1. Introduction
In 2021, we presented [
1] a potential new pharmaceutical
E, see
Scheme 1, developed for treatment of antibiotic resistant
Pseudomonas aeruginosa biofilm infections. We showed that the compound
E interferes with c-di-GMP signalling to induce dispersal of
P. aeruginosa biofilms [
1], which allow the standard of care antibiotics to destroy the bacterial infection in vivo [
2]. In the meantime, we published a synthetic route for the compound
E [
3], as well as a study of its mode of action [
4]. To increase water solubility, we formulated compound E as the HCl salt and now named it Disperazol [
2].
To further advance Disperazol as a novel pharmaceutical, a large-scale synthesis was pursued. This letter delineates the work done to go from laboratory scale (lab scale) to large scale, more specifically from a 1 g-scale to a 400 g-scale. Also, an evaluation of the physicochemical stability of the active compound E is presented.
1.1. Safety Assessment of the Active Compound.
Compound
E has a relatively high ‘nitrogen to carbon’-ratio (N:C; 6:9, see
Scheme 1) making it potentially explosive[
5] and therefore it is important to study the compounds physicochemical stability before initiating a large-scale synthesis. While preliminary tests, including mechanical stress (friction by mortar and shock by wrench) and thermal stability (boiling in sulfuric acid) did not show any significant chemical degradation or decomposition, an extended investigation is needed.
Table 1 presents the results from the ‘OSHA
1 Combustible Dust National Emphasis Program (NEP) Recommended Tests’ done by Fauske and Associates, LLC.
The safety assessment concludes that the active compound, E, is not sensitive to mechanical stress, neither shock nor friction. Overall, it was stated that the UN Manual of Tests and Criteria for Transportation of Dangerous Goods consider compound E ‘safe to transport’, Looking at the minimum explosible concentration (MEC), minimum ignition energy (MIE), explosion sensitivity (ES) and ignition sensitivity (IS), it can be concluded that a dust cloud of E is sensitive to electrical sparks and classifies as an St3 explosive (deflagration index, Kst >300). ES and IS for E are calculated using a minimum autoignition temperature (MIT) of 601°C. These values classify E as a Class II dust according to the National Electrical Code ranking, and proper dust control and ventilation should be ensured in a large-scale facility. Dust clouds of E are not heat sensitive below 600°C but show decomposition above 700°C. When heated above 700°C the monitored heat flow is positive, indicating that a reaction is taking place; probably a decomposition to CO2 and NOX.
1.2. Synthetic Route
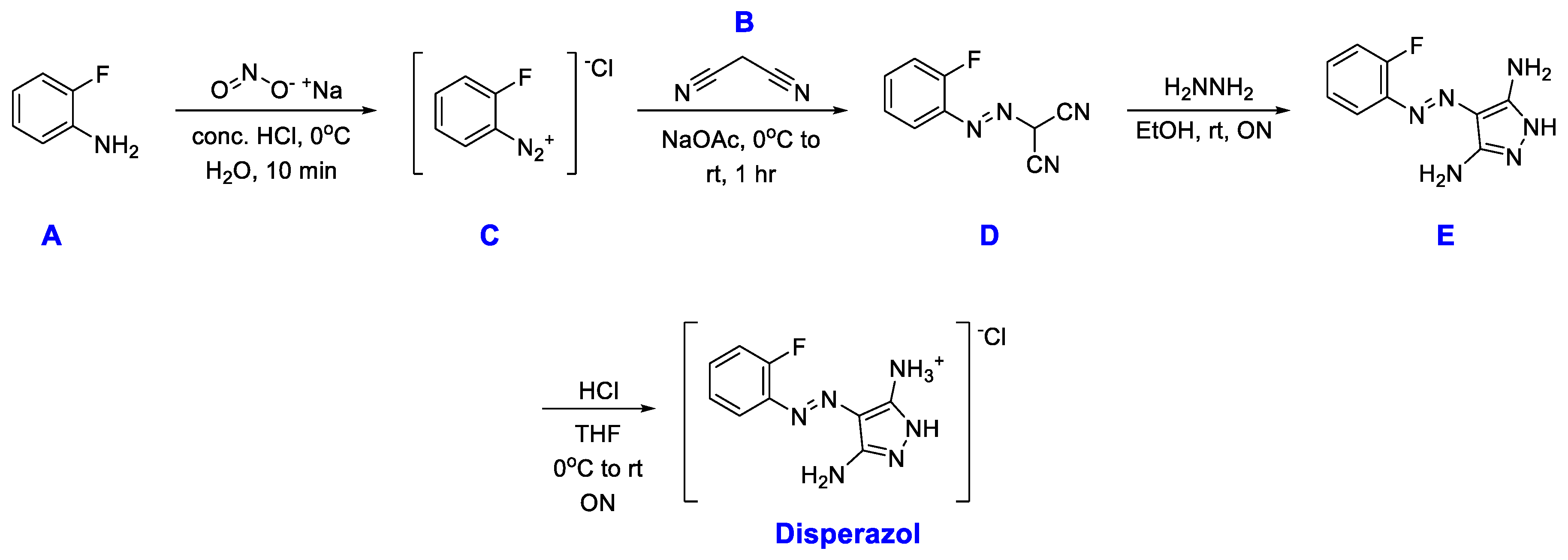
During the initial development and optimisation, Disperazol was synthesised through a 3-step synthesis[
3] shown in
Scheme 2, without isolation of the diazonium-ion
C. Initially, the diazonium-ion is formed from the aniline
A and stabilised under cold acidic conditions. The diazotised aniline
C is then quenched by addition of malononitrile (
B), which reacts to give the intermediate
D. Intermediate
D is isolated by precipitation and then dissolved in EtOH to undergo ring-closure upon addition of hydrazine, leading to formation of the active compound
E. The last step is the HCl-salt formation to give Disperazol with improved water solubility[
2].
2. Results and Discussion
It is important to consider physical parameters when scaling up a synthesis[
6]. The surface area to mass relationship, especially for heat/cold transfer, along with mixing, and chemical and physical properties, all play an important role in organic chemistry and can be significantly different in a large-scale synthesis compared to a batch reaction. Therefore, care should be taken when developing a large-scale process.
2.1. Step 1 – Diazotisation
When scaling up a diazotisation reaction, the instability of the formed diazonium ion must be acknowledged. The current reaction involves the formation of the aromatic diazonium-ion
C, which is unstable if not handled correctly. Upon decomposition the diazonium-species rapidly losses N
2 (gas), which can build pressure inside the reaction container[
7]. Ensuring low temperature (< 5°C)[
8] is crucial for the safety of this step, which can be difficult in a large-scale production. Historically, most incidents with diazo coupling reactions have resulted from isolation and/or accumulation of a diazonium species where decomposition can be caused by shock, friction, heat, light and a number of other factors[
8,
9,
10,
11,
12,
13]. One of the main reasons for the limited stability is the low bond strength between the diazo group and the aromatic ring[
9], therefore both the substitution pattern and counterion will affect the stability[
14]. In general, smaller counterions like chlorides and acetates has a low stability whereas larger counterions like tetrafluoroborates and tosylates provide higher stability[
15].
To eliminate the potential danger of the diazotization step, we pursued to develop a safe and ‘easy to handle’ procedure to form the intermediate
D. Inspired by the work of Jacq and Pasau[
16] this involved 1) Substituting sodium nitrite (NaNO
2) with tert-butyl nitrite (TBN) as a safer diazonium reagent, which would also enable the reaction to be run safely at higher temperatures. 2) Transferring the first step of the synthesis from batch to flow synthesis, hereby avoiding accumulation of the unstable intermediate
C. TBN has been reported as a mild, nonexplosive and stable diazonium reagent[
17,
18,
19,
20] with a high safety profile in both batch and flow processes[
16,
21] from small to bulk multikilogram scale[
22,
23]. Diazotization reactions using TBN have been run at temperatures ranging from room temperature to 80°C[
24,
25]. The higher stability of the
in-situ formed diazonium species could arise from the lager counterion in
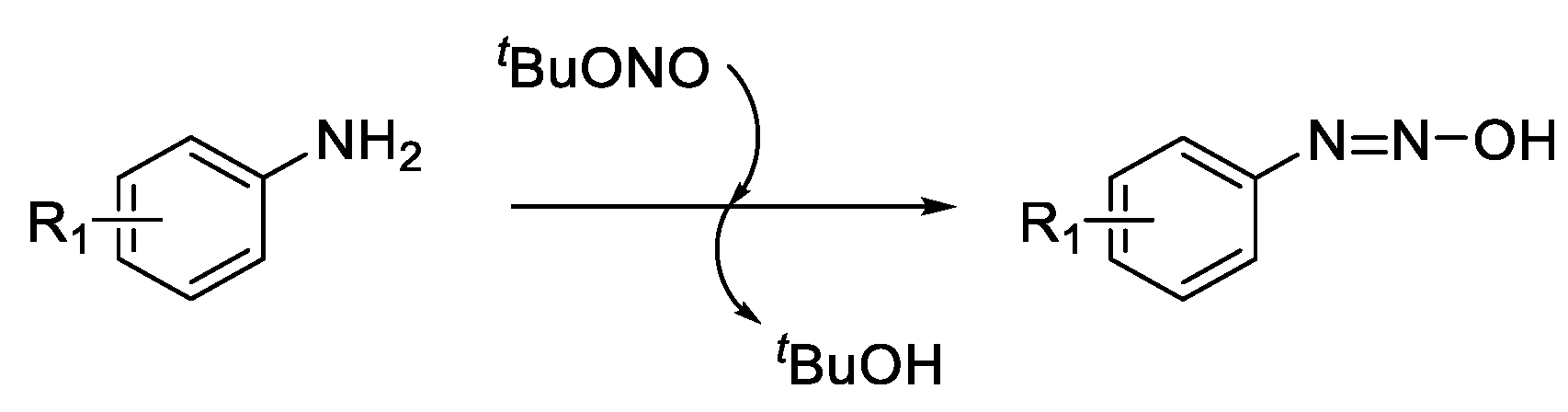
tert-butyl oxide but it has also been suggested that a hydroxydiazene intermediate is formed (
Scheme 3)[
26,
27].
Reactions that involve formation of hazardous intermediates are advantageously run using flow chemistry, to ensure the reactive hazardous species is continuously formed in small amounts. Immediate consumption will then eliminate the need for stockpiling[
9,
15,
28,
29]. As accumulation of large quantities of the hazardous compound is avoided, flow synthesis also eliminates the need for cooling and even heating of diazotization reactions has been done safely[
30]. The many advantages in flow synthesis provide an opportunity for both the academic and industrial chemical sectors to safely run chemical reaction with hazardous compounds. Flow chemistry is for example applied for diazotization reactions by established companies as Lundbeck[
31] and Pfizer[
32].
Inspired by Jacq and Pasau[
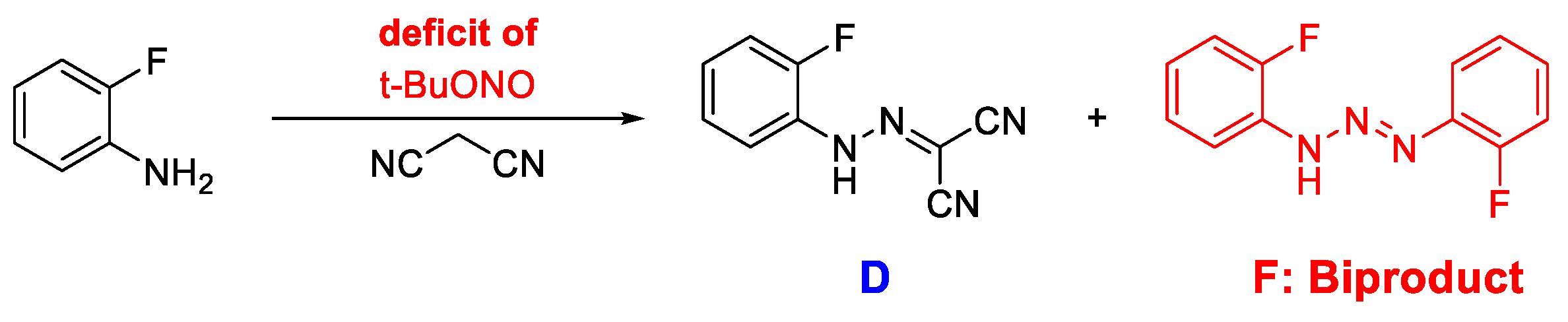
16]we initiated our work towards establishing a flow synthesis of Disperazol by setting up the Uniqsis FlowSyn machine at room temperature with a reagent ratio of 1:1:1.3 (aniline:malononitrile:TBN) with an aniline concentration of 0.4 M, and a coil resident time of 5 min. However, these conditions resulted in both lower yield and the formation of impurities. Therefore, the flow reaction required some optimisation. One of the main impurities being formed was the biproduct
F resulting from the condensation of the aryl diazonium species with another aniline species, see
Scheme 4. This was not observed under the original lab scale synthesis and is most likely a result of changing the reaction conditions. The diazotisation reaction is no longer run under acidic conditions, which will make the aniline nucleophile. To optimize the reaction, a range of parameters was investigated, see
Table 2. By changing them one at a time, the effect of each parameter on the purity of the crude product was determined. All reactions were analysed with HPLC.
Through the investigations it was found that a temperature of 28°C in the reaction coil gave high product purity, with low reaction time (3 min) and with high concentrations of reactants (2 M) in a 1:1:1 ratio. After an extensive number of reactions (see
Table 2), an optimised flow procedure was identified, allowing formation of pure intermediate
D in high yields in a short time. In the optimised flow design, see
Scheme 5, the 2-fluoro aniline (
A) and malononitrile (
B) were mixed in one flask (ratio 1:1) in acetonitrile (ACN, 2 M), while the other flask contained the TBN dissolved in ACN (2 M). The two streams are telescoped in a T-mixer unit before being introduced to the PTFE coil reactor (14 mL) at 28°C. Here the diazonium specie is formed
in situ and instantly consumed by the malononitrile present in the same stream[
16,
25]. It was found that the reaction was slightly exothermic, so to ensure a constant temperature in the coil at 28°C, a fan was placed next to the coil. This enabled the production of approximately 420 g (280 mmol) of
D in 8 hrs.
2.2. Step 2 – Ring closure of Pyrazole
Step 2 and 3 of the synthesis were both done as batch chemistry. The second step of the small-scale synthesis proceeds by dissolving the intermediate
D in ethanol (0.34 M), followed by addition of hydrazine hydrate (1.2 equiv.). The mixture was allowed to stir overnight at room temperature, resulting in precipitation of the active compound
E[
3]. To ensure full precipitation and herby improve the yield on larger scale a series of solvents was investigated as compound
E is partly soluble in ethanol. The solvents investigated included ACN, water, acidic and basic aqueous solutions, diethyl ether, toluene, THF, heptane, dichloromethane (DCM) and acetone. From the solvent tests, it was found that
E nicely precipitated in diethyl ether, while solubility of the intermediate
D was achievable upon heating. When running the reaction at large scale, it was observed that the ring closure was rather exothermic. While this required slow addition of hydrazine hydrate (1.15 equiv.), it was fortunately found to help dissolving
D without the need for external heating. Diethyl ether was found to be a good solvent with a concentration of intermediate
D at 0.5 M. For step 2 the largest amount of compound
E synthesised in one round was 652 g.
2.3. Step 3 – Formulation as a salt
To improve the water solubility of the active compound
E, it was formulated as the hydrochloride-salt[
2], E•HCl, denominated Disperazol. For the lab scale synthesis[
2] of Disperazol the active compound
E was dissolved in dry 1,4-dioxane (0.1 M), followed by addition of a solution of HCl (4 M in dioxane, 5 equiv.) under a nitrogen atmosphere and stirred overnight. For the large-scale synthesis, again different solvents were investigated, as 1,4-dioxane is carcinogenic. Also, different reaction concentrations were tested as large-scale reactions are preferable run at higher concentrations than 0.1 M to limit the reaction volume. The investigation included diethyl ether, acetone, ethyl acetate and tetrahydrofuran (THF), where THF was found to give the most reliable results and a concentration of 0.2 M was found to give good conversion and high purity. For the large-scale synthesis compound
E is dissolved in dry THF (0.2 M) under a nitrogen atmosphere with gentle heating to fully dissolve the active compound. The mixture is then cooled to 0°C using an ice bath, before addition of HCl (2 M in diethyl ether, 1 equiv.), and afterwards allowed to stir overnight at room temperature. The next day, the reaction mixture is cooled to 0°C for two hours to ensure full product precipitation, before isolating the product by suction filtration. In case the precipitation does not start upon cooling, dry diethyl ether is added to start the precipitation. The largest scale synthesised formed 43 g of Disperazol (80%).
2.4. Summary
The synthetic route for Disperazol has successfully been scaled from a 9 mmol scale to a 3.6 mol with satisfying yields and easy purification. To eliminate the potential danger of the diazotization-step a flow-strategy was implemented and optimised, resulting in a clean reaction with good yields. Additionally, the physicochemical stability properties of the active compound E were evaluated for both the solid powder and the dust particles, providing insight to the future implementation in an industrial production facility. It was found that the solid powder of E was non-explosive to all tested parameters.
3. Materials and Methods
Flow synthesis was performed on an Uniqsis FlowSyn System with FlowSyn Steel™, 316L flow path, 2 x 10 ml/min, Pmax=100bar, Tmax=260°C and a 14 mL PTFE coil.
Procedure for large-scale synthesis of Disperazol. For step 1; synthesis of
D; the two solutions are prepared for the flow system. Flask A: 2-fluoroaniline (CAS 348-54-9, 444.44 g, 1 equiv.), malononitrile (CAS 109-77-3, 264.39 g, 1 equiv.) and acetonitrile (75-05-8, fill up to 2000 mL). Flask B: tert-butyl nitrite (540-80-7, 475.7 mL,1 equiv.) and acetonitrile (75-05-8, fill up to 2000 mL). The flow system is configured as shown on
Scheme 5.
For the reaction using FlowSyn, 99% of the prepared solutions (Flask A and B) is used (1980 mL), the coil residence time is set to 3 min giving a total flow rate of 4.67 mL/min. The coil temperature is set to 28°C, and pre- and post-collect is set to 0.5 and 8.5 mL respectively. Additionally, a small fan is installed next to the coil to keep the temperature constant at 28°C of the slightly exothermic reaction. Total operation time is approximately 14 hours without the final washing of the system. Then the solvent is removed in vacuo to give the crude intermediate D in quantitative yield (approx. 745 g, 3.96 mol), which is used directly in the next step.
In step 2; synthesis of E; the ring-closure. The solid intermediate D from step 1 (745 g, 1 equiv.) was transferred to a conical flask (not round bottomed) with a stir bar. Solvent (Et2O, CAS 60-29-7, 2.02 mL/mmol, corresponding to 8000 mL) was added to give a concentration of 0.5 M and to the slurry was then added hydrazine hydrate (CAS 10217-52-4, 221.4 mL,1.15 equiv.) dropwise under constant stirring, as the reaction is highly exothermic. Upon addition of hydrazine the intermediate will fully dissolve, and the product will start to precipitate. When all hydrazine has been added, the reaction is left stirring overnight at room temperature. Next day the product was isolated by suction filter and wash with cold Et2O to give the active compound E as and orange solid (652 g, 75%).
For the third step; synthesis of Disperazol; salt formation. For better water solubility the active compound E is formulated as a hydrochloride-salt. This is achieved by dissolving E from step 2 (46 g, 1 equiv.) in dry THF (CAS 109-99-9, 0.2 M, 5.32 mL/mmol, corresponding to 1110 mL) under a nitrogen atmosphere, with gentle heating to fully dissolve the active compound. The solution is then cooled to 0°C in an ice bath and HCl (2 M in Et2O, CAS 7647-01-0, 105 mL, 1 equiv.) was slowly added. The reaction is allowed to heat to room temperature and left overnight. Next day the reaction mixture is again cooled to 0°C for two hours. The product is isolated by suction filtration and washed with small amount of cold Et2O, to give Disperazol (43 g, 80%). In case the salt does not precipitate dry Et2O can be added to the reaction mixture before filtration.
Author Contributions
Conceptualization, K.Q., C.U.J and K.E.G.; methodology, C.U.J and K.E.G.; validation, C.U.J., K.E.G., J.B.A., L.D.H. and M.N.; investigation, C.U.J and K.E.G.; data curation, C.U.J and K.E.G.; writing—original draft preparation, K.Q., C.U.J and K.E.G.; writing—review and editing, all authors; visualization, C.U.J.; supervision, K.Q. and M.Gz.; project administration, K.Q., T.T.N, M.G., M.Gz and C.E.M.; funding acquisition, K.Q., T.T.N, M.G., M.Gz and C.E.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Sygeforsikringen, grant number 2021-0300.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data was created.
Conflicts of Interest
The authors declare no conflicts of interest.
Note
| 1 |
Occupational Safety and Health Administration |
References
- J.B. Andersen, L.D. Hultqvist, C.U. Jansen, T.H. Jakobsen, M. Nilsson, M. Rybtke, J. Uhd, B.G. Fritz, R. Seifert, J. Berthelsen, T.E. Nielsen, K. Qvortrup, M. Givskov, T. Tolker-Nielsen, Identification of small molecules that interfere with c-di-GMP signaling and induce dispersal of Pseudomonas aeruginosa biofilms, Npj Biofilms Microbiomes 7 (2021) 59. [CrossRef]
- L.D. Hultqvist, J.B. Andersen, C.M. Nilsson, C.U. Jansen, M. Rybtke, T.H. Jakobsen, T.E. Nielsen, K. Qvortrup, C. Moser, M. Graz, K. Qvortrup, T. Tolker-Nielsen, M. Givskov, High efficacy treatment of murine Pseudomonas aeruginosa catheter-associated urinary tract infections using the c-di-GMP modulating anti-biofilm compound Disperazol in combination with ciprofloxacin, Antimicrob. Agents Chemother. (2024) e01481-23. [CrossRef]
- C.U. Jansen, J. Uhd, J.B. Andersen, L.D. Hultqvist, T.H. Jakobsen, M. Nilsson, T.E. Nielsen, M. Givskov, T. Tolker-Nielsen, K.M. Qvortrup, SAR study of 4-arylazo-3,5-diamino-1 H -pyrazoles: identification of small molecules that induce dispersal of Pseudomonas aeruginosa biofilms, RSC Med. Chem. 12 (2021) 1868–1878. [CrossRef]
- C. Manner, R. Dias Teixeira, D. Saha, A. Kaczmarczyk, R. Zemp, F. Wyss, T. Jaeger, B.-J. Laventie, S. Boyer, J.G. Malone, K. Qvortrup, J.B. Andersen, M. Givskov, T. Tolker-Nielsen, S. Hiller, K. Drescher, U. Jenal, A genetic switch controls Pseudomonas aeruginosa surface colonization, Nat. Microbiol. (2023). [CrossRef]
- M.A. Tarselli, Life and death with nitrogen, Nat. Chem. 4 (2012) 686–686. [CrossRef]
- M.G. Warawdekar, Challenges in Scale-Up of Specialty Chemicals—A Development Chemist’s Perspective, in: Ind. Catal. Process. Fine Spec. Chem., Elsevier, 2016: pp. 721–736. [CrossRef]
- S. Partington, S.P. Waldram, Runaway Reaction During Production of an Azo Dye Intermediate, Process Saf. Environ. Prot. 80 (2002) 33–39. [CrossRef]
- M. Sheng, D. Frurip, D. Gorman, Reactive chemical hazards of diazonium salts, J. Loss Prev. Process Ind. 38 (2015) 114–118. [CrossRef]
- V.D. Filimonov, E.A. Krasnokutskaya, A.A. Bondarev, Structures, Stability, and Safety of Diazonium Salts, in: M.M. Chehimi, J. Pinson, F. Mousli (Eds.), Aryl Diazonium Salts Relat. Compd., Springer International Publishing, Cham, 2022: pp. 35–57. [CrossRef]
- L. Bretherick, Bretherick’s handbook of reactive chemical hazards: an indexed guide to published data, Eighth edition, Elsevier, Amsterdam Oxford Cambridge, MA, 2017.
- R. Ullrich, Th. Grewer, Decomposition of aromatic diazonium compounds, Thermochim. Acta 225 (1993) 201–211. [CrossRef]
- S. Kittsley, Need for minimum standards in evaluating doctoral programs, J. Chem. Educ. 48 (1971) 419. [CrossRef]
- J.D. Firth, I.J.S. Fairlamb, A Need for Caution in the Preparation and Application of Synthetically Versatile Aryl Diazonium Tetrafluoroborate Salts, Org. Lett. 22 (2020) 7057–7059. [CrossRef]
- A.A. Bondarev, E.V. Naumov, A.Zh. Kassanova, E.A. Krasnokutskaya, K.S. Stankevich, V.D. Filimonov, First Study of the Thermal and Storage Stability of Arenediazonium Triflates Comparing to 4-Nitrobenzenediazonium Tosylate and Tetrafluoroborate by Calorimetric Methods, Org. Process Res. Dev. 23 (2019) 2405–2415. [CrossRef]
- B.J. Deadman, S.G. Collins, A.R. Maguire, Taming Hazardous Chemistry in Flow: The Continuous Processing of Diazo and Diazonium Compounds, Chem. - Eur. J. 21 (2015) 2298–2308. [CrossRef]
- J. Jacq, P. Pasau, Multistep Flow Synthesis of 5-Amino-2-aryl-2 H -[1,2,3]-triazole-4-carbonitriles, Chem. - Eur. J. 20 (2014) 12223–12233. [CrossRef]
- F.P. Crisóstomo, T. Martín, R. Carrillo, Ascorbic Acid as an Initiator for the Direct C-H Arylation of (Hetero)arenes with Anilines Nitrosated In Situ, Angew. Chem. Int. Ed. 53 (2014) 2181–2185. [CrossRef]
- D. Qiu, H. Meng, L. Jin, S. Wang, S. Tang, X. Wang, F. Mo, Y. Zhang, J. Wang, Synthesis of Aryl Trimethylstannanes from Aryl Amines: A Sandmeyer-Type Stannylation Reaction, Angew. Chem. Int. Ed. 52 (2013) 11581–11584. [CrossRef]
- A. Chakraborty, S. Jana, G. Kibriya, A. Dey, A. Hajra, tert-Butyl nitrite mediated azo coupling between anilines and imidazoheterocycles, RSC Adv. 6 (2016) 34146–34152. [CrossRef]
- L. He, G. Qiu, Y. Gao, J. Wu, Removal of amino groups from anilines through diazonium salt-based reactions, Org. Biomol. Chem. 12 (2014) 6965. [CrossRef]
- T. Hu, I. Baxendale, M. Baumann, Exploring Flow Procedures for Diazonium Formation, Molecules 21 (2016) 918. [CrossRef]
- M. Mihelač, A. Siljanovska, J. Košmrlj, A convenient approach to arenediazonium tosylates, Dyes Pigments 184 (2021) 108726. [CrossRef]
- N. Oger, M. d’Halluin, E. Le Grognec, F.-X. Felpin, Using Aryl Diazonium Salts in Palladium-Catalyzed Reactions under Safer Conditions, Org. Process Res. Dev. 18 (2014) 1786–1801. [CrossRef]
- F. Mo, Y. Jiang, D. Qiu, Y. Zhang, J. Wang, Direct Conversion of Arylamines to Pinacol Boronates: A Metal-Free Borylation Process, Angew. Chem. Int. Ed. 49 (2010) 1846–1849. [CrossRef]
- N. Oger, E. Le Grognec, F.-X. Felpin, Handling diazonium salts in flow for organic and material chemistry, Org. Chem. Front. 2 (2015) 590–614. [CrossRef]
- F.L. Callonnec, E. Fouquet, F.-X. Felpin, Unprecedented Substoichiometric Use of Hazardous Aryl Diazonium Salts in the Heck-Matsuda Reaction via a Double Catalytic Cycle, Org. Lett. 13 (2011) 2646–2649. [CrossRef]
- N. Susperregui, K. Miqueu, J.-M. Sotiropoulos, F. Le Callonnec, E. Fouquet, F.-X. Felpin, Sustainable Heck-Matsuda Reaction with Catalytic Amounts of Diazonium Salts: An Experimental and Theoretical Study, Chem. - Eur. J. 18 (2012) 7210–7218. [CrossRef]
- B. Ahmed-Omer, D.A. Barrow, T. Wirth, Heck reactions using segmented flow conditions, Tetrahedron Lett. 50 (2009) 3352–3355. [CrossRef]
- M. Movsisyan, E.I.P. Delbeke, J.K.E.T. Berton, C. Battilocchio, S.V. Ley, C.V. Stevens, Taming hazardous chemistry by continuous flow technology, Chem. Soc. Rev. 45 (2016) 4892–4928. [CrossRef]
- C.J. Smith, C.D. Smith, N. Nikbin, S.V. Ley, I.R. Baxendale, Flow synthesis of organic azides and the multistep synthesis of imines and amines using a new monolithic triphenylphosphine reagent, Org. Biomol. Chem. 9 (2011) 1927. [CrossRef]
- M.A. Nielsen, M.K. Nielsen, T. Pittelkow, Scale-Up and Safety Evaluation of a Sandmeyer Reaction, Org. Process Res. Dev. 8 (2004) 1059–1064. [CrossRef]
- B. Li, D. Widlicka, S. Boucher, C. Hayward, J. Lucas, J.C. Murray, B.T. O’Neil, D. Pfisterer, L. Samp, J. VanAlsten, Y. Xiang, J. Young, Telescoped Flow Process for the Syntheses of N -Aryl Pyrazoles, Org. Process Res. Dev. 16 (2012) 2031–2035. [CrossRef]
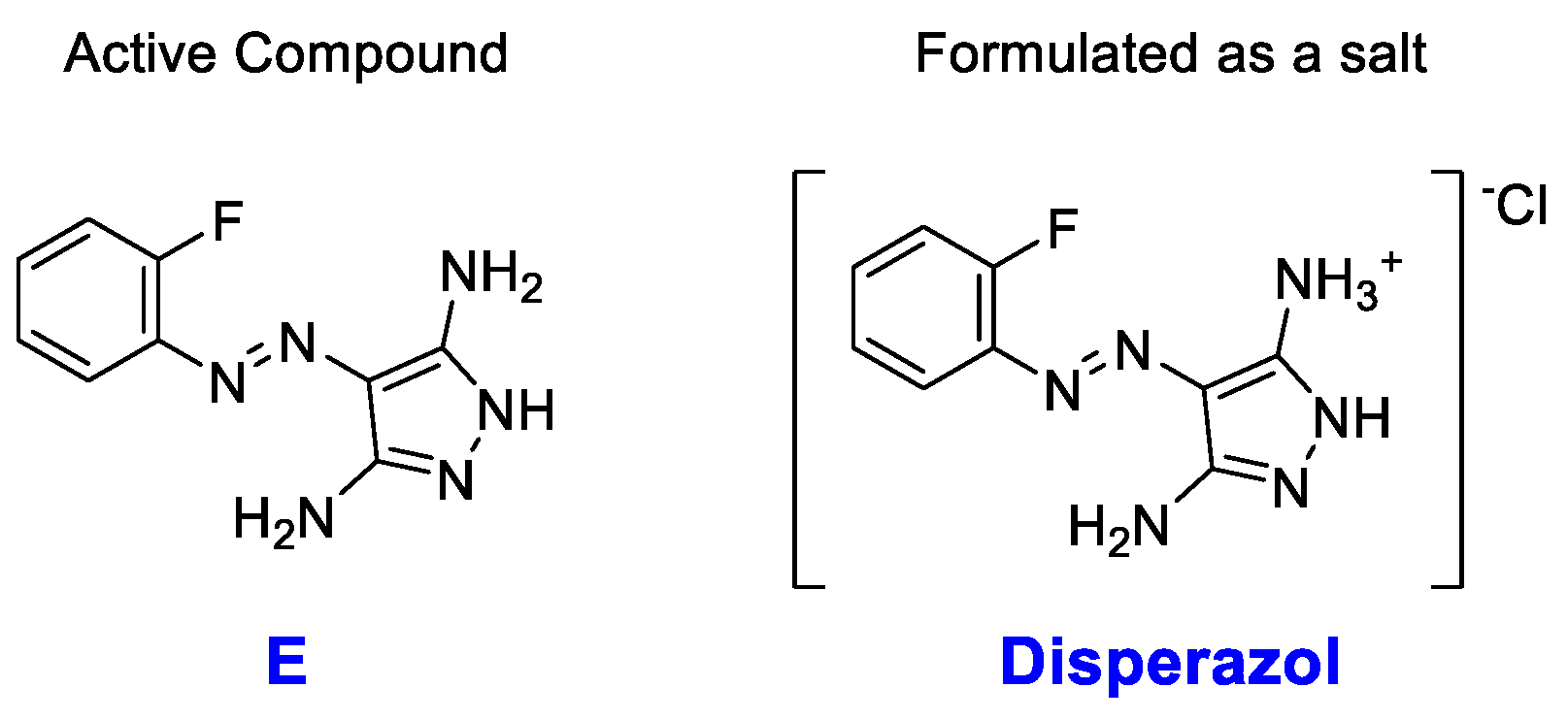
Scheme 1.
Chemical structure of the active compound E and the formulated Disperazol.
Scheme 1.
Chemical structure of the active compound E and the formulated Disperazol.
Scheme 2.
Lab scale synthetic route[
2,
3], where rt is room temperature and ON is overnight.
Scheme 2.
Lab scale synthetic route[
2,
3], where rt is room temperature and ON is overnight.
Scheme 3.
Formation of hydroxydiazene intermediate[
26,
27].
Scheme 3.
Formation of hydroxydiazene intermediate[
26,
27].
Scheme 4.
Reaction scheme for the formation of biproduct F under the conditions where TBN is in deficit.
Scheme 4.
Reaction scheme for the formation of biproduct F under the conditions where TBN is in deficit.
Scheme 5.
Schematic of the flow design for step 1. Made with BioRender and PowerPoint.
Scheme 5.
Schematic of the flow design for step 1. Made with BioRender and PowerPoint.
Table 1.
OSHA recommended safety assessment of the active compound E, conducted by Fauske and Associates, LLC.
Table 1.
OSHA recommended safety assessment of the active compound E, conducted by Fauske and Associates, LLC.
| TEST |
Compound E |
| Drop-weight impact/Fallhammer (bulk sample) |
> 100 J |
| Friction sensitivity (bulk sample) |
> 360 N |
| Explosion severity, ES, and Ignition sensitivity, IS (dust cloud) |
Pmax = 9.6 bar
(dP/dt)max = 1128 bar/s
Kmax =306 bar·m/s
ES = 2.9
IS = 13.1
|
| Minimum explosible concentration, MEC (dust cloud) |
50-60 g/m3
Estimate: 56 g/m3
|
| Minimum ignition energy, MIE (dust cloud) |
3-10 mJ
Estimate: 8 mJ
|
| Minimum autoignition temperature, MIT (dust cloud) |
> 600°C |
| Total combustible content (bulk sample) |
100 wt% > 700°C |
Table 2.
Parameters alternated during optimisation of the flow-setup.
Table 2.
Parameters alternated during optimisation of the flow-setup.
| Parameters |
|
| Concentration of A |
0.3 M, 0.4 M, 0.6 M, 0.8 M, 1.0 M, 2.0 M |
| Temperature |
Room temperature (ranging from 23-26°C), 28°C, 30°C |
| Coil resident time |
2.5 min, 3 min, 4 min, 5 min, 6.5 min, 7.5 min, 8 min, 10 min, 12 min, 15 min |
| Reagents ratio (A:B:TBN) |
1:1:1, 1:1:1.3, 1:1:1.7, 1:1:2, 1:1:2.3, 1:1.1:1, 1:1.2:1, 1:1.3:2, 1:1.3:1.3, 1:1.5:1.3, 1:2:1.3 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).