1. Introduction
1.1. Overview of Cosmetic and Dermatology Sciences
Cosmetics may be generally categorized as health and beauty products and are used to improve the appearance of a person’s skin [
1]. Although cosmetics are used for beautifying and hygiene purposes, they may also be used to maintain and enhance skin beauty[
2,
3,
4]. Currently, there is an increasing interest in cosmetic and antiaging preparation techniques as people are becoming more concerned regarding their appearance when signs of aging commence. The increased interest is due to the need for individuals to have resources by utilising anti-aging products [
5]. Cosmetics make the skin smooth and supple, hence providing a youthful appearance. Skin aging is a process that takes place due to chronological and photo-aging resulting from the influence of external factors. The skin produces antioxidants as a defensive mechanism. Therefore, many products have antioxidants as a core ingredient that assists in the reduction of wrinkles hence maintaining the smoothness of the skin[
6,
7].
Cosmetology has a wide range of products with various types of cosmetics used for specific purposes such as cleansing, moisturizing, facial toner, shampoos, hair care products, and oily cosmetics that do not contain water, etc. [
2,
8,
9]. While cosmetics are used for the purpose of beautifying, perfuming, and cleansing, and have been employed since the early origin of world civilization, huge progress has been made during the 20th century in the diversification of these types of products in addition to the safety and protection of the consumer [
2].
Cosmetic products have a long history and have been noted to be present in every ancient society around the world [
1,
10]. After 1939, cosmetics were started to be regulated as drugs and products used for health. This was followed by, the Food and Drug Administration (FDA), through the Federal Food, Drug, and Cosmetic Act, which had the authority to regulate cosmetics that were required to be safe for the consumer and hypoallergic [
1,
2,
11,
12].
The term “cosmeceutical” refers to the cosmetic-pharmaceutical hybrids intended to enhance the health and beauty of the skin. Due to cosmeceuticals promising future in skin care products, this area has made significant advances within the world of dermatological products. They are either naturally derived or synthetic, but both have functional ingredients with either therapeutic, disease-fighting or healing properties [
13,
14]. The role of cosmetics as positive healing products might be exploited by developing formulations to improve the appearance of UV- damaged and wrinkled skin in persons with hypersensitive skin. It can be concluded that cosmetic products play a key role in everyday life and are used by all genders, thereby creating a significant increase in cosmetic usage and scope also [
13,
15] . Furthermore, cosmetic, are products that are applied daily on parts or all parts of the human body and therefore require special knowledge and care when designing appropriate formulations. The ingredients used should be combined to achieve the desired therapeutic or cosmetic effects and be appropriate for the purpose. Additionally, all cosmetic products must meet the standard requirements, such as, being temperature resistant and stable over a sufficient period of time, and therefore preservative usage, pH regulators, chelating agents, and antioxidants should be considered [
9,
16].
The fact that many cosmetic and skin care products consist of synthetic chemicals may cause side effects such as skin irritation, allergic reactions, and dermatitis. Therefore, investigating the safety of these products and the protection of the customer is one of the extremely important issues in quality control analysis for skin application formulations [
17]. However, stability must also be controlled, to minimise changes in the colour, smell and viscosity of the product for the users. They should have antimicrobial ingredients such as preservatives that maintain the stability of the product and last long over the shelf life and avoid secondary pollution after opening the pack [
9].
Dermatology is the study of everything related to the skin, which includes thousands of skin diseases and skin conditions, that vary from mild to severe or even fatal. According to Global Burden of Disease (GBD) evaluation, the list of diseases are noted over all the globe, and include eczema, psoriasis, acne vulgaris, pruritus, alopecia areata, decubitus ulcer, urticaria, scabies, fungal skin diseases, impetigo, abscess, and other bacterial skin diseases, cellulitis, viral warts, molluscum contagiosum, and non-melanoma skin cancer [
18]. Melanoma is of concern in UK with due to increasing incidence in white populations, suspected to be caused by increased sun exposure [
19,
20]. Dermatological treatments vary from mild oral supplementations to enhance skin health to more traditional drugs used to treat the skin diseases, while more concerns go to chronic skin diseases that are often difficult to treat them such as skin pigmentation disorders, onychomycosis, psoriasis, and atopic dermatitis. However, considerable pharmaceutical developments have been achieved in recent years [
8,
18] pharmaceutical industries have put more effort in developing new and more efficient topical formulation to treat skin disorder such as skin inflammatory diseases [
21]. Hence having knowledge and of skin physiology is essential to designing new approaches and developing better topical products for skin conditions [
8,
22]
1.2. The Skin and Skin Anatomy
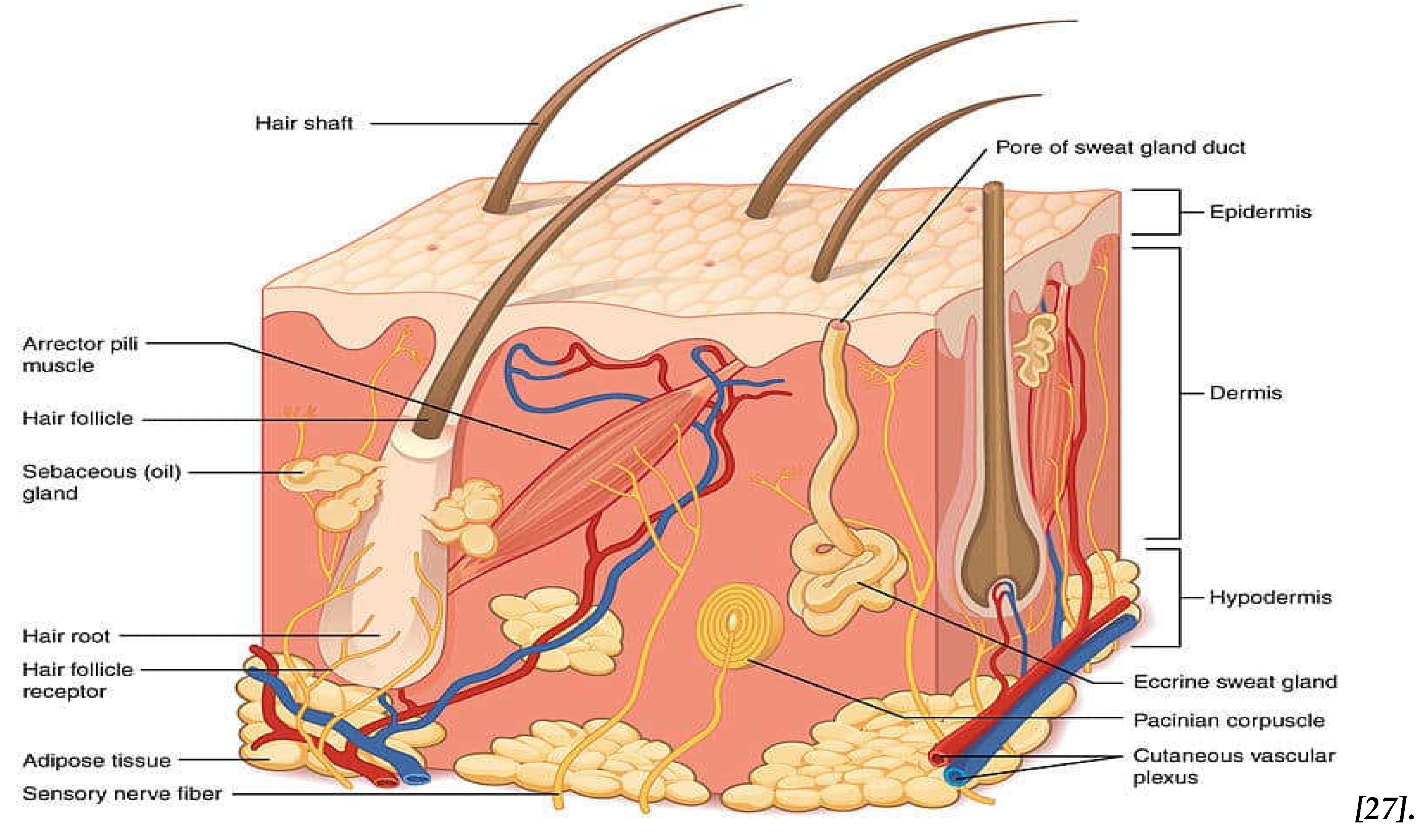
The skin is the interface between the body and the environment. It is composed of the epidermis, dermis and hypodermis [
23,
24]. The epidermis is the outer structure, and it is a vascular multilamellar multi-layered epithelial tissue divided into several layers. The epidermis, mostly consists of epidermal cells, known as keratinocytes. The stratum corneum (SC), (
Figure 1), is the outer layer of the epidermis and outlines the epidermal permeability barrier that prevents water and electrolytes being lost from the skin [
25,
26] . SC is therefore the most important layer involved in skin hence, it is important to understand the structure and function of the stratum corneum and the epidermal barrier. SC is a heterogeneous tissue in its structure and consists of non-nucleated, flat, protein-enriched corneocytes and lipid-enriched intercellular areas [
25,
26]. These lipids that act as barrier function are synthesized in the keratinocytes of the nucleated epidermal layers, then their storage is held in the lamellar bodies, while, during the transition, expel out into the intercellular spaces from the stratum granulosum to the stratum corneum forming a system of continuous membrane bilayers [
25]. However, other composites such as melanin’s, proteins of the SC and epidermis, and many other small components have essential responsibilities in the protective barrier of the skin [
25] . This barrier acts as defence against sunlight, insects, bacteria, toxins, and allergens and is also responsible for variations in skin appearance [
26].
The dermis layer is approximately 1–3 mm in thickness and it determines elastic properties and the flexibility of the skin, whilst also it provides physical strength to the skin. The major component of this layer is a protein in the form of collagen fibers (75%) and elastin fibres (2–4%) containing blood vessels and lymphatic channels [
8,
28,
29]. The dermis is composed of the papillary dermis and the reticular dermis with the papillary dermis lying in direct contact with the epidermis [
23,
26].
Furthermore, in addition to the connective tissue cells, this layer of skin also contains other substances, such as glycosaminoglycans and hyaluronic acid, that regulate the amount of water in the skin and maintain skin hydration [
28,
29]. The high-water content in the dermis, comprising around 60-70%, is crucial for maintaining skin hydration. As age advances, the skin tends to experience a decline in water content, contributing to increased water loss and potentially leading to the formation of wrinkles. Skin with lower water levels is more prone to dehydration and the development of visible signs of aging [
29,
30,
31]. The dermis has additional glands such as sebaceous glands and sweat glands. These two glands are different in their shape and size in different parts of the skin, but they act similarly. Sebaceous glands are distributed throughout the body, but they secrete sebum, hence on the skin surface which performs as a preservative and lubricant and protects against biological infections [
28,
30].
Hydrogels have gained attention in addressing skin hydration problems. These water-based materials can provide a moist environment, helping to replenish and retain skin moisture. Hydrogels may be used in skincare products and transdermal applications, offering a potential solution for improving skin hydration and combating issues related to water loss, which is often associated with dryness and wrinkles. Furthermore, hydrogels serve as effective delivery systems for various substances, including skincare ingredients and medications. Their high-water content and ability to hold and release substances make them ideal for immediate and controlled release applications. In skincare, hydrogels can deliver hydrating compounds, antioxidants, or other active ingredients to the skin, promoting better absorption and targeted delivery. This makes hydrogels valuable in enhancing the efficacy of skincare products and ensuring a more sustained impact on skin hydration and health.
2. Overview of Hydrogels
2.1. Introduction to Hydrogels
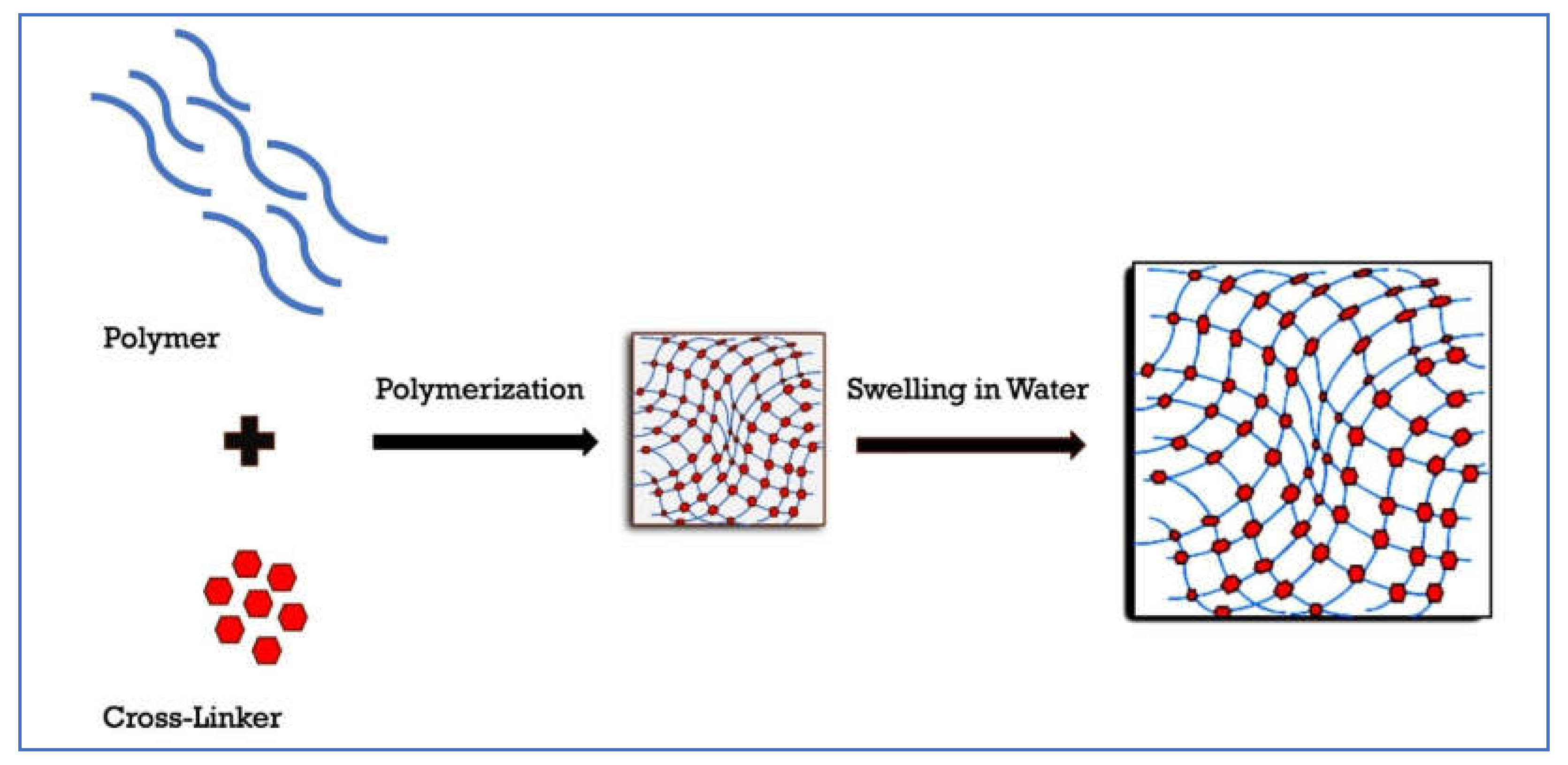
Hydrogels are cross-linked polymers that swell in water and retain it within their structure without dissolving [
32]. Hydrogels may be made up of one or more polymers, which are three-dimensional chains that are cross-linked [
33]. The spaces between the macromolecules fill with water swelling the compound to form a hydrogel. The polymers have hydrophilic functional groups which hydrate in aqueous media and are responsible for the hydrogel’s ability to absorb water [
33,
34]. Covalent crosslinks prevent the dissolution of the hydrogel in water [
32,
35](
Figure 2).
2.2. Types of Hydrogels
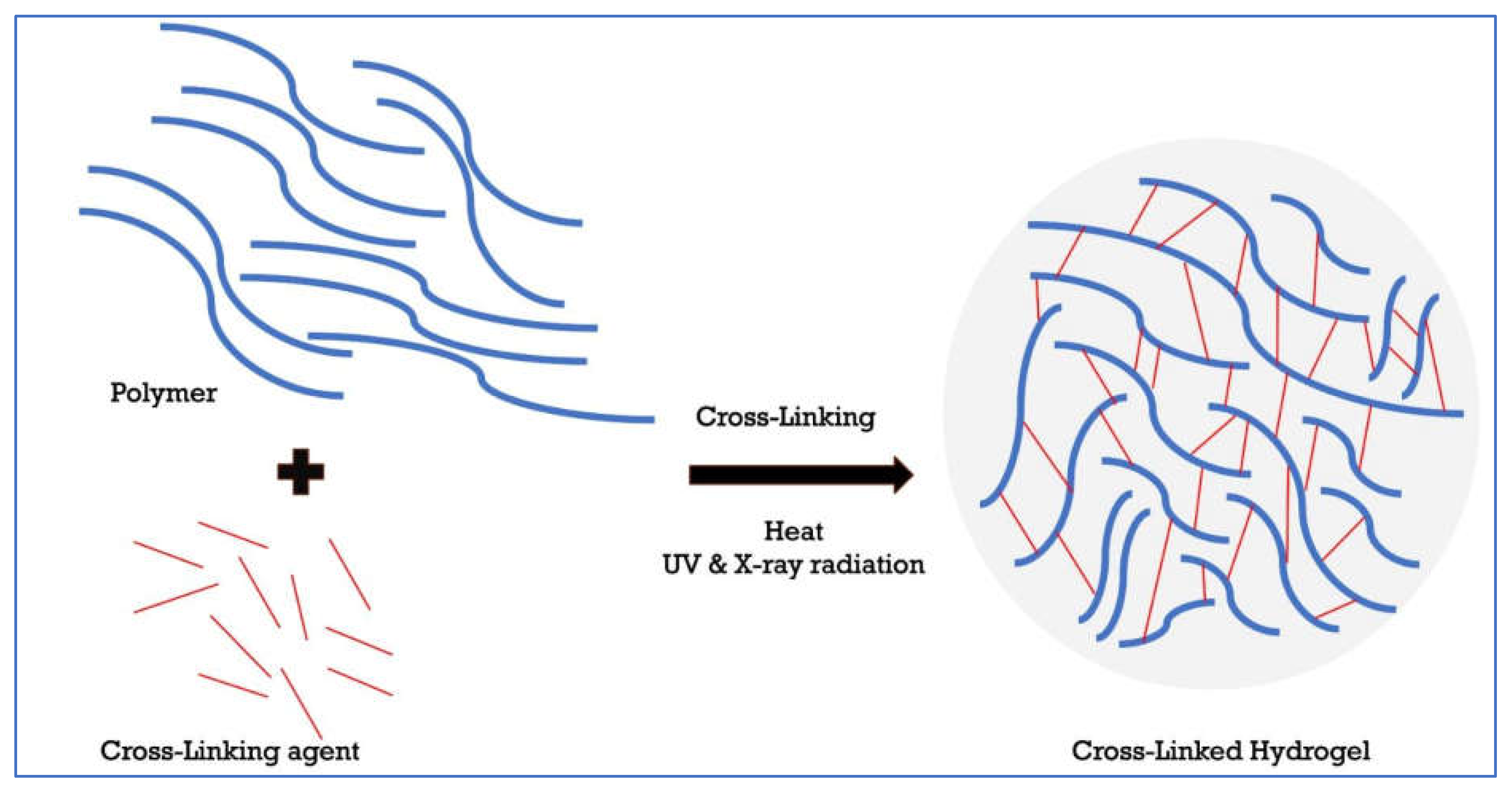
There are different types of hydrogels depending on their origin (natural versus synthetic hydrogels), polymer composition i.e., one or more monomer types (e.g., copolymer, and homopolymeric hydrogels), biodegradability (biodegradable hydrogels versus non-biodegradable hydrogels) and configuration (crystalline, non-crystalline, hydrocolloid aggregates, and semi-crystalline) [
36,
37]. Hydrogels can also be classified according to their type of cross-linking (chemically, and physically cross-linked hydrogels), physical appearance (matrix and microsphere) as well as the network’s electrical charge (neutral, anionic, cationic) [
36,
37]. Hydrogels can also be classified according to their method of preparation (e.g., irradiation, UV radiation) [
36] (
Figure 3).
2.3. Rheological Properties of Hydrogels
The rheology of hydrogels is mostly dependent on the molecular structure of its polymers [
38]. According to [
39], the consistency and the elasticity of hydrogel improve, when polymer concentration increases. While a higher shear thinning behaviour is indicated with decreasing in polymer concentration. In Liao et al. it was shown that an increase in HA molecular weight from (134.000 to 4 million Da) or decreasing the pH to acidic leads to an increase the rheological behaviour [
40].
2.4. Characterization of the Physical and Chemical Properties of the Hydrogels
The characterization of hydrogels depends on the gel bonding type. Physical or reversible hydrogels are formed by molecular entanglements or secondary bonds (e.g., ionic, H-bonding, stereo-complexation, and hydrophobic forces), while chemical hydrogels are irreversible and formed by covalent cross - linking (chemical functionalization), which are stronger and have higher mechanical properties [
41]. The chemical composition also influences the response of the hydrogel to stimuli such as pH, temperature, and light [
42].
3. Hyaluronic Acid
3.1. Introduction on Hyaluronic Acid
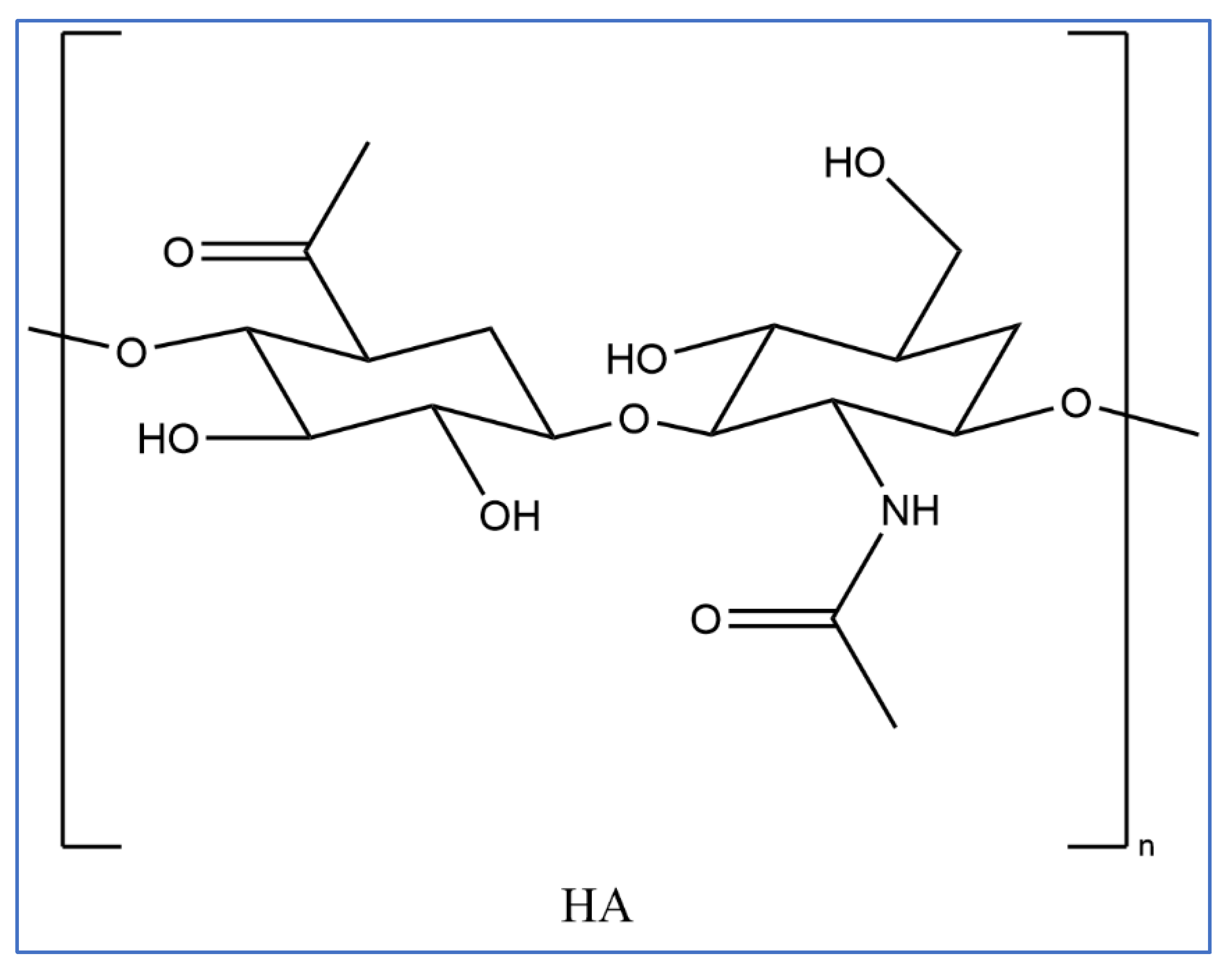
Hyaluronic acid (HA) is a non-sulphated glycosaminoglycan comprising repeating polymeric disaccharides of N-acetyly-D-glucosamine and D-glucuronic acid [
43,
44]. These are linked together by alternative beta-1, 3, and beta-1,4 glycosidic bonds [
45]. It is a naturally occurring biopolymer identical in molecular and chemical form in all tissues and has significant biological functions in all living cells. It is found on the body in connective tissues, joints, and skin. HA contributes to tissue hydration, lubrication, and cellular signalling. In the skin, it helps maintain moisture and elasticity. Additionally, HA is involved in wound healing, inflammation regulation, and other physiological processes across different cell types, making it a versatile and significant component in the body. [
46]. (
Figure 4 ) shows the chemical structure of HA [
47,
48].
3.2. Classification of Hyaluronic Acid
Based on molecular weight, HA can be classified into low molecular weight and high molecular weight HA. High molecular weight HA has a molecular mass of more than 1000 kDa [
49]. Low molecular weight HA is HA with a molecular mass of less than 500 kDa [
50] . The molecular weight of HA is mostly dependent on its source [
40,
51]. For example, the weight of HA in rabbit vitreous is mostly high (2000-3000 kDa)[
52]. The biological effects of HA may differ depending on the molecular weight [
46].Different molecular weights may influence how HA interacts with cells and tissues. Generally, high Molecular Weight (HMW) HA is known for its hydrating and lubricating properties. It forms a protective barrier on the skin’s surface, helping to retain moisture and maintain elasticity. While low Molecular Weight (LMW) HA is believed to penetrate the skin more easily. It may have anti-inflammatory effects and could potentially influence cellular processes, such as promoting wound healing and modulating inflammation[
53].
3.3. Physical and Chemical Properties of Hyaluronic Acid
HA has hydroxyl and carboxyl groups, and it can form a hydrogel under mild conditions such as mixing it with water [
54,
55]. The degree of cross-linking and modification determines its physical and chemical properties. Hydrogen atoms around the axis of the structure are non-polar and hydrophobic while the side chains are more polar and hydrophilic [
45]. Hydrogen bonding stabilizes the conformation of HA in solution. However, these bonds are easily disrupted by temperature and pH. At intermediate pH levels, HA behaves like most other polymers with no strong intermolecular interactions, but as pH reduces towards 2.5, HA forms a gel due to decreasing carboxylate dissociation and increased intermolecular interaction [
56]. HA must be chemically modified to form a less degradable gel that is used in many biomedical applications [
56].
3.4. Types of Gelling Agents Used for Preparation of Hyaluronic Acid Hydrogels
3.4.1. Specific Types of Cross-Linking Agents
While HA itself is a good gelling agent, some literature reports the use of other gelling agents that facilitate cross-linking of polymers, which leads to viscoelasticity and gives a gel its structure and elasticity for different applications [
57]. Cross-linkers or other polymers mostly used to enhance the crosslinking, in addition to some effect as gelling agents. Polymers such as alginate, gelatine, and chitosan can be used as gelling agents for other polymers to form hydrogels [
32] . Gum Arabic aggregates its protein components after exposure to heat, which increases its molecular weight to produce a hydrogel with enhanced water binding capability and mechanical properties [
57,
58]. Cross-linkers such as glutaraldehyde, adipic acid dihyadrizide, epichlorohydrin, and aldehyde induce reaction of the functional groups of polymers which result in cross-linking and subsequently, hydrogel formation [
57]. Others include dextran, xanthan gum and hydrocolloids (carrageenan) [
57,
58].
3.4.2. Temperature Dependence of Gelation Mechanism to Prepare HA Hydrogels.
Gelation of HA is temperature sensitive which is a typical characteristic of thermo-reversible (sol-gel-sol) hydrogels. They have a gelation temperature and a gelation concentration. HA is non-gelling, but as Fujiwara et al. found, it can form hydrogels if annealed in the sol. State [
59]. The authors studied the effect of annealing on the gelation of HA in aqueous solution and found that HA solutions of higher concentration (3%wt) formed a gel at 60 ˚C, if given more than 6 hours, but at lower concentrations (1%wt and 2%wt) they did not [
59].
3.4.3. pH Dependence of Gelation Mechanism to Prepare HA Hydrogels
HA degrades slightly in acidic and basic conditions (pH 1.6 and 12.6 respectively). It may undergo hydrolysis, depolymerization, and breaking down of its molecular structure into shorter fragments, although rheological behaviour is not affected much [
60]. However, at pH 2.5, a reduction in the net charge of the polymer increases interchain interactions causing the polymer to become gel-like. Above a pH of 12, the hydrogen bond network partially breaks, which reduces the stiffness of the polymer and subsequently, the viscosity [
60]. The extent of degradation is greatest at high pH [
61].
3.5. Preparation of Hyaluronic Acid Hydrogels Using Cross-Linking Agents
Preparation of HA hydrogels involves the addition of an agent to promote cross-linking of polymer molecules to form a gel. Gelling agents can be natural or synthetic. Methylcellulose (cellulose gum) is a gelling agent that facilitates gel formation through thermally induced physical crosslinks. The first step in the preparation of hydrogel is to sterilize the HA and methylcellulose. Caicco et al. sterilized their polymers by dissolving them in a filtration system comprising of a 0.22µm polyether sulfone membrane [
62]. Lyophilization processes are then conducted to recover the solid polymers. HA (1500) kDa and methylcellulose hydrogel were prepared by dissolving the two components together. Different amounts were used, but the gel was strongest when the total polymer content was highest [
62]. According to Parhi et.al, HA and methylcellulose blends formed H-bonding interactions between the two polymers chains. This interaction in addition to the compatibility in the geometry of the two compounds improves the hydrogel’s viscoelastic properties [
41,
63].
Lv et al. investigated cross-linked HA and a synthetic tri-block copolymer poly(-caprolactone-co-1,4,8-trioxa [4.6]spiro-9- undecanone)–poly(ethylene glycol)–poly(-caprolactone-co-1,4,8-trioxa [4.6]spiro-9-undecanone) triblock copolymers (PECT). The researchers synthesized PECT and freeze-dried it into a powder to enhance its dispersibility [
64]. Sodium hyaluronate was deprotonated dissolving in 100ml deionized water and then dialyzed in dilute acid for 12 hours after which it was lyophilized [
64]. They found combining HA with PECT enhanced the mechanical strength, viscosity, and morphological appearance of the hydrogel. [
64].
3.6. Methods of Preparation for Hyaluronic Acid Hydrogels in Different
3.6.1. Hyaluronic Acid Molecular Weight
The molecular weight of HA is one of the factors that determines the viscosity as well as the elasticity of the gel formed. Mostly HA of high MW can be used as a gelling agent. These gels can be either reversible (pH or temperature –induced gelation) or irreversible (covalent cross-links using cross-linking agents), whereas HA of low MW is used as active ingredient [
65].
Preparation of HA hydrogels using HA of different molecular weights differs in the type of material being produced and the gelling agent used. In Chun et al., HA hydrogels of different molecular weights were prepared. The concentration of the solutions was 0.5% w/v; the molecular weights were low (69 kDa) and high (1058 kDa) [
66]. The sodium hyaluronate for the two compounds of different molecular weights is dissolved in sodium hydroxide and the pH is adjusted. A cross-linker was added, and the hydrogels dried at 60 ˚C for two hours. The cross-linked HA microspheres of the lower molecular HA were larger than those of the higher molecular weight HA [
66].
3.6.2. pH
The pH of the medium partly influences the uptake of water and thus, the swelling of the HA hydrogel. Preparation methods of HA hydrogels in mediums of different pH should be carefully done because the behaviour of the gel changes with pH. Larraneta et al., prepared HA hydrogels using 5 %w/w of HA and varying concentrations of Gantrez S97 (cross-linker). The polymer with a lower concentration of cross-linker had a higher swelling capacity in water than PBS while those with higher concentrations of the cross-linker had lower swelling capacity in water than PBS. The cross-linker is comprised of poly-acids (p
Ka1 = 3.47 and p
Ka2= 6.47), but only a few groups can react with the HA to form the hydrogel [
67]. The remaining functional groups are ionized depending on the pH of the medium. Deionized water is slightly acidic compared to PBS, which explains the different swelling behaviour [
67].
The type of medium is also affected by the pH, whereby, for example, the preparation of HA hydrogels in NaOH is likely to result in a product which has different properties than when prepared in HCl or any other medium. In [
68],HA hydrogels were prepared in aqueous NaOH solutions to provide a high pH and facilitate easier disruption of intramolecular hydrogen bonds of the HA molecule. This enhances chain flexibility, solubility in water, and cross-linking between HA’s functional groups and the cross-linker [
68].
3.6.3. Temperature
Temperature influences the formation of hydrogels, particularly for temperature-sensitive polymers. These polymers have a lower critical solution temperature above which they become less soluble or insoluble in water [
69,
70]. For example, a thermo-responsive hydrogel comprising of HA and poly(N-isopropylacrylamide) (PNIPAAm) phase transition occurred at around 30-33 ˚C [
71]. Above, 35 ˚C, the viscosity of the hydrogel increased [
71]. HA hydrogels (cross-linked with thiol end capped Pluronic F127 copolymer) were solid at room temperature but transitioned to gel state at body temperature [
51]. Such materials have numerous applications in drug and cell delivery [
51].
3.7. Preparation of High Molecular Weight Hydrogel Platform Loaded with Low Molecular Weight Hyaluronic Acid
High molecular weight hydrogel platforms can be loaded with low molecular weight HA in skin care products and procedures. The numerous benefits of HA on the skin are limited by the molecular size of HA, which is usually so large that it cannot penetrate the skin [
72]. However, fragmenting high molecular weight HA into smaller fragments of smaller molecular weight can overcome this limitation [
72].
3.8. Pharmaceutical and Cosmetic Uses of Hyaluronic Acid
3.8.1. Uses of Hyaluronic Acid in Pharmaceutical Applications and its Advantages
Due to its chemical and physical properties as well as functions in biological tissues, HA has numerous pharmaceutical and cosmetic uses. HA is used as a drug delivery system due to its biocompatibility, non-immunogenicity, and biodegradability [
73]. HA can interact with receptors such as CD44 (cluster of differentiation 44) antigen a cell-surface glycoprotein, which is expressed in many types of tumor cells and thus, it is a candidate for the delivery of imaging and anticancer agents [
74]. HA can embed fibroblast growth factor and b’e used to make scaffolds, which can be used to fasten wound healing [
75].
3.8.2. Uses of Hyaluronic Acid in the Cosmetic Field and its Advantages
HA in the cosmetic industry has gained widespread usage in limiting or reducing skin aging [
76]. Hyaluronic acid formulations of different molecular weights (50, 130, 300, 800, and 2000 kDa) respectively, is also used in products that promote skin hydration, which is found low molecular weight HA (50, 130 kDa) are a significant improvement in wrinkle reduction. It is also used as filler to shape lips, model cheeks, and correct facial lines [
77].
3.9. Hydrogel Based Hyaluronic Acid Phase Transition by Changing the pH for Full or Completed Absorption via Skin
Some HA hydrogel formulations for use on the skin are made to change phase when applied to the skin after or during the application by pH or temperature (thermo-responsive). Hydrogels with thermo-induced gelation systems consist of interpenetrating networks of polymer chains that transition into a gel-sol state due to temperature change. The drugs are loaded at room temperature, but once the formulation is injected into the body, the gel shrinks, traps the contents, and facilitates their release [
78]. Poly(N-isopropylacrylamide) (PNIPAAm) was discussed in Section 1.4.6.3. Other mechanisms include pH change, solvent exchange or crystallization, UV light exposure, thickening after removal of injection shear, and ionic cross-linking [
71]. Ionic cross-linking of hydrogels is cross-linking under mild conditions such as physiological pH and room temperature [
79]. They can be easily reversed with a slight change in the conditions, leading to the release of the loaded materials. HA hydrogels have specific behaviour in low, high, and intermediate pH. This can be exploited to facilitate the release of drugs, for example, in the stomach where the pH is low. Electro-sensitive hydrogels respond to changes in the electric field and may shrink or swell when the surface of the hydrogel is in contact with the electric field [
80]. Light-sensitive hydrogels change phases due to changes in light and have applications in ophthalmic drug delivery systems [
80].
4. Overview of the Development of Transdermal Delivery Systems
The most common routes of drug and actives delivery are via oral and parenteral routes, and more conventional route is oral delivery for small molecule drugs [
81,
82]. The main pros of the oral route are pre-determined doses, portability, and patient self-administration, which explains why the oral route is the most convenient route for delivering medications[
83,
84]. In other cases, some drugs such as therapeutic peptides and proteins are not suitable to be delivered via the oral route, because they rapidly degrade in the stomach, and in addition, have size-limited transport across the epithelium [
85]. Therefore, these macromolecules could be delivered by parenteral route [
81,
85,
86]. However, the parenteral route also has some limitations, such as the invasive nature of injections, the pain and lower compliance by patients. Additionally, the administrator requires to be officially trained for parenteral administration [
86,
87]. Reasonably, to overcome these disadvantages, other advanced drug delivery methods have been developed such as transdermal drug delivery (TDD) [
26].
Transdermal drug delivery (TDD) is a method of delivering drugs and actives to the body system by applying a drug/active formulation onto intact and healthy skin thereby mitigating painful injection [
82,
85]. Firstly, the drug or active will penetrate outer layer of skin through the stratum corneum and pass through the deeper layers such as epidermis and dermis without accumulation of the drug/active in any of the dermal layer of skin. Once the drug reaches the dermal layer, it will be absorbed systemically via the dermal microcirculation [
88,
89].
Furthermore, the transdermal delivery route has many benefits over other routes such as supplying a non-invasive technique as an alternative to parenteral routes, hence avoiding patient needle phobia problems [
66]. The large surface area of the human skin provides a wide scope of opportunities for transdermal absorption of applied drugs /actives [
85]. Looking to the pharmacokinetic aspects of drugs and actives, the TDD provides lower risk of toxic side effects due to more consistent drug release and absorption it [
82]. Other advantages of the TDD are enhanced patient compliance, because it minimizes dose frequencies and it is suitable for administration to unconscious patient or those who are unable to swallow, and who rely on self-administration [
26]. The transdermal drug delivery system avoids pre-systemic metabolism (first pass hepatic metabolism), and it therefore improves the bioavailability[
82,
84] .
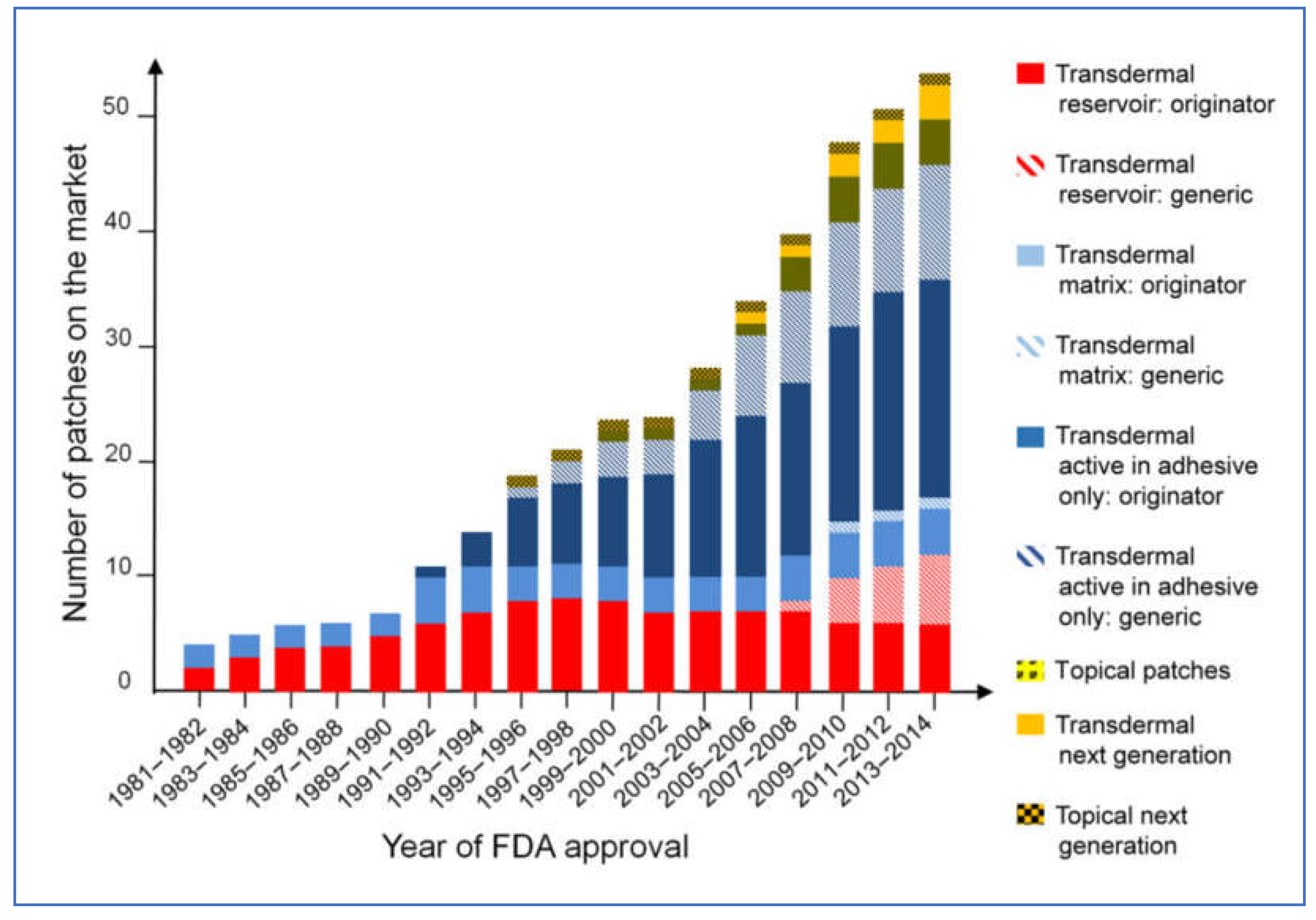
Transdermal patches have gained a reputation to be used as potential cosmetic, topical, and transdermal delivery systems with major consideration given to the diffusion area and level of skin hydration [
79]. They have evolved from the growth in skin science, technology, and development trials [
90]. The transdermal delivery system is well defined as a delivery system and transfer of the drug depending on the (dose, area, vehicle, and device) through the skin to the body is well understood [
91]. The transdermal drug delivery system has undergone significant evolution and market growth since its early stages in the 1980s, (
Figure 5) represents the evolution of TDD system over the years.
Drug Delivery via Skin Patches
Although many actives need to be delivered via a patch to the skin, not all may be suitable to load in the patches due to incompatibilities and physicochemical properties, for instance, large peptides, certain lipophilic drugs, and actives that are susceptible to degradation in the patch matrix due to pH instability. The drug’s potency and the clinical requirements are among the main points that regulatory authorities need to look to approve for transdermal application and release to the market [
90,
92]. Many parameters of the active ingredients determine the skin penetration and flux from the stratum corneum such as the molecular weight, and solubility[
93,
94]. All these directly affect drug diffusion and penetration via the SC. The molecular size of any active is very important with regards to percutaneous penetration of the skin, especially for the drugs with large molecules such as large proteins [
90,
95]. For the drug to be able to penetrate the skin layers it should have low molecular weight (MW < 500 Da), a balanced lipophilicity, partition coefficient (log P 1–3), and authors suggest that a high rate of penetration of the drug and actives may depend on a two-pathway (polar and lipid) model for drug transportation through the stratum corneum layer of the skin [
90,
96]. In addition, for a drug to be a feasible candidate for transdermal delivery, is preferable to possess high pharmacological potency. This helps in minimizing the amount of the drug loaded in the patch, reducing the risk of skin irritation and enhancing patient compliance. Examples of such drugs are fentanyl, and nitroglycerin, which have high potency and they are commonly delivered via transdermal patches [
76].
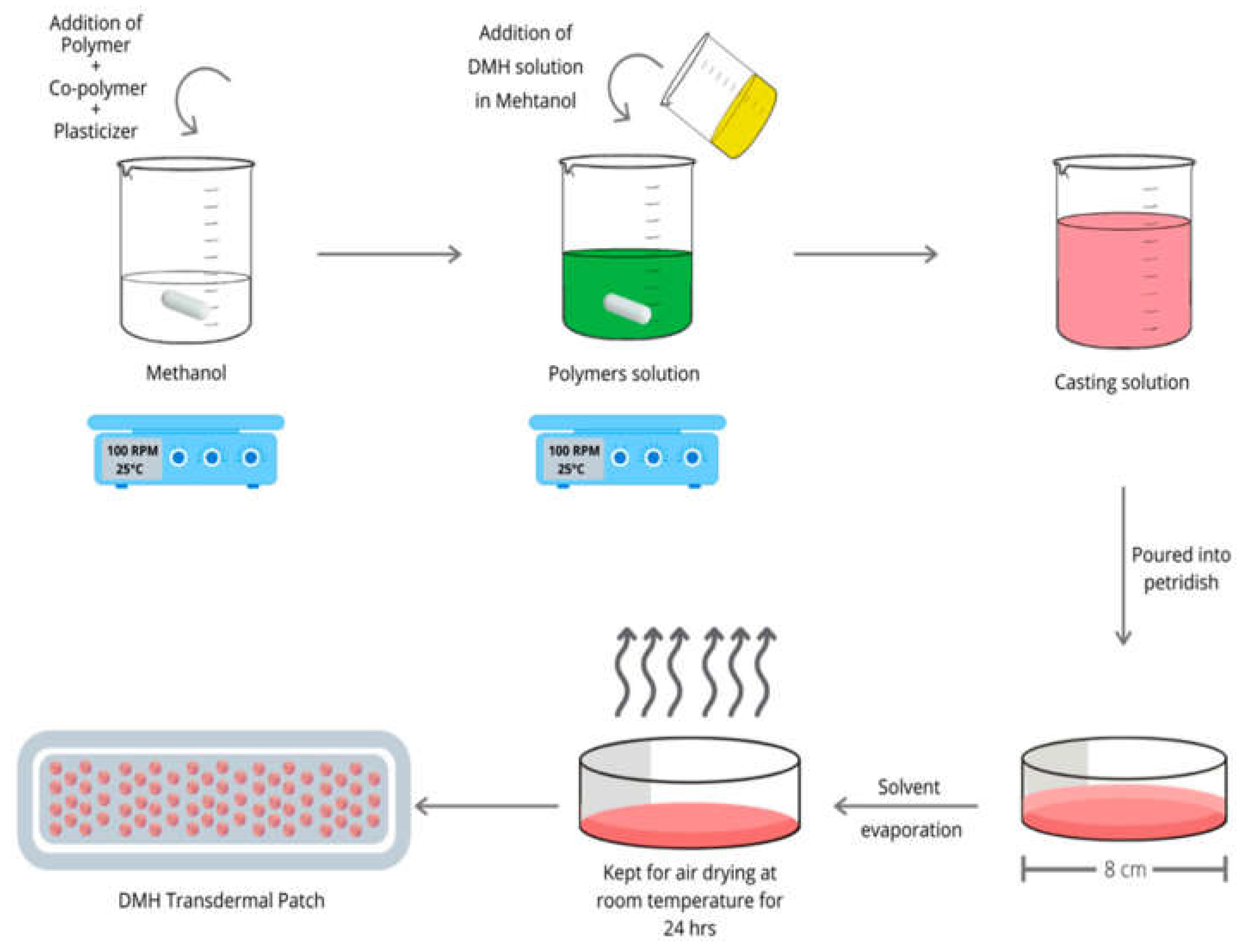
5. Drug and Actives Loading in Hydrogel Films
Hydrogels provide delivery of the pharmaceutical agents (drugs or actives), to specific sites within the body at a more controlled rate. The drug or active molecules distribution within a hydrogel structure can be manipulated and therefore modified or immediate drug release can be achieved [
97]. The drug entrapment is directly connected with the process of swelling/deswelling mechanisms of the hydrogel films [
98]. Thus, swelling governs the uptake of the drug into the hydrogel and influences the release of the drug from the same hydrogel matrix. The drugs and active ingredients in the hydrogels can be loaded in different methods. However, the main two methods for drug loading in hydrogel film matrices are post-loading and in situ loading [
98,
99].(
Figure 6) represent a diagram of preparation of drug loaded patch.
5.1. Post-Loading (Osmosis Dependent Loading) Method in the Hydrogel
Commonly, with post loading method, the drug-loading process is performed by immersing the hydrogel films in the dry state into the drug solution (usually aqueous solution), that contains the drug, depending on their swelling behaviour in the solution [
100,
101,
102]. The drug particles will diffuse and incorporate into the hydrogel structure via the osmosis process. Diffusion is the major force of drug uptake inside the hydrogel system which depends on the hydrogel swelling rate and the time it takes to reach equilibrium [
98,
103]. Hence, many factors affect the amount of drug that incorporates into the hydrogel, such as the molecular weight of the desired incorporated substances and the physicochemical properties. However, the disadvantages of this method appear particularly when the drug molecules are too large, since the drug will be unable to move around through the hydrogel polymer network which results in the drug loading process failing. Moreover, when loading hydrophobic drugs which are solubilised only dissolved in organic solvents (such as ethanol, methanol, and dimethyl sulfoxide (DMSO), it’s essential to include an organic solvent removal process in order to minimize the potentially harmful effects induced by organic solvents, in addition, to ensure good biocompatibility of drug loaded [
99]. Therefore, it’s crucial to optimize the drug-loading method of hydrogels to maximize the drug-loading capacity because it will directly affect the swelling and drug release [
97].
5.2. In-Situ Loading Method of the Actives and Drugs in the Hydrogel
The in-situ loading method involves initially adding the drug or active ingredient to the solution during the preparation of the hydrogel which incorporates the drug into the hydrogel network. This means in the in-situ loading, a polymer precursor solution is mixed with a drug or drug-polymer conjugated solution with or without a crosslinker and allowed to polymerize. Incorporating the drug within the matrix allows hydrogel network formation and drug or actives encapsulation to be achieved simultaneously [
104,
105,
106] see (
Figure 6),which represents the in-situ loading of dimenhydrinate (DMH) to form DMH transdermal patch . This method has the advantage of overcoming many osmosis loading limitations. The in-situ loading method allows the loading and incorporation of higher amounts of drugs and actives than the post loading method, thus achieving more drug delivery to the site. Additionally, it will overcome the physicochemical incompatibilities that occur during the osmosis loading such as pH [
104]. Furthermore, this method enables drug delivery in controlled or modified release due to the entrapment of the loaded drug in the hydrogel polymer network, leading to slower diffusing of the drug out from the hydrogel structure [
97].
6. Drugs and Actives Release from the Hydrogel Film
There are three major mechanisms of drug delivery or release: diffusion, erosion, and swelling [
98]. Hydrogels have various characteristics, such as water absorption, swelling, and degradation aspects, and these properties can greatly improve the utilization rate of drugs and actives and help to control their release as desired [
63]. An example of the advantages of the hydrogels based on drug-controlled-release systems is demonstrated in tissue engineering [
98,
108]. Many articles describe the connection between the duration and release rate of a drug from a hydrogel matrix with some of the structural properties of the hydrogel network, such as (crystallinity of the substances, swelling degree, molecular weight (MW) between the crosslinking points, cross-link density and chemical structure of polymer chains inside the hydrogel) [
104,
109]. The influence of one or more of the hydrogel polymer network parameters upon the release of the drug such as network structure, made up of meshes of different sizes, as a result of a crosslinking process (for example crosslinking copolymerization or irradiation), and chains with functional groups that distribute randomly are also reported [
110,
111,
112,
113,
114]
Generally, drug release studies are carried in vitro, then in vivo. In vitro release studies are connected to the release kinetics where a range of mathematical equations are used as a means of modelling the release kinetics of drugs from polymeric carriers. The drug release follows either Fick’s first law of diffusion or Fick’s second law of diffusion [
115,
116]. According to Fick’s first law of diffusion, the diffusion from a reservoir environment of the hydrogel has a direct relationship with time without depending on the drug concentration, this was referred to as zero order release kinetics [
83,
100]. Fick’s second law of diffusion explains that diffusion from a matrix-style and swellable system when the rapid uptake from surrounding media happens, reaches the equilibrium of swelling immediately. In addition, the dispersed drug will then diffuse through the swollen network. Equation 2 defines this mechanism of drug release, and the equation must be modified relative to the geometry [
98,
117].
7. Conclusions
Hydrogels and transdermal delivery systems have gained significant attention in the field of cosmeceuticals. However, the development of these polymeric carriers as active/drug delivery systems is considered an alternative way for many conventional drug formulations. Mainly used to address inadequate local active/drug availability and challenges associated with delivery in a specific site.
Generally, hydrogel patches are used in a wide range of applications. One of the most frequently used in the cosmetic sector for delivering actives that enhance skin hydration, and beautifying, and a system for delivering the antioxidants. While pharmaceutically, it is used as a treatment for many dermatological conditions. Additionally, Hydrogel transdermal patches are utilized in tissue engineering, wound healing, biomedical applications, and the delivery of drugs to targeted sites. They are used in these applications due to their unique properties. These hydrogels are effective carriers for therapeutic agents, they help minimize invasiveness, adaptability to irregular delivery sites, and biocompatibility. Furthermore, they are employed in controlling the release of therapeutic agents, enhancing the treatment efficacy in diseases, cancers, and tissue regeneration. All these allowed them to be promising biomaterials for scaffolds and carriers in the biomedical field such as applications in skin injury repair, angiogenesis, and targeted drug delivery systems.
Author Contributions
Conceptualization, F.R.; Investigation, F.R., S.C.; Supervision, S.C., P.C.; Validation, F.R., S.C.; Visualization, F.R.; Writing – original draft, F.R.; Writing – review & editing, F.R., S.C., P.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Surya, M. and S. Gunasekaran, A Review on Recent Scenario of Cosmetics. International Journal of Pharmaceutical Sciences Review and Research, 2021.
- Barel, A.O. , Handbook of Cosmetic Science and Technology. 2009, CRC: Press EBooks.
- Cosmeceuticals and Active Cosmetics, Drugs vs. Cosmetics. 2005.
- Raja, S. , et al., Cosmeceuticals and Active Cosmetics. 2015.
- Sharma, A.; Kuhad, A.; Bhandari, R. Novel nanotechnological approaches for treatment of skin-aging. J. Tissue Viability 2022, 31, 374–386. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, D.H.; Neudecker, B.A.; Dinardo, J.C.; Lewis, J.A., II; Maibach, H.I. Clinical efficacy assessment in photodamaged skin of 0.5% and 1.0% idebenone. J. Cosmet. Dermatol. 2005, 4, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Milani, M.; Colombo, F. Skin Anti-Aging Effect of Oral Vitamin A Supplementation in Combination with Topical Retinoic Acid Treatment in Comparison with Topical Treatment Alone: A Randomized, Prospective, Assessor-Blinded, Parallel Trial. Cosmetics 2023, 10, 144. [Google Scholar] [CrossRef]
- Handbook of Cosmetic Science and Technology. 2014.
- Iwata, H. and K. Shimada, Formulas, Ingredients and Production of Cosmetics: Technology of Skin- and Hair-Care Products in Japan. Formulas, Ingredients and Production of Cosmetics, 2012.
- McMullen, R.L.; Dell’acqua, G. History of Natural Ingredients in Cosmetics. Cosmetics 2023, 10, 71. [Google Scholar] [CrossRef]
- Polymers for Personal Care Products and Cosmetics, ed. X.J. Loh. 2016: The Royal Society of Chemistry.
- Patil, A.A. and M. S. Ferritto. Polymers for personal care and cosmetics. 2013.
- Wanjari, N. and J.S. Waghmare, A Review on Latest Trend of Cosmetics-Cosmeceuticals. International Journal of Pharma Research & Review, 2015.
- Rashid, F.; Childs, S.; Dodou, K. Comparison of Analytical Methods for the Detection of Residual Crosslinker in Hyaluronic Acid Hydrogel Films. Cosmetics 2023, 10, 70. [Google Scholar] [CrossRef]
- Gupta, V.; Mohapatra, S.; Mishra, H.; Farooq, U.; Kumar, K.; Ansari, M.J.; Aldawsari, M.F.; Alalaiwe, A.S.; Mirza, M.A.; Iqbal, Z. Nanotechnology in Cosmetics and Cosmeceuticals—A Review of Latest Advancements. Gels 2022, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Manful, M.E.; Ahmed, L.; Barry-Ryan, C. Cosmetic Formulations from Natural Sources: Safety Considerations and Legislative Frameworks in the European Union. Cosmetics 2024, 11, 72. [Google Scholar] [CrossRef]
- Sarkic, A.; Stappen, I. Essential Oils and Their Single Compounds in Cosmetics—A Critical Review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef]
- Hay, R.J.; Johns, N.E.; Williams, H.C.; Bolliger, I.; Dellavalle, R.P.; Margolis, D.J.; Marks, R.; Naldi, L.; Weinstock, M.A.; Wulf, S.K.; et al. The Global Burden of Skin Disease in 2010: An Analysis of the Prevalence and Impact of Skin Conditions. J. Investig. Dermatol. 2014, 134, 1527–1534. [Google Scholar] [CrossRef]
- Pacifico, M.D.; Pearl, R.; Grover, R. The UK Government Two-Week Rule and its Impact on Melanoma Prognosis: An Evidence-Based Study. Ind. Mark. Manag. 2007, 89, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Conforti, C.; Zalaudek, I. Epidemiology and Risk Factors of Melanoma: A Review. Dermatol. Pr. Concept. 2021, 11, 2021161S–2021161S. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-W.; Jee, S.-H. Strategies to Develop a Suitable Formulation for Inflammatory Skin Disease Treatment. Int. J. Mol. Sci. 2021, 22, 6078. [Google Scholar] [CrossRef] [PubMed]
- Textbook of Cosmetic Dermatology. 2017.
- Moser, K.; Kriwet, K.; Naik, A.; Kalia, Y.N.; Guy, R.H. Passive skin penetration enhancement and its quantification in vitro. Eur. J. Pharm. Biopharm. 2001, 52, 103–112. [Google Scholar] [CrossRef]
- C, M.B.Z., S. Grushchak, and J. Newman, Skin Anatomy and Analysis. Facial Plast Surg Clin North Am, 2023. 31(4): p. 433-442.
- Draelos, Z.D. Cosmetics and Dermatologic Problems and Solutions; Taylor & Francis Ltd: London, United Kingdom, 2011. [Google Scholar]
- Alkilani, A.Z.; McCrudden, M.T.C.; Donnelly, R.F. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the Stratum Corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [PubMed]
- Skin Ultrastructure Epidermis - Dermis [cited 2023 27/Nov./2023]; - TeachMeAnatomy]. Available from: https://teachmeanatomy.info/the-basics/ultrastructure/skin/.
- Rostkowska, E.; Poleszak, E.; Wojciechowska, K.; Szewczyk, K.D.S. Dermatological Management of Aged Skin. Cosmetics 2023, 10, 55. [Google Scholar] [CrossRef]
- Rahrovan, S.; Fanian, F.; Mehryan, P.; Humbert, P.; Firooz, A. Male versus female skin: What dermatologists and cosmeticians should know. Int. J. Women's Dermatol. 2018, 4, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Abdo, J.M.; Sopko, N.A.; Milner, S.M. The applied anatomy of human skin: A model for regeneration. Wound Med. 2020, 28, 100179. [Google Scholar] [CrossRef]
- Lai-Cheong, J.E. and J.A. McGrath, Structure and function of skin, hair and nails. Medicine, 2013. 41(6): p. 317-320.
- Ahmed, E.M. , Hydrogel: Preparation, characterization, and applications: A review. Journal of Advanced Research, 2015. 6(2): p. 105-121.
- Akhtar, M.F., M. Hanif, and N.M. Ranjha, Methods of synthesis of hydrogels … A review. Saudi Pharmaceutical Journal, 2016. 24(5): p. 554-559.
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Morteza, B., M. Naimeh, and M. Mehdi, An Introduction to Hydrogels and Some Recent Applications, in Emerging Concepts in Analysis and Applications of Hydrogels, M. Sutapa Biswas, Editor. 2016, IntechOpen: Rijeka. p. Ch. 2.
- Singh, S.K., A. Dhyani, and D.S. Juyal, Hydrogel: Preparation, Characterization and Applications. The Pharma Innovation Journal, 2017. 6: p. 25-32.
- Tang, G.; Du, B.; Stadler, F.J. A novel approach to analyze the rheological properties of hydrogels with network structure simulation. J. Polym. Res. 2017, 25, 1–10. [Google Scholar] [CrossRef]
- Di Giuseppe, E.; Corbi, F.; Funiciello, F.; Massmeyer, A.; Santimano, T.; Rosenau, M.; Davaille, A. Characterization of Carbopol® hydrogel rheology for experimental tectonics and geodynamics. Tectonophysics 2015, 642, 29–45. [Google Scholar] [CrossRef]
- Liao, Y.-H.; Jones, S.A.; Forbes, B.; Martin, G.P.; Brown, M.B. Hyaluronan: Pharmaceutical Characterization and Drug Delivery. Drug Deliv. 2005, 12, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R. Cross-Linked Hydrogel for Pharmaceutical Applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, A.K.; Shukla, S.K.; Bhanu, S.; Kankane, S. Responsive polymers in controlled drug delivery. Prog. Polym. Sci. 2008, 33, 1088–1118. Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermato-Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Iaconisi, G.N.; Lunetti, P.; Gallo, N.; Cappello, A.R.; Fiermonte, G.; Dolce, V.; Capobianco, L. Hyaluronic Acid: A Powerful Biomolecule with Wide-Ranging Applications—A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 10296. [Google Scholar] [CrossRef] [PubMed]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Maneiro, E.; De Andres, M.C.; Fernández-Sueiro, J.L.; Galdo, F.; Blanco, F.J. The biological action of hyaluronan on human osteoartritic articular chondrocytes: the importance of molecular weight. . 2004, 22, 307–12. [Google Scholar]
- Chen, L.H.; Xue, J.F.; Zheng, Z.Y.; Shuhaidi, M.; Thu, H.E.; Hussain, Z. Hyaluronic acid, an efficient biomacromolecule for treatment of inflammatory skin and joint diseases: A review of recent developments and critical appraisal of preclinical and clinical investigations. Int. J. Biol. Macromol. 2018, 116, 572–584. [Google Scholar] [CrossRef]
- Rashid, F.; Carter, P.; Childs, S. Novel Injectable Hydrogel Formulations and Gas Chromatography Analysis of the Residual Crosslinker in Formulations Intended for Pharmaceutical and Cosmetic Applications. Gels 2024, 10, 280. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, A.S.; Pilling, D.; Gomer, R.H. High and Low Molecular Weight Hyaluronic Acid Differentially Regulate Human Fibrocyte Differentiation. PLOS ONE 2011, 6, e26078. [Google Scholar] [CrossRef] [PubMed]
- Albano, G.D. , et al., A 3D “In Vitro” Model to Study Hyaluronan Effect in Nasal Epithelial Cell Line Exposed to Double-Stranded RNA Poly(I:C). Biomol Ther (Seoul), 2020. 28(3): p. 272-281.
- Lee, Y. , et al., Thermo-sensitive, injectable, and tissue adhesive sol–gel transition hyaluronic acid/pluronic composite hydrogels prepared from bio-inspired catechol-thiol reaction. Soft Matter, 2010. 6(5): p. 977-983.
- Cowman, M.K.; Lee, H.-G.; Schwertfeger, K.L.; McCarthy, J.B.; Turley, E.A. The Content and Size of Hyaluronan in Biological Fluids and Tissues. Front. Immunol. 2015, 6, 261. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, F.; Babaeipour, V.; Saharkhiz, S. Comprehensive review on biosynthesis of hyaluronic acid with different molecular weights and its biomedical applications. Int. J. Biol. Macromol. 2023, 240, 124484. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wang, Y.-M.; Yang, J.; Luo, X.-S. Hyaluronic acid: a versatile biomaterial in tissue engineering. Plast. Aesthetic Res. 2017, 4, 219. [Google Scholar] [CrossRef]
- Rashid, F.; Albayati, M.; Dodou, K. Studies on Novel Methods for Formulating Novel Cross-Linked Hydrogel Films of Hyaluronic Acid. Cosmetics 2019, 6, 59. [Google Scholar] [CrossRef]
- Ström, A.; Larsson, A.; Okay, O. Preparation and physical properties of hyaluronic acid-based cryogels. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Gulrez, S., S. Al-Assaf, and G. Phillips, Hydrogels: Methods of Preparation, Characterisation and Applications in Molecular and Environmental Bioengineering. 2011.
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Versatility of Hydrogels: From Synthetic Strategies, Classification, and Properties to Biomedical Applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef]
- Fujiwara, J.; Takahashi, M.; Hatakeyama, T.; Hatakeyama, H. Gelation of hyaluronic acid through annealing. Polym. Int. 2000, 49, 1604–1608. [Google Scholar] [CrossRef]
- Gatej, I.; Popa, M.; Rinaudo, M. Role of the pH on Hyaluronan Behavior in Aqueous Solution. Biomacromolecules 2004, 6, 61–67. [Google Scholar] [CrossRef]
- Maleki, A.; Kjøniksen, A.; Nyström, B. Effect of pH on the Behavior of Hyaluronic Acid in Dilute and Semidilute Aqueous Solutions. Macromol. Symp. 2008, 274, 131–140. [Google Scholar] [CrossRef]
- Caicco, M.J.; Zahir, T.; Mothe, A.J.; Ballios, B.G.; Kihm, A.J.; Tator, C.H.; Shoichet, M.S. Characterization of hyaluronan–methylcellulose hydrogels for cell delivery to the injured spinal cord. J. Biomed. Mater. Res. Part A 2012, 101A, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Bisht, A.; Alexander, A.; Dave, V.; Sharma, S. Biomedical applications of hydrogels in drug delivery system: An update. J. Drug Deliv. Sci. Technol. 2021, 66, 102914. [Google Scholar] [CrossRef]
- Lv, Z.; Chang, L.; Long, X.; Liu, J.; Xiang, Y.; Liu, J.; Liu, J.; Deng, H.; Deng, L.; Dong, A. Thermosensitive in situ hydrogel based on the hybrid of hyaluronic acid and modified PCL/PEG triblock copolymer. Carbohydr. Polym. 2014, 108, 26–33. [Google Scholar] [CrossRef]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Hyaluronic Acid: The Influence of Molecular Weight on Structural, Physical, Physico-Chemical, and Degradable Properties of Biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef] [PubMed]
- Chun, C.; Lee, D.Y.; Kim, J.-T.; Kwon, M.-K.; Kim, Y.-Z.; Kim, S.-S. Effect of molecular weight of hyaluronic acid (HA) on viscoelasticity and particle texturing feel of HA dermal biphasic fillers. Biomater. Res. 2016, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Larraneta, E.; Henry, M.; Irwin, N.J.; Trotter, J.; Perminova, A.A.; Donnelly, R.F. Synthesis and characterization of hyaluronic acid hydrogels crosslinked using a solvent-free process for potential biomedical applications. Carbohydr. Polym. 2018, 181, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Tavsanli, B.; Okay, O. Preparation and fracture process of high strength hyaluronic acid hydrogels cross-linked by ethylene glycol diglycidyl ether. React. Funct. Polym. 2016, 109, 42–51. [Google Scholar] [CrossRef]
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J. Tissue Eng. 2017, 8. [Google Scholar] [CrossRef]
- Xu, L.; Zhong, S.; Gao, Y.; Cui, X. Thermo-responsive poly(N-isopropylacrylamide)-hyaluronic acid nano-hydrogel and its multiple applications. Int. J. Biol. Macromol. 2022, 194, 811–818. [Google Scholar] [CrossRef]
- Ha, D.I.; Lee, S.B.; Chong, M.S.; Lee, Y.M.; Kim, S.Y.; Park, Y.H. Preparation of thermo-responsive and injectable hydrogels based on hyaluronic acid and poly(N-isopropylacrylamide) and their drug release behaviors. Macromol. Res. 2006, 14, 87–93. [Google Scholar] [CrossRef]
- Farwick, M., P. Lersch, and G. Strutz, Low molecular weight hyaluronic acid: Its effects on epidermal gene expression & skin ageing. SÖFW J., 2008. 11: p. 134-137.
- Huang, G.; Huang, H. Application of hyaluronic acid as carriers in drug delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Tripodo, G.; Trapani, A.; Torre, M.L.; Giammona, G.; Trapani, G.; Mandracchia, D. Hyaluronic acid and its derivatives in drug delivery and imaging: Recent advances and challenges. Eur. J. Pharm. Biopharm. 2015, 97, 400–416. [Google Scholar] [CrossRef]
- Neuman, M.G.; Nanau, R.M.; Oruña-Sanchez, L.; Coto, G. Hyaluronic Acid and Wound Healing. J. Pharm. Pharm. Sci. 2015, 18, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Galvez-Martin, P.; Soto-Fernandez, C.; Romero-Rueda, J.; Cabañas, J.; Torrent, A.; Castells, G.; Martinez-Puig, D. A Novel Hyaluronic Acid Matrix Ingredient with Regenerative, Anti-Aging and Antioxidant Capacity. Int. J. Mol. Sci. 2023, 24, 4774. [Google Scholar] [CrossRef] [PubMed]
- Gold, M. Use of hyaluronic acid fillers for the treatment of the aging face. Clin. Interv. Aging 2007, ume 2, 369–376. [Google Scholar] [CrossRef]
- Kondiah, P.J. , et al., A Review of Injectable Polymeric Hydrogel Systems for Application in Bone Tissue Engineering. Molecules, 2016. 21(11).
- Maitra, J. and V. Shukla, Cross-linking in hydrogels - a review. Am J Polym Sci, 2014. 4: p. 25-31.
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. An overview of clinical and commercial impact of drug delivery systems. J. Control. Release 2014, 190, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Das, D.B. Potential of combined ultrasound and microneedles for enhanced transdermal drug permeation: A review. Eur. J. Pharm. Biopharm. 2015, 89, 312–328. [Google Scholar] [CrossRef]
- Brambilla, D.; Luciani, P.; Leroux, J.-C. Breakthrough discoveries in drug delivery technologies: The next 30 years. J. Control. Release 2014, 190, 9–14. [Google Scholar] [CrossRef]
- Ita, K. Transdermal drug delivery: progress and challenges. J. Drug Deliv. Sci. Technol. 2014, 24, 245–250. [Google Scholar] [CrossRef]
- Schoellhammer, C.M.; Blankschtein, D.; Langer, R. Skin permeabilization for transdermal drug delivery: recent advances and future prospects. Expert Opin. Drug Deliv. 2014, 11, 393–407. [Google Scholar] [CrossRef]
- McCrudden, M.T.; Singh, T.R.R.; Migalska, K.; Donnelly, R.F. Strategies for Enhanced Peptide and Protein Delivery. Ther. Deliv. 2013, 4, 593–614. [Google Scholar] [CrossRef] [PubMed]
- Kermode, M. Unsafe injections in low-income country health settings: need for injection safety promotion to prevent the spread of blood-borne viruses. Heal. Promot. Int. 2004, 19, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Singh, T.R.R.; Morrow, D.I.J.; Woolfson, A.D. Microneedle-Mediated Transdermal and Intradermal Drug Delivery; Wiley, 2012;
- Kretsos, K.; Kasting, G.B. A geometrical model of dermal capillary clearance. Math. Biosci. 2007, 208, 430–453. [Google Scholar] [CrossRef] [PubMed]
- Pastore, M.N.; Kalia, Y.N.; Horstmann, M.; Roberts, M.S. Transdermal patches: history, development and pharmacology. Br. J. Pharmacol. 2015, 172, 2179–2209. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M. Solute-Vehicle-Skin Interactions in Percutaneous Absorption: The Principles and the People. Ski. Pharmacol. Physiol. 2013, 26, 356–370. [Google Scholar] [CrossRef] [PubMed]
- Benson, H.A.E. and A.C. Watkinson. Topical and Transdermal Drug Delivery: Principles and Practice. 2011.
- Kasting, G. Lipid Solubility and Molecular Weight: Whose Idea Was That. Ski. Pharmacol. Physiol. 2013, 26, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Walters, K.A. Dermatological And Transdermal Formulations. 2002.
- Magnusson, B.M.; Anissimov, Y.G.; Cross, S.E.; Roberts, M.S. Molecular Size as the Main Determinant of Solute Maximum Flux Across the Skin. J. Investig. Dermatol. 2004, 122, 993–999. [Google Scholar] [CrossRef]
- Wiedersberg, S. and R.H. Guy, Transdermal drug delivery: 30+ years of war and still fighting! Journal of Controlled Release, 2014. 190: p. 150-156.
- Penn, M.J.; Hennessy, M.G. Optimal loading of hydrogel-based drug-delivery systems. Appl. Math. Model. 2022, 112, 649–668. [Google Scholar] [CrossRef]
- Singh, B.; Chauhan, G.; Kumar, S.; Chauhan, N. Synthesis, characterization and swelling responses of pH sensitive psyllium and polyacrylamide based hydrogels for the use in drug delivery (I). Carbohydr. Polym. 2007, 67, 190–200. [Google Scholar] [CrossRef]
- Dong, K. , et al., Assessment of the safety, targeting, and distribution characteristics of a novel pH-sensitive hydrogel. Colloids Surf B Biointerfaces, 2014. 123: p. 965-73.
- Chaturvedi, K.; Ganguly, K.; Nadagouda, M.N.; Aminabhavi, T.M. Polymeric hydrogels for oral insulin delivery. J. Control. Release 2013, 165, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, F.; Chiessi, E.; Villa, R.; Viganò, L.; Zaffaroni, N.; Telling, M.F.; Paradossi, G. Novel PVA-Based Hydrogel Microparticles for Doxorubicin Delivery. Biomacromolecules 2008, 9, 1967–1973. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Ding, J.; Fei, L.; He, M.; Cui, F.; Tang, C.; Yin, C. Beneficial properties for insulin absorption using superporous hydrogel containing interpenetrating polymer network as oral delivery vehicles. Int. J. Pharm. 2008, 350, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Rashid, F.; Albayati, M.; Dodou, K. Novel Crosslinked HA Hydrogel Films for the Immediate Release of Active Ingredients. Cosmetics 2022, 10, 6. [Google Scholar] [CrossRef]
- Cursaru, B.; Teodorescu, M.; Boscornea, C.; Stanescu, P.O.; Stoleriu, S. Drug absorption and release properties of crosslinked hydrogels based on diepoxy-terminated poly(ethylene glycol)s and aliphatic polyamines — a study on the effect of the gel molecular structure. Mater. Sci. Eng. C 2013, 33, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J. , et al., Swelling Kinetics of Interpenetrating Polymer Hydrogels Composed of Poly(Vinyl Alcohol)/Chitosan. Journal of Macromolecular Science, Part A, 2003. 40(5): p. 501-510.
- Kim, S.W., Y. H. Bae, and T. Okano, Hydrogels: swelling, drug loading, and release. Pharm Res, 1992. 9(3): p. 283-90.
- Rasool, B.K.A.; Mohammed, A.A.; Salem, Y.Y. The Optimization of a Dimenhydrinate Transdermal Patch Formulation Based on the Quantitative Analysis of In Vitro Release Data by DDSolver through Skin Penetration Studies. Sci. Pharm. 2021, 89, 33. [Google Scholar] [CrossRef]
- Lei, L.; Bai, Y.; Qin, X.; Liu, J.; Huang, W.; Lv, Q. Current Understanding of Hydrogel for Drug Release and Tissue Engineering. Gels 2022, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Serra, L.; Doménech, J.; Peppas, N.A. Drug transport mechanisms and release kinetics from molecularly designed poly(acrylic acid-g-ethylene glycol) hydrogels. Biomaterials 2006, 27, 5440–5451. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Liu, W.-Q.; Chen, W.-X.; Sun, L.; Zhang, G.-B. Investigation of swelling and controlled-release behaviors of hydrophobically modified poly(methacrylic acid) hydrogels. Polymer 2007, 48, 2665–2671. [Google Scholar] [CrossRef]
- Reddy, O.S.; Subha, M.C.S.; Jithendra, T.; Madhavi, C.; Rao, K.C. Fabrication and characterization of smart karaya gum/sodium alginate semi-IPN microbeads for controlled release of D-penicillamine drug. Polym. Polym. Compos. 2021, 29, 163–175. [Google Scholar] [CrossRef]
- Chen, J.; Liu, M.; Liu, H.; Ma, L. Synthesis, swelling and drug release behavior of poly(N,N-diethylacrylamide-co-N-hydroxymethyl acrylamide) hydrogel. Mater. Sci. Eng. C 2009, 29, 2116–2123. [Google Scholar] [CrossRef]
- Dadsetan, M.; Liu, Z.; Pumberger, M.; Giraldo, C.V.; Ruesink, T.; Lu, L.; Yaszemski, M.J. A stimuli-responsive hydrogel for doxorubicin delivery. Biomaterials 2010, 31, 8051–8062. [Google Scholar] [CrossRef] [PubMed]
- Sullad, A.G.; Manjeshwar, L.S.; Aminabhavi, T.M. Blend microspheres of chitosan and polyurethane for controlled release of water-soluble antihypertensitive drugs. Polym. Bull. 2014, 72, 265–280. [Google Scholar] [CrossRef]
- Kim, I.S. and I.J. Oh, Drug release from the enzyme-degradable and pH-sensitive hydrogel composed of glycidyl methacrylate dextran and poly(acrylic acid). Arch Pharm Res, 2005. 28(8): p. 983-7.
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Fu, Y.; Kao, W.J. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin. Drug Deliv. 2010, 7, 429–444. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).