Sceptics stimulate the quest for better models of the world we inhabit. The medieval sceptic William of Occam (1285-1347) was excommunicated, or in modern parlance, non-platformed, for his controversial views that remain the bedrock of modern scientific enquiry. Entia non sunt multiplicanda praeter necessitatem... [Occam]
Today we face complex issues with climate change paramount. How can plant scientists contribute to the debate (Eckardt et al. 2023)? Which has the greater influence? Water deficit or CO2 abundance? Inspired by Occam we suggest a simple approach based on plant biochemistry at the single cell level, specifically stomatal guard cells. This involves the novel role of classical AGPs juxtaposed with the plasma membrane proton pump that dissociates AGP-Ca2+ and thus underpins Ca2+ homeostasis; the profound ecological consequences prompt this brief essay. Our working hypothesis suggests that AGP adaptations extend the habitable space in several ways; First and foremost stomatal regulation is a crucial component of global homeostasis; and secondly AGPs enable growth under highly saline conditions. Therefore, we describe those adaptations and detailed evidence for the growth oscillator to show how they may enhance plant survival under the extremes induced by climate change. These adaptations also support the Gaia hypothesis of James Lovelock and Lynn Margulis; i.e. regulation of the environment is homeostatic.
Figure 1.
Photograph of the Earth taken on December 7, 1972, by the crew of the Apollo 17 spacecraft en route to the Moon at a distance of about 29,400 kilometres (18,300 mi). Planet Earth appears as a single cell floating in space.
Figure 1.
Photograph of the Earth taken on December 7, 1972, by the crew of the Apollo 17 spacecraft en route to the Moon at a distance of about 29,400 kilometres (18,300 mi). Planet Earth appears as a single cell floating in space.
Despite recent advances many intractable problems remain in major fields including both physics and biology. Both involve protons as primary players.
James Bonner (1934) first noted that protons enhance extension growth of oat coleoptiles but the precise mechanism of such “acid growth” remains speculative focussed largely on biophysical cell wall properties assuming they depend directly on protons secreted by the proton pump. Although that has revealed tantalising results involving the role of pectin, definitive biochemical description remains elusive. Surprisingly, an alternative approach involving the recent growth oscillator hypothesis is relevant to climate change ... it proposes that protons affect wall properties indirectly rather than directly, but how is Bonner’s problem relevant to climate change?
While much work invokes Ca2+ as a regulator of plant growth, the plasma membrane proton pump also plays an essential role in “acid growth”. Recently, a direct connection between proton efflux and Ca2+ influx suggests that in addition to auxin efflux “PIN” proteins and Ca2+ channels, AGPs and proton pumps embedded in the plasma membrane comprise a simple oscillator that regulates cytosolic Ca2+. Arguably the oscillator is involved in numerous dynamic aspects of plant biology from tip growth of pollen tubes to stomatal regulation. This revisionist view of proton pump and AGP interactions has widespread consequences that require critical analysis; the biochemical evidence summarised here leads us to consider the crucial role of stomata in the water cycle and its contribution to global homeostasis.
First we consider how the structure of classical arabinogalactan glycoproteins (AGPs) connect proton efflux with Ca2+ influx.
The isolation of cell walls of algae like Chlorella (Northcote 1958) led to the isolation of cell suspension cultures of higher plants and their pure primary cell wall containing hydroxyproline rich proteins extensins. AGPs isolated later by iso-electric focusing as an acidic protein-polysaccharide complex (Lamport 1970) with non-contiguous Hyp differed from the contiguous Hyp of the extensin Ser-Hyp4 signature sequence. Thus, Hyp contiguity encodes glycosylation with short arabinosides in extensin but small acidic arabinogalactan-oligosaccharides in AGPs suggesting quite a different signalling function rather than purely structural.
Classical AGPs are predominantly cell surface and readily solubilised although a substantial proportion is GPI-anchored to the plasma membrane (Lamport et al. 2006). Classical AGPs are thus in prime position as major components covering the outer surface of the plasma membrane. Faced with the huge multiplicity of AGPs in the genome our conclusions might appear over simplified! However, classical AGPs purified by Yariv precipitation represent by far the major bulk of AGPs; most other AGPs actually minor cellular components confuse the issue that was resolved by structural elucidation of the classical AGP glycomodule.
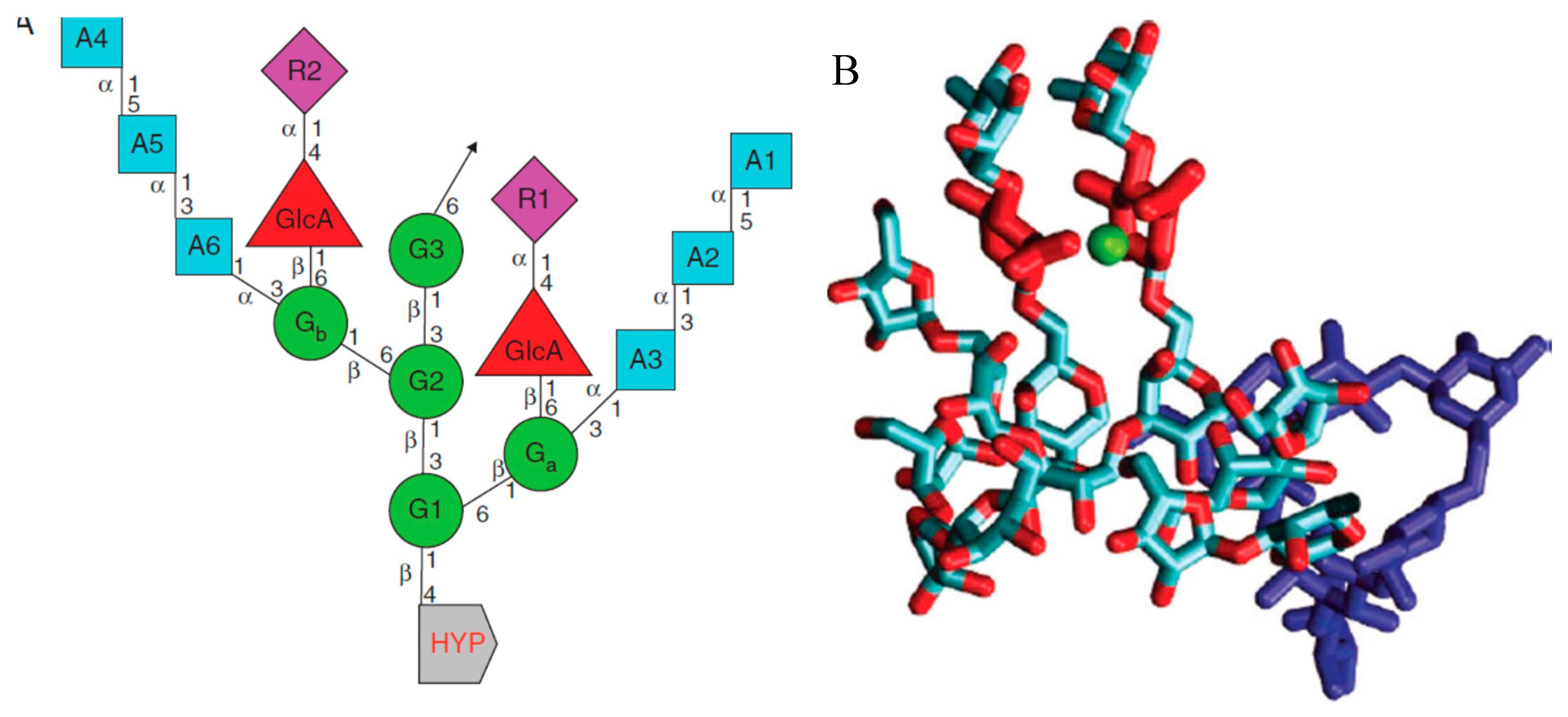
AGP arabinogalactan was long considered an intractable mixture resistant to structural elucidation until the structural analysis of small Hyp-oligosaccharide fragments purified from alkaline hydrolysates of intact AGPs. Further analysis of these fragments by high resolution nuclear magnetic resonance techniques identified the precise linkage analysis of a 15-residue consensus structure (
Figure 2.) consisting of a beta1,3 galactan backbone with two sidechains terminated by glucuronic acid residues. Molecular modelling and molecular dynamics simulations showing the close proximity of their twin carboxyls identified potential intramolecular Ca
2+-binding sites. (
Figure 2B). That Eureka moment of truth was verified by quantitative analysis showed quantitative binding of Ca
2+ by AGPs. Further assay of Ca
2+-AGP binding showed its pH dependence, thus juxtaposition of AGPs and proton pump makes dissociation inevitable under “acid growth” conditions and identified an extracellular source of cytosolic Ca
2+ that established a major role for cell surface AGPs as a dynamic Ca
2+ reservoir. Indeed, the tip-focused Ca
2+ gradient of oscillatory pollen tube growth (Hepler et al. 2006) shows that such cytosolic oscillations are widespread including stomata. This novel role for proton pumps and AGPs as membrane embedded components of a global growth oscillator represents a paradigm shift currently overlooked by several recent reviews with the notable exception of (Hromadova et al. 2021) (Boerjan et al. 2024; Delmer et al. 2024; Domozych, LoRicco 2024; Geitmann & Bacic 2024; Jobert et al. 2023; Jonsson et al. 2021; Yu, Zhang, Cosgrove. 2024). This may imply legitimate scepticism based on lack of experimental evidence for Gaia as a verifiable hypothesis. However, the pivotal role of stomata and their underlying function in the global water cycle depends on extensive evidence for an essential AGP-Ca
2+ growth oscillator discussed here. Finally we consider how the growth oscillator and its ramifications enable plant adaptation and contribute to global homeostasis.
The Eureka moment of truth revealed by molecular modelling (
Figure 2.) identified twin glucuronic acid residues in AGP glycomodule subunits (Tan et al. 2010). A 2:1 glucuronic acid:Ca
2+ binding stoichiometry (Lamport & Varnai 2013) implied a role in Ca
2+ signalling/homeostasis where classical AGPs act as a dynamic Ca
2+ reservoir at the cell surface as the source of cytosolic Ca oscillations. These observations arguably solve the major problem of classical AGP function further corroborated by the use of multiple glucuronosyl transferase knockouts that severely impair Ca
2+ homeostasis, indicating that glucuronic-bound Ca
2+ is an essential source of cytosolic Ca
2+ (Lopez-Hernandez et al. 2020; Ajayi, et al. 2021) discussed further in (Lamport 2023). This may help to explain the ubiquity of classical AGPs in the Plant Kingdom and their Ca
2+-binding properties: “
AGPs are implicated in many developmental processes. (Cosgrove 2023). We hypothesize that classical AGPs of the cell surface define
the primary role of an auxin-activated proton pump as an essential component of calcium homeostasis that regulates many aspects of plant biology that include plant tropisms, pollen tube growth, gravitropism, phyllotaxis and stomatal dynamics, as follows:
The power of movement in plants (Darwin 1880) describes how plants respond to specific stimuli from gravity to light with characteristic tropes. Such expression defined by botanists as tropisms are auxin dependent and the result of unequal growth by cell extension.
Numerous auxin-dependent tropisms range from thigmotropism to gravitropism and phototropism summarised by Table 3 of (Lamport et al 2014) where expression of AGPs, Ca2+ signalling and proton pump activity is evidence of widespread oscillator activity in many cells and tissues discussed as follows:
The simplest cell wall model exemplifies Occam: At the pollen tube tip the cell wall is a simple pectin matrix that lacks a cellulosic framework. This model stimulated much research particularly by Hepler and his colleagues. They noted that ions play a crucial role in the control of pulsatile oscillatory pollen tube growth (Hepler et al. 2006) with the crucial observation that cytosolic Ca2+oscillations form a steep intracellular tip-focused gradient with H+ also forming an intracellular gradient with a slightly acidic domain at the extreme apex....their additional insights also noted the.... “transmembrane pH gradients, driven by the H+-ATPase” suggesting “a close coupling between the intracellular [Ca2+] gradient and extracellular influx” and pectin de-esterification providing numerous possible binding sites, hence the possible source of Ca2+ influx “that must be considered in any model of pollen tube growth” summarising these observations quite prophetically: “it is clear that some form of Ca2+ storage is required to account for the marked phase separation in the expression of the intracellular Ca2+ gradient and the influx of extracellular Ca2+. Remarkably, that inference predates AGP-Ca2+ binding (Lamport & Varnai 2013) and is consistent with the current model that involves Ca2+ binding by twin glucuronic acid carboxylate ions of classical AGPs.
Our current model postulates that a growth oscillator regulates tip growth by involving a plasma membrane proton pump that dissociates cell surface AGP-Ca2+ as the source of cytosolic Ca2+. Indeed, proton pumps and AGPs invariably coincide in all metabolically active tissues. Thus the presence of tissue AGPs act as a proxy for growth oscillator activity, depicted metaphorically as a molecular pinball machine (Lamport et al. 2021), widespread throughout the Plant Kingdom briefly summarised below!
Root gravitropism: (Lamport et al. 2022) Close cooperation between the two major cell surface glycoproteins, extensins and AGPs resolves the root-shoot gravitropism paradox. Auxin enhances cell extension of shoots but inhibits root cell extension! Thus the presence of root AGPs (Hromadová et al. 2021) suggests that regulation of growth in roots involves interaction between both extensins and the growth oscillator: The role of hydroxyproline-containing protein (extensin) in the cessation of cell elongation” proposed by Cleland & Karlesnes (1967) implies that a protein network of crosslinked extensin slows cell expansion by stiffening the wall in both stems and roots or even loosening the wall (Rodriguez-Garcia et al 2024). The difference in the auxin response depends on crucial differences between the biochemistry of roots and shoots. Notably, roots express NADPH oxidase, and superoxide dismutase which generate hydrogen peroxide [H2O2] (Foreman et al. 2003) a co-substrate of root peroxidases (Dunand et al. 2007) (Krieger et al. 2016). They also include extensin peroxidase (Everdeen et al. 1988) that generates di-isodityrosine crosslinked extensin networks (Held et al. 2004) that decrease cell expansion. Epidermal cells in the root elongation zone express a specific extensin LeExt1. Thus increased extensin crosslinking on the lower concave side decreases its growth rate and the root bends down. Numerous papers invoke a role of Ca2+ in root gravitropism including AGPs (Hromadová et al. 2021) hence evidence of an active growth oscillator.
An additional crucial role of the growth oscillator arguably involves phyllotaxis and the origin of floral symmetry in the stem apical meristem SAM (Lamport et al. 2020). Cytosolic Ca2+ waves activate exocytosis of wall precursors. AGPs and PIN proteins are critical determinants of an algorithm that generates the phyllotaxis spiral. Primordia are initiated in a “generative annulus”, a thin band of AGP-rich protodermal cells that encircle the outermost cell layer of the stem apical meristem. This may account for the rotational symmetry that predominates in dicot floral phyllotaxis: Speculatively, Fibonacci hybridisation between plants with one, two, three, five and eight petals yields the classic Fibonacci series of up to thirteen and more!!
Rapidly expanding cells of the protoderm transmit the stress vector via anticlinal walls towards slower expanding cells; that stress relocates the PIN proteins so that auxin moves against its concentration gradient towards cells that initiate primordia when auxin reaches a critical threshold level while depleting the auxin of less rapidly expanding cells. Attenuation of the stress vector by intervening auxin depleted distal cells slows their expansion until a boundary “tipping point” of minimum cell expansion appears where the stress vector reverses its direction with concomitant reversal of PIN protein polarity (Feraru et al 2011). Thus, a new auxin gradient increases towards a new stress vector initiated by cell expansion of a newly formed primordium. Hence, auxin waves appear as peaks that generate new primordia separated by auxin troughs.
Stomata provide a critical test of the growth oscillator (Lamport 2023) and their global significance. Stomatal Ca2+ oscillations (Minguet-Parramona et al. 2016) and the abundance of stomatal AGPs (Giannoutsou et al. 2016) suggest their role in Ca2+ K+ and malate ion fluxes that regulate guard cell turgor and stomatal aperture. The growth oscillator is itself regulated by turgor as a direct result of the K+ osmoticum (Humble & Raschke 1971). This may also involve feedback via Hechtian adhesion (Yoneda et al. 2020) of the plasma membrane to the cell wall typically mediated by terminal rhamnose of Arabidopsis AGP57c linked to the reducing end of Rhamnogalacturonan-I pectin (Tan et al. 2013)
As prominent components of guard cells, AGPs are therefore the source of Ca2+ oscillations; their frequency and amplitude trigger opening and closure by cation osmolyte influx and efflux, respectively. Not surprisingly, metabolic activity, highest in turgid cells, triggers the highest cytosolic Ca2+ oscillations (Young et al.2006). In open stomata, the high malate concentration (25 µM cytosol; 464µM vacuole (Jezek & Blatt 2017) is of particular significance; as malate is the counter ion for massive K+ influx; malate also chelates excess Ca2+ that might otherwise overwhelm the cytosol. However, flaccid guard cells of closed stomata are virtually quiescent with low metabolic demands just sufficient to open K+ efflux channels and convert malate to starch. Such low demands presumably reflect the much weaker Ca2+ oscillations hence a low level of metabolic activity. This may resolve the apparent paradox of Ca2+ required for both opening and closing stomata (Kim et al. 2010; Minguet-Parramona 2016; Ng et al. 2001; Young et al. 2006.)
Stomata evolved from Occam simplicity. Competing demands have driven the evolution of extreme complexity in stomatal regulation exemplified by (Hossain et al. 2011 and Chen et al 2024)! How are the single guard cells of stomata particularly relevant to climate change and water conservation? At the single cell level the first land plants developed a waterproof cuticle at the cell surface. It had the obvious drawback that restricting water loss also restricts CO
2 entry. Water is the single most obvious factor limiting the growth of plants that responded by evolving Bryophytes and their sporophyte with efficient spore dispersal via an upright growth habit with a transpiration stream that increased water dependency requiring water conservation. Hence the evolution of novel epidermal stomata consisting of two kidney-shaped guard cells (well-named) whose turgor opens or closes a stomatal pore. Ion fluxes determine the size of this pore that regulates a trade-off between CO
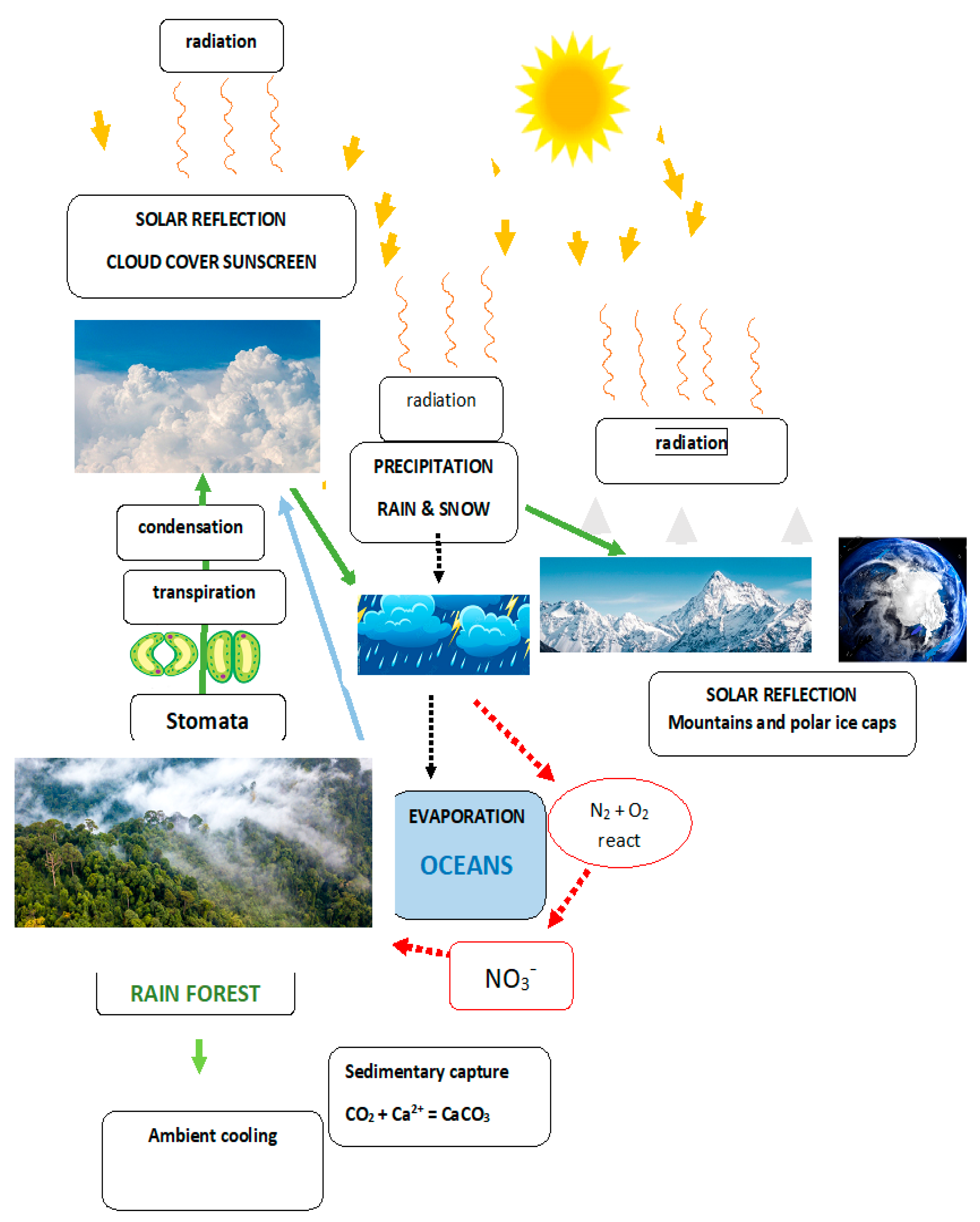
2 entry and water loss. Stomata optimise plant growth and reproduction by detecting the water status. The stomata of forests thus appear as major contributors to planetary homeostasis as follows: Initial evapotranspiration has a local cooling effect; its condensation forms a cloud heat shield reflecting solar insolation; finally precipitation generates snow-capped mountain ranges and polar ice-caps with the highest and most reflective albedo. (
Figure 3.) Green life belts encircle the globe; tropical rain forests in the South and arboreal forests in the North. Both are compromised by deforestation that leads to reduced rainfall tipping points: drought exacerbates prolonged stomatal closure with concomitant hydraulic collapse of xylem vessels (Mantova et al. 2023).
The subtle connection between AGPs, stomata and climate is consistent with the holistic feedback system described by James Lovelock and Lynn Margulis (1972). The Gaia Hypothesis postulates a protective atmosphere generated by life that enables life itself to exist on Planet Earth. Indeed, the current search for extra-terrestrial life focuses on the aerobiology of exoplanetary atmospheres compromised by the solar wind but stabilised by the magnetosphere generated by an iron core that maintains the delicate balance between a living planet and a dead planet like Mars. Indeed, the significance of cloud cover also includes the nitrogen cycle dependent on electric discharge of a negatively charged storm cloud base to fix nitrogen as nitrogen oxides and nitrates essential to plant growth. Clouds involve beautifully intertwined factors summarised poetically by William Wordsworth’s Daffodils: I wandered lonely as a cloud.
Oceans contain more than 97% of the Earth’s water and cover more than 70% of the earth surface, yet water deficit is a greater problem than CO2 abundance.
Thus, higher plants including most crop plants generally cannot tolerate high salt levels and are restricted to a relatively small amount (~3%) of “fresh water”. Escaping that limitation is of great interest. Halophytes have adapted by excretion of excess salt or sequestration in the vacuole. Many Succulents such as cacti have compromised by slower growth but with efficient water storage exemplified by Baobab trees; xerophytes have developed thickened lignified cell walls, deeply sunken stomata and a fortified sculptured leaf surface densely covered by waxes, hairs etc.
Remarkably, despite the oceanic high salt level, two small groups have returned to a marine or semi-marine habitat. They include mangroves and sea grasses. Both form complex ecosystems. Both use different strategies to cope with high salt. Mangroves and other halophytes secrete salt (facilitate Na+ removal from the cytosol to the apoplast). However, Sea grasses like Zostera are uniquely adapted to a totally submerged marine habitat and form extensive intertidal sea meadows of considerable ecological significance. High levels of Na+ compete with AGP-bound Ca2+ and thus disrupt Ca2+ homeostasis. Zostera has solved this problem by evolving modified AGPs with high levels of glucuronic acid that enhance Ca2+binding (Pfeifer et al. 2020) The overall versatility of AGPs is truly astonishing. This raises the question how do marine algae cope with high salt yet presumably maintain Ca2+ homeostasis? Their sulfated polysaccharides with a higher affinity for Ca2+ than glucuronate carboxyls suggest an answer. Indeed, the intertidal chlorophyte Ulva lactuca (sea lettuce) has arabinogalactan proteins as well as sulphated polysaccharides (ulvans) (Reis et al. 2020). This suggests an ecological compromise as the intertidal zone of a rocky shore often contains brackish pools. Thus Ulva may switch from sulphated polysaccharides that enable Ca2+ homeostasis in sea water to AGPs under brackish conditions. SO42- binds Ca2+ more strongly than COO- thus enhancing Ca2+ binding under saline conditions.
Self-regulation involves Ca
2+ homeostasis in both Plant and Animal kingdoms but with profound differences. Plants store dynamic Ca
2+-binding AGPs at the cell surface; animals lack AGPs and store dynamic Ca
2+ in the ER. However, in both plants and animals, intracellular EF-hand calcium binding proteins are central players in all aspects of Ca
2+ homeostasis. Their relevance to climate change becomes clear when considering the balance between water loss and CO
2 uptake is regulated by stomatal guard cells and their abundant AGPs. Planet Earth has evolved incredibly diverse homeostasis based on a protective green layer of plant life...that not only regulates oxygen levels and carbon capture but also a global thermal equilibria: huge forests provide evaporative cooling by transpiration that results in reflective cloud cover enhanced by polar ice caps, snow-capped mountain ranges with oceans as a heat sink. Forests also act as a carbon sink that mitigates increased atmospheric CO
2. Finally vast chalk and limestone deposits of fossilised calcium carbonate have removed excess Ca
2+ and CO
2 from the environment, that include sequestration of coal, oil and gas deposits. Gaia is an increasingly acceptable metaphor for planetary self-regulation. Unfortunately,
Homo sapiens has only recently recognised that self-regulation begins at home; some believe the “fantasy of fossil-fuel phaseout” and climate change as “junk science”! However, despite our propensity for self-destruction (Tainter 1988), as planetary custodians we have good reasons for some optimism including the
goal of net zero by
balancing; CO2 production and its removal. Alternative energy sources involve a worldwide dramatic increase in the production of solar panels about 80% by China! Even in the UK a projected green hydrogen plant in Grangemouth, near Falkirk Scotland, involving 62,000 solar panels will produce 3.6 tonnes of hydrogen per hour. The U.K. is also well placed to exploit large wind driven turbines with over 2000 wind farms that contribute ~20% of UK electricity! International cooperation can make better decisions to avoid the 1.5
0C tipping point
Table 1. (irreversible climate change) and subsequent scenarios; for example by reversing deforestation (Kirschbaum et al. 2024) (
Figure 4.) along with other major interventions: Beef production, palm oil, Sugarcane farms etc are responsible for massive deforestation. That includes worldwide coal mining and the continuous quest to extract oil from the Bakken shale formation, which stretches across the US midwest and Canada. Massive ecological damage has resulted in current legal challenges to specific fossil fuel projects in Alaska, Montana, Hawaii, Florida, Utah and Virginia. We can learn from dystopian poets like T.S. Eliot “Waste Land” and John Betjeman “Slough” and Thomas Gray “Elegy”.
An increase of 1.50C Heatwaves and storms intensify, tropical corals die off and tipping points for ice sheet collapses and permafrost thawing may be triggered.
An increase of 20C The brutal heatwave that struck the the Pacific north-west in 2021 would be 100-200 times more likely. Increases in direct flood damage around the world doubles.
An increase of 2.70C Two billion people would be pushed outside humanity’s “climate niche”, i.e. the benign conditions in which the whole of civilisation arose over the past 10,000 years.
An increase of 30C Cities including Shanghai, Rio de Janeiro, Miami and The Hague would end up below sea level.
An increase above 30C The impact of climate shocks in one place will cascade around the world, through food price spikes, food and water shortages, broken supply chains, and refugees in the millions.
Table 1. Predicted Climate tipping points.
Based on predictions of the IPCC Intergovernmental Panel on Climate Change
Figure 4.
Finally, we can learn to avoid fossil fuels and use the new powerful techniques of genetic engineering to increase the habitable range of crop plants. For example adaptation to high salinity marine conditions such as sea grass Zostera AGPs have increased glucuronic acid levels that enhance Ca2+ binding. The possibility of transgenic crop plants with modified AGPs may be quite beneficial. AGPs as keystone proteins of the Ca2+ signalling cascade play an essential role in global homeostasis....AGP location at the cell surface mirrors the role of the atmosphere in homeostasis of Planet Earth as a single cell floating in space.
Figure 4.
Finally, we can learn to avoid fossil fuels and use the new powerful techniques of genetic engineering to increase the habitable range of crop plants. For example adaptation to high salinity marine conditions such as sea grass Zostera AGPs have increased glucuronic acid levels that enhance Ca2+ binding. The possibility of transgenic crop plants with modified AGPs may be quite beneficial. AGPs as keystone proteins of the Ca2+ signalling cascade play an essential role in global homeostasis....AGP location at the cell surface mirrors the role of the atmosphere in homeostasis of Planet Earth as a single cell floating in space.
More than fifty years after their discovery AGPs provide a sense of completion!
Acknowledgments
Dedicated to the memory of Professor Klaus Raschke (1928-2022) a former colleague in the Plant Research Laboratory at Michigan State University. His work established the pivotal role of potassium and anion channels in regulating guard cell osmotic pressure that opens stomata refuting the then prevailing opinions.
Conflicts of Interest
The author declares no conflict of interest.
References
- Ajayi OO, Held MA, Showalter AM. Three β-Glucuronosyltransferase Genes Involved in Arabinogalactan Biosynthesis Function in Arabidopsis Growth and Development. Plants. 2021:10:1172.
- Bonner J. The relation of hydrogen ions to the growth rate of the Avena coleoptile. Protoplasma 1934:21:406-423.
- Boerjan W, Burlat V, Cosgrove DJ, Dunand C, Dupree P, Haas KT, Ingram G, Jamet E, Moussu S, Peaucelle SA, Persson S, Cătălin C, Höfte H. Top five unanswered questions in plant cell surface research. The Cell Surface. 2024: in press.
- Cleland RE, Karlsnes A. A possible role for hydroxyproline-containing proteins in the cessation of cell elongation. Plant Physiology. 1967:42:669-671.
- Chen G, Qin Y, Wang J, Li S, Zeng F, Deng F, Chater C, Xu S, Chen Z-H. Stomatal evolution and plant adaptation to future climate. Plant Cell Environ. 2024:1-17. [CrossRef]
- Cosgrove D. Structure and growth of plant cell walls. Nature Reviews Molecular Cell Biology.
- Darwin,C. The Power of movement in plants 1880: 592pp. Publ. John Murray, London.
- Delmer D, Dixon RA, Keegstra K, Mohnen D. The plant cell wall—dynamic, strong, and adaptable—is a natural shapeshifter. The Plant Cell. 2024: 36:(5):1257–1311.
- Domozych DS, LoRicco JG. The extracellular matrix of green algae. Plant Physiology 2024: 194:15–32.
- Dunand C, Crevecoeur M, and Penel C Distribution of superoxide and hydrogen peroxide in arabidopsis root and their influence on root development: possible interaction.
- with peroxidases. New Phytologist. 2007:174:332-341.
- Eckardt NA. et al. Climate change challenges, plant science solutions. The Plant Cell. 2023:35: 24–66.
- Everdeen DS, Kiefer S, Willard JJ, Muldoon EP, Dey PM, Li X-B, and Lamport DTA. Enzymic crosslinkage of monomeric extensin precursors in vitro. Plant Physiology. 1988:87:616-621.
- Feraru E, Feraru MI, Kleine-Vehn J, Martinie A, Mouille G, Vanneste S, Vernhettes S, Runions J, Friml J. PIN Polarity Maintenance by the Cell Wall in Arabidopsis. Curr. Biol. 2011:21: 338–343.
- Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P,.
- Costa S, Brownlee C, Jones JDG, Davies JM, and Dolan L (2003) Reactive oxygen species.
- produced by NADPH oxidase regulate plant cell growth. Nature. 422, 442-446.
- Geitmann A, Bacic A. Focus on Cell Walls. Plant Physiology. 2024:194:1–4.
- Giannoutsou E, Apostolakos P, Galatis B. Spatio-temporal diversification of the cell wall matrix materials in the developing stomatal complexes of Zea mays. Planta. 2016:244:1125–1143.
- Held MA, Tan L, Kamyab A, Hare M, Shpak E, Kieliszewksi MJ. Di-isodityrosine is the intermolecular cross-link of isodityrosine-rich extensin analogs cross-linked in vitro. J.Biol. Chem. 2004:279:(53):55474-554782.
- Humble GD, Raschke K. Stomatal opening quantitatively related to potassium transport. Plant Physiology. 1971:48:447-453.
- Jermyn M. A class of lectins present in the tissues of seed plants. Aust J. Plant Physiol. 1975:2:501-531.
- Hepler PK, Lovy-Wheeler A, McKenna ST, Kunkel, JG. Ions and Pollen Tube Growth. Plant Cell Monograph (3) R. Malhó: The Pollen Tube. Published online: 26 January 2006 Springer-Verlag Berlin Heidelberg. [CrossRef]
- Hossain MA, Munemasa S, Uraji M, Yoshimasa Nakamura Y, Mori IC, and Murata Y. Involvement of Endogenous Abscisic Acid in Methyl Jasmonate-Induced Stomatal Closure in Arabidopsis. Plant Physiology 2011:156:430–438,.
- Hromadová D, Soukup A, Tylová E. Arabinogalactan Proteins in Plant Roots – An Update on Possible Functions. Frontiers in Plant Science. 2021:12: Article 674010.
- Jonsson K, Lathe RS, Kierzkowski D, Routier-Kierzkowska A-L, Hamant O, Bhalerao RP. Mechanochemical feedback mediates tissue bending required for seedling emergence. Current Biology 2021:31: 1154–1164.
- Jezek M, Blatt MR. The membrane transport system of the guard cell and its integration for stomatal dynamics. Plant Physiology. 2017:174:487–519.
- Jobert F, Yadav S, Robert S. Auxin as an architect of the pectin matrix. Journal of Experimental Botany. 2023:74:6933–6949.
- Kim, T-H, Bohmer M, Hu H, Nishimura N, Schroeder JI. Guard Cell Signal Transduction Network: Advances in Understanding Abscisic Acid, CO2, and Ca2+ Signaling. Annu. Rev. Plant Biol. 2010:61: 561–591.
- Kirschbaum MUF, Cowie AL, Penuelas J, Smith P, Conant RT, Sage RF, Brandao M, Cotrufo F, Luo Y,.
- Way DA, Robinson SA. Is tree planting an effective strategy for climate change mitigation? Science of the Total Environment. 2024:909: article 168479.
- Krieger G, Shkolnik D, Miller G, and Fromm H. Reactive Oxygen Species Tune Root.
- Tropic Responses. Plant Physiology. 2016:172:1209-1220.
- Lamport, DTA. Cell wall metabolism, Ann. Rev. Plant Physiol. 1970:21:235-270.
- Lamport DTA, Tan L, Held MA, Kieliszewksi MJ. Phyllotaxis Turns Over a New Leaf-A New Hypothesis. Int.J.Mol.Sci. 2020:21:1-15.
- Lamport DTA, Tan L, Kieliszewski MJ. A Molecular Pinball Machine of the Plasma Membrane Regulates Plant Growth—A New Paradigm. Cells. 2021:10:1935.
- Lamport DTA, Tan L, Held MA, Kieliszewski MJ. Root-shoot gravitropism paradox resolved. Academia Letters. 2022: April Article 4998.
- Lamport DTA, Kieliszewski MJ, Showalter AM. Salt stress upregulates periplasmic arabinogalactan proteins: using salt stress to analyze AGP function. New Phytologist. 2006:169:479–492.
- Lamport DTA. The Growth Oscillator and Plant Stomata: An Open and Shut Case. Plants. 2023:12:2531.
- Lamport DTA, Northcote DH. Hydroxyproline in primary cell walls of higher plants Nature. 1960:188:665-666.
- Lamport D.T.A, Tan Li. Held M.A, Kieliszewksi MJ. The Role of the Primary Cell Wall in Plant Morphogenesis. Int.J.Mol.Sci. 2018:19: 2674.
- Lamport DTA, Varnai P, Seal CE. Back to the future with the AGP-Ca2+ flux capacitor.
- Annals of Botany. 2014:114:1069–1085.
- Lamport DTA, Varnai P. Periplasmic arabinogalactan glycoproteins act as a calcium capacitor that regulates plant growth and development. New Phytologist. 2013:197:58-64.
- Lopez-Hernandez F, Tryfona T, Rizza A, Yu XL. Harris MOB, Webb AAR, Kotake T, Dupree P. Calcium Binding by Arabinogalactan Polysaccharides Is Important for Normal Plant Development. Plant Cell. 2020:32:3346–3369.
- Lovelock JE, Margulis L. Atmospheric homeostasis by and for the biosphere: the gaia hypothesis. Tellus, 1974: 26:1-2:2-10. [CrossRef]
- Mantova M, Cochard H, Burlett R, Delzon S, King A, Rodriguez-Dominguez CM, Ahmed MA, Trueba S, Torres-Ruiz JM. On the path from xylem hydraulic failure to downstream cell death. New Phytologist. 2023:237:793–806.
- Minguet-Parramona C, Wang Y, Hills A, Vialet-Chabrand S, Griffiths H, Rogers S, Lawson T, Lew VL, Blatt MR. An optimal frequency in Ca2+ oscillations for stomatal closure Is an emergent property of ion transport in guard cells. Plant Physiology. 2016:170:33–42.
- Ng, C.K.-Y, Mcainsh MR, Gray J.E, Hunt L, Leckie CP, Mills S, Hetherington AM. Calcium-based signalling systems in guard cells. New Phytologist. 2001:151: 109–120.
- Northcote DH, Goulding KJ, Horne RW. The chemical composition and structure of the cell wall of Chlorella pyrenoidosa, Biochem.J. 1958:70:391-397.
- Pfeifer L, Shafee T, Johnson KL, Bacic A, Classen B. Arabinogalactan-proteins of Zostera marina L. contain unique glycan structures and provide insight into adaption processes to saline environments. Nature Scientific Reports. 2020:10:8232.
- Reis, SE, et al. Influence of sulfated polysaccharides from Ulva lactuca L. upon Xa and IIa coagulation factors and on venous blood clot formation. Algal Research. 2020:45: 101750.
- Rodríguez-García et al. Transcription factor NAC1 activates expression of peptidase-encoding AtCEPs in roots to limit root hair growth. Plant Physiology. 2024:194:81–93.
- Tainter J. The collapse of complex societies. 1988: Cambridge University Press 1-217.
- Tan Li, Varnai P, Lamport DTA, Yuan C, Xu J, Q F, Kieliszewski MJ. Plant O-Hydroxyproline arabinogalactans are composed of repeating trigalactosyl subunits with short bifurcated side chains. J.Biol. Chem. 2010:285:(32): 24575-24583.
- Tan L, Eberhard S, Pattathil S, Warder C, Glushka J, Yuan C, Hao Z, Zhu X,.
- Avci U, Miller J S, Baldwin D, Pham C, Orlando R, Darvill A, Hahn MG, Kieliszewski MJ, Mohnen D.
- An Arabidopsis cell wall proteoglycan consists of pectin and arabinoxylan covalently linked to an arabinogalactan protein. Plant Cell. 2013:25:270–287.
- Young JJ, Mehta S, Israelsson M, Godoski J, Grill E, Schroeder JI. CO2 signaling in guard cells: Calcium sensitivity response modulation, a Ca2+-independent phase, and CO2 insensitivity of the gca2 mutant. PNAS. 2006:163: 7506–7511.
- Yu J, Zhang Y, Cosgrove DJ. The nonlinear mechanics of highly extensible plant epidermal cell walls. PNAS 2024:121: No. 2 e2316396121.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).