Submitted:
23 May 2024
Posted:
24 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Reactive Oxygen Species (ROS) and Oxidative Stress (OS)
2.1. Physiological and Pathological Origin of ROS
2.2. Pathogens Responses to OS
3. Antimicrobial Oxidative Therapies: Available Methods to Induce ROS Formation
3.1. ROS Formation Induced by Conventional, Alternative and Natural Antimicrobials
3.1.2. ROS Formation Induced by Alternative Antimicrobials
Ebselen
Nanoparticles (NPs)
Nanozymes (NZs)
AGXX®
3.1.3. ROS Formation Induced by Natural Compounds
3.2. ROS Formation Induced by Antibacterial Photodynamic Therapy
3.2.1. Basic Principles of Photodynamic Therapy
3.2.2. Photosensitizers Used in Clinical Trials
3.2.3. Antibacterial PDT vs. Antibiotics
| Advantages | Limitations | Discussion on limitations |
|---|---|---|
| ↑ BS action than antibiotics including bacteria, protozoa, fungi |
Light limited penetration capabilities |
Possible problems in reaching deep seated infections by scattering phenomena [122] Bacterial colonies located beneath the skin’s surface or within organs could be difficult to reach * [123] |
| ↓ Adverse effects and damage to the host tissue | Potential lack of target specificity |
↑ Selectivity and efficiency of PSs toward bacteria and ↓ toxicity on mammalian cells can be achieved using proper vectors or by co-administration, conjugation or incorporation with polycationic materials, bacterial-targeting peptides, polymers, antibiotics, or antibodies [124,125,126,127] |
| Bactericidal effects independent of antibiotic resistance pattern | Risk of antibiotic inactivation |
When in combination with certain antibiotics APDT can inactivate the antibiotics [123] |
| No resistance following multiple sessions of therapy | Potential side effects | Emergence of skin sensitivity, redness, and pain at the treatment site [128] |
3.2.4. Light Sources
| Light source | LASER | Argon |
Monochromatic, coherent, and collimated light High irradiance Couplable into optical fiber bundles Expansive, cumbersome |
Refs. | |
| Diode | [133] | ||||
| Neodymium doped | Yttrium Aluminum Garnet lasers |
||||
| Light-emitting diodes (LEDs) | Deliver a slightly wider emission spectrum than LASER Low costs No monochromatic, no coherent |
[133] | |||
| Gas-discharge lamps | Quartz-tungsten-halogen lamps Xenon-discharge lamps Sodium lamps |
They can be spectrally filtered to match any PS No efficiently couplable into optical fiber bundles Cause more heating as compared to LASERs and LEDs |
[134] | ||
| Daylight | Broad-spectral range from UV to IR region Free of cost Can illuminate a very large area with high uniformity Variable in irradiance, radiant exposure is poorly controlled |
[133,135] | |||
3.2.5. Clinical Trials
3.3. ROS Formation Induced by Antibacterial Honey Therapy
| Country of Origin | Honey Sample | Organisms | Ref. |
|---|---|---|---|
| Australia | |||
| New Zealand | Manuka | S. aureus, P. aeruginosa | [153] |
| New Zealand | Manuka |
S. aureus, MRSA, MSSA Coagulase-negative S. epidermidis K. pneumonia, ESBL E. coli |
[154] |
| Australia | Leptospermum based honey | S. aureus | [155] |
| North America | |||
| Canada | Canadian honey | E. coli, Bacillus subtilis | [156] |
| Cuba | Christmas vine, Morning glory Black mangrove Linen vine, Singing bean |
S. aureus, P. aeruginosa E. coli, B. subtilis |
[157] |
| South America | |||
| Chile | Ulmo honey | MRSA, E. coli, P. aeruginosa | [158] |
| Argentina | Algarrobo, citrus and multifloral honey |
S. aureus, E. faecalis, E. coli Morganella morganii P. aeruginosa |
[159] |
| Europe | |||
| Scotland | Blossom, heather, Highland, Portobello Orchard |
Acinetobactor calcoaceticus S. aureus, P. aeruginosa, E. coli |
[160] |
| Northwest Spain | Rubus honey |
S. aureus, S. epidermidis Micrococcus luteus, E. faecalis B. cereus, Proteus mirabilis, E. coli P. aeruginosa Salmonella typhimurium |
[161] |
| Denmark | Heather, raspberry, rapeseed, hawthorn White clover | S. aureus, P. aeruginosa, E. coli | [162] |
| Slovakia | Honeydew honey | P. aeruginosa, S. aureus | [163] |
| Asia | |||
| China | Buckwheat honey | S. aureus, P. aeruginosa | [153] |
| Saudi Arabia | Sider honey |
S. aureus, Streptococcus pyogenes Corynebacteria pseudotuberculosis K. pneumonia, P. aeruginosa E. coli |
[164] |
| Africa | |||
| Algeria | Astragalus, wall-rocket, eucalyptus Legume, peach, juniper, buckthorn multifloral |
Clostridium perfringens, S. aureus, E. coli, B. subtili. | [165] |
| Nigeria | Wildflower and bitter leaf honey |
S. typhimurium Shigella dysenteries, E. coli B. cereus, S. aureus |
[166] |
| Egypt | Cotton, blackseed, orange, eucalyptus Sider, clover honey |
E. coli, S. aureus Streptococcus mutans, P. mirabilis P. aeruginosa K. pneumoniae |
[167] |
| Egypt | Acacia, citrus, clover, coriander, cotto palm honey |
S. aureus, S. pyogenes C. pseudotuberculosis K. pneumonia, P. aeruginosa, E. coli |
[164] |
3.3.1. Medical Application of Honey
The Case of Surgihoney Reactive Oxygen (SHRO)
Medical Grade Honey for Clinical Applications
3.4. ROS Formation Induced by Antibacterial Hyperbaric Oxygen Therapy
3.4.1. Clinical Application of HBOT in Infections
4. Possible Novel Methods to Induce ROS Formation: Our Proposal
4.1. Environmental Persistent Free Radicals (EPFRs)
4.2. Biochar-Derived Persistent Free Radicals (PFRs)
4.1.1. Proposed Mechanisms for PFRs Formation During Biomass Pyrolysis
4.1.2. Possible Activities of PFRs and Our Proposal
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Saliba, R.; Zahar, J.-R.; Dabar, G.; Riachy, M.; Karam-Sarkis, D.; Husni, R. Limiting the Spread of Multidrug-Resistant Bacteria in Low-to-Middle-Income Countries: One Size Does Not Fit All. Pathogens 2023, 12, 144. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Courvalin, P.; Dantas, G.; Davies, J.; Eisenstein, B.; Huovinen, P.; Jacoby, G.A.; Kishony, R.; Kreiswirth, B.N.; Kutter, E.; et al. Tackling Antibiotic Resistance. Nat Rev Microbiol 2011, 9, 894–896. [Google Scholar] [CrossRef] [PubMed]
- Franco, B.E.; Altagracia Martínez, M.; Sánchez Rodríguez, M.A.; Wertheimer, A.I. The Determinants of the Antibiotic Resistance Process. Infect Drug Resist 2009, 2, 1–11. [Google Scholar] [PubMed]
- Dryden, M. Reactive Oxygen Species: A Novel Antimicrobial. Int J Antimicrob Agents 2018, 51, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.C.; Martinez, L.; Kirsner, R. The Diabetic Foot: The Importance of Biofilms and Wound Bed Preparation. Curr Diab Rep 2006, 6, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Caviglia, D. Prevention and Eradication of Biofilm by Dendrimers: A Possibility Still Little Explored. Pharmaceutics 2022, 14, 2016. [Google Scholar] [CrossRef]
- Dryden, M.; Cooke, J.; Salib, R.; Holding, R.; Pender, S.L.F.; Brooks, J. Hot Topics in Reactive Oxygen Therapy: Antimicrobial and Immunological Mechanisms, Safety and Clinical Applications. J Glob Antimicrob Resist 2017, 8, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Dryden, M. Reactive Oxygen Therapy: A Novel Therapy in Soft Tissue Infection. Curr Opin Infect Dis 2017, 30, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Dryden, M.S.; Cooke, J.; Salib, R.J.; Holding, R.E.; Biggs, T.; Salamat, A.A.; Allan, R.N.; Newby, R.S.; Halstead, F.; Oppenheim, B.; et al. Reactive Oxygen: A Novel Antimicrobial Mechanism for Targeting Biofilm-Associated Infection. J Glob Antimicrob Resist 2017, 8, 186–191. [Google Scholar] [CrossRef]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive Oxygen Species (ROS) and Wound Healing: The Functional Role of ROS and Emerging ROS-modulating Technologies for Augmentation of the Healing Process. Int Wound J 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Zuccari, G. Oxidative Stress, Antioxidant Capabilities, and Bioavailability: Ellagic Acid or Urolithins? Antioxidants 2020, 9, 707. [Google Scholar] [CrossRef] [PubMed]
- Genestra, M. Oxyl Radicals, Redox-Sensitive Signalling Cascades and Antioxidants. Cell Signal 2007, 19, 1807–1819. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, K.; Khurana, S.; Tai, T. Oxidative Stress in Aging-Matters of the Heart and Mind. Int J Mol Sci 2013, 14, 17897–17925. [Google Scholar] [CrossRef] [PubMed]
- Marengo, B.; Nitti, M.; Furfaro, A.L.; Colla, R.; Ciucis, C. De; Marinari, U.M.; Pronzato, M.A.; Traverso, N.; Domenicotti, C. Redox Homeostasis and Cellular Antioxidant Systems: Crucial Players in Cancer Growth and Therapy. Oxid Med Cell Longev 2016, 2016, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Marengo, B.; Raffaghello, L.; Pistoia, V.; Cottalasso, D.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Reactive Oxygen Species: Biological Stimuli of Neuroblastoma Cell Response. Cancer Lett 2005, 228, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; N. Setzer, W.; Fazel Nabavi, S.; Erdogan Orhan, I.; Braidy, N.; Sobarzo-Sanchez, E.; Mohammad Nabavi, S. Insights Into Effects of Ellagic Acid on the Nervous System: A Mini Review. Curr Pharm Des 2016, 22, 1350–1360. [Google Scholar] [CrossRef]
- Kaneto, H.; Katakami, N.; Matsuhisa, M.; Matsuoka, T. Role of Reactive Oxygen Species in the Progression of Type 2 Diabetes and Atherosclerosis. Mediators Inflamm 2010, 2010, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Park, C.; Jang, H.-J.; Kim, B.; Bae, H.-W.; Chung, I.-Y.; Kim, E.S.; Cho, Y.-H. Antibacterial Strategies Inspired by the Oxidative Stress and Response Networks. Journal of Microbiology 2019, 57, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Gaupp, R.; Ledala, N.; Somerville, G.A. Staphylococcal Response to Oxidative Stress. Front Cell Infect Microbiol 2012, 2. [Google Scholar] [CrossRef]
- Hong, Y.; Zeng, J.; Wang, X.; Drlica, K.; Zhao, X. Post-Stress Bacterial Cell Death Mediated by Reactive Oxygen Species. Proceedings of the National Academy of Sciences 2019, 116, 10064–10071. [Google Scholar] [CrossRef]
- Dryden, M.; Lockyer, G.; Saeed, K.; Cooke, J. Engineered Honey: In Vitro Antimicrobial Activity of a Novel Topical Wound Care Treatment. J Glob Antimicrob Resist 2014, 2, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Vaishampayan, A.; Grohmann, E. Antimicrobials Functioning through ROS-Mediated Mechanisms: Current Insights. Microorganisms 2021, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Pandoli, O.G. Bamboo-Based Biochar: A Still Too Little-Studied Black Gold and Its Current Applications. J Xenobiot 2024, 14, 416–451. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Pandoli, O.G. Biochar-Derived Persistent Free Radicals: A Plethora of Environmental Applications in a Light and Shadows Scenario. Toxics 2024, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A Review of Biochars’ Potential Role in the Remediation, Revegetation and Restoration of Contaminated Soils. Environmental Pollution 2011, 159, 3269–3282. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A Quantitative Review of the Effects of Biochar Application to Soils on Crop Productivity Using Meta-Analysis. Agric Ecosyst Environ 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Kinney, T.J.; Masiello, C.A.; Dugan, B.; Hockaday, W.C.; Dean, M.R.; Zygourakis, K.; Barnes, R.T. Hydrologic Properties of Biochars Produced at Different Temperatures. Biomass Bioenergy 2012, 41, 34–43. [Google Scholar] [CrossRef]
- Qin, Y.; Li, G.; Gao, Y.; Zhang, L.; Ok, Y.S.; An, T. Persistent Free Radicals in Carbon-Based Materials on Transformation of Refractory Organic Contaminants (ROCs) in Water: A Critical Review. Water Res 2018, 137, 130–143. [Google Scholar] [CrossRef]
- Vejerano, E.P.; Rao, G.; Khachatryan, L.; Cormier, S.A.; Lomnicki, S. Environmentally Persistent Free Radicals: Insights on a New Class of Pollutants. Environ Sci Technol 2018, 52, 2468–2481. [Google Scholar] [CrossRef]
- Chen, Y.; Duan, X.; Zhang, C.; Wang, S.; Ren, N.; Ho, S.-H. Graphitic Biochar Catalysts from Anaerobic Digestion Sludge for Nonradical Degradation of Micropollutants and Disinfection. Chemical Engineering Journal 2020, 384, 123244. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, J.; Cai, J.; Liu, Q.; Zhang, X. Visible-Light-Driven Photocatalytic Degradation of Dye and Antibiotics by Activated Biochar Composited with K+ Doped g-C3N4: Effects, Mechanisms, Actual Wastewater Treatment and Disinfection. Science of The Total Environment 2022, 839, 155955. [Google Scholar] [CrossRef]
- Shi, J.; Wang, J.; Liang, L.; Xu, Z.; Chen, Y.; Chen, S.; Xu, M.; Wang, X.; Wang, S. Carbothermal Synthesis of Biochar-Supported Metallic Silver for Enhanced Photocatalytic Removal of Methylene Blue and Antimicrobial Efficacy. J Hazard Mater 2021, 401, 123382. [Google Scholar] [CrossRef] [PubMed]
- Arshad, U.; Altaf, M.T.; Liaqat, W.; Ali, M.; Shah, M.N.; Jabran, M.; Ali, M.A. Biochar: Black Gold for Sustainable Agriculture and Fortification Against Plant Pathogens—A Review. Gesunde Pflanzen 2023. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Z.; Lu, S.; Shi, X.; Qu, F.; Cheng, D.; Wei, W.; Shon, H.K.; Ni, B.-J. Persistent Free Radicals on Biochar for Its Catalytic Capability: A Review. Water Res 2024, 250, 120999. [Google Scholar] [CrossRef]
- Fang, G.; Liu, C.; Gao, J.; Dionysiou, D.D.; Zhou, D. Manipulation of Persistent Free Radicals in Biochar To Activate Persulfate for Contaminant Degradation. Environ Sci Technol 2015, 49, 5645–5653. [Google Scholar] [CrossRef]
- Odinga, E.S.; Waigi, M.G.; Gudda, F.O.; Wang, J.; Yang, B.; Hu, X.; Li, S.; Gao, Y. Occurrence, Formation, Environmental Fate and Risks of Environmentally Persistent Free Radicals in Biochars. Environ Int 2020, 134, 105172. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Hypochlorous Acid Chemistry in Mammalian Cells-Influence on Infection and Role in Various Pathologies. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Adams, L.; Franco, M.C.; Estevez, A.G. Reactive Nitrogen Species in Cellular Signaling. Exp Biol Med 2015, 240, 711–717. [Google Scholar] [CrossRef]
- Salisbury, D.; Bronas, U. Reactive Oxygen and Nitrogen Species. Nurs Res 2015, 64, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Genestra, M. Oxyl Radicals, Redox-Sensitive Signalling Cascades and Antioxidants. Cell Signal 2007, 19, 1807–1819. [Google Scholar] [CrossRef]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical Relevance of Biomarkers of Oxidative Stress. Antioxid Redox Signal 2015, 23, 1144–1170. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, E. Role of Protein Carbonylation in Skeletal Muscle Mass Loss Associated with Chronic Conditions. Proteomes 2016, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Trpkovic, A.; Resanovic, I.; Stanimirovic, J.; Radak, D.; Mousa, S.A.; Cenic-Milosevic, D.; Jevremovic, D.; Isenovic, E.R. Oxidized Low-Density Lipoprotein as a Biomarker of Cardiovascular Diseases. Crit Rev Clin Lab Sci 2015, 52, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Reynaert, N.L.; Gopal, P.; Rutten, E.P.A.; Wouters, E.F.M.; Schalkwijk, C.G. Advanced Glycation End Products and Their Receptor in Age-Related, Non-Communicable Chronic Inflammatory Diseases; Overview of Clinical Evidence and Potential Contributions to Disease. Int J Biochem Cell Biol 2016, 81, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Jacob, K.D.; Noren Hooten, N.; Trzeciak, A.R.; Evans, M.K. Markers of Oxidant Stress That Are Clinically Relevant in Aging and Age-Related Disease. Mech Ageing Dev 2013, 134, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Gaupp, R.; Ledala, N.; Somerville, G.A. Staphylococcal Response to Oxidative Stress. Front Cell Infect Microbiol 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Doukyu, N.; Taguchi, K. Involvement of Catalase and Superoxide Dismutase in Hydrophobic Organic Solvent Tolerance of Escherichia Coli. AMB Express 2021, 11, 97. [Google Scholar] [CrossRef]
- Chiang, S.M.; Schellhorn, H.E. Regulators of Oxidative Stress Response Genes in Escherichia Coli and Their Functional Conservation in Bacteria. Arch Biochem Biophys 2012, 525, 161–169. [Google Scholar] [CrossRef]
- Battesti, A.; Majdalani, N.; Gottesman, S. The RpoS-Mediated General Stress Response in Escherichia Coli. Annu Rev Microbiol 2011, 65, 189–213. [Google Scholar] [CrossRef]
- Nguyen, G.T.; Green, E.R.; Mecsas, J. Neutrophils to the ROScue: Mechanisms of NADPH Oxidase Activation and Bacterial Resistance. Front Cell Infect Microbiol 2017, 7. [Google Scholar] [CrossRef]
- Li, H.; Zhou, X.; Huang, Y.; Liao, B.; Cheng, L.; Ren, B. Reactive Oxygen Species in Pathogen Clearance: The Killing Mechanisms, the Adaption Response, and the Side Effects. Front Microbiol 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhu, L.; Pan, D.; Li, S.; Xiao, H.; Zhang, Z.; Shen, X.; Wang, Y.; Long, M. Siderophore-Mediated Iron Acquisition Enhances Resistance to Oxidative and Aromatic Compound Stress in Cupriavidus Necator JMP134. Appl Environ Microbiol 2019, 85. [Google Scholar] [CrossRef] [PubMed]
- Peralta, D.R.; Adler, C.; Corbalán, N.S.; Paz García, E.C.; Pomares, M.F.; Vincent, P.A. Enterobactin as Part of the Oxidative Stress Response Repertoire. PLoS One 2016, 11, e0157799. [Google Scholar] [CrossRef] [PubMed]
- Vaishampayan, A.; de Jong, A.; Wight, D.J.; Kok, J.; Grohmann, E. A Novel Antimicrobial Coating Represses Biofilm and Virulence-Related Genes in Methicillin-Resistant Staphylococcus Aureus. Front Microbiol 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; El Meouche, I.; Dunlop, M.J. Bacterial Persistence Induced by Salicylate via Reactive Oxygen Species. Sci Rep 2017, 7, 43839. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.S.; Hung, D.T. Persistent Bacterial Infections, Antibiotic Tolerance, and the Oxidative Stress Response. Virulence 2013, 4, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Memar, M.Y.; Ghotaslou, R.; Samiei, M.; Adibkia, K. Antimicrobial Use of Reactive Oxygen Therapy: Current Insights. Infect Drug Resist 2018, 11, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Liu, X.; Schroeder, M.R.; Kraft, C.S.; Burd, E.M.; Weiss, D.S. Rapid Killing of Acinetobacter Baumannii by Polymyxins Is Mediated by a Hydroxyl Radical Death Pathway. Antimicrob Agents Chemother 2012, 56, 5642–5649. [Google Scholar] [CrossRef]
- Arriaga-Alba, M.; Rivera-Sánchez, R.; Parra-Cervantes, G.; Barro-Moreno, F.; Flores-Paz, R.; Garcı́a-Jiménez, E. Antimutagenesis of β-Carotene to Mutations Induced by Quinolone on Salmonella Typhimurium. Arch Med Res 2000, 31, 156–161. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, X. Contribution of Oxidative Damage to Antimicrobial Lethality. Antimicrob Agents Chemother 2009, 53, 1395–1402. [Google Scholar] [CrossRef]
- Rasouly, A.; Nudler, E. Reactive Oxygen Species as the Long Arm of Bactericidal Antibiotics. Proceedings of the National Academy of Sciences 2019, 116, 9696–9698. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Zeng, J.; Wang, X.; Drlica, K.; Zhao, X. Post-Stress Bacterial Cell Death Mediated by Reactive Oxygen Species. Proceedings of the National Academy of Sciences 2019, 116, 10064–10071. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Zhou, J.; Wang, P.; Li, T.; Zhao, Y.; Ren, X.; Lu, J.; Wang, J.; Holmgren, A.; Zou, L. Topical Therapeutic Efficacy of Ebselen Against Multidrug-Resistant Staphylococcus Aureus LT-1 Targeting Thioredoxin Reductase. Front Microbiol 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Mourenza, Á.; Gil, J.A.; Mateos, L.M.; Letek, M. Oxidative Stress-Generating Antimicrobials, a Novel Strategy to Overcome Antibacterial Resistance. Antioxidants 2020, 9, 361. [Google Scholar] [CrossRef] [PubMed]
- Díaz-García, D.; Ardiles, P.; Prashar, S.; Rodríguez-Diéguez, A.; Páez, P.; Gómez-Ruiz, S. Preparation and Study of the Antibacterial Applications and Oxidative Stress Induction of Copper Maleamate-Functionalized Mesoporous Silica Nanoparticles. Pharmaceutics 2019, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Shao, T.; Yu, Y.; Xiong, Y.; Yang, L. Surface-Bound Reactive Oxygen Species Generating Nanozymes for Selective Antibacterial Action. Nat Commun 2021, 12, 745. [Google Scholar] [CrossRef] [PubMed]
- Linzner, N.; Antelmann, H. The Antimicrobial Activity of the AGXX® Surface Coating Requires a Small Particle Size to Efficiently Kill Staphylococcus Aureus. Front Microbiol 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Clauss-Lendzian, E.; Vaishampayan, A.; de Jong, A.; Landau, U.; Meyer, C.; Kok, J.; Grohmann, E. Stress Response of a Clinical Enterococcus Faecalis Isolate Subjected to a Novel Antimicrobial Surface Coating. Microbiol Res 2018, 207, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Loi, V. Van; Busche, T.; Preuß, T.; Kalinowski, J.; Bernhardt, J.; Antelmann, H. The AGXX® Antimicrobial Coating Causes a Thiol-Specific Oxidative Stress Response and Protein S-Bacillithiolation in Staphylococcus Aureus. Front Microbiol 2018, 9. [Google Scholar] [CrossRef]
- Loi, V. Van; Huyen, N.T.T.; Busche, T.; Tung, Q.N.; Gruhlke, M.C.H.; Kalinowski, J.; Bernhardt, J.; Slusarenko, A.J.; Antelmann, H. Staphylococcus Aureus Responds to Allicin by Global S-Thioallylation – Role of the Brx/BSH/YpdA Pathway and the Disulfide Reductase MerA to Overcome Allicin Stress. Free Radic Biol Med 2019, 139, 55–69. [Google Scholar] [CrossRef]
- Paulander, W.; Wang, Y.; Folkesson, A.; Charbon, G.; Løbner-Olesen, A.; Ingmer, H. Bactericidal Antibiotics Increase Hydroxyphenyl Fluorescein Signal by Altering Cell Morphology. PLoS One 2014, 9, e92231. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A Common Mechanism of Cellular Death Induced by Bactericidal Antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Chua, N.G.; Zhou, Y.P.; Tan, T.T.; Lingegowda, P.B.; Lee, W.; Lim, T.P.; Teo, J.; Cai, Y.; Kwa, A.L. Polymyxin B with Dual Carbapenem Combination Therapy against Carbapenemase-Producing Klebsiella Pneumoniae. Journal of Infection 2015, 70, 309–311. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Schito, A.M. Positively Charged Polymers as Promising Devices against Multidrug Resistant Gram-Negative Bacteria: A Review. Polymers (Basel) 2020, 12, 1195. [Google Scholar] [CrossRef] [PubMed]
- Ezraty, B.; Vergnes, A.; Banzhaf, M.; Duverger, Y.; Huguenot, A.; Brochado, A.R.; Su, S.-Y.; Espinosa, L.; Loiseau, L.; Py, B.; et al. Fe-S Cluster Biosynthesis Controls Uptake of Aminoglycosides in a ROS-Less Death Pathway. Science (1979) 2013, 340, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Keren, I.; Wu, Y.; Inocencio, J.; Mulcahy, L.R.; Lewis, K. Killing by Bactericidal Antibiotics Does Not Depend on Reactive Oxygen Species. Science (1979) 2013, 339, 1213–1216. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Imlay, J.A. Cell Death from Antibiotics Without the Involvement of Reactive Oxygen Species. Science (1979) 2013, 339, 1210–1213. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field. Nanoscale Res Lett 2020, 15, 115. [Google Scholar] [CrossRef] [PubMed]
- Linzner, N.; Antelmann, H. The Antimicrobial Activity of the AGXX® Surface Coating Requires a Small Particle Size to Efficiently Kill Staphylococcus Aureus. Front Microbiol 2021, 12. [Google Scholar] [CrossRef]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial Photodynamic Therapy – What We Know and What We Don’t. Crit Rev Microbiol 2018, 44, 571–589. [Google Scholar] [CrossRef]
- Mitton, D.; Ackroyd, R. A Brief Overview of Photodynamic Therapy in Europe. Photodiagnosis Photodyn Ther 2008, 5, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic Therapy for Cancer. Nat Rev Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Ackroyd, R.; Kelty, C.; Brown, N.; Reed, M. The History of Photodetection and Photodynamic Therapy. Photochem Photobiol 2001, 74, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M. Dyes, Flies, and Sunny Skies: Photodynamic Therapy and Neglected Tropical Diseases. Coloration Technology 2017, 133, 3–14. [Google Scholar] [CrossRef]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials—Are We Afraid of the Light? Lancet Infect Dis 2017, 17, e49–e55. [Google Scholar] [CrossRef] [PubMed]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- Lutkus, L. V.; Rickenbach, S.S.; McCormick, T.M. Singlet Oxygen Quantum Yields Determined by Oxygen Consumption. J Photochem Photobiol A Chem 2019, 378, 131–135. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, R.; Zaat, S.A.J.; Breukink, E.; Heger, M. Antibacterial Photodynamic Therapy: Overview of a Promising Approach to Fight Antibiotic-Resistant Bacterial Infections. J Clin Transl Res 2015, 1, 140–167. [Google Scholar]
- Wainwright, M. Photoantimicrobials and PACT: What’s in an Abbreviation? Photochemical & Photobiological Sciences 2019, 18, 12–14. [Google Scholar] [CrossRef]
- Yan, E.; Kwek, G.; Qing, N.S.; Lingesh, S.; Xing, B. Antimicrobial Photodynamic Therapy for the Remote Eradication of Bacteria. Chempluschem 2023, 88. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Abrahamse, H. Oxygen-Independent Antimicrobial Photoinactivation: Type III Photochemical Mechanism? Antibiotics 2020, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, J.; Rahban, D.; Aghamiri, S.; Teymouri, A.; Bahador, A. Photosensitizers in Antibacterial Photodynamic Therapy: An Overview. Laser Ther 2018, 27, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Youf, R.; Müller, M.; Balasini, A.; Thétiot, F.; Müller, M.; Hascoët, A.; Jonas, U.; Schönherr, H.; Lemercier, G.; Montier, T.; et al. Antimicrobial Photodynamic Therapy: Latest Developments with a Focus on Combinatory Strategies. Pharmaceutics 2021, 13, 1995. [Google Scholar] [CrossRef] [PubMed]
- Fontana, C.R.; Abernethy, A.D.; Som, S.; Ruggiero, K.; Doucette, S.; Marcantonio, R.C.; Boussios, C.I.; Kent, R.; Goodson, J.M.; Tanner, A.C.R.; et al. The Antibacterial Effect of Photodynamic Therapy in Dental Plaque-derived Biofilms. J Periodontal Res 2009, 44, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Zanin, I.C.J.; Lobo, M.M.; Rodrigues, L.K.A.; Pimenta, L.A.F.; Höfling, J.F.; Gonçalves, R.B. Photosensitization of in Vitro Biofilms by Toluidine Blue O Combined with a Light-emitting Diode. Eur J Oral Sci 2006, 114, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Fekrazad, R.; Zare, H.; Vand, S.M.S. Photodynamic Therapy Effect on Cell Growth Inhibition Induced by Radachlorin and Toluidine Blue O on Staphylococcus Aureus and Escherichia Coli: An in Vitro Study. Photodiagnosis Photodyn Ther 2016, 15, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Voos, A.C.; Kranz, S.; Tonndorf-Martini, S.; Voelpel, A.; Sigusch, H.; Staudte, H.; Albrecht, V.; Sigusch, B.W. Photodynamic Antimicrobial Effect of Safranine O on an Ex Vivo Periodontal Biofilm. Lasers Surg Med 2014, 46, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Collins, T.L.; Markus, E.A.; Hassett, D.J.; Robinson, J.B. The Effect of a Cationic Porphyrin on Pseudomonas Aeruginosa Biofilms. Curr Microbiol 2010, 61, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Di Poto, A.; Sbarra, M.S.; Provenza, G.; Visai, L.; Speziale, P. The Effect of Photodynamic Treatment Combined with Antibiotic Action or Host Defence Mechanisms on Staphylococcus Aureus Biofilms. Biomaterials 2009, 30, 3158–3166. [Google Scholar] [CrossRef]
- Cieplik, F.; Späth, A.; Regensburger, J.; Gollmer, A.; Tabenski, L.; Hiller, K.-A.; Bäumler, W.; Maisch, T.; Schmalz, G. Photodynamic Biofilm Inactivation by SAPYR—An Exclusive Singlet Oxygen Photosensitizer. Free Radic Biol Med 2013, 65, 477–487. [Google Scholar] [CrossRef]
- Malá, Z.; Žárská, L.; Bajgar, R.; Bogdanová, K.; Kolář, M.; Panáček, A.; Binder, S.; Kolářová, H. The Application of Antimicrobial Photodynamic Inactivation on Methicillin-Resistant S. Aureus and ESBL-Producing K. Pneumoniae Using Porphyrin Photosensitizer in Combination with Silver Nanoparticles. Photodiagnosis Photodyn Ther 2021, 33, 102140. [Google Scholar] [CrossRef] [PubMed]
- Board-Davies, E.L.; Rhys-Williams, W.; Hynes, D.; Williams, D.; Farnell, D.J.J.; Love, W. Antibacterial and Antibiofilm Potency of XF Drugs, Impact of Photodynamic Activation and Synergy With Antibiotics. Front Cell Infect Microbiol 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Karygianni, L.; Ruf, S.; Follo, M.; Hellwig, E.; Bucher, M.; Anderson, A.C.; Vach, K.; Al-Ahmad, A. Novel Broad-Spectrum Antimicrobial Photoinactivation of In Situ Oral Biofilms by Visible Light plus Water-Filtered Infrared A. Appl Environ Microbiol 2014, 80, 7324–7336. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, M.Q.; Menezes, J.C.J.M.D.S.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Almeida, A.; Hackbarth, S.; Röder, B.; Faustino, M.A.F. Photodynamic Inactivation of Bioluminescent Escherichia Coli by Neutral and Cationic Pyrrolidine-Fused Chlorins and Isobacteriochlorins. Bioorg Med Chem Lett 2014, 24, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Souza, B.M.N.; Pinto, J.G.; Pereira, A.H.C.; Miñán, A.G.; Ferreira-Strixino, J. Efficiency of Antimicrobial Photodynamic Therapy with Photodithazine® on MSSA and MRSA Strains. Antibiotics (Basel) 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Bertoloni, G.; Rossi, F.; Valduga, G.; Jori, G.; Ali, H.; van Lier, J.E. Photosensitizing Activity of Water- and Lipid-Soluble Phthalocyanines on Prokaryotic and Eukaryotic Microbial Cells. Microbios 1992, 71, 33–46. [Google Scholar] [PubMed]
- Fan, B.; Peng, W.; Zhang, Y.; Liu, P.; Shen, J. ROS Conversion Promotes the Bactericidal Efficiency of Eosin Y Based Photodynamic Therapy. Biomater Sci 2023, 11, 4930–4937. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.L.L.; Sobral, A.P.T.; Gallo, J.M.A.S.; Gimenez, T.; Ferri, E.P.; Ianello, S.; Motta, P. de B.; Motta, L.J.; Horliana, A.C.R.T.; Santos, E.M.; et al. Antimicrobial Photodynamic Therapy with Erythrosine and Blue Light on Dental Biofilm Bacteria: Study Protocol for Randomised Clinical Trial. BMJ Open 2023, 13, e075084. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Kishen, A. Polycationic Chitosan-Conjugated Photosensitizer for Antibacterial Photodynamic Therapy †. Photochem Photobiol 2012, 88, 577–583. [Google Scholar] [CrossRef]
- Spesia, M.B.; Milanesio, M.E.; Durantini, E.N. Synthesis, Properties and Photodynamic Inactivation of Escherichia Coli by Novel Cationic Fullerene C60 Derivatives. Eur J Med Chem 2008, 43, 853–861. [Google Scholar] [CrossRef]
- Wang, M.; Huang, L.; Sharma, S.K.; Jeon, S.; Thota, S.; Sperandio, F.F.; Nayka, S.; Chang, J.; Hamblin, M.R.; Chiang, L.Y. Synthesis and Photodynamic Effect of New Highly Photostable Decacationically Armed [60]- and [70]Fullerene Decaiodide Monoadducts To Target Pathogenic Bacteria and Cancer Cells. J Med Chem 2012, 55, 4274–4285. [Google Scholar] [CrossRef]
- Cieplik, F.; Pummer, A.; Regensburger, J.; Hiller, K.-A.; Späth, A.; Tabenski, L.; Buchalla, W.; Maisch, T. The Impact of Absorbed Photons on Antimicrobial Photodynamic Efficacy. Front Microbiol 2015, 6. [Google Scholar] [CrossRef]
- Maisch, T.; Eichner, A.; Späth, A.; Gollmer, A.; König, B.; Regensburger, J.; Bäumler, W. Fast and Effective Photodynamic Inactivation of Multiresistant Bacteria by Cationic Riboflavin Derivatives. PLoS One 2014, 9, e111792. [Google Scholar] [CrossRef] [PubMed]
- Najafi, S.; Khayamzadeh, M.; Paknejad, M.; Poursepanj, G.; Kharazi Fard, M.J.; Bahador, A. An In Vitro Comparison of Antimicrobial Effects of Curcumin-Based Photodynamic Therapy and Chlorhexidine, on Aggregatibacter Actinomycetemcomitans. J Lasers Med Sci 2016, 7, 21–25. [Google Scholar] [CrossRef]
- Dahl, T.A.; McGowan, W.M.; Shand, M.A.; Srinivasan, V.S. Photokilling of Bacteria by the Natural Dye Curcumin. Arch Microbiol 1989, 151, 183–185. [Google Scholar] [CrossRef]
- García, I.; Ballesta, S.; Gilaberte, Y.; Rezusta, A.; Pascual, Á. Antimicrobial Photodynamic Activity of Hypericin against Methicillin-Susceptible and Resistant Staphylococcus Aureus Biofilms. Future Microbiol 2015, 10, 347–356. [Google Scholar] [CrossRef]
- Yow, C.M.N.; Tang, H.M.; Chu, E.S.M.; Huang, Z. Hypericin-mediated Photodynamic Antimicrobial Effect on Clinically Isolated Pathogens †. Photochem Photobiol 2012, 88, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, K.; Ozawa, T.; Awazu, K.; Ito, N.; Honda, N.; Matsumoto, S.; Tsuruta, D. Photodynamic Therapy Using Systemic Administration of 5-Aminolevulinic Acid and a 410-Nm Wavelength Light-Emitting Diode for Methicillin-Resistant Staphylococcus Aureus-Infected Ulcers in Mice. PLoS One 2014, 9, e105173. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, B.; Zhang, L.; Liu, X.; Shi, L.; Kang, X.; Lei, X.; Chen, K.; Chen, Z.; Li, C.; et al. Clinical Practice Guidelines for 5-Aminolevulinic Acid Photodynamic Therapy for Acne Vulgaris in China. Photodiagnosis Photodyn Ther 2023, 41, 103261. [Google Scholar] [CrossRef]
- Polat, E.; Kang, K. Natural Photosensitizers in Antimicrobial Photodynamic Therapy. Biomedicines 2021, 9, 584. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New Photosensitizers for Photodynamic Therapy. Biochemical Journal 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy-Current Limitations and Novel Approaches. Front Chem 2021, 9, 691697. [Google Scholar] [CrossRef] [PubMed]
- Clément, S.; Winum, J.-Y. Photodynamic Therapy Alone or in Combination to Counteract Bacterial Infections. Expert Opin Ther Pat 2024, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Embleton, M.L. Selective Lethal Photosensitization of Methicillin-Resistant Staphylococcus Aureus Using an IgG-Tin (IV) Chlorin E6 Conjugate. Journal of Antimicrobial Chemotherapy 2002, 50, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Dosselli, R.; Gobbo, M.; Bolognini, E.; Campestrini, S.; Reddi, E. Porphyrin−Apidaecin Conjugate as a New Broad Spectrum Antibacterial Agent. ACS Med Chem Lett 2010, 1, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, F.; Huang, Y.-Y.; Hamblin, M. Antimicrobial Photodynamic Therapy to Kill Gram-Negative Bacteria. Recent Pat Antiinfect Drug Discov 2013, 8, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.R.; Gan, H.; Smith, B.D. Bacterial Imaging and Photodynamic Inactivation Using Zinc(Ii)-Dipicolylamine BODIPY Conjugates. Photochemical & Photobiological Sciences 2015, 14, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Soto-Moreno, A.; Montero-Vilchez, T.; Diaz-Calvillo, P.; Molina-Leyva, A.; Arias-Santiago, S. The Impact of Photodynamic Therapy on Skin Homeostasis in Patients with Actinic Keratosis: A Prospective Observational Study. Skin Res Technol 2023, 29, e13493. [Google Scholar] [CrossRef]
- Rapacka-Zdończyk, A.; Woźniak, A.; Michalska, K.; Pierański, M.; Ogonowska, P.; Grinholc, M.; Nakonieczna, J. Factors Determining the Susceptibility of Bacteria to Antibacterial Photodynamic Inactivation. Front Med (Lausanne) 2021, 8. [Google Scholar] [CrossRef]
- Klausen, M.; Ucuncu, M.; Bradley, M. Design of Photosensitizing Agents for Targeted Antimicrobial Photodynamic Therapy. Molecules 2020, 25, 5239. [Google Scholar] [CrossRef]
- Zhou, W.; Jiang, X.; Zhen, X. Development of Organic Photosensitizers for Antimicrobial Photodynamic Therapy. Biomater Sci 2023, 11, 5108–5128. [Google Scholar] [CrossRef] [PubMed]
- Badran, Z.; Rahman, B.; De Bonfils, P.; Nun, P.; Coeffard, V.; Verron, E. Antibacterial Nanophotosensitizers in Photodynamic Therapy: An Update. Drug Discov Today 2023, 28, 103493. [Google Scholar] [CrossRef] [PubMed]
- Piksa, M.; Lian, C.; Samuel, I.C.; Pawlik, K.J.; Samuel, I.D.W.; Matczyszyn, K. The Role of the Light Source in Antimicrobial Photodynamic Therapy. Chem Soc Rev 2023, 52, 1697–1722. [Google Scholar] [CrossRef] [PubMed]
- Usui, Y. DETERMINATION OF QUANTUM YIELD OF SINGLET OXYGEN FORMATION BY PHOTOSENSITIZATION. Chem Lett 1973, 2, 743–744. [Google Scholar] [CrossRef]
- Wiegell, S.R.; Skødt, V.; Wulf, H.C. Daylight-mediated Photodynamic Therapy of Basal Cell Carcinomas – an Explorative Study. Journal of the European Academy of Dermatology and Venereology 2014, 28, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Al-Mutairi, R.; Tovmasyan, A.; Batinic-Haberle, I.; Benov, L. Sublethal Photodynamic Treatment Does Not Lead to Development of Resistance. Front Microbiol 2018, 9. [Google Scholar] [CrossRef]
- Nakonieczna, J.; Wozniak, A.; Pieranski, M.; Rapacka-Zdonczyk, A.; Ogonowska, P.; Grinholc, M. Photoinactivation of ESKAPE Pathogens: Overview of Novel Therapeutic Strategy. Future Med Chem 2019, 11, 443–461. [Google Scholar] [CrossRef] [PubMed]
- Songca, S.P.; Adjei, Y. Applications of Antimicrobial Photodynamic Therapy against Bacterial Biofilms. Int J Mol Sci 2022, 23, 3209. [Google Scholar] [CrossRef]
- Ribeiro, M.; Gomes, I.B.; Saavedra, M.J.; Simões, M. Photodynamic Therapy and Combinatory Treatments for the Control of Biofilm-Associated Infections. Lett Appl Microbiol 2022, 75, 548–564. [Google Scholar] [CrossRef]
- Barolet, D.; Boucher, A. Radiant near Infrared Light Emitting Diode Exposure as Skin Preparation to Enhance Photodynamic Therapy Inflammatory Type Acne Treatment Outcome. Lasers Surg Med 2010, 42, 171–178. [Google Scholar] [CrossRef]
- Sakamoto, F.H.; Torezan, L.; Anderson, R.R. Photodynamic Therapy for Acne Vulgaris: A Critical Review from Basics to Clinical Practice. J Am Acad Dermatol 2010, 63, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Boen, M.; Brownell, J.; Patel, P.; Tsoukas, M.M. The Role of Photodynamic Therapy in Acne: An Evidence-Based Review. Am J Clin Dermatol 2017, 18, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Wojewoda, K.; Gillstedt, M.; Tovi, J.; Salah, L.; Wennberg Larkö, A.-M.; Sjöholm, A.; Sandberg, C. Optimizing Treatment of Acne with Photodynamic Therapy (PDT) to Achieve Long-Term Remission and Reduce Side Effects. A Prospective Randomized Controlled Trial. J Photochem Photobiol B 2021, 223, 112299. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; He, G.; Zeng, W.; Xia, Y. The Photosensitizer-Based Therapies Enhance the Repairing of Skin Wounds. Front Med (Lausanne) 2022, 9. [Google Scholar] [CrossRef]
- Brown, S. Clinical Antimicrobial Photodynamic Therapy: Phase II Studies in Chronic Wounds. Journal of the National Comprehensive Cancer Network 2012, 10, S–80. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Laguna, V.; Gilaberte, Y.; Millán-Lou, M.I.; Agut, M.; Nonell, S.; Rezusta, A.; Hamblin, M.R. A Combination of Photodynamic Therapy and Antimicrobial Compounds to Treat Skin and Mucosal Infections: A Systematic Review. Photochemical & Photobiological Sciences 2019, 18, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Prażmo, E.; Mielczarek, A.; Kwaśny, M.; Łapiński, M. Photodynamic Therapy As a Promising Method Used in the Treatment of Oral Diseases. Advances in Clinical and Experimental Medicine 2016, 25, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Dragana, R.; Jelena, M.; Jovan, M.; Biljana, N.; Dejan, M. Antibacterial Efficiency of Adjuvant Photodynamic Therapy and High-Power Diode Laser in the Treatment of Young Permanent Teeth with Chronic Periapical Periodontitis. A Prospective Clinical Study. Photodiagnosis Photodyn Ther 2023, 41, 103129. [Google Scholar] [CrossRef] [PubMed]
- Vohra, F.; Akram, Z.; Safii, S.H.; Vaithilingam, R.D.; Ghanem, A.; Sergis, K.; Javed, F. Role of Antimicrobial Photodynamic Therapy in the Treatment of Aggressive Periodontitis: A Systematic Review. Photodiagnosis Photodyn Ther 2016, 13, 139–147. [Google Scholar] [CrossRef]
- Bechara Andere, N.M.R.; dos Santos, N.C.C.; Araujo, C.F.; Mathias, I.F.; Rossato, A.; de Marco, A.C.; Santamaria, M.; Jardini, M.A.N.; Santamaria, M.P. Evaluation of the Local Effect of Nonsurgical Periodontal Treatment with and without Systemic Antibiotic and Photodynamic Therapy in Generalized Aggressive Periodontitis. A Randomized Clinical Trial. Photodiagnosis Photodyn Ther 2018, 24, 115–120. [Google Scholar] [CrossRef]
- Ohba, S.; Sato, M.; Noda, S.; Yamamoto, H.; Egahira, K.; Asahina, I. Assessment of Safety and Efficacy of Antimicrobial Photodynamic Therapy for Peri-Implant Disease. Photodiagnosis Photodyn Ther 2020, 31, 101936. [Google Scholar] [CrossRef]
- Yupanqui Mieles, J.; Vyas, C.; Aslan, E.; Humphreys, G.; Diver, C.; Bartolo, P. Honey: An Advanced Antimicrobial and Wound Healing Biomaterial for Tissue Engineering Applications. Pharmaceutics 2022, 14, 1663. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Liu, R.; Lu, Q.; Hao, P.; Xu, A.; Zhang, J.; Tan, J. Biochemical Properties, Antibacterial and Cellular Antioxidant Activities of Buckwheat Honey in Comparison to Manuka Honey. Food Chem 2018, 252, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Girma, A.; Seo, W.; She, R.C. Antibacterial Activity of Varying UMF-Graded Manuka Honeys. PLoS One 2019, 14, e0224495. [Google Scholar] [CrossRef] [PubMed]
- Cokcetin, N.; W.S.; B.S.; C.D.; B.P.; H.L. Cokcetin, N.; Williams, S.; Blair, S.; Carter, D.; Brooks, P.; Harry, L.Active Australian Leptospermum Honey: New Sources and Their Bioactivity; Agrifutures Australia:Canberra, Australia,.; 2019;
- Brudzynski, K. Effect of Hydrogen Peroxide on Antibacterial Activities of Canadian Honeys. Can J Microbiol 2006, 52, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Tulipani, S.; Díaz, D.; Estevez, Y.; Romandini, S.; Giampieri, F.; Damiani, E.; Astolfi, P.; Bompadre, S.; Battino, M. Antioxidant and Antimicrobial Capacity of Several Monofloral Cuban Honeys and Their Correlation with Color, Polyphenol Content and Other Chemical Compounds. Food and Chemical Toxicology 2010, 48, 2490–2499. [Google Scholar] [CrossRef]
- Sherlock, O.; Dolan, A.; Athman, R.; Power, A.; Gethin, G.; Cowman, S.; Humphreys, H. Comparison of the Antimicrobial Activity of Ulmo Honey from Chile and Manuka Honey against Methicillin-Resistant Staphylococcus Aureus, Escherichia Coli and Pseudomonas Aeruginosa. BMC Complement Altern Med 2010, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Isla, M.I.; Craig, A.; Ordoñez, R.; Zampini, C.; Sayago, J.; Bedascarrasbure, E.; Alvarez, A.; Salomón, V.; Maldonado, L. Physico Chemical and Bioactive Properties of Honeys from Northwestern Argentina. LWT - Food Science and Technology 2011, 44, 1922–1930. [Google Scholar] [CrossRef]
- Fyfe, L.; Okoro, P.; Paterson, E.; Coyle, S.; McDougall, G.J. Compositional Analysis of Scottish Honeys with Antimicrobial Activity against Antibiotic-Resistant Bacteria Reveals Novel Antimicrobial Components. LWT - Food Science and Technology 2017, 79, 52–59. [Google Scholar] [CrossRef]
- Escuredo, O.; Silva, L.R.; Valentão, P.; Seijo, M.C.; Andrade, P.B. Assessing Rubus Honey Value: Pollen and Phenolic Compounds Content and Antibacterial Capacity. Food Chem 2012, 130, 671–678. [Google Scholar] [CrossRef]
- Matzen, R.D.; Zinck Leth-Espensen, J.; Jansson, T.; Nielsen, D.S.; Lund, M.N.; Matzen, S. The Antibacterial Effect In Vitro of Honey Derived from Various Danish Flora. Dermatol Res Pract 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bucekova, M.; Jardekova, L.; Juricova, V.; Bugarova, V.; Di Marco, G.; Gismondi, A.; Leonardi, D.; Farkasovska, J.; Godocikova, J.; Laho, M.; et al. Antibacterial Activity of Different Blossom Honeys: New Findings. Molecules 2019, 24, 1573. [Google Scholar] [CrossRef]
- Ahmed, G. Hegazi Antimicrobial Activity of Different Egyptian Honeys as Comparison of Saudi Arabia Honey. Res J Microbiol 2011, 6, 488–495. [Google Scholar]
- Laallam, H.; Boughediri, L.; Bissati, S.; Menasria, T.; Mouzaoui, M.S.; Hadjadj, S.; Hammoudi, R.; Chenchouni, H. Modeling the Synergistic Antibacterial Effects of Honey Characteristics of Different Botanical Origins from the Sahara Desert of Algeria. Front Microbiol 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- John-Isa, J.F.; Adebolu, T.T.; Oyetayo, V.O. Antibacterial Effects of Honey in Nigeria on Selected Diarrhoeagenic Bacteria. South Asian Journal of Research in Microbiology 2019, 1–11. [Google Scholar] [CrossRef]
- ElBorai, A. Antibacterial and Antioxidant Activities of Different Varieties of Locally Produced Egyptian Honey. Egyptian Journal of Botany 2018, 0, 0–0. [Google Scholar] [CrossRef]
- Kwakman, P.H.S.; Zaat, S.A.J. Antibacterial Components of Honey. IUBMB Life 2012, 64, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Moore, O.A.; Smith, L.A.; Campbell, F.; Seers, K.; McQuay, H.J.; Moore, R.A. Systematic Review of the Use of Honey as a Wound Dressing. BMC Complement Altern Med 2001, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Jull, A.B.; Rodgers, A.; Walker, N. Honey as a Topical Treatment for Wounds. In Cochrane Database of Systematic Reviews; Jull, A.B., Ed.; John Wiley & Sons, Ltd: Chichester, UK, 2008. [Google Scholar]
- Johnson, D.W.; van Eps, C.; Mudge, D.W.; Wiggins, K.J.; Armstrong, K.; Hawley, C.M.; Campbell, S.B.; Isbel, N.M.; Nimmo, G.R.; Gibbs, H. Randomized, Controlled Trial of Topical Exit-Site Application of Honey (Medihoney) versus Mupirocin for the Prevention of Catheter-Associated Infections in Hemodialysis Patients. Journal of the American Society of Nephrology 2005, 16, 1456–1462. [Google Scholar] [CrossRef]
- Love, N.R.; Chen, Y.; Ishibashi, S.; Kritsiligkou, P.; Lea, R.; Koh, Y.; Gallop, J.L.; Dorey, K.; Amaya, E. Amputation-Induced Reactive Oxygen Species Are Required for Successful Xenopus Tadpole Tail Regeneration. Nat Cell Biol 2013, 15, 222–228. [Google Scholar] [CrossRef]
- Hixon, K.R.; Klein, R.C.; Eberlin, C.T.; Linder, H.R.; Ona, W.J.; Gonzalez, H.; Sell, S.A. A Critical Review and Perspective of Honey in Tissue Engineering and Clinical Wound Healing. Adv Wound Care (New Rochelle) 2019, 8, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Li, W.; Zhang, F.; Liu, Z.; Zanjanizadeh Ezazi, N.; Liu, D.; Santos, H.A. Electrospun Fibrous Architectures for Drug Delivery, Tissue Engineering and Cancer Therapy. Adv Funct Mater 2019, 29. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat Rev Mater 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Marrazzo, P. The Potential of Honeybee Products for Biomaterial Applications. Biomimetics 2021, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Minden-Birkenmaier, B.; Bowlin, G. Honey-Based Templates in Wound Healing and Tissue Engineering. Bioengineering 2018, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Halstead, F.D.; Webber, M.A.; Rauf, M.; Burt, R.; Dryden, M.; Oppenheim, B.A. In Vitro Activity of an Engineered Honey, Medical-Grade Honeys, and Antimicrobial Wound Dressings against Biofilm-Producing Clinical Bacterial Isolates. J Wound Care 2016, 25, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.; Dryden, M.; Patton, T.; Brennan, J.; Barrett, J. The Antimicrobial Activity of Prototype Modified Honeys That Generate Reactive Oxygen Species (ROS) Hydrogen Peroxide. BMC Res Notes 2015, 8, 20. [Google Scholar] [CrossRef]
- Wilkinson, J.M.; Cavanagh, H.M.A. Antibacterial Activity of 13 Honeys Against Escherichia Coli and Pseudomonas Aeruginosa. J Med Food 2005, 8, 100–103. [Google Scholar] [CrossRef]
- Mullai, V.; Menon, T. Bactericidal Activity of Different Types of Honey against Clinical and Environmental Isolates of Pseudomonas Aeruginosa. The Journal of Alternative and Complementary Medicine 2007, 13, 439–442. [Google Scholar] [CrossRef]
- Percival, S.L.; Hill, K.E.; Williams, D.W.; Hooper, S.J.; Thomas, D.W.; Costerton, J.W. A Review of the Scientific Evidence for Biofilms in Wounds. Wound Repair and Regeneration 2012, 20, 647–657. [Google Scholar] [CrossRef]
- Dryden, M.; Dickinson, A.; Brooks, J.; Hudgell, L.; Saeed, K.; Cutting, K.F. A Multi-Centre Clinical Evaluation of Reactive Oxygen Topical Wound Gel in 114 Wounds. J Wound Care 2016, 25, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Dryden, M.; Milward, G.; Saeed, K. Infection Prevention in Wounds with Surgihoney. Journal of Hospital Infection 2014, 88, 121–122. [Google Scholar] [CrossRef] [PubMed]
- Dryden, M.; Tawse, C.; Adams, J.; Howard, A.; Saeed, K.; Cooke, J. The Use of Surgihoney to Prevent or Eradicate Bacterial Colonisation in Dressing Oncology Long Vascular Lines. J Wound Care 2014, 23, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Nair, H.K.R.; Tatavilis, N.; Pospíšilová, I.; Kučerová, J.; Cremers, N.A.J. Medical-Grade Honey Kills Antibiotic-Resistant Bacteria and Prevents Amputation in Diabetics with Infected Ulcers: A Prospective Case Series. Antibiotics 2020, 9, 529. [Google Scholar] [CrossRef] [PubMed]
- Hermanns, R.; Mateescu, C.; Thrasyvoulou, A.; Tananaki, C.; Wagener, F.A.D.T.G.; Cremers, N.A.J. Defining the Standards for Medical Grade Honey. J Apic Res 2020, 59, 125–135. [Google Scholar] [CrossRef]
- Kwakman, P.H.S.; Velde, A.A. te; Boer, L.; Speijer, D.; Christina Vandenbroucke-Grauls, M.J.; Zaat, S.A.J. How Honey Kills Bacteria. The FASEB Journal 2010, 24, 2576–2582. [Google Scholar] [CrossRef] [PubMed]
- Kwakman, P.H.S.; te Velde, A.A.; de Boer, L.; Vandenbroucke-Grauls, C.M.J.E.; Zaat, S.A.J. Two Major Medicinal Honeys Have Different Mechanisms of Bactericidal Activity. PLoS One 2011, 6, e17709. [Google Scholar] [CrossRef] [PubMed]
- Advancis Medical Activon® Manuka Honey. Available online: Https://Uk.Advancismedical.Com/Products/Activon-Manuka-Honey(accessed on 5 May 2024).
- Rafter, L.; Reynolds, T.; Collier, M.; Rafter, M.; West, M. A Clinical Evaluation of Algivon® Plus Manuka Honey Dressings for Chronic Wounds. Wounds UK ; 2017; Vol. 13, pp. 132–140;
- Edwards, J. A Prospective Evaluation of the Use of Honey Dressings to Manage Burn Wounds. Wounds UK ; 2013; Vol. 9, pp. 102–106;
- Cryer, S. The Use of Manuka Honey to Facilitate the Autoamputation of Fingertip Necrosis. Wounds UK ; 2016; Vol. 12, pp. 66–70;
- Welland Medical Aurum with Manuka Honey. Available online: Https://Wellandmedical.Com/Brand_type/Aurum/(accessed on 6 May 2024).
- Martin-Skurr, K. Case Study: Pyoderma Gangrenosum and the Effects of Manuka Honey. Available online: Https://Wellandmedical.Com/Wp-Content/Uploads/2019/02/Case-Study_NZ-Pyoderma-Gangrenosum-Manuka-Honey.Pdf(accessed on 6 May 2024).
- L-Mesitran. Products. Available online: Https://L-Mesitran.Com/Eu/En/Products(accessed on 6 May 2024).
- Boggust, A. Evaluation of L-Mesitran® Dressings in the Treatment of Minor Burns and Scalds at a Paediatric Emergency Department. Wounds UK 2013, 9, 114–117. [Google Scholar]
- Stephen-Haynes, J.; Callaghan, R. Properties of Honey: Its Mode of Action and Clinical Outcomes. Wounds UK 2011, 7, 50–57. [Google Scholar]
- Callaghan, R. Treating Difficult-to-Debride Wounds Using a Manuka Honey Dressing: A Case Study Evaluation. Wounds UK 2014, 10, 104–109. [Google Scholar]
- Bradbury, S.; Callaghan, R.; Ivins, N. ManukaDress Made Easy. Wounds UK 2014, 10, 1–6. [Google Scholar]
- Derma Science Europe Antibacterial Dressing Medihoney®: Antibacterial Medical Honey. Available online: Https://Media.Supplychain.Nhs.Uk/Media/Documents/ELY302/Marketing/48251_ELY302_3.Pdf.Pdf(accessed on 6 May 2024).
- Robson, V.; Dodd, S.; Thomas, S. Standardized Antibacterial Honey (MedihoneyTM) with Standard Therapy in Wound Care: Randomized Clinical Trial. J Adv Nurs 2009, 65, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.W.; van Eps, C.; Mudge, D.W.; Wiggins, K.J.; Armstrong, K.; Hawley, C.M.; Campbell, S.B.; Isbel, N.M.; Nimmo, G.R.; Gibbs, H. Randomized, Controlled Trial of Topical Exit-Site Application of Honey (Medihoney) versus Mupirocin for the Prevention of Catheter-Associated Infections in Hemodialysis Patients. Journal of the American Society of Nephrology 2005, 16, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Kestrel Health Information MEDIHONEY® Calcium Alginate Dressing. Available online: Https://Www.Woundsource.Com/Product/Medihoney-Calcium-Alginate-Dressing(accessed on 6 May 2024).
- Jull, A.; Walker, N.; Parag, V.; Molan, P.; Rodgers, A. Randomized Clinical Trial of Honey-Impregnated Dressings for Venous Leg Ulcers. British Journal of Surgery 2008, 95, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Derma Sciences Europe Antibacterial Dressing Medihoney®: Barrier Cream. Available online: Https://Media.Supplychain.Nhs.Uk/Media/Documents/ELY289/Marketing/48248_ELY289.Pdf.Pdf(accessed on 6 May 2024).
- Nijhuis, W.; Houwing, R.; Van der Zwet, W.; Jansman, F. A Randomised Trial of Honey Barrier Cream versus Zinc Oxide Ointment. British Journal of Nursing 2012, 21, S10–S13. [Google Scholar] [CrossRef]
- Comvita Antibacterial Wound GelTM 25g. Available online: Https://Www.Comvita.Co.Uk/Product/Antibacterial-Wound-Gel%E2%84%A2-25g/6011(accessed on 6 May 2024).
- Robson, V.; Yorke, J.; Sen, R.A.; Lowe, D.; Rogers, S.N. Randomised Controlled Feasibility Trial on the Use of Medical Grade Honey Following Microvascular Free Tissue Transfer to Reduce the Incidence of Wound Infection. British Journal of Oral and Maxillofacial Surgery 2012, 50, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Matoke Holdings Surgihoney, RO. Available Online:. Available online: Https://Www.Surgihoneyro.Com/(accessed on 6 May 2024).
- Dryden, M.; Goddard, C.; Madadi, A.; Heard, M.; Saeed, K.; Cooke, J. Using Antimicrobial Surgihoney to Prevent Caesarean Wound Infection. Br J Midwifery 2014, 22, 111–115. [Google Scholar] [CrossRef]
- Molan, P. Why Honey Is Effective as a Medicine. Bee World 2001, 82, 22–40. [Google Scholar] [CrossRef]
- Molan, P.C. The Antibacterial Activity of Honey. Bee World 1992, 73, 5–28. [Google Scholar] [CrossRef]
- Mandal, M.D.; Mandal, S. Honey: Its Medicinal Property and Antibacterial Activity. Asian Pac J Trop Biomed 2011, 1, 154–160. [Google Scholar] [CrossRef]
- Nolan, V.C.; Harrison, J.; Cox, J.A.G. Dissecting the Antimicrobial Composition of Honey. Antibiotics 2019, 8, 251. [Google Scholar] [CrossRef] [PubMed]
- Bucekova, M.; Valachova, I.; Kohutova, L.; Prochazka, E.; Klaudiny, J.; Majtan, J. Honeybee Glucose Oxidase—Its Expression in Honeybee Workers and Comparative Analyses of Its Content and H2O2-Mediated Antibacterial Activity in Natural Honeys. Naturwissenschaften 2014, 101, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Cebrero, G.; Sanhueza, O.; Pezoa, M.; Báez, M.E.; Martínez, J.; Báez, M.; Fuentes, E. Relationship among the Minor Constituents, Antibacterial Activity and Geographical Origin of Honey: A Multifactor Perspective. Food Chem 2020, 315, 126296. [Google Scholar] [CrossRef]
- Wong, C.M.; Wong, K.H.; Chen, X.D. Glucose Oxidase: Natural Occurrence, Function, Properties and Industrial Applications. Appl Microbiol Biotechnol 2008, 78, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Kuś, P.M.; Szweda, P.; Jerković, I.; Tuberoso, C.I.G. Activity of Polish Unifloral Honeys against Pathogenic Bacteria and Its Correlation with Colour, Phenolic Content, Antioxidant Capacity and Other Parameters. Lett Appl Microbiol 2016, 62, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.; McBride, M.; Dahiya, D.; Owusu-Apenten, R.; Singh Nigam, P. Antibacterial Activity of Manuka Honey and Its Components: An Overview. AIMS Microbiol 2018, 4, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Albaridi, N.A. Antibacterial Potency of Honey. Int J Microbiol 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Güneş, M.E.; Şahin, S.; Demir, C.; Borum, E.; Tosunoğlu, A. Determination of Phenolic Compounds Profile in Chestnut and Floral Honeys and Their Antioxidant and Antimicrobial Activities. J Food Biochem 2017, 41, e12345. [Google Scholar] [CrossRef]
- Ball, D.W. The Chemical Composition of Honey. J Chem Educ 2007, 84, 1643. [Google Scholar] [CrossRef]
- McLoone, P.; Warnock, M.; Fyfe, L. Honey: A Realistic Antimicrobial for Disorders of the Skin. Journal of Microbiology, Immunology and Infection 2016, 49, 161–167. [Google Scholar] [CrossRef]
- Ganz, T. Defensins: Antimicrobial Peptides of Innate Immunity. Nat Rev Immunol 2003, 3, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Bucekova, M.; Sojka, M.; Valachova, I.; Martinotti, S.; Ranzato, E.; Szep, Z.; Majtan, V.; Klaudiny, J.; Majtan, J. Bee-Derived Antibacterial Peptide, Defensin-1, Promotes Wound Re-Epithelialisation in Vitro and in Vivo. Sci Rep 2017, 7, 7340. [Google Scholar] [CrossRef]
- Memar, M.Y.; Yekani, M.; Alizadeh, N.; Baghi, H.B. Hyperbaric Oxygen Therapy: Antimicrobial Mechanisms and Clinical Application for Infections. Biomedicine & Pharmacotherapy 2019, 109, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Çimşit, M.; Uzun, G.; Yıldız, Ş. Hyperbaric Oxygen Therapy as an Anti-Infective Agent. Expert Rev Anti Infect Ther 2009, 7, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Fu, D.; Yan, L.; Xie, L. The Role of Hyperbaric Oxygen Therapy in the Treatment of Surgical Site Infections: A Narrative Review. Medicina (B Aires) 2023, 59, 762. [Google Scholar] [CrossRef] [PubMed]
- Chmelař, D.; Rozložník, M.; Hájek, M.; Pospíšilová, N.; Kuzma, J. Effect of Hyperbaric Oxygen on the Growth and Susceptibility of Facultatively Anaerobic Bacteria and Bacteria with Oxidative Metabolism to Selected Antibiotics. Folia Microbiol (Praha) 2024, 69, 101–108. [Google Scholar] [CrossRef]
- Kolpen, M.; Mousavi, N.; Sams, T.; Bjarnsholt, T.; Ciofu, O.; Moser, C.; Kühl, M.; Høiby, N.; Jensen, P.Ø. Reinforcement of the Bactericidal Effect of Ciprofloxacin on Pseudomonas Aeruginosa Biofilm by Hyperbaric Oxygen Treatment. Int J Antimicrob Agents 2016, 47, 163–167. [Google Scholar] [CrossRef]
- Kolpen, M.; Lerche, C.J.; Kragh, K.N.; Sams, T.; Koren, K.; Jensen, A.S.; Line, L.; Bjarnsholt, T.; Ciofu, O.; Moser, C.; et al. Hyperbaric Oxygen Sensitizes Anoxic Pseudomonas Aeruginosa Biofilm to Ciprofloxacin. Antimicrob Agents Chemother 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Weaver, L.K. Hyperbaric Oxygen Therapy for Carbon Monoxide Poisoning. Undersea Hyperb Med 2014, 41, 339–354. [Google Scholar]
- Ortega, M.A.; Fraile-Martinez, O.; García-Montero, C.; Callejón-Peláez, E.; Sáez, M.A.; Álvarez-Mon, M.A.; García-Honduvilla, N.; Monserrat, J.; Álvarez-Mon, M.; Bujan, J.; et al. A General Overview on the Hyperbaric Oxygen Therapy: Applications, Mechanisms and Translational Opportunities. Medicina (B Aires) 2021, 57, 864. [Google Scholar] [CrossRef]
- Harnanik, T.; Soeroso, J.; Suryokusumo, M.G.; Juliandhy, T. Effects of Hyperbaric Oxygen on T Helper 17/Regulatory T Polarization in Antigen and Collagen-Induced Arthritis: Hypoxia-Inducible Factor-1α as a Target. Oman Med J 2020, 35, e90–e90. [Google Scholar] [CrossRef] [PubMed]
- Baiula, M.; Greco, R.; Ferrazzano, L.; Caligiana, A.; Hoxha, K.; Bandini, D.; Longobardi, P.; Spampinato, S.; Tolomelli, A. Integrin-Mediated Adhesive Properties of Neutrophils Are Reduced by Hyperbaric Oxygen Therapy in Patients with Chronic Non-Healing Wound. PLoS One 2020, 15, e0237746. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yi, H.; Kato, M.; Suzuki, H.; Kobayashi, S.; Takahashi, H.; Nakashima, I. Differential Sensitivities to Hyperbaric Oxygen of Lymphocyte Subpopulations of Normal and Autoimmune Mice. Immunol Lett 1997, 59, 79–84. [Google Scholar] [CrossRef] [PubMed]
- SAITO, K.; TANAKA, Y.; OTA, T.; ETO, S.; YAMASHITA, U. Suppressive Effect of Hyperbaric Oxygenation on Immune Responses of Normal and Autoimmune Mice. Clin Exp Immunol 2008, 86, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Schottlender, N.; Gottfried, I.; Ashery, U. Hyperbaric Oxygen Treatment: Effects on Mitochondrial Function and Oxidative Stress. Biomolecules 2021, 11, 1827. [Google Scholar] [CrossRef] [PubMed]
- Růžička, J.; Dejmek, J.; Bolek, L.; Beneš, J.; Kuncová, J. Hyperbaric Oxygen Influences Chronic Wound Healing - a Cellular Level Review. Physiol Res 2021, 70, S261–S273. [Google Scholar] [CrossRef] [PubMed]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—a Scale for the Quality Assessment of Narrative Review Articles. Res Integr Peer Rev 2019, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Lerche, C.J.; Schwartz, F.; Pries-Heje, M.M.; Fosbøl, E.L.; Iversen, K.; Jensen, P.Ø.; Høiby, N.; Hyldegaard, O.; Bundgaard, H.; Moser, C. Potential Advances of Adjunctive Hyperbaric Oxygen Therapy in Infective Endocarditis. Front Cell Infect Microbiol 2022, 12. [Google Scholar] [CrossRef]
- Löndahl, M.; Boulton, A.J.M. Hyperbaric Oxygen Therapy in Diabetic Foot Ulceration: Useless or Useful? A Battle. Diabetes Metab Res Rev 2020, 36. [Google Scholar] [CrossRef]
- Brouwer, R.J.; Lalieu, R.C.; Hoencamp, R.; van Hulst, R.A.; Ubbink, D.T. A Systematic Review and Meta-Analysis of Hyperbaric Oxygen Therapy for Diabetic Foot Ulcers with Arterial Insufficiency. J Vasc Surg 2020, 71, 682–692. [Google Scholar] [CrossRef]
- Lalieu, R.C.; Brouwer, R.J.; Ubbink, D.T.; Hoencamp, R.; Bol Raap, R.; van Hulst, R.A. Hyperbaric Oxygen Therapy for Nonischemic Diabetic Ulcers: A Systematic Review. Wound Repair and Regeneration 2020, 28, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Lerche, C.J.; Christophersen, L.J.; Kolpen, M.; Nielsen, P.R.; Trøstrup, H.; Thomsen, K.; Hyldegaard, O.; Bundgaard, H.; Jensen, P.Ø.; Høiby, N.; et al. Hyperbaric Oxygen Therapy Augments Tobramycin Efficacy in Experimental Staphylococcus Aureus Endocarditis. Int J Antimicrob Agents 2017, 50, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-E.; Ko, J.-Y.; Fong, C.-Y.; Juhn, R.-J. Treatment of Diabetic Foot Infection with Hyperbaric Oxygen Therapy. Foot and Ankle Surgery 2010, 16, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.J.; Chen, T.X.; Wald, K.J.; Tooley, A.A.; Lisman, R.D.; Chiu, E.S. Hyperbaric Oxygen Therapy in Ophthalmic Practice: An Expert Opinion. Expert Rev Ophthalmol 2020, 15, 119–126. [Google Scholar] [CrossRef]
- Oguz, H.; Sobaci, G. The Use of Hyperbaric Oxygen Therapy in Ophthalmology. Surv Ophthalmol 2008, 53, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Khalatbary, A. A Review on the Neuroprotective Effects of Hyperbaric Oxygen Therapy. Med Gas Res 2021, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Moen, I.; Stuhr, L.E.B. Hyperbaric Oxygen Therapy and Cancer—a Review. Target Oncol 2012, 7, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Borab, Z.; Mirmanesh, M.D.; Gantz, M.; Cusano, A.; Pu, L.L.Q. Systematic Review of Hyperbaric Oxygen Therapy for the Treatment of Radiation-Induced Skin Necrosis. Journal of Plastic, Reconstructive & Aesthetic Surgery 2017, 70, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Song, Z.; Zhou, Y.; Pan, S.; Wang, F.; Guo, Z.; Jiang, M.; Wang, G.; Kong, R.; Sun, B. The Apoptosis of Peripheral Blood Lymphocytes Promoted by Hyperbaric Oxygen Treatment Contributes to Attenuate the Severity of Early Stage Acute Pancreatitis in Rats. Apoptosis 2014, 19, 58–75. [Google Scholar] [CrossRef]
- Godman, C.A.; Chheda, K.P.; Hightower, L.E.; Perdrizet, G.; Shin, D.-G.; Giardina, C. Hyperbaric Oxygen Induces a Cytoprotective and Angiogenic Response in Human Microvascular Endothelial Cells. Cell Stress Chaperones 2010, 15, 431–442. [Google Scholar] [CrossRef]
- Fielden, M.P.; Martinovic, E.; Ells, A.L. Hyperbaric Oxygen Therapy in the Treatment of Orbital Gas Gangrene. Journal of American Association for Pediatric Ophthalmology and Strabismus 2002, 6, 252–254. [Google Scholar] [CrossRef] [PubMed]
- Bumah, V.V.; Whelan, H.T.; Masson-Meyers, D.S.; Quirk, B.; Buchmann, E.; Enwemeka, C.S. The Bactericidal Effect of 470-Nm Light and Hyperbaric Oxygen on Methicillin-Resistant Staphylococcus Aureus (MRSA). Lasers Med Sci 2015, 30, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Mogami, H.; Hayakawa, T.; Kanai, N.; Kuroda, R.; Yamada, R.; Ikeda, T.; Katsurada, K.; Sugimoto, T. Clinical Application of Hyperbaric Oxygenation in the Treatment of Acute Cerebral Damage. J Neurosurg 1969, 31, 636–643. [Google Scholar] [CrossRef]
- Edwards, M.L. Hyperbaric Oxygen Therapy. Part 2: Application in Disease. Journal of Veterinary Emergency and Critical Care 2010, 20, 289–297. [Google Scholar] [CrossRef]
- Wang, R.-Y.; Yang, Y.-R.; Chang, H.-C. The SDF1-CXCR4 Axis Is Involved in the Hyperbaric Oxygen Therapy-Mediated Neuronal Cells Migration in Transient Brain Ischemic Rats. Int J Mol Sci 2022, 23, 1780. [Google Scholar] [CrossRef] [PubMed]
- Helms, A.K.; Whelan, H.T.; Torbey, M.T. Hyperbaric Oxygen Therapy of Cerebral Ischemia. Cerebrovascular Diseases 2005, 20, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Matchett, G.A.; Martin, R.D.; Zhang, J.H. Hyperbaric Oxygen Therapy and Cerebral Ischemia: Neuroprotective Mechanisms. Neurol Res 2009, 31, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Cevolani, D.; Di Donato, F.; Santarella, L.; Bertossi, S.; Cellerini, M. Functional MRI (FMRI) Evaluation of Hyperbaric Oxygen Therapy (HBOT) Efficacy in Chronic Cerebral Stroke: A Small Retrospective Consecutive Case Series. Int J Environ Res Public Health 2020, 18, 190. [Google Scholar] [CrossRef] [PubMed]
- Thiankhaw, K.; Chattipakorn, N.; Chattipakorn, S.C. The Effects of Hyperbaric Oxygen Therapy on the Brain with Middle Cerebral Artery Occlusion. J Cell Physiol 2021, 236, 1677–1694. [Google Scholar] [CrossRef]
- Chiang, I.-H.; Chen, S.-G.; Huang, K.-L.; Chou, Y.-C.; Dai, N.-T.; Peng, C.-K. Adjunctive Hyperbaric Oxygen Therapy in Severe Burns: Experience in Taiwan Formosa Water Park Dust Explosion Disaster. Burns 2017, 43, 852–857. [Google Scholar] [CrossRef]
- M. Misiuga; J. Glik; M. Kawecki; I. Dziurzyńska; M. Ples; W. Łabuś; M. Nowak The Effect of Hyperbaric Oxygen Therapy on Burn Wounds Covered With Skin Allografts. JOTSRR 2016, 1, 17–27. [Google Scholar]
- Bartek, J.; Jakola, A.S.; Skyrman, S.; Förander, P.; Alpkvist, P.; Schechtmann, G.; Glimåker, M.; Larsson, A.; Lind, F.; Mathiesen, T. Hyperbaric Oxygen Therapy in Spontaneous Brain Abscess Patients: A Population-Based Comparative Cohort Study. Acta Neurochir (Wien) 2016, 158, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Inanmaz, M.E.; Kose, K.C.; Isik, C.; Atmaca, H.; Basar, H. Can Hyperbaric Oxygen Be Used to Prevent Deep Infections in Neuro-Muscular Scoliosis Surgery? BMC Surg 2014, 14, 85. [Google Scholar] [CrossRef] [PubMed]
- Barili, F.; Polvani, G.; Topkara, V.K.; Dainese, L.; Cheema, F.H.; Roberto, M.; Naliato, M.; Parolari, A.; Alamanni, F.; Biglioli, P. Role of Hyperbaric Oxygen Therapy in the Treatment of Postoperative Organ/Space Sternal Surgical Site Infections. World J Surg 2007, 31, 1702–1706. [Google Scholar] [CrossRef]
- Larsson, A.; Uusijärvi, J.; Lind, F.; Gustavsson, B.; Saraste, H. Hyperbaric Oxygen in the Treatment of Postoperative Infections in Paediatric Patients with Neuromuscular Spine Deformity. European Spine Journal 2011, 20, 2217–2222. [Google Scholar] [CrossRef]
- George, M.E.; Rueth, N.M.; Skarda, D.E.; Chipman, J.G.; Quickel, R.R.; Beilman, G.J. Hyperbaric Oxygen Does Not Improve Outcome in Patients with Necrotizing Soft Tissue Infection. Surg Infect (Larchmt) 2009, 10, 21–28. [Google Scholar] [CrossRef]
- Wilkinson, D. Hyperbaric Oxygen Treatment and Survival From Necrotizing Soft Tissue Infection. Archives of Surgery 2004, 139, 1339. [Google Scholar] [CrossRef] [PubMed]
- Massey, P.R.; Sakran, J. V.; Mills, A.M.; Sarani, B.; Aufhauser, D.D.; Sims, C.A.; Pascual, J.L.; Kelz, R.R.; Holena, D.N. Hyperbaric Oxygen Therapy in Necrotizing Soft Tissue Infections. Journal of Surgical Research 2012, 177, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Shupak, A.; Oren, S.; Goldenberg, I.; Barzilai, A.; Moskuna, R.; Bursztein, S. Necrotizing Fasciitis: An Indication for Hyperbaric Oxygenation Therapy? Surgery 1995, 118, 873–878. [Google Scholar] [CrossRef]
- Duzgun, A.P.; Satır, H.Z.; Ozozan, O.; Saylam, B.; Kulah, B.; Coskun, F. Effect of Hyperbaric Oxygen Therapy on Healing of Diabetic Foot Ulcers. The Journal of Foot and Ankle Surgery 2008, 47, 515–519. [Google Scholar] [CrossRef]
- Löndahl, M.; Katzman, P.; Nilsson, A.; Hammarlund, C. Hyperbaric Oxygen Therapy Facilitates Healing of Chronic Foot Ulcers in Patients With Diabetes. Diabetes Care 2010, 33, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Faglia, E.; Favales, F.; Aldeghi, A.; Calia, P.; Quarantiello, A.; Oriani, G.; Michael, M.; Campagnoli, P.; Morabito, A. Adjunctive Systemic Hyperbaric Oxygen Therapy in Treatment of Severe Prevalently Ischemic Diabetic Foot Ulcer: A Randomized Study. Diabetes Care 1996, 19, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Kessler, L.; Bilbault, P.; Ortéga, F.; Grasso, C.; Passemard, R.; Stephan, D.; Pinget, M.; Schneider, F. Hyperbaric Oxygenation Accelerates the Healing Rate of Nonischemic Chronic Diabetic Foot Ulcers. Diabetes Care 2003, 26, 2378–2382. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, P.; Shi, Z.; Hou, T.; Chen, X.; Du, J. A Prospective, Randomized, Controlled Study of Hyperbaric Oxygen Therapy: Effects on Healing and Oxidative Stress of Ulcer Tissue in Patients with a Diabetic Foot Ulcer. Ostomy Wound Manage 2013, 59, 18–24. [Google Scholar] [PubMed]
- Kalani, M.; Jörneskog, G.; Naderi, N.; Lind, F.; Brismar, K. Hyperbaric Oxygen (HBO) Therapy in Treatment of Diabetic Foot Ulcers. J Diabetes Complications 2002, 16, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Abidia, A.; Laden, G.; Kuhan, G.; Johnson, B.F.; Wilkinson, A.R.; Renwick, P.M.; Masson, E.A.; McCollum, P.T. The Role of Hyperbaric Oxygen Therapy in Ischaemic Diabetic Lower Extremity Ulcers: A Double-Blind Randomised-Controlled Trial. European Journal of Vascular and Endovascular Surgery 2003, 25, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Severson, M.A.; Traynelis, V.C. Role of Hyperbaric Oxygen Therapy in the Treatment of Bacterial Spinal Osteomyelitis. J Neurosurg Spine 2009, 10, 16–20. [Google Scholar] [CrossRef]
- Chen, C.-E.; Shih, S.-T.; Fu, T.-H.; Wang, J.-W.; Wang, C.-J. Hyperbaric Oxygen Therapy in the Treatment of Chronic Refractory Osteomyelitis: A Preliminary Report. Chang Gung Med J 2003, 26, 114–121. [Google Scholar]
- Delasotta, L.A.; Hanflik, A.; Bicking, G.; Mannella, W.J. Hyperbaric Oxygen for Osteomyelitis in a Compromised Host. Open Orthop J 2013, 7, 114–117. [Google Scholar] [CrossRef]
- Yu, W.-K.; Chen, Y.-W.; Shie, H.-G.; Lien, T.-C.; Kao, H.-K.; Wang, J.-H. Hyperbaric Oxygen Therapy as an Adjunctive Treatment for Sternal Infection and Osteomyelitis after Sternotomy and Cardiothoracic Surgery. J Cardiothorac Surg 2011, 6, 141. [Google Scholar] [CrossRef]
- Petzold, T.; Feindt, P.R.; Carl, U.M.; Gams, E. Hyperbaric Oxygen Therapy in Deep Sternal Wound Infection After Heart Transplantation. Chest 1999, 115, 1455–1458. [Google Scholar] [CrossRef] [PubMed]
- Siondalski, P.; Keita, L.; Sićko, Z.; Zelechowski, P.; Jaworski, Ł.; Rogowski, J. [Surgical Treatment and Adjunct Hyperbaric Therapy to Improve Healing of Wound Infection Complications after Sterno-Mediastinitis]. Pneumonol Alergol Pol 2003, 71, 12–16. [Google Scholar] [PubMed]
- Sun, I.; Lee, S.; Chiu, C.; Lin, S.; Lai, C. Hyperbaric Oxygen Therapy with Topical Negative Pressure: An Alternative Treatment for the Refractory Sternal Wound Infection. J Card Surg 2008, 23, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Larsson, A.; Uusijärvi, J.; Lind, F.; Gustavsson, B.; Saraste, H. Hyperbaric Oxygen in the Treatment of Postoperative Infections in Paediatric Patients with Neuromuscular Spine Deformity. European Spine Journal 2011, 20, 2217–2222. [Google Scholar] [CrossRef] [PubMed]
- Egito, J.G.T. do; Abboud, C.S.; Oliveira, A.P.V. de; Máximo, C.A.G.; Montenegro, C.M.; Amato, V.L.; Bammann, R.; Farsky, P.S. Evolução Clínica de Pacientes Com Mediastinite Pós-Cirurgia de Revascularização Miocárdica Submetidos à Oxigenoterapia Hiperbárica Como Terapia Adjuvante. Einstein (São Paulo) 2013, 11, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Litwinowicz, R.; Bryndza, M.; Chrapusta, A.; Kobielska, E.; Kapelak, B.; Grudzień, G. Hyperbaric Oxygen Therapy as Additional Treatment in Deep Sternal Wound Infections – a Single Center’s Experience. Polish Journal of Cardio-Thoracic Surgery 2016, 3, 198–202. [Google Scholar] [CrossRef]
- Bartek Jr., J.; Skyrman, S.; Nekludov, M.; Mathiesen, T.; Lind, F.; Schechtmann, G. Hyperbaric Oxygen Therapy as Adjuvant Treatment for Hardware-Related Infections in Neuromodulation. Stereotact Funct Neurosurg 2018, 96, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Copeland, H.; Newcombe, J.; Yamin, F.; Bhajri, K.; Mille, V.A.; Hasaniya, N.; Bailey, L.; Razzouk, A.J. Role of Negative Pressure Wound Care and Hyperbaric Oxygen Therapy for Sternal Wound Infections After Pediatric Cardiac Surgery. World J Pediatr Congenit Heart Surg 2018, 9, 440–445. [Google Scholar] [CrossRef]
- Stizzo, M.; Manfredi, C.; Spirito, L.; Sciorio, C.; Romero Otero, J.; Martinez Salamanca, J.I.; Crocetto, F.; Verze, P.; Imbimbo, C.; Fusco, F.; et al. Hyperbaric Oxygen Therapy as Adjuvant Treatment for Surgical Site Infections after Male-to-female Gender Affirmation Surgery: A 10-year Experience. Andrology 2022, 10, 1310–1316. [Google Scholar] [CrossRef]
- Pan, B.; Li, H.; Lang, D.; Xing, B. Environmentally Persistent Free Radicals: Occurrence, Formation Mechanisms and Implications. Environmental Pollution 2019, 248, 320–331. [Google Scholar] [CrossRef]
- Vinayak, A.; Mudgal, G.; Singh, G.B. Environment Persistent Free Radicals: Long-Lived Particles. In; 2021; pp. 1–19.
- Saravia, J.; Lee, G.I.; Lomnicki, S.; Dellinger, B.; Cormier, S.A. Particulate Matter Containing Environmentally Persistent Free Radicals and Adverse Infant Respiratory Health Effects: A Review. J Biochem Mol Toxicol 2013, 27, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Yao, D.; Qian, Y.; Zhong, S.; Zhang, L.; Xue, G.; Jia, H. Factors Controlling the Formation of Persistent Free Radicals in Hydrochar during Hydrothermal Conversion of Rice Straw. Environ Chem Lett 2018, 16, 1463–1468. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, L.; Wang, X.; Zheng, M.; Li, C.; Zhang, A.; Fu, J.; Yang, Y.; Qin, L.; Liu, X.; et al. Risk Evaluation of Environmentally Persistent Free Radicals in Airborne Particulate Matter and Influence of Atmospheric Factors. Ecotoxicol Environ Saf 2020, 196, 110571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Sun, P.; Faye, M.C.A.S.; Zhang, Y. Characterization of Biochar Derived from Rice Husks and Its Potential in Chlorobenzene Degradation. Carbon N Y 2018, 130, 730–740. [Google Scholar] [CrossRef]
- Kumbhar, D.; Palliyarayil, A.; Reghu, D.; Shrungar, D.; Umapathy, S.; Sil, S. Rapid Discrimination of Porous Bio-Carbon Derived from Nitrogen Rich Biomass Using Raman Spectroscopy and Artificial Intelligence Methods. Carbon N Y 2021, 178, 792–802. [Google Scholar] [CrossRef]
- Wu, C.; Fu, L.; Li, H.; Liu, X.; Wan, C. Using Biochar to Strengthen the Removal of Antibiotic Resistance Genes: Performance and Mechanism. Science of The Total Environment 2022, 816, 151554. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Qin, F.; Zhang, C.; Huang, D.; Tang, L.; Yan, M.; Wang, W.; Song, B.; Qin, D.; Zhou, Y.; et al. Effects of Heterogeneous Metals on the Generation of Persistent Free Radicals as Critical Redox Sites in Iron-Containing Biochar for Persulfate Activation. ACS ES&T Water 2023, 3, 298–310. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, Q.; Shi, L.; Liu, Z. Electron Spin Resonance Studies of Coals and Coal Conversion Processes: A Review. Fuel Processing Technology 2019, 188, 212–227. [Google Scholar] [CrossRef]
- Ruan, X.; Sun, Y.; Du, W.; Tang, Y.; Liu, Q.; Zhang, Z.; Doherty, W.; Frost, R.L.; Qian, G.; Tsang, D.C.W. Formation, Characteristics, and Applications of Environmentally Persistent Free Radicals in Biochars: A Review. Bioresour Technol 2019, 281, 457–468. [Google Scholar] [CrossRef]
- Jothirani, R.; Kumar, P.S.; Saravanan, A.; Narayan, A.S.; Dutta, A. Ultrasonic Modified Corn Pith for the Sequestration of Dye from Aqueous Solution. Journal of Industrial and Engineering Chemistry 2016, 39, 162–175. [Google Scholar] [CrossRef]
- S. , S.; P., S.K.; A., S.; P., S.R.; C., R. Computation of Adsorption Parameters for the Removal of Dye from Wastewater by Microwave Assisted Sawdust: Theoretical and Experimental Analysis. Environ Toxicol Pharmacol 2017, 50, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Kumar, P.S.; Renita, A.A. Hybrid Synthesis of Novel Material through Acid Modification Followed Ultrasonication to Improve Adsorption Capacity for Zinc Removal. J Clean Prod 2018, 172, 92–105. [Google Scholar] [CrossRef]
- Luo, Z.; Yao, B.; Yang, X.; Wang, L.; Xu, Z.; Yan, X.; Tian, L.; Zhou, H.; Zhou, Y. Novel Insights into the Adsorption of Organic Contaminants by Biochar: A Review. Chemosphere 2022, 287, 132113. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Tang, L.; Jiang, H.; Mao, Y.; Song, W.; Chen, H. Prediction of Heavy Metals Adsorption by Hydrochars and Identification of Critical Factors Using Machine Learning Algorithms. Bioresour Technol 2023, 383, 129223. [Google Scholar] [CrossRef]
- Chauhan, S.; Shafi, T.; Dubey, B.K.; Chowdhury, S. Biochar-Mediated Removal of Pharmaceutical Compounds from Aqueous Matrices via Adsorption. Waste Dispos Sustain Energy 2023, 5, 37–62. [Google Scholar] [CrossRef]
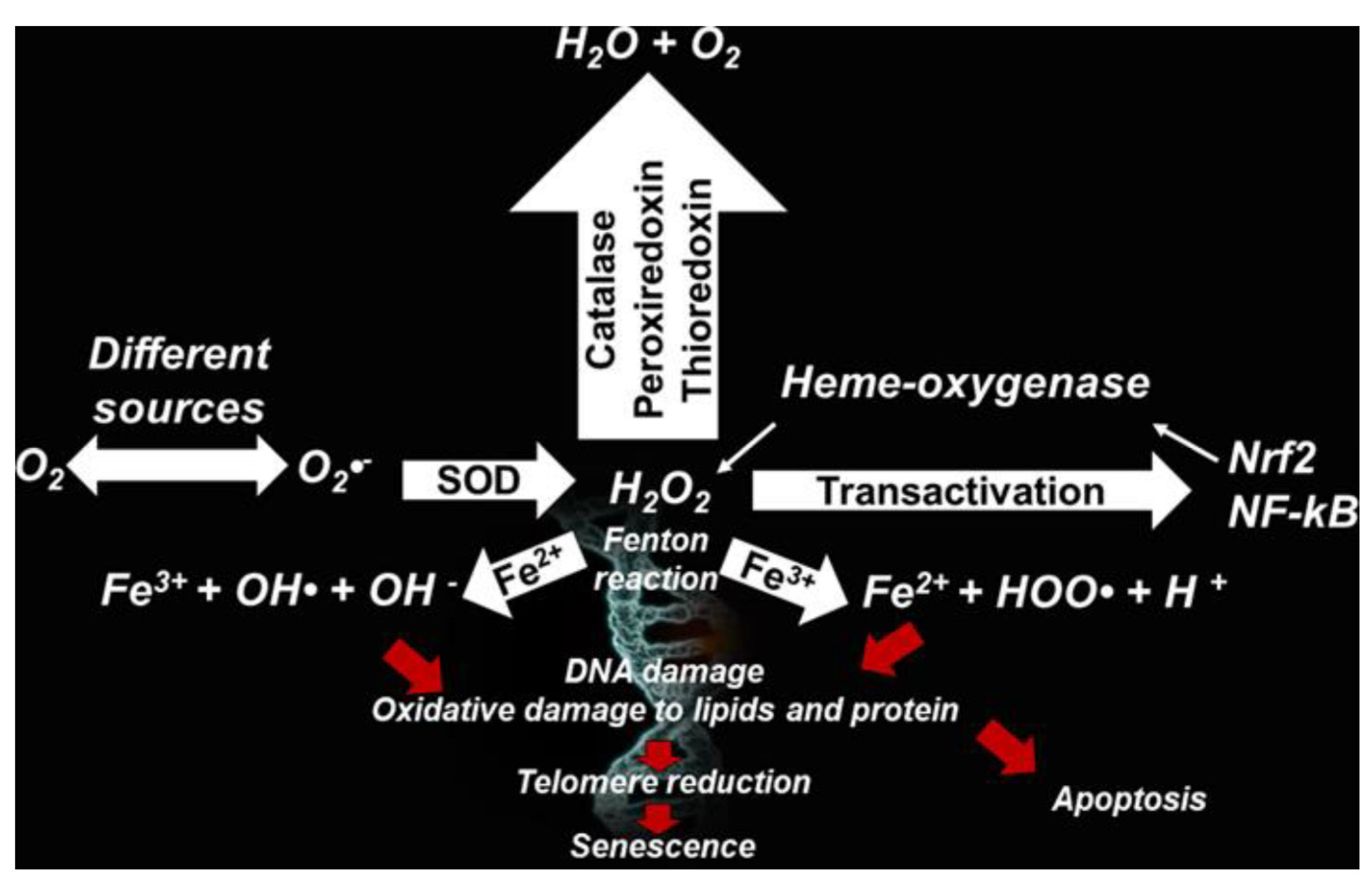
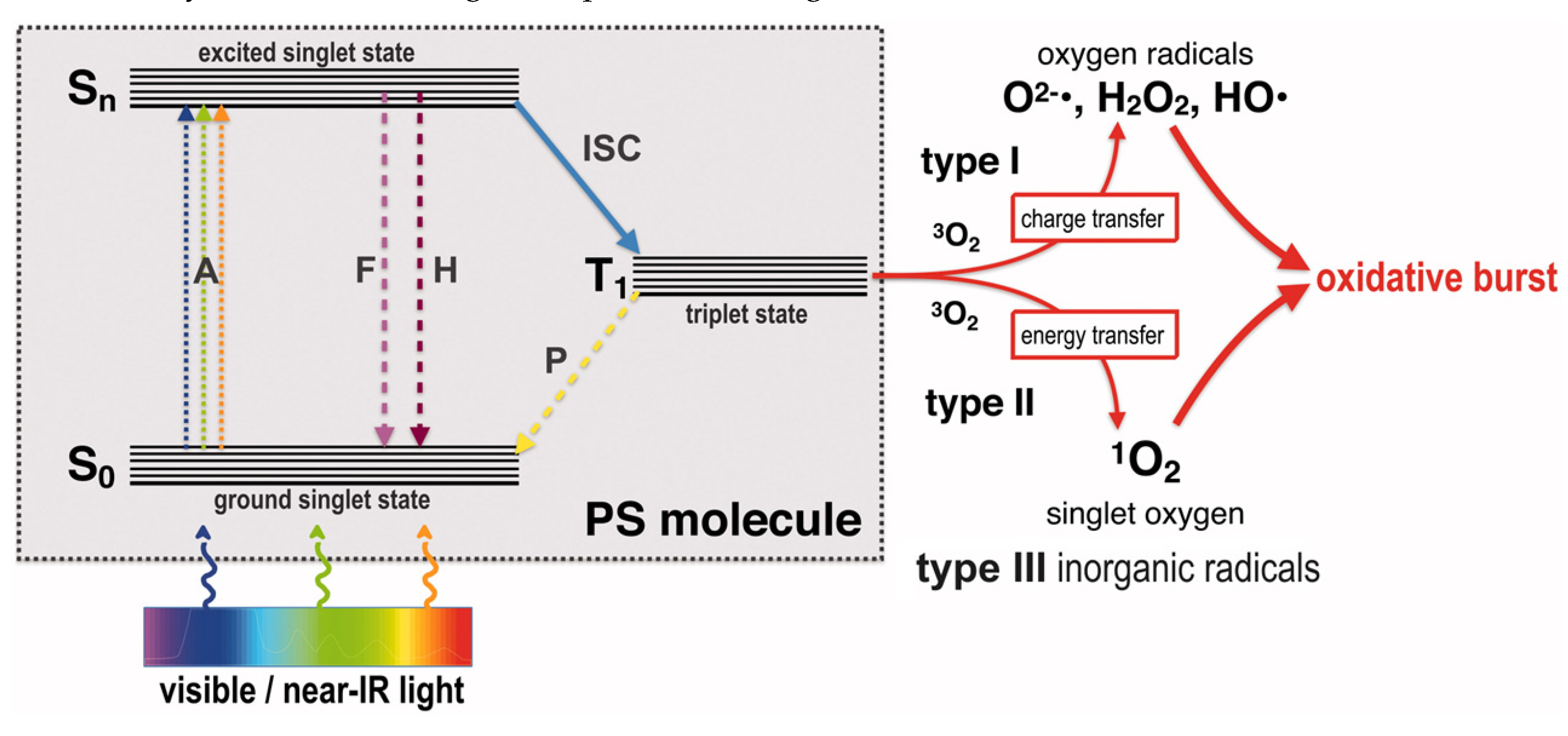
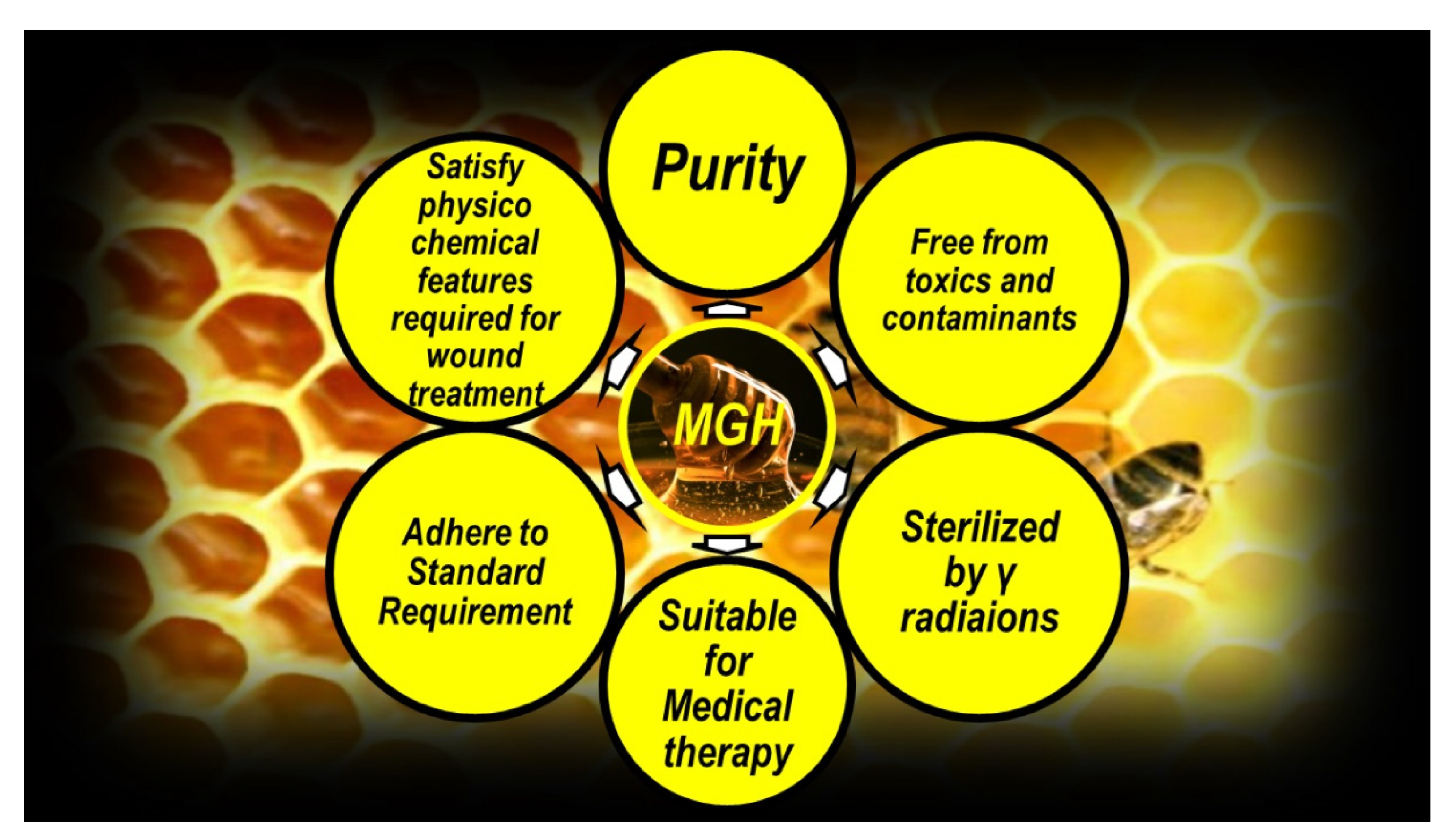
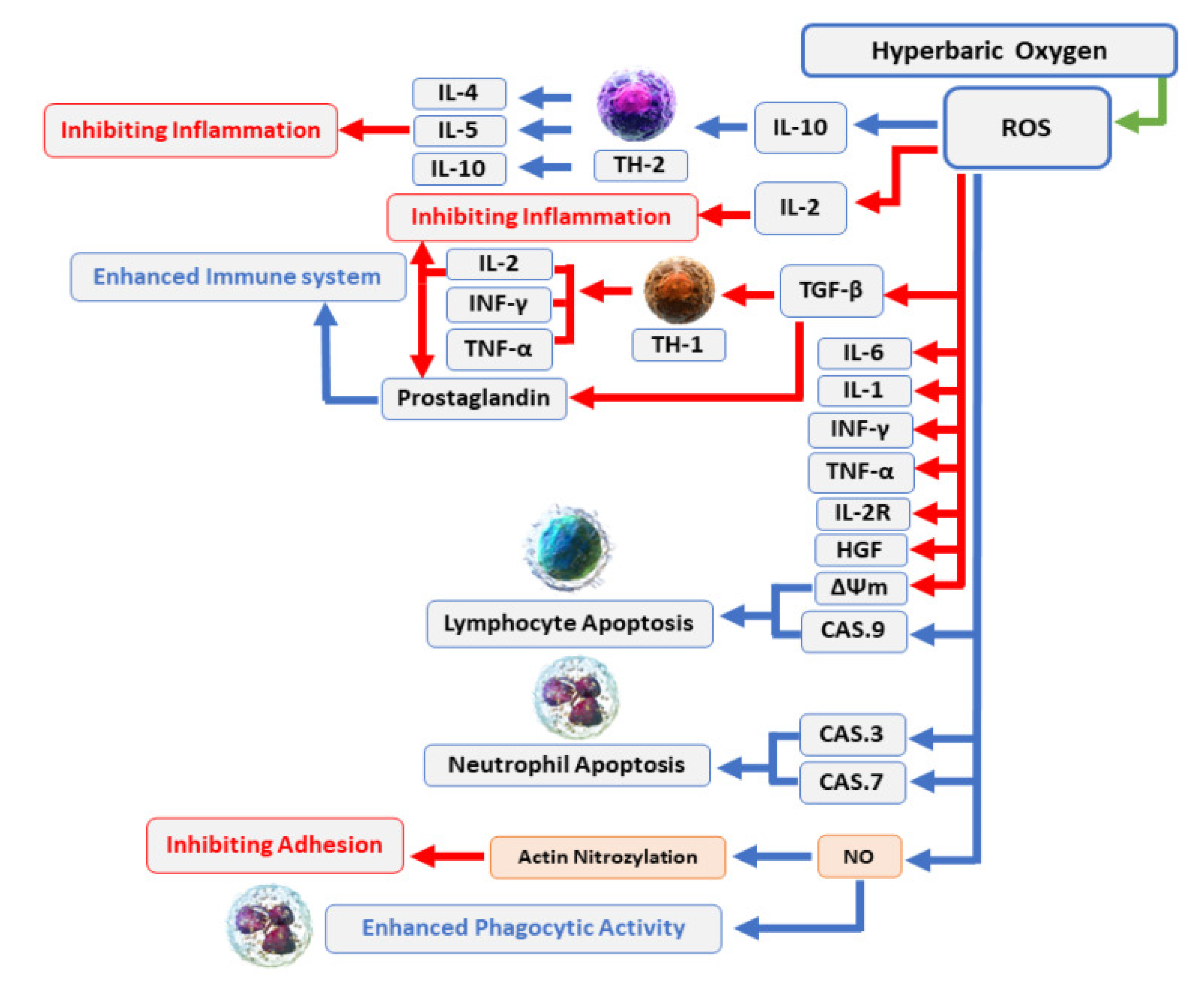
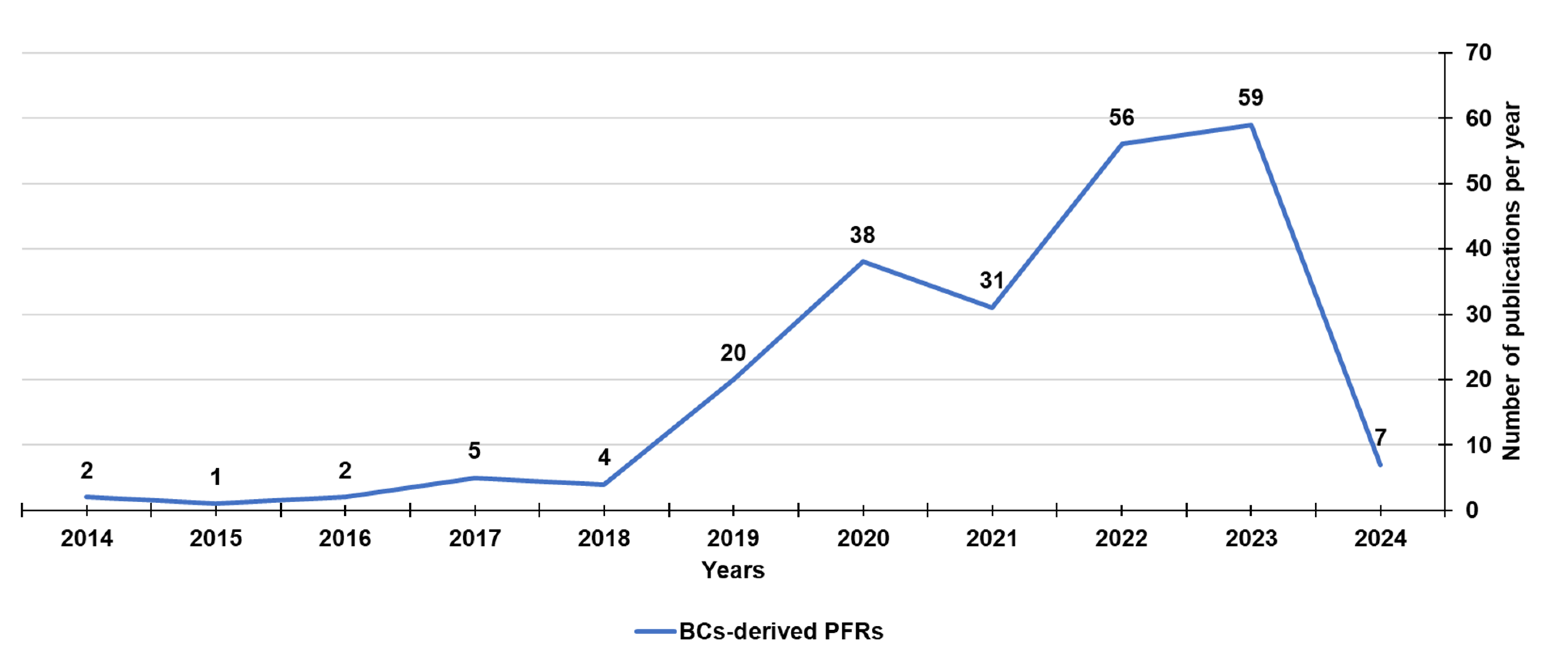
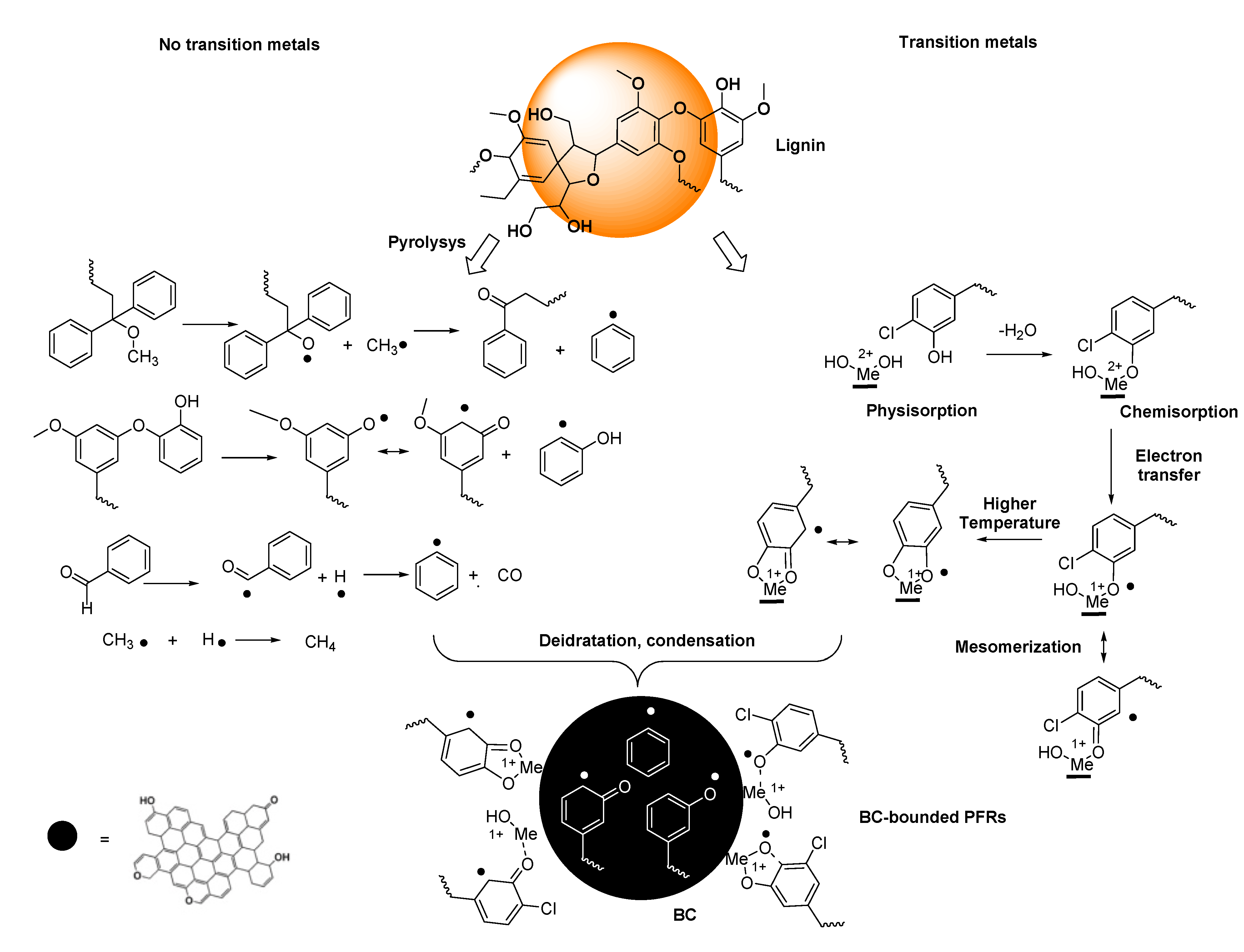
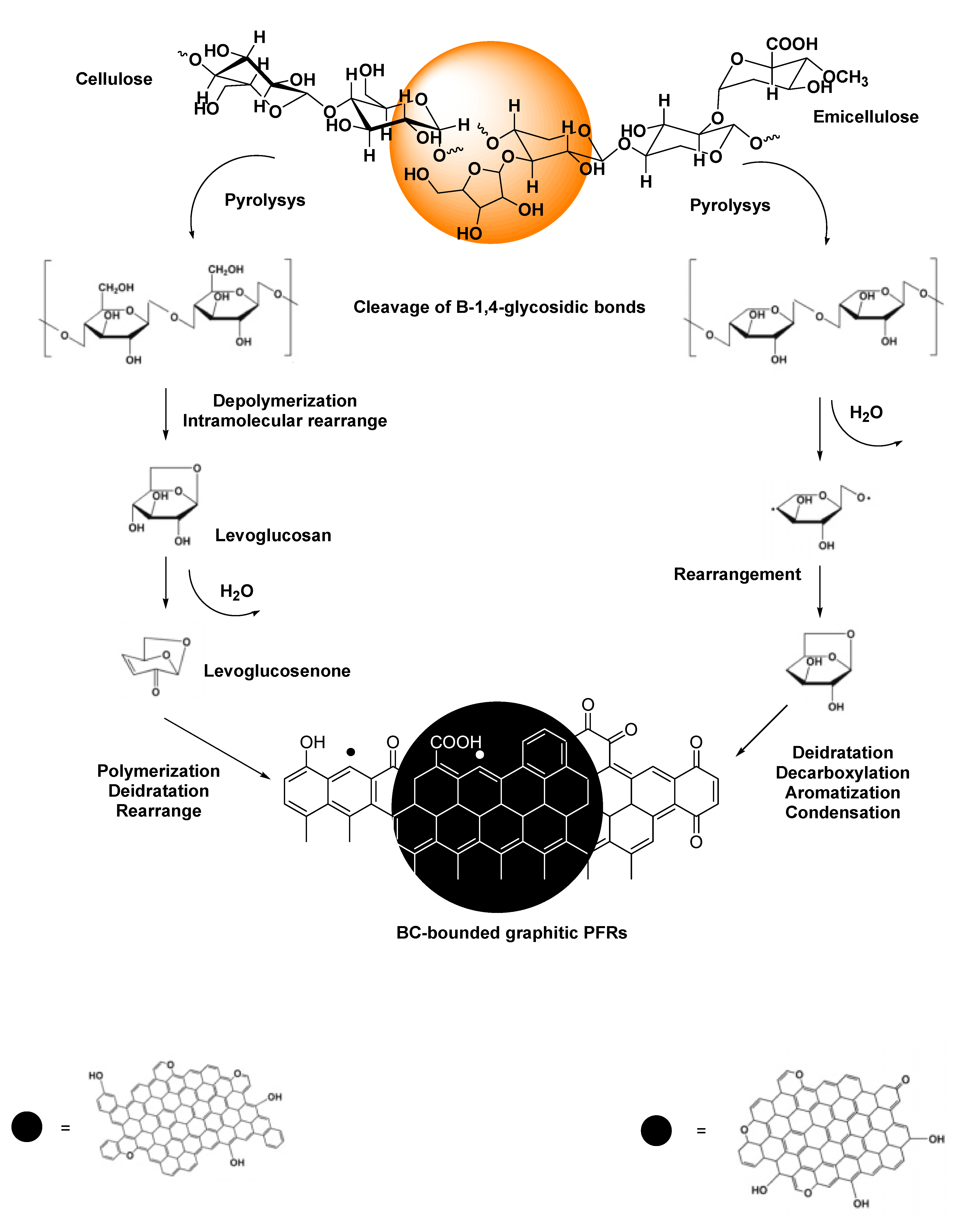
| Endogenous Sources | Exogenous Sources | Reactive Species | |
|---|---|---|---|
| Enzymatic | Non-Enzymatic | ||
| NOX MPO Cytochrome P450 Lipoxygenase Angiotensin II Xanthene oxidase Cyclooxygenase FpH• |
Mitochondria Respiratory chain Glucose auto-oxidation NAD• Semiquinone radicals Radical pyridinium Hemoproteins |
Air Water pollution Tobacco Alcohol Heavy/transition metals Drugs Industrial solvents Cooking Radiation EPFRs BC-PFRs |
O2•− H2O2 •OH •OOH ONOO• NO2• NO• ONOOCO2− NO2+ ONOOH N2O3 ONOO− ONOOCO2− CO3•− |
| Reactive Specie | Source | Function |
|---|---|---|
| O2•− | Enzymatic process Autoxidation reactions Non-enzymatic electron transfer reactions |
Reduces iron complexes such as cytochrome C Oxidizes ascorbic acid and α-tocopherol |
| HOO• | Protonation of O2•− | Initiates fatty acid peroxidation |
| HO• | By H2O2 via Fenton reaction and HWR | Reacts with organic and inorganic molecules * |
| NO• |
L-arginine (substrate) NADPH (electron source) Nitric oxide-synthase |
Intracellular second messenger Stimulates GC and PK Causes smooth muscle relaxation in blood vessels |
| NO2• | Protonation of ONOO− Homolytic fragmentation of ONOOCO2− |
Acts on the antioxidant mechanism ↓ Ascorbate and α-tocopherol in plasma |
| ONOO• | Reaction of O2 with NO• | Oxidizes and nitrates methionine and L-tyrosine Oxidizes DNA to form nitroguanine |
| CO3•− | (SOD)-Cu2+ By reaction of •OH reacts with HCO3- |
Oxidizes proteins and nucleic acids |
| ONOOCO2− | Reaction of ONOO− with CO2 | Promotes nitration of oxyhaemoglobin’s tyrosine of the via free radicals |
| Cellular Macromolecules | Reactions | OS biomarkers | Ref |
|---|---|---|---|
| Proteins | RNS with L-tyrosine | NT | [41] |
| Fenton reaction of ROS with L-lysine, L-arginine L-proline, L-threonine |
PC | [42] | |
| Proteins/lipids | Michael-addition of aldehydic lipid oxidation products to L-lysine, L-cysteine, L-histidine | PC | [42] |
| Proteins/lipids | Complex oxidative process | Ox-LDL | [43] |
| Proteins/carbohydrates | Glyco-oxidation between L-lysine amino groups and L-arginine carbonyl groups linked to carbohydrates |
AGEs | [44] |
| Lipids | •OH and HOO• mediated lipid peroxidation of poly-unsaturated fatty acids * | 4-HNE, MDA, F2-IsoPs | [41] |
| DNA | Mutagenic oxidation | 2-Hydroxy adenine 8-Oxoadenine 5-Hydroxycytosine Cytosine glycol Thymine Glycol 8-OHGua 8-OHdG |
[45] |
| Process | Endogenous Molecules | Actions | Exogenous Molecules | Effect |
|---|---|---|---|---|
| NE | Vitamin E Vitamin C Carotenes Ferritin Ceruloplasmin Selenium GSH Manganese Ubiquinone Zinc Flavonoids Coenzyme Q Melatonin Bilirubin Taurine Cysteine Albumin Uric acid |
Interact with ROS and RNS and terminate the free radical chain reactions | Vitamin C | ↓O2• ↓•OH |
| Vitamin E | ↓Lipid peroxidation | |||
| Resveratrol Phenolic acids Flavonoids |
↓O2• ↓•OH ↓Lipid peroxidation |
|||
| Oil Lecithin |
↓O2• ↓•OH ↓Lipid peroxidation |
|||
| Selenium Zinc |
Antioxidant | |||
| Acetylcysteine | Antioxidant | |||
| E | SOD | Converts O2 to H2O2 ↓Hydroxyl radical production |
||
| CAT | Decomposes H2O2 to H2O+O2 ↓Hydroxyl radical production |
|||
| GSH-Px | Converts peroxides and hydroxyl radicals into nontoxic forms by the oxidation of GSH into GSSG | |||
| GR | Converts glutathione disulphide to GSH | |||
| GSTs | Catalyses the conjugation of GSH to xenobiotic substrate | |||
| G6PD | Catalyses the dehydrogenation of G6P to 6-phosphoglucono-Δ-lactone | |||
| Nrf2 | Regulates the expression of antioxidant proteins | |||
| ARE | Encodes for detoxification enzymes and cytoprotective proteins | |||
| NQO1 | Catalyses the reduction of quinones and quinonoids to hydroquinone molecules | |||
| MSR | Carries out the enzymatic reduction of the oxidized form of methionine to methionine | |||
| Type | Name | Mechanism of Action | Clinical Application | Target | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| Antibiotics | Nitrofurantoin | Autooxidation of nitroaromatic anion radicals * in presence of O2 providing O2− and then ROS thus causing OS and toxicity to bacteria | UTI | E. coli | [18] | ||||
| Polymyxin B | Accumulation of OH• | Untreatable infections |
Acinetobacter baumannii Pseudomonas aeruginosa Enterobacteriaceae ** |
[58] | |||||
| Nalidixic acid | Mutagenesis by oxygen free radical generation |
Gastroenteritis Enteric fever Bacteremia |
Salmonella typhimurium | [59] | |||||
| Norfloxacin | |||||||||
| Norfloxacin Ampicillin Kanamycin Fluoroquinolones β-Lactams Aminoglycosides |
↑ Superoxide levels ↑ H2O2 ↑ Lethality by accumulation of OH• |
UTI | E. coli | [60] | |||||
| Nalidixic acid Trimethoprim Ampicillin Aminoglyoside |
Post-stress ROS-mediated toxicity | [61,62] | |||||||
|
Alternative antimicrobials |
Organo Metals |
OSECs | Ebselen *** | By inhibiting TrxR in bacteria laching glutathione thus triggering OS. |
Skin infections Bacteremia Endocarditis Food poisoning Pneumonia TSS |
S. aureus | [63] | ||
| Tuberculosis | M. tuberculosis | [64] | |||||||
| Nanomaterials | NPs | MSNP-maleamic | ↑ ROS (40% E. coli, 50% S. aureus) | UTI | E. coli | [65] | |||
| MSNPs-maleamic-Cu | ↑ ROS (40% E. coli, 30% S. aureus) | Skin infections Bacteremia Endocarditis Food poisoning Pneumonia TSS |
S. aureus | ||||||
| Metal oxide NPs | ↑ ROS ↑ RNS |
Skin infections Bacteremia Endocarditis Food poisoning Pneumonia TSS UTI |
E. coli S. aureus S. epiderdimis Photobacterium hosphoreum |
[64] | |||||
| NZs | AgPd0.38 | By ROS produced on Ag and Pd bimetallic alloy |
Severe infections |
S. aureus Bacillus subtilis E. coli P. aeruginosa |
[66] | ||||
| Metal based | AgRuSCs | AGXX® | By ROS catalytical production | UTI | Enterococcus faecalis | [54,67,68,69] | |||
| Skin infections Bacteremia Endocarditis Food poisoning Pneumonia TSS |
MRSA | ||||||||
| Natural Compounds | Allicin | OS by ↑ ROS via ↓ of low MW thiols | Skin infections Bacteremia Endocarditis Food poisoning Pneumonia TSS |
S. aureus | [70] | ||||
| Class | Compound | Description | Target | Abs max (nm) |
Impact in the field | Refs. |
|---|---|---|---|---|---|---|
| Phenotiazinium derivatives |
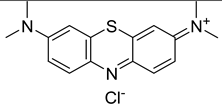 Methylene Blue |
3-ring p-system Auxochromic side groups Single positive charge SOQY < 0.5 Type I reactions |
Dental plaque | 632 | 1st clinically approved PS (dentistry) Standard PS in vitro |
[94] |
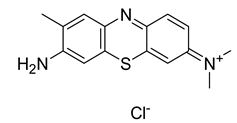 Toluidine Blue |
Streptococcus mutans | 410 | [95] | |||
| E. coli | [96] | |||||
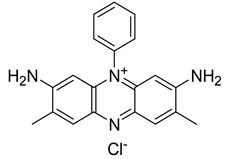 Safranine O |
F. nucleatum P. gingivalis |
520 | [97] | |||
| Porphyrin derivatives |
 Porphyrin |
Four pyrrole cycles Up to eight positive charges SOQY = 0.5-0.8 Occurring in nature Type II reactions |
S. aureus P. aeruginosa E. faecalis |
446 | Widely used as standard PS in vitro | [98,99,100] |
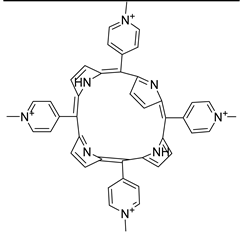 TMPyP * |
MRSA ESBL K. pneumoniae |
421 | N.R. | [101] | ||
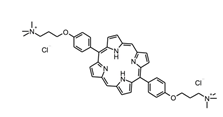 XF-73 * |
Staphylococci Enterococci Streptococci S. aureus biofilm |
380-480 | [102] | |||
| Chlorin derivatives |
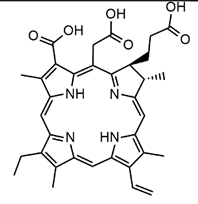 Chlorin e6 |
Like heterocyclic-macrocyclic compounds Neutral or up to eight positive charges SOQY = 0.5-0.8 3 pyrrole and 1 pyrroline subunits Type II reactions |
S. aureus E. coli |
660 (neutral) |
[103] | |
| E. coli | 532 (cationic) |
[104] | ||||
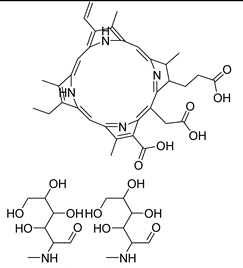 Photodithazine® |
MRSA MSSA |
660 | [105] | |||
| Phthalocyanin derivatives |
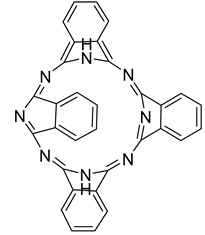 Phthalocyanine |
4 pyrrole cycles Hydrophobic and uncharged Type II reactions |
A. hidrophila | 670 | [106] | |
| Xanthene derivatives |
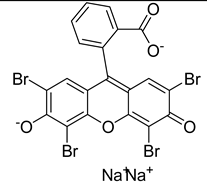 Eosin Y |
Anionic xanthene dyes Fluorescein derivatives SOQY = 0.5-0.6 Type II reactions |
MRSA MRSA biofilm S. aureus |
N.R. | Sparse studies in recent years | [107] |
 Erythrosine |
S. mutans Lactobacillus casei Candida albicans |
470 | [108] | |||
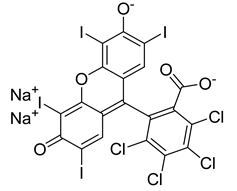 Rose Bengal |
E. faecalis P. aeruginosa |
532 | [109] | |||
| Nanomaterials |
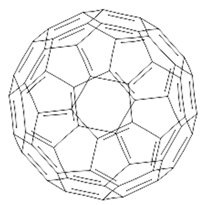 Fullerene C60 *** |
Soccer-ball shaped cage molecules Made exclusively from carbon atoms Neutral Extended p-conjugated system Type I and II reactions |
S. aureus E. coli |
532 | Unique class of PS Sparse studies on effect on biofilms | [110] [111] |
| Phenalenones |
 SAPYR |
Biosynthesized by plants to defend against pathogens using sun to generate singlet oxygen SOQY > 0.9 Positively charged pyridinium-methyl moiety Type II reactions |
E. faecalis Actinomyces naeslundii |
360-420 | First water-soluble exclusive type-II PS | [112] |
| Riboflavin derivatives |
 Vitamin B2 |
In PDT cationic derivatives with up to eight positive charges SOQY = 0.7-0.8 Type II reactions |
MRSA EHEC |
450 (cationic) |
N.R.° | [113] |
| Curcumins |
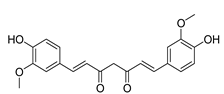 Curcumin (neutral) |
Naturally occurring yellow dye from the rootstocks of Curcuma longa Approved as food additive (E100) For use in PDT positive charges have been included in derivatives structures Type I reactions |
S. mutans L. acidophilus |
547 | Novel positively charged derivatives with enhanced water solubility |
[114] [115] |
| Natural compounds |
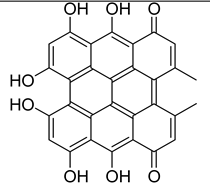 Hypericin (neutral) |
Naturally occurring For use in PDT positive charges have been included in derivatives structures Type II reactions |
S. aureus E. coli |
593 | N.R. | [116] [117] |
| 5-ALA |
 5-amminolevulinic acid |
δ-amino acid in which the hydrogens at the γ position are replaced by an oxo group Metabolized to protoporphyrin IX |
MRSA | 410 | Optical imaging agent | [118] |
| Clinical site | Dosing regimen | Clinical details | Outcome | Adverse reports |
|---|---|---|---|---|
| Respiratory tract | Daily nebulized SHRO in respiratory nebulizer | Bronchiectasis, Several patients One patient—recurrent exacerbations with secondary infection with Mycobacterium avium |
Reduction in bacterial load and temporary eradication of M. avium (1 patient) | None |
| Scalp | Daily topical application for 6 weeks | Fungal kerion, T. tonsurans Patient intolerant to oral antifungals |
Complete resolution | None |
| Intraperitoneal | 50–100 g daily via abdominal drain | Severe four-quadrant peritonitis following intraabdominal infection and corrective surgery Patients also on systemic antibiotics Often polymicrobial infections with MDR strains and Candida spp. |
Variable, but general peritonitis Control |
N.R. to SHRO use |
| Abdominal wall Deep soft tissue |
SHRO into open cavity with each dressing | Used both prophylactically and therapeutically in around 20 patients | Prevention of infection and effective therapy in infected cavities | None |
| Prosthetic joints | Single dose around prosthetic joint at surgery Numerous patients |
Mixed microbiology including S. ludenensis Given in conjunction with systemic antibiotics | Good adjunct to existing management | None |
| Prepatellar bursitis |
Single application at debridement |
On immunosuppression for psoriasis M. malmoense isolated from prepatellar pus Put on clarithromycin, rifampicin and ethambutol + SHRO topically |
Complete healing and no further isolation of M. malmoense | None |
| Bladder | Twice-weekly instillation via suprapubic catheter | Several patients with long-term urethral or suprapubic catheters | Reduction in urosepsis | None |
| External auditory canal |
Daily with wick or cotton wool | Pseudomonas otitis externa | Resolved | None |
| Oral infections | Daily oral application of 10 g SHRO |
Recurrent aphthous ulcer, gingivitis, geographic tongue. No microbiology |
Reported reduction in duration of symptoms | None |
|
Helicobacter gastritis |
Once daily 10 g SHRO for 10 days | Confirmed Helicobacter pylori gastritis MDR strain and no response to antibiotic eradication regimens |
Continuation of symptoms Therapeutic failure |
None |
| Product | Description | Indications | Mechanism of Action | Ref. | Clinical Evidence |
|---|---|---|---|---|---|
| Activon® Manuka Honey Tube AM |
100% MGMH | Sloughy, necrotic wounds # Malodorous wounds # |
Debrides necrotic tissue Can be used in dressings or directly into cavities |
[190] | Blistering and cellulitis on a type 2 diabetic patient Pediatric burn Foot ulceration Grade 5 sacral wound [190] |
| Activon® Tulle AM | Knitted viscose mesh dressing with 100% MH | Granulating or shallow wounds Debriding or de-sloughing small areas of necrotic or sloughy tissue |
Creates a moist healing environment Eliminates wound odor Antibacterial action |
[190] | Over-granulated grade 3 and 4 pressure ulcers Extensive leg cellulitis Venous ulcer, chronic wound Infections, necrotic foot [190] |
| Algivon® Plus AM | Reinforced alginate dressing with 100% MH |
Cavities, sinuses, pressure, leg, diabetic ulcers Surgical, infected wounds Burns, graft sites Ideal for wetter wounds |
Absorbs exudate Debrides, removes slough Reduces bacterial load |
[190] | Chronic wounds [191] Burn wound management [192] |
| Algivon® Plus Ribbon AM | [190] | Autoamputation of fingertip necrosis [193] |
|||
| Aurum® ostomy bags WM | MGMH added to hydrocolloids |
Stoma care | Kills bacteria Suppresses inflammation Promote healthy skin around the stoma |
[194] | Pyoderma gangrenosum around ileostomy [195] |
| L-Mesitran® Border AME | Hydrogel + honey (30%) pad on a fixation layer | Chronic wounds $ | Exudate absorption Re-hydration of dry tissue Antibacterial properties |
[196] | Pediatric minor burns and scalds [197] |
| L-Mesitran® Hydro AME | Sterile, semi-permeable hydrogel dressing ## |
Chronic wounds * Superficial and acute wounds ** Superficial and partial-thickness burns *** Fungating wounds, donor sites Surgical wounds, cuts, abrasions |
Donates moisture to rehydrate dry tissue Antibacterial properties |
[196] | Pediatric minor burns and scalds [197] Fungating wounds [198] |
| L-Mesitran® Ointment AME |
Ointment $$ | Aids debridement and reduce bacterial colonization | [196] | Skin tears; irritation and inflammation [198] | |
| ManukaDress IG MPI | Wound dressing made with 100% Leptospermum scoparium @ | Leg and pressure ulcers First- and second-degree burns Diabetic foot ulcers, surgical and trauma wounds |
Osmotic activity that promotes autolytic debridement and helps maintain a moist wound environment | [152] | Burn management [152] Difficult-to-debride wounds [199] Necrotic pressure ulcer Recurrent venous leg ulceration [200] |
| Medihoney® Antibacterial Honey DSC |
100% sterilized MGMH | Deep, sinus, necrotic, infected surgical and malodorous wounds |
Creates an antibacterial environment Debridement on sloughy and necrotic tissue, removes malodor Provides a moist environment |
[201] | Wound healing [202] Prevention of catheter-associated infections in hemodialyzed patients [203] |
| Medihoney® Apinate Dressing DSC |
Calcium alginate dressing with 100% MGMH |
Diabetic foot, leg, pressure ulcers First- and second-degree partial-thickness burns Donor sites, traumatic, surgical wounds. |
Provides a moist environment Osmotic potential Draws fluid through the wound to the surface Low pH of 3.5–4.5. |
[204] | Venous leg ulcers [205] |
| Medihoney® Barrier Cream DSC |
Barrier cream with 30% MGMH | Protects skin damaged by irradiation treatment or in wet areas Prevents damage caused by shear and friction |
Maintains skin moisture and pH. | [206] | Treatment for intertrigo in large skin folds [207] |
| Medihoney® Antibacterial Wound Gel™ DSC |
Antibacterial wound gel & | Burns, cuts, grazes, and eczema wounds | Creates a moist, low-pH environment Cleans the wound by osmotic effect Reduces the risk of infection |
[208] | Reduction in incidence of wound infection after microvascular free tissue reconstruction [209] |
| SurgihoneyRO™ MH | Antimicrobial wound gel && | Infected, chronic wounds | Antimicrobial activity by controlled release of H2O2 Promotes debridement and new tissue growth |
[210] | Prevention of caesarean wound infection Prevention/eradication of bacterial colonies in dressing oncology long vascular lines; ulcers, surgical wounds and trauma wounds [183,185,211] In vitro activity against biofilm-producing clinical bacterial isolates [178] |
| Antibacterial factors | Sources | Mechanism of formation | Effects on Bacteria | Ref. |
|---|---|---|---|---|
| H2O2 | Glu | Oxidation of Glu deriving by sucrose captured by bee from flowers | OS DNA damage |
[152] |
| Bee Def-1 | Bee’s hypopharyngeal gland | Innate immune response | Create pores in membrane Interferes with bacterial adhesion Alters the production of EPSs |
[215] |
| Acidic pH (3.4-6.1) |
GluLac GluA |
By enzymatic oxidation of Glu | Prevents bacterial growth | [152] |
| MGO * | Dihydroxyacetone | Heating | Alters bacteria fimbria and flagella |
[220] |
| Osmotic pressure |
Super concentration of sugars | N.A. | ↓ Availability of free water molecules ↓ Bacterial growth |
[221] |
| Polyphenols | Flowers as secondary metabolites | Flowers metabolism | Pro-oxidative properties Accelerate HO• formation Oxidative DNA breakage Non-enzymatic ↑ H2O2 |
[222] |
| Disease | Cause */Circumstances ° | Ref |
|---|---|---|
| CO poisoning | [233] | |
| Acute anemia | [234] | |
| Rheumatoid arthritis Cell hypoxia |
Polarization of Th17 cells to T reg * | [235] |
| Inflammatory disorders (by ↓ inflammatory mediators) |
Ischemic circumstances ° Compartment syndrome ° |
[227] |
| Microcirculatory disorder | ||
| ↓ Leucocyte chemotaxis and adhesion ↑ Proliferation of neutrophiles |
[236] | |
| Autoimmune syndrome Immune reactions in antigens Autoimmune symptoms. |
Proteinuria ° Facial erythema ° Lymphadenopathy ° |
[237,238] |
| ↓ Lymphocytes and leukocytes | [234] | |
| ↑ Mitochondrial Function | [239] | |
| Chronic skin damage healing (by angiogenesis) |
[240] | |
| Recalcitrant infections | Necrotizing fasciitis ° Osteomyelitis ° Chronic soft tissue infections ° Infective endocarditis ° Acute or chronic wounds ° Diabetic foot ulcers ° |
[227,241,242,243,244,245] [246,247] |
| Ocular disorders | Cystoid macular edema ° Scleral thinning ° Necrosis faced after pterygium surgery ° Nonhealing corneal edema ° Anterior segment ischemia ° Some blinding diseases ° |
[248,249] |
| Brain/cerebral injuries | Ischemic-reperfusion damage ° | [250] |
| Cancer | [251] | |
| Complications by radiotherapy | Radiation-induced skin necrosis ° | [252] |
| Infections | Study papulation | Treatment sessions * | Pressure ** | Exposure Time (min) | Main findings | Ref |
|---|---|---|---|---|---|---|
| Burns | 53 | Based on outcomes | 2.5 | 90 | All patients survived | [264] |
| Burn | 40 | 10 | 2.5 | 80 | Faster healing, shorter hospitalization | [265] |
| Brain abscess | 41 | 4–52 | 2.5–2.8 | 25– | ↓ Treatment failures, ↑ outcomes | [266] |
| SSIs | 42 | 30 | 2.4 | 90 | ↓ Post-surgical deep infections in CSD | [267] |
| SSIs | 32 | Based on outcomes | 2-3 | 90 | Valuable AT for PO organ/space S-SSI | [268] |
| SSIs | 6 | 28–106 | 2.5–2.8 | 75 | AT for early PO deep infections | [269] |
| NSTI | 48 | Based on outcomes | 3 | 90 | Not ↓ mortality rate, number of debridement, hospital stay, antibiotic use |
[270] |
| NSTI | 44 | 2.8 | 60 | ↑ Survival and limb salvage | [271] | |
| NSTI | 32 | 2.8 | 45 | AT | [272] | |
| NSTI | 37 | 2.5 | 45 | Doubtful advantage of using HBOT as AT for NF in ↓ mortality and morbidity | [273] | |
| DFIs | 100 | 20 to 30 | 2–3 | 90 | Useful AT for nonhealing DFIs | [274] |
| DFIs | 42 | Group1:<10 Group 2:>10 |
2.5 | 120 | ↓ Amputation rate | [247] |
| DFIs | 94 | 40 | 2.5 | 85 | Facilitates healing of chronic DFIs | [275] |
| DFIs | 35 | 38±8 | 2.2–2.5 | 90 | ↓ Amputations | [276] |
| DFIs | 28 | 20 | 2.5 | 90 | ↑ Healing rate of nonischemic chronic DFIs | [277] |
| DFIs | 36 | 2.5 | 90 | Healing response in chronic DFIs | [278] | |
| DFIs | 38 | 40–60 | 2.5 | 90 | ↑ Healing rate ↓ Amputation rate |
[279] |
| DFIs | 18 | 30 | 2.4 | 90 | AT when reconstructive surgery is not possible | [280] |
| Osteomyelitis | 6 | 2.0–2.4 | 30 | Effective following failure of primary therapy of osteomyelitis | [281] | |
| Osteomyelitis | 14 | 2.5 | 120 | Effective and safe for chronic refractory osteomyelitis |
[282] | |
| Osteomyelitis | 1 | 2 | – | Early use of HBOT for a compromised host who develops recurrent osteomyelitis | [283] | |
| Osteomyelitis | 12 | Based on outcomes | 2.5 | 90 | AT for patients who develop S-SSI and osteomyelitis after CTS |
[284] |
| Study | Surgery | SSI | Population ***/# |
ATA Time (min) |
Outcomes and Conclusion | Refs. |
|---|---|---|---|---|---|---|
| Case report | CTS | S-SSI | 1 | 2.40 90 |
Rapid healing and epithelialization * | [285] |
| Retrospective | Sternotomy | S-SSI | 55 | 2.50 90 |
S-SSI cured in all patients within an average of 8 weeks No in-hospital death ↑ Clinical outcome in patients with sterno-mediastinis and post-sternotomy wound infection after CTS |
[286] |
| Prospective trial | CTS | S-SSI | 32; 14/18 | 2–3 90 |
S. aureus was the most common pathogen #, *** Infection duration was similar #,***,** Infection relapse rate was significantly ↓ in *** Intravenous antibiotic use duration ↓ in *** Total hospital stay ↓ in *** HBOT could be a valuable AT for treating PO organ/space S-SSI |
[268] |
| Case report | CTS | S-SSI | 1 | 2.50 90 |
Sternal wounds totally healed and epithelized in 9 weeks HBOT with TNP dressing is a good alternative method for patients who cannot tolerate or refuse any surgical reconstruction |
[287] |
| Retrospective | NMSS | DWI | 6 | 2.50 3 × 25 |
All infections resolved Wound healing in an average of 3 months Minor side effects of HBOT HBOT is a safe AT for early deep PO infections in case of spinal implants in HR pediatric patients |
[288] |
| Retrospective | CTS | S-SSI | 12; 6/6 | 2.50 90 |
No treatment-related complication in *** Length of stay in ICU ↓ in *** Invasive and noninvasive positive pressure ventilation ↓ in *** Hospital mortality ↓ in *** HBOT may be used as a safe AT to ↑ clinical outcomes in patients with S-SSI and osteomyelitis after sternotomy and CTS |
[284] |
| Retrospective | CABS | Mediastinitis | 18 | 2.50 90 |
1 HBOT-unrelated death caused by sepsis HBOT was well-tolerated Favorable clinical outcomes using HBOT as AT for treating mediastinis patients after CABS |
[289] |
| Retrospective | NMSS | DWI | 42; 18/24 | 2.40 90 |
11.9% (5/42) Incidence of infection in both ***,# 5.5% (1/18) Infection rate in *** 6.6% (4/24) Infection rate in # HBOT significantly ↓ PO infections in NMSS patients HBOT is a safe AT to prevent PO deep infections in complex spine deformity in HR NM patients |
[267] |
| Retrospective | CTS | S-SSI | 10 | 2.50 92 |
70% complete wound healing with fibrous scar formation in 4 weeks HBOT *** HBOT had 80% success as AT in DSWI *** No complications were observed |
[290] |
| Retrospective | NM | HRI | 14 | 2.0–2.8 75 |
86% of HRI successfully treated without hardware removal *** Hardware was removed following HBOT failure in 2 infections Observed intrathecal pump malfunction caused by HBOT (2.8 bars) HBOT has potential as AT in the treatment of HRI in NM Diminished need for hardware removal and treatment interruption |
[291] |
| Retrospective | CTS | S-SSI | 53 | Readmitted with infected sternotomies discharged in 2–39 days Time for wounds healing using NPWT alone was 21–42 days in # Time for wounds healing using NPWT+HBOT was 28–42 days in *** HBOT was 5–35 days HBO treatments were 22.6 (+11.06) Restore time 21–98 days in *** (NPWT+HBOT) Multimodality therapy of incision and drainage using NPWT+HBOT+antibiotics is successful for treat complex deep sternal wound infections in the pediatric population after congenital heart surgery |
[292] | |
| Retrospective | MtF-GAS | SSI | 33; 15/18 | 2.2–3.0/90 | 100% Complete wound healing *** 94.4% Complete wound healing # ↓ Duration of antibiotic therapy *** ↓ Perineal drain time *** ↓ Bladder catheter time *** ↓ Hospital stay *** HBOT as effective adjuvant treatment for SSIs in patients undergoing MtF GAS |
[293] |
| Parameter | Influencing Factors | Specifications | Observations | Ref. |
|---|---|---|---|---|
| PFRs concentration |
Biomass type | Cow manure, rice husk, others (< 500°C) | ≠ Concentrations | [305,306] |
| Non-lignocellulosic biomass with ⇓ H/C and O/C | ⇓ Concentration | [307] | ||
| Lignocellulosic biomass | ⇑ Concentration | |||
| Temperature | 300°C, 700°C | ≠ Concentrations | [305] | |
| Maximum concentration at 600°C | [308] | |||
| Maximum of concentration at 500-600°C | [309] | |||
| Transition metals | Adsorb onto biomass and transfers electrons from polymer to metal center during pyrolysis | ⇑ Concentration | [25] | |
| Type of PFRs | Temperature | 200-300°C | OCR | [309] |
| 400°C | OCR + CCR | |||
| 500-700°C | CCR |
| Biomass | Pyrolysis °C/Time | BC-name | Active radicals | Radical Mechanisms | Degraded compound | Refs. |
|---|---|---|---|---|---|---|
|
Anaerobic
digestion sludge |
400°C 600°C 800°C 1000°C |
ADSBC 400 ADSBC 600 ADSBC 800 ADSBC 1000 |
SO4•- PFRs OH• | BC-mediated activation of PDS | Dyes, Estrogens Sulfonamides, E. coli Others |
[30] |
|
Caragana korshinskii |
650 °C/3h | ACB-K-gC3N | PFRs h+•OH• O2- | Electron photogeneration and PFRs mediated H2O and O2 activation |
S. aureus, E. coli RhB, TC, NOR, CAP |
[31] |
| Pinewood | 600°C | Ag0-PBC | PFRs •OH • O2- | UV-light promoted excitation of the electron-hole pairs and subsequently, the production of ROS Enhanced ROS generation by PFRs |
MB, E. coli | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





