1. Introduction
Carotenoids are widespread and versatile compounds that are precursors of derivatives termed apocarotenoids. Both carotenoids and apocarotenoids play key roles in processes that are essential for plant development and resilience (light collection and photoprotection in photosynthesis, communication between plants, pollinators, and seed dispersers, regulation of many plant processes, etc.) and are therefore essential for food security. Carotenoids also function as pigments contributing to the colours of many vegetables and fruits, and as important nutritional components of foods, e.g. as precursors of vitamin A. Many studies indicate that carotenoids have health-promoting properties and contribute to the amelioration or the reduction of the risk of developing diverse diseases (cancer, cardiovascular disease, skin and bone conditions, eye disorders, metabolic disorders, age-related macular degeneration, cognitive impairment, etc.). They can also contribute to skin health, colour and other aesthetic parameters. Therefore, carotenoids are of great interest for the development of a variety of products for human consumption including functional foods, nutraceuticals, supplements, botanicals, cosmeceuticals, or (nutri)cosmetics [
1].
The main bulk of dietary intake of carotenoids comes from plant-derived foods (mainly fruits and vegetables), although they are also present in animal-derived foods (e.g., egg yolk, salmon, mussels, dairy), food colorants, and supplements [
1]. Microalgae also synthesise carotenoids, as well as many other bioactive compounds with health benefits, and are rich in polysaturated fatty acids and in essential amino acids. Compared to terrestrial plants, cultivation of microalgae is more sustainable due to their rapid growth, ease of cultivation, and non-need for agricultural land, and the commercial importance of microalgae for sustainable production of health-promoting foods is growing. Thus microalgae are a potential source of nutrition that would reduce the need for agricultural land use, decreasing the environmental impact of food production, while improving health [
2].
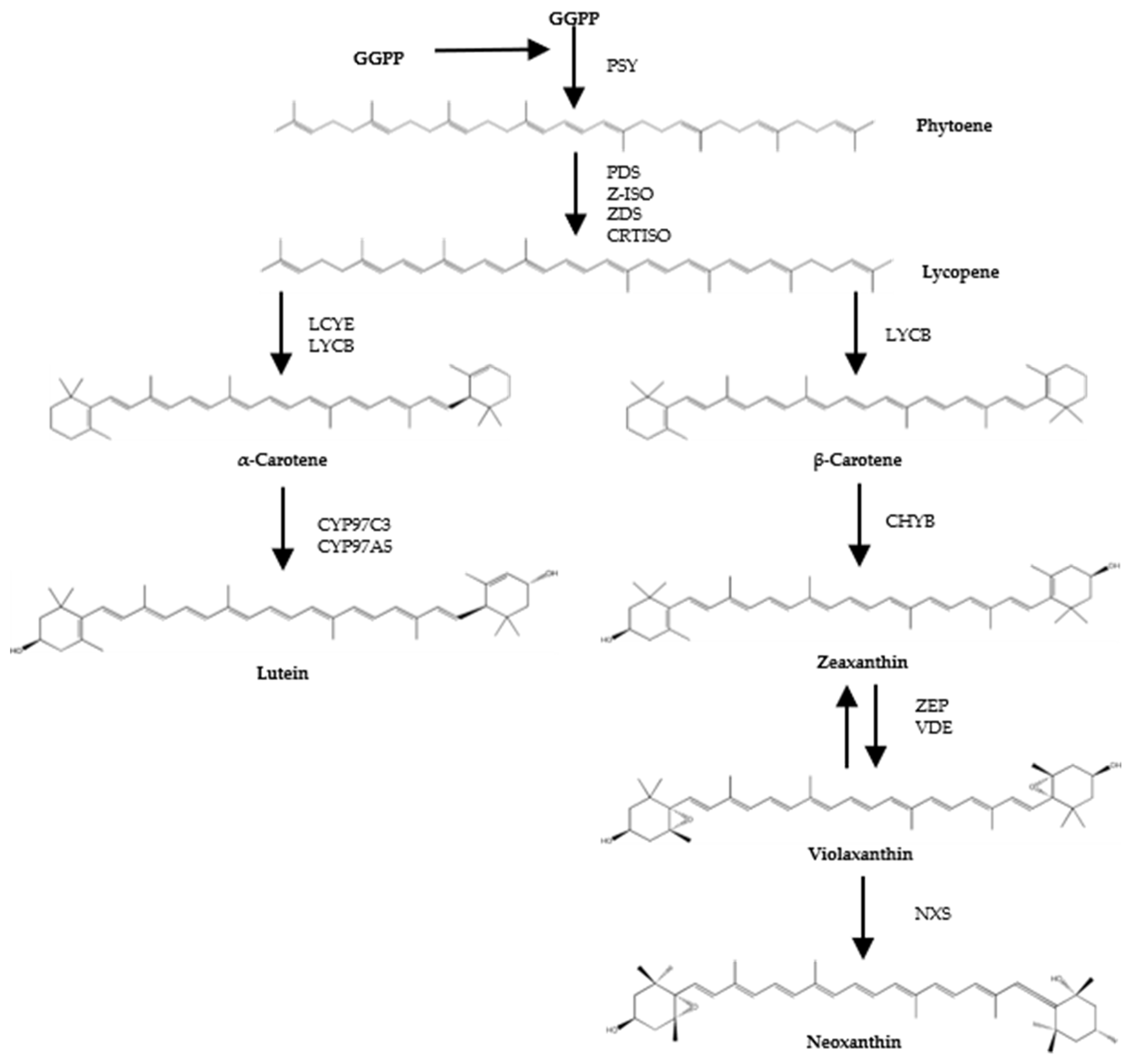
Figure 1.
Scheme of biosynthetic steps of microalgal carotenoids (adapted from Valera J.C. et al. (2015) [
3], and Lichtenthaler H.K. et al (2012) [
4]). GGPP: Geranylgeranyl pyrophosphate; PSY: Phytoene synthase; PDS: Phytoene desaturase; Z-ISO: ζ-Carotene isomerase; ZDS: ζ-Carotene desaturase; CRTISO: Carotene isomerase; LCYE: Lycopene ε-cyclase; LCYB: Lycopene β-cyclase; CYP97C3: Cytochrome P450 ε-hydroxylase; CYP97A5: Cytochrome P450 β-hydroxylase; CHYB: Carotene β-hydroxylase; BKT: β-Carotene oxygenase; ZEP: Zeaxanthin epoxidase; VDE: Violaxanthin de-epoxidase; NXS: Neoxanthin synthase.
Figure 1.
Scheme of biosynthetic steps of microalgal carotenoids (adapted from Valera J.C. et al. (2015) [
3], and Lichtenthaler H.K. et al (2012) [
4]). GGPP: Geranylgeranyl pyrophosphate; PSY: Phytoene synthase; PDS: Phytoene desaturase; Z-ISO: ζ-Carotene isomerase; ZDS: ζ-Carotene desaturase; CRTISO: Carotene isomerase; LCYE: Lycopene ε-cyclase; LCYB: Lycopene β-cyclase; CYP97C3: Cytochrome P450 ε-hydroxylase; CYP97A5: Cytochrome P450 β-hydroxylase; CHYB: Carotene β-hydroxylase; BKT: β-Carotene oxygenase; ZEP: Zeaxanthin epoxidase; VDE: Violaxanthin de-epoxidase; NXS: Neoxanthin synthase.
Biosynthesis of carotenoids occurs through the production of phytoene from geranygeranyl pyrophosphate by the enzyme phytoene synthase. Phytoene subsequently undergoes consecutive desaturation and isomerization steps to form lycopene, which is later cyclised to α- and β-carotene. The latter two can be oxygenated to form xanthophylls such as lutein and zeaxanthin. Zeaxanthin is precursor of other typical carotenoids of green microalga, such as violaxanthin and neoxanthin (
Figure 1). In contrast to most other carotenoids, phytoene is colourless, does not contribute to the characteristic colours of carotenoid-rich foods and is generally viewed as a precursor without bioactivity in the human body. Phytoene is typically not reported in epidemiological studies and the understanding of its impact on physiology is minimal. However, a few studies have shown that daily intake of phytoene is higher than most other carotenoids, and have detected phytoene in plasma and tissues at concentrations suggesting high levels of bioavailability and bioaccumulation [
5,
6,
7].
Here we examine the bioactivity of phytoene-rich carotenoid extractions from the microalgae Dunaliella bardawil and Chlorella sorokiniana, and pure phytoene, in the model organism C. elegans. We show that both microalgae extracts, as well as phytoene on its own, protect against oxidative stress and amyloid-β toxicity in a model of Alzheimer’s disease, and extend lifespan.
2. Materials and Methods
2.1. Reagents
Tert-butyl methyl ether (HPLC-grade) and CaCl2 were purchased from Honeywell (Seelze, Germany); MeTHF, KH2PO4, Na2HPO4, KPO4, 5-fluoro-2′-deoxyuridine (FUDR), cholesterol, phytoene standard, and 5-hydroxy-1,4-naphthoquinone (juglone) from Sigma-Aldrich (Steinheim, Germany); methanol (HPLC-grade) and ethyl acetate (HPLC-grade) from VWR Chemicals (Leuven, Belgium); dimethyl sulfoxide (DMSO) was purchased from Duchefa Biochemie (Haarlem, Netherlands); sodium chloride obtained from Fisher Chemical (Hampton, EEUU). Agar, peptone, and LB media from Thermo Fisher Scientific (Hampshire, UK); MgSO4 from Melford (Suffolk, UK).
2.2. Microalgae Cultivation
Chlorella sorokiniana (211-32) was kindly provided by the algal collection of the Institute of Plant Biochemistry and Photosynthesis (IBVF-CSIC, Seville, Spain) and cultured photo-mixotrophically in liquid Tris-acetate phosphate (TAP) medium [
8].
Dunaliella bardawil (UTEX 2538) was supplied from the UTEX Culture Collection of Microalgae (University of Texas, Austin), and cultured using Johnson's modified medium for
Dunaliella, as described by Johnson et al. (1968) [
9].
2.3. Phytoene Microalgae Enrichment
Cultures of
C. sorokiniana in the middle of the exponential phase were harvested by centrifugation, resuspended in fresh TAP culture medium, divided into 50 mL-cultures, and incubated with 1 µg/mL of norflurazon, an inhibitor of the carotenoid pathway, to induce accumulation of phytoene [
10].
D. bardawil cultures were harvested in the middle of the exponential growth phase and incubated with 10 µg/mL of norflurazon [
11].
2.4. Extraction of Carotenoids from Microalgae
2-MeTHF, an emerging green solvent that has proved appropriate for the extraction of carotenoids in microalga was used ([
10,
12]). For extraction, ultrasound-assisted extraction was applied using a frequency of 20 kHz, and amplitude of 30%, and treatment time of 2 min. Samples were centrifuged and the supernatant was transferred to another tube. The procedure was repeated until the sample showed no colour. Subsequently, the samples were concentrated in a rotary evaporator (Eppendorf Concentrator plus™, Eppendorf, Hamburg, Germany), either for use as a worm supplementation or for HPLC analysis.
2.5. HPLC Analysis
Quantification of carotenoids was carried out using an Agilent (Waldbronn, Germany) 1260 Infinity II Prime LC system. This system was equipped with a diode array detector and a C
30 column (3 µm, 150 × 4.6 mm) (YMC, Wilmington, NC). Carotenoid extracts were dissolved in 500 µL of ethyl acetate and 10 µL were injected into the system for analysis. Phytoene was detected at 285 nm. The mobile phase consisted of a mixture of methanol,
tert-butyl methyl ether, and water, delivered at a flow rate of 1 mL/min via a linear gradient, as described in a study by Stinco et al. (2019) [
13]. The quantification was performed by external calibration as explained in that study.
2.6. Bacterial Growth
Bacterial growth curve methodology was implemented in order to ensure no effects of carotenoids in the growth of Escherichia coli (OP50). A growth curve was created over 24 hours. The 96-wells microplate assay was employed using OP50 bacteria, and absorbance was measured using a SPECTROstar Nano spectrophotometer (BMG Labtech, Germany). The plate was incubated at 37 °C with shaking for 25 hours. The absorbance was measured at 600 nm every 100 seconds for 900 cycles, and measurements repeated three time for accuracy. Three biological replicates were performed for each condition.
2.7. C. elegans Culture Methods and Strains
C. elegans were maintained at 20 ˚C on Nematode Growth Medium (NGM) plates seeded with Escherichia coli OP50. C. elegans strains used in this study included: N2 and GMC101 dvIs100 [unc-54p::A-beta-1-42::unc-54 3'-UTR + mtl-2p::GFP], both obtained from Caenorhabditis Genetics Center (CGC, University of Minnesota).
2.8. Preparation of Experimental Plates
35 mm NGM plates were seeded 150 µL OP50 and supplemented with either 50 µL of DMSO control, or 50 µL of 0.2 µg/mL, 1 µg/mL, 2 µg/mL extraction or phytoene standard 48 h later and allowed to dry overnight.
2.9. Developmental Assays
Thirty adult C. elegans were placed on experimental plates and allowed to lay eggs for 2 h. After 52-54 hours, the number of animals at L4 and adult stages was counted to evaluate effects on developmental rate. The experiment was repeated three times.
2.10. Oxidative Stress Assays
Assays were performed as previously described [
14]. Egg lays were performed on experimental plates, approximately 60 h after the egg lay, 50 nematodes at L4 stage were transferred to plates with 400 µM juglone. Survival was recorded hourly over a period of 8 hours. Nematodes were counted as dead if they failed to respond after stimulation with a platinum wire. Any animals that died due to bagging or crawling off the plate were censored. Experiments were carried out in triplicate.
2.11. Proteotoxicity Assay
The GMC101 strain was used in this assay. Egg lays were performed on experimental plates. Approximately 60 h after the egg lay, animals at L4s stage were transferred to new experimental plates and shifted to 25°C to induce expression of amyloid-β42, and kept at 25°C for the remainder of the experiment. Animals were scored every day for four days, and the number of paralysed and non-paralysed animals were counted. Nematodes were counted as paralysed if they were alive but failed to move forwards or backwards after stimulation with a platinum wire. Three biological replicates were performed with three plates containing 30 animals each for each condition.
2.12. Lifespan Assay
Egg lays were performed on supplemented plates for 2 hours. 60 h later thirty, animals at L4 stage were transferred onto experimental plates treated with 100 µM FUDR. Survival was scored every two days until all animals were dead. Any animals that died due to bagging or crawling off the plate were censored. Three biological replicates were conducted, and each contained four technical replicates.
2.13. Statistical Analysis
Data processing and statistical evaluation were performed with GraphPad Prism, version 10.1.1 (270) (San Diego, California, USA;
www.graphpad.com). Oxidative stress and proteotoxicity assays were analysed using two-way ANOVA. Survival assays were analysed using Kaplan-Meier and Gehan-Breslow-Wilcoxon tests applied for statistical comparison.
3. Results
3.1. Characterisation of the Carotenoid Profile of the Extracts
To establish if phytoene has effects on health and ageing, we first prepared phytoene-rich extractions from the microalgae
C. sorokiniana and
D. bardawil. Phytoene enrichment was induced in
C. sorokiniana and
D. bardawil cultures by the addition of norflurazon. We extracted carotenoids from the cultures using the green solvent 2-methyltetrahydrofuran and ultrasound-assisted extraction, as this provides a sustainable extraction method while still giving a high yield. Carotenoid content was quantified by HPLC analysis and showed high levels of phytoene present in the extracts. Phytoene constituted 47% and 45% of the total carotenoid content in
C. sorokiniana and
D. bardawil extracts, respectively (
Table 1). The
C. sorokiniana and
D. bardawil extracts, and a phytoene standard, were used to test for bioactivity throughout this study.
3.2. Effects of Phytoene-Rich Extracts on Growth of E. coli OP50
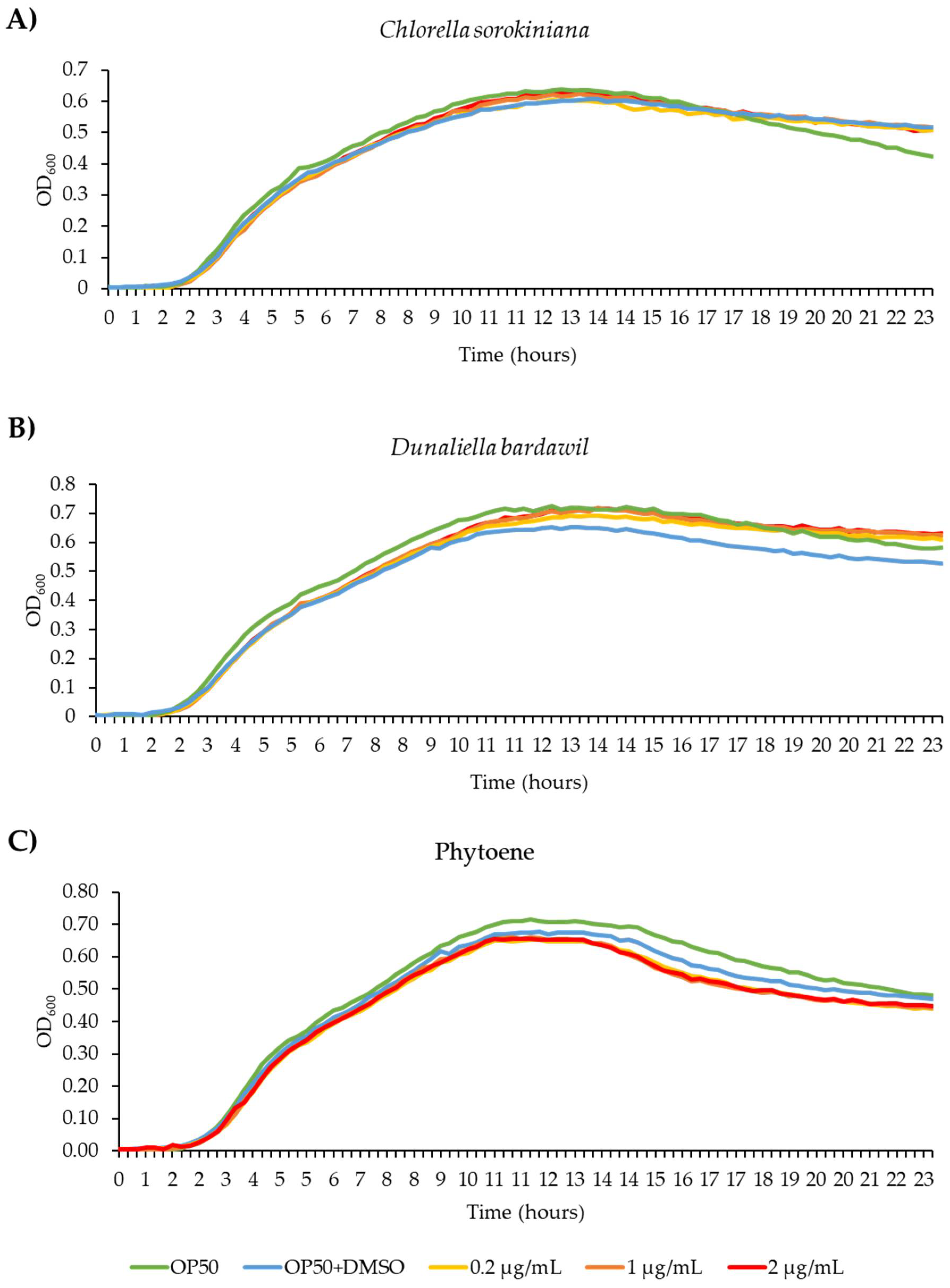
Before performing tests in
C. elegans, we wanted to rule out the possibility that the extracts inhibit growth of the
C. elegans food source
E. coli OP50, as this could lead to reduced food availability and dietary restriction. We conducted bacterial growth assays, treating OP50 bacteria with
C. sorokiniana and
D. bardawil phytoene-rich extracts, and with phytoene, at concentrations of 0.2 µg/mL, 1 µg/mL and 2 µg/mL. OD
600 of the bacterial cultures were measured and used to generate growth curves. OP50 growth was not affected by any of the concentrations (
Figure 2), showing that treatment with the phytoene-rich extracts, or with phytoene, does not affect the amount of food available to
C. elegans.
3.3. Effects of Phytoene-Rich Extracts on Development of C. elegans
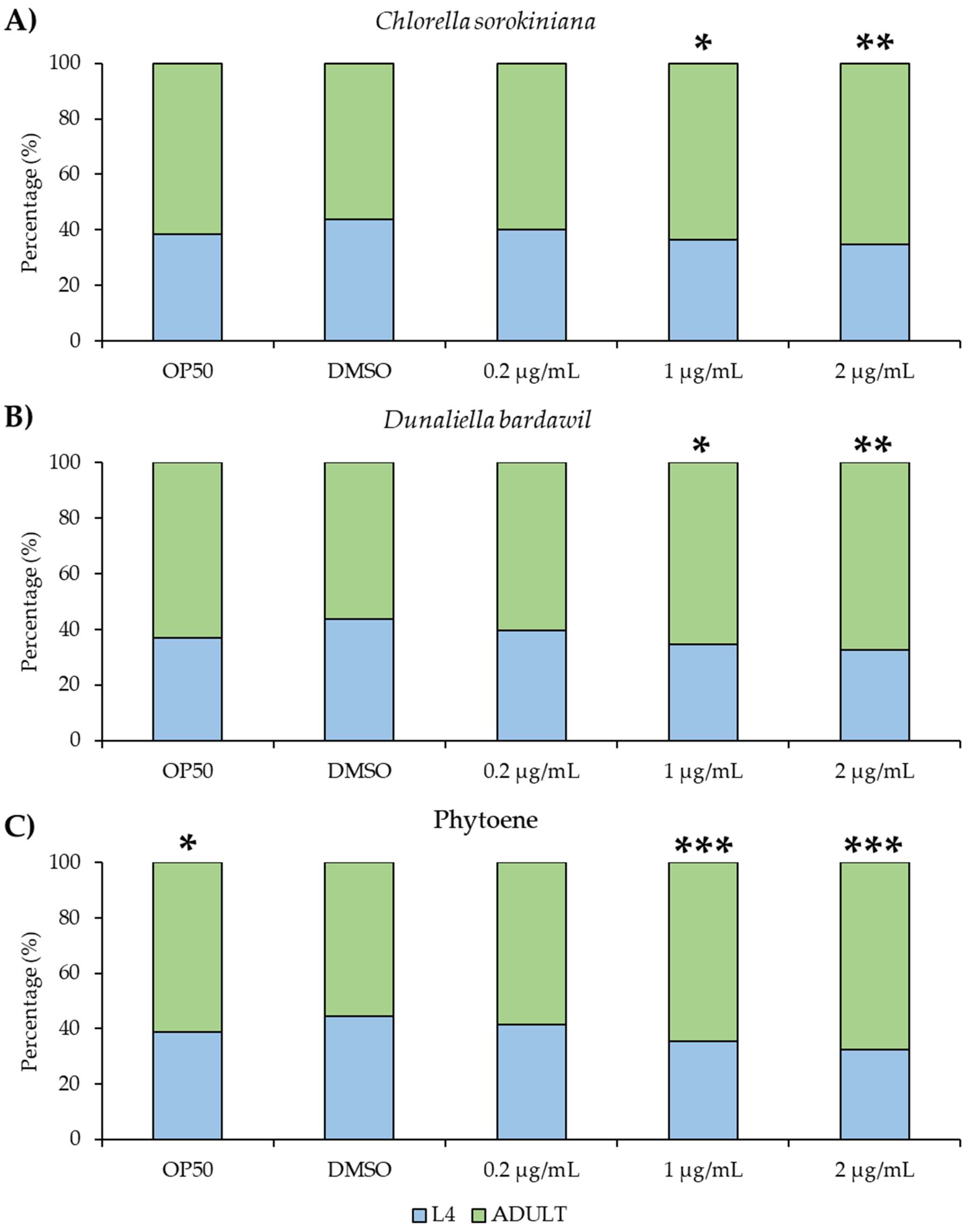
Because natural compounds can have toxic effects, we next asked if the phytoene-rich extracts have detrimental effects on
C. elegans growth and development. We conducted developmental assays to detect any effects on the development of larvae into adulthood. Extracts at concentrations of 0.2 µg/mL, 1 µg/mL, and 2 µg/mL were tested and no differences were found between the extracts and the controls. Phytoene at the same concentrations did not affect development either (
Figure 1), showing that phytoene-rich extracts and phytoene do not exhibit any major toxic effects at the tested concentrations.
Figure 3.
Phytoene-rich extracts and phytoene do not alter development of C. elegans (a-c) Percentage of animals that have reached L4 (larval, pre-adulthood) stage and adulthood. Animals were treated with 0.2 µg/mL, 1 µg/mL and 2 µg/mL C. sorokiniana extracts (a), D. bardawil extracts (b) and phytoene standard (c). Three biological replicates, n=200-300 per condition. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns: Not significant with two-way ANOVA compared to DMSO.
Figure 3.
Phytoene-rich extracts and phytoene do not alter development of C. elegans (a-c) Percentage of animals that have reached L4 (larval, pre-adulthood) stage and adulthood. Animals were treated with 0.2 µg/mL, 1 µg/mL and 2 µg/mL C. sorokiniana extracts (a), D. bardawil extracts (b) and phytoene standard (c). Three biological replicates, n=200-300 per condition. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns: Not significant with two-way ANOVA compared to DMSO.
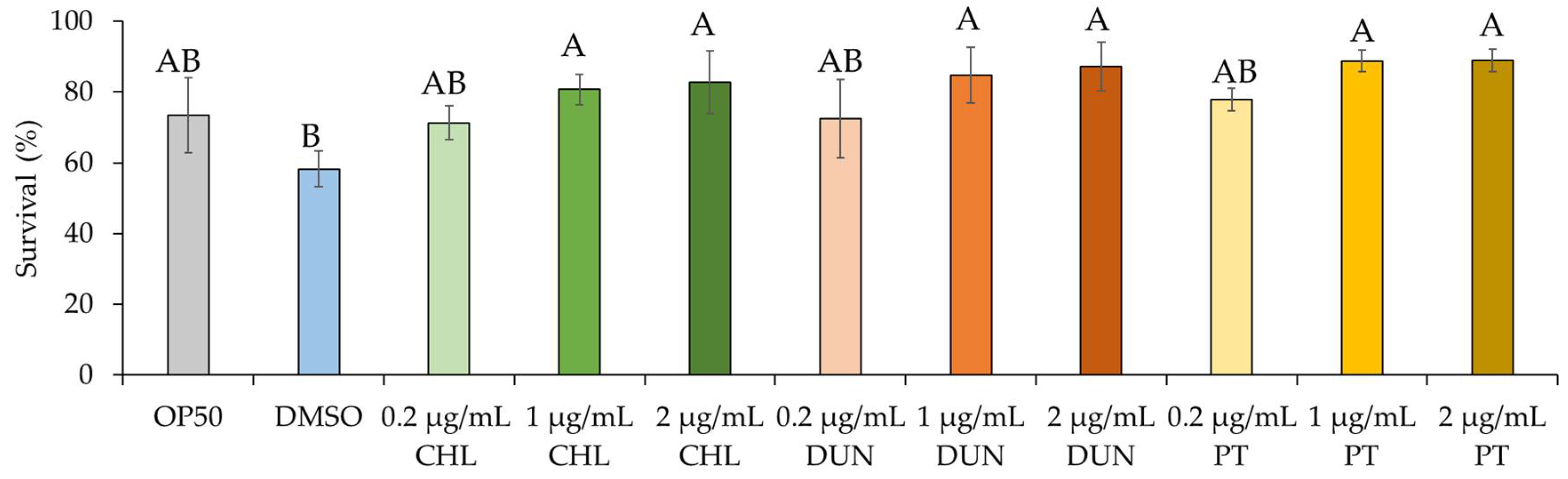
3.4. Phytoene and Phytoene-Rich Extracts Protect against Oxidative Stress
Next, we asked if the extracts have beneficial effects on health of the animal. Carotenoids are known to have antioxidant properties due to mechanisms including quenching, scavenging, or modulation of gene expression [
1]. We therefore tested if the phytoene-rich extracts affect sensitivity to oxidative stress in
C. elegans using juglone, a naturally occurring mitochondrial toxin that generates superoxide anion radicals [
15,
16]. We exposed animals to 400 µM juglone and compared survival after 8 hours. We found that in comparison to control conditions, supplementation with the extracts at 0.2 µg/mL) did not affect survival. In contrast, at supplementation with concentrations of 1 and 2 µg/mL, the survival rate of
C. elegans was increased by 39-53%, depending on the extract and the concentration (
p < 0.05) (
Figure 3). Phytoene only also increased survival at the same concentrations, and to a similar extent, as the
C. sorokiniana and
D. bardawil extracts. These findings show that phytoene-rich microalgae extracts, as well as phytoene on its own, have antioxidant properties leading to increased resistance to oxidative stress
in vivo.
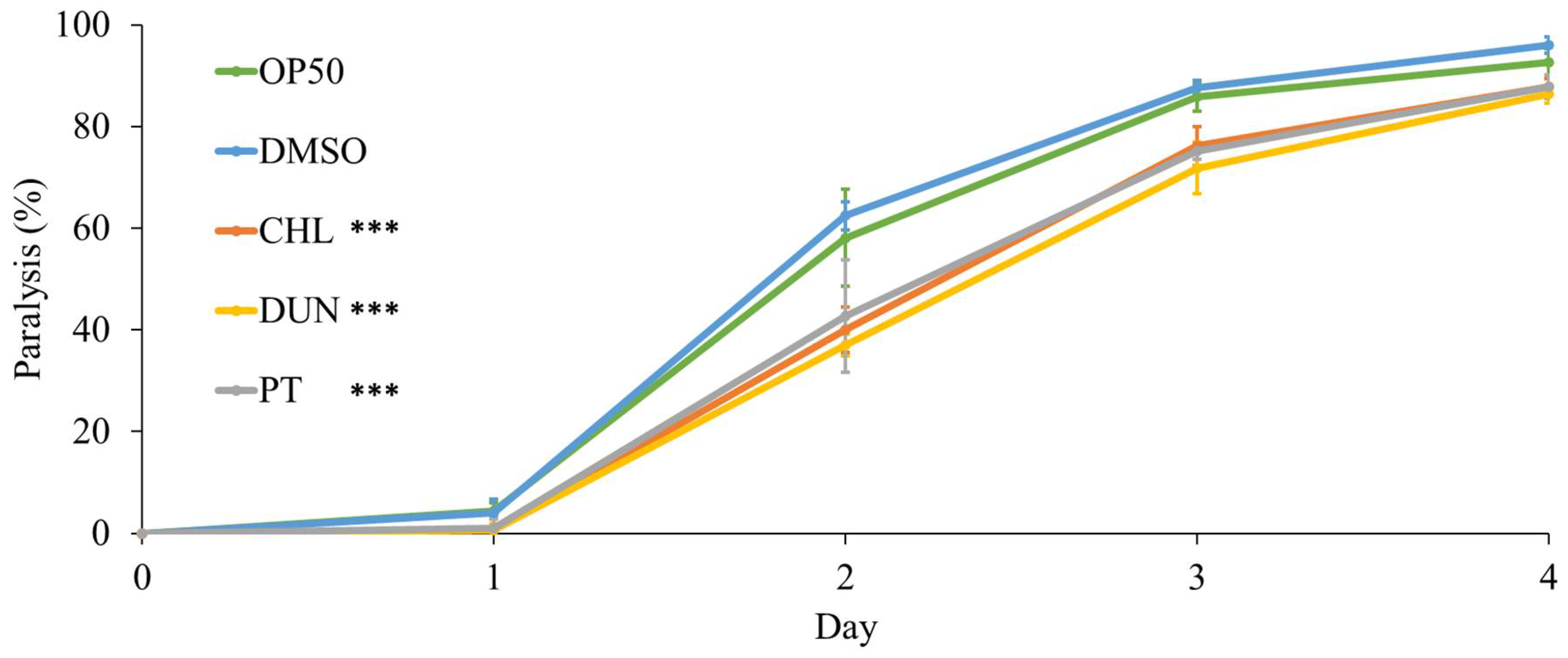
3.5. Phytoene and Phytoene-Rich Extracts Protect against Proteotoxicity
To further investigate protective effects of microalgae extracts on health, we used a humanised
C. elegans model of proteotoxicity expressing human amyloid-β
42. In this model, amyloid-β
42 leads to protein aggregation and formation of amyloid plaques, the main pathology in Alzheimer’s disease. Because the amyloid-β
42 is expressed in body-wall muscle, protein aggregation leads to muscle dysfunction and paralysis of the animal. We evaluated proteotoxicity by measuring paralysis in animals supplemented with microalgae extracts at 1 µg/mL. Compared to control, supplementation with the extracts, and with phytoene, had a protective effect. On day 2 of the experiment, paralysis was reduced from 62% in the DMSO control to 37%, 40%, and 43% for treatments with
D. bardawil and
C. sorokiniana phytoene rich-extracts, and phytoene standard, respectively (
p > 0.05) (
Figure 4). Thus, phytoene protects
C. elegans against proteotoxicity resulting from human amyloid-β
42.
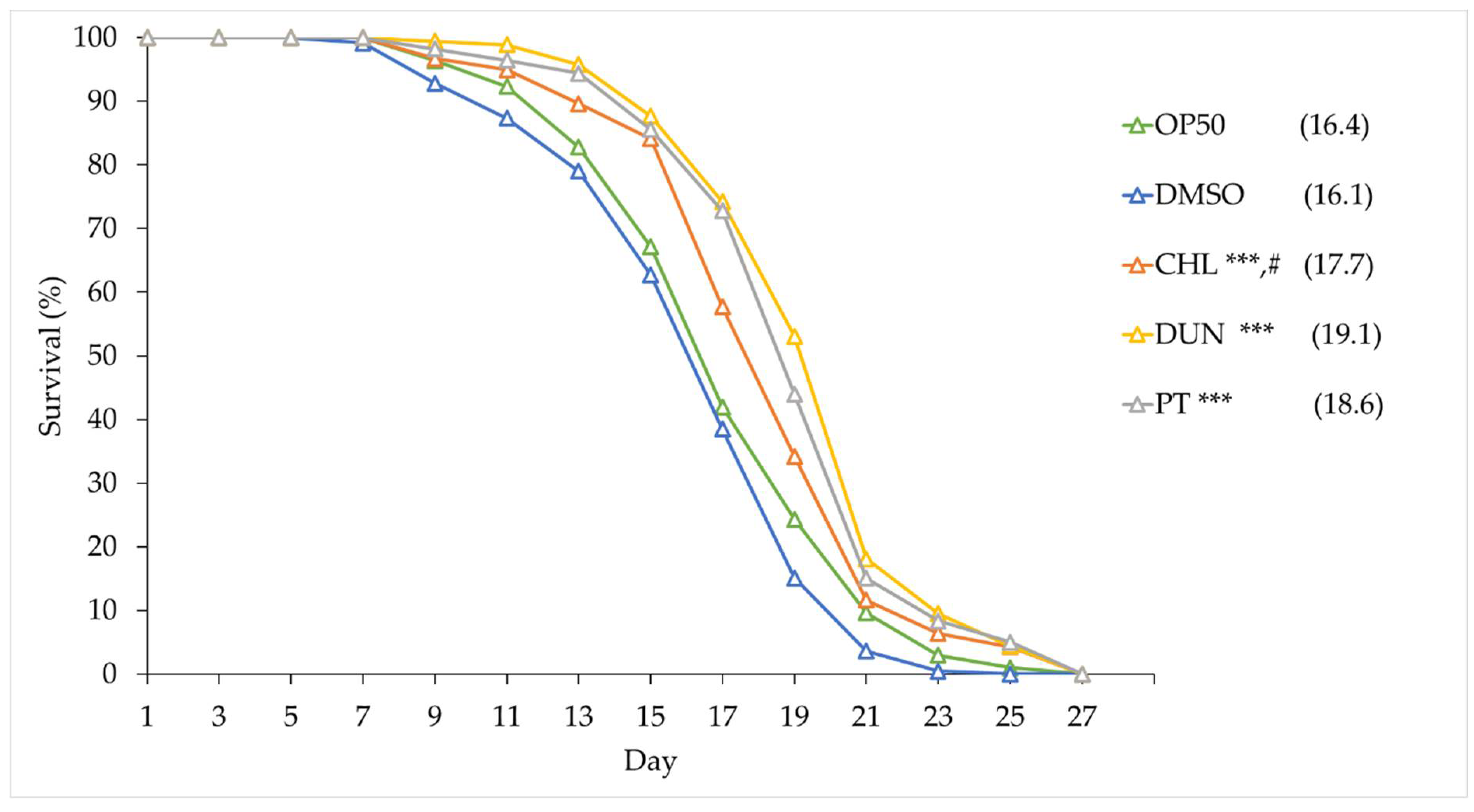
3.6. Phytoene and Phytoene-Rich Extracts Extend Lifespan
Both oxidative stress and proteotoxicity play central roles in ageing, and the protective effects we observed suggest that phytoene might affect ageing and longevity. We tested this by supplementing animals with the microalgae extracts and with phytoene at 1 µg/ml and conducted lifespan assays. Animals on OP50 had a median lifespan of 16.4 days, similarly to animals on DMSO, which had a median lifespan of 16.1 days. Animals supplemented with extracts from
C. sorokiniana and
D. bardawil had median lifespans of 17.7 and 19.1 days, an increase of 10% and 18.6%, respectively. Phytoene also extended lifespan, animals treated with phytoene had a median lifespan of 18.6 days and increase of 15.5% (
Figure 5 and
Table S1). Our findings suggest that supplementation with phytoene, and phytoene-rich extracts from microalgae, results in lifespan extension in
C. elegans.
4. Discussion
4.1. Insights from Analysing Bioactivity of Phytoene
In this study we have described how phytoene, and phytoene-rich extracts from microalgae, affect resistance to oxidative stress, resistance to amyloid-β42 proteotoxicity, and lifespan in C. elegans. Our findings show that microalgae are a source of health-promoting compounds and have potential to be developed into functional foods and other products promoting health. Our study also suggests that phytoene is not only a precursor for the biosynthesis of other carotenoids, but also has biological activity in its own right, with beneficial effects on health and longevity. In our study we showed that treatment with phytoene results in anti-ageing effects to the same extent as extracts containing a mixed pool of carotenoids. To our knowledge, no other studies have directly shown that phytoene intake promotes longevity.
Epidemiological studies show that greater intake of foods rich in carotenoids is linked to improved health outcomes. For example, tomatoes are associated with reduced risks of cardiovascular disease and cancer, with these health effects mostly attributed to lycopene. Tomatoes also have high levels of phytoene, but measures of phytoene are not included in most epidemiological studies [
17,
18], despite some studies showing that phytoene intake is higher than other carotenoids traditionally studied in relation to health, such as lutein, zeaxanthin or β-cryptoxanthin [
5,
6]. Including a wider range of measures in future epidemiological studies would uncover links between phytoene and health outcomes, and determine if lycopene is the main bioactive component in foods such as tomatoes, or a marker for consumption of foods containing other health-promoting phytochemicals.
4.2. Mechanisms by which Phytoene Impacts Ageing
By which mechanisms does phytoene protect against oxidative stress, proteotoxicity and ageing? Carotenoids have antioxidant effects that have been well described in plants, as well as in a large number of
in vitro and
in vivo experiments with human relevance [
19]. Carotenoids protect against oxidative damage caused by reactive free radicals, including in disease states where oxidative damage occurs such as cancer, diabetes, atherosclerosis, obesity, arthritis and neurodegeneration [
20,
21]. As already mentioned, carotenoids can act as antioxidants by directly quenching or scavenging reactive oxygen species or through the induction of the expression of genes [
1]. In a study the free radical scavenging properties of lycopene, phytofluene, and phytoene were evaluated experimentally and in silico. It was concluded that, although lycopene was the best antiradical, phytoene and phytofluene exhibited a higher antioxidant capacity than could be expected from their fewer number of conjugated double bonds (3 and 5, respectively, compared to 11 for lycopene) [
22].
There is growing evidence supporting that carotenoids in addition to directly interacting with reactive oxygen species, also interact with cellular targets, leading to broader effects mediated by endogenous proteins [
19]. For example, lycopene and astaxanthin have been shown to inhibit the NF-κB pathway, reducing the activation of downstream pro-inflammatory genes and inflammation caused by obesity, cancer, and other diseases [
23]. β-carotene, lycopene, and astaxanthin activate the transcription factor NRF2, resulting in increased expression of antioxidant and detoxification enzymes. Activation of these endogenous defence systems leads to a reduction of intracellular reactive oxygen species, carcinogens and other toxins, and may reduce progression of cancers, cardiovascular diseases, and neurodegeneration [
19]. Future studies will show if phytoene also has bioactive properties beyond scavenging reactive oxygen species.
In vivo studies in model organisms have directly demonstrated that carotenoids protect against ageing. Several studies used
C. elegans and showed that supplementation with carotenoid-rich extracts from plant sources not only protect against oxidative stress but also increase lifespan [
20,
24,
25,
26,
27,
28,
29]. Moreover, individual carotenoids improve health in
C. elegans. e.g. astaxanthin protects against oxidative stress [
30] and extends lifespan [
31,
32,
33] and lycopene protects against amyloid-β
42 proteotoxicity [
26]. Several studies in
C. elegans also confirmed that the anti-ageing effects of carotenoids require cellular targets and do not only act by directly quenching free radicals. Protection against oxidative stress by carotenoid-rich supplementation is dependent of the Nrf2 orthologue SKN-1 [
28]. Lifespan extension by supplementation with Vitamin A is also dependent on the Nrf2/SKN-1 pathway [
34], suggesting an important role for Nrf-2/SKN-1 in lifespan extension by carotenoid supplementation. However, individual carotenoids may act through different cellular pathways. Interestingly, astaxanthin promotes longevity in
C .elegans not through Nrf2/SKN-1, but requires the transcription factor DAF-16, homolog of mammalian FoxO, a critical longevity factor across species [
35]. One possibility is that phytoene also stimulates longevity pathways by activation of cellular targets that either overlap, or do not overlap, with those of other carotenoids. This warrants further investigation.
4.3. Microalgae – A Sustainable Source of Bioactive Compounds to Improve Healthy Ageing?
The role of foods is shifting from focusing on the provision of energy and basic nutrients to also include the supply of bioactive compounds capable of offering protection against the development of diseases. With the global population increasing, and demographics rapidly shifting towards ageing societies with increasing numbers of people suffering from multiple age-related chronic diseases, there is a growing demand for production of foods that meet our growing health needs, while being sustainable [
36]. Microalgae are a heterogeneous group of photosynthetic microorganisms with enormous ecological importance that can be harnessed for the sustainable production of foods or ingredients. Thus, they account for approximately half of the global CO
2 fixation, their cultivation does not require arable land and they grow 10-50-fold faster than plants. Moreover, they are adapted to diverse environments and can thrive in abiotic stressing conditions, including high and low temperatures, nutrient depletion, high salinity or the presence of pollutants. In addition, microalgae have lower nutritional and water requirements compared to terrestrial plants, no requirements of herbicides or pesticides, cultivation produces less wastewater and modulation of biosynthesis of diverse metabolites is easier. Their nutritional value (high quality proteins, unsaturated fatty acids, minerals, vitamins, bioactives) is remarkable, being used as nutritional supplements in aquaculture, human foods, or for the obtaining of food, pharmaceutical, or cosmetic ingredients [
2]. In our study we used phytoene-rich cultures of the microalgae species
Chlorella sorokiniana and
Dunaliella bardawil and sustainable extraction methods to demonstrate the potential of microalgae as sources for functional ingredients that promote healthy ageing, while reducing land use and increasing sustainability of food production.
5. Conclusions
Our study aimed to evaluate the effects of phytoene, and phytoene-rich microalgae extracts, on health in the model organism C. elegans. Supplementation with 0.2, 1 and 2 µg/mL of phytoene and phytoene-rich extracts did not affect development, suggesting absence of toxic effects and dietary restriction. Supplementation with 1 and 2 µg/mL, but not 0.2 µg/mL, of the extracts and pure phytoene increased resistance to juglone, a mitochondrial toxin that generates superoxide anion radicals, by 39-53%, showing that phytoene, like other carotenoids, protects against oxidative stress. To assess the effects of phytoene in the context of age-related health, we used a humanised C. elegans model of amyloid-β42 toxicity, in which in amyloid plaques, a landmark of Alzheimer disease, are formed. We found that 1 µg/mL phytoene and phytoene-rich extracts reduced proteotoxic effects by 30-40%. Finally, we tested the effects of phytoene on lifespan and found that 1 µg/mL phytoene and phytoene-rich extracts increased lifespan by 10-18.6%. Together our results demonstrate that phytoene is a bioactive compound with anti-ageing effects that promote longevity.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1: Lifespan data of C. elegans treated with C. sorokiniana and D. bardawil extracts and phytoene.
Author Contributions
Conceptualization, A.J.M.M. and M.E.; methodology, M.E.; investigation, A.M.O., A.A.K. and P.M.B.; resources, A.J.M.M. and M.E; data curation, A.M.O.; writing—original draft preparation, A.M.O.; writing—review and editing, M.E. and A.J.M.M.; supervision, P.M.B.; project administration, A.J.M.M. and M.E.; funding acquisition, A.J.M.M. and M.E. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by grants PID2019-110438RB-C21 (NEWCARFOODS), funded by MCIN/AEI/10.13039/501100011033 and ERDF/EU, and BB/V011243/1 funded by BBSRC. PMB was supported by a postdoc fellowship from Consejería de Transformación Económica, Industria, Conocimiento y Universidades de la Junta de Andalucía (PAIDI 2020). AMO was supported by a grant associated to grant PID2019-110438RB-C21 (NEWCARFOODS). AAK was supported by a studentship awarded by Global Challenges Doctoral Centre, University of Kent. AJMM, AMO and PMB are members of the Spanish Carotenoid Network (CaRed), grant RED2022-134577-T, funded by MCIN/AEI/10.13039/501100011033 and ERDF/EU.
Acknowledgments
The authors are very grateful to Drs. Rosa León-Bañares and Antonio León-Vaz for the obtaining and provision of phytoene-rich microalgal biomass.
Conflicts of Interest
AJMM carries out consultancy work for diverse companies. AAK, PMB, AMO and ME declare no conflict of interest.
References
- Meléndez-Martínez AJ, Böhm V, Borge GI, Cano MP, Fikselová M, Gruskiene R, et al. Carotenoids: Considerations for their Use in Functional Foods, Nutraceuticals, Nutricosmetics, Supplements, Botanicals and Novel Foods in the Context of Sustainability, Circular Economy and Climate Change. Annu Rev Food Sci Technol 2021;12:433–460. [CrossRef]
- Mapelli-Brahm P, Gómez-Villegas P, Gonda ML, León-Vaz A, León R, Mildenberger J, et al. Microalgae, Seaweeds and Aquatic Bacteria, Archaea, and Yeasts: Sources of Carotenoids with Potential Antioxidant and Anti-Inflammatory Health-Promoting Actions in the Sustainability Era. Mar Drugs 2023;21:340. [CrossRef]
- Varela JC, Pereira H, Vila M, León R. Production of carotenoids by microalgae: Achievements and challenges. Photosynth Res 2015;125. [CrossRef]
- Lichtenthaler HK. Biosynthesis, Localization and Concentration of Carotenoids in Plants and Algae, 2012. [CrossRef]
- Biehler E, Alkerwi A, Hoffmann L, Krause E, Guillaume M, Lair ML, et al. Contribution of violaxanthin, neoxanthin, phytoene and phytofluene to total carotenoid intake: Assessment in Luxembourg. J Food Compos Anal 2012;25:56–65. [CrossRef]
- Olmedilla-Alonso B, Benítez-González AM, Estévez-Santiago R, Mapelli-Brahm P, Stinco CM, Meléndez-Martínez AJ. Assessment of Food Sources and the Intake of the Colourless Carotenoids Phytoene and Phytofluene in Spain. Nutrients 2021;13:4436. [CrossRef]
- Meléndez-Martínez AJ, Stinco CM, Mapelli-Brahm P. Skin carotenoids in public health and nutricosmetics: The emerging roles and applications of the UV radiation-absorbing colourless carotenoids phytoene and phytofluene. Nutrients 2019;11. [CrossRef]
- The Chlamydomonas Sourcebook. 1989. [CrossRef]
- Johnson MK, Johnson EJ, MacElroy RD, Speer HL, Bruff BS. Effects of salts on the halophilic alga Dunaliella viridis. J Bacteriol 1968;95. [CrossRef]
- Morón-Ortiz Á, Mapelli-Brahm P, León-Vaz A, Benitez-González AM, León R, Meléndez-Martínez AJ. Ultrasound-assisted extraction of carotenoids from phytoene-accumulating Chlorella sorokiniana microalgae: Effect of milling and performance of the green biosolvents 2-methyltetrahydrofuran and ethyl lactate. Food Chem 2024;434. [CrossRef]
- León R, Vila M, Hernánz D, Vílchez C. Production of phytoene by herbicide-treated microalgae Dunaliella bardawil in two-phase systems. Biotechnol Bioeng 2005;92. [CrossRef]
- Morón-Ortiz Á, Mapelli-Brahm P, Meléndez-Martínez AJ. Sustainable Green Extraction of Carotenoid Pigments : Innovative Technologies and Bio-Based Solvents. Antioxidants 2024;13:239. [CrossRef]
- Stinco CM, Benítez-González AM, Meléndez-Martínez AJ, Hernanz D, Vicario IM. Simultaneous determination of dietary isoprenoids (carotenoids, chlorophylls and tocopherols) in human faeces by Rapid Resolution Liquid Chromatography. J Chromatogr A 2019;1583. [CrossRef]
- González-Peña MA, Lozada-ramírez JD, Ortega-regules AE. Carotenoids from mamey (Pouteria sapota) and carrot (Daucus carota) increase the oxidative stress resistance of Caenorhabditis elegans 2021;26. [CrossRef]
- Paulsen MT, Ljungman M. The natural toxin juglone causes degradation of p53 and induces rapid H2AX phosphorylation and cell death in human fibroblasts. Toxicol Appl Pharmacol 2005;209. [CrossRef]
- Ahmad T, Suzuki YJ. Juglone in oxidative stress and cell signaling. Antioxidants 2019;8. [CrossRef]
- Engelmann NJ, Clinton SK, Erdman JW. Nutritional aspects of phytoene and phytofluene, carotenoid precursors to lycopene. Adv Nutr 2011;2. [CrossRef]
- Mapelli-Brahm P, Meléndez-Martínez AJ. The colourless carotenoids phytoene and phytofluene: sources, consumption, bioavailability and health effects. Curr Opin Food Sci 2021;41. [CrossRef]
- Bohn T, Bonet ML, Borel P, Keijer J, Landrier JF, Milisav I, et al. Mechanistic aspects of carotenoid health benefits - Where are we now? Nutr Res Rev 2021;34. [CrossRef]
- Hernández-Cruz EY, Eugenio-Pérez D, Ramírez-Magaña KJ, Pedraza-Chaverri J. Effects of Vegetal Extracts and Metabolites against Oxidative Stress and Associated Diseases: Studies in Caenorhabditis elegans. ACS Omega 2023;8. [CrossRef]
- Pérez-gálvez A, Viera I, Roca M. Carotenoids and chlorophylls as antioxidants. Antioxidants 2020;9. [CrossRef]
- Martínez A, Stinco CM, Meléndez-Martínez AJ. Free radical scavenging properties of Phytofluene and Phytoene isomers as compared to Lycopene: A combined experimental and theoretical study. J Phys Chem B 2014;118. [CrossRef]
- Sharoni Y, Linnewiel-Hermoni K, Khanin M, Salman H, Veprik A, Danilenko M, et al. Carotenoids and apocarotenoids in cellular signaling related to cancer: A review. Mol Nutr Food Res 2012;56. [CrossRef]
- Ahrazem O, Diretto G, Rambla JL, Rubio-Moraga Á, Lobato-Gómez M, Frusciante S, et al. Engineering high levels of saffron apocarotenoids in tomato. Hortic Res 2022;9. [CrossRef]
- Valdés A, Sánchez-martínez JD, Gallego R, Ibáñez E, Cifuentes A. In vivo neuroprotective capacity of a Dunaliella salina extract - Comprehensive transcriptomics and metabolomics study. Npj Sci Food 2024;8:23968370. [CrossRef]
- Chen W, Mao L, Xing H, Xu L, Fu X, Huang L, et al. Lycopene attenuates Aβ1-42 secretion and its toxicity in human cell and Caenorhabditis elegans models of Alzheimer disease. Neurosci Lett 2015;608. [CrossRef]
- Moraes L de LS, Rodrigues NR, Dal Forno AH, Tambara AL, Boldori JR, Vizzotto M, et al. Araçá (Psidium Cattleianum Sabine) ethanol extracts increase lifespan and alleviate oxidative stress in Caenorhabditis elegans. J Agric Food Res 2023;11. [CrossRef]
- De Oliveira Caland RB, Cadavid COM, Carmona L, Peña L, De Paula Oliveira R. Pasteurized orange juice rich in carotenoids protects caenorhabditis elegans against oxidative stress and β-amyloid toxicity through direct and indirect mechanisms. Oxid Med Cell Longev 2019;2019. [CrossRef]
- Raquel Ferreira Paulo I, Basílio de Oliveira Caland R, Orlando Muñoz Cadavid C, Martins Melo G, Soares De Castro Bezerra L, Pons E, et al. β-carotene genetically-enriched lyophilized orange juice increases antioxidant capacity and reduces β-amyloid proteotoxicity and fat accumulation in Caenorhabditis elegans. Food Chem Mol Sci 2022;5. [CrossRef]
- Liu X, Luo Q, Cao Y, Goulette T, Liu X, Xiao H. Mechanism of Different Stereoisomeric Astaxanthin in Resistance to Oxidative Stress in Caenorhabditis elegans. J Food Sci 2016;81. [CrossRef]
- Ding F, Zhao Y. Astaxanthin Induces Transcriptomic Responses Associated with Lifespan Extension in Caenorhabditis elegans. Antioxidants 2022;11. [CrossRef]
- Fu M, Zhang X, Zhang X, Yang L, Luo S, Liu H. Autophagy Plays a Role in the Prolongation of the Life Span of Caenorhabditis elegans by Astaxanthin. Rejuvenation Res 2021;24. [CrossRef]
- Liu X, Chen X, Liu H, Cao Y. Antioxidation and anti-aging activities of astaxanthin geometrical isomers and molecular mechanism involved in Caenorhabditis elegans. J Funct Foods 2018;44. [CrossRef]
- Sirakawin C, Lin D, Zhou Z, Wang X, Kelleher R, Huang S, et al. SKN-1/NRF2 upregulation by vitamin A is conserved from nematodes to mammals and is critical for lifespan extension in Caenorhabditis elegans. Aging Cell 2024;23. [CrossRef]
- Yazaki K, Yoshikoshi C, Oshiro S, Yanase S. Supplemental cellular protection by a carotenoid extends lifespan via Ins/IGF-1 signaling in caenorhabditis elegans. Oxid Med Cell Longev 2011. [CrossRef]
- Willett W, Rockström J, Loken B, Springmann M, Lang T, Vermeulen S, et al. Food in the Anthropocene: the EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019;393. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).