Submitted:
25 May 2024
Posted:
27 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Part
2.1. Reagents
2.2. (A) Synthesized of ZnO NPs and ZnO NPS composite with ITO NPs
2.2. (B) Preparation of ITO Nanoparticles by Sol-Gel Method
2.3. Preparation of Electrode
2.4. Measurements of Dye Adsorption Levels
2.5. Electrochemical Measurements (I-V)
3. Results and Discussions
3.1. Characterizations of Composites
3.2. Optical Properties
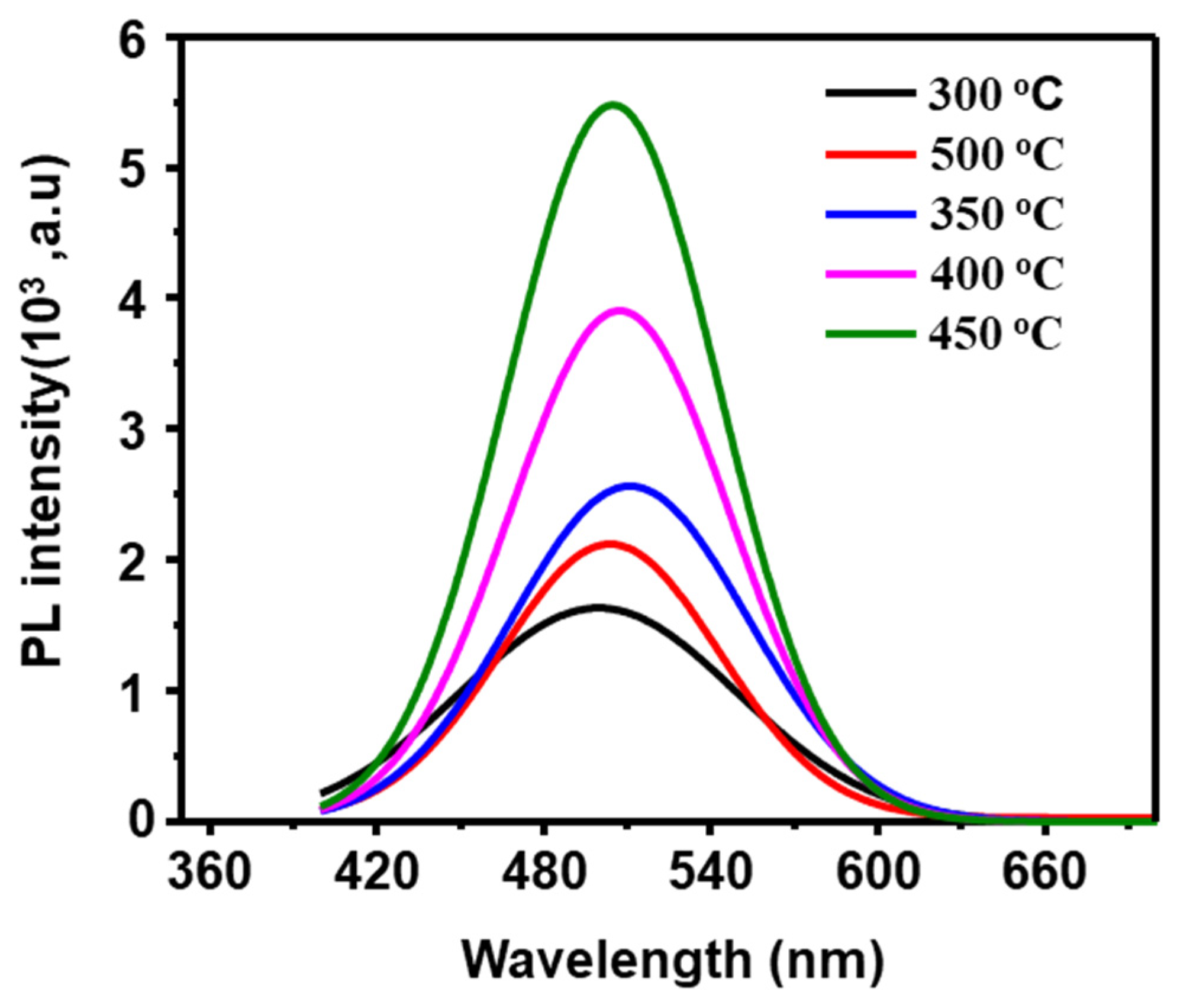
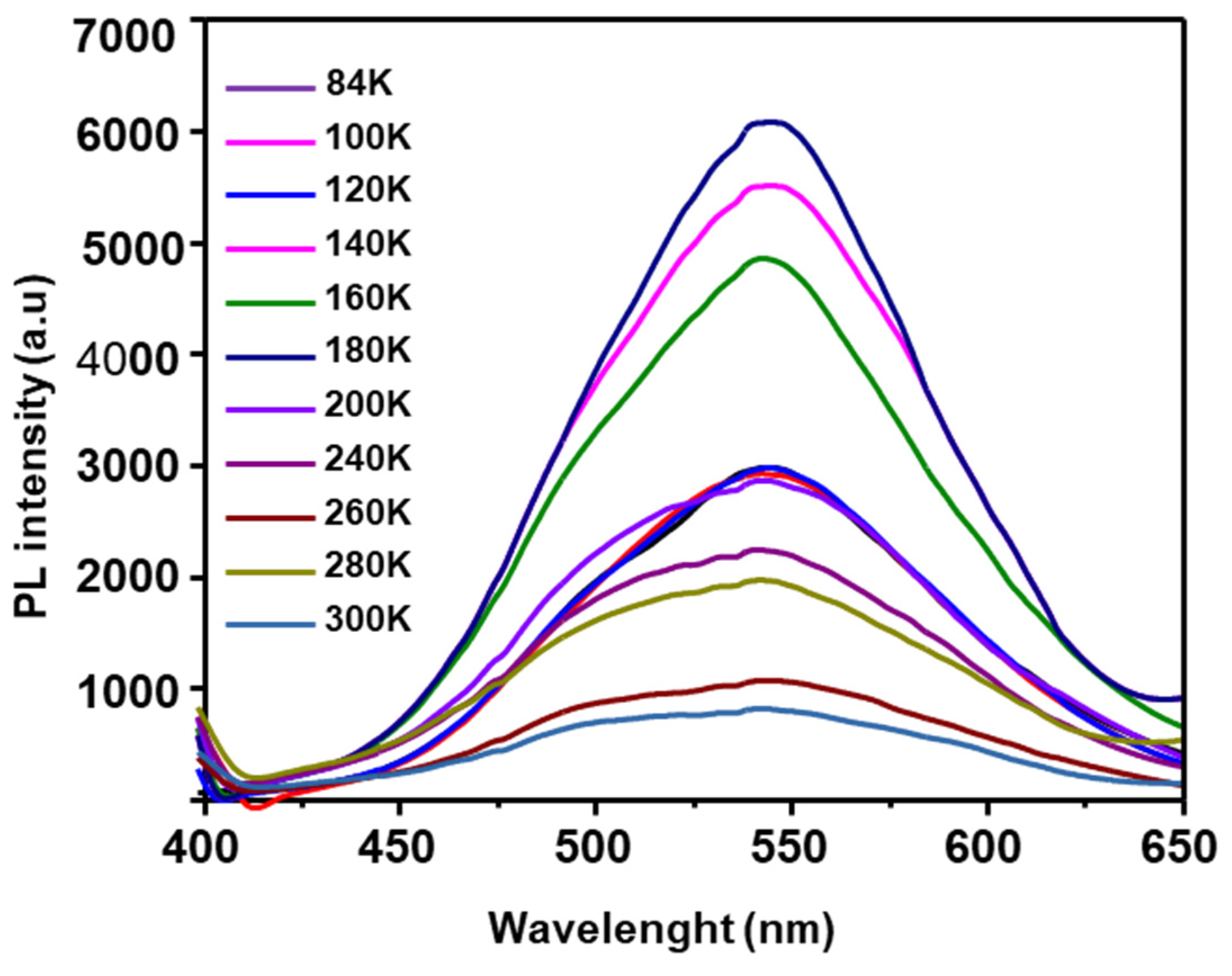
3.3. Photoluminescence Properties
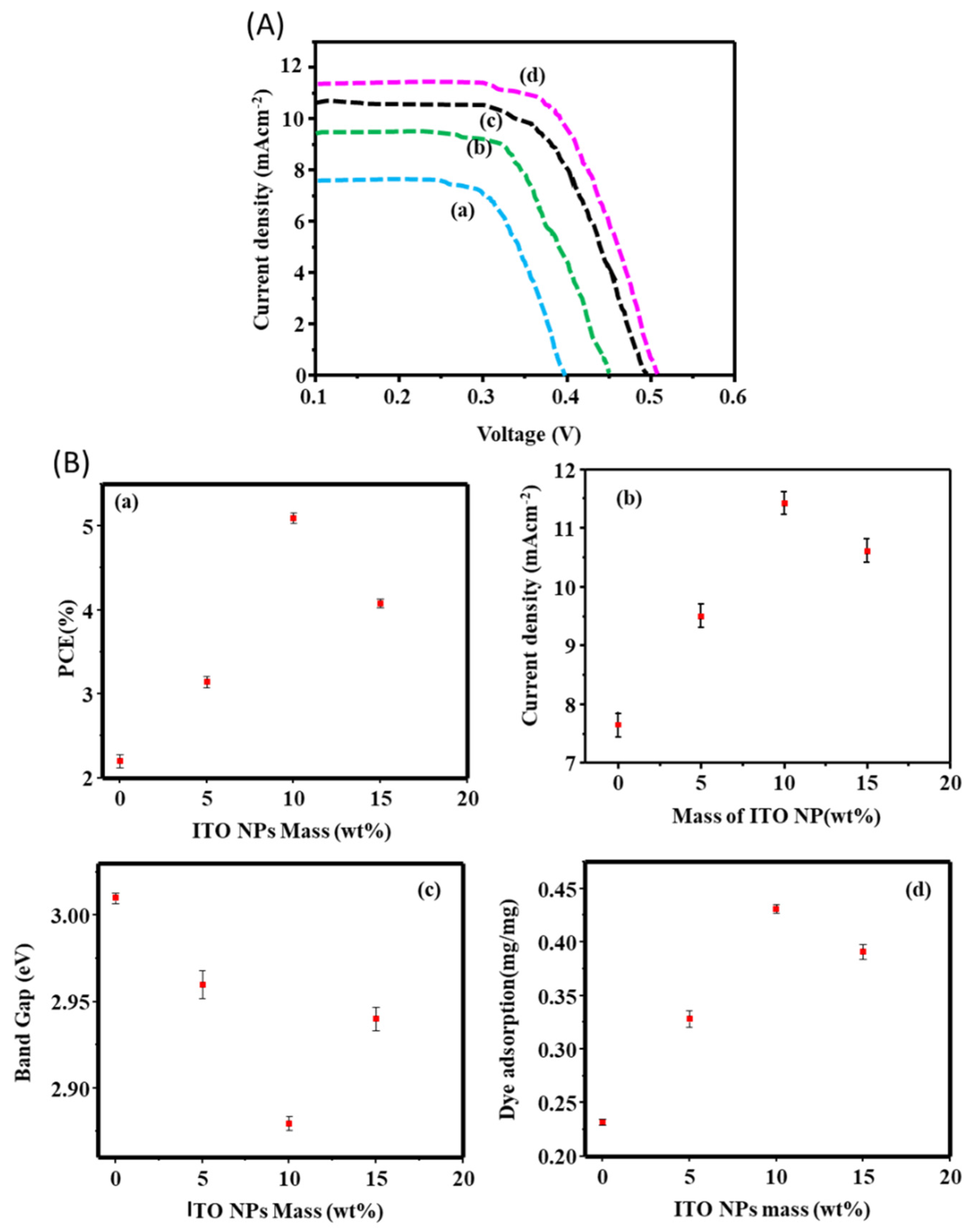
3.4. I-V Performance of F DSSCs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- G. Li, L. Sheng, T. Li, J. Hu, P. Li, K. Wang, Engineering flexible dye-sensitized solar cells for portable electronics, Solar Energy 177 (2019) 80-98. [CrossRef]
- D. Devadiga, M. Selvakumar, P. Shetty, M.S. Santosh, The integration of flexible dye-sensitized solar cells and storage devices towards wearable self-charging power systems: A review, Renewable and Sustainable Energy Reviews 159 (2022) 112252. [CrossRef]
- A.K. Shukla, K. Sudhakar, P. Baredar, A comprehensive review on design of building integrated photovoltaic system, Energy and Buildings 128 (2016) 99-110. [CrossRef]
- H.M. Ahmed, A. Roy, M. Wahab, M. Ahmed, G. Othman-Qadir, B.H. Elesawy, M.U. Khandaker, M.N. Islam, T.B. Emran, Applications of nanomaterials in agrifood and pharmaceutical industry, Journal of Nanomaterials 2021 (2021) 1-10. [CrossRef]
- J. Hänig, B. Weller, Experimental investigations and numerical simulations of innovative lightweight glass–plastic-composite panels made of thin glass and PMMA, Glass Structures & Engineering 6 (2021) 249-271. [CrossRef]
- H. Hassan, P. Sharma, M.R. Hasan, S. Singh, D. Thakur, J. Narang, Gold nanomaterials–The golden approach from synthesis to applications, Materials Science for Energy Technologies 5(2022) 375-390. [CrossRef]
- P. Pandey, Role of nanotechnology in electronics: A review of recent developments and patents, Recent Patents on Nanotechnology 16 (2022) 45-66. [CrossRef]
- A.H.H. Asal, S.N.T. Al-Rashid, Effects of Quantum Confinement Energy on the Transmittance of Cadmium Telluride (CdTe) Within the Near Infrared Region (700-2500nm), East European Journal of Physics (2023) 329-333. [CrossRef]
- H.F. Etefa, V. Kumar, Hybrid Photocatalyst Nanomaterials in Solar Cell Applications, Multifunctional Hybrid Semiconductor Photocatalyst Nanomaterials: Application on Health, Energy and Environment, Springer, Switzerland AG,2023, pp. 221-238. [CrossRef]
- D. Sudha, E.R. Kumar, S. Shanjitha, A.M. Munshi, G.A. Al-Hazmi, N.M. El-Metwaly, S.J. Kirubavathy, Structural, optical, morphological and electrochemical properties of ZnO and graphene oxide blended ZnO nanocomposites, Ceramics International 49 (2023) 7284-7288. [CrossRef]
- R. Suganya, A. Revathi, D. Sudha, V. Sivaprakash, E.R. Kumar, Evaluation of structural, optical properties and photocatalytic activity of Ag2O coated ZnO nanoparticles, Journal of Materials Science: Materials in Electronics 33 (2022) 23224-23235. [CrossRef]
- Z.M. Almarhoon, T. Indumathi, E.R. Kumar, Optimized green synthesis of ZnO nanoparticles: evaluation of structural, morphological, vibrational and optical properties, Journal of Materials Science: Materials in Electronics 33 (2022) 23659-23672. [CrossRef]
- B. Ranjithkumar, E.R. Kumar, M. Srinivas, H. Ramalingam, C. Srinivas, G. Magesh, A. Balamurugan, C.S. Rahale, B. ChandarShekar, Evaluation of structural, surface morphological and thermal properties of Ag-doped ZnO nanoparticles for antimicrobial activities, Physica E: Low-dimensional Systems and Nanostructures 133 (2021) 114801. [CrossRef]
- J. Sahu, S. Kumar, V. Vats, P. Alvi, B. Dalela, S. Kumar, S. Dalela, Lattice defects and oxygen vacancies formulated ferromagnetic, luminescence, structural properties and band-gap tuning in Nd3+ substituted ZnO nanoparticles, Journal of Luminescence 243 (2022) 118673. [CrossRef]
- T. Indumathi, C. Theivarasu, I. Pradeep, M.T. Rani, G. Magesh, C.S. Rahale, E.R. Kumar, Effects of Nd doping on structural, optical, morphological and surface-chemical state analysis of ZnO nanoparticles for antimicrobial and anticancer activities, Surfaces and Interfaces 23 (2021) 101000. [CrossRef]
- S.C.C. Arruda, A.L.D. Silva, R.M. Galazzi, R.A. Azevedo, M.A.Z. Arruda, Nanoparticles applied to plant science: a review, Talanta 131 (2015) 693-705. [CrossRef]
- A. Ali, A.-R. Phull, M. Zia, Elemental zinc to zinc nanoparticles: Is ZnO NPs crucial for life? Synthesis, toxicological, and environmental concerns, Nanotechnology Reviews 7 (2018) 413-441. [CrossRef]
- A.B. Djurišić, X. Chen, Y.H. Leung, A.M.C. Ng, ZnO nanostructures: growth, properties and applications, Journal of Materials Chemistry 22 (2012) 6526-6535. [CrossRef]
- G. Magesh, G. Bhoopathi, A. Arun, E.R. Kumar, C. Srinivas, S. Sathiyaraj, Study of structural, morphological, optical and biomedical properties of pH based ZnO nanostructures, Superlattices and Microstructures 124 (2018) 41-51. [CrossRef]
- R. Mahdavi, S.S.A. Talesh, The effect of ultrasonic irradiation on the structure, morphology and photocatalytic performance of ZnO nanoparticles by sol-gel method, Ultrasonics Sonochemistry 39 (2017) 504-510. [CrossRef]
- H.F. Etefa, D.J. Nemera, F.B. Dejene, Green Synthesis of Nickel Oxide NPs Incorporating Carbon Dots for Antimicrobial Activities, ACS omega 8 (2023) 38418-38425. [CrossRef]
- V. Kumar, H.F. Etefa, L.T. Jule, H.C. Swart, Mechanism, properties and applications of phosphors, Phosphor Handbook, Elsevier, 2023, pp. 33-45. [CrossRef]
- V. Kumar, H.F. Etefa, M.T. Efa, L.T. Jule, Hybrid 1D Semiconducting ZnO and GaN Nanostructures for Light-Emitting Devices, 1D Semiconducting Hybrid Nanostructures: Synthesis and Applications in Gas Sensing and Optoelectronics (2023) 205-216. [CrossRef]
- H.F. Etefa, V. Kumar, F.B. Dejene, M.T. Efa, L.T. Jule, Modification of Flexible Electrodes for P-Type (Nickel Oxide) Dye-Sensitized Solar Cell Performance Based on the Cellulose Nanofiber Film, ACS omega 8 (2023) 15249-15258. [CrossRef]
- A. Umar, R. Kumar, G. Kumar, H. Algarni, S. Kim, Effect of annealing temperature on the properties and photocatalytic efficiencies of ZnO nanoparticles, Journal of Alloys and Compounds 648 (2015) 46-52. [CrossRef]
- ] S. Azizi, R. Mohamad, A. Bahadoran, S. Bayat, R.A. Rahim, A. Ariff, W.Z. Saad, Effect of annealing temperature on antimicrobial and structural properties of bio-synthesized zinc oxide nanoparticles using flower extract of Anchusa italica, Journal of Photochemistry and Photobiology B: Biology 161 (2016) 441-449. [CrossRef]
- D.J. Nemera, H.F. Etefa, V. Kumar, F.B. Dejene, Hybridization of nickel oxide nanoparticles with carbon dots and its application for antibacterial activities, Luminescence 37 (2022) 965-970. [CrossRef]
- K. Thangavel, A. Balamurugan, T. Venkatachalam, E.R. Kumar, Structural, morphological and optical properties of ZnO nano-fibers, Superlattices and Microstructures 90 (2016) 45-52. [CrossRef]
- D.A.M. Osman, M.A. Mustafa, Synthesis and characterization of zinc oxide nanoparticles using zinc acetate dihydrate and sodium hydroxide, J. Nanosci. Nanoeng 1 (2015) 248-251. Available online: http://creativecommons.org/licenses/by-nc/4.0/.
- A. Rahman, M.H. Harunsani, A.L. Tan, M.M. Khan, Zinc oxide and zinc oxide-based nanostructures: biogenic and phytogenic synthesis, properties and applications, Bioprocess and Biosystems Engineering 44 (2021) 1333-1372. [CrossRef]
- V. Sabaghi, F. Davar, P. Rashidi-Ranjbar, A. Abdi, Synthesis and evaluation of pH-responsive mesoporous ZnO/PEG/DOX nanocomposite based on Zn-HKUST-1 MOF nanostructure for targeted drug delivery, Journal of Porous Materials 30 (2023) 201-209. [CrossRef]
- P. Rajiv, S. Rajeshwari, R. Venckatesh, Bio-Fabrication of zinc oxide nanoparticles using leaf extract of Parthenium hysterophorus L. and its size-dependent antifungal activity against plant fungal pathogens, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 112 (2013) 384-387. [CrossRef]
- A.K.Q. Nguyen, T.T. Huynh, V.T.T. Ho, Preparation and characterization of indium doped tin oxide (ITO) via a non-aqueous sol-gel, Molecular Crystals and Liquid Crystals 635 (2016) 32-39. [CrossRef]
- A. Rasool, M. Santhosh Kumar, M. Mamat, C. Gopalakrishnan, R. Amiruddin, Analysis on different detection mechanisms involved in ZnO-based photodetector and photodiodes, Journal of Materials Science: Materials in Electronics 31 (2020) 7100-7113. [CrossRef]
- L. Saikia, D. Bhuyan, M. Saikia, B. Malakar, D.K. Dutta, P. Sengupta, Photocatalytic performance of ZnO nanomaterials for self sensitized degradation of malachite green dye under solar light, Applied Catalysis A: General 490 (2015) 42-49. [CrossRef]
- N. Li, T. Stubhan, N.A. Luechinger, S.C. Halim, G.J. Matt, T. Ameri, C.J. Brabec, Inverted structure organic photovoltaic devices employing a low temperature solution processed WO3 anode buffer layer, Organic Electronics 13 (2012) 2479-2484. [CrossRef]
- S. Padmanathan, A. Prakasam, Incorporation of carbon dots on the ZnO Nanosheets as metal–organic framework Photoanodes for high efficient dye sensitized solar cell applications, Journal of Cluster Science 32 (2021) 795-804. [CrossRef]
- X. Atanacio-Sánchez, W. Pech-Rodríguez, E. Armendáriz-Mireles, J. Castillo-Robles, P. Meléndez-González, E. Rocha-Rangel, Improving performance of ZnO flexible dye sensitized solar cell by incorporation of graphene oxide, Microsystem Technologies (2020) 1-9. [CrossRef]
- C. Bonilha, J.o.E. Benedetti, A.F. Nogueira, A. de Souza Gonçalves, Transparent conducting oxide-free dye-sensitized solar cells based solely on flexible foils, Industrial & engineering chemistry research 51 (2012) 9700-9703. [CrossRef]
- H. Abdullah, S. Mahalingam, K.J. Xian, A. Manap, M.H.D. Othman, M. Akhtaruzzaman, Impedance analysis of charge transfer upon nickel doping in Tio 2-based flexible dye-sensitized solar cell, Polymer Bulletin (2020) 1-14. [CrossRef]
- L. Zhang, A. Konno, Development of flexible dye-sensitized solar cell based on predyed zinc oxide nanoparticle, Int. J. Electrochem. Sci 13 (2018) 344-352. [CrossRef]
- G.F. Gemeda, H.F. Etefa, C.-C. Hsieh, M.A. Kebede, T. Imae, Y.-W. Yen, Preparation of ZnO/NiO-loaded flexible cellulose nanofiber film electrodes and their application to dye-sensitized solar cells, Carbohydrate Polymer Technologies and Applications 3 (2022) 100213. [CrossRef]
- M.T. Efa, T. Imae, Effects of carbon dots on ZnO nanoparticle-based dye-sensitized solar cells, Electrochimica Acta 303 (2019) 204-210. [CrossRef]
- M. Kumari, V.S. Kundu, S. Kumar, S. Siwatch, N. Chauhan, Nitrogen and silver codoped one-dimensional ZnO nanostructure for optoelectronic application, Journal of Sol-Gel Science and Technology 93 (2020) 302-308. [CrossRef]
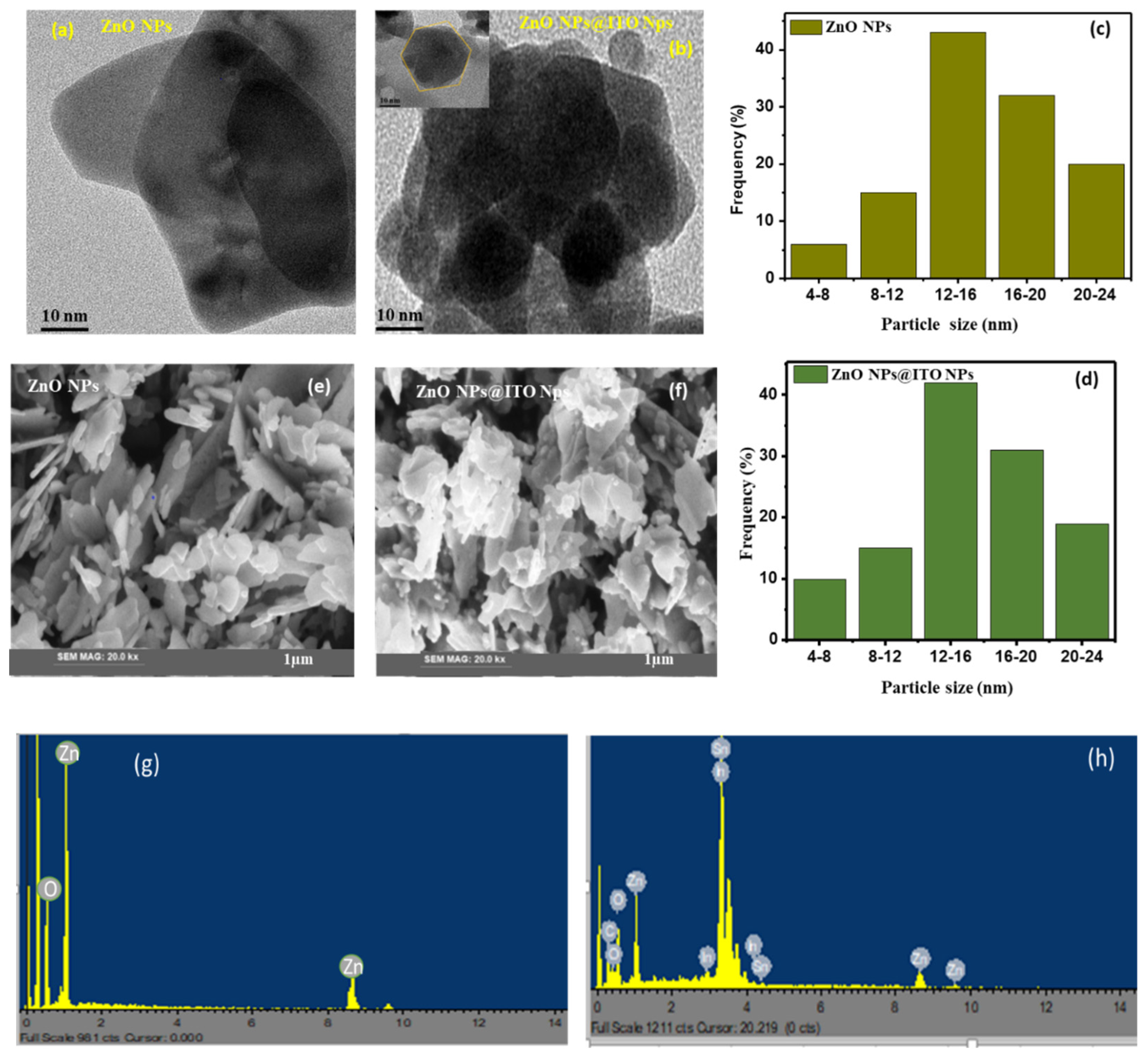
| Materials | Elementa | Wt% | At% |
| ZnO | Zn | 59.99 | 63.24 |
| O | 48.01 | 36.76 | |
| ZnO/ITO | Zn | 34.19 | 37.25 |
| O | 29.14 | 21.23 | |
| Sn | 5.15 | 13.51 | |
| In | 30.02 | 26.20 | |
| C | 1.5 | 1.81 | |
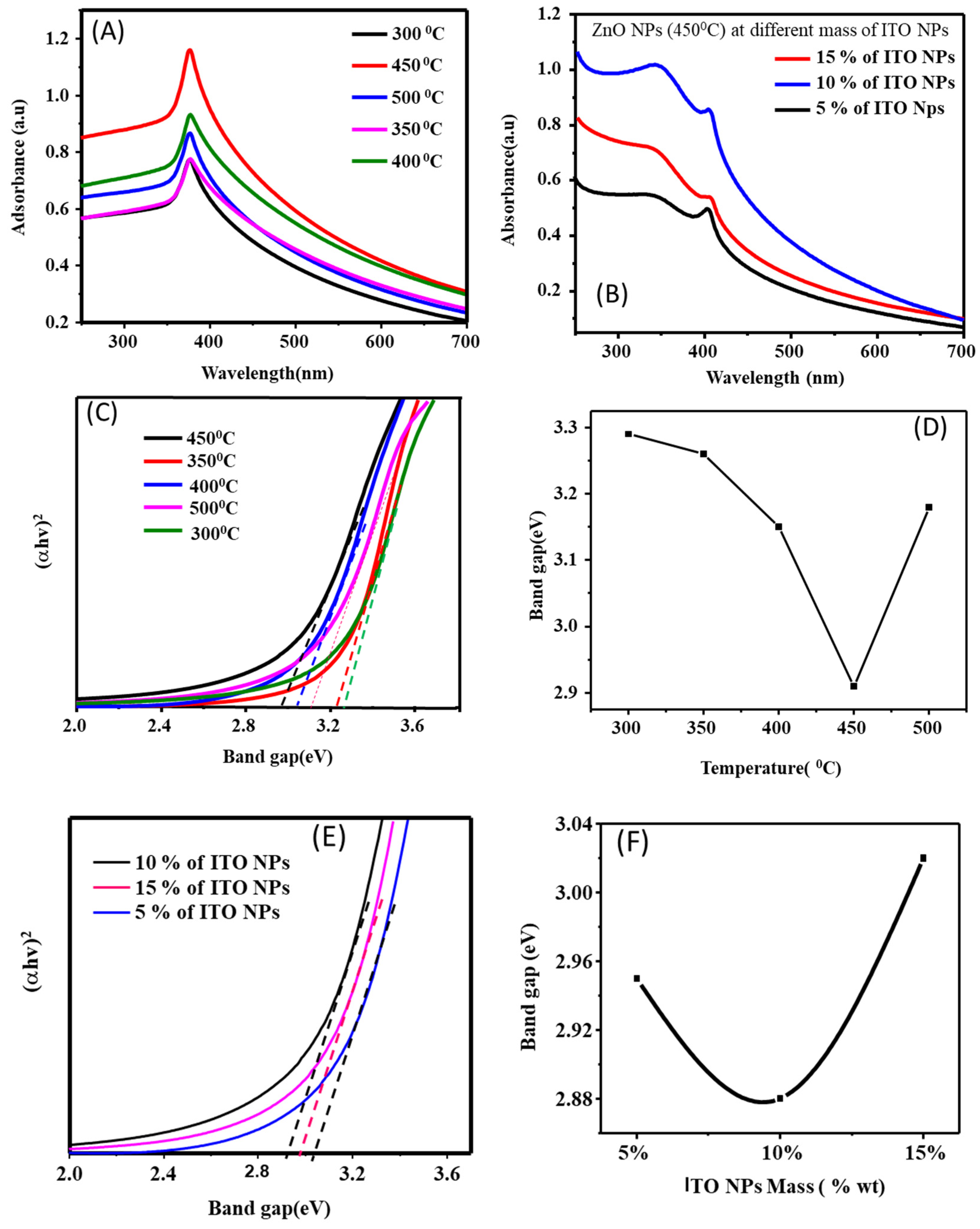
| Materials | Annealing temperature | Band gap(eV) | |
| ZnO NPs | 300 0C | 3.24 | |
| 350 0C | 3.22 | ||
| 400 0C | 3.09 | ||
| 450 0C | 3.01 | ||
| 500 0C | 3.12 | ||
| ZnO NPs at different wt % ITO NPs |
At 450 0C | ||
| 5 wt% | 2.96 | ||
| 10 wt% | 2.88 | ||
| 15 wt% | 2.94 | ||
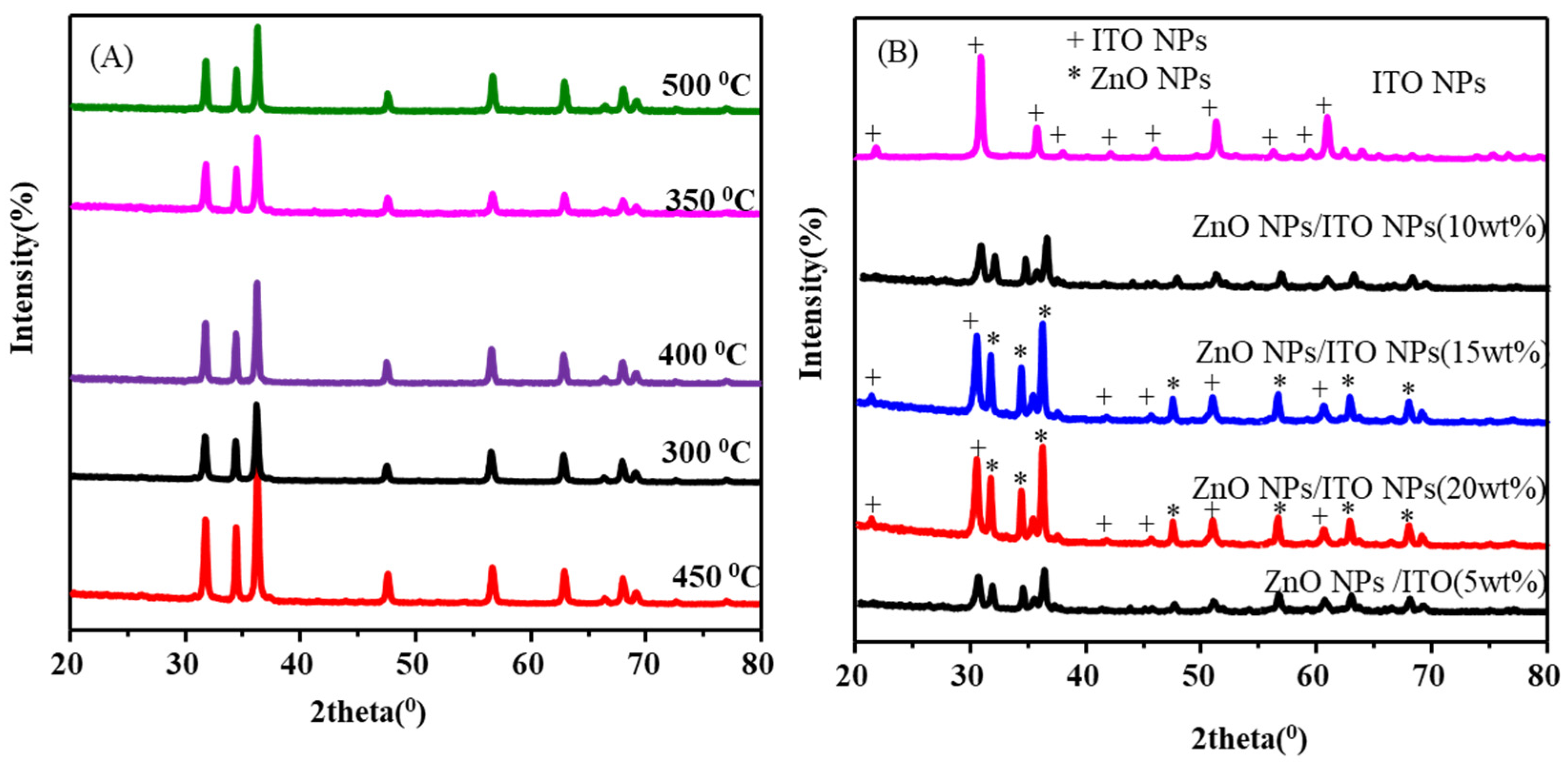
| (A) | ZnO NPs (d-spacing, indices, and 2theta) at the different annealing temperatures | ||||||||||
| 300oC | d-(Ȧ) | 2.811 | 2.551 | 2.465 | 1.923 | 1.642 | 1.467 | 1.430 | 1.373 | 1.362 | |
| hkl | 100 | 002 | 101 | 102 | 110 | 103 | 200 | 112 | 201 | ||
| 2θ(0) | 31.72 | 34.44 | 36.25 | 47.55 | 56.51 | 62.85 | 66.34 | 67.87 | 69.02 | ||
| 350oC | d-(Ȧ) | 2.831 | 2.622 | 2.456 | 1.931 | 1.621 | 1.466 | 1.421 | 1.372 | 1.363 | |
| hkl | 100 | 002 | 101 | 102 | 110 | 103 | 200 | 112 | 201 | ||
| 2θ(0) | 31.74 | 34.45 | 36.24 | 47.54 | 56.56 | 62.85 | 66.33 | 67.88 | 69.06 | ||
| 400oC | d-(Ȧ) | 2.822 | 2.630 | 2.448 | 1.940 | 1.651 | 1.471 | 1.450 | 1.376 | 1.365 | |
| hkl | 100 | 002 | 101 | 102 | 110 | 103 | 200 | 112 | 201 | ||
| 2θ(0) | 31.71 | 34.48 | 36.27 | 47.56 | 56.55 | 62.84 | 66.42 | 67.83 | 69.12 | ||
| 450oC | d-(Ȧ) | 2.821 | 2.612 | 2.466 | 1.921 | 1.640 | 1.468 | 1.420 | 1.374 | 1.361 | |
| hkl | 100 | 002 | 101 | 102 | 110 | 103 | 200 | 112 | 201 | ||
| 2θ(0) | 31.73 | 34.42 | 36.26 | 47.53 | 56.53 | 62.87 | 66.35 | 67.86 | 69.04 | ||
| 500oC | d-(Ȧ) | 2.801 | 2.60 | 2.460 | 1.925 | 1.646 | 1.462 | 1.421 | 1.375 | 1.357 | |
| hkl | 100 | 002 | 101 | 102 | 110 | 103 | 200 | 112 | 201 | ||
| 2θ(0) | 31.69 | 34.38 | 36.27 | 47.60 | 56.55 | 62.82 | 66.41 | 67.91 | 69.30 | ||
| ITO NPs | d-(Ȧ) | 4.079 | 2.906 | 2.511 | 2.372 | 2.139 | 1.969 | 1.777 | 1.633 | 1.551 | 1.519 |
| hkl | 211 | 222 | 400 | 411 | 332 | 431 | 440 | 433 | 611 | 622 | |
| 2θ(0) | 21.77 | 30.74 | 35.72 | 37.90 | 42.21 | 46.04 | 51.35 | 56.27 | 59.55 | 60.92 | |
| (B) | ZnO NPs/ITO NPs (d-spacing, indices and 2theta) | ||||||||||
| ZnO NPs/ ITO NPs |
d-(Ȧ) | 4.124 | 2.924 | 2.820 | 2.607 | 2.473 | 2.387 | 2.156 | 1.981 | 1.906 | 1.786 |
| hkl | 211 | 222 | 100 | 002 | 101 | 411 | 332 | 431 | 102 | 440 | |
| 2θ(0) | 21.53 | 30.54 | 31.70 | 34.37 | 36.29 | 37.65 | 41.86 | 45.76 | 47.67 | 51.09 | |
| d-(Ȧ) | 1.623 | 1.525 | 1.474 | 1.402 | 1.376 | 1.355 | |||||
| hkl | 110 | 622 | 103 | 200 | 112 | 201 | |||||
| 2θ(0) | 56.66 | 60.65 | 62.98 | 66.65 | 68.04 | 69.28 | |||||
| D/t Wt% of ITO NPs deposited on ZnO NPs | Isc(mAcm-2) | Vov(V) | FF | η% | N719 adsorption (mg/mg) |
| 0 | 7.65±0.13 | 0.395±0.0 | 0.291±0.03 | 2.198±0.14 | 0.232 |
| 5% | 9.500±0.02 | 0.450±0.04 | 0.294±0.04 | 3.142±0.04 | 0.3281 |
| 10 wt% | 11.420±0.14 | 0.52±0.0 | 0.450±0.02 | 6.680±0.12 | 0.431 |
| 15 wt% | 10.610±0.02 | 0.4930±0.0 | 0.312±0.03 | 4.079±0.03 | 0.391 |
| Materials | WE | CE | Method | PCE (%) | Reference |
| Zn2SnO4 | StSta/Zn2SnO4 nanoparticle | Pt-foil | calcination | 0.55 | [36] |
| Polymer dye A (conc.) + ZnO | ITO/Glass | carbondust flamed/ITO glass | doctor blade | 3.5 | [37] |
| ZnO | ZnO/PET | Graphite | electrophoretic deposition | 0.4499 | [38] |
| TNPs (TiO2)85-Ni15 |
StSta/TNPs ITO/PET |
StSt/Pt Pt |
deposition Sol-gel |
1.34 0.92 |
[39] [40] |
| ZnO | PET | Graphite | calcination | 0.45 | [41] |
| ZnONP@Cdot | ZnONW/Cdot/TOCNF | Pt-PPy/TOCNF | 1.34 | [42] | |
| ZnO@C-dots | ITO/PET | Pt/FTO | doctor blade | 5.81 | [37] |
| ZnO | ITO/PET | Pt/FTO | doctor blade | 2.45 | [37] |
| ZnO NP | ITO/glass | Pt/glass | Sola-gel | 2.90 | [43] |
| ZnO NP@C-dots | ITO/glass | Pt/glass | Sola-gel | 5.92 | [43] |
| NiO/C-dots | TOCNF/PPY | Ni(OH)2/TOCNF | deposition | 1.3 | [24] |
| ZnO | S5-ZnO/ITO/glass | Pt/ITO /glass | Spin Caoting | 5.105 | [44] |
| ZnO NPs | ZnO NPs /PET | Pt /PET | Spin coating | 2.198 | This work |
| ZnO NPs/ITO NPs | ZnO NPs/ITO NPs /PET | Pt /PET | Spin Coating | 6.680 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





