Submitted:
27 May 2024
Posted:
28 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
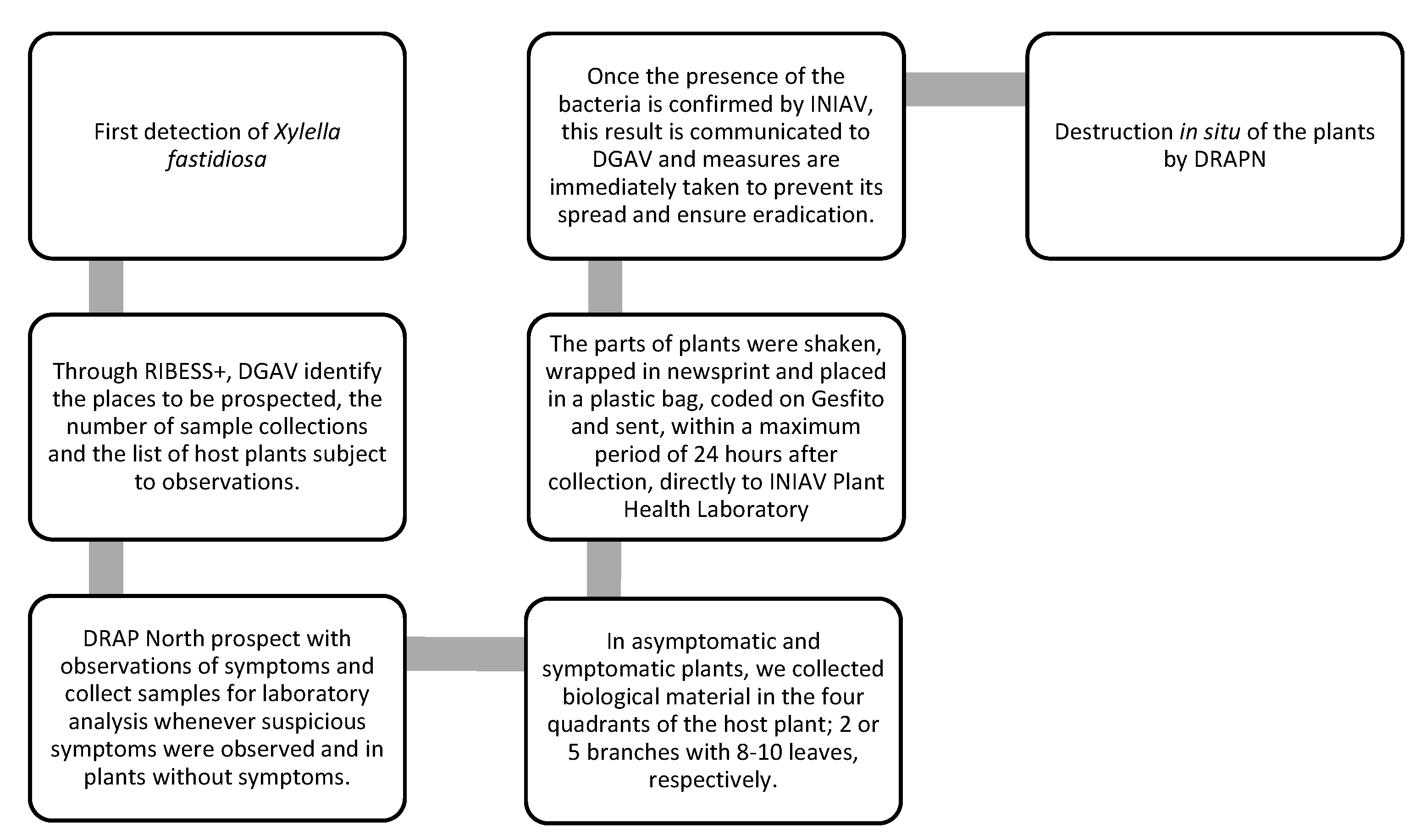
2.1. Prospection Phase
2.1.1. Sample Collection
2.1.2. Results Reception
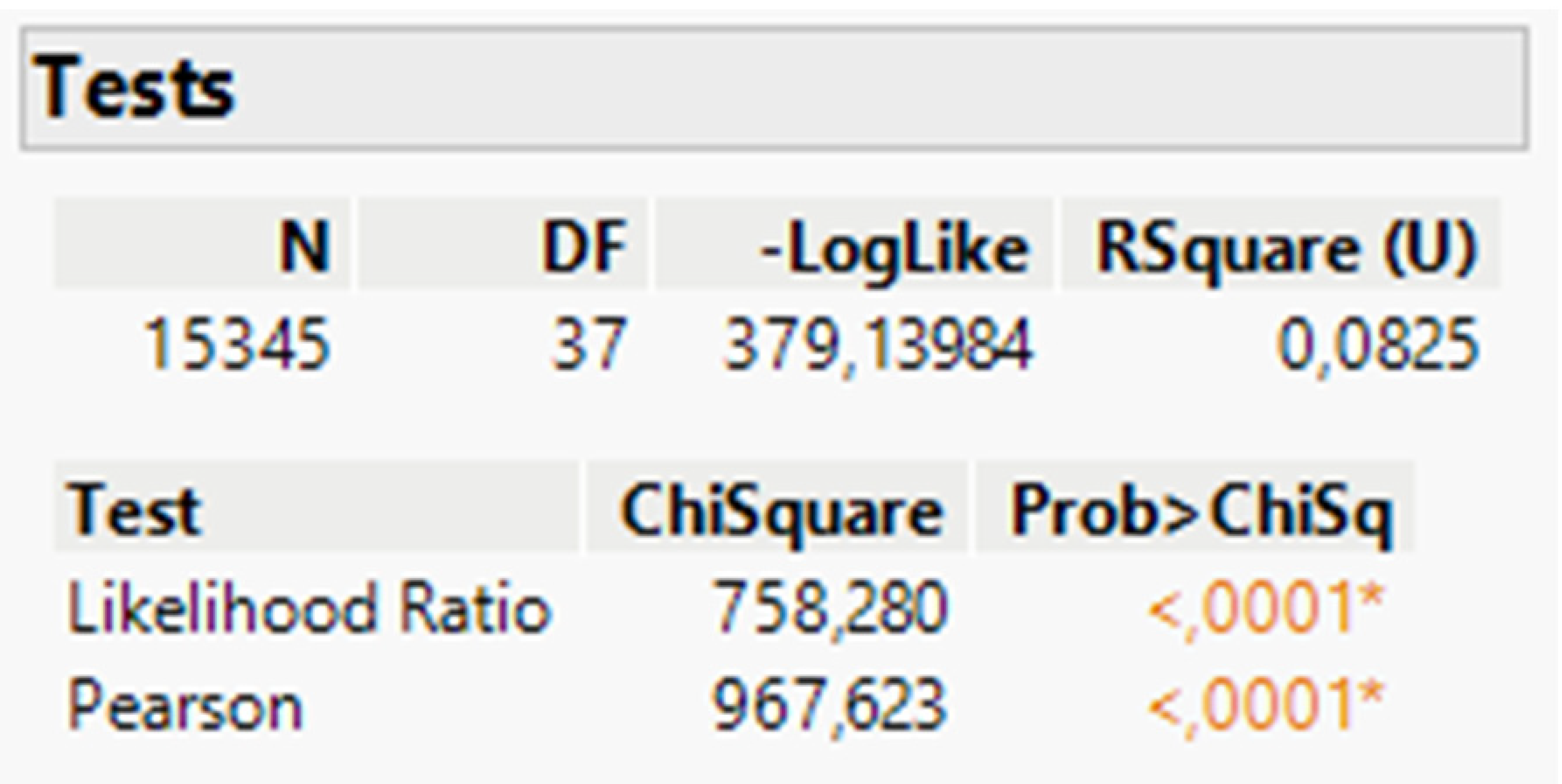
2.2. Collecting, Organizing and Statistical Treatment
3. Results and Discussion
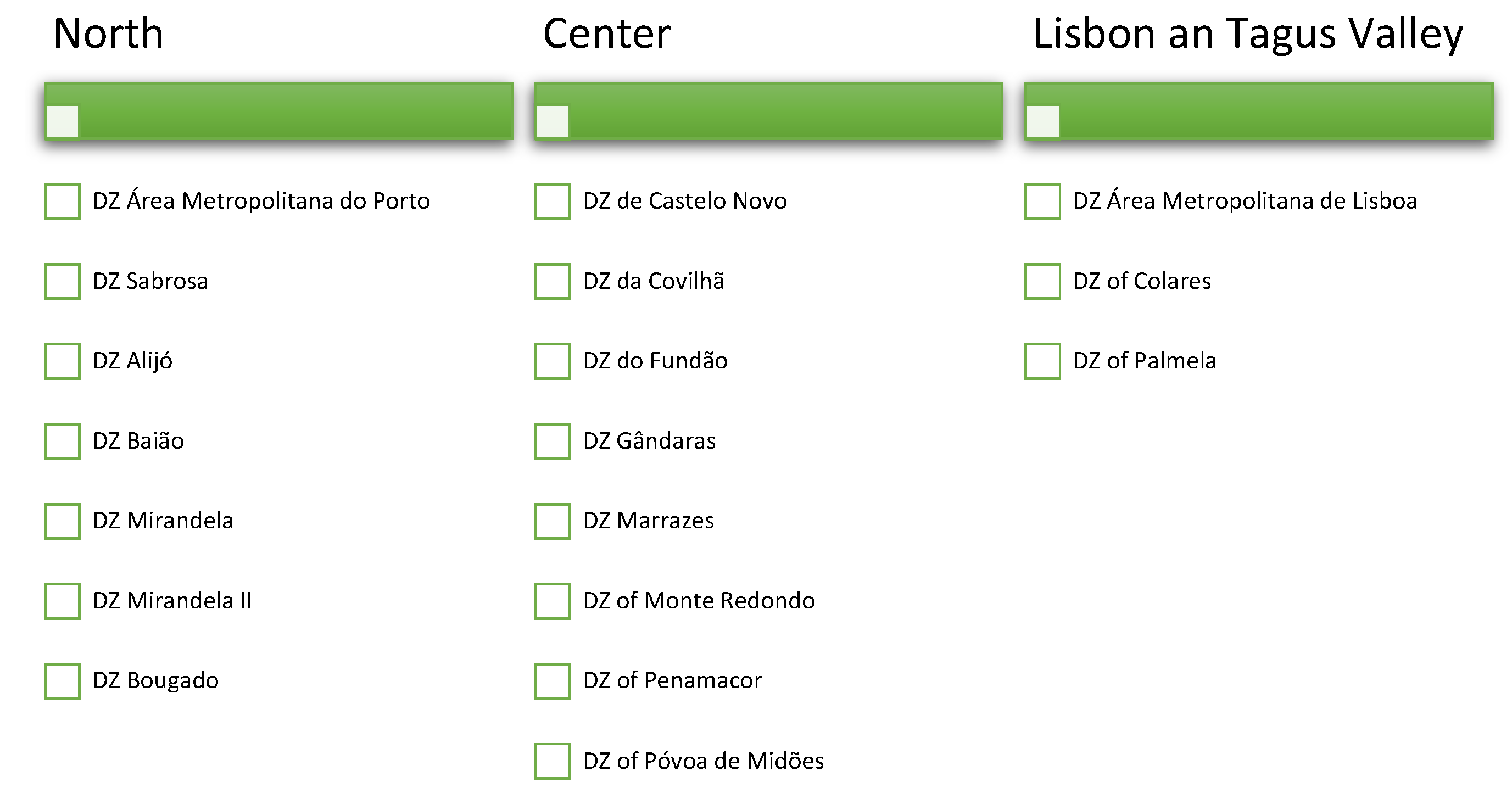
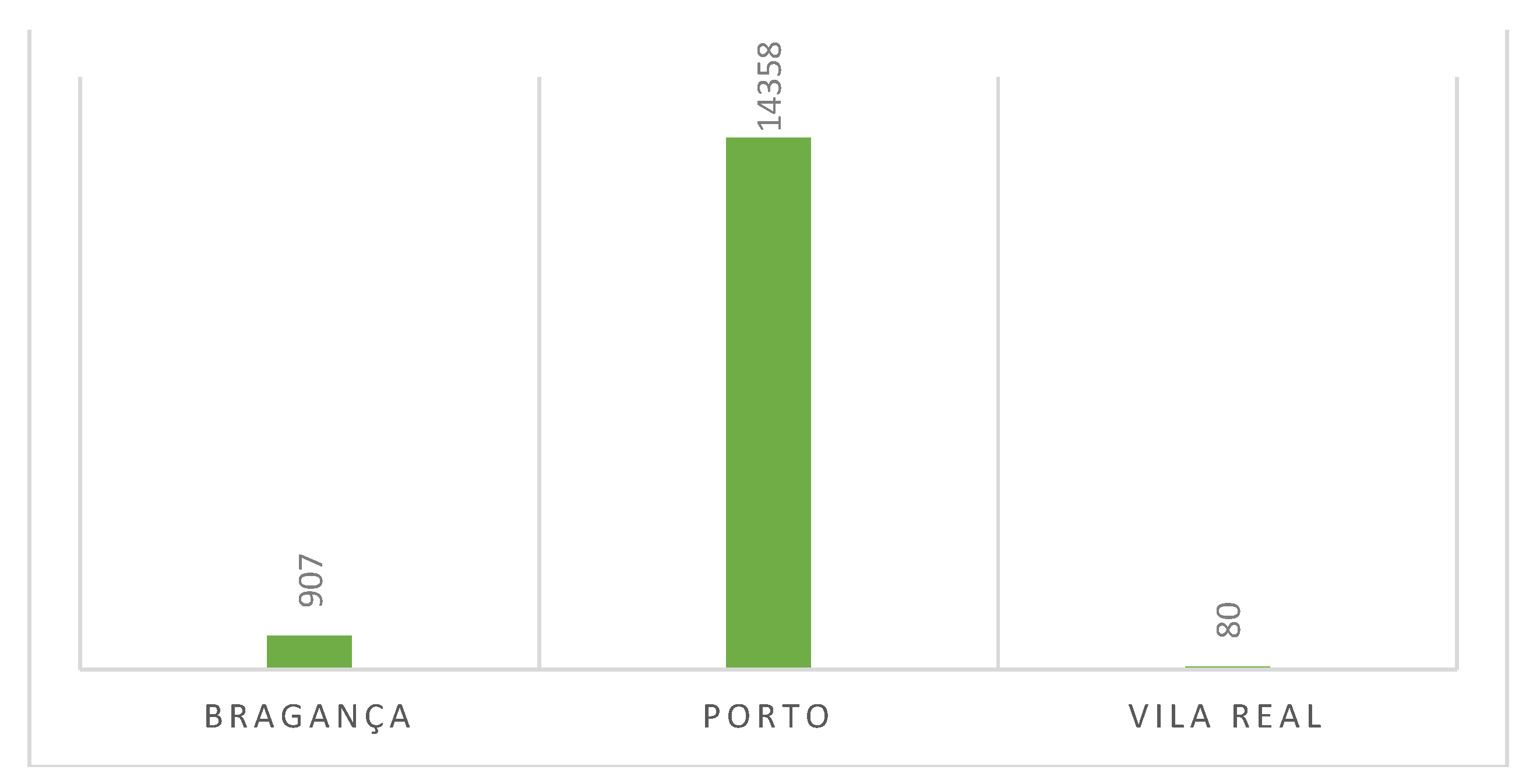
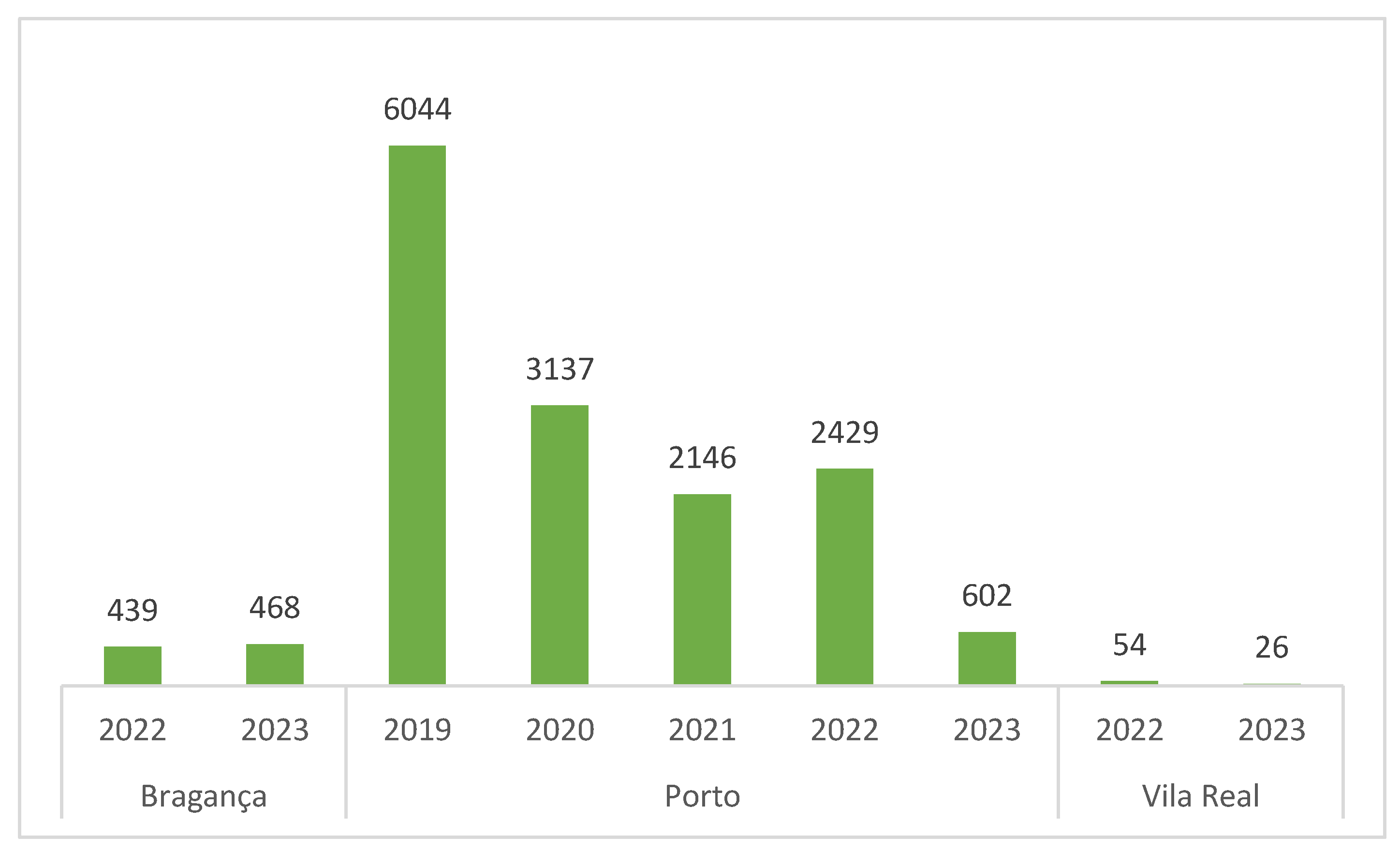
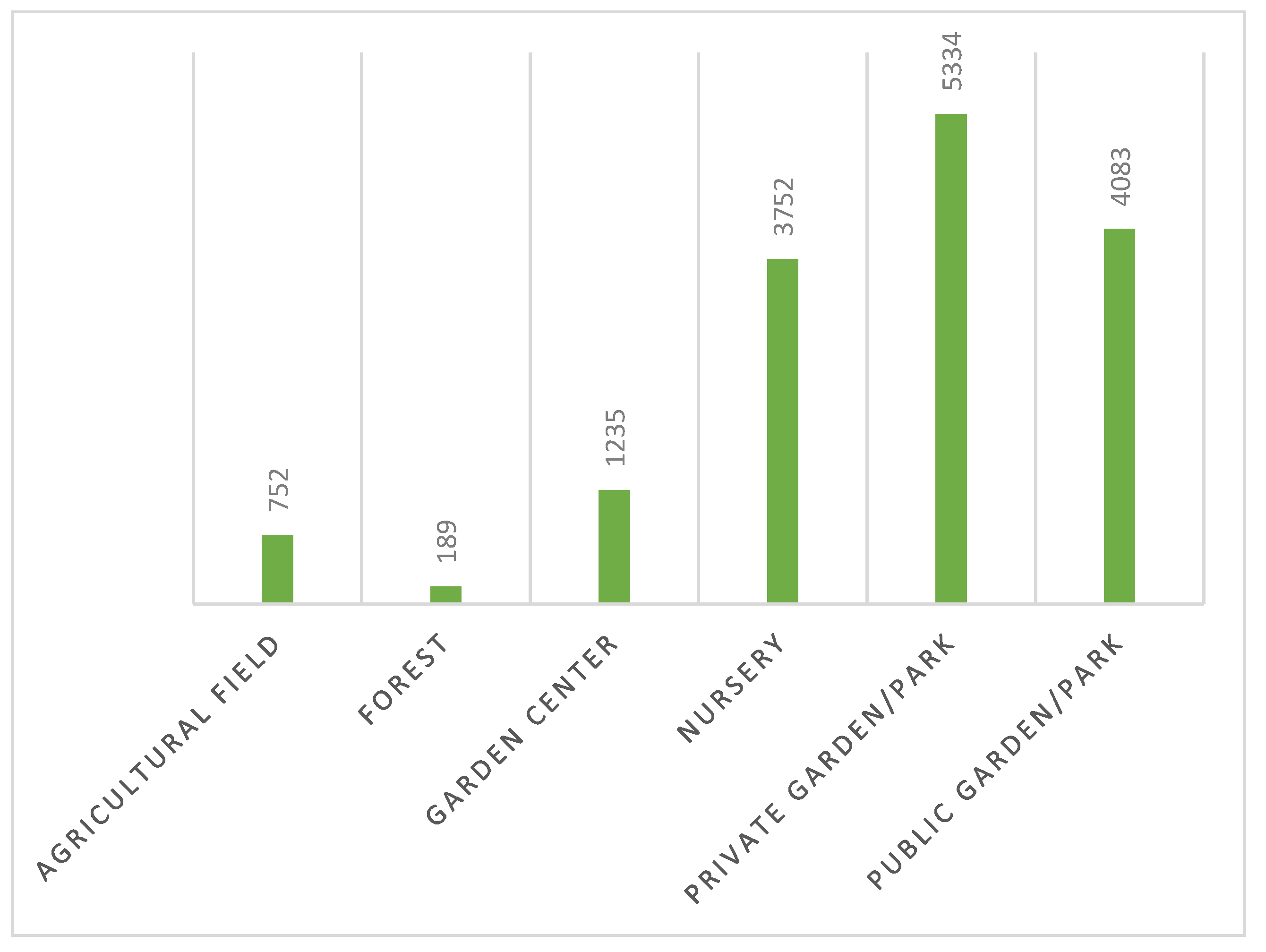
3.1. Number of Locations Observed
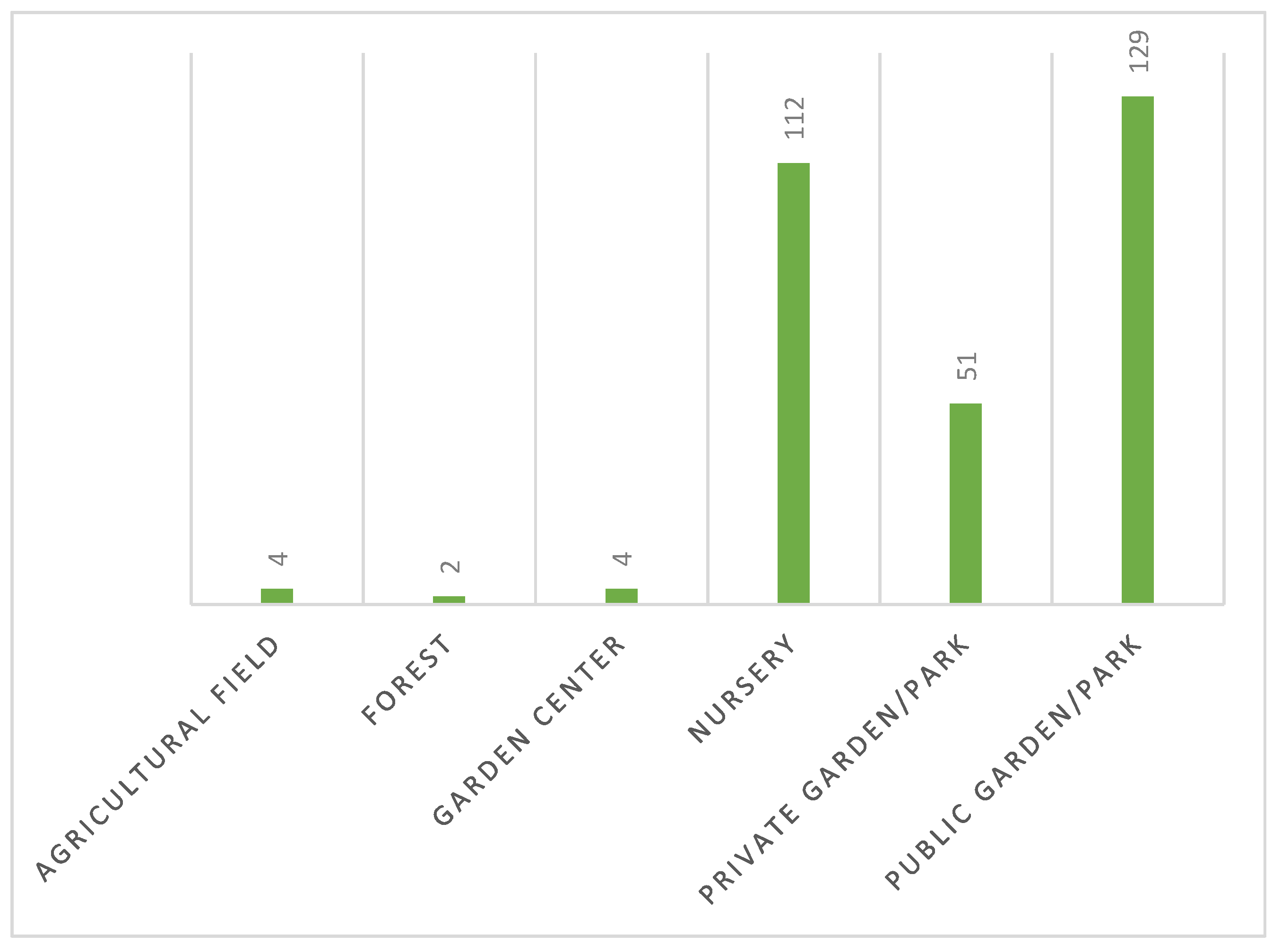
3.2. Type of Locations Observed
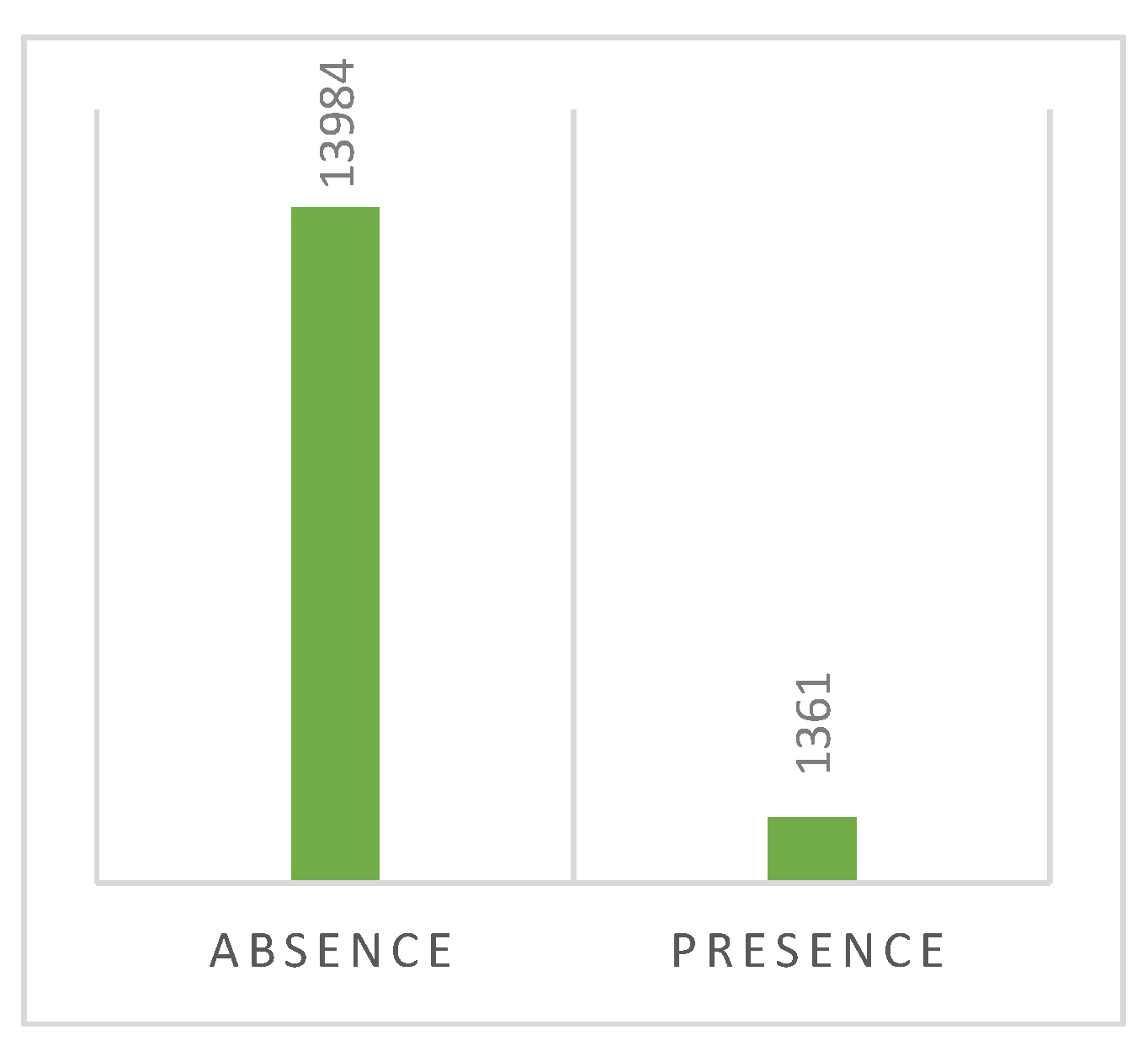
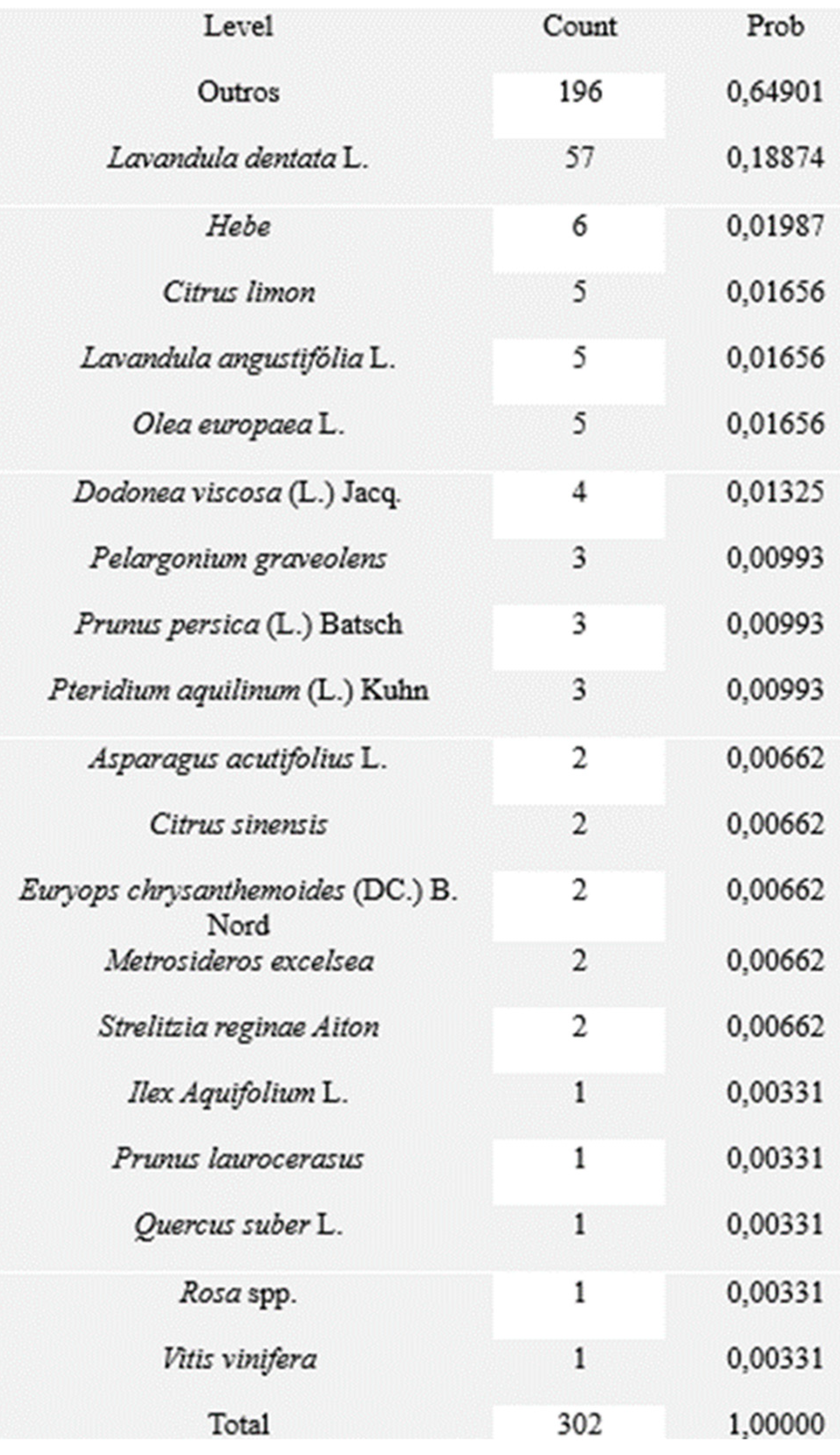
3.3. Presence of Symptoms
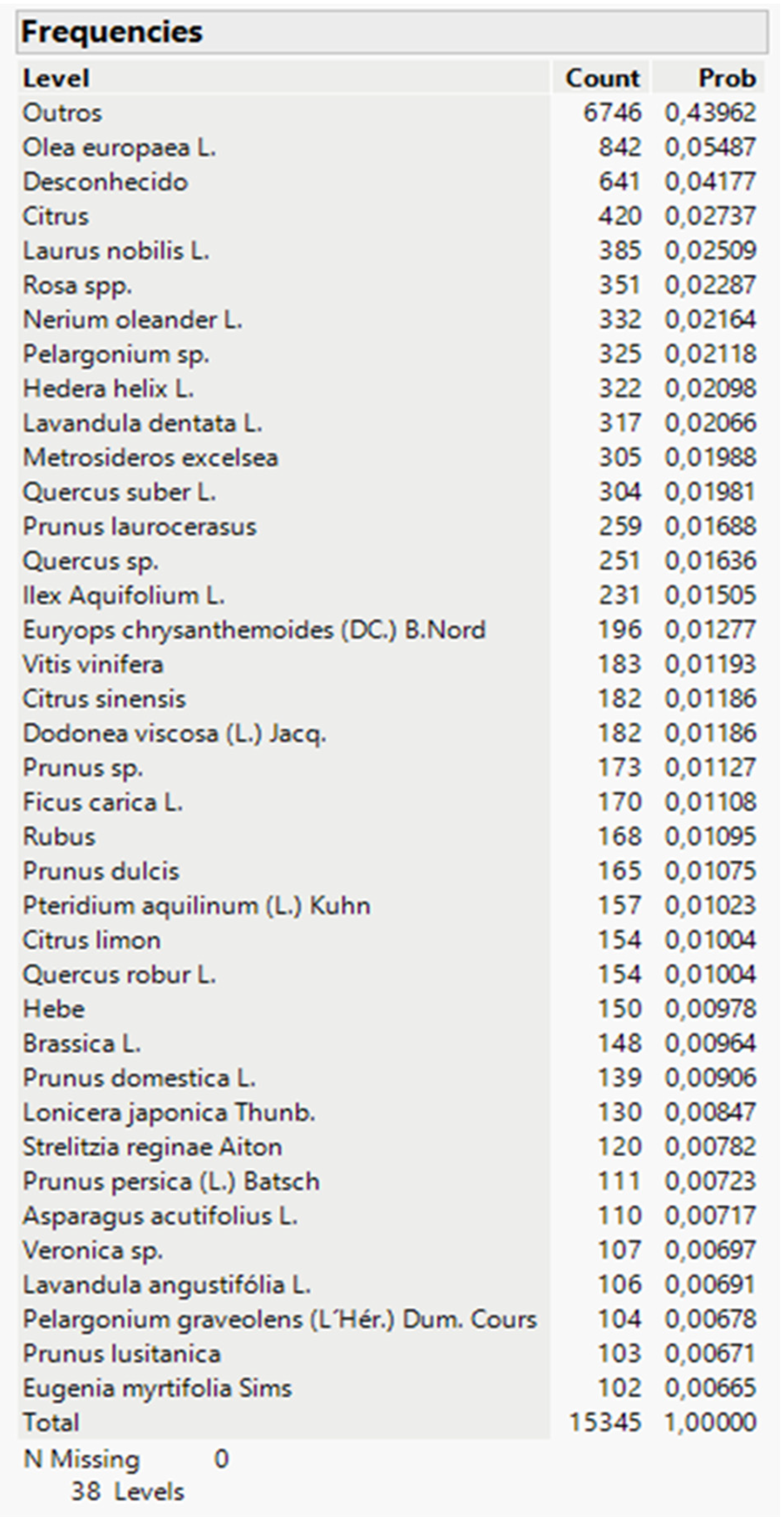
3.4. Hosts Observed
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- K. Schneider, M. Mourits, W. van der Werf, and A. O. Lansink, “On consumer impact from Xylella fastidiosa subspecies pauca,” Ecological Economics, vol. 185, p. 107024, Jul. 2021. [CrossRef]
- B. M. Ali, W. van der Werf, and A. Oude Lansink, “Assessment of the environmental impacts of Xylella fastidiosa subsp. pauca in Puglia,” Crop Protection, vol. 142, p. 105519, Apr. 2021. [CrossRef]
- V. Montilon et al., “Xylella fastidiosa subsp. pauca ST53 exploits pit membranes of susceptible olive cultivars to spread systemically in the xylem,” Plant Pathol, vol. 72, no. 1, pp. 144–153, Jan. 2023. [CrossRef]
- E. Food et al., “Pest survey card on Xylella fastidiosa,” EFSA Supporting Publications, vol. 16, no. 6, p. 1667E, Jun. 2019. [CrossRef]
- G. Petit et al., “Susceptibility to Xylella fastidiosa and functional xylem anatomy in Olea europaea: revisiting a tale of plant-pathogen interaction,” AoB Plants, vol. 13, no. 4, 2021. [CrossRef]
- Delbianco, D. Gibin, L. Pasinato, D. Boscia, and M. Morelli, “Update of the Xylella spp. host plant database – systematic literature search up to 30 June 2022,” EFSA Journal, vol. 21, no. 1, Jan. 2023. [CrossRef]
- Baù, A. Delbianco, G. Stancanelli, and S. Tramontini, “Susceptibility of Olea europaea L. varieties to Xylella fastidiosa subsp. pauca ST53: systematic literature search up to 24 March 2017,” EFSA Journal, vol. 15, no. 4, Apr. 2017. [CrossRef]
- G. P. Martelli, D. Boscia, F. Porcelli, and M. Saponari, “The olive quick decline syndrome in south-east Italy: a threatening phytosanitary emergency,” Eur J Plant Pathol, vol. 144, no. 2, pp. 235–243, Feb. 2016. [CrossRef]
- D. Martinetti and S. Soubeyrand, “Identifying lookouts for epidemio-surveillance: Application to the emergence of xylella fastidiosa in France,” Phytopathology, vol. 109, no. 2, pp. 265–276, Feb. 2019. [CrossRef]
- EPPO Bulletin, “PM 7/24 (4) Xylella fastidiosa,” EPPO Bulletin, vol. 49, no. 2, pp. 175–227, Aug. 2019. [CrossRef]
- G. Gilioli et al., “An eco-epidemiological model supporting rational disease management of Xylella fastidiosa. An application to the outbreak in Apulia (Italy),” Ecol Modell, vol. 476, Feb. 2023. [CrossRef]
- O. G. Hernández and L. V. García, “Incidencia de Xylella fastidiosa en las Islas Baleares y distribución potencial en la península ibérica,” Investigaciones Geográficas, no. 69, pp. 55–72, Jun. 2018. [CrossRef]
- D. Olmo et al., “Landscape Epidemiology of Xylella fastidiosa in the Balearic Islands,” Agronomy 2021, Vol. 11, Page 473, vol. 11, no. 3, p. 473, Mar. 2021. [CrossRef]
- DGAV, Plano de Contingência Xylella fastidiosa e seus vetores. 2022.
- “Xylella fastidiosa – DGAV.” Accessed: Mar. 25, 2023. [Online]. Available: https://www.dgav.pt/plantas/conteudo/sanidade-vegetal/inspecao-fitossanitaria/informacao-fitossanitaria/xylella-fastidiosa/.
- RE (UE) 2020/1201, “REGULAMENTO DE EXECUÇÃO (UE) 2020/1201 DA COMISSÃO de 14 de agosto de 2020 relativo às medidas para impedir a introdução e a propagação na União de Xylella fastidiosa (Wells et al.),” Accessed: Nov. 06, 2022. [Online]. Available: https://eur-lex.europa.eu/legal-content/PT/TXT/PDF/?uri=CELEX:32020R1201&from=EN.
- Paula, A. Cruz De Carvalho, A. Cruz De Carvalho Dn: C=pt, Title=subdiretora Geral, G. de Alimentação E Veterinária, P. de Almeida, and C. de Carvalho, “PLANO DE AÇÃO PARA ERRADICAÇÃO DE Xylella fastidiosa e controlo dos seus vetores ZONA DEMARCADA DA ÁREA METROPOLITANA DO PORTO Atualizado em fevereiro de 2022 Aprovado,” 2022.
- “Portaria n.o 243/2020, de 14 de outubro | DRE.” Accessed: Apr. 23, 2023. [Online]. Available: https://dre.pt/dre/detalhe/portaria/243-2020-145359683.
- “Decreto-Lei n.o 67/2020, de 15 de setembro | DRE.” Accessed: Apr. 23, 2023. [Online]. Available: https://dre.pt/dre/detalhe/decreto-lei/67-2020-142870334.
- M. Frem et al., “Xylella fastidiosa invasion of new countries in Europe, the Middle East and North Africa: Ranking the potential exposure scenarios,” NeoBiota 59: 77-97, vol. 59, pp. 77–97, Aug. 2020. [CrossRef]
- H. Purcell and S. R. Saunders, “Fate of Pierce’s Disease Strains of Xylella fastidiosa in Common Riparian Plants in California,” https://doi.org/10.1094/PDIS.1999.83.9.825, vol. 83, no. 9, pp. 825–830, Feb. 2007. [CrossRef]
- “Phyto71n04_429.pdf | Enhanced Reader.
- T. Cao, J. H. Connell, M. Wilhelm, and B. C. Kirkpatrick, “Influence of Inoculation Date on the Colonization of Xylella fastidiosa and the Persistence of Almond Leaf Scorch Disease Among Almond Cultivars,” https://doi.org/10.1094/PDIS-05-10-0327, vol. 95, no. 2, pp. 158–165, Jan. 2011. [CrossRef]
- A. Ledbetter, J. Chen, S. Livingston, and R. L. Groves, “Winter curing of prunus dulcis cv ‘Butte,’ P. webbii and their interspecific hybrid in response to xylella fastidiosa infections,” Euphytica, vol. 169, no. 1, pp. 113–122, May 2009. [CrossRef]
- R. P. P. Almeida and A. H. Purcell, “Biological Traits of Xylella fastidiosa Strains from Grapes and Almonds,” Appl Environ Microbiol, vol. 69, no. 12, pp. 7447–7452, Dec. 2003. [CrossRef]
- H. Feil, W. S. Feil, and A. H. Purcell, “Effects of Date of Inoculation on the Within-Plant Movement of Xylella fastidiosa and Persistence of Pierce’s Disease Within Field Grapevines,” Phytopathology, vol. 93, no. 2, pp. 244–251, Feb. 2003. [CrossRef]
- M. Saponari, A. Giampetruzzi, G. Loconsole, D. Boscia, and P. Saldarelli, “Xylella fastidiosa in olive in apulia: Where we stand,” Phytopathology, vol. 109, no. 2, pp. 175–186, Feb. 2019. [CrossRef]
- de N. G. dos Santos, M. Anguita-Maeso, and H. D. Coletta-Filho, “Transmission and distribution of Xylella fastidiosa subsp. pauca in olive trees as a parameter for managing olive quick decline syndrome,” Plant Pathol, vol. 71, no. 9, pp. 1849–1858, Dec. 2022. [CrossRef]
- N. Amanifar, M. Taghavi, and M. Salehi, “<em>Xylella fastidiosa</em> from almond in Iran: overwinter recovery and effects of antibiotics,” Phytopathol Mediterr, vol. 55, no. 3, pp. 337–345, Jul. 2016. [CrossRef]
- M. J. Davis, W. J. French, and N. W. Schaad, “Axenic culture of the bacteria associated with phony disease of peach and plum leaf scald,” Curr Microbiol, vol. 6, no. 5, pp. 309–314, 1981. [CrossRef]
- J. H. Aldrich, “ Distribution of Xylella fastidiosa Within Roots of Peach,” Plant Dis, vol. 76, no. 9, p. 885, 1992. [CrossRef]
- R. M. Holland, R. S. C. Christiano, E. Gamliel-Atinsky, and H. Scherm, “Distribution of Xylella fastidiosa in Blueberry Stem and Root Sections in Relation to Disease Severity in the Field,” https://doi.org/10.1094/PDIS-06-13-0680-RE, vol. 98, no. 4, pp. 443–447, Mar. 2014. [CrossRef]
- T. S. M. Henneberger, K. L. Stevenson, K. O. Britton, and C. J. Chang, “Distribution of Xylella fastidiosa in Sycamore Associated with Low Temperature and Host Resistance,” https://doi.org/10.1094/PDIS.2004.88.9.951, vol. 88, no. 9, pp. 951–958, Feb. 2007. [CrossRef]
- L. Hopkins, “ Seasonal Fluctuation in the Occurrence of Xylella fastidiosa in Root and Stem Extracts from Citrus with Blight,” Plant Dis, vol. 75, no. 2, p. 145, 1991. [CrossRef]
- X. He, W. B. Li, A. J. Ayres, J. S. Hartung, V. S. Miranda, and D. C. Teixeira, “Distribution of Xylella fastidiosa in Citrus Rootstocks and Transmission of Citrus Variegated Chlorosis Between Sweet Orange Plants Through Natural Root Grafts,” https://doi.org/10.1094/PDIS.2000.84.6.622, vol. 84, no. 6, pp. 622–626, Feb. 2007. [CrossRef]
- J. N. Rapicavoli et al., “Lipopolysaccharide O-antigen delays plant innate immune recognition of Xylella fastidiosa,” Nature Communications 2018 9:1, vol. 9, no. 1, pp. 1–12, Jan. 2018. [CrossRef]
- Purcell, “Paradigms: Examples from the Bacterium Xylella fastidiosa,” https://doi.org/10.1146/annurev-phyto-082712-102325, vol. 51, pp. 339–356, Aug. 2013. [CrossRef]
- Bragard et al., “Update of the Scientific Opinion on the risks to plant health posed by Xylella fastidiosa in the EU territory,” EFSA Journal, vol. 17, no. 5, May 2019. [CrossRef]
- R. B. Queiroz-Voltan, L. Perosin Cabral, and O. Paradela Filho, “Severidade do sintoma da bactéria Xylella fastidiosa em cultivares de cafeeiro,” Bragantia, vol. 63, no. 3, pp. 395–404, 2004. [CrossRef]
- L. Hopkins, “Biological Control of Pierce’s Disease in the Vineyard with Strains of Xylella fastidiosa Benign to Grapevine,” https://doi.org/10.1094/PD-89-1348, vol. 89, no. 12, pp. 1348–1352, Feb. 200. [CrossRef]
- P. P. Kandel, R. P. P. Almeida, P. A. Cobine, and L. De La Fuente, “Natural competence rates are variable among xylella fastidiosa strains and homologous recombination occurs in vitro between subspecies fastidiosa and multiplex,” Molecular Plant-Microbe Interactions, vol. 30, no. 7, pp. 589–600, Jul. 2017. [CrossRef]
- Gibin, L. Pasinato, and A. Delbianco, “Update of the Xylella spp. host plant database – systematic literature search up to 31 December 2022,” EFSA Journal, vol. 21, no. 6, p. e08061, Jun. 2023. [CrossRef]
- Niza, H. D. Coletta-Filho, M. V. Merfa, M. A. Takita, and A. A. de Souza, “Differential colonization patterns of Xylella fastidiosa infecting citrus genotypes,” Plant Pathol, vol. 64, no. 6, pp. 1259–1269, Dec. 2015. [CrossRef]
- Baccari, E. Antonova, and S. Lindow, “Biological control of Pierce’s disease of grape by an endophytic bacterium,” Phytopathology, vol. 109, no. 2, pp. 248–256, Feb. 2019. [CrossRef]
- Direção Geral de Alimentação e Veterinária, “XYLELLA FASTIDIOSA GÉNEROS E ESPÉCIES VEGETAIS DETETADOS INFETADOS NA ZONA DEMARCADA DA ÁREA METROPOLITANA DO PORTO,” Mar. 2023.
- P. S. Pereira, “Xylella fastidiosa - a new menace for Portuguese agriculture and forestry.,” Revista de Ciências Agrárias (Portugal), vol. 38, no. 2, pp. 149–154, 2015.
- V. Cavalieri et al., “Transmission of Xylella fastidiosa Subspecies Pauca Sequence Type 53 by Different Insect Species,” Insects, vol. 10, no. 10, Oct. 2019. [CrossRef]
- H. Dietrich, “KEYS TO THE FAMILIES OF CICADOMORPHA AND SUBFAMILIES AND TRIBES OF CICADELLIDAE (HEMIPTERA: AUCHENORRHYNCHA)”, Accessed: Apr. 23, 2023. [Online]. Available: https://bioone.org/journals/florida-entomologist/volume-88/issue-4/0015-4040_2005_88_502_KTTFOC_2.0.CO_2/KEYS-TO-THE-FAMILIES-OF-CICADOMORPHA-AND-SUBFAMILIES-AND-TRIBES/10.1653/0015-4040(2005)88[502:KTTFOC]2.0.CO;2.full.
- N. Bodino et al., “Spittlebugs of Mediterranean Olive Groves: Host-Plant Exploitation throughout the Year,” Insects 2020, Vol. 11, Page 130, vol. 11, no. 2, p. 130, Feb. 2020. [CrossRef]
- S. Yoon and W. H. Lee, “Spatial analysis of climatic and dispersion characteristics of Xylella fastidiosa outbreak by insect vectors,” J Asia Pac Entomol, vol. 26, no. 1, p. 102011, Mar. 2023. [CrossRef]
- Lago, D. Cornara, S. A. Minutillo, A. Moreno, and A. Fereres, “Feeding behaviour and mortality of Philaenus spumarius exposed to insecticides and their impact on Xylella fastidiosa transmission,” Pest Manag Sci, vol. 78, no. 11, p. 4841, Nov. 2022. [CrossRef]
- S. DROSOPOULOS and M. ASCHE, “Biosystematic studies on the spittlebug genus Philaenus with the description of a new species,” Zool J Linn Soc, vol. 101, no. 2, pp. 169–177, Feb. 1991. [CrossRef]
- M. Godefroid and J. M. Durán, “Composition of landscape impacts the distribution of the main vectors of Xylella fastidiosa in southern Spain,” Journal of Applied Entomology, vol. 146, no. 6, pp. 666–675, Jul. 2022. [CrossRef]
- R. Karban and S. Y. Strauss, “Physiological tolerance, climate change, and a northward range shift in the spittlebug, Philaenus spumarius,” Ecol Entomol, vol. 29, no. 2, pp. 251–254, Apr. 2004. [CrossRef]
- S. M. Chmiel and M. Curtis Wilson, “Estimation of the Lower and Upper Developmental Threshold Temperatures and Duration of the Nymphal Stages of the Meadow Spittlebug, Philaenus spumarius,” Environ Entomol, vol. 8, no. 4, pp. 682–685, Aug. 1979. [CrossRef]
- D. Ahmed and R. H. Davidson, “Life History of the Meadow Spittlebug in Ohio,” J Econ Entomol, vol. 43, no. 6, pp. 905–908, Dec. 1950. [CrossRef]
- S. Drosopoulos, “New data on the nature and origin of colour polymorphism in the spittlebug genus Philaenus (Hemiptera: Aphorophoridae),” Annales de la Société Entomologique de France, vol. 39, no. 1, pp. 31–42, Jan. 2003. [CrossRef]
- M. Chartois et al., “Environmental factors driving the abundance of Philaenus spumarius in mesomediterranean habitats of Corsica (France),” Scientific Reports 2023 13:1, vol. 13, no. 1, pp. 1–11, Feb. 2023. [CrossRef]
- Cornara et al., “Natural areas as reservoir of candidate vectors of Xylella fastidiosa,” Bull Insectology, vol. 74, no. 2, pp. 173–180, 2021.
- Dongiovanni et al., “Plant Selection and Population Trend of Spittlebug Immatures (Hemiptera: Aphrophoridae) in Olive Groves of the Apulia Region of Italy,” J Econ Entomol, vol. 112, no. 1, pp. 67–74, Feb. 2019. [CrossRef]
- H. Séverine and N. Casarin, “Belgian Journal of Entomology Distribution, adult phenology and life history traits of potential insect vectors of Xylella fastidiosa in Belgium”, Accessed: Oct. 25, 2023. [Online]. Available: https://www.researchgate.net/publication/352903222.
- X. Mesmin, M. Chartois, S. Borgomano, J. Y. Rasplus, J. P. Rossi, and A. Cruaud, “Interaction networks between spittlebugs and vegetation types in and around olive and clementine groves of Corsica; implications for the spread of Xylella fastidiosa,” Agric Ecosyst Environ, vol. 334, p. 107979, Aug. 2022. [CrossRef]
- J. Albre, J. M. G. Carrasco, and M. Gibernau, “Ecology of the meadow spittlebug Philaenus spumarius in the Ajaccio region (Corsica) – I: spring,” Bull Entomol Res, vol. 111, no. 2, pp. 246–256, Apr. 2021. [CrossRef]
- “(PDF) Xylella fastidiosa: AN OVERVIEW OF RESEARCH AT SASA.” Accessed: Oct. 24, 2023. [Online]. Available: https://www.researchgate.net/publication/344376341_Xylella_fastidiosa_AN_OVERVIEW_OF_RESEARCH_AT_SASA.
- N. Bodino et al., “Phenology, seasonal abundance and stage-structure of spittlebug (Hemiptera: Aphrophoridae) populations in olive groves in Italy,” Scientific Reports 2019 9:1, vol. 9, no. 1, pp. 1–17, Nov. 2019. [CrossRef]
- Dongiovanni et al., “Plant Selection and Population Trend of Spittlebug Immatures (Hemiptera: Aphrophoridae) in Olive Groves of the Apulia Region of Italy,” J Econ Entomol, vol. 112, no. 1, pp. 67–74, Feb. 2019. [CrossRef]
- M. Villa, I. Rodrigues, P. Baptista, A. Fereres, and J. A. Pereira, “Populations and Host/Non-Host Plants of Spittlebugs Nymphs in Olive Orchards from Northeastern Portugal,” Insects 2020, Vol. 11, Page 720, vol. 11, no. 10, p. 720, Oct. 2020. [CrossRef]
- M. Morente et al., “Distribution and Relative Abundance of Insect Vectors of Xylella fastidiosa in Olive Groves of the Iberian Peninsula,” Insects 2018, Vol. 9, Page 175, vol. 9, no. 4, p. 175, Dec. 2018. [CrossRef]
- S. Antonatos, D. P. Papachristos, K. Varikou, P. Vahamidis, A. Kapranas, and P. Milonas, “Seasonal Appearance, Abundance, and Host Preference of Philaenus spumarius and Neophilaenus campestris (Hemiptera: Aphrophoridae) in Olive Groves in Greece,” Environ Entomol, vol. 50, no. 6, pp. 1474–1482, Dec. 2021. [CrossRef]
- X. Yuan, L. Morano, R. Bromley, S. Spring-pearson, R. Stouthamer, and L. Nunney, “Multilocus sequence typing of Xylella fastidiosa causing Pierce’s disease and oleander leaf scorch in the United States,” Phytopathology, vol. 100, no. 6, pp. 601–611, Jun. 2010. [CrossRef]
- L. Nunney, S. Elfekih, and R. Stouthamer, “The importance of multilocus sequence typing: cautionary tales from the bacterium Xylella fastidiosa,” Phytopathology, vol. 102, no. 5, pp. 456–462, May 2012. [CrossRef]
- L. Nunney et al., “Population Genomic Analysis of a Bacterial Plant Pathogen: Novel Insight into the Origin of Pierce’s Disease of Grapevine in the U.S.,” PLoS One, vol. 5, no. 11, p. e15488, 2010. [CrossRef]
- N. Denancé et al., “Several subspecies and sequence types are associated with the emergence of Xylella fastidiosa in natural settings in France,” Plant Pathol, vol. 66, no. 7, pp. 1054–1064, Sep. 2017. [CrossRef]
- Cunty et al., “Update of the Xylella fastidiosa outbreak in France: two new variants detected and a new region affected,” Eur J Plant Pathol, vol. 163, no. 2, pp. 505–510, Jun. 2022. [CrossRef]
- B. Landa et al., “Emergence of a plant pathogen in europe associated with multiple intercontinental introductions,” Appl Environ Microbiol, vol. 86, no. 3, Feb. 2020. [CrossRef]
- Direção Geral de Alimentação e Veterinária, “Despacho no6/G/2022 - ATUALIZAÇÃO DA ZONA DEMARCADA PARA Xylella fastidiosa DA ÁREA METROPOLITANA DO PORTO.
- Dupas et al., “Suspicions of two bridgehead invasions of Xylella fastidiosa subsp. multiplex in France,” Communications Biology 2023 6:1, vol. 6, no. 1, pp. 1–13, Jan. 2023. [CrossRef]
- Direção Geral de Alimentação e Veterinária, “Atualização da Zona Demarcada para Xylella fastidiosa da Área Metropolitana de Lisboa.
- Giampetruzzi et al., “Genome-Wide Analysis Provides Evidence on the Genetic Relatedness of the Emergent Xylella fastidiosa Genotype in Italy to Isolates from Central America,” Phytopathology, vol. 107, no. 7, pp. 816–827, Jul. 201. [CrossRef]
- M. Godefroid, A. Cruaud, J.-C. Streito, J.-Y. Rasplus, and J.-P. Rossi, “Climate change and the potential distribution of Xylella fastidiosa in Europe,” bioRxiv, p. 289876, Mar. 2018. [CrossRef]
- Piyapong, C. Tattoni, M. Ciolli, S. Dembski, and E. Paradis, “Modelling the geographical distributions of one native and two introduced species of crayfish in the French Alps,” Ecol Inform, vol. 60, p. 101172, Nov. 2020. [CrossRef]
- M. S. Hoddle, “The potential adventive geographic range of glassy-winged sharpshooter, Homalodisca coagulata and the grape pathogen Xylella fastidiosa: implications for California and other grape growing regions of the world,” Crop Protection, vol. 23, no. 8, pp. 691–699, Aug. 2004. [CrossRef]
- V. Chikwendu and H. Tochi, “Aspects of the ecology of spittlebugs (Homoptera: Cercopidae) in Nsukka, south east, Nigeria,” Animal Research International, vol. 7, no. 3, pp. 1242–1252, 2010. [CrossRef]
- H. Purcell and D. L. Hopkins, “FASTIDIOUS XYLEM-LIMITED BACTERIAL PLANT PATHOGENS,” https://doi.org/10.1146/annurev.phyto.34.1.131, vol. 34, pp. 131–151, Nov. 2003. [CrossRef]
- H. Feil and A. H. Purcell, “Temperature-Dependent Growth and Survival of Xylella fastidiosa in Vitro and in Potted Grapevines,” Plant Dis, vol. 85, no. 12, pp. 1230–1234, 2001. [CrossRef]
- “Temperaturas médias do ar em Portugal 2019-2022.” Accessed: Dec. 17, 2023. [Online]. Available: https://www.ine.pt/xportal/xmain?xpid=INE&xpgid=ine_indicadores&indOcorrCod=0009895&contexto=bd&selTab=tab2&xlang=pt.
- L. Bosso, D. Russo, M. Di Febbraro, G. Cristinzio, and A. Zoina, “Potential distribution of Xylella fastidiosa in Italy: A maximum entropy model,” Phytopathol Mediterr, vol. 55, no. 1, pp. 62–72, 2016. [CrossRef]
- S. Avosani, C. Tattoni, V. Mazzoni, and M. Ciolli, “Occupancy and detection of agricultural threats: The case of Philaenus spumarius, European vector of Xylella fastidiosa,” Agric Ecosyst Environ, vol. 324, p. 107707, Feb. 2022. [CrossRef]
- M. Castro, J. Castro, and A. Gómez Sal, “The role of black oak woodlands (Quercus pyrenaica Willd.) in small ruminant production in Northeast Portugal,” Sustainability of Agrosilvopastoral Systems, pp. 221–229, 2004, Accessed: Dec. 07, 2023. [Online]. Available: https://bibliotecadigital.ipb.pt/handle/10198/4447.
- L. Bosso, M. Di Febbraro, G. Cristinzio, A. Zoina, and D. Russo, “Shedding light on the effects of climate change on the potential distribution of Xylella fastidiosa in the Mediterranean basin,” Biol Invasions, vol. 18, no. 6, pp. 1759–1768, Jun. 2016. [CrossRef]
- H. Feil and A. H. Purcell, “Temperature-Dependent Growth and Survival of Xylella fastidiosa in Vitro and in Potted Grapevines,” https://doi.org/10.1094/PDIS.2001.85.12.1230, vol. 85, no. 12, pp. 1230–1234, Feb. 2007. [CrossRef]
- Cardone et al., “Socio-Economic Risks Posed by a New Plant Disease in the Mediterranean Basin,” Diversity (Basel), vol. 14, no. 11, p. 975, Nov. 2022. [CrossRef]

 |
 |
 |
| 1. Acacia longifolia (Andrews) Wild. | 41. Lagerstroemia indica L. | 38. Hypericum androsaemum L. |
|---|---|---|
| 2. Acacia melanoxylon R. Br. | 42. Laurus nobilis | 39. Hypericum perforatum L. |
| 3. Acacia dealbata Link. | 43. Lavandula angustifólia L. | 40. Ilex aquifolium L. |
| 4. Adenocarpus lainzii (Castrov.) | 44. Lavandula dentata L. | 78. Vinca |
| 5. Argyranthemum frutescens L. | 45. Lavandula stoechas L. | 79. Vitis spp. |
| 6. Artemisia arborescens L. | 46. Lavatera cretica L. | |
| 7. Asparagus acutifolius L. | 47. Liquidambar styraciflua L. | |
| 8. Athyrium filix-femina (L.) Roth. | 48. Lonicera periclymenum L. | |
| 9. Berberis thunbergii DC. | 49. Magnolia grandiflora L. | |
| 10. Calluna vulgaris (L.) Hull. | 50. Magnolia x soulangeana Soul.-Bod. | |
| 11. Castanea sativa Mill. | 51. Mentha suaveolens Ehrh. | |
| 12. Cistus psilosepalus Sweet. | 52. Medicago sativa L. | |
| 13. Cistus salviifolius L. | 53. Metrosideros excelsea Sol. Ex Gaertn. | |
| 14. Citrus limon (L.) N. Burman | 54. Myrtus communis L. | |
| 15. Citrus paradisii Macfadyen | 55. Nerium oleander L. | |
| 16. Citrus reticulata Blanco | 56. Olea europaea L. | |
| 17. Citrus sinensis (L.) Osbeck | 57. Pelargonium graveolens (L´Hér.) Dum. | |
| 18. Coprosma repens A. Rich. | 58. Pyracantha coccinea M. Römer | |
| 19. Cortaderia selloana | 59. Plantago lanceolata L. | |
| 20. Cytisus scoparius (L.) Link. | 60. Platanus x hispanica | |
| 21. Dimorphoteca ecklonis (DC.) Norl. | 61. Prunus laurocerasus L. | |
| 22. Dodonea viscosa (L.) Jacq. | 62. Prunus persica (L.) Batsch | |
| 23. Echium plantagineum L. | 63. Prunus cerasifera Ehrh. | |
| 24. Elaeagnus x submacrophylla | 64. Pteridium aquilinum (L.) Kuhn | |
| 25. Erica cinerea L. | 65. Quercus coccinea Münchh. | |
| 26. Erigeron canadensis (L.) | 66. Quercus robur L. | |
| 27. Erodium moschatum (L.) L. Her. | 67. Quercus rubra L. | |
| 28. Euryops chrysanthemoides (DC.) B. Nord. | 68. Quercus suber L. | |
| 29. Frangula alnus Mill. | 69. Rosa | |
| 30. Gazania rigens (L.) Gaertn. | 70. Rubus idaeus L. | |
| 31. Genista triacanthos Brot. | 71. Rubus ulmifolius Schott. | |
| 32. Genista tridentata (L.) | 72. Ruta graveolans L. | |
| 33. Gleditsia triacanthos L. | 73. Salvia rosmarinus Spenn. | |
| 34. Grevillea rosmarinifolia | 74. Sambucus nigra L. | |
| 35. Hebe | 75. Santolina chamaecyparissus L. | |
| 36. Helichrysum italicum (Roth) G.Don | 76. Strelitzia reginae Ait. | |
| 37. Hibiscus syriacus L. | 77. Ulex spp. |
| Sequence Types | Subspecies | Country most frequently found | Number of records |
|---|---|---|---|
| ST53 | pauca | Italy, France, Costa Rica | 475 |
| ST11 | pauca | Brazil | 52 |
| ST1 | fastidiosa | US, Spain, Mexico | 201 |
| ST06 | multiplex | France, Spain, US | 160 |
| ST07 | multiplex | Portugal, US, France | 142 |
| ST81 | multiplex | Spain | 100 |
| ST87 | multiplex | Italy | 91 |
| ST29 | morus | US | 10 |
| ST05 | sandyi | US | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





