1. Introduction
Around the world, experts from various disciplines are calling for a major overhaul of indoor air quality (IAQ). High health-related costs from infectious and chronic respiratory disease as well as human performance loss associated with a variety of indoor air contaminants were already being discussed prior to the COVID-19 pandemic [
1]. Now, the need for clean indoor air has culminated in calls for a total revolution of the indoor environment akin to what once was achieved for safe drinking water many decades ago [
2].
The significant contribution of building air conditioning to global greenhouse gas emissions adds to the concern. Thus, there is now the triple challenge of improving IAQ, reducing airborne viral disease transmission and reducing the carbon footprint of heating, ventilation, and air conditioning (HVAC) systems [
3]. Energy-efficient air purification systems, based on multiple modes of action to deal with various air contaminants including toxic particles and volatile organic compounds, are required to meet this challenge.
During the first year of the COVID-19 pandemic, the airborne pathway was gradually accepted, recognising that infectious particles in the range of <5 micrometres (μm) can stay in the air and impact over large distances [
4,
5,
6]. This also applies to a range of other infectious particles that transmit via the airways including influenza, tuberculosis, measles and many more [
7]. Recently, the increased risk of hospital acquired COVID-19 infections has been shown which is concerning for staff and vulnerable patients [
8,
9].
Historically the main functions of building HVAC systems were to provide occupant thermal comfort and to introduce fresh air from the outside which assists in alleviating odours and the built up of environmental pollutants. In the context of infectious aerosols and COVID-19, international ventilation guidelines were developed [
10,
11]. While there are no specific legislated standards in Australia, the Victorian Department of Health suggests that building systems should attain more than six air changes per hour (ACH) to achieve a meaningful reduction or dilution in the numbers of particles, including viruses such as SARS CoV-2 [
12]. For standard hospital rooms a minimum of 5 ACH is required and for negative-pressure isolation rooms 12 ACH [
12]
An increase in ventilation can assist in reducing the spread of infectious disease, but it cannot eliminate the risk of airborne diseases, for example by close range ballistic exposure [
13]. Increasing the amount of outside air leads to significantly higher energy usage and CO
2 emissions, along with increased implementation costs. These factors create obstacles to its practical application in many scenarios. Some healthcare spaces have even more stringent requirements such as high efficiency particulate air (HEPA) filters either in-built into the HVAC systems or as a freestanding room air cleaner with HEPA filters. These HEPA filters have the capacity to filter out 99.97% of particles, down to the size of particles of 300 nm as defined in the Australian Standard AS 4260 [
14].The HEPA filter extends its usefulness to other non-infectious particles such as pollen and fungi, and outdoor air pollution-related fine particulate matter such as those emitted from traffic, wind-blown dust or smoke particles created by wild fires. Air pollution related particles have been shown to cause short-term and long -term effects, including cardiopulmonary disease. The World Health Organisation (WHO) and the International Agency for Cancer (IARC) have both deemed the relationship between fine particles, those that are smaller than 10 micrometres in diameter) (<PM
10), as sufficient to cause cardiorespiratory disease, and cancer of the lung [
15]
However, there are limitations to HEPA filtration alone. The energy requirement for air to be pumped through the restrictive filters is extremely high, as is the requirements for exchanging the filters regularly as they pose a health hazard in relation to viable microorganisms [
16].
The Australian Commission on Safety and Quality in Health Care provides guidance in relation to optimising the mechanical ventilation and filtration systems for infection prevention and control in healthcare settings but has yet not extended its guidance to a system combining ventilation and filtration with an added-on disinfection system [
17]. This is most likely due to the reality that emerging air disinfection techniques have not been yet trialled for their efficiency in real life settings. Recently, here have been advanced prototype investigations into air disinfection using UV-C techniques with providing good results [
18] Advanced HVAC-integrated air purification systems have been reported in comparative studies, and these have seen a reduction in hospital stays [
19,
20]. Arikan et al reported a reduction in surface microbial levels following air treatment in an intensive care unit [
21]. There was a significant positive correlation between the number of colonies detected and the rate of hospital-acquired infections. However, the evidence base is incomplete [
22], and further trials are under way [
23].
PlasmaShield® is a medical grade multi-modal air purifier fitted to a new or existing HVAC system. Laboratory tests demonstrate effective particle removal and the mitigation of microbial contamination via multiple modes of action (electron beam irradiation, electric field electroporation and final filtration) [
24,
25].
This study evaluates the PlasmaShield ® technology in the real-life situation of a post-surgical recovery suite within a pediatric hospital in Adelaide, South Australia. The aim of the study is to compare, in an intervention trial design, its performance with a pre-existing system, based on Minimum Efficiency Reporting Value (MERV) 13 filtration [
26]. The evaluation entails multi-size airborne particle monitoring, cultural bacteria and fungi assessment as well as monitoring of key IAQ parameters.
2. Materials and Methods
2.1. Study Location
The Women’s and Children’s Hospital was the location for the intervention study. Following uncomplicated surgery in adjacent operating theatres, mothers and newborn babies are transferred to beds in a recovery suite, where they are kept under observation and care by nursing staff. Infection prevention and control is an imperative. The suite is operational from about 7.30 am to 4.30 pm, although staff are generally present for slightly longer periods of time.
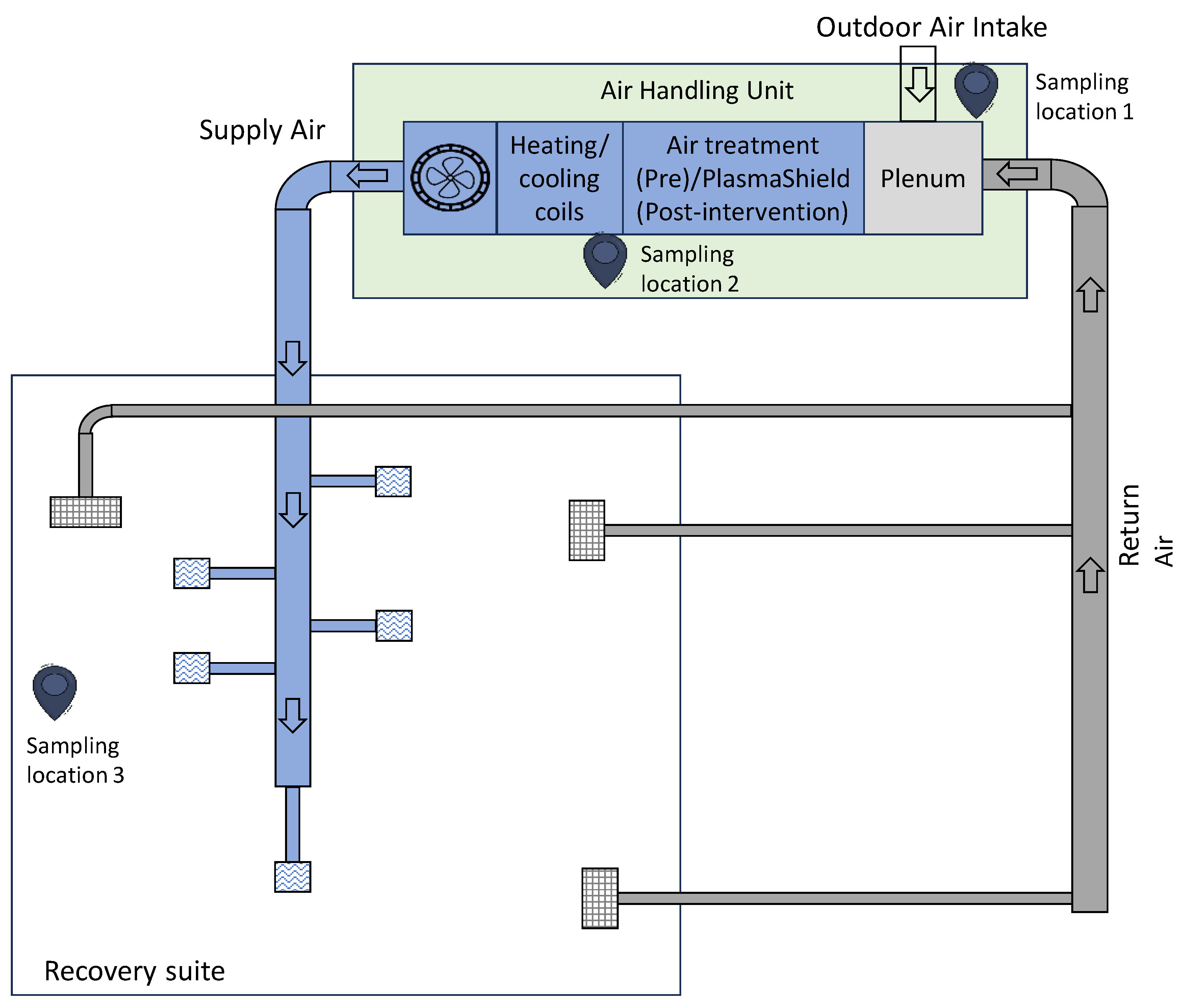
Figure 1 is a simplified schematic diagram of HVAC layout.
It also indicates the three main sampling points for (1) outdoor air intake, (2) supply air and (3) room air.
2.2. Pre-and Post-Intervention Trial
Each arm of the trial was conducted over a three-day period. The three-day span allowed for differing weather conditions and inter-day exposures. The HVAC system operated 24 hrs per day, and periods between 7am and 7pm were classified as work periods whereas 7 pm - 7 am were classified as night-time periods (unoccupied area).
The pre-intervention monitoring period was 9/08/2023 to 11/08/2023. During this period, the standard MERV-13 filter was in place. The MERV-13 filter has a 50-75% particle collection efficiency for 0.3-1.0μm, >90% for particles 1.0-3.0μm, and >90% for 3-10 microns [
24,
26]
The post-intervention monitoring period was 06/09/2023- 08/09/2023.
Here, a bank of six MMD-600 PlasmaShield units substituted for the existing MERV-13 filter (see
Figure 2) that supplied air to the recovery unit.
2.3. Air Sampling
Continuous multi-size particle counting was conducted at the sampling points described above, along with assessment of other air quality parameters, including temperature, humidity, volatile organic compounds (VOC), formaldehyde and carbon dioxide. Supplementary measurements were also taken in the outside areas adjacent to the building housing the recovery suite, as well as in the recovery suite.
Calibrated instrumentation and standardised methods were used.
Periodic grab sampling of airborne culturable bacteria and fungi was also undertaken. One purpose of the grab sampling was to identify microbial species, complementing the generic airborne particle assessment.
2.3.1. Sampling methods
2.3.1.1. Air Quality Assessments (Non-Biological)
Airborne particle counts in one-minute intervals at the three locations were measured continuously and simultaneously using TSI AeroTrak Handheld Particle Counters (Model 9306 v2).
Particle numbers in the range of 0.3-10 μm were measured, i.e. 0.3, 0.5, 1, 3, 5 and 10 μm. The particle number concentrations were the number of particles counted per cubic metre of air.
On a size basis, particles could be broadly classified as virus-like (VLP, 0.3 – 0.5 um), and bacteria-like (BLP 0.5 – 5 µm
post hoc [
27].
This size range fits well within the published size distributions of exhaled viruses and bacteria, as well as with aerosol measurements observed during vocalisation in an experimental setting [
28].
A handheld TSI DustTrak DRX was also used periodically as a complementary device at the various locations in the recovery suite and external to the building.
Carbon dioxide (CO2), temperature, humidity, and carbon monoxide (CO) were measured continuously using TSI Q-Track 7575 multifunction air quality monitors. Spot checks of nitrogen dioxide utilised a Aeroqual - Series 500 instrument. Spot checks for ozone and sulphur dioxide utilised a InDevR 2B Technologies 202 instrument and an Industrial Scientific MX6 Ibrid instrument respectively.
VOCs and formaldehyde were sampled over 6–8-hour periods using SKC Airchek XR5000 air sampling pumps with flow rates of 1 L/minute and 0.5 L/minute, respectively. Flow rates were checked with a Defender 510 calibrator. For VOCs, sampling was carried out using SKC sorbent tubes packed with coconut charcoal (Anasorb CSC, 226-09). For formaldehyde, SKC Sorbent Tubes (226-119) containing high purity silica gel treated with 2,4-dinitrophenyl hydrazine were employed.
VOCs and formaldehyde samples were analysed by TestSafe Australia using accredited standard methods.
Volumetric airflows through air supply registers were checked with a TSI 8380 Accubalance flow hood. Air movement (speed and directionality) in the occupied space was assessed using a Drager Cumulus air flow indicator. Real time VOC assessment was undertaken with a Ion Science Tiger XT photoionisation detector (10.6 eV).
Surface temperatures were assessed with a Digitech QM 7226 non-contact infrared thermometer.
Simultaneous monitoring of environmental parameters such as CO2 concentration, temperature, humidity, and CO levels provided a holistic view of the indoor and outdoor air conditions and the level of occupancy during the study.
2.3.1.2. Microbial Assessments
Microbial measurements were undertaken by Bacterial and Mould Services, an expert microbial exposure assessment company. Air grab samples were taken for bacterial and fungal assessment, pre and post intervention, daily in the morning and in the afternoon, from the outside air, in the outside air intake, supply air and inside the recovery suite. Five hundred litre air samples were taken with a Spin Air sampler (IUL5532) on to separate bacterial and fungal media.
Incubation was conducted over 3 days for bacterial load at 30-35 °C and over 5 days for the fungal load at 26-28°C. Basic identification techniques were subsequently used.
Bacterial, and fungal load were expressed as colony-forming units per cubic metre (CFU/m3). For microbial assessment, bacterial and fungal load were combined.
2.4. Statistics
Statistical assessment was mainly of a descriptive nature stating summary statistics for particle number concentrations using the six pre-described size ranges by time of the day, during pre-and post-intervention.
Box and whisker plots were used to describe the distribution in particle number concentrations for VLP and BLP.
Reduction in particle number concentrations (VLP and BLP) in the occupied recovery suite was expressed as percent reduction from pre-intervention to post intervention. The non-parametric Wilcoxon signed-rank test was used to test the significance of differences, utilising Stata 18 software [
29].
3. Results
3.1. General Observations
The recovery suite was essentially an open room space of 220 m2 and a volume of approximately 660 m3. Air to the recovery suite was supplied at 4300 m3/hr, giving a theoretical air exchange rate of 6.5 ACH on total air and 1.6 ACH based on outside air.
Assessment of air mixing with the smoke generator indicated reasonable air mixing, with air flowing from the recovery suite to the corridor adjacent to the operating theatres.
There were no obvious odours. However, staff were using disinfectant wipes and placing them into open bins, potentially contributing to VOC levels. This was confirmed by spot checks with the photoionisation detector, and also with the results of the 8-hr sampling with charcoal tubes. The key VOCs were ethyl alcohol and isopropyl alcohol.
Parts per million levels of VOCs were recorded around the rubbish bins. Very low levels (<0.05 ppm) were recorded in outside areas and in the plant room.
Room temperature was around 25°C and relative humidity ranged from 27-47%.
3.2. Airborne Particles
Raw graphical data for each of the six size channels (
Figures S1–S12), and detailed statistics (
Table S1) are provided in the Supplementary Material and tabulated summary data are given in
Table 1 below.
Table 1 summarizes the VLP and the BLP-sized particle concentrations for outdoor air, supply air and the occupied space air during pre-and post-intervention, for all day (total), during work shifts and during night-times, by the mean, the standard deviation (SD) and the median concentrations of particles per cubic metre.
For both categories, VLP and BLP, particle concentrations were reduced for the total 24-hour day, during the work shift, and during night-time in relation to the supply and the recovery suite air when compared to outdoor air by a large magnitude. Outside air quality indicated higher VLP during pre-intervention, and higher BLP concentration during intervention.
The reduction in mean total particle number concentrations between the occupied room air before and after the intervention was of high magnitude for the channels measuring 0.3 μm (>14 fold), 0.5μm (6-fold) and 1μm (>2 fold). Using the mean values, the decrease of VLP in the occupied space post-intervention compared to pre-intervention during work-shifts was 8-fold and for BLP it was 2.7-fold.
For the particle sizes 3-10 μm, no further major reductions were achieved indicating that PlasmaShield, compared with MERV-13 is mostly relevant for reducing VLP and BLP particles.
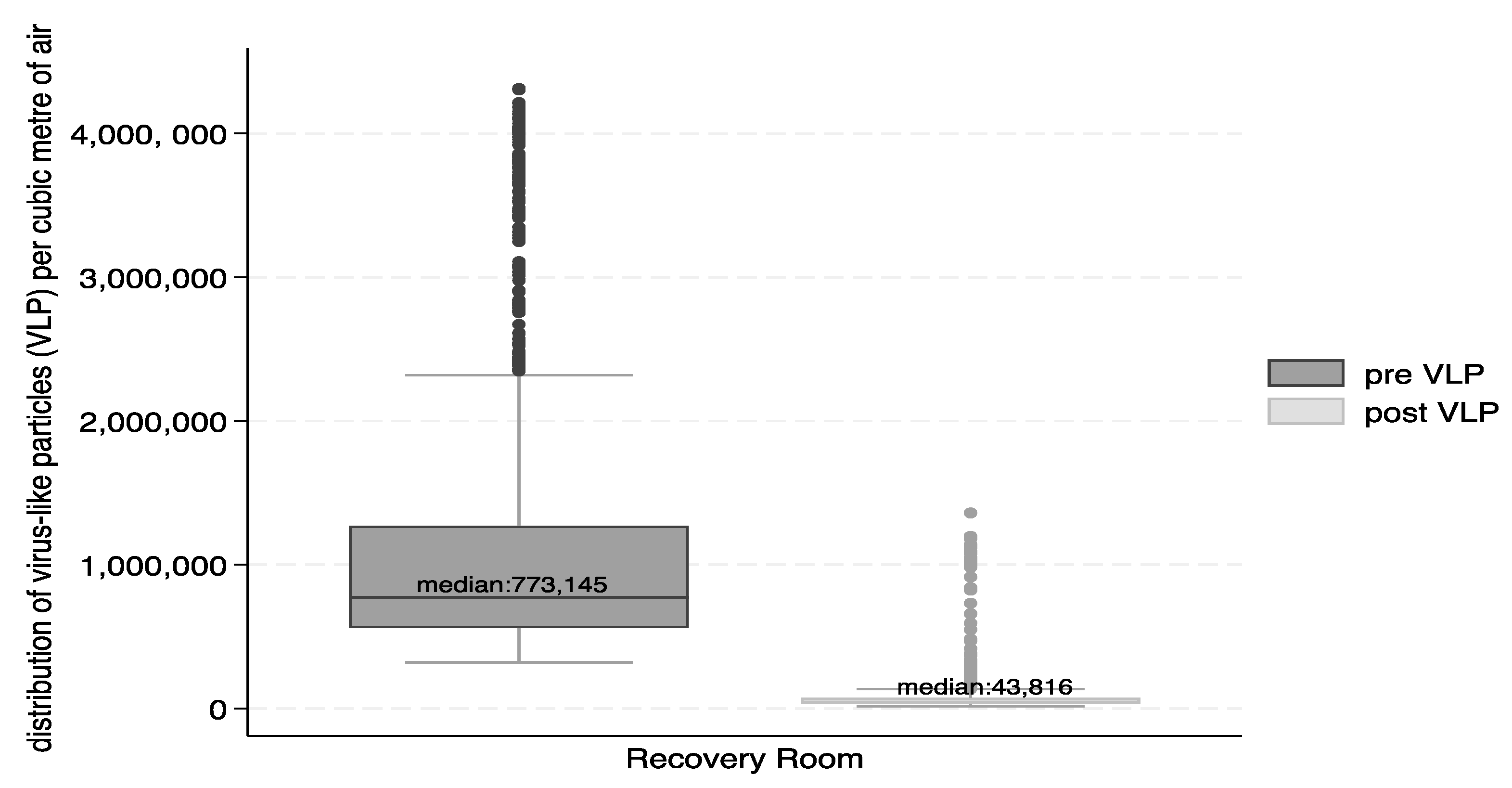
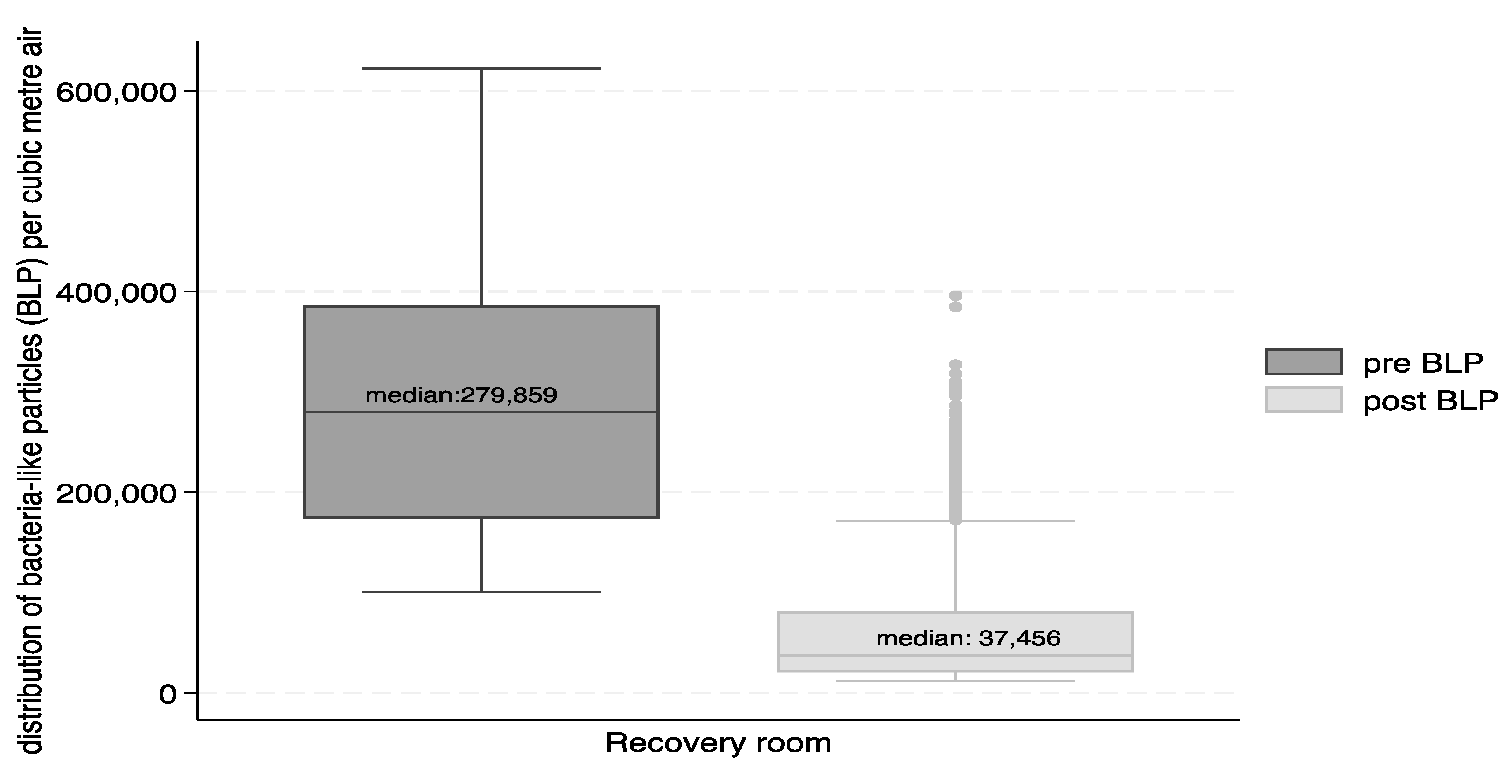
Figure 3 and
Figure 4 show the reductions in form of box and whisker plots. The median number of VLP particles per cubic metre of air were reduced from 773,145 to 43,816 and for BLP from 279,859 to 37,456.
The percent reduction for the total number of particles in the recovery suite between the pre- and post-intervention was 94% for VLP and 87% for BLP. The Wilcoxon sign-rank test indicated that the differences were significant (p<0.0001) for total VLP and BLP, as well as for VLP and BLP differences in the recovery suite during the work shifts and during night times.
3.3. Culturable Microbial Counts
For all grab samples, the types of bacteria and fungi cultured were common environmental saprophytes which are widespread throughout many environments and can be readily disseminated by ventilation systems and indoor occupants.
Compared with outdoor air, significant reductions in culturable bacteria and fungi were observed at the exit point of the PlasmaShield unit.
From
Table 2, it can be seen that in the case of bacteria the PlasmaShield unit was able to reduce the total microbial load by 98% compared to only 41% the original MERV-13.
In the case of fungi, PlasmaShield unit reduced the total microbial load by 96% compared to 90% the original MERV-13.
Overall, the PlasmaShield unit reduced the total microbial load by 96% compared to 71% in the original MERV-13.
When comparing PlasmaShield with the original MERV-13 filter, further reductions in culturable bacteria and fungi were observed in the occupied space but these were modest and variable and can be attributed to the human occupancy and activity as described in the Discussion.
3.4. VOCs, Formaldehyde, Ozone, Nitrogen Dioxide, Sulphur Dioxide, Carbon Monoxide and Carbon Dioxide
Volatile organic compound and formaldehyde concentrations were low, and the biggest contributions probably arose from the regular use of cleaning agents and disinfectants and off gassing from indoor surfaces.
3.4.1. Volatile Organic Compounds
VOC concentrations were low/ There was a slight reduction in VOCs post intervention in the recovery suite. Pre-intervention: 1282 µg/m3 (n=5); post-intervention: 1046 µg/m3 (n=5)
The VOCs were predominantly ethyl alcohol and isopropyl alcohol, deriving from the use of disinfecting agents and the work practice of disposing disinfectant materials in the open bins.
3.4.2. Formaldehyde
The measured values were low, below the odour threshold of approximately 50 µg/m3.
Pre-intervention: 7.6 µg/m3 (n=3) post-intervention: 8.3 µg/m3 (n=3)
The WHO IAQ guideline for formaldehyde is 100 µg/m
3 [
30].
3.4.3. Ozone
Ozone levels in the recovery suite were below the limit of detection (<1 ppb)
3.4.4. Nitrogen Dioxide, Sulphur Dioxide and Carbon Monoxide
The levels were low and below accepted standards. Nitrogen dioxide levels were typically less than 10 ppb (maximum was 30 ppb). Carbon monoxide and sulphur dioxide levels were generally too low for reliable detection (< 1 ppm and 0.1 ppm)
3.4.5. Carbon Dioxide
These were variable in the normal range and similar pre- and post-intervention (400-750 ppm).
As the predominant source of carbon dioxide are humans, the data indicated that the occupancy was similar both pre- and post-intervention.
4. Discussion
The PlasmaShield system is a new technology. Unlike UV/HEPA systems it uses electron beams and electric fields to inactivate bioaerosols and which also serve to aggregate very small particles so that they can be captured efficiently by filters that do not suffer from the large pressure drops associated with HEPA [
24]. It is the first study of such a device in a real-life setting, in this case, a post-operative paediatric clinical setting. As such, it adds to the existing evidence base on advanced air treatment systems that address the triple challenge of improved IAQ, infectious disease reduction and energy efficiency.
The trial was not able to clearly demonstrate reductions in VOCs, gases in the occupied space, as these were at very low levels, below accepted IAQ standards and odour thresholds. The activity and use of chemicals in the occupied space generated VOCs and bioaerosols.
There are a number of limitations to this study.
Surface sampling was not conducted, and it was not feasible to attach personal air sampling devices to nursing staff and monitor for a shift. Viruses were not directly measured and “grab” sampling for culturable airborne bacteria and fungi could only be conducted twice per day when there was reduced activity and occupancy. This is therefore a gross measure and unlikely to be representative of the average microbial load in the air.
Mitigating the limitations above, continuous real time multi-size particle counting was used providing rich and representative data, but without microbial speciation. Before and after measurements in the same environmental conditions with particle counting instrumentation is a robust reflection of the effectiveness of the intervention and has been used in multiple studies of air cleaning systems [
22,
24].
Evidence of transmission of respiratory viral disease in form of aerosols has been widely acknowledged [
4,
5,
7]. It has been recognised that virus- containing aerosols can be ejected during respiratory exhalations from proximity (close range, ballistic) to far distance (>2 m). Depending on the activities, particle number concentrations change with a significant difference between breathing and vocalisation, as well as based on the sound volume measured in decibels (dB) [
28]. Moreover, in an experimental study with children and adults, vocalisation seems to produce a bimodal aerosol size distribution with the size of the smaller particles being 0.50-0.64 μm in diameter, while the larger particles were around 1.39-1.94 μm [
28]. Aerosol collection experiments with COVID-19 patients found that SARS-CoV-2 RNA during vocalisation spanned 0.34->8.1μm with 90% of the particles being <4.5μm [
31]. A sub-micron range in particles virus ejection due to vocalisation has been supported elsewhere [
32]. This evidence plus research on outdoor air suggests that a focus of indoor clean air should be on the sub-micron particles [
33].
With the hindsight of the COVID-19 pandemic, and in the absence of any relevant guidance, ASHRAE recently developed clean air indoor standards that are relevant for infection risk [
34].
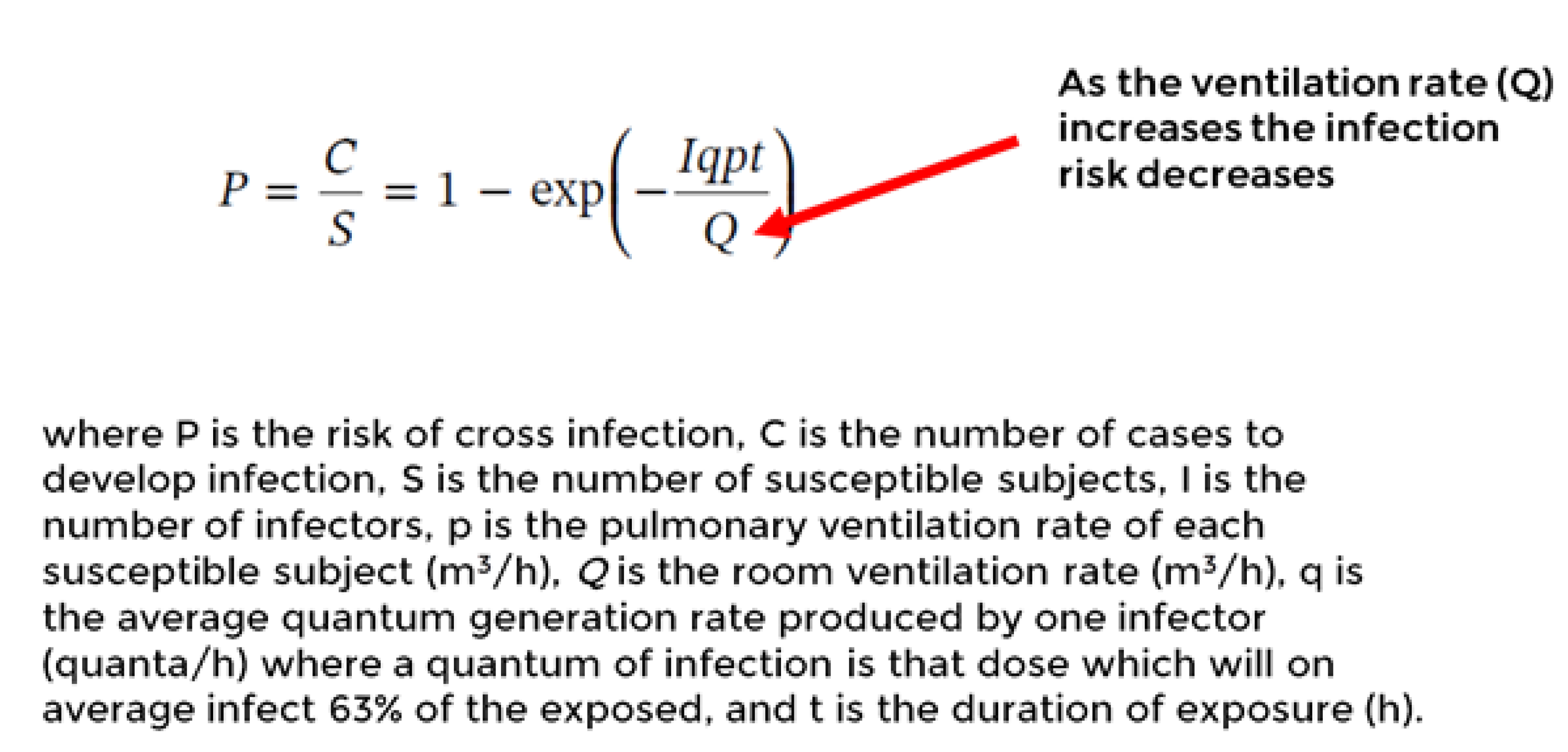
The Wells-Riley equation as in
Figure 5 can used to predict the relative infection risk reduction in this study [
23].
To evaluate the effectiveness of control measures, the eight-fold reduced VLP (886,302 vs 108,630 particles) particle number concentration post-intervention compared to pre- intervention during work shifts was assessed via Wells-Riley equation. Thus, it is inferred that the 8-fold post intervention reduction in VLP serves as the equivalent to an 8-fold increased ventilation rate (m
3/h) in clean air. Adding the increased ventilation rate into the Wells-Riley formula P=1-e
(-1/8) results in a 5.33 -fold risk reduction. This calculation provides an example of a solution for equivalent clean airflow through air cleaning devices as described in the new ASHRAE standard [
34]. In the case of BLP it is a 2.7-fold concentration reduction, which translates to about a two-fold risk reduction.
HEPA filters are widely used in critical environments affording more than 99% protection from sub-micron particles. However, as shown in a hypothetical office environment study, HEPA-filters compared to MERV-filters are costly due to high energy requirements to overcome airflow restrictions [
35]. Unforeseen environmental indoor aerosol production associated with the UV-C ionising lamps has also recently been raised [
36].
This study was not designed to demonstrate reductions in infectious health effects in patients, visitors, or staff. In future, health outcome improvements collected from a similar clinical setting or from a GP clinic would provide further validation of PlasmaShield. This is especially pertinent considering that there are an estimated 165,000 healthcare associated infections in Australia per year, many of them preventable [
37]. Furthermore, submicron airborne particles derived from general air pollution or major events, such as from wildfires, could be captured and removed from airstreams by this device which has an applicability far beyond the clinical care space. Further studies in buildings are needed to evaluate the health and productivity benefits for occupants.
5. Conclusions
Compared with a standard approach using MERV-13 filtration, the PlasmaShield air purifier incorporated into a HVAC system yields significant improvement in respect of submicron particles, bacteria and fungi. Performance with respect to particles is HEPA-like. Disinfection via electron beam irradiation also mitigates the risks associated with filter replacement and disposal.
The technology addresses a number of key challenges in IAQ. The multiple modes of action are of relevance for a variety of air contaminants, as evidenced by laboratory studies, with the potential for reduced energy costs.
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1, Figure S1–12: caption: The 12 time series figures describe the pre-and post-intervention particle number concentrations (/m
3 air) by the measurement channels for particles 0.3, 0.5, 1, 3, 5 and 10 µm. Work periods are indicated. Table S1: Mean and standard deviation (SD) of particles by channel size (0.3, 0.5, 1, 3, 5 and 10 µm) during pre and post intervention for outdoor, supply and recovery room air, summarized by the total time, by work hours and by night.
Author Contributions
Conceptualization, D.P.; methodology, D.P.; validation, D.P.; formal analysis, M.N.; investigation, D.P.; resources, D.P.; data curation, D.P., M.N.; writing—original draft preparation, M.N., D.P.; writing—review and editing, M.N., D.P.; project administration, D.P.; funding acquisition, N/A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable for studies not involving humans or animals. The participating hospital deemed that there are no ethics committee requirements as no human data were collected. Assessments of indoor air quality were conducted by an experienced occupational hygienist (D.P.) using standard procedures.
Informed Consent Statement
N/A
Data Availability Statement
Raw dataset supporting the conclusion will be available on request from the authors.
Acknowledgments
1. Support and access to facilities was provided by Mr. Jack Noonan, Director of Engineering and Capital Projects at the Women’s and Children’s Health Network. 2. We thank PlasmaShield for the supply of equipment for the evaluation.
Conflicts of Interest
No conflicts of interest are declared. A Public Health Partnership Agreement exists between the School of Public Health at the University of Adelaide and SA Health. The authors conducted research under this partnership in a hospital administered by SA Health.
References
- Allen, J.G.; MacNaughton, P.; Satish, U.; Santanam, S.; Vallarino, J.; Spengler, J.D. Associations of cognitive function scores with carbon dioxide, ventilation, and volatile organic compound exposures in office workers: A controlled exposure study of green and conventional office environments. Environ Health Perspect 2016, 124, 805–812. [Google Scholar] [CrossRef]
- Morawska, L.; Marks, G.B.; Monty, J. Healthy indoor air is our fundamental need: the time to act is now. Med J Aust 2022, 217, 578–581. [Google Scholar] [CrossRef] [PubMed]
- Bolten, A.; Kringos, D.S.; Spijkerman, I.J.B.; Sperna Weiland, N.H. The carbon footprint of the operating room related to infection prevention measures: a scoping review. J Hosp Infect 2022, 128, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Morawska, L.; Milton, D.K. It is time to address airborne transmission of coronavirus disease 2019 (COVID-19). Clin Infect Dis 2020, 71, 2311–2313. [Google Scholar] [CrossRef] [PubMed]
- Nissen, K.; Krambrich, J.; Akaberi, D.; Hoffman, T.; Ling, J.; Lundkvist, A.; Svensson, L.; Salaneck, E. Long-distance airborne dispersal of SARS-CoV-2 in COVID-19 wards. Sci Rep 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global technical consultation report on proposed terminology for pathogens that transmit through the air.; Geneva, 2024. Available online: https://cdn.who.int/media/docs/default-source/documents/emergencies/global-technical-consultation-report-on-proposed-terminology-for-pathogens-that-transmit-through-the-air.pdf?sfvrsn=de07eb5f_1&download=true (accessed on 17 May 2024).
- Tang, J.; Tellier, R.; Yuguo, L. Hypothesis: All respiratory viruses (including SARS-C0V-2) are aerosol-transmitted. Indoor Air 2022, 32:e12937. [CrossRef]
- Christie, B. COVID-19: Bring back mandatory mask wearing in health settings, say Scottish workers. BMJ 2023, 382, 1648. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C. Concerns over catching covid-19 in hospital. New Sci 2022, 255. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention (CDC 2023). Ventilation in Buildings. Available online: https://www.cdc.gov/coronavirus/2019-ncov/community/ventilation.html (accessed on 17 May 2024).
- World Health Organization. Roadmap to improve and ensure good indoor ventilation in the context of Covid-19.; Geneva, 2021. Available online: https://www.who.int/publications/i/item/9789240021280. (accessed on 17 May 2024).
- Victorian Department of Health. 2024. Ventilation: Guidelines for optimising ventilation to reduce the risk of transmission of COVID-19 in healthcare settings. 2024. Available online: https://www.health.vic.gov.au/covid-19-infection-prevention-control-guidelines/ventilation (accessed on 17 May 2024).
- National Health and Medical Research Committee (NHMRC.) The role of airflow and ventilation in relation to SARS-CoV-2 transmission in quarantine assessment. In National COVID-19 Health and Research Advisory Committee, Australian Government: Canberra, 2021. URL: https://www.nhmrc.gov.au/sites/default/files/2023-02/NCHRAC-Advice-18-Airflow-and-ventilation-in-quarantine-arrangements.
- Standards Australia, AS 4260-1997 High efficiency particulate air (HEPA) filters —Classification,construction and performance. Available online: https://www.standards.org.au/standards-catalogue/standard-details?designation=as-4260-1997. (accessed on 17 May 2024).
- International Agency for Research and Cancer (IARC) 2016. Outdoor air pollution. IARC Scientific Publication No. 16. Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Outdoor-Air-Pollution-2015. (accessed on 17 May 2024).
- Fernández de Mera, I.G.; Granda, C.; Villanueva, F. et al. HEPA filters of portable air cleaners as a tool for the surveillance of SARS-CoV-2. Indoor Air. 2022; 32:e13109. [CrossRef]
- Australian Commission on Safety and Quality in Health Care. Optimising ventilation for infection prevention and control in healthcare settings. 2022. Available online. URL https://www.safetyandquality.gov.au/our-work/infection-prevention-and-control/optimising-ventilation-infection-prevention-and-control-healthcare-settings.
- Li P., K.J., Macedo N, Zimmerman JJ, Wrzesinski D, Sobotka E, Balderas M, Walz WB, Paris RV, Lee M, Liu D, Yedilbayev B, Ramirez BC, Jenks WS. Evaluation of an air cleaning device equipped with filtration and uv: comparison of removal efficiency on particulate matter and viable airborne bacteria in the inlet and treated air. Int J Environ Res Public Health 2022, 19, 16135. [Google Scholar] [CrossRef] [PubMed]
- Stawicki, S.P.; Wolfe, S.; Brisendine, C.; Eid, S.; Zangari, M.; Ford, F.; Snyder, B.; Moyer, W.; Levicoff, L.; Burfeind, W.R. The impact of comprehensive air purification on patient duration of stay, discharge outcomes, and health care economics: A retrospective cohort study. Surgery 2020, 168, 968–974. [Google Scholar] [CrossRef]
- Urrutia, A.R.; Schlener, S.D.; Eid, S.; Bock, K.A.; Worrilow, K.C. The effects of an advanced air purification technology on environmental and clinical outcomes in a long-term care facility. J Gerontol A Biol Sci Med Sci 2023, 78, 2325–2332. [Google Scholar] [CrossRef]
- Arikan, I.; Genc, O.; Uyar, C.; Tokur, M.E.; Balci, C.; Percin Renders, D. Effectiveness of air purifiers in intensive care units: an intervention study. J Hosp Infect 2022, 120, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Brainard, J.; Jones, N.R.; Swindells, I.C.; Archer, E.J.; Kolyva, A.; Letley, C.; Pond, K.; Lake, I.R.; Hunter, P.R. Effectiveness of filtering or decontaminating air to reduce or prevent respiratory infections: A systematic review. Prev Med 2023, 177, 107774. [Google Scholar] [CrossRef] [PubMed]
- Noakes, C.J.; Sleigh, P.A. (2008) Applying the Wells-Riley equation to the risk of airborne infection in hospital environments: the importance of stochastic and proximity effects. Proceedings of the11th International Conference on Indoor Air Quality. Indoor Air 2008, 17-22 Aug 2008, Copenhagen, Denmark.Available online :URL https://eprints.whiterose.ac.uk/7702/. (accessed 17/May/2024).
- Lau, T.; Belusko, M. Airborne particulate matter filtration using non-thermal plasma air purification. ASHRAE Journal 2023, 65 (12) 38-48.
- Whiley, H.; Keerthirathne, T.; Kuhn, E. .; Nisar, M.; Sibley, A.; Speck, P.; Ross, K.E. Efficacy of the Plasmashield®, a non-thermal, plasma-basedair purification device, in removing airborne microorganisms. Electrochem 2022, 3, 276–284. [Google Scholar] [CrossRef]
- ANSI/ASHRAE. Standard 52.2-2017: Method of testing general ventilation air-cleaning devices for removal efficiency by particle size; 2017. Available online: https://www.ashrae.org/File%20Library/Technical%20Resources/COVID-19/52_2_2017_COVID-19_20200401.pdf. (accessed on 17 May 2024).
- Prussin, A.J., 2nd; Garcia, E.B.; Marr, L.C. Total virus and bacteria concentrations in indoor and outdoor air. Environ Sci Technol Lett 2015, 2, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Archer, J.; McCarthy, L.P.; Symons, H.E.; Watson, N.A.; Orton, C.M.; Browne, W.J.; Harrison, J.; Moseley, B.; Philip, K.E.J.; Calder, J.D.; et al. Comparing aerosol number and mass exhalation rates from children and adults during breathing, speaking and singing. Interface Focus 2022, 12. [Google Scholar] [CrossRef] [PubMed]
-
Stata Statistical Software: Release 18; StataCorp LLC: College Station, TX, 2023.
- World Health Organization. WHO guidelines for indoor air quality: selected pollutants; WHO, 2010.
- Alsved, M.; Nygren, D.; Thuresson, S.; Fraenkel, C.J.; Medstrand, P.; Londahl, J. Size distribution of exhaled aerosol particles containing SARS-CoV-2 RNA. Infect Dis (Lond) 2023, 55, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Shah, Y. ,;K. J., Peterson, S.D.; Yarusevych, S. Experimental investigations of indoor aerosol dispersion and accumulation in the context of COVID-19: Effects of masks and ventilation. Phys Fluids 2021, 33. [Google Scholar] [CrossRef] [PubMed]
- Schraufnagel, D.E. The health effects of ultrafine particles. Exp Mol Med 2020, 52, 311–317. [Google Scholar] [CrossRef]
- American Society of Heating, Refrigerating and Air Conditioning Engineers (ASHRAE). ASHRAE Standard 241 Factsheet, Control of Infectious Aerosols; Washington, 2023. Available online: https://www.ashrae.org/file%20library/about/government%20affairs/advocacy%20toolkit/virtual%20packet/standard-241-fact-sheet.pdf. (accessed on 17 May 2024).
- Azimi, P.; Stephens, B. HVAC filtration for controlling infectious airborne disease transmission in indoor environments: Predicting risk reductions and operational costs. Building and Environment 2013, 70, 150–160. [Google Scholar] [CrossRef]
- Graeffe, F., L. Y., Guo, Y., Ehn, M. Unwanted indoor air quality effects from using ultraviolet c lamps for disinfection. Environ Sci Technol Lett 2023, 10. [Google Scholar] [CrossRef]
- Mitchell, B.G.; Shaban, R.Z.; MacBeth, D.; Wood, C.J.; Russo, P.L. The burden of healthcare-associated infection in Australian hospitals: A systematic review of the literature. Infect Dis Health 2017, 22, 117–128. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).