1. Introduction
Alcoholic liver injury (ALI) is a liver disease associated with long-term alcoholism. The initial manifestation of ALI is alcoholic fatty liver, which can gradually progress into liver failure and liver cancer. With increasing incidence, ALI is becoming a public health concern globally [
1,
2]. It is estimated that about 20% of drinkers worldwide suffer from varying degrees of alcoholic liver diseases, and three million die each year from excessive alcohol consumption [
3]. Considering the increasing socioeconomic costs, there is a high clinical need to prevent the occurrence and development of ALI of all stages [
4].
Currently, there is a lack of standard guidance on the prevention and treatment of chronic ALI. It is still an unmet challenge to effectively alleviate the symptoms. Existing therapeutic approaches include the abstinence from alcohol, nutritional support, medication, and liver transplantation [
5]. Besides modern medication and surgery, traditional Chinese medicine (TCM) provides a low-cost, non-invasive option. TCM offers diverse strategies targeting multiple components, pathways, and molecular targets, with the potential of avoiding drug-induced liver injury. Some clinical trials have initially demonstrated the treatment efficacy of TCM for ALI [
6,
7]. With its unique advantages, TCM has become a research hotspot in the treatment of ALI.
Cistanche tubulosa (Schenk) Wight is a parasitic plant, which is mainly distributed in the deserts of Northwest China. The dried succulent stems of
Cistanche tubulosa (CT) and
Cistanche deserticola (CD) are used as a famous tonic medicine known as Cistanches Herba, or Rou Cong Rong in the Chinese Pharmacopoeia. It has been known as “desert ginseng” with potentials for the treatment of ALI. [
8,
9]. It was various pharmacological activities, such as the functions of enhancing immune function, anti-aging, anti-radiation, anti-oxidation, anti-lipid peroxidation, which commonly play a protective role against ALI [
10,
11]. Evidence showed that a polysaccharide component isolated from the CD could reduce the levels malondialdehyde (MDA) and triglycerides in blood by regulating the activity of related enzymes to relieve ALI [
12]. Wang et al. reported that CD significantly restored the intestinal microbiota composition in mice with ALI, which highlights the potential of CD and CT in preventing and treating ALI by modulating the gut-liver axis [
13]. Yan et al. performed in vivo experiment in mice and found that the extracts of CD might regulate lipid metabolism by activating PPAR α, promoting β-oxidative metabolism, and reducing fatty acids accumulation [
14]. Despite the potentials of CT for treating ALI, the active components and molecular mechanisms underlying the hepatoprotective effects are not clear.
Network pharmacology provides a powerful tool for analyzing the mechanisms of TCM [
15]. By integrating bioinformatics, biochemistry, pharmacology and computational science, network pharmacology uses computational methods to simulate complex drug metabolisms, analyze mechanisms of action, and predict treatment efficacy. It provides important support for the development of new drugs and the medicines optimization. The core of network pharmacology is to establish a network model between the drugs and the molecules in organisms, in order to reveal the interrelationship between drugs and target proteins, metabolic pathways, and cell signaling pathways. With recent advances in computational sciences, network pharmacology is playing an increasingly important role in the field of medicine, contributing the improvement of treatment efficacy and in-depth pathophysiological research [
16].
In this study, network pharmacology was applied to construct the "composition-disease-target-pathway" network diagram, and then protein interaction and target enrichment analysis were conducted to predict the relevant targets and pathways of CT in the treatment of ALI-induced liver fibrosis. Through molecular docking technology and animal experiments, the pharmacological effects of CT's active components in the treatment of liver fibrosis were investigated.
2. Materials and Methods
2.1. Collection of CT Ingredients and Target Genes
Using “Citicola tubulosa” as a keyword, we searched the Traditional Chinese Medicine Systems Pharmacology database and BATMAN TCM Analysis Platform database to identify the bioactive components and target proteins under the condition of Scorecutoff higher than 0.9 (LR=124) and
P value less than 0.05. The obtained targets were calibrated by Uniprot (
https://www.uniprot.org/) data, with non-human genes and invalid duplicates deleted to obtain standardized gene names.
2.2. Acquisition of ALI-Associated Target Genes and Disease-Drug Intersection
Using ‘alcohol liver injury’ as a keyword, we searched the Online Mendelian Inheritance in Man (OMIM,
https://omim.org/) and GeneCards (
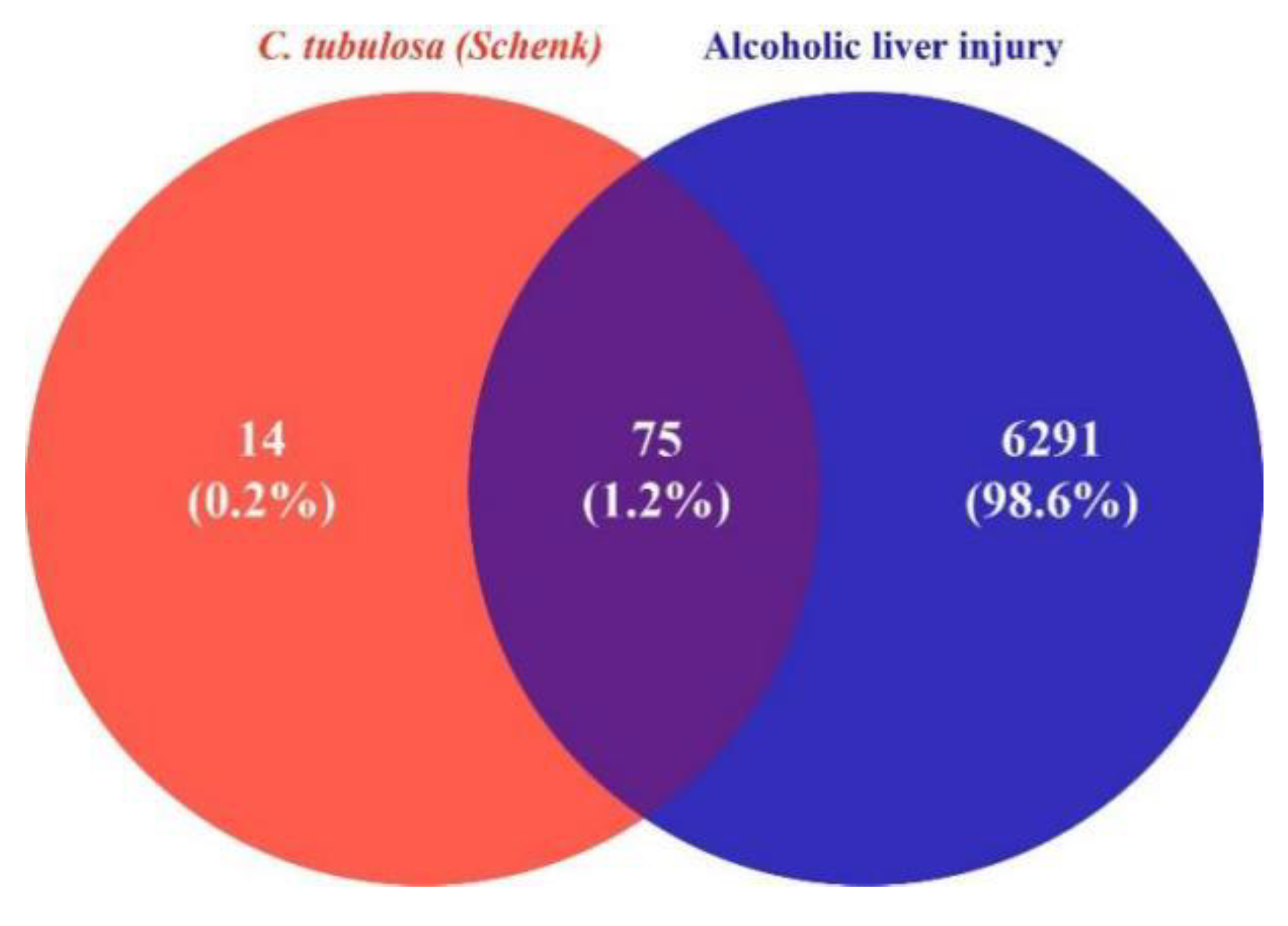
https://www.genecards.org/) databases to determine ALI-associated target genes. All targets of the two databases were integrated into an excel table with duplicate genes removed, and corrected by Uniprot database to obtain the gene information of disease targets. R language was used to create a script that compared the CT- and ALI-associated target genes, generating a Venn diagram to obtain the common targets, i.e., the disease-drug intersection.
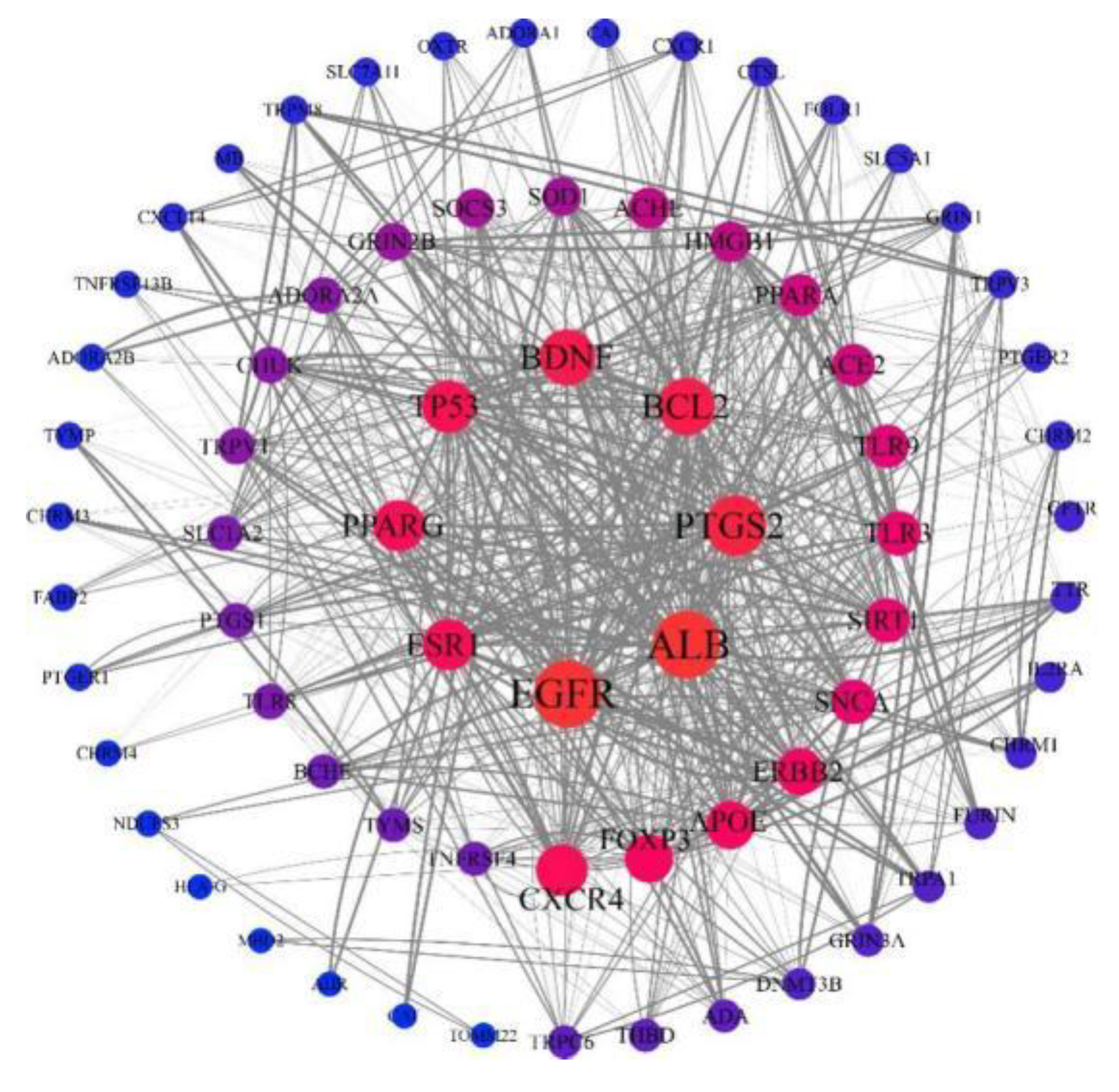
2.3. Construction of Target Protein Interaction Network for Drug-Disease Target Prediction
To predict the protein-protein interactions (PPI) during the treatment, the target genes of disease-drug intersection were input into string database (
https://string-db.org/) for PPI analysis. The visualization and topology analysis of the PPI network were carried out on Cytoscape software (version: 3.8.2,
www.cytoscape.org) which is an open-source bioinformatics software platform for visualizing molecular interaction networks.
2.4. Gene Ontology Enrichment Analysis and KEGG Pathway Analysis
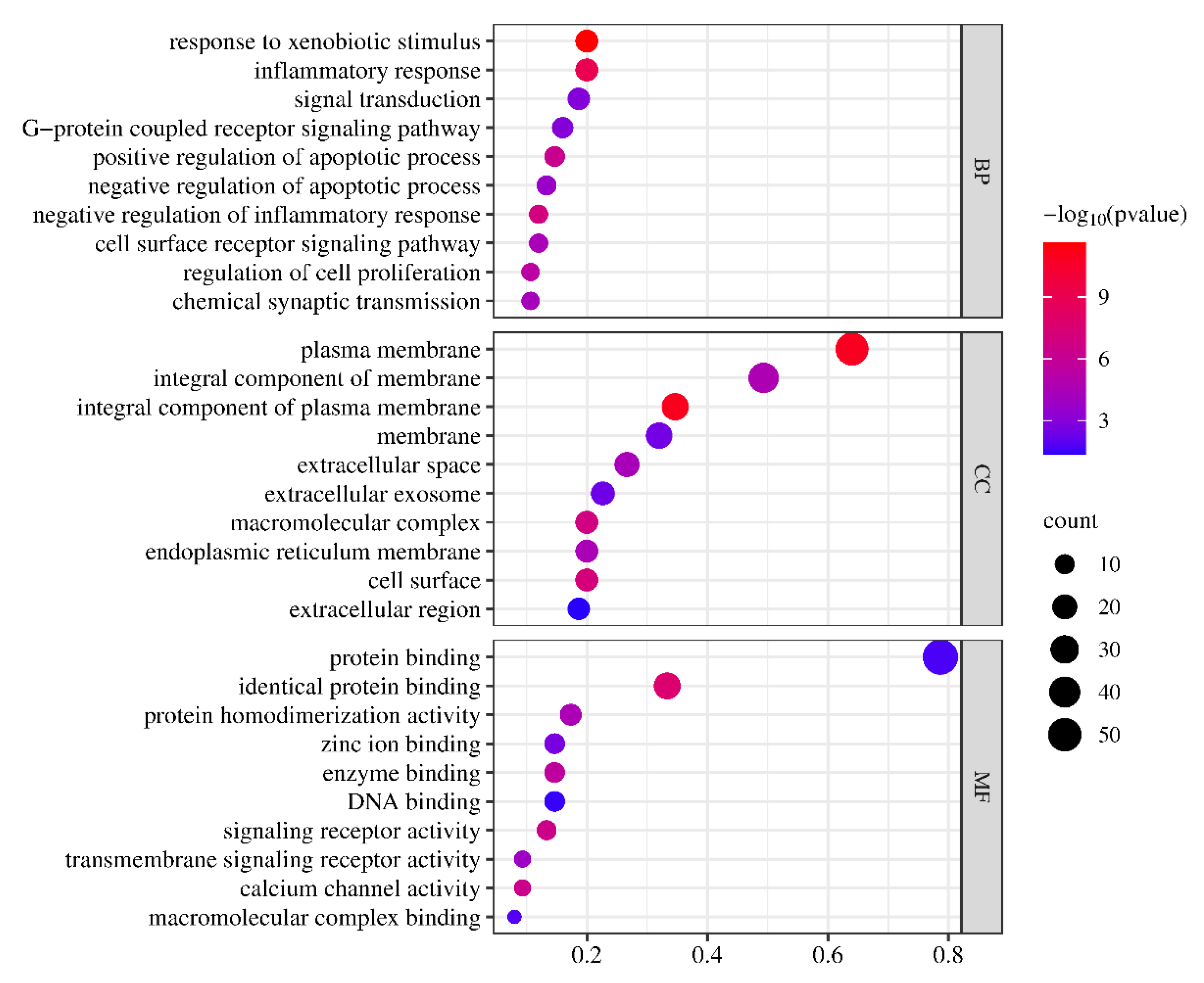
The obtained key target proteins in PPI were input into the DAVID database (version: 6.8,
https://david.ncifcrf.gov/summary.jsp) to retrieve relevant data on biological processes (BPs), cellular components (CCs), and molecular functions (MFs), which commonly reflected the role of target protein and gene function during the treatment of ALI by CT. To elucidate the target genes of CT, gene ontology (GO) enrichment analysis of KEGG pathway was performed. The top 10 BPs, CCs, and MFs in GO function, and 20 related pathways identified from the Kyoto Encyclopedia of Genes and Genomes (KEGG) database ranked by
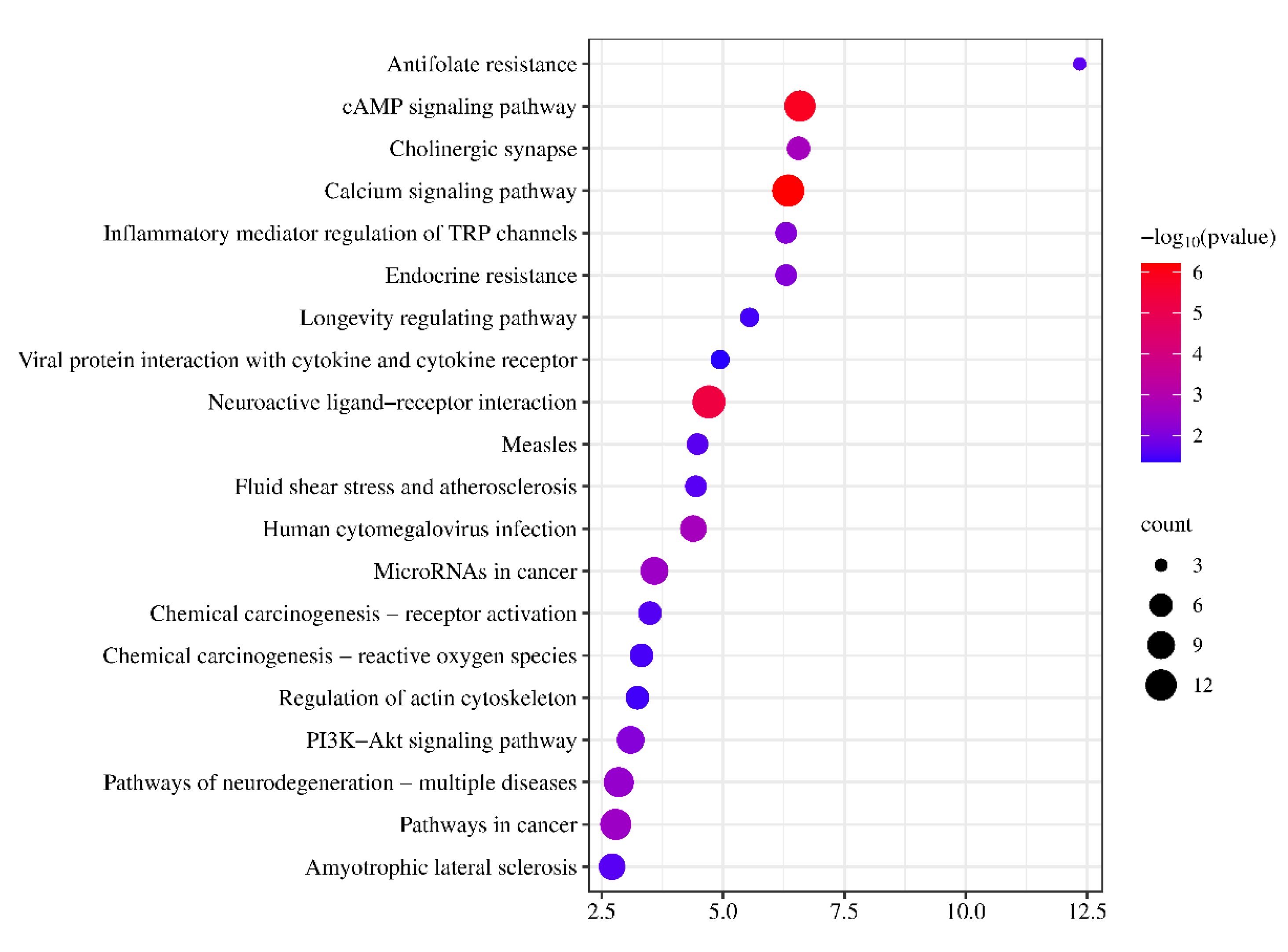
P values were selected to predict the mechanism of CT in treating ALI. KEGG is a collection of pathway maps including metabolic pathways, signaling pathways, pathways involved in various cellular processes and organismal systems, and perturbed pathways associated with human diseases.
2.5. Molecular Docking
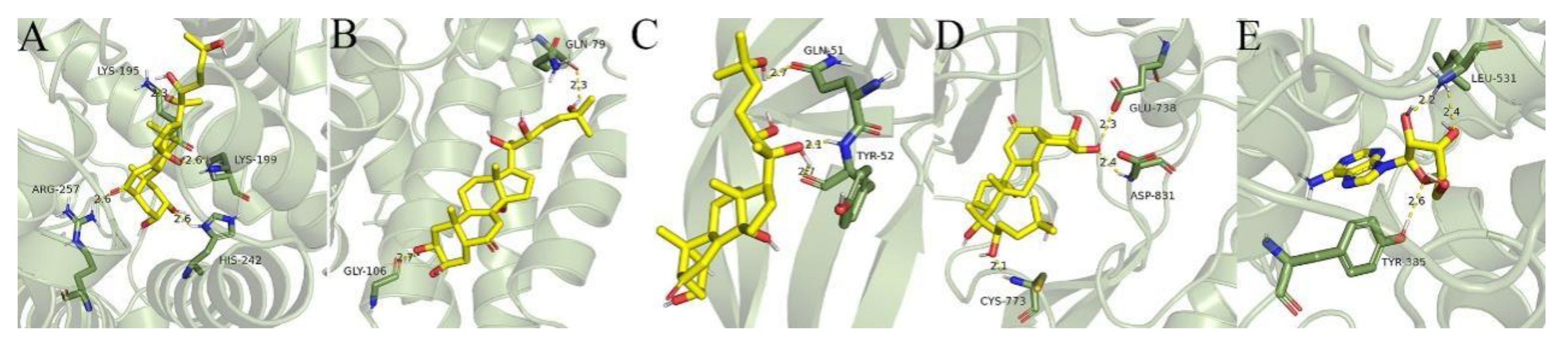
In general, proteins with high degrees of connection in the PPT network play an important role in the treatment [
19]
. The first 5 important targets were selected for semi-flexible docking with compounds with high degree values, and the binding energy (affinity) was used to indicate the quality of the binding of small molecules and target proteins. The negative binding energy indicates that small molecules and target proteins can be freely bound, and the smaller the value, the higher the possibility of binding.
The AutoDock Vina 1.1.2 software was used for molecular docking of active ingredients and key targets to investigate their interactions. The steps are as follows: (1) Download the compounds (i.e., active ingredients) in SDF format from PubChem database (
https://pubchem.ncbi.nlm.nih.gov) and import them into Chembio3D (version 12 for Windows, CambridgeSoft, Cambridge, MA, USA) for energy minimization. The processed compounds were import into AutodockTools software (version: 1.5.6) for hydrogenation, charge calculation, and charge distribution, with the rotable key set. The results were saved in "pdbqt" format. (2) Download key target proteins from PDB database (
http://www.rcsb.org/). The human proteins with high structural similarity between the original ligand and the active ingredient to be docked were preferred. The one with the highest resolution was selected. (3) The selected protein was imported into PyMoL (The PyMOL Molecular Graphics System, version: 2.3.0, Schrödinger, LLC) to remove the original ligand and water molecules. Then the protein was imported into AutoDocktools for hydrogenation, charge calculation, charge allocation, and atom type designation, with results saved as "pdbqt" format. (4) POCASA (version: 1.1,
http://g6altair.sci.hokudai.ac.jp/g6/service/pocasa/) was used to predict protein binding sites. The size of the lattice box was set to 60×60×60 (spacing of each lattice was 0.375A), with remaining parameters set as default. (5) Interaction simulation.
2.6. Animal Experiment
A mouse model of ALI was established, and 30 Kunming mice with average body mass of (25±2) g were randomly divided into normal control (NC) group, model group and CT extract (300 mg/kg) group. CT extract was administrated intragastrically for 15 days. The NC group and the model group were injected with equal volume normal saline by gavage. 1 h after the last administration, the model and CT extract groups were given 10 mL/kg ethanol with a volume fraction of 56%. After 12 hours, orbital blood was taken and cervical vertebrae was dislocated, and the liver was immediately dissected and stored at -80 ℃.
2.7. Pathological Observation Based On Liver Histopathology
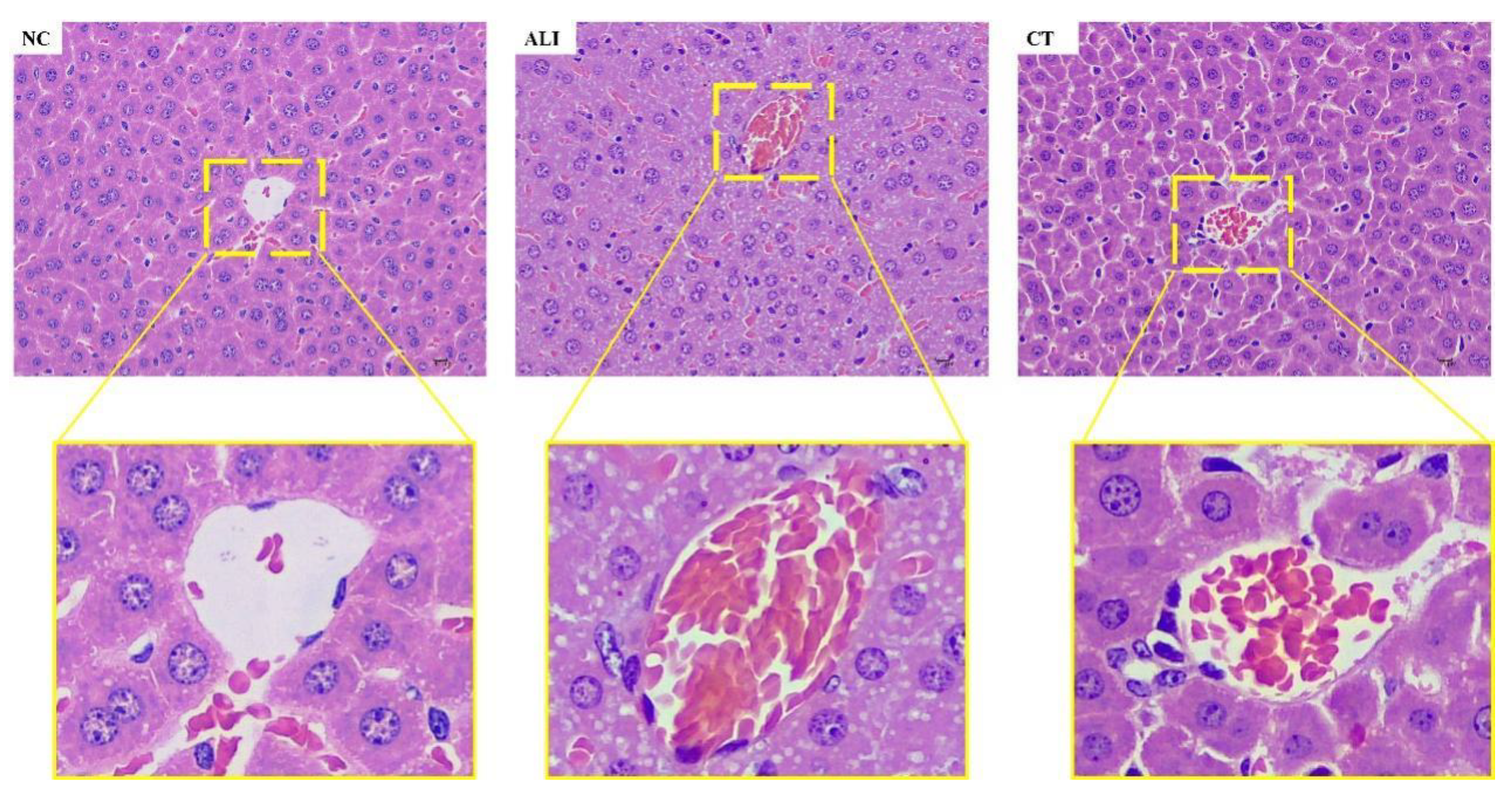
Liver histopathology is the gold standard to judge the degree of liver injury and has been widely used in various liver injury models. HE stained pathological section of liver tissue can directly reveal liver tissue damage for clinical evaluation.
Liver tissue was taken, fixed with 4% formaldehyde solution, rinsed with water, dehydrated, transparentized, impregnated with wax, embedded, sliced, and stained by haematoxylin and eosin (HE). The pathological changes of liver tissues were observed with a 40-fold objective microscope.
2.8. Cell Experiment
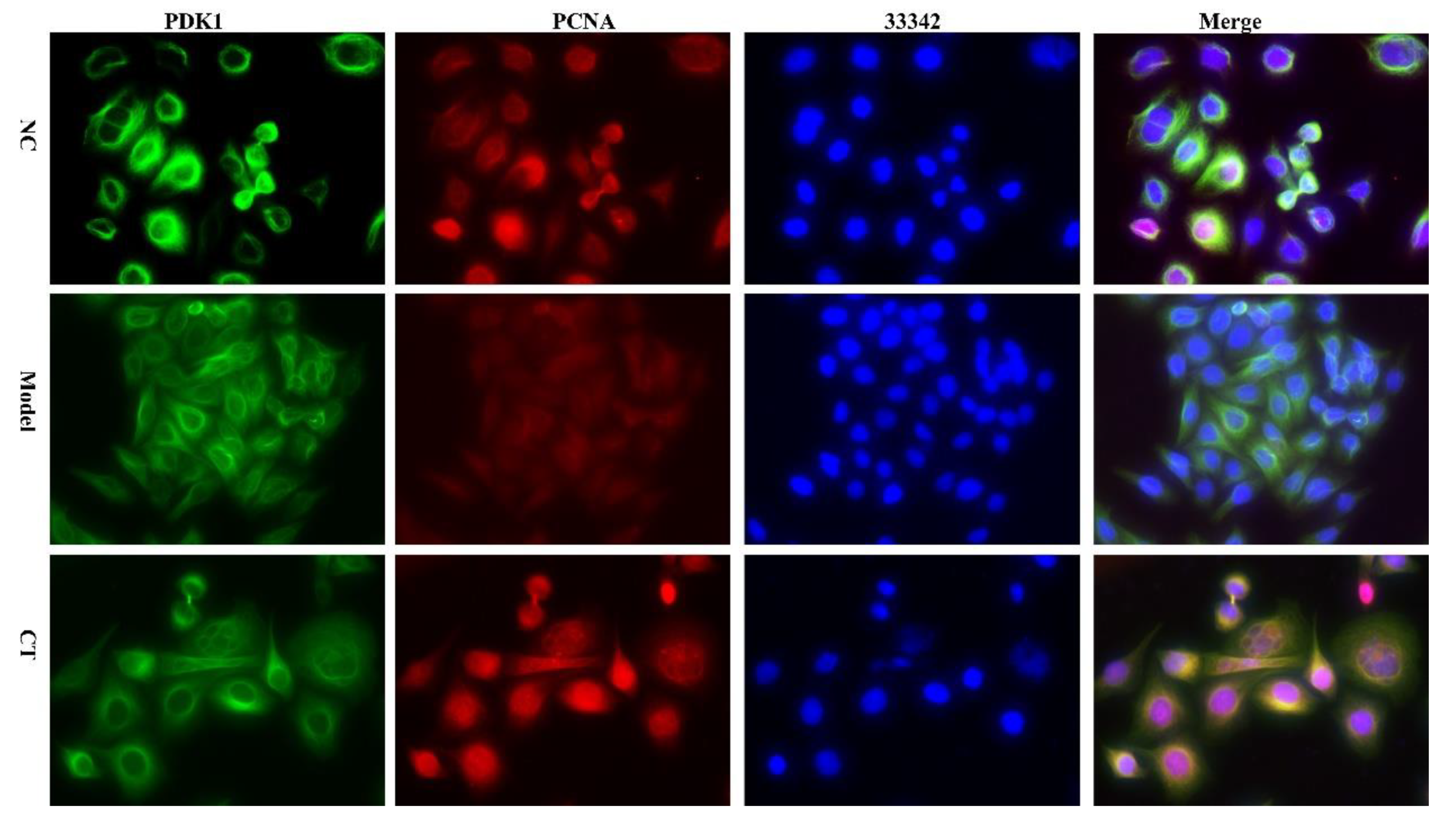
HepG2 cells were inoculated in a 96-well plate with 1.5×104 cells per well. After 12 h, the control group was added with normal medium, the drug administration group and the alcohol model group were pretreated with medium containing 732 mmol/L ethanol per well for 6 h, then the drug administration group was treated with medium containing CT extract (10 μg/mL) for 18 h, and the model group was treated with medium containing 732 mmol/L ethanol for 18 h.
2.9. Statistical Analysis
The data were expressed as the mean ± standard deviation. All the graphing and statistical analyses were performed using GraphPad Prism software (version: 8.0, Graph Pad Software Inc., San Diego, CA, USA). Multiple groups were compared using one-way ANOVA, followed by the least significant difference post hoc test, and p < 0.05 was considered to indicate statistical significance.
4. Discussion
Drinking is prevalent globally and there have been different forms of alcohol comsumption throughout human civilization [
18]. According to the 2018 National Survey on Drug Abuse and Health, more than 80 percent of adults in the USA have drunk alcohol at least once in their lives, and 25 percent of those surveyed admitted to binge drinking. Currently, alcoholic liver diseases are also emerging as a new public health problem in China [
20]. It is difficult to fully estimate the actual amount of alcohol consumed worldwide with uniform figures, but recent studies have defined about 2.3 billion people worldwide as alcoholics [
19]. The evolution of alcoholic liver disease from ALI has become a recognized disease burden and there is a crying need for efficient treatment [
21].
The initial damage of chronic ALI generally starts from hepatic fatty lesions. The accumulation of alcohol in the liver induces the increase of peroxidation products and fat production, resulting in the accumulation of fat molecules in the liver tissue. Hepatic steatosis is a reversible stage, and the liver can regenerate and repair itself after alcohol withdrawal. On the other hand, an untreated steatosis of the liver can lead to alcoholic hepatitis, a persistent inflammation of the liver and the appearance of Molly bodies in the liver tissue. Caused by chronic inflammation, long-term hepatitis can lead to liver fibrosis, in which collagen tissue replaces healthy cells in the liver, leading to the formation of scar tissue characterized by tissue dysfunction. Cirrhosis is often the last stage before liver failure. The only viable treatment option for liver failure is a liver transplant supported by alcohol withdrawal and steroid therapy. In addition, long-term alcohol consumption can lead to hepatocellular carcinoma, which often develops at the same time as advanced cirrhosis.
Chinese herbal medicine is effective in clinical practice but mechanisms are difficult to clarify due to the complexity of components, which limits the widespread applications in the context of precise medicine [
22]. In recent years, network pharmacology has been developed into an effective way to observe the interactions between drugs and diseases, providing a power tool for TCM investigation. Some researchers have used network pharmacology to identify active ingredients of TCM and predict the mechanisms. For example, Wang et al. found that Shicao could prevent acute liver injury through metabolomics and network pharmacology [
23]. Li et al. proposed an integrated strategy of network pharmacology, serum pharmacochemistry and animal experiment to verify the treatment efficacy of polyphenols extracted from Penthorum chinense Pursh against ALI [
21].
As shown in PPI network screening, the core targets of CT on acute ALI include EGFR, ALB, PTGS2, BCL2, BDNF, TP53, AKT, ESR1, etc. AKT regulates metabolism, proliferation, cell survival, growth, angiogenesis and other biological processes during cancer progression. BCL-2 inhibits cell death caused by a variety of cytotoxic factors. Overexpression of BCL-2 can enhance the resistance of observed cells to most cytotoxins. This discovery suggests that the various signal transduction pathways of apoptosis share a common pathway or junction that is regulated by BCL-2. BCL-2 could enhance the resistance of cells to most DNA damage factors and inhibit the apoptosis of target cells induced by chemotherapy drugs, but it could not inhibit the consequent damages. Likewise, it does not promote DNA repair. The TP53 gene is a tumor suppressor protein that plays a role in inducing cell cycle arrest, apoptosis, senescence, DNA repair or metabolic changes [
24]. A number of studies have confirmed that pro-inflammatory cytokines BCL2 and AKT participate in the whole course of liver injury and development, and play a key role in the deterioration and severity of liver disease. Studies have shown that the concentration of pro-inflammatory cytokines is positively associated with the severity of liver injury. In summary, the TC can treat acute alcoholic liver injury in an all-round way through multi-target treatment.
As the GO enrichment analysis shows, oxidative stress response is an important factor in the course of liver disease and plays a key role in the progression of liver injury. Cell injury, inflammatory response, oxidative stress, regeneration, and bacterial translocation are key drivers of steatosis to cirrhosis throughout the course of alcoholic liver injury. The analysis of KEGG pathway showed that the local tissue injury was caused by the interaction between the end product of glycation (AGE) and its receptor RAGE, which mediated intracellular oxidative stress and inflammatory response, so the anti-oxidative stress and inflammatory response had a positive effect in the early stage of acute ALI. cAMP signaling pathway is widely distributed in mammalian cells, microorganisms and other organisms, and is involved in the regulation of growth, differentiation, metabolism, immunity, nerve and other physiological processes. The composition of cAMP signaling pathway includes membrane receptors, AC, cAMP, PKA, etc.. Membrane receptors include G protein-coupled receptors (GPCRS) and ion channel receptors, which activate AC when specific external stimuli act on the receptor, resulting in the production of cAMP [
25]. cAMP molecules are able to bind to two CAMP-binding domains in the subunits of PKA and release two catalytic subunits of PKA that activate and phosphorylate downstream target proteins. In addition, the cAMP signaling pathway is also very important for the regulation of the immune system. On T cells, the activation of cAMP signaling pathway inhibits the activation of the cells, thus exerting the immune tolerance. In our study, the animal and cell experiments suggested that CT relieves the inflammation in ALI. On macrophages, the activation of cAMP signaling pathway inhibits the production of NO, thus playing an anti-inflammatory role. cAMP induces the differentiation of cytokines IL-6 and TNF through the release of proinflammatory cytokines, which promotes inflammatory response and the development of acute ALI [
26]. The PI3K/AKT signaling pathway is involved in the process of cell proliferation, differentiation and apoptosis, and is one of the important pathways in the progression from liver injury to liver cancer. Moreover, studies have shown that inflammatory response and oxidative stress are closely related to NF⁃ Sin B/p38MAPK signaling pathway [
27].
Based on computer experiments simulating the docking of core targets and small molecules, the protein pathways related to acute ALI were found, including straight chains and mosaics. This approach may be modified for the optimization of TC-enhanced medication for ALI.
There are limitations in this study. Network pharmacology is based on a network database of identified components, and does not include the interaction between components of different herbal medicines during decoctions and other processing methods. In addition, the animal and cell experiments are for initial validation but did not provide the evidences on the modular level. In future research, it is necessary to include the more secondary components, and add both molecular observation and large-scale clinical studies to validate the results and discovery more TCM components in treating ALI.