Submitted:
27 May 2024
Posted:
28 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Data Analysis
3. Results
3.1. Study Population
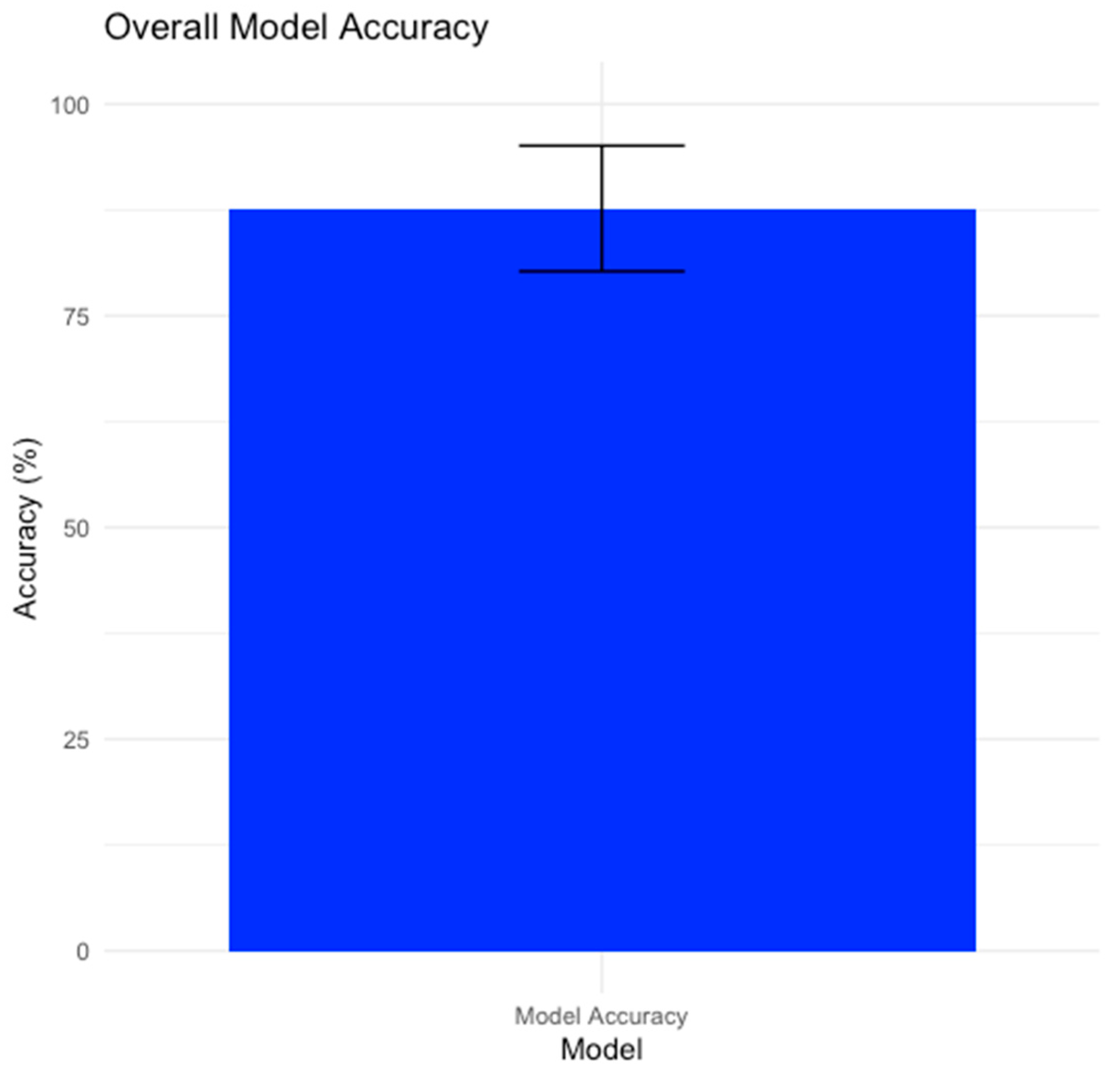
3.2. Overall Model Performance
3.3. Confusion Matrix and Performance Metrics
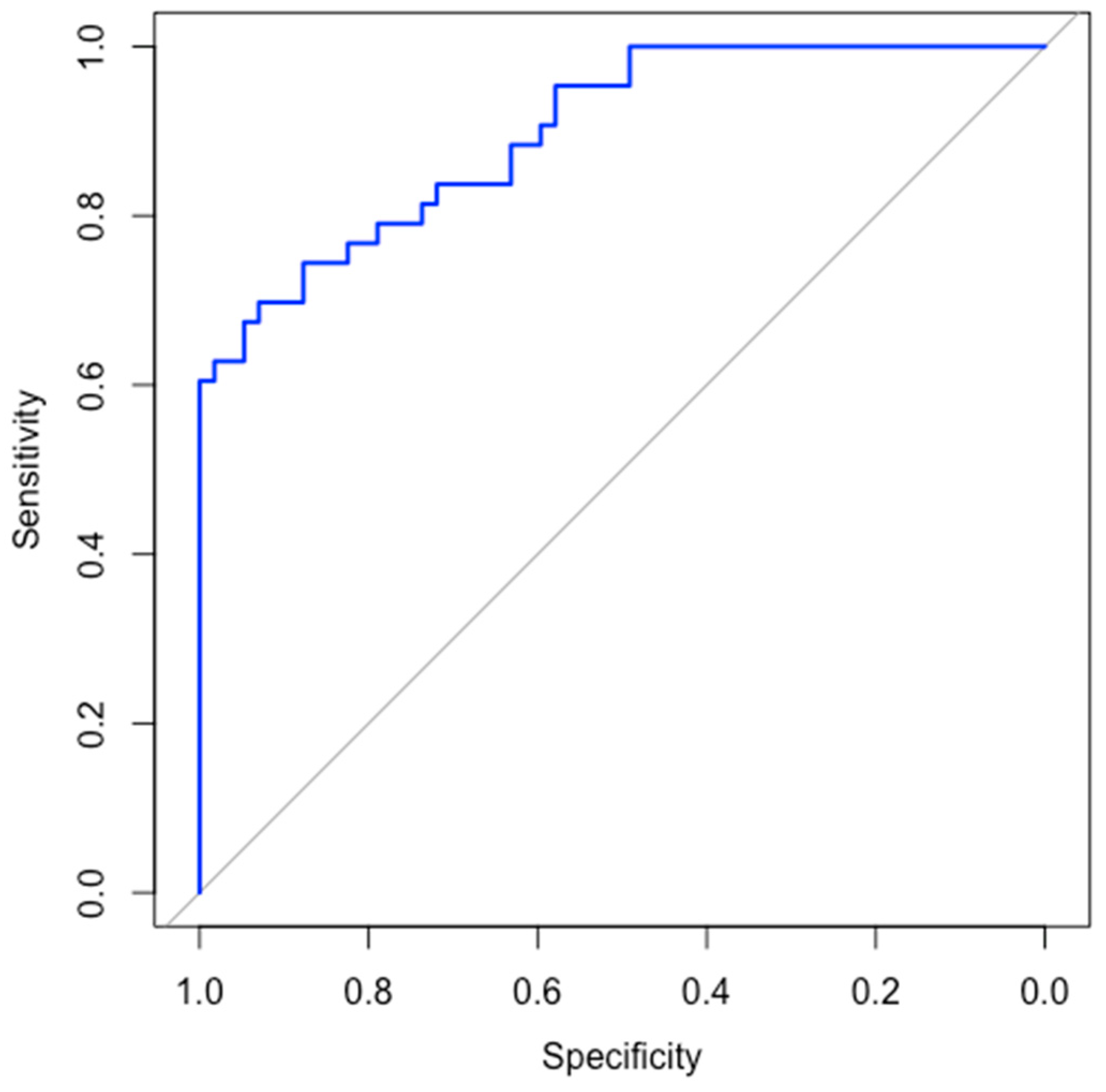
3.4. ROC Curve and AUC
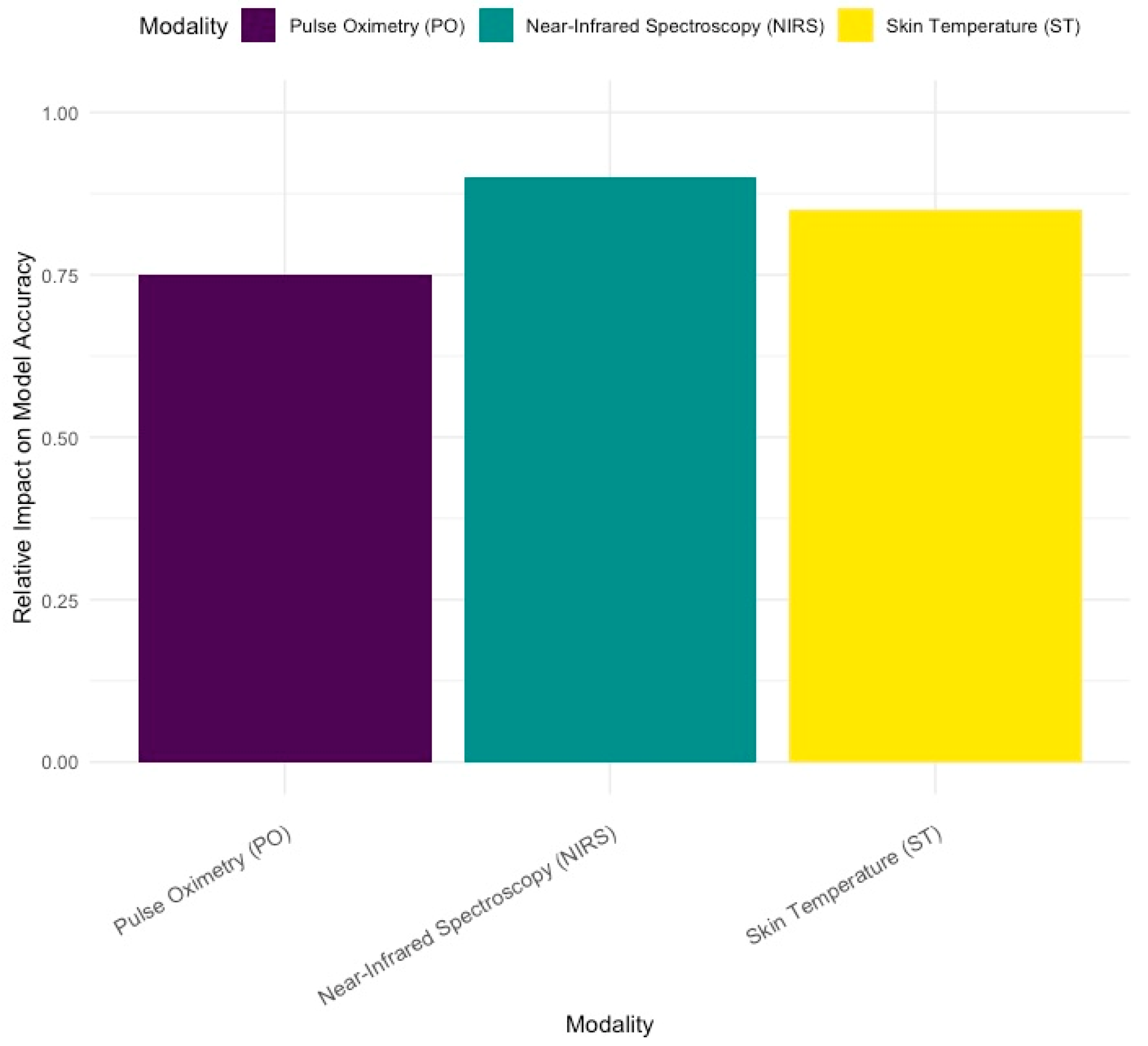
3.5. Impact of Signal Modalities
3.6. Improvement in Accuracy with Multimodal Monitoring
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Beć, K.B.; Grabska, J.; Huck, C.W. Near-Infrared Spectroscopy in Bio-Applications. Molecules 2020, 25, 2948. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.J.; Cowan, H.J.; Lord, J.A.; Zuege, D.J.; Zygun, D.A. The Accuracy of Pulse Oximetry in Emergency Department Patients with Severe Sepsis and Septic Shock: A Retrospective Cohort Study. BMC Emerg Med 2010, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Zareef, M.; Chen, Q.; Hassan, M.M.; Arslan, M.; Hashim, M.M.; Ahmad, W.; Kutsanedzie, F.Y.H.; Agyekum, A.A. An Overview on the Applications of Typical Non-Linear Algorithms Coupled With NIR Spectroscopy in Food Analysis. Food Eng Rev 2020, 12, 173–190. [Google Scholar] [CrossRef]
- Mekonnen, B.K.; Yang, W.; Hsieh, T.-H.; Liaw, S.-K.; Yang, F.-L. Accurate Prediction of Glucose Concentration and Identification of Major Contributing Features from Hardly Distinguishable Near-Infrared Spectroscopy. Biomed. Signal Process. Control. 2020, 59, 101923. [Google Scholar] [CrossRef]
- Sokou, R.; Ioakeimidis, G.; Piovani, D.; Parastatidou, S.; Konstantinidi, A.; Tsantes, A.G.; Lampridou, M.; Houhoula, D.; Iacovidou, N.; Kokoris, S.; et al. Development and Validation of a Sepsis Diagnostic Scoring Model for Neonates with Suspected Sepsis. Front Pediatr 2022, 10, 1004727. [Google Scholar] [CrossRef] [PubMed]
- You, T.; Zhou, Y.-R.; Liu, X.-C.; Li, L.-Q. Risk Factors and Clinical Characteristics of Neonatal Acute Respiratory Distress Syndrome Caused by Early Onset Sepsis. Front. Pediatr. 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Maddahi, A.; Leach, T.R.; Saeedi, M.; Dhannapuneni, P.R.; Maddahi, Y.; Choukou, M.-A.; Zareinia, K. Roboethics in Remote Human Interactions and Rehabilitative Therapeutics. Applied Sciences 2022, 12, 6033. [Google Scholar] [CrossRef]
- Peng, Z.; Varisco, G.; Long, X.; Liang, R.-H.; Kommers, D.; Cottaar, W.; Andriessen, P.; van Pul, C. A Continuous Late-Onset Sepsis Prediction Algorithm for Preterm Infants Using Multi-Channel Physiological Signals From a Patient Monitor. IEEE J. Biomed. Health Inform. 2023, 27, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Tian, M.; Zhang, J.; Li, J.; Tan, C.; Ren, C.; Feng, J.; Cai, Y.; Gao, J.; Ma, Y.; et al. IEMS: An IoT-Empowered Wearable Multi-Modal Monitoring System in Neurocritical Care. IEEE Internet of Things Journal 2022, 10, 1860–1875. [Google Scholar] [CrossRef]
- Sun, L.; Joshi, M.; Khan, S.N.; Ashrafian, H.; Darzi, A. Clinical Impact of Multi-Parameter Continuous Non-Invasive Monitoring in Hospital Wards: A Systematic Review and Meta-Analysis. J R Soc Med 2020, 113, 217–224. [Google Scholar] [CrossRef]
- Tindal, E.W.; Armstead, B.; Monaghan, S.F.; Heffernan, D.S.; Ayala, A. Emerging Therapeutic Targets for Sepsis. Expert Opin Ther Targets 2021, 25, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Harper, A.; Vijayakumar, V.; Ouwehand, A.C.; ter Haar, J.; Obis, D.; Espadaler, J.; Binda, S.; Desiraju, S.; Day, R. Viral Infections, the Microbiome, and Probiotics. Front Cell Infect Microbiol 2021, 10, 596166. [Google Scholar] [CrossRef] [PubMed]
- Ristovska, S.; Stomnaroska, O.; Danilovski, D. Hypoxic Ischemic Encephalopathy (HIE) in Term and Preterm Infants. PRILOZI 2022, 43, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Bobba, P.S.; Malhotra, A.; Sheth, K.N.; Taylor, S.N.; Ment, L.R.; Payabvash, S. Brain Injury Patterns in Hypoxic Ischemic Encephalopathy of Term Neonates. J. Neuroimaging 2023, 33, 79–84. [Google Scholar] [CrossRef]
- Dubois, J.; Alison, M.; Counsell, S.J.; Hertz-Pannier, L.; Hüppi, P.S.; Benders, M.J.N.L. MRI of the Neonatal Brain: A Review of Methodological Challenges and Neuroscientific Advances. J Magn Reson Imaging 2021, 53, 1318–1343. [Google Scholar] [CrossRef] [PubMed]
- Panigrahy, A.; Votava-Smith, J.K.; Licht, D.J. Need for ‘One-Stop-Shop’ Heart-Brain-Placental Imaging in Fetal Congenital Heart Disease: Fetal Hemodynamics Portend Neurodevelopmental Outcome∗. Journal of the American College of Cardiology 2024, 83, 1240–1242. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Reddy, K. Pulse Oximetry for the Measurement of Oxygen Saturation in Arterial Blood. In; 2021; pp. 51–78 ISBN 9789811554476.
- Davies, H.J.; Williams, I.; Peters, N.S.; Mandic, D.P. In-Ear SpO2: A Tool for Wearable, Unobtrusive Monitoring of Core Blood Oxygen Saturation. Sensors 2020, 20, 4879. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H. Investigation of the Mechanisms Underlying the Development and Evolution of Folds of the Cerebrum Using Gyrencephalic Ferrets. Journal of Comparative Neurology 2024, 532, e25615. [Google Scholar] [CrossRef] [PubMed]
- Jeon, G.W. Clinical Application of Near-Infrared Spectroscopy in Neonates. Neonatal Med 2019, 26, 121–127. [Google Scholar] [CrossRef]
- Tanaka, Y.; Suzuki, M.; Yoshitani, K.; Sakamoto, A.; Bito, H. Anatomical and Physiological Variables Influencing Measurement of Regional Cerebral Oxygen Saturation by near Infrared Spectroscopy Using the Sensmart Model X-100TM. J Clin Monit Comput 2021, 35, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Claassen, J.A.H.R.; Thijssen, D.H.J.; Panerai, R.B.; Faraci, F.M. Regulation of Cerebral Blood Flow in Humans: Physiology and Clinical Implications of Autoregulation. Physiol Rev 2021, 101, 1487–1559. [Google Scholar] [CrossRef] [PubMed]
- Boscarino, G.; Migliorino, R.; Carbone, G.; Davino, G.; Dell’Orto, V.G.; Perrone, S.; Principi, N.; Esposito, S. Biomarkers of Neonatal Sepsis: Where We Are and Where We Are Going. Antibiotics 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Greisen, G.; Hansen, M.L.; Rasmussen, M.I.S.; Vestager, M.; Hyttel-Sørensen, S.; Hahn, G.H. Cerebral Oximetry in Preterm Infants–To Use or Not to Use, That Is the Question. Front Pediatr 2022, 9, 747660. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Kirpalani, H. Safe and Sound Oxygen Therapy for Extremely Preterm Infants: A Literature Review. Pediatr. Med. 2022, 5. [Google Scholar] [CrossRef]

| Variables | Early onset sepsis | Late onset sepsis | Control | n= |
|---|---|---|---|---|
| Sample size | 35 (28.9%) | 39 (32.2%) | 47 (38.8%) | 121 |
| Vaginal delivery | 17 (24.6%) | 13 (18.8%) | 39 (56.5%) | 69 |
| Cesarian section | 18 (35.3%) | 25 (49%) | 8 (15.7%) | 51 |
| 25-28 weeks GA1 | 4 (30.7%) | 8 (61.5%) | 1 (7.6%) | 13 |
| 29-32 weeks GA1 | 10 (25.6%) | 12 (30.7%) | 17 (43.5%) | 39 |
| 33-37 weeks GA1 | 21 (30.4%) | 19 (27.5%) | 29 (40.1%) | 69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





