Submitted:
24 May 2024
Posted:
27 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Asymmetric Synthesis Exploiting Substrate Directed Stereoselectivity
2.1. Polyfunctionalized Pyrrolidines
2.2. Pyrrolidines within Polycyclic Structures
2.3. Piperidine, Morpholine and Piperazine Derivatives
2.4. Substituted Guanidines
2.5. Penems and Lactams
2.6. 1,3-Oxazolidin-2-Ones and 4,5-Dihydrooxazoles
2.7. 4,5-Dihydroimidazoles
3. Asymmetric Synthesis Exploiting Stereoselectivity Directed by an Added Chiral Catalyst
3.1. N-Sulfonyl and Carbamoyl Pyrrolidines, Indolines and Hexahydropyrrolo [2,3,-b]indoles (HPI)
3.2. N-Sulfonyl Piperidines
3.3. [1,2,3] Oxathiazine 2,2-dioxides
3.4. N-Sulfonyl 4,5-dihydro-1H-pyrazoles
3.5. N-Tosyl Lactams
3.6. N-Tosyl 1,3-oxazolidin-2-ones and 1,3-oxazin-2-ones
3.7. 1,3-Imidazolidin-2-Ones and Tetrahydropyrimidin-2(1H)-Ones
4. Conclusions
Funding
Conflicts of Interest
References
- Saikia, I.; Borah, A.J.; Phukan, P. Use of bromine and bromo-organic compounds in organic synthesis. Chem. Rev., 2016, 116, 6837–7042. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, V.S.C.; de Mattos, M.C.S. N-Halo reagents: modern synthetic approaches for heterocyclic synthesis. Synthesis, 2019, 51, 1841–1870. [Google Scholar] [CrossRef]
- Lyubchuk, T.V.; Hordiyenko, O.V. The use of N-halosuccinimides for cyclization with the formation of five-membered heterocyclic compounds. Chem. Heterocycl. Comp., 2020, 56, 1–29. [Google Scholar] [CrossRef]
- Powell, W.H. Revision of the extended Hantzsch-Widman system of nomenclature for heteromonocycles. Pure Appl. Chem., 1983, 55, 409–416. [Google Scholar] [CrossRef]
- Moss, G.P.; Smith, P.A.S.; Tavernier, D. Glossary of class names of organic compounds and reactive intermediates based on structure. Pure Appl. Chem., 1995, 67, 1307–1375. [Google Scholar] [CrossRef]
- Baldwin, J.E. Rules for ring closure. J. Chem. Soc., Chem. Commun. 1976, 734. [CrossRef]
- Baldwin, J.E. 5-Endo-trigonal reactions: a disfavoured ring closure. J. Chem. Soc., Chem. Commun., 1976, 736-738. [CrossRef]
- Piccirilli, J.A. Do enzymes obey the Baldwin rules? A mechanistic imperative in enzymatic cyclization reactions. Chem. Biol., 1999, 6, R59–R64. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, K.; Alabugin, I.V. Cyclizations of alkynes: revisiting Baldwin’s rules for ring closure. Chem. Rev., 2011, 111, 6513–6556. [Google Scholar] [CrossRef]
- Alabugin, I.V.; Gilmore, K. Finding the right path: Baldwin “Rules for Ring Closure” and stereoelectronic control of cyclizations. J. Chem. Soc., Chem. Commun., 2013, 49, 11246–11250. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, K.; Mohamed, R.K.; Alabugin, I.V. The Baldwin rules: revised and extended. WIREs Comput. Mol. Sci., 2016, 6, 487–514. [Google Scholar] [CrossRef]
- Cardillo, G.; Orena, M. Stereocontrolled cyclofunctionalizations of double bonds through heterocyclic intermediates. Tetrahedron, 1990, 46, 3321–3408. [Google Scholar] [CrossRef]
- Orena, M. Amination reactions promoted by electrophiles. In Houben-Weyl Methods in Organic Chemistry: Stereoselective Synthesis, Vol. e-21e, , Helmchen, G., 4th Ed. ed; Hoffmann, R.W.: Mulzer, J.; Schaumann, E., Eds.; Thieme, Stuttgart, 1996; pp. 5291–5355. [Google Scholar]
- Frederickson, M.; Grigg, R. Electrophile mediated heteroatom cyclizations onto C=C π-bonds. Part 1: halogen and chalcogen mediated cyclization Org. Prep. Proc. Int., 1997, 29, 33–62. [Google Scholar] [CrossRef]
- Ranganathan, S.; Muraleedharan, K.M.; Vaisha, N.K.; Jayaraman, N. Halo- and selenolactonisation: the two major strategies for cyclofunctionalisation. Tetrahedron, 2004, 60, 5273–5308. [Google Scholar] [CrossRef]
- Mphahlele, M.J. Molecular iodine-mediated cyclization of tethered heteroatom-containing alkenyl or alkynyl systems. Molecules, 2009, 14, 4814–4837. [Google Scholar] [CrossRef] [PubMed]
- Ashtekar, K.D.; Jaganathan, A.; Borhan, B.; Whitehead, D.C. Enantioselective halofunctionalization of alkenes. In: Organic Reactions, 2021, 105, Evans, P.A., Ed., Wiley, pp. 1–266. [Google Scholar]
- Ladenburg, A. Versuche zur Synthese von Tropin und dessen Derivate. Chem. Ber., 1881, 14, 1342–1349. [Google Scholar]
- https://gallica.bnf.fr/ark:/12148/bpt6k90692z?rk=21459;2.
- Merling, G. Ueber Bromsubstitutionsprodukte des Dimethylpiperidins und einige sich von diesen ableitende Verbingdungen. Chem. Ber., 1884, 17(pt6), 2139–2143. [Google Scholar] [CrossRef]
- Merling, G. Ueber die bei Einwirkung von Brom auf Dimethylpiperidin enstehenden Verbindungen. - Neue Synthese von Piperidinderivaten. Chem. Ber., 1886, 19(pt6), 2628–2632. [Google Scholar] [CrossRef]
- Chemler, S.R.; Bovino, M.T. Catalytic aminohalogenation of alkenes and alkynes. ACS Catal., 2013, 3, 1076–1091. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, C.B.; Mukherjee, S. Catalytic enantioselective halocyclizations beyond lactones: emerging routes to enantioenriched nitrogenous heterocycles. Synlett, 2014, 25, 163–169. [Google Scholar] [CrossRef]
- Mizar, P.; Wirth, T. Iodoaminations of alkenes. Synthesis, 2017, 49, 981–986. [Google Scholar] [CrossRef]
- China, H.; Kumar, R.; Kikushima, K.; Dohi, T. Molecules, 2020, 25, 6007–6040. 25. [CrossRef]
- Liu, S.; Zhang, B.-Q.; Xiao, W.-Y.; Li, Y.-L.; Deng, J. Recent advances in catalytic asymmetric syntheses of functionalized heterocycles via halogenation/chalcogenation of carbon-carbon unsaturated bonds. Adv. Synth. Catal., 2022, 364, 3974–4005. [Google Scholar] [CrossRef]
- Yan, J.; Zhou, Z.; He, Q.; Chen, G.; Wei, H.; Xie, W. The applications of catalytic asymmetric halocyclization in natural product synthesis. Org. Chem. Front., 2022, 9, 499–516. [Google Scholar] [CrossRef]
- Yao, C.-Z.; Tu, X.-Q.; Jiang, H.-J.; Li, Q.; Yu, J. Recent advances in catalytic asymmetric haloamination and haloetherification of alkenes. Tetrahedron Lett., 2023, 126, 154639. [Google Scholar] [CrossRef]
- La Ferla, B.; Nicotra, F. Synthetic methods for the preparation of iminosugars. In: Iminosugars as Glycosidase Inibitors - Nojirimycin and Beyond. Stiitz, A.E. Ed.; Wiley-VCH Verlag GmbH, Weinheim, Germany, 1999, pp. 68–92.
- Matassini, C.; Cardona, F. Recent synthetic strategies to access diverse iminosugars. In: Synthetic Strategies in Carbohydrate Chemistry, Tiwari, V.K., Ed.; Elsevier, Amsterdam, 2024, pp. 335–364. [CrossRef]
- Compain, P.; Martin, O.R. Design, synthesis and biological evaluation of iminosugar-based glycosyltransferase inhibitors. Curr. Top. Med. Chem., 2003, 3, 541–560. [Google Scholar] [CrossRef]
- Conforti, I.; Marra, A. Iminosugars as glycosyltransferase inhibitors. Org. Biomol. Chem., 2021, 19, 5439–5475. [Google Scholar] [CrossRef] [PubMed]
- . [CrossRef]
- Chamberlin, A.R.; Dezube, M.; Dussault, P.; McMills, M.C. Iodocyclization of allylic alcohol derivatives containing internal nucleophiles. Control of stereoselectivity by substituents in the acyclic precursors. J. Am. Chem. Soc., 1983, 105, 5819–5825. [Google Scholar] [CrossRef]
- . [CrossRef]
- Kahn, S.D.; Pau, C.F.; Chamberlin, A.R.; Hehre, W.J. Modeling chemical reactivity. 4. Regiochemistry and stereochemistry of electrophilic additions to allylic double bonds. J. Am. Chem. Soc., 1987, 109, 650–663. [Google Scholar] [CrossRef]
- Chamberlin, A.R.; Mulholland, R.L.; Kahn, S.D.; Hehre, W.J. Modeling chemical reactivity. 7. The effect of a change in rate-limiting step on the stereoselectivity of electrophilic addition to allylic alcohols and related chiral alkenes. J. Am. Chem. Soc., 1987, 109, 672–677. [Google Scholar] [CrossRef]
- Tamaru, Y.; Kawamura, S.-I.; Bando, T.; Tanaka, K.; Hojo, M.; Yoshida, Z.-I. Stereoselective intramolecular haloamidation of N-protected 3-hydroxy-4-pentenylamines and 4-hydroxy-5-hexenylamines. J. Org. Chem., 1988, 53, 5491–5501. [Google Scholar] [CrossRef]
- . [CrossRef]
- Labelle, M.; Guindon, Y. Diastereoselective synthesis of 2,3-disubstituted tetrahydrofuran synthons via the iodoetherification reaction. A transition state model based rationalization of the allylic asymmetric induction. J. Am. Chem. Soc., 1989, 111, 2204–2210. [Google Scholar] [CrossRef]
- Tredwell, M.; Luft, J.A.; Schuler, M.; Tenza, K.; Houk, K.N.; Gouverneur, V. Fluorine-directed diastereoselective iodocyclizations. Angew. Chem. Int. Ed., 2008, 47, 357–360. [Google Scholar] [CrossRef]
- Zhi-cai, S.; Chun-min, Z.; Guo-qiang. L. A synthesis of the polyhydroxylated pyrrolidines: synthesis of 1,4-dideoxy-1,4-imino-D-lyxitol and N-benzyl-4-epi-(-)-anisomycin. Heterocycles, 1995, 41, 277–287. [Google Scholar] [CrossRef]
- Verhelst, S.H.L.; Paez Martinez, B.; Timmer, M.S.M.; Lodder, G.; van der Marel, G.A.; Overkleeft, H.S.; van Boom, J.H. A short route toward chiral, polyhydroxylated indolizidines and quinolizidines. J. Org. Chem., 2003, 68, 9598–9603. [Google Scholar] [CrossRef]
- . [CrossRef]
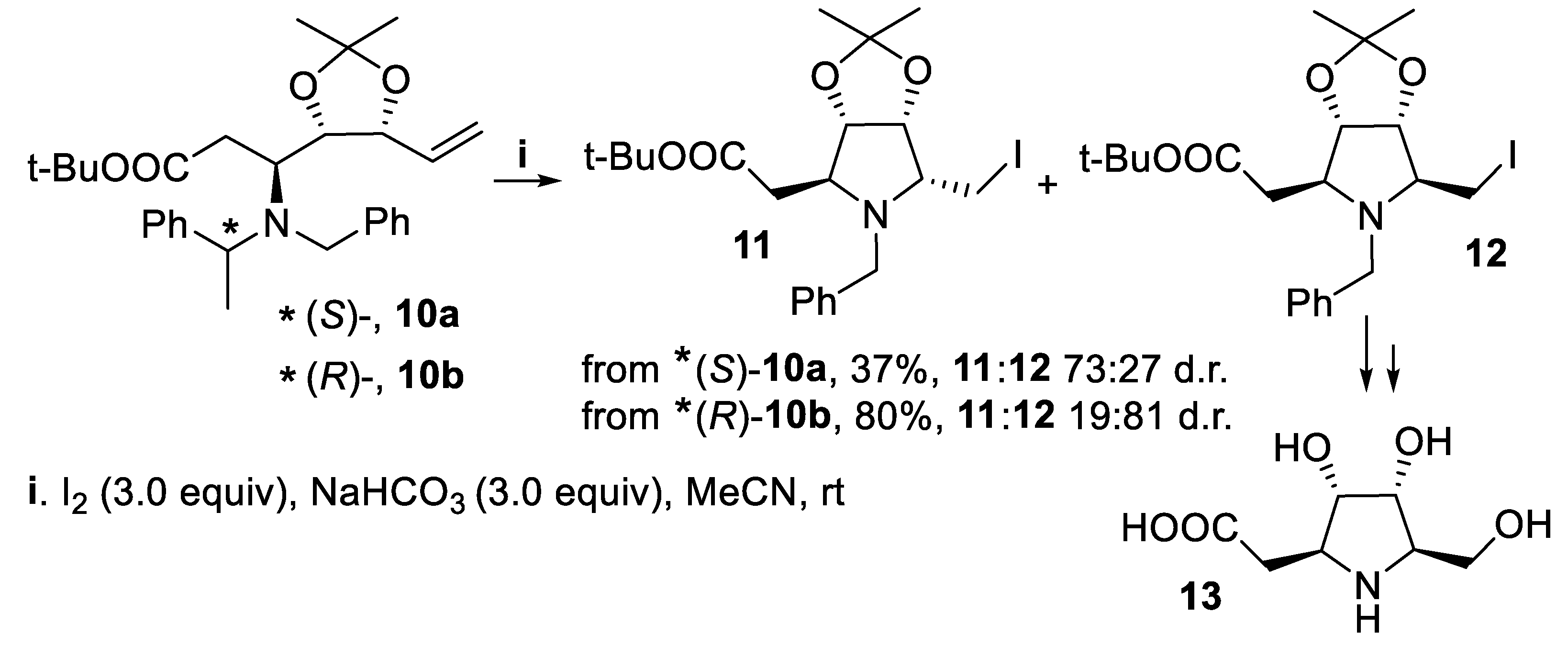
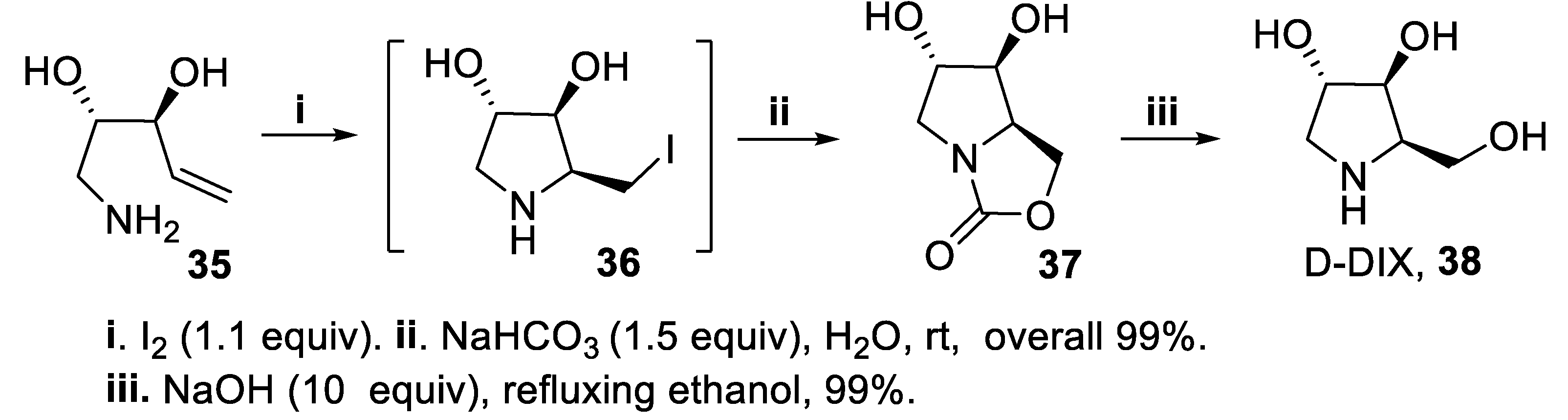
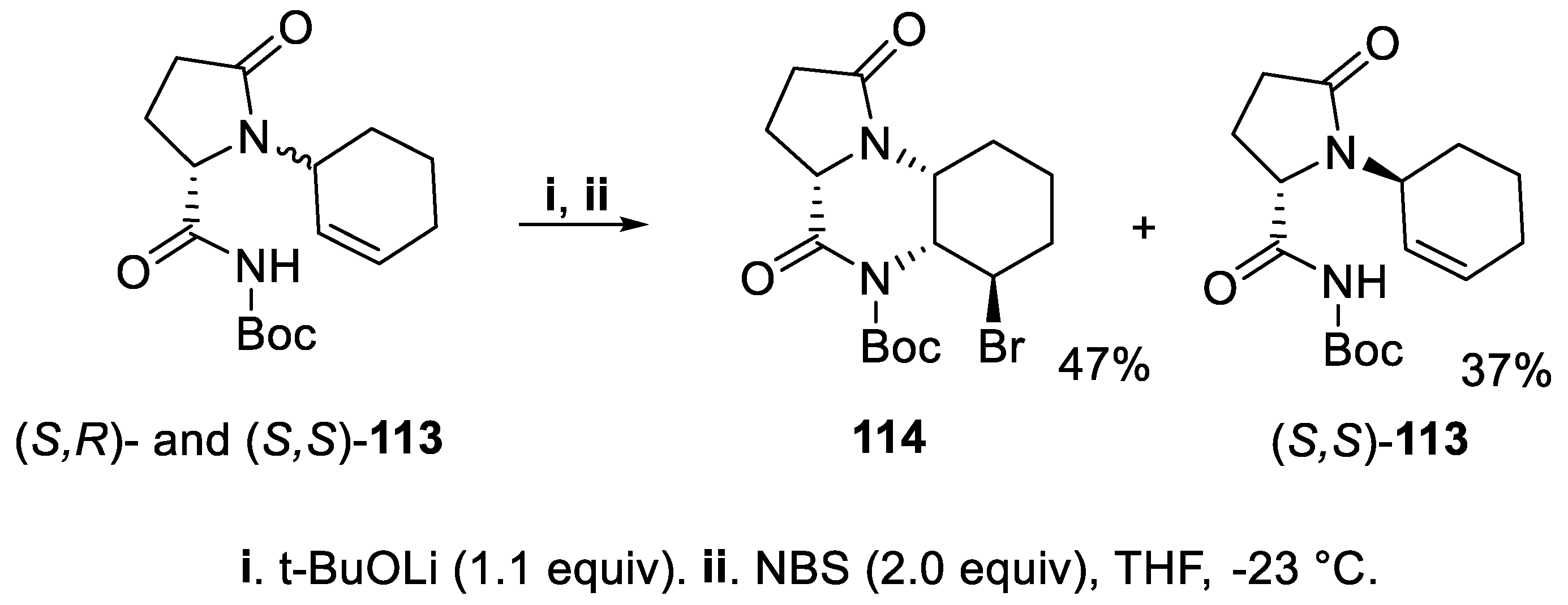
- Davies, S.G.; Nicholson, R.L.; Price, P.D.; Roberts, P.M.; Smith, A.D. Iodine-mediated ring closing alkene iodoamination with N-debenzylation for the asymmetric synthesis of polyhydroxylated pyrrolidines. Synlett, 1055. [Google Scholar]
- Davies, S.G.; Nicholson, R.L.; Price, P.D.; Roberts, P.M.; Russell, A.J.; Savory, E.D.; Smith, A.D.; Thomson, J.E. Iodine-mediated ring-closing iodoamination with concomitant N-debenzylation for the asymmetric synthesis of polyhydroxylated pyrrolidines. Tetrahedron: Asymmetry, 2009, 20, 758–772. [Google Scholar] [CrossRef]
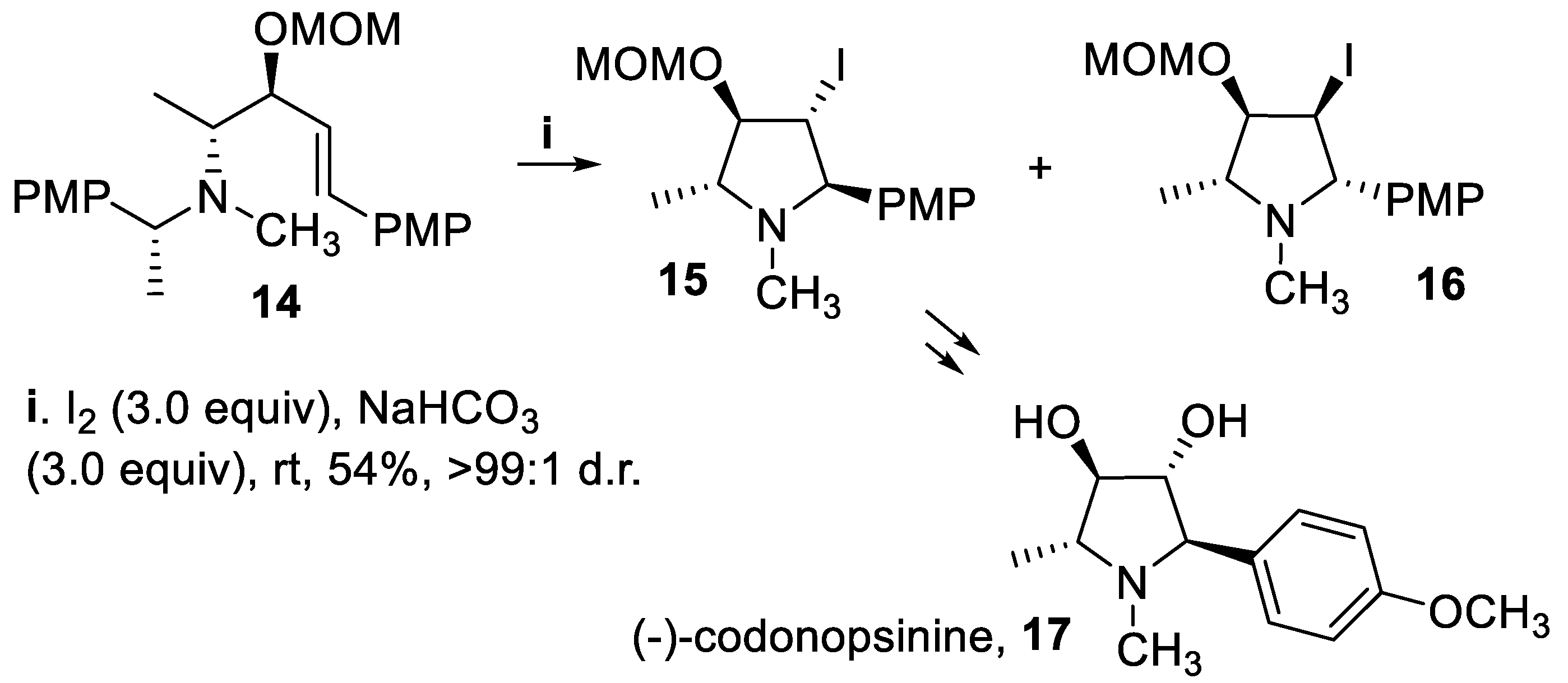
- Davies, S.G.; Lee, J.A.; Roberts, P.M.; Thomson, J.E.; West, C.J. Asymmetric synthesis of (-)-codonopsinine. Tetrahedron Lett., 2011, 52, 6477–6480. [Google Scholar] [CrossRef]
- Davies, S.G.; Lee, J.A.; Roberts, P.M.; Thomson, J.E.; West, C.J. Ring-closing iodoamination of homoallylic amines for the synthesis of polysubstituted pyrrolidines: application to the asymmetric synthesis of (-)-codonopsinine. Tetrahedron, 2012, 68, 4302–4319. [Google Scholar] [CrossRef]
- Kondo, Y.; Suzuki, N.; Takahashi, M.; Kumamoto, T.; Masu, H.; Ishikawa, T. Enantioselective construction of a polyhydroxylated pyrrolidine skeleton from 3-vinylaziridine-2-carboxylates: synthesis of (+)-DMDP and a potential common intermediate for (+)-hyacinthacine A1 and (+)-1-epi-australine. J. Org. Chem., 2012, 77, 7988–7999. [Google Scholar] [CrossRef] [PubMed]
- Elbein, A.D.; Mitchell, M.; Sanford, B.A.; Fellows, L.E.; Evans, S.V. The pyrrolidine alkaloid, 2,5-dihydroxymethyl-3,4-dihydroxypyrrolidine, inhibits glycoprotein processing. J. Biol. Chem., 1984, 259, 12409–12413. [Google Scholar] [CrossRef]
- Welter, A.; Jadot, J.; Dardenne, G.; Marlier, M.; Casimir, J. 2,5-Dihydroxymethyl 3,4-dihydroxypyrrolidine dans les feuilles de Derris elliptica. Phytochemistry, 1976, 15, 747–749. [Google Scholar] [CrossRef]
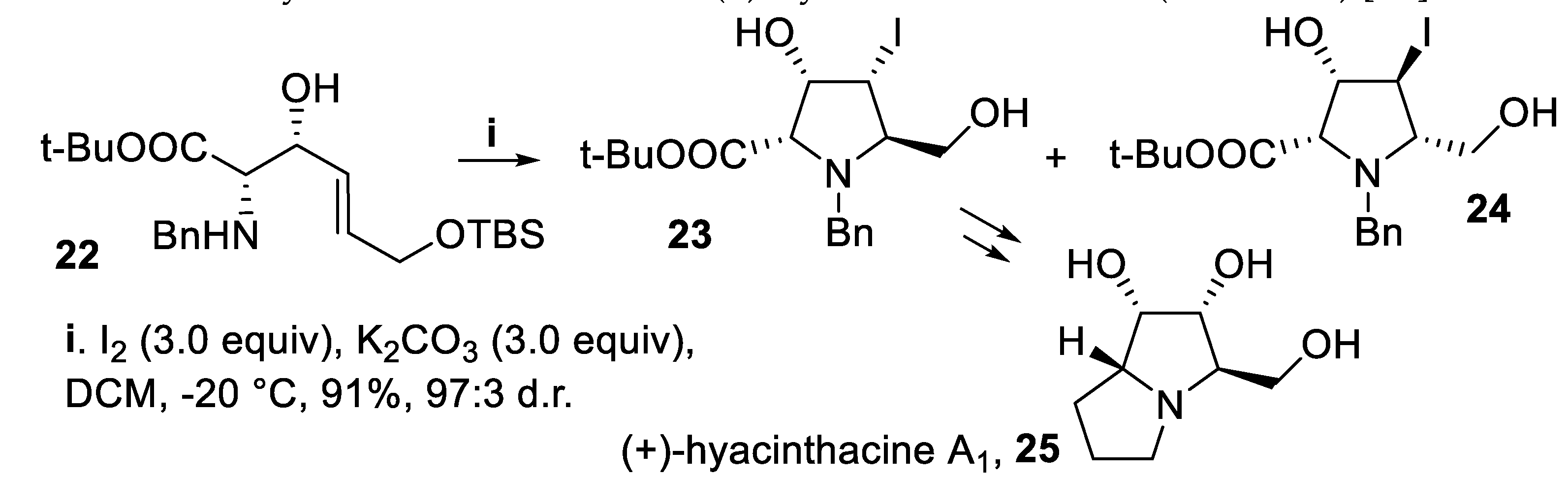
- Donohoe, T.J.; Sintim, H.O.; Hollinshead, J. A noncarbohydrate based approach to polyhydroxylated pyrrolidizines: total syntheses of the natural products hyacinthacine A1 and 1-epiaustraline. J. Org. Chem., 2005, 70, 7297–7304. [Google Scholar] [CrossRef]
- . [CrossRef]
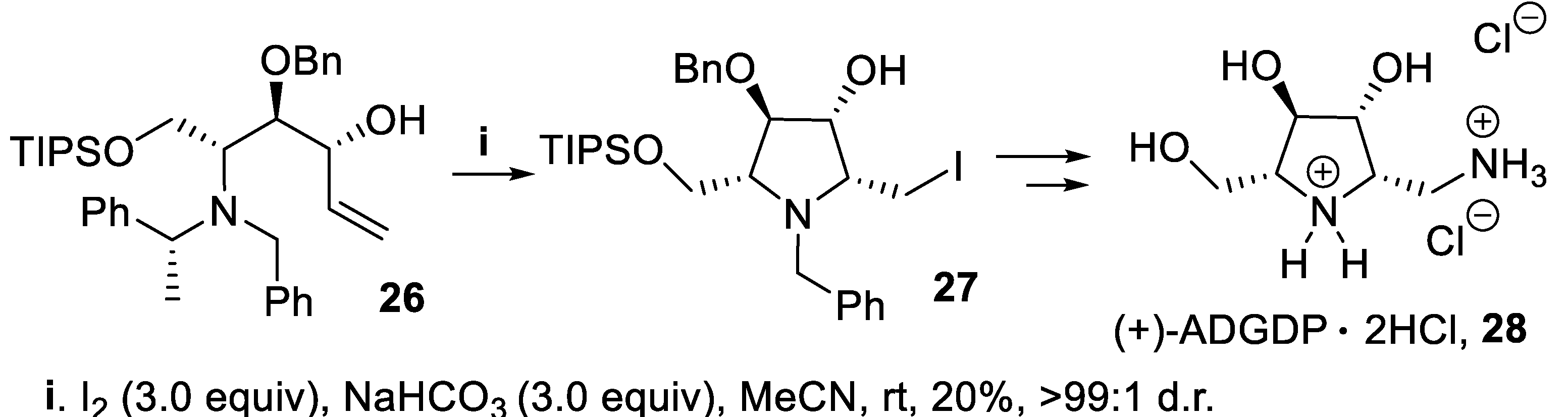
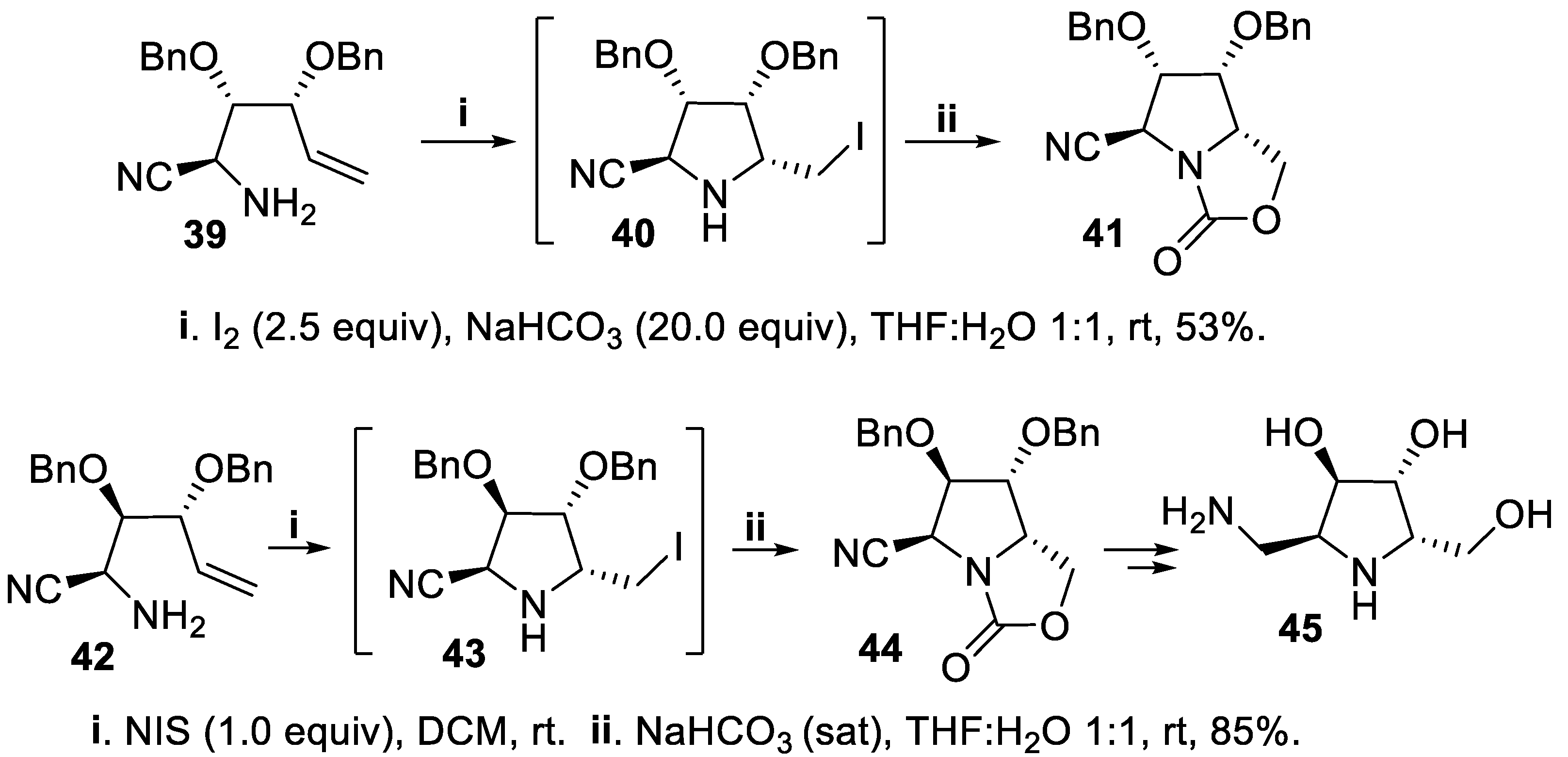
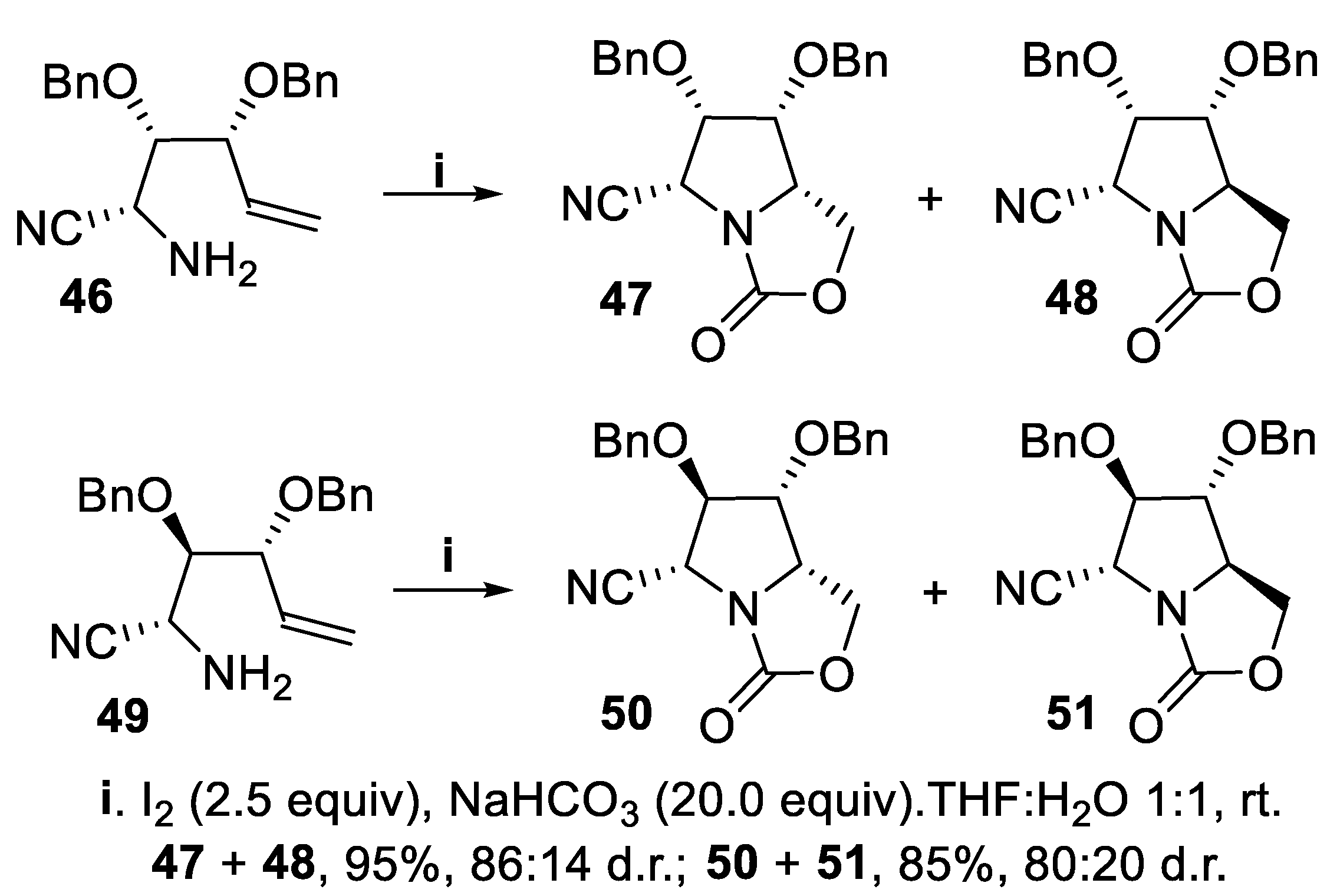
- Davies, S.G.; Figuccia, A.L.A.; Fletcher, A.M.; Roberts, P.M.; Thomson, J.E. Asymmetric syntheses of 2,5-dideoxy-2,5-imino-d-glucitol [(+)-DGDP] and 1,2,5-trideoxy-1-amino-2,5-imino-d-glucitol [(+)-ADGDP]. Tetrahedron, 2014, 70, 3601–3607. [Google Scholar] [CrossRef]
- . [CrossRef]
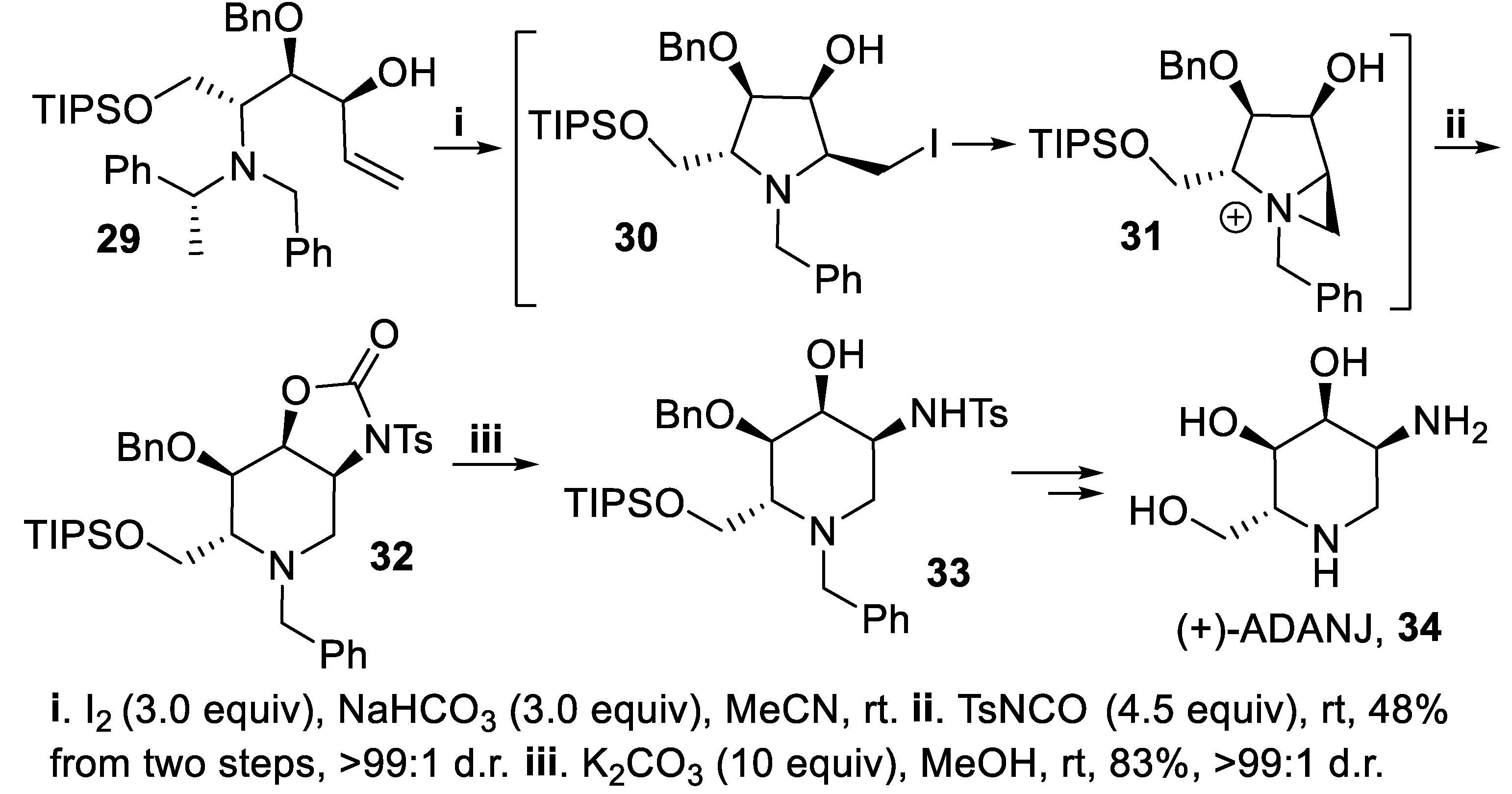
- Davies, S.G.; Figuccia, A.L.A.; Fletcher, A.M.; Roberts, P.M.; Thomson, J.E. Asymmetric syntheses of (−)-ADMJ and (+)-ADANJ: 2-deoxy-2-amino analogues of (−)-1-deoxymannojirimycin and (+)-1-deoxyallonojirimycin. J. Org. Chem., 2016, 81, 6481–6495. [Google Scholar] [CrossRef] [PubMed]
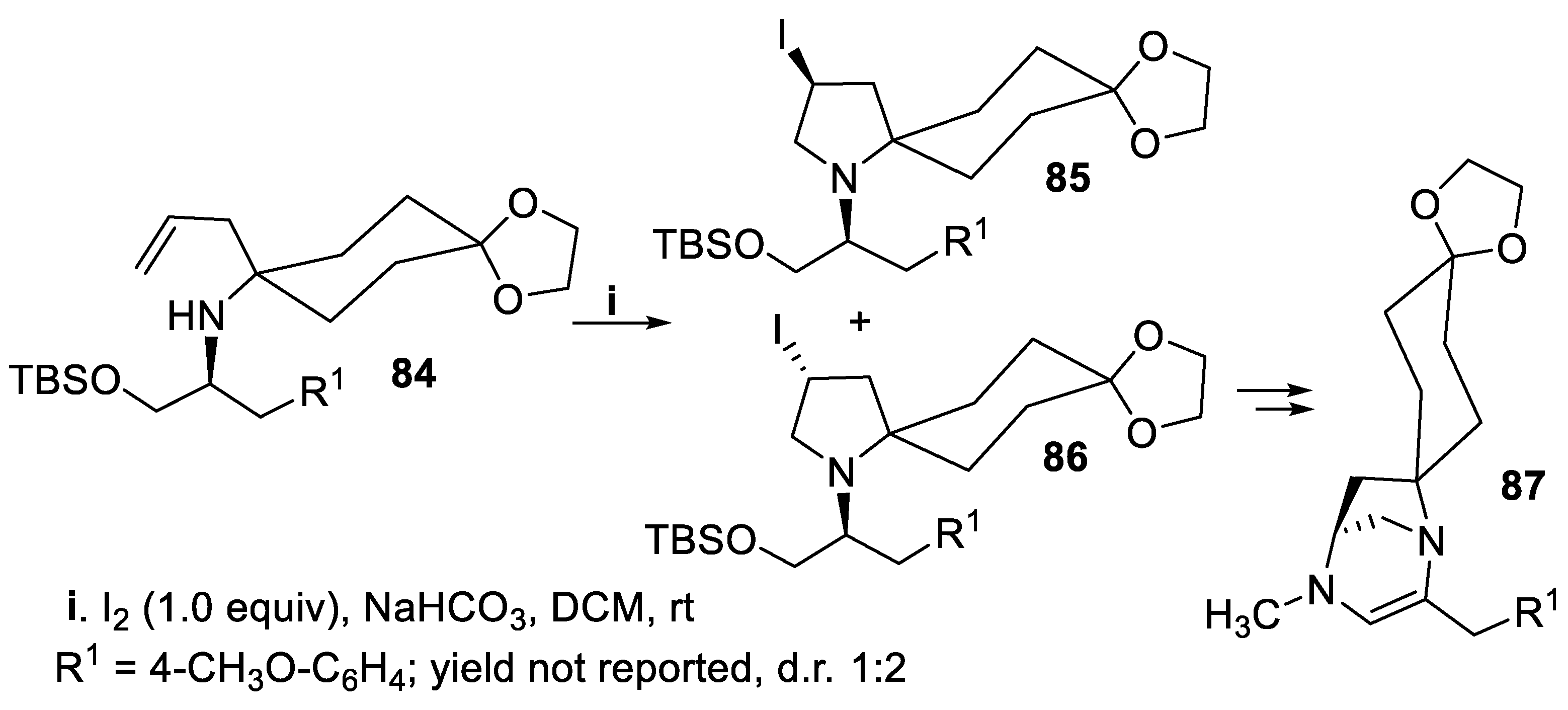
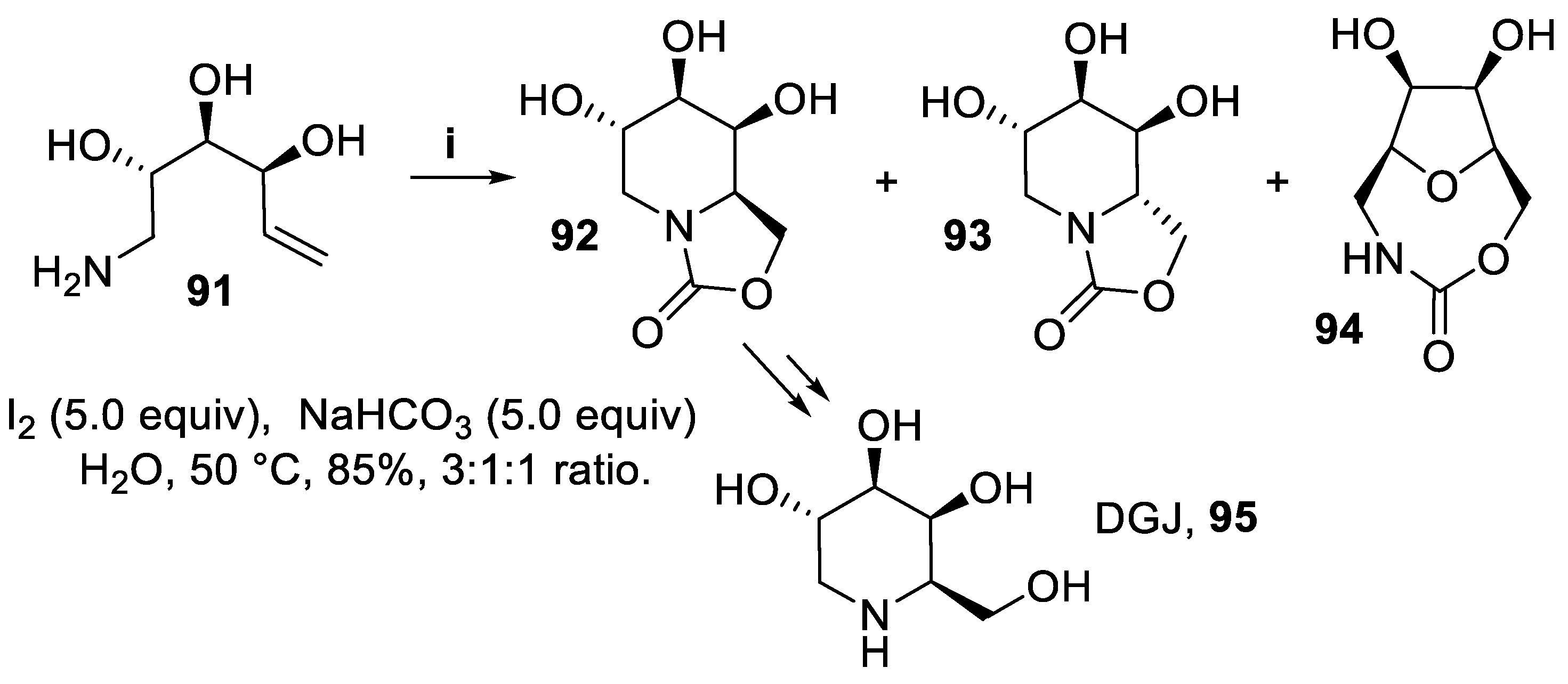
- Dangerfield, E.M.; Timmer, M.S.M.; Stocker, B.L. Total synthesis without protecting groups: pyrrolidines and cyclic carbamates. Org. Lett., 2009, 11, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Stocker, B.L.; Win-Mason, A.L.; Timmer, M.S.M. I2-mediated carbamate annulation: scope and application in the synthesis of azasugars. Carb. Res., 2012, 356, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Saludes, J.P.; Lievens, S.C.; Molinski, T.F. Occurrence of the α-glucosidase inhibitor 1,4-dideoxy-1,4-imino-d-arabinitol and related iminopentitols in marine sponges. J. Nat. Prod., 2007, 70, 436–438. [Google Scholar] [CrossRef] [PubMed]
- Win-Mason, A.L.; Jongkees, S.A.K.; Withers, S.G.; Tyler, P.C.; Timmer, M.S.M.; Stocker, B.L. Stereoselective total synthesis of aminoiminohexitols via carbamate annulation. J. Org. Chem., 2011, 76, 9611–9621. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Yudin, A.K. Oxidative cycloamination of olefins with aziridines as a versatile route to saturated nitrogen-containing heterocycles. J. Am. Chem. Soc., 2003, 125, 14242–14243. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.S.; Li, J.; Navarro, A. Total synthesis of azinomycin A. Angew. Chem. Int. Ed., 2001, 40, 1736–1739. [Google Scholar] [CrossRef]
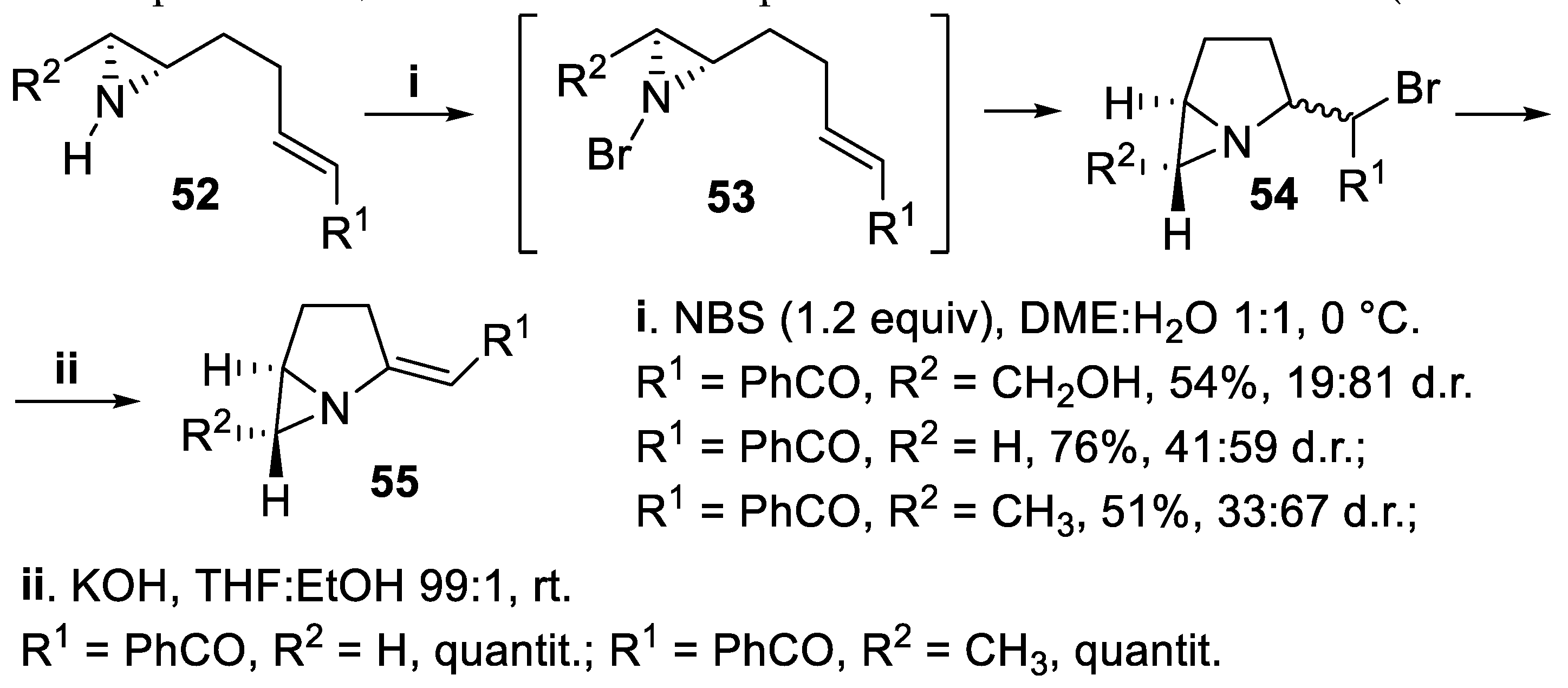
- Zhou, J.; Yeung, Y.-Y. Diastereoselective synthesis of functionalized pyrrolidines through N-bromosuccinimide induced aziridine ring expansion cascade of cinnamylaziridine. Org. Biomol. Chem., 2014, 12, 7482–7485. [Google Scholar] [CrossRef]
- Zhou, J.; Yeung, Y.-Y. N-bromosuccinimide-induced aminocyclization-aziridine ring-expansion cascade: an asymmetric and highly stereoselective approach toward the synthesis of azepane. Org. Lett., 2014, 16, 2134–2137. [Google Scholar] [CrossRef]
- . [CrossRef]
- Asano, N. Glycosidase-inhibiting alkaloids: isolation, structure, and application. In: Modern alkaloids: structure, isolation, synthesis and biology, Fattorusso, E.; Taglialatela-Scafati, O., Eds.; Wiley-VCH, Weinheim, 2008, pp. 111–138.
- Salunke, R.V.; Ramesh, N.G. Divergent synthesis of amino-substituted indolizidine alkaloids, decahydropyrazino [2,1,6-cd]pyrrolizine triols, and (-)-pochonicine stereoisomers. Eur. J. Org. Chem, 2020; 2626–2640. [Google Scholar] [CrossRef]
- Itoh, A.; Miura, T.; Tada, N. Oxidation of carbon-halogen bonds. In: Comprehensive organic synthesis, Knochel, P., Ed.; Second Edition, Elsevier Science Ltd., Amsterdam, 2014, Vol. VII, pp. 744–769.
- Volpin, G.; Vepřek, N.A.; Bellan, A.B.; Trauner, D. Enantioselective synthesis and racemization of (-)-sinoracutine. Angew. Chem. Int. Ed., 2017, 56, 897–901. [Google Scholar] [CrossRef]
- Beshore, D.C.; Smith, A.B. III The lyconadins: enantioselective total syntheses of (+)-lyconadin A and (-)-lyconadin B. J. Am. Chem. Soc., 2008, 130, 13778–13789. [Google Scholar] [CrossRef]
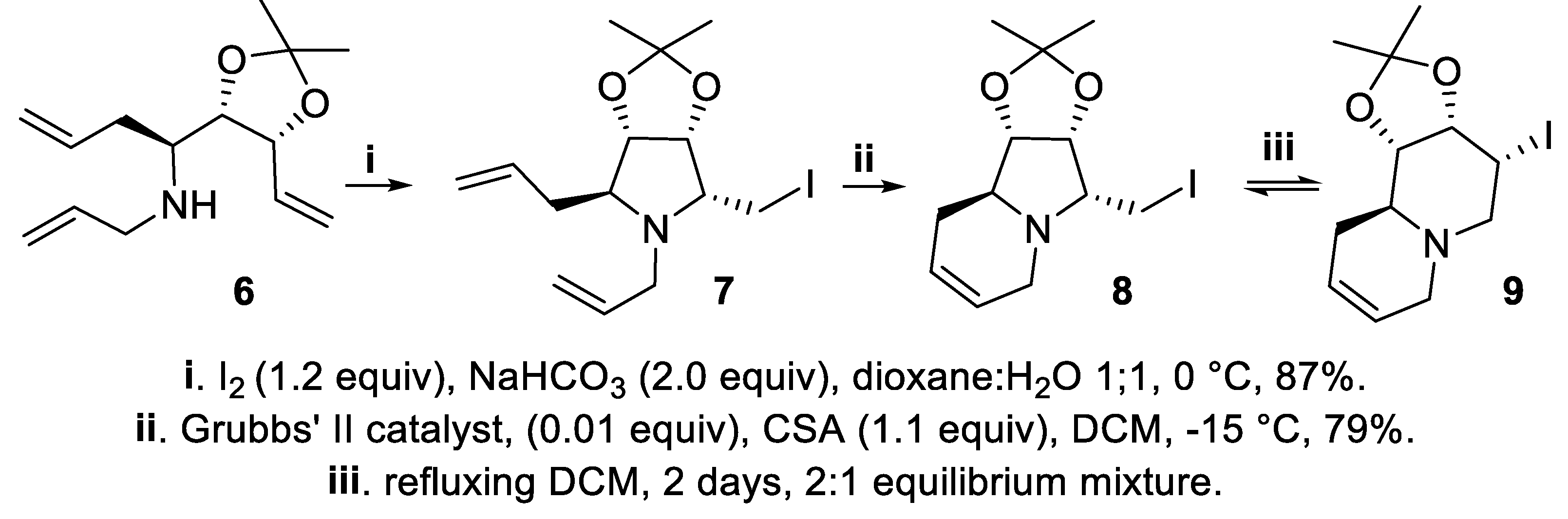
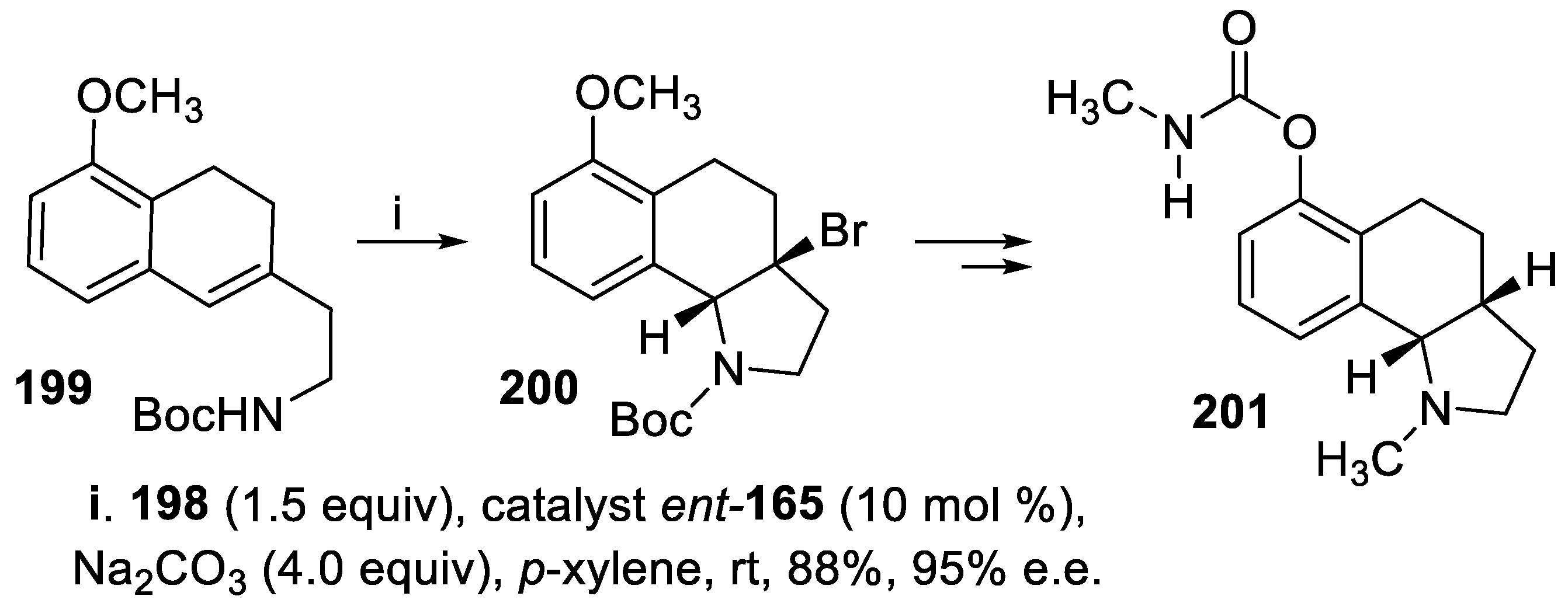
- Brock, E.A.; Davies, S.G.; Lee, J.A.; Roberts, P.M.; Thomson, J.E. Asymmetric synthesis of the tropane alkaloid (+)-pseudococaine via ring-closing iodoamination. Org. Lett., 2012, 14, 4278–4271. [Google Scholar] [CrossRef]
- Robertson, J.; Stevens, K. Pyrrolizidine alkaloids. Nat. Prod. Rep., 2014, 31, 1721–1788. [Google Scholar] [CrossRef] [PubMed]
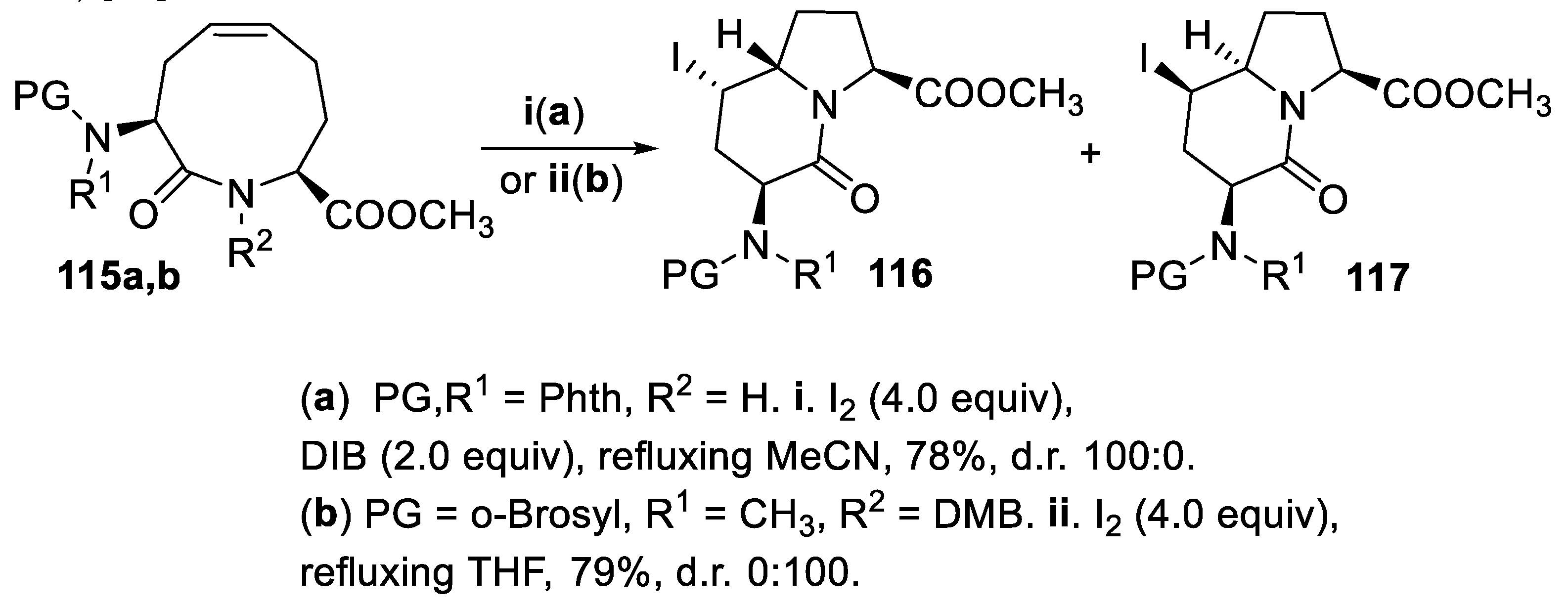
- Brock, E.A.; Davies, S.G.; Lee, J.A.; Roberts, P.M.; Thomson, J.E. Asymmetric synthesis of polyhydroxylated pyrrolizidines via transannular iodoamination with concomitant N-debenzylation. Org. Lett., 2011, 13, 1594–1597. [Google Scholar] [CrossRef]
- . [CrossRef]
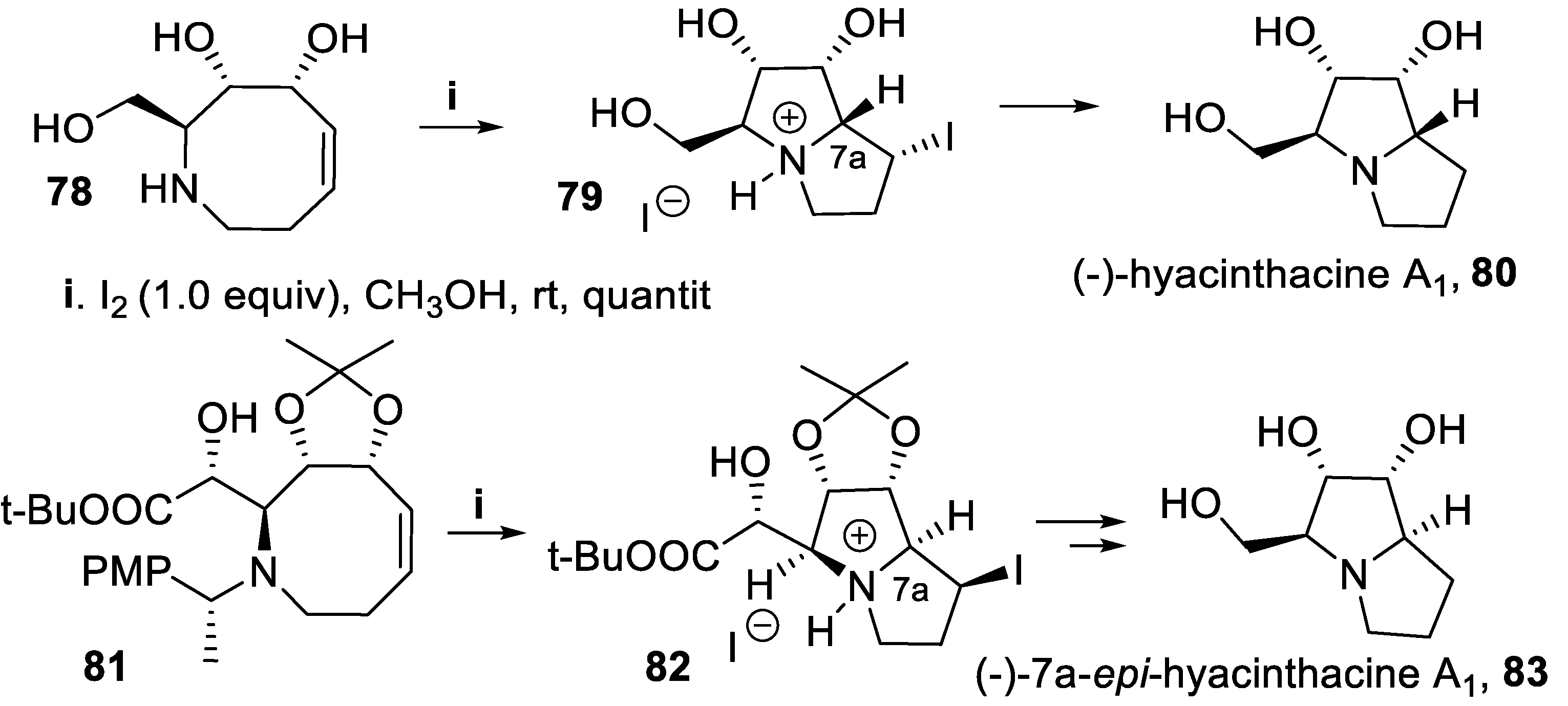
- Brock, E.A.; Davies, S.G.; Lee, J.A.; Roberts, P.M.; Thomson, J.E. Polyhydroxylated pyrrolizidine alkaloids from transannular iodoaminations: application to the asymmetric syntheses of (-)-hyacinthacine A1, (-)-7a-epi-hyacinthacine A1, (-)-hyacinthacine A2, and (-)-1-epi-alexine. Org. Biomol. Chem., 2013, 11, 3187–3202. [Google Scholar] [CrossRef] [PubMed]
- Ciufolini, M.A. Synthetic studies on heterocyclic natural products. Farmaco, 2005, 60, 627–641. [Google Scholar] [CrossRef] [PubMed]
- . [CrossRef]
- Zhang, H.; Lin, Z.; Huang, H.; Huo, H.; Huang, Y.; Ye, J.; Huang, P. Enantioselective Synthesis of the diazatricyclic core of alkaloid TAN1251C via an iodoaminocyclization reaction. Chin. J. Chem., 2010, 28, 1717–1724. [Google Scholar] [CrossRef]
- . [CrossRef]
- Wada, M.; Murata, T.; Oikawa, H.; Oguri, H. Nickel-catalyzed dimerization of pyrrolidinoindoline scaffolds: systematic access to chimonanthines, folicanthines and (+)-WIN 64821. Org. Biomol. Chem., 2014, 12, 298–306. [Google Scholar] [CrossRef]
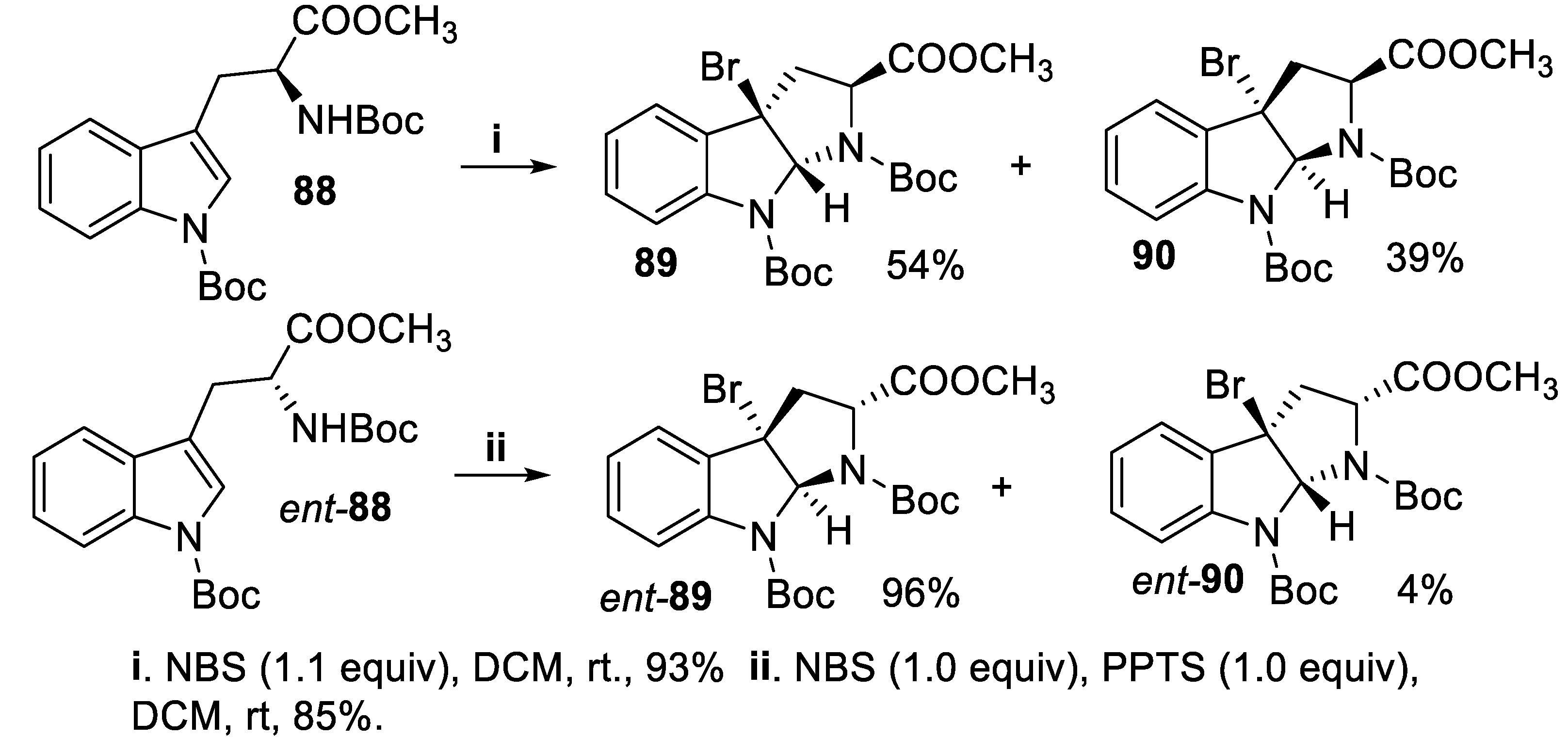
- Silva López, C.; Pérez-Balado, C.; Rodríguez-Graña, P.; de Lera, A.R. Mechanistic insights into the stereocontrolled synthesis of hexahydropyrrolo [2,3-b]indoles by electrophilic activation of tryptophan derivatives. Org. Lett., 2008, 10, 77–80. [Google Scholar] [CrossRef]
- . [CrossRef]
- Fan, J.-Q.; Ishii, S.; Asano, N.; Suzuki, Y. Accelerated transport and maturation of lysosomal α–galactosidase A in Fabry lymphoblasts by an enzyme inhibitor. Nat. Med., 1999, 5, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Wijtmans, R.; Vink, M.K.S.; Schoemaker, H.E.; van Delft, F.L.; Blaauw, R.H.; Rutjes, F.P.J.T. Biological relevance and synthesis of C-substituted morpholine derivatives. Synthesis. [CrossRef]
- Shcherbatiuk, A.V.; Shyshlyk, O.S.; Yarmoliuk, D.V.; Shishkin, O.V.; Shishkina, S.V.; Starova, V.S.; Zaporozhets, O.A.; Zozulya, S.; Moriev, R.; Kravchuk, O.; Manoilenko, O.; Tolmachev, A.A.; Mykhailiuk, P.K. Synthesis of 2- and 3-trifluoromethylmorpholines: useful building blocks for drug discovery. Tetrahedron, 2013, 69, 3796–3804. [Google Scholar] [CrossRef]
- Nonnenmacher, J.; Grellepois, F.; Portella, C. Synthesis of enantiopure 2-aryl(alkyl)-2-trifluoromethyl-substituted morpholines and oxazepanes. Eur. J. Org. Chem, 3726. [Google Scholar] [CrossRef]
- Magriotis, P.A. Recent progress toward the asymmetric synthesis of carbon-substituted piperazine pharmacophores and oxidative related heterocycles. RSC Med. Chem., 2020, 11, 745–759. [Google Scholar] [CrossRef]
- Bera, S.; Pandai, G. I2-Mediated diversity oriented diastereoselective synthesis of amino acid derived trans-2,5-disubstituted morpholines, piperazines, and thiomorpholines. ACS Comb. Sci., 2012, 14, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, J.; Ishiwata, T.; Shirai, K.; Koshino, H.; Tanatani, A.; Nakata, T.; Hashimoto, Y.; Nagasawa, K. Total synthesis of (+)-batzelladine A and (-)-batzelladine D, and identification of their target protein. Chem. Eur. J., 2005, 11, 6878–6888. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.A.; Durón, S.G.; Gin, D.Y. Diastereoselective [4+2] annulation of vinyl carbodiimides with N-alkyl imines. Asymmetric synthetic access to the batzelladine alkaloids. J. Am. Chem. Soc., 2005, 127, 6924–6925. [Google Scholar] [CrossRef]
- Arnold, M.A.; Day, K.A.; Durón, S.G.; Gin, D.Y. Total synthesis of (+)-batzelladine A and (-)-batzelladine D via [4+2]-annulation of vinyl carbodiimides with N-alkyl imines. J. Am. Chem. Soc., 2006, 128, 13255–13260. [Google Scholar] [CrossRef]
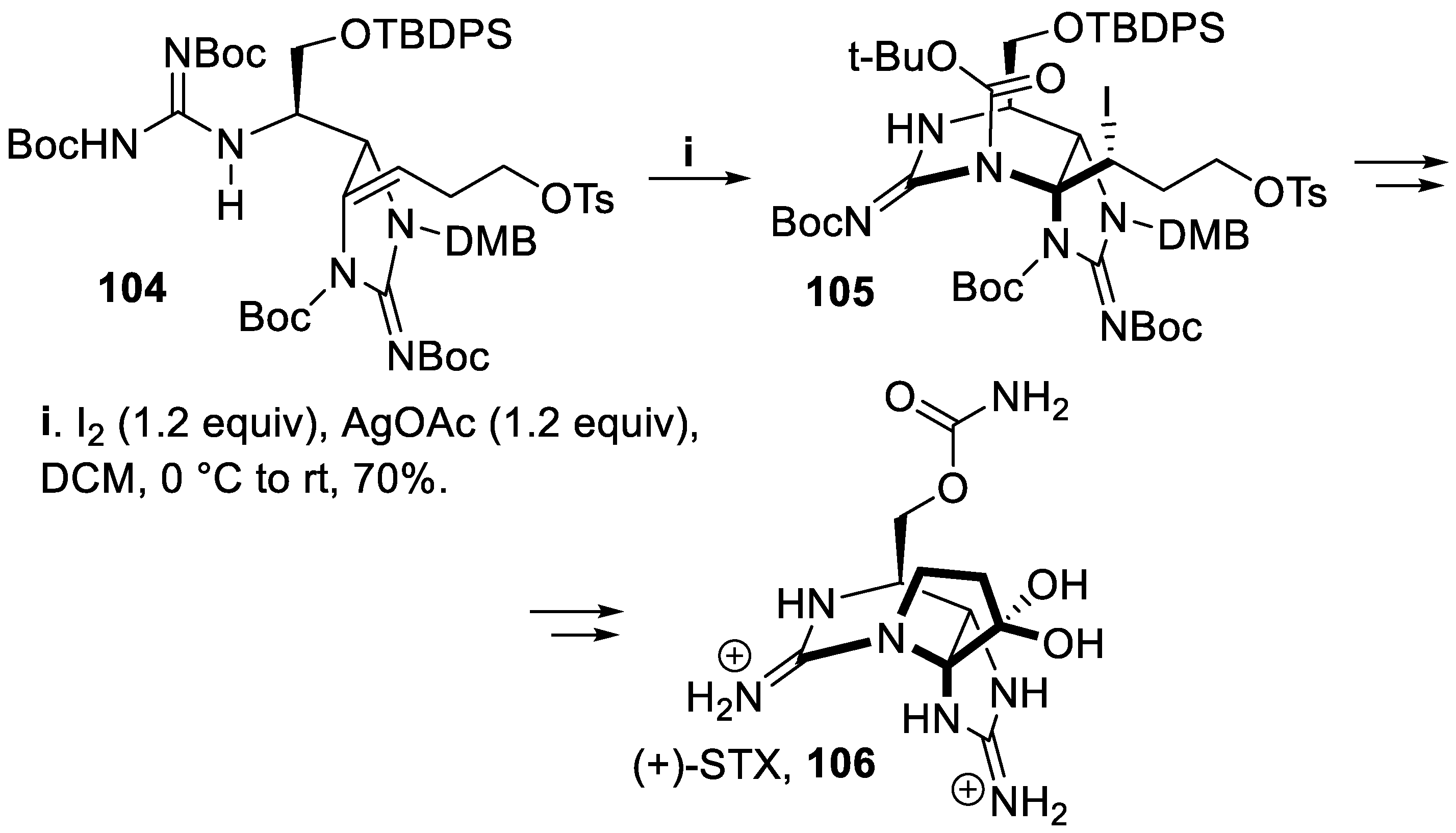
- Durán-Riveroll, L.M.; Cembella, A.D. Guanidinium toxins and their interactions with voltage-gated sodium ion channels. Mar. Drugs, 2017, 15, 303–331. [Google Scholar] [CrossRef]
- Paladugu, S.R.; James, C.K.; Looper, R.E. A direct C11 alkylation strategy on the saxitoxin core: a synthesis of (+)-11-saxitoxinethanoic acid. Org. Lett., 2019, 21, 7999–8002. [Google Scholar] [CrossRef]
- Sakurai, O.; Takahashi, M.; Ogiku, T.; Hayashi, M.; Horikawa, H.; Iwasaki, T. A new synthesis of 1β-methylcarbapenems using NBS-promoted cyclization as a key step. Tetr. Lett., 1994, 35, 6317–6320. [Google Scholar] [CrossRef]
- Bateson, J.H.; Roberts, P.M.; Snale, T.C.; Southgate, R. Synthesis of 7-oxo-3-sulphinyl-1-azabicyclo [3.2.0]hept-2-ene-2-carboxylates: olivanic acid analogues. J. Chem. Soc., Chem. Commun 1980, 185–186. [Google Scholar] [CrossRef]
- Biloski, A.J.; Wood, R.D.; Ganem, B. A new beta-lactam synthesis. J. Am. Chem. Soc., 1982, 104, 3233–3235. [Google Scholar] [CrossRef]
- . [CrossRef]
- Krook, M.A.; Miller, M.J. The direct cyclization of alpha-acylamino-substituted hydroxamates to beta-lactams. J. Org. Chem., 1985, 50, 1126–1128. [Google Scholar] [CrossRef]
- Rajendra, G.; Miller, M.J. Oxidative cyclization of β,γ-unsaturated O-acyl hydroxamates to β-lactams. Tetr. Lett., 1985, 26, 5385–5388. [Google Scholar] [CrossRef]
- Cheng, Y.A.; Yu, W.Z.; Yeung, Y.Y. An unexpected bromolactamization of olefinic amides using a three-component co-catalyst system. J. Org. Chem., 2016, 81, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Pazos, M.; González, B.; Suescun, L.; Seoane, G.; Carrera, I. Production of enantiopure β-amino-γ-hydroxyesters from benzoic acid by a selective formal aminohydroxylation. Tetr. Lett., 2017, 58, 2182–2185. [Google Scholar] [CrossRef]
- Schulte, A.S.; Janich, S.; Würthwein, E.-U.; Saito, S.; Wünsch, B. Investigation of the Corey bromolactamization with N-functionalized allylamines. J. Heterocycl. Chem., 2016, 53, 1827–1837. [Google Scholar] [CrossRef]
- Schulte, A.; Situ, X.; Saito, S.; Wünsch, B. Bromolactamization: key step in the stereoselective synthesis of enantiomerically pure, cis-configured perhydropyrroloquinoxalines. Chirality, 2014, 26, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Atmuri, P.N.D.; Lubell, W.D. Stereo- and regiochemical transannular cyclization of a common hexahydro-1H-azonine to afford three different indolizidinone dipeptide mimetics. J. Org. Chem., 2020, 85, 1340–1351. [Google Scholar] [CrossRef]
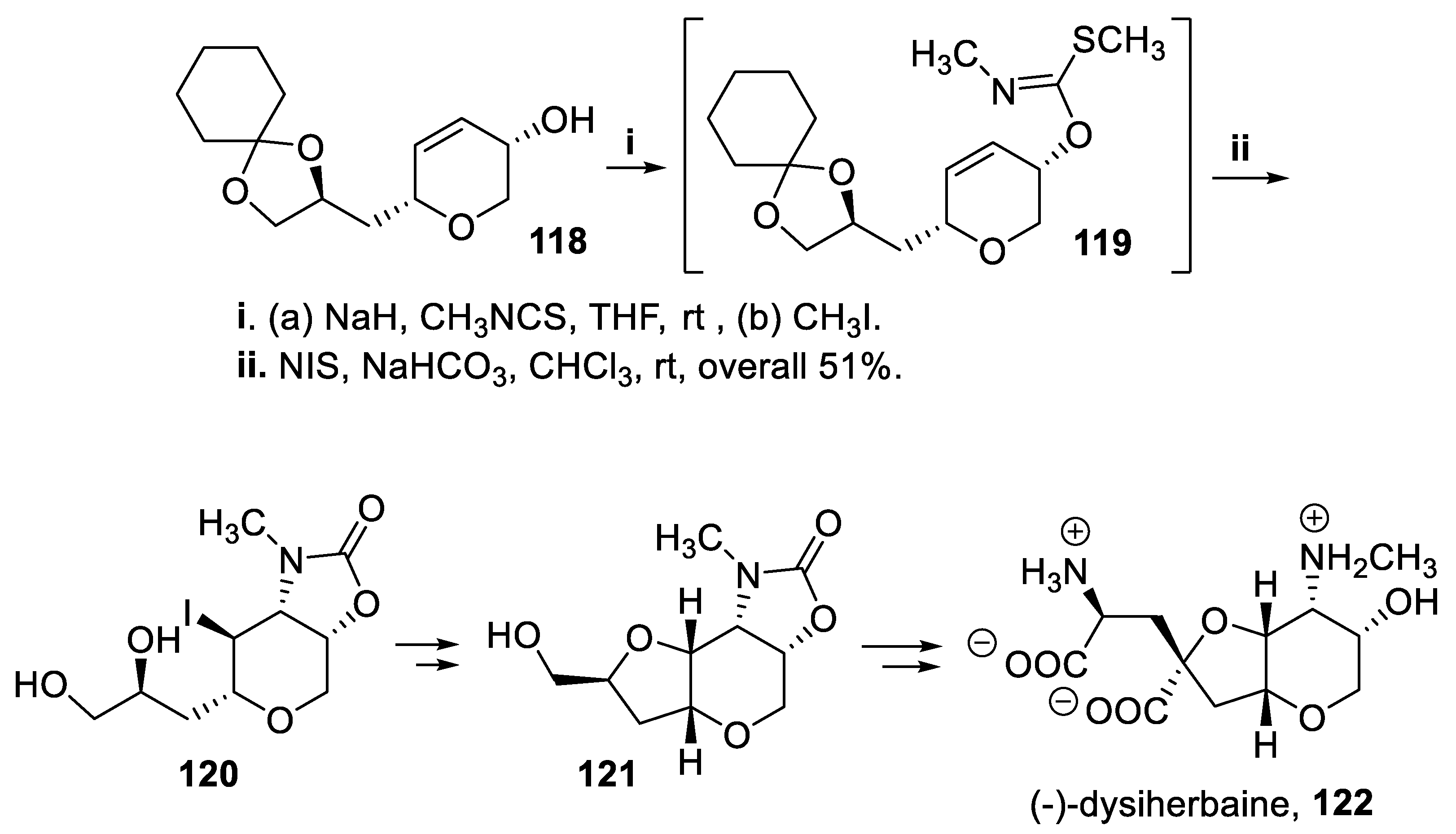
- Snyder, B.B.; Hawryluk, N.A. Synthesis of (-)-dysiherbaine. Org. Lett., 2000, 2, 635–638. [Google Scholar] [CrossRef]
- Kang, S.H.; Lee, Y.M. A formal total synthesis of (-)-dysiherbaine. Synlett, 2003, 993-99. [CrossRef]
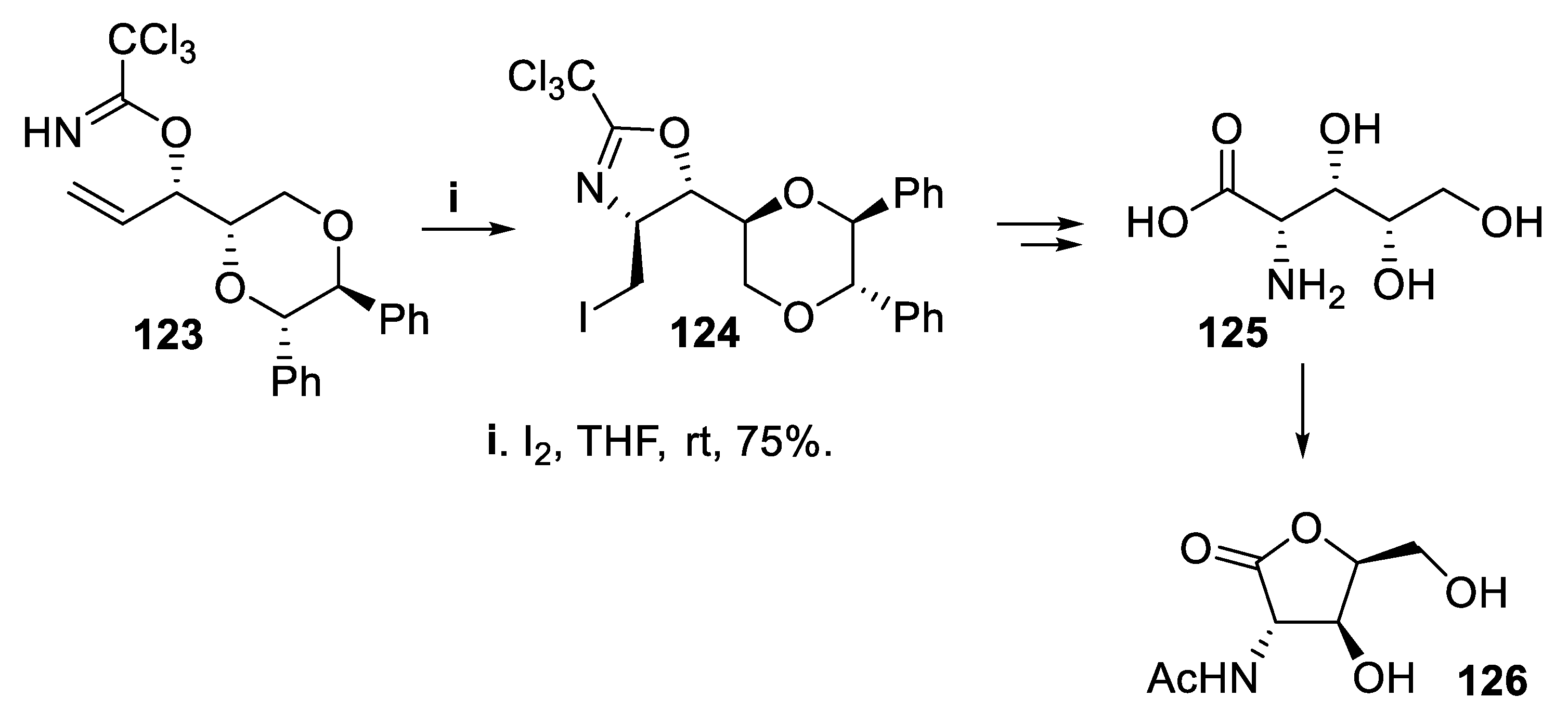
- Fujioka, H.; Murai, K.; Ohba, Y.; Hirose, H. ; Kita, Y.; Kim, K.K.; Lee, Y.J.; Kim, J.K.; Sung, D.K. Synthesis of (+)-polyoxamic acid and D-sorbitol from simple achiral allylic halides employing (S,S)-hydrobenzoin as a chiral source. Chem. Commun. 2002, 1116–1117. [Google Scholar] [CrossRef] [PubMed]
- Poss, C.S.; Schreiber, S.L. Two-directional chain synthesis and terminus differentiation. Acc. Chem. Res., 1994, 27, 9–17. [Google Scholar] [CrossRef]
- Hoffmann, R.W. Stereoselective synthesis using diastereotopic groups. Synthesis, 2004, 2075-2090.
- . [CrossRef]
- Studer, A.; Schleth, F. Desymmetrization and diastereotopic group selection in 1,4-cyclohexadienes. Synlett 2005, 3033–3041. [Google Scholar] [CrossRef]
- Nakahara, K.; Fujioka, H. Diastereoselective desymmetrization of symmetric dienes and its synthetic application.
- Symmetry2010, 2, 437–454. [CrossRef]
- Horwitz, M.A.; Johnson, J.S. Local desymmetrization through diastereotopic group selection: an enabling strategy for natural product synthesis. Eur. J. Org. Chem 2017, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
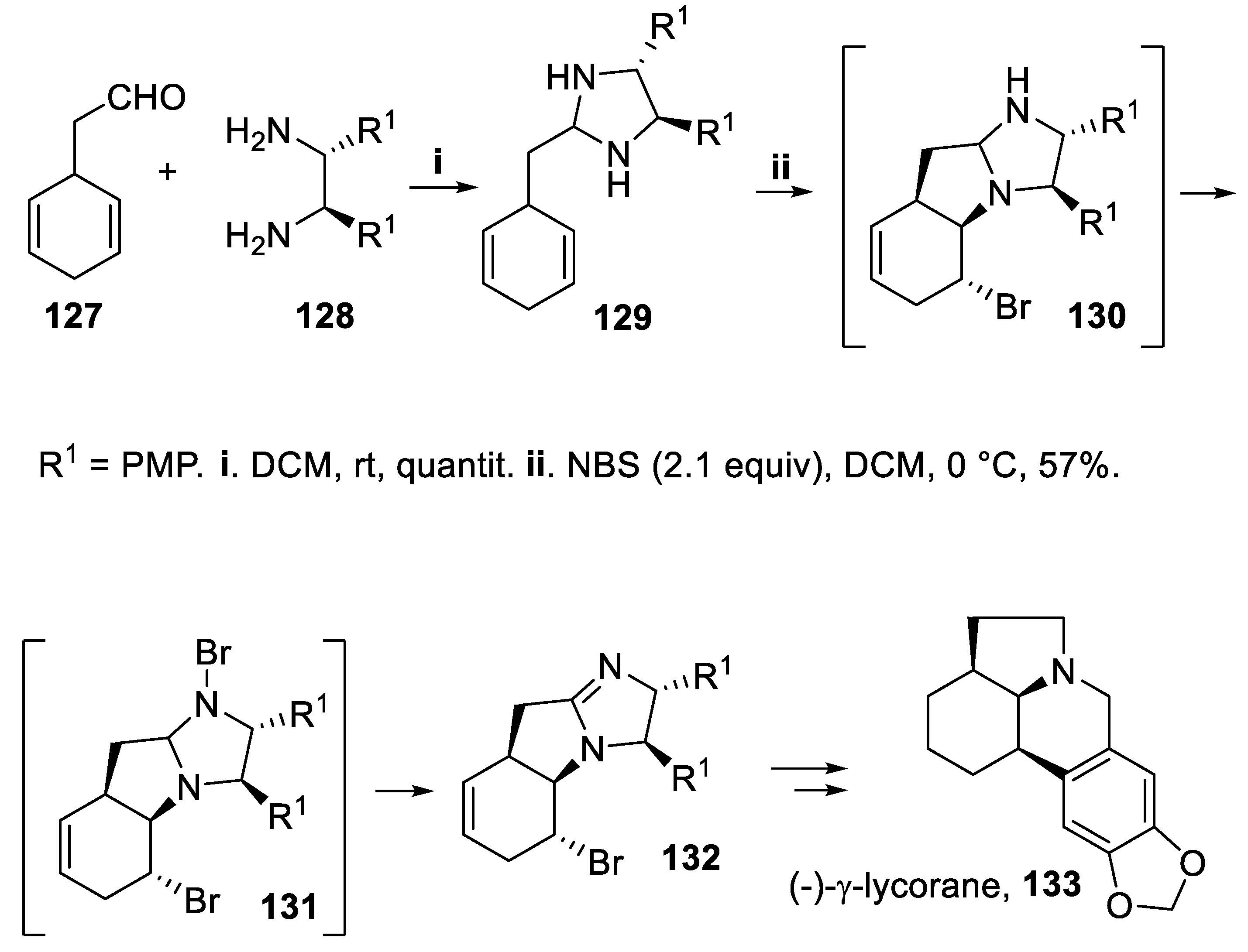
- Fujioka, H.; Murai, K.; Ohba, Y.; Hirose, H.; Kita, Y. Intramolecular bromo-amination of 1,4-cyclohexadieneaminal: one-pot discrimination of two olefins and concise asymmetric synthesis of (-)-γ-lycorane. Chem. Commun., 2006, 832-834.
- . [CrossRef]
- Hall, C.J.J.; Marriott, I.S.; Christensen, K.E.; Day, A.J.; Goundry, W.R.F.; Donohoe, T.J. Extension of hydrogen borrowing alkylation reactions for the total synthesis of (-)-γ-lycorane. Chem. Commun., 2022, 58, 4966–4968. [Google Scholar] [CrossRef] [PubMed]
- . [CrossRef]
- O’Hagan, D. Pyrrole, pyrrolidine, pyridine, piperidine and tropane alkaloids. Nat. Prod. Rep., 2000, 17, 435–446. [Google Scholar] [CrossRef] [PubMed]
- . [CrossRef]
- Felpin, F.-X.; Lebreton, J. Recent advances in the total synthesis of piperidine and pyrrolidine natural alkaloids with Ring-Closing Metathesis as a key step. Eur. J. Org. Chem 2003, 3693–3712. [Google Scholar] [CrossRef]
- Wen-Fang, X.; Xian-Chao, C.; Qiang, W.; Hao, F. Advances in matrix metalloproteinase inhibitors based on pyrrolidine scaffold. Curr. Med. Chem., 2008, 15, 374–385. [Google Scholar] [CrossRef]
- Harris, L.D.; Harijan, R.K.; Ducati, R.G.; Evans, G.B.; Hirsch, B.M.; Schramm, V.L. Synthesis of bis-phosphate iminoaltritol enantiomers and structural characterization with adenine phosphoribosyltransferase. ACS Chem. Biol., 2018, 13, 152–160. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, L.; Yeung, Y.-Y. A highly enantioselective approach towards 2-substituted 3-bromopyrrolidines. Org. Biomol. Chem., 2012, 10, 3808–3811. [Google Scholar] [CrossRef]
- Lloyd, J.; Finlay, H.J.; Vacarro, W.; Hyunh, T.; Kover, A.; Bhandaru, R.; Yan, L.; Atwal, K.; Conder, M.L.; Jenkins-West, T.; Shi, H.; Huang, C.; Li, W.D.; Sun, H.; Levesque, P. Pyrrolidine amides of pyrazolodihydropyrimidines as potent and selective KV1.5 blockers. Bioorg. Med. Chem. Lett., 2010, 20, 1436–1439. [Google Scholar] [CrossRef] [PubMed]
- Floyd, D.M.; Kimball, S.D.; Krapcho, J.; Das, J.; Turk, C.F.; Moquin, R.V.; Lago, M.W.; Duff, K.J.; Lee, V.G. Benzazepinone calcium channel blockers. 2. Structure-activity and drug metabolism studies leading to potent antihypertensive agents. Comparison with benzothiazepinones. J. Med. Chem., 1992, 35, 756–772. [Google Scholar] [CrossRef] [PubMed]
- Cushing, T.D.; Baichwal, V.; Berry, K.; Billedeau, R.; Bordunov, V.; Broka, C.; Browner, M.F.; Cardozo, M.; Cheng, P.; Clark, D.; Dalrymple, S.; DeGraffenreid, M.; Gill, A.; Hao, X.; Hawley, R.C.; He, X.; Labadie, S.S.; Labelle, M.; Lehel, C.; Lu, P.-P.; McIntosh, J.; Miao, S.; Parast, C.; Shin, Y.; Sjogren, E.B.; Smith, M.-L.; Talamas, F.X.; Tonn, G.; Walker, K.M.; Walker, N.P.C.; Wesche, H.; Whitehead, C.; Wright, M.; Jaen, J.C.A. A novel series of IKKβ inhibitors part II: Description of a potent and pharmacologically active series of analogs. Bioorg. Med. Chem. Lett., 2011, 21, 423–426. [Google Scholar] [CrossRef]
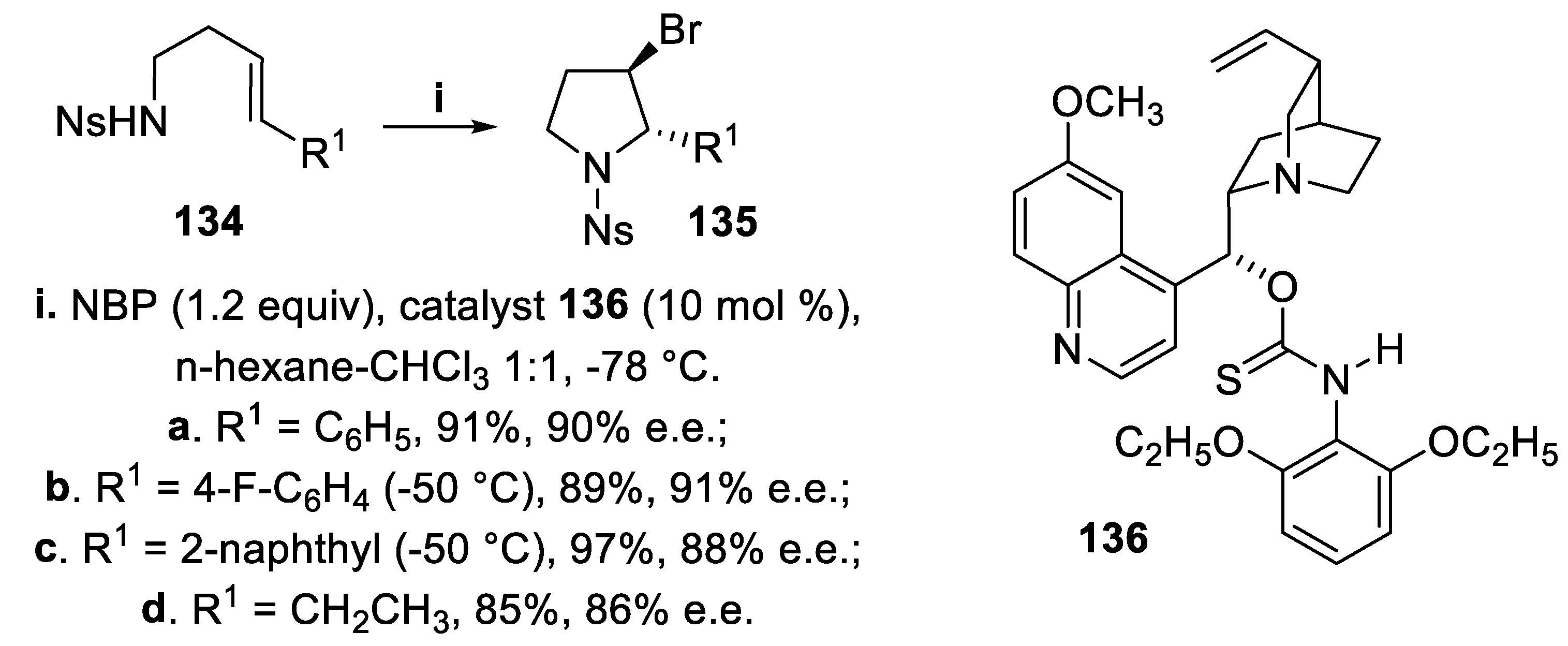
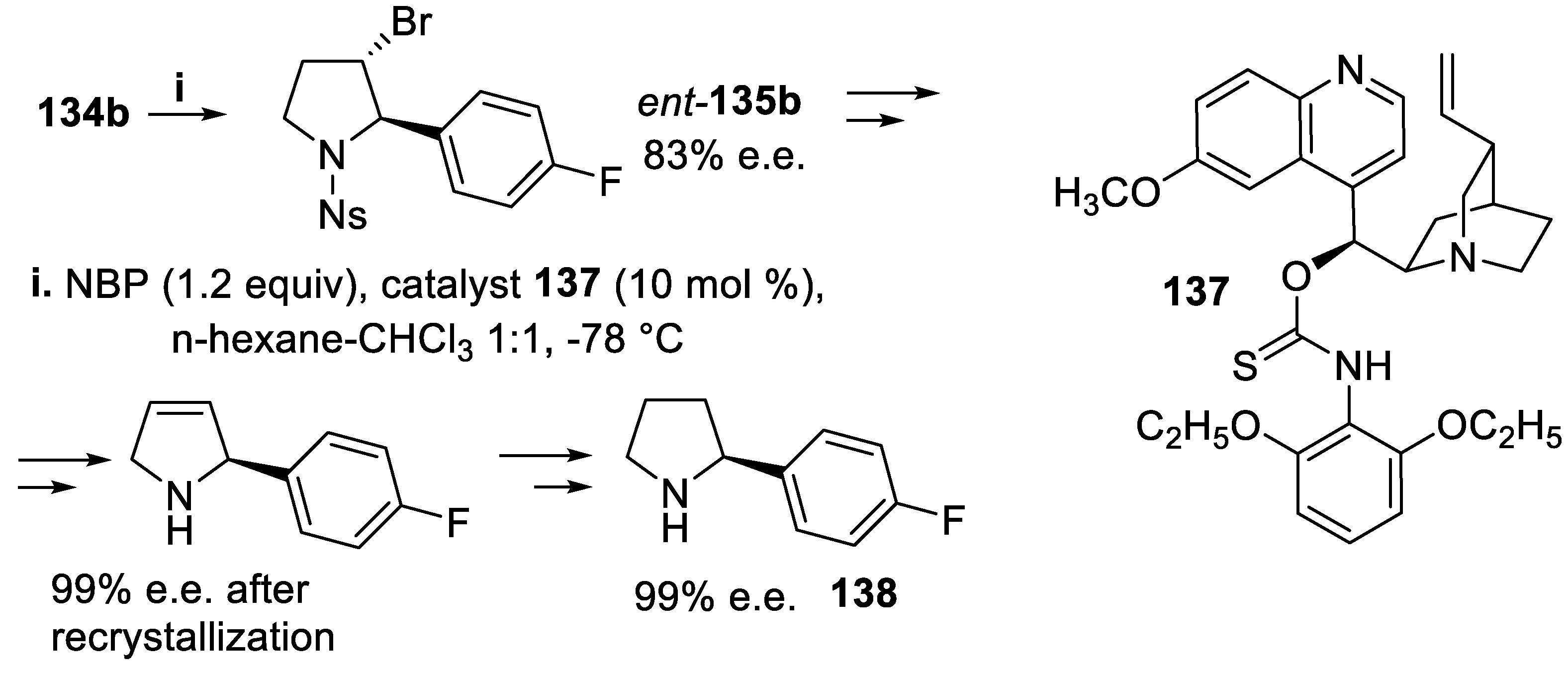
- Mizar, P.; Burrelli, A.; Günther, E.; Söftje, M.; Farooq, U.; Wirth, T. Organocatalytic stereoselective iodoamination of alkenes. Chem. Eur. J., 2014, 20, 13113–13116. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.S.; Rodrigues, M.T.J.; Santo, H.; Zeoly, L.A.; Almeida, W.P.; Barcelos, R.C.; Gomes, R.C.; Fernandes, F.S.; Coelho, F. Recent advances in indoline synthesis. Tetrahedron, 2019, 75, 2063–2097. [Google Scholar] [CrossRef]
- Hua, T.-B.; Xiao, C.; Yang, Q.-Q.; Chen, J.-R. Recent advances in asymmetric synthesis of 2-substituted indoline derivatives. Chin. Chem. Lett., 2020, 31, 311–323. [Google Scholar] [CrossRef]
- Liu, F.; Su, M. Indole and indoline scaffolds in drug discovery. In: Privileged Scaffolds in Drug Discovery, Yu, B.; Li, N.; Fu, C. Eds.; Academic Press, Cambridge, MA, 2023, Chapter 8, Pages 147-161.
- Breugst, M.; von der Heiden, D. Mechanisms in iodine catalysis. Chem. Eur. J., 2018, 24, 9187–9199. [Google Scholar] [CrossRef]
- . [CrossRef]
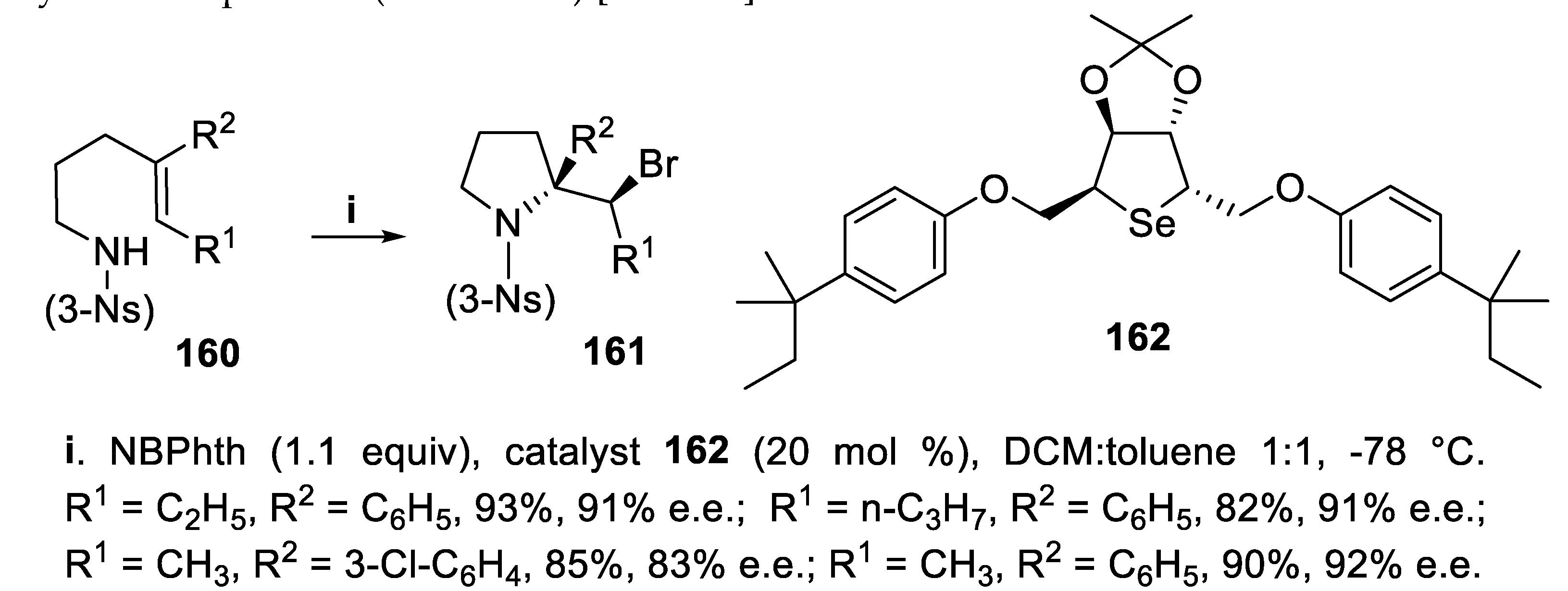
- Yu, S.-N.; Li, Y.-L.; Deng, J. Enantioselective synthesis of 2-bromomethyl indolines via BINAP(S)-catalyzed bromoaminocyclization of allyl aniline. Adv. Synth. Catal., 2017, 359, 2499–2508. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, J.; Tan, C.K.; Yeung, Y.-Y. Enantioselective bromoaminocyclization using amino-thiocarbamate catalysts. J. Am. Chem. Soc., 2011, 133, 9164–9167. [Google Scholar] [CrossRef]
- Hu, S.; Yuan, L.; Yan, H.; Li, Z. Design, synthesis and biological evaluation of lenalidomide derivatives as tumor angiogenesis inhibitor. Bioorg. Med. Chem. Lett., 2017, 27, 4075–4081. [Google Scholar] [CrossRef]
- Xiao, D.; Wang, Y.-j.; Hu, X.-b.; Kan, W.-j.; Zhang, Q.; Jiang, X.; Zhou, Y.-b.; Li, J.; Lu, W. Design, synthesis and biological evaluation of the thioether-containing lenalidomide analogs with anti-proliferative activities. Eur. J. Med. Chem., 2019, 176, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.P.; Thapa, P.; Sharma, R.; Sharma, M. 1-Isoindolinone scaffold-based natural products with a promising diverse bioactivity. Fitoterapia, 2020, 146, 104722. [Google Scholar] [CrossRef]
- Nakatsuji, H.; Sawamura, Y.; Sakakura, A.; Ishihara, K. Cooperative activation with chiral nucleophilic catalysts and N-haloimides: enantioselective iodolactonization of 4-arylmethyl-4-pentenoic acids. Angew. Chem., Int. Ed., 2014, 53, 6974–6977. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Nakatsuji, H.; Okumura, Y.; Yao, L.; Ishihara, K. Enantioselective halo-oxy- and halo-azacyclizations induced by chiral amidophosphate catalysts and halo-Lewis acids. J. Am. Chem. Soc., 2018, 140, 6039–6043. [Google Scholar] [CrossRef] [PubMed]
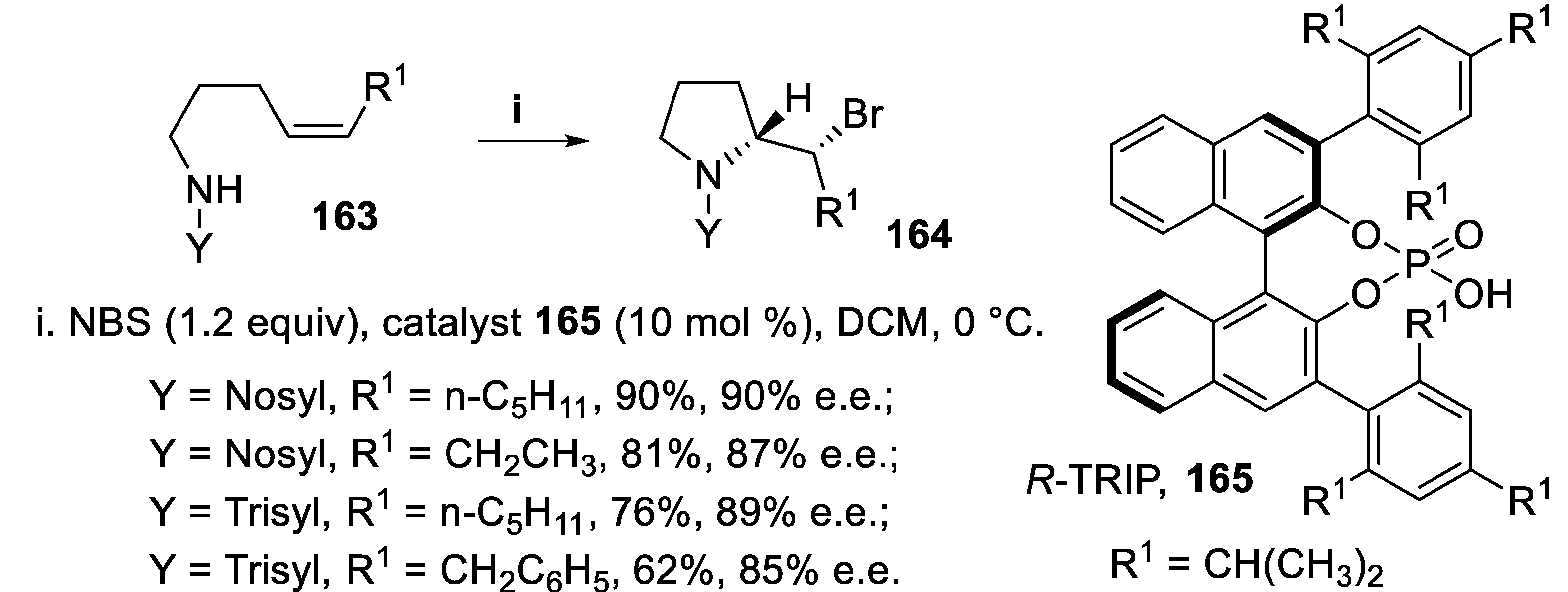
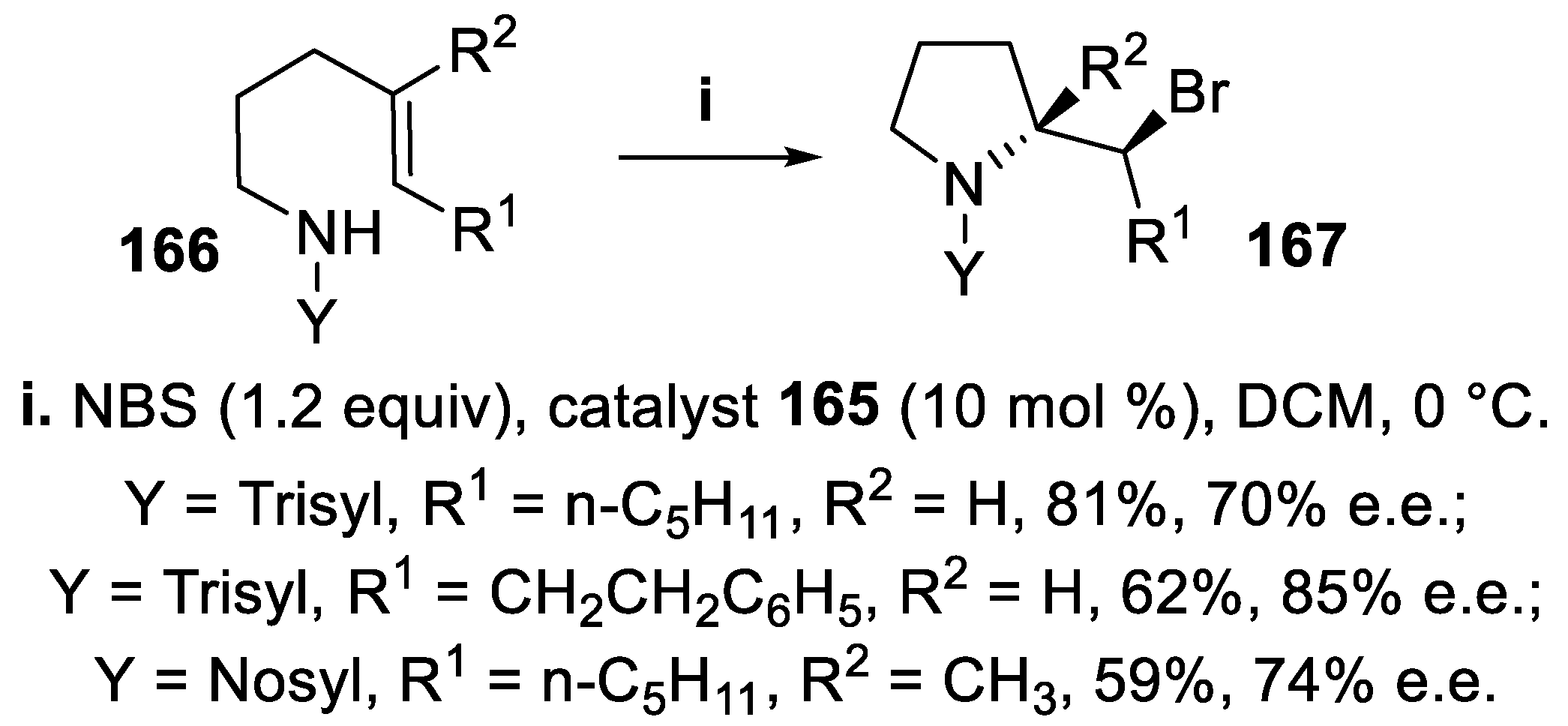
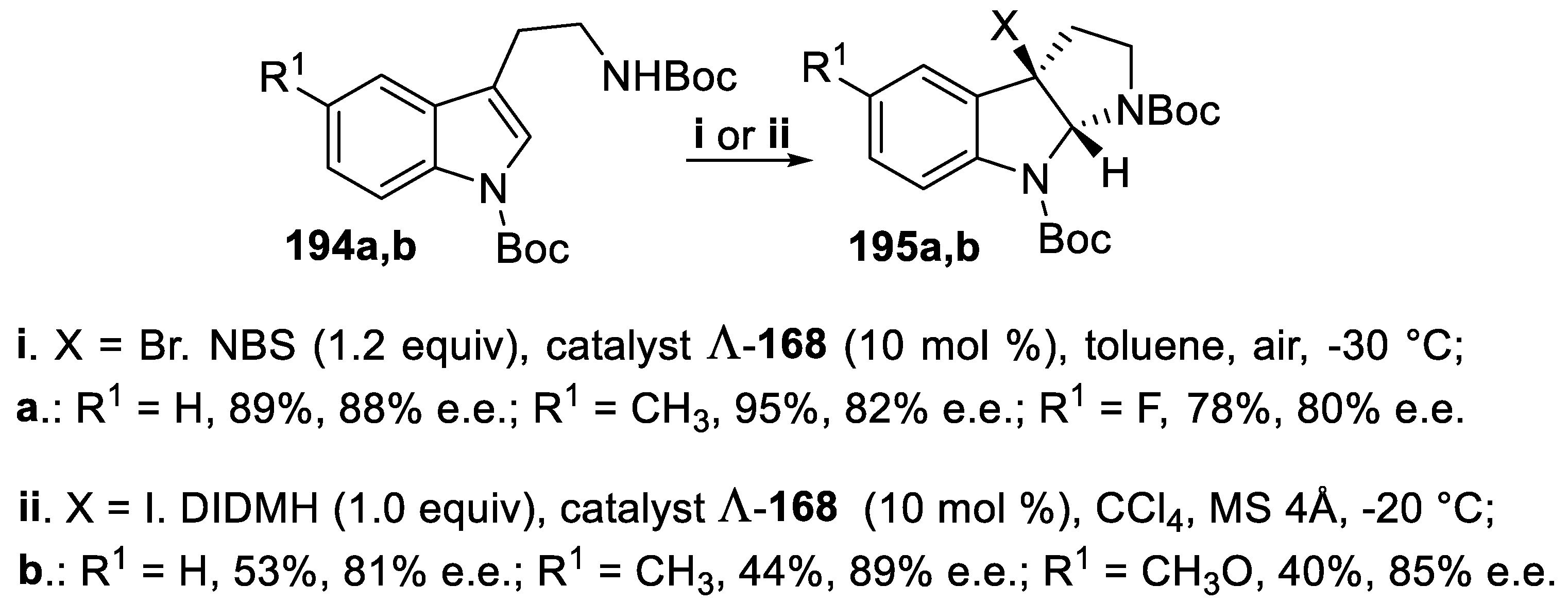
- Chen, F.; Tan, C.K.; Yeung, Y.-Y. C2-symmetric cyclic selenium-catalyzed enantioselective bromoaminocyclization. J. Am. Chem. Soc., 2013, 135, 1232–1235. [Google Scholar] [CrossRef] [PubMed]
- Gieuw, M.H.; Ke, Z.; Yeung, Y.-Y. Lewis base catalyzed stereo- and regioselective bromocyclization. Chem. Rec., 2017, 17, 287–311. [Google Scholar] [CrossRef] [PubMed]
- Sunggi, L.; Chung, W.-j. Enantioselective halogenation via asymmetric phase-transfer catalysis. Bull. Korean Chem. Soc., 2022, 43, 896–911. [Google Scholar] [CrossRef]
- Huang, D.; Wang, H.; Xue, F.; Guan, H.; Li, L.; Peng, X.; Shi, Y. Enantioselective bromocyclization of olefins catalyzed by chiral phosphoric acid. Org. Lett., 2011, 13, 6350–6353. [Google Scholar] [CrossRef] [PubMed]
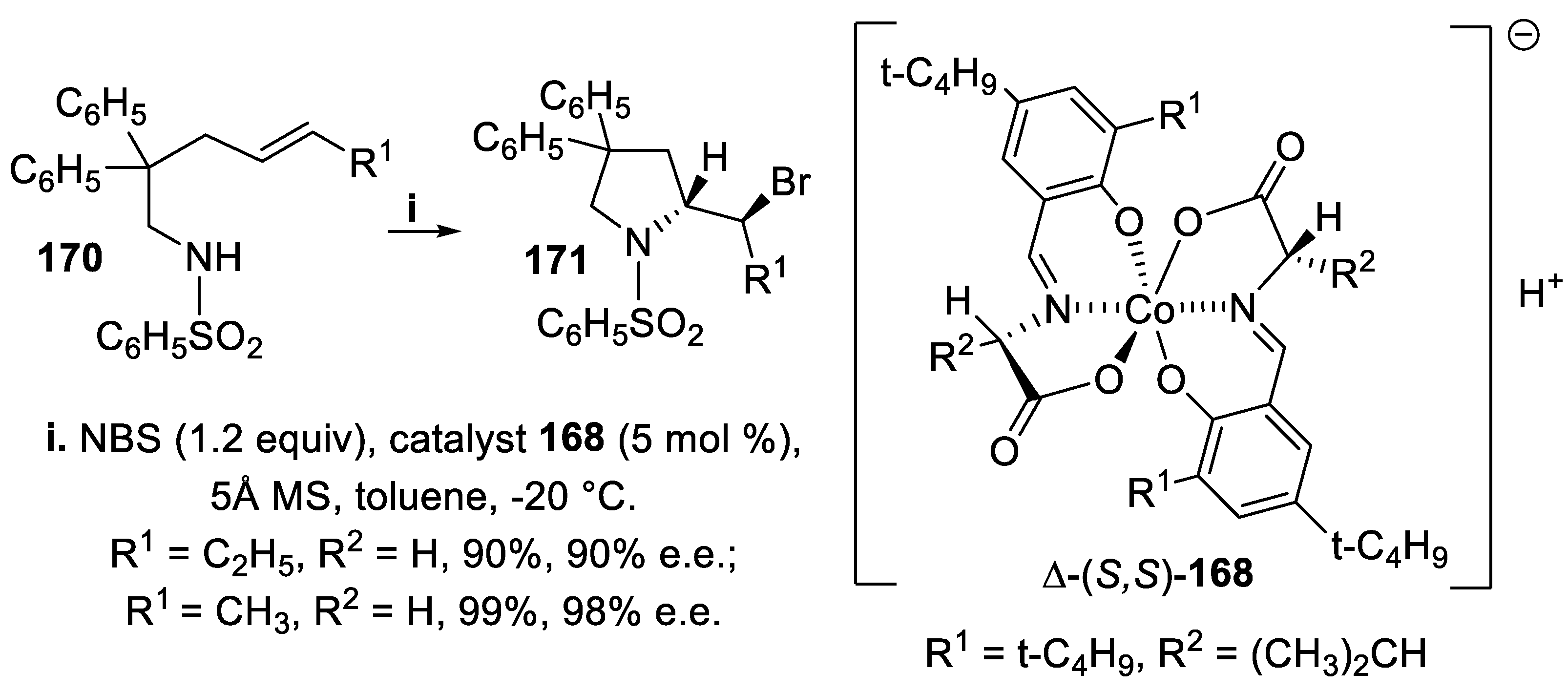
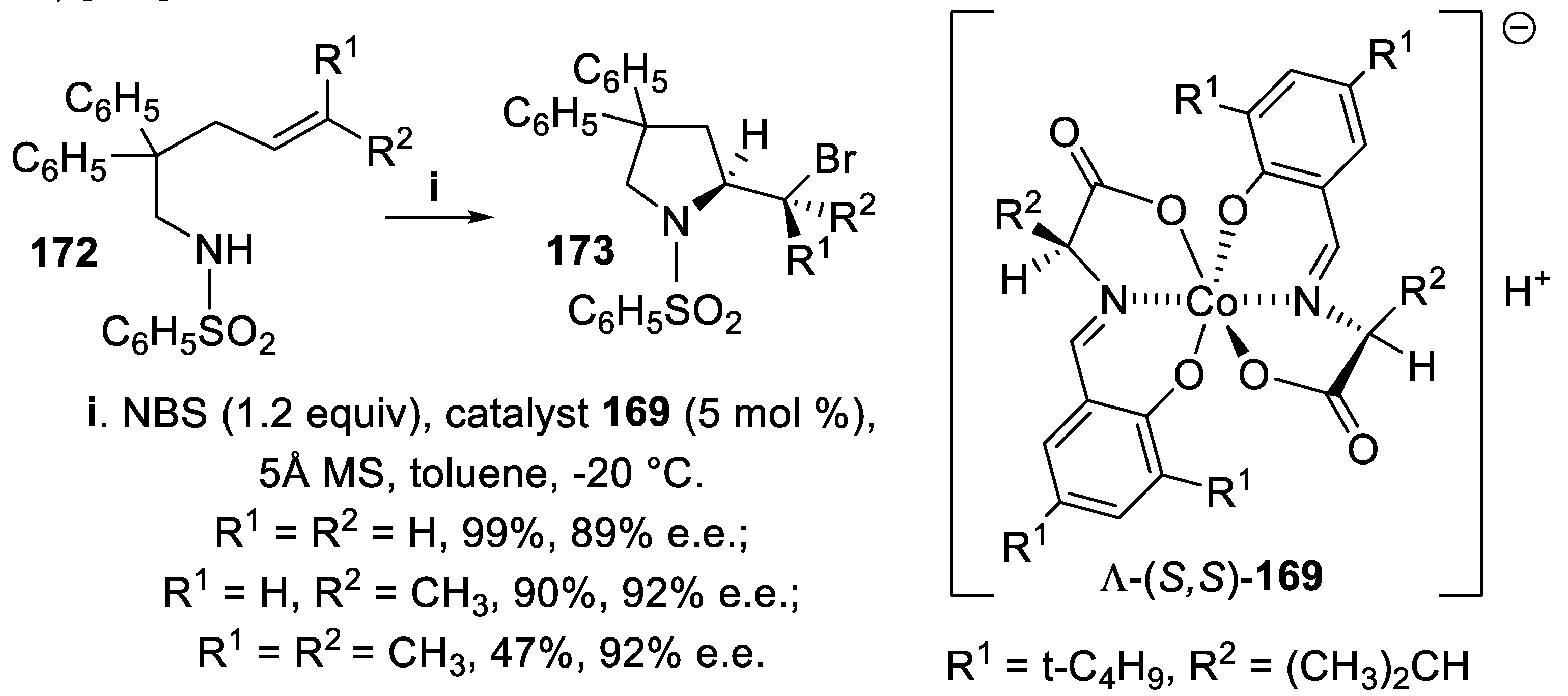
- Jiang, H.-J.; Liu, K.; Yu, J.; Zhang, L.; Gong, L.-Z. Switchable stereoselectivity in bromoaminocyclization of olefins: using Brønsted acids of anionic chiral Cobalt(III) complexes. Angew. Chem. Int. Ed., 2017, 56, 11931–11935. [Google Scholar] [CrossRef]
- . [CrossRef]
- Liang, X.-W.; Zheng, C.; You, S.-L. Dearomatization through halofunctionalization reactions. Chem. Eur. J., 2016, 22, 11918–11933. [Google Scholar] [CrossRef]
- Crich, D.; Banerjee, A. Chemistry of the hexahydropyrrolo [2,3-b]indoles: configuration, conformation, reactivity, and applications in synthesis. Acc. Chem. Res., 2007, 40, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Yin, Q.; You, S.-L. Chiral-amine-catalyzed asymmetric bromocyclization of tryptamine derivatives. Asian J. Org. Chem., 2014, 3, 408–411. [Google Scholar] [CrossRef]
- Qian, D.; Sun, J. Recent progress in asymmetric ion-pairing catalysis with ammonium salts. Chem. Eur. J., 2019, 25, 3740–3751. [Google Scholar] [CrossRef]
- Xie, W.; Jiang, G.; Liu, H.; Hu, J.; Pan, X.; Zhang, H.; Wan, X.; Lai, Y.; Ma, D. Highly enantioselective bromocyclization of tryptamines and its application in the synthesis of (-)-chimonanthine. Angew. Chem. Int. Ed., 2013, 52, 12924–12927. [Google Scholar] [CrossRef] [PubMed]
- . [CrossRef]
- Morikawa, T.; Nakanishi, Y.; Ninomiya, K.; Matsuda, H.; Nakashima, S.; Miki, H.; Miyashita, Y.; Yoshikawa, M.; Hayakawa, T.; Muraoka, O. Dimeric pyrrolidinoindoline-type alkaloids with melanogenesis inhibitory activity in flower buds of Chimonanthus praecox. J. Nat. Med., 2014, 68, 539–549. [Google Scholar] [CrossRef] [PubMed]
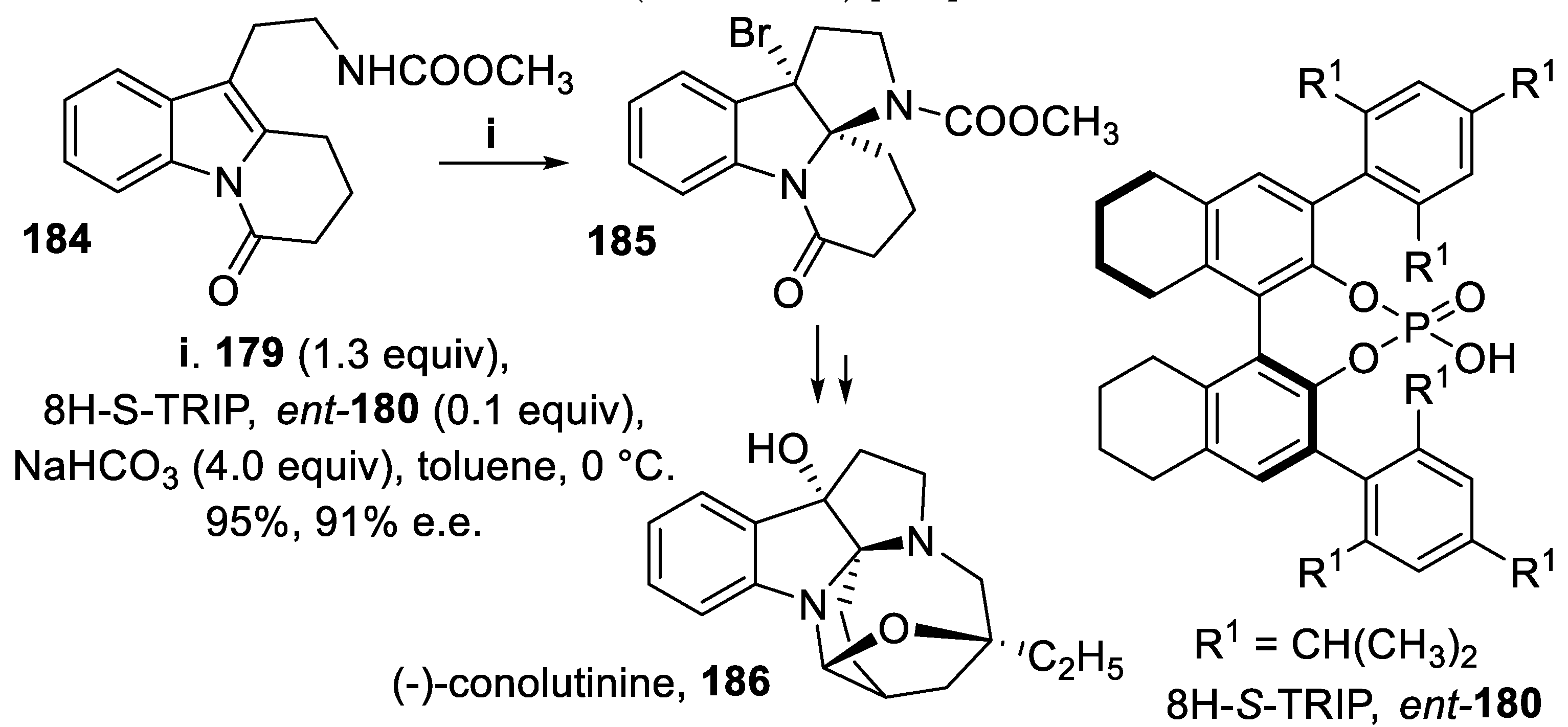
- Feng, X.; Jiang, G.; Xia, Z.; Hu, J.; Wan, X.; Gao, J.-M.; Lai, Y.; Xie, W. Total synthesis of (-)-conolutinine. Org. Lett., 2015, 17, 4428–4431. [Google Scholar] [CrossRef] [PubMed]
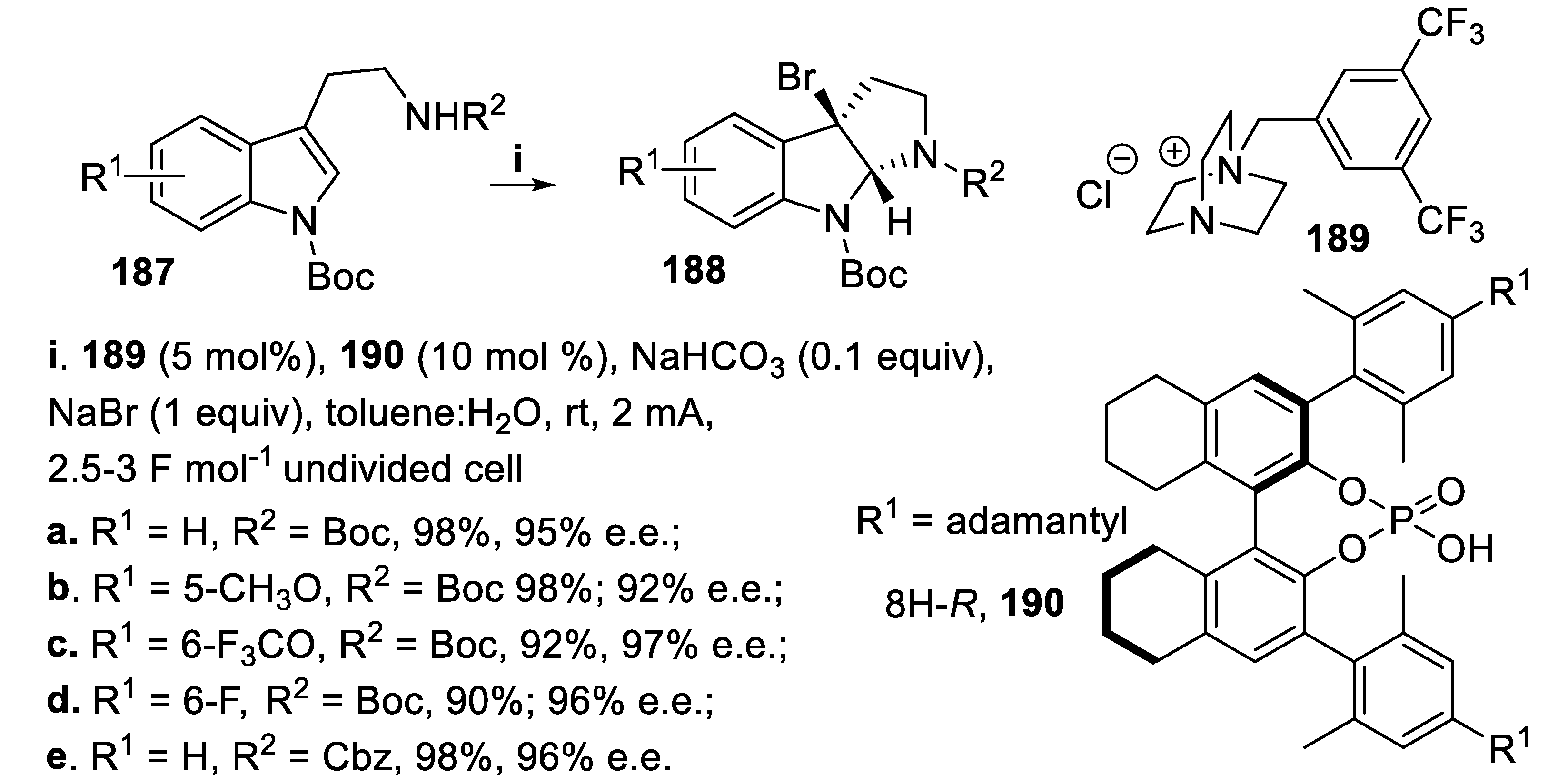
- Tan, X.; Wang, Q.; Sun, J. Electricity-driven asymmetric bromocyclization enabled by chiral phosphate anion phase-transfer catalysis. Nat. Commun., 2023, 14, 357. [Google Scholar] [CrossRef] [PubMed]
- Lindovska, P.; Movassaghi, M. Concise synthesis of (-)-hodgkinsine,(-)-calycosidine, (-)-hodgkinsine B, (-)-quadrigemine C, and (-)-psycholeine via convergent and directed modular assembly of cyclotryptamines. J. Am. Chem. Soc., 2017, 139, 17590–17596. [Google Scholar] [CrossRef] [PubMed]
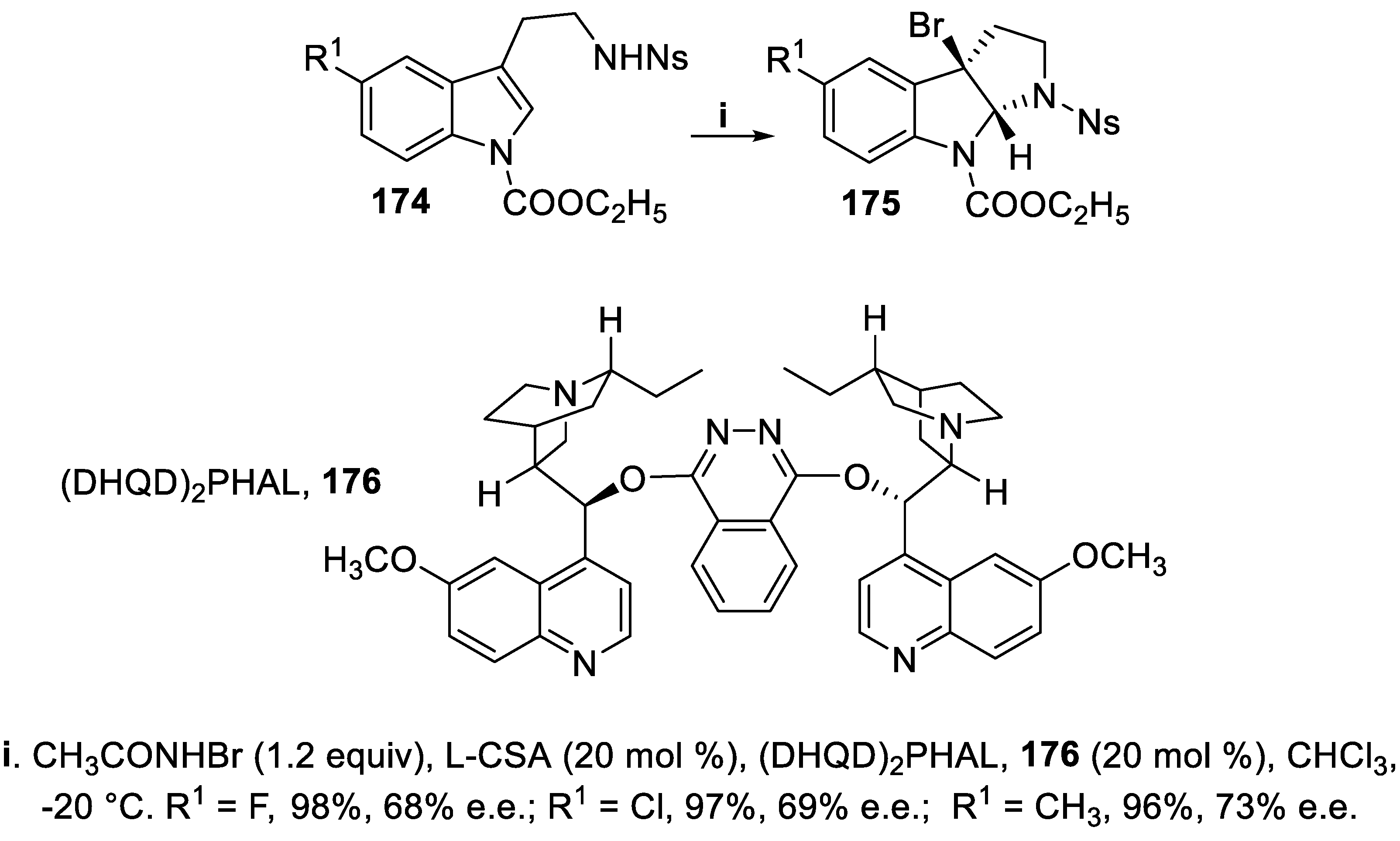
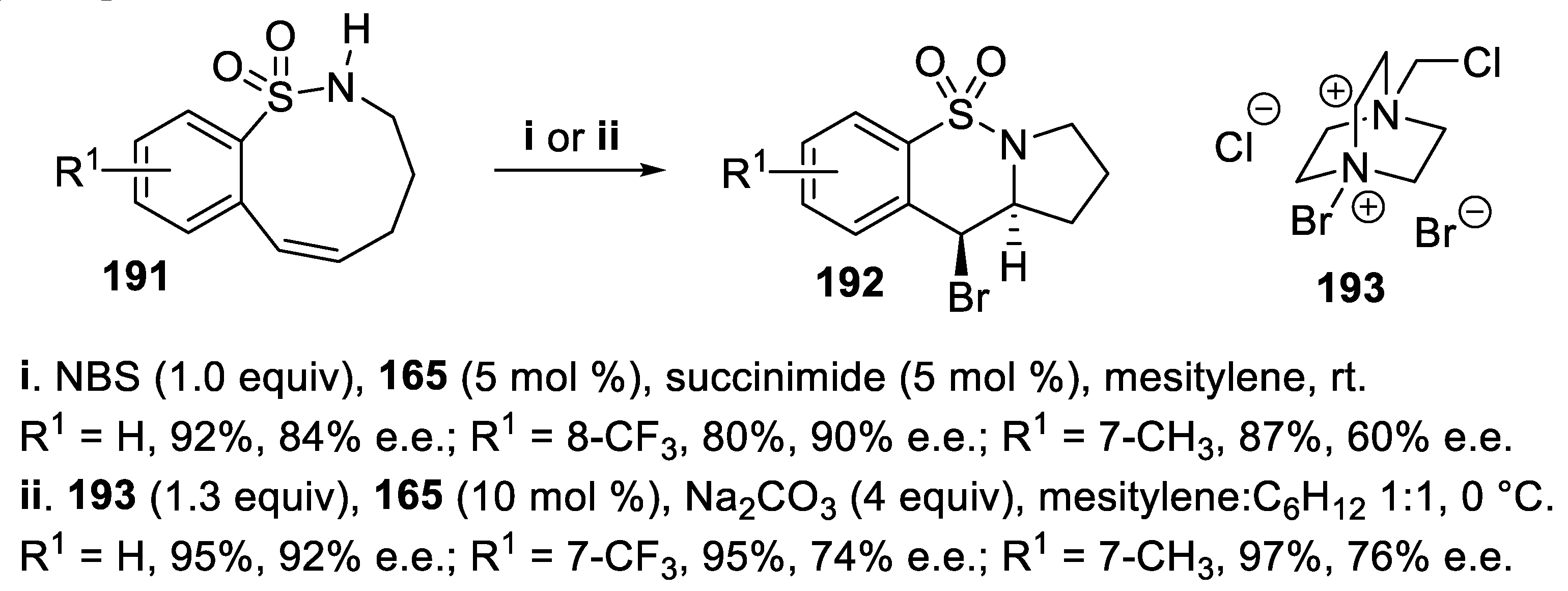
- Luis-Barrera, J.; Rodriguez, S.; Uria, U.; Reyes, E.; Prieto, L.; Carrillo, L.; Pedrón, M.; Tejero, T.; Merino, P.; Vicario, J.L. Brønsted acid versus Phase-Transfer Catalysis in the enantioselective transannular aminohalogenation of enesultams. Chem. Eur. J., 2022, 28, e202202267. [Google Scholar] [CrossRef]
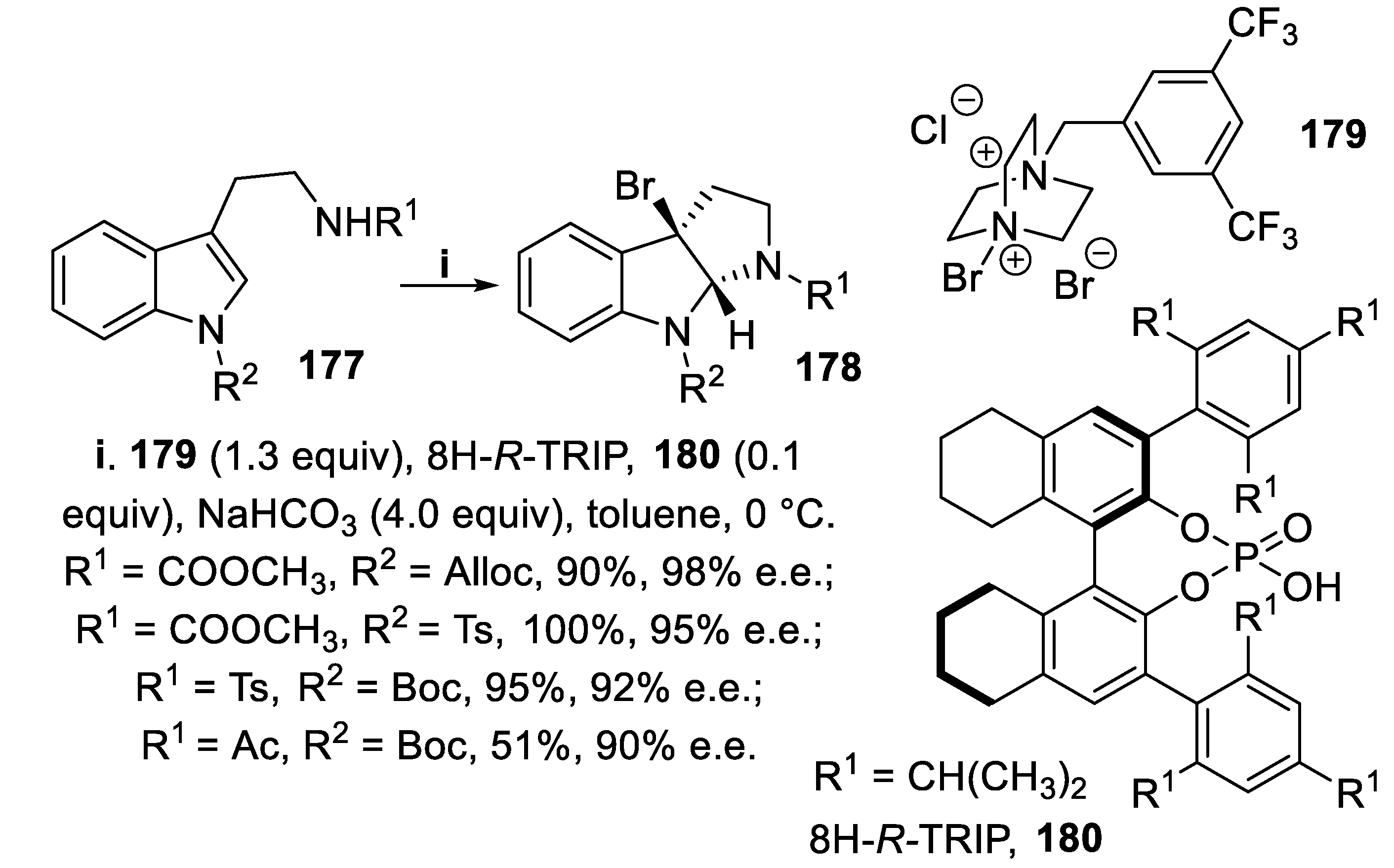
- Liu, K.; Jiang, H.-J.; Li, N.; Li, H.; Wang, J.; Zhang, Z.-Z.; Yu, J. Enantioselective bromocyclization of tryptamines induced by chiral Co(III)-complex-templated Brønsted acids under an air atmosphere. J. Org. Chem., 2018, 83, 6815–6823. [Google Scholar] [CrossRef]
- . [CrossRef]
- Sun, T.-T.; Liu, K.; Zhang, S.-X.; Wang, C.-R.; Yao, C.-Z.; Yu, J. Enantioselective halocyclization of indole derivatives: using 1,3-dihalohydantoins with anionic chiral Co(III) complexes. Synlett, 2021, 32, 701–707. [Google Scholar] [CrossRef]
- Wang, H.; Zhong, H.; Xu, X.; Xu, W.; Jiang, X. Catalytic enantioselective bromoaminocyclization and bromocycloetherification. Adv. Synth. Catal., 2020, 362, 5358–5362. [Google Scholar] [CrossRef]
- Bolognesi, M.L.; Bartolini, M.; Cavalli, A.; Andrisano, V.; Rosini, M.; Minarini, A.; Melchiorre, C. Design, synthesis, and biological evaluation of conformationally restricted rivastigmine analogues. J. Med. Chem., 2004, 47, 5945–5953. [Google Scholar] [CrossRef] [PubMed]
- . [CrossRef]
- Ward, R.S. Non-enzymatic asymmetric transformations involving symmetrical bifunctional compounds. Chem. Soc. Rev., 1990, 19, 1–19. [Google Scholar] [CrossRef]
- Magnuson, S.R. Two-directional synthesis and its use in natural product synthesis. Tetrahedron, 1995, 51, 2167–2213. [Google Scholar] [CrossRef]
- Willis, M.C. Enantioselective desymmetrisation. J. Chem. Soc. Perkin Trans. 1, 1999, 1765-1784. [CrossRef]
- García-Urdiales, E.; Alfonso, I.; Gotor, V. Enantioselective enzymatic desymmetrizations in organic synthesis. Chem. Rev., 2005, 105, 313–354. [Google Scholar] [CrossRef]
- Wang, M.; Feng, M.; Tang, B.; Jiang, X. Recent advances of desymmetrization protocol applied in natural product total synthesis. Tetrahedron Lett., 2014, 55, 7147–7155. [Google Scholar] [CrossRef]
- Borissov, A.; Davies, T.Q.; Ellis, S.R.; Fleming, T.A.; Richardson, M.S.W.; Dixon, D.J. Organocatalytic enantioselective desymmetrisation. Chem. Soc. Rev., 2016, 45, 5474–5540. [Google Scholar] [CrossRef]
- Zeng, X.-P.; Cao, Z.-Y.; Wang, Y.-H.; Zhou, F.; Zhou, J. Catalytic enantioselective desymmetrization reactions to all-carbon quaternary stereocenters. Chem. Rev., 2016, 116, 7330–7396. [Google Scholar] [CrossRef]
- Long, H.-J.; Li, Y.-L.; Zhang, B.-Q.; Xiao, W.-Y.; Zhang, X.-Y.; He, L.; Deng, J. Asymmetric bromoaminocyclization and desymmetrization of cyclohexa-1,4-dienes through anion phase-transfer catalysis. Org. Lett., 2021, 23, 8153–8157. [Google Scholar] [CrossRef]
- . [CrossRef]
- Jin, Z. Amaryllidaceae and Sceletium alkaloids. Nat. Prod. Rep., 2009, 26, 363–381. [Google Scholar] [CrossRef] [PubMed]
- Das, M.K.; De, S.; Shubhashish; Bisai, A. Concise total syntheses of (±)mesembrane and (±)-crinane. Org. Biomol. Chem., 2015, 13, 3585–3588. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Tay, D.W.; Chen, J.; Leung, G.Y.C.; Yeung, Y.-Y. Enantioselective synthesis of 2-substituted and 3-substituted piperidines through a bromoaminocyclization process. Chem. Commun., 2013, 49, 4412–4414. [Google Scholar] [CrossRef] [PubMed]
- Lindström, E.; von Mentzer, B.; Påhlman, I.; Ahlstedt, I.; Uvebrant, A.; Kristensson, E.; Martinsson, R.; Novén, A.; de Verdier, J.; Vauquelin, G. Neurokinin 1 receptor antagonists: correlation between in vitro receptor interaction and in vivo efficacy. J. Pharmacol. Exp. Ther., 2007, 322, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhou, P.; Liu, X.; Zhao, J.; Lin, L.; Feng, X. Diastereoselectively switchable asymmetric haloaminocyclization for the synthesis of cyclic sulfamates. Chem. Eur. J., 2015, 21, 6386–6389. [Google Scholar] [CrossRef] [PubMed]
- Rueping, M.; Maji, M.S.; Küçük, H.B.; Atodiresei, I. Asymmetric Brønsted acid catalyzed cycloadditions - Efficient enantioselective synthesis of pyrazolidines, pyrazolines, and 1,3-diamines from N-acyl hydrazones and alkenes. Angew. Chem. Int. Ed., 2012, 51, 12864–12868. [Google Scholar] [CrossRef] [PubMed]
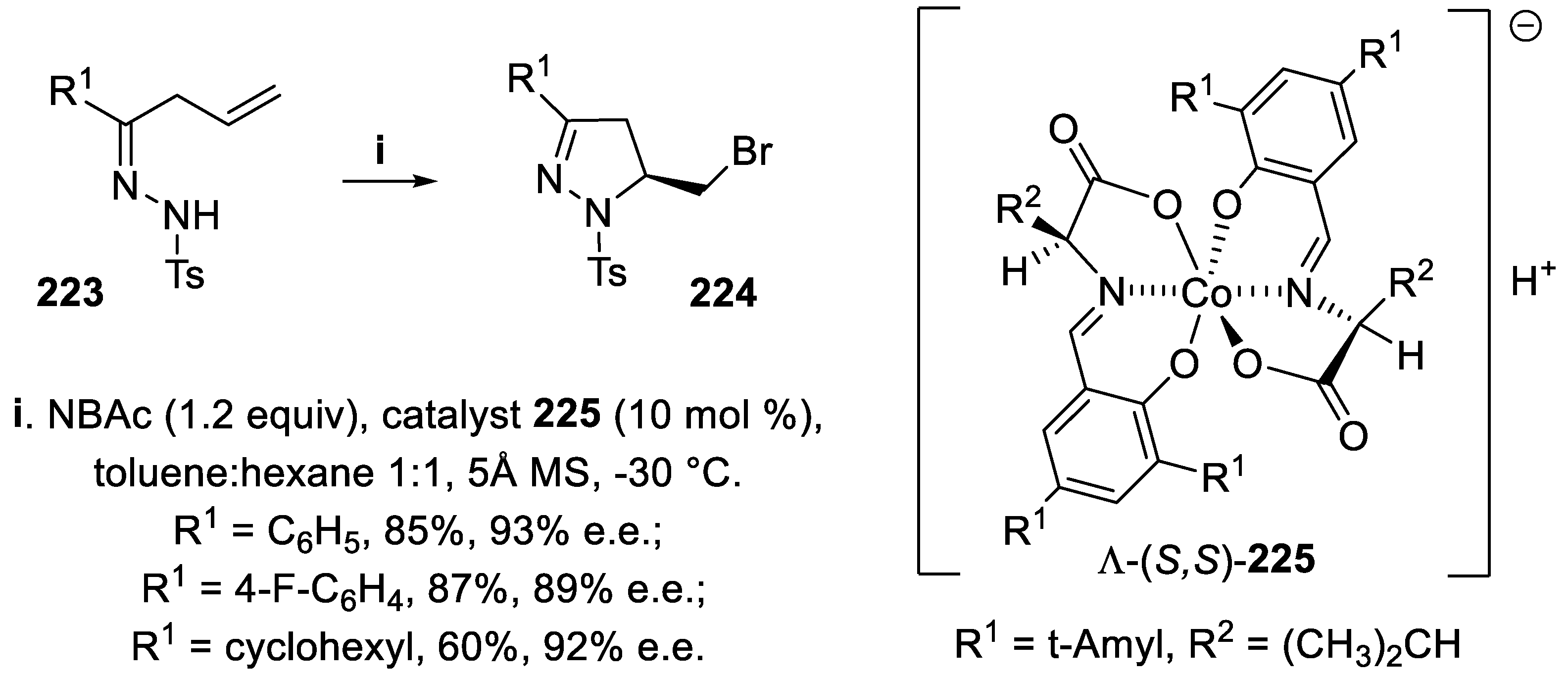
- Wu, X.-B.; Gao, Q.; Fan, J.-J.; Zhao, Z.-Y.; Tu, X.-Q.; Cao, H.-Q.; Yu, J. ; Yu, J. Anionic chiral Co(III) complexes mediated asymmetric halocyclization - Synthesis of 5-halomethyl pyrazolines and isoxazolines. Org. Lett., 2021, 23, 9134–9139. [Google Scholar]
- . [CrossRef]
- Sebastian, J. Dihydropyrazole and dihydropyrrole structures based design of Kif15 inhibitors as novel therapeutic agents for cancer. Comput. Biol. Chem., 2017, 68, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Roecker, A.J.; Coleman, P.J.; Mercer, S.P.; Schreier, J.D.; Buser, C.A.; Walsh, E.S.; Hamilton, K.; Lobell, R.B.; Tao, W.; Diehl, R.E.; South, V.J.; David, J.P.; Kohl, N.E.; Yan, Y.; Kuo, L.C.; Li, C.; Fernandez-Metzler, C.; Mahan, E.A.; Prueksaritanont, T.; Hartman, G.D. Kinesin spindle protein (KSP) inhibitors. Part 8: Design and synthesis of 1,4-diaryl-4,5-dihydropyrazoles as potent inhibitors of the mitotic kinesin KSP. Bioorg. Med. Chem. Lett., 2007, 17, 5677–5682. [Google Scholar] [CrossRef]
- Tripathi, C.B.; Mukherjee, S. Catalytic enantioselective iodoaminocyclization of hydrazones. Org. Lett., 2014, 16, 3368–3371. [Google Scholar] [CrossRef]
- Tripathi, C.B.; Mukherjee, S. Catalytic enantioselective 1,4-iodofunctionalizations of conjugated dienes. Org. Lett., 2015, 17, 4424–4427. [Google Scholar] [CrossRef] [PubMed]
- Coleman, P.J.; Schreier, J.D.; Cox, C.D.; Fraley, M.E.; Garbaccio, R.M.; Buser, C.A.; Walsh, E.S.; Hamilton, K.; Lobell, R.B.; Rickert, K.; Tao, W.; Diehl, R.E.; South, V.J.; David, J.P.; Kohl, N.E.; Yan, Y.; Kuo, L.; Prueksaritanont, T.; Li, C.; Mahan, E.A.; Fernandez- Metzler, C.; Salata, J.J.; Hartman, G.D. Kinesin spindle protein (KSP) inhibitors. Part 6: Design and synthesis of 3,5-diaryl-4,5-dihydropyrazole amides as potent inhibitors of the mitotic kinesin KSP. Bioorg. Med. Chem. Lett., 2007, 17, 5390–5395. [Google Scholar] [CrossRef] [PubMed]
- Richter, H.G.F.; Freichel, C.; Huwyler, J.; Nakagawa, T.; Nettekoven, M.; Plancher, J.-M.; Roche, S.O.; Schuler, F.; Taylor, S.; Ullmer, C.; Wiegand, R. Discovery of potent and selective histamine H3 receptor inverse agonists based on the 3,4-dihydro-2H-pyrazino [1,2-a]indol-1-one scaffold. Bioorg. Med. Chem. Lett., 2010, 20, 5713–5717. [Google Scholar] [CrossRef] [PubMed]
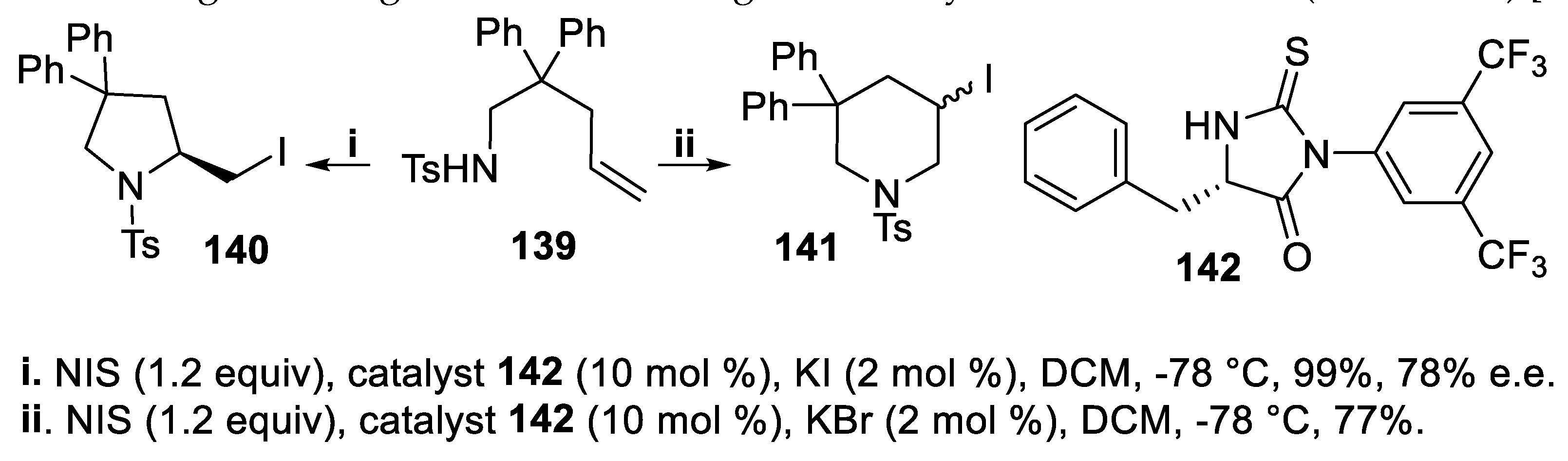
- Cheng, Y.A.; Yu, W.Z.; Yeung, Y.-Y. Carbamate-catalyzed enantioselective bromolactamization. Angew. Chem. Int. Ed., 2015, 54, 12102–12106. [Google Scholar] [CrossRef] [PubMed]
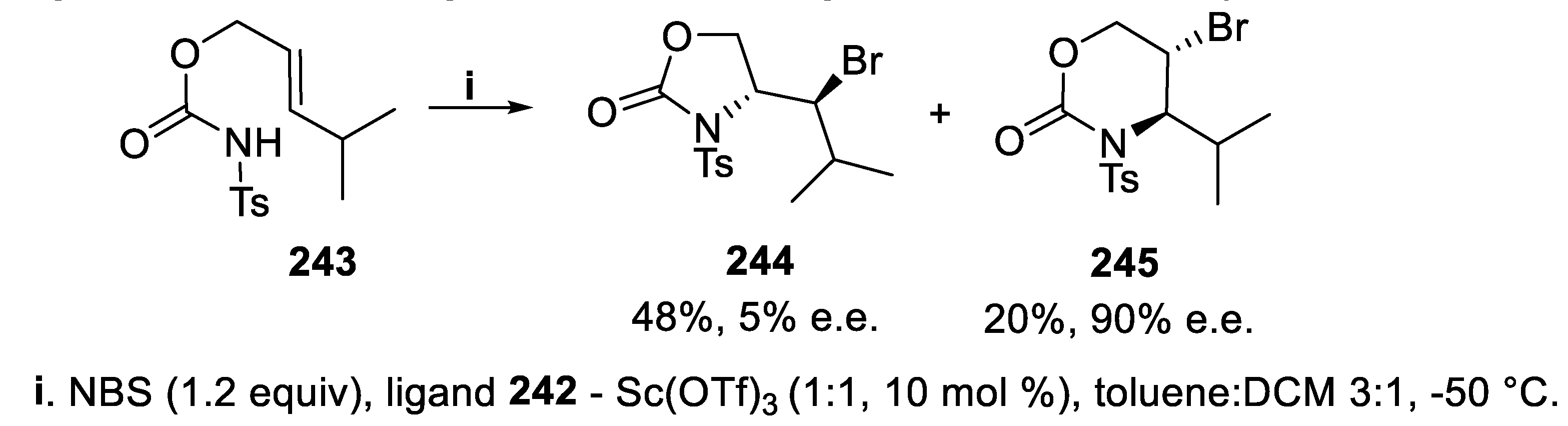
- Huang, D.; Liu, X.; Li, L.; Cai, Y.; Liu, W.; Shi, Y. Enantioselective bromoaminocyclization of allyl N-tosylcarbamates catalyzed by a chiral phosphine–Sc(OTf)3 complex. J. Am. Chem. Soc., 2013, 135, 8101–8104. [Google Scholar] [CrossRef] [PubMed]
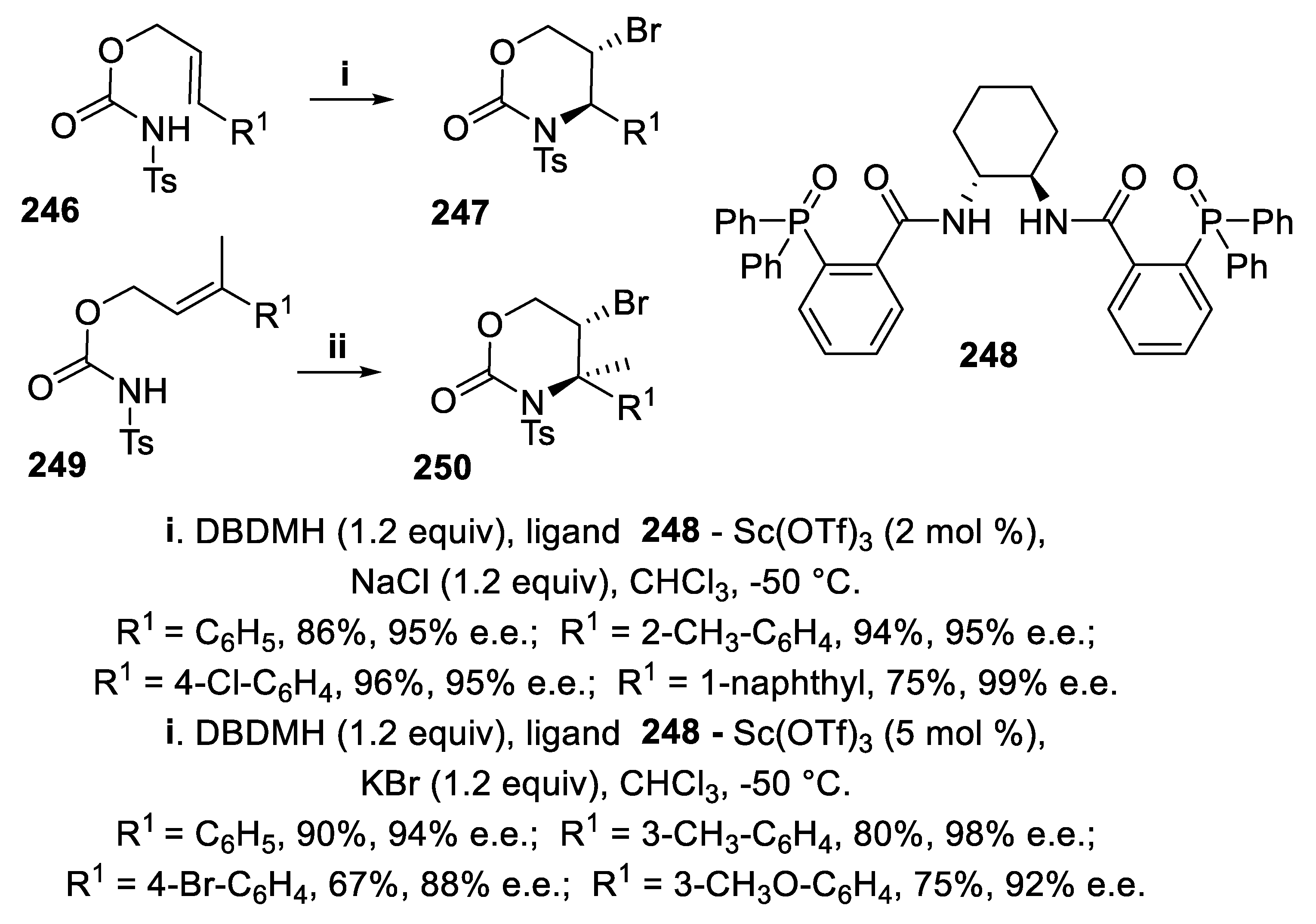
- Pan, H.; Huang, H.; Liu, W.; Tian, H.; Shi, Y. Phosphine oxide–Sc(OTf)3 catalyzed highly regio- and enantioselective bromoaminocyclization of (E)-cinnamyl tosylcarbamates. An approach to a class of synthetically versatile functionalized molecules. Org. Lett., 2016, 18, 896–899. [Google Scholar] [CrossRef]
- Pan, H.; Tian, H.; Shi, Y. Phosphine oxide-Sc(OTf)3 catalyzed enantioselective bromoaminocyclization of tri-substituted allyl N-tosylcarbamates. Sci. China Chem., 2018, 61, 656–659. [Google Scholar] [CrossRef]
- Huang, H.; Pan, H.; Cai, Y.; Liu, M.; Tian, H.; Shi, Y. Enantioselective 6-endo bromoaminocyclization of 2,4-dienyl N-tosylcarbamates catalyzed by a chiral phosphine oxide-Sc(OTf)3 complex. A dramatic additive effect. Org. Biomol. Chem., 2015, 13, 3566–3570. [Google Scholar] [CrossRef] [PubMed]
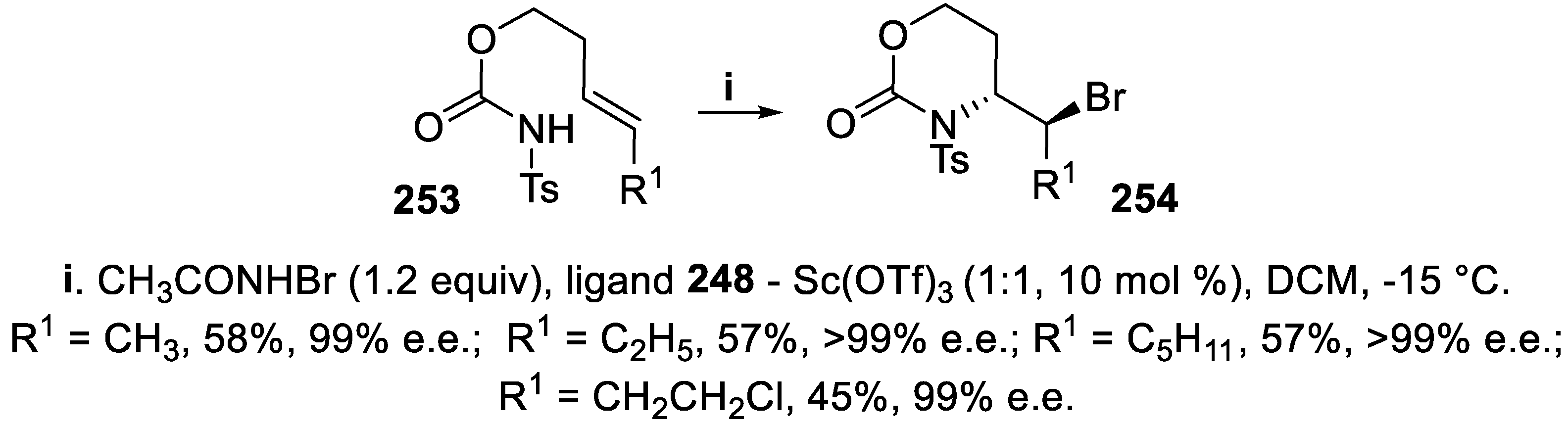
- Liu, W.; Pan, H.; Tian, H.; Shi, Y. Enantioselective 6-exo-bromoaminocyclization of homoallylic N-tosylcarbamates catalyzed by a novel monophosphine-Sc(OTf)3 complex. Org. Lett., 2015, 17, 3956–3959. [Google Scholar] [CrossRef]
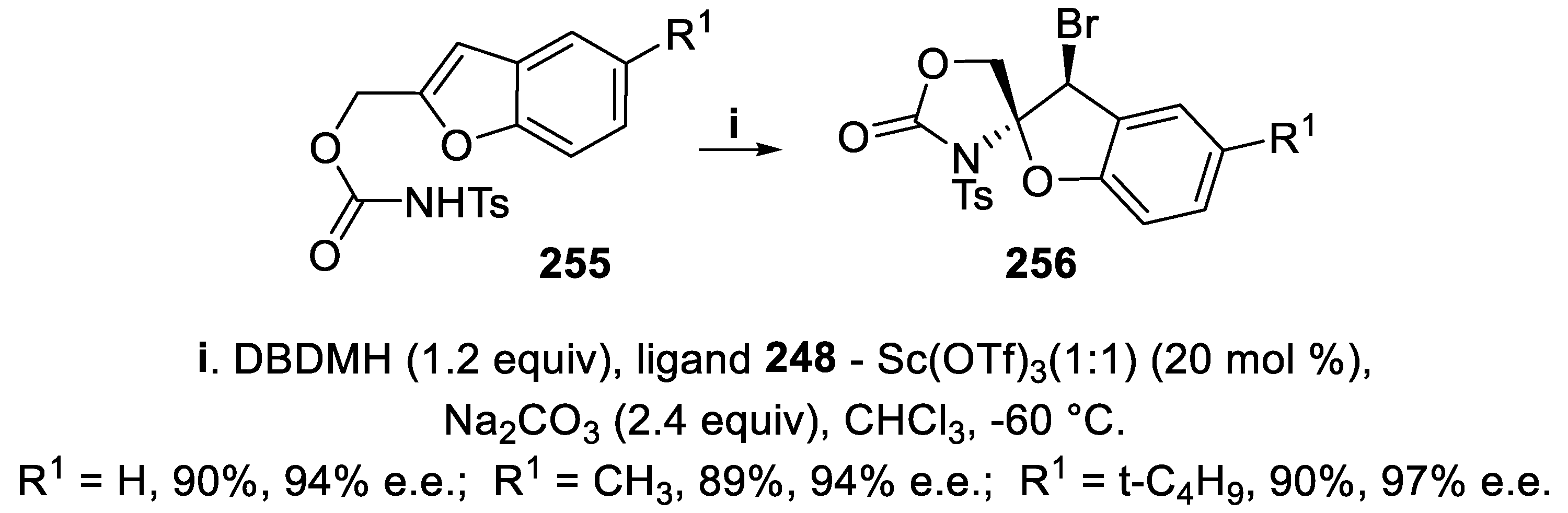
- Li, Z.; Shi, Y. Chiral phosphine oxide–Sc(OTf)3 complex catalyzed enantioselective bromoaminocyclization of 2-benzofuranylmethyl N-tosylcarbamates. Approach to a novel class of optically active spiro compounds. Org. Lett., 2015, 17, 5752–5755. [Google Scholar] [CrossRef]
- . [CrossRef]
- Struble, T.J.; Lankswert, H.M.; Pink, M.; Johnston, J.N. Enantioselective organocatalytic amine-isocyanate capture-cyclization: regioselective alkene iodoamination for the synthesis of chiral cyclic ureas. ACS Catal., 2018, 8, 11926–11931. [Google Scholar] [CrossRef] [PubMed]
- . [CrossRef]
- Shue, H.-J.; Chen, X.; Schwerdt, J.H.; Paliwal, S.; Blythin, D.J.; Lin, L.; Gu, D.; Wang, C.; Reichard, G.A.; Wang, H.; Piwinski, J.J.; Duffy, R.A.; Lachowicz, J.E.; Coffin, V.L.; Nomeir, A.A.; Morgan, C.A.; Varty, G.B.; Shih, N.-Y. Cyclic urea derivatives as potent NK1 selective antagonists.Part II: Effects of fluoro and benzylic methyl substitutions. Bioorg. Med. Chem. Lett., 2006, 16, 1065–1069. [Google Scholar] [CrossRef] [PubMed]
- Naapuri, J.; Rolfes, J.D.; Keil, J.; Manzuna Sapu, C.; Deska, J. Enzymatic halocyclization of allenic alcohols and carboxylates: a biocatalytic entry to functionalized O-heterocycles. Green Chem., 2017, 19, 447–452. [Google Scholar] [CrossRef]
- Younes, S.H.H.; Tieves, F.; Lan, D.; Wang, Y.; Süss, P.; Brundiek, H.; Wever, R.; Hollmann, F. Chemoenzymatic halocyclization of γ,δ-unsaturated carboxylic acids and alcohols. ChemSusChem, 2020, 13, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Hoefler, G.T.; But, A.; Younes, S.H.H.; Wever, R.; Paul, C.E.; Arends, I.W.C.E.; Hollmann, F. Chemoenzymatic halocyclization of 4-pentenoic acid at preparative scale. ACS Sustainable Chem. Eng., 2020, 8, 2602–2607. [Google Scholar] [CrossRef]
- . [CrossRef]
- Mondal, D.; Fisher, B.F.; Jiang, Y.; Lewis, J.C. Flavin-dependent halogenases catalyze enantioselective olefin halocyclization. Nat. Commun., 2021, 12, 3268. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Mondal, D.; Lewis, J.C. Expanding the reactivity of flavin-dependent halogenases toward olefins via enantioselective intramolecular haloetherification and chemoenzymatic oxidative rearrangements. ACS Catal., 2022, 12, 13501–13505. [Google Scholar] [CrossRef] [PubMed]
- . [CrossRef]
- Jiang, Y.; Lewis, J.C. Asymmetric catalysis by flavin-dependent halogenases. Chirality, 2023, 35, 452–460. [Google Scholar] [CrossRef] [PubMed]
- . [CrossRef]
- Murray, J.; Hodgson, D.R.W.; O’Donoghue, A.C. Going full circle with organocatalysis and biocatalysis: the latent potential of cofactor mimics in asymmetric synthesis. J. Org. Chem., 2023, 88, 7619–7629. [Google Scholar] [CrossRef]
- Qi, C.; Force, G.; Gandon, V.; Leboeuf, D. Hexafluoroisopropanol-promoted haloamidation and halolactonization of unactivated alkenes. Angew. Chem. Int. Ed., 2021, 60, 946–953. [Google Scholar] [CrossRef]
- Stini, N.A.; Gkizis, P.L.; Kokotos, C.G. Cyrene: a bio-based novel and sustainable solvent for organic synthesis. Green Chem., 2022, 24, 6435–6449. [Google Scholar] [CrossRef]
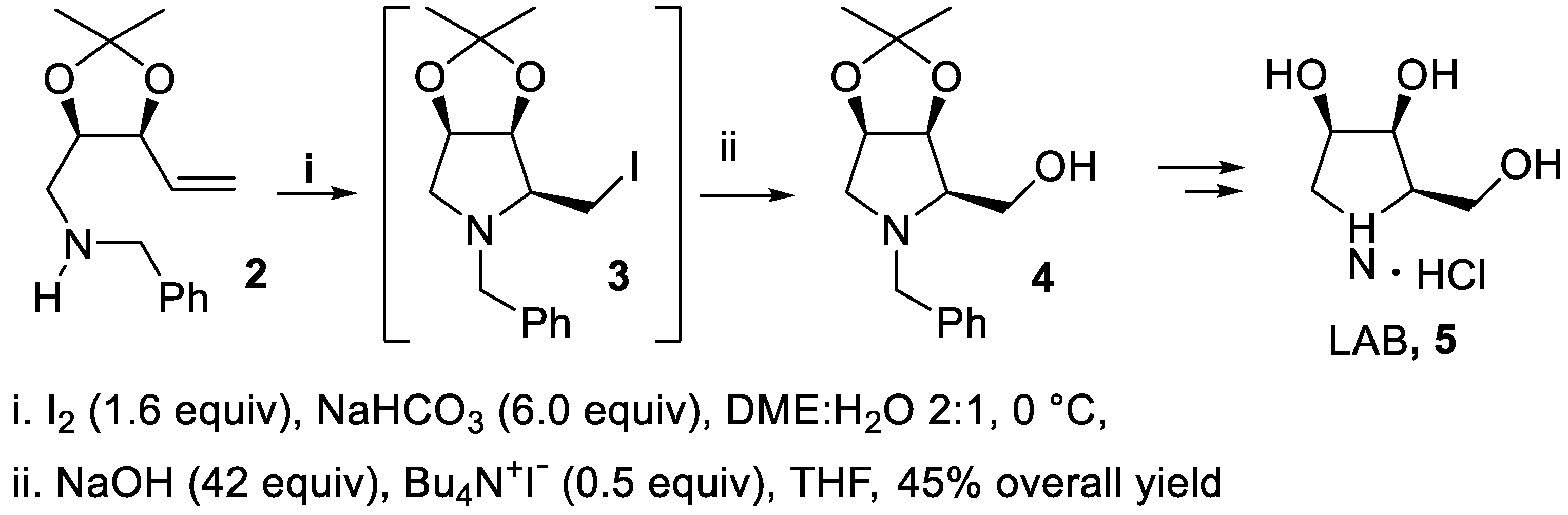
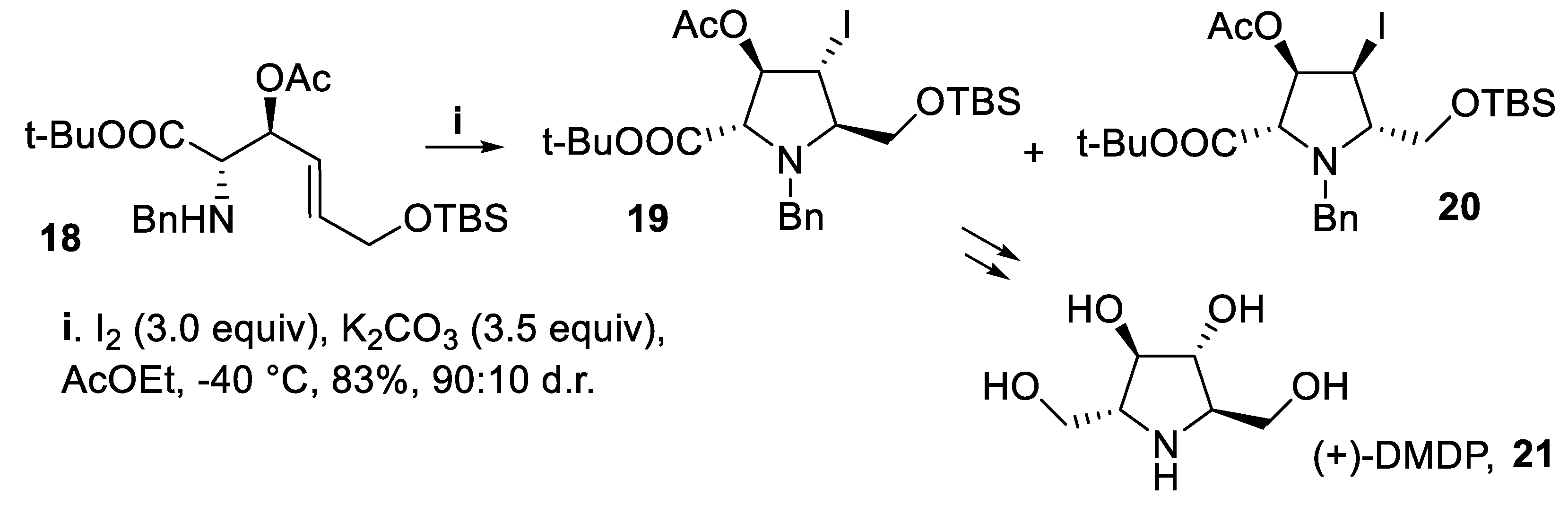













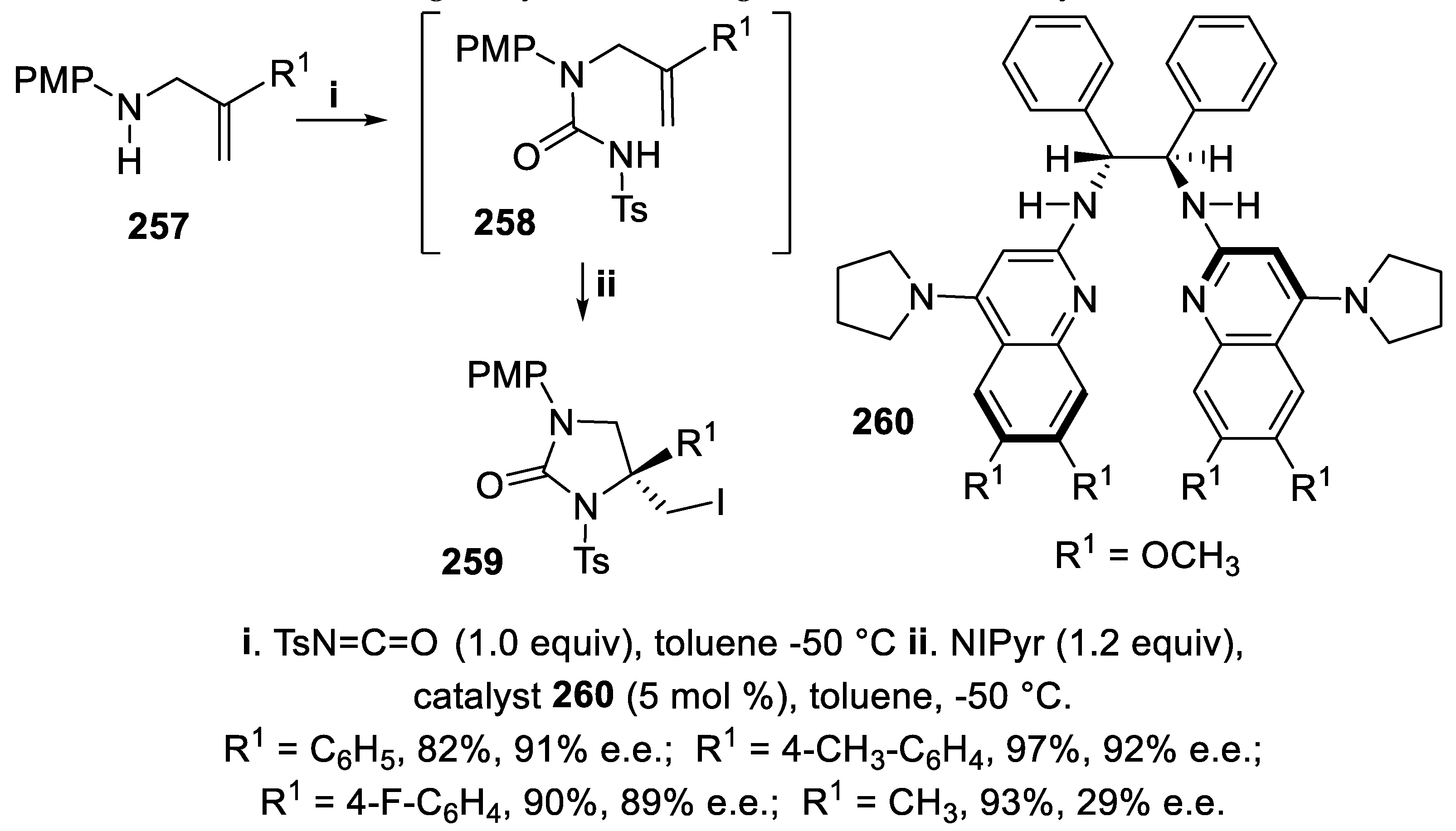
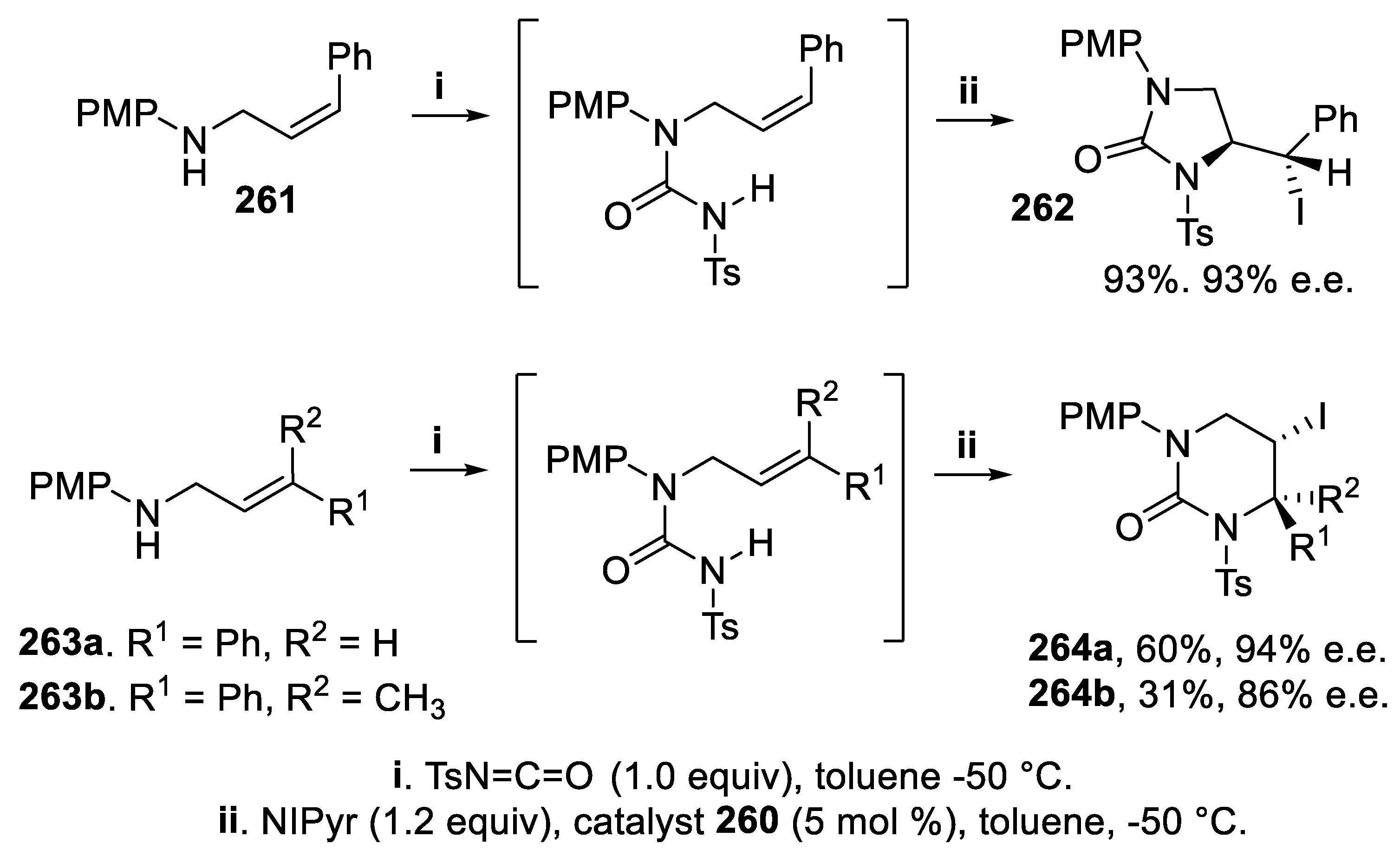
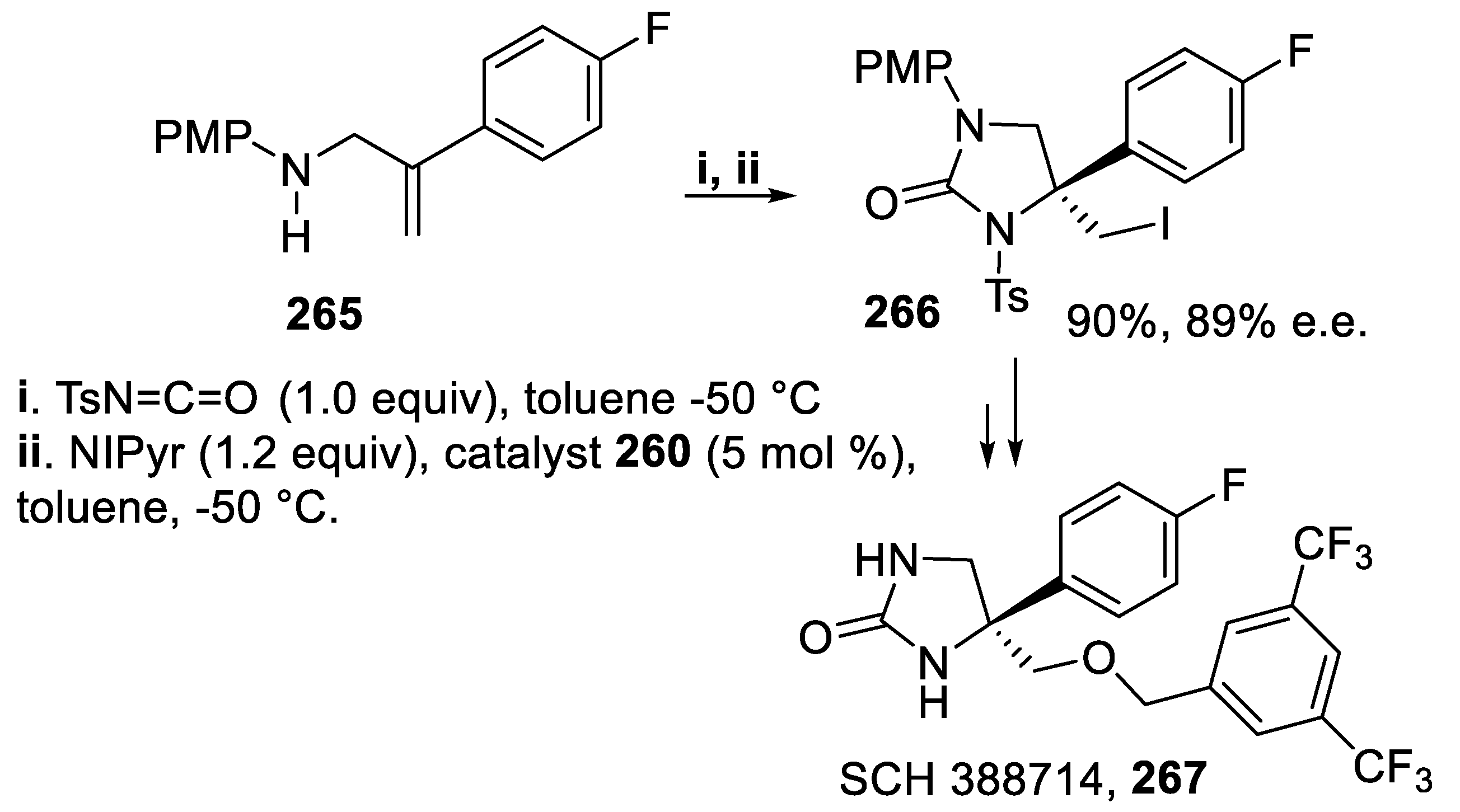
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).