Submitted:
23 May 2024
Posted:
24 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
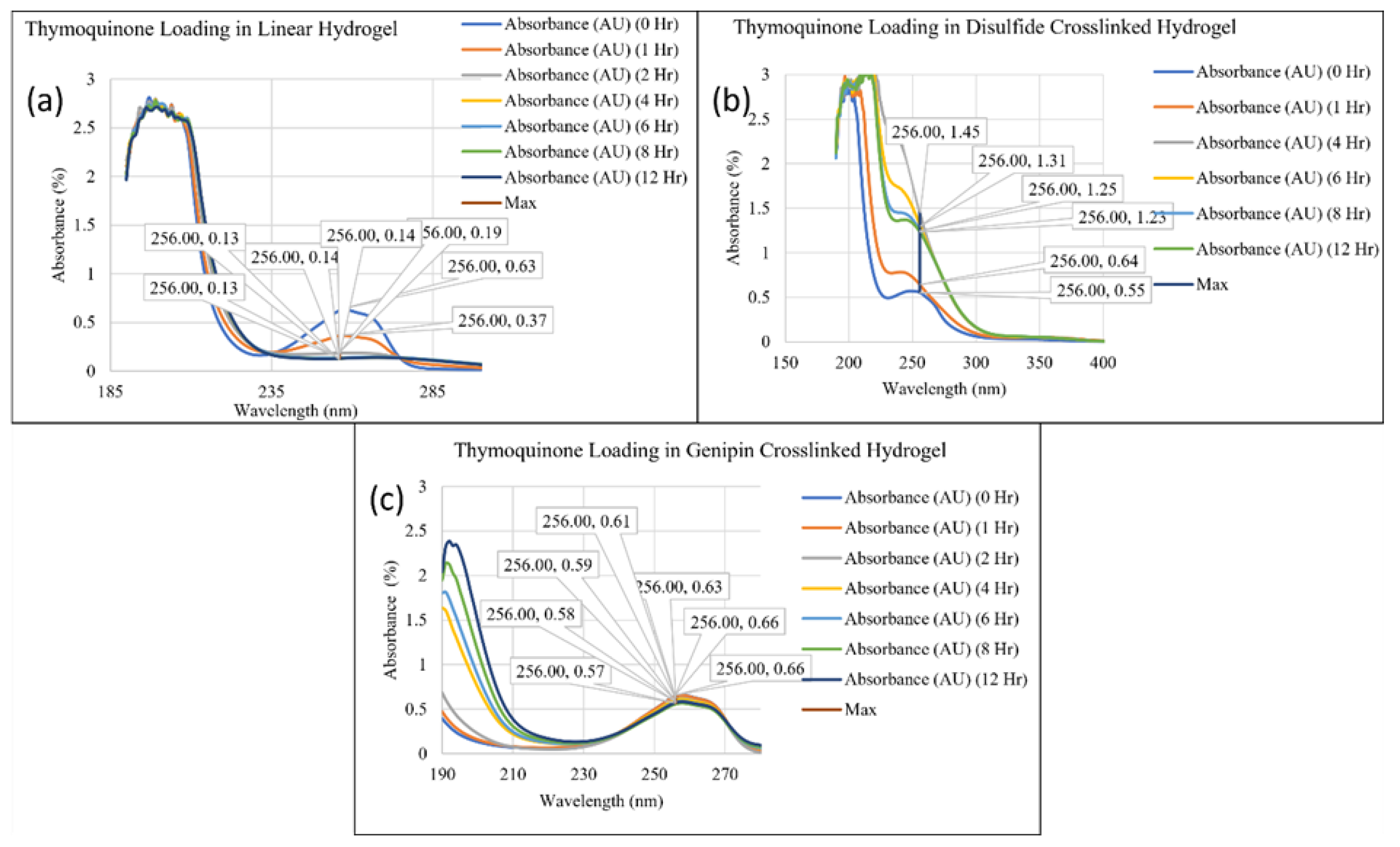
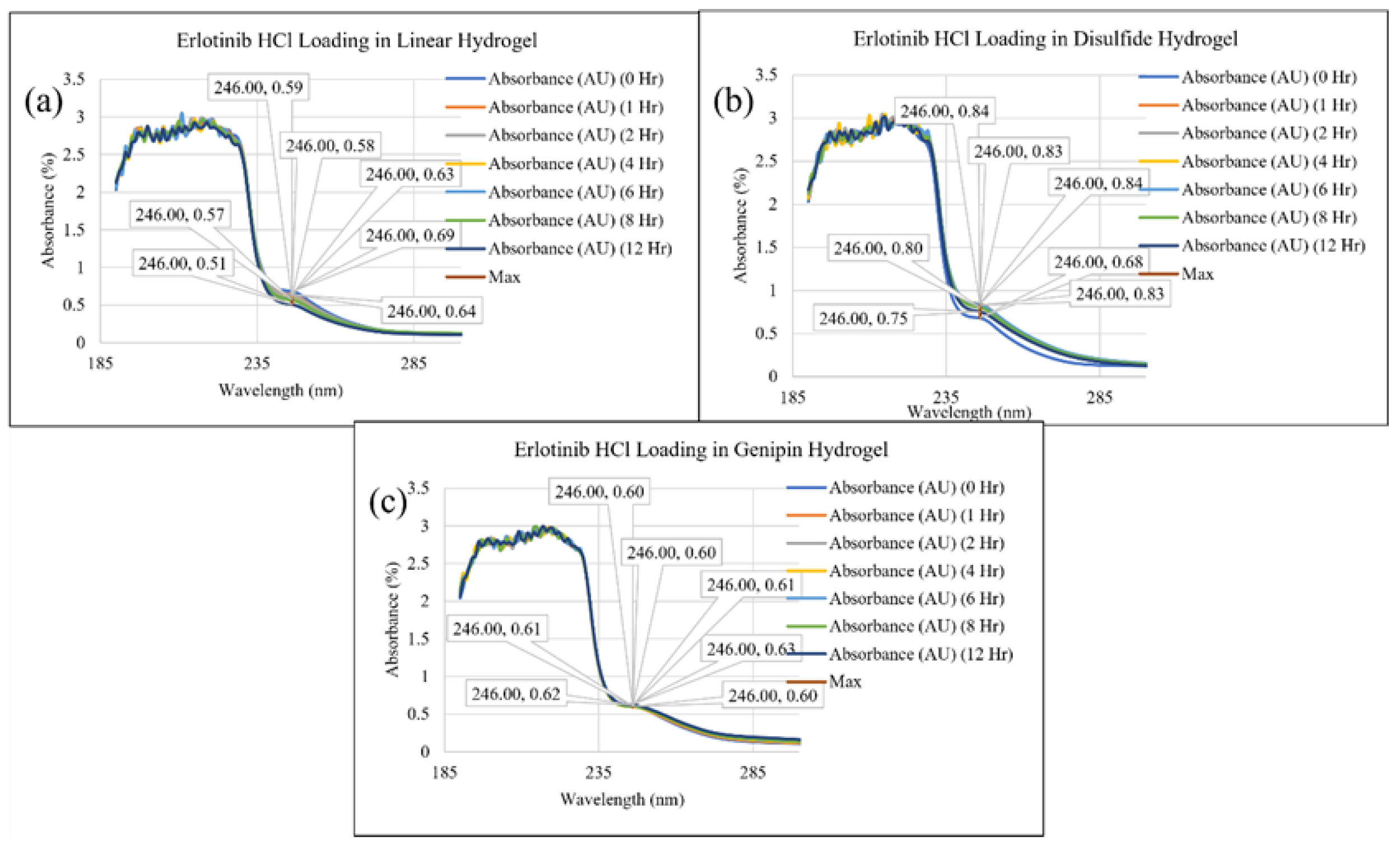
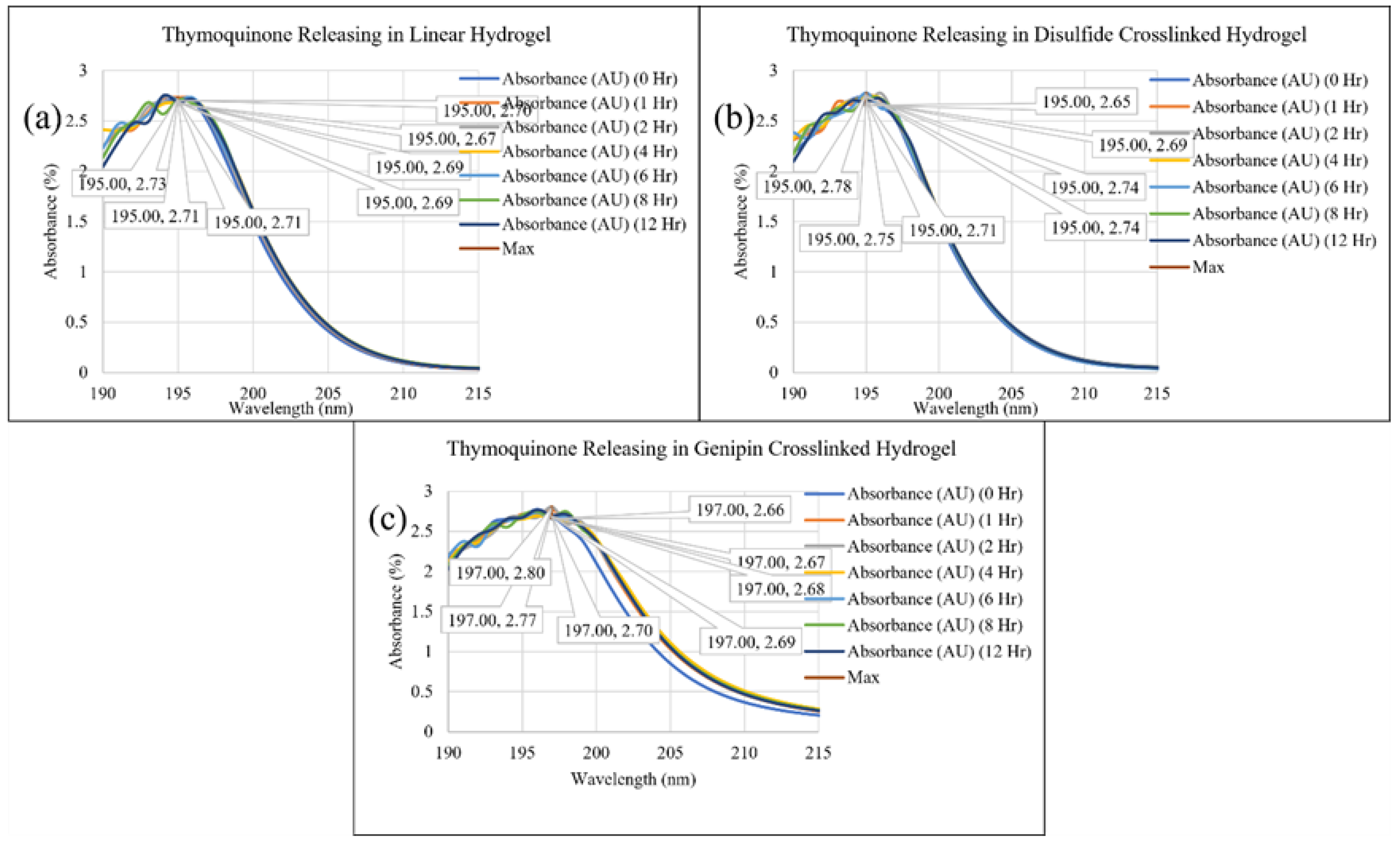
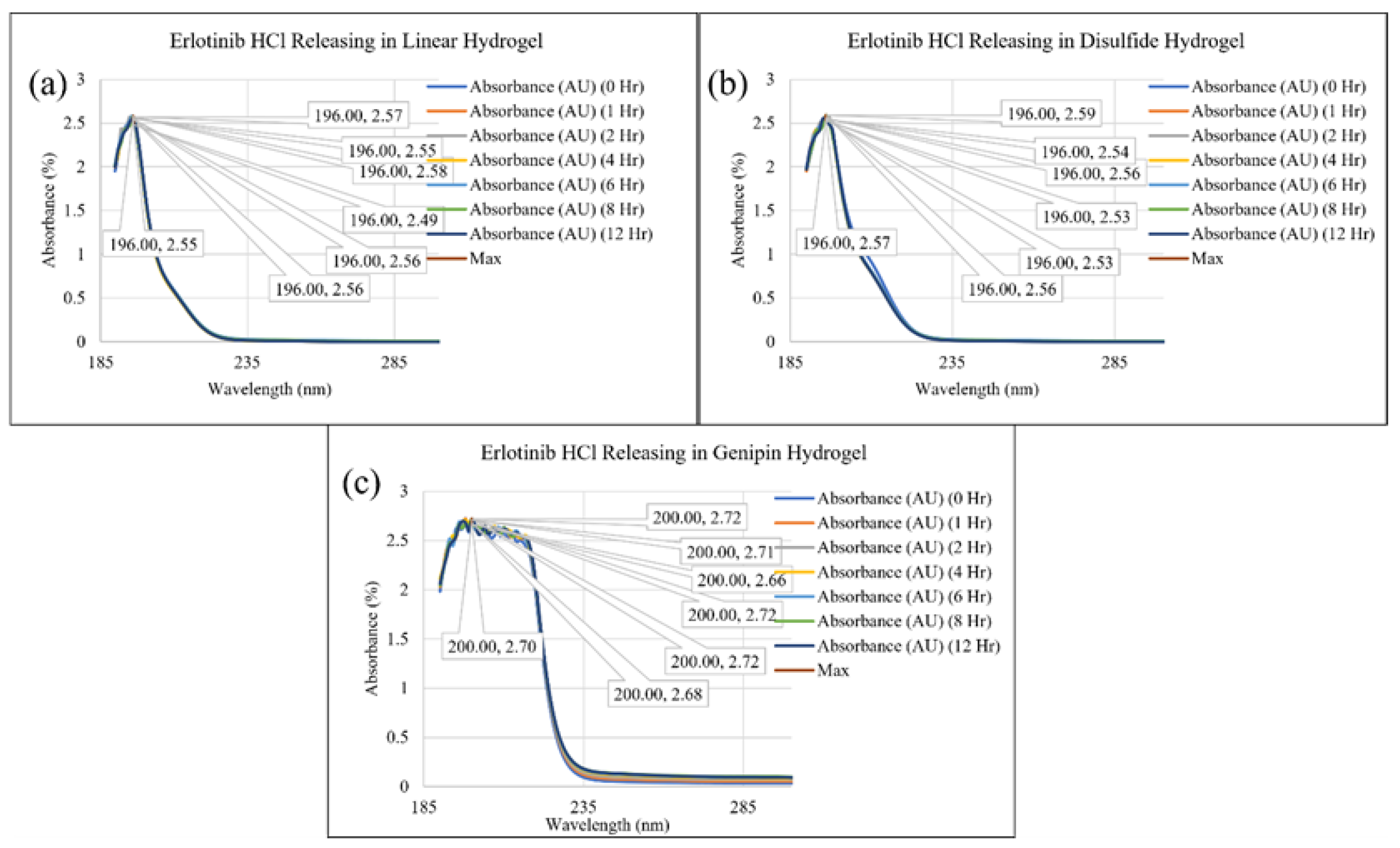
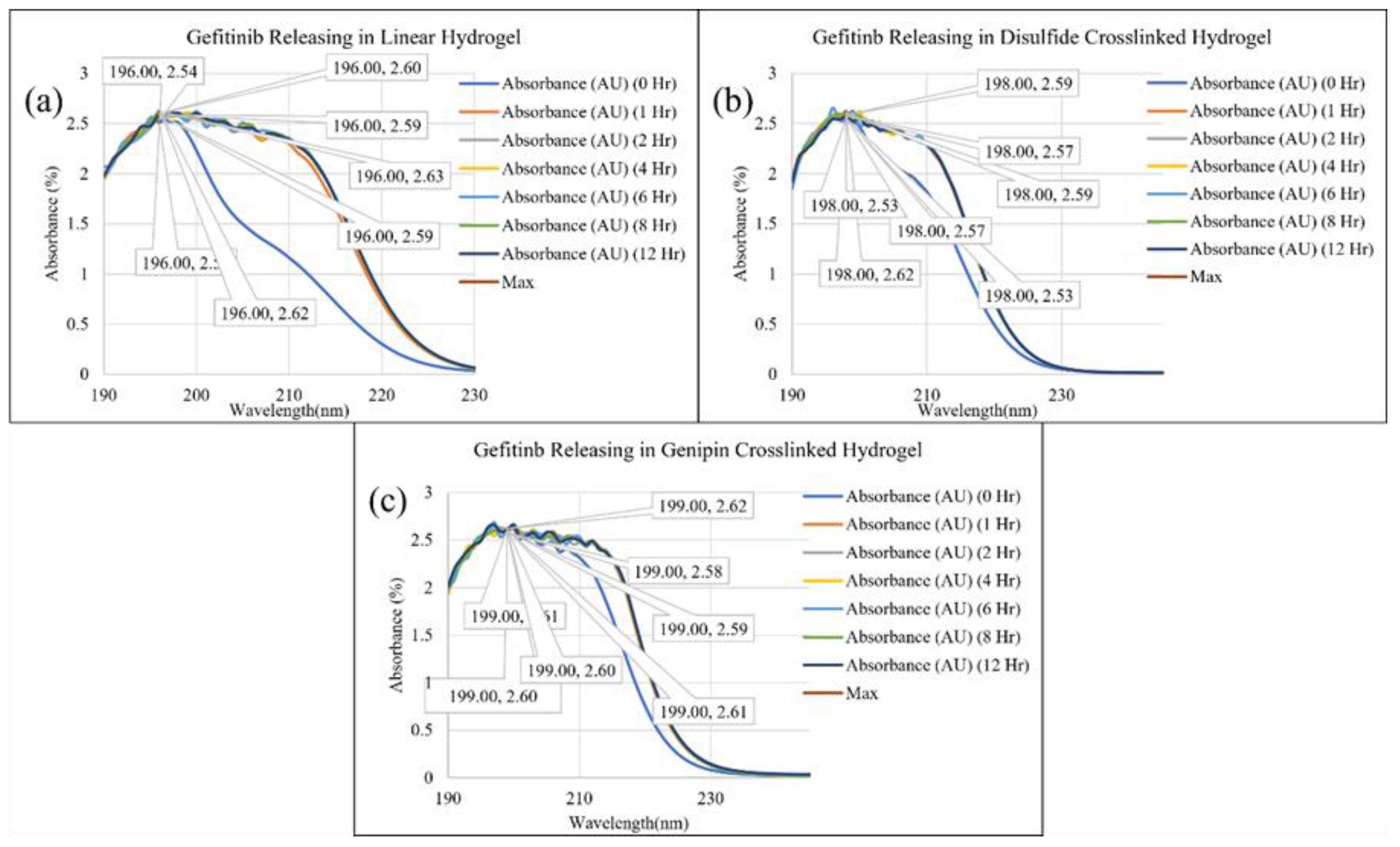
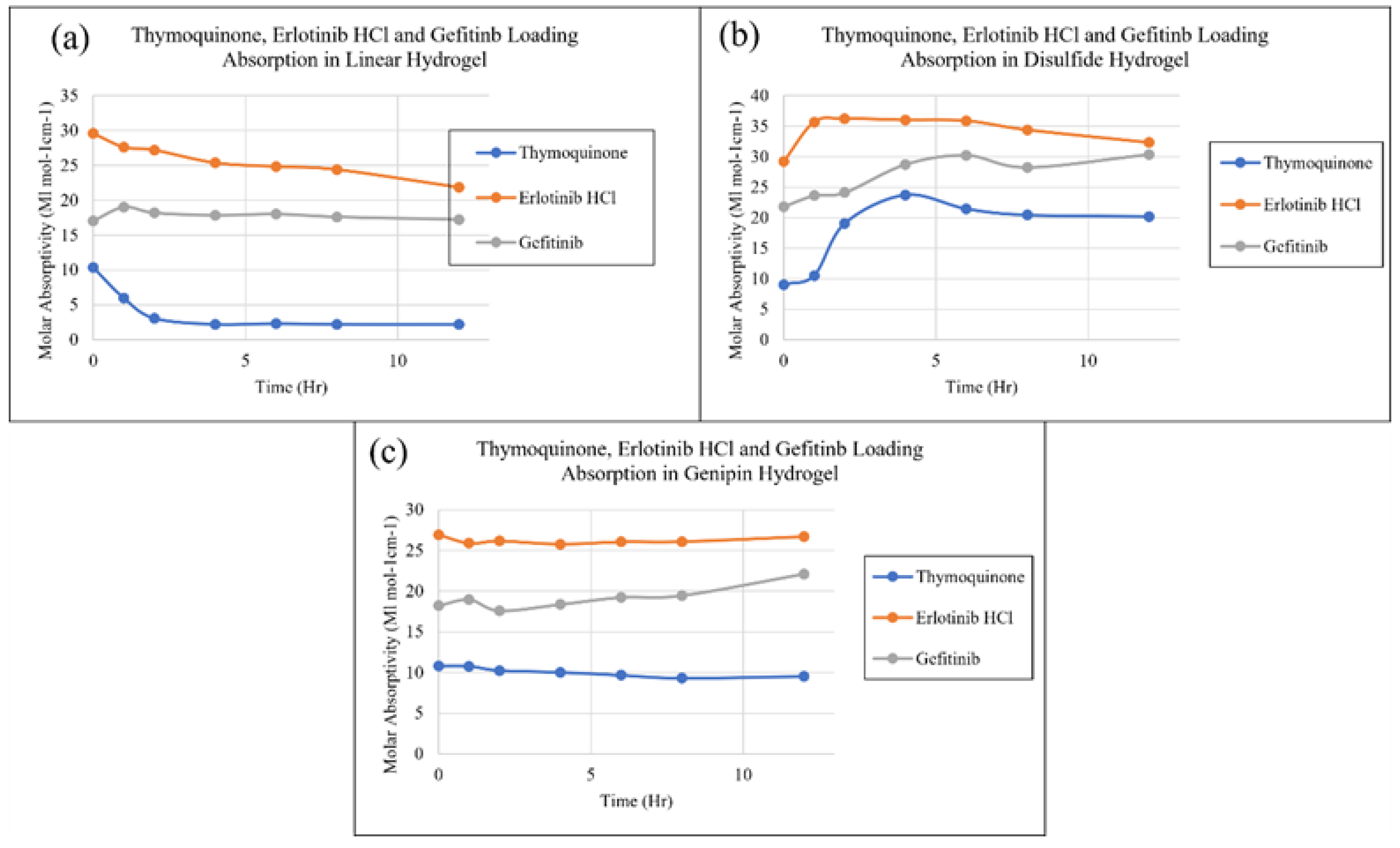
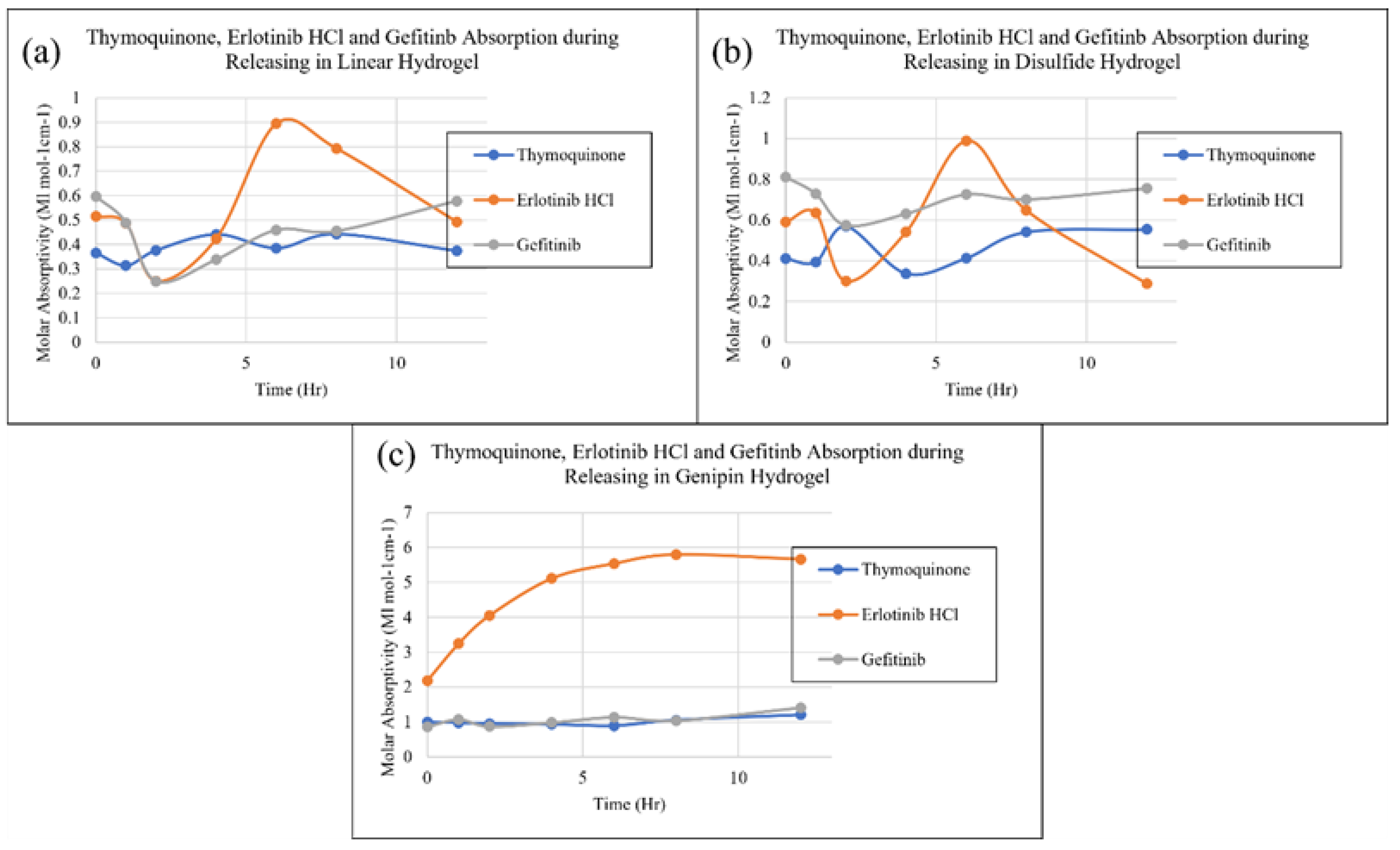
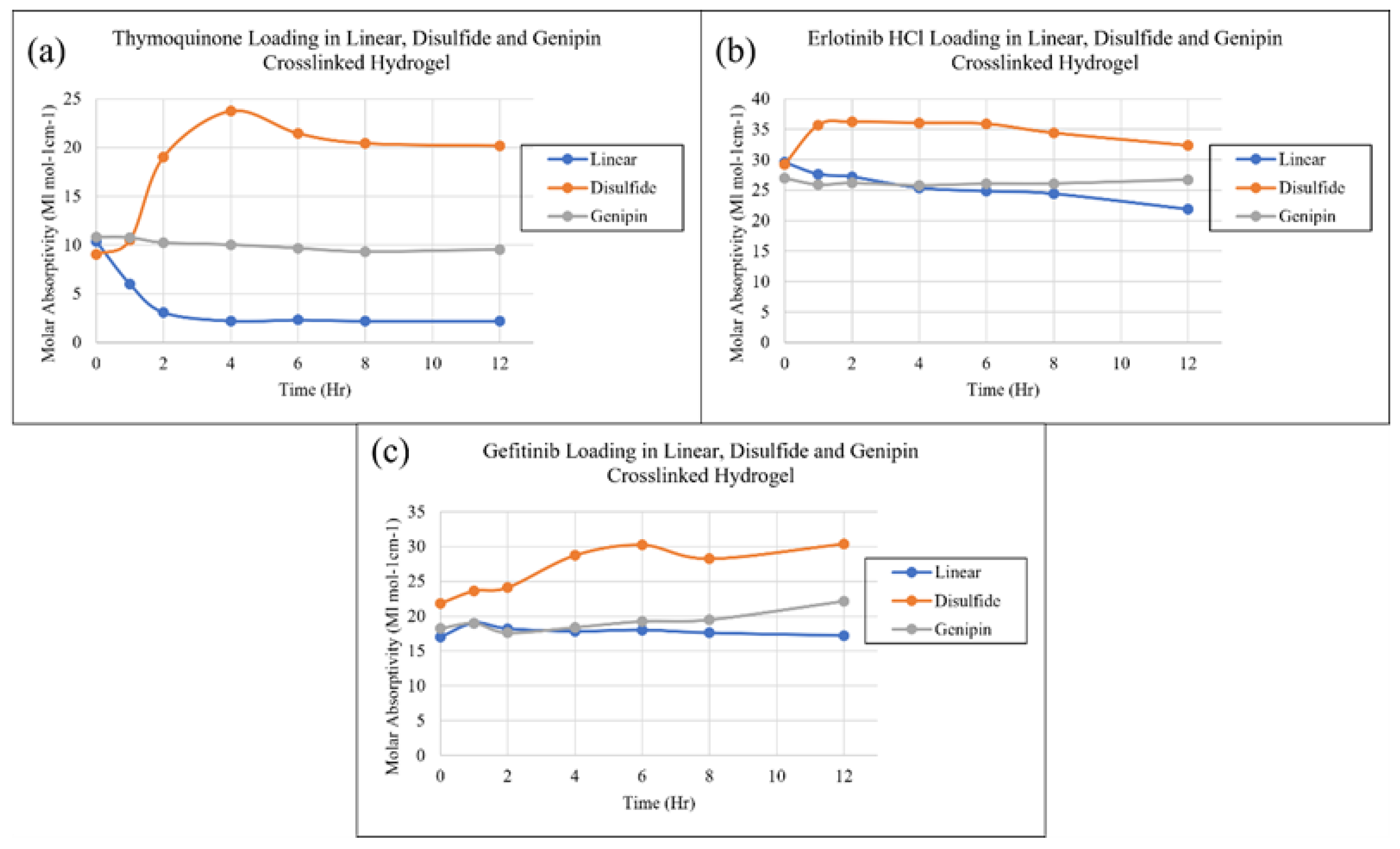
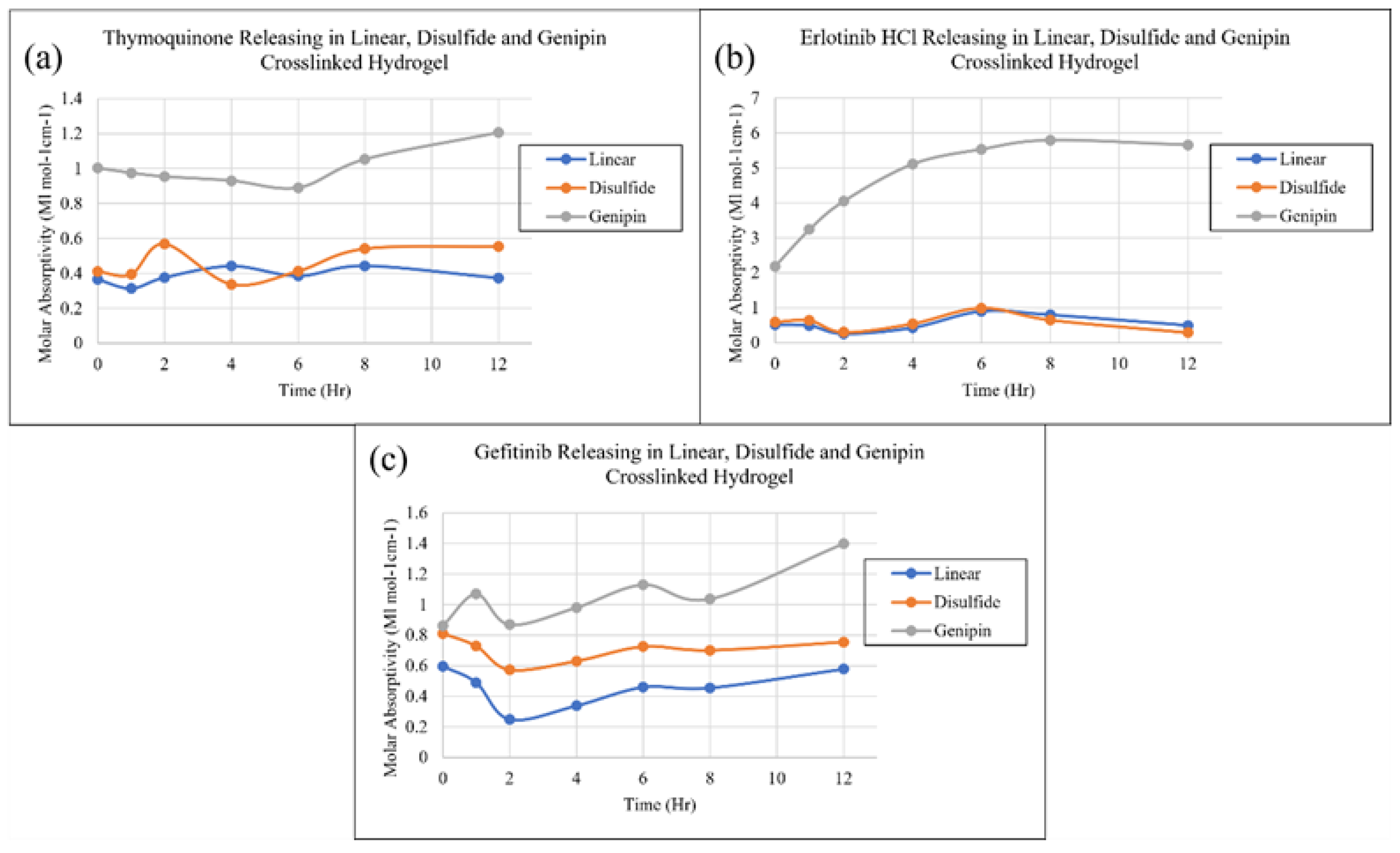
2. Results and Discussion
3. Conclusions
4. Materials and Methods
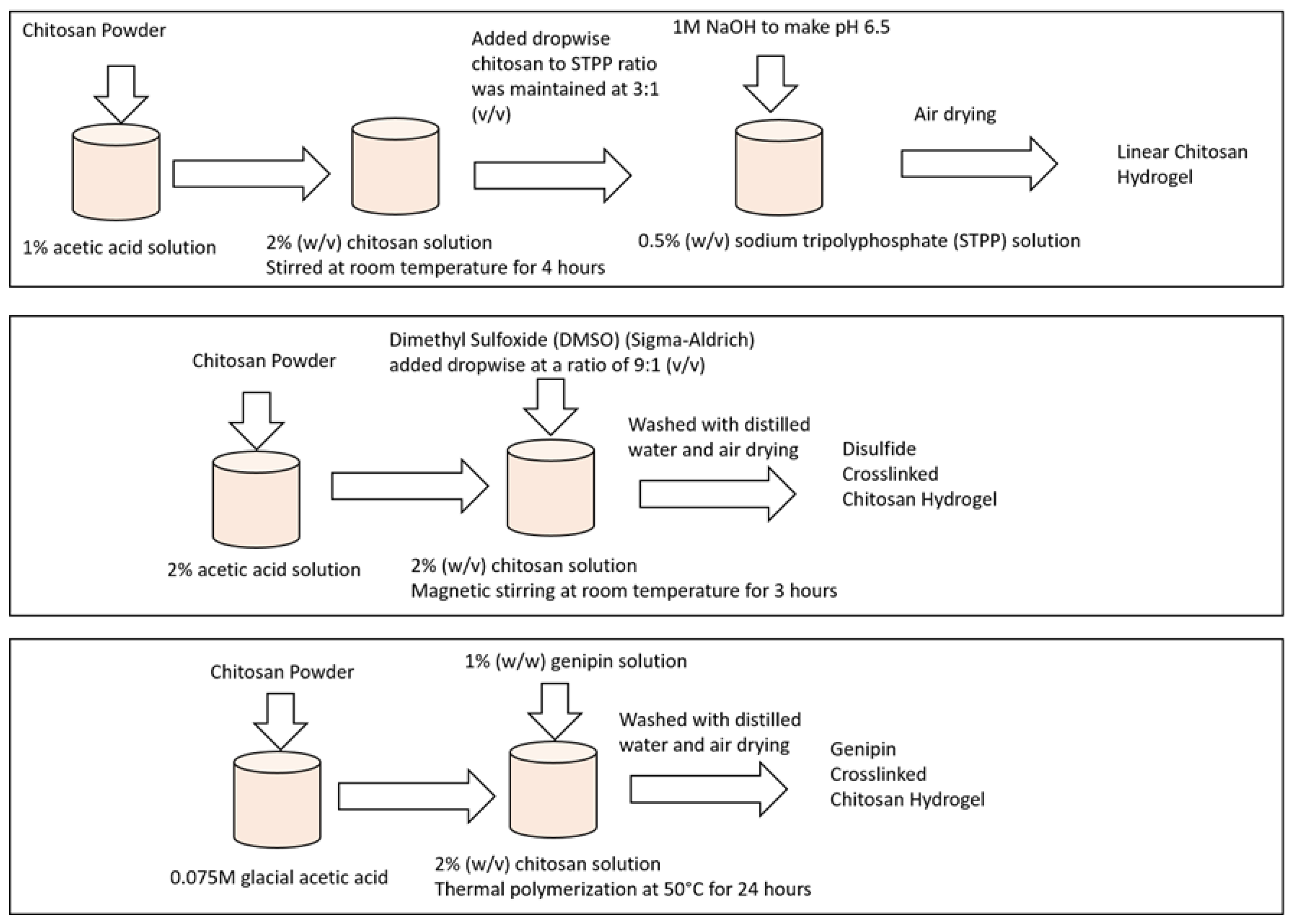
4.1. Sample Preparation
4.1.1. Preparation of Linear Chitosan Hydrogel Sample
4.1.2. Preparation of Disulfide Crosslinked Chitosan Hydrogel Sample
4.1.3. Preparation of Genipin Crosslinked Chitosan Hydrogel Sample
4.2. Drug Selection
4.2.1. Thymoquinone
4.2.2. Erlotinib Hydrochloride
4.2.3. Gefitinib
4.3. Ultraviolet–Visible (Uv-Vis) Spectroscopy
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torchilin, V. P. (2000). Drug targeting. European Journal of Pharmaceutical Sciences, 11, S81-S91.
- Torchilin, V. P. (1995). Handbook of targeted delivery of imaging agents (Vol. 26). CRC Press.
- Buxton, I. L. (2006). Pharmacokinetics and pharmacodynamics. Goodman and Gilman’s the pharmacologic basis of therapeutics, 11th Ed. New York: McGraw-Hill, 1-52.
- Sonker, M. , Bajpai, S., Khan, M. A., Yu, X., Tiwary, S. K., & Shreyash, N. (2021). Review of Recent Advances and Their Improvement in the Effectiveness of Hydrogel-Based Targeted Drug Delivery: A Hope for Treating Cancer. ACS Applied Bio Materials, 4(12), 8080-8109.
- Madhumathi, K. , & Tamura, H. (2015). Chitosan-based hydrogels for drug delivery and tissue engineering. International Journal of Biological Macromolecules, 80, 737–749. [CrossRef]
- Dash, M. , Chiellini, F., Ottenbrite, R.M., & Chiellini, E. (2011). Chitosan—a versatile semi-synthetic polymer in biomedical applications. Progress in Polymer Science, 36(8), 981-1014. [CrossRef]
- Roy, D., & Gupta, M. N. (2017). Modeling the Formation of Hydrogels Using Density Functional Theory. Journal of Physical Chemistry B, 121(47), 10609–10616. [CrossRef]
- Zhao, Y. , Yang, J., Zhang, C., & Cheng, R. (2017). Theoretical investigation of non-covalent interactions in hydrogels. Journal of Materials Chemistry B, 5(29), 5739–5746. [CrossRef]
- Abitha M. Heimbuck, Tyler R. Priddy-Arrington, Benjamin J. Sawyer, Mary E. Caldorera-Moore, Effects of post-processing methods on chitosan-genipin hydrogel properties, Materials Science and Engineering: C, Volume 98,2019, Pages 612-618, ISSN 0928-4931. [CrossRef]
- Yew, M. Y. , Basri, M., Masoumi, H. R. F., Ahmad, M. B., Ismail, M., & Abdul Rahman, M. B. (2016). Fabrication of chitosan hydrogel beads cross-linked with genipin for controlled release of vitamin C. International journal of biological macromolecules, 93, 1478-1486. [CrossRef]
- Abdel-Bar, H. M. , El-Magd, A. A., & Al-Mahallawi, A. M. (2018). Effect of chitosan concentration on the performance of ibuprofen-loaded chitosan hydrogels. International Journal of Biological Macromolecules, 117, 41–49. [CrossRef]
- Hu, Q. , Zhu, Y., Zhang, X., & Liu, Y. (2014). pH-sensitive chitosan-based hydrogel microspheres for doxorubicin delivery. Carbohydrate Polymers, 101, 1235–1243. [CrossRef]
- Uddin, M. S., & Ju, J. (2020). Effect of crosslinking agents on drug distribution in chitosan hydrogel for targeted drug delivery to treat cancer. Journal of Polymer Research, 27, 1-10.
- E. S. De Alvarenga, “Characterization and Properties of Chitosan,” in Biotechnology of Biopolymers, InTech, 2011. doi 10.5772/17020.
- F. Ahmadi, Z. Oveisi, S. M. Samani, and Z. Amoozgar, "Chitosan-based hydrogels: characteristics and pharmaceutical applications.," Res. Pharm. Sci., vol. 10, no. 1, pp. 1–16, 2015, [Online]. Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4578208/.
- K. Miller, “Chitosan,” WebMD, 2023. https://www.webmd.com/vitamins-and- supplements/chitosan-uses-and-risks.
- L. L. Palmese, M. Fan, R. A. Scott, H. Tan, and K. L. Kiick, “Multi-stimuli-responsive, liposome-crosslinked poly (ethylene glycol) hydrogels for drug delivery,” J. Biomater. Sci. Polym. Ed., vol. 32, no. 5, pp. 635–656, Mar. 2021. [CrossRef]
- M. Prabaharan and J. F. Mano, “Chitosan-Based Particles as Controlled Drug Delivery Systems,” Drug Deliv., vol. 12, no. 1, pp. 41–57, Jan. 2004. [CrossRef]
- L. Upadhyaya, J. Singh, V. Agarwal, and R. P. Tewari, "The implications of recent advances in carboxymethyl chitosan-based targeted drug delivery and tissue engineering applications," J. Control. Release, vol. 186, pp. 54–87, Jul. 2014. [CrossRef]
- Wang, Q., Wang, X., & Feng, Y. (2023). Chitosan hydrogel as tissue engineering scaffolds for vascular regeneration applications. Gels, 9(5), 373.
- Qu C, Li X, Zhao Y, Han X, Liu Y, Du Y. Preparation and characterization of chitosan hydrogel containing silver nanoparticles for wound dressing application. J Biomater Appl. 2016;31(4):524-534.
- Gali-Muhtasib, H. , Roessner, A., & Schneider-Stock, R. (2006). Thymoquinone: a promising anti-cancer drug from natural sources. International journal of biochemistry and cell biology, 38(8), 1249-1253. [CrossRef]
- https://pubchem.ncbi.nlm.nih.gov/compound/123631#section=Structures.
- Moore, M. J. , Goldstein, D., Hamm, J., et al. (2007). Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. Journal of Clinical Oncology, 25(15), 1960-1966.
- https://pubchem.ncbi.nlm.nih.gov/compound/176871#section=Structures.
- Li, W., Ma, G., & Zhou, L. (2016). In situ crosslinked chitosan hydrogel for the controlled release of gefitinib. Carbohydrate Polymers, 138, 194-202. [CrossRef]
- National Center for Biotechnology Information. "PubChem Compound Summary for CID 123631, Gefitinib" PubChem, https://pubchem.ncbi.nlm.nih.gov/compound/Gefitinib. Accessed 10 March, 2022.
- Kim YJ, Yoon JJ, Park JH. Drug release from electro spun fibers - a review. J Ind Eng Chem. 2015; 29:1-11.
- La WG, Kang SW. Effect of crosslinking degree and pH on chitosan hydrogel for drug release. Korean J Chem Eng. 2014;31(8):1373-1379.
- Yao, F., & Zhang, L. (2011). Swelling behaviors of chitosan hydrogels in different pH and salt solutions. Journal of Applied Polymer Science, 119(4), 2244-2251.


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




