1. Introduction
Cocoyam is a non-woody monocotyledonous perennial plant, which belongs to the
Araceae family and it is one of the six root and tuber crops with great research interest in the world [
1]. Some cocoyam varieties such as
Xanthosoma sagittifolium, popularly known as
tannia, and
Colocasia esculenta, called taro, are important and indispensable plants grown expansively in South-eastern Nigeria. Meanwhile, they are classified as less important humid root crops than cassava, yam and sweet potato. However, it assumes the third position based on its importance after cassava and yam among the root and tuber crops that are cultivated [
2]. Nigeria is the leading producer of cocoyam in the world with an estimated 10 million metric tons in 2016, which was about 40% of the world’s cocoyam production [
3]. However, postharvest losses result in the wastage of a large percentage of the cocoyam produced.
Conversion of harvested cocoyam into some of its products such as flakes, chips and flour, with better storage stability and shelf life is, therefore, recommended to reduce the postharvest losses. The desired end-product determines the unit operations that cocoyam cormel is subjected to. Various pre-treatments and processing methods can be adopted with varying influences on the quality attributes of the final products. Some of these pre-treatments for root and tuber crops processing include soaking, blanching, steaming, fermentation, etc., while common processing methods include cleaning, drying, milling, packaging etc [
4,
5]. Several studies have been carried out to investigate the influence of various pre-treatments, processing, packaging and storage methods on the quality attributes of flours and flour blends from various agricultural materials, to optimise the processing parameters, retain and enhance the product quality over a long period, as well as enhancing the safety of the final consumers. Some of these studies include those carried out on flours from water yam [
6], tannia cormel [
7,
8,
9] Bambara nut [
10], wheat and barley [
11], cassava [
12], soybean [
13], taro [
14] etc. This study, therefore, was designed to investigate the effect of selected pre-treatments and processing methods on the proximate composition of cocoyam (
Xanthosoma sagittifolium) cormel flour.
2. Materials and Methods
2.1. Sample Preparation
Cocoyam (
Xanthosoma sagittifolium) cormels used for this study were purchased from Bodija market, Ibadan, Oyo State, Nigeria. The cocoyam cormels were cleaned to remove the soil and the petiole. It was washed, peeled and sliced into chips of 2-3 cm thickness as shown in
Figure 1. The sliced cocoyam chips were divided into four parts, each weighing 12 kg, for different treatments (soaking, fermentation and steaming) and the control sample. The first portion of the sliced cocoyam was soaked in fresh water at room temperature (28±2
oC) for 18 hours. The second portion of the sliced cocoyam was naturally fermented at room temperature (28±2
oC) for 72 hours. The third portion of the sliced cocoyam was steamed in boiling water with a pressure cooker for 15 minutes while the fourth portion of the sliced cocoyam was not subjected to any pre-treatment and used as the control.
Each of the four portions of the pre-treated cocoyam (soaked, fermented, steamed) and the control were sub-divided into two portions for wet- and dry-milling processes. Dry milling was facilitated by oven drying the pretreated cormels as well as the control using a laboratory oven (Universal Oven UF55, Memmert, Germany) at 70℃ for 10 hours. The flour obtained was sieved using 300 µm laboratory sieve and stored in a low-density polythene (LDPE) bag to minimize interaction with the immediate environment. The wet-milling process was carried out by milling the pre-treated cocoyam cormels after the pre-treatment processes without drying.
2.2. Proximate Analysis of the Cocoyam Flour
2.2.1. Moisture Content Determination
The moisture content of the cocoyam flour was determined by using the vacuum oven method. Five grams of the flour was placed in a drying dish and dried at about 95-100oC, not to exceed a pressure of 100 mm Hg for about 5 hours. When drying was complete, the dish was placed in a desiccator to cool, after which it was reweighed until a constant value was obtained and the loss in weight was reported as the moisture content.
2.2.2. Crude Protein Determination
The crude protein was determined by converting the nitrogen obtained from the flour into ammonia by boiling it with concentrated sulphuric acid in the presence of sodium sulphate as a catalyst. About 2 grams of each sample was weighed and poured into a Kjeldahl flask. Five grams of anhydrous sodium sulphate was added and followed up with the addition of 1 g of copper sulphate and a speck of selenium. Afterward, 25cc concentrated acid and 5 glass beads which prevent bumping during heating were introduced. The solution was heated in the fume cupboard; it was gently heated at first and then the heat was increased with an occasional shaking until the solution assumed a green colour. After cooling, the digest was transferred into a 250cc volumetric flask which was made up to the mark of the distilled water. The digest was distilled using a Markham distillation apparatus.
2.2.3. Crude Fat Determination
The crude fat was determined using the Soxhlet method. Five grams of each sample was weighed into a beaker containing diethyl ether. It was agitated for 5 minutes in a Waring blender. The solvent layer was filtered, and the supernatant was evaporated over low heat to a small volume.
.
It was dried to constant weight in a hot air oven at 65℃. The dried sample was weighed. The difference in weight of the later and the sample weight is the fat content.
2.2.4. Crude Fibre Determination
The crude fibre was determined by weighing 1g of each sample (well ground) into 500 ml conical flask, after which 100 ml of the digestion reagent (trichloroacetic acid) was added by washing it down the sides of the flask. Boiling and refluxing were carried out for 40 minutes, and a water-jacketed condenser was used to prevent loss of liquid. The flask was removed from the heater and cooled under a cold tap. Then filtration was done through a 15 cm of No. 4 Whatman paper. Washing was done 6 times with hot water once in a methylated spirit, the paper was opened and the residue was removed with a spatula. The fibre was transferred to a silica dish. The fibre was dried overnight at 105℃. It was transferred to a desiccator, weighed and cooled. Afterwards, it was ash at 600℃ (overnight) in a muffle furnace. It was cooled in a desiccator and reweighed. The percentage crude fibre was calculated by calculating the percentage of the difference in weighing divided by the weight of the sample. The percentage ash was obtained by weighing accurately 2-5 grams of each finely ground samples into a porcelain crucible, while dust contamination was avoided. The sample was charred on an electric heater to drive off most of the smoke. The sample was transferred into a preheated muffle furnace at 600℃ and heated at the same temperature for 2 hours. The crucible was transferred to a desiccator to cool and then the grey or white ash was weighed. The % Ash was calculated by finding the percentage of the ash weight divided by the sample weight using Equation 1.
2.2.5. Carbohydrate Analysis
Percentage carbohydrate was determined by difference i.e. by subtracting the percentage of water, ash, protein and fat from 100.
2.2.6. Statistical Analysis
The data obtained were analyzed using statistical package for the social sciences, SPSS 20.0 software. The data were treated applying Analysis of variance. The means were separated with the use of Duncan multiple Difference range test, compared by Least Significant Difference (LSD) with mean square error at 5% probability.
3. Results and Discussion
3.1. Moisture Content of Dry and Wet-Milled Flour
The proximate composition of the dry-milled and wet-milled cocoyam flour samples are shown in
Table 1 and
Table 2. The moisture contents of the dry-milled flour samples ranged from 1.17± 0.01% to 5.65 ±0.3%; the maximum value being 5.65 ± 0.03% was obtained in the control followed by the dried soaked sample being 3.64 ± 0.05% while the lowest value was obtained in the dried fermented sample being 1.17±0.01%. This is similar to the trend reported by some researchers [
15], who deduced that the longer the fermentation time, the lesser the moisture content. Hence the fermented sample has the highest storage life, and the highest resistance to microbial spoilage than the other samples, because of its low moisture content. The moisture contents of the wet-milled samples were quite high with a range of 81.16
0.10 % to 89.75
0.07%. The steamed and wet-milled sample had the highest moisture content (89.75
0.07%), followed by the soaked and wet-milled sample (84.16
0.02%), while the fermented and wet-milled sample and the control and wet-milled sample had 81.16
0.02% and 81.72
0.10% respectively. The Fermented and wet-milled sample had the lowest moisture content, which indicates that it has a better storage life and the lowest microbial spoilage capability than the others.
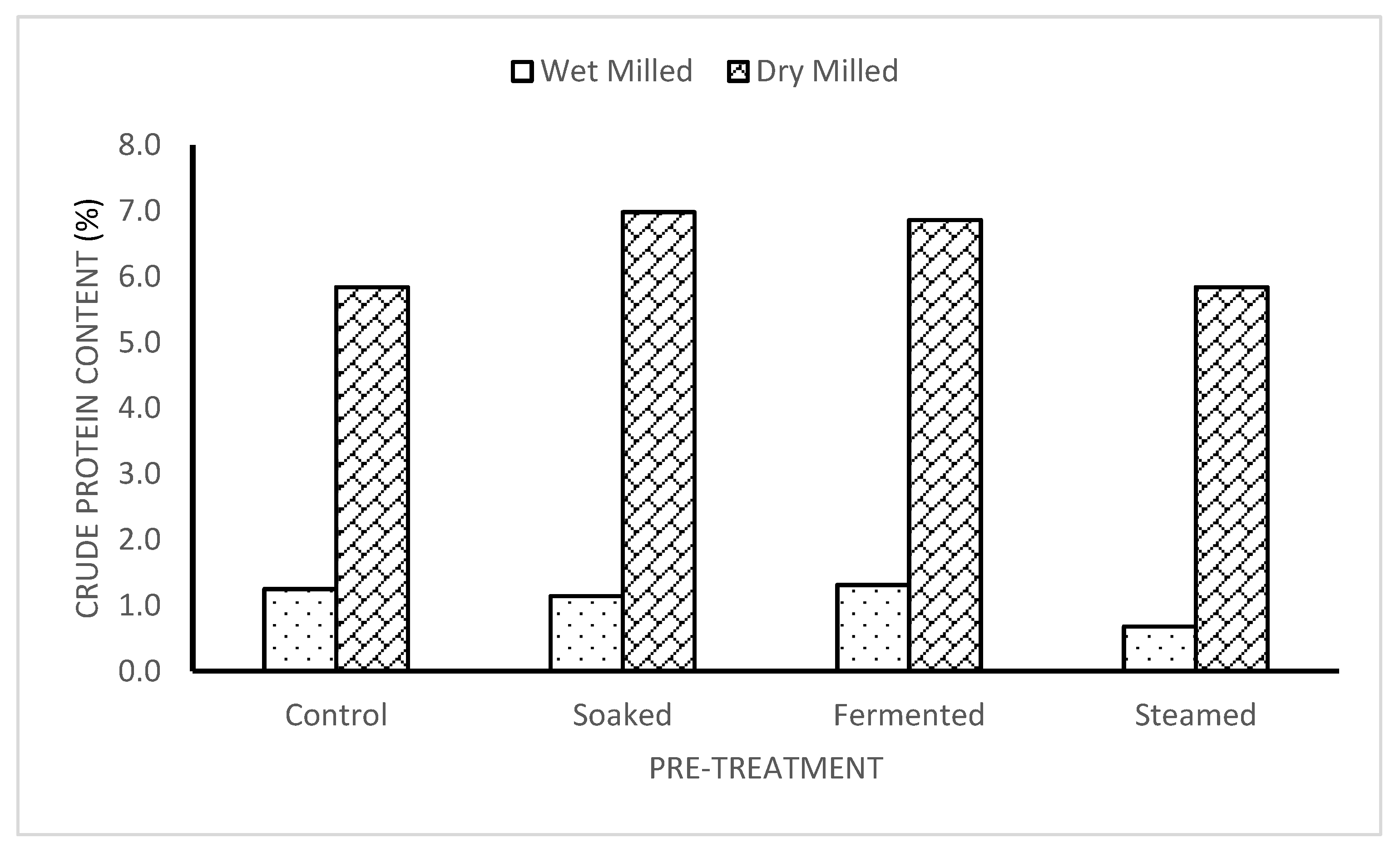
3.2. Crude Protein Content of Dry and Wet-Milled Flour
The crude protein ranged from 5.83±0.10 to 6.98±0.84%. It had the lowest value in the dried steamed sample being 5.83 ± 0.10% while the maximum value was obtained in the dried soaked sample being 6.98± 0.84%, the Control and the dry-milled sample had the same value with the Steamed and dry-milled sample (5.83
0.10). As observed from the work of some researchers [
16,
17], the higher the fermentation time, the higher the crude protein content in the
Colocasia species which is opposite to the result obtained in the
Xanthosoma species, which revealed that the soaked sample (18 hours) had a higher crude protein than the fermented sample (72 hours). The values of the crude fat for the dry-milled samples were very close and were within the range of 0.55
0.04% to 0.61
0.03%. The crude protein of the wet-milled cocoyam samples ranged from 0.68
0.07% to 1.31
0.02%. The crude protein was highest in the fermented wet-milled sample (1.31
0.02%) and had the lowest value in the steamed wet-milled samples (0.68
0.01%).
.
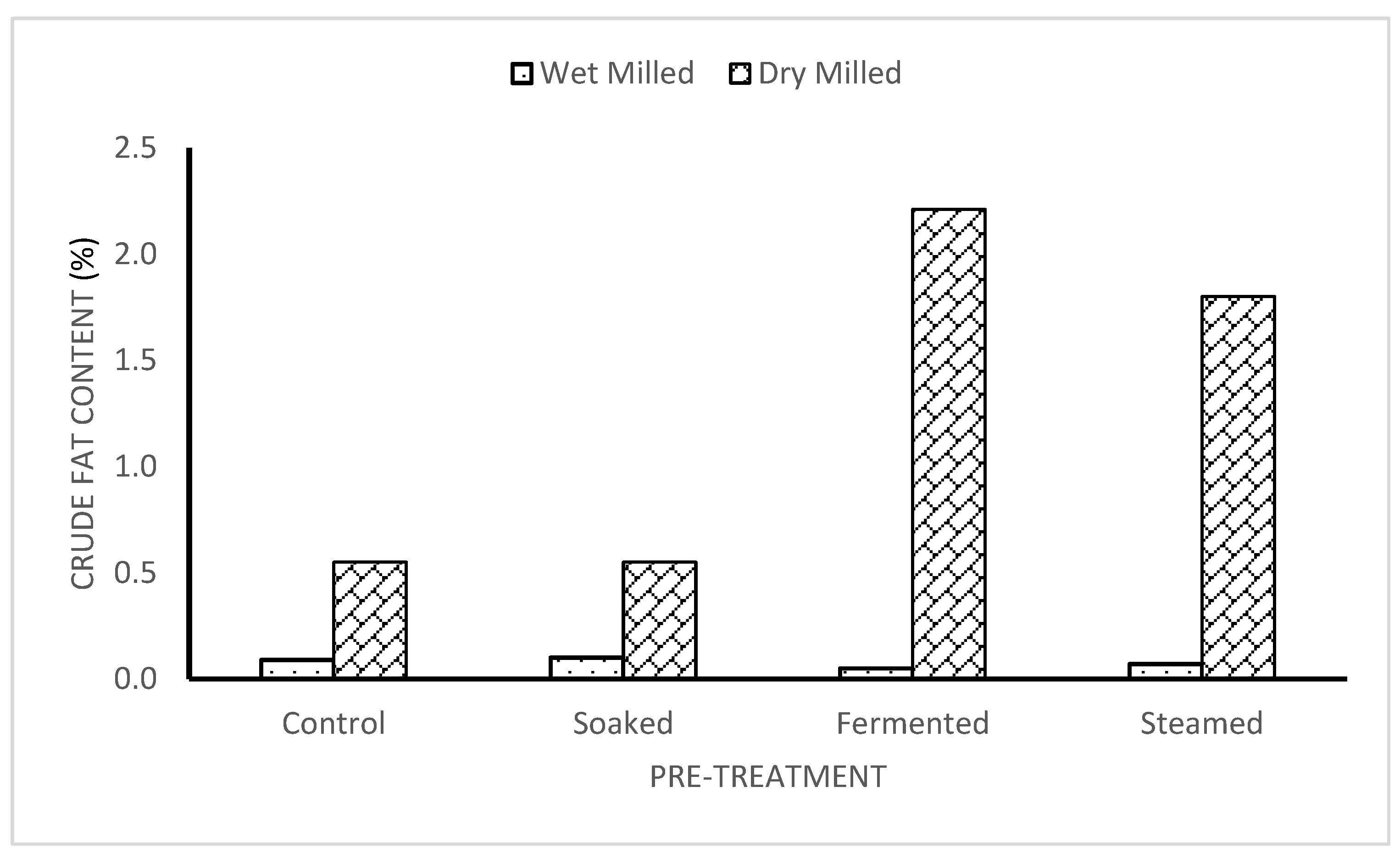
3.3. Crude Fat of Dry and Wet-Milled Flour
The control and dry-milled sample with the soaked and dry-milled sample had the same crude fat content of 0.55
0.04%, which were the lowest. It was revealed from the study that the steamed sample had the highest crude fat content being 0.61
0.03%. This is because foods processed with heat treatments without the immersion of the tuber in water produces the best fat nutrients. The crude fat contents of the wet-milled samples were lower than the dry-milled samples having the range of 0.05
0.01% to 0.1
0.00%. The soaked wet-milled sample retained the highest crude fat, closely followed by the control wet-milled sample (0.09
0.05%). The steamed wet-milled sample had a value of 0.07
0.01%, while the fermented wet-milled sample had the lowest crude fat (0.05
0.01%) which is similar to values obtained by some researchers [
18,
19].
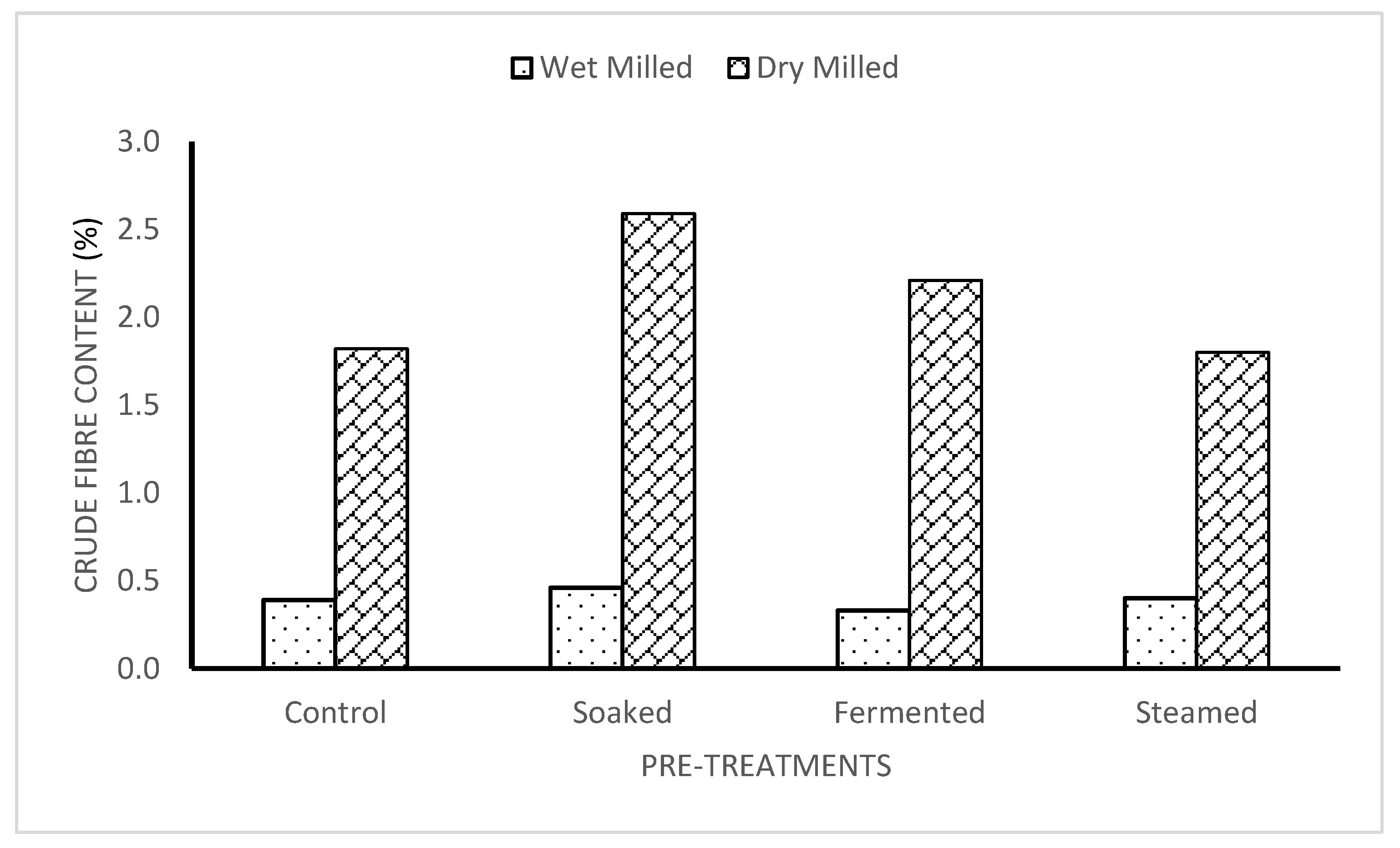
3.4. Crude Fibre of Dry and Wet-Milled Flour
The crude fibre for the dry-milling method was highest in the soaked and dry-milled sample being 2.59
0.02%, followed by the fermented dry-milled and control dry-milled samples which was 2.21
0.02%. The steamed dry-milled sample had the lowest crude fibre content of 1.80
0.02%. The crude fibre of the wet-milled samples had a range of 0.40
0.00% to 0.33
0.01%. The soaked wet-milled sample retained the highest percentage of crude fibre, while the lowest percentage was found in the fermented wet-milled sample [
20,
21,
22].
3.5. Ash Content of Dry and Wet-Milled Flour
The ash contents of the dry-milled flours was found to have the highest value in the steamed dry-milled sample being 4.29
0.01%, followed by the control dry-milled and the soaked dry-milled samples which have the same value (4.21
0.01%) and then lowest in the fermented dry-milled sample (3.75
0.04%). The ash contents of the wet-milled flour ranged from 0.91
0.02% to 0.51
0.01%. The highest percentage was obtained in the control wet-milled sample, followed by the soaked wet-milled sample (0.79
0.01%), while the lowest percentage was in the Steamed and wet-milled sample (0.51
0.01%) [
23].
3.6. Carbohydrate Content of Dry and Wet-Milled Flour
The carbohydrate contents of the dry-milled flour ranged from 83.75
0.11% to 87.62
0.10%. The percentage was highest in the fermented sample being 87.62
0.10%, followed by the steamed sample (87.34
0.06%), soaked sample (84.62
0.03%) and lowest in the control sample (83.75
0.11%) [
24]. The carbohydrate contents of the wet-milled samples ranged from 16.84
0.02% to 8.98
0.07%. The fermented sample possessed the highest carbohydrate content while the steamed had the lowest carbohydrate content [
25,
26].
4. Conclusions
Effects of pre-treatments and milling methods on the proximate composition of cocoyam flour were investigated in this study. Different heat and non-heat treatment methods significantly affected the proximate composition of the cocoyam flour. It was established that cocoyam is not an excellent source of protein and fat, regardless of the processing methods employed. Soaked dry-milled samples can be recommended for patients with high cholesterol, high blood sugar level, irritable bowel syndrome, and other digestive and gastrointestinal diseases because of its high fibre content which aids food digestion and reduces constipation risk. Cocoyam flour from fermented samples had the best nutrients retention for both wet and dry milling methods.
Authors’ contributions:
B.O.O., Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Supervision; Writing original draft; Investigation; E.O.A., Methodology; Review; Execution of research; O.K.F., Review and editing; Data curation; Formal analysis.
Availability of data and materials:
Data will be shared upon request by the readers.
Acknowledgments
The authors wish to express their gratitude to Departments of Agricultural and Environmental Engineering and Food Technology, University of Ibadan, for accessibility to their facilities in the course of this research.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Ekanem, A.M.; Osuji, J.O. Mitotic index studies on edible cocoyams (Xanthosoma and Colocasia species). African journal of Biotechnology 2006, 5, 846–849. [Google Scholar]
- Olayiwola, I.O.; Folaranmi, F.; Adebowale, A.; Onabanjo, O.O.; Sanni, S.A.; Afolabi, W.A.O. Nutritional Composition and Sensory Qualities of Cocoyam-Based Recipes Enriched with Cowpea Flour. Journal of Nutrition & Food Sciences 2012, 2, 2155–9600. [Google Scholar]
- FAO, 2016).
- Owusu-Darko, P.G.; Paterson, A.; Omenyo, E.L. Cocoyam (corms and cormels)—An underexploited food and feed resource. J. Agric. Chem. Environ. 2014, 03, 22–29. [Google Scholar] [CrossRef]
- Oyefeso, B.; Raji, A. Effective Moisture Diffusivity and Activation Energy of Tannia Cormels: Influence of Temperature, Pre-Treatment and Slice Thickness. Niger. J. Technol. 2021, 40, 140–145. [Google Scholar] [CrossRef]
- Adebowale, A.; Owo, H.; Sobukola, O.; Obadina, O.; Kajihausa, O.; Adegunwa, M.; Sanni, L.; Tomlins, K. Influence of storage conditions and packaging materials on some quality attributes of water yam flour. Cogent Food Agric. 2017, 3. [Google Scholar] [CrossRef]
- Oyefeso, B.O.; Ayorinde, M.O.; Ogunlade, C.A.; Fadele, O.K.; Akintola, A. Effect of Blanching Period and Drying Temperature on Selected Physicochemical Properties of Cocoyam (Xanthosoma sagittifolium) Flour. Agric. Food Sci. J. Ghana 2022, 14, 1457–1467. [Google Scholar] [CrossRef]
- Aremu, A.; Oyefeso, B.; Molehin, K. Quality of Cocoyam (Xanthosoma sagittifolium) Flour in Different Packaging Materials. Croat. J. Food Sci. Technol. 2022, 14, 253–260. [Google Scholar] [CrossRef]
- Oyefeso, B.O. , Akintola, A., Akintunde, M.M., Ayandokun, O.C., Fadele, O.K. and Ogunlade, C.A. (2023). Proximate composition of tannia (Xanthosoma sagittifolium) flour as influenced by pretreatment and drying temperature. LAUTECH Journal of Engineering and Technology, 17(2): 58-66.
- Abiodun, A. , and Adepeju, A.B. (2011). Effect of Processing on the Chemical, Pasting and Anti-Nutritional Composition of Bambara Nut (Vigna subterranea L. Verdc) Flour. Advance Journal of Food Science and Technology, 3(4): 224-227.
- nbsp; Narwal, S. ; Gupta, O.P.; Pandey, V., Kumar, D., Ram, S. Effect of storage and processing conditions on nutrient composition of wheat and barley. In Wheat and Barley Grain Biofortification, Eds.; Woodhead Publishing: Sawston/Cambridge, UK, 2020. [Google Scholar]
- Ekwu, F.C.; Ngoddy, P.O.; Uvere, P.O. Effect of processing on the quality of flour, abacha slices and its flour derived from cassava (Manihot esculenta Crantz) TMS 97/4779. Afr. J. Food Sci. 2014, 8, 476–483. [Google Scholar] [CrossRef]
- Agrahar-Murugkar, D.; Jha, K. Influence of storage and packaging conditions on the quality of soy flour from sprouted soybean. J. Food Sci. Technol. 2011, 48, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Ogundare-Akanmu, O.A. , Akande, S.A., Inana, M.E., Israel, D. and Adindu, M.N. (2015). Quality Attributes of Heat Treated Cocoyam (Colocasia esculenta) Flour. Food Science and Quality Management, 37(1): 79-81.
- Igbabul, B.; Hiikyaa, O.; Amove, J. Effect of Fermentation on the Proximate Composition and Functional Properties of Mahogany Bean (Afzelia africana) Flour. Curr. Res. Nutr. Food Sci. J. 2014, 2, 01–07. [Google Scholar] [CrossRef]
- Bolarin, F.M. , Olotu, F.B., Alfa S., Afolabi, R.A. (2017). Effect of Processing Methods on the Functional Properties of Cocoyam Flour. American Journal of Food Science and Nutrition Research; 4(6): 203-206.
- Coronell-Tovar, D.C. Chavez-Jauregui, R.N. Bosques-Vega, A. and Lopez-Moreno, M.L. (2019). Characterization of cocoyam (Xanthosoma spp.) corm flour from the Nazareno cultivar. Journal for Food Science and Technology Campinas, 39(2): 349-357.
- Ejoh, S.I. (2013). Extending the use of an underutilized tuber I: Physicochemical and pasting properties of cocoyam (Xanthosoma sagittifolium) flour and its suitability for making biscuits. African Journal of Food Science, 7(9):264-273.
- Mepba, H.D.; Eboh, L.; Eko, C.B.; Ukpabi, U.J. Composition and pasting properties of starch from two cocoyam cultivars. J. Food Qual. 2009, 32, 522–537. [Google Scholar] [CrossRef]
- Iwuoha, C.I.; Kalu, F.A. Calcium oxalate and physico-chemical properties of cocoyam (Colocasia esculenta and Xanthosoma sagittifolium) tuber flours as affected by processing. Food Chem. 1995, 54, 61–66. [Google Scholar] [CrossRef]
- Nnabuk, O.E. , Emmanuel, E., Eno, E., and Richard, U. (2012). Industrial Potential of Two Varieties of Cocoyam in Bread Making. European Journal of Chemistry 9(1).
- Okpala, L. C. and Ekwe, O. O. (2011).Nutritional quality of cookies produced from mixtures of fermented pigeon pea, germinated sorghum and cocoyam flours. European Journal of Food Research and Review 3(1): 38-49.
- Sefa-Dedeh, S.; Agyir-Sackey, E.K. Chemical composition and the effect of processing on oxalate content of cocoyam Xanthosoma sagittifolium and Colocasia esculenta cormels. Food Chem. 2004, 85, 479–487. [Google Scholar] [CrossRef]
- Ogunlakin, G.; Oke, M.; Babarinde, G.; Olatunbosu, D. Effect of Drying Methods on Proximate Composition and Physico-chemical Properties of Cocoyam Flour. Am. J. Food Technol. 2012, 7, 245–250. [Google Scholar] [CrossRef]
- Ogundare, O.A. Akande, S.A. Inana,M.E.Israel D., Adindu, M.N. (2015). Quality Attributes of Heat Treated Cocoyam (Colocasia esculenta) flour. Food Science and Quality Management.
- Rita, E.S. (2010). Production of Cocoyam, Cassava and Wheat flour Composite rock cake. Pakistan Journal of Nutrition, 9(8):810-814.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).