1. Introduction
Wettability is an essential property of solid surfaces which refers to how a liquid spreads out when placed on a solid (or liquid) substrate. The wettability phenomenon is used in several fields and is an area where chemistry, physics, and engineering overlap. Many applications employ wettability properties such as printing, painting, adhesion, lubrication, cleaning, coating, soldering, brazing, and composite processing [
1]. For example, wetting is used to enhance the spreading of paint and adhesives on solid surfaces or the application of cosmetics onto human skin. On the contrary, dewetting is used to reduce surface contaminations; hence contaminating liquids such as oil and water are repelled and no longer attracted to that surface [
2].
Nonreactive wettability depends on the surface energy that is affected by the materials of spreading liquid and substrate (i.e., wetting system). In general, the wettability of a particular wetting system is affected by many factors [
3], such as the effects of surface roughness and heterogeneity, surface preparation, the presence of contaminants, the vapor environment, pressure and temperature, droplet size, and electrical charge. The surface energy is an indication of surface wetting capability, hence when the surface energy is lowered, the hydrophobicity is enhanced. In other words, a liquid with a low surface energy is able to wet a material with a high surface energy [
4,
5].
The latest development in material fabrication and synthesis techniques allowed scientists to control and form variable surface energy patterns and it enabled scientists to develop a gradient in the surface energy. The surface energy patterns allow fluid droplets to form and move passively and purely in the desired direction due to the surface energy gradient [
6]. Different surface energy patterning techniques are investigated in the literature that utilize these techniques for surface self-cleaning or to enhance heat transfer and condensation [
7,
8,
9].
Surface energy depends on surface roughness, for simply rough surfaces, Wenzel’s theory can be used cos (
θA) = r · cos(
θ), where
θA is the apparent contact angle,
θ is the contact angle, and r is the ratio of the real rough surface area to the projected perfectly smooth surface. In other words, r is proportional to the extension of surface area due to the roughness. Hence, r is greater than one for a rough surface and is equal to one for a perfectly smooth surface. In practice, this theory applies to a contact angle range of less than 90
o [
10].
PVA is the most readily biodegradable of vinyl polymers [
11,
12]. This type of biodegradable and water-soluble polymer is used in textile processing, frequently for nylon and in fiber manufacture as a raw material for the production of PVA fiber [
13]. Due to its distinguished properties, it has been reported in [
14,
15,
16,
17,
18] that PVA is used as a semiconducting polymer in fabricating storage devices and memory sensors with the use of various nanoparticles as storage elements. PVA alone is non-conductive, however, when mixed with organic plasticizers increasing their ionic conductivity thus making them suitable to be used as semiconducting polymers. When glycerol is added to a rigid polymer, such as PVA, changes the polymeric properties and increases the processability, making it easier to change the molecular conformation of the polymer. Researchers have revealed a decrease in hardness and elastic modulus with an increase in plasticizer concentrations as the polymer matrix becomes more permeable [
19,
20] and thus is susceptible to various applications such as sensors and actuators, batteries and capacitors, and biomedical applications [
21].
In this paper, the blending combinations of polyvinyl alcohol (PVA) with glycerol-choline chloride (GCC) ionic liquid are selected and tested for surface wettability with de-ionized water. The surface energy of PVA-GCC films with different GCC contents is evaluated using the sessile drop technique. The GCC content was varied from 0% to 10% per weight of the PVA solution. The surface roughness of the films has been evaluated using SEM and nanoindenter Stylus.
2. Experimental Analysis
2.1. Material and Preparation Methods
The PVA films doped with glycerol-choline chloride (GCC) ionic liquid are prepared by solution casting method. In this method, a stock solution is prepared by adding 5 g of PVA (Mw ~ 61,000 g/mol) to 100 mL de-ionized water and sonicated for 4 hours to ensure homogenous formation of the PVA solution. The ionic liquid is prepared by mixing 1:1 glycerol to choline chloride (GCC). The 0% film was prepared first by pouring 2 mL of the PVA solution on a 1 in x 1 in glass slide. To prepare the PVA with 1% GCC solution, 0.05 g of GCC was added to 5 mL of the stock solution and kept on the Incu-shaker at 37oC and 250 rpm for 4 hours. The same procedure was followed for preparing the other samples of 2% to 10% of the PVA-GCC mixture while adding the appropriate weight of GCC to the PVA solution. After the 4-hour shaking, 2 mL of each solution is poured on a glass slide and placed in an oven at 55oC for 4 hours to dry. Finally, the films were left outside the oven for testing.
2.2. Contact Angle Measurements
The contact angle of water on PVA and doped PVA film surfaces was measured using the sessile drop method. The de-ionized water was charged into a 10.0 µL syringe. The syringe was attached to a metal stand and suspended vertically by a micromanipulator on top of the glass slide. The micromanipulator was used to adjust the position of the needle tip of the syringe carefully above the clean film sample. The tip of the syringe was positioned a few micrometers from the film to eliminate the impact effect when the droplet was released. The drop volume was taken within the range where the contact angle did not change with the modification of the volume, being 10±1 µL for water.
The film was placed on an optical stand within the focus of a two-inch CCD digital video camera. The contact angle measurements were taken for each drop. Each data point presented an average value of at least 10 measurements. The standard deviation calculated for each data point was found to be less than 7.6°C. All experiments were carried out at ambient conditions, i.e., 25±1°C and 47%±3% RH.
Eleven films were used in this study, which consisted of PVA doped with different GCC concentrations. The films were cleaned by thoroughly rinsing with distilled water. The clean slides are subsequently dried in fresh air just before the start of the experiment. Each cleaned, dried film was used only once. The quality of surface roughness and heterogeneity was inspected using scratch scans on a nanoindenter (Micro Materials Ltd, Wrexham, UK).
2.3. Roughness Measurements
Surface roughness is a measure of the texture of a surface which was measured using a nanoindenter, Micro Materials Ltd, Wrexham, UK, instrument. The nano-scratch tests for the films were performed using the scratch option. During the test, the specimen was moved against a static and loaded diamond indenter, which has a radius of 25 µm. A single pass scratch mode was applied using a constant load of 0.5 mN at a speed of 1 μm/s for a total length of 2000 µm. The scratch was repeated three times for each film and the average of the three scratches was reported in
Table 1. The testing load was applied to the indenter after a 20 μm pre-scan under a small load of 0.1 mN. The roughness is measured in terms of arithmetic average (
), root mean square roughness (
), maximum peak height (R
p), maximum valley depth (R
v), maximum peak-to-valley (R
t), and average peak-to-valley height (R
z).
The statistics were calculated over the scan length of 2000 µm. Where Ra is the arithmetic average, Rq is the root mean square roughness, Rv is the maximum valley depth, Rt is the maximum peak to valley, and Rz is the average peak to valley height.
2.4. Material Surface Evaluation Through SEM Imaging
To verify the surface topology, scanning electron microscopy (Jeol JSM-5600) imaging was utilized. Literature reports [
22,
23,
24] showed that SEM images of hydrophobic leaf surface microstructure have demonstrated the importance of having multiple characteristic length scales to achieve lower contact angle hysteresis. The SEM micrograph was produced for current films at an acceleration voltage of 5 kV.
3. Results and Discussion
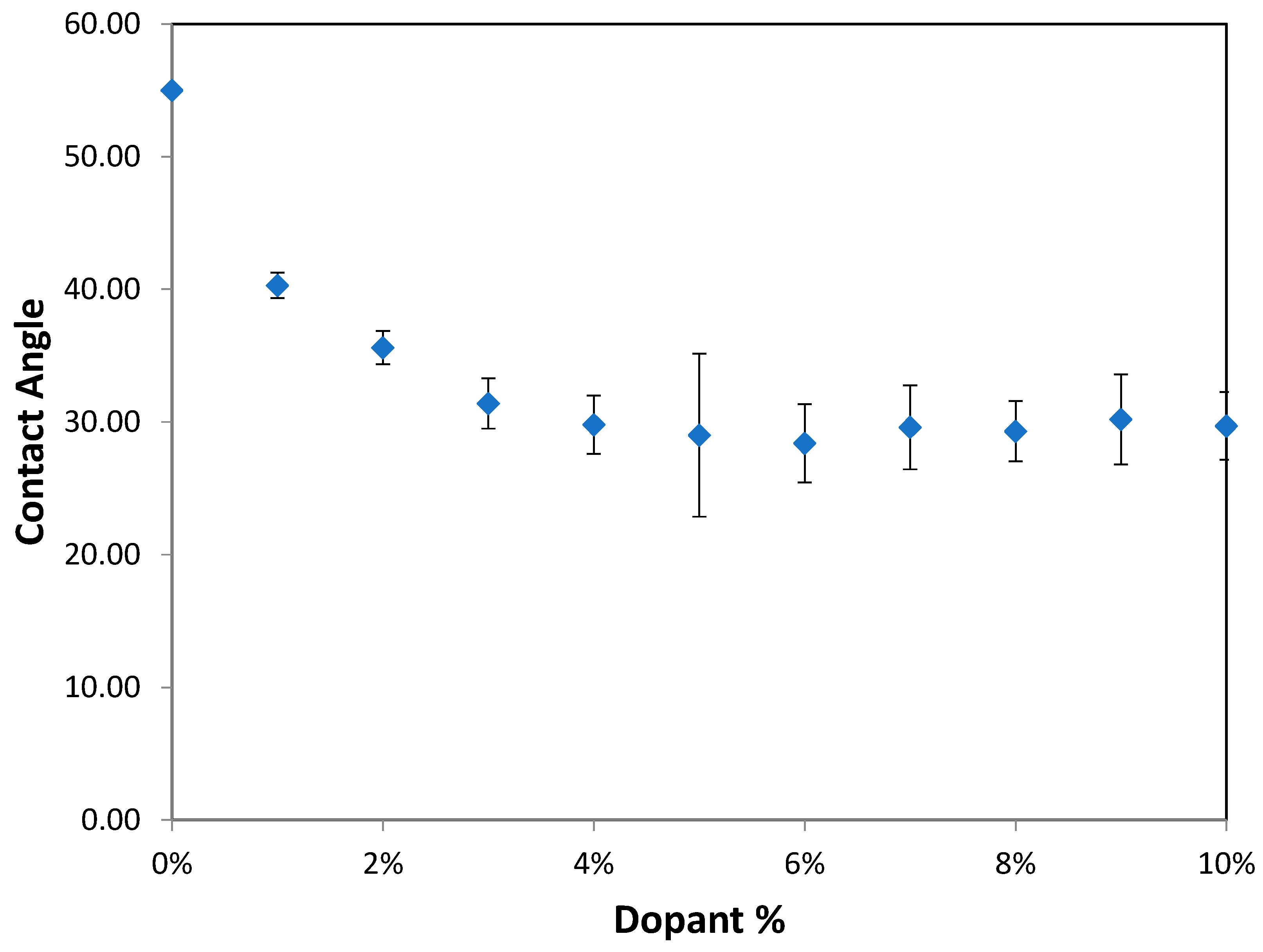
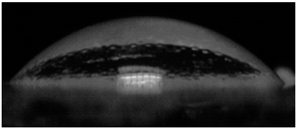
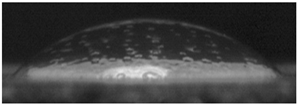
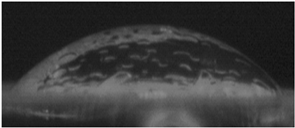
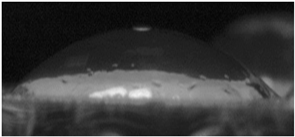
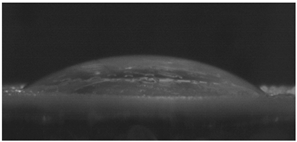
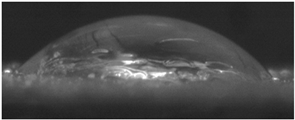
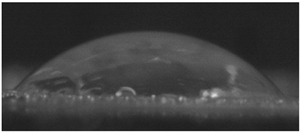
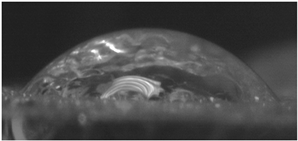
The effects of varying GCC content on altering the surface energy of PVA-GCC films are shown in
Table 2 and the effect on contact angle is shown in
Figure 1.
The surface tensions on the films were evaluated by measuring the contact angle formed by a 10.0 µL droplet placed on PVA-GCC films. Different PVA-GCC films were cast by altering the content of GCC from 0% to 10%. The results show that increasing plasticizer, ionic liquid, (GCC) content in the film enhances wettability, or in other words, it increases the surface energy and hence decreases contact angle. As the GCC content increases, the wettability and the surface energy of the films increase.
The contact angle for PVA without dopant closely matches that reported by Tezuka [
25], who also used the sessile drop method on PVA. He reported a contact angle of 51
o at room temperature.
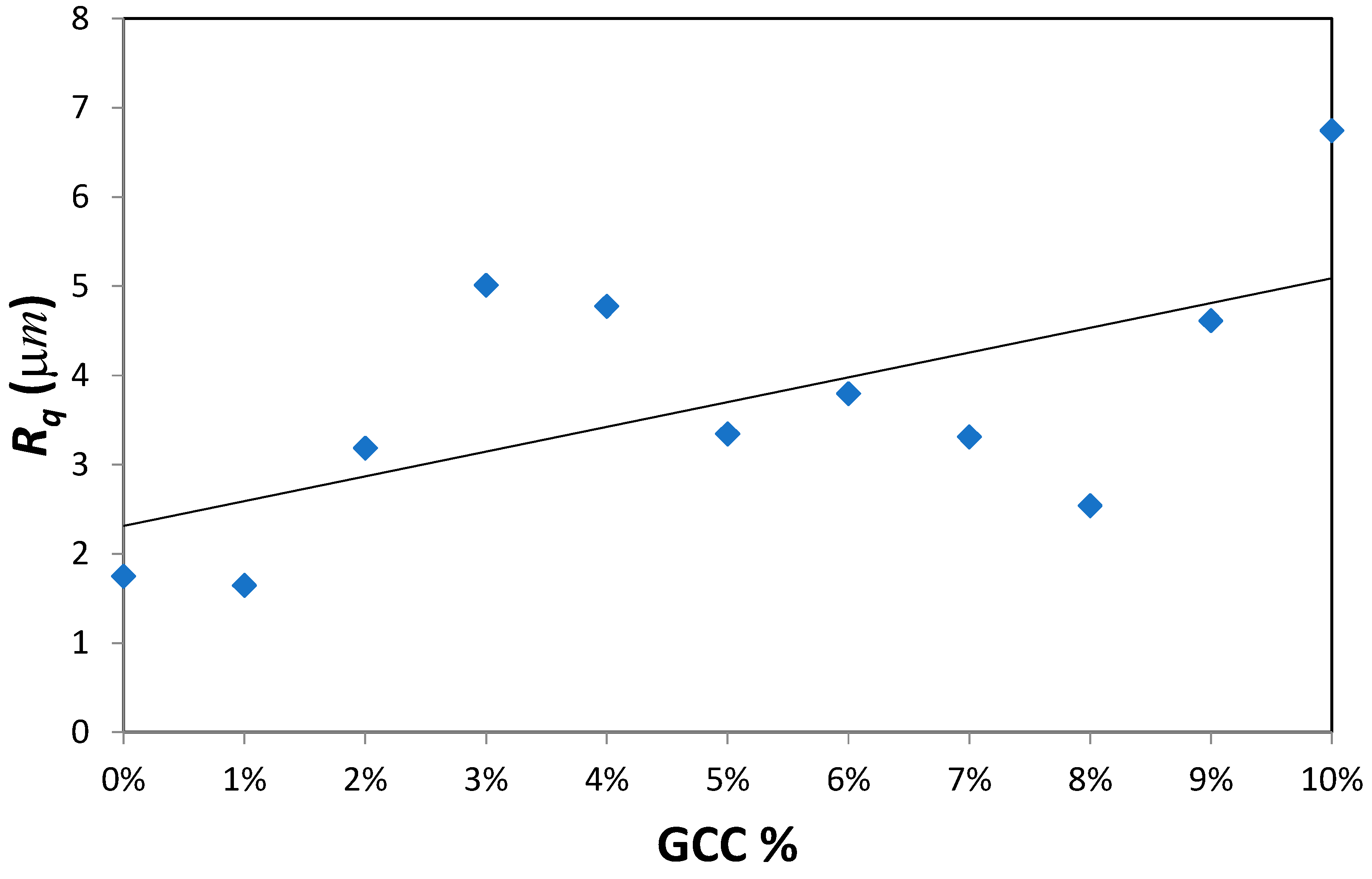
As mentioned earlier, the surface energy depends on many factors and most importantly the surface roughness. Therefore, the effect of GCC on surface roughness was evaluated using a nanoindenter for a length of 2 mm. The results are shown in
Table 1 and
Figure 2.
Figure 2 shows the variation of the root mean square roughness as GCC content in PVA film was altered. A linear curve fitting is plotted for all films excluding the pure PVA film.
The results show that as GCC content increases from 0% to 10%, the surface roughness (R
q) almost tripled from 1.748 µm to 6.746 µm hence the wettability was enhanced. It is reported in the literature that lipophilicity and hydrophobicity are reinforced by roughness [
26,
27]. Hence for contact angles less than 90° such as the one observed in the PVA-GCC films, the apparent contact angle decreases with an increase in roughness. On the contrary, the apparent contact angle tends to increase with increasing roughness for contact angles greater than 90°.
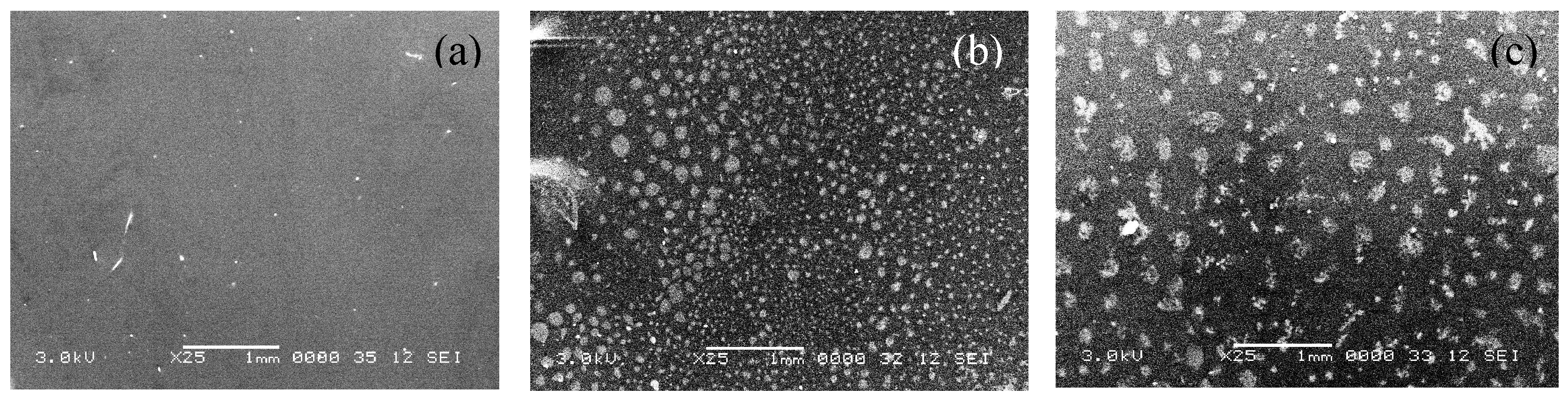
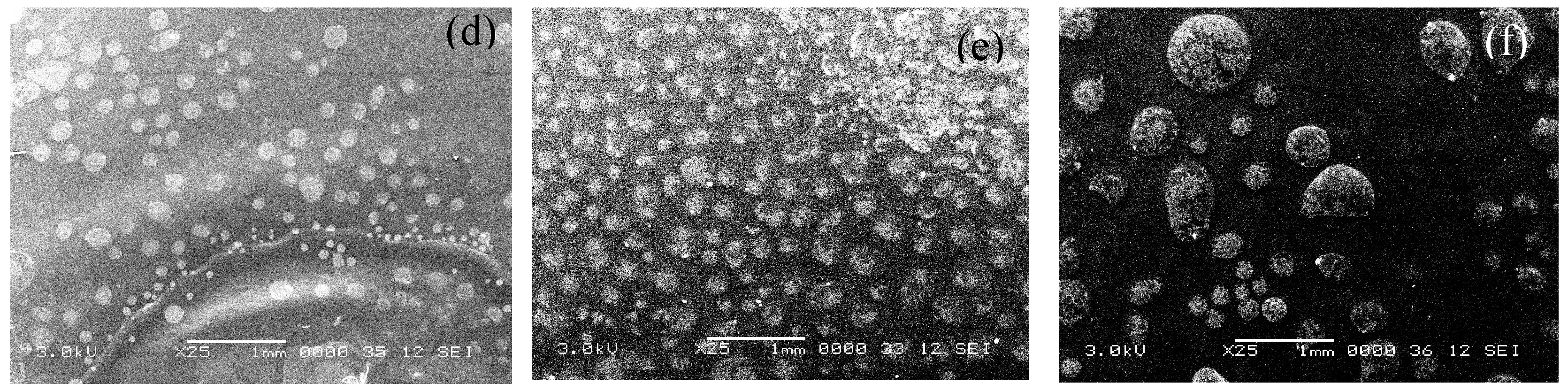
Figure 3 shows the surface topology through SEM images for the 11 films that were evaluated in this study. It is clear that as the content of GCC increases the contrast of the grooves and surface roughness are more pronounced and become darker which indicates a rougher surface. The same pattern surface features were observed in all the samples, however with a different average diameter size as shown in
Table 3.
4. Conclusions
This work shows that increasing GCC content from 0% to 10% in PVA-GCC films increases the surface energy and hence enhances the wettability of the films. The contact angle of 10 µL deionized water droplet placed on the surface of PVA-GCC film decreases as GCC decreases. However, after GCC content reaches 5%, the contact angle does not change anymore. This enhancement is due to a change in the surface roughness which has increased due to the addition of the GCC. All the PVA-GCC films produced in this study are clear and see-through films. It is found that roughness has a strong influence on the wettability of the PVA-GCC films.
Author Contributions
Conceptualization, Yousef Haik and Mohammad O. Hamdan.; methodology, Asma Al Hatti; validation, Asma Al Hatti, Mohammad O. Hamdan and Yousef Haik; formal analysis, Mohammad O. Hamdan and Yousef Haik; investigation, All Authors; resources, Yousef Haik; data curation, Asma Al Hatti; writing—original draft preparation, Mohammad Y. Al-Haik and Mohammad M. Kabir; writing—review and editing, Mohammad Y. Al-Haik, Mohammad M. Kabir and Md Mainul Islam; supervision, Yousef Haik and Mohammad O. Hamdan; project administration, Yousef Haik; funding acquisition, No Funding Available.
Funding
This research received no external funding
Institutional Review Board Statement
“Not applicable” for studies not involving humans or animals.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bonn, Daniel, Jens Eggers, Joseph Indekeu, Jacques Meunier, and Etienne Rolley. “Wetting and spreading.” Reviews of modern physics 81, no. 2 (2009): 739. [CrossRef]
- Tang, Lei, Zhixiang Zeng, Gang Wang, Luli Shen, Lijing Zhu, Yingxin Zhang, and Qunji Xue. “Study of oil dewetting ability of superhydrophilic and underwater superoleophobic surfaces from air to water for high-effective self-cleaning surface designing.” ACS applied materials & interfaces 11, no. 20 (2019): 18865-18875. [CrossRef]
- Zhen, Q. I., L. I. A. O. Liang, Rong-yue Wang, Yan-gang Zhang, and Zhang-fu Yuan. “Roughness-dependent wetting and surface tension of molten lead on alumina.” Transactions of Nonferrous Metals Society of China 31, no. 8 (2021): 2511-2521. [CrossRef]
- Rücker, M. “Measuring three-phase contact angle in porous media from 3D images.” (2022).
- Laurén, Susanna. “Why is surface tension important.” Biolin Scientific. Available source: https://www. biolinscientific. com/blog/why-is-surface-tensionimportant#:~: text= High% 20surface% 20tension% 20of% 20water, can% 20stay 2 (2017).
- Holmes, Hal R., and Karl F. Böhringer. “Transporting droplets through surface anisotropy.” Microsystems & Nanoengineering 1, no. 1 (2015): 1-8. [CrossRef]
- El Fil, Bachir, Girish Kini, and Srinivas Garimella. “A review of dropwise condensation: Theory, modeling, experiments, and applications.” International Journal of Heat and Mass Transfer 160 (2020): 120172. [CrossRef]
- Khandekar, Sameer, and Krishnamurthy Muralidhar. Dropwise condensation on inclined textured surfaces. New York, NY: Springer New York, 2014.
- Zheng, Shao-Fei, Ulrich Gross, and Xiao-Dong Wang. “Dropwise condensation: From fundamentals of wetting, nucleation, and droplet mobility to performance improvement by advanced functional surfaces.” Advances in Colloid and Interface Science 295 (2021): 102503. [CrossRef]
- Lauren, Susanna. “Why is Contact Angle Important?.” Biolin Scientific, Surface Science Blog, https://www. biolinscientific. com/blog/why-is-contact-angle-important (2018).
- Amann, Manfred, and Oliver Minge. “Biodegradability of Poly (vinyl acetate) and Related Polymers.” Synthetic biodegradable polymers (2011): 137-172.
- Halima, Nihed Ben. “Poly (vinyl alcohol): review of its promising applications and insights into biodegradation.” RSC advances 6, no. 46 (2016): 39823-39832. [CrossRef]
- Lin, Chin-An, and Te-Hsing Ku. “Shear and elongational flow properties of thermoplastic polyvinyl alcohol melts with different plasticizer contents and degrees of polymerization.” Journal of Materials Processing Technology 200, no. 1-3 (2008): 331-338. [CrossRef]
- Al-Haik, Mohammad Y., Yousef Haik, and Muhammad R. Hajj. “Characterization of CdS and AgPt nanofillers used in organic capacitors.” Synthetic Metals 223 (2017): 26-33. [CrossRef]
- Al-Haik, Mohammad Y., Mohammad M. Kabir, Ahmad I. Ayesh, Yousef Haik, and Saud H. Aldajah. “Doped conductive polymers and single-walled carbon nanotubes as charge storage devices.” Materials Research Express 5, no. 9 (2018): 095023. [CrossRef]
- Al-Haik, Mohammad Y., Mohamed Y. Zakaria, Muhammad R. Hajj, and Yousef Haik. “Storage of energy harvested from a miniature turbine in a novel organic capacitor.” Journal of Energy Storage 6 (2016): 232-238. [CrossRef]
- Haik, Mohammad Y., Ahmad I. Ayesh, Tahir Abdulrehman, and Yousef Haik. “Novel organic memory devices using Au–Pt–Ag nanoparticles as charge storage elements.” Materials Letters 124 (2014): 67-72. [CrossRef]
- Al-Haik, Mohammad Y., Abdulmohsen A. Alothman, and Muhammad R. Hajj. “Integrated Thermoelectric Energy Generator and Organic Storage Device.” Energy Harvesting and Systems 5, no. 3-4 (2018): 73-79. [CrossRef]
- Mohsin, Mahmood, Asiful Hossin, and Yousef Haik. “Thermomechanical properties of poly (vinyl alcohol) plasticized with varying ratios of sorbitol.” Materials Science and Engineering: A 528, no. 3 (2011): 925-930. [CrossRef]
- Josh, Vinitha, Mohammad Y. Haik, Ahmad I. Ayesh, Mahmoud A. Mohsin, and Yousef Haik. “Electrical properties of sorbitol-doped poly (vinyl alcohol)–poly (acrylamide-co-acrylic acid) polymer membranes.” Journal of applied polymer science 128, no. 6 (2013): 3861-3869. [CrossRef]
- Correia, Daniela Maria, Liliana Correia Fernandes, Pedro Manuel Martins, Clara García-Astrain, Carlos Miguel Costa, Javier Reguera, and Senentxu Lanceros-Méndez. “Ionic liquid–polymer composites: A new platform for multifunctional applications.” Advanced Functional Materials 30, no. 24 (2020): 1909736. [CrossRef]
- Gao, Lichao, and Thomas J. McCarthy. “The “lotus effect” explained: two reasons why two length scales of topography are important.” Langmuir 22, no. 7 (2006): 2966-2967. [CrossRef]
- Ebert, Daniel, and Bharat Bhushan. “Durable Lotus-effect surfaces with hierarchical structure using micro-and nanosized hydrophobic silica particles.” Journal of colloid and interface science 368, no. 1 (2012): 584-591. [CrossRef]
- Feng, Lin, Yanan Zhang, Yingze Cao, Xinxia Ye, and Lei Jiang. “The effect of surface microstructures and surface compositions on the wettabilities of flower petals.” Soft Matter 7, no. 6 (2011): 2977-2980. [CrossRef]
- Tezuka Y, Fukushima A, Matsui S, Imai K “Surface Studies on Poly(vinyl Alcholo)-Poly(dimethylsiloxane) Graft Copolymers), Journal of Colloid and Interfcase Science, 114 (1), 1986. [CrossRef]
- Wang, Li, Yong Zhao, Ye Tian, and Lei Jiang. “A general strategy for the separation of immiscible organic liquids by manipulating the surface tensions of nanofibrous membranes.” Angewandte Chemie 127, no. 49 (2015): 14945-14950. [CrossRef]
- Mei, Yang, Jiahui Zhou, Yutong Hao, Xin Hu, Jiao Lin, Yongxin Huang, Li Li, Changgen Feng, Feng Wu, and Renjie Chen. “High-Lithiophilicity Host with Micro/Nanostructured Active Sites based on Wenzel Wetting Model for Dendrite-Free Lithium Metal Anodes.” Advanced Functional Materials 31, no. 50 (2021): 2106676. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).