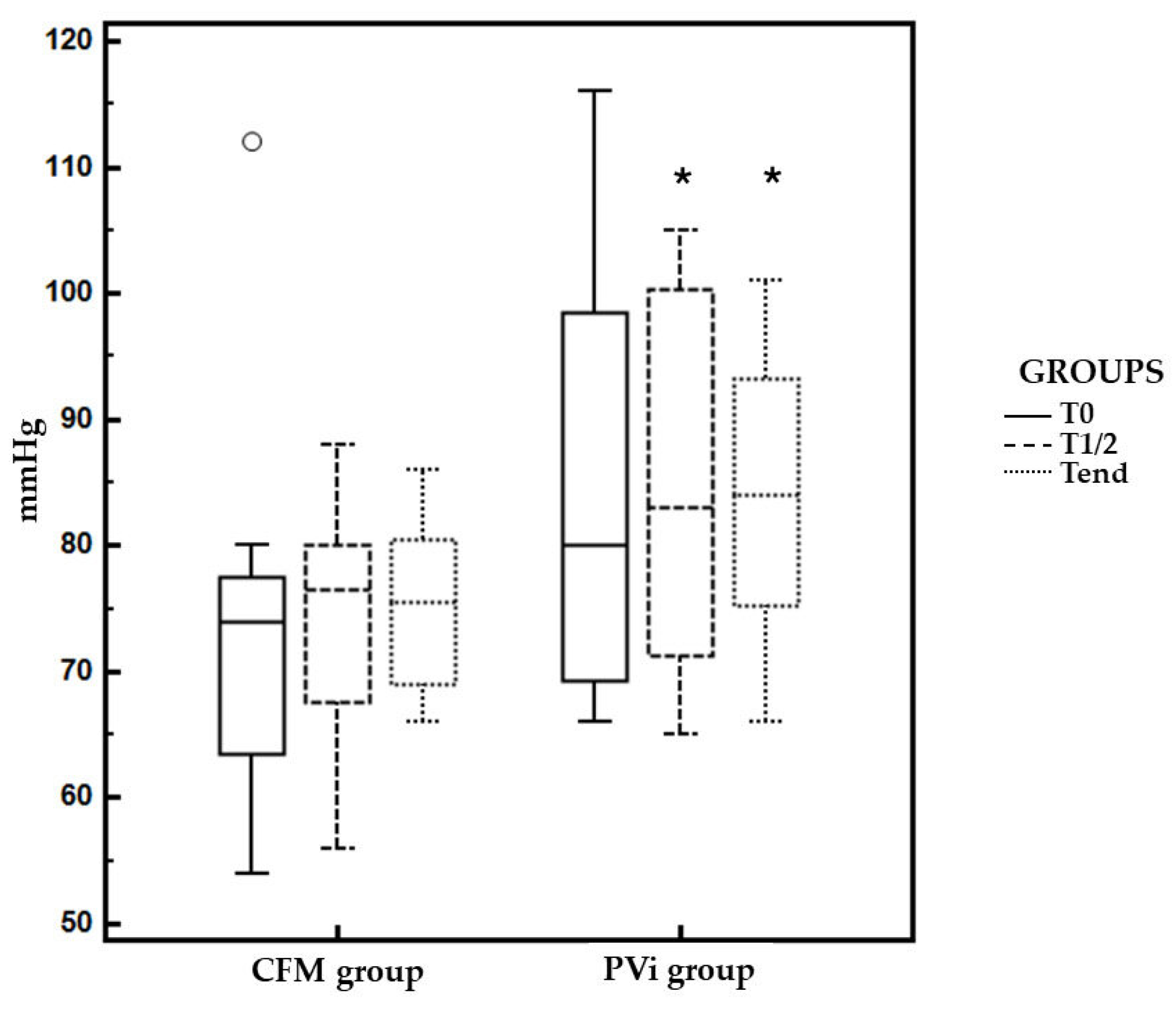
Figure 3). The duration of anesthesia, surgery, PVi study and value of MAP at T0 were similar in both groups (
Table 2). Frequency of hypotension in CFM group was 41.6%, while in PVi group was 0% (p = 0.023). MAP was significantly higher at T1/2 and Tend in the PVi group compared to the CFM (p < 0.05) (
Table 3). Dobutamine and atropine were not use in both groups.
4. Discussion
The results of the present study showed that PVI-based intraoperative fluid therapy is able to provide better hemodynamic conditions (lower incidence of hypotension and higher MAP values) with lower average total fluid administration compared to traditional fluid management.
Fluid management during anesthesia and surgery remains a highly controversial topic. Perioperative morbidity has been associated with the amount of intravenous fluid administered, with both inadequate and, more commonly, excessive fluid administration leading to an increase in postoperative complications [
19]. Intraoperative fluid administration based on a generalized equation that relies on body weight per unit time and modified based on specific requirements of the animals and the surgical procedure [
20] is not supported by known physiologic principles.
Insufficient fluid therapy can lead to various perioperative complications, including low blood pressure, acute kidney injury, irregular heartbeats and tissue hypoperfusion/ischemia. Conversely, excessive fluid intake may result in prolonged need for mechanical ventilation, delayed healing of wounds, increased risk of infection, and longer hospital stays due to fluid overload [
21,
22].
Several variables could impact the cardiovascular status of a dog during general anesthesia, such as the types of anesthetic agents administered, the positioning of the body and the specific surgical and anesthetic techniques employed [
23,
24]. In this study, the administration of propofol and isoflurane might have caused vasodilation, potentially resulting in a slight reduction in stressed venous blood volume. Consequently, this could lead to a decrease in SV and cardiac index (CI), shifting the heart towards the steeper segment of the Frank-Starling curve. Additionally, the use of intermittent positive pressure ventilation (IPPV) could exacerbate this condition [
25,
26]. Suboptimal SV and CI values are likely responsible for inadequate oxygen delivery (DO
2), as suggested by previous human studies. Optimizing DO
2 during surgery has been proposed to enhance patient outcomes [
27].
Therefore, it could be hypothesized that monitoring dynamic preload indices during anesthesia might provide prevention of hemodynamic instability, even in cases not associated with alterations in classical parameters (e.g., MAP and HR), and could be a valid tool to guide fluid therapy [
26,
28].
MAP is usually considered the reference hemodynamic parameter during anesthesia, with the goal of maintaining an adequate hemodynamic state. It is the main cardiovascular parameter that guides fluid therapy and the use of sympathomimetic drugs. However, it is well known that this parameter has several limitations in terms of providing an effective hemodynamic picture of the patient [
31]. A recent study in dogs proved that MAP has the lowest accuracy in predicting fluid responsiveness in dogs as compared to other dynamic hemodynamic parameters (SVV, PPV, PVi) in dogs [
15]. The compensatory mechanisms related to myocardial contractility and peripheral resistances may compensate for MAP even in patients that have a fluid deficit (absolute or relative) [
32].
The preliminary results of this study demonstrate that PVI monitoring may be helpful in identifying patients in need of fluid to prevent the occurrence of hemodynamic instability. In addition, its dynamic nature makes it an ideal tool for adjusting the fluid rate based on patient and/or surgical needs. Using a multimodal approach to hemodynamic monitoring may help to prevent complications related to both hypotension and inappropriate fluid management. In our study the PVi approach resulted in no hypotensive events, in contrast, in the CFM group, hypotension occurred in 41% of cases, requiring additional fluid administration and resulting in a higher average fluid rate. This result may have a very important impact on the clinical management of fluids in the perioperative period. The reference values of PVi used in this study were taken from Skouropoulou et al. where 14% was identified as the cutoff value for fluid responsiveness [
15]. In addition, the limit of 20% of PVi to increase the fluid rate was arbitrary chosen considering the direct correlation observed between PVi and the entity of responsiveness (variation of SV) in dogs without any major systemic disease. Indeed, we may suppose different thresholds in dogs with cardiovascular or renal pathologies. MAP was significantly higher during surgery in the PVi group, further supporting the effectiveness of PVi-guided fluid therapy in maintaining adequate blood pressure.
Among all other dynamic hemodynamic parameters, PVi is by far the less invasive and more convenient in terms of cost [
33]. In addition, several veterinary and human studies have demonstrated its accuracy compared to invasive methods [
15]. PVi is only valid during mechanical ventilation and therefore cannot be used in spontaneously breathing cases. The PVi requires a minimum of 1% of the PI to produce a reliable value, which may be the main limitation of this technology [
34], so in dogs with low peripheral perfusion or minimal changes in pulse volume might be less accurate than other hemodynamic indices such as SVV and PPV.
In humane medicine, the use of PVi for goal-directed fluid therapy (GDFT) management has been investigated in several studies. PVi seems to guide GDFT similarly to PPV regarding hospital length of stay, amount of fluid, and incidence of postoperative complications [
35]. Our results are consistent with a recent study in pediatric patients with a protocol very similar to this study [
36].
This study has some limitations: its preliminary nature and the small sample size, necessitating larger-scale studies to confirm the results. The study focused on ASA 1-2 dogs undergoing surgery, limiting applicability to more diverse patient populations. The duration of anesthesia and surgery varied among patients, which could affect fluid requirements and hemodynamic stability. Furthermore, the premedication protocol was not standardized, which could have resulted in different hemodynamic effects depending on the molecules used.
5. Conclusion
The preliminary findings of this study support the potential utility of PVi-guided fluid therapy in dogs undergoing general anesthesia. Larger, multicenter trials are warranted to validate these findings and explore the broader applicability of PVi in clinical practice. Further research should also investigate the optimal PVi thresholds and fluid management protocols to maximize patient outcomes while minimizing complications.
In summary, the study sheds light on the potential benefits of incorporating dynamic indicators like PVi into intraoperative fluid management strategies, paving the way for more individualized and effective patient care in veterinary anesthesia.
Author Contributions
Conceptualization, F.S. and C.V.; methodology, C.V., N.R. and F.S.; software, C.V. and N.R.; validation, C.V., N.R., M.S., C.L., C.P., A.F., A.C., L.L. and F.S.; formal analysis, C.V., F.S., M.S. and C.P.; investigation, C.V., N.R., C.L., F.S., A.F., A.C., L.L. and F.S.; resources, F.S. and A.C.; data curation, F.S., C.V., M.S. and C.P.; writing—original draft preparation, C.V., F.S. and N.R.; writing—review and editing, M.S., C.L. and C.P.; visualization, C.L., C.P. and M.S.; supervision, N.R. and F.S.; project administration, F.S. and N.R.; funding acquisition, F.S. and N.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by the Ethical Committee of the Clinical and Zootechnical Studies in Animals of the Department of Emergency and Organ Transplantation of the University of Bari, Italy (n. D.E.T.O.), and it was conducted in two centers: University of Bari (Bari, Italy), University of Bologna (Bologna, Italy). This article is reported in accordance with the Consolidated Standard of Reporting Trials (CONSORT) Statement for the reporting of randomized controlled trials.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available contacting the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Al-Ghamdi, A. Intraoperative Fluid Management: Past and Future, Where Is the Evidence? Saudi J Anaesth 2018, 12, 311. [CrossRef]
- Le Manach, Y.; Hofer, C.K.; Lehot, J.-J.; Vallet, B.; Goarin, J.-P.; Tavernier, B.; Cannesson, M. Can Changes in Arterial Pressure Be Used to Detect Changes in Cardiac Output during Volume Expansion in the Perioperative Period? Anesthesiology 2012, 117, 1165–1174. [CrossRef]
- Marik, P.E.; Cavallazzi, R. Does the Central Venous Pressure Predict Fluid Responsiveness? An Updated Meta-Analysis and a Plea for Some Common Sense*: Critical Care Medicine 2013, 41, 1774–1781. [CrossRef]
- McDermid, R.C. Controversies in Fluid Therapy: Type, Dose and Toxicity. WJCCM 2014, 3, 24. [CrossRef]
- Hofer, C.K.; Cannesson, M. Monitoring Fluid Responsiveness. Acta Anaesthesiologica Taiwanica 2011, 49, 59–65. [CrossRef]
- Pinsky, M.R. Heart???Lung Interactions: Current Opinion in Critical Care 2007, 13, 528–531. [CrossRef]
- Cannesson, M.; Attof, Y.; Rosamel, P.; Desebbe, O.; Joseph, P.; Metton, O.; Bastien, O.; Lehot, J.-J. Respiratory Variations in Pulse Oximetry Plethysmographic Waveform Amplitude to Predict Fluid Responsiveness in the Operating Room. Anesthesiology 2007, 106, 1105–1111. [CrossRef]
- Desebbe, O.; Cannesson, M. Using Ventilation-Induced Plethysmographic Variations to Optimize Patient Fluid Status. Current Opinion in Anaesthesiology 2008, 21, 772–778. [CrossRef]
- Zimmermann, M.; Feibicke, T.; Keyl, C.; Prasser, C.; Moritz, S.; Graf, B.M.; Wiesenack, C. Accuracy of Stroke Volume Variation Compared with Pleth Variability Index to Predict Fluid Responsiveness in Mechanically Ventilated Patients Undergoing Major Surgery: European Journal of Anaesthesiology 2010, 27, 555–561. [CrossRef]
- Michard, F.; Giglio, M.T.; Brienza, N. Perioperative Goal-Directed Therapy with Uncalibrated Pulse Contour Methods: Impact on Fluid Management and Postoperative Outcome. British Journal of Anaesthesia 2017, 119, 22–30. [CrossRef]
- Davis, H.; Jensen, T.; Johnson, A.; Knowles, P.; Meyer, R.; Rucinsky, R.; Shafford, H. 2013 AAHA/AAFP Fluid Therapy Guidelines for Dogs and Cats*. Journal of the American Animal Hospital Association 2013, 49, 149–159. [CrossRef]
- Mohamed, Z.U.; Mullenheim, J.W. Predicting Fluid Responsiveness. Trends in Anaesthesia and Critical Care 2012, 2, 15–19. [CrossRef]
- Forget, P.; Lois, F.; De Kock, M. Goal-Directed Fluid Management Based on the Pulse Oximeter–Derived Pleth Variability Index Reduces Lactate Levels and Improves Fluid Management. Anesthesia & Analgesia 2010, 111, 910–914. [CrossRef]
- Tsuchiya, M.; Yamada, T.; Asada, A. Pleth Variability Index Predicts Hypotension during Anesthesia Induction. Acta Anaesthesiol Scand 2010, 54, 596–602. [CrossRef]
- Skouropoulou, D.; Lacitignola, L.; Di Bella, C.; Stabile, M.; Acquafredda, C.; Brienza, N.; Grasso, S.; Crovace, A.; Iarussi, F.; Staffieri, F. Intraoperative Assessment of Fluid Responsiveness in Normotensive Dogs under Isoflurane Anaesthesia. Veterinary Sciences 2021, 8, 26. [CrossRef]
- Dwan, K.; Li, T.; Altman, D.G.; Elbourne, D. CONSORT 2010 Statement: Extension to Randomised Crossover Trials. BMJ 2019, l4378. [CrossRef]
- Portier, K.; Ida, K.K. The ASA Physical Status Classification: What Is the Evidence for Recommending Its Use in Veterinary Anesthesia?—A Systematic Review. Front. Vet. Sci. 2018, 5, 204. [CrossRef]
- Reed, R.; Doherty, T. Minimum Alveolar Concentration: Key Concepts and a Review of Its Pharmacological Reduction in Dogs. Part 1. Research in Veterinary Science 2018, 117, 266–270. [CrossRef]
- Doherty, M.; Buggy, D.J. Intraoperative Fluids: How Much Is Too Much? British Journal of Anaesthesia 2012, 109, 69–79. [CrossRef]
- Holte, K.; Sharrock, N.E.; Kehlet, H. Pathophysiology and Clinical Implications of Perioperative Fluid Excess. British Journal of Anaesthesia 2002, 89, 622–632. [CrossRef]
- Chong, M.A.; Wang, Y.; Berbenetz, N.M.; McConachie, I. Does Goal-Directed Haemodynamic and Fluid Therapy Improve Peri-Operative Outcomes?: A Systematic Review and Meta-Analysis. European Journal of Anaesthesiology 2018, 35, 469–483. [CrossRef]
- Alimian, M.; Mohseni, M.; Moradi Moghadam, O.; Seyed Siamdoust, S.A.; Moazzami, J. Effects of Liberal Versus Restrictive Fluid Therapy on Renal Function Indices in Laparoscopic Bariatric Surgery. Anesth Pain Med 2020, 10. [CrossRef]
- Noel-Morgan, J.; Muir, W.W. Anesthesia-Associated Relative Hypovolemia: Mechanisms, Monitoring, and Treatment Considerations. Front. Vet. Sci. 2018, 5, 53. [CrossRef]
- Fantoni, D.; Shih, A.C. Perioperative Fluid Therapy. Veterinary Clinics of North America: Small Animal Practice 2017, 47, 423–434. [CrossRef]
- Bundgaard-Nielsen, M.; Jørgensen, C.C.; Secher, N.H.; Kehlet, H. Functional Intravascular Volume Deficit in Patients before Surgery. Acta Anaesthesiol Scand 2010, 54, 464–469. [CrossRef]
- Bennett, V.; Cecconi, M. Perioperative Fluid Management: From Physiology to Improving Clinical Outcomes. Indian J Anaesth 2017, 61, 614. [CrossRef]
- Miller, T.E.; Roche, A.M.; Mythen, M. Fluid Management and Goal-Directed Therapy as an Adjunct to Enhanced Recovery After Surgery (ERAS). Can J Anesth/J Can Anesth 2015, 62, 158–168. [CrossRef]
- Aditianingsih, D.; George, Y.W.H. Guiding Principles of Fluid and Volume Therapy. Best Practice & Research Clinical Anaesthesiology 2014, 28, 249–260. [CrossRef]
- Cecconi, M.; Parsons, A.K.; Rhodes, A. What Is a Fluid Challenge?: Current Opinion in Critical Care 2011, 17, 290–295. [CrossRef]
- Araos, J.; Kenny, J.-E.S.; Rousseau-Blass, F.; Pang, D.Sj. Dynamic Prediction of Fluid Responsiveness during Positive Pressure Ventilation: A Review of the Physiology Underlying Heart–Lung Interactions and a Critical Interpretation. Veterinary Anaesthesia and Analgesia 2020, 47, 3–14. [CrossRef]
- Sedgwick, S.; Lorenzutti, A.M.; Araos, J.B.; Gleed, R.D.; Martin-Flores, M. Evaluation of an Oscillometric Blood Pressure Monitor in Anesthetized Dogs: Agreement with Direct Measurements and Ability to Detect Hypotension. Research in Veterinary Science 2021, 135, 162–166. [CrossRef]
- Fine, D.M.; Durham Jr, H.E.; Rossi, N.F.; Spier, A.W.; Selting, K.; Rubin, L.J. Echocardiographic Assessment of Hemodynamic Changes Produced by Two Methods of Inducing Fluid Deficit in Dogs. Journal of Veterinary Internal Medicine 2010, 24, 348–353. [CrossRef]
- Urhan, G.; Demirel, İ.; Deniz, A.; Aksu, A.; Altun, A.Y.; Bolat, E.; Beştaş, A.; Altuntaş, G. Comparison of Dynamic Measures in Intraoperative Goal-Directed Fluid Therapy of Patients with Morbid Obesity Undergoing Laparoscopic Sleeve Gastrectomy. OBES SURG 2024, 34, 1600–1607. [CrossRef]
- Cannesson, M.; Desebbe, O.; Rosamel, P.; Delannoy, B.; Robin, J.; Bastien, O.; Lehot, J.-J. Pleth Variability Index to Monitor the Respiratory Variations in the Pulse Oximeter Plethysmographic Waveform Amplitude and Predict Fluid Responsiveness in the Operating Theatre. British Journal of Anaesthesia 2008, 101, 200–206. [CrossRef]
- Coeckelenbergh, S.; Delaporte, A.; Ghoundiwal, D.; Bidgoli, J.; Fils, J.-F.; Schmartz, D.; Van Der Linden, P. Pleth Variability Index versus Pulse Pressure Variation for Intraoperative Goal-Directed Fluid Therapy in Patients Undergoing Low-to-Moderate Risk Abdominal Surgery: A Randomized Controlled Trial. BMC Anesthesiol 2019, 19, 34. [CrossRef]
- Mathew, P.J.; Sharma, S.; Bhardwaj, N.; Ashok, V.; Malik, M.A. Goal-Directed Fluid Therapy Guided by Plethysmographic Variability Index (PVI) versus Conventional Liberal Fluid Administration in Children during Elective Abdominal Surgery: A Randomized Controlled Trial. Journal of Pediatric Surgery 2023, 58, 735–740. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).