1. Introduction
The coronavirus disease 2019 (COVID-19) has spread and caused high morbidity and mortality worldwide since it was first reported in Wuhan in the Chinese province of Hubei in late 2019 [
1,
2]. Gabon is the 3rd country most affected by COVID-19 behind Cameroon and the Democratic Republic of Congo in Central Africa, with 48980 cases and 306 deaths in critically ill patients since the first confirmed case of COVID-19 on the 12th of March 2020 (
https://africacdc.org/COVID-19/).
The response strategy against COVID-19 in Gabon was based on tests performed on travellers, suspected cases, and direct contacts of people who tested positive in the country [
3]. Although massive screening campaigns have been occasionally organized in the country, the actual infection rate of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of COVID-19, remains unclear, since asymptomatic individuals may represent the hidden face of iceberg and therefore a major source of viral spread and continuously spread the virus. Case reporting is influenced by strategies implemented for case finding, testing, and contact tracing and may underestimate the true burden of SARS-CoV-2 infection.
As recommended by the World Health Organization (WHO), measuring the extent of seropositivity could estimate the proportion of individuals positive for SARS-CoV-2 antibodies in the population and could further indicate the rate of disease transmission over time [
4]. Moreover, as the extent of infection is heavily related to social interactions, population density and the assessment of the proportion of potentially protected individuals in the population are crucial. Population-based seroepidemiological studies are useful for measuring the extent of SARS-CoV-2 infection and the effect of ongoing public health responses on controlling the pandemic4 (A Coordinated Global Research Roadmap, 2022). Owing to the increased number of cases and patients in hospitals, restrictions on mass gatherings were initiated in April 2020. These restrictions were accompanied by a vaccination campaign as a complementary measure to control the pandemic.
This study aimed to conduct a national household serosurvey to estimate the nationwide seroprevalence of SARS-CoV-2 antibodies in the general population, including age group, sex, area of residence, and COVID-19 related characteristics, and to determine trends in infection since the implementation of restrictive measures and vaccination campaigns.
2. Materials and Methods
Study Design and Population
This study was designed in accordance to WHO united studies [
5]. This population-based cross-sectional study was conducted across the entire Gabonese Territory (the main cities of all provinces and villages in rural areas drawn by lot) between November 28 and December 20, 2021.
Gabon is a Central African country with an area of approximately 268,000 km2, located on the edge of the Atlantic Ocean and bordering Congo-Brazzaville, Cameroon, and mainland Equatorial Guinea. Its population, one of the smallest in Central Africa, is estimated at 1811079 inhabitants. Gabon is subdivided into nine provinces, with capital in each province and 52 departments.
Sample Collection
We randomly selected and invited participants from the general population and areas included in the study, according to specific inclusion and exclusion criteria. Individuals of both sexes aged one year and over, and residing for more than three months in the study area were eligible to participate in the study. Individuals who are not residing in the locality surveyed, or those residing for less than three months, and those who refuse to provide informed consent and contraindications for venipuncture, were excluded.
Random selection was performed at two levels. The first category concerns the areas to be surveyed. This random selection was made from the national census file of 2013 households distributed in the enumeration areas. Subsequently, in each of the areas selected in the first stage, a sampling interval was calculated with a fixed number of 20 households to be visited. The survey teams randomly chose a starting household and visited it at an established sampling interval. All eligible permanent-resident household members present at the time of the survey team visits were invited to participate. At least one individual from each random household was enrolled in a serological survey.
Written informed consent from individuals aged 18 years or older, or assent from children aged between 12 and 17 years, with written informed consent from their parents or guardians, was obtained before their enrollment in the study.
The study was conducted in accordance with the Declaration of Helsinki, ICH-GCP guidelines, and local regulations. The study protocol was approved by the National Ethics Committee for Research (0056/2021/P/SG/CNER).
Procedures
Eligible participants were interviewed to collect information on their sociodemographic characteristics, medical history, recent COVID-19-related symptoms (e.g., fever, cough, fatigue, loss of taste or smell, sore throat) and COVID-19 related exposure and SARS-CoV-2 vaccination using the KOBOCOLLECT mobile phone application (kc.kobotoolbox.org) on a questionnaire that included GPS data. Paper questionnaires were also made available to the teams to address difficulties in the operation of tablets and telephones, particularly in rural areas. After completing the questionnaire, a research nurse collected 5 mL of venous blood in dry tubes. WHO-recommended WANTAI Elisa Kits were used according to the manufacturer’s instructions to assess the presence of SARS-CoV-2 spike protein-specific antibodies in serum samples. This assay has a sensitivity of 94.36% and a specificity of 100% (
https://www.ystwt.cn/wp-content/uploads/2020/05/Brochure-Wantai-SARS-CoV-2-Ab-ELISA.pdf). The assay was calibrated with positive and negative quality controls before sample analyses. Assay results higher than or equal to the cutoff index value were interpreted as positive for SARS-CoV-2 antibodies. As part of the quality control, 5% of the 3455 serum samples were re-tested using the same assay by two different laboratory technicians.
Concordance Test
The manufacturer-reported sensitivity and specificity of the ELISA kits (WANTAI) were 96.7% (95% confidence interval [CI], 83.3–99.4) and 97.5% (95% CI, 91.3–99.3), respectively. These values were based on 30 SARS-CoV-2 antibody-positive serum samples confirmed by a nucleic acid amplification test (NAAT) for both IgM and IgG antibodies and 80 antibody-negative serum samples collected before the SARS-CoV-2 pandemic.
Independent test of concordance was performed between WANTAI Elisa and Elecsys®Anti-SARS-CoV-2 using randomly selected 100 positive and 85 negative serum samples to the WANTAI Elisa kits. All of the 100 samples positive for WANTAI Elisa were positive for Elecsys®Anti-SARS-CoV-2 corresponding to a collective sensitivity of 100%; 77 of 85 samples were tested negative for both of the ELISA kits used, corresponding to a collective specificity of 90,6%.
Sample size Estimation and Statistical Analyses
The sample size needed to estimate prevalence in the study was calculated to be 3455 on the basis of a seroprevalence rate of 10%, with a confidence level of 95%, precision of 2%, and calculation factor of 4 (
https://www.openepi.com/SampleSize/SSPropor.htm). The required sample size for each area was proportional to the population of each area relative to the total population of the country.
Demographic variables included age, sex, area of residence, and school level. COVID-19 related symptoms included cough, fever, sore throat, headache, diarrhea, altered level of consciousness, and chest pain experienced during the 12 weeks preceding questionnaire completion. Moreover, data on vaccination status were recorded.
The dependent variable was the presence or absence of anti-SARS-CoV-2 antibodies, and the independent variables were the other collected variables.
Data were analyzed using the R statistical software (The R Foundation for Statistical Computing, Vienna, Austria). Qualitative variables were described using percentage and quantitative variables using mean ± SD and median (min and max). A 95% CI was calculated for all the prevalence values. The significance level was set at 5%.
3. Results
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
3.1. Study Profile
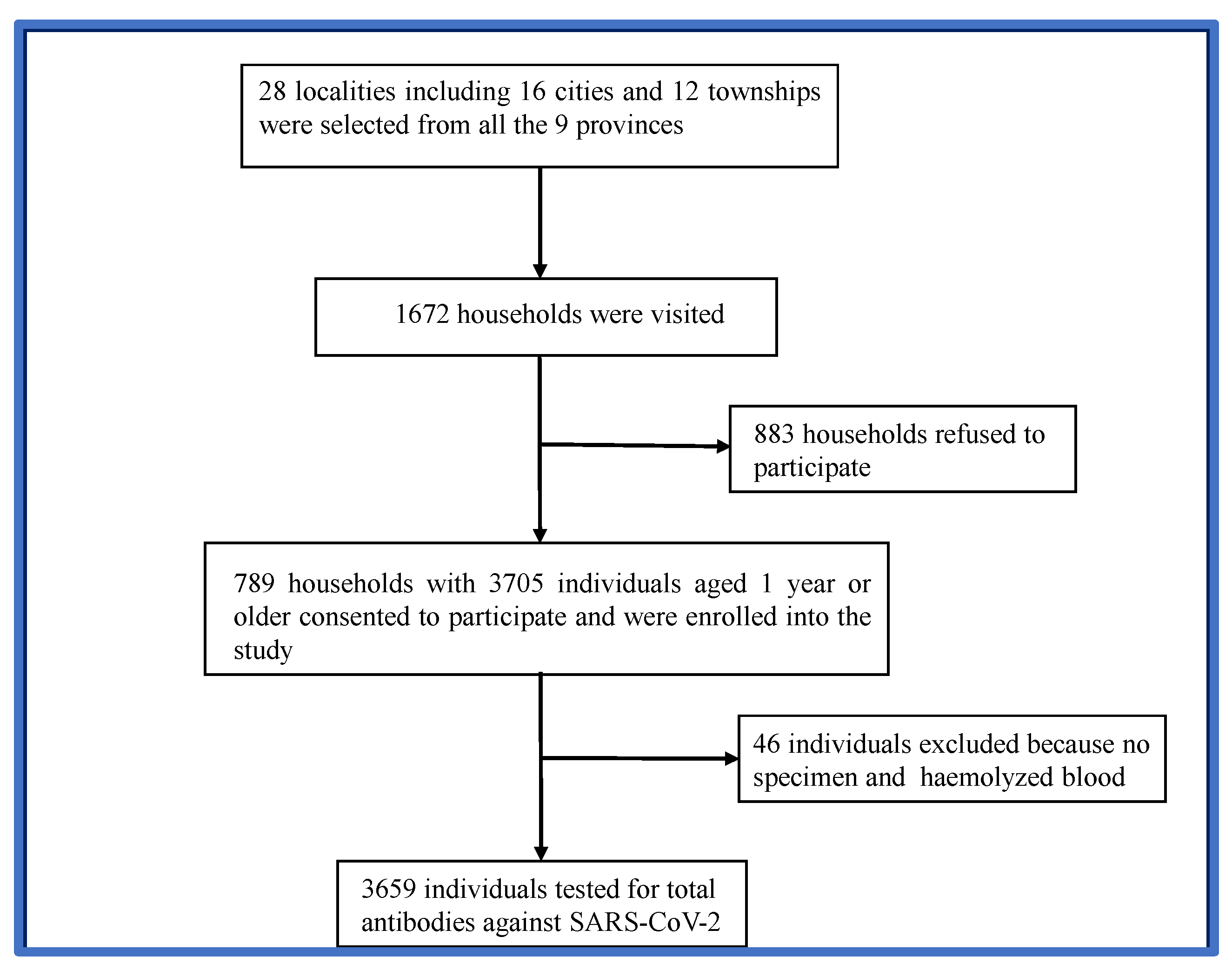
Based on the size of the Gabonese population in the nine provinces, 28 localities, including 16 urban and 12 rural areas, were randomly selected to participate in the study. In total, 1672 households were visited during the study period. Approximately 47.2% (789) of the households consented to participate in the study. Of these, 3705 eligible individuals aged 1 year and above were enrolled (
Figure 1). Forty-six (46) individuals were excluded from the analysis because of the poor quality of the samples provided. Therefore, 3659 participants were examined for the presence of SARS-CoV-2 antibodies.
3.2. Demographic Characteristics of Study Participants
Participants’ main characteristics are shown in
Table 1. Analysis of the study population showed that the 30–39 age group (20.6%) was the most representative, followed by the 20–29 (19.5%), 40–49 (15.2%) and 50–59 (11.2%) age groups. Children aged 0–4 (2.7%) and those aged > 70 years (3.9%) were the least represented age groups. The mean age was 32.6 years (standard deviation [SD]:23). The sex ratio was 1 (50.6 F/49.6 M). The sex profile of our baseline population was consistent with that of the entire Gabon population. However, the age profile of our baseline population was not consistent with that of the whole population, which was younger, with a mean age of 22 years and a median age of 26 years. Among all participants, 14.88% resided in rural areas while 85.46% resided in urban areas. This is consistent with the general population wherein 87% of whom live in urban areas and the 13% in rural areas.
3.3. Seroprevalence in Study Population
SARS-CoV-2 antibodies tested positive in 3175 individual samples. Only 484 participants tested negative for SARS-CoV-2. The national seroprevalence was 86.8% (95% CI: [86–88]). No statistical difference in seroprevalence was found between women (87.82%, 95% CI: [86.2–89.2]) and men (86.45%, 95% CI: [84.8–87.9]). Stratification of study participants by age group has shown highest seroprevalences in 20–29 and 40–49 age groups at 91.78% (95% CI:89.5–93.6) and 91.42% (95% CI:88.7–93.5), respectively (
Table 2).
The prevalence of SARS-CoV-2 antibodies was similar among age groups [10,14], [15,19], and [30,39]:87.6% (95% CI:83–91), 87.5% (95% CI:83–91) and 88.3% (95% CI:84–91), respectively. The seroprevalence was higher in the most active study groups from 10 to 49 years of age (school-age and young workers) and was found to be between 87 and 91%. This seroprevalence was reduced in participants aged 50 years and older, and was found to be between 81 and 82.6%. The lowest seroprevalence was found in children with 72.6% (95% CI:62–91) for those aged 0–4 years and 79.6% (95% CI:74–85) for those aged 5–9 years.
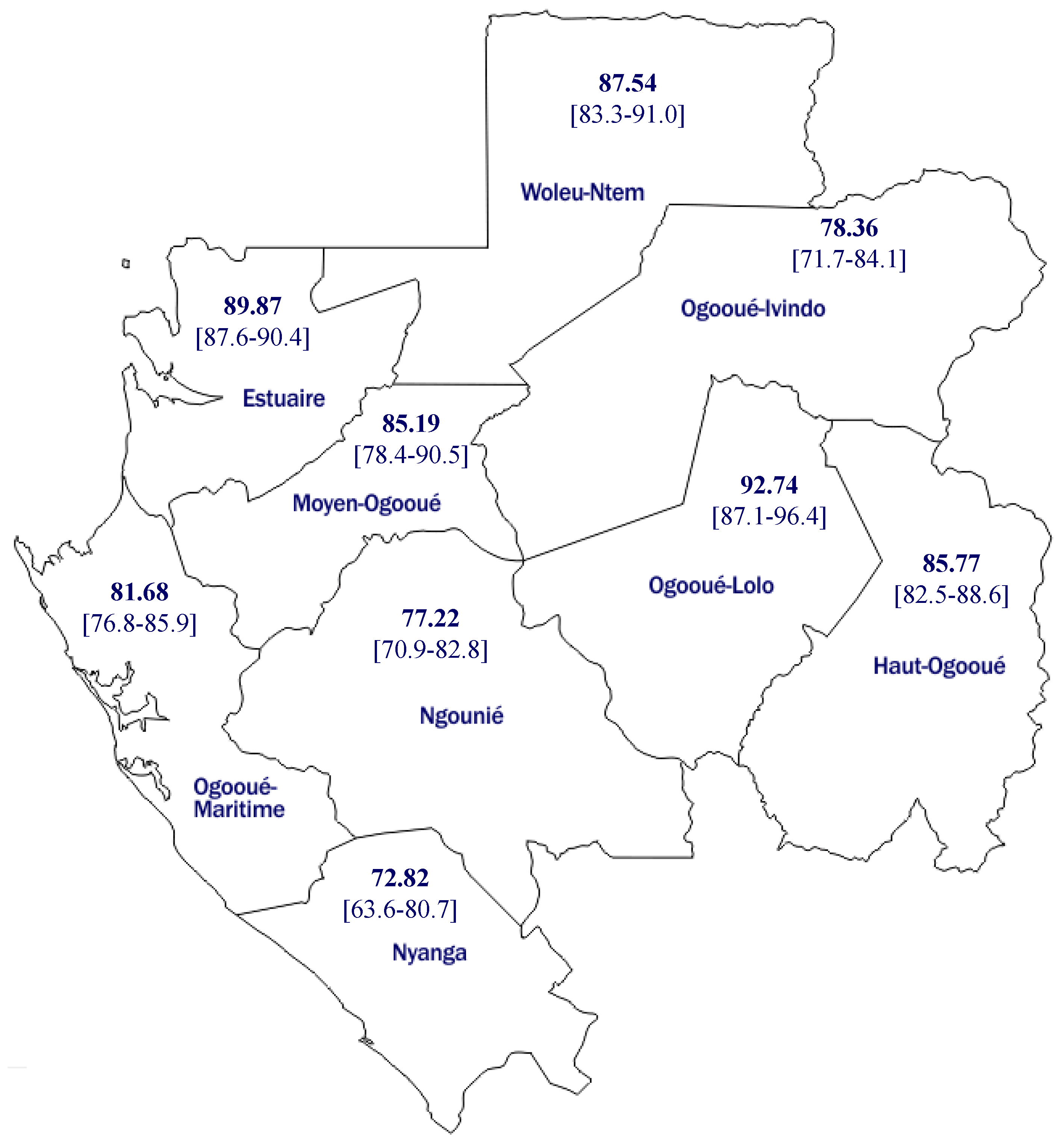
Nyanga is a less-populated province in Gabon (
Table 3 and
Figure 2). Only 2% of the study population were enrolled in this province. It also presented the lowest vaccine coverage, with only 4% (three participants) of the study participants from this province were vaccinated. The provinces with the lowest seroprevalence in Gabon, Nyanga, and Ngounié (72, 8, and 77.2%, respectively) were located in the sud (. Moyen Ogooué had the highest vaccine coverage.
Twelve of 134 participants (9%) in the province were vaccinated. The comorbidity rate was also high in this province, with more than 15% of the participants having at least one comorbidity. Estuaire is the most populated province in Gabon, and more than 50% of the study population was recruited from this province. Vaccine coverage was 7.4%, and 9.2% of the study participants had at least one comorbidity.
The four main cities in this province were selected for this study: Akanda, Owendo, Libreville, and Ntoum (
Table 4). Owendo had the highest rate of comorbidities, with 19% of participants from that city presenting at least one comorbidity. The seroprevalence was also high in that city (92.8%). Akiéni is a small rural area within the Haut-Ogooué province. Eleven participants were enrolled in the study. All the patients tested positive for SARS-CoV-2 antibodies (100% seroprevalence). Only Lambaréné (Moyen-Ogooué) had a seroprevalence < 70% in Gabon. The remaining cities had seroprevalence rates ranging from 84% to 100%.
4. Discussion
Gabon is one of the countries most affected by SARS-CoV-2 in Central Africa. However, in the absence of seroprevalence studies, the true infection rate in Gabon remains unclear. This study aimed at estimating the seroprevalence of SARS-CoV-2 antibodies across Gabon. This study sampled largely populated and geographically distributed regions. All main Gabonese cities (urban) and randomly selected rural (village) areas were visited within 15 days. The National seroprevalence was estimated to be 86%, demonstrating the immunization status of the Gabonese population. This is the first study to reveal high SARS-CoV-2 seroprevalence at the population or country level with remarkably low vaccine coverage (
https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html). The results presented here are not surprising, as many studies have shown the rapid progression of SARS-CoV-2 antibody seroprevalence in the African population. The data in this study were collected 21 months after the SARS-CoV-2 infection in Gabon (March 2020 to November 2021). The seroprevalence observed in our study might be explained by the low compliance of the general population with applied health regulations such as physical distancing and wearing masks [
7].
Sagara et al. collected blood samples from one urban and two rural communities in Mali at two separate visits within six months [
8]. Within this period, the seroprevalence was multiplied by more than three in urban areas, from 19% during the first visit (July to October 2020) to 70% during the second visit (December 2020 to January 2021). A ten folds increase was observed in rural areas during the same period. This corroborates the observation in South Africa where the authors collected blood from urban and rural cohorts every two months from July 2020 to March 2021 [
9]. During this nine-month study, the seroprevalence increased from 1% to 26% in rural settings and from 15% to 41% in urban communities after the two waves of COVID-19. A hospital-based study enrolling patients and parents visiting the health center in Libreville (Gabon) revealed a seroprevalence of 36% six months after the first detection of SARS-CoV-2 in Gabon [
10]. Although morbidity and mortality were low in Central Africa, this region was found to have the highest seroprevalence in a systematic review aimed at estimating global and regional SARS-CoV-2 seroprevalence worldwide [
11]. The data presented in the current study, showing naturally acquired immunity in the Gabonese population after 21 months of COVID-19 emergence, corroborates with the previous study. The SARS-CoV-2 transmission rate in urban and rural settings is high in Central Africa, especially in Gabon. Further studies are needed to determine the source of the observed resistance to COVID-19 in the Gabonese population. The current data showed the highest transmission rate in school-aged and young workers from 10 to 49 years old. This observation can be explained by the fact that the younger population may have adopted fewer safety measures to limit transmission. Notably, the morbidity and mortality rates due to COVID-19 are low in this age range. Morbidity and mortality due to COVID-19 has been associated with age structure and the co-endemicity with other pathogens, including helminths and other related viruses [
12]. This profile was already shown in the early stage of the COVID-19 pandemic in a seroprevalence study conducted in Kenya eight months after the introduction of SARS-CoV-2 in this country [
13]. The seroprevalence was found to be between 30 and 39% in age group 10–59 years old, but 22% in participants older than 60 years. The lowest prevalence was observed in the younger population (< 10 years old) at 19%. Some studies have shown the highest seroprevalence in older population [
14].
5. Conclusions
Current data show a high transmission rate of the first SARS-CoV-2 in Gabon. The virus was first detected in March 2020 in Gabon. After 21 months, the population presented with a high SARS-CoV-2 seroprevalence, indicating a high prevalence of asymptomatic or mildly unreported COVID-19 cases in the country.
Supplementary Materials
A subset of the key-anonymized individual data collected during the study, along with a data dictionary, is available upon request from the corresponding author at paulinessone@gmail.com after approval of a proposal with a signed data access agreement.
Author Contributions
Conceptualization, PNE, RA,SZA,SLOL, AMN, BRA, BI,MMB, IA, ANM, JBLD, KMMNM, JFDS, AAA, and EBN.; methodology, PNE, RA,SZA,SLOL, AMN, BRA, BI,MMB, IA, ANM, JBLD, KMMNM, JFDS, AAA, and EBN.; software, IB and EBN.; validation, SZA, PNE, RA, SLOL, BRA, JFDS, BI, AAA, and EBN.; formal analysis, SZA, PNE, RA, SLOL, BRA, JFDS, BI, AAA, and EBN.; investigation, PNE, RA,SZA,SLOL, AMN, AKAE, MSH, JNT, HMK, AY, MJVMM, IPMB, ACMS, JA, AOM, RB, RENS, ULDM, DCAN, AMM, AM, FM, LCK, CMMN, JCBBE and RKIL.; resources, IA and AM.; data curation, EBN.; writing—original draft preparation, SZA, PNE, RA, SLOL, BRA, JFDS, BI, BEN, and AAA.; writing—review and editing, SZA, PNE, RA, SLOL, BRA, JFDS, BI, BEN, and AAA.; visualization, SZA, PNE, RA, SLOL, BRA, JFDS, BI, BEN, and AAA.; supervision, SZA, PNE, RA, SLOL, BRA, JFDS, BI, BEN, and AAA.; project administration, PNE, SLOL,SZA and EBN.; funding acquisition, SZA, PNE, RA, SLOL, BRA, JFDS, BI, AAA, and EBN. All authors have read and agreed to the published version of the manuscript.
Funding
This study received funding and technical support from the WHO (Unity Studies).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, ICH-GCP guidelines, and local regulations. The study protocol was approved by the National Ethics Committee for Research (0056/2021/P/SG/CNER).
Informed Consent Statement
Written informed consent from individuals aged 18 years or older, or assent from children aged between 12 and 17 years, with written informed consent from their parents or guardians, was obtained before their enrollment in the study.
Data Availability Statement
A subset of the key-anonymized individual data collected during the study, along with a data dictionary, is available upon request from the corresponding author at ngoungou2001@yahoo.fr after approval of a proposal with a signed data access agreement.
Acknowledgments
We thank all personnel who helped with data and sample gathering in the areas involved and the participants, without whom this study would not have been possible.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis 2020, 34:101623. [CrossRef]
- Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323(13):1239–1242. [CrossRef]
- Zoa-Assoumou S, Ndeboko B, Manouana GP, et al. SARS-CoV-2 emerging variants in Africa: view from Gabon. Lancet Microbe 2021, 2(8):e349. [CrossRef]
- WHO. World Health Organization. Geneva:2020. Coordinated global research roadmap:2019 novel coronavirus; March 2020. Accessed 14/05/2022.
- Bergeri I, Lewis HC, Subissi L, et al. Early epidemiological investigations: World Health Organization UNITY protocols provide a standardized and timely international investigation framework during the COVID-19 pandemic. Influenza Other Respir. Viruses 2022, 16(1):7–13. [CrossRef]
- Murhekar MV, Bhatnagar T, Selvaraju S, et al. SARS-CoV-2 antibody seroprevalence in India, August-September, 2020: findings from the second nationwide household serosurvey. Lancet Glob Health 2021, 9(3):e257–e266. [CrossRef]
- Sagara I, Woodford J, Kone M, et al. Rapidly Increasing Severe Acute Respiratory Syndrome Coronavirus 2 Seroprevalence and Limited Clinical Disease in 3 Malian Communities: A Prospective Cohort Study. Clin Infect Dis 2022, 74(6):1030–1038. [CrossRef]
- leynhans J, Tempia S, Wolter N, et al. SARS-CoV-2 Seroprevalence in a Rural and Urban Household Cohort during First and Second Waves of Infections, South Africa, July 2020-March 2021. Emerg Infect Dis 2021, 27(12):3020–3029. [CrossRef]
- Mveang Nzoghe A, Leboueny M, Kuissi Kamgaing E, et al. Circulating anti-SARS-CoV-2 nucleocapsid (N)-protein antibodies and anti-SARS-CoV-2 spike (S)-protein antibodies in an African setting: herd immunity, not there yet! BMC Res Notes 2021, 14(1):152. [CrossRef]
- Rostami A, Sepidarkish M, Fazlzadeh A, et al. Update on SARS-CoV-2 seroprevalence: regional and worldwide. Clin Microbiol Infect 2021, 27(12):1762–1771. [CrossRef]
- Mbow M, Lell B, Jochems SP, et al. COVID-19 in Africa: Dampening the storm? Science 2020, 369(6504):624–626. [CrossRef]
- Ngere I, Dawa J, Hunsperger E, et al. High seroprevalence of SARS-CoV-2 but low infection fatality ratio eight months after introduction in Nairobi, Kenya. Int J Infect Dis 2021, 112:25–34. [CrossRef]
- Poustchi H, Darvishian M, Mohammadi Z, et al. SARS-CoV-2 antibody seroprevalence in the general population and high-risk occupational groups across 18 cities in Iran: a population-based cross-sectional study. Lancet Infect Dis 2021, 21(4):473–481. [CrossRef]
- Nwosu K, Fokam J, Wanda F, et al. SARS-CoV-2 antibody seroprevalence and associated risk factors in an urban district in Cameroon. Nat Commun 2021, 12(1):5851. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).