1. Introduction
Current study is not a typical review in a canonical sense in which content of the article would largely depend on the expertise of the author. The contents of this article primarily rely on the data derived from the MADS box related curated local reference database, hence the term ‘meta-review’ in the article title. While the study has taken the MADS-box studies as a test case, the devised approach is expectedly applicable to any other study-to-(key-of-interest) associations.
MADS represents the ‘fabulous four founder proteins’- MCM1 (from Saccharomyces cerevisiae), AGAMOUS (from Arabidopsis thaliana), DEFICIENS (from Antirrhinum majus) and SRF (from Homo sapiens) [
1]. Studies suggest that a common ancestor of fern and seed plants already constituted at least two flowering-related MADS-box genes (MIKC-type) around 400 MYA [
2,
3]. There have been several prominent studies on the phylogenetic classification of the MADS-box gene members [
1,
4,
5,
6,
7,
8,
9,
10]. The studies suggest diverse roles of the genes during plant growth and development. While majority of the studies are associated with flowering, a comprehensive understanding of the genes would offer a broader perspective on their functional evolution and diversification. Huge number of independent studies on the MADS box member genes are available in model as well as non-model plants. Utilization of their holistic information in a single manuscript is relatively daunting yet seems essential to have an ‘aerial’ perspective regarding the progress on the subject, which may offer initial ground for experimental design to the experts and non-experts alike. With such an intent in mind, we have carried out a meta-review of the MADS associated studies.
Here, we have discussed on the MADS box member genes and their roles regarding plant growth phases, interplay of known members to the hormonal cues, potential involvement of the known members in bridging multiple traits and/or factors based on the information retrieved from the curated reference database containing 773 independent literatures.
2. Study-Based Meta-Review: Basic Strategy
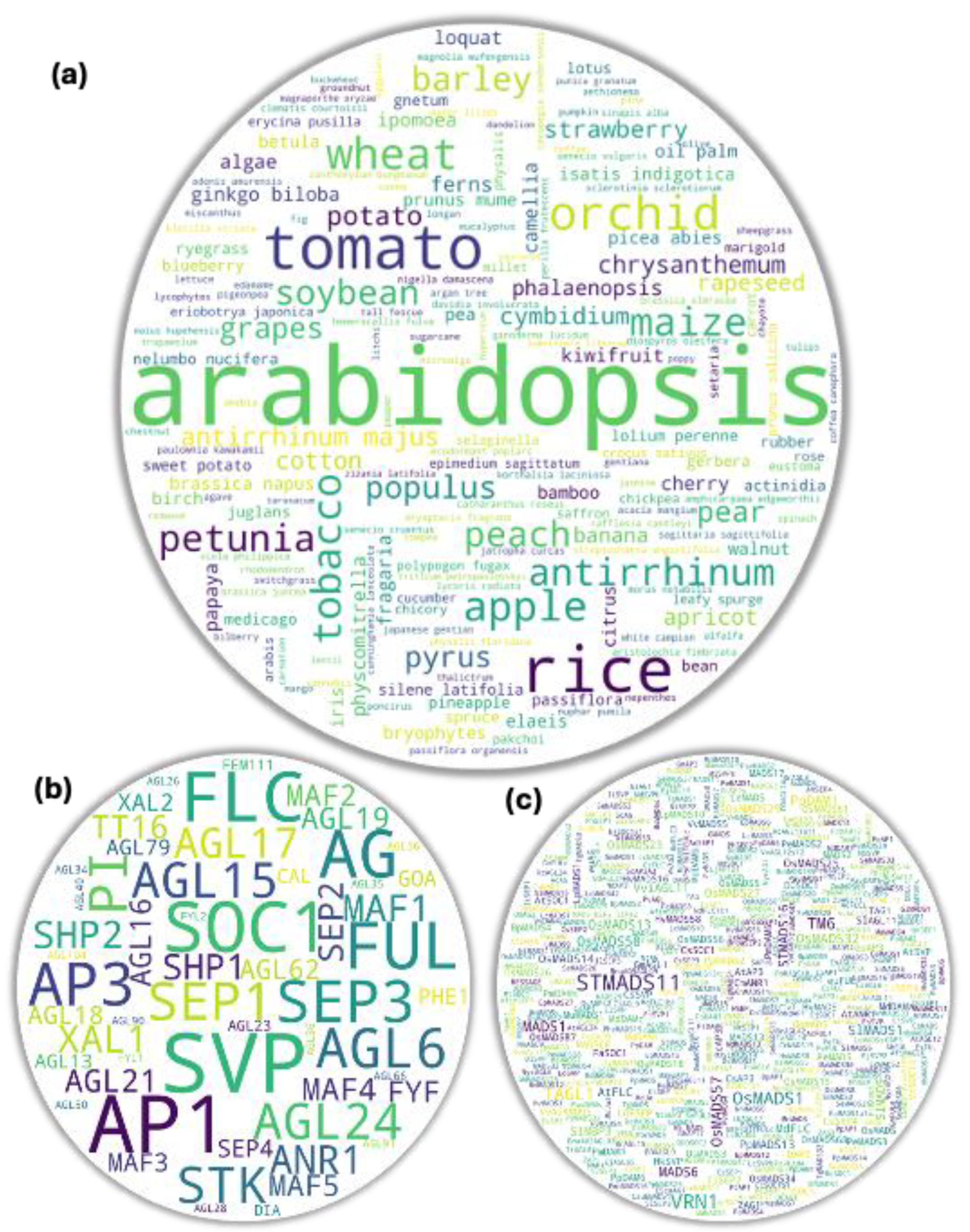
Since their first discovery in 1990, there have been plethora of studies in MADS box genes in several plants as shown in
Figure 1 [
11]. Most of those studies have been meticulously planned, conducted, peer-reviewed, and published. Using them as a direct reference to have broader understanding on the roles of genes-of-interest in plants would offer an advantage to the researchers in study design regardless of their depth of knowledge in the study subject at the beginning. With such concept in mind, references were fetched from PubMed, Google Scholar, and Semantic Scholars using main keyword ‘MADS box’ with or without either of the additional keywords- ‘flowering’, ‘genome-wide’, ‘vegetative’, ‘seed germination’, ‘seed development’, etc. They were screened for gene-specific experiments excluding most of the broader studies like genome-wide studies and reviews except for the tissue-specific and/or gene/clade-specific ones. Studied organism names were manually extracted from the remaining 773 references (published from 1992 to 2024) (Supplementary Dataset 1) and proceeded for the gene-to-study association analyses. We used an in-house script for pooling keywords/search term with or without constraints and generated word-cloud for each gene pool using wordcloud 1.9.3 python library [
12]. Threshold of 3 was set during the analysis to reduce potential false positive hits unless mentioned otherwise.
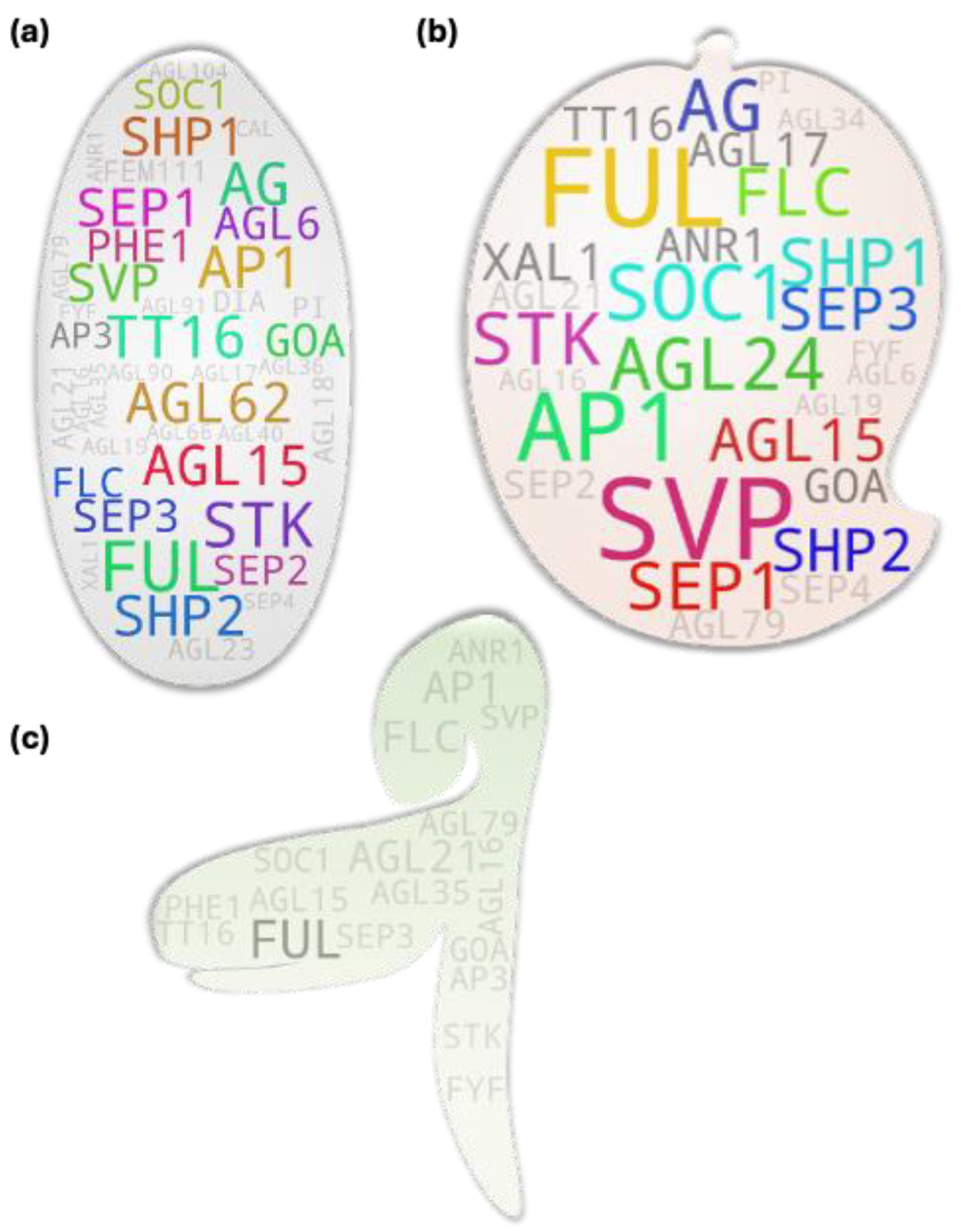
3. General Overview: Plants and Genes of Studies
Our very initial question to the database was, ‘in which plant the MADS box member genes have been studied the most?’ It was not unusual for Arabidopsis to appear as the first hit being a model plant. Excluding it and another model plant Nicotiana, cereals (rice, maize, wheat), vegetables (tomato in particular), fruits (apple, peach etc.), ornamental plant (Orchids) were some of the top hits. Rice, tomato, and apple have frequently been used as model plants in monocot, vegetable, and fruit tree studies. In total, 188 organisms were recorded to have studied directly or indirectly by the studies used for the analysis (
Figure 1a). Since Arabidopsis was the most studied plant, we next checked which were the most studied MADS gene members amont the studies. Interestingly, most studied genes were among the known flowering repressors (
FLC,
AGL15,
AGL24,
SVP,
AGL18,
MAF3/4/5, etc.) and promoters (
STK,
SEP3,
AG,
AGL17,
PI, etc.) (
Figure 1b). Since several gene MADS box IDs are identical to their associated clade IDs, such genes often showed higher hits (
Figure 1b,
Figure S1). Additionally, when checked using only clade IDs, SVP returned with the highest hits followed by AP1, SOC1, FLC, AG, SEP, AP3, etc. (
Figure S2). Interestingly, our analysis showed a stark disparity between the studies in type I and type II MADS box members. Among 69 type I members only 15 showed study association hits while 41 showed such hits among only 46 type II members (Supplementary table S1). We further checked potential MADS box members studied in other organism using a wild-card keywords for MADS, AGL, DAM, RIN, etc. related genes. Among the 560 putative gene terms retrieved,
StMADS11, an
SVP member from potato and
VRN1 a
FUL homolog from wheat and relatives, were the most studied genes followed by
TAGL1 (an AG-clade member from tomato),
OsMADS1 (a SEP-clade member from rice), and
TM6 (an AP3-clade member often from tomato) (
Figure 1c).
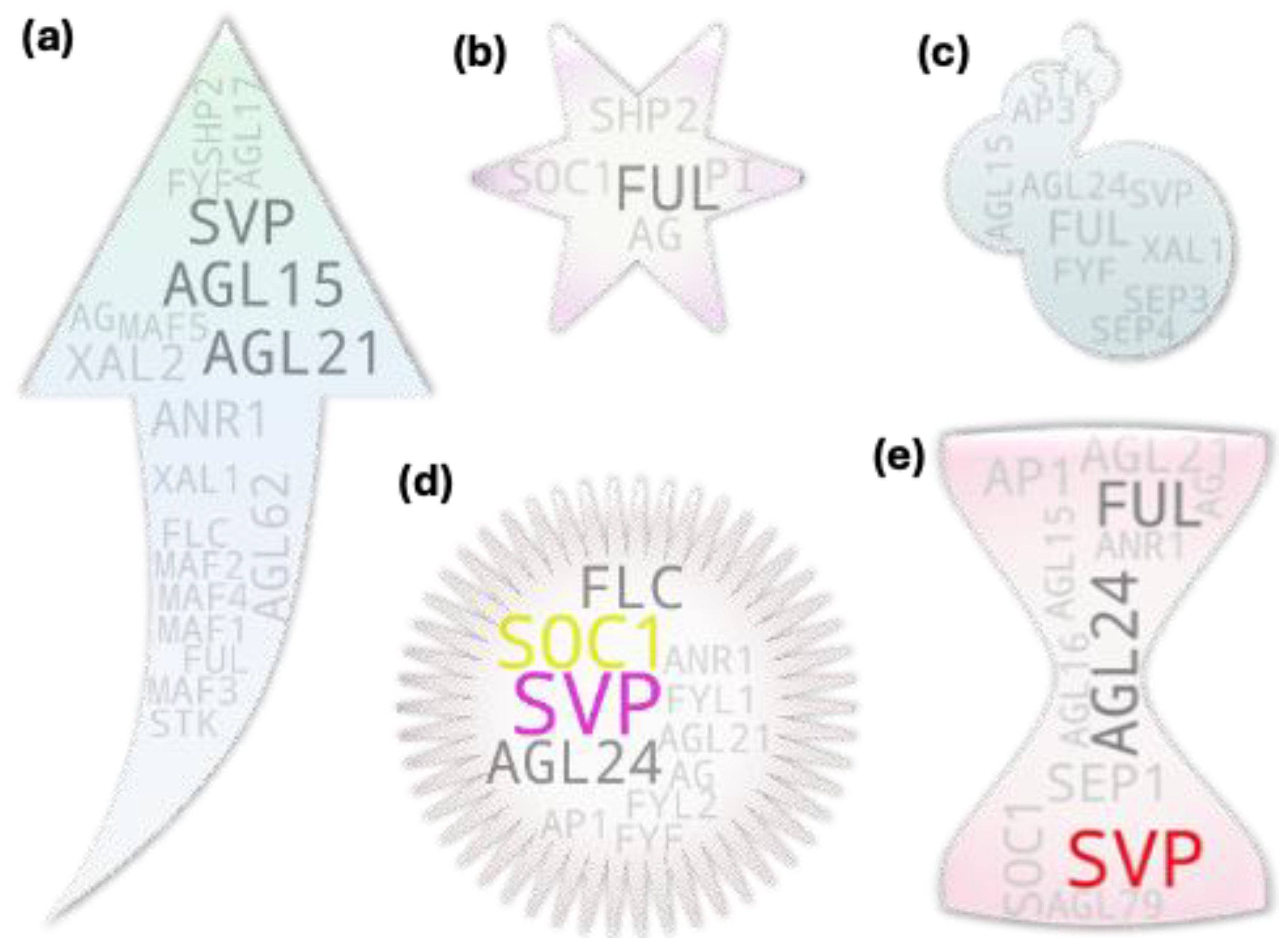
4. Screening Potential Pleiotropics: Sorting Threads from Haystack
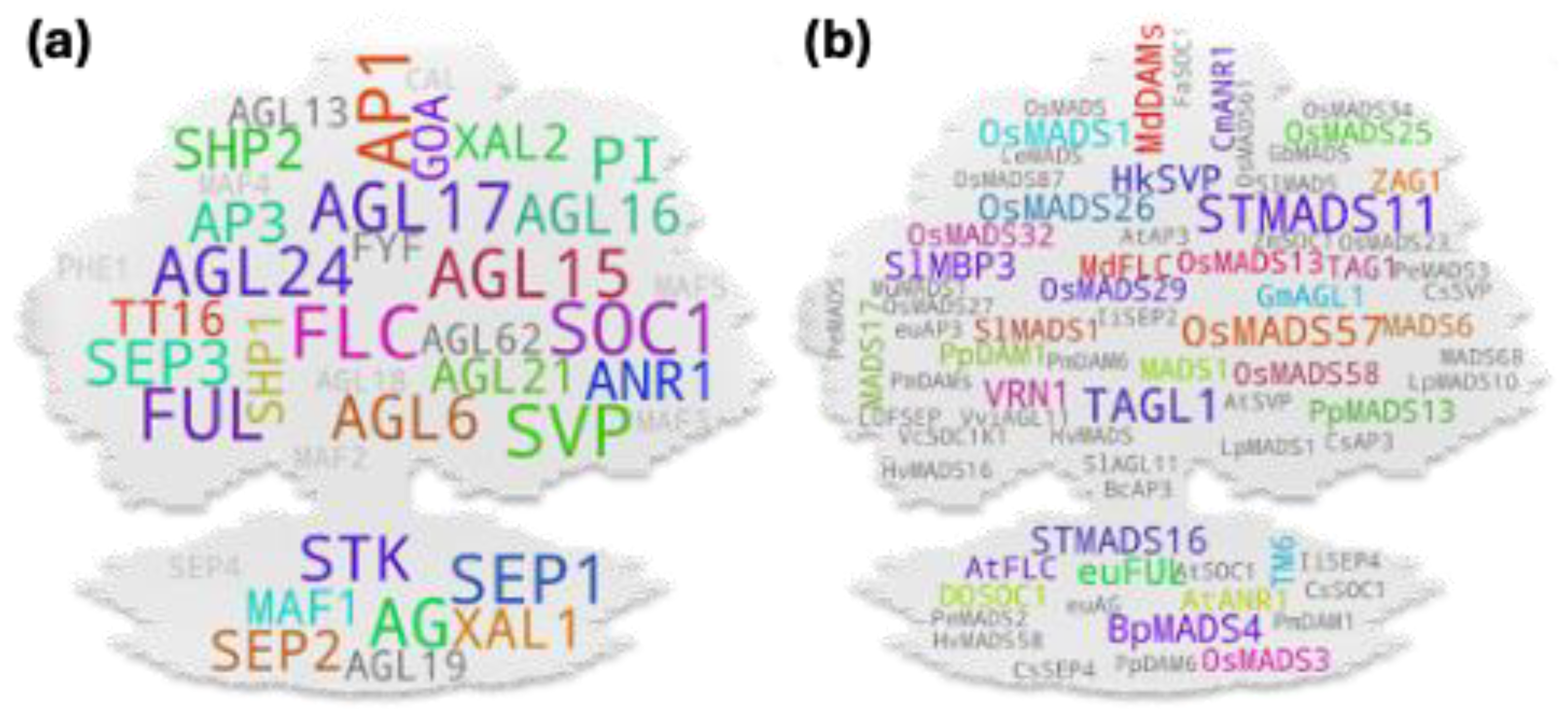
Majority of the genes closer to the terminal end of genetic/physiological pathways often tend to be less pleiotropic in nature due to their narrowly specified functions and hence generally tend to have association to a single if not closely associated traits. Pleiotropic genes on the other hand often have multi-trait associations. To retrieve such MADS box members, we initially set out to assess the tissues-to-studies association for root, shoot, leaf, apical meristem/SAM, flower, fruit, and seed. Later, we checked for the recurring genes among those independently sorted gene pools (
Figure 2). which were presumed to have pleiotropic function in plants.
Most of the highest hit IDs were of such mutual IDs (e.g.,
FLC,
SOC1,
SVP,
AP1,
AG,
AGL15, etc.) as observed in earlier case except for
FUL,
AGL17,
SEP1, and
AGL24 in the Arabidopsis gene tree (
Figure 2a). Our analysis result aligns with the genes’ pleiotropic role during plant growth and development. Taking
FUL as an example, initial observations made on the mutants of FUL gene reported disorder in the silique (fruit) development (shorter silique with frequent premature dehiscence) in Arabidopsis due to the absence of cell expansion and selective restriction of the cell division. The mutant siliques at mature stage contained highly compacted seeds within the short silique- hence the name ‘
FRUITFULL’ [
13]. The study additionally reported difference in cauline leaf shape (more round in the mutant). Latter studies showed that
FUL directly represses downstream MADS box members
SHP1 and
SHP2 which is crucial for the lignification and formation of the silique dehiscence region as the siliques remain ‘shatter-proof’ in their cumulative mutants [
14,
15]. In addition to its role in fruit development,
FUL has been attributed for other roles as well. Such examples include its Involvement in meristem determinacy by negatively regulating AP2 in the developing inflorescence [
16] and in apical hook opening modulation by negatively regulating the expression of growth promoting genes in Arabidopsis [
17]; involvement of its homolog from birch [
18] and several other plants in precocious flowering, role of its rice homolog in normal seed development by regulating at least two key genes involved in starch synthesis-
OsAGPL2 and
WAXY [
17]; crucial role of its tomato orthologs
FUL1 and
FUL2 in tomato fruit ripening potentially by forming a tetramer complex with additional MADS box members- RIN and AGL1 [
19]; involvement of them (
FUL1/2) along with an additional MADS member
MBP20 (a SEP-like gene) in the tomato vegetative-to-reproductive transition and inflorescence architecture regulation [
20]; similar role for rice
AP1/FUL homologs (
OsMADS14,
OsMADS15, and
OsMADS18) in addition to a
SEP homolog (
PAP2) in floral meristem identity [
21]; ABA-responsiveness of the
OsMADS18 and its involvement in diverse development features from germination to tillering and inflorescence architecture [
22]; involvement of a
FUL homolog haplotype
GmFULa in plant biomass and seed yield without affecting flowering time in soybean [
23], etc.
Among the cross-species MADS box gene pool (
Figure 2b),
STMADS11 and
TAGL1- both tomato-derived gene IDs- were of the highest hit. It should be noted that like that for several Arabidopsis gene IDs,
STMADS11 has often been used as clade ID (synonymous to SVP-clade). Regardless, the gene itself has been attributed for diverse developmental roles in plants. Here, while taking
TAGL1 (an AG-clade member and SHP homolog) as a test example, unlike that observed for the Arabidopsis gene ID-derived top hits, the gene-to-phenotype coverage for it was relatively narrow most likely due to the lower threshold (2 hits) and lax parameters used during the screening process of the cross-species derived gene IDs.
TAGL1 has been attributed for its direct involvement in the regulation of chloroplast synthesis [
24] and fruit ripening [
25] in tomato; its potential involvement in tomato seed size control via interaction with another MADS box member
SlMBP21 [
26]; and potential involvement in ethylene biosynthesis and carotenoid accumulation in ripening fruit via interaction with yet another MADS box member SlCMB1 [
27]. Due to the relatively higher hits accompanied with stronger reliability for Arabidopsis-derived gene IDs as compared to the cross-species derived ones, we carried out downstream analyses using the former.
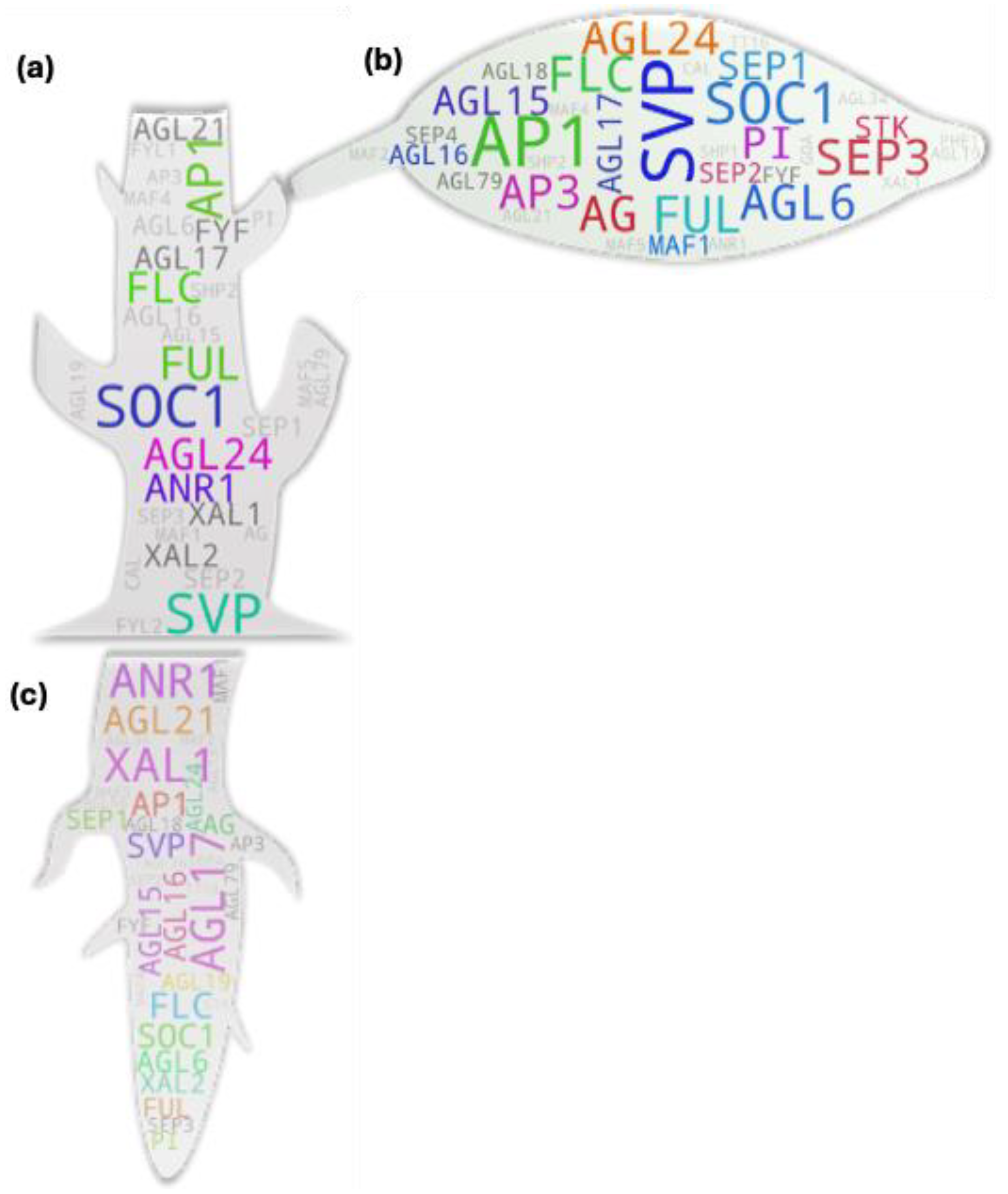
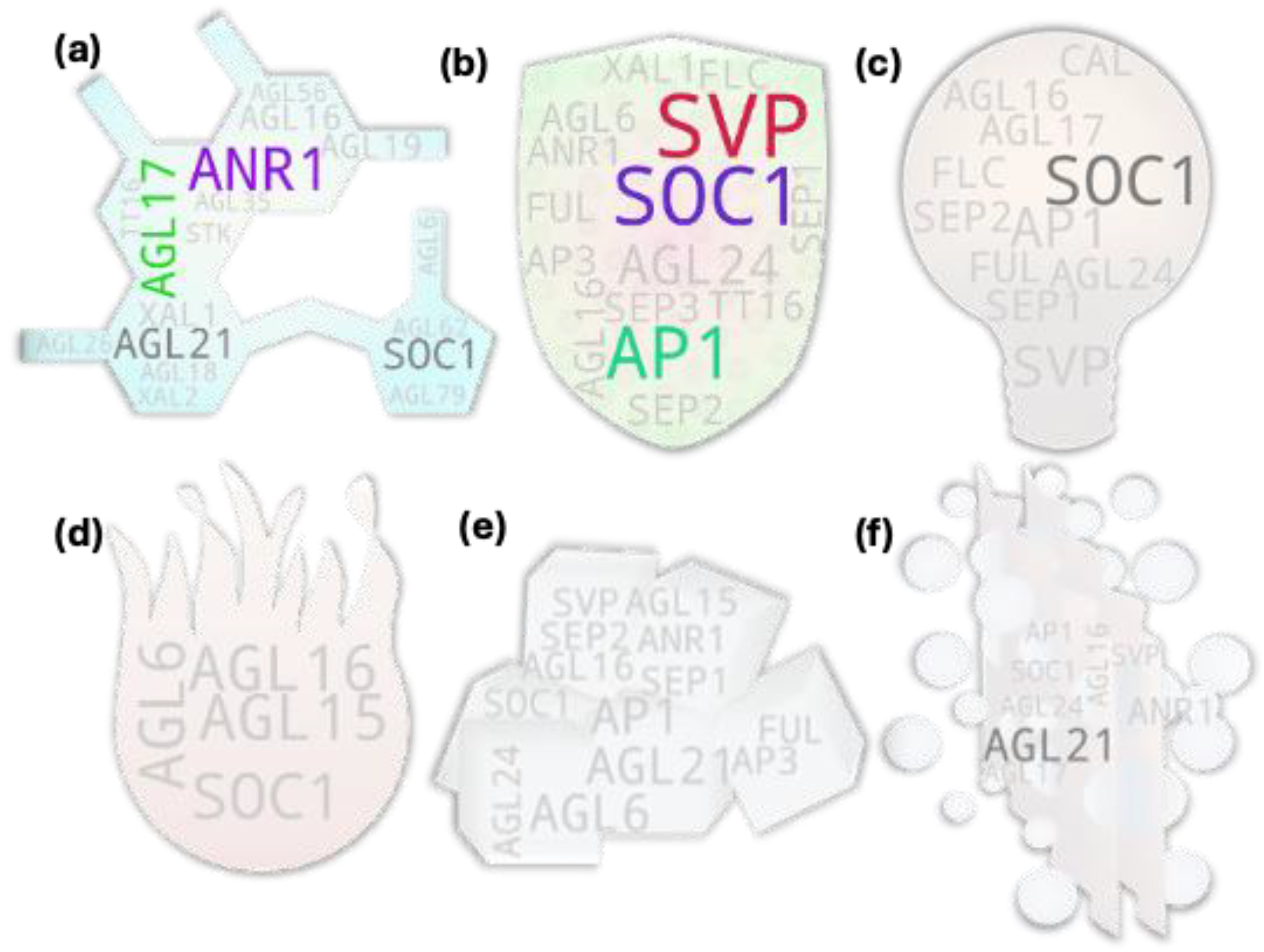
5. Gene-to-Major Tissues Growth Associations
5.1. Shoots
Apparently, there are not much shoot-focused studies on MADS box gene members. Our analysis with shoot/stem keyword and some exclusion terms (shoot meristem, shoot apex, stem cell, etc.) returned 30 MADS box member with direct/indirect study association with shoot among which only seven were above threshold (
Figure 3a). We compared our gene pool with the tissue-specific expression analysis derived pools of Parenicova
, et al. [
28]. Even though the study showed several type I MADS box genes expressed at shoot, our analysis returned none. It was mainly because of the much lesser studies on the type I MADS box members (Supplementary table S1), which have skewed the local reference database towards type II members. Among them as well, our analysis returned only 14 out of 23 shoot expressed type II members reported by Parenicova
, et al. [
28]. Such disparity was expected as the analysis approach and objectives were different for these studies. Interestingly, of the seven genes above threshold, only two (
FUL and
AGL24) were common with the reported study. The former reportedly affects branch angle by negatively modulating the expression of
SAUR10 and influences other genes involved in hormone and light signaling pathways in Arabidopsis [
29].
AGL24 on the other hand, is a flowering promoter and its overexpression lines flower at much shorter heights as in the case of majority of other MADS box members promoting precocious flowering. A study showed that the phenotype of
svp mutant is epistatic to
agl24 as the genes are involved in recruiting the co-repressor complex [
30].
SVP and
FLC are the key MADS box members associated with positive regulation of the vegetative growth in plants which is often positively correlated with the shoot growth. Even though there are not much studies on other members of the FLC clade, they have been attributed for their
FLC-like role in delaying flowering process
via repression of
FT expression in leaf in response to the endogenous and exogenous cues [
31].
5.2. Leaves
Fifteen out of 36 MADS box IDs showed above threshold hits in the leaf-associated gene pool suggesting for their potential direct or indirect involvement in leaf or leaf associated growth and development processes in plant. It included most of the FLC-clade members even though only
FLC showed above threshold hit. Among all,
SVP and
AP1 were the two members with the excessively higher hits (
Figure 3b). While there are no reports on the involvement of
AP1 in leaf associated features in model plants, some cross-species studies suggest its such potential if not the gene has been neo-functionalized in them. One of such studies in barley reported that
PHOTOPERIOD-H1 (
Pdp-H1), a
PRR7 gene encoding a component of circadian clock, -regulated reduction in leaf size and number was correlated with the
Pdp-H1-dependent induction of barley
AP1 and
FUL like homologs
BM3 and
BM8 indicating their potential involvement in the process [
32]. Regarding
SVP, mutant study in Arabidopsis reported that its dysfunctional state brings changes to leaf size [
33] and leaf shape prior to the first flower formation in addition to the changes in numbers of rosette and cauline leaves [
34].
A common leaf features observed with the flower promoting genes is that vegetative-to-reproductive phase transition is often directly correlated with the trichome development in higher density at the abaxial side of the cauline leaves. A study reported that
AG, one of the IDs with above threshold hits, is directly involved in repressing the development of branched trichome, a key aspect of leaf development, in gynoecium [
35] by regulating cytokinin responses and genetically interacting with
KANADI1, an organ polarity gene suggesting that the genetic program for leaf development have been rewired during flower formation process mainly
via MADS box member floral homeotic proteins [
36]. Yet additional study has reported that normal expression of
AGL15,
AGL18,
AGL24, and
SVP is essential to block floral programs in the vegetative tissues in absence of which leaves show aberrant morphology (upward curling) due to the de-repression of
FT, a known florigen, and a MADS box member
SEP3 [
37].
AGL6, a member MADS box gene in the gene pool reportedly affects leaf movement, an active process regulating its circadian clock in plants, by modulating the expression of
ZEITLUPE, a blue-light photoreceptor governing circadian rhythm and repressing photoperiodic flowering [
38]. Regarding
AGL24, an additional MADS member with above threshold hit in the gene pool, a recent study demonstrated that it confers floral organ identity speciation
via long distance movement of its mRNA from leaf to shoot apex. Furthermore, its encoded protein is actively degraded in the leaf itself to avoid misexpression of its downstream genes in the tissue [
39].
5.3. Roots
Our analysis derived root-associated gene pool encompassed all known root-expressed or root-specific genes [
40,
41] except for
SHP1 and
SHP2 (
Figure 3c). However, some of them showed hits below threshold which include
AGL18,
AGL26,
AGL42, and
AGL56. Interestingly, our analysis returned additional MADS box members with above threshold hits which include
FLC,
SVP,
AP1,
AGL6,
AGL15,
FUL,
AG,
SEP1, and
AGL24. Such occurrence is supported by other studies like that in sweet potato for
SVP, and
AGL24 [
42],
Medicago sativa for
AGL6 [
43] and
AP1 [
44], Arabidopsis for
FLC [45] and
AGL15 [
46] etc.
Commonly known root-associated genes have been well-described by some of the published reviews [
41,
47]. To briefly mention some of their known functional roles,
ANR1 and
AGL21 are involved in nitrate foraging dependent lateral root growth and development [
48,
49];
FYF/
AGL42, despite its unclear functional relevance, is often used as a quiescent center marker due of its exclusive expression pattern at the tissue [
50];
AGL17 exhibits its highest expression with yet unknown function in roots [
51,
52];
AGL16 reportedly confers stress tolerance during root elongation [
53];
XAL1 and
XAL2 are involved in root meristem proliferation and patterning by modulating auxin transport [
54,
55],
AGL15 may play role in ROS signaling in developing roots. Other root associated MADS members confer more indirect effect on root growth and development.
5.4. Apical Meristem
A studies-based report earlier from 2002 showed that majority of the MADS box members exhibit expression in Arabidopsis shoot apical meristem (SAM) among the assessed genes [
56]. Later studies have further expanded the range. However, our analysis with the SAM-to-studies association returned only 16 member genes among which six were above threshold, that include
SOC1,
AGL24,
AP1,
SVP,
FUL, and
FLC (
Figure 4a).
SOC1 and
SVP have been attributed for their involvement in dynamic regulation of gibberellin biosynthesis and catabolism by increasing cell size and numbers at the site during apical meristem to floral meristem transition in Arabidopsis [
57]. Furthermore, according to an earlier study, SVP and AGL24 can redundantly dimerize with AP1 to recruit the LUG-SEU co-repressor complex to repress class E member (
SEP3), class B (
PI and
AP3) and class C (
AG) during the transition process to prevent precocious floral meristem differentiation [
58].
FUL on the other hand has been attributed for its role in inducing global proliferation arrest of active meristems by directly repressing member genes of
AP2 clade, the ERF members, negatively regulating flowering and flower development process, which would otherwise repress the repressors of
WUSCHEL, a key gene behind meristem maintenance [
59]. Regarding
FLC, its regulation of maintaining vegetative state of the apical meristem is at least partly mediated
via the repression of its target gene
TFS1, a B3-type REM member gene. Furthermore,
SVP acts redundantly (to
FLC) in the process. In other case, SOC1 recruits REF6, a histone demethylase, and BRM, the SWI/SNF chromatin remodeler ATPase, to activate
TFS1 during floral transition [
60].
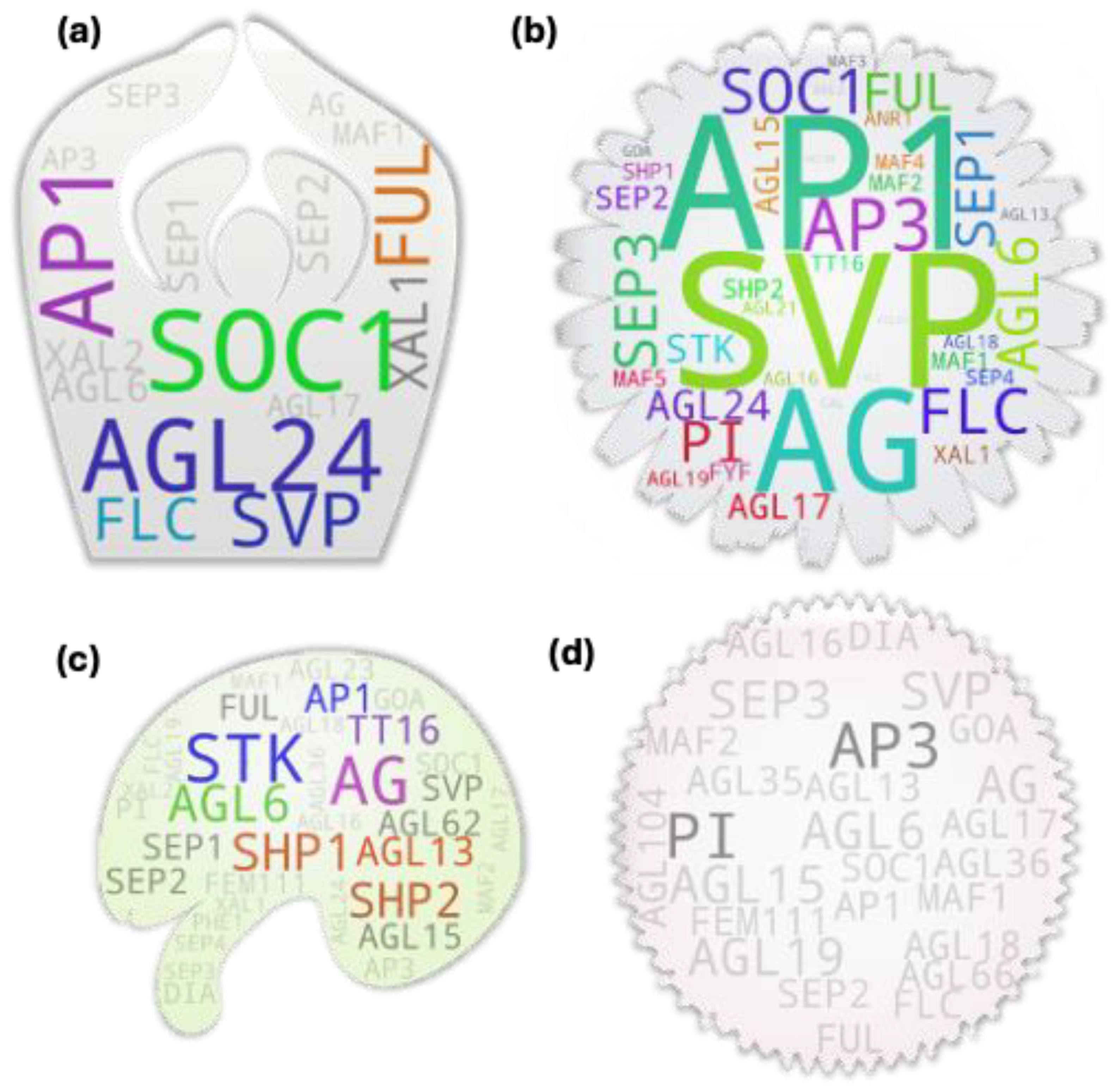
5.5. Flowers
Majority of the MADS box member associated studies are flowering focused. Hence the flower/flowering associated gene pool encompassed the gene IDs with the highest hits among all assessed gene pools in the study. In total, 40 genes were returned among which 31 were above threshold (
Figure 4b). Except two, all members belonged to type II group. Interestingly, regardless of the threshold, all but one member (
GOA) have reported florigenic function. Additionally, despite their absence in the derived pool, the missing type II members (
AGL30,
AGL33,
AGL65,
AGL66,
AGL67,
AGL79,
AGL94, and
AGL104) too reportedly have florigenic potential. Absence of related studies in our local database could be the reason behind such occurrence.
Functional roles of the type II members on floral induction have been extensively studied and there are well versed evolutionary as well as review studies on the topic. Some of them include Gramzow and Theissen [
61] on both functional and evolutionary aspects of MADS-box members; simultaneous independent studies by Becker and Theissen [
62] and Nam
, et al. [
63] on detailed dated evolutionary studies regarding MADS box gene origin and divergence. We briefly touched on the topic in our earlier review [
64]. To describe the functional roles of some of the representative MADS box members in floral development here, we will simply use the ABCDE model often taken as a reference in flowering associated studies. The sepal, petal, stamens, carpel, and ovule development depend on the A-, A + B-, B + C-, C-, and C + D- function genes respectively each in association with an E-function member. In Arabidopsis,
AP1 functions as A;
AP3 or
PI function as B;
AG functions as C;
STK,
SHP1 or
SHP2 functions as D; and either of the
SEP members functions as E class genes. A study additionally has proposed AGL6 members, which are closely clustered with the
SEP members (
Figure S1), as additional putative E class genes based on their functional analyses in petunia, maize, and rice [
65]. As described in earlier sections, several MADS box members in the gene pool play role in floral transition, inflorescence architecture regulation, and floral meristem modulation.
5.6. Ovules
Our analysis returned 35 MADS box members to have study associations with ovules among which eight showed above threshold hits (
Figure 4c). However, majority of the genes with at least two hits (21 in total) reportedly have direct or indirect function in ovule development. The genes from the lower hit spectrum (with 2 hits each) include
SVP,
FEM111/
AGL80,
DIA/
AGL61,
AGL23,
SOC1,
AP3,
PI, and
GOA. As mentioned earlier, SVP-AP1 dimer reportedly forms a repressor complex by recruiting the co-repressors SEU-LUG and represses the expression of one of the ovule identity genes-
STK in floral meristems by binding to its promoter. The process is mediated by BASIC PENTACYSTEINE (BPC) transcription factors to potentially bring changes to the bound promoter region during the repression process [
66]. A study in
Ginkgo biloba, one of the oldest living tree species, reported that ectopic expression of its natively flower and ovule-expressed AP1/SQUA clade member
GbMADS9 downregulates its
SVP homolog [
67] roughly indicating potential state of similar
SVP repression mechanism during ovule development. In other cases,
AGL61/
DIA and
AGL80 are crucial MADS box members for central cell development [
68,
69];
AGL23 plays crucial role in female gametophyte development and its dysfunction renders the ovule sterile [
70];
SOC1 reportedly binds to the promoter of
SUPERMAN (
SUP) gene encoding C2H2-type zinc finger protein which is involved in the cell proliferation in ovule in addition to its similar role in stamen and carpel primordia [
71,
72];
AP3, even though a B class member, plays crucial role in ovule development and defect in the gene leads to the development of ovule out of its native site of development [
73].
5.7. Pollens
Total 26 genes were returned for the pollen associated gene pool among which four belong to type I MADS box group. Interestingly however, none of the members in the pool had hit value above threshold (
Figure 4d). Apparently however, there are not much detailed studies regarding the roles of MADS box members in pollen development. Nevertheless, a study in Arabidopsis reported that AGL13, one of the member gene in the pool, plays role in anther, pollen, and ovule development potentially by forming heterodimer with other MADS box members, AP3, PI, and AG as it cannot form homodimer [
74]. Furthermore, the study showed that
AGL13 affects the expression of
AG,
AP3, and
PI via positive feedback loop and represses its own expression by activating its repressor
AGL6. Additional study in Chinese fir reported relative upregulated status of
AP3,
PI, and
AGL15, downregulated status of
SVP, and non-differential expression of
AG in male cones as compared to the female cone [
75] suggesting their functional relevance in the male and female cone development. An
AGL15 ortholog,
AGL18, has also been reported to exhibit its expression in developing Arabidopsis pollen at the time of mitosis and even stronger later during the maturation stage in addition to the gene’s expression in the developing female gametophyte and endosperm [
45].
5.8. Seeds
Seed-associated gene pool contained 41 MADS box members in total among which, 18 returned above threshold hits. Overall, the pool encompassed 10 and 31 type I and type II members with only two of the former (
AGL62 and
PHE1) above threshold. Some of the genes from the lower spectrum above threshold include
SEP2,
SOC1, and
FLC. Among them,
SEP2 mainly plays role in floral development and a study in cotton-tobacco reported its down-regulation along with other florigenic MADS box members
AP1,
AP3,
AGL8,
AGL6, and
SEP1 upon ectopic expression of seed yield enhancing gene
GhKTI12, an elongator-associated protein encoding gene, in tobacco [
76] suggesting for negative feedback signal from the developing seed on the expression of the genes associated with floral development. Such case comes in agreement with a grape-tomato study which reported decrease in seed size and numbers in tomato upon ectopic expression of the grape-derived
SEP2 homolog
VvMADS39 [
77]. Negative effect of seed-derived signals on inflorescence architecture and fruit/seed yield has been observed in Arabidopsis [
59], field pea [
78], and rapeseed [
79] by modulating the expression of
FUL and
AP1, two of the MADS box members with higher hits in the seed-associated gene pool (
Figure 5a). Regarding
SOC1, a study stated a failure of seed development in Arabidopsis lines constitutively expressing the gene [
80]. However, SOC1 clade members have potentially neofunctionalized and subfunctionalized roles in Arabidopsis flower development [
81] and flower senescence [
82] as well as in seed development as reported in
Medicago truncalata [
83], barley [
84], etc.
The seed-associated gene pool additionally contained several other members that are also associated with their positive and negative regulation on flowering. Their expression in plants expected to have respective negative and positive correlation to the seed yield. Such case has been observed for
FLC homologs in barley [
85] and a
SVP homolog (
SVP-A1) in
Triticum ispahanicum [
86]. Within the developing seed itself, a soybean genome-wide expression study observed the elevated expression of AG, SEP, and FLC clade members when assessed at the globular, heart, cotyledonary, and early maturation stages [
87] suggesting their positive regulatory role in seed development process, even though their direct role in the process has not been reported yet except for
FLC. Expression of
FLC peaks at seed maturity unlike
FT,
SOC1, and
AP1 which reportedly show opposite expression trend with seed maturity in Arabidopsis. The seed-expressed
FLC confers risk-aversion of the seeds after maturity by controlling germination based on ambient temperature through modulated expression of hormonal genes [
88]. Additional notable MADS box member in the gene pool include
AGL15 which is reportedly involved in phase transition from seed maturity to germination and seedling growth.
AGL15 repression brought upon by HSI2/VAL1, a B3-domain protein, leads to down-regulation of seed maturity associated genes by depositing the H3K27me3 at the AGL15 locus. The study further observed interaction between HSI2 and MSI1, a PRC2 repressive complex member, and suggested potential recruitment of MSI1, by HSI2 to form a PRC2 nucleation site at the
AGL15 promoter [
89].
Some of the studies on relevant to seeds associated MADS members include Ehlers
, et al. [
90] on the roles of
SHP1 and
SHP2; Bemer
, et al. [
91] on expression pattern of type I MADS box members; Coen
, et al. [
92] on roles of
TT16 and
STK etc.
5.9. Fruits
In total 28 MADS box members were returned in the fruits associated gene pool, which contained all but one type II members. Thirteen of them- all type II members- were above threshold with
SVP,
FUL, and
AP1 at the highest and
AGL15,
SEP3, and
SHP2 at the lowest spectrum above threshold (
Figure 5b). Some of the members in the gene pool reportedly have relatively subtle and indirect effect, which include
SVP. As reported in self-abscission apple, its
SVP homolog
MdJOINTLESS is associated with the abscission zone often developed in the pedicel of the lateral fruits and suggested its potential involvement in regulating auxin gradient in the developing fruit [
93]. Similar case has been attributed for its tomato homolog regarding flower and fruit abscission zone development [
94,
95]. Among some of the genes from the lower spectrum,
SEP3- a gene often linked with flowering promotion- plays dynamic role in pollination-dependent fruit growth and contributes on fruit ripening as reported in strawberry [
96]. It should be noted that similar to seed set, fruit set and its growth exert negative effect on floral induction [
97] suggesting potential involvement of flowering related MADS box members in the gene pool in fruit dependent feedback loop. Regarding
AGL15, it affects fruit maturity process if rendered active during fruit development as observed in the transgenic Arabidopsis with its constitutive expression [
98]. Those plants exhibit retention of petals and sepals long after pollination (and silique development) and brings significant delays in fruit/silique and seed maturity/desiccation. Latter study by the group further showed that such delayed floral organ senescence is correlated with the increase in
AGL15 expression around the time of floral opening, before the onset of senescence and abscission [
99]. Embryo expressed
AGL15 however, confers no significant effect on seed desiccation.
Some of the published studies dedicated to fruit-associated MADS box members include Busi
, et al. [
100] profiling MADS box members during tomato fruit and seed development, Wang
, et al. [
101] profiling MADS box members during longan flower and fruit development, Li
, et al. [
102] reviewing the MADS box members regulated fruit ripening process in plants,
etc.
5.10. Seed Germination
We chose seed germination instead of seedling to pool the MADS box members potentially involved in transitioning seeds to seedlings. Seed germination returned 18 MADS box members in total among which, two (
AGL35 and
PHE1) belonged to type I. Interestingly however, none of the members returned the hits above threshold (3) (
Figure 5c) which could be because of relatively less studies on this aspect of their role.
FUL, the only member with the threshold hit, reportedly plays positive role in seed germination as down-regulation of its
AP1/
FUL homolog
OsMADS18 causes delay in germination and lower germination rate in rice [
22]. The study further showed that its overexpression lines exhibit reduced auxin content and diminished expression of strigolactone signaling associated genes,
D14 and
OsTB1. The expression of
OsMADS18 was positively affected by ABA which triggered the re-localization of otherwise plasma membrane localized MADS18 protein to the nucleus [
22]. Earlier growth architecture-focused study additionally reported that
FUL represses the expression of
SAUR10, an auxin and brassinosteroids inducible gene in Arabidopsis [
29]. However, a recent rice study observed slightly reduced germination rate in the
ossaur10 mutants even though not all transgenic lines exhibited significantly different germination rates (as compared to WT) [
103] indicating potential of
SAUR10-independent
FUL-regulated genetic network in seed germination.
Among other members in the gene pool, role of
AGL15 in germination process has been discussed earlier.
ANR1 and
AGL21 act synergistically to repress seed germination in response to ABA and salinity to avoid germination at the unfavorable condition. The process is facilitated by the respective regulation of
ABI3 and
ABI5 by
ANR1 and
AGL21 [
104,
105].
FLC affects seed germination and dormancy, however studies on its role in the process have been contradictory [
106] suggesting potential yet unknown variable mediating the
FLC effect.
AGL16 on the other hand hinders Arabidopsis seed germination at higher salinity but suppresses ABA-sensitivity during the process [
107]. The suppression of its targets
HEAT SHOCK TRANSCRIPTION FACTOR A6A (
HSFA6A) and
MYB102 by binding to CArG elements of their respective promoters is associated with the reduced germination under salt stress condition and ABA treatment respectively. It is notable that similar to OsMADS18, HSFA6A localizes to nucleus at stress condition, which would otherwise exhibit cytoplasmic localization [
108].
TT16, a MADS box member involved in pigmentation of seed coat, contributes to seed dormancy by maintaining normal seed coat. When it is defective, the seeds exhibit premature germination in Arabidopsis [
109]. A papaya study additionally showed that its
TT16 ortholog and
FUL/
AGL8 ortholog exhibit higher expression during germination suggesting their potential roles in the process [
110]. Additional MADS box members,
STK and
GOA in combination with an auxin response factor,
ARF2, control polyamines accumulation and mucilage release in the seed coat.
STK in particular controls pectin methylesterase (PME) activity and pectin maturation, defect in which leads to delay in germination at drought condition in Arabidopsis [
111].
STK apparently contributes to salt and oxidative stresses tolerance as well by the enhanced ROS scavenging potential and ABA sensitivity as reported in a rice study by Zhou
, et al. [
112] which observed respectively decreased and increased germination rates in
STK-OE and
STK-KO lines as compared to the WT under ABA treatment (1-6 μM). The study suggested that
STK overexpression-mediated upregulation of stress/ABA-activated protein kinase10 (
OsSAPK10) could be behind the severe ABA-mediated seed germination repression in the
STK-OE lines.
AGL35, yet another MADS box gene reportedly affects germination rates in certain hybrids only by affecting the endosperm cellularization process. The hybrid seeds derived from
AGL35 defective
A. thaliana (♀) and normal
A. arenosa (♂) exhibit much reduced germination rate while those derived from the
AGL35 defective
A. thaliana (♀) and normal
A. lyrata (♂) show much higher as compared to the respective hybrid seeds derived from normal
A. thaliana (♀) [
113].
6. Genes-to-Factors Associations
To have general overview on some of the major factors affecting plant growth and development, we chose hormones and biotic/abiotic factors to extract associated MADS box members in respective gene pools from the local reference database.
6.1. MADS Members-Hormones Association
We generated five hormones-associated gene pools each on auxin, cytokinin, ethylene, gibberellin, and abscisic acid (
Figure 6). Due to the low abundance of hormone associated MADS studies, few of the gene pools showed MADS members with above threshold hits. Nevertheless, the genes with as low as two hits, in most cases, appear to have functions true to the associated gene pool.
To mention few of such example, an auxin-associated gene pool member,
AGL62, which is known to induce auxin in the syncytial endosperm of newly fertilized ovule (seed) defect of which brings impaired auxin transport from the developing endosperm to integuments leading to seed abortion [
114].
XAL2/
AGL14 reportedly plays role on auxin transport during Arabidopsis root development by upregulating
PIN1 and
PIN4 expression. Furthermore, its own expression is positively regulated by the auxin level in a positive feedback loop [
54].
SVP showed at least one hit in all gene pools except cytokinin associated one (
Figure 6b). Ethylene-associated gene pool had its single hit. Nevertheless, a SVP-focused study reported that its clade members show discrepancy in ethylene response-related ERE elements in their promoter with the SVP3-members- which are absent in Brassicaceae- harboring the most suggesting its ethylene-dependent regulation [
115]. Association of
SVP member to auxin has been briefly discussed earlier in the ‘Fruits’ section. Regarding its association with other hormones, we can take an apple study as an example which showed that its
SVP homologs, often referred to as
DORMANCY ASSOCIATED MADS-BOX (
DAM), exhibit highest expression- brought upon mostly by the higher level of H3K4me3- during autumn. Their expression is positively affected by ABA level in a positive feedback loop [
116]. Furthermore, the study observed a significant overlap between the
SVP/
DAM target genes and the genes with differential H3K4me3 levels among the simulated-season-derived samples. The overlapped members included auxin and gibberellin (GA) biosynthesis as well as cell cycle and cell wall expansion associated genes among others indicating role of
SVP/DAM in regulating H3K4me3 level itself in a positive feedback loop. The study concluded that the elevated levels of auxin and GA as well as increased cell cycle progression are key to bud break during spring [
116]. Notably, our analysis shows
SVP hit above threshold in the gene pools associated with GA and ABA, and a threshold-level hit in the auxin associated one (
Figure 6).
Ethylene associations to the MADS members were the lowest among all gene pools.
STK which returned a single hit, is often associated with the seed development and is an unusual gene to have association with ethylene. However, a tomato study with modulated expression of its homolog
Sl-AGL11 showed that apart from obvious differences in the floral and fruit morphologies, the timing of ethylene peak and ethylene level during the peak were widely different between the WT and
Sl-AGL11 overexpressing lines which were correlated with the significant difference in the expression of the ripening associated genes [
117].
6.1. MADS Members-Biotic/Abiotic Factors Association
Local reference database-derived independent gene pools were developed for biotic and abiotic factors each associated with nutrients, defense (tolerance/resistance/ susceptibility), light (response), salt/salinity, and osmotic (response). While the latter four did not return any MADS box members above threshold, few were returned for the former two (
Figure 7). Interestingly, all the hits at and above threshold in the nutrient gene pool were associated with the ANR1 clade except
SOC1. As mentioned in earlier section,
ANR1 and homologs play role in nitrogen foraging.
SOC1 on the other hand reportedly responds to the changes in phosphorus and Sulphur [
40].
STK, one of the members with the lowest hit in the pool, is often associated with ovule development and seed coat formation, is one of the unlikely occurrences. However, a study associated with cell wall invertase (CWIN) reported that,
STK and other genes involved in ovule development are dependent on sugar signaling cues potentially received by the RLK members at the intracellular space [
118]. The study proposed that CWIN may play role on hydrolyzing the sucrose molecules at the intracellular spaces into glucose and fructose which in turn may be sensed by the membrane-bound RLKs to regulate downstream genes involved in ovule development.
Among the genes associated with the defense,
SVP returned with the highest hit (
Figure 7b). The gene is known to play role in age related-resistance (ARR) in Arabidopsis [
119]. However, its role on biotic/abiotic stress has not been explored much. Nevertheless, a study related to the ACCase inhibitor herbicide (clodinafop-propargyl) tolerance by
Polygon fugax, a weedy plant belonging to the Poaceae family, showed that the plant reportedly exhibits positive correlation of its herbicide tolerance to
PfMADS11 expression and precocious flowering, even though the molecular mechanism behind the process remains yet to be elucidated [
120]. Overexpression of
SOC1-like gene,
VcSOC1K in blueberry reportedly confers high pH tolerance to the plant [
121]. Regarding
AP1, a study on shade-tolerant orchid species
Cymbidium sinense reported to have expansion of AP1, SOC1, and SVP members [
122]. However, whether such case has any direct association to the shade tolerance remains unexplored. Regarding the light associated MADS box members, single gene
SOC1 was returned at threshold level hit. The gene is well known for its photoperiod-response and expression fluctuations with the circadian rhythm. As reported in a poplar study, it plays an active role in seasonal ecodormant bud break as well. Furthermore, the study showed that plants overexpressing its
SOC1 homolog,
MADS12, significantly induces much precocious budbreak at long day conditions without pre-chilling treatment
via downregulation of
GA2ox4, a gene actively involved in GA degradation, during the process [
123].
Our analysis returned
SOC1 hits in heat associated gene pool as well albeit below threshold. Its temperature responsiveness is often not highlighted. However, studies show that its photoperiodic response is further enhanced at the warmer temperature in plants [
123]. Interestingly,
SOC1 showed hits to the salt/salinity associated gene pool as well although below threshold. As reported in a study, stress-dependent dual localizing
OXS2, a zinc finger transcription factor essential for salt tolerance [
124], plays active role in activating
SOC1 by directly binding to its promoter during stress condition in Arabidopsis. In normal state however, OXS2 is localized at the cytoplasm and promotes vegetative growth [
125].
SOC1 additionally showed a hit for the osmotic response associated gene pool. The associated study carried out a functional characterization of
Ginkgo biloba derived TT16/GGM13 clade member
GbMADS9, which showed that the plants overexpressing the gene exhibit better growth under high osmotic stress (as compared to WT) and leads to precocious flowering due to the increased expression of florigenic genes
FT,
AP1,
LFY, and
SOC1 [
67]. However, a relatively recent study suggests that
SOC1 itself may not have direct effect on the process [
126]. The involvement of
AGL21 in regulation of osmotic stress is well studied. One of such examples include an Arabidopsis study by Yu
, et al. [
105], which reported the hypersensitivity of the
AGL21 overexpressing lines to osmotic, ABA, and salt stresses during seed germination.
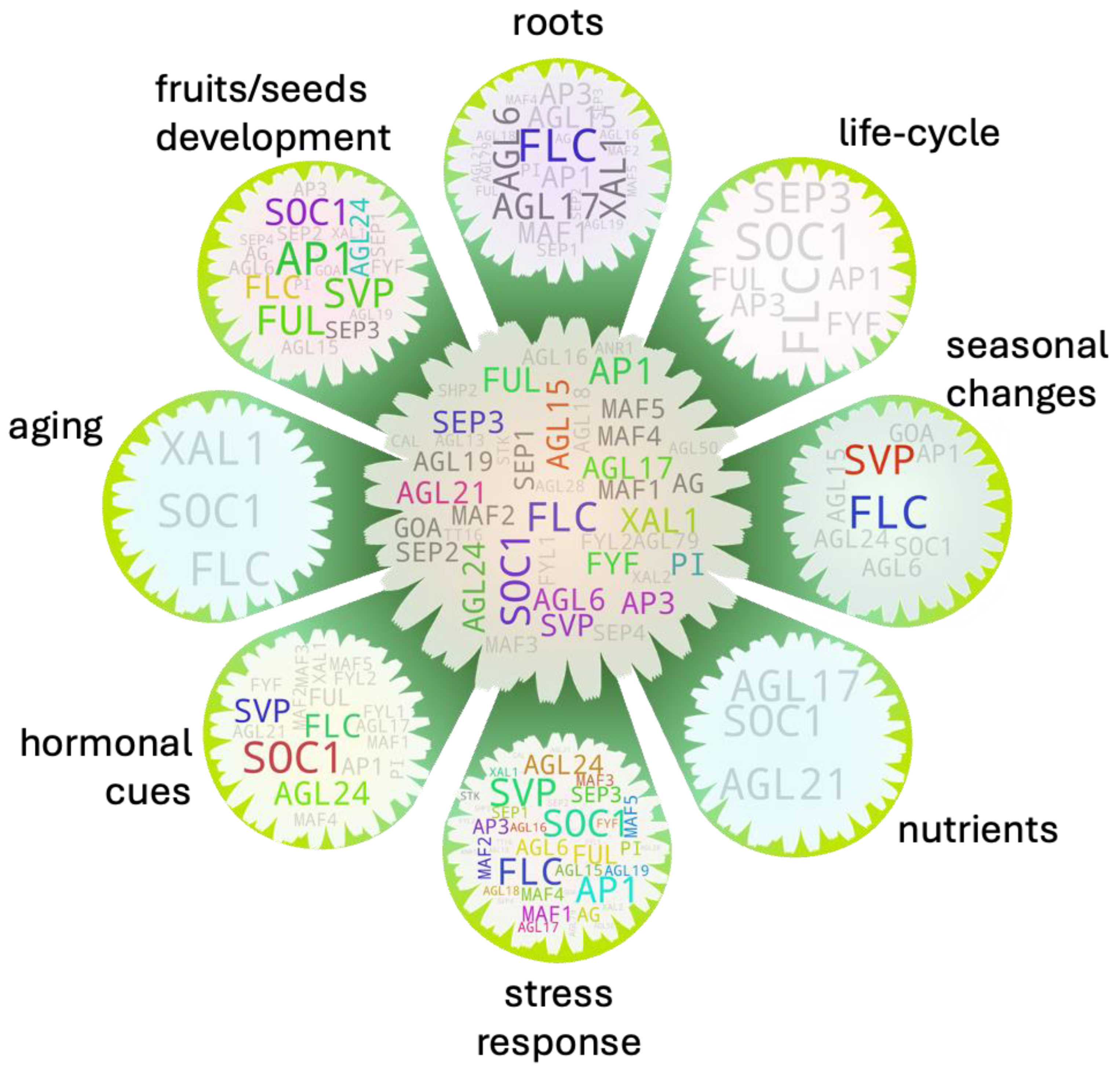
7. Traits-to-Factors Associations Bridged by MADS
While working with a specific phenotype, general overview of potential genes linked with the factors associated to the phenotype would offer information on genetic layers and potential directionality of the genes’ action. Direct literature-derived information would be very helpful in such case. Being one of the heavily studied gene groups in association to flowering, MADS box members are expected to have relatively richer information regarding their role on bridging the biotic and abiotic factors derived cues to the process.
Flowering is a complex process. However, studies have often demonstrated that ectopic expression of florigenic terminal genes is sufficient for floral induction in many cases, which often renders the transgenic plant phenotypically different/deformed as compared to its wild-type counterpart indicating potential genetic bottleneck behind the phenomenon. Such effect is more pronounced in the perennials [
127,
128,
129,
130]. Plants respond to the biotic and abiotic cues to allocate their resources according to their physiological need. When those processes are cut short or abruptly disturbed
via transgenic approach, such cues are less likely to be aligned in in the plant, which could be the main reason behind such aberrant phenotype.
Being a terminal developmental process in a plant’s life cycle, flowering commences either when the plant is fully mature or if there is risk-to-perish prior to its maturity due to unavoidable biotic/abiotic factors [
131,
132]. In other case, the flowering frequency and/intensity may decrease when there is ample fruit/seed-set to secure next generation through negative feedback loop which we discussed earlier in the ‘fruits’ and ‘seeds’ sections. We screened MADS box members with such potentials of bridging external/internal cues to the flowering process. In total, eight separate gene-pools- each with potential role in bridging flowering process to fruit/seed development, root development/biomass, nutrients, stress response, hormonal cues, seasonal changes, aging, and plant’s life cycle (
Figure 8, outer gene pools). Even though five out of them returned genes above threshold, majority of the genes returned in each gene pool apparently had their functional relevance to their associated gene pools.
We additionally checked potential multi-factor integrator MADS box members (
Figure 8, central gene pool) based on their frequency of occurrences in the aforementioned independent gene pools.
SOC1 and
FLC showed the highest hit (7 each) followed by
AP1 (6), and
FUL and
XAL1 (5 each), roughly suggesting that their ectopic expression modulation may bring at least less phenotypic abnormalities in the transgenic plants. Such assumption is partly corroborated by a transgenic study with
MtSOC1a in Medicago (perennial plant) in which the overexpression lines not only exhibited precocious flowering phenotype but showed increased shoot growth as well [
133]. In a different study on soybean (annual pant) however, a maize derived
ZmSOC1 conferred shorter plant height with frequent abnormal flower development, but increased branching and pod numbers per plant among the overexpression lines as compared to wild-type [
134]. As mentioned earlier, its constitutive expression in Arabidopsis reportedly causes failure of seed development. It should be noted that
SOC1 is one of the key flowering pathway integrator [
81].
FLC along with majority of its clade members play role in temperature/vernalization dependent flowering process.
AP1 and its clade members (including
FUL) function terminally in the flowering pathway, and
XAL1 mainly contributes to root growth and development as well as in flowering process. While its defect brings significant delay in the process, its overexpression effect on flowering is not as significant likely because
XAL1 itself may not be sufficient to activate its target genes involved in the process [
55,
135].
Even though majority of the MADS box members play a direct crucial role in floral development and some in vegetative-to-reproductive phase transition, they are not the only major players behind flowering associated physiological processes. While modulated expression of florigenic MADS box members often triggers plants to produce new sink (flower), its state and further developmental progression would still necessitate proper alignment of the underlying physiological processes in the plant system. Similar comprehensive assessment particularly focusing on flowering rather than a particular gene group may offer relatively robust find returning with additional key players involved in the physiological processes during flowering.
8. Optimization Considerations for the Approach
During literature data extraction and analysis, we customized our approach to better fit its result with the study findings. Below are some of those key customization parameters considered-
Threshold calibration: Thresholds for each analysis may depend on the volumes of the studies in the local reference database. Larger volumes of references along with higher threshold hit assignment may enhance reliability of the assessment. From our analysis, threshold hit of at least 3 is sufficient to return a workable result from a representative local reference database.
Choice of keywords/terms: As observed in MADS box members assessment, several gene IDs may match with their respective clade IDs (e.g.,
FLC,
AP1,
SOC1, etc.). Hence, such IDs often return with higher hits. In such case, their respective association to a particular trait could equally be trait-to-clade association in addition to trait-to-gene association. Furthermore, use of dual-meaning terms (e.g., light) may include higher false positive hits. Use of exclusion for the search-term associated unwanted phrases could circumvent the case. In rare cases, search terms may match with the unintended annotations used in the studies. One of such examples include the occurrence of “AG” in naming an allele in a rice study [
136], which was picked up in the gene pools associated with the gene
AG. Use of suitable (higher) threshold level would help reduce such unwanted ‘noise’ data.
Analysis skewdness: Pleiotropic gene pooling approach used in current study basically depends on the independent trait-based gene pools used for the analysis and tends to have skewedness towards the most studied members as the prediction circles back to the holistic assessment of those independent gene pools derived from the same local reference database. Expanding reference database size may certainly help circumventing such case to some level. However, allowing some buffer zone (gray area) at both sides of the threshold and manual inspection of the genes within the area are expected to enhance the analysis strength.
9. Significance and Application of the Approach
Research studies are often carried out at narrower niche of the fields with narrower objectives as the knowledge and technology advances with time. While it is beneficial to have narrow study focus, it may sometime leave obvious blind spot which would otherwise have been noticed. In other cases, not all studies are equally legible to all the researchers. Additionally, while we gain expertise with knowledge and experiences, experts of the subject matter of interest may not always be available or reachable. The study approach devised in the current study aims to circumvent such cases.
With the use of relevant keywords and constraints along with suitable threshold assignment, current approach would offer an alternative to have expert-like glimpse on the subject matter of interest. Furthermore, it would offer an opportunity to have a quick overview on the subject matter from multiple perspectives, which is often deemed crucial for initial phases of research and experimental design. Current approach is also useful to have a data-based overview on any potential study biases as we observed between type I and type II MADS box members in current study.
10. Conclusions
Our assessment showed a clear disparity between studies associated with type I and type II MADS box members. While most of the MADS box associated studies are flower and fruit focused and MADS box members indeed have played significant role in the evolution of Angiosperms, our study suggests there are more avenues to its functional relevance in plant. We devised and used an approach to extract gene associations to various factors and developmental stages from the manually curated MADS-focused local reference database (all the retrieved gene pools associated data are provided in Supplementary Dataset 2). Such approach is equally applicable for any other study of interest be it a particular gene focused, a specific trait focused, or any other topic of interest (for non-biological disciplines).
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: MADS box member TCOFFEE-alignment derived ML phylogeny; Figure S2: Gene clades-to-studies association with hits ranging from 6 (Mδ) to 76 (SVP); Supplementary Table S1: MADS box member genes along with their respective study-association hit counts on the used local reference database; Supplementary Dataset 1: Hits data of the term pools derived in the study; Supplementary Dataset 2: References used to prepare the local database to carry out current study
Author Contributions
P.B.A. conceived and prepared the manuscript. R.D.K. assisted during editing process.
Funding
This work was supported by the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research (Grant #: 22K21366 to R.D.K.).
Data Availability Statement
All the data used for and produced during the analysis have been included in the manuscript. The in-house script prepared during the analysis can be provided to researchers upon request.
Acknowledgments
ChatGPT [
137] was used for scripting assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Theissen, G.; Kim, J.T.; Saedler, H. Classification and phylogeny of the MADS-box multigene family suggest defined roles of MADS-box gene subfamilies in the morphological evolution of eukaryotes. J Mol Evol 1996, 43, 484–516. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Winter, K.-U.; Meyer, B.; Saedler, H.; Theißen, G. MADS-Box Gene Diversity in Seed Plants 300 Million Years Ago. Molecular Biology and Evolution 2000, 17, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Buylla, E.R.; Corvera-Poiré, A.; Garay-Arroyo, A.; García-Ponce, B.; Jaimes-Miranda, F.; Pérez-Ruiz, R.V. Alvarez-Buylla, E.R.; Corvera-Poiré, A.; Garay-Arroyo, A.; García-Ponce, B.; Jaimes-Miranda, F.; Pérez-Ruiz, R.V. A MADS view of plant development and evolution. In Topics in Animal and Plant Development: From Cell Differentiation to Morphogenesis, Chimal-Monroy, J., Ed.; Transworld Research Network: Kerala, India, 2011; Jaimes-Miranda, F.; pp. 181–220. [Google Scholar]
- Shen, G.; Yang, C.H.; Shen, C.Y.; Huang, K.S. Origination and selection of ABCDE and AGL6 subfamily MADS-box genes in gymnosperms and angiosperms. Biol Res 2019, 52, 25. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Jia, Y.; Wang, W.L. Evolutionary divergence of motifs in B-class MADS-box proteins of seed plants. J Biol Res (Thessalon) 2021, 28, 12. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Zahn, L.; Guindon, S.; Wall, P.K.; Kong, H.; Ma, H.; DePamphilis, C.W.; Leebens-Mack, J. Evolution of plant MADS box transcription factors: evidence for shifts in selection associated with early angiosperm diversification and concerted gene duplications. Mol Biol Evol 2009, 26, 2229–2244. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, Z.; Walther, D.; Kohler, C. Updated phylogeny and protein structure predictions revise the hypothesis on the origin of MADS-box transcription factors in land plants. Mol Biol Evol 2023, 40. [Google Scholar] [CrossRef] [PubMed]
- Preston, J.C.; Christensen, A.; Malcomber, S.T.; Kellogg, E.A. MADS-box gene expression and implications for developmental origins of the grass spikelet. Am J Bot 2009, 96, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Yanofsky, M.F. Function and evolution of the plant MADS-box gene family. Nat Rev Genet 2001, 2, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Kim, J.; Lee, S.; An, G.; Ma, H.; Nei, M. Type I MADS-box genes have experienced faster birth-and-death evolution than type II MADS-box genes in angiosperms. Proc Natl Acad Sci U S A 2004, 101, 1910–1915. [Google Scholar] [CrossRef]
- Smaczniak, C.; Immink, R.G.; Angenent, G.C.; Kaufmann, K. Developmental and evolutionary diversity of plant MADS-domain factors: insights from recent studies. Development 2012, 139, 3081–3098. [Google Scholar] [CrossRef]
- Mueller, A.; Fillion-Robin, J.-C.; Boidol, R.; Tian, F.; Nechifor, P.; Rampin, R.; Corvellec, M.; Medina, J.; Dai, Y.; Petrushev, B. Amueller/word_cloud-1.9.3. Zenodo 2018. [Google Scholar] [CrossRef]
- Gu, Q.; Ferrandiz, C.; Yanofsky, M.F.; Martienssen, R. The FRUITFULL MADS-box gene mediates cell differentiation during Arabidopsis fruit development. Development 1998, 125, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Ferrándiz, C.; Liljegren, S.J.; Yanofsky, M.F. Negative regulation of the SHATTERPROOF genes by FRUITFULL during Arabidopsis fruit development. Science 2000, 289, 436–438. [Google Scholar] [CrossRef] [PubMed]
- Liljegren, S.J.; Ditta, G.S.; Eshed, Y.; Savidge, B.; Bowman, J.L.; Yanofsky, M.F. SHATTERPROOF MADS-box genes control seed dispersal in Arabidopsis. Nature 2000, 404, 766–770. [Google Scholar] [CrossRef]
- Balanzà, V.; Martínez-Fernández, I.; Sato, S.; Yanofsky, M.F.; Ferrándiz, C. Inflorescence meristem fate is dependent on seed development and fruitfull in Arabidopsis thaliana. Frontiers in Plant Science 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Fuhrer, M.; Gaidora, A.; Venhuizen, P.; Dobrogojski, J.; Beziat, C.; Feraru, M.I.; Kleine-Vehn, J.; Kalyna, M.; Barbez, E. FRUITFULL Is a Repressor of Apical Hook Opening in Arabidopsis thaliana. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Elo, A.; Lemmetyinen, J.; Turunen, M.L.; Tikka, L.; Sopanen, T. Three MADS-box genes similar to APETALA1 and FRUITFULL from silver birch (Betula pendula). Physiol Plant 2001, 112, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, M.; Shima, Y.; Nakagawa, H.; Kitagawa, M.; Kimbara, J.; Nakano, T.; Kasumi, T.; Ito, Y. Transcriptional regulation of fruit ripening by tomato FRUITFULL homologs and associated MADS box proteins. Plant Cell 2014, 26, 89–101. [Google Scholar] [CrossRef]
- Jiang, X.; Lubini, G.; Hernandes-Lopes, J.; Rijnsburger, K.; Veltkamp, V.; de Maagd, R.A.; Angenent, G.C.; Bemer, M. FRUITFULL-like genes regulate flowering time and inflorescence architecture in tomato. Plant Cell 2022, 34, 1002–1019. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Yasuno, N.; Sato, Y.; Yoda, M.; Yamazaki, R.; Kimizu, M.; Yoshida, H.; Nagamura, Y.; Kyozuka, J. Inflorescence meristem identity in rice is specified by overlapping functions of three AP1/FUL-like MADS box genes and PAP2, a SEPALLATA MADS box gene. Plant Cell 2012, 24, 1848–1859. [Google Scholar] [CrossRef]
- Yin, X.; Liu, X.; Xu, B.; Lu, P.; Dong, T.; Yang, D.; Ye, T.; Feng, Y.Q.; Wu, Y. OsMADS18, a membrane-bound MADS-box transcription factor, modulates plant architecture and the abscisic acid response in rice. J Exp Bot 2019, 70, 3895–3909. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Sun, S.; Li, J.; Yu, H.; Wu, H.; Sun, B.; Li, T.; Han, T.; Jiang, B. GmFULa improves soybean yield by enhancing carbon assimilation without altering flowering time or maturity. Plant Cell Rep 2021, 40, 1875–1888. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, C.; Yu, H.; Tao, P.; Yuan, L.; Ye, J.; Chen, W.; Wang, Y.; Ge, P.; Zhang, J.; Zhou, G.; Zheng, W.; Ye, Z.; Zhang, Y. GREEN STRIPE, encoding methylated TOMATO AGAMOUS-LIKE 1, regulates chloroplast development and Chl synthesis in fruit. New Phytol 2020, 228, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Cui, X.; Qin, G.; Chen, T.; Tian, S. SlFERL interacts with S-adenosylmethionine synthetase to regulate fruit ripening. Plant Physiol 2020, 184, 2168–2181. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.s.; Guo, P.y.; Zhang, J.l.; Xie, Q.l.; Shen, H.; Hu, Z.l.; Chen, G.p. Overexpression of the MADS-box gene SIMBP21 alters leaf morphology and affects reproductive development in tomato. Journal of Integrative Agriculture 2021, 20, 3170–3185. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Z.; Yao, Q.; Guo, X.; Nguyen, V.; Li, F.; Chen, G. A tomato MADS-box protein, SlCMB1, regulates ethylene biosynthesis and carotenoid accumulation during fruit ripening. Sci Rep 2018, 8, 3413. [Google Scholar] [CrossRef] [PubMed]
- Parenicova, L.; de Folter, S.; Kieffer, M.; Horner, D.S.; Favalli, C.; Busscher, J.; Cook, H.E.; Ingram, R.M.; Kater, M.M.; Davies, B.; Angenent, G.C.; Colombo, L. Molecular and phylogenetic analyses of the complete MADS-box transcription factor family in Arabidopsis: new openings to the MADS world. Plant Cell 2003, 15, 1538–1551. [Google Scholar] [CrossRef] [PubMed]
- Bemer, M.; van Mourik, H.; Muiño, J.M.; Ferrándiz, C.; Kaufmann, K.; Angenent, G.C. FRUITFULL controls SAUR10 expression and regulates Arabidopsis growth and architecture. Journal of Experimental Botany 2017, 68, 3391–3403. [Google Scholar] [CrossRef]
- Gregis, V.; Sessa, A.; Colombo, L.; Kater, M.M. AGL24, SHORT VEGETATIVE PHASE, and APETALA1 redundantly control AGAMOUS during early stages of flower development in Arabidopsis. Plant Cell 2006, 18, 1373–1382. [Google Scholar] [CrossRef]
- Gu, X.; Le, C.; Wang, Y.; Li, Z.; Jiang, D.; Wang, Y.; He, Y. Arabidopsis FLC clade members form flowering-repressor complexes coordinating responses to endogenous and environmental cues. Nat Commun 2013, 4, 1947. [Google Scholar] [CrossRef]
- Digel, B.; Tavakol, E.; Verderio, G.; Tondelli, A.; Xu, X.; Cattivelli, L.; Rossini, L.; von Korff, M. Photoperiod-H1 (Ppd-H1) Controls Leaf Size. Plant Physiol 2016, 172, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Willmann, M.R.; Poethig, R.S. The effect of the floral repressor FLC on the timing and progression of vegetative phase change in Arabidopsis. Development 2011, 138, 677–685. [Google Scholar] [CrossRef]
- Hartmann, U.; Höhmann, S.; Nettesheim, K.; Wisman, E.; Saedler, H.; Huijser, P. Molecular cloning of SVP: a negative regulator of the floral transition in Arabidopsis. The Plant Journal 2000, 21, 351–360. [Google Scholar] [CrossRef]
- Ó’Maoiléidigh, D.S.; Wuest, S.E.; Rae, L.; Raganelli, A.; Ryan, P.T.; Kwaśniewska, K.; Das, P.; Lohan, A.J.; Loftus, B.; Graciet, E.; Wellmer, F. Control of reproductive floral organ identity specification in Arabidopsis by the C function regulator AGAMOUS The Plant Cell 2013, 25, 2482-2503. 25. [CrossRef]
- O’Maoileidigh, D.S.; Stewart, D.; Zheng, B.; Coupland, G.; Wellmer, F. Floral homeotic proteins modulate the genetic program for leaf development to suppress trichome formation in flowers. Development 2018, 145. [Google Scholar] [CrossRef]
- Fernandez, D.E.; Wang, C.T.; Zheng, Y.; Adamczyk, B.J.; Singhal, R.; Hall, P.K.; Perry, S.E. The MADS-domain factors AGAMOUS-LIKE15 and AGAMOUS-LIKE18, along with short vegetative phase and AGAMOUS-LIKE24, are necessary to block floral gene expression during the vegetative phase. Plant Physiol 2014, 165, 1591–1603. [Google Scholar] [CrossRef]
- Yoo, S.K.; Hong, S.M.; Lee, J.S.; Ahn, J.H. A genetic screen for leaf movement mutants identifies a potential role for AGAMOUS-LIKE 6 (AGL6) in circadian-clock control. Molecules and Cells 2011, 31, 281–287. [Google Scholar] [CrossRef]
- Huang, N.C.; Tien, H.C.; Yu, T.S. Arabidopsis leaf-expressed AGAMOUS-LIKE 24 mRNA systemically specifies floral meristem differentiation. New Phytol 2024, 241, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Filleur, S.; Rahman, A.; Gotensparre, S.; Forde, B.G. Nutritional regulation of ANR1 and other root-expressed MADS-box genes in Arabidopsis thaliana. Planta 2005, 222, 730–742. [Google Scholar] [CrossRef]
- Alvarez-Buylla, E.R.; Garcia-Ponce, B.; Sanchez, M.P.; Espinosa-Soto, C.; Garcia-Gomez, M.L.; Pineyro-Nelson, A.; Garay-Arroyo, A. MADS-box genes underground becoming mainstream: plant root developmental mechanisms. New Phytol 2019, 223, 1143–1158. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Mizuno, K.; Fujimura, T. Isolation of MADS-box genes from sweet potato (Ipomoea batatas (L.) Lam.) expressed specifically in vegetative tissues. Plant Cell Physiol 2002, 43, 314–322. [Google Scholar] [CrossRef]
- Nasrollahi, V.; Yuan, Z.C.; Lu, Q.S.M.; McDowell, T.; Kohalmi, S.E.; Hannoufa, A. Deciphering the role of SPL12 and AGL6 from a genetic module that functions in nodulation and root regeneration in Medicago sativa. Plant Mol Biol 2022, 110, 511–529. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Hamada, T.; Otani, M.; Shimada, T. Isolation and characterization of MADS box genes possibly related to root development in sweetpotato (Ipomoea batatas L. Lam.). Journal of Plant Biology 2005, 48, 387–393. [Google Scholar] [CrossRef]
- Alvarez-Buylla, E.R.; Liljegren, S.J.; Pelaz, S.; Gold, S.E.; Burgeff, C.; Ditta, G.S.; Vergara-Silva, F.; Yanofsky, M.F. MADS-box gene evolution beyond flowers: expression in pollen, endosperm, guard cells, roots and trichomes. The Plant Journal 2008, 24, 457–466. [Google Scholar] [CrossRef]
- Adamczyk, B.J.; Lehti Shiu, M.D.; Fernandez, D.E. The MADS domain factors AGL15 and AGL18 act redundantly as repressors of the floral transition in Arabidopsis. Plant J 2007, 50, 1007–1019. [Google Scholar] [CrossRef]
- Shah, L.; Sohail, A.; Ahmad, R.; Cheng, S.; Cao, L.; Wu, W. The roles of MADS-box genes from root growth to maturity in Arabidopsis and rice. Agronomy 2022, 12. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, Y.; Yu, M.; Su, B.; Gong, W.; Baluška, F.; Komis, G.; Šamaj, J.; Shan, X.; Lin, J. Phosphorylation-mediated dynamics of nitrate transceptor NRT1.1 regulate auxin flux and nitrate signaling in lateral root growth. Plant Physiol 2019, 181, 480–498. [Google Scholar] [CrossRef]
- Gan, Y.; Zhou, Z.; An, L.; Bao, S.; Liu, Q.; Srinivasan, M.; Goddard, P. The effects of fluctuations in the nutrient supply on the expression of ANR1 and 11 other MADS box genes in shoots and roots of Arabidopsis thaliana. Botany 2010, 88, 1023–1031. [Google Scholar] [CrossRef]
- Nawy, T.; Lee, J.Y.; Colinas, J.; Wang, J.Y.; Thongrod, S.C.; Malamy, J.E.; Birnbaum, K.; Benfey, P.N. Transcriptional profile of the Arabidopsis root quiescent center. Plant Cell 2005, 17, 1908–1925. [Google Scholar] [CrossRef] [PubMed]
- Burgeff, C.; Liljegren, S.J.; Tapia-Lopez, R.; Yanofsky, M.F.; Alvarez-Buylla, E.R. MADS-box gene expression in lateral primordia, meristems and differentiated tissues of Arabidopsis thaliana roots. Planta 2002, 214, 365–372. [Google Scholar] [CrossRef]
- Han, P.; Garcia-Ponce, B.; Fonseca-Salazar, G.; Alvarez-Buylla, E.R.; Yu, H. AGAMOUS-LIKE 17, a novel flowering promoter, acts in a FT-independent photoperiod pathway. Plant J 2008, 55, 253–265. [Google Scholar] [CrossRef]
- Zhao, P.X.; Zhang, J.; Chen, S.Y.; Wu, J.; Xia, J.Q.; Sun, L.Q.; Ma, S.S.; Xiang, C.B. AGL16 negatively modulates stress response to balance with growth. bioRxiv, 2002; 2021.2002.2016.431464. [Google Scholar] [CrossRef]
- Garay-Arroyo, A.; Ortiz-Moreno, E.; de la Paz Sánchez, M.; Murphy, A.S.; García-Ponce, B.; Marsch-Martínez, N.; de Folter, S.; Corvera-Poiré, A.; Jaimes-Miranda, F.; Pacheco-Escobedo, M.A.; Dubrovsky, J.G.; Pelaz, S.; Álvarez-Buylla, E.R. The MADS transcription factor XAL2/AGL14 modulates auxin transport during Arabidopsis root development by regulating PIN expression. Embo j 2013, 32, 2884–2895. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Lopez, R.; Garcia-Ponce, B.; Dubrovsky, J.G.; Garay-Arroyo, A.; Perez-Ruiz, R.V.; Kim, S.H.; Acevedo, F.; Pelaz, S.; Alvarez-Buylla, E.R. An AGAMOUS-related MADS-box gene, XAL1 (AGL12), regulates root meristem cell proliferation and flowering transition in Arabidopsis. Plant Physiol 2008, 146, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Johansen, B.; Pedersen, L.B.; Skipper, M.; Frederiksen, S. MADS-box gene evolution-structure and transcription patterns. Mol Phylogenet Evol 2002, 23, 458–480. [Google Scholar] [CrossRef]
- Kinoshita, A.; Vayssieres, A.; Richter, R.; Sang, Q.; Roggen, A.; van Driel, A.D.; Smith, R.S.; Coupland, G. Regulation of shoot meristem shape by photoperiodic signaling and phytohormones during floral induction of Arabidopsis. Elife 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Gregis, V.; Sessa, A.; Dorca-Fornell, C.; Kater, M.M. The Arabidopsis floral meristem identity genes AP1, AGL24 and SVP directly repress class B and C floral homeotic genes. The Plant Journal 2009, 60, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Fernandez, I.; Menezes de Moura, S.; Alves-Ferreira, M.; Ferrandiz, C.; Balanza, V. Identification of players controlling meristem arrest downstream of the FRUITFULL-APETALA2 pathway. Plant Physiol 2020, 184, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Richter, R.; Kinoshita, A.; Vincent, C.; Martinez-Gallegos, R.; Gao, H.; van Driel, A.D.; Hyun, Y.; Mateos, J.L.; Coupland, G. Floral regulators FLC and SOC1 directly regulate expression of the B3-type transcription factor TARGET OF FLC AND SVP 1 at the Arabidopsis shoot apex via antagonistic chromatin modifications. PLoS Genet 2019, 15, e1008065. [Google Scholar] [CrossRef] [PubMed]
- Gramzow, L.; Theissen, G. A hitchhiker’s guide to the MADS world of plants. Genome Biol 2010, 11, 214. [Google Scholar] [CrossRef]
- Becker, A.; Theissen, G. The major clades of MADS-box genes and their role in the development and evolution of flowering plants. Mol Phylogenet Evol 2003, 29, 464–489. [Google Scholar] [CrossRef]
- Nam, J.; dePamphilis, C.W.; Ma, H.; Nei, M. Antiquity and evolution of the MADS-box gene family controlling flower development in plants. Mol Biol Evol 2003, 20, 1435–1447. [Google Scholar] [CrossRef]
- Adhikari, P.B.; Liu, X.; Wu, X.; Zhu, S.; Kasahara, R.D. Fertilization in flowering plants: an odyssey of sperm cell delivery. Plant Molecular Biology 2020, 103, 9–32. [Google Scholar] [CrossRef] [PubMed]
- Dreni, L.; Zhang, D. Flower development: the evolutionary history and functions of the AGL6 subfamily MADS-box genes. J Exp Bot 2016, 67, 1625–1638. [Google Scholar] [CrossRef]
- Simonini, S.; Roig-Villanova, I.; Gregis, V.; Colombo, B.; Colombo, L.; Kater, M.M. BASIC PENTACYSTEINE proteins mediate MADS domain complex binding to the DNA for tissue-specific expression of target genes in Arabidopsis The Plant Cell 2012, 24, 4163-4172. [CrossRef]
- Yang, F.; Xu, F.; Wang, X.; Liao, Y.; Chen, Q.; Meng, X. Characterization and functional analysis of a MADS-box transcription factor gene (GbMADS9) from Ginkgo biloba. Scientia Horticulturae 2016, 212, 104–114. [Google Scholar] [CrossRef]
- Portereiko, M.F.; Lloyd, A.; Steffen, J.G.; Punwani, J.A.; Otsuga, D.; Drews, G.N. AGL80 Is required for central cell and endosperm development in Arabidopsis. The Plant Cell 2006, 18, 1862–1872. [Google Scholar] [CrossRef] [PubMed]
- Steffen, J.G.; Kang, I.H.; Portereiko, M.F.; Lloyd, A.; Drews, G.N. AGL61 interacts with AGL80 and is required for central cell development in Arabidopsis. Plant Physiol 2008, 148, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Masiero, S.; Vanzulli, S.; Lardelli, P.; Kater, M.M.; Colombo, L. AGL23, a type I MADS-box gene that controls female gametophyte and embryo development in Arabidopsis. Plant J 2008, 54, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Immink, R.G.; Posé, D.; Ferrario, S.; Ott, F.; Kaufmann, K.; Valentim, F.L.; de Folter, S.; van der Wal, F.; van Dijk, A.D.; Schmid, M.; Angenent, G.C. Characterization of SOC1’s central role in flowering by the identification of its upstream and downstream regulators. Plant Physiol 2012, 160, 433–449. [Google Scholar] [CrossRef]
- Ito, T.; Sakai, H.; Meyerowitz, E.M. Whorl-specific expression of the SUPERMAN gene of Arabidopsis is mediated by cis elements in the transcribed region. Current Biology 2003, 13, 1524–1530. [Google Scholar] [CrossRef]
- Bowman, J.L.; Smyth, D.R.; Meyerowitz, E.M. Genes directing flower development in Arabidopsis. Plant Cell 1989, 1, 37–52. [Google Scholar] [CrossRef]
- Hsu, W.H.; Yeh, T.J.; Huang, K.Y.; Li, J.Y.; Chen, H.Y.; Yang, C.H. AGAMOUS-LIKE13, a putative ancestor for the E functional genes, specifies male and female gametophyte morphogenesis. Plant J 2014, 77, 1–15. [Google Scholar] [CrossRef]
- Wang, D.; Hao, Z.; Long, X.; Wang, Z.; Zheng, X.; Ye, D.; Peng, Y.; Wu, W.; Hu, X.; Wang, G.; Zheng, R.; Shi, J.; Chen, J. The transcriptome of Cunninghamia lanceolata male/female cone reveal the association between MIKC MADS-box genes and reproductive organs development. BMC Plant Biol 2020, 20, 508. [Google Scholar] [CrossRef] [PubMed]
- Myat, A.A.; Zhou, Y.; Gao, Y.; Zhao, X.; Liang, C.; Abid, M.A.; Wang, P.; Akram, U.; Abbas, M.; Askari, M.; Guo, S.; Zhang, R.; Meng, Z. Overexpression of GhKTI12 enhances seed yield and biomass production in Nicotiana tabacum. Genes (Basel) 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yao, J.; Wang, L.; Wu, N.; van Nocker, S.; Li, Z.; Gao, M.; Wang, X. Role of grapevine SEPALLATA-related MADS-box gene VvMADS39 in flower and ovule development. Plant J 2022, 111, 1565–1579. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Fernández, I.; Fourquin, C.; Lindsay, D.; Berbel, A.; Balanzà, V.; Huang, S.; Dalmais, M.; LeSignor, C.; Bendahmane, A.; Warkentin, T.D.; Madueño, F.; Ferrándiz, C. Analysis of pea mutants reveals the conserved role of FRUITFULL controlling the end of flowering and its potential to boost yield. Proceedings of the National Academy of Sciences 2024, 121, e2321975121. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Karunarathna, N.L.; Jung, C.; Emrani, N. An APETALA1 ortholog affects plant architecture and seed yield component in oilseed rape (Brassica napus L.). BMC Plant Biology 2018, 18, 380. [Google Scholar] [CrossRef] [PubMed]
- Samach, A.; Onouchi, H.; Gold, S.E.; Ditta, G.S.; Schwarz-Sommer, Z.; Yanofsky, M.F.; Coupland, G. Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science 2000, 288, 1613–1616. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, I. Regulation and function of SOC1, a flowering pathway integrator. Journal of Experimental Botany 2010, 61, 2247–2254. [Google Scholar] [CrossRef]
- Chen, W.H.; Lin, P.T.; Hsu, W.H.; Hsu, H.F.; Li, Y.C.; Tsao, C.W.; Hsu, M.C.; Mao, W.T.; Yang, C.H. Regulatory network for FOREVER YOUNG FLOWER-like genes in regulating Arabidopsis flower senescence and abscission. Commun Biol 2022, 5, 662. [Google Scholar] [CrossRef]
- Yuan, J.; Long, H.; Qiu, F.; Wang, Y.; Zhang, M.; Chao, Y.; Chen, L. MADS-box protein MtSOC1c regulates flowering and seed development in Medicago truncatula. Industrial Crops and Products 2023, 193, 116125. [Google Scholar] [CrossRef]
- Papaefthimiou, D.; Kapazoglou, A.; Tsaftaris, A.S. Cloning and characterization of SOC1 homologs in barley (Hordeum vulgare) and their expression during seed development and in response to vernalization. Physiologia Plantarum 2012, 146, 71–85. [Google Scholar] [CrossRef]
- Kennedy, A.; Geuten, K. The Role of FLOWERING LOCUS C Relatives in Cereals. Front Plant Sci 2020, 11, 617340. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Y.; Zhang, J.; Torrance, A.; Watanabe, N.; Adamski, N.M.; Uauy, C. The Triticum ispahanicum elongated glume locus P2 maps to chromosome 6A and is associated with the ectopic expression of SVP-A1. Theor Appl Genet 2022, 135, 2313–2331. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.M.; Wang, X.; Wang, Y.W.; Hu, R.B.; Zhang, X.M.; Chen, J.X.; Fu, Y.F. Genome-wide expression analysis of soybean MADS genes showing potential function in the seed development. PLoS One 2013, 8, e62288. [Google Scholar] [CrossRef] [PubMed]
- Chiang, G.C.; Barua, D.; Kramer, E.M.; Amasino, R.M.; Donohue, K. Major flowering time gene, FLOWERING LOCUS C, regulates seed germination in Arabidopsis thaliana. Proc Natl Acad Sci U S A 2009, 106, 11661–11666. [Google Scholar] [CrossRef]
- Chen, N.; Veerappan, V.; Abdelmageed, H.; Kang, M.; Allen, R.D. HSI2/VAL1 silences AGL15 to regulate the developmental transition from seed maturation to vegetative growth in Arabidopsis. Plant Cell 2018, 30, 600–619. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, K.; Bhide, A.S.; Tekleyohans, D.G.; Wittkop, B.; Snowdon, R.J.; Becker, A. The MADS box genes ABS, SHP1, and SHP2 are essential for the coordination of cell divisions in ovule and seed coat development and for endosperm formation in Arabidopsis thaliana. PLoS One 2016, 11, e0165075. [Google Scholar] [CrossRef] [PubMed]
- Bemer, M.; Heijmans, K.; Airoldi, C.; Davies, B.; Angenent, G.C. An atlas of type I MADS box gene expression during female gametophyte and seed development in Arabidopsis. Plant Physiol 2010, 154, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Coen, O.; Fiume, E.; Xu, W.; De Vos, D.; Lu, J.; Pechoux, C.; Lepiniec, L.; Magnani, E. Developmental patterning of the sub-epidermal integument cell layer in Arabidopsis seeds. Development 2017, 144, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.; Chung, Y.S. Validation of MADS-box genes from apple fruit pedicels during early fruit abscission by transcriptome analysis and real-time PCR. Genes Genomics 2019, 41, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Begum, D.; Chuang, H.W.; Budiman, M.A.; Szymkowiak, E.J.; Irish, E.E.; Wing, R.A. JOINTLESS is a MADS-box gene controlling tomato flower abscission zone development. Nature 2000, 406, 910–913. [Google Scholar] [CrossRef]
- Nakano, T.; Kimbara, J.; Fujisawa, M.; Kitagawa, M.; Ihashi, N.; Maeda, H.; Kasumi, T.; Ito, Y. MACROCALYX and JOINTLESS Interact in the transcriptional regulation of tomato fruit abscission zone development. Plant Physiology 2011, 158, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Pi, M.; Hu, S.; Cheng, L.; Zhong, R.; Cai, Z.; Liu, Z.; Yao, J.L.; Kang, C. The MADS-box gene FveSEP3 plays essential roles in flower organogenesis and fruit development in woodland strawberry. Hortic Res 2021, 8, 247. [Google Scholar] [CrossRef] [PubMed]
- Kofler, J.; Milyaev, A.; Capezzone, F.; Stojnić, S.; Mićić, N.; Flachowsky, H.; Hanke, M.-V.; Wünsche, J.-N. High crop load and low temperature delay the onset of bud initiation in apple. Scientific Reports 2019, 9, 17986. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, D.E.; Heck, G.R.; Perry, S.E.; Patterson, S.E.; Bleecker, A.B.; Fang, S.C. The embryo MADS domain factor AGL15 acts postembryonically. Inhibition of perianth senescence and abscission via constitutive expression. Plant Cell 2000, 12, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.-C.; Fernandez, D.E. Effect of regulated overexpression of the MADS domain factor AGL15 on flower senescence and fruit maturation. Plant Physiology 2002, 130, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Busi, M.V.; Bustamante, C.; D’Angelo, C.; Hidalgo-Cuevas, M.; Boggio, S.B.; Valle, E.M.; Zabaleta, E. MADS-box genes expressed during tomato seed and fruit development. Plant Molecular Biology 2003, 52, 801–815. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Hu, W.; Fang, Y.; Feng, X.; Fang, J.; Zou, T.; Zheng, S.; Ming, R.; Zhang, J. Comparative analysis of the MADS-box genes revealed their potential functions for flower and fruit development in Longan (Dimocarpus longan). Front Plant Sci 2021, 12, 813798. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lu, X.; Xu, J.; Liu, Y. Regulation of fruit ripening by MADS-box transcription factors. Scientia Horticulturae 2023, 314, 111950. [Google Scholar] [CrossRef]
- Huang, X.; Lu, Z.; Zhai, L.; Li, N.; Yan, H. The Small Auxin-Up RNA SAUR10 is involved in the promotion of seedling growth in rice. Plants (Basel) 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Yu, L.H.; Xiang, C.B. ARABIDOPSIS NITRATE REGULATED 1 acts as a negative modulator of seed germination by activating ABI3 expression. New Phytol 2020, 225, 835–847. [Google Scholar] [CrossRef]
- Yu, L.H.; Wu, J.; Zhang, Z.S.; Miao, Z.Q.; Zhao, P.X.; Wang, Z.; Xiang, C.B. Arabidopsis MADS-box transcription factor AGL21 acts as environmental surveillance of seed germination by regulating ABI5 expression. Mol Plant 2017, 10, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Soppe, W.J.J.; Vinegra de la Torre, N.; Albani, M.C. The diverse roles of FLOWERING LOCUS C in annual and perennial brassicaceae species. Front Plant Sci 2021, 12, 627258. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.X.; Zhang, J.; Chen, S.Y.; Wu, J.; Xia, J.Q.; Sun, L.Q.; Ma, S.S.; Xiang, C.B. Arabidopsis MADS-box factor AGL16 is a negative regulator of plant response to salt stress by downregulating salt-responsive genes. New Phytol 2021, 232, 2418–2439. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.M.; Kim, D.W.; Woo, M.S.; Jeong, H.S.; Son, Y.S.; Akhter, S.; Choi, G.J.; Bahk, J.D. Functional characterization of Arabidopsis HsfA6a as a heat-shock transcription factor under high salinity and dehydration conditions. Plant, Cell & Environment 2014, 37, 1202–1222. [Google Scholar] [CrossRef] [PubMed]
- Nesi, N.; Debeaujon, I.; Jond, C.; Stewart, A.J.; Jenkins, G.I.; Caboche, M.; Lepiniec, L. The TRANSPARENT TESTA16 locus encodes the ARABIDOPSIS BSISTER MADS domain protein and is required for proper development and pigmentation of the seed coat. Plant Cell 2002, 14, 2463–2479. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Wang, Y.; Zeng, L.; Jia, R.; He, L.; Huang, X.; Zhao, H.; Liu, D.; Zhao, H.; Hu, S.; Gao, L.; Guo, A.; Xia, W.; Ji, C. Genomic andtranscriptomic insights into the evolution and divergence of MIKC-type MADS-box genes in Carica papaya. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Ezquer, I.; Mizzotti, C.; Nguema-Ona, E.; Gotté, M.; Beauzamy, L.; Viana, V.E.; Dubrulle, N.; Costa de Oliveira, A.; Caporali, E.; Koroney, A.-S.; Boudaoud, A.; Driouich, A.; Colombo, L. The developmental regulator SEEDSTICK controls structural and mechanical properties of the Arabidopsis seed coat. The Plant Cell 2016, 28, 2478–2492. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, Z.; Zhao, X.; Liu, L.; Tang, Q.; Fu, J.; Tang, X.; Yang, R.; Lin, J.; Liu, X.; Yang, Y. Receptor-Like cytoplasmic kinase stk confers salt tolerance in rice. Rice 2023, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Bjerkan, K.N.; Alling, R.M.; Myking, I.V.; Brysting, A.K.; Grini, P.E. Genetic and environmental manipulation of Arabidopsis hybridization barriers uncovers antagonistic functions in endosperm cellularization. Front Plant Sci 2023, 14, 1229060. [Google Scholar] [CrossRef]
- Figueiredo, D.D.; Batista, R.A.; Roszak, P.J.; Hennig, L.; Kohler, C. Auxin production in the endosperm drives seed coat development in Arabidopsis. Elife 2016, 5. [Google Scholar] [CrossRef]
- Liu, X.; Sun, Z.; Dong, W.; Wang, Z.; Zhang, L. Expansion and functional divergence of the SHORT VEGETATIVE PHASE (SVP) genes in eudicots. Genome Biol Evol 2018, 10, 3026–3037. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Tamada, Y.; Yamane, H.; Matsushita, M.; Osako, Y.; Gao-Takai, M.; Luo, Z.; Tao, R. H3K4me3 plays a key role in establishing permissive chromatin states during bud dormancy and bud break in apple. Plant J 2022, 111, 1015–1031. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Routaboul, J.M.; Liu, M.; Deng, W.; Maza, E.; Mila, I.; Hu, G.; Zouine, M.; Frasse, P.; Vrebalov, J.T.; Giovannoni, J.J.; Li, Z.; van der Rest, B.; Bouzayen, M. Overexpression of the class D MADS-box gene Sl-AGL11 impacts fleshy tissue differentiation and structure in tomato fruits. J Exp Bot 2017, 68, 4869–4884. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Wang, L.; Li, J.; Ruan, Y.L. Cell wall invertase is essential for ovule development through sugar signaling rather than provision of carbon nutrients. Plant Physiol 2020, 183, 1126–1144. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.C.; Kempthorne, C.J.; Carella, P.; Liscombe, D.K.; Cameron, R.K. Age-related resistance in Arabidopsis thaliana involves the MADS-domain transcription factor SHORT VEGETATIVE PHASE and direct action of salicylic acid on Pseudomonas syringae. Molecular Plant-Microbe Interactions® 2017, 30, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.Y.; Yu, Q.; Zhang, Y.; Yao, C.C.; Han, Y.J. StMADS11 Subfamily Gene PfMADS16 From Polypogon fugax Regulates Early Flowering and Seed Development. Front Plant Sci 2020, 11, 525. [Google Scholar] [CrossRef] [PubMed]
- Song, G.Q.; Chen, Q. Overexpression of the MADS-box gene K-domain increases the yield potential of blueberry. Plant Sci 2018, 276, 22–31. [Google Scholar] [CrossRef]
- Yang, F.X.; Gao, J.; Wei, Y.L.; Ren, R.; Zhang, G.Q.; Lu, C.Q.; Jin, J.P.; Ai, Y.; Wang, Y.Q.; Chen, L.J.; Ahmad, S.; Zhang, D.Y.; Sun, W.H.; Tsai, W.C.; Liu, Z.J.; Zhu, G.F. The genome of Cymbidium sinense revealed the evolution of orchid traits. Plant Biotechnol J 2021, 19, 2501–2516. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Soto, D.; Ramos-Sanchez, J.M.; Alique, D.; Conde, D.; Triozzi, P.M.; Perales, M.; Allona, I. Overexpression of a SOC1-related gene promotes bud break in ecodormant poplars. Front Plant Sci 2021, 12, 670497. [Google Scholar] [CrossRef]
- Jing, Y.; Shi, L.; Li, X.; Zheng, H.; Gao, J.; Wang, M.; He, L.; Zhang, W. OXS2 is required for salt tolerance mainly through associating with salt inducible genes, CA1 and ARAPORT11, in Arabidopsis. Scientific Reports 2019, 9, 20341. [Google Scholar] [CrossRef]
- Blanvillain, R.; Wei, S.; Wei, P.; Kim, J.H.; Ow, D.W. Stress tolerance to stress escape in plants: role of the OXS2 zinc-finger transcription factor family. The EMBO Journal 2011, 30, 3812–3822. [Google Scholar] [CrossRef]
- Castañón-Suárez, C.A.; Arrizubieta, M.; Castelán-Muñoz, N.; Sánchez-Rodríguez, D.B.; Caballero-Cordero, C.; Zluhan-Martínez, E.; Patiño-Olvera, S.C.; Arciniega-González, J.A.; García-Ponce, B.; Sánchez, M.P.; Álvarez-Buylla, E.R.; Garay-Arroyo, A. The MADS-box genes SOC1 and AGL24 antagonize XAL2 functions in Arabidopsis thaliana root development. Front Plant Sci 2024, 15, 1331269. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Shimada, T.; Fujii, H.; Kobayashi, Y.; Araki, T.; Omura, M. Ectopic expression of an FT homolog from citrus confers an early flowering phenotype on trifoliate orange (Poncirus trifoliata L. Raf.). Transgenic Research 2005, 14, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Schlathölter, I.; Jänsch, M.; Flachowsky, H.; Broggini, G.A.L.; Hanke, M.-V.; Patocchi, A. Generation of advanced fire blight-resistant apple (Malus × domestica) selections of the fifth generation within 7 years of applying the early flowering approach. Planta 2018, 247, 1475–1488. [Google Scholar] [CrossRef] [PubMed]
- Flachowsky, H.; Szankowski, I.; Waidmann, S.; Peil, A.; Tränkner, C.; Hanke, M.-V. The MdTFL1 gene of apple (Malus × domestica Borkh.) reduces vegetative growth and generation time. Tree Physiology 2012, 32, 1288–1301. [Google Scholar] [CrossRef] [PubMed]
- Flachowsky, H.; Hättasch, C.; Höfer, M.; Peil, A.; Hanke, M.-V. Overexpression of LEAFY in apple leads to a columnar phenotype with shorter internodes. Planta 2010, 231, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Guardiola, J.L. Overview of flower bud induction, flowering and fruit set. In Proceedings of the Proceedings of citrus flowering and fruit short course.; pp. 19975–21.
- Jones, H.G. Repeat flowering in apple caused by water stress or defoliation. Trees 1987, 1, 135–138. [Google Scholar] [CrossRef]
- Jaudal, M.; Zhang, L.; Che, C.; Li, G.; Tang, Y.; Wen, J.; Mysore, K.S.; Putterill, J. A SOC1-like gene MtSOC1a promotes flowering and primary stem elongation in Medicago. Journal of Experimental Botany 2018, 69, 4867–4880. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, D.; Song, G.Q. Expression of a maize SOC1 gene enhances soybean yield potential through modulating plant growth and flowering. Sci Rep 2021, 11, 12758. [Google Scholar] [CrossRef]
- Rodriguez-Bolanos, M.; Martinez, T.; Juarez, S.; Quiroz, S.; Dominguez, A.; Garay-Arroyo, A.; Sanchez, M.P.; Alvarez-Buylla, E.R.; Garcia-Ponce, B. XAANTAL1 reveals an additional level of flowering regulation in the shoot apical meristem in response to light and increased temperature in Arabidopsis. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Dang, X.; Zhang, Y.; Li, Y.; Chen, S.; Liu, E.; Fang, B.; Liu, Q.; She, D.; Dong, Z.; Fan, Z.; Li, D.; Wang, H.; Zhu, S.; Hu, X.; Li, Y.; Jiang, J.; Hong, D. SYL3-k increases style length and yield of F(1) seeds via enhancement of endogenous GA(4) content in Oryza sativa L. pistils. Theor Appl Genet 2022, 135, 321–336. [Google Scholar] [CrossRef] [PubMed]
- OpenAI. ChatGPT (GPT 4) [Large language model]. 2023.
Figure 1.
General overview of MADS-box studies. (a) Study-to-organism association ranging from 1 (several species) to 363 (Arabidopsis). (b) MADS box gene-IDs-to-study association ranging from 1 (several genes) to 76 (SVP), (c) putative cross-species MADS box gene-IDs-to-study association ranging from 1 (several genes) to 12 (STMADS11). The gene-word sizes are relative to their frequencies in each gene pool.
Figure 1.
General overview of MADS-box studies. (a) Study-to-organism association ranging from 1 (several species) to 363 (Arabidopsis). (b) MADS box gene-IDs-to-study association ranging from 1 (several genes) to 76 (SVP), (c) putative cross-species MADS box gene-IDs-to-study association ranging from 1 (several genes) to 12 (STMADS11). The gene-word sizes are relative to their frequencies in each gene pool.
Figure 2.
Genes with multi-organ associations. (a) Arabidopsis gene IDs associated with at least two of the seven plant organs (root, shoot, leaf, apical meristem/SAM, flower, fruit, and seed). The shown genes had at least three hits for each of their respective organ. The IDs with two hits among organs are in gray and those with only one hit are in light gray. (b) Cross-species gene IDs associated with at least two of the seven plant organs. The shown genes had at least two hits for each of their respective organ. The IDs with only one hit are in gray.
Figure 2.
Genes with multi-organ associations. (a) Arabidopsis gene IDs associated with at least two of the seven plant organs (root, shoot, leaf, apical meristem/SAM, flower, fruit, and seed). The shown genes had at least three hits for each of their respective organ. The IDs with two hits among organs are in gray and those with only one hit are in light gray. (b) Cross-species gene IDs associated with at least two of the seven plant organs. The shown genes had at least two hits for each of their respective organ. The IDs with only one hit are in gray.
Figure 3.
MADS box members associated with shoots (a), leaves (b), and roots (c). The IDs with 1-2 hits are in light gray and those three hits are in dark gray. The IDs with >3 hits are in any other random color. Their text size are relative to their respective hit frequencies.
Figure 3.
MADS box members associated with shoots (a), leaves (b), and roots (c). The IDs with 1-2 hits are in light gray and those three hits are in dark gray. The IDs with >3 hits are in any other random color. Their text size are relative to their respective hit frequencies.
Figure 4.
MADS box members associated with SAM (a), flower (b), ovule (c), and pollen (d). The IDs with 1-2 hits are in light gray and those three hits are in dark gray. The IDs with >3 hits are in any other random color. Their text sizes are relative to their respective hit frequencies.
Figure 4.
MADS box members associated with SAM (a), flower (b), ovule (c), and pollen (d). The IDs with 1-2 hits are in light gray and those three hits are in dark gray. The IDs with >3 hits are in any other random color. Their text sizes are relative to their respective hit frequencies.
Figure 5.
MADS box members associated with seeds (a), fruits (b), and seed germination (c). The IDs with 1-2 hits are in light gray and those three hits are in dark gray. The IDs with >3 hits are in any other random color. Their text sizes are relative to their respective hit frequencies.
Figure 5.
MADS box members associated with seeds (a), fruits (b), and seed germination (c). The IDs with 1-2 hits are in light gray and those three hits are in dark gray. The IDs with >3 hits are in any other random color. Their text sizes are relative to their respective hit frequencies.
Figure 6.
MADS box members associated with hormones. (a) auxin, (b) cytokinin, (c) ethylene, (d) gibberellin, (e) abscisic acid. The IDs with 1-2 hits are in light gray and those three hits are in dark gray. The IDs with >3 hits are in any other random color. Their text sizes are relative to their respective hit frequencies.
Figure 6.
MADS box members associated with hormones. (a) auxin, (b) cytokinin, (c) ethylene, (d) gibberellin, (e) abscisic acid. The IDs with 1-2 hits are in light gray and those three hits are in dark gray. The IDs with >3 hits are in any other random color. Their text sizes are relative to their respective hit frequencies.
Figure 7.
MADS box members associated with biotic and abiotic factors. (a) nutrient response, (b) tolerance, resistance, or susceptibility response, (c) light response, (d) salt response, (e) osmotic response.
Figure 7.
MADS box members associated with biotic and abiotic factors. (a) nutrient response, (b) tolerance, resistance, or susceptibility response, (c) light response, (d) salt response, (e) osmotic response.
Figure 8.
Trait-to-factors bridging MADS box members with ‘flowering’ trait. Gene pools at the circles represent respective factors-to-flowering associated MADS box members. The central gene pool was generated from all other gene pools to assess most frequent MADS box members among them. The IDs with 1-2 hits are in light gray and those three hits are in dark gray. The IDs with >3 hits are in any other random color. Their text sizes are relative to their respective hit frequencies within each gene pool.
Figure 8.
Trait-to-factors bridging MADS box members with ‘flowering’ trait. Gene pools at the circles represent respective factors-to-flowering associated MADS box members. The central gene pool was generated from all other gene pools to assess most frequent MADS box members among them. The IDs with 1-2 hits are in light gray and those three hits are in dark gray. The IDs with >3 hits are in any other random color. Their text sizes are relative to their respective hit frequencies within each gene pool.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).