1. Introduction
Aflatoxins are highly toxic secondary metabolites produced primarily by certain species of fungi, particularly Aspergillus flavus and Aspergillus parasiticus. These fungi commonly contaminate agricultural commodities such as maize, peanuts, cottonseed, and tree nuts, especially under conditions of high temperature and humidity. Aflatoxins alone contaminateone-fourth of the world’s food grain (Chatterjee et al., 2023). There are several types of aflatoxins, with aflatoxin B1 being the most potent carcinogen. Consumption of aflatoxin-contaminated food and feed poses significant health risks, including hepatotoxicity, carcinogenicity, immunosuppression, and growth impairment in humans and animals (Bennett and Klich, 2003) due to this, aflatoxin contamination is a significant concern in global trade. In agricultural settings, aflatoxin contamination can lead to economic losses due to rejected crops, reduced market value, and trade restrictions. Therefore, research efforts in molecular biology and plant biotechnology often focus on understanding the mechanisms of aflatoxin biosynthesis, developing strategies for aflatoxin management, and enhancing crop resistance to fungal contamination through breeding or biotechnological approaches.
The Food and Agriculture Organization (FAO) of the United Nations has reported that approximately 25% of crops worldwide are impacted by mycotoxins (FAO, 2017). Aflatoxins, produced by the food-borne fungi Aspergillus flavus and A. parasiticus, primarily contaminate food crops like maize, peanuts, and tree nuts in tropical and subtropical regions across the globe (Bandyopadhyay et al., 2007). These crops often face unfavourable storage conditions, leading to the accumulation of aflatoxins (Strosnider et al., 2006; Williams et al., 2004). Exposure to dietary aflatoxins is a concern for over 5 billion people worldwide (Strosnider et al., 2006). These naturally occurring mixes of aflatoxins, including aflatoxins B1, B2, G1, and G2, are known to be highly potent liver carcinogens. The International Agency for Research on Cancer has classified them as a Group 1 human carcinogen (IARC, 2002).In individuals exposed to chronic hepatitis B virus (HBV) infection and aflatoxin, the risk of developing liver cancer is significantly higher than in those exposed to either risk factor alone (Groopmanet al., 2008). The risk can be up to 30 times greater in individuals exposed to both factors and it also shows a multiplicative relationship between aflatoxin and HBV in the induction of liver cancer. As per the report of Food and Agriculture Organization (FAO, 2004) of the United Nations, more than 120 countries worldwide have established regulations regarding aflatoxins in food. This represents a 30% increase from 1995 (FAO, 1997) in terms of the number of countries with aflatoxin maximum levels (MLs). These regulations aim to safeguard human health by reducing dietary exposure to aflatoxin (FAO, 2004; Wu and Guclu, 2012). While these measures are crucial for protecting human and animal health, they can also significantly influence global food trade activities (Wu, 2004).
Mycotoxins in general or aflatoxins specifically offerdetrimental effects in two different ways. The first one iseconomic losses by virtue of production of contaminated food products while the other in the form of health implications upon consuming such contaminated foods. Such implications may arise during the course of ingestion of infected foods like food grains, milk, meat etc. The toxicity principle behind such health issues lies not only due to the parent toxin molecule i.e. aflatoxin but mainly due to its toxic metabolites such as its epoxides or diols, damaging the liver in majority of the cases but often damagingthe kidney or adrenal too. Further, such chronic exposure may lead to liver cancer, convulsions and death in severe cases (Leeson et al., 1995; Eaton and Groopman, 1994; Heathcote and Hibbert, 1978; Goldbatt, 1969).
Aflatoxins (AF) along with other mycotoxins are commonly suspected to play a crucial role in the development of oedema in malnourished people as well as in the pathogenesis of kwashiorkor in malnourished kids (Coulter et al., 1986). More than this, AF contamination negatively affects crop and animal production leading not only to natural resource waste but also decreased market value that may cause significant economic losses. Due to these impacts, several countries and some international organizations have established strict rules and regulations to manage AF contamination in food and feeds and also to prohibit the trade of contaminated products (Juan et al., 2012). The regulations on “acceptable health risk” usually depend on a country’s economic level and development, the amount of consumption of high-risk crops, and the susceptibility to contamination of crops that have to be regulated (Kendra and Dyer, 2007). The established safe limit of AFs for human consumption ranges from 4 to 30µg/kg (Mahato et al., 2019). The EU has set very strict standards, which establish that any product for direct human consumption cannot be marketed with a concentration of AF-B1 and total AFs greater than 2 µg/kg and 4 µg/kg, respectively. Likewise, US regulations have also specified the maximum acceptable limit for AFs at 20 µg/kg (Wu, 2006).
However, if the EU aflatoxin standard is adopted worldwide, developing countries of Asia and Sub-Saharan Africa will face both economic losses and additional costs related to meeting those standards. This situation requires alternative technologies at pre- and post-harvest levels that will help to minimize contamination of commercial foods and feeds, at least to ensure that AF levels remain below safe limits (Prietto et al., 2015). Implementation of innovative technologies is invaluable to address the challenges related to AFs and their effects. Reduction of AF contamination through knowledge of pre- and post-harvest management is one of the first and most important steps toward an appropriate strategy to improve agricultural production and productivity in a sustainable way. This has a direct positive effect on improving the quality and nutritional value of foods, conserving natural resources, as well as improving local and international trade by increasing competitiveness. It is important to identify and document available technologies that can effectively control and minimize aflatoxin contamination to sustain healthy living and socioeconomic development. There exists ample literature on tools for AF control and their benefits. Therefore, this review compiles data on innovative pre- and post-harvest technologies developed that can manage AF contamination in foods. The benefits of these technologies are also discussed in terms of food security, human health, and economic value. Most importantly, implications for research and management policies addressing AF issues are emphasized.
2. Global Trade Hindered by Aflatoxin
To address this issue, international organizations like the Codex Alimentarius Commission, jointly established by the Food and Agriculture Organization (FAO) and the World Health Organization (WHO), develop standards and guidelines for food safety. These standards include maximum allowable limits for aflatoxin levels in various foods and feed products, ensuring that they are safe for consumption. Additionally, the World Trade Organization (WTO) oversees trade agreements and regulations among its member countries. Aflatoxin regulations may be included in trade agreements or enforced through sanitary and phytosanitary measures to protect human, animal, or plant health. Overall, global trade regulations regarding aflatoxin aim to safeguard public health while facilitating the movement of safe agricultural products across borders (
Table 1).
- a.
Regulation and global trade
Mycotoxins are a significant category of food contaminants that are closely monitored in international trade. Aflatoxins, which have been identified as carcinogenic and immunosuppressive (Dash et al., 2007; Wild and Hall, 1999; Williams et al., 2004), are a prominent example of mycotoxins. The detrimental health effects associated with mycotoxins are the main driving force behind the increasing concerns regarding food safety among researchers and policymakers. To address the health risks posed by aflatoxins, the World Trade Organization's (WTO) Sanitary and Phytosanitary Standards (SPS) agreement empowers member countries to establish their own food standards for the protection of consumers (Yue et al., 2006). The precautionary principle, also known as the SPS policy, is the term used by the WTO to describe this approach. Over the years, food standards in developed countries have undergone changes. For instance, the European Commission has recently introduced new regulations regarding aflatoxin in imported food items (Otsuki et al., 2001a; Otsuki et al., 2001b). In the United States, the US Food and Drug Administration (US FDA) has issued a Compliance Policy Guide for Aflatoxins in Peanuts and Peanut Products, Sec. 570.375 (FDA, 1980). Similarly, in Canada, the Canadian Food Inspection Agency (CFIA) has established regulations concerning aflatoxin levels in food and feeds (CFIA, 2017). In Australia and New Zealand, the regulation of maximum levels (MLs) for aflatoxins in peanuts falls under the jurisdiction of Food Standards Australia New Zealand (FSANZ) through Schedule 19: Maximum levels of contaminants and natural toxicants (FSANZ, 2017). On the other hand, the Food Safety and Standards Authority of India (FSSAI) introduced the Food Safety and Standards (Contaminants, toxins and Residues) Regulations (F. No. 3/15015/30/2011) in 2011, which specifies MLs for aflatoxins in all foods (FSSAI, 2011).The National Health and Family Planning Commission (NHFPC) and the China Food and Drug Administration (CFDA) in China have released the National Food Safety Standard of Maximum Levels of Mycotoxin in Foods, outlining a specific limit for AFB1 in peanuts and their derivatives (USDA, 2018). Meanwhile, Japan's Food and Agricultural Materials Inspection Centre (FAMIC) has set regulatory thresholds for AFB1 in all food categories (FAMIC, 2011). Furthermore, MERCOSUR, a trade agreement comprising Argentina, Brazil, Paraguay, Uruguay, and Venezuela, has adopted uniform maximum levels for aflatoxins in peanuts and peanut products (FAO/WHO 2004).
Numerous countries have responded by implementing stringent legal limits on Aflatoxin in various food products. In 2006, the European Commission established the maximum allowable level of Aflatoxin B1 (AFB1) at 2.0 mg/kg for grain and grain products, and at 0.1 mg/kg for processed cereal-based baby foods (European Commission, 2006). Conversely, the Food and Drug Administration (FDA) in the United States of America (USA) tolerates higher levels of AFB1, allowing up to 20 mg/kg. Additionally, the FDA has set the maximum level of Aflatoxin M1 (AFM1) in milk at 0.5 mg/kg (FDA, 2000). The implementation of strict regulations on trading practices, food monitoring, and storage methods in industrialized nations has proven successful in reducing the health risks associated with Aflatoxin (Brown et al., 1999).
- b.
Legislation in feed and feed ingredients
Mycotoxins are estimated to affect approximately 25% of the world's food crops (Kumar et al., 2008). Aflatoxin, a major contaminant, has been found in maize, wheat, rice, barley, and oilseeds such as peanut, sunflower, soybeans, mustard, cottonseed, and their products in many countries across Asia, Latin America, and sub-Saharan Africa (Filazi and Sireli, 2013; Lee et al., 2015). The impact of this contamination can result in significant economic losses for developing nations, particularly due to the lack of drying equipment and high humidity conditions. Aflatoxin B1, among various types of aflatoxins, is considered the most dangerous toxin, with the upper safe limit regulated by BIS at a narrow margin of 20 ppb in most animal feedstuffs (BIS, 2021). Aflatoxin-M1, a type of aflatoxin, can be transmitted from the feed consumed by ruminant animals to their milk, posing a significant risk to human health (FSSAI, 2020). The presence of aflatoxin is not limited to milk alone; it has also been found in other dairy products, eggs, and edible animal products. This has led to the development of regulations aimed at minimizing aflatoxin exposure in food animals. Surprisingly, aflatoxin has even been detected in pasteurized ultra-high temperature milk, broiler chicken meat, eggs, and sausage (Siddappa et al., 2012; Shaltout et al., 2014). Although the financial impact of aflatoxin contamination in animal feeds is not specifically determined in India, the U.S. corn industry in the southern region experiences considerable annual losses estimated at US$ 1.68 billion due to climatic conditions (Mitchell et al., 2016). The presence of aflatoxin and ochratoxin, natural contaminants commonly found in animal feeds, can have a significant negative impact on animal production, leading to substantial economic losses (Battacone et al., 2010). Among these contaminants, aflatoxin poses the greatest risk to human health when it enters the food or feed chain. The exposure to mycotoxins can vary significantly depending on various factors, including the source of contamination, the type of food, storage conditions, and climatic factors (Sanders et al., 1984). It is crucial to carefully evaluate the consumption of maize, cereals, peanuts, and milk through the feed-food carryover, as these commodities are widely consumed globally and contribute to elevated levels of aflatoxin exposure in humans (Wild & Gong, 2010).A significant concern has arisen in Africa's dairy sector regarding the contamination of milk with Aflatoxin M1 (AFM1) through animal feeds that are contaminated with Aflatoxin B1 (Abebe et al., 2020; Mulunda et al., 2013; Voth-Gaeddert et al., 2019). AFM1 is a metabolite of Aflatoxin B1 (AFB1), which is a potent hepatocarcinogen produced by Aspergillus fungi found in certain dairy animal feed ingredients. While the consumption of high-quality dairy products could potentially address Africa's malnutrition problems, the presence of contaminated milk may undermine these benefits and even have more severe consequences on child morbidity or mortality (Grace et al., 2020; SahaTurna et al., 2023; SahaTurna and Wu, 2022).
The U.S. Food and Drug Administration (FDA, 2019) has established the acceptable threshold for aflatoxin M1 (AFM1) in milk and other dairy products at 0.50 μg/L. Additionally, the maximum level of total aflatoxins (AFB1+AFB2+AFG1+AFG2) in feed ingredients provided to dairy animals and foods intended for human consumption is set at 20 μg/kg (FDA, 2019). On the other hand, the European Commission has implemented strict regulations, setting an action level of 0.05 μg/L for AFM1 in liquid milk. Furthermore, the limits for AFB1 are 20 μg/kg in all feedstuffs, 10 μg/kg in complete feeds, and 5 μg/kg in complete feeds for dairy animals (European Commission, 2006). In India, the Bureau of Indian Standards (BIS) has established a maximum permissible level of 20 μg/kg or 20 ppb for aflatoxin B1 in all animal feeds. However, the Food Safety and Standards Authority of India (FSSAI, 2020) recommends a maximum permissible limit of 10 μg/kg for aflatoxin B1 in ready-to-eat oilseeds and oils and 0.5 μg/kg for aflatoxin M1 in milk intended for human consumption.
Iran and the United States dominate the global pistachio market, with a combined contribution of over 70% to the world's pistachio exports. According to FAOSTAT (2011), Iran accounts for 47% of the exports, while the US contributes 25%. However, pistachios also pose a significant risk in terms of dietary aflatoxin exposure. They are responsible for 7-45% of the total aflatoxin exposure from all sources for humans, as stated by JECFA (2007). This has led to several instances of aflatoxin contamination in pistachios, causing trade disruptions. In 1997, the European Union imposed a ban on pistachio imports from Iran due to high aflatoxin levels ranging from 11-400 ng/g in consignments intended for European import. The United Kingdom also called for a reinstatement of the ban in 2002, as over 10% of sampled consignments were found to be contaminated. More recently, in 2010, the US implemented a ban on all Iranian pistachios, as reported by JECFA (2007).
Aflatoxin, known for its hepatotoxic and immunosuppressive properties, is classified as a group-1 carcinogen, leading to strict regulations on both agricultural goods and animal products. The levels of aflatoxin M1 in dairy items and aflatoxin B1 in eggs and meat are influenced by the contamination levels in animal feed. Aflatoxin M1 has been found in human breast milk and animals exposed to contaminated feed. The transfer rate of aflatoxin M1 ranges from 0.8-6.5% based on species, milk production, and lactation stage (Churchill, 2017). High levels of aflatoxins have also been detected in sausages and various meat products worldwide (Aziz and Youssef, 1991; Shaltout et al., 2014).
- c.
Regulation and control
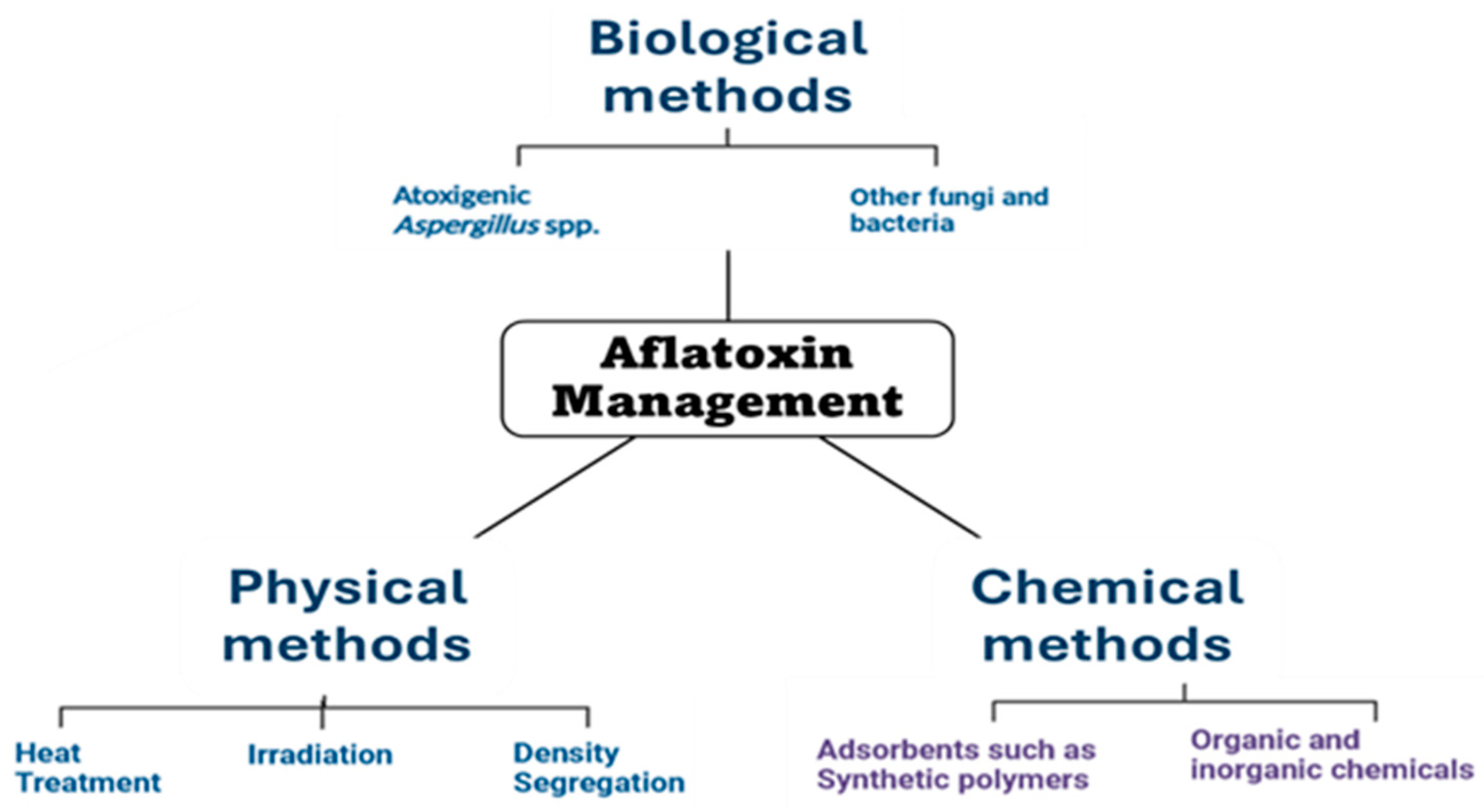
Governments and international organizations establish regulations and guidelines to safeguard public health by limiting the levels of aflatoxin in food and feed. These regulations often include maximum allowable limits (MRLs) for different aflatoxins in various foods and feed commodities. For example, the European Union has established strict MRLs for aflatoxins in foods such as nuts, dried fruits, and cereals. Prevention is a key aspect of aflatoxin control. Agricultural practices such as crop rotation, proper storage facilities, timely harvesting, and use of resistant crop varieties can help reduce fungal contamination and aflatoxin production in the field. Regular monitoring and surveillance programs are conducted to assess the levels of aflatoxin contamination in food and feed. Sampling and analysis are carried out using validated methods to ensure accurate detection and quantification of aflatoxin. If aflatoxin contamination is detected above regulatory limits, various control measures may be implemented. These can include sorting and cleaning of contaminated grains, use of chemical and biological agents to inhibit fungal growth, and application of physical methods such as irradiation or thermal treatments to reduce aflatoxin levels. Educating farmers, food producers, and consumers about the risks associated with aflatoxin contamination is crucial for effective control. Training programs, informational campaigns, and dissemination of best practices can help stakeholders understand how to prevent, detect, and manage contamination throughout the food supply chain.
- d.
Risk assessment
Understanding the framework used for aflatoxin risk assessment is equally important, which involves hazard identification, exposure assessment, dose-response assessment, and risk characterization. Recognizing the various pathways through which humans and animals are exposed to aflatoxin, including ingestion of contaminated food and feed, inhalation of fungal spores, and dermal exposure are the keys to assessing the risk. Gaining insights into the adverse health effects associated with aflatoxin exposure, including acute toxicity, carcinogenicity, immunosuppression, reproductive disorders, and developmental abnormalities may give an idea of the potentiality of the risk emanating from consumption of the aflatoxin. Vulnerability of different population groups to aflatoxin exposure, such as infants, children, pregnant women, immunocompromised individuals, and livestock species may also be taken into consideration. Learning about the international regulatory standards and guidelines established by organizations such as the Codex Alimentarius Commission, the World Health Organization (WHO), and the Food and Agriculture Organization (FAO) to ensure food safety and protect public health is necessary. One has to understand the sources of uncertainty and variability in aflatoxin risk assessment, including variability in toxin levels, exposure patterns, susceptibility factors, and data limitations. Risk management options available for mitigating aflatoxin contamination in food and feed, including good agricultural practices (GAPs), hazard analysis and critical control points (HACCP), maximum allowable limits (MRLs), and post-harvest interventions may be explored. Developing a comprehensive understanding of aflatoxin risk assessment and its role in setting international regulatory standards to safeguard food safety and public health is, therefore, crucial.
- e.
Legislative framework
The legislative framework for food safety has evolved over centuries, with scientific advancements leading to formal regulations aimed at protecting consumers from chemical and microbiological hazards. Natural toxins, especially mycotoxins like aflatoxins, are among the most regulated contaminants globally. Initially, only a few developed countries had such regulations, but by 2003, around 100 countries had adopted specific guidelines, covering about 85% of the world's population (Van Egmond and Jonker, 2004). The international trade in food and feed has driven the need for consistent global standards to ensure consumer safety and fair trade, with the food industry valued at approximately 2000 billion dollars annually.
The Codex Alimentarius Commission (CAC), a joint effort of the FAO and WHO, develops international food standards, including for contaminants in food and feed (Eskola et al., 2020). The Codex Committee for Contaminants in Foods (CCCF) manages risk by setting maximum permitted levels (MLs), prioritizing risk assessments, and creating sampling and analysis methods. The Joint FAO/WHO Expert Committee on Food Additives (JECFA) conducts scientific evaluations to inform these decisions. The Codex General Standard for Contaminants and Toxins in Food and Feed outlines principles for setting MLs to protect consumers without creating trade barriers. These standards are integrated into the WTO’s Agreement on Sanitary and Phytosanitary Measures, aiding in trade dispute resolution. Specific limits for aflatoxins in various commodities, including peanuts, are provided, along with sampling plans.
In the European Union, the legislation for food safety, including contaminant limits, follows principles defined in Council Regulation (EEC) No 315/93. The European Commission (EC) sets maximum limits for contaminants and specifies detection and sampling methods. Risk management is handled by the Directorate-General for Health and Food Safety (DG SANTE). The European Food Safety Authority (EFSA) now conducts risk assessments, a role previously held by the Scientific Committee on Food. EFSA's various panels, like the CONTAM Panel, evaluate toxicological data and methods for contaminants in food and feed. Initial regulations, such as Commission Regulation (EC) No 1525/98, were updated by Regulation (EC) No 466/2001 and later by Regulation (EC) No 1881/2006 to align with new developments and Codex guidelines (Eskola et al., 2018).
- f.
Global health impact, economic risks and vulnerable regions
Governments and international organizations establish regulatory standards and quality control measures to limit aflatoxin contamination in food and feed. Maximum allowable limits (MRLs) are set for aflatoxin levels in agricultural commodities, and food safety regulations are enforced to ensure compliance throughout the supply chain. These regulatory interventions aim to safeguard public health and maintain consumer confidence in food safety. Aflatoxin exposure poses significant health risks to both humans and animals. Acute toxicity can lead to symptoms such as vomiting, abdominal pain, and liver damage, while chronic exposure is associated with serious health conditions like liver cancer, immune suppression, and stunted growth in children. Recognizing these health impacts underscores the importance of stringent food safety regulations and public health interventions. Researchers employ various methodologies to quantify the social costs associated with fungi and aflatoxins in maize and peanuts. This involves estimating both direct costs, such as losses incurred from contaminated crops and medical expenses related to aflatoxin poisoning, and indirect costs, which encompass broader economic impacts like reduced agricultural productivity and trade disruptions due to contaminated exports.
Aflatoxins are widely recognized as the most crucial mycotoxins in terms of safeguarding human and animal health (Marshall et al., 2020). Discovered in the 1960s, aflatoxin was the first mycotoxin to be identified and remains the most extensively researched (Bennett and Klich, 2003; Richard, 2008). These toxins are known to be carcinogenic, immunogenic, and teratogenic to both humans and animals (Marshall et al., 2020), with potential impacts on immunity, fertility, and child growth (IARC, 2012a). The prevalence of chronic undernourishment affecting 821 million individuals globally (United Nations, 2019), coupled with reports linking aflatoxins to child stunting in Africa (IARC, 2015; Matacic, 2016), highlights the significant food and feed losses resulting from mycotoxin contamination, particularly in developing countries and Sub-Saharan Africa. Aflatoxins present a significant challenge when crops are stored in unfavourable conditions that promote the growth of moulds (Bennett and Klich, 2003; CAST, 2003). In farm animals, the consumption of Aflatoxin affects both growth and the immune system, leading to a strain on local meat production, the national economy, and the global food supply (Wild and Gong, 2010). The IARC (International Agency for Research on Cancer) has classified these fungal metabolites as class-1 agents due to their carcinogenic properties. Aflatoxins are known to cause cancer, especially when combined with a chronic Hepatitis B virus (HBV) infection. Although the causal relationship with Hepatitis C virus is likely, it lacks strong evidence (Wild and Montesano, 2009). Aflatoxins are statistically associated with a significant risk of developing Hepatocellular Carcinoma (HCC) (Qian et al., 1994; Ross et al., 1992). The WHO's 2017 report revealed 788,000 global deaths from HCC in 2015, making it the second leading cause of cancer-related deaths worldwide after lung cancer. In developing nations, HBV and Aflatoxin e are common risk factors for HCC (Liu and Wu, 2010). Dietary exposure to aflatoxins has a significant human health consequence, leading to hepatocellular carcinoma (HCC). This disease causes the death of about 250,000 people annually in Sub-Saharan Africa, with the high intake of aflatoxins in food and the presence of the Hepatitis B virus (HBV) being identified as key factors (Ladeira et al. 2017).A massive outbreak of hepatitis with an estimated 106 deaths of tribal people due to consumption of aflatoxin-contaminated maize was reported in 1974 in the Indian states (Gujarat and Rajasthan) (Krishnamachari et al., 1975). Most recently, aflatoxicosis outbreaks occurred in Kenya in 2004 and 2005, following the consumption of mouldy maize that had been harvested early and stored improperly (Strosnider et al., 2006). In the 2004 outbreak, the largest ever recorded, 317 people became ill and 125 died (Azziz-Baumgartner et al., 2005). Two additional human diseases have also been associated with the ingestion of food contaminated with aflatoxin. These include Kwashiorkor, a form of malnutrition commonly seen in children, and Reye's syndrome, which is characterized by encephalopathy and fatty degeneration of the organs in children. The exact cause of Reye's syndrome remains unknown (CAST 2003; et and Klich 2003).
Various symptoms of chronic aflatoxicoses have been documented in livestock, including decreased rate of weight gain in cattle, pigs, and poultry, as well as reduced feed intake and conversion in pigs and poultry. Additionally, cows may experience reduced milk yields, while poultry may suffer from immunosuppression and decreased egg production (CAST 2003; Diaz 2005; EC 1994). On the other hand, acute exposure to aflatoxins can result in liver necrosis and eventual fatality (EC 1994; CAST 2003). Nevertheless, it is the long-term exposure to these toxins in animals that poses the most significant economic impact, surpassing the consequences of acute animal mortality (CAST 2003).
Aflatoxin contamination in agricultural products has far-reaching consequences that extend beyond public health concerns. It also impacts trade and economic aspects for both developed and developing nations. For instance, maize farmers in the United States face a yearly loss of $160 million due to aflatoxin issues (Wu, 2015). In developing countries, particularly in sub-Saharan Africa, the losses are even more significant, totalling $450 million, which accounts for 38% of global agricultural losses caused by aflatoxin (Gbashi et al., 2018). Moreover, aflatoxins play a crucial role in causing a significant drop in agricultural trade between developed and developing countries (Wu, 2015). The direct economic consequences of aflatoxin pollution in crops primarily stem from a decline in marketable products due to rejection in the global market, as well as losses incurred from livestock diseases, resulting in morbidity and mortality, leading to reduced volume and value in national markets, ultimately causing substantial economic harm (Wagacha and Muthomi, 2008).
The socio-economic consequences of aflatoxin contamination extend beyond individual health impacts to affect entire communities and livelihoods, particularly in regions where maize and peanuts are staple crops. Smallholder farmers, in particular, face reduced income and food insecurity when their crops are contaminated, while trade disruptions limit market access and economic opportunities. Fungal contamination and aflatoxin presence in maize and peanuts are influenced by a range of factors including environmental conditions (such as temperature and humidity), agricultural practices (such as crop rotation and pesticide usage), and post-harvest handling and storage techniques. Understanding the dynamics of contamination is crucial for implementing effective control measures. Effective risk management strategies encompass both pre-harvest and post-harvest interventions.
3. Factors Influencing Toxicity for Aflatoxin Affecting Health
Aflatoxins are the mycotoxins that pose the most threat to food safety because of their high toxicity and widespread presence in foods and feeds. Geographically, aflatoxins are most prevalent in tropical and subtropical areas. They are contaminants of food and feed like peanuts, maize, and their derivatives Aflatoxin-related health issues have been linked since its discovery; it significantly increases the risk of certain chronic diseases that can be fatal, including aflatoxicosis outbreaks.It is now widely documented that aflatoxins induce a variety of different acute and chronic disorders, most of which are severe, even though they have been primarily linked to cancer-related illnesses. Aflatoxin carcinogenicity has long been linked to the liver, where the metabolization process releases reactive intermediate metabolites (Benkerroum, 2020). Nevertheless, later animal and epidemiological research showed that they could cause cancer in organs other than the liver, such as the kidney, pancreas, bladder, bone, viscera, central nervous system, etc.
- a.
Chemistry of aflatoxin toxicity and degradation mechanism
Aflatoxin is a group of 20 fungal metabolites, viz., B1, B2, G1, G2, M1 and M2 etc. Of them, Aflatoxin B1 (AFB1) and aflatoxin B2 (AFB2) are produced by both A. flavus and A. parasiticus; AFB1 is believed to be the most potent and common food contaminants among all aflatoxins.Aflatoxin G1 (AFG1) and aflatoxin G2 (AFG2) are also produced by A. parasiticus, found in the foodstuff (Vankayalapati, 2018). Whereas, Aflatoxin M1 (AFM1) is produced in the fermentation broth ofA. parasiticus; AFM1 and AFM2 also metabolized from AFB1 and AFB2 in the infected liver cell (OwuorLalah et al., 2020) and are found in human and animal milk (Kamkar et al., 2014).
Aflatoxins are crystalline, colourless to pale yellow compounds that are easily soluble in solvents that are somewhat polar, such as methanol, chloroform, and dimethyl sulfoxide, and they have a water solubility of 10–20 μg/mL (Sarmento Amoras and Pena Costa, 2020). Aflatoxin molecules become unstable when exposed to certain conditions, such as oxygen-rich ultraviolet light, extreme pH values (pH < 3 or > 10), oxidizing agents, and high temperatures that cause ammonization (Dhakal et al., 2023). This irreversible reaction leads to the opening of the lactone ring and the decarboxylation of the aflatoxin molecule. These poisons belong to a special class of naturally occurring heterocyclic chemicals that are highly oxygenated.
The most studied aflatoxin, AFB1-exo 8,9-epoxide, is produced by the microsomal cytochrome enzyme (CYP450) at the beginning of AFB1 metabolism (Guengerich et al., 1998). In the instance of aflatoxin B1, it has been established that this aflatoxin is ultimately responsible for genotoxicity (Benkerroum, 2020). There is mounting evidence that the oxidative stress caused by AFB1 is equally, if not more, responsible for the genotoxicity of aflatoxin (Ma et al., 2021). The mechanisms underlying aflatoxin immunotoxicity, which is likely the second most well-studied toxicological impact, are becoming understood. Other aflatoxin-induced acute and chronic health issues, such as malnutrition diseases, delayed physical and mental maturity, reproduction, nervous system diseases, etc., have been demonstrated in humans or animals in addition to these major toxicological effects (Peles et al., 2019); however, more clarification is needed regarding their mechanisms of action. Although reports of its immunostimulatory effects have also been produced, aflatoxin is most recognized for its immunosuppressive characteristics.
- b.
Toxicity and health implications of aflatoxin exposure
Numerous toxicological effects of aflatoxin processes are produced, many of which are yet not well known. Numerous investigations have been carried out to investigate the mechanisms behind the toxicity of aflatoxins, providing a scientific basis for the creation of control and prevention measures. Food safety authorities can also utilize their expertise as a scientific tool to create regulations. The mutagenesis effects of aflatoxin AFB1 have been extensively studied since its discovery, and these effects have primarily been linked to the intermediate metabolite AFB1-exo-8,9 epoxide (AFBO) (Bedard and Massey, 2006). When AFBO combines with cellular macromolecules including proteins, phospholipids, and nucleic acids (Zhuang et al., 2016), it can alter genetic, metabolic, signalling, and cell structural aspects of the cell because of its severe instability. However, an increasing amount of evidence is being gathered that demonstrates how AFB1 causes oxidative stress (OS), which has effects on cell integrity and function that are at least as substantial if not more so (Kövesi et al., 2021). AFB1's several toxicity pathways produce genotoxicity, immunotoxicity, and acute poisoning when they interact with genomic DNA, other functional macromolecules, and immunocompetent cells (Dabuo et al., 2022). OS and AFBO are involved in these mechanisms. The refractory nature of AFB1-FAPy lesions to NER repair may not be the only explanation for their enhanced mutagenicity, since these lesions can also be repaired through the less sensitive to helix distortion mechanism of base excision repair (BER) (McCullough and Lloyd, 2019). Glycosylases in BER identify the damaged bases on a site-specific basis, allowing them to be eliminated and replaced with the correct base (Dianov and Hübscher, 2013). However, it is now well established that exposure to aflatoxins alters repair genes in a variety of ways, including through epigenetic alterations that stop the BER pathway.
For example, it has recently been shown that transcriptional inhibition of the gene caused by hypermethylation of the promoter of the NEIL1 (Nei Like 1) gene, which codes for a DNA glycosylase (NEIL1) crucial for BER, lowers the excision efficacy in AFB1-FAPy adducts (Hildrestrand et al., 2021). The prevalent polymorphisms that result in catalytically inactive NEIL1 enzyme may also make it more difficult for people's AFB1-FAPy lesions to repair (Zuckerman et al., 2023). Aflatoxin-induced HCC in high-exposure scenarios has also been demonstrated to be associated with polymorphism in multiple human DNA repair genes, such as XPC, XPD, XRCC1, XRCC3, XRCC4, XPD, and XRCC7 (Long et al., 2013).
This risk is exacerbated with concurrent polymorphism of repair genes and phase II-enzyme detoxification genes, as was demonstrated for the combined polymorphisms of XRCC1 (involved in BER repair) with GSTM1 and HYL1*2 (coding for GST and microsomal epoxide hydrolase, respectively) (Tiemersma et al., 2001). However, the effect of the AFB1-detoxifying gene polymorphism alone on the increased risk of HCC remains a matter of continuous controversy.
- c.
Aflatoxicosis outbreaks
Aflatoxicosis is a global mycotoxicosis that produces strong hepatotoxins on animal feed during hot weather (drought) and in storage. It frequently happens at the same time as agricultural pest damage. Usually invading peanuts, almonds, corn (maize), and cottonseed, Aspergillus flavus and A. parasiticus can swiftly produce significant amounts of aflatoxins, with aflatoxin B being the most dangerous (Kumar et al., 2017). In the liver, aflatoxins are broken down into an epoxide that attaches to macromolecules, such as nucleic acids. Mutagenesis, cancer, teratogenesis, immunosuppression, and liver damage with decreased protein synthesis are a few examples of the harmful effects. Patients may exhibit poor growth, bleeding, icterus, sadness, unthriftiness, and inappetence to food. Aspergillus flavus and A.parasiticus infestation of corn (maize), peanuts, almonds, rice, cottonseed, and other crops can lead to aflatoxicosis, a global health concern (Sarma et al., 2017).
Aflatoxin severely affects fish, humans, animals, birds, dogs, cats, and rodents; the young are especially vulnerable. Aflatoxin targets the liver, kidney, spleen, brain, gut, skin, testis, and cardiac tissue and is mutagenic, carcinogenic, teratogenic, and immunosuppressive (Dai et al., 2022). Adverse consequences can result in low production, food residues, and even death. They are associated with liver damage. Aflatoxin contamination in feed should be avoided by nursing animals due to aflatoxin M1 residual in milk, and most countries have regulatory limitations on the amount of aflatoxin in animal feeds, human food, and milk (Akbar et al., 2020). The feed should be chemically evaluated before use. The hydrated sodium calcium aluminosilicates can function as aflatoxin binders in feed and minimize harmful effects and milk aflatoxin M1 residue. The treatment is supportive.
Around the world, aflatoxicosis affects growing fowl, particularly ducklings and turkey poults, as well as young pigs, pregnant sows, calves, and canines (Dalvi et al., 1986). Though they are susceptible if toxic diets are fed for extended periods, adult cattle, sheep, and goats are rather resistant to the acute phase of the disease. All examined animal species have demonstrated some degree of vulnerability in experiments (Allah Ditta et al., 2019). Aflatoxin dietary quantities that are often tolerated in animals include dogs, cats, cattle, and weaner pigs, 100 ppb in calves, < 50 ppb in dogs, < 100 ppb in calves, and < 300 ppb in cattle (Jallow et al., 2021).
Clinical illness, including some mortality, is anticipated to occur at about double the specified acceptable levels. Aflatoxin M1 and M2, two detectable metabolites, are excreted in the milk of nursing animals at dietary levels as low as 10–20 ppb (Zentai et al., 2023); dairy cows, goats, or sheep should not be fed feedstuffs containing aflatoxin. Different nations have varying acceptable legal limits for milk, which can range from 0.05 ppb to 0.5 ppb (Aslam et al., 2015). When contamination happens, state or federal regulatory organizations should be consulted. In the liver, aflatoxins are broken down into an epoxide that attaches itself to macromolecules, particularly nucleic acids and nucleoproteins. Their harmful consequences include immunosuppression, decreased protein synthesis, teratogenesis, carcinogenesis, and mutagenesis brought on by the alkylation of nuclear DNA (Bou Zerdan et al., 2021). Decreased synthesis of proteins leads to a decrease in vital metabolic enzymes and growth-promoting structural proteins. The primary organ impacted is the liver. Aflatoxin dosages that are too high cause hepatic necrosis, while doses that are too low over time cause immunosuppression, decreased growth rate, and enlarged liver.
- d.
Aspergillosis and cancer
Species of Aspergillus, a fungus or mould, can lead to the development of aspergillosis in individuals. The symptoms and severity of aspergillosis-related infections can vary widely, but they generally affect the respiratory system. Aspergillus, can grow both indoors and outdoors. While the majority of these mould strains are harmless to breathe in, a small number can pose a significant risk to individuals with compromised immune systems, underlying lung conditions, or asthma (Vanfleteren et al., 2018). The symptoms and signs of aspergillosis differ based on the type of infection. Some individuals with cystic fibrosis or asthma may have allergic reactions to Aspergillus mould. This condition, also known as allergic bronchopulmonary aspergillosis, is characterized by fever, a cough that may produce mucus plugs or blood, as well as exacerbation of chronic lung diseases such as severe sarcoidosis, emphysema, and tuberculosis (Chen et al., 2022). In individuals with aspergillus infections, fungus fibres may enter lung cavities and form tangled masses (fungus balls) known as aspergillomas. Initially, aspergillomas may cause a mild cough or no symptoms at all (Dib et al., 2019). However, over time and without treatment, aspergillomas have the potential to worsen underlying chronic lung conditions and lead to a cough that frequently produces blood (hemoptysis), as well as symptoms such as sighing, breathing difficulty, and unintentional weight loss.
4. Aflatoxin Contamination Contributors
Mycotoxins, which are fungal toxins naturally present, pose a threat to food staples during their production and storage, resulting in health concerns for humans and animals alike (Diao et al., 2014; Villers, 2014). The contamination of food items by mycotoxins occurs throughout the pre-harvest and postharvest stages, particularly when they are exposed to moisture or stored under conditions of high temperature or humidity (Villers,2014). Aflatoxins (AFs) are particularly noteworthy among mycotoxins due to their prevalence, toxicity, and economic consequences. Aflatoxin B1 (AFB1) is the most commonly found AF in contaminated agricultural products, alongside approximately 20 other identified AFs. These toxins, including AFB2, AFG1, and AFG2, are primarily produced by toxigenic strains of Aspergillus fungi, notably Aspergillus flavus, Aspergillus parasiticus, and Aspergillus nomius. Additionally, aflatoxin M1 (AFM1), a metabolite of AFB1, holds significance within the AFs group, particularly for its occurrence in human and animal milk when contaminated food or feed contains AFB1.For decades, aflatoxin contamination in peanuts has remained a persistent global concern, garnering widespread attention since the 1960s. These fungal byproducts pose substantial health risks to both humans and animals, thereby imposing significant economic burdens on stakeholders across the production, handling, processing, and distribution chains of contaminated peanuts. The inevitability of aflatoxin contamination is rooted in the diverse array of factors influencing peanuts at various stages, from pre-harvest conditions to harvesting and postharvest practices (Diao et al., 2014).
- g.
Environmental factors
As climatic conditions undergo significant shifts, ecosystems experience alterations in temperature, precipitation, and humidity, all of which create conducive environments for fungal proliferation and subsequent mycotoxin synthesis (Achaglinkame et al., 2017; Nleya et al., 2020). Fungi inhabit numerous crops and exhibit adaptability to diverse environmental conditions, occupying distinct ecological niches that may overlap (Perrone et al., 2020). The proliferation of fungi and the production of aflatoxins in cereals are contingent upon factors such as temperature, moisture levels, soil composition, and storage conditions (Achaglinkame et al., 2017). Various abiotic factors (temperature, water activity, pH levels, carbon availability, and nitrogen content) significantly impact the pathway of aflatoxin biosynthesis (Liu et al., 2017). However, aflatoxin contamination is particularly influenced by temperature and water activity. These factors not only stimulate the growth of aflatoxin-producing fungi, primarily A. flavus, but also significantly impact the activation of the gene cluster responsible for aflatoxin production (Abdel-Hadi et al., 2011; Bernáldez et al., 2017; Tejero et al., 2021). Increased water activity promotes enhanced fungal growth and the synthesis of toxins (Bhatnagar et al., 2006). It's been determined that a water activity of approximately 0.99 aw and temperatures ranging from 29 to 30°C are conducive to aflatoxin production (Tejero et al., 2021). These two factors, namely temperature and water activity (aw), are pivotal in regulating the transcription of two significant genes (aflR and aflS) in the pathway of aflatoxin biosynthesis (Liu et al., 2017). Medina et al. (2014) explored how key environmental factors affect the molecular ecology, growth, and aflatoxin production by Aspergillus flavus, both in vitro and in maize grains. Recent studies on water activity × temperature interactions and aflatoxin biosynthesis are examined, revealing a direct correlation between gene expression levels and aflatoxin B1 production. A model integrating the expression of 10 biosynthetic genes, growth, and AFB1 production was validated under elevated temperature and water stress conditions. The impact of water activity × temperature × elevated CO2 levels is detailed for the first time, showing significant effects on aflatoxin biosynthetic gene expression (including structural aflD and regulatory aflR genes) and AFB1 production. Baazeem et al. (2021) demonstrated that A. flavus strains isolated from pistachio nuts exhibited optimal growth at approximately 35 °C on both pistachio nut-based media and raw pistachio nuts. AFB1 production, however, peaked at around 30 °C. Under climate change conditions with elevated temperatures (35 vs. 37 °C) and CO2 levels (400 vs. 1000 ppm), growth of A. flavus strains showed minimal variation, regardless of water availability. Notably, AFB1 production was stimulated under these conditions both in vitro and on raw pistachio nuts (Baazeem et al.,2021), accompanied by changes in the expression of the structural and regulatory genes (aflD and aflR) involved in toxin biosynthesis, with some strain variability observed. This integrative approach provides crucial insights into the effects of climate change on mycotoxigenicity. Temperatures outside the range of 25 to 37°Care unsuitable for the growth and synthesis of aflatoxins, while moisture levels below 0.85 aw hinder growth and toxin production, ceasing completely at 0.70–0.75 aw (Bhatnagar et al.,2006). Elevated temperatures, higher levels of carbon dioxide (CO2), drought, and rainfall directly influence maize crops, altering the prevalence of Aspergillus flavus. These environmental factors stimulate fungal growth, conidiation, and spore dispersal, while also hindering the growth and maturation of maize (Ojiamboet al., 2018; Nleya et al., 2018). Extended periods of frequent droughts stimulate mycotoxin production both before and after harvest (Ojiambo et al., 2018; Nleya et al., 2018; Kachapulula et al., 2017; Medina et al., 2017; Dövényi-Nagy et al., 2020). The impact of climate change on maize production is multifaceted, with factors such as rainfall distribution, prevalence of pests and diseases, and temperature fluctuations playing significant roles (Mapfumo et al.,2020). According to research by Zuma-Netshiukhwi et al. (2021), even a modest temperature rise of 1°C to 2°C can lead to a substantial decrease in grain yield, estimated at around 20–25%. Other studies suggest even higher reductions in yield, possibly up to 50%, contingent upon the plant's reproductive stage (Adisa et al., 2019; Jain et al., 2019).
As projected, a reduction of approximately 15% in rainfall is anticipated by the close of the 21st century (Jury et al., 2013). Boko et al. (2007) envisage a 5% to 8% augmentation in aridity and semi-aridity throughout Africa, positing potential implications such as heightened drought occurrences and ensuing crop stress. These circumstances may contribute to an elevation in mycotoxin contamination levels (Kachapulula et al., 2017; Hajnal et al., 2017). Climate projections suggest that atmospheric CO2 levels are expected to increase two to threefold (from 350-400 ppm to 800-1200 ppm) over the next 25 to 50 years. This escalation in CO2, along with rising temperatures and frequent drought episodes, is likely to have significant impacts on pest dynamics, disease prevalence, and crop yields across various regions. Climate projections indicate that atmospheric CO2 levels are anticipated to rise two to threefold (from 350-400 ppm to 800-1200 ppm) within the next 25 to 50 years. This increase, coupled with higher temperatures and more frequent droughts, is expected to significantly affect pest dynamics, disease incidence, and crop yields in various regions. Baazeem et al. (2021) found that acclimatizing A. flavus for five generations in elevated CO2 on raw pistachio nuts caused different strains to exhibit varied growth and AFB1 production. Both the original and acclimatized AB3 and B10 strains showed similar colonization under different climate-related conditions of temperature, CO2, and water availability. The effect of these abiotic factors on AFB1 production varied by strain. For AB3, the acclimatized strain produced more AFB1 than the original at 37°C, 1000 ppm CO2, and 0.98 aw. For AB10, AFB1 production increased at 0.93 aw after 5 and 10 days at 37°C and 1000 ppm CO2.Storage conditions of ≥0.90 aw, especially at temperatures ≥25-30 °C, significantly increase the risk of AFB1 contamination to levels that could lead to product rejection, even after just 10-20 days of storage. This underscores the need for optimal storage conditions to minimize aflatoxin contamination in chillies (Al-Jaza et al.,2022). Aflatoxin-producing moulds can grow in a pH range of 1.7 to 9.3, with an optimal range of 3 to 7 (Yoshinari et al. 2010). Lower pH levels (1 to 3) reduce fungal growth, while a slightly higher pH (3 to 6) promotes both fungal growth and aflatoxin production (Eshelli et al. 2015). An initial pH of 5 enhances AFB production, while a pH of 7 favours AFG production. The media composition also affects the pH (Dalié et al. 2010).
- h.
Nutritional factors
Aflatoxin production is significantly influenced by the substrate and various nutritional factors, including carbon, amino acids, nitrogen, lipids, and trace elements. Carbohydrate-rich substrates enhance aflatoxin production more than oil-rich substrates because carbohydrates readily supply the carbon necessary for optimal fungal growth (Ma et al.,2014). According to Liu et al. (2016), glucose, ribose, sucrose, xylose, and glycerol are effective substrates for aflatoxin production. In contrast, peptone, lactose, and sorbose do not promote aflatoxin production. Most previous research has focused on controlling AFB1 production in crops, with few studies examining the factors responsible for different levels of AFB1 contamination in various grains. While some studies have suggested a connection between lipids and AFB1 production in crops, the complex composition of grains means that AFB1 production is also influenced by other nutrients, including starch, proteins, saccharides, and trace elements (Liu et al.,2016). Nitrogen, when provided as nitrite and nitrate, significantly enhances aflatoxin production by A. flavus through various mechanisms (Wang et al.,2017). Lipids play a significant role in the production of aflatoxins. The biosynthesis of aflatoxins in toxigenic fungi is initiated by lipophilic epoxy fatty acids and fungal growth, as well as aflatoxin production, is triggered by ergosterol oxidation (Reverberi et al., 2014). Consequently, lipids serve both as a substrate for obtaining an acyl-CoA starter and as a signalling molecule. Aflatoxin production and accumulation are higher in full-fat substrates compared to low-fat ones. Adding corn oil to defatted wheat infected with A. flavus promotes more aflatoxin production than in media without corn oil (Liu et al., 2016). Vitamins, amino acids, and metal ions collectively enhance aflatoxin production. Amino acids such as glycine, glutamate, and alanine, along with bivalent metals like zinc and magnesium, are known to promote aflatoxin production (Bolu et al., 2014). Liu et al. (2016, 2016a) worked on maize and other grains and reported aflatoxin production increased by 4, 5, and 19 times with zinc concentrations of 20, 50, and 100 mg/L, respectively (Liu et al., 2016). Amino acids like tyrosine promote aflatoxin production, whereas tryptophan inhibits it (Chang et al., 2015). AFB1 production is supported by arginine, glycine, glutamic acid, and aspartic acid at a concentration of 0.5%.
- i.
Biological factors
Biological factors such as fungal species, weeds, and insect damage affect aflatoxin production. Weeds compete with crops and cause stress, increasing aflatoxin levels. The amount of aflatoxin produced depends largely on the fungal species involved. Insect wounds create stress and entry points for aflatoxigenic fungi, leading to contamination (Kinyungu et al., 2019). Aflatoxin production varies by strain, with A. flavus producing fewer aflatoxins than A. parasiticus (Manjunatha et al., 2018). Despite various factors, A. flavus is the primary species responsible for aflatoxin contamination due to its abundance in soil and its ability to thrive on diverse organic substrates such as compost, plant debris, cotton, dead insects, stored grains, field crops, animal corpses, and fodder (Kakde, 2012). Pre-harvest contamination of crops is common due to presence of A. flavus in soil, while post-harvest contamination occurs during storage as it spoils food grains. A. flavus lacks host specificity, contaminating both monocot and dicot seeds (St. Leger et al. 2000).
- j.
Agricultural practices
Conventional methods are ineffective in preventing mycotoxin formation during the pre-harvest stages of crop production; however, several non-conventional techniques and good agricultural practices have demonstrated efficacy. These pre-harvest strategies encompass crop rotation, intercropping, residue management, optimal sowing times, early harvesting, and the judicious use of fertilizers, insecticides, and herbicides. By disrupting the infectious cycle, crop rotation and intercropping significantly reduce mycotoxin contamination. Therefore, identifying appropriate crop combinations is essential for effective mycotoxin management. For instance, rotating legume crops such as cowpea and soybean with maize can disrupt pest and disease cycles and enhance soil fertility (Logrieco et al., 2021; Abbas et al., 2012). Mutiga et al. (2014) observed that intercropping cowpea, beans, and maize lowers aflatoxin levels in maize compared to growing maize as a sole crop, owing to enhanced soil nitrogen content. Moreover, residues or debris from prior harvests can carry fungal spores that remain dormant in the soil between crop cycles. Therefore, effective management of residues from previous harvests, either by removal or burial, can significantly reduce fungal infections in the field (Blandino et al., 2008). Additionally, adjusting sowing times or selecting cultivars suited to lower temperatures and reduced water stress can help mitigate mycotoxin contamination. This strategy also helps to avoid wet periods during early flowering. Early harvesting is also strongly recommended in high-risk areas as it reduces the time fungal pathogens have to develop and produce aflatoxins (Dövényi-Nagy et al., 2020; Mondaet al., 2020).
5. Aflatoxin: Impact on Human and Animal Health
Effects on Human Health
The major source of aflatoxin exposure in the human body is through ingestion of aflatoxin-contaminated food products such as meat, eggs, milk, milk products contaminated grains etc. (Tchana et al., 2010). Agricultural harvests including cotton seeds, coffee and spices along with major food crops such as rice and wheat are the major sources of aflatoxin contamination in the human body (Refai, 1988). These crops can be infected prior to harvesting or post-harvesting during storage. Warm and humid environments in the storage area result in enhanced concentrations of aflatoxins in food grains (Awuchi et al., 2020; Jajic et al., 2018).
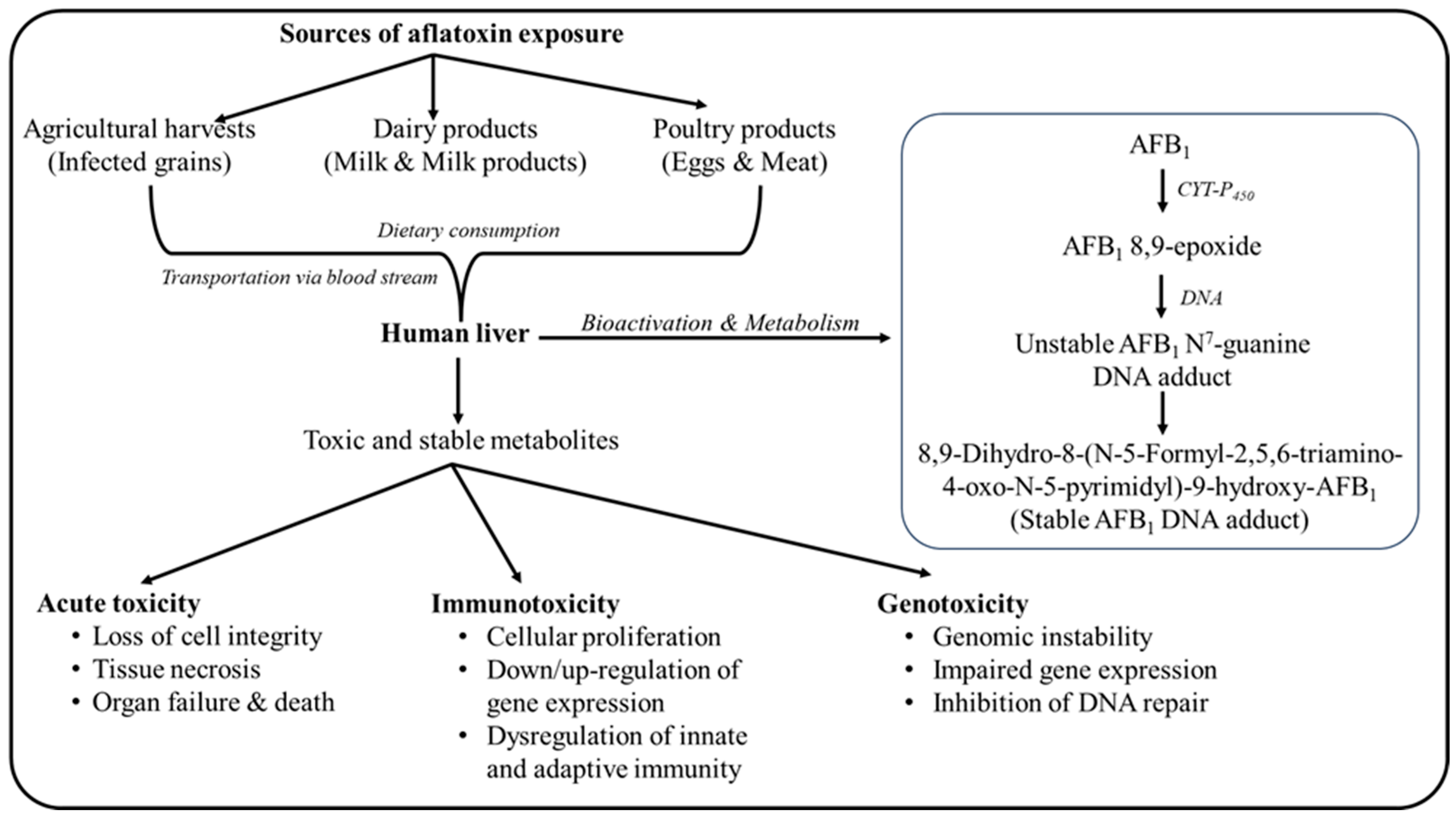
Post consumption human body particularly the liver metabolizes the aflatoxin present in bloodstream manifolds. Cytochrome P-450 enzyme present in the human liver bioactivates the aflatoxin and metabolizes into its toxic, unstable and reactive epoxides, responsible for its toxicity principle (Usha
et al., 2017). Post activation and metabolism the resulting aflatoxin epoxides form adduct with the DNA, RNA and proteins resulting in its inhibition leading to acute toxicity, genotoxicity and immunotoxicity (Smith
et al., 2018; Smela et al., 2001; Gouas et al., 2009). The generalized toxicity mechanism of aflatoxin in the human body has been depicted in
Figure 1. Often high exposure of aflatoxin in human body leads to acute aflatoxicosis which ultimately leads to liver cirrhosis or liver cancer. Frequent and chronic exposure leads to increased concentrations of its epoxide moieties resulting in DNA intercalation causing p53 gene mutation, a vital enzyme responsible for cell cycle progression. Such metabolism often leads to signalling apoptosis resulting in programmed cell death (Bedard and Massey, 2006). Although dietary consumption of aflatoxin-contaminated foods results in severe liver cancer but other organs such as the pancreas, bladder, gastrointestinal tract and kidney have also been reported to develop such tumours upon prolonged exposure (Fouad
et al., 2019; Williams
et al., 2004). In fact, there are several reports claiming symptoms of teratogenicity, immunosuppression, cytotoxicity and mutagenicity in humans due to chronic exposure to aflatoxin. The mechanism behind every form of aflatoxin toxicity in human body lies in the chemistry of aflatoxin metabolites. Covalent bonding of aflatoxin epoxide to DNA leads to alkylation at N
7 of guanine residues to form 8,9-dihydro-8-(N7-guanyl)- 9-hydroxy-AFB1 (a stereoselective alkylated aflatoxin adduct (Perri et al., 2016; Coskun et al., 2019). The adduct developed is highly unstable and during stabilization releases itself leaving behind an apurinic DNA molecule. Further imidazole ring opening of the toxic metabolite in the alkaline medium of liver cells results in the development of AFB1-formamidopyrimidine in the body responsible for every proliferation mechanism in any cell of the human body (Chawanthayatham et al., 2017; Croy and Wogan, 1981).
Table 2 enlists the toxic metabolites and symptoms of aflatoxin toxicity in the human body.
Effects on Animal Health
Animals are fed with crops that are usually unfit for human consumption due to the presence of some insects, moulds or rotten feeds etc. The feed that is usually served to animals in developing nations are cotton seeds, groundnuts, maize and copra. Such crop leftovers or feeds are a major source of aflatoxin contamination in animals (FAO, 2008). There are several reports which establish the fact that most of the feed materials meant for animals are usually contaminated with high concentrations of aflatoxin, even more than 52% of the feed materials used in Europe, the Mediterranean region and African nations were reported to be contaminated with aflatoxin (Binder et al., 2007; Rodrigues and Naehrer, 2012). Such contaminations have been reported to possess several health implications for animal health. Susceptibility doses of aflatoxin have been reported to vary as per the difference in animal body weight. Rabbits, ducks, and cats have been reported to be highly susceptible to aflatoxin exposure having LC50 values as low as less than 1 mg/kg body weight while some animals such are dogs, horses, pigs and sheep are moderately susceptible with relatively high concentrations of aflatoxin such as 1-2 mg/kg body weight (Grace, 2013; Hamilton, 1986).
Many experimental studies suggest possible health implications in poultry animals. Some report suggests broiler chicken to be more susceptible than that of layer chicken. Chicken health has been reported to be severely deteriorated upon aflatoxin exposure up to 1000 ppb. Several symptoms have been diagnosed post-aflatoxin exposure such as reduced egg weight, frequency of egg laying, egg shell thinning, yolk weight and color etc. Sometimes impaired immune responses have been diagnosed upon heavy exposure to aflatoxin through contaminated feed with more than 200 ppb concentration (Dersjant-Li et al., 2003; Gabal and Azzam, 1998; Otim et al., 2005). Sheep and goats, moderately susceptible animals have been reported to show symptoms like decreased body weight, liver and nasal tumours and often mortality upon exposure at concentration levels up to 1750 ppb for a period of up to 3.5 years (Lewis et al., 1967; Ewuola et al., 2013). Susceptibility towards aflatoxin in fishes varies from species to species. In the majority of the cases, most usual symptoms involve weight loss, anorexia, liver malfunction, hepatic injury etc. Susceptible species like rainbow trout develop such symptoms at a very low concentration of 0.4 ppb while most resistant fish species like catfish are deprived of such symptoms at very high concentrations of 10,000 ppb. Chances of liver damage and tumour development are most common in the case of susceptible ones while mere weight reduction was observed in the case of resistant species (Jantrarotai and Lovell, 1990; Tuan et al., 2002).
Overall Impact of Aflatoxin on Human and Animal Health
Aspergillus flavus and Aspergillus parasiticus can contaminate crops like maize, peanuts, cottonseed and various spices. The toxins produced are strong carcinogens that endanger both human as well as animal health (Shabeer et al., 2022). Aspergillosis is a fungal infection caused by the genus Aspergillus, namely A. fumigatus, A. flavus, and A. niger (Garbino, 2004). Aspergillosis is one of the top four diseases that lead to death in immune-compromised persons worldwide (Winters et al., 2012). This illness frequently affects the respiratory system, particularly in people with impaired immune systems or underlying lung disorders such as asthma or cystic fibrosis. Aspergillosis affects rabbits, chickens, turkeys, and geese also. In addition, A. flavus produces stone brood sickness in honeybees (Shabeer et al., 2022). Inhaling Aspergillus spores can cause a wide range of clinical symptoms, from moderate allergic reactions to severe invasive illness. The second leading cause of infection is the spread of spores through infected wounds and the smoking of contaminated tobacco or marijuana plants. Aspergillosis manifests clinically in a variety of ways, including extrinsic asthma, allergic bronchopulmonary aspergillosis, extrinsic allergic alveolitis, saprophytic pulmonary and extra-pulmonary colonization (Amaike and Keller, 2011). Allergic bronchopulmonary aspergillosis (ABPA) and allergic fungal sinusitis (AFS) are two of the less severe Aspergillosis, affecting 1- 15% of the world's population and characterized by respiratory symptoms such as coughing, wheezing and nasal congestion. In contrast, invasive aspergillosis is a serious hazard to immunocompromised people, causing pneumonia with symptoms such as fever, chest pain, and difficulty breathing, and it can spread to other organs if not treated.
Aflatoxicosis is another serious health condition resulting from the ingestion of the toxic metabolites produced by
Aspergillus flavus and
Aspergillus parasiticus. The occurrence of various Aflatoxins is common in wide varieties of food and feed (
Table 3). Aflatoxicoses can manifest in both acute and chronic forms, with symptoms ranging from mild gastrointestinal disturbances to severe liver damage and even death. A few studies suggest that 1000 µg/kg of aflatoxin in food can cause toxicity in humans (WHO, 2018), while the acceptable level for animals is 50-300 µg/kg (Grace et al., 2015). Acute aflatoxicosis typically occurs after consuming high levels of aflatoxin-contaminated food, leading to symptoms such as nausea, vomiting, abdominal pain, and liver failure. Chronic exposure to lower levels of aflatoxins can result in long-term health effects, including an increased risk of liver cancer, immune system suppression, growth impairment, and developmental delays (Dhanasekaran et al., 2011). Prevention strategies focus on minimizing aflatoxin contamination in food and feed through proper storage, handling, and agricultural practices, alongside regulatory measures and routine monitoring to ensure food safety and protect public health.
6. Detection of Aflatoxin
Aflatoxin contamination is the primary problem with food safety in both developed and developing countries. Accurate and effective detection is essential to reducing the risk of mycotoxin contamination in food and feed before it affects human and animal health. Although the discovery of aflatoxins dates back to the early 1960s (Pickova et al., 2021), concomitant technological advancements increased the efficiency and precision of aflatoxin detection. While culture-based approaches were employed in the past, many developing nations now frequently employ assays based on high-performance liquid chromatography (HPLC) and enzyme-linked immunosorbent assay (ELISA). A range of technologies have been developed to date for the detection of aflatoxins, such as fluorescence spectroscopy (FS) (Bertania et al. 2020; Wu and Xu 2020), immunology-based enzyme-linked immunosorbent assay (Nardo et al., 2020), lateral flow immunoassay (Di Nardo et al., 2019), etc. These technologies can be grouped into different forms of aflatoxin detection strategies.
- k.
Culture-based techniques
Traditionally, different culture-dependent techniques have been used to detect aflatoxins in foods and feeds. This approach includes (1) fluorescence test and (2) ammonium hydroxide vapour test to detect aflatoxigenic isolates of Aspergillus spp. In the fluorescence test, the detection of blue fluorescence of aflatoxin B1 is done under UV light (365 nm) particularly when the culture medium is supplemented with β-cyclodextrin (Ordaz, 2003). The presence of a blue fluorescent zone around the growing fungal colony visualized on the reverse side indicates the aflatoxin-producing ability of the fungal isolates. Further, yellow pigment (anthraquinones) production on the reverse side of colonies is used for the rapid identification of aflatoxigenic strains of A. flavus, as the yellow pigment was associated with the aflatoxin biosynthetic pathway (Shier et al., 2005). The ammonium hydroxide vapour test can also be employed to detect the aflatoxin-producing ability of A. flavus isolates based on the intensity of colour changing from yellow to red (Saito and Machida, 1999). These culture-based processes can be easily performed as these are visualization-based detection approaches, but very labour-intensive, time-consuming, and less accurate; sometimes yield false-positive or false-negative results.
- l.
Molecular techniques
Different molecular approaches, viz., Polymerase chain reaction (PCR), loop-mediated isothermal amplification (LAMP), and other DNA-based techniques can be used to detect genes involved in aflatoxin production in mould samples. These methods can provide rapid screening for aflatoxin-producing moulds in food and feed samples. For the conventional PCR and multiplex PCR-based detection, different housekeeping genes coding for ribosomal RNA and or genes, viz., aflM, aflP, aflR, and aflOinvolved in the aflatoxins biosynthesis (Adetunji et al., 2019) can be amplified to detect the contamination of Aspergillus flavus in food grains (Reddy et al., 2009; Mangal et al., 2016); whereas for the quantitative estimation using real-time PCR (qPCR) the Aspergillus norsolorinic acid reductase gene (NOR1) can be selected (Sardiñas et al., 2011; Kumsiri and Kanchanaphum, 2020). Similarly, LAMP-based strategy was developed to amplify the Norsolorinic Acid gene (nor1) to distinguish aflatoxigenic and non-aflatoxigenic fungal strains in rice grain (Douksouna et al., 2020). Further, a nor1 gene-specific single LAMP assay was developed for rapid and sensitive detection of several aflatoxin-producing species within Aspergillus section Flavi in food raw materials, spices, and dried fruit (Niessen et al., 2018). For the rapid evaluation of mycotoxin synthesis by fungi, PCR-based methods are generally used because of their simplicity, speed, cost-effectiveness, and reliability.
- m.
Chromatography and spectroscopy
Spectrophotometry and chromatography are two efficient techniques that are widely used for aflatoxin detection in food and agricultural items because of their sensitivity and accuracy. Chromatography uses features like size, charge, and polarity to distinguish aflatoxin molecules from other chemicals in a sample (Abbas, 2022), while spectroscopy uses electromagnetic spectra to identify individual atoms and molecules (Dadi and Yasir, 2022).
Chromatography techniques
Thin-Layer Chromatography (TLC): A chromatographic technique called TLC can be used to identify, isolate, and assess the purity of aflatoxin (Wacoo et al., 2014). The components are then separated based on how effectively they bind to the stationary phase after the sample is spotted onto a thin film of adsorbent material. Mycotoxins were easily separated by TLC using a range of solvents (Odhav and Naicker, 2002), such as acetic acid, acetone, acetonitrile, chloroform, ether peroxide free, ethyl alcohol, hexane, isopropanol, toluene, etc.
High-Performance Liquid Chromatography(HPLC): Aflatoxin can be precisely quantified using HPLC, a technology that is frequently used (Yazdanpanah et al., 2013). In HPLC methods, you can alter the elution mixes and gradients, detectors, and column types (normal or reverse phase, different packing, particle size, etc.) (Kizis et al., 2021). Most HPLC processes use an acidic mobile phase, such as C18 columns, acetonitrile, methanol, water, or mixtures of these that have been acidified with phosphoric or acetic acid, and are performed in reversed-phase (Afsah-Hejri et al., 2011). When components of a sample are arranged based on their affinities for a stationary phase and a mobile phase, aflatoxin separation takes place.
Gas Chromatography (GC): GC distinguishes volatile compounds because their retention times vary. Mycotoxin detection in food products can also be achieved through mycotoxin conversion into volatile compounds. GC is not frequently employed for mycotoxin analysis since most mycotoxin components are extremely polar and non-volatile. Detectors for electron capture (ECD), flame ionization (FID), or mass spectrometry (MS) are frequently used with gas chromatography (GC) to identify volatile mycotoxins, including patulin and trichothecenes (Yang et al., 2020). Aflatoxin analysis can also be done using GC-MS, or gas chromatography coupled with mass spectrometry (Janik et al., 2021) and MS locates and categorizes the chemicals based on their mass-to-charge ratios.
Spectroscopy Techniques
Spectroscopy is a useful tool for studying the interaction between matter and electromagnetic radiation. Its basic concepts are derived from the properties of matter and light. Spectroscopy studies a wide range of electromagnetic spectrum wavelengths, from radio waves to gamma rays. Different parts of this spectrum are the focus of different spectroscopic techniques. When matter interacts with electromagnetic radiation, it can either emit or absorb photons. Absorption spectroscopy measures the light that a sample absorbs, as opposed to emission spectroscopy, which measures the light that a material emits. This technique quantifies aflatoxins, which are toxic metabolites produced by certain moulds, primarily A. flavus and A. parasiticus.
Mass spectrometry: Mass spectrometry (MS) techniques, offer high sensitivity and selectivity for aflatoxin detection. These methods can identify and quantify aflatoxins in complex samples with high accuracy (Ahuja et al., 2023). The process typically involves several steps:
(1) Sample preparation through solvent extraction, solid-phase extraction (SPE), and QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) can be used depending on the sample type and the desired sensitivity.
(2) Chromatographic separation of aflatoxins from other components present in the sample using High-performance liquid chromatography (HPLC), ultra-high-performance liquid chromatography (UHPLC) or liquid chromatography-mass spectrometry (LC-MS).
(3) Ionization of aflatoxins to generate charged species via electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI).
(4) Mass analysis of the ionized aflatoxin molecules in the mass spectrometer, where they are separated based on their mass-to-charge ratio (m/z). The mass spectrometer detects these ions and generates mass spectra, which provide information about the molecular weight and fragmentation pattern of the aflatoxins.
(5) Analysis of acquired mass spectra using specialized software to compare the observed mass spectra with reference spectra of known aflatoxin standards and identify and quantify the aflatoxins present in the sample.
Mass spectrometry offers several advantages for aflatoxin detection, including high sensitivity, selectivity, and the ability to analyze multiple aflatoxin types simultaneously (Liu et al., 2024). Additionally, mass spectrometry can be coupled with various chromatographic techniques to enhance separation and detection capabilities, making it a versatile tool for food safety testing and regulatory compliance.
Nuclear magnetic resonance (NMR) spectroscopy: Nuclear Magnetic Resonance (NMR) spectroscopy is another analytical technique used in the detection and characterization of aflatoxins, complementing mass spectrometry approaches (Kluczkovski et al., 2022). While mass spectrometry relies on the mass-to-charge ratio of ions, NMR spectroscopy exploits the magnetic properties of certain atomic nuclei, such as hydrogen (^1H) and carbon-13 (^13C), to provide information about the molecular structure of compounds. NMR spectroscopy can be used to detect aflatoxins based on their characteristic chemical shifts in the NMR spectrum. This method provides structural information about aflatoxin molecules, aiding in their identification. In aflatoxin analysis, NMR spectroscopy can be employed in several ways:
Structural Identification: NMR spectroscopy can provide detailed structural information about aflatoxins, including the connectivity of atoms within the molecule, the types of functional groups present, and the configuration of stereocenters (Xu et al., 2023). By comparing the NMR spectra of unknown samples with reference spectra of known aflatoxins, analysts can confirm the presence of aflatoxins and determine their chemical structure.
Metabolite Profiling: NMR spectroscopy is valuable for studying the metabolic fate of aflatoxins in biological systems. By analyzing the NMR spectra of biological samples, researchers can identify and quantify aflatoxin metabolites formed during detoxification or biotransformation processes in organisms (Ogunade et al., 2018).
Quality Control: NMR spectroscopy can be used for quality control purposes in food safety testing and regulatory compliance. By establishing NMR-based methods for aflatoxin analysis, laboratories can ensure the accuracy, reliability, and reproducibility of their analytical results (Sobolev et al., 2022).
NMR spectroscopy offers several advantages for aflatoxin analysis, including non-destructive analysis, high structural specificity, and the ability to analyze complex mixtures without the need for extensive sample preparation. However, NMR spectroscopy generally has lower sensitivity compared to mass spectrometry, which may limit its utility for trace-level detection of aflatoxins in highly contaminated samples. Nonetheless, when combined with other analytical techniques, such as mass spectrometry and chromatography, NMR spectroscopy can provide comprehensive information about aflatoxin contamination in food and feed samples.
Fluorescence Spectroscopy: Aflatoxins exhibit natural fluorescence under certain conditions. Fluorescence spectroscopy can exploit this property for detection. Aflatoxins can be excited by a specific wavelength of light, and the emitted fluorescence can be measured to quantify their presence (Bartolić et al., 2022).
The process typically involves several steps:
1. Sample preparation for the extraction of aflatoxins from the sample matrix and purifying the extract to remove any interfering substances that could affect the fluorescence signal.
2. Excitation of aflatoxin at the specific wavelength of light to excite the molecules in the sample and to move to higher energy states.
3. Emission of light (fluorescence signal) from the aflatoxin at a longer wavelength.
4. Detection of the emitted fluorescence signal and its intensity and wavelength.
5. Quantitative measurement of the concentration of aflatoxins in the sample based on the intensity of the fluorescence signal using calibration curves.
Fluorescence spectroscopy is rapid, highly sensitive and specific, allowing for the detection of aflatoxins even at very low concentrations (Chen et al., 2021). Additionally, it can distinguish between different types of aflatoxins, providing valuable information for food safety and quality control.
Infrared Spectroscopy (IR): IR spectroscopy is another analytical technique that can be utilized for aflatoxin detection (Freitag et al., 2022). IR spectroscopy involves the interaction of infrared radiation with molecules. When infrared light is passed through a sample, certain wavelengths of the light are absorbed by the sample's molecules, causing them to vibrate. Different functional groups within molecules absorb infrared radiation at characteristic frequencies, leading to the generation of an infrared spectrum. Aflatoxins have specific functional groups, such as carbonyl, alkene, and aromatic groups that can be detected using IR spectroscopy (Tao et al., 2019). By analyzing the infrared spectrum of a sample containing aflatoxins, characteristic peaks corresponding to these functional groups can be identified. It involves the following steps: (1) Preparation of a solid homogeneous sample as a thin film or mixing the sample with a suitable matrix to avoid interfering substances, (2) Detection of specific peaks of aflatoxin samples in the infrared spectrum (3) Quantification of aflatoxins by correlating the intensity of infrared absorption peaks with the calibration curves (Wang et al., 2015). IR spectroscopy offers a label-free and non-destructive method for aflatoxin detection, based on the characteristic absorption of infrared radiation by functional groups within the aflatoxin molecules. While it may not be as sensitive as fluorescence spectroscopy, it can still be a valuable tool for aflatoxin analysis, particularly in research and laboratory settings (Yao et al., 2022). These techniques can be used individually or in combination to achieve better sensitivity and selectivity in aflatoxin detection. They are essential tools in ensuring food safety and regulatory compliance in the agricultural and food industries.
- n.
Immunochemical methods
The specific antigen-antibody reaction-based immunochemical methods are extensively used for the detection of mycotoxins. One of the most important steps in developing immunoassay techniques is the production of high-quality antibodies. Because aflatoxins are tiny chemicals that do not elicit an immune response, they cannot, through any method, directly induce the antibodies production within the body. Therefore, to create artificial antigens with a high level of immunogenicity, they must be combined with macromolecular protein carriers. Thus, highly specific antibodies are required for their employment in different diagnostic systems, viz., enzyme-linked immunosorbent assay (ELISA), lateral flow immunoassay (LFA), etc which are frequently employed to quickly detect aflatoxins in food samples (Kuntalee et al., 2021). These qualitative or semi-quantitative tests rely on specific antibodies that bind to aflatoxin molecules, producing a detectable signal. In ELISAs, samples are immobilised in microtiter plates and the presence of aflatoxins is detected based on the specificity and sensitivity of the antibody. To identify mycotoxins, different ELISA formats are utilized; among these, traditional competitive and competitive inhibition formats work better for identifying low molecular weight compounds, such as mycotoxins with restricted epitope site display (Xu et al., 2018; Han et al., 2019).ELISA offers several advantages for mycotoxin detection, including high sensitivity, specificity, and the ability to analyze multiple samples simultaneously (Oplatowska-Stachowiak et al., 2016). However, ELISA requires specialized equipment (e.g., microplate reader) reagents and skill, along with a time-consuming assay procedure; but onsite rapid monitoring is not possible. Nonetheless, its accuracy and reliability make it a valuable tool for mycotoxin analysis in food safety laboratories and quality control processes.
For rapid and on-site detection of mycotoxins, lateral flow immunoassays (LFAs) can be employed. LFAs operate on the principle of immunochromatography using specific labelled antibodies to capture target analytes (mycotoxins) present in the sample and to produce signals (Tripathi, et al., 2017). It offers several advantages like rapid results (typically within minutes), simplicity of operation, portability, and cost-effectiveness (Li et al., 2019; Liu et al., 2020). However, they may have limitations in terms of sensitivity and specificity compared to laboratory-based methods like ELISA (Enzyme-Linked Immunosorbent Assay). Nonetheless, LFAs are valuable tools, especially for preliminary screening and field testing of the contaminants in food and feed.
To avoid the shortcomings of ‘onsite’ detection, immunosensors are now in use (Moon et al, 2018). These are analytical devices that combine the specificity of immunoassays with the sensitivity of modern sensor technologies to detect and quantify target analytes, such as aflatoxins. Immunosensors offer several advantages for aflatoxin detection, including high sensitivity, specificity, rapid analysis times, and potential for miniaturization and portability (Kinnamon et al., 2022; Viola et al., 2024). They are suitable for on-site and real-time monitoring of aflatoxin contamination in food and feed samples (Janik-Karpinska et al., 2022). Additionally, immunosensors can be integrated into automated systems for high-throughput analysis (Kinnamon et al., 2022). However, challenges such as stability, reproducibility, and interference from matrix components need to be addressed for widespread adoption in practical applications.
- o.
Nanotechnological interventions
Nanotechnology offers innovative solutions for the detection of aflatoxins, toxic compounds produced by certain moulds that can contaminate food products (Mohammad et al., 2022). To detect aflatoxin, nanotechnology has produced a wide variety of nanoparticles/materials, including AuNPs, CBNs, MNPs, QDs, and UCNPs, as well as their mixtures (Xue et al., 2019). Functional nanomaterials and conventional detection techniques can be combined to create innovative detection strategies with increased sensitivity, high throughput, and quick detection times. These strategies have garnered a lot of interest in the detection of aflatoxin (Yan et al., 2020). The development of sensitive alternative techniques for the quick identification of mycotoxins would be nanobiosensors. The focus of this study is on the use of nanomaterials in the creation of nanobiosensors and their use in the identification of mycotoxins in food and feed. In order to weave nanobiosensors for aflatoxin detection, scientists have inventoried nano-scale materials (Hu et al., 2023) such as carbon nanotubes, nanowires, nanoparticles, quantum dots, nanorods, and nanofibers.
Colourimetric Assays: Gold and nano nanoparticles are used as probes in colourimetric assays where the presence of aflatoxins causes a colour change visible to the naked eye. These assays are simple and do not require sophisticated instruments (Majdinasab et al., 2020).
Fluorescent Probes: Quantum dot-based fluorescent probes can detect aflatoxins with high sensitivity. The fluorescence intensity changes in the presence of aflatoxins, providing a quantitative measurement (Liao et al., 2023).
Electrochemical Sensors: Carbon nanotube-based electrochemical sensors detect aflatoxins by measuring changes in electrical conductivity when aflatoxins bind to the nanotubes (Le et al., 2020).
Nanoparticle-based biosensors: These are analytical devices that combine a biological recognition element (such as antibodies or enzymes) with a transducer to detect target molecules, including aflatoxins, in real time (Wang et al., 2016). Biosensors offer advantages such as portability, rapid analysis, and low sample volume requirements.
7. Postharvest Management of Aflatoxins
Postharvest peanut seed storage is a critical aspect of ensuring grain quality and preventing aflatoxin contamination. Proper storage conditions, including clean and dry environments with low kernel moisture content and protection from insect infestation, are recommended to minimize the risk of moulding and aflatoxin accumulation (Mutiga et al., 2015. Additionally, early detection of grain and mould respiration can help anticipate and prevent mycotoxigenic fungi spoilage. Furthermore, the use of drying systems and equipment, such as solar energy-based systems, can contribute to maintaining grain quality and reducing the growth of mycotoxigenic fungi. Proper storage practices, including the use of protectants and monitoring of temperature and relative humidity, can significantly impact mycotoxin incidences in stored peanuts and other grains (Lavkor and Var, 2017). In addition to these preventive measures, the use of hermetic storage technologies has shown promise in inhibiting mould development and preventing significant increases in aflatoxin levels (Villers, 2014).
Although the prevention of pre-harvest infection in peanuts is the best control strategy, aflatoxin also accumulates during harvesting and postharvest processing. Pre-harvest peanut seeds contain mycelia and spores of aflatoxigenic fungi, which can result in a significant decrease in grain quality when they are stored. If the storage conditions are not good, these seeds may cause serious damage and aflatoxin accumulation at higher than internationallyaccepted levels. Waliyar et al. (2008) reported that in West Africa aflatoxin accumulates, especially in susceptible cultivars, when pod removal from lifted plants is delayed. According to them the toxin content in peanut kernels increased from 4 ng/g when tested immediately after lifting the plants to 6.8 ng/g two weeks after cropharvesting in resistant cultivars 55-437 and J11. In the susceptible cultivars JL24 and Fleur 11, aflatoxin levels were 105 ng/g immediately after harvesting and 270 ng/g two weeks after harvesting. Farmers conventionally dry harvested plants in heaps, which increases humidity due to poor ventilation and favours fungal proliferation and toxin accumulation. These problems create conditions conducive to the accumulation of aflatoxin even in products that were uncontaminated when harvested.To lessen these problems, postharvest recommendations include the removal of pods soon after harvesting of plants. Replacing farmers’ conventional practice of drying in heaps with a “batch” drying process (where pods are kept facing the sun for rapid drying and easing good aeration) gradually reduces aflatoxin buildup.
- i.
Segregation of contaminated seeds: Kaaya and Warren (2005) demonstrated that uprooting groundnuts with hand hoes causes significant damage to the shells and kernels, making them susceptible to fungal infection during storage. Products contaminated with visible moulds, such as Aspergillus flavus or Aspergillus parasiticus, must be stored separately and not consumed. Sorting of kernels should be the initial step in postharvest management for small, shrivelled seeds (Davidson et al., 1982a), mouldy, discoloured seeds (Turner et al., 2005), and damaged seeds (Hamid, 1997), which account for around 80% of aflatoxin contamination (Afolabi et al. 2006). Following lot segregation, off-coloured, infected kernels are screened out by manual sorting, seed size and density separation, or electronic colour sorting. Floating and density segregation also reduce aflatoxins in storage units; kernels that are floating in water contain 95% of aflatoxins (Phillips et al., 1994). Electronic colour sorting has been reported to reduce 70% aflatoxin content in commercial shelling plants (Cole et al., 1995; Dorner, 2008), but this method has an inherent disadvantage because all the altered kernels are not contaminated, so this technology is never 100% effective in aflatoxin removal (Waliyar et al., 2008). The best method to effectively reduce aflatoxin contamination is blanching followed by photoelectric colour sorting and hand-picking (Dorner, 2008), but this comes with increased cost, weight loss during blanching, and the loss of kernels by sorting (Dorner and Lamb, 2006). Aflatoxin-contaminated product inspection may now be done on a broad scale thanks to advancements in sorting methods, such as infrared and UV sorting combined with color-detection technology (Womack et al., 2014).
The first step of correct postharvest management is lot segregation, which means that lots with visible moulds, confirming the presence of A. flavus or A. parasiticus, or with aflatoxin contamination above the legal limit must be stored separately and not used for edible purposes. When aflatoxin contamination occurs, there are usually only a few highly contaminated seeds irregularly distributed in the peanut lots while most of the harvested seeds are free of contamination. Kernels that differ substantially in colour (i.e., are darker, lighter or moulded) from the standard for the particular cultivar or cultivars being examined, should be discarded. Applicable methods include manual sorting, seed size and density separation, or electronic colour sorting. The most effective technique for managing aflatoxin contamination in commercial shelling plants is electronic colour sorting (Dorner, 2008), reported to produce a 70% reduction for aflatoxin, reported to produce a 70% reduction for aflatoxin (Cole et al., 1995). This approach has a disadvantage: the altered kernels are linked to aflatoxin contamination, but they are not necessarily contaminated. This means that yield losses due to different sorting approaches are not always justified by aflatoxin reduction (Waliyaret al., 2008). In recent years, continued advances in electronic colour sorting technology have improved sorter efficiency; however, not all aflatoxin-contaminated kernels are discoloured, so this technology is never 100% effective in aflatoxin removal. Finally, to reduce effectively the aflatoxin concentration in shelled peanut lots the best method is blanching followed by photoelectric color sorting and hand-picking (Dorner, 2008). The major disadvantage to this form of aflatoxin reduction is the cost, including direct charges, weight loss during blanching, and the loss of kernels by sorting (Dorner and Lamb, 2006).
- ii.
Moisture management in harvested seeds: Heathcote and Hibbert (1978) found that excessive grain moisture enhances postharvest moulding and aflatoxin contamination. Proper grain drying after harvest to ≤7% moisture levels is optimum to limit fungus growth, particularly aflatoxigenic strains (Dick, 1987). Moisture content, sanitary conditions and temperature in the environment are the key parameters for aflatoxin contamination in both storage and transit (Abramson, 1998). It is also well known that stockpiling peanuts can lead to heat buildup and moisture accumulation, resulting in mould growth and aflatoxin contamination. The relative humidity and moisture content are mainly associated with aflatoxin production; at relative humidity levels above 82% and moisture contents of 10% or higher, aflatoxin is produced in elevated amounts. (Diener and Davis, 1970). On the other hand, peanuts can be stored for almost a year at a temperature of 25 to 27 °C and a relative humidity of 70% (Odogola, 1994; Waliyar et al., 2008). It is safe to use these moisture levels on both shelled and unshelled groundnuts. When storing unshelled groundnuts, the maximum moisture content is 9%, which is higher than when storing shelled groundnuts (7%). After harvest, inverted windrowing increased air circulation, which sped up the drying of the pods and kernels while reducing the growth of mould and aflatoxin contamination. Similar results were obtained after 4 to 6 days of field drying the pods. Both strategies are financially effective for managing aflatoxin contamination (A’Brook, 1963; Devi and Hall, 2000).
According to the guide of Codex, to prevent an increase in aflatoxin contamination occurring during storage and transportation, it is important to control the moisture content, the temperature in the environment, and the hygienic conditions. Postharvest aflatoxin contamination is most attributable to improper storage of the pods and seeds. It is well established that mould invasion is facilitated because of increased moisture levels of stored commodities (Abramson, 1998). Inappropriate kernel moisture during storage can proceed from leaky roofs, condensation because of improper ventilation in the warehouse, high-moisture foreign material associated with stored peanuts, and high-moisture peanuts initially going into storage (Davidson et al., 1982). The minimum moisture content for A. flavus growth on peanut is 8–10% at around 82% relative humidity, and aflatoxin production is generally correlated with kernel moisture contents of 10% or higher (Diener and Davis, 1970). It is well known also that stockpilingpeanuts can cause heat build-up and moisture accumulation, resulting in mould growth and aflatoxin contamination. Other studies reported that the maximum moisture content for storage of groundnuts (unshelled) is 9% while that for shelled peanut is 7%. At these moisture contents, if the environment's relative humidity is maintained at 70% and temperature at 25–27 °C, safe storage of nuts is guaranteed for approximately one year (Waliyaret al., 2008).
- iii.
Cleaning and disinfecting of storage house and handling equipment: All handling equipment and the warehouse system as a whole need to be thoroughly cleaned using compressed air and water. The groundnut must be completely cleaned of all dust, dirt, and residue before the farmer's stock may enter the shelling mechanism, because this sanitation results in reduced aflatoxin levels in kernels (Hell et al., 2000). According to Rahmianna et al. (2007), storing dry pods in airy, dry, and clean rooms can help decrease aflatoxin accumulation. Loose-shelled kernels, high-moisture components, and dirt should be removed with extra care during cleaning since these materials will probably increase the danger of insect damage and mould contamination, which could result in the development of aflatoxin during storage. Regular inspections are crucial to prevent the growth of mould. If Aspergillus growth is visible right away, corrective action must be taken. The mold-affected kernels must be removed for consumption. Paramawati et al. (2006) showed postharvest machines such as threshers, dryers, and shellers improve yield and reduce processing and drying time. As a result, they are often linked to lower aflatoxin contamination in groundnuts.
During the postharvest stage, thorough drying, prompt storage and transport using clean, dry containers are the basic elements of aflatoxin prevention and control. Timely harvest is also critical for aflatoxin prevention. Clean, dry, insect and rodent-free storage conditions are critical to prevent aflatoxin growth as noted by the USAID desk review. Making storage options inexpensive and accessible is of paramount importance for consistent, long-term utilization. The USAID synthesis outlines broadly the various pre-harvest and postharvest methods (USAID and Danya International, 2012). A study on low-technology postharvest handling options for groundnut in an aflatoxin-pronearea in Guinea was successfully achieved. The package of interventions investigated included: hand sorting, storage in jute bags, education on improved sun drying, wooden pallets for drying, locally-made natural fibre mats and insecticides.
- iv.
Modification of air-gas composition: Hell and Mutegi (2011) showed that smoking pods and kernels during storage reduces moisture content and prevents mould infestation. As most Aspergillus species are obligate aerobes, mould growth and aflatoxin production can be controlled by modifying atmospheric gases such as carbon dioxide, nitrogen, and sulphur dioxide in storage godowns and transportation (Kabak et al., 2006). Heathcote and Hibbert (1978) found that increasing the concentration of CO2 in storage godowns resulted in significant reductions in aflatoxin production. Chemical fumigants such as ethylene oxide and methyl bromide have been shown to effectively reduce toxic mould and aflatoxin production, but due to health concerns and non-target effects, these fumigants are slowly phased out of agriculture (Bankole et al., 1996).
Since mycotoxin-producing moulds are obligate aerobes, it seems likely that mycotoxin production could be prevented or at least reduced by modification of atmospheric gasses in storage silos; such as by using carbon dioxide, nitrogen, carbon monoxide, and sulfur dioxide (Kabak et al., 2006).Torlaket al. (2016)suggested that significant (P < 0.05) reductions in the AFB1 level and microbial population can be achieved in poultry feed by ozonation with an acceptable change in lipid oxidation.According to Shi et al.(2017), ozone applications have demonstrated great potential for the detoxification of aflatoxins in some foods.It was found that ClO2 gas could inhibit mycelium growth, spore germination, and AFB1-producing ability of Aspergillus flavus.
- v.
Application of biocontrol agents:The most effective strategy for managing aflatoxin in the field and storage is to use naturally occurring atoxigenic strains through competitive exclusion. Dorner and Cole (2002) demonstrated that field application of non-aflatoxin-forming biocontrol strains of
A. flavus had long-term field protection with a carryover effect in reducing mould growth of
Aspergillus and aflatoxin contamination in storage. The study results of Kong Q et al. (2010) showed that the antagonist of marine
Bacillus megaterium had a significant effect on biocontrol effectiveness in vivo, significantly reducing the biosynthesis of aflatoxins and the expression of the
aflR gene and
aflS gene. Aflasafe™ (
www.aflasafe.com) is an indigenous aflatoxin biocontrol product that uses nontoxigenic strains of
A. flavus to out-compete aflatoxin-producing strains in the field (Atehnkeng et al., 2008). According to Grace et al. (2015), Aflasafe™ effectively reduced aflatoxin contamination in maize and groundnut by 80–99% across various stages of crop growth and the value chain in several African countries. The authors reported case studies on aflatoxins and investigated the aflatoxin standards for feed (Grace et al., 2015, 2015a).
Using non-aflatoxigenic strains of A. flavus (AF−) as biocontrol agents is the most promising method to control AFs’ contamination in cereal crops. AF− strains cannot produce AFs due to the absence of polyketide synthase genes or genetic mutation. AF− strains competitively exclude the AF+ strains in the field, giving an extra advantage to the stored grains. Several microbiological, molecular, and field-based approaches have been used to select a suitable biocontrol agent. The effectiveness of biocontrol agents in controlling AF contamination could reach up to 99.3% (Khan et al., 2021). Dorner (2009, 2009a) working on biological control strategy of aflatoxin contamination reported a reduction of AFs concentrations between 74.3% and 99.8% in peanut crops when they applied the AF− strains with non-aflatoxigenic strains of A. parasiticus. Peanuts produce fruiting bodies below the soil and hence increase the chances of biological control of AFs.Kong et al. (2010) examined the potential antifungal activity of B. megaterium against the growth of A. flavus in groundnut kernels in vitro and in vivo. However, it has been found that biological control of AFs using AF− strains is more productive compared to bacterial strains (Khan et al., 2021).
- vi
Detoxification of peanuts: Despite improved handling, processing, and storage, aflatoxin contamination is still a major problem in the groundnut industry. Though a lot of physical methods such as cooking, roasting, frying, spray drying, and baking can effectively deactivate aflatoxins, new methods of detoxifying contaminated products are required to mitigate economic and health consequences while also adding value to the groundnut industry. Proctor et al. (2004) studiedthe effectiveness of ozonation and mild heat in breaking down aflatoxins in peanut kernels and flour and showed that ozonation efficiency increased with higher temperatures and longer treatment times. Regardless of treatment combinations, aflatoxins B1 (reduced in groundnuts by 77% with 10 min of ozonation at 75 °C) and G1 had the highest degradation rates, with a more efficient degradation achieved in peanut kernels than in flour. The temperature effect decreased as the exposure time increased, implying that ozonation at room temperature for 10–15 minutes could yield degradation levels comparable to those achieved at higher temperatures with a shorter exposure time while being more economical. The aflatoxin contamination can be reduced to a great extent when the produce is exposed to ammonia at a high temperature and pressure (Gomaaet al., 1997). Sodium chloride (Scott, 1984), sodium bisulphite (Doyle and Marth, 1978), and potassium bisulphite (Doyle et al., 1982) are also reported as chemical detoxifiers.
H2O2 treatment fulfils the requirement of environmentally friendly and safety concerns since it can be easily removed or decomposed into water and oxygen. Even though the high efficiency in detoxifying aflatoxins (AF) in foods by H2O2 was reported by some studies, the information to utilize this reagent for practical application is very limited (Shen and Singh, 2022). They reported that immersing peanuts in 30 g/hg H2O2 at 50 °C for 24 h successfully reduced the AF from about 2.8 μg/g to less than 100 ng/g in severely contaminated peanuts.Liu et al. (2018)reported that electron beam irradiation (EBI) treatment EBI treatment is a potentially applicable method to effectively and safely degrade the AFB1 in the peanut meal.
- vii
Chemical control: Spraying chemicals onto freshly harvested groundnut pods in the field reduces A. flavus invasion and aflatoxin contamination in kernels during storage (Bell and Doupnik Junior, 1972). Spraying natural antifungals and chemical preservatives can prevent postharvest aflatoxin contamination in groundnuts (Haciseferogullaryet al., 2005; Onyeagbaet al., 2004). The US Food and Drug Administration (FDA) allowed the use of formulations containing antioxidants such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and propylparaben (PP) for the effective control of moulds such as Aspergillus flavus in natural and inoculated stored peanuts by preventing the oxidation of groundnut and delaying their oxidative rancidity. Passone et al. (2009) showed that when stored groundnut was treated with a ternary mixture of food-grade antioxidants, mould growth was inhibited with no trace of aflatoxin. Aside from that, plant-based phytochemicals are being developed to manage aflatoxin contamination in response to consumer and food industry demands (Etcheverry et al., 2011). Natural products based on cinnamon and clove oil have shown great results in reducing mould growth, as has aflatoxin, but they are very expensive. However, the alternatives to these, such as methyl eugenol (which is less expensive), when sprayed in 0.5% concentration, show protection to groundnut pods and kernels from aflatoxin contamination (Sudhakar et al., 2009).
Several chemicals such as acids, alkalis, oxidizing agents, aldehydes, and several gasses are also proved to mitigate the aflatoxigenic fungal growth and aflatoxin production when used in appropriate quantities (Udomkun et al., 2017). In peanut and maize, AFB1 production was also inhibited by the treatment of sodium chloride (10%), acetic acid (5%), and propionic acid (5%) (Brožkováet al. 2015). Even under favourable conditions for the growth of A. flavus, several salts of propionic acid-like calcium, and sodium propionates were able to reduce the aflatoxin formation in maize (Hassan et al. 2015). Azole fungicides are also used as a tool to control fungus growth and aflatoxin production with prochloraz being more effective than tebuconazole (Mateo et al. 2017).
8. Aflatoxin Management and Future Strategies
Widespread occurrence of aflatoxin is reported in groundnut, rice and nuts (pistachios, hazelnuts, and almonds), spices, and dried figs, with concentrations up to 1000 µg/kg (Shabeer et al., 2022) necessitating management of aflatoxins imperative for maintaining nutritional security. This is aggravated due to insufficient knowledge among farmers about aflatoxins and dependency on laboratory techniques for their detection (Udomkun et al., 2017a). Further, there is a need to educate all the stakeholders about the role of good agricultural practices (GAPs), good manufacturing practices (GMPs) and good storage practices (GSPs) in mitigation of mycotoxin contamination.Major factors affecting colonization and toxin production by
A. flavus are temperature, humidity, environmental stress, injury caused by insects or birds on the host, and post-harvest practices like improper handling and storage conditions (Amaike and Keller, 2011; Callicott etal., 2018). Due to adverse climatic conditions and climate change scenarios aflatoxin contamination is being reported from the countries previously not affected by the problem. Due to the determinantal effects of aflatoxins on human and animal health, it is necessary to develop strategies for the economical and sustainable management of these toxins. These strategies include biological, chemical, and physical management practices (
Figure 2).
- a.
-
Biological management
- i.
Utilizing atoxigenic strains of Aspergillus spp.: This is the most potential method for the management of aflatoxins and is commercially viable in many countries (Ortega-Beltran and Bandyopadhyay, 2021). Atoxigenic strains do not produce aflatoxins and compete with the aflatoxin-producing strains, thereby negatively affecting the multiplication of toxigenic fungi and reducing aflatoxin accumulation in the target crops/ produce. The atoxigenic nature of these strains is attributed to deletion in the gene or of genes that are involved in the aflatoxin biosynthesis pathway or SNP (single nucleotide polymorphism) that initiates a stop codon in the aflC (pksA) gene that plays a role in the polyketide pathway of aflatoxin (Atehnkengetal., 2016; Grubishaetal., 2015). Isolate of A. flavus, AF36, is registered with the US Environmental Protection Agency (USEPA) for the management of aflatoxins in maize and nuts etc., Another biocontrol product—with a different active ingredient registered with USEPA is Afla-Guard®, for use in maize and groundnut in the US (Ortega-Beltran and Bandyopadhyay, 2021).
- ii.
Biological management by other microorganisms is at the experimental stage and no commercial product is available for the management of aflatoxins. Further, it is reported that these microorganisms may reduce conidial production along with aflatoxin control. Antagonistic strains of Pseudomonas, bacillus, and Trichodermaspp. (Mahato etal., 2019, Shabeer et al., 2022) and yeast (Shabeer et al., 2022) may be applied in preharvest conditions for reducing infection by Aspergillus spp. and reduction in aflatoxins.
- b.
Chemical management
Chemicals are used for the treatment of the crop produce for reducing post-harvest contamination of aflatoxins (
Table 4). In addition to these chemicals adsorbents synthetic polymers including polyvinyl pyrrolidione, cholestyramine, complex polysaccharides etc. are used for removing aflatoxins from the diet (Shabeer et al., 2022).
Ozone can be produced from UV irradiation, electrical discharge in oxygen and electrolysis of water and is granted “Generally Recognised as Safe” (GRAS) status for use in food and water. Aflatoxins are degraded by electrophilic attack on the double-bonded carbons in the furan ring by ozone resulting in the formation of primary ozonides followed by rearrangement into monozonide derivatives like aldehydes, ketones and organic acids (Jalili, 2016). The efficiency of ozone in degrading aflatoxins depends upon exposure time and moisture content of the produce. Also, though found effective at the laboratory scale, scaling up ozone for aflatoxin management requires further development of equipment of high capacity for commercial facilities.
- c.
-
Physical management
- i.
Heat treatment: Aflatoxins are generally stable at high temperatures but inactivation is reported for a few aflatoxins (Shabeer et al., 2022).
- ii.
Irradiation: Irradiation can be classified into two forms: ionizing (e.g. X-rays, ultraviolet rays, gamma rays, and electron beams) and non-ionizing irradiation (e.g. radio waves, microwaves, infrared waves, and visible light waves). Gamma rays and electron beams are ionizing radiation employed for aflatoxin degradation. Gamma rays are emitted from decaying unstable radioactive isotopes and are highly reactive and penetrating. These rays may cause lipid peroxidation and hence are not suitable for food with high lipid and vitamin content. The aflatoxin degradation capacity of gamma rays may range up to 95% and is dependent on radiation dose, aflatoxin concentration, water content and matrix composition. Other types of irradiation are electronic beams and UV irradiation. Among the two, UV irradiation is more preferred method as it is non-thermal food decontamination technology, and is cost-effective and ecofriendly with no toxic effects and waste generation (Shabeer et al., 2021; Guo et al., 2020). Other methodologies viz. Pulsed light treatment and visible light treatment for aflatoxin are also found effective and reviewed recently by (Guo et al., 2020; Kutasi et al., 2021)
- d.
Integrated Aflatoxin Management (IAM)
Integrated aflatoxin management includes pre-harvest prevention of aflatoxin production and post-harvest aflatoxin elimination. This can be achieved through following GAPs i.e. use of resistant varieties, balanced use of fertilisers, pest management, harvesting at the optimum time and maintaining optimum temperature and moisture during storage, these practices may reduce the growth of aflatoxin-producing fungi and aflatoxin contamination in food and feed (Guo etal., 2020). Further, awareness creation about the aflatoxin contamination may reduce the harmful effects on animal and human health. Also, the development of sensitive detection assays for aflatoxins in feed and food followed by treatment procedures will facilitate safer food availability. One such initiative is the ‘Aflasafe Initiative’ operating in Sub-Saharan Africa (SSA). The initiative is facilitated by the International Institute of Tropical Agriculture (IITA), along with USDA–ARS, and national and international institutions in maize, groundnut, sorghum, sesame, millet, and sunflower in SSA countries (
Figure 3). The description of the initiative is summarized by Ortega-Beltran and Bandyopadhyay (2021), and it is evident that each strategy synergises the complete process of producing aflatoxin-safe produce in targeted crops.
9. Conclusion
During this phase of One Health, the relationship between human, animal and environmental health is acknowledged. The Wildlife Conservation Society (WCS) has introduced the term “One World-One Health,” which when combined with twelve proposals known as the Manhattan Principles, focuses on developing a more comprehensive strategy for averting epidemic disease and pre serving ecosystem integrity.The existence of aflatoxin in food and animal feed has far-reaching implications for human and animal health, as well as trade-related concerns that lead to substantial economic losses. It is of utmost importance to enforce stringent regulatory measures to address aflatoxin contamination and to educate the public about the toxicity of aflatoxins. These measures are essential in order to protect individuals from the detrimental impacts caused by aflatoxins. Furthermore, extensive research is needed to develop advanced techniques or kits capable of detecting trace amounts of aflatoxin in food and food products.
Pre-harvest measures include implementing good agricultural practices (GAPs) to minimize fungal contamination in the field, while postharvest technologies such as proper drying, storage, and sorting techniques help prevent further aflatoxin production during storage and processing. Public awareness campaigns educate stakeholders about the risks of aflatoxin contamination and promote the adoption of best practices throughout the agricultural and food industries. By addressing each of these aspects comprehensively, stakeholders can develop holistic strategies to mitigate the social and economic impacts of fungi and aflatoxins in maize and peanuts, ultimately promoting food security, economic stability, and public health.
Advanced technology offers powerful tools for the detection of aflatoxins, improving food safety through enhanced sensitivity, specificity, and rapid response. Different molecular, serological, and the combination of chromatography-spectroscopy-based tools can detect aflatoxins at very low concentrations, ensuring food safety. Further, nanotechnology-based tools often provide faster results compared to traditional methods. Many nanosensors can be integrated into portable devices, allowing for on-site testing. Some nanotechnology-based methods can be more cost-effective than conventional laboratory techniques. The ongoing research and development in this field are likely to yield even more advanced and accessible detection methods in the future.
Postharvest management is a critical step in ensuring food safety and minimizing aflatoxin contamination (Cheli et al., 2017). By implementing proper storage and handling practices, as well as employing strategies such as drying, sorting, cleaning, detoxification, and biological and chemical control, producers can significantly reduce the risk of aflatoxin contamination and protect the health of consumers (Miller, 2008). Therefore, a comprehensive approach that combines effective postharvest management practices with government regulations and monitoring is essential in the fight against aflatoxin contamination. Aflatoxin contamination in food crops and their derived products has become a serious threat to human and animal lives.Aflatoxin-producingmoulds, including A. flavus, contaminate these commodities at different stages within the food web.Research efforts in the prevention and management of aflatoxin contamination in peanuts are much required. Looking into the seriousness of the matter postharvest practices should be followed by farmers and food industries to minimize and degrade the aflatoxin content in food crops and their derived products.
It could be concluded that aflatoxins are toxic compounds produced by certain moulds (Aspergillus species) that commonly contaminate crops such as maize, peanuts, and tree nuts. The toxicity and health implications of aflatoxin exposure are significant and multifaceted, encompassing acute and chronic health effects.The chemistry of aflatoxin toxicity involves bioactivation to reactive intermediates that damage DNA and proteins, leading to severe health effects, including cancer and immune suppression. The toxicity and health implications of aflatoxin are profound, affecting both individual health and broader socio-economic conditions. Managing aflatoxin exposure through regulatory and agricultural practices is crucial to mitigate these risks.Addressing aflatoxin contamination requires a comprehensive approach involving regulatory oversight, public health initiatives, and ongoing research to develop better detection, prevention, and mitigation strategies. Ensuring food safety and reducing exposure to aflatoxins remain critical to protecting public health, particularly in vulnerable populations.
Aflatoxins are a serious threat to food and nutritional security globally. The problem is multifaceted in developing and underdeveloped countries, due to lack of information about aflatoxin contamination and its deleterious impacts on health. Integrated aflatoxin management is the most suited strategy currently for aflatoxin management as this will facilitate synergizing individual intervention to effective management. Aflatoxin and the related food-borne diseases pose a significant global health issue. It is imperative to enhance the awareness levels of food producers, food processors, consumers, and public authorities to effectively minimize Aflatoxin exposure, especially in developing countries. It is crucial to adapt national and international regulatory policies to guarantee consumer safety.
Author Contributions
Conceptualization, JPR and AG; methodology, AG and JN; software, RK, SK, AC, SKH, KB and JN; validation, NP, VK, PP, NK and NA; formal analysis, AKS and MWS; investigation, MWS, UBS, RK; resources, JPR, AG, and RB; writing—original draft preparation, SA, AG and SSB; writing—review and editing, JPR, AG, MWS and SSB; visualization, JPR, AG and MWS; supervision, JPR, AG and MWS. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- A’Brook, J., 1963. Artificial drying of groundnut: a method suitable for small farmer. Tropical Agriculture 40: 2412-2415.
- Abbas, H.K.; Jr., H.J.M.; Bruns, H.A.; Shier, W.T. Effect of Planting Density, Irrigation Regimes, and Maize Hybrids with Varying Ear Size on Yield, and Aflatoxin and Fumonisin Contamination Levels. Am. J. Plant Sci. 2012, 03, 1341–1354, . [CrossRef]
- Abbas, M. (2022). Chromatographic Techniques for Estimation of Aflatoxins in Food Commodities. IntechOpen. [CrossRef]
- Abdel-Hadi, A.; Schmidt-Heydt, M.; Parra, R.; Geisen, R.; Magan, N. A systems approach to model the relationship between aflatoxin gene cluster expression, environmental factors, growth and toxin production by Aspergillus flavus. J. R. Soc. Interface 2011, 9, 757–767, doi:10.1098/rsif.2011.0482.
- Abramson, D. Mycotoxin formation and environmental factors. In K. K. Sinha, & D. Bhatnagar (Eds.), Mycotoxins in agriculture and food safety, 1998, 255–277.
- Adetunji, M.C.; Ngoma, L.; Atanda, O.O.; Mwanza, M. A polyphasic method for the identification of aflatoxigenic Aspergilla from cashew nuts. World J. Microbiol. Biotechnol. 2019, 35, 15, . [CrossRef]
- Adisa, O.M.; Botai, J.O.; Adeola, A.M.; Hassen, A.; Botai, C.M.; Darkey, D.; Tesfamariam, E. Application of Artificial Neural Network for Predicting Maize Production in South Africa. Sustainability 2019, 11, 1145, . [CrossRef]
- Afolabi, C.G., Bandyopadhyay, R., Leslie, J.F. and Ekpo, E.J.A., 2006. Effect of sorting on incidence and occurrence of fumonisin and fumonisin-producing Fusarium species on maize (Zea mays L.) in Nigeria. Journal of Food Protection 69: 2019-2023.
- Afsah-Hejri, L.; Jinap, S.; Arzandeh, S.; Mirhosseini, H. Optimization of HPLC conditions for quantitative analysis of aflatoxins in contaminated peanut. Food Control. 2011, 22, 381–388, . [CrossRef]
- Agag, B. (2004). Mycotoxins in foods and feeds: Aflatoxins. Assiut University Bulletin for Environmental Research, 7(1), 173–205.
- Ahuja, V.; Singh, A.; Paul, D.; Dasgupta, D.; Urajová, P.; Ghosh, S.; Singh, R.; Sahoo, G.; Ewe, D.; Saurav, K. Recent Advances in the Detection of Food Toxins Using Mass Spectrometry. Chem. Res. Toxicol. 2023, 36, 1834–1863, . [CrossRef]
- Akbar N, Nasir M, Naeem N, Ahmad MU, Saeed F, Anjum FM, Iqbal S, Imran M, Tufail T, Shah FU, Atif M. Assessment of aflatoxin in milk and feed samples and impact of seasonal variations in the Punjab, Pakistan. Food Sci Nutr. 2020;8(6):2699-2709. [CrossRef]
- Alameri, M.M.; Kong, A.S.-Y.; Aljaafari, M.N.; Ali, H.A.; Eid, K.; Sallagi, M.A.; Cheng, W.-H.; Abushelaibi, A.; Lim, S.-H.E.; Loh, J.-Y.; et al. Aflatoxin Contamination: An Overview on Health Issues, Detection and Management Strategies. Toxins 2023, 15, 246. [CrossRef]
- Al-Jaza, D.; Medina, A.; Magan, N. Abiotic factors affect growth and aflatoxin B-1 production by Aspergillus flavus strains on chilli powder and red chillies. World Mycotoxin J. 2022, 15, 251–260, . [CrossRef]
- Allah Ditta, Y., Mahad, S., & Bacha, U. (2019). Aflatoxins: Their Toxic Effect on Poultry and Recent Advances in Their Treatment. IntechOpen. [CrossRef]
- Alsayyah, A., ElMazoudy, R., Al-Namshan, M., Al-Jafary, M., &Alaqeel, N. (2019). Chronic neurodegeneration by aflatoxin B1 depends on alterations of brain enzyme activity and immunoexpression of astrocyte in male rats. Ecotoxicology and Environmental Safety, 182, 109407. [CrossRef]
- Amaike, S.; Keller, N.P. Aspergillus flavus. Annu. Rev. Phytopathol. 2011, 49, 107–133. [CrossRef]
- Ammann, H.M. (2003). Is indoor mold contamination a threat to health? Journal of Environmental Health, 66(2), 47–49.
- Manjunatha, A.S., Manjunath, K., & Mohana, D. C. (2018). Assessment of contamination levels and characterization of aflatoxin B1 producing Aspergillus flavus strain from food and feed samples from local markets of South India. Int. J. of Life Sciences, 6(1), 326-334.
- Aslam, N.; Wynn, P.C. Aflatoxin Contamination of the Milk Supply: A Pakistan Perspective. Agriculture 2015, 5, 1172–1182, . [CrossRef]
- Atehnkeng, J., Ojiambo, P.S., Ikotun, T., Sikora, R. A., Cotty, P. J., &Bandyopadhyay, R. (2008). Evaluation of atoxigenic isolates of Aspergillus flavus as potential biocontrol agents for aflatoxin in maize. Food Additives and Contaminants Part A, 25(10), 1264–1271. [CrossRef]
- Atehnkeng, J.; Donner, M.; Ojiambo, P.S.; Ikotun, B.; Augusto, J.; Cotty, P.J.; Bandyopadhyay, R. Environmental distribution and genetic diversity of vegetative compatibility groups determine biocontrol strategies to mitigate aflatoxin contamination of maize by Aspergillus flavus. Microb. Biotechnol. 2016, 9, 75–88, . [CrossRef]
- Achaglinkame, M.A.; Opoku, N.; Amagloh, F.K. Aflatoxin contamination in cereals and legumes to reconsider usage as complementary food ingredients for Ghanaian infants: A review. J. Nutr. Intermed. Metab. 2017, 10, 1–7, . [CrossRef]
- Awuchi, C.G., Amagwula, I.O., Priya, P., et al. (2020). Aflatoxins in foods and feeds: A review on health implications, detection, and control. Bulletin of Environment, Pharmacology and Life Sciences.
- Aycicek, H.; Aksoy, A.; Saygi, S. Determination of aflatoxin levels in some dairy and food products which consumed in Ankara, Turkey. Food Control. 2005, 16, 263–266, . [CrossRef]
- Aziz, N. and Youssef, A., 1991. Occurrence of aflatoxin producing moulds in fresh and processed meat in Egypt. Food AdditContam, 8: 321-331.
- Azziz-Baumgartner, E., Lindblade, K., Gieseker, K., Rogers, H.S., Kieszak, S., Njapau, H., Schleicher, R., McCoy, L.F., Misore, A., DeCock, K., Rubin, C. and Slutsker, L., 2005. Case-control study of an acute aflatoxicosis outbreak, Kenya, 2004. Environmental Health Perspective 113: 1779-1783.
- Baazeem, A.; Medina, A.; Magan, N. Effect of Acclimatization in Elevated CO2 on Growth and Aflatoxin B1 Production by Aspergillus flavus Strains on Pistachio Nuts. Microorganisms 2021, 10, 49, . [CrossRef]
- Bandyopadhyay, R.; Kumar, M.; Leslie, J.F. Relative severity of aflatoxin contamination of cereal crops in West Africa. Food Addit. Contam. 2007, 24, 1109–1114, . [CrossRef]
- Bankole, S.A., Eseigbe, D.A. and Enikuomehin, O.A., 1996. Mycoflora and aflatoxin production in pigeonpea stored in jute sacks and iron bins. Mycopathologia 132: 155-160.
- Bartolić, D.; Mutavdžić, D.; Carstensen, J.M.; Stanković, S.; Nikolić, M.; Krstović, S.; Radotić, K. Fluorescence spectroscopy and multispectral imaging for fingerprinting of aflatoxin-B1 contaminated (Zea mays L.) seeds: a preliminary study. Sci. Rep. 2022, 12, 1–8, . [CrossRef]
- Battacone G, Nudda A and Pulina G (2010). Effects of ochratoxinA on livestock production. Toxins 2: 1796- 1824.
- Küçükersan, B.E. Comparison of nutritional composition (moisture, ash, crude protein, nitrogen) and safety (aflatoxin, nitrate/nitrite) of organic and conventional rice and lentil samples consumed in Ankara. Ank. Univ. Veter- Fak. Derg. 2016, 63, 365–370, . [CrossRef]
- Bbosa, S.G., David, K.A., David, K.A., ... Ssenyonga, R. (2013). Review of the biological and health effects of aflatoxin on body organ and systems. In M. Sajid & F. Masood (Eds.), Aflatoxins - Recent Advances and Future Prospects (pp. 239–265).
- Bedard, L.L.; Massey, T.E. Aflatoxin B1-induced DNA damage and its repair. Cancer Lett. 2006, 241, 174–183, . [CrossRef]
- Bell, D.K. and Doupnik Jr., B., 1972. Chemicals in the windrow for controlling aflatoxins in peanuts. Journal of American Peanut Research and Education Association 4: 18-20.
- Benkerroum, N. Chronic and Acute Toxicities of Aflatoxins: Mechanisms of Action. Int. J. Environ. Res. Public Heal. 2020, 17, 423, . [CrossRef]
- Bennett JW, Klich M. (2003). Mycotoxins. ClinMicrobiol Rev 16(3):497. https:// doi. org/ 10. 1128/ cmr. 16.3. 497- 516. 2003.
- Bernáldez, V.; Córdoba, J.J.; Magan, N.; Peromingo, B.; Rodriguez, A. The influence of ecophysiological factors on growth, aflR gene expression and aflatoxin B 1 production by a type strain of Aspergillus flavus. LWT Food Sci. Technol. 2017, 83, 283–291, doi:10.1016/j.lwt.2017.05.030.
- Bhatnagar, D.; Cary, J.W.; Ehrlich, K.; Yu, J.; Cleveland, T.E. Understanding the genetics of regulation of aflatoxin production and Aspergillus flavus development. Mycopathologia 2006, 162, 155–166, . [CrossRef]
- Bianco, G.; Russo, R.; Marzocco, S.; Velotto, S.; Autore, G.; Severino, L. Modulation of macrophage activity by aflatoxins B1 and B2 and their metabolites aflatoxins M1 and M2. Toxicon 2012, 59, 644–650, . [CrossRef]
- Binder, E.; Tan, L.; Chin, L.; Handl, J.; Richard, J. Worldwide occurrence of mycotoxins in commodities, feeds and feed ingredients. Anim. Feed. Sci. Technol. 2007, 137, 265–282, . [CrossRef]
- BIS, 2021. Bureau of Indian Standards (Animal Husbandry, Feeds and Equipment Sectional Committee, FAD 5), Specification for cottonseed oil cake as livestock feed ingredient. Third Revision of IS 1712, ICS code 65.120.
- Blandino, M.; Reyneri, A.; Vanara, F. Influence of nitrogen fertilization on mycotoxin contamination of maize kernels. Crop. Prot. 2008, 27, 222–230, . [CrossRef]
- Boko, M.; Niang, I.; Nyong, A.; Vogel, C.; Githeko, A.; Medany, M.; Osman-Elasha, B.; Tabo, R.; Yanda, P. Africa. In ClimateChange: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the IntergovernmentalPanel on Climate Change (IPCC); Parry, M.L., Canziani, O.F., Palutikof, J.P., Linden, P.J., van der Hanson, C.E., Eds.; CambridgeUniversity Press: Cambridge, UK, 2007; pp. 433–467.
- Bolu, S. A., Elelu, N., Ahmed, R. N., Solaojo, F. E., Daramola, K. F., Omotosho, V. S., Atitebi, A. G., &Afeni, M. (2014). Effect of vitamins, amino acids and phyto-active biomolecules on Aspergillus flavus in poultry production. Pharmacology and Therapeutics. [CrossRef]
- Zerdan, M.B.; Moussa, S.; Atoui, A.; Assi, H.I. Mechanisms of Immunotoxicity: Stressors and Evaluators. Int. J. Mol. Sci. 2021, 22, 8242, . [CrossRef]
- Brown R L, Chen Z Y, Cleveland T E, Russin J S (1999). Advances in the development of host resistance in corn to aflatoxin contamination by Aspergillusflavus. Phytopathology, 89(2), 113-117. [CrossRef]
- Brožková, I., Šmahová, P., Vytřasová, J., Moťková, P., Pejchalová, M., &Šilha, D. Influence of chosen microbes and some chemical substances on the production of aflatoxins. Potravinarstvo Slovak Journal of Food Sciences, 2015, 9(1), 9–17.
- Callicott, K.A.; Kachapalula, P.; Edmunds, D.; Singh, P.; Jaime, R.; Islam, M.S.; Shenge, K.; Adhikari, B.N.; Arone-Maxwell, L.; Ching’anda, C.; et al. Brief Protocols for Research on Management of Aflatoxin-Producing Fungi, 2nd ed.; ARS, USDA: Phoenix, AZ, USA, 2018.
- CAST. (2003). Mycotoxins: risks in plant, animal and human systems. Task Force Report, No. 139. The Council for Agricultural Science and Technology Agricultural Science. Iowa, USA. https:// www. cast-science. org/ publication/ mycotoxins- risks- in- plantanimal-and- human- systems/. Accessed 19 July 2021.
- Chatterjee, S.; Dhole, A.; Krishnan, A.A.; Banerjee, K. Mycotoxin Monitoring, Regulation and Analysis in India: A Success Story. Foods 2023, 12, 705, . [CrossRef]
- CFIA. (2017). Canadian Food Inspection Agency (CFIA) RG-8 Regulatory guidance. Contaminants in feed (formerly RG-1, Chapter 7), Section 1: mycotoxins in livestock feed. http:// www. Inspection. gc. ca/ anima ls/ feeds/ regulatory- guidance/ rg-8/ eng/ 13473 83943 203/ 13473 84015 909. Accessed 19 July 2021.
- Chala, A.; Mohammed, A.; Ayalew, A.; Skinnes, H. Natural occurrence of aflatoxins in groundnut (Arachis hypogaea L.) from eastern Ethiopia. Food Control. 2013, 30, 602–605, . [CrossRef]
- Chandra, P. Aflatoxins: Food Safety, Human Health Hazards and Their Prevention. In Aflatoxins—Occurrence, Detoxification, Determination and Health Risks; IntechOpen: London, UK, 2021.
- Chang, P.-K.; Hua, S.S.T.; Sarreal, S.B.L.; Li, R.W. Suppression of Aflatoxin Biosynthesis in Aspergillus flavus by 2-Phenylethanol Is Associated with Stimulated Growth and Decreased Degradation of Branched-Chain Amino Acids. Toxins 2015, 7, 3887–3902, . [CrossRef]
- Chatterjee, S.; Dhole, A.; Krishnan, A.A.; Banerjee, K. Mycotoxin Monitoring, Regulation and Analysis in India: A Success Story. Foods 2023, 12, 705, . [CrossRef]
- Chawanthayatham, S.; Valentine, C.C.; Fedeles, B.I.; Fox, E.J.; Loeb, L.A.; Levine, S.S.; Slocum, S.L.; Wogan, G.N.; Croy, R.G.; Essigmann, J.M. Mutational spectra of aflatoxin B 1 in vivo establish biomarkers of exposure for human hepatocellular carcinoma. Proc. Natl. Acad. Sci. 2017, 114, E3101–E3109, . [CrossRef]
- Cheli, F., Pinotti, L., Novacco, M., Ottoboni, M., Tretola, M., &Dell’Orto, V. 2017. Mycotoxins in wheat and mitigation measures. Wheat improvement, management and utilization. Croatia: IntechOpen: London, 227-251.
- Chen, C.-A.; Ho, C.-H.; Wu, Y.-C.; Chen, Y.-C.; Wang, J.-J.; Liao, K.-M. Epidemiology of Aspergillosis in Cancer Patients in Taiwan. Infect. Drug Resist. 2022, ume 15, 3757–3766, . [CrossRef]
- Chen, M.; He, X.; Pang, Y.; Shen, F.; Fang, Y.; Hu, Q. Laser induced fluorescence spectroscopy for detection of Aflatoxin B1 contamination in peanut oil. J. Food Meas. Charact. 2021, 15, 2231–2239, . [CrossRef]
- Churchill, K.A., 2017. The carry-over of aflatoxins in dairy feed to milk of modern Holstein dairy cows. Cornell University.
- Cole , R. J; Dorner, J.W.; Holbrook, C.C. 1995 . “ Advances in mycotoxin elimination and resistance ” . In Advances in peanut science , Edited by: Stalker , HT and Pattee , HE,1995, 456 – 474 . Stillwater, OK : American Peanut Research and Education Society .
- Cole, R. J., Dorner, J. W., & Holbrook, C. C. (1995). Advances in mycotoxin elimination and resistance. In H. E. Pattee, & H. T. Stalker (Eds.), Advances in peanut science (pp. 456–474). Stillwater, OK, USA: American Peanut Research Educational Society.
- Corrier, D. Mycotoxicosis: mechanisms of immunosuppression. Veter- Immunol. Immunopathol. 1991, 30, 73–87, . [CrossRef]
- Coskun, E.; Jaruga, P.; Vartanian, V.; Erdem, O.; Egner, P.A.; Groopman, J.D.; Lloyd, R.S.; Dizdaroglu, M. Aflatoxin-Guanine DNA Adducts and Oxidatively Induced DNA Damage in Aflatoxin-Treated Mice in Vivo as Measured by Liquid Chromatography-Tandem Mass Spectrometry with Isotope Dilution. Chem. Res. Toxicol. 2019, 32, 80–89, . [CrossRef]
- Coulter, J.B., Hendrickse, R.G., Lamplugh, S.M., Macfarlane, S.B., Moody, J.B., Omer, M.I.A., Suliman, G.E., Williams, T.E. (1986). Aflatoxins and kwashiorkor: Clinical studies in Sudanese children. Transactions of the Royal Society of Tropical Medicine and Hygiene, 80, 945–951. [CrossRef]
- Croy, R.G., &Wogan, G.N. (1981). Temporal patterns of covalent DNA adducts in rat liver after single and multiple doses of aflatoxin B1. Cancer Research, 41, 197–203.
- Grace, D.,F. Wu, A.H. Havelaar. (2020). Milk symposium review: foodborne diseases from milk and milk products in developing countries—review of causes and health and economic implications, J. Dairy Sci. 103 :9715–9729. [CrossRef]
- Dabuo, B., WesomeAvogo, E., Owusu Koomson, G., Akantibila, M., &AyendoGbati, D. (2022). Aflatoxins: Toxicity, Occurrences and Chronic Exposure. IntechOpen. [CrossRef]
- Dadi, M., & Yasir, M. (2022). Spectroscopy and Spectrophotometry: Principles and Applications for Colorimetric and Related Other Analysis. IntechOpen. [CrossRef]
- Dai, C.; Tian, E.; Hao, Z.; Tang, S.; Wang, Z.; Sharma, G.; Jiang, H.; Shen, J. Aflatoxin B1 Toxicity and Protective Effects of Curcumin: Molecular Mechanisms and Clinical Implications. Antioxidants 2022, 11, 2031, . [CrossRef]
- Dalié, D.; Deschamps, A.; Richard-Forget, F. Lactic acid bacteria – Potential for control of mould growth and mycotoxins: A review. Food Control. 2010, 21, 370–380, . [CrossRef]
- Dalvi, R.R. An overview of aflatoxicosis of poultry: Its characteristics, prevention and reduction. Veter- Res. Commun. 1986, 10, 429–443, . [CrossRef]
- Dash, B., Afriyie-Gyawu, E., Huebner, H. J., Porter, W., Wang, J. S., Jolly, P. E., & Phillips, T. D. (2007). Determinantsof the variability of aflatoxin–albumin adduct levels in Ghanaians. Journal of Toxicology and Environmental Health,Part A, 70, 58–66. [CrossRef]
- Davidson, J. I.; Hill, R. A.; Cole, R. A.;Mixoon, A. C.; Henning, R. U. Field performance of two peanut cultivars relative to resistance to invasion by A. flavus and subsequent aflatoxin contamination. Proceedings of the American Peanut Research and Education Society, 1982, 14, 74-78. [CrossRef]
- Davidson, Jr, J.I., Whitaker, T.B. and Dickens, J.W., 1982a. Grading, cleaning, storage, shelling, and marketing of peanuts in the United States. In: Pattee, H.E., Young, C.T. and Yoakum, T.X. (eds.) Peanut science and technology. American Peanut Research and Education Society, Inc., Yoakum, TX, USA, pp. 571-623.
- Dersjant-Li, Y., Martin, W., Verstegen, M., Walter, J., &Gerrits, J. (2003). The Impact of Low Concentrations of Aflatoxin, Deoxynivalenol or Fumonisin in Diets on Growing Pigs and Poultry. Nutrition Research Reviews, 16(2), 223–239.
- Devi, R. and Hall, A., 2000. Strategies for reducing aflatoxin levels in groundnut based foods and feeds in India: a step towards improving health of humans and livestocks. International Crops Research Institute for Semi-Arid Tropics, Patancheru, India, pp. 9-17.
- Dhakal A, Hashmi MF, Sbar E. Aflatoxin Toxicity. [Updated 2023 Feb 19]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557781/.
- Dhanasekaran, D.; Shanmugapriya, S.; Thajuddin, N.; Panneerselvam, A. Aflatoxins and aflatoxicosis in human and animals. In Aflatoxins: Biochemistry and Molecular Biology; Guevara-González, R.G., Ed.; BoD–Books on Demand: London, UK, 2011; Volume 10, pp. 221–254.
- Di Nardo, F.; Alladio, E.; Baggiani, C.; Cavalera, S.; Giovannoli, C.; Spano, G.; Anfossi, L. Colour-encoded lateral flow immunoassay for the simultaneous detection of aflatoxin B1 and type-B fumonisins in a single Test line. Talanta 2019, 192, 288–294, . [CrossRef]
- Dianov, G.L.; Hübscher, U. Mammalian Base Excision Repair: the Forgotten Archangel. Nucleic Acids Res. 2013, 41, 3483–3490, . [CrossRef]
- Diao, E.; Dong, H.; Hou, H.; Zhang, Z.; Ji, N.; Ma, W. Factors Influencing Aflatoxin Contamination in Before and After Harvest Peanuts: A Review. J. Food Res. 2014, 4, . [CrossRef]
- Diaz, D.E. (2005). Themycotoxin blue book. Nottingham University Press, Nottingham.
- Dib, R.; Khalil, M.; Fares, J.; Hachem, R.; Jiang, Y.; Dandachi, D.; Chaftari, A.-M.; Raad, I. Invasive pulmonary aspergillosis: comparative analysis in cancer patients with underlying haematologic malignancies versus solid tumours. J. Hosp. Infect. 2019, 104, 358–364, . [CrossRef]
- Dick, K.M., 1987. Pest management in stored groundnuts. Information Bulletin 22. International Crops Research Institute for Semi-Arid Tropics, Patancheru, India, 28 pp.
- Diener, U.L.; Davis, N.D. Limiting temperature and relative humidity for aflatoxin production byAspergillus flavus in stored peanuts. J. Am. Oil Chem. Soc. 1970, 47, 347–351, . [CrossRef]
- Dorner, J.W. Development of Biocontrol Technology to Manage Aflatoxin Contamination in Peanuts. Peanut Sci. 2009, 36, 60–67, . [CrossRef]
- Dorner, J.W. Management and prevention of mycotoxins in peanuts. Food Addit. Contam. Part A 2008, 25, 203–208, . [CrossRef]
- Dorner, J.W.; Cole, R.J. Effect of application of nontoxigenic strains of Aspergillus flavus and A. parasiticus on subsequent aflatoxin contamination of peanuts in storage. J. Stored Prod. Res. 2002, 38, 329–339, . [CrossRef]
- Dorner, J.W.; Lamb, M.C. Development and commercial use of afla-Guard®, an aflatoxin biocontrol agent. Mycotoxin Res. 2006, 22, 33–38, . [CrossRef]
- Dorner, J.W. Biological Control of Aflatoxin Contamination in Corn Using a Nontoxigenic Strain of Aspergillus flavus. J. Food Prot. 2009, 72, 801–804, . [CrossRef]
- Douksouna, Y.; Kwallah, A.O.; Nyerere, A.; Runo, S.; Ambang, Z. Application and Evaluation of the Loop-Mediated Isothermal Amplification assay for the Detection of Aflatoxigenic Fungi Contaminants of Rice Grains in Kenya. 2020, 11 , 1–8, . [CrossRef]
- Dövényi-Nagy, T.; Rácz, C.; Molnár, K.; Bakó, K.; Szláma, Z.; Jóźwiak, .; Farkas, Z.; Pócsi, I.; Dobos, A.C. Pre-Harvest Modelling and Mitigation of Aflatoxins in Maize in a Changing Climatic Environment—A Review. Toxins 2020, 12, 768, . [CrossRef]
- Dövényi-Nagy, T., Rácz, C., Molnár, K., Bakó, K., Szláma, Z., Jóźwiak, Á., ... &Dobos, A. C. (2020). Pre-harvest modelling and mitigation of aflatoxins in maize in a changing climatic environment—A review. Toxins, 12(12), 768.
- Doyle, M.P.; Marth, E.H. Bisulfite Degrades Aflatoxin: Effect of Temperature and Concentration of Bisulfite. J. Food Prot. 1978, 41, 774–780, . [CrossRef]
- Doyle, M.P.; Applebaum, R.S.; Brackett, R.E.; Marth, E.H. Physical, Chemical and Biological Degradation of Mycotoxins in Foods and Agricultural Commodities. J. Food Prot. 1982, 45, 964–971, . [CrossRef]
- Abebe, E.; Gugsa, G.; Ahmed, M. Review on Major Food-Borne Zoonotic Bacterial Pathogens. J. Trop. Med. 2020, 2020, 1–19, . [CrossRef]
- Eaton, D.L., &Groopman, J.D. (1994). The Toxicology of Aflatoxins. Academic Press.
- EC. (1994). Mycotoxins in human nutrition and health. Agro-Industrial Research Division. Directorate-General XII for Scientific Research and Development.
- El-Azab, S.M.; Abdelhamid, A.; Shalaby, H.A.; Mehrim, A.; Ibrahim, A.H. Study of aflatoxin B1as a risk factor that impairs the reproductive performance in females – Egypt. Toxicol. Environ. Chem. 2009, 92, 383–389, . [CrossRef]
- Eshelli, M.; Harvey, L.; Edrada-Ebel, R.; McNeil, B. Metabolomics of the Bio-Degradation Process of Aflatoxin B1 by Actinomycetes at an Initial pH of 6.0. Toxins 2015, 7, 439–456, . [CrossRef]
- Eskola, M.; Altieri, A.; Galobart, J. Overview of the activities of the European Food Safety Authority on mycotoxins in food and feed. World Mycotoxin J. 2018, 11, 277–289, . [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789, . [CrossRef]
- Etcheverry, M., Nesci, A., &Passone, A. (2011). Chemical strategies applied to maize and peanut storage agroecosystem in Argentina to prevent aflatoxin contamination. In G. E. Cohen, & C. M. Levin (Eds.), Food storage (pp. 65–98). NY, USA: Nova Science Publishers, Inc.
- European Commission, 2006. Setting maximum levels for certain contaminants in foodstuffs. Off J Eur Union, 364: 5-24.
- Ewuola, E.O., Jimoh, O.A., & Bello, A.D. (2013). Growth Response and Nutrient Digestibility of West African Dwarf Goats Fed Micro Doses of Dietary Aflatoxin. Scientific Journal of Animal Science, 2, 316–322.
- Bertania,F. R., Businaroa,L., Gambacortab,L., Mencattinic,A., Brendac,D., Di Giuseppec,D., De Ninnoa,A., Solfrizzob,M., Martinellic,E., Gerardinoa,A. Optical detection of Aflatoxins B in grained almonds using fluorescence spectroscopy and machine learning algorithms 2020.
- FAMIC. (2011). Aflatoxin. Food and Agricultural Materials Inspection Centre (FAMIC). http:// www. famic. go. jp/ ffis/ oie/ obj/ hc_ aflatoxin. pdf. Accessed 19 July 2021.
- FAO (1997). Worldwide regulations for mycotoxins - 1995 - a compendium. FAO Food and Nutrition Rome, Italy.
- FAO (2004). Worldwide regulations for mycotoxins in food and feed in 2003. Rome, Italy: FAO.
- FAO, Food and Agriculture Organization of the United Nations, URL: http://www.fao.org/home/en/ (Accessed Aug 2017).
- FAO. (2008). Animal Feed Impact on Food Safety. Food and Agriculture Organization of the United Nations.
- FAO/WHO. (2004). Worldwide regulations for mycotoxins in food and feed, FAO Food and Nutrition Paper 81. Food and Agriculture Organisation of the United Nations, Rome, Italy. http:// www. fao. org/3/ y5499e/ y5499e. pdf. Accessed 19 July 2021.
- FAOSTAT (2011) Top Production - Pistachios 2010. In: FAO, editor.
- FDA (1980) Food and Drug Administration of the United States Compliance Policy Guide Sec. 570.375 Aflatoxins in Peanuts and Peanut Products: Guidance for FDA Staff. https:// www. fda. gov/media/ 72073/ download. Accessed 19 July 2021.
- FDA (Food and Drug Administration US), 2019. Action Levels for Aflatoxins in Animal Food, Compliance Policy Guide. Available in https://www.fda.gov/ media/121202/download [5th December, 2021].
- Filazi A and Sireli UT, 2013. Occurrence of aflatoxins in food. In: Aflatoxins: Recent Advances and Future Prospects, pp 143-170. [CrossRef]
- Fouad, A.M.; Ruan, D.; El-Senousey, H.K.; Chen, W.; Jiang, S.; Zheng, C. Harmful Effects and Control Strategies of Aflatoxin B1 Produced by Aspergillus flavus and Aspergillus parasiticus Strains on Poultry: Review. Toxins 2019, 11, 176, . [CrossRef]
- Freitag, S.; Sulyok, M.; Logan, N.; Elliott, C.T.; Krska, R. The potential and applicability of infrared spectroscopic methods for the rapid screening and routine analysis of mycotoxins in food crops. Compr. Rev. Food Sci. Food Saf. 2022, 21, 5199–5224, . [CrossRef]
- FSANZ. (2017). Australia New Zealand Food Standards Code, 2017,Schedule 19, Maximum levels of contaminants and natural toxicants. Prepared by Food Standards Australia New Zealand (FSANZ) on 13 April 2017. Australian Government, Federal Register of Legislation. https:// www. legislation. gov. au/ Details/ F2017 C00333. Accessed 19 July 2021.
- FSSAI, 2020. Manual of methods of analysis of foods- Mycotoxins. Food Safety and Standards Authority of India. Ministry of Health and Family Welfare, Govt. of India, pp 1-101.
- FSSAI. (2011). Food Safety and Standards Authority of India (FSSAI) Food Safety and Standards (Contaminants, Toxins and Residues) Regulations, 2011, F. No. 2-15015/30/2010. https:// www. Fssai.
- Gabal, M.A.; Azzam, A.H. Interaction of aflatoxin in the feed and immunization against selected infectious diseases in poultry. II. Effect on one-day-old layer chicks simultaneously vaccinated against Newcastle disease, infectious bronchitis and infectious bursal disease. Avian Pathol. 1998, 27, 290–295, . [CrossRef]
- Garbino, J. (2004). Aspergillosis. Orphanet, 1-10.
- Gbashi, S., Madala, N. E., De Saeger, S., De Boevre, M., Adekoya, I., Adebo, O. A., &Njobeh, P. B. (2018). The socio-economic impact of mycotoxin contamination in Africa. Fungi and mycotoxins-their occurrence, impact on health and the economy as well as pre-and postharvest management strategies (ed. Njobeh, PB), 1-20.
- Ghali, R.; Khlifa, K.H.; Ghorbel, H.; Maaroufi, K.; Hedilli, A. Aflatoxin determination in commonly consumed foods in Tunisia. J. Sci. Food Agric. 2010, 90, 2347–2351, . [CrossRef]
- Gibb, H.; Devleesschauwer, B.; Bolger, P.M.; Wu, F.; Ezendam, J.; Cliff, J.; Zeilmaker, M.; Verger, P.; Pitt, J.; Baines, J.; et al. World Health Organization estimates of the global and regional disease burden of four foodborne chemical toxins, 2010: a data synthesis. F1000Research 2015, 4, 1393, . [CrossRef]
- Goldbatt, L.A. (1969). Aflatoxin. Academic Press.
- Gomaa, M.N.E.; Ayesh, A.M.; Galil, M.M.A.; Naguib, K. Effect of high pressure ammoniation procedure on the detoxification of aflatoxins. Mycotoxin Res. 1997, 13, 23–34, . [CrossRef]
- Gouas, D.; Shi, H.; Hainaut, P. The aflatoxin-induced TP53 mutation at codon 249 (R249S): Biomarker of exposure, early detection and target for therapy. Cancer Lett. 2009, 286, 29–37, . [CrossRef]
- Grace, D. (2013). Aflatoxins: Finding Solutions for Improved Food Safety Animals and Aflatoxins, Brief number 5. Animals and Aflatoxins. Focus 2020. International Food Policy Research Institute.
- Grace, D.; Mahuku, G.; Hoffmann, V.; Atherstone, C.; Upadhyaya, H.D.; Bandyopadhyay, R. International agricultural research to reduce food risks: case studies on aflatoxins. Food Secur. 2015, 7, 569–582, . [CrossRef]
- Grace, D.; Lindahl, J.F.; Atherstone, C.; Kang’ethe, E.; Nelson, F.; Wesonga, T.; Manyong, V. (2015a). Aflatoxin Standards for Feed. Building an Aflatoxin Safe East African Community Technical Policy Paper 7; International Institute of Tropical Agriculture: Ibadan, Nigeria, 2015.
- Granados-Chinchilla, F.; Molina, A.; Chavarría, G.; Alfaro-Cascante, M.; Bogantes-Ledezma, D.; Murillo-Williams, A. Aflatoxins occurrence through the food chain in Costa Rica: Applying the One Health approach to mycotoxin surveillance. Food Control. 2017, 82, 217–226, . [CrossRef]
- Groopman, J.D.; Kensler, T.W.; Wild, C.P. Protective Interventions to Prevent Aflatoxin-Induced Carcinogenesis in Developing Countries. Annu. Rev. Public Heal. 2008, 29, 187–203, . [CrossRef]
- Grubisha, L.C.; Cotty, P.J. Genetic Analysis of the Aspergillus flavus Vegetative Compatibility Group to Which a Biological Control Agent That Limits Aflatoxin Contamination in U.S. Crops Belongs. Appl. Environ. Microbiol. 2015, 81, 5889–5899, . [CrossRef]
- Guengerich, F.; Johnson, W.W.; Shimada, T.; Ueng, Y.-F.; Yamazaki, H.; Langouët, S. Activation and detoxication of aflatoxin B1. Mutat. Res. Mol. Mech. Mutagen. 1998, 402, 121–128, . [CrossRef]
- Guo, Y.; Zhao, L.; Ma, Q.; Ji, C. Novel strategies for degradation of aflatoxins in food and feed: A review. Food Res. Int. 2020, 140, 109878, . [CrossRef]
- Gupta, R.C. (2011). Aflatoxins, Ochratoxins and Citrinins. Reproductive and Developmental Toxicology, 55, 753–763.
- Gürses, M. Mycoflora and Aflatoxin Content of Hazelnuts, Walnuts, Peanuts, Almonds and Roasted Chickpeas (LEBLEBI) Sold in Turkey. Int. J. Food Prop. 2006, 9, 395–399, . [CrossRef]
- Haciseferogullary, H., Ozcan, M., Demir, F. and Calysyr, S., 2005. Some nutritional and technological properties of garlic (Allium sativum). Journal of Food Engineering 68: 463-469.
- Hamid, A.B., 1997. Present status and future prospects of research on the groundnut aflatoxin problem in Malaysia. In: Mehan, V.K. and Gowda, C.L.L. (eds.) Aflatoxin contamination problem in groundnut in Asia. Proceedings of the first Asia Working Group Meeting. May 27-29, 1996. Hanoi, Vietnam, pp. 32-35.
- Hamilton, P.B. (1986). Aflatoxicosis in farm animals. International Maize and Wheat Improvement Center (CIMMYT).
- Han, L.; Li, Y.-T.; Jiang, J.-Q.; Li, R.-F.; Fan, G.-Y.; Lv, J.-M.; Zhou, Y.; Zhang, W.-J.; Wang, Z.-L. Development of a Direct Competitive ELISA Kit for Detecting Deoxynivalenol Contamination in Wheat. Molecules 2019, 25, 50, . [CrossRef]
- Hassan, R., El-Kadi, S., & Sand, M. Effect of some organic acids on some fungal growth and the production of their toxins. International Journal of Advances in Biology, 2015, 2(1), 1–11.
- Hatem, N.L.; Hassab, H.M.A.; Al-Rahman, E.M.A.; El-Deeb, S.A.; Ahmed, R.L.E.-S. Prevalence of Aflatoxins in Blood and Urine of Egyptian Infants with Protein–Energy Malnutrition. Food Nutr. Bull. 2005, 26, 49–56, . [CrossRef]
- Heathcote, J.G. and Hibbert, J.R., 1978. Aflatoxins: chemical and biological aspects. Elsevier, New York, NY, USA. Hell, K. and Mutegi, C., 2011. Aflatoxin control and prevention strategies in key crops of sub-Saharan Africa. African Journal of Microbiology Research 5: 459-466.
- Hell, K., &Mutegi, C. (2011). Aflatoxin control and prevention strategies in key crops of Sub-Saharan Africa. African Journal of Microbiology Research.
- Hell, K.; Cardwell, K.; Setamou, M.; Poehling, H.-M. The influence of storage practices on aflatoxin contamination in maize in four agroecological zones of Benin, west Africa. J. Stored Prod. Res. 2000, 36, 365–382, . [CrossRef]
- Henry, S.H., Bosch, F.X., & Bowers, J.C. (2002). Aflatoxin, hepatitis and worldwide liver cancer risks. Advances in Experimental Medicine and Biology, 504, 229–233.
- Hildrestrand, G.A.; Rolseth, V.; Kunath, N.; Suganthan, R.; Jensen, V.; Bugaj, A.M.; Fernandez-Berrocal, M.S.; Sikko, S.B.; Vetlesen, S.; Kuśnierczyk, A.; et al. NEIL1 and NEIL2 DNA glycosylases modulate anxiety and learning in a cooperative manner in mice. Commun. Biol. 2021, 4, 1–14, . [CrossRef]
- Hu, X.; Li, H.; Yang, J.; Wen, X.; Wang, S.; Pan, M. Nanoscale Materials Applying for the Detection of Mycotoxins in Foods. Foods 2023, 12, 3448, . [CrossRef]
- IARC (2002). Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 82: 171–300.
- IARC (International Agency for Research on Cancer). Chemical agents and related occupations. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon (Fr), France, 2012; Volume 100F, pp. 225–248.
- IARC. (2012a). Improving public health through mycotoxin control. IARC Scientific Publication No. 158. Lyon, France: International Agency for Research on Cancer. https:// publicatio ns. iarc. fr/ Book- And- Report- Series/ Iarc- Scientific- Publications/ Improving- Public- Health- Through- Mycotoxin- Control- 2012a. Accessed 19 July 2021.
- IARC. (2015) Mycotoxin control in low- and middle-income countries. IARC Working Group Report Volume 9. Lyon, France: International Agency for Research on Cancer. https:// publicatio ns. iarc. fr/ Book- And- Report- Series/ Iarc- Working- Group- Reports/ Mycotoxin- Control- In- Low-- And- Middle- income- Count ries- 2015.
- Iqbal, S.Z.; Asi, M.R.; Hanif, U.; Zuber, M.; Jinap, S. The presence of aflatoxins and ochratoxin A in rice and rice products; and evaluation of dietary intake. Food Chem. 2016, 210, 135–140, . [CrossRef]
- Ordaz, J.J.; Abuín, C.M.F.; Fente, C.; Vázquez, B.; Franco, C.; Cepeda, A. Development of a method for direct visual determination of aflatoxin production by colonies of the Aspergillus flavus group. Int. J. Food Microbiol. 2003, 83, 219–225, . [CrossRef]
- Jain, L. K., Parewa, H. P., &Ratnoo, S. (2019). Impact of frontline demonstration on productivity and profitability analysis of cluster bean in Barmer district of Rajasthan. Forage Res, 44(4), 282-285.
- Jajić, I.; Glamočić, D.; Krstović, S.; Horvatović, M.P. Aflatoxin M1 occurrence in Serbian milk and its impact on legislative. J. Hell. Veter- Med Soc. 2018, 69, 1283, . [CrossRef]
- Jalili, M. (2016). A review on aflatoxins reduction in food. Iran. J. Health Saf. Environ. 3, 445–459.
- Jallow, A.; Xie, H.; Tang, X.; Qi, Z.; Li, P. Worldwide aflatoxin contamination of agricultural products and foods: From occurrence to control. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2332–2381, . [CrossRef]
- Hajnal, E.J.; Kos, J.; Krulj, J.; Krstović, S.; Jajić, I.; Pezo, L.; Šarić, B.; Nedeljković, N. Aflatoxins contamination of maize in Serbia: the impact of weather conditions in 2015. Food Addit. Contam. Part A 2017, 34, 1999–2010, . [CrossRef]
- Janik, E.; Niemcewicz, M.; Podogrocki, M.; Ceremuga, M.; Gorniak, L.; Stela, M.; Bijak, M. The Existing Methods and Novel Approaches in Mycotoxins’ Detection. Molecules 2021, 26, 3981, . [CrossRef]
- Janik-Karpinska, E.; Ceremuga, M.; Niemcewicz, M.; Podogrocki, M.; Stela, M.; Cichon, N.; Bijak, M. Immunosensors—The Future of Pathogen Real-Time Detection. Sensors 2022, 22, 9757, . [CrossRef]
- Jantrarotai, W.; Lovell, R.T. Subchronic Toxicity of Dietary Aflatoxin B1to Channel Catfish. J. Aquat. Anim. Heal. 1990, 2, 248–254, . [CrossRef]
- Joint FAO/WHO Expert Committee on Food Additives (JECFA) (2007). Evaluation of certain food additives and contaminants: sixty-eighth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO technical report series 947.
- Juan C., Ritieni A., Mañes J. Determination of trichothecenes and zearalenones in grain cereal, flour and bread by liquid chromatography tandem mass spectroscopy. Food Chemistry. 2012;134(4):2389–2397.
- Jury, M.R. Climate trends in southern Africa (with erratum). South Afr. J. Sci. 2013, 109, 111, . [CrossRef]
- Reddy, K.; Reddy, C.; Muralidharan, K. Detection of Aspergillus spp. and aflatoxin B1 in rice in India. Food Microbiol. 2009, 26, 27–31, . [CrossRef]
- Kaaya, N.A. and Warren, H.L., 2005. A review of past and present research on aflatoxin in Uganda. African Journal of Food Agriculture and Nutritional Development 5: 1-17.
- Kabak, B. Aflatoxins in hazelnuts and dried figs: Occurrence and exposure assessment. Food Chem. 2016, 211, 8–16, . [CrossRef]
- Kabak, B.; Dobson, A.D.W.; Var, I. Strategies to Prevent Mycotoxin Contamination of Food and Animal Feed: A Review. Crit. Rev. Food Sci. Nutr. 2006, 46, 593–619, . [CrossRef]
- Kachapulula, P.; Akello, J.; Bandyopadhyay, R.; Cotty, P. Aflatoxin contamination of groundnut and maize in Zambia: observed and potential concentrations. J. Appl. Microbiol. 2017, 122, 1471–1482, . [CrossRef]
- Kamkar, A.; Yazdankhah, S.; Nafchi, A.M.; Nejad, A.S.M. Aflatoxin M1in raw cow and buffalo milk in Shush city of Iran. Food Addit. Contam. Part B 2014, 7, 21–24, . [CrossRef]
- Kamle, M.; Mahato, D.K.; Devi, S.; Lee, K.E.; Kang, S.G.; Kumar, P. Fumonisins: Impact on Agriculture, Food, and Human Health and their Management Strategies. Toxins 2019, 11, 328, . [CrossRef]
- Kara, G.N.; Ozbey, F.; Kabak, B. Co-occurrence of aflatoxins and ochratoxin A in cereal flours commercialised in Turkey. Food Control. 2015, 54, 275–281, . [CrossRef]
- Kendra D.F., Dyer R.B. Opportunities for biotechnology and policy regarding mycotoxin issues in international trade. International Journal of Food Microbiology. 2007;119 (1–2):147–151.
- Khan, R.; Ghazali, F.M.; Mahyudin, N.A.; Samsudin, N.I.P. Biocontrol of Aflatoxins Using Non-Aflatoxigenic Aspergillus flavus: A Literature Review. J. Fungi 2021, 7, 381, . [CrossRef]
- Kharbach, M.; Mansouri, M.A.; Taabouz, M.; Yu, H. Current Application of Advancing Spectroscopy Techniques in Food Analysis: Data Handling with Chemometric Approaches. Foods 2023, 12, 2753, . [CrossRef]
- Kinnamon, D.S.; Heggestad, J.T.; Liu, J.; Chilkoti, A. Technologies for Frugal and Sensitive Point-of-Care Immunoassays. Annu. Rev. Anal. Chem. 2022, 15, 123–149, . [CrossRef]
- Kizis, D.; Vichou, A.-E.; Natskoulis, P.I. Recent Advances in Mycotoxin Analysis and Detection of Mycotoxigenic Fungi in Grapes and Derived Products. Sustainability 2021, 13, 2537, . [CrossRef]
- Kluczkovski, A.; Bezerra, L.; Januário, B.; Lima, E.; Campelo, P.; Machado, M.; Bezerra, J. Nuclear Magnetic Resonance Approach in Brazil Nut Oil and the Occurrence of Aflatoxins. J. Oleo Sci. 2022, 71, 1439–1444, . [CrossRef]
- Kong, Q.; Shan, S.; Liu, Q.; Wang, X.; Yu, F. Biocontrol of Aspergillus flavus on peanut kernels by use of a strain of marine Bacillus megaterium. Int. J. Food Microbiol. 2010, 139, 31–35, . [CrossRef]
- Kövesi, B.; Kulcsár, S.; Ancsin, Z.; Zándoki, E.; Erdélyi, M.; Mézes, M.; Balogh, K. Individual and Combined Effects of Aflatoxin B1 and Sterigmatocystin on Lipid Peroxidation and Glutathione Redox System of Common Carp Liver. Toxins 2021, 13, 109, . [CrossRef]
- Krishnamachari, K. A. V. R., Nagarajan, V., Bhat, R., &Tilak, T. B. G. (1975). Hepatitis due to aflatoxicosis: an outbreak in western India. The Lancet, 305(7915), 1061-1063.
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A Global Concern for Food Safety, Human Health and Their Management. Front. Microbiol. 2017, 07, 2170, . [CrossRef]
- Kumsiri R, Kanchanaphum P. A Comparison of Four Molecular Methods for Detection of Aflatoxin-Producing Aspergillus in Peanut and Dried Shrimp Samples Collected from Local Markets around Pathum Thani Province, Thailand. Scientifica (Cairo). 2020 Dec 24;2020:8580451. [CrossRef]
- Kumsiri, R.; Kanchanaphum, P. A Comparison of Four Molecular Methods for Detection of Aflatoxin-Producing Aspergillus in Peanut and Dried Shrimp Samples Collected from Local Markets around Pathum Thani Province, Thailand. Scientifica 2020, 2020, 1–6, . [CrossRef]
- Rangnoi, K.; Rüker, F.; Wozniak-Knopp, G.; Cvak, B.; O’kennedy, R.; Yamabhai, M. Binding Characteristic of Various Antibody Formats Against Aflatoxins. ACS Omega 2021, 6, 25258–25268, . [CrossRef]
- Kutasi, K.; Recek, N.; Zaplotnik, R.; Mozetič, M.; Krajnc, M.; Gselman, P.; Primc, G. Approaches to Inactivating Aflatoxins—A Review and Challenges. Int. J. Mol. Sci. 2021, 22, 13322, . [CrossRef]
- Voth-Gaeddert, L.E.; Torres, O.; Maldonado, J.; Krajmalnik-Brown, R.; Rittmann, B.E.; Oerther, D.B. Aflatoxin Exposure, Child Stunting, and Dysbiosis in the Intestinal Microbiome Among Children in Guatemala. Environ. Eng. Sci. 2019, 36, 958–968, . [CrossRef]
- Ladeira, C.; Frazzoli, C.; Orisakwe, O.E. Engaging One Health for Non-Communicable Diseases in Africa: Perspective for Mycotoxins. Front. Public Heal. 2017, 5, 266, . [CrossRef]
- Lavkor, I., &Var, I. (2017). The Control of Aflatoxin Contamination at Harvest, Drying, Pre- Storage and Storage Periods in Peanut: The New Approach. InTech. [CrossRef]
- Le, V.T.; Vasseghian, Y.; Dragoi, E.-N.; Moradi, M.; Khaneghah, A.M. A review on graphene-based electrochemical sensor for mycotoxins detection. Food Chem. Toxicol. 2020, 148, 111931, . [CrossRef]
- Lee, K.-M.; Davis, J.; Herrman, T.J.; Murray, S.C.; Deng, Y. An empirical evaluation of three vibrational spectroscopic methods for detection of aflatoxins in maize. Food Chem. 2015, 173, 629–639, . [CrossRef]
- Leeson, S., Diaz, G.J., & Summers, J.D. (1995). Poultry Metabolic Disorders and Mycotoxins. University Books.
- Lewis, G., Markson, L.M., &Allcroft, R. (1967). The Effect of Feeding Toxic Groundnut Meal to Sheep over a Period of Five Years. Veterinary Record.
- Li, R.; Meng, C.; Wen, Y.; Fu, W.; He, P. Fluorometric lateral flow immunoassay for simultaneous determination of three mycotoxins (aflatoxin B(1), zearalenone and deoxynivalenol) using quantum dot microbeads. Mikrochim. Acta 2019, 186, 748.
- Liao, X.; Liu, Y.-M.; Qiu, L.; Cao, L.; Yang, X.; Hu, X.-G. A quantum dot aptamer fluorescent sensor based on magnetic graphene oxide for the detection of zearalenone. Anal. Methods 2023, 15, 4946–4953, . [CrossRef]
- Lippolis, V., Irurhe, O., Porricelli, A. C. R., Cortese, M., Schena, R., Imafidon, T., et al. (2017). Natural co-occurrence of aflatoxins and ochratoxin A in ginger (Zingiber officinale) from Nigeria. Food control 73, 1061–1067. [CrossRef]
- Liu, J.; Yang, M.; He, M.; Xu, M.; Chen, Y.; Zhang, F. Rapid and selective detection of AFB1 by direct mass spectrometry using immunoaffinity paper substrate. Microchem. J. 2024, 196, . [CrossRef]
- Liu, J.; Sun, L.; Zhang, N.; Zhang, J.; Guo, J.; Li, C.; Rajput, S.A.; Qi, D. Effects of Nutrients in Substrates of Different Grains on Aflatoxin B1Production byAspergillus flavus. BioMed Res. Int. 2016, 2016, 1–10, . [CrossRef]
- Liu, X.; Guan, X.; Xing, F.; Lv, C.; Dai, X.; Liu, Y. Effect of water activity and temperature on the growth of Aspergillus flavus, the expression of aflatoxin biosynthetic genes and aflatoxin production in shelled peanuts. Food Control. 2017, 82, 325–332, . [CrossRef]
- Liu, Y.; Wu, F. Global Burden of Aflatoxin-Induced Hepatocellular Carcinoma: A Risk Assessment. Environ. Heal. Perspect. 2010, 118, 818–824, . [CrossRef]
- Liu, Z.; Zhang, G.; Zhang, Y.; Jin, Q.; Zhao, J.; Li, J. Factors controlling mycotoxin contamination in maize and food in the Hebei province, China. Agron. Sustain. Dev. 2016, 36, 1–10, . [CrossRef]
- Liu, Z.; Hua, Q.; Wang, J.; Liang, Z.; Li, J.; Wu, J.; Shen, X.; Lei, H.; Li, X. A smartphone-based dual detection mode device integrated with two lateral flow immunoassays for multiplex mycotoxins in cereals. Biosens. Bioelectron. 2020, 158, 112178, . [CrossRef]
- Logrieco, A.; Battilani, P.; Leggieri, M.C.; Jiang, Y.; Haesaert, G.; Lanubile, A.; Mahuku, G.; Mesterházy, A.; Ortega-Beltran, A.; Pasti, M.; et al. Perspectives on Global Mycotoxin Issues and Management From the MycoKey Maize Working Group. Plant Dis. 2021, 105, 525–537, . [CrossRef]
- Long, X.-D.; Zhao, D.; Wang, C.; Huang, X.-Y.; Yao, J.-G.; Ma, Y.; Wei, Z.-H.; Liu, M.; Zeng, L.-X.; Mo, X.-Q.; et al. Genetic Polymorphisms in DNA Repair Genes XRCC4 and XRCC5 and Aflatoxin B1–related Hepatocellular Carcinoma. Epidemiology 2013, 24, 671–681, . [CrossRef]
- Mulunda, M.; Ngoma, L.; Nyirenda, M.; Motsei, L.; Bakunzi, F. A Decade of Aflatoxin M1 Surveillance in Milk and Dairy Products in Developing Countries (2001-2011): A Review. In Mycotoxin and Food Safety in Developing Countries; Hussaini, A.M., Ed.; The Publisher : Rijeka, Croatia, 2013; pp. 39–60, ISBN 978-953-51-1096-5.
- Ma, J.; Liu, Y.; Guo, Y.; Ma, Q.; Ji, C.; Zhao, L. Transcriptional Profiling of Aflatoxin B1-Induced Oxidative Stress and Inflammatory Response in Macrophages. Toxins 2021, 13, 401, . [CrossRef]
- Ma, X.; Wang, W.; Chen, X.; Xia, Y.; Wu, S.; Duan, N.; Wang, Z. Selection, identification, and application of Aflatoxin B1 aptamer. Eur. Food Res. Technol. 2014, 238, 919–925, . [CrossRef]
- Mahato, D.K.; Lee, K.E.; Kamle, M.; Devi, S.; Dewangan, K.N.; Kumar, P.; Kang, S.G. Aflatoxins in Food and Feed: An Overview on Prevalence, Detection and Control Strategies. Front. Microbiol. 2019, 10, 2266, . [CrossRef]
- Majdinasab, M.; Ben Aissa, S.; Marty, J.L. Advances in Colorimetric Strategies for Mycotoxins Detection: Toward Rapid Industrial Monitoring. Toxins 2020, 13, 13, . [CrossRef]
- Malachová, A., Sulyok, M., Beltr´ an, E., Berthiller, F., Krska, R., 2014. Optimization and validation of a quantitative liquid chromatography–tandem mass spectrometric method covering 295 bacterial and fungal metabolites including all regulated mycotoxins in four model food matrices. Journal of Chromatography A 1362.
- Mangal, M.; Khan, F.; Bansal, S.; Oberoi, H. Validation of PCR based detection system for aflatoxin producing molds.. 2016, 54, 472–476.
- Mapfumo, P.; Chagwiza, C.; Antwi, M. Impact of rainfall variability on maize yield in the KwaZulu-Natal, North-West and Free State provinces of South Africa (1987–2017). J. Agribus. Rural. Dev. 2020, 58, 359–367, . [CrossRef]
- Marshall, H.; Meneely, J.P.; Quinn, B.; Zhao, Y.; Bourke, P.; Gilmore, B.F.; Zhang, G.; Elliott, C.T. Novel decontamination approaches and their potential application for post-harvest aflatoxin control. Trends Food Sci. Technol. 2020, 106, 489–496, . [CrossRef]
- Matacic C. (2016). Fungal toxins are poisoning Africa's children, says new report. "Invisible" epidemic could lead to stunting and delayed development. https:// www. science. org/ content/ artic le/ fungal- toxins- are- poisoning- africa- s- child ren- says- new- report. Accessed 16 Dec 2021.
- Mateo, E. M., Gómez, J. V., Gimeno-Adelantado, J. V., Romera, D., Mateo-Castro, R., & Jiménez, M. (2017). Assessment of azole fungicides as a tool to control growth of Aspergillus flavus and aflatoxin B1 and B2 production in maize. Food Additives & Contaminants: Part A, 34(6), 1039–1051.
- McCullough, A.K.; Lloyd, R.S. Mechanisms underlying aflatoxin-associated mutagenesis – Implications in carcinogenesis. DNA Repair 2019, 77, 76–86, . [CrossRef]
- Medina, A.; Akbar, A.; Baazeem, A.; Rodriguez, A.; Magan, N. Climate change, food security and mycotoxins: Do we know enough? Fungal Biol. Rev. 2017, 31, 143–154, . [CrossRef]
- Milhome, M.; Lima, C.; de Lima, L.; Lima, F.; Sousa, D.; Nascimento, R. Occurrence of aflatoxins in cashew nuts produced in northeastern brazil. Food Control. 2014, 42, 34–37, . [CrossRef]
- Miller, J.D. Mycotoxins in small grains and maize: Old problems, new challenges. Food Addit. Contam. Part A 2008, 25, 219–230, . [CrossRef]
- Mitchell NJ, Bowers E, Hurburgh C and Wu F, 2016. Potential economic losses to the US corn industry from aflatoxin con-tamination. Food AdditContam Part A, 33(3): 540-550. [CrossRef]
- Mohammad, Z.H.; Ahmad, F.; Ibrahim, S.A.; Zaidi, S. Application of nanotechnology in different aspects of the food industry. Discov. Food 2022, 2, 1–21, . [CrossRef]
- Monda, E., Masanga, J., &Alakonya, A. (2020). Variation in occurrence and aflatoxigenicity of Aspergillus flavus from two climatically varied regions in Kenya. Toxins, 12(1), 34.
- Moon, J.; Byun, J.; Kim, H.; Lim, E.-K.; Jeong, J.; Jung, J.; Kang, T. On-Site Detection of Aflatoxin B1 in Grains by a Palm-Sized Surface Plasmon Resonance Sensor. Sensors 2018, 18, 598, . [CrossRef]
- Mutiga, S.K.; Hoffmann, V.; Harvey, J.W.; Milgroom, M.G.; Nelson, R.J. Assessment of Aflatoxin and Fumonisin Contamination of Maize in Western Kenya. Phytopathology® 2015, 105, 1250–1261, . [CrossRef]
- Mutiga, S.K.; Were, V.; Hoffmann, V.; Harvey, J.W.; Milgroom, M.G.; Nelson, R.J. Extent and Drivers of Mycotoxin Contamination: Inferences from a Survey of Kenyan Maize Mills. Phytopathology 2014, 104, 1221–1231, . [CrossRef]
- Turna, N.S.; Wu, F. Estimation of Tolerable Daily Intake (TDI) for Immunological Effects of Aflatoxin. Risk Anal. 2022, 42, 431–438, . [CrossRef]
- Turna, N.S.; Comstock, S.S.; Gangur, V.; Wu, F. Effects of aflatoxin on the immune system: Evidence from human and mammalian animal research. Crit. Rev. Food Sci. Nutr. 2023, 1–19, . [CrossRef]
- Di Nardo, F.; Cavalera, S.; Baggiani, C.; Chiarello, M.; Pazzi, M.; Anfossi, L. Enzyme Immunoassay for Measuring Aflatoxin B1 in Legal Cannabis. Toxins 2020, 12, 265, . [CrossRef]
- National Dairy Development Board (2019). In: Devegowda, G., Sridhar, V. and Sherasia, P. L. Aflatoxin: Prevenance and Control in dairy feed and milk: 10.
- Niessen, L.; Bechtner, J.; Fodil, S.; Taniwaki, M.H.; Vogel, R.F. LAMP-based group specific detection of aflatoxin producers within Aspergillus section Flavi in food raw materials, spices, and dried fruit using neutral red for visible-light signal detection. Int. J. Food Microbiol. 2018, 266, 241–250, . [CrossRef]
- Nleya, N.; Adetunji, M.C.; Mwanza, M. Current Status of Mycotoxin Contamination of Food Commodities in Zimbabwe. Toxins 2018, 10, 89, . [CrossRef]
- Nleya, N., Ngoma, L., & Mwanza, M. (2020). Aflatoxin occurrence in dairy feeds: A case of Bulawayo, Zimbabwe. Aflatoxin B1 Occurrence, Detection and Toxicological Effects. [CrossRef]
- Odhav, B.; Naicker, V. Mycotoxins in South African traditionally brewed beers. Food Addit. Contam. 2002, 19, 55–61, . [CrossRef]
- Odogola, W. R. (1994). Postharvest management and storage of food legumes. Technical systems for agriculture. UNDP/OPS Regional Programme, RAF/92/R51. Harare.
- Ogunade, I.; Jiang, Y.; Adeyemi, J.; Oliveira, A.; Vyas, D.; Adesogan, A. Biomarker of Aflatoxin Ingestion: 1H NMR-Based Plasma Metabolomics of Dairy Cows Fed Aflatoxin B1 with or without Sequestering Agents. Toxins 2018, 10, 545, . [CrossRef]
- Ortega-Beltran, A.; Bandyopadhyay, R. Contributions of integrated aflatoxin management strategies to achieve the sustainable development goals in various African countries. Glob. Food Secur. 2021, 30, 100559, . [CrossRef]
- Ojiambo, P.S.; Battilani, P.; Cary, J.W.; Blum, B.H.; Carbone, I. Cultural and Genetic Approaches to Manage Aflatoxin Contamination: Recent Insights Provide Opportunities for Improved Control. Phytopathology® 2018, 108, 1024–1037, . [CrossRef]
- Ojiambo, P.S.; Battilani, P.; Cary, J.W.; Blum, B.H.; Carbone, I. Cultural and Genetic Approaches to Manage Aflatoxin Contamination: Recent Insights Provide Opportunities for Improved Control. Phytopathology® 2018, 108, 1024–1037, . [CrossRef]
- Onyeagba, R.A., Ugbogu, O.C., Okeke, C.U. and Iroakasi, O., 2004. Studies on the microbial effects of garlic (Allium sativum Linn), ginger (Zingiberofficinale Roscoe) and lime (Citrus aurantifolia Linn.). African Journal of Biotechnology 3: 552-554.
- Oplatowska-Stachowiak, M.; Sajic, N.; Xu, Y.; Haughey, S.A.; Mooney, M.H.; Gong, Y.Y.; Verheijen, R.; Elliott, C.T. Fast and sensitive aflatoxin B1 and total aflatoxins ELISAs for analysis of peanuts, maize and feed ingredients. Food Control. 2016, 63, 239–245, . [CrossRef]
- Otim, M.O.; Mukiibi-Muka, G.; Christensen, H.; Bisgaard, M. Aflatoxicosis, infectious bursal disease and immune response to Newcastle disease vaccination in rural chickens. Avian Pathol. 2005, 34, 319–323, . [CrossRef]
- Otsuki, T., Wilson, J. S., &Sewadeh, M. (2001a). Saving two in a billion: Quantifying the trade effect of European food safety standards on African exports. Food Policy, 26, 495–514. [CrossRef]
- Otsuki, T., Wilson, J. S., &Sewadeh, M. (2001b). What price precaution? European harmonisation of aflatoxinregulationsand African groundnut exports. European Review of Agricultural Economics, 28, 263–283. [CrossRef]
- OwuorLalah, J., Omwoma, S., & A.O. Orony, D. (2020). Aflatoxin B1: Chemistry, Environmental and Diet Sources and Potential Exposure in Human in Kenya. IntechOpen. [CrossRef]
- Paramawati, R., Widodo, P., Budiharti, U. and Handaka., 2006. The role of postharvest machineries and packaging in minimizing aflatoxin contamination in peanut. Indonesian Journal of Agricultural Science 7: 15-19. DOI: 10.21082/ijas.v7n1.2006.p15-19.
- Passone, M.A., Ruffino, M., Ponzio, V., Resnik, S., &Etcheverry, M. G. (2009). Postharvest control of peanut Aspergillus section Flavi populations by a formulation of food-grade antioxidants. International Journal of Food Microbiology, 131(2–3), 211–217. [CrossRef]
- Peles, F.; Sipos, P.; Győri, Z.; Pfliegler, W.P.; Giacometti, F.; Serraino, A.; Pagliuca, G.; Gazzotti, T.; Pócsi, I. Adverse Effects, Transformation and Channeling of Aflatoxins Into Food Raw Materials in Livestock. Front. Microbiol. 2019, 10, 2861, . [CrossRef]
- Perri, F.; Pisconti, S.; Scarpati, G.D.V. P53 mutations and cancer: a tight linkage. Ann. Transl. Med. 2016, 4, 522–522, . [CrossRef]
- Perrone, G.; Ferrara, M.; Medina, A.; Pascale, M.; Magan, N. Toxigenic Fungi and Mycotoxins in a Climate Change Scenario: Ecology, Genomics, Distribution, Prediction and Prevention of the Risk. Microorganisms 2020, 8, 1496, . [CrossRef]
- Phillips, T.D.; Clement, B.A.; Park, D.L. Approaches to Reduction of Aflatoxins in Foods and Feeds. In The Toxicology of Aflatoxins: Human Health, Veterinary, and Agricultural Significance; David, L., Eaton, J.D.G., Eds.; Academic Press: Cambridge, MA, USA, 1994; pp. 383–406, . [CrossRef]
- Pickova, D.; Ostry, V.; Toman, J.; Malir, F. Aflatoxins: History, Significant Milestones, Recent Data on Their Toxicity and Ways to Mitigation. Toxins 2021, 13, 399, . [CrossRef]
- Prietto L., Moraes P.S., Kraus R.B., Meneghetti V., Fagundes C.A.A., Furlong E.B. Post-harvest operations and aflatoxin levels in rice (Oryza sativa) Crop Protection. 2015;78:172–177. [CrossRef]
- Proctor, A.D., Ahmedna, M., Kumar, J.V., and Goktepe, I., 2004. Degradation of aflatoxins in peanut grains/flour by gaseous ozonation and mild heat treatment. Food Additives and Contaminants 21: 786-793. [CrossRef]
- Qian, G.S.; Ross, R.K.; Yu, M.C.; Yuan, J.M.; Gao, Y.T.; Henderson, B.E.; Wogan, G.N.; Groopman, J.D. A follow-up study of urinary markers of aflatoxin exposure and liver cancer risk in Shanghai, People's Republic of China. Cancer Epidemiol. Biomark. Prev. 1994, 3, 3–10.
- Wu, Q.; Xu, H. Design and development of an on-line fluorescence spectroscopy system for detection of aflatoxin in pistachio nuts. Postharvest Biol. Technol. 2020, 159, 111016, . [CrossRef]
- Rahmianna, A.A., Taufiq, A. and Yusnawan, E., 2007. Effect of harvest timing and postharvest storage conditions on aflatoxin contamination in groundnuts harvested from the Wonogiri regency in Indonesia. SAT eJournal 5: 1-3.
- Refai, M. (1988). Aflatoxin and Aflatoxicosis. Journal of Egypt Veterinary Medical Association, 48(1), 1–19.
- Richard, J.L. DISCOVERY OF AFLATOXINS AND SIGNIFICANT HISTORICAL FEATURES. Toxin Rev. 2008, 27, 171–201, . [CrossRef]
- Ríos-Reina, R.; Azcarate, S.; Camiña, J.; Callejón, R. Assessment of UV–visible spectroscopy as a useful tool for determining grape-must caramel in high-quality wine and balsamic vinegars. Food Chem. 2020, 323, 126792, . [CrossRef]
- Rodrigues, I., &Naehrer, K. (2012). Three-year survey on the worldwide occurrence of mycotoxins in feedstuffs and feed. Toxins (Basel), 9, 663–675. [CrossRef]
- Ross, R.K.; Yu, M.C.; Henderson, B.E.; Yuan, J.M.; Qian, G.S.; Tu, J.T.; Gao, Y.T.; Wogan, G.N.; Groopman, J.D. Urinary aflatoxin biomarkers and risk of hepatocellular carcinoma. Lancet 1992, 339, 943–946, . [CrossRef]
- Liu, R., MengyaoLu ,Ruiqi Wang , Shanshan Wang , Ming Chang , QingzheJina , and XingguoWanga Degradation of aflatoxin B1 in peanut meal by electron beam irradiation. International journal of food properties. 2018, 21 (1), 892–901. [CrossRef]
- Saito, M.; Machida, S. A rapid identification method for aflatoxin-producing strains of Aspergillus flavus and A. parasiticus by ammonia vapor. Mycoscience 1999, 40, 205–208, . [CrossRef]
- Sanders, T. H., Blankenship, P. D., Cole, R. J., & Hill, R. A. (1984). Effect of soil temperature and drought on peanut pod and stem temperatures relative to Aspergillusflavus invasion and aflatoxin contamination. Mycopathologia, 86(1), 51-54. [CrossRef]
- Sardiñas, N.; Vázquez, C.; Gil-Serna, J.; González-Jaén, M.T.; Patiño, B. Specific detection and quantification of Aspergillus flavus and Aspergillus parasiticus in wheat flour by SYBR® Green quantitative PCR. Int. J. Food Microbiol. 2011, 145, 121–125, . [CrossRef]
- Sarma, U.P.; Bhetaria, P.J.; Devi, P.; Varma, A. Aflatoxins: Implications on Health. Indian J. Clin. Biochem. 2017, 32, 124–133, . [CrossRef]
- Amoras, E.S.; Costa, A.L.P. Aflatoxins: A Brief Review of their Chemical Properties, Toxicological Effects and Control Measures. Arch. Ecotoxicol. 2020, 2, 43–46, . [CrossRef]
- Scarpari, M.; Punelli, M.; Scala, V.; Zaccaria, M.; Nobili, C.; Ludovici, M.; Camera, E.; Fabbri, A.A.; Reverberi, M.; Fanelli, C. Lipids in Aspergillus flavus-maize interaction. Front. Microbiol. 2014, 5, 74, . [CrossRef]
- Scott, P.M., 1984. Effect of food processing on mycotoxins. Journal of Food Protection 47: 489-499.
- Shabeer, S.; Asad, S.; Jamal, A.; Ali, A. Aflatoxin Contamination, Its Impact and Management Strategies: An Updated Review. Toxins 2022, 14, 307. [CrossRef]
- Shaltout FA, Amin RA, Nassif MZ and Abd-Elwahab SA, 2014. Detection of aflatoxins in some meat products. Benha Vet Med J, 27(2): 368-674.
- Shen, M.-H.; Singh, R.K. Determining aflatoxins in raw peanuts using immunoaffinity column as sample clean-up method followed by normal-phase HPLC-FLD analysis. Food Control. 2022, 139, . [CrossRef]
- Shi, H.; Cooper, B.; Stroshine, R.L.; Ileleji, K.E.; Keener, K.M. Structures of degradation products and degradation pathways of aflatoxin B-1 by high-voltage atmospheric cold plasma (HVACP) treatment Journal of Agricultural and Food Chemistry, 2017, 65 (30), 6222-6230.
- Shier, W.T.; Lao, Y.; Steele, T.W.; Abbas, H.K. Yellow pigments used in rapid identification of aflatoxin-producing Aspergillus strains are anthraquinones associated with the aflatoxin biosynthetic pathway. Bioorganic Chem. 2005, 33, 426–438, . [CrossRef]
- Siddappa V, Nanjegowda DK and Viswanath P, 2012. Occurrence of aflatoxin M1 in some samples of UHT, raw and pasteurized milk from Indian states of Karnataka and Tamilnadu. Food Chem Toxicol,50(11): 4158-4162. [CrossRef]
- Singh, P.; Cotty, P.J. Aflatoxin contamination of dried red chilies: Contrasts between the United States and Nigeria, two markets differing in regulation enforcement. Food Control. 2017, 80, 374–379, . [CrossRef]
- Smela, M.E., Currier, S.S., Bailey, E.A., &Essigmann, J.M. (2001). The chemistry and biology of aflatoxin B(1): From mutational spectrometry to carcinogenesis. Carcinogenesis, 22, 535–545. [CrossRef]
- Smith, J., &Groopman, J. (2018). Aflatoxin. In P. Boffetta& P. Hainaut (Eds.), Encyclopedia of Cancer (pp. 30–43). Academic Press.
- Sobolev, A.P.; Ingallina, C.; Spano, M.; Di Matteo, G.; Mannina, L. NMR-Based Approaches in the Study of Foods. Molecules 2022, 27, 7906, . [CrossRef]
- Leger, R.J.S.; Screen, S.E.; Shams-Pirzadeh, B. Lack of Host Specialization inAspergillus flavus. Appl. Environ. Microbiol. 2000, 66, 320–324, . [CrossRef]
- Strosnider, H.; Azziz-Baumgartner, E.; Banziger, M.; Bhat, R.V.; Breiman, R.; Brune, M.-N.; Decock, K.; Dilley, A.; Groopman, J.; Hell, K.; et al. Workgroup Report: Public Health Strategies for Reducing Aflatoxin Exposure in Developing Countries. Environ. Health Perspect. 2006, 114, 1898–1903, doi:10.1289/ehp.9302.
- Sudhakar, P.; Latha, P.; Sreenivasulu, Y.; Reddy, B.V.B.; Hemalatha, T.M.; Balakrishna, M.; Reddy, K.R. Inhibition of Aspergillus flavus colonization and aflatoxin (AfB1) in peanut by methyleugenol. Indian Journal of Experimental Biology 2009, 47, 63–7.
- Tao, F.; Yao, H.; Hruska, Z.; Liu, Y.; Rajasekaran, K.; Bhatnagar, D. Use of Visible–Near-Infrared (Vis-NIR) Spectroscopy to Detect Aflatoxin B1 on Peanut Kernels. Appl. Spectrosc. 2019, 73, 415–423, . [CrossRef]
- Tchana, A.N., Moundipa, P.F., &Tchouanguep, F.M. (2010). Aflatoxin contamination in food and body fluids in relation to malnutrition and cancer status in Cameroon. International Journal of Environmental Research and Public Health, 7(1), 178–188. [CrossRef]
- Tejero, P.; Martín, A.; Rodríguez, A.; Galván, A.I.; Ruiz-Moyano, S.; Hernández, A. In Vitro Biological Control of Aspergillus flavus by Hanseniaspora opuntiae L479 and Hanseniaspora uvarum L793, Producers of Antifungal Volatile Organic Compounds. Toxins 2021, 13, 663, . [CrossRef]
- Tiemersma, E.W.; E Omer, R.; Bunschoten, A.; Veer, P.V.; Kok, F.J.; O Idris, M.; Kadaru, A.M.; Fedail, S.S.; Kampman, E. Role of genetic polymorphism of glutathione-S-transferase T1 and microsomal epoxide hydrolase in aflatoxin-associated hepatocellular carcinoma.. 2001, 10, 785–91.
- Torlak, E.; Akata, I.; Erci, F.; Uncu, A.T. Use of gaseous ozone to reduce aflatoxin B1 and microorganisms in poultry feed. J. Stored Prod. Res. 2016, 68, 44–49, . [CrossRef]
- Tripathi, P.; Upadhyay, N.; Nara, S. Recent advancements in lateral flow immunoassays: A journey for toxindetection in food. Crit. Rev. Food Sci. Nutr. 2017, 58, 1715–1734. [CrossRef]
- Tuan, N.A.; Grizzle, J.M.; Lovell, R.T.; Manning, B.B.; E Rottinghaus, G. Growth and hepatic lesions of Nile tilapia (Oreochromis niloticus) fed diets containing aflatoxin B1. Aquaculture 2002, 212, 311–319, . [CrossRef]
- Turner, P.; Sylla, A.; Gong, Y.; Diallo, M.S.; Sutcliffe, A.; Hall, A.; Wild, C. Reduction in exposure to carcinogenic aflatoxins by postharvest intervention measures in west Africa: a community-based intervention study. Lancet 2005, 365, 1950–1956, . [CrossRef]
- Udomkun, P.; Wiredu, A.N.; Nagle, M.; Müller, J.; Vanlauwe, B.; Bandyopadhyay, R. Innovative technologies to manage aflatoxins in foods and feeds and the profitability of application – A review. Food Control. 2017, 76, 127–138, . [CrossRef]
- Udomkun, P.; Wiredu, A.N.; Nagle, M.; Bandyopadhyay, R.; Müller, J.; Vanlauwe, B. Mycotoxins in Sub-Saharan Africa: Present situation, socio-economic impact, awareness, and outlook. Food Control. 2017, 72, 110–122, . [CrossRef]
- Kakde, U.B.K.U.B.; Ijrbat STUDY OF BIODIVERSITY OF FUNGAL BIOAEROSOLS IN MUMBAI METROPOLIS. 2014, . [CrossRef]
- United Nations (2019). UN News global perspective human stories: can we feed the world and ensure no one goes hungry? https:// news. un. org/ en/ story/ 2019/ 10/ 10484 52. Accessed 16 Dec 2021.
- USAID and Danya International. Aflatoxin: A Synthesis of the Research on Health, Agriculture and Trade. 2012.
- USDA. (2018). United States Department of Agriculture. China Releases Standard for Maximum Levels of Mycotoxins in Foods. USDA Foreign Agriculture Service, Global Agriculture Information Network (GAIN) Report CH18026. https:// apps. fas. usda. gov/ newgainapi/ api/ report/ downloadreportbyfile name? filename= China% 20Rel eases% 20Sta ndard% 20for% 20Max imum% 20Lev els% 20of% 20Myc otoxi ns% 20in% 20Foo ds% 20_ Beijing_ China% 20-% 20Peo ples% 20Rep ublic% 20of_5- 9- 2018. pdf. Accessed 19 July 2021.
- Usha, P.S., Preetida, J.B., Prameela, D., et al. (2017). Aflatoxins: Implications on health. Indian Journal of Clinical Biochemistry, 32(2), 124–133. [CrossRef]
- Valtchev, I., Koynarski, T., Sotirov, L., Nikolov, Y., &Petkov, P. (2015). Effect of aflatoxin B1 on moulard duck’s natural immunity. Pakistan Veterinary Journal, 35, 67–70.
- van Egmond, H.P.; Jonker, M.A. Worldwide Regulations on Aflatoxins—The Situation in 2002. J. Toxicol. Toxin Rev. 2004, 23, 273–293, . [CrossRef]
- Vanfleteren, M.J.E.G.W.; Dingemans, A.-M.C.; Surmont, V.F.; Vermaelen, K.Y.; Postma, A.A.; Lashof, A.M.L.O.; Pitz, C.C.M.; Hendriks, L.E.L. Invasive Aspergillosis Mimicking Metastatic Lung Cancer. Front. Oncol. 2018, 8, 188, . [CrossRef]
- Kumar, V.V. Aflatoxins: Properties, Toxicity and Detoxification. Nutr. Food Sci. Int. J. 2018, 6, 1–4, . [CrossRef]
- Verma, R.J. (2004). Aflatoxin cause DNA damage. International Journal of Human Genetics, 4, 231–236. [CrossRef]
- Verma, R.J. (2004). Aflatoxin cause DNA damage. International Journal of Human Genetics, 4(4), 231–236. [CrossRef]
- Villers, P. Aflatoxins and safe storage. Front. Microbiol. 2014, 5, 158, . [CrossRef]
- Okechukwu, V.O.; Adelusi, O.A.; Kappo, A.P.; Njobeh, P.B.; Mamo, M.A. Aflatoxins: Occurrence, biosynthesis, mechanism of action and effects, conventional/emerging detection techniques. Food Chem. 2024, 436, 137775, . [CrossRef]
- Wacoo Alex P., Wendiro Deborah, Vuzi Peter C., Hawumba Joseph F. 2014. Methods for Detection of Aflatoxins in Agricultural Food Crops. Journal of Applied Chemistry, 2014:1-15. [CrossRef]
- Wagacha, J. M., &Muthomi, J. W. (2008). Mycotoxin problem in Africa: current status, implications to food safety and health and possible management strategies. International journal of food microbiology, 124(1), 1-12. [CrossRef]
- Waliyar, F., Kumar, P.L., Ntare, B.R., Diarra, B. and Kodio, O. 2008. Pre- and post-harvest management of aflatoxin contamination in peanuts. In J. F. Leslie et al. (Eds.), Mycotoxins: Detection methods, management, public health and agricultural trade. Wallingford: CABI. Pp. 209–218.
- Wang, X.; Niessner, R.; Tang, D.; Knopp, D. Nanoparticle-based immunosensors and immunoassays for aflatoxins. Anal. Chim. Acta 2016, 912, 10–23, . [CrossRef]
- Wang, B.; Han, X.; Bai, Y.; Lin, Z.; Qiu, M.; Nie, X.; Wang, S.; Zhang, F.; Zhuang, Z.; Yuan, J.; et al. Effects of nitrogen metabolism on growth and aflatoxin biosynthesis in Aspergillus flavus. J. Hazard. Mater. 2017, 324, 691–700, . [CrossRef]
- Wang, W.; Lawrence, K.C.; Ni, X.; Yoon, S.-C.; Heitschmidt, G.W.; Feldner, P. Near-infrared hyperspectral imaging for detecting Aflatoxin B1 of maize kernels. Food Control. 2015, 51, 347–355, . [CrossRef]
- Wild, C.P.; Gong, Y.Y. Mycotoxins and human disease: a largely ignored global health issue. Carcinog. 2010, 31, 71–82, . [CrossRef]
- Wild, C. P., & Hall, A. J. (1999). Hepatitis B virus and liver cancer: Unanswered questions. Cancer Survey, 33, 35–54.
- Wild, C.P.; Montesano, R. A model of interaction: Aflatoxins and hepatitis viruses in liver cancer aetiology and prevention. Cancer Lett. 2009, 286, 22–28, . [CrossRef]
- Wild, C.P., Miller, J.D., &Groopman, J.D. (2015). Effects of aflatoxins on aflatoxicosis and liver cancer. In C.P. Wild, J.D. Miller, & J.D. Groopman (Eds.), Mycotoxin Control in Low- and Middle-Income Countries (Chapter 3). IARC Working Group Reports, No. 9. International Agency for Research on Cancer.
- Williams JH, Phillips TD, Jolly PE, Stiles JK, Jolly CM, et al. (2004). Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. The American Journal of Clinical Nutrition,80 (5):2004,1106-1122,ISSN 0002-9165. [CrossRef]
- Winters, B.; Custer, J.; Galvagno, S.M., Jr.; Colantuoni, E.; Kapoor, S.G.; Lee, H.; Goode, V.; Robinson, K.; Nakhasi, A.; Pronovost, P.; et al. Diagnostic errors in the intensive care unit: a systematic review of autopsy studies. BMJ Qual. Saf. 2012, 21, 894–902, doi:10.1136/bmjqs-2012-000803.
- Womack, E.D.; E Brown, A.; Sparks, D.L. A recent review of non-biological remediation of aflatoxin-contaminated crops. J. Sci. Food Agric. 2014, 94, 1706–1714, . [CrossRef]
- World Health Organization (WHO). Aflatoxins; WHO: Geneva, Switzerland, 2018. Available online: https://www.who.int/ foodsafety/FSDigest_Aflatoxins_EN.pdf (accessed on 19 December 2021).
- Wu F (2004). Mycotoxin risk assessment for the purpose of setting international regulatory standards. Environ SciTechnol 38: 4049–4055.
- Wu, F.; Guclu, H. Aflatoxin Regulations in a Network of Global Maize Trade. PLOS ONE 2012, 7, e45151, . [CrossRef]
- Wu, F. Mycotoxin Reduction in Bt Corn: Potential Economic, Health, and Regulatory Impacts. Transgenic Res. 2006, 15, 277–289, . [CrossRef]
- Wu Q and Xu H. (2020) Design and development of an on-line fluorescence spectroscopy system for detection of aflatoxin in pistachio nuts. Postharvest Biology and Technology, 159, 111016.
- Wu, F. (2015). Global impacts of aflatoxin in maize: Trade and human health. World Mycotoxin Journal, 8(2), 137–142. [CrossRef]
- Xu, G.; Wang, C.; Yu, H.; Li, Y.; Zhao, Q.; Zhou, X.; Li, C.; Liu, M. Structural basis for high-affinity recognition of aflatoxin B1 by a DNA aptamer. Nucleic Acids Res. 2023, 51, 7666–7674, . [CrossRef]
- Xu, H.; Dong, Y.; Guo, J.; Jiang, X.; Liu, J.; Xu, S.; Wang, H. Monoclonal antibody production and the development of an indirect competitive enzyme-linked immunosorbent assay for screening T-2 toxin in milk. Toxicon 2018, 156, 1–6, . [CrossRef]
- Xue, Z.; Zhang, Y.; Yu, W.; Zhang, J.; Wang, J.; Wan, F.; Kim, Y.; Liu, Y.; Kou, X. Recent advances in aflatoxin B1 detection based on nanotechnology and nanomaterials-A review. Anal. Chim. Acta 2019, 1069, 1–27, . [CrossRef]
- Yan, C.; Wang, Q.; Yang, Q.; Wu, W. Recent Advances in Aflatoxins Detection Based on Nanomaterials. Nanomaterials 2020, 10, 1626, . [CrossRef]
- Yang, Y.; Li, G.; Wu, D.; Liu, J.; Li, X.; Luo, P.; Hu, N.; Wang, H.; Wu, Y. Recent advances on toxicity and determination methods ofmycotoxins in foodstuffs. Trends Food Sci. Technol. 2020, 96, 233–252.
- Yao, W.; Liu, R.; Xu, Z.; Zhang, Y.; Deng, Y.; Guo, H. Rapid Determination of Aflatoxin B1 Contamination in Peanut Oil by Fourier Transform Near-Infrared Spectroscopy. Spectrosc. 2022, 2022, 1–9, . [CrossRef]
- Yazdanpanah, H.; Zarghi, A.; Shafaati, A.R.; Foroutan, S.M.; Aboul-Fathi, F.; Khoddam, A.; Nazari, F.; Shaki, F. Analysis of aflatoxin b1 in Iranian foods using HPLC and a monolithic column and estimation of its dietary intake.. 2013, 12, 83–9.
- Yoshinari, T.; Noda, Y.; Yoda, K.; Sezaki, H.; Nagasawa, H.; Sakuda, S. Inhibitory activity of blasticidin A, a strong aflatoxin production inhibitor, on protein synthesis of yeast: selective inhibition of aflatoxin production by protein synthesis inhibitors. J. Antibiot. 2010, 63, 309–314, . [CrossRef]
- Yue, C., Beghin, J., & Jensen, H. H. (2006). Tariff equivalent of technical barriers to trade with imperfect substitution andtrade costs. American Journal of Agricultural Economics, 88(4), 947–960. [CrossRef]
- Zentai, A.; Jóźwiak, .; Süth, M.; Farkas, Z. Carry-Over of Aflatoxin B1 from Feed to Cow Milk—A Review. Toxins 2023, 15, 195, . [CrossRef]
- Zhuang, Z.; Huang, Y.; Yang, Y.; Wang, S. Identification of AFB1-interacting proteins and interactions between RPSA and AFB1. J. Hazard. Mater. 2016, 301, 297–303, . [CrossRef]
- Zuckerman, J.T.; Minko, I.G.; Kant, M.; Jaruga, P.; Stone, M.P.; Dizdaroglu, M.; McCullough, A.K.; Lloyd, R.S. Functional analyses of single nucleotide polymorphic variants of the DNA glycosylase NEIL1 in sub-Saharan African populations. DNA Repair 2023, 129, 103544–103544, . [CrossRef]
- Zuma-Netshiukhwi, G.; Hlazo, O.M.; Motholo, S.A. Evaluating the Effect of Climate Variability on Zea Mays Productivity over Glen Research Station: South Africa. Eur. J. Agric. Food Sci. 2021, 3, 110–120, . [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).