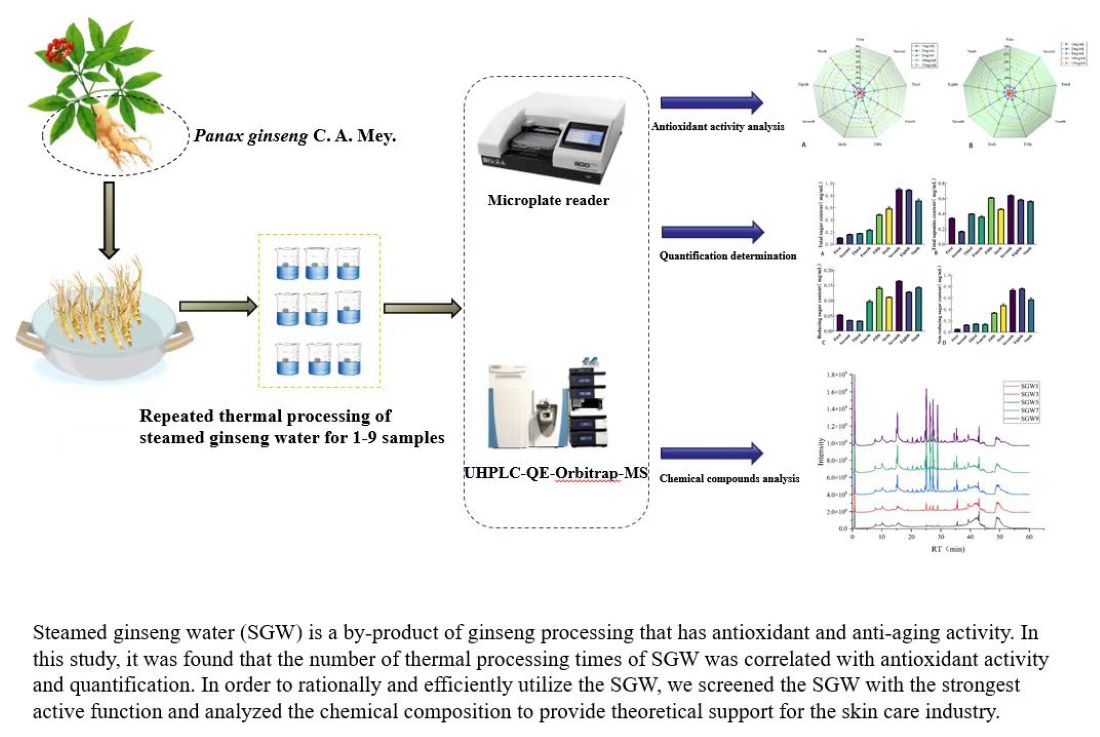
1. Introduction
Panax ginseng C. A. Mey., which belongs to the Araliaceous family, is a perennial herbaceous plant and is one of the most famous Chinese folk medicines [
1]. According to the different processing processes, ginseng products can be classified as sundew ginseng, Dali ginseng, red ginseng, and black ginseng, etc..[
2,
3]. It is reported that there are nearly 13000 tons fresh ginseng are processed into red ginseng in Jilin Province, China recent years, which is the most well-known ginseng producing district and produces 1/3 output of the worldwide [
4,
5,
6]. The SGW is a main liquid byproduct occurring at the red ginseng thermal manufacturing process, with light-yellow color and strong ginseng flavor, accumulated during the evaporating process [
7,
8]. In Chinese folklore, people usually gathering the SGW as natural anti-aging, whitening and anti-wrinkling skincare material, yet it is often discarded as waste material under the most of industrial process state, caused the environment pollution and resource waste.
Excessive exposure to solar UV irradiation and oxidizing free radicals could lead to human skin aging. It’s been a long time for people looking forward natural and effective skincare materials. Ginseng, as the most popular cosmetical botanical materials, was found has better skincare activities after appropriate heat treatment[
9,
10]. During the evaporating process, hydrolysis, isomerization, and decarboxylation degradation reactions could occur simultaneously or solely, for instance, the major ginsenosides which is the most functional ingredients in ginseng could be converted the into rare ginsenosides , the activities are thus getting better [
11]. Besides, many sesquiterpenes, sesquiterpene polyacetylene and the products of the Maillard reaction are found in steamed ginseng water extracts, other types of glycosides, like phenolics, lignans, amino acids, polysaccharides, aliphatic, aromatic compounds, glycosides, phenolics, lignans, amino acids, and polysaccharides were also found in the water extract [
12]. Up to now, it’s still vague while people stressed to make effective use SGW resource, while there were few documents reported on repeated thermal process on variation trend of composition and antioxidant activities of SGW.
Although red ginseng has high medicinal value. It is necessary to screen out an alternative to red ginseng that is high yielding and low cost. As the heat treatment time was extended, the chemical composition and activity of SGW changed dynamically. In the current study, we investigated the variation trend of the contents from 1-9 times repeated thermal process of the SGW through the observation on the total sugar, total saponins and reducing sugar contents. Using by the UHPLC-Q-Exactive-MS liquid mass spectrometry, the chemical constituents of SGW were identified combing with principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA) methodologies. A total of eight ginsenosides shared Q-markers were identified by comparing the four model groups of SGW (1- and 3-, 1- and 5-, 1- and 7-, and 1- and 9). Thermal processing has effects not only on SGW chemical composition changes but also on antioxidant activities. To evaluate the variation trends on antioxidant activities of 1-9 times SGW, the DPPH, ABTS+, Fe3+ reducing capacity, and hydroxyl radicals were carried to be monitored. Our findings showed the scientific way to effective usage of by-products of red ginseng by repeated thermal processing and a promising way to provide a theoretical basis for the potential on cosmetic materials.
2. Results
2.1. Plotting of Standard Curves
For calculating the Fe+ reduction ability, the regression equation was Y=3.762x+0.1627(r=0.9994).
For calculating the total sugar content, the regression equation was Y=3.456x+0.128(r=0.9958).
For calculating the reducing sugar content, the regression equation was Y=1.1634 x-0.0404(r=0.9936).
For calculating the total saponins content, the regression equation was Y=0.5801x+ 0.0196(r=0.9995).
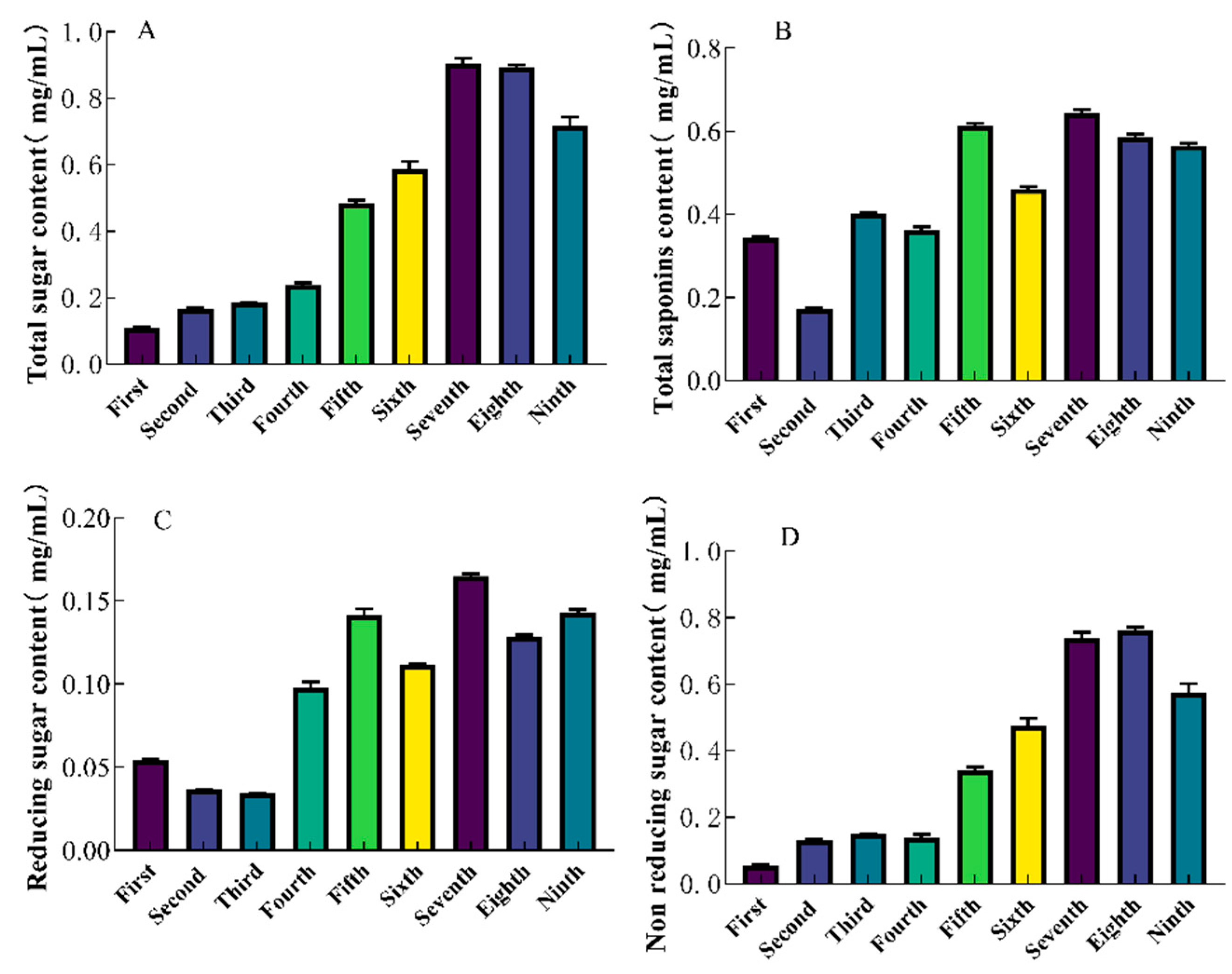
2.2. Quantitative Determination of SGW
The total sugar content of SGW was determined by phenol sulfate method. With increasing steaming times, the total sugar content increased, with the 7th SGW having the highest total sugar content of 0.90 mg/mL. (
Figure 1A). The ginsenoside content showed fluctuating changes during repeated thermal processing, in which the 7th SGW had the highest total saponins content of 0.64 mg/mL. (
Figure 1B). The 1st-9th time SGW was selected for the determination of reducing and non-reducing sugar content. Among them, the 7th SGW had the highest reducing and non-reducing sugar contents of 0.16 mg/mL and 0.74 mg/mL. (
Figure 1C,D).
2.3. Results of Chemical Composition
2.3.1. Multivariate Statistical Analysis of Chemical Composition of Repeated Thermal Processed on SGW.
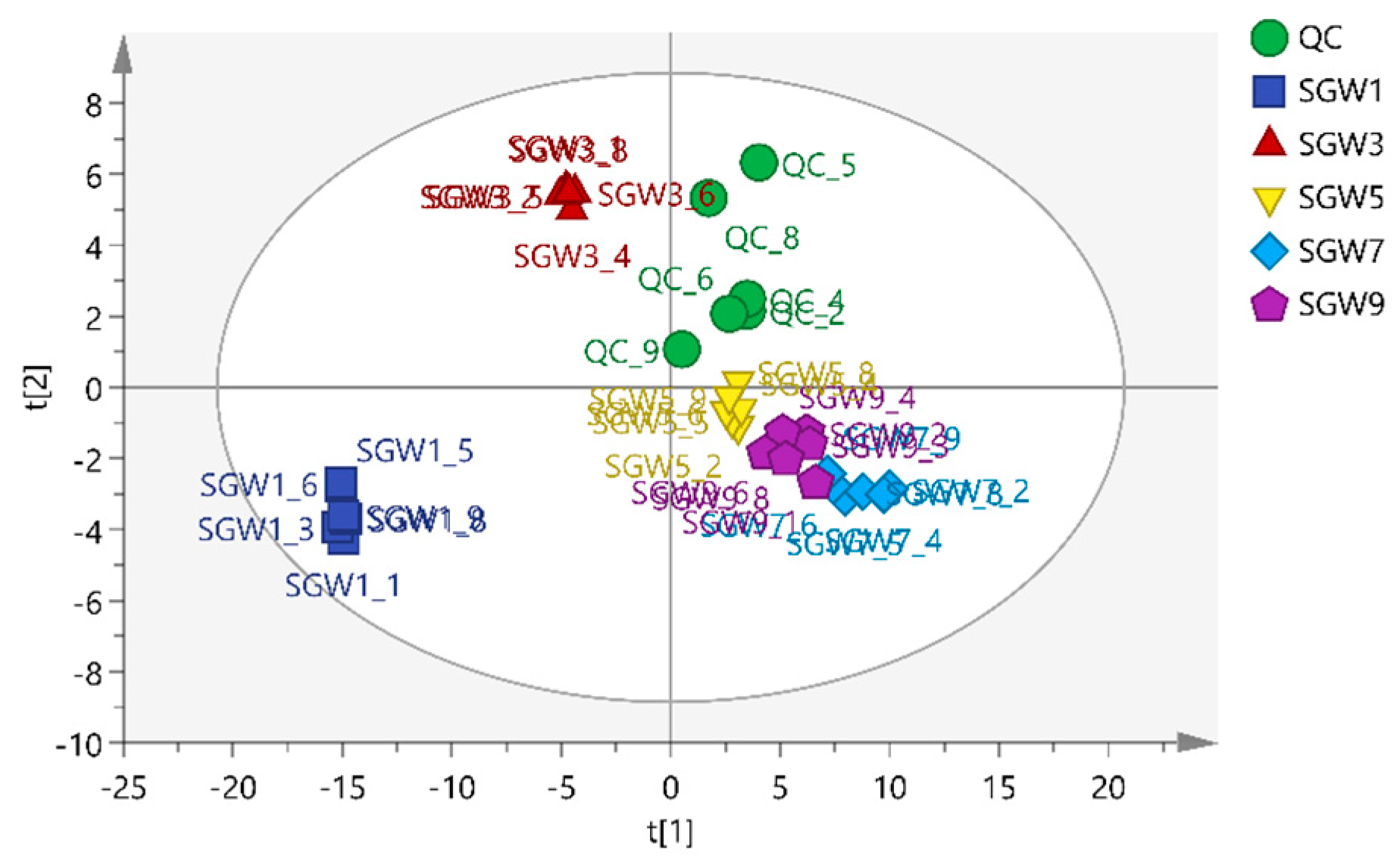
PCA is a different model based on the projection to provide a path for the data observation in the most informatics viewpoint of the data [
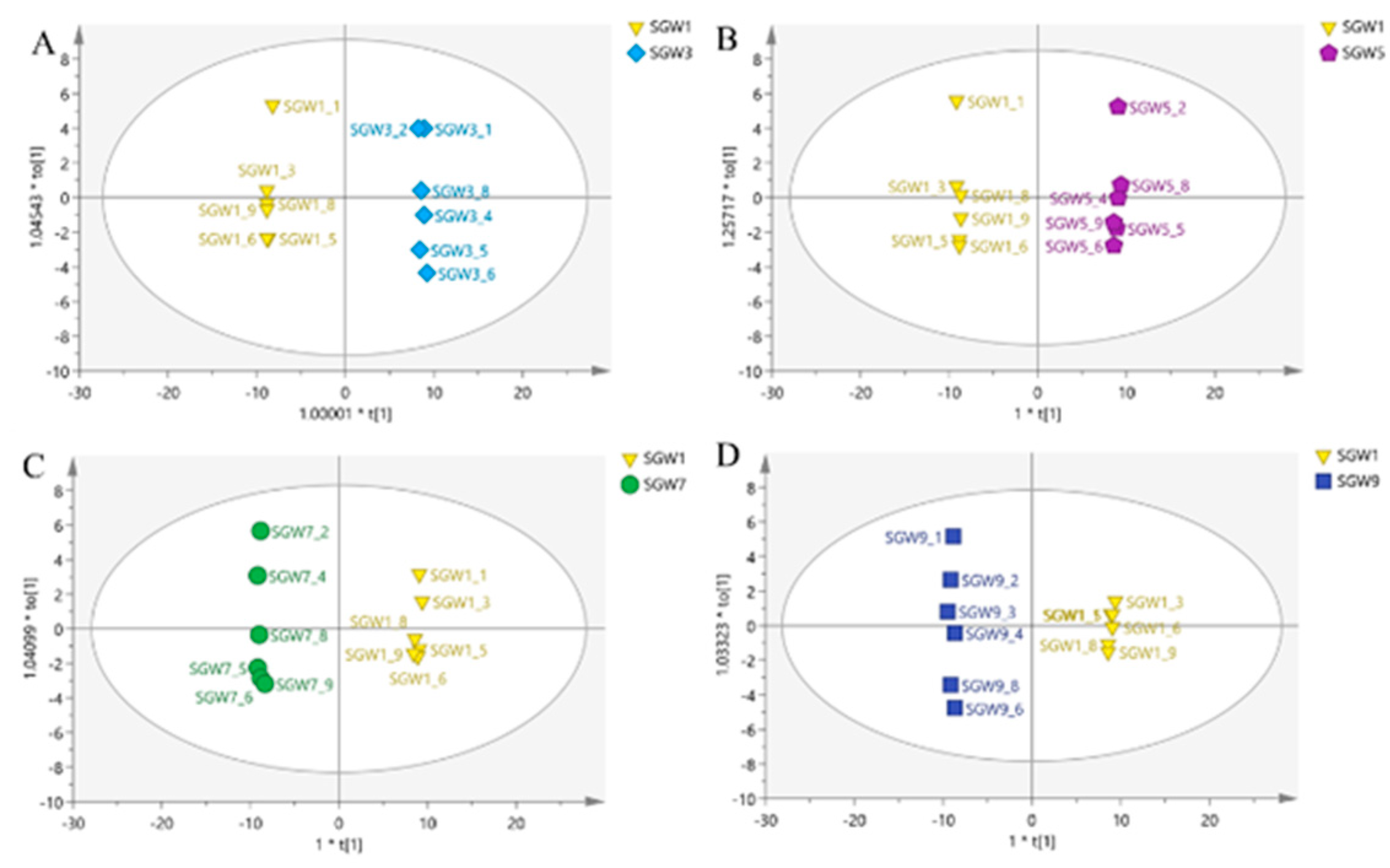
13]. PCA analysis was performed on 5 groups of samples and QC samples of repeated thermal processed on SGW. PCA was used to intuitively refine group differences among thermal heated treatment with 1st, 3rd,5th,7th,9th times SGW.As shown in
Figure 2, it can be seen that the QC samples were closely clustered, indicating good fitness and prediction of the established PCA model
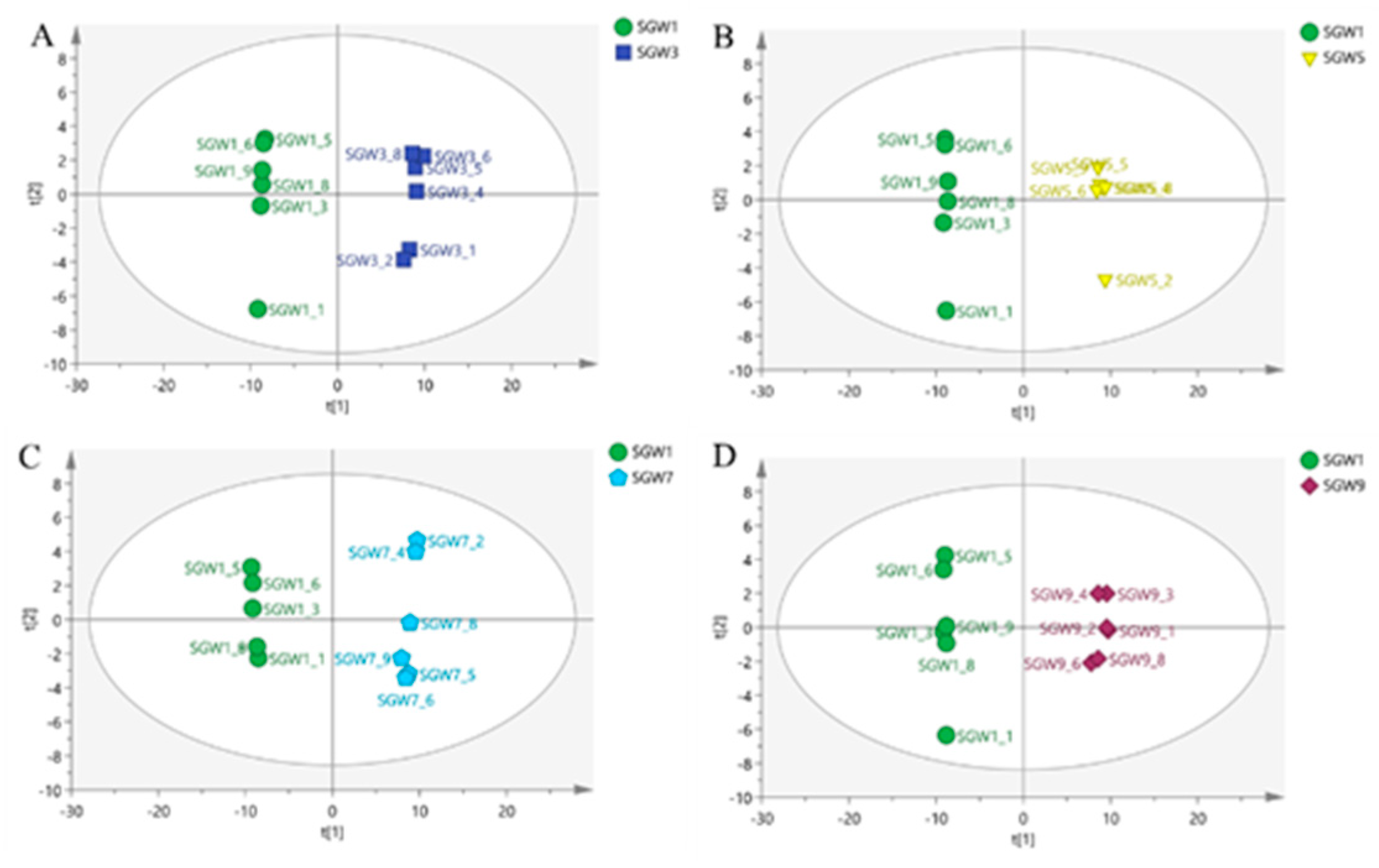
A clear separation of the two classes was observed in
Figure 3, the two classes located in the end of the t[1] and t[2] direction. The PCA score plot was obtained by comparing the 1st SGW sample as a control with the 3rd, 5th, 7th and 9th SGW samples in turn in two-by-two comparisons, which it can be seen that the 1st SGW sample can be differentiated from the 3rd, 5th, 7th and 9th SGW samples.
2.3.2. Discovery and Identification of Biomarkers for Chemical Composition of Repeated Thermal Processed on SGW
The OPLS-DA model was utilized to generate the score plots from which it can be seen that the model has good predictive ability (
Figure 4). Additional validation was then performed using a permutation test (300 times) to assess the validity of the OPLS-DA model.All R
2 (cum) and Q
2 (cum) values calculated from the data for each mutation were lower than the original values of the model developed and the inner cross section of the regression line for Q
2 on the y-axis was negative, indicating that the model developed was not overfitted and had good fitting and predictive ability. Based on the importance values of the projected variables (VIP) in the OPLS-DA model, combined with the t-test results, potential candidate biomarkers in the 1st, 3rd, 5th, 7th and 9th evaporated ginseng water were screened out with VIP >1 and
P<0.05. The results showed that 330 differential compounds were screened in the 1st vs. 3rd ratio; 250 differential compounds were screened in the 1st vs.5th ratio; 289 differential compounds were screened in the 1st vs. 7th ratio; 250 differential compounds were screened in by the 1st vs. 9th ratio; and another 37 differential compounds were screened with the condition of FC >2 and
P<0.01. The total ion chromatograms (TIC) of SGW samples after different repeated thermal processes by UHPLC-Q-Exactive-MS (
Figure 5).
2.4. Radical Scavenging Activity
2.4.1. Evaluation of DPPH Antioxidant Activities of SGW
The free radical scavenging capacity was evaluated according to the IC
50 value and scavenging percentage (IC
50 value is defined as the concentration of the antioxidant needed to scavenge 50% of free radicals present in the test solution). The 7th SGW IC
50 was 8.012 mg/mL, the smaller the IC
50 value, the higher the antioxidant capacity[
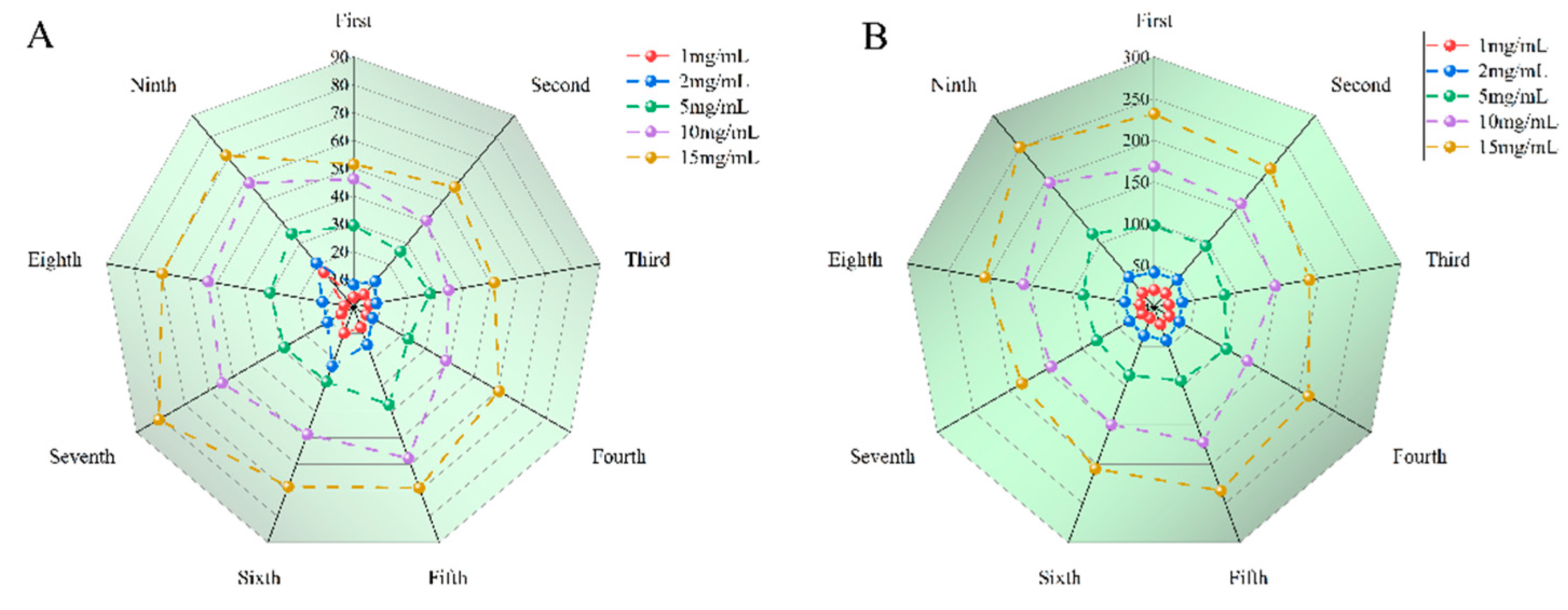
14]. The antioxidant capacity of SGW samples with 1-9 times of cumulative steaming was determined, respectively. SGW with different numbers of repeated thermal processing had significant scavenging capacity for DPPH radicals, and the higher the sample concentration, the stronger the scavenging capacity. In
Figure 6A, the 7th SGW exhibits the greatest antioxidant activity.
Table 1.
Identification of shared differential ginsenoside compounds by UHPLC-Q-Exactive-MS/MS analysis.
Table 1.
Identification of shared differential ginsenoside compounds by UHPLC-Q-Exactive-MS/MS analysis.
| Pattern |
No. |
Extract m/z |
t/min |
Metabolites |
Formula |
Monoisotopic Mass |
Adduct |
Mass Error (mDa) |
1vs.3 |
1vs.5 |
1vs.7 |
1vs.9 |
| ESI-
|
1 |
435.0563 |
0.82 |
3,4-dihydroxyphenylpyruvate |
C15H20O10
|
195.0299 |
2M+FA-H |
4 |
-1.59 |
-2.21 |
-2.49 |
-2.42 |
| 2 |
777.1628 |
1.16 |
3-Methylbutyl glucosinolate |
C6H12N3PS |
389.0814 |
2M-H |
9 |
-2.36 |
-3.26 |
-3.54 |
-3.32 |
| 3 |
779.1603 |
1.16 |
Dimethyl 2-galloylgalactarate |
C30H40O6
|
390.0798 |
2M-H |
10 |
-2.30 |
-3.20 |
-3.47 |
-3.28 |
| 4 |
719.2043 |
1.17 |
3-Methoxy-4-hydroxyphenylglycol glucuronide |
C54H92O23
|
360.1056 |
2M-H |
0 |
-2.06 |
-3.02 |
-3.30 |
-2.96 |
| 5 |
539.14 |
1.24 |
Phenylglucuronide |
C29H40N8O5
|
270.0740 |
2M-H |
1 |
-1.28 |
-1.97 |
-2.21 |
-1.99 |
| 6 |
767.5313 |
1.26 |
Persicachrome |
C58H98O26
|
384.2664 |
2M-H |
7 |
0.26 |
0.83 |
1.58 |
1.49 |
| 7 |
387.1176 |
5.84 |
Ferulic acid |
C54H92O24
|
194.0579 |
2M-H |
23 |
4.14 |
1.60 |
3.44 |
3.61 |
| 8 |
455.1047 |
5.84 |
Epicatechin 3-O-(4-methylgallate) |
C53H90O22
|
456.1056 |
M-H |
14 |
8.87 |
1.94 |
7.44 |
7.80 |
| 9 |
767.5309 |
5.88 |
Persicaxanthin |
C48H82O18
|
384.2664 |
2M-H |
7 |
1.34 |
1.32 |
2.11 |
1.69 |
| 10 |
377.0881 |
5.94 |
3,3',5-Trihydroxy-4',7-dimethoxyflavanone |
C42H66O14
|
332.0896 |
M+FA-H |
1 |
7.14 |
1.30 |
6.45 |
6.64 |
| 11 |
991.5515 |
15.38 |
Ginsenoside Re |
C10H10O4
|
946.5501 |
M+FA-H |
3 |
-2.28 |
-3.39 |
-3.99 |
-3.67 |
| 12 |
815.4829 |
21.62 |
Majonoside R1 |
C12H14O7
|
816.4871 |
M-H |
4 |
-2.01 |
-3.27 |
-4.01 |
-3.62 |
| 13 |
1107.596 |
24.96 |
Ginsenoside Rb1 |
C12H23NO9S2
|
1108.6029 |
M-H |
0 |
-2.70 |
-4.30 |
-4.96 |
-4.70 |
| 14 |
955.4933 |
26.04 |
Ginsenoside Ro |
C29H46O3
|
956.4981 |
M-H |
3 |
-1.87 |
-2.80 |
-3.01 |
-1.89 |
| 15 |
561.293 |
26.23 |
Hordatine B |
C15H18O12
|
580.3122 |
M-H2O-H |
1 |
-2.87 |
-4.20 |
-4.77 |
-4.51 |
| 16 |
1209.629 |
26.43 |
Ginsenoside Ra1 |
C25H36O3
|
1210.6346 |
M-H |
1 |
-2.73 |
-4.30 |
-4.85 |
-4.41 |
| 17 |
1123.594 |
27.05 |
Ginsenoside Rb2 |
C23H20O10
|
1078.5924 |
M+FA-H |
3 |
-2.32 |
-3.63 |
-4.23 |
-3.97 |
| 18 |
1077.584 |
27.24 |
Ginsenoside Rc |
C25H36O3
|
1078.5924 |
M+FA-H |
3 |
-2.50 |
-4.02 |
-4.75 |
-4.42 |
| |
19 |
945.5425 |
28.76 |
Ginsenoside Rd |
C12H22O11
|
946.5501 |
M-H |
0 |
-2.37 |
-3.98 |
-4.76 |
-4.37 |
| 20 |
793.4408 |
34.44 |
Spinasaponin A |
C42H66O14
|
794.4453 |
M-H |
4 |
-2.35 |
-3.45 |
-4.38 |
-4.34 |
| 21 |
333.2318 |
42.87 |
(S)-10,16-Dihydroxyhexadecanoic acid |
C16H32O4
|
288.2301 |
M+FA-H |
11 |
-0.62 |
3.28 |
0.17 |
0.95 |
| 22 |
369.2081 |
42.87 |
Ecgonine |
C9H15NO3
|
185.1052 |
2M-H |
14 |
-0.46 |
2.85 |
0.13 |
0.74 |
| 23 |
423.33 |
44.04 |
Camellenodiol |
C29H46O3
|
442.3447 |
M-H2O-H |
9 |
-1.05 |
2.79 |
-0.02 |
1.48 |
| ESI+
|
24 |
462.3461 |
1.39 |
Galactosylsphingosine |
C24H47NO7
|
461.3353 |
M+H |
8 |
0.42 |
0.89 |
1.14 |
1.13 |
| 25 |
367.2169 |
1.44 |
Demethoxyfumitremorgin C |
C21H23N3O2
|
349.1790 |
M+NH4
|
11 |
0.00 |
0.24 |
0.69 |
0.43 |
| 26 |
300.2 |
1.92 |
Miltirone |
C19H22O2
|
282.1620 |
M+NH4
|
14 |
-0.55 |
2.29 |
0.65 |
0.23 |
| 27 |
328.2313 |
1.97 |
Menaquinol |
C21H26O2
|
310.1933 |
M+NH4
|
13 |
-0.35 |
2.68 |
1.02 |
0.63 |
| 28 |
344.2258 |
2.12 |
Isopiperolein B |
C19H30O5
|
343.2147 |
M+H |
11 |
-0.46 |
2.65 |
0.69 |
0.24 |
| 29 |
327.2 |
2.18 |
Heptaethylene glycol |
C14H30O8
|
326.1941 |
M+H |
6 |
-0.50 |
2.60 |
0.67 |
0.20 |
| 30 |
388.2518 |
2.44 |
Octaethylene glycol |
C16H34O9
|
370.2203 |
M+NH4
|
6 |
-0.43 |
2.92 |
0.77 |
0.30 |
| 31 |
460.3084 |
2.85 |
Muzanzagenin |
C27H38O5
|
442.2719 |
M+NH4
|
4 |
-0.41 |
3.55 |
0.85 |
0.29 |
| 32 |
476.3039 |
3.34 |
Lucidenic acid A |
C27H38O6
|
458.2668 |
M+NH4
|
5 |
-0.46 |
3.14 |
0.83 |
0.32 |
| 33 |
605.3827 |
3.92 |
Ginsenoyne H |
C19H26O3
|
302.1882 |
2M+H |
2 |
1.06 |
-0.45 |
2.17 |
1.04 |
| 34 |
567.4277 |
3.92 |
Cryptocapsone |
C40H54O2
|
566.4124 |
M+H |
14 |
0.92 |
-0.18 |
1.35 |
0.96 |
| 35 |
679.5078 |
3.92 |
Avocadene 1-acetate |
C19H36O4
|
328.2614 |
2M+Na |
6 |
-0.17 |
0.13 |
-0.11 |
-0.03 |
| 36 |
358.2571 |
43.05 |
2-alpha-Ethoxydihydrophytuberin |
C19H32O5
|
340.2250 |
M+NH4
|
5 |
-0.13 |
-0.01 |
-0.21 |
0.09 |
| 37 |
359.2593 |
43.16 |
Isolinderanolide |
C21H36O3
|
336.2664 |
M+Na |
10 |
0.07 |
0.09 |
0.02 |
0.09 |
2.4.2. Evaluation of FRAP Antioxidant Activities of SGW
SGW with different numbers of repeated thermal processing had obvious reducing ability for Fe
3+ ions, and the higher the concentration of the samples, the stronger the reducing ability (
Figure 6B).
2.4.3. Evaluation of ABTS+ Antioxidant Activities of SGW
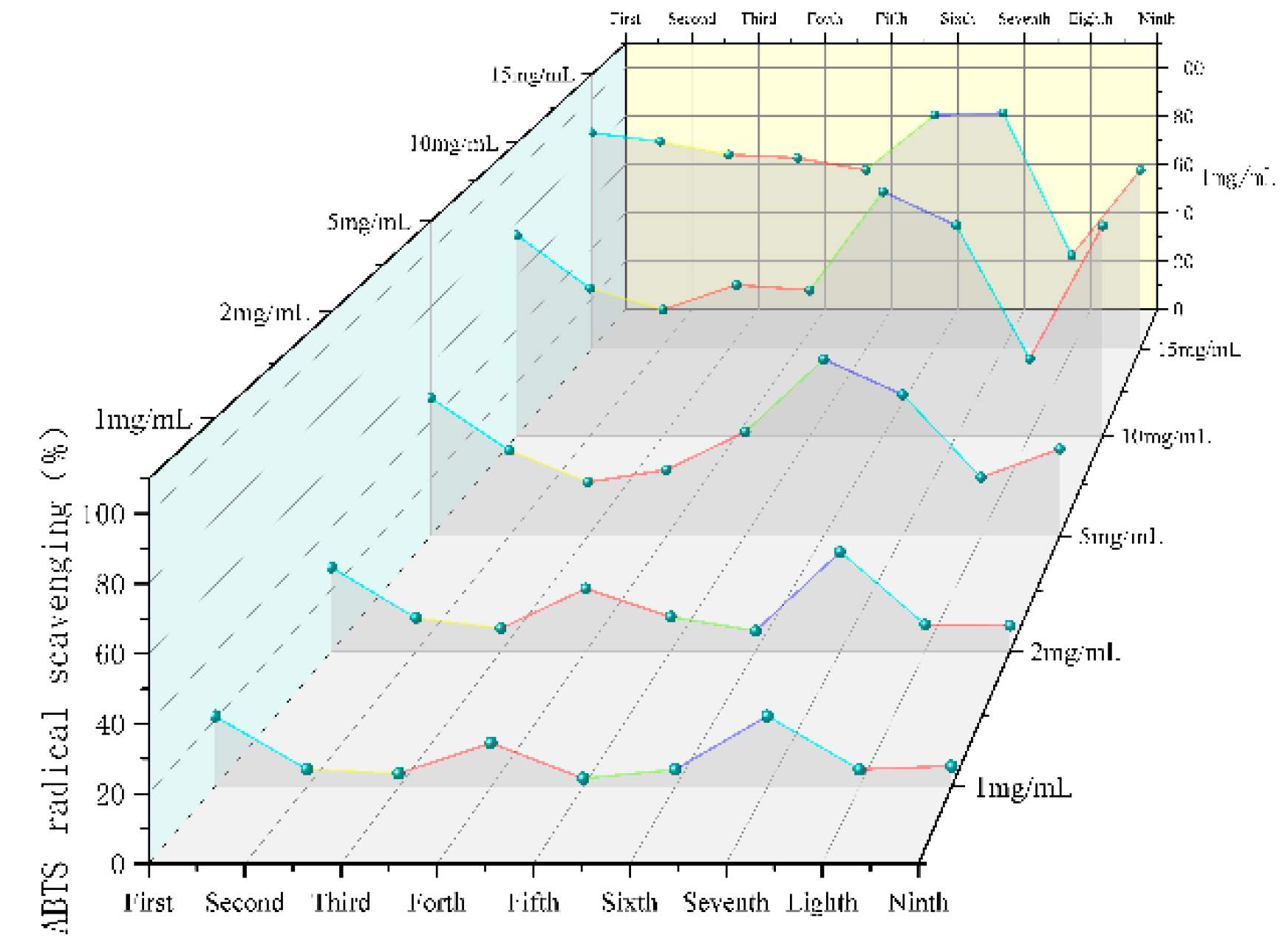
SGW with different numbers of repeated thermal processing had significant scavenging ability for ABTS
+ radicals, and the higher the concentration of the samples, the stronger the scavenging ability (
Figure 7). Overall the 7th collection of SGW had a higher scavenging rate.
2.4.4. Evaluation of Hydroxyl Radical Scavenging Ability of SGW
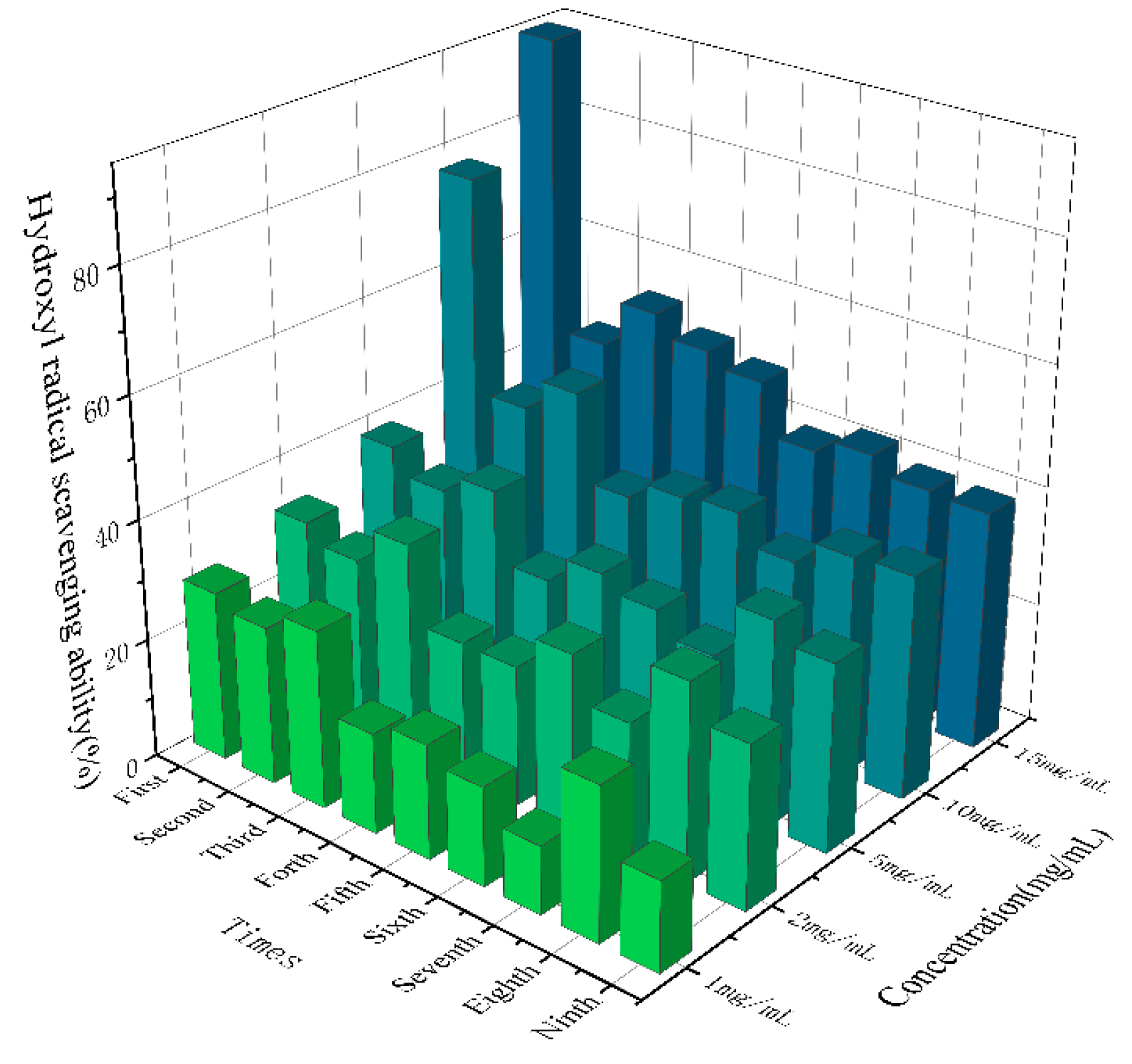
As shown in
Figure 8, SGW with different numbers of repeated thermal processing had significant scavenging ability for hydroxyl radicals, and the higher the sample concentration, the stronger the scavenging ability. Overall, the 1st SGW had the greatest scavenging ability for hydroxyl radicals.
2.4.5. Correlation analysis of antioxidant activity with total saponins and total sugar
According to the above results combined with
Figure 9. It can be found that the hydroxyl radical scavenging ability of SGW was highly linearly correlated with the total sugar content of steamed ginseng water (0.8<|r|≤1); the scavenging ability of DPPH/ABTS
+/FRAP and the correlation co-efficient of total saponins (0.8<|r|≤1) were highly linearly correlated. Based on the sugar and saponins content of SGW, it was cleared to conclude that the antioxidant capacity might be affected by thermal processed times.
3. Discussion
The quantitative determination, chemical composition and antioxidant activity of SGW in repeated heat treatment were studied in this paper. The effects of different heating times on SGW activity were compared. SGW selected for optimal heating times was used as raw material for cosmetics development and industrial preparation. After 1-9 heat treatments, the contents of total sugar, total saponins, reducing sugar and non-reducing sugar were found to be the highest at the 7th heat treatment, and their contents were closely related to the way, amount, temperature and time of heat treatment [
15,
16,
17]. Thermal instability degraded ginsenoside, which was responsible for the reduction of ginsenoside content in the 2nd heat treatment. Not only the polar ginsenosides were not completely degraded, but also a few ginsenosides were degraded on the basis of the 2nd heat treatment to produce secondary ginsenosides, which was the 3rd heating treatment with an increase in ginsenoside content (
Figure 3) [
18]. The ginsenoside content was the highest in the 7th, and gradually decreased in the 8th and 9th times, which was caused by excessive processing. At the initial stage of ginseng heating, the change in temperature caused malonyl-ginsenoside to be hydrolyzed due to the instability of the malonyl-ginsenoside side chain molecules. Malonyl-ginsenoside was hydrolyzed by acid, alkali or high temperature in which process it was converted to neutral saponins [
19]. Furthermore, the polar ginsenosides Rg1/Re, Rc, Rb2, and Rd were gradually reduced, and the rare ginsenosides Rg2, Rg3, F4, Rg5, Rk1, Rh2, Rh1, and Rh4 were progressively generated, which led to the enhancement of antioxidant activity during heat treatment. [
20,
21,
22,
23].
The reducing sugars mainly contained glucose, fructose, galactose, maltose and so on. The change of reducing sugar content was consistent with the trend of ginsenoside content. After frequent heat treatment, ginsenosides were hydrolyzed to glycosides and sugars, and the SGW reducing sugar content varied with the ginsenoside content [
24]. The non-reducing sugars mainly contained starch, sucrose, arabinogalactan and so on. SGW was repeatedly heated, it was constantly reduced and concentrated, resulting in the highest concentration of non-reducing sugars when it was heated to the 7th time (
Figure 1C,D) [
25]. Thermal processing can cause a decrease in water-soluble polysaccharides and an increase in acidic polysaccharides [
26]. Starch is the main component of ginseng polysaccharides, which are extracted from ginseng roots. In the process of repeated heating and steaming ginseng starch was dextrinated to form monosaccharides and oligosaccharides. After the 7th steaming, the viscosity of ginseng starch gradually increased[
27]. Antioxidant activity and SGW quantitative assay were correlated. The antioxidant activity of the 7th SGW samples was better, the ability to scavenge hydroxyl radicals was highly linearly correlated with the total sugar content, and the total saponin content was linearly correlated with the scavenging ability of DPPH, ABTS
+ and FRAP.
According to related studies, the C-20 sugar in the side chain of ginsenosides is hydrolyzed first, followed by the C-6 or C-3 sugar. In addition, C-20 stereo isomerization a significant structural change [
28,
29]. SGW contained 38 active ingredients including ginsenosides Re, Rb1, Rb2, Rc, Rd and Ro, which were determined by UHPLC-Q-Exactive-MS liquid mass spectrometry. By multivariate statistical analysis of SGW 1st vs. 3rd, 5th, 7th, and 9th, respectively. PCA and OPLS-DA were well separated. Ginseng was heat-treated to produce maltol, salicylic acid, vanillic acid, and p-coumaric acid, which scavenge free radicals and improve antioxidant activity [
30,
31,
32]. In addition, our group also compared the 7th measured SGW with steamed red ginseng, and found that the total saponin content could be up to 63.4% of that of red ginseng. In the future, the comprehensive utilization of SGW could be used as a potential raw material for cosmetic and food development, which could reduce the waste of ginseng resources on one hand, and improve the economic benefits on the other hand.
4. Materials and Methods
4.1. Materials
Fresh roots of 5-year-old ginseng was purchased from Wanliang Town, Fusong County, Jilin Province, and identified by Professor Weng Lili from Changchun University of Traditional Chinese Medicine. UPLC-grade acetonitrile and methanol were obtained from Tedia Company Incorporated (Fairfield, OH, USA). Purified water was made by a water purifier (Global Water Solution Ltd., Randolph, MA, USA).
4.2. Extraction and Preparation of Steamed Ginseng Extract
Fresh ginseng was washed and neatly arranged with the reeds facing down. The ginseng was steamed with 2000 mL of distilled water at 100°C for 2 hours. At the end of steaming, the water was replenished to 2000 mL. Meanwhile, the process was repeated 9 times by replacing fresh ginseng of the same size and quality. The 9 groups of SGW treated by repeated steaming were concentrated to 200 mL under a vacuum pressure of minus 0.08 Mpa at 55℃. Then the samples were centrifuged at 1000 x g for 5 min and then the supernatant was freeze-dried for spare.
4.3. Sample Preparation for Mass Spectrometry
5 mg SGW lyophilized powder was accurately weighted and dissolved in 50 mL methanol to make a concentration of 0.1 mg/mL, 10 µL sample solution was added into 0.99 mL methanol in 2.5 mL eppendorf tube, vibrated for 30 seconds, then filtered by 0.22 μM PTFE membrane and refrigerating at 4 °C for spare.
4.4. The Quantitative Analysis of SGW
4.4.1. Total Sugar Content Assay
Accurately weigh glucose and dry it in a constant-weight oven at 105 °C. Prepared a glucose solution of 0.1 mg/mL. 2 mL glucose diluent of different concentrations, 1 mL 5% phenol solution and 5 mL sulfuric acid concentrated solution were added [
33]. The water bath was heated at 40℃ for 30 minutes and cooled to room temperature. Absorbance was measured at 490 nm and a standard curve was drawn. The glucose content of SGW samples of different times was calculated by regression equation.
4.4.2. Total Saponins Content Assay
The activated D101 macroporous adsorption resin column was prepared and activated again with ethanol and distilled water. First, different repeated heat treatment SGW 1mL and 25mL distilled water washing column were precisely added twice, and the eluent was discarded. Then, the ginsenosides were slowly washed off with 25 mL 70% ethanol, and the eluent was collected in the evaporating dish. Finally, the water bath evaporates and dries at 60°C.
Prepare ginsenoside Re standard diluted to 1 mg/mL. First, it was placed into a solution of 0 mg/mL, 0.02 mg/mL, 0.04 mg/mL, 0.06 mg/mL, 0.08 mg/mL and 1 mg/mL in a tube with plugs. Then, 0.2mL of vanillin and 0.8mL of perchloric acid were added to the solution. Finally, the water bath was heated at 60℃ for 10min. After cooling, add glacial acetic acid constant volume to 5.0 mL. The ginsenoside content of SGW samples was calculated by substituting the absorbance value into the standard curve.
4.4.3. Total Reducing Sugar Content Assay
Reducing sugar content in SGW was determined by using reducing sugar kit and spectrophotometry. Different times SGW samples of 100μL and 100μL reagent 1 (colorimetric solution) were thoroughly mixed in a centrifuge tube. 95°C water bath heated for 10 minutes before cooling. The control group was replaced with distilled water and the absorbance was measured at 500nm.
4.5. UHPLC-Q-Exactive-MS/MS Conditions
Chromatographic conditions: Separation was performed using an Ascentis® Express C18 (3.0 mm × 500 mm, 2.7 μm). The mobile phase consisted of 0.1% formic acid (solvent A) and acetonitrile (solvent B). The gradient elution program was as follows: 0–3 min 25% B, 3–20 min 25%–32% B, 20–55 min 32%–40% B, 55–56 min 40%–95% B, and56-60 min 95%-25%B with a flow rate at 0.3 mL /min. Column and sampler temperatures were set at 35 °C and 4°C, respectively. The injection volume was 5 μL.
Mass spectrometry conditions: A UHPLC-Q- Exactive -MS/MS (Thermo Fisher Scientific, USA) equipped with a Xcalibur data acquisition system and electrospray ionization source were used in both positive and negative ion mode. Electrospray ion source (ESI), positive and negative ion mode detection, scanning type: Full scan-ddMS2, mass scanning range: 150.0-2000.0 m/z, the flow rate of sheath gas was 40 arb, the aux gas was 10 arb, spray voltage: (±) 3.5KV, capillary temperature: 350 °C, fragmentation voltage: 30ev, mass axis was calibrated before detection.
4.6. Antioxidant Activity Assays
4.6.1. DPPH Scavenging Activity Assay
The DPPH scavenging activity was assayed according to reference with a few minor adjustments [
34]. The method involves the repeated thermal processing of SGW with 0.2 mM of DPPH alcohol solution. Briefly, 80µL of diluted DPPH solution and 50µL of sample solutions were mixed in a microplate and incubated for 30 min in the dark. The absorbance of the sample was recorded at 520 nm.
4.6.2. FRAP Scavenging Activity Assay
FRAP solution was obtained by mixing sodium acetate buffer (pH 3.6), sodium acetate buffer (0.3 mol/L), TPTZ solution (10 mmol/L) and FeCl
3 solution (20 mmol/L) at a ratio of 10:1:1[
35]. Briefly, 200µL of diluted FRAP solution and 50µL of sample solutions were mixed in a microplate and incubated for 10 min at 37 °C. The absorbance of the sample was recorded at 592 nm. The antioxidant capacity was expressed as the mass concentration of ferrous sulfate (mg/L), using ferrous sulfate solution as the standard curve.
4.6.3. ABTS+ Scavenging Activity Assay
A solution of 7.0 mM ABTS
+ and 2.45 mM potassium persulfate was mixed evenly in equal proportions and stored in the dark for 12–16 hours [
36]. The absorbance of the diluent at 734nm was 0.70±0.02. The sample solution of 50 μL was added into the diluted ABTS
+ solution of 80 μL and reacted in the dark for 6 minutes, then measured at 734 nm.
4.6.4. Hydroxyl Radical Assay
Hydroxyl radical scavenging experiments were performed by Fenton reaction method [
37]. A solution of 5.0 mM FeSO
4, 5.0 mM ethanolic salicylic acid, and 3 mM H
2O
2 were mixed and reacted for 30 min at 37°C. The absorbance of the sample was recorded at 510 nm.
4.7. Data Processing and Multivariate Analysis
SPSS 23.0 (SPSS Inc., Chicago, IL, USA) was used for one-way ANOVA, and Bonferroni was used for significance testing to analyze the significant differences (p < 0.05) in the peak areas of chemical composition in four groups of SGW. Statistical analysis was performed from triplicate results and the mean and standard deviation (mean±SD) were listed.
All original MS spectrometry data were preprocessed and exported. Then, retention time calibration, peak alignment, baseline calibration, normalization, and logarithmic conversion were conducted. Multivariate analyses were performed by using SIMCA-P software 17.0 (Umetrics, Umea, Sweden) The data sets of peak areas were scaled (UV or Pareto) prior to multivariate analysis by PCA or OPLS-DA. The variable importance in the projection (VIP) values indicate the major compounds contributing to the separation of each sample in the OPLS-DA score plots. The VIP value is a weighted sum of squares of the OPLS-DA weights that takes the explained Y variance in each dimension into account. The OPLS-DA models were validated with permutation tests。The biomarkers were analyzed by MS/MS analysis and the metabolite database HMDB (
http://www.hmdb.ca/).
5. Conclusions
SGW, as a valuable resource during the red ginseng manufacture processing, is widely used for skin care and health enhancement in folklore of the Changbai mountain region in the northeast of Jilin province, China. Since the composition and activity are vague, the scientific basis for being recycled is insufficient, In the present study, we investigated the correlation between the frequency of sample reheating and skincare properties, including anti-aging effects and composition content level trends. The result showed that the times of heating cycles significantly impacts the content of saponins and sugars(including polysaccharides, reducing sugar and non-reducing sugar). The results of the frequency of heating thermal and compositional analyses proved that the process of generating SGW, if it is desired to maintain its antioxidant activity, minimize its repeated heating process in the equipment and try not to exceed 7 times. In summary, this study may provide a solid scientific proof to the high-quality recycling of red ginseng processed by-products, and deepen the application value the SGW in the cosmetic industry.
Author Contributions
All authors contributed to the study’s conception and design. Original draft preparation, Y.-D.W.; review and editing, project administration, and funding acquisition, E.-P.W. All authors have read and agreed to the published version of the manuscript
Funding
This work was sponsored by the Jilin Province Science and Technology Development Plan Project (No. 20210401108YY, No. YDZJ202201ZYTS207), the Key Technology Research Project of Chang-chun Science and Technology Bureau (No. 21ZGY10), Jiayi Biochemical Research and Development Center (Suzhou Industrial Park) Co., Ltd., and the National Natural Science Foundation of China (No. 82073969).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.
Acknowledgments
We appreciate the Jilin Province Science and Technology Development Plan Project (No. 20210401108YY, No. YDZJ202201ZYTS207), the Key Technology Research Project of Chang-chun Science and Technology Bureau (No. 21ZGY10), Jiayi Biochemical Research and Development Center (Suzhou Industrial Park) Co., Ltd., and the National Natural Science Foundation of China (No. 82073969). for financial support. The authors also appreciate the anonymous reviewers for their valuable comments and suggestions on this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yu, W.; Cai, S.; Zhao, J.; Hu, S.; Zang, C.; Xu, J.; Hu, L. Beyond genome: Advanced omics progress of Panax ginseng. Plant Sci. 2024, 341, 112022. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Ji, S.H.; Choi, B.R.; Choi, D.J.; Lee, Y.G.; Kim, H.G.; Kim, G.S.; Kim, K.; Lee, Y.H.; Baek, N.I.; Lee, D.Y. UPLC-QTOF/MS-Based Metabolomics Applied for the Quality Evaluation of Four Processed Panax ginseng Products. Molecules. 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ren, C.; Li, H.J.; Wu, Y.C. Recent Progress on Processing Technologies, Chemical Components, and Bioactivities of Chinese Red Ginseng, American Red Ginseng, and Korean Red Ginseng. Food and Bioprocess Technology. 2022, 15, 47–71. [Google Scholar] [CrossRef]
- Peng, X.; Hao, M.; Zhao, Y.; Cai, Y.; Chen, X.; Chen, H.; Zhang, Y.; Dong, L.; Liu, X.; Ding, C.; Liu, W.; Yang, M.; Luo, Y. Red ginseng has stronger anti-aging effects compared to ginseng possibly due to its regulation of oxidative stress and the gut microbiota. Phytomedicine. 2021, 93, 153772. [Google Scholar] [CrossRef] [PubMed]
- Min, S.J.; Kim, H.; Yambe, N.; Shin, M.S. Ameliorative Effects of Korean-Red-Ginseng-Derived Polysaccharide on Antibiotic-Associated Diarrhea. Polymers (Basel). 2024, 16. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.Y.; Zhang, S.; Skinner, D.; Koch, C.G.; Smith, M.J.; Lim, D.J.; Grayson, J.W.; Tearney, G.J.; Rowe, S.M.; Woodworth, B.A. Red ginseng aqueous extract improves mucociliary transport dysfunction and histopathology in CF rat airways. J Cyst Fibros. 2023, 22, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Jeddy, N.; Saravanan, R.; Natrajan, R.; Sai Lakshmi, L.J.; Ashwath, V.; Singhal, I. Comparison of the effectiveness of red ginseng herbal mouth rinse with chlorhexidine and saline in oral cancer patients: A pilot double-blinded randomized control trial. J Oral Maxillofac Pathol. 2023, 27, 778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.E.; Chu, M.Y.; Jiang, T.; Song, X.H.; Hou, J.F.; Cheng, L.Y.; Feng, Y.; Chen, C.B.; Wang, E.P. By-Product of the Red Ginseng Manufacturing Process as Potential Material for Use as Cosmetics: Chemical Profiling and In Vitro Antioxidant and Whitening Activities. Molecules. 2022, 27. [Google Scholar] [CrossRef]
- Kang, K.A.; Piao, M.J.; Fernando, P.; Herath, H.; Yi, J.M.; Hyun, J.W. Korean Red Ginseng Attenuates Particulate Matter-Induced Senescence of Skin Keratinocytes. Antioxidants (Basel). 2023, 12. [Google Scholar] [CrossRef]
- Lee, D.Y.; Arndt, J.; O'Connell, J.F.; Egan, J.M.; Kim, Y. Red Ginseng Attenuates the Hepatic Cellular Senescence in Aged Mice. Biology (Basel). 2024, 13. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Zhang, X.; Yang, L.; Wang, Z. Ginsenoside Contents in Ginseng: Quality by Design-Coupled Two-Dimensional Liquid Chromatography Technique. J Chromatogr Sci. 2022, 60, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Zhang, C.F.; Zhang, Q.H.; Yuan, C.S. Phytochemistry of Red Ginseng, A Steam-Processed Panax ginseng. Am J Chin Med. 2024, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.M.; Chen, T.B.; Xiao, S.Y.; Zha, Q.L.; Luo, P.; Wang, Y.P.; Cui, X.M.; Liu, L.; Zhou, H. A new approach for authentication of four ginseng herbs and their related products based on the simultaneous quantification of 19 ginseng saponins by UHPLC-TOF/MS coupled with OPLS-DA. Rsc Advances. 2017, 7, 46839–46851. [Google Scholar] [CrossRef]
- Li, X.; Lin, J.; Gao, Y.; Han, W.; Chen, D. Antioxidant activity and mechanism of Rhizoma Cimicifugae. Chem Cent J. 2012, 6, 140. [Google Scholar] [CrossRef]
- Kim, C.J.; Kim, B.M.; Kim, C.S.; Baek, J.Y.; Jung, I.C. Variations in Ginsenosides of Raw Ginseng According to Heating Temperature and Time. J Pharmacopuncture. 2020, 23, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.F.; Gao, Y.; Xu, S.Y.; Liu, H.; Xue, X.; Zhang, Y.; Zhang, H.; Liu, M.N.; Xiong, H.; Lin, R.C.; Li, X.R. Remarkable impact of steam temperature on ginsenosides transformation from fresh ginseng to red ginseng. Journal of Ginseng Research. 2018, 42, 277–287. [Google Scholar] [CrossRef]
- Hwang, C.R.; Lee, S.H.; Jang, G.Y.; Hwang, I.G.; Kim, H.Y.; Woo, K.S.; Lee, J.; Jeong, H.S. Changes in ginsenoside compositions and antioxidant activities of hydroponic-cultured ginseng roots and leaves with heating temperature. J Ginseng Res. 2014, 38, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, Y.; Dou, D. Anti-prostate cancer mechanism of black ginseng during the "nine steaming and nine sun-drying" process based on HPLC analysis combined with vector space network pharmacology. Discov Oncol. 2024, 15, 12. [Google Scholar] [CrossRef]
- Liu, Z.; Xia, J.; Wang, C.Z.; Zhang, J.Q.; Ruan, C.C.; Sun, G.Z.; Yuan, C.S. Remarkable Impact of Acidic Ginsenosides and Organic Acids on Ginsenoside Transformation from Fresh Ginseng to Red Ginseng. J Agric Food Chem. 2016, 64, 5389–5399. [Google Scholar] [CrossRef]
- Gao, D.; Kim, J.H.; Vinh, L.B.; Seo, E.Y.; Yang, S.Y.; Cho, C.W.; Kim, Y.H.; Kim, K.T.; Sim, J.; Kang, J.S. Effect of citric acid and heat treatment on the content of less-polar ginsenosides in flower buds of Panax ginseng. Prep Biochem Biotechnol. 2022, 52, 144–153. [Google Scholar] [CrossRef]
- Chen, W.; Balan, P.; Popovich, D.G. Changes of Ginsenoside Composition in the Creation of Black Ginseng Leaf. Molecules. 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.B.; Jeong, D.E.; Lee, D.E.; Yoo, J.H.; Kim, Y.S.; Kim, T.Y. Structural Identification of Ginsenoside Based on UPLC-QTOF-MS of Black Ginseng (Panax Ginseng C.A. Mayer). Metabolites. 2024, 14. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Jo, H.K.; Im, B.O.; Kim, S.; Whang, W.K.; Ko, S.K. Changes in the Contents of Prosapogenin in the Red Ginseng (Panax ginseng) Depending on Steaming Batches. J Ginseng Res. 2012, 36, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Qin, K.M.; Li, W.D.; Yin, F.Z.; Cai, H.; Cai, B.C. [Research on chemical reactions during ginseng processing]. Zhongguo Zhong Yao Za Zhi. 2014, 39, 3701–3706. [Google Scholar] [PubMed]
- Du, Q.Q.; Liu, S.Y.; Xu, R.F.; Li, M.; Song, F.R.; Liu, Z.Q. Studies on structures and activities of initial Maillard reaction products by electrospray ionisation mass spectrometry combined with liquid chromatography in processing of red ginseng. Food Chem. 2012, 135, 832–838. [Google Scholar] [CrossRef]
- Fan, J.; Liu, F.; Ji, W.; Wang, X.; Li, L. Comprehensive Investigation of Ginsenosides in the Steamed Panax quinquefolius with Different Processing Conditions Using LC-MS. Molecules. 2024, 29. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.R.; Cheng, Y.; Zhang, X.; Wang, Y.P.; Zhao, H. Comparative analysis of physicochemical properties, ginsenosides content and α-amylase inhibitory effects in white ginseng and red ginsen. Food Science and Human Wellness. 2023, 12, 14–27. [Google Scholar] [CrossRef]
- Xie, Y.Y.; Luo, D.; Cheng, Y.J.; Ma, J.F.; Wang, Y.M.; Liang, Q.L.; Luo, G.A. Steaming-induced chemical transformations and holistic quality assessment of red ginseng derived from Panax ginseng by means of HPLC-ESI-MS/MS(n)-based multicomponent quantification fingerprint. J Agric Food Chem. 2012, 60, 8213–8224. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Kim, Y.J.; Jeon, J.N.; Wang, C.; Min, J.W.; Noh, H.Y.; Yang, D.C. Effect of white, red and black ginseng on physicochemical properties and ginsenosides. Plant Foods Hum Nutr. 2015, 70, 141–145. [Google Scholar] [CrossRef]
- Oh, C.H.; Kim, G.N.; Lee, S.H.; Lee, J.S.; Jang, H.D. Effects of Heat Processing Time on Total Phenolic Content and Antioxidant Capacity of Ginseng <i>Jung Kwa</i>. Journal of Ginseng Research. 2010, 34, 198–204. [Google Scholar]
- Kang, K.S.; Kim, H.Y.; Pyo, J.S.; Yokozawa, T. Increase in the free radical scavenging activity of ginseng by heat-processing. Biol Pharm Bull. 2006, 29, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Choi, J.S.; Yokozawa, T. Increase in the hydroxyl radical-scavenging activity of Panax ginseng and ginsenosides by heat-processing. Drug Discov Ther. 2018, 12, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Yue, F.F.; Zhang, J.R.; Xu, J.X.; Niu, T.F.; Lu, X.; Liu, M.S. Effects of monosaccharide composition on quantitative analysis of total sugar content by phenol-sulfuric acid method. Frontiers in Nutrition. 2022, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Syed Salleh, S.N.A.; Mohd Hanapiah, N.A.; Ahmad, H.; Wan Johari, W.L.; Osman, N.H.; Mamat, M.R. Determination of Total Phenolics, Flavonoids, and Antioxidant Activity and GC-MS Analysis of Malaysian Stingless Bee Propolis Water Extracts. Scientifica (Cairo). 2021, 2021, 3789351. [Google Scholar] [CrossRef]
- Jerković, I.; Marijanović, Z. Oak (Quercus frainetto Ten.) honeydew honey--approach to screening of volatile organic composition and antioxidant capacity (DPPH and FRAP assay). Molecules. 2010, 15, 3744–3756. [Google Scholar] [CrossRef] [PubMed]
- Ying, A.; Yu, Q.T.; Guo, L.; Zhang, W.S.; Liu, J.F.; Li, Y.; Song, H.; Li, P.; Qi, L.W.; Ge, Y.Z.; Liu, E.H.; Liu, Q. Structural-Activity Relationship of Ginsenosides from Steamed Ginseng in the Treatment of Erectile Dysfunction. Am J Chin Med. 2018, 46, 137–155. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: an updated overview. Arch Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).