1. Introduction
The prevalence of overweight and obesity is a growing public health problem worldwide (1,2). This pathophysiological condition is associated with metabolic disturbance and increased insulin resistance (3). Moreover, during mid-pregnancy, placental hormones change maternal physiology to achieve a state of insulin resistance to support fetal growth. Thus, starting pregnancy with pre-existing obesity-related insulin resistance poses an increased risk of developing GDM (4). It is well documented that both GDM and obesity are independently associated with adverse maternal and infant outcomes such as pre-eclampsia, emergency caesarean delivery, fetal macrosomia and neonatal hypoglycemia (5–8). Previous studies in our health-care area have observed a significant increase in the risk of GDM, with its associated maternal and fetal adverse events (9,10). Consequently, the development of preventive strategies become a priority.
Numerous randomized controlled trials (RCTs) have been conducted to attempt a reduction in the incidence of GDM. These trials have explored the effect of dietary modifications, physical activity, combined interventions, and medication. However, the results remain controversial (11), and very few have demonstrated efficacy in high-risk populations (12–14). Heterogeneities of the population included, the types of intervention evaluated and the time of their initiation are some of the main causes that explain the inconsistencies observed (15). Treatment of GDM has focused on proper glycemic control and adequate weight gain during pregnancy using diet and exercise (4,15,16), and, if lifestyle approaches alone are not sufficient, insulin or other antihyperglycemic drug therapies may be prescribed (17). Nutritional intervention may include a wide range of possibilities. Although no specific diet has been described for the management of GDM, previous studies have shown that large amounts of carbohydrates (CHO), especially rapidly absorbed CHO, have a negative impact on glycemic control (18–21). Therefore, individualized moderation of CHO intake is reasonable, and the focus should turn to the type of CHO, prioritizing those high in fiber.
MedDiet is high in complex CHO and healthy fats. It has demonstrated many health benefits for people with obesity, including weight loss and reduction of associated comorbidities (22–24). In this regard, the San Carlos study has demonstrated a decrease in GDM in its cohort of pregnant women using a prompt (12th GW) nutritional intervention, based on a MedDiet, with free supply of extra virgin olive oil (EVOO) and nuts (10). However, prevention strategies in the specific setting of overweight and obesity has not been fully explored.
In this context, the main aim of this study is to investigate if early nutritional treatment in overweight or obese women reduces the incidence of GDM and its associated complications, including maternal-fetal adverse effects during pregnancy and at 3 years postpartum.
2. Materials and Methods
2.1. Study Design
The Hospital Clínico San Carlos is a recognized healthcare, education and research center in Madrid, Spain. Pregnant women have their first clinical visit between 8th and 12th GW, coinciding with the first ultrasound scan. At this visit, the first trimester's blood tests are reviewed, and general recommendations are given for a good pregnancy. The oral glucose tolerance test (OGTT) to detect GDM is performed between 24-28 weeks of gestation, using the IADPSG criteria. With the aim of preventing GDM, the San Carlos study, a single-center, prospective, RCT, was launched in 2015. It included all pregnant women attending their first gestational visit between 8th and 12th GW with FBG <92 mg/dL. This cohort encompasses three main research studies that we have used in our analysis, including only women with a BMI≥ 25 kg/m2 from each of the three cohorts/studies:
The first study (Study 1) was a RCT (ISRCTN84389045, ethic code CI 13/296-E), in which women were randomly assigned before the 12th GW to either the CG or the IG. In this way, nutritional intervention could be ensured for at least 3 months. The IG was recommended a MedDiet guideline: insisting on increased consumption of EVOO (>40 ml/day) and pistachios (1 handful per day), with both foods provided free of charge. In contrast, women in the CG group were advised to limit their daily EVOO intake to less than 40 ml and to avoid eating nuts more than three times a week.
The second study (Study 2) (ISRCTN13389832, ethic code CI 16/442-E) assessed the effects of giving MedDiet-based recommendations in early pregnancy in a single group based on usual clinical practice, Real World (RW). These women were encouraged to follow the same nutritional guidelines as the RCT IG of Study 1, but without providing them with free EVOO and nuts.
And the last study (Study 3) was a RCT (ISRCTN16896947, ethic code CI 16/316) in which women with a body mass index (BMI) ≥ 25 kg/m2 were randomized into CG and IG. In this case, intervention consisted in increasing the consumption of nuts and EVOO, but only pistachios were administered free of charge, not EVOO.
A post hoc analysis of this sample was performed for women with overweight or obesity (BMI≥ 25 kg/m2). IGs from both RCTs and the group participating in the practice-based study underwent the analysis as IGs. This was because they were all encouraged to follow a MedDiet guideline, including specific dietary advice related to nuts and EVOO. These IGs were compared with the two CGs in the RCTs. Women diagnosed with GDM were closely monitored by the Department of Endocrinology and received a consistent protocolized treatment, regardless of whether they were assigned to the control or intervention group.
During the study, pregnant women were followed-up uniformly to reinforce the nutritional intervention. Three visits were made during pregnancy, coinciding with the third, fifth and ninth month of gestation. At 3 months postpartum, another motivational interview was conducted to encourage all patients, regardless of the group they belonged to, to follow the previously proposed nutritional recommendations. After 3 years of the study, patients were invited again for a voluntary follow-up visit to evaluate the results. However, several women refused to participate for different reasons, such as being pregnant again or health problems, among others.
2.2. Patients
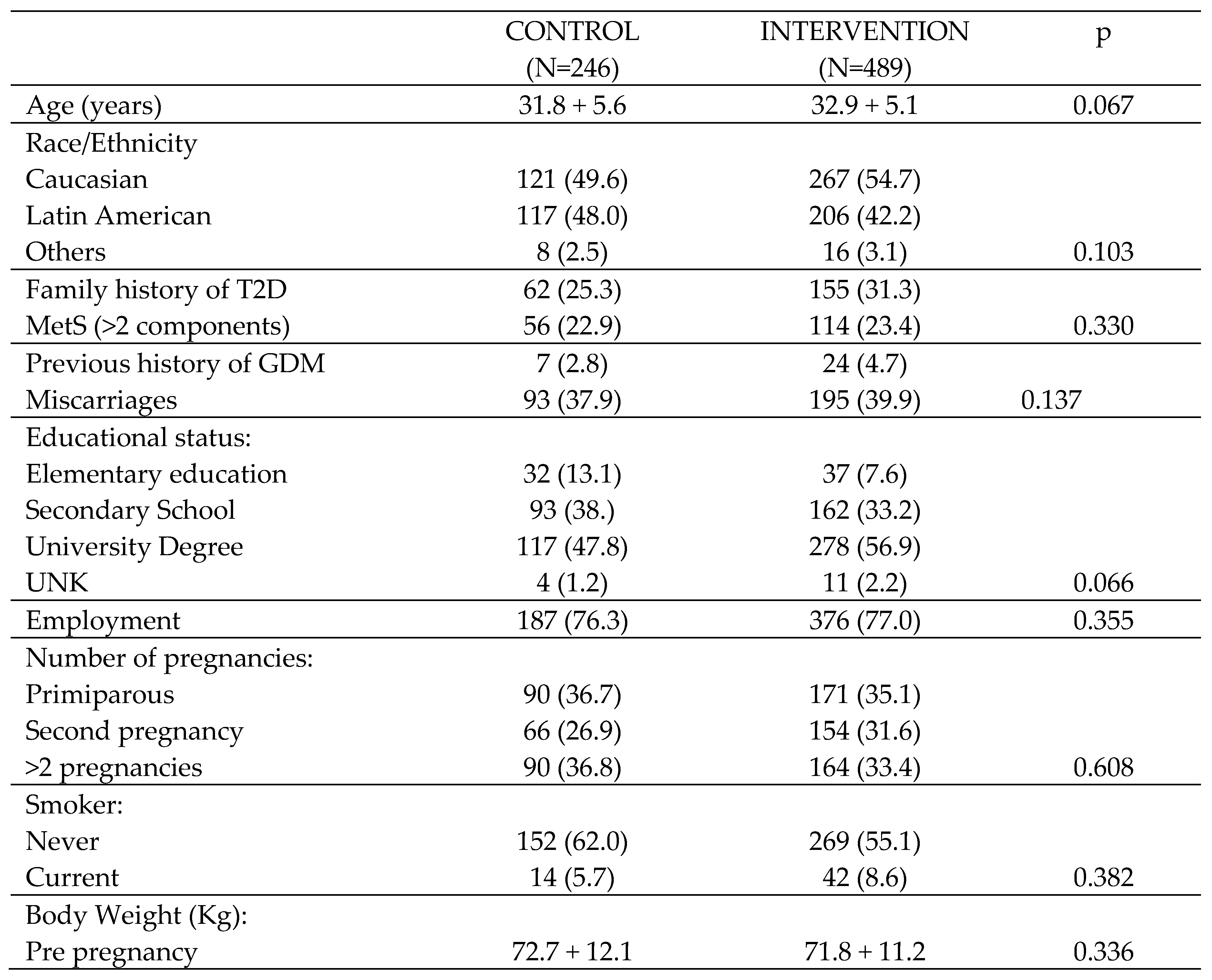
A total of 735 pregnant women: 246/CG (144/RCT + 102/pistachios) and 489/IG (120 RCT + 162 RW + 207 Pistachios) were included in the present study. All agreed to participate in the study and signed the informed consent. Women’s demographic and clinical characteristics at baseline can be seen in
Table 1.
2.3. Studied Variables:
2.3.1. Clinical and Demographic Characteristics:
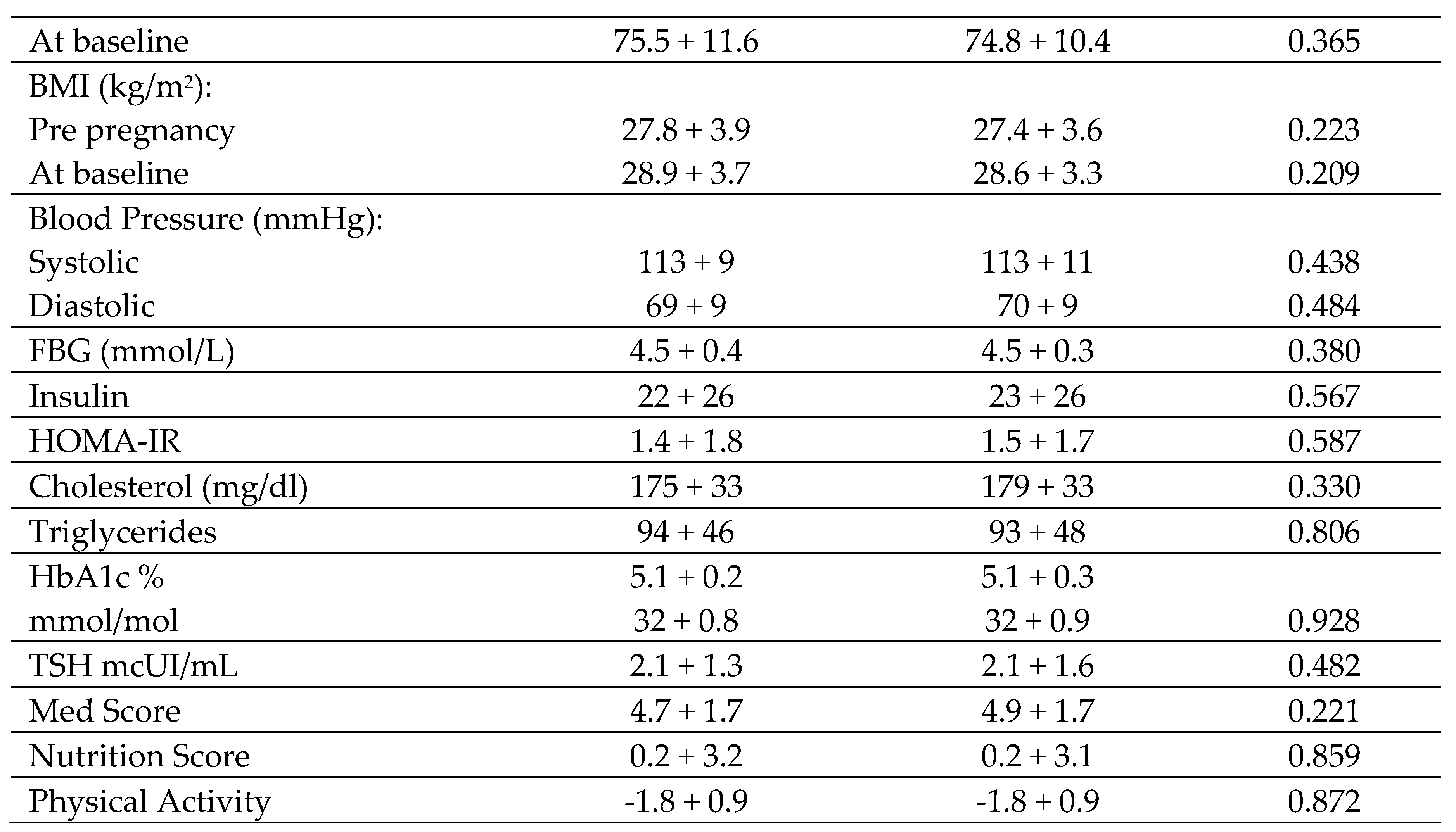
At the first visit, a brief medical history was taken, including age, ethnicity (Caucasian, Latin American or others), family history of Type 2 diabetes mellitus (T2DM), having more than two components of the MetS (BMI≥30 kg/m2, WC ≥89.5 cm, systolic blood pressure (SBP)≥ 130 mm Hg, diastolic blood pressure (DBP) ≥85 mmHg, triglycerides (TG) ≥150 mg, HDL-Chol <50 mg/dL, abnormal glucose regulation (AGR) and HOMA-IR ≥ 3.5), previous miscarriages, educational level (elementary education, secondary school, university degree), employment status, number of previous pregnancies and smoking habits (never or currently). Anthropometric data such as weight (kg) and BMI (kg/m2) were also collected, and blood pressure was measured using a digital sphygmomanometer (Omron 705IT, Omron Global, Kyoto, Japan). In patients who were followed up at 3 years postpartum, it was possible to analyze body composition by means of electrical bioimpedance (SECA mBCA 514), obtaining weight (kg), fat mass (FF) (kg) and BMI (kg/m2). WC was measured with a non-stretch tape measure using ISAK criteria.
2.3.2. Laboratory Parameters:
Blood and urine samples were obtained after an overnight fast of at least 8 hours. Fasting blood glucose (mmol/L) was measured in serum by the Glucose-Hexokinase method in an AU5800-Beckman Diagnostic. To measure HbA1c as a percentage, ion exchange high-performance liquid chromatography (HPLC) was used with a Tosoh G8 analyzer (Tosoh Co., Tokyo, Japan). The method is standardized against the International Federation of Clinical Chemistry and has an imprecision of 1.23% for values of 32.23 mmol/mol (5.1% NGSP) and 1.36% for values of 85.24 mmol/mol (10% NGSP). To measure fasting serum insulin (FSI), a chemiluminescence immunoassay on an IMMULITE 2000 Xpi (Siemens, Healthcare Diagnostics, Munich, Germany) was used, with an imprecision of 21 IUU/mL concentrations of 5.91%. Homeostasis for insulin resistance (HOMA) was calculated as glucose (mmol/L) × insulin (μIU/mL)/22.7. Total cholesterol was quantitatively assessed using the colorimetric enzymatic test method (CHOD-PAP). In a similar way, the colorimetric enzymatic method using glycerol phosphate oxidase p-amino phenazone (GPO-PAP) was used to determine serum TG. Fasting serum insulin (FSI) levels were determined using a chemiluminescence immunoassay performed on an IMMULITE 2000 Xpi analyzer from Siemens Healthcare Diagnostics in Munich, Germany. The inter-assay accuracy was 6.3% for concentrations of 11 uIU/mL and 5.91% for insulin concentrations of 21uIU/mL. Thyroid-stimulating hormone (TSH) levels were assessed using a third-generation sandwich-chemiluminescence immunoassay with magnetic particles. This method employed human TSH mouse monoclonal antibodies and the DXI-800® analyzer from Beckman–Coulter. The manufacturer’s specified reference range for TSH in non-pregnant adults is 0.38–5.33 μIU/mL, with a sensitivity of 0.01 μIU/mL. The intraassay coefficient of variation (CV) is less than 10%, and the TSH measurement range spans from 0.01 to 50.0 μIU/mL. Intra-assay CVs are 4.9% for a concentration of 0.69 μIU/mL, 5,8% for 5.47 μIU/mL, and 6.2% for 29 μIU/mL. In addition, to ensure the quality of the procedures, an External Quality Assurance Programme of the SEQC (Spanish Society of Clinical Chemistry) evaluates the methods monthly and performs a review of the methods.
2.3.3. Lifestyle and Data Collection:
The Mediterranean diet adherence screener (MEDAS), a validated semiquantitative questionnaire derived from the PREDIMED study, was used to evaluate the nutritional intervention and adherence to the MedDiet (25). A score above 5 indicated adequate adherence, although the aim was to achieve a minimum score of 7. The second questionnaire applied was the Nutrition and Diabetes Complications Trial (DNCT), which more precisely reflects the consumption of specific food groups per week, as well as the physical activity carried out on a regular basis. This questionnaire evaluates 15 items: three of them focused on physical activity and the remaining twelve on diet. Factors that prevent T2DM were given an A (+1 point); neutral factors were given a B, which do not prevent T2DM but do not increase the risk (+0 points); and a C was given when the factor increased the risk of developing T2DM (-1 point). Further details of the assessment are given in the pilot study (26).
2.4. Statistical Analysis
Categorical variables are represented as percentages, while continuous variables are expressed as median (standard deviation) and interquartile range (IQR). Differences between groups were compared using X2 tests, Student's t-test, or the Mann-Whitney U-test, depending on the type of variable and its normal or non-normal distribution. Logistic regression was used to assess the impact of nutritional treatment on adverse maternal, neonatal and delivery outcomes that showed significant differences in univariate analysis. The control group served as the reference group. Relative risk (RR) was adjusted, and the 95% confidence interval (95% CI) was calculated considering age, parity, and BMI. All p-values were two-tailed and were considered statistically significant if less than 0.05. These analyses were performed using SPSS software, version 21, based in Chicago, IL, USA.
3. Results
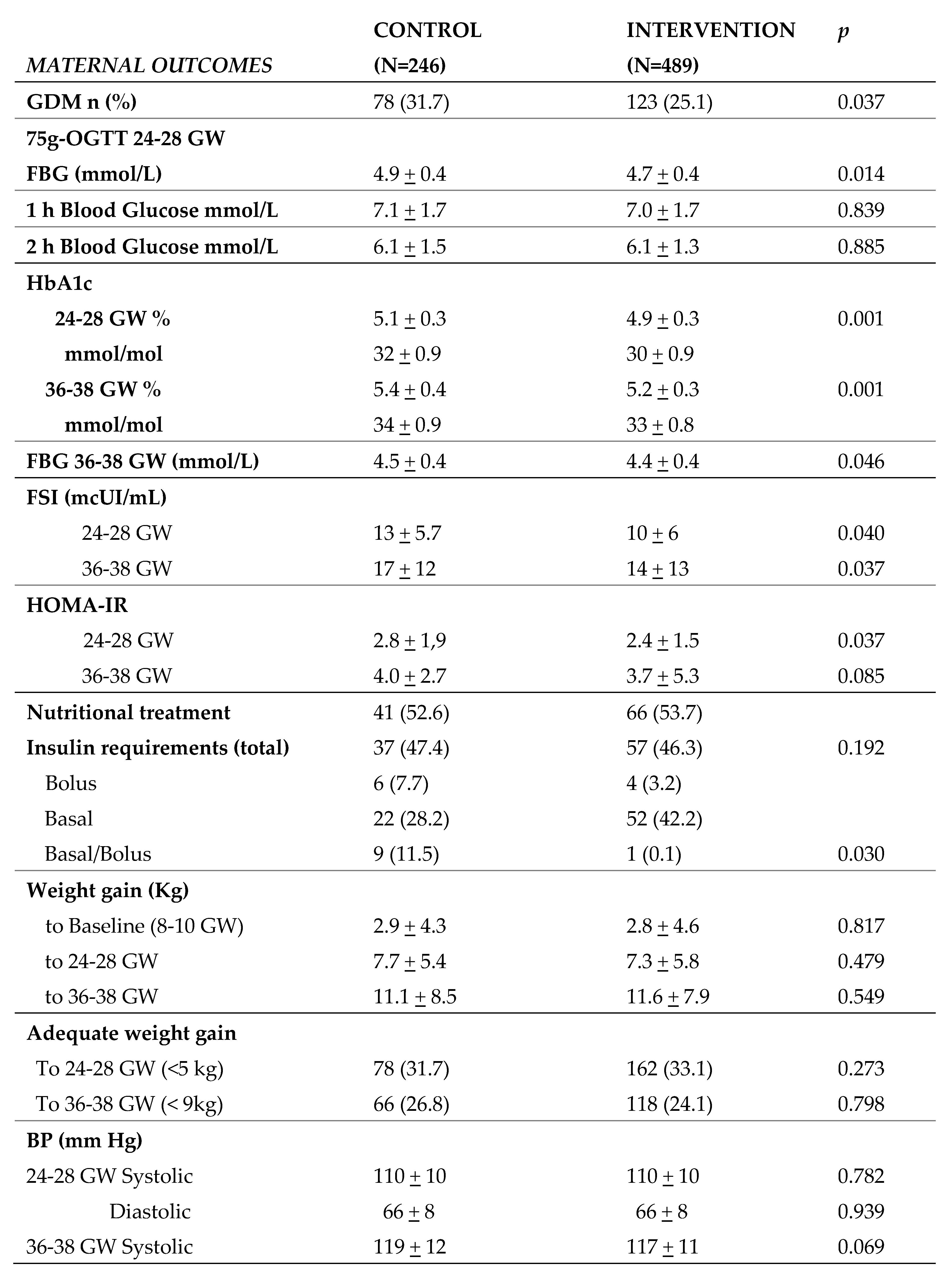
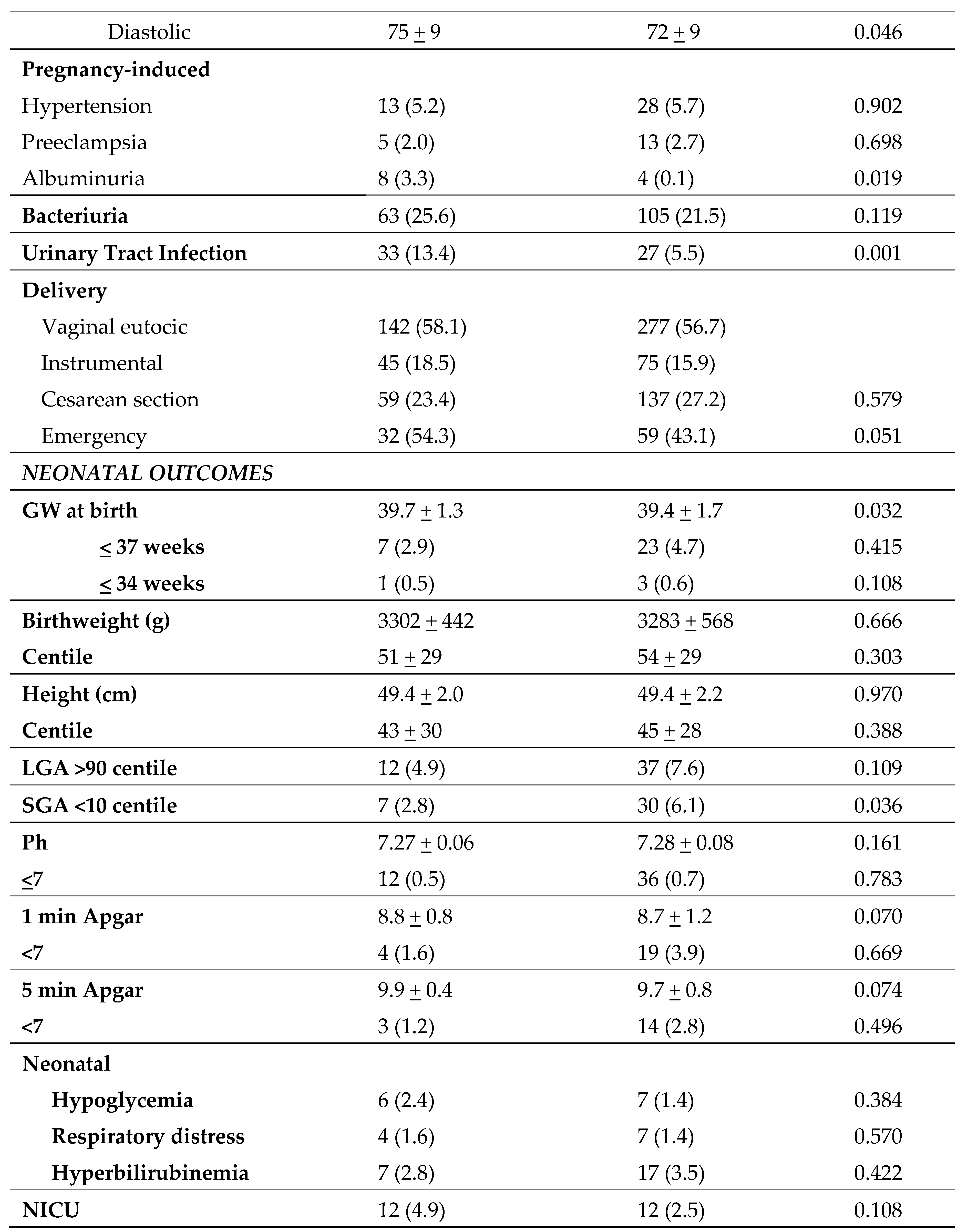
Table 2 shows maternal and fetal outcomes during pregnancy in the CG and IG. The rate of GDM was lower in the CG (31.7%) in comparison to the IG (25.1%) (p=0.037), which was associated with lower fasting basal glucose. HbA1c was also lower in both the second and third trimesters of pregnancy (5.1 vs 4.9; p=0.001 and 5.4 vs 5.2; p=0.001, respectively), although neither value conferred a diagnosis of pre-diabetes (HbA1c≥5.7%). No significant differences were observed in weight gain during pregnancy or adequate weight gain from baseline BMI. In terms of treatment, no significant changes were observed in the number of patients who had nutritional intervention or in total insulin doses between the two groups. However, there were differences in basal insulin requirements, with lower doses needed in IG versus CG (11,5% vs 0,1%; p=0,030), probably due to lower fasting basal glucose levels. IG women had lower rates of other associated comorbidities such as diastolic blood pressure (DBP) between 36-38
th GW, and urinary tract infection (UTI) and albuminuria. There were no differences in blood pressure between 24-28
th GW, in the diagnosis of hypertension or preeclampsia, or in bacteriuria. Regarding neonatal outcomes, there was an increase in small-for-gestational-age (SGA) neonates in the IG relative to the CG (6,1 vs 2,8%; p=0,036). However, there were no differences in other anthropometric variables, such as weight and length, nor in large-for-gestational-age (LGA) infants. There were also no differences in rates of prematurity or dystocia, umbilical cord pH, APGAR, or other adverse effects in the newborn (hypoglycemia, respiratory problems, fever).
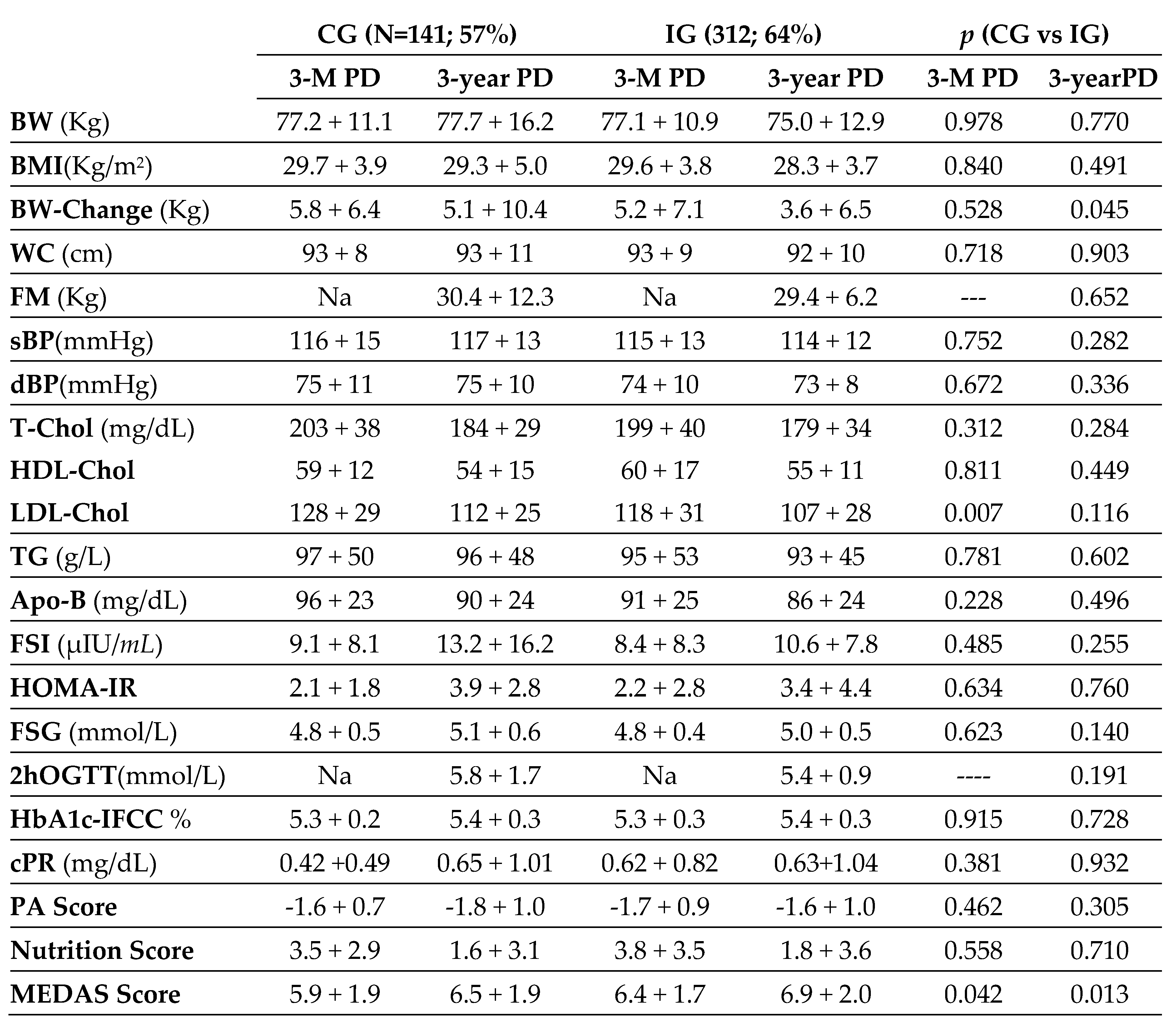
Table 3 shows biochemical, anthropometric, and clinical data of women at postnatal, 3-month and 3-year follow-up. This follow-up was completed by a total of 141 women of the CG women (57%) and 312 of the IG women (64%). We found a higher weight gain at 3 years postpartum in the CG compared to the IG (5.1±10.4 vs 3.6±6.5; p=0.045). There were no significant differences in other anthropometric measures. In analytical parameters, no differences were seen except for higher LDL-Chol in CG against IG at 3 months postpartum (128±29 vs 118±31; p=0.007). In terms of lifestyle, differences in MedDiet adherence were observed, with higher scores in IG than in CG at both 3 months and 3 years postpartum (6.4±1.7 vs 5.9±1.9; p=0.042 and 6.9±2.0 vs 6.5±1.9; p=0.013). However, no differences were found in the level of physical activity.
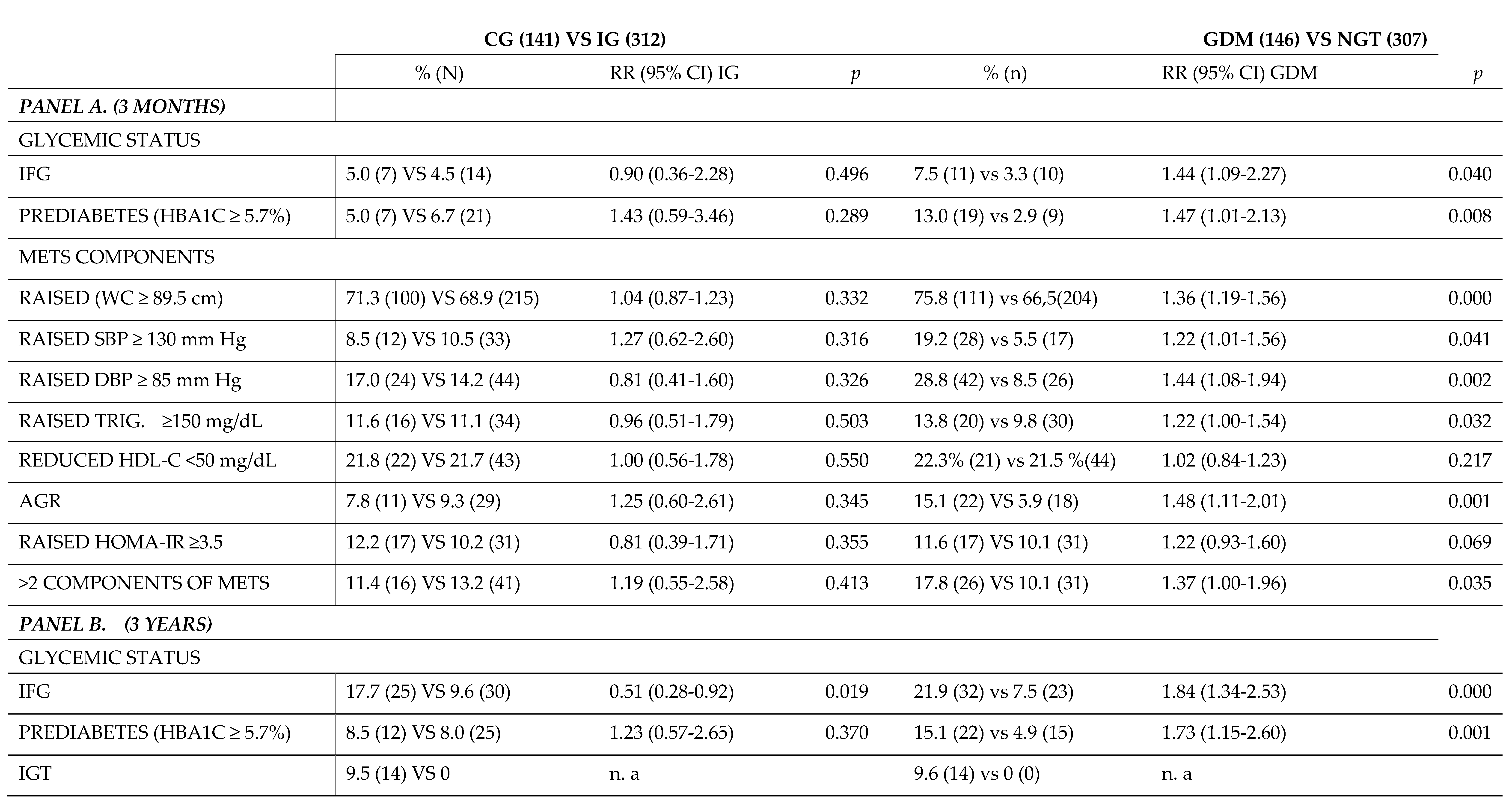
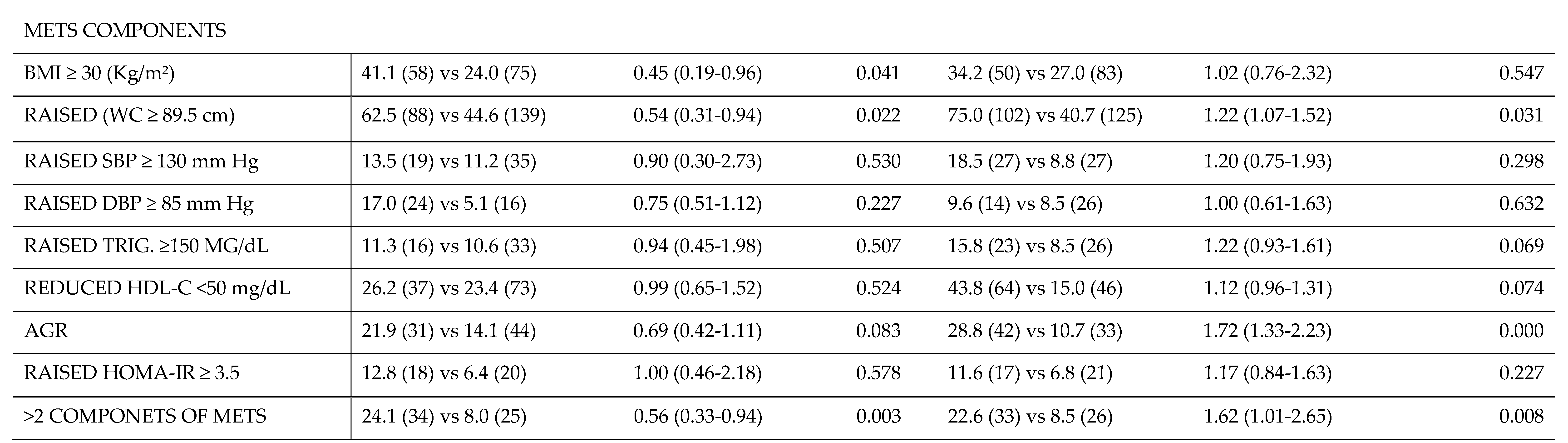
A detailed comparison of the postpartum rate of MetS components between overweight and obese women separated by IG vs CG and woman with GDM vs NGT is shown in
Table 4. We compared the relative risk (RR) of developing MetS and its components according to the nutritional intervention at 3 months (panel A) and 3 years (panel B) after delivery. No significant differences were found at 3 months postpartum between IG and CG in any of the MetS components. However, as expected, at 3 months postpartum there was a reduction in RR (95% CI) in the GDM group versus the NGT group in the rates of IFG, prediabetes, and all MetS components (WC ≥89.5 cm, SBP of ≥130 mmHg, DBP ≥85 mmHg, and AGR), except for HOMA-IR in which there were no different between groups.
In contrast, at 3 years postpartum (panel B) there was a reduction in the RR (95% CI) of the IFG rate (0.51 (0.28-0.92); p=0.019). Also, there were significant differences in the risk of developing MetS between both groups. This would imply that the nutritional intervention could be a protective factor against long-term MetS, as there was a higher rate of women with obesity in the CG (0.51 (0.28-0.92); p=0.041), as well as a higher rate of women with a WC ≥89.5 cm (0.54 (0.31-0.94); p=0.022). There were no significant differences for the other components of the MetS or in the RR of developing prediabetes.
Finally, comparing the GDM group with the group of women with GTN at 3 years postpartum, a decrease in the RR of both IFG (1.84 (1.34-2.53); p=0.000) and prediabetes (1.73 (1.15-2.60); p=0.001) was observed. Regarding the components of MetS, a higher risk of developing MetS for having more than 2 components was found in the GDM group vs. women with NGT (1.62 (1.01-2.65); p=0.008). This was due to the increased rate of women with WC ≥89.5 cm and AGR. In contrary, there was no significant difference in the rate of women with BMI >30 kg/m2, nor with HOMA-IR≥3.5, nor elevated blood pressure (SBP ≥130 mmHg, nor DBP≥85 mmHg), nor in a dysregulated lipid profile (TG ≥150 mg/dL and HDL<50 mg/dL) between both groups.
4. Discussion
This study shows that a nutritional intervention based on MedDiet significantly reduces the rate of GDM and the RR of developing MetS at 3 years postpartum, when implemented at the beginning of pregnancy (before 12 weeks of gestation) in women with BMI≥25kg/m2. In fact, this is because FBG and HBA1c levels were significantly lower in the IG group.
A recent systematic review suggested that nutritional intervention during pregnancy and postpartum may improve glucose regulation in patients with GDM (27). However, nutritional intervention covers a wide range of possibilities. This is why many studies insist on focusing on the type of nutritional intervention recommended, and aim to develop specific guidelines for a standardized approach (4,28,29). Our study has provided evidence for using a nutritional intervention with MedDiet based on vegetables, fruits, legumes, whole grains, lean meats instead of processed meats, oily fish, EVOO and nuts, as well as reducing consumption of processed and sugary foods as early as possible in pregnant women to reduce the likelihood of developing GDM and the long-term negative postnatal metabolic impact. In addition, the dietary recommendations given are specific, measurable, and assessed through validated semi-quantitative questionnaires. This allows not only to reproduce the study, but also to establish specific food consumption frequencies for dietary recommendations.
We found no significant differences in the rate of maternal-fetal adverse events. This may be because the total sample included women who are already at high prior risk of developing adverse events in pregnancy due to their own excess weight. In fact, all patients studied presented a BMI≥25 kg/m2 both at baseline and after intervention. A recent RCT in pregnant women at risk of hyperglycemia (29) showed a significant, but modestly smaller, decrease in adverse neonatal events. This study performed the intervention later, before 20 weeks' gestation, and has the limitation of non-standardized treatment of GDM. Perhaps these differences are explained by the fact that this study included overweight patients with additional risk factors for developing GDM, such as previous diagnosis of GDM, high gestational age, first-degree relatives with diabetes, polycystic ovarian syndrome, and non-European ancestry. In addition, the sample included a limited number of Hispanic women.
Previous studies have focused on the beneficial effects of the prevention of GDM on the control of total weight gain during pregnancy (4,28,30,31). However, in this study, we observed a reduction in the rate of GDM, even in the setting of no significant differences in total weight gain between women in the GC and the IG. This could be because of the influence of the complex interrelationship between weight, fat distribution and diet. In fact, we did observe a higher rate of women with WC≥89.5 cm at 3 years postpartum in the CG, drawing the attention to the importance of body composition and distribution in the follow-up of weight gain during pregnancy. Thus, our study reinforces what other large studies such as PREDIMED have shown: MedDiet prevents the development of MetS in the long-term follow-up due to a lower central distribution of adipose tissue. It would be interesting to validate methods for the assessment of body composition in pregnant women, in order to develop more personalized nutritional recommendations in the specific setting of pregnancy (32).
A topic of great interest concerns the timing of initiation and duration of the nutritional intervention set for pregnant women for the prevention of GDM. Many studies argue in favor of its implementation as soon as possible, especially in high-risk patients (31,33). In this regard, previous reports suggest the need for initiating the intervention in the 20th GW to achieve a relevant reduction of GDM (29), but not all reports have observed a clear benefit when implementing it at this time (12). There is no doubt that appropriately designed and targeted interventions can be effective tools for the management of pregnancies which are complicated by overweight and obesity. However, demonstration of its efficacy is somehow blurred because of lack of resources and failure of previous studies to establish the appropriate time at which this personalized nutritional care should be provided to effectively prove benefit.
Our study entails some limitations. Firstly, this is a post-hoc study, meaning that the initial main objective was not to evaluate women with overweight or obesity. This limits the sample size; however, we have managed to group enough patients to obtain significant data. On the other hand, postnatal follow-up was performed in approximately 60% of women, mainly due to the difficulty of attendance because of the recent delivery, having other small children, and unavailability to attend medical follow-ups. Other causes were a change of community, follow-up in a private hospital or not wishing to continue participating in the study. However, this proportion is still considered acceptable and is descriptive of our population, representing more than half of the total sample. This highlights the challenge of adhering to the recommended postnatal follow-up by all scientific guidelines. Another limitation is that diet is a subjective, complex, and changing parameter and questionnaire responses could be biased. However, semi-quantitative questionnaires are a standardized, quick, and non-invasive tool to adequately represent food intake, and are always administered by specialized professionals to minimize bias. Therefore, the use of validated questionnaires allows us to overcome this limitation, and provides a genuine character to our study, by objectively quantifying and measuring the nutritional intervention carried out.
5. Conclusions
In conclusion, our research demonstrates that an early nutritional intervention using MedDiet, along with supplementation of EVOO and nuts, in women with a BMI≥25kg/m2 and initiated during early pregnancy, decreases the rate of GDM and positively influences the risk of developing MetS over a three-year period after delivery. Therefore, indicating MedDiet-based dietary patterns in women with excess weight from early pregnancy can be considered a preventive strategy for the development of postnatal GDM and MetS. Future studies with a defined and measurable nutritional intervention in high-risk women are needed to further confirm our findings.
Author Contributions
Conceptualization, Rocio Martin-O´Connor, Ana Ramos-Leví, María Arnoriaga-Rodríguez, Johanna Valerio, Laura Del Valle, Angel Díiaz, Cristina Familiar, Alejandra Durán, Maria Jose Torrejón, Miguel Ángel Rubio-Herrera, Pilar Matía-Martín and Alfonso Luis Calle-Pascual; Data curation, Pilar Matía-Martín and Alfonso Luis Calle-Pascual; Formal analysis, Maria Jose Torrejón, Mercedes Martinez-Novillo and Alfonso Luis Calle-Pascual; Funding acquisition, Alfonso Luis Calle-Pascual; Investigation, Ana Ramos-Leví, Verónica Melero, María Arnoriaga-Rodríguez, Ana Barabash, Johanna Valerio, Laura Del Valle, María Paz De Miguel Novoa, Angel Díiaz, Cristina Familiar, Inmaculada Moraga, Alejandra Durán, Martín Cuesta, Maria Jose Torrejón, Clara Marcuello, Mario Pazos, Miguel Ángel Rubio-Herrera and Alfonso Luis Calle-Pascual; Methodology, Rocio Martin-O´Connor, Ana Ramos-Leví, Verónica Melero, María Arnoriaga-Rodríguez, Ana Barabash, Johanna Valerio, Laura Del Valle, María Paz De Miguel Novoa, Cristina Familiar, Inmaculada Moraga, Martín Cuesta, Maria Jose Torrejón, Mercedes Martinez-Novillo, Clara Marcuello, Mario Pazos, Miguel Ángel Rubio-Herrera, Pilar Matía-Martín and Alfonso Luis Calle-Pascual; Validation, Angel Díiaz; Writing – original draft, Rocio Martin-O´Connor, Ana Ramos-Leví, Pilar Matía-Martín and Alfonso Luis Calle-Pascual; Writing – review & editing, Rocio Martin-O´Connor, Ana Ramos-Leví, Pilar Matía-Martín and Alfonso Luis Calle-Pascual. All authors will be informed about each step of manuscript processing including submission, revision, revision reminder, etc. via emails from our system or assigned Assistant Editor. All authors have seen and agree with the content of the full last version of manuscript.
Funding
This research was funded by grants from the Instituto de Salud Carlos III/MICINN of Spain under grant number PI20/01758, and European Regional Development Fund (FEDER) ‘‘A way to build Europe’’ and Fundación para Estudios Endocrino-Metabolicos. The design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication are the responsibilities of the authors alone and independent of the funders.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. The Institutional Review Board and the Clinical Ethics Committee of the Hospital Clínico San Carlos approved the studies (CI 13/296-E: 22/07/2013; CI 16/442-E: 08/11/2016; and CI 16/316: 15/07/2016). Written informed were provided and signed by all participants.
Informed Consent Statement
Written informed were provided and signed by all participants.
Data Availability Statement
The datasets generated during and/or analyzed in the current study are available from the corresponding author upon reasonable request
Acknowledgments
We wish to acknowledge our deep appreciation to the administrative personnel and nurses from the Laboratory Department (María Victoria Saez de Parayuelo), the Pregnancy and Diabetes Unit and to all members of the Endocrinology and Nutrition and Obstetrics and Gynecology departments of the San Carlos Clinical Hospital, Madrid, Spain.
Conflicts of Interest
The authors declare no potential conflict relevant to this article. The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Chen C, Xu X, Yan Y. Estimated global overweight and obesity burden in pregnant women based on panel data model. PloS One. 2018;13(8):e0202183.
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet Lond Engl. 16 de diciembre de 2017;390(10113):2627-42.
- Barazzoni R, Gortan Cappellari G, Ragni M, Nisoli E. Insulin resistance in obesity: an overview of fundamental alterations. Eat Weight Disord EWD. abril de 2018;23(2):149-57.
- Parrettini S, Caroli A, Torlone E. Nutrition and Metabolic Adaptations in Physiological and Complicated Pregnancy: Focus on Obesity and Gestational Diabetes. Front Endocrinol. 2020;11:611929.
- McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primer. 11 de julio de 2019;5(1):47.
- Farahvar S, Walfisch A, Sheiner E. Gestational diabetes risk factors and long-term consequences for both mother and offspring: a literature review. Expert Rev Endocrinol Metab. enero de 2019;14(1):63-74.
- Ye W, Luo C, Huang J, Li C, Liu Z, Liu F. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. BMJ. 25 de mayo de 2022;377:e067946.
- Owens LA, O’Sullivan EP, Kirwan B, Avalos G, Gaffney G, Dunne F, et al. ATLANTIC DIP: the impact of obesity on pregnancy outcome in glucose-tolerant women. Diabetes Care. marzo de 2010;33(3):577-9.
- Catalano PM, McIntyre HD, Cruickshank JK, McCance DR, Dyer AR, Metzger BE, et al. The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care. abril de 2012;35(4):780-6.
- Assaf-Balut C, Familiar C, García de la Torre N, Rubio MA, Bordiú E, Del Valle L, et al. Gestational diabetes mellitus treatment reduces obesity-induced adverse pregnancy and neonatal outcomes: the St. Carlos gestational study. BMJ Open Diabetes Res Care. 2016;4(1):e000314.
- Shepherd E, Gomersall JC, Tieu J, Han S, Crowther CA, Middleton P. Combined diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database Syst Rev. 13 de noviembre de 2017;11(11):CD010443.
- Wu S, Jin J, Hu KL, Wu Y, Zhang D. Prevention of Gestational Diabetes Mellitus and Gestational Weight Gain Restriction in Overweight/Obese Pregnant Women: A Systematic Review and Network Meta-Analysis. Nutrients. 9 de junio de 2022;14(12):2383.
- Koivusalo SB, Rönö K, Klemetti MM, Roine RP, Lindström J, Erkkola M, et al. Gestational Diabetes Mellitus Can Be Prevented by Lifestyle Intervention: The Finnish Gestational Diabetes Prevention Study (RADIEL): A Randomized Controlled Trial. Diabetes Care. enero de 2016;39(1):24-30.
- H Al Wattar B, Dodds J, Placzek A, Beresford L, Spyreli E, Moore A, et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): A pragmatic multicentre randomised trial. PLoS Med. julio de 2019;16(7):e1002857.
- Bruno R, Petrella E, Bertarini V, Pedrielli G, Neri I, Facchinetti F. Adherence to a lifestyle programme in overweight/obese pregnant women and effect on gestational diabetes mellitus: a randomized controlled trial. Matern Child Nutr. julio de 2017;13(3):e12333.
- Herring SJ, Cruice JF, Bennett GG, Rose MZ, Davey A, Foster GD. Preventing excessive gestational weight gain among African American women: A randomized clinical trial. Obes Silver Spring Md. enero de 2016;24(1):30-6.
- Brown J, Alwan NA, West J, Brown S, McKinlay CJ, Farrar D, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 4 de mayo de 2017;5(5):CD011970.
- Mahajan A, Donovan LE, Vallee R, Yamamoto JM. Evidenced-Based Nutrition for Gestational Diabetes Mellitus. Curr Diab Rep. 31 de agosto de 2019;19(10):94.
- Filardi T, Panimolle F, Crescioli C, Lenzi A, Morano S. Gestational Diabetes Mellitus: The Impact of Carbohydrate Quality in Diet. Nutrients. 9 de julio de 2019;11(7):1549.
- ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 15. Management of Diabetes in Pregnancy: Standards of Care in Diabetes—2023. Diabetes Care. 12 de diciembre de 2022;46(Supplement_1):S254-66.
- Rasmussen L, Poulsen CW, Kampmann U, Smedegaard SB, Ovesen PG, Fuglsang J. Diet and Healthy Lifestyle in the Management of Gestational Diabetes Mellitus. Nutrients. 6 de octubre de 2020;12(10):3050.
- Estruch R, Ros E. The role of the Mediterranean diet on weight loss and obesity-related diseases. Rev Endocr Metab Disord. septiembre de 2020;21(3):315-27.
- Muscogiuri G, Verde L, Sulu C, Katsiki N, Hassapidou M, Frias-Toral E, et al. Mediterranean Diet and Obesity-related Disorders: What is the Evidence? Curr Obes Rep. diciembre de 2022;11(4):287-304.
- Guasch-Ferré M, Willett WC. The Mediterranean diet and health: a comprehensive overview. J Intern Med. septiembre de 2021;290(3):549-66.
- Schröder H, Fitó M, Estruch R, Martínez-González MA, Corella D, Salas-Salvadó J, et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J Nutr. junio de 2011;141(6):1140-5.
- Assaf-Balut C, Torre NG de la, Durán A, Fuentes M, Bordiú E, Valle L del, et al. A Mediterranean diet with additional extra virgin olive oil and pistachios reduces the incidence of gestational diabetes mellitus (GDM): A randomized controlled trial: The St. Carlos GDM prevention study. PLOS ONE. 19 de octubre de 2017;12(10):e0185873.
- Huang S, Magny-Normilus C, McMahon E, Whittemore R. Systematic Review of Lifestyle Interventions for Gestational Diabetes Mellitus in Pregnancy and the Postpartum Period. J Obstet Gynecol Neonatal Nurs. 1 de marzo de 2022;51(2):115-25.
- Champion ML, Harper LM. Gestational Weight Gain: Update on Outcomes and Interventions. Curr Diab Rep. 27 de febrero de 2020;20(3):11.
- Simmons D, Immanuel J, Hague WM, Teede H, Nolan CJ, Peek MJ, et al. Treatment of Gestational Diabetes Mellitus Diagnosed Early in Pregnancy. N Engl J Med. 8 de junio de 2023;388(23):2132-44.
- Chiefari E, Arcidiacono B, Foti D, Brunetti A. Gestational diabetes mellitus: an updated overview. J Endocrinol Invest. septiembre de 2017;40(9):899-909.
- Guo XY, Shu J, Fu XH, Chen XP, Zhang L, Ji MX, et al. Improving the effectiveness of lifestyle interventions for gestational diabetes prevention: a meta-analysis and meta-regression. BJOG Int J Obstet Gynaecol. febrero de 2019;126(3):311-20.
- Most J, Marlatt KL, Altazan AD, Redman LM. Advances in assessing body composition during pregnancy. Eur J Clin Nutr. mayo de 2018;72(5):645-56.
- Langley-Evans SC, Pearce J, Ellis S. Overweight, obesity and excessive weight gain in pregnancy as risk factors for adverse pregnancy outcomes: A narrative review. J Hum Nutr Diet Off J Br Diet Assoc. abril de 2022;35(2):250-64.
- Guo XY, Shu J, Fu XH, Chen XP, Zhang L, Ji MX, et al. Improving the effectiveness of lifestyle interventions for gestational diabetes prevention: a meta-analysis and meta-regression. BJOG Int J Obstet Gynaecol. 2019;126(3):311-20.
Table 1.
Characteristics of maternal population with pregnancy BMI≥25 kg/m2 at baseline visit (8-10th GW) assessed in the clinical trial population by groups.
Table 1.
Characteristics of maternal population with pregnancy BMI≥25 kg/m2 at baseline visit (8-10th GW) assessed in the clinical trial population by groups.
Table 2.
Maternal, pregnancy and neonatal outcomes.
Table 2.
Maternal, pregnancy and neonatal outcomes.
Table 3.
Postnatal biochemical, anthropometric, and clinical data of women during postnatal follow-up, at 3 months and 3 years post-delivery by groups.
Table 3.
Postnatal biochemical, anthropometric, and clinical data of women during postnatal follow-up, at 3 months and 3 years post-delivery by groups.
Table 4.
Comparison of post-delivery rate of metabolic syndrome (MetS) components between obese/overweight women from IG vs CG and women with GDM vs normal glucose tolerance (NGT).
Table 4.
Comparison of post-delivery rate of metabolic syndrome (MetS) components between obese/overweight women from IG vs CG and women with GDM vs normal glucose tolerance (NGT).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).